Abstract
Background
Urothelial carcinoma (UC) is the fifth most common malignancy that accounts for 5% of all cancers. Diagnostic markers that predict UC progressions are inadequate. NF-κB contributes towards disease progression upon constitutive activation in many solid tumors. The nuclear localization of NF-κB indicates increased transcriptional activity while cytoplasmic localization indicates the inactive protein repository that can be utilized readily by a malignant cell. This study delineates the nuclear and cytoplasmic differential expression of NF-κB heterodimers in UC progression.
Methods
The involvement of the NF-κB proteins in UC was analyzed in silico using cytoscape. The expression of NF-κB heterodimers was analyzed by immunohistochemistry.
Results
PINA4MS app in cytoscape revealed over expression of RelA and suppression of NF-κB1 (p50 precursor) in UC whereas the expression of NF-κB target proteins remained unhindered. Immunohistochemical localization showed nuclear RelA/p50 in low grade UC whereas in high grade only RelA expression was observed. Conversely, cytoplasmic expression of RelA/p50 remained extensive across high and low grade UC tissues (p < 0.005). RelA nuclear and cytoplasmic expression (p < 0.005) was directly proportional to the disease progression. In our study, some of the high-grade UC tissues with squamous differentiation and muscle invasion had extensive nuclear p50 localization. The phenomenon of RelA/p50 expression seen increased in low-grade UC than high grade UC might be due to their interaction with other members of NF-κB family of proteins. Thus, NF-κB RelA/p50 differential expression may play a unique role in UC pathogenesis and can serve as a biomarker for diagnosis.
Keywords: NF-κB, RelA (p65), p50, Differential expression, Urothelial carcinoma, Diagnosis, Cytoscape, Immunohistochemistry
Introduction
In industrialized countries, urothelial carcinoma (UC) is the fifth most common cancer and second most common genitourinary tract cancer (Schulz, 2006; Lee et al., 2014). UC classification has an important role in determining treatment for patients and their prognosis (Öztürk, 2015). According to the World Health Organization grading in 2004, there are papilloma, papillary urothelial neoplasm of low malignant potential, low-grade UC and high-grade UC (Babjuk et al., 2017). The extent of tumor tissue invasion is defined by the stages Ta, T1, T2, T3 and T4. Most stages comprising Ta and T1 are composed of non-muscle invasive tumor confined to only the epithelium and lamina propria. Muscle invasive tumors invade the detrusor smooth muscle or beyond and they belong to the stages T2 to T4 (Kobayashi et al., 2014). Since bladder neoplasm is morphologically and genetically heterogeneous, it is important to differentiate these lesions as their treatment outcomes differ (Hodges et al., 2010). Low-grade UC with Ta and T1 stages require long term treatment and surveillance while high-grade T1–T4 stages would require meticulous strategizing for effective treatment inhibiting metastasis (Abol-Enein, 2008; Bellmunt et al., 2014; Miller et al., 2016). Hence, biomarkers that could predict UC progression are warranted.
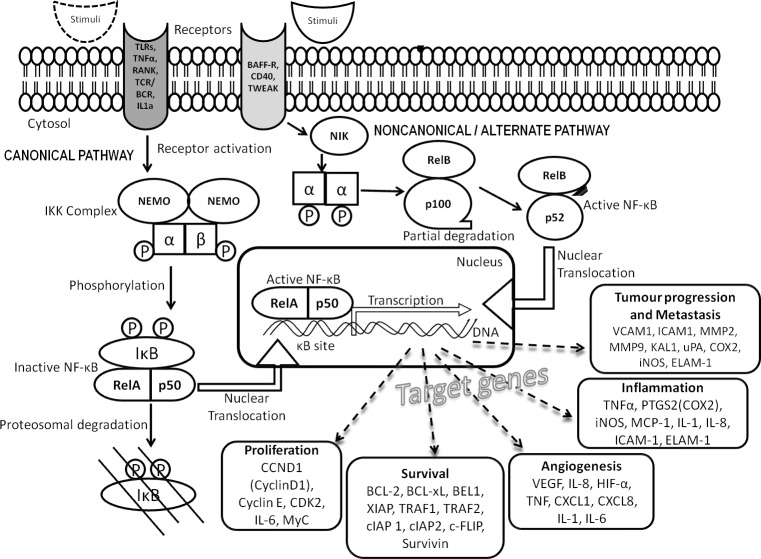
Nuclear factor kappa B (NF-κB) transcription factor is an assembly of protein subunits as heterodimers that bind to a specific region on genes called the κB site for its function. Each dimer (RelA/p50; RelA/p52, RelB/p50, RelB/p52, RelA/RelB and p50/p52) has a distinct function in the cell acting upon specific stimuli. Upon induction, they act to modulate the immune system, inflammation, angiogenesis, survival and proliferation of cells (Gilmore, 2006). These effects are brought about by orchestrating more than 200 genes by the canonical pathway (classical) or the non-canonical pathway (alternate) as illustrated in Fig. 1. These important genes are tightly regulated under normal conditions, however, constitutive activation of NF-κB pathway occurs during tumorigenesis leading