Abstract
Background
Kudiezi injection is a traditional Chinese medicine for acute cerebral infarction, but the exact mechanisms are poorly understood.
Objective
To investigate the mechanisms of Kudiezi injection on the inflammatory response in the treatment of acute cerebral infarction.
Methods
This was a prospective study of patients with acute cerebral infarction within 48 h of onset and treated between July 2012 and July 2016 at three hospitals in China. The patients were randomized to routine treatments (control group) versus routine treatments and Kudiezi injection (Kudiezi group). The National Institutes of Health Stroke Score was assessed on days 1, 3, 5, 7, and 14. The patients were tested for serum levels of pro- and anti-inflammatory cytokines (S100 calcium-binding protein B, neuron-specific enolase, interleukin-6, interleukin-10, interleukin-18, and matrix metaloproteinase-9; by enzyme-linked immunosorbent assay) immediately after admission and on days 3, 5, and 14.
Results
Stroke scores were improved in both groups from days 1 to 14. On days 5 and 7, stroke scores in the Kudiezi group were lower than in the control group (P < 0.05). Compared with controls, the Kudiezi group had lower serum S100 calcium-binding protein B on day 14; higher interleukin-6 and interleukin-10 on day 3; lower interleukin-6 and interleukin-18 on day 5; and lower interleukin-18 and matrix metaloproteinase-9 on day 14.
Conclusion
Kudiezi injection could lead to early reduction of interleukin-6, interleukin-18, matrix metaloproteinase-9, neuron-specific enolase, and S100 calcium-binding protein B levels and increases of interleukin-10 levels in patients with acute ischemic stroke. This trial is registered with ClinicalTrials.gov NCT01636154.
1. Introduction
Inflammation plays a key role in the pathogenesis of acute cerebral infarction and leads to brain damage [1, 2]. Numerous cytokines, both pro- and anti-inflammatory, are involved in this process. Interleukin- (IL-) 6 is one of the most important proinflammatory cytokines; it is expressed within a few hours after cerebral ischemia and is an acknowledged biomarker for long- and short-term neurological outcomes after ischemic stroke [3, 4]. IL-18 is a proinflammatory cytokine that stimulates the production of a large number of inflammatory factors after cerebral ischemia; it is also involved in brain edema and damage [5]. On the other hand, IL-10 is an anti-inflammatory cytokine that inhibits the inflammatory response, playing protective effects after stroke [6, 7].
Markers are also available to measure the extent of neurological damage after stroke. Indeed, changes of serum S100 calcium-binding protein B (S100B) levels can reflect the severity of neuroglial cell injury [8]. Serum neuron-specific enolase (NSE) is a specific indicator of brain neuronal damage and necrosis. High levels of serum S100B and NSE are associated with disease severity and poor patient prognosis [9, 10].
Kudiezi injection is the first-line traditional Chinese medicine (TCM) treatment for acute cerebral infarction and has been shown to improve the outcomes of patients with stroke [11–13]. The Kudiezi injection is a preparation made of Ixeris sonchifolia (Bge.) Hance and is made using the whole herb. In clinical practice, it is mainly used for the treatment of coronary heart disease, angina, and cerebral infarction. Its main components are amino acids, flavonoids, saponins, and sesquiterpene lactones [14–16]. Sesquiterpene lactones are considered as the major active compounds in Kudiezi injection because of their special structures and activities [15]. Nevertheless, the exact mechanisms of action of Kudiezi injection remain elusive. The protection mechanism of Kudiezi injection against ischemic reperfusion (I/R) cerebral damage may possibly be related to the biosynthesis of phenylalanine, tyrosine, and tryptophan [17]. KDZ protects the blood-brain barrier from disruption and improves cerebral outcomes following I/R by preventing the degradation of tight junction proteins, increasing caveolin-1 expression, and inhibiting p-caveolin-1 and p-Src, probably because of the ability of its main ingredients to bind to Src and inhibit its phosphorylation [18]. KDZ protects the brain against acute focal ischemic injury in vivo and in vitro. The underlying mechanisms might be associated with the anti-inflammatory effect of KDZ through the TLR4/NF-κB signaling pathway [19]. Studies have shown that Kudiezi injection can reduce inflammatory markers such as IL-1β, IL-6, tumor necrosis factor (TNF)-α, C-reactive protein (CRP), toll-like receptor 4 (TLR4), and NADPH oxidase 4 (NOX4) in patients with acute cerebral infarction [12, 13, 20, 21]. Animal studies also showed that Kudiezi injection can downregulate the expression of nuclear factor (NF)-κB in the cerebral cortex of rats with acute cerebral infarction and modulate the expression of a number of proteins involved in neuronal damage [20, 22]. Moreover, Kudiezi injection downregulates the expression of adhesion molecules (intercellular adhesion molecule- (ICAM-) 1 and vascular cell adhesion molecule- (VCAM-) 1) involved in the recruitment of inflammatory cells in human brain microvascular endothelial cells injured by high glucose concentrations [23]. In addition, the balance between oxidative stress and antioxidant capacity is involved in the extent of damage after ischemic stroke [24]. Kudiezi injection has been shown to shift the balance toward decreased oxidative stress in rats [25].
In order to improve our understanding of the mechanisms of Kudiezi injection in the treatment of stroke, patients with cerebral infarction within 48 h of onset were recruited to investigate the effects of Kudiezi injection on the inflammatory response during the treatment of acute cerebral infarction.
2. Study Subjects and Methods
2.1. Study Design and Patients
This was a prospective study of patients with acute cerebral infarction within 48 h of onset and treated between July 2012 and July 2016 (ClinicalTrials.gov NCT01636154). This study was approved by the Ethics Committee of Dongfang Hospital, Beijing University of Chinese Medicine.
The study patient population was from the Dongfang Hospital affiliated to Beijing University of Chinese Medicine, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, and Beijing Huairou District Hospital of Chinese Medicine. The inclusion criteria were (1) diagnosis of acute cerebral infarction according to the “Chinese treatment guidelines of acute ischemic stroke 2010” [26]; (2) ≤48 h from onset; (3) National Institutes of Health Stroke Scale (NIHSS) scores of 5–25 points [27]; (4) male or female patients of ≥18 years of age; and (5) provided informed consent. The exclusion criteria were (1) cardiogenic brain embolism, cerebral infarction due to other causes, or unknown causes; (2) eligible and ready for thrombolytic therapy; (3) eligible and ready for endovascular treatment or had already undergone endovascular treatment; (4) severe systemic diseases of the heart, lung, liver, or kidney (alanine transaminase (ALT) or aspartate transaminase (AST) >2 times the upper limit of normal, creatinine > 1.5 times the upper limit of normal, acute attack of asthma or chronic obstructive pulmonary disease (COPD), or heart function of grade IV); (5) hemorrhage or tendency to bleed; (6) pregnant or lactating women; (7) limb dysfunction, mental disorders, or cognitive impairment affecting adequate patient evaluation; (8) allergy or contraindications to Kudiezi injection or its components; or (9) participated in another clinical trial within 3 months.
2.2. Grouping
Randomization was performed using a central computer system. The recruited patients were randomized to the routine treatment group (control group) and Kudiezi injection group (KDZ group).
2.3. Treatments
Treatments for improving cerebral blood circulation (including antiplatelet, anticoagulant, fibrinolysis, and dilation) and neuroprotective therapy were performed for patients in the control group according to the “Chinese treatment guidelines of acute ischemic stroke 2010” [26]. TCM injection, oral administration of TCM decoctions, and acupuncture were not performed.
The patients in the KDZ group received the same routine treatments as in the control group, as well as intravenous infusion of Kudiezi injection (Tonghua Huaxia Pharmaceutical Co. Ltd., Jilin, China). Kudiezi injection (40 ml in 250 ml of 0.9% sodium chloride) was intravenously injected once daily at 40 drops/min for 14 days.
2.4. Concomitant Medications
Drugs that had to be administrated due to comorbidities could remain unchanged, based on the physicians' experience. Any TCM that would affect the adequate evaluation of Kudiezi injection was prohibited.
2.5. Data Collection
Demographic data (age, gender, height, weight, previous disease history, drugs, smoking, and drinking) were collected. General physical examination (body temperature, breathing, resting heart rate, and blood pressure) was performed at admission.
2.6. Clinical Outcomes
NIHSS score was assessed on days 1, 3, 5, 7, and 14 [27]. Peripheral venous blood was sampled in the fasting state on days 1, 3, 5, and 14 to measure S100B, NSE, IL-6, IL-10, IL-18, and matrix metalloproteinase- (MMP-) 9 levels using commercial ELISA kits.
2.7. Blood Sampling
Peripheral venous blood (4 ml) was sampled from each patient in the fasting state, in the morning, on days 1, 3, 5, and 14 after admission. The blood was clotted at room temperature for 2 h and centrifuged at room temperature at 3000 rpm for 10 min. The supernatant was aliquoted (200 μl/vial) and stored at −80°C. Repeated freeze thawing was avoided.
2.8. ELISA
S100B, NSE, IL-6, IL-10, IL-18, and MMP-9 were measured according to the kit's instructions. The absorbance of each well was measured at 450 nm using a microplate reader. The human IL-18 ELISA kit (D7620; R&D Systems, Minneapolis, MN, USA), human IL-10 ELISA kit (D1000B; R&D Systems, Minneapolis, MN, USA), human MMP9 ELISA kit (D6000; R&D Systems, Minneapolis, MN, USA), human S100B ELISA kit (DY1820-05; R&D Systems, Minneapolis, MN, USA), and human NSE ELISA kit (DENL20; R&D Systems, Minneapolis, MN, USA) were used.
2.9. Statistical Analysis
Statistical analysis was performed using the FAS population and GraphPad Prism version 6.0 (GraphPad Software Inc., San Diego, CA, USA). Categorical data were presented as frequency, percentage, or ratio and analyzed using the chi-square test. Continuous data were presented using means ± standard deviations (SD) and analyzed using the Student t-test. Two-sided P values < 0.05 were considered statistically significant.