to aberrant expression of many target proteins (Meteoglu et al., 2008). Like other solid tumors, UC usually has a poorly understood association with an inflammatory-phenotype regulated by pro-inflammatory cytokine secretion (Masilamoni et al., 2006; Lee et al., 2012). NF-κB being the major inflammatory transcription factor, its role in UC needs exploration (Mukherjee et al., 2015). All NF-κB heterodimers act together for gene transcription while some homodimers of NF-κB are repressors of transcription. Hence we hypothesize analyzing the nuclear and cytoplasmic localization of the heterodimers in low- and high-grade UC will reveal the degree of transcriptional activation and thus the disease progression and would serve as novel biomarkers.
Figure 1. NF-κB signal transduction pathway.
Signaling cascade comprises the canonical (classical) and non-canonical (alternate) pathways involving NF-κB family proteins and their target proteins.
Material and Methods
Cytoscape analysis of NF-κB heterodimers (RelA/p50)
The NF-κB family of proteins and their representative target protein list were created by text mining of literature and from the KEGG database for NF-κB signaling pathway of Homo sapiens (04064). Uniprot IDs (provided as supplemental files, datasets) were identified for the list of proteins and analyzed in Cytoscape. This tool helps in visualization and analysis of the tissue specific expression data, RNA sequence data and interactions between/within multiple protein datasets. Protein Interaction Network Analysis for Multiple Sets (PINA4MS) app is a plug-in for cytoscape that uses protein interaction data from six public databases that are curated manually: IntAct, MINT, BioGRID, DIP, HPRD and MIPS MPact data, thus increasing the efficiency of the tool (Cowley et al., 2012; Shannon et al., 2003). The physical interactions among proteins of interest were retrieved from the Protein Interaction Network Analysis (PINA) platform, and kinase–substrate relationships were downloaded from the PhosphoSitePlus database.
Using the UniProt IDs as input we utilized the pre-existing UC tissue specific expression data set in the app to analyze the NF-κB family of proteins and their targets. Comparative analysis was performed with those proteins expressed on normal tissues and urothelial carcinoma tissues using PINA4MS app. Thresholds and limits (protein interactions only with the range 0.01–2; inter and intra spread value was set to 1 and circular cluster layout was chosen) set as default parameters of the tool were used while constructing the figure. This app uses the hypergeometric test to identify and eliminate the false discovery rate. It identifies the overrepresented terms with a correction for multiple testing using false discovery rate (the P-value < 0.05 was adjusted). The hypergeometric test was applied to test statistical enrichment of identified KEGG and reactome pathways and the P values were corrected for multiple comparisons using the Benjamini and Hochberg method. The output file of each tool was created and the functional enrichment analyses were performed on an SGE cluster using GOstats. For pathway enrichment analysis, the KEGG Orthology Based Annotation System (KOBAS) was used. The bubble diameter of the protein analyzed is representative of the degree of protein expression.
Urothelial tissues
The study was designed with 121 cases under two main groups: (i) normal tissues from subjects with no malignancy but presented with an inflammatory condition of the bladder; n = 60 and (ii) urothelial carcinoma of low grade and high grade; n = 61. The retrospective study design waived the need for obtaining informed consent from the patients, however the Institutional Ethical Committee of Sri Ramachandra Medical College and Research Institute (Deemed to be University), Chennai, India reviewed and accepted the study approving the use of FFPE tissues deposited at Sri Ramachandra Medical Centre from the year 2008 for this retrospective study (CSP/14/FEB/33/25).