3. Results
3.1. Characteristics of the Patients
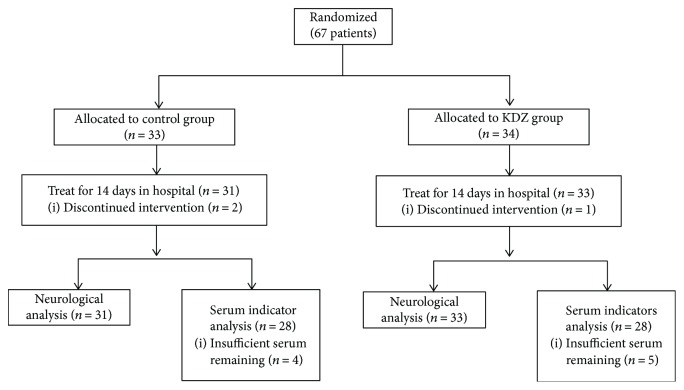
Figure 1 presents the patient flowchart. Sixty-seven patients were randomized to the control (n = 33) and KDZ (n = 34) groups. Two patients discontinued intervention in the control group and one in the KDZ group because they refused to repeat blood sample collection. Neurological assessment was performed in 31 and 33 patients in the control and KDZ groups, respectively. After excluding those with missing data or blood samples, 28 patients were analyzed in each group. The baseline characteristics of the patients are shown in Table 1. There were no differences between the two groups regarding age, gender, risk factors, complications, and distribution of stroke subtypes (all P > 0.05).
Figure 1.
Patient flowchart.
Table 1.
Baseline characteristic of the patients.
| Controls | KDZ | P | |
|---|---|---|---|
| n | 28 | 28 | |
| Age, mean (years) | 62.0 ± 12.1 | 62.2 ± 16.0 | 0.963 |
| Gender (F : M) | 11 : 17 | 10 : 18 | 1.000 |
| Risk factors, n (%) | |||
| Hypertension | 15 (53.6) | 17 (60.7) | 0.787 |
| Diabetes | 9 (32.1) | 9 (32.1) | 1.000 |
| Hypercholesterolemia | 9 (32.1) | 7 (25.0) | 0.554 |
| Dyslipidemia | 9 (32.1) | 7 (25.0) | 0.768 |
| Stroke history | 6 (21.4) | 4 (14.3) | 0.729 |
| Smoking | 27 (96.4) | 28 (100.0) | 0.313 |
| Complications, n (%) | |||
| Chronic kidney disease | 1 (3.6) | 2 (7.1) | 1.000 |
| Chronic lung disease | 2 (7.1) | 1 (3.6) | 1.000 |
| Coronary heart disease | 5 (17.9) | 3 (10.7) | 0.705 |
| Atrial fibrillation | 1 (3.6) | 1 (3.6) | 1.000 |
| Valvular heart disease/peripheral arterial disease | 0/0 | 0/0 | — |
| Stroke subtypes, n (%) | |||
| Large-artery atherosclerosis | 3 (10.7) | 1 (3.6) | |
| Small blood vessel occlusion | 12 (42.9) | 14 (50.0) | |
| Other or unknown causes | 13 (46.4) | 13 (46.4) | |
3.2. Effects of Kudiezi Injection on Neurological Deficits
In both groups, NIHSS score was improved from day 1 to day 14 (both P < 0.05). There were no differences in NIHSS scores between the two groups on days 1, 3, and 14 (all P > 0.05), but on days 5 and 7, NIHSS scores in the KDZ group were lower than in the control group (day 5: P = 0.030; day 7: P = 0.042) (Figure 2).
Figure 2.
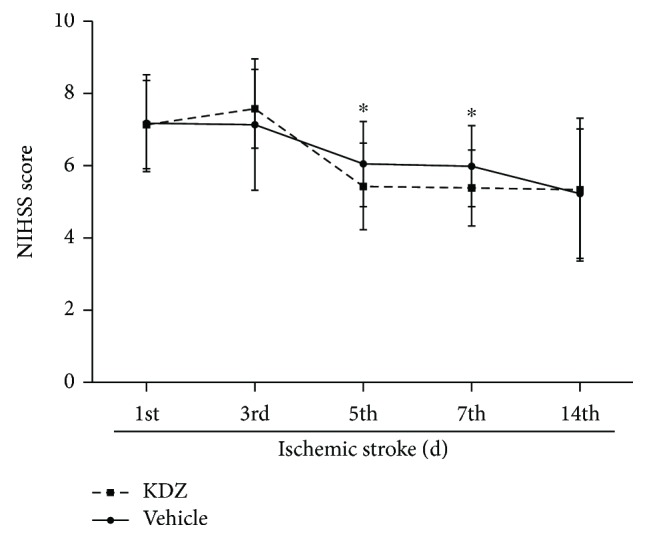
NIHSS score after acute ischemic stroke. NIHSS scores were decreased in both groups from day 1 to day 14. There were no differences between the two groups, except on days 5 and 7. n = 28/group. ∗P < 0.05 versus day 1. NIHSS: National Institutes of Health Stroke Scale.
3.3. Effects of Kudiezi Injection on Serum S100B and NSE
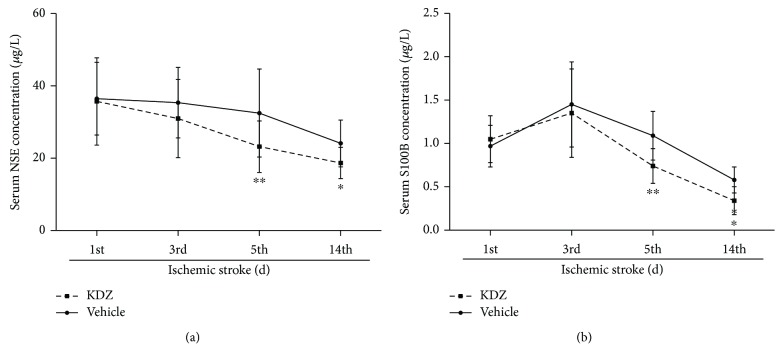
NSE and S100B levels decreased in both groups from day 1 to day 14 (all P < 0.05). There were no differences of serum levels of NSE and S100B between the two groups on days 1 and 3 (all P > 0.05). From day 5, the KDZ group showed lower serum levels of NSE and S100B compared with controls (NSE: P = 0.001 and P = 0.0006; S100B: P < 0.0001 and P < 0.0001) (Figure 3).
Figure 3.
Serum NSE and S100B levels in acute ischemic stroke patients. (a) NSE levels. (b) S100B levels. n = 28/group. ∗P < 0.05, ∗∗P < 0.01, t-test, versus the control group at the same time point. NSE: neuron-specific enolase; S100B: S100 calcium-binding protein B; KDZ: Kudiezi injection.
3.4. Effects of Kudiezi Injection on Serum Inflammatory Factors
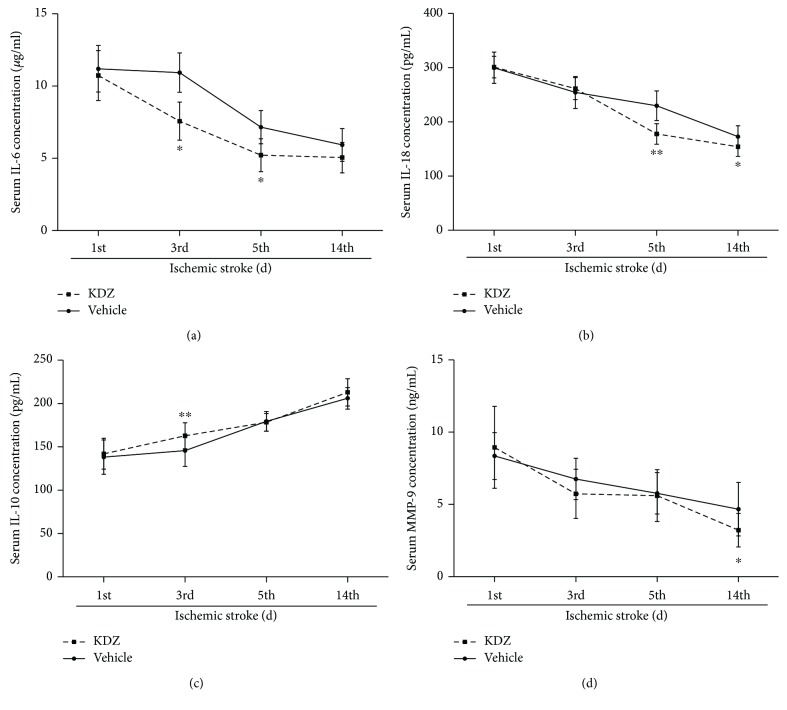
From day 1 to day 14, the levels of IL-6, Il-18, and MMP-9 decreased in both groups, while IL-10 levels increased (all P < 0.05). Compared with the control group, IL-6 levels were lower in the KDZ group on days 3, 5, and 14 (P < 0.0001, P < 0.0001, and P = 0.0006); IL-18 levels were lower in the KDZ group on days 5 and 14 (P < 0.0001 and P = 0.0005); IL-10 levels were higher in the KDZ group on day 3 (P = 0.0003); and finally, MMP-9 levels were lower on days 3 and 14 (P = 0.02 and P = 0.0009) (Figure 4).
Figure 4.
Serum inflammatory markers in acute ischemic stroke patients. (a) IL-6 levels. (b) IL-18 levels. (c) IL-10 levels. (d) MMP-9 levels. n = 28/group. ∗P < 0.05, ∗∗P < 0.01, t-test, versus the control group on the same time point. IL: interleukin; MMP: matrix metalloproteinase; KDZ: Kudiezi injection.
3.5. Correlations
On day 5, the NIHSS scores were positively correlated with IL-18 (r = 0.329, P = 0.013) and S100B (r = 0.379, P = 0.004). On day 14, the NIHSS scores were negatively correlated with IL-10 (r = −0.447, P = 0.013). No other correlations were observed between NIHSS scores at different time points and biomarkers.