Statistical analysis
Clinicopathological features were compared between the normal group and cancer group of patients. Low- and high-grades of UC were also compared to derive useful statistical associations using Mann Whitney U test and Fisher’s exact test. Pearson’s chi-square test was used for assessing differential expression of NF-κB heterodimers between the normal and cancer group. Any p value <0.05 was considered to be statistically significant. All the statistical analyses were performed using IBM SPSS v23.
Immunohistochemical analysis
Urothelial tissues fixed in 10% buffered formalin for 24 h at 4 °C were processed and embedded in paraffin as tissue blocks. FFPE tissue sections with 4 µM thickness were deparaffinized, rehydrated and incubated in Citrate buffer at pH 6.0 for 30 min under pressure for antigen retrieval. Once when the tissue sections were brought to ambient temperature, endogenous peroxidase was quenched using hydrogen peroxide (10%) for 20 min. The sections were further washed twice with tris buffered saline (TBS) at pH 7.3 and blocked with normal goat serum (Biolegend, San Diego, CA, USA) to reduce non-specific background staining. Tissue sections were incubated overnight with a rabbit polyclonal IgG anti-NF-κB p65 antibody (622602; Biolegend, San Diego, CA, USA) and with rabbit polyclonal IgG anti-NF-κB p50 antibody (sc-7178, Santa Cruz, USA) respectively, at a dilution of 1:200 each. Sections were then washed in TBS and incubated with HRP conjugated secondary antibody (goat anti rabbit IgG-HRP: sc-2004; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 2 h and visualized using 3, 3′-diaminobenzidine (Sigma Aldrich, St. Louis, MO, USA). Finally, the sections were counterstained with Mayer’s hematoxylin, dehydrated and cover-slipped. Meteoglu et al. (2008) method was followed with slight modifications. The lymphocytes in the UC tissue sections were found to be positive for the NF-κB heterodimers (RelA/p50) and hence served as positive controls. Further, normal urothelium devoid of blood vessels served as negative control (No primary antibody, reagent control).
The immunohistochemical staining was documented using Olympus BX43 microscope with a QImaging Micropublisher 3.3 RTV camera and QCapture Pro 7 software (QImaging, Surrey, BC, Canada). Each section was assessed for the immunoperoxidase staining and scored by a pathologist. The scoring includes RelA and p50 nuclear and cytoplasmic staining positivity. RelA and p50 staining in the nucleus and cytoplasm were scored as follows (i) absence of staining (no or non-specific), (ii) low (<20%), (iii) moderate (21–60%) and (iv) extensive (>60%) staining.
Results
In silico differential expression of NF-κB family of proteins and their targets in UC and normal urothelium
Cytoscape app PINA4MS extracted the differentially expressed proteins of normal and UC tissues. NF-κB family proteins (marked in red) like RelA, IKBKB (IKKβ), NF-κBIE (IκBε), IKBKG (NEMO), CHUK (IKKα) were over expressed in UC when compared to normal urothelial cells. However, expressions of other NF-κB family proteins like BCL3, NF-κBIA (IκBα), NF-κBIB (IκBβ), RelB, NF-κB1 (p105) and NF-κB2 (p100) were suppressed as depicted by the diameter of the bubble (Figs. 2 and 3). The NF-κB target proteins (marked in green), like CFLAR (c-FLIP), TRAF2, XIAP, MYC, BIRC3, TRAF1, BCL2, CCND1, IL8, ICAM1 and VCAM1 showed altered expression (Figs. 2 and 3). These proteins are either direct or indirect targets of NF-κB signal transduction. Of the target proteins, VEGF-A, SOD2, and PTGS2 (COX2) showed over expression, however, TRAF2, CFLAR (c-FLIP), XIAP, BIRC3 (cIAP-2), BIRC2 (cIAP-1), MYC and BAD proteins showed homogenous expressions while CCL4 (MIP-1β), CCND1 (Cyclin D1), ICAM1, BCL2, MMP9 and TRAF9 proteins showed a more suppressed state (Figs. 2 and 3). The app resulted in a direct association between RelA and NF-κB1 (p50 precursor) with a difference in their expression levels between UC and normal tissues. Hence, the localization of RelA/ p50 heterodimers was further analyzed using immunohistochemistry in UC and normal urothelium.
Figure 2. Cytoscape-PINA4MS result of NF-κB family proteins expression and targets in urothelial carcinoma.