4. Discussion
Kudiezi injection is the first-line traditional Chinese medicine treatment for acute cerebral infarction, but its exact mechanisms of action are still poorly understood. Therefore, this study aimed to investigate the effects of Kudiezi injection on the inflammatory response in the treatment of acute cerebral infarction. The results showed that Kudiezi injection could significantly decrease serum IL-6, IL-18, MMP-9, NSE, and S100B levels and increase IL-10 levels in patients with acute stroke. These changes occurred earlier than with conventional stroke treatment.
S100B is mainly released by astrocytes [28]. Under physiological conditions, S100B is a neurotrophic cytokine that regulates intracellular signals, cell structure, and energy metabolism [29]. In addition, S100B is closely associated with glial cell proliferation, axonal growth, and calcium homeostasis, but excessive S100B levels lead to increased release of proinflammatory cytokines such as IL-6, IL-1β, and TNF-α. High S100B levels also lead to increased inflammatory stress-related enzymes such as inducible nitric oxide synthase and upregulated NF-κB pathway, leading to neuronal death [30]. Hence, as a clinical biomarker, S100B, is highly sensitive for brain tissue damage. Indeed, in patients with mild brain injury, CT scan may be normal but serum S100B levels may be elevated significantly [31]. Elevated S100B is a marker of poor prognosis. In stroke, S100B is released in the cerebrospinal fluid at the beginning of the ischemic period and enters the bloodstream, where the levels peak within 24–120 h after ischemia [8]. NSE is a glycolytic enzyme found primarily in neuroendocrine cells and neuronal cytoplasm and is a marker of acute brain injury severity and clinical status [32]. NSE levels in the cerebrospinal fluid are elevated after cerebral ischemia. Serum NSE levels can be detected in the initial stage of the disease, peaking within 24–96 h after ischemia, and showing a decreasing trend when the symptoms disappear [33]. Serum NSE can be used as a marker of neurological function after stroke [34]. A previous animal study showed that Kudiezi injection could downregulate NF-κB-related neuronal death after ischemic injury [19]. This could explain why NSE and S100B levels started to decrease earlier in the Kudiezi group than in the control group.
Serum levels of NSE and S100B in patients with acute cerebral infarction are positively correlated with the NIHSS [29]. In the present study, serum NSE and S100B in the KDZ group were decreased 5 days after admission and they remained lower than in the control group until the end of treatment (day 14), suggesting that Kudiezi injection can reduce the NSE and S100B levels. Although the NIHSS scores were not different between the two groups, Kudiezi injection could improve the patients' NIHSS score after 14 days of treatment, suggesting that it can be used to treat acute cerebral infarction and alleviate the symptoms of nerve injury of the patients. The lack of change of NIHSS could be because it is a somewhat suggestive and lacks sensitivity, as it is based on observations and not on quantitative measurements.
Inflammatory regulators (including proinflammatory and anti-inflammatory factors) play important roles in the pathogenesis of stroke. Inflammatory processes and injury-induced changes in neurotransmitter may further increase tissue damage [1, 35, 36]. IL-6 is a multifunctional factor produced by various types of cells that regulate immune responses, acute phase responses, and inflammation in general [37]. After cerebral ischemia, IL-6 levels increase and peak around 3 days after ischemia. IL-6 levels are closely associated with stroke severity, infarct volume, and poor prognosis [38, 39]. The results of the present study suggest that serum IL-6 levels in the KDZ group within 3–5 days after admission (i.e., 5–7 days after onset) were significantly lower compared to the control group.
In the brain, microglia and astrocytes are the main sources of IL-18 [40]. Elevated IL-18 levels after stroke are closely associated with ischemic brain injury [41] and increase with the aggravation of neurological impairments [42]. Elevated IL-18 levels in patients with acute cerebral infarction may be due to cytokine leakage from the infarct area but may also be from cerebrospinal fluid circulation [43]. In the present study, serum IL-18 levels in patients with acute cerebral infarction started to decrease after 5 days of Kudiezi injection and were still decreasing by day 14.
MMPs attack various extracellular matrixes. In the brain, they can promote neuronal cell death [44]. In the ischemic phase, MMP-9 is closely associated with the permeability and function of the blood-brain barrier [44]. In the early stages of cerebral ischemia (ranging from a few hours to a few days), MMP-9 damages the blood-brain barrier, causing leakage, leukocyte infiltration, cerebral edema, and even bleeding [44]. MMP-9 levels are closely associated with the degree of neurological deficit and infarct volume in patients with acute cerebral infarction [45]. Elevated MMP-9 levels can be used as predictor of poor prognosis and stroke-related death [46]. A previous study showed that Kudiezi injection alleviated the dysfunction of the blood-brain barrier in rats with cerebral ischemia through the inhibition of the degradation of a number of proteins [18]; unfortunately, MMPs were not assessed. The present study showed that MMP-9 level started to decline from day 3 after admission in the KDZ group and that the MMP-9 levels were lower than in the control group.
IL-10 is an anti-inflammatory cytokine synthesized in the central nervous system. IL-10 reduces IL-1 and TNF-α production by inhibiting the expression and activation of cytokine receptors, and IL-10 has a protective effect in ischemic injuries [36]. In addition, low levels of peripheral serum IL-10 can increase the risk of stroke [35]. IL-10 levels in patients with acute cerebral infarction can increase at the initial stage of stroke, and IL-10 levels are positively correlated with disease severity, which is in line with the results of the present study. Indeed, IL-10 levels in patients with acute cerebral infarction at admission were higher than the normal reference values. After 3 days of Kudiezi injection, IL-10 levels increased significantly, but there was no difference with the control group. This is supported by a previous study in rats [19].
The present study is not without limitations. Only levels of various serum inflammatory factors were measured, and other pathological processes involved in ischemic damage (e.g., oxidative stress) were not assessed. A previous study in rats showed that Kudiezi injection could decrease oxidative stress after stroke, as well as secondary myocardial damage in rats [25]. This aspect could be worth exploring in humans. In addition, the mid- and long-term levels of the serum inflammatory markers after Kudiezi injection treatment are unknown. The small sample size may also affect the statistical results. Finally, the NIHSS could be not sensitive enough to detect differences in neurological function between the two groups. Additional studies are needed to overcome these limitations.
5. Conclusion
Taken together, those results suggested that Kudiezi injection could lead to early reduction of IL-6, IL-18, MMP-9, NSE, and S100B levels and increase of IL-10 levels in patients with acute ischemic stroke. Future studies will be performed in larger samples of patients and from different regions of China in order to improve generalizability. Additional biomarkers will also be assayed in order to uncover the exact mechanisms of Kudiezi injection in patients with stroke. Moreover, further analysis about the correlations between inflammatory biomarkers and NIHSS scores will be conducted. Longer-term follow-up will also be performed to examine the effects of Kudiezi injection on stroke recurrence and mortality.
Acknowledgments
This work was supported by the International Science and Technology Cooperation Program of China (no. 2015DFA31130), the Beijing National Science Foundation (no. 7182099), and the National Basic Research Program of China (973 Program) (no. 2012CB518406).
Conflicts of Interest
All authors declare that they have no competing interests.
References
- 1.Iadecola C., Anrather J. The immunology of stroke: from mechanisms to translation. Nature Medicine. 2011;17(7):796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jafarinaveh H. R., Allahtavakoli M., Rezazadeh H., et al. Proinflammatory cytokines in the embolic model of cerebral ischemia in rat. Iranian Journal of Allergy, Asthma, and Immunology. 2014;13(2):125–130. [PubMed] [Google Scholar]
- 3.Park S. Y., Kim J., Kim O. J., et al. Predictive value of circulating interleukin-6 and heart-type fatty acid binding protein for three months clinical outcome in acute cerebral infarction: multiple blood markers profiling study. Critical Care. 2013;17(2, article R45) doi: 10.1186/cc12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bustamante A., Sobrino T., Giralt D., et al. Prognostic value of blood interleukin-6 in the prediction of functional outcome after stroke: a systematic review and meta-analysis. Journal of Neuroimmunology. 2014;274(1-2):215–224. doi: 10.1016/j.jneuroim.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Kang H. J., Bae K. Y., Kim S. W., et al. Effects of interleukin-6, interleukin-18, and statin use, evaluated at acute stroke, on post-stroke depression during 1-year follow-up. Psychoneuroendocrinology. 2016;72:156–160. doi: 10.1016/j.psyneuen.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Protti G. G., Gagliardi R. J., Forte W. C. N., Sprovieri S. R. S. Interleukin-10 may protect against progressing injury during the acute phase of ischemic stroke. Arquivos de Neuro-Psiquiatria. 2013;71(11):846–851. doi: 10.1590/0004-282X20130168. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez-Yáñez M., Castellanos M., Sobrino T., et al. Interleukin-10 facilitates the selection of patients for systemic thrombolysis. BMC Neurology. 2013;13(1):p. 62. doi: 10.1186/1471-2377-13-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nash D. L., Bellolio M. F., Stead L. G. S100 as a marker of acute brain ischemia: a systematic review. Neurocritical Care. 2008;8(2):301–307. doi: 10.1007/s12028-007-9019-x. [DOI] [PubMed] [Google Scholar]