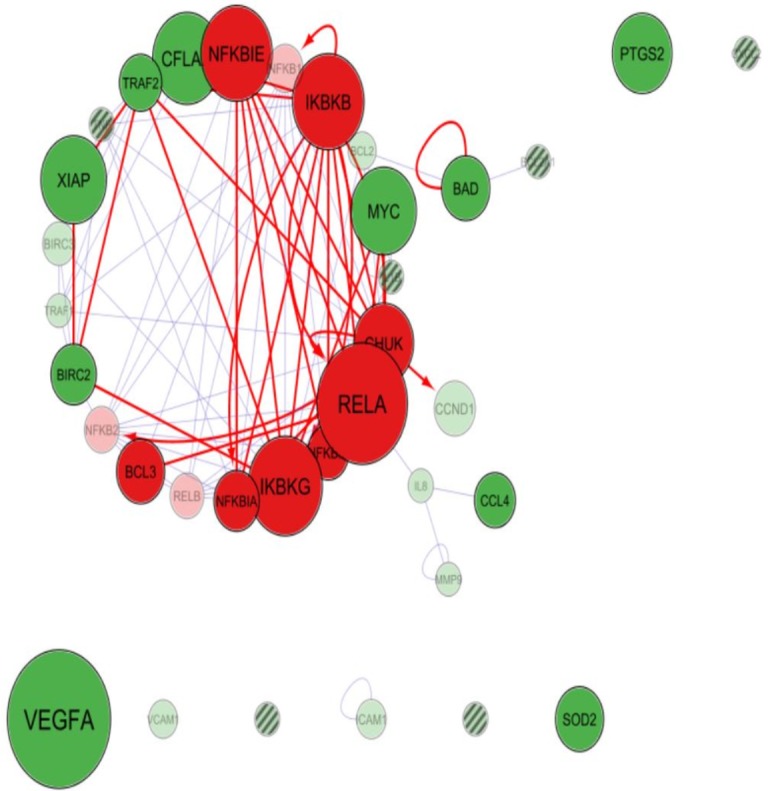
NF-κB proteins (red bubbles) and their target proteins (green bubbles) expressed or repressed in UC tissues. Size of the bubble represents the expression at mentioned condition. Transparency of the bubble is proportional to the secretion and subsequent expression or suppression. Purple arrows indicate protein–protein interactions whereas magenta arrows indicate the substrate-kinase interactions. All interactions in UC are marked red irrespective of their interaction mode. Proteins with no arrows (stand alone) indicate indirect interaction with the members of the network. Proteins with line fill indicate those with no human protein atlas data.
Figure 3. Cytoscape-PINA4MS result of NF-κB family proteins expression and targets in Normal Urothelium.
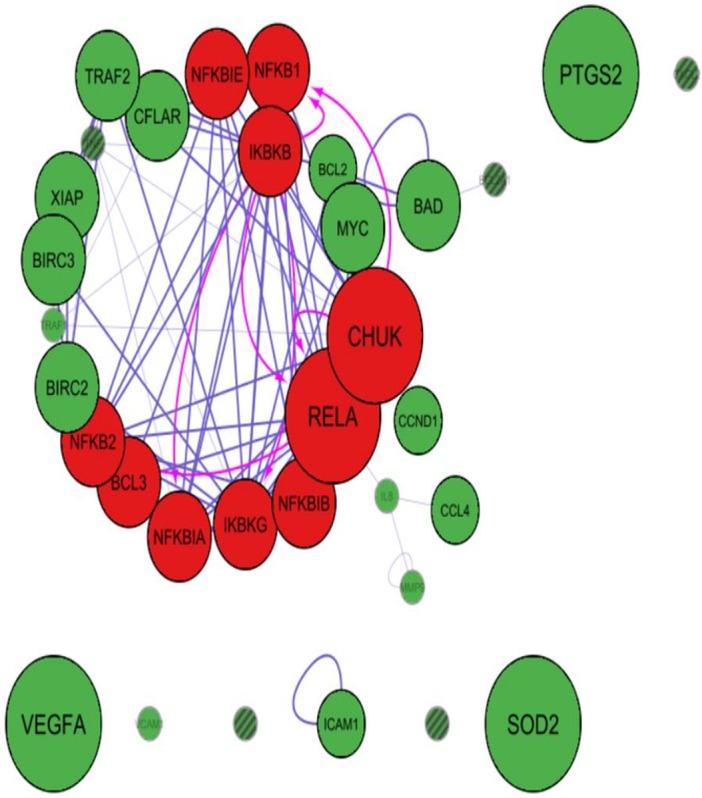
NF-κB proteins (red bubbles) and their target proteins (green bubbles) expressed in normal urothelial tissues. Size of the bubble represents the expression at mentioned condition. Transparency of the bubble is proportional to the secretion and subsequent expression or suppression. Purple arrows indicate protein–protein interactions whereas magenta arrows indicate the substrate-kinase interactions. All interactions in UC are marked red irrespective of their interaction mode. Proteins with no arrows (stand alone) indicate indirect interaction with the members of the network. Proteins with line fill indicate those with no human protein atlas data.
Clinicopathological correlations with NF-κB heterodimer expression in UC and normal urothelium
The clinicopathological parameters like age, gender, tumor size and grades of UC were assessed to find the significance between nuclear and cytoplasmic localization of NF-κB heterodimers. The age of patients in the normal group ranged from 20 to 79 years and those in the cancer group ranged from 18 to 88 years. It was a heterogeneous population consisting of 32 males and 28 females in the normal group whereas 49 males and 12 females in cancer group. UC occurrence was higher in the age category of 61 to 70 years and found to have a high incidence in the male population with a statistical significance of p < 0.05. Comparison of tumor size, grades had high significance (p < 0.005) and revealed significant association with RelA nuclear (p < 0.005) and cytoplasmic localization (p < 0.005). However, there was no association with p50 nuclear localization (p > 0.05) but there was a significant association with the cytoplasmic expression (p < 0.05). Those cases that had muscle invasive areas showed differential uptake of the NF-κB subunits irrespective of grade.
The nuclear expression of RelA when compared between normal and UC groups by Pearson’s chi-square test (χ2) revealed a strong association with UC and nuclear localization (Table 1). Comparison of the high and low grades of UC by Fisher’s exact test showed no association with RelA nuclear localization and UC grades (Table 2). The cytoplasmic expression of RelA was compared with normal and UC group by Pearson’s chi-square test and it revealed a significant association with UC (Table 3). Comparing high and low grades of UC and the cytoplasmic localization of RelA by Fisher’s exact test revealed significant associations for RelA cytoplasmic localization (Table 4). Similarly, the nuclear expression of p50 was compared between normal and UC groups by Pearson’s chi-square test which had significant associations (Table 1) with UC and nuclear localization. Comparison of high and low grades of UC by Fisher’s exact test revealed no significant associations with p50 nuclear localization and UC grades (Table 2). When cytoplasmic expression of p50 was compared in normal and UC group by Pearson’s chi-square test it exhibited high statistical significance in UC (Table 3). Comparing the cytoplasmic expression of p50 within high and low grades of UC using Fisher’s exact test, showed significant association for p50 cytoplasmic localization (Table 4). The normal urothelium had no significant associations in the localization of the NF-κB heterodimers. The inflammatory regions had weak to moderately stained heterodimers in the cytoplasm and the nucleus had only negligible staining, unlike neoplastic cells (Tables 1 and 3).
Table 1. Nuclear localization of NF-κB heterodimers in urothelial tissues: n (%).
| Tissue | NF-κB heterodimers | χ2 test/Fisher’s test | p value | ||
|---|---|---|---|---|---|
| Absent | Present | ||||
| Normal | RelA | 60 (100%) | 0 (0%) | 39.236 | <0.001 |
| Cancer | 31 (50.8%) | 30 (49.2%) | |||
| Normal | p50 | 60 (100%) | 0 (0%) | 9.564 | <0.005 |
| Cancer | 52 (85.2%) | 9 (14.8%) | |||
Table 2. Nuclear localization of NF-κB heterodimers in urothelial carcinoma tissues: n (%).
| Cancer grade | NF-κB heterodimers | χ2test/Fisher’s test | p value | ||
|---|---|---|---|---|---|
| Absent | Present | ||||
| Low | RelA | 18 (64.3%) | 10 (35.7%) | 3.755 | 0.073 |
| High | 13 (39.4%) | 20 (60.6%) | |||
| Low | p50 | 24 (85.7%) | 4 (14.3%) | 0.009 | 1.000 |
| High | 28 (84.8%) | 5 (15.2%) | |||
Table 3. Cytoplasmic localization of NF-κB heterodimers in urothelial tissues: n (%).
| Tissue | I | II | III | χ2test/Fisher’s test | p value | |
|---|---|---|---|---|---|---|
| Normal | RelA | 24 (40%) | 36 (60%) | 0 (0%) | 67.944 | <0.001 |
| Cancer | 0 (0%) | 21 (34.4%) | 40 (65.6%) | |||
| Normal | p50 | 24 (40%) | 36 (60%) | 0 (0%) | 78.644 | <0.001 |
| Cancer | 0 (0%) | 15 (24.6%) | 46 (75.4%) |
Notes.
Cytoplasmic positivity, I, low (≤20%); II, moderate (21–60%); III, extensive (≥61%) was based on the staining intensity of the cell cytoplasm with immunoperoxidase positivity.
Table 4. Cytoplasmic localization of NF-κB heterodimers in urothelial carcinoma tissues: n (%).
| Cancer grade | II | III | χ2 test/Fisher’s test | p value | |
|---|---|---|---|---|---|
| Low | RelA | 14 (50%) | 14 (50%) | 5.561 | 0.030 |
| High | 7 (21.2%) | 26 (78.8%) | |||
| Low | p50 | 11 (39.3%) | 17 (60.7%) | 6.028 | 0.019 |
| High | 4 (12.1%) | 29 (87.9%) |
Notes.
Cytoplasmic positivity, I, low (≤20%); II, moderate (21–60%); III, extensive (≥61%) was based on the staining intensity of the cell cytoplasm with immunoperoxidase positivity.
There were no cases in the “low cytoplasmic positivity (I)” category.
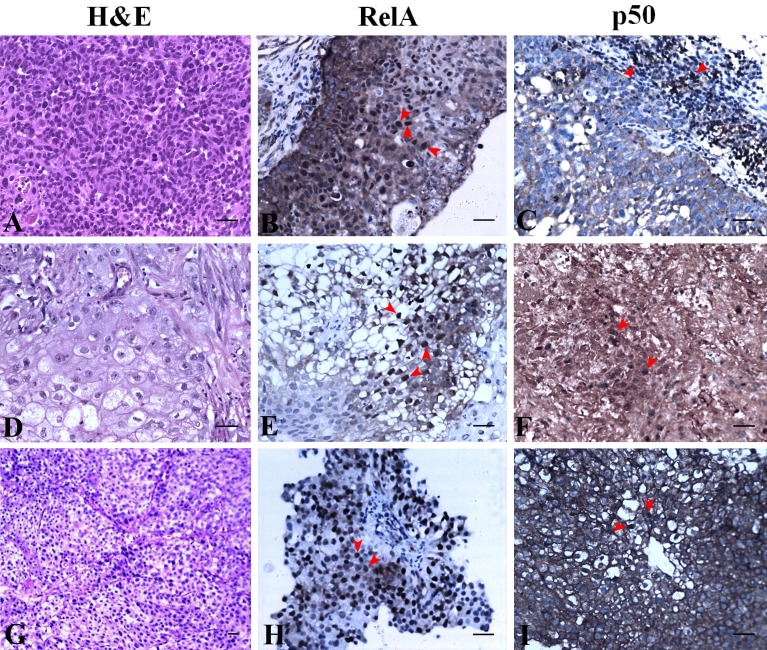
Differential expression of NF-κB subunits in high grade UC
The expression of RelA and p50 are described based on the grade and stage of UC for clarity. H&E staining revealed histology of a non-invasive high grade UC with Ta stage (Fig. 4A). RelA expression in high grade UC at Ta stage had most of the urothelial cells positive for nuclear expression along with extensive cytoplasmic expression (Fig. 4B). However, nuclear staining was absent for p50 in high grade UC at Ta stage but all urothelial cells had moderate to extensive cytoplasmic expression (Fig. 4C). The focal areas of squamous cell differentiation in high grade UC (5%) also showed nuclear positivity (Fig. 4D). RelA nuclear expression in high grade UC at T1 stage was low whereas its cytoplasmic expression remained extensive (Fig. 4E). In high grade UC at T1 stage, p50 expression was moderately positive in the nucleus with extensive cytoplasmic expression (Fig. 4F). H&E staining revealed the histology of high grade invasive UC with focal squamous differentiation and muscle invasion at T2b stage (Fig. 4G). Nuclear RelA expression in high-grade UC at T2 stage was low and the cytoplasmic expression was moderate to extensive (Fig. 4H). Nuclear p50 expression was low but had extensive cytoplasmic staining in T2 stage of UC. Muscle invasive areas and regions with squamous differentiation had intense staining of p50 subunit (Fig. 4I). High-grade UC at T3 stage showed moderate expression of RelA in the nucleus and cytoplasm. However, p50 nuclear staining was absent and showed moderate cytoplasmic positivity.
Figure 4. NF-κB heterodimer expression: High grade urothelial carcinoma.
(A) High grade non invasive urothelial carcinoma H&E × 200. (B) Immunostaining with RelA (p65) showed nuclear positivity (brown colour) indicated by arrow IHC × 200. (C) NF-κB p50 showed moderate cytoplasmic positivity IHC × 200. Note: adjacent lymphocytes (in-built control) have taken up nuclear staining indicated by arrows. (D) High grade papillary invasive urothelial carcinoma with squamous differentiation H&E × 200. (E) Immunostaining with RelA (p65) showed nuclear positivity (brown colour) as indicated by arrows IHC × 200. (F) NF-κB p50 showed few nuclear and cytoplasmic positivity in squamous regions which are indicated by arrows IHC × 200. (G) High grade invasive urothelial carcinoma H&E × 100. (H) Immunostaining with RelA (p65) showed nuclear positivity (brown colour) as indicated by arrows IHC × 200. (I) NF-κB p50 showed extensive cytoplasmic positivity as indicated by arrows IHC × 200. Scale bar indicates 50 µM.
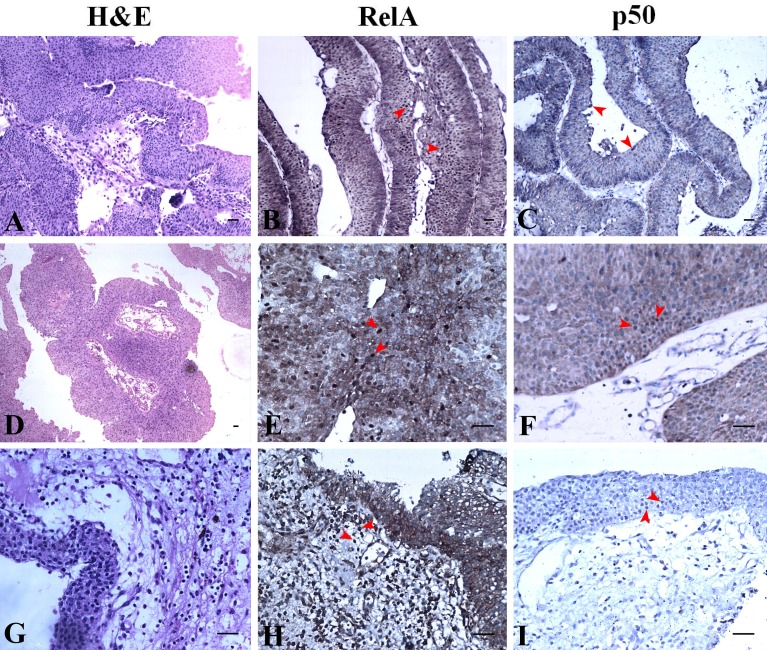
NF-κB heterodimer expression varies in low-grade UC and normal urothelium
H&E staining revealed the histology of low-grade papillary UC at Ta stage (Fig. 5A). The expression of NF-κB heterodimers was prominent with extensive nuclear and cytoplasmic expression of RelA (Fig. 5B). However, nuclear expression of p50 in low-grade UC at Ta stage was observed along with extensive cytoplasmic expression (Fig. 5C). H&E staining revealed tumor histology suggestive of low-grade papillary UC with T1 stage (Fig. 5D). Also, low-grade papillary UC at T1 stage had extensive nuclear staining of RelA with equivalent expression in the cytoplasm (Fig. 5E). Nuclear expression of p50 in low-grade UC at T1 stage was absent but it expressed moderate to extensive cytoplasmic staining (Fig. 5F). RelA/p50 subunits had negligible nuclear positivity and low to moderate cytoplasmic positivity in normal urothelial tissues. The regions of inflammation had some prominent nuclear positive cells of RelA/p50 subunits but they did not show any significance. However, the cytoplasm showed a difference in the staining intensity for both subunits when compared to their nuclear staining which was restricted to cells with inflammation (Figs. 5H, 5I).
Figure 5. NF-κB heterodimer expression: low grade urothelial carcinoma with normal urothelium.
(A) Low grade papillary urothelial carcinoma H&E ×