- 9.Wunderlich M. T., Lins H., Skalej M., Wallesch C. W., Goertler M. Neuron-specific enolase and tau protein as neurobiochemical markers of neuronal damage are related to early clinical course and long-term outcome in acute ischemic stroke. Clinical Neurology and Neurosurgery. 2006;108(6):558–563. doi: 10.1016/j.clineuro.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Mir I. N., Chalak L. F. Serum biomarkers to evaluate the integrity of the neurovascular unit. Early Human Development. 2014;90(10):707–711. doi: 10.1016/j.earlhumdev.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Zhang B., Wang Y. National 'Tenth five-Year' scientific and technological projects "comprehensive treatment program of acute stroke"-- clinical study on treatment of ischemic stroke with dish Mai Ling Kudiezi injection. Guide to Chinese Medicine. 2006;2006(1):112–117. [Google Scholar]
- 12.Zhai Q. Effect of Kudiezi injection in treating middle-aged and young patients with acute cerebral infarction and its influence on Interleukin-6 and C-reactive protein. Hebei Journal of Traditional Chinese Medicine. 2011;33(6):900–902. [Google Scholar]
- 13.Zhou Y., Liu D. Effect of Kudiezi injection on serum inflammatory factors, efficacy and quality of life in patients with acute cerebral infarction. Chinese Archives of Traditional Chinese Medicine. 2016;34(4):900–903. [Google Scholar]
- 14.Sun L., Liu R. R., Zhao W., Li Y. B., Zhang Y. J. Determination of four amino acids in Kudiezi injection. Tianjin Journal of Traditional Chinese Medicine. 2015;2015(2):106–109. [Google Scholar]
- 15.Liu R.-R., Zhang X.-P., Wang F., et al. Rapid screening and identification of sesquiterpene lactones in Kudiezi injection based on high-performance liquid chromatography coupled with linear ion trap-orbitrap mass spectrometry. Chinese Journal of Natural Medicines. 2018;16(2):150–160. doi: 10.1016/S1875-5364(18)30042-6. [DOI] [PubMed] [Google Scholar]
- 16.Yin P., Liu Y., Zhang J., Cai W., Li Y., Lu J. Rapid content determination of four flavones in Ku-die-zi injection by UPLC-ESI-MS/MS. World Science and Technology - Modernization of Traditional Chinese Medicine. 2015;2015(1):119–123. [Google Scholar]
- 17.Liu S.-Y., Cai W., Wang F., et al. UHPLC-LTQ-Orbitrap-based metabolomics coupled with metabolomics pathway analysis method for exploring the protection mechanism of Kudiezi injection in a rat anti-ischemic cerebral reperfusion damage model. Chinese Journal of Natural Medicines. 2017;15(12):955–960. doi: 10.1016/S1875-5364(18)30013-X. [DOI] [PubMed] [Google Scholar]
- 18.Chen F.-Q., Li Q., Pan C.-S., et al. Kudiezi injection® alleviates blood–brain barrier disruption after ischemia-reperfusion in rats. Microcirculation. 2016;23(6):426–437. doi: 10.1111/micc.12288. [DOI] [PubMed] [Google Scholar]
- 19.Liu X., Zhang X., Wang F., et al. Improvement in cerebral ischemia–reperfusion injury through the TLR4/NF-κB pathway after Kudiezi injection in rats. Life Sciences. 2017;191:132–140. doi: 10.1016/j.lfs.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 20.Liang X., Zhang Y., Wang X., et al. Effects of heat-clearing and blood-activating combination on acute cerebral ischemia of heat toxin pattern in rats via NF-κB signaling pathway. Journal of Beijing University Traditional Chinese Medicine. 2015;38:377–382. [Google Scholar]
- 21.Wang J. Qing heat and activating blood component treat acute cerebral infarction by TLR4-NOX4 signaling pathway in rats, [M.S. thesis] Beijing University of Chinese Medicine; 2015. [Google Scholar]
- 22.Wang F., Zhang Y., Liu X., et al. Study on the differential proteins of Kudiezi injection on rats with Huoxue syndrome of ischemic stroke. China Journal of Chinese Material Medicine. 2014;39(10):1874–1879. [PubMed] [Google Scholar]
- 23.Liu Y., Zhu L., Zhang Y., Zheng H., Liu X. Effect of Kudiezi injection on the expression of nuclear factor-κB and adhesion molecules after high glucose injury in human brain microvascular endothelial cells. China Journal of Traditional Chinese Medicine and Pharmacy. 2010;25(2):204–207. [Google Scholar]
- 24.Kopani M., Celec P., Danisovic L., Michalka P., Biro C. Oxidative stress and electron spin resonance. Clinica Chimica Acta. 2006;364(1-2):61–66. doi: 10.1016/j.cca.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Liu X., Tao Y., Wang F., et al. Kudiezi injection mitigates myocardial injury induced by acute cerebral ischemia in rats. BMC Complementary and Alternative Medicine. 2017;17(1):p. 8. doi: 10.1186/s12906-016-1514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chinese Medical Association Neurology Branch Cerebrovascular Disease Study Group. Chinese treatment guidelines of the acute ischemic stroke 2010. Chinese Journal of Neurology. 2010;43:146–154. [Google Scholar]
- 27.Goldstein L. B., Bertels C., Davis J. N. Interrater reliability of the NIH stroke scale. Archives of Neurology. 1989;46(6):660–662. doi: 10.1001/archneur.1989.00520420080026. [DOI] [PubMed] [Google Scholar]
- 28.Hu Y. Y., Dong X. Q., Yu W. H., Zhang Z. Y. Change in plasma S100B level after acute spontaneous basal ganglia hemorrhage. Shock. 2010;33(2):134–140. doi: 10.1097/SHK.0b013e3181ad5c88. [DOI] [PubMed] [Google Scholar]
- 29.Alatas Ö. D., Gürger M., Ateşçelik M., et al. Neuron-specific enolase, S100 calcium-binding protein B, and heat shock protein 70 levels in patients with intracranial hemorrhage. Medicine. 2015;94(50):p. 1. doi: 10.1097/01.md.0000476046.44577.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunswick A. S., Hwang B. Y., Appelboom G., Hwang R. Y., Piazza M. A., Connolly E. S., Jr. Serum biomarkers of spontaneous intracerebral hemorrhage induced secondary brain injury. Journal of the Neurological Sciences. 2012;321(1-2):1–10. doi: 10.1016/j.jns.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Raabe A., Kopetsch O., Woszczyk A., et al. Serum S-100B protein as a molecular marker in severe traumatic brain injury. Restorative Neurology and Neuroscience. 2003;21(3-4):159–169. [PubMed] [Google Scholar]
- 32.Meric E., Gunduz A., Turedi S., Cakir E., Yandi M. The prognostic value of neuron-specific enolase in head trauma patients. The Journal of Emergency Medicine. 2010;38(3):297–301. doi: 10.1016/j.jemermed.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 33.Lamers K. J. B., Vos P., Verbeek M. M., Rosmalen F., van Geel W. J. A., van Engelen B. G. M. Protein S-100B, neuron-specific enolase (NSE), myelin basic protein (MBP) and glial fibrillary acidic protein (GFAP) in cerebrospinal fluid (CSF) and blood of neurological patients. Brain Research Bulletin. 2003;61(3):261–264. doi: 10.1016/S0361-9230(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 34.González-García S., González-Quevedo A., Fernández-Concepción O., et al. Short-term prognostic value of serum neuron specific enolase and S100B in acute stroke patients. Clinical Biochemistry. 2012;45(16-17):1302–1307. doi: 10.1016/j.clinbiochem.2012.07.094. [DOI] [PubMed] [Google Scholar]
- 35.van Exel E., Gussekloo J., de Craen A. J. M., Bootsma-van der Wiel A., Frolich M., Westendorp R. G. J. Inflammation and stroke: the Leiden 85-plus study. Stroke. 2002;33(4):1135–1138. doi: 10.1161/01.STR.0000014206.05597.9E. [DOI] [PubMed] [Google Scholar]
- 36.Wang Q., Tang X., Yenari M. The inflammatory response in stroke. Journal of Neuroimmunology. 2007;184(1-2):53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mihara M., Hashizume M., Yoshida H., Suzuki M., Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clinical Science (London, England) 2012;122(4):143–159. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- 38.Vila N., Reverter J. C., Yague J., Chamorro A. Interaction between interleukin-6 and the natural anticoagulant system in acute stroke. Journal of Interferon & Cytokine Research. 2000;20(3):325–329. doi: 10.1089/107999000312478. [DOI] [PubMed] [Google Scholar]
- 39.Perini F., Morra M., Alecci M., Galloni E., Marchi M., Toso V. Temporal profile of serum anti-inflammatory and pro-inflammatory interleukins in acute ischemic stroke patients. Neurological Sciences. 2001;22(4):289–296. doi: 10.1007/s10072-001-8170-y. [DOI] [PubMed] [Google Scholar]
- 40.Kohno K., Kurimoto M. Interleukin 18, a cytokine which resembles IL-1 structurally and IL-12 functionally but exerts its effect independently of both. Clinical Immunology and Immunopathology. 1998;86(1):11–15. doi: 10.1006/clin.1997.4475. [DOI] [PubMed] [Google Scholar]
- 41.Yang L., Zhang Z., Sun D., Xu Z., Zhang X., Li L. The serum interleukin-18 is a potential marker for development of post-stroke depression. Neurological Research. 2010;32(4):340–346. doi: 10.1179/016164110X12656393665080. [DOI] [PubMed] [Google Scholar]
- 42.Pan Y., Tang R., Huang J., Wei Y. Detection of IL-18 and CRP in stroke patients. Chinese Journal of Laboratory Diagnosis. 2010;14(1):93–95. [Google Scholar]
- 43.Hedtjarn M., Leverin A. L., Eriksson K., Blomgren K., Mallard C., Hagberg H. Interleukin-18 involvement in hypoxic-ischemic brain injury. The Journal of Neuroscience. 2002;22(14):5910–5919. doi: 10.1523/JNEUROSCI.22-14-05910.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sapojnikova N., Kartvelishvili T., Asatiani N., et al. Correlation between MMP-9 and extracellular cytokine HMGB1 in prediction of human ischemic stroke outcome. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2014;1842(9):1379–1384. doi: 10.1016/j.bbadis.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 45.Montaner J., Alvarez-Sabin J., Molina C., et al. Matrix metalloproteinase expression after human cardioembolic stroke: temporal profile and relation to neurological impairment. Stroke. 2001;32(8):1759–1766. doi: 10.1161/01.STR.32.8.1759. [DOI] [PubMed] [Google Scholar]
- 46.Graham C. A., Chan R. W. Y., Chan D. Y. S., Chan C. P. Y., Wong L. K. S., Rainer T. H. Matrix metalloproteinase 9 mRNA: an early prognostic marker for patients with acute stroke. Clinical Biochemistry. 2012;45(4-5):352–355. doi: 10.1016/j.clinbiochem.2011.12.006. [DOI] [PubMed] [Google Scholar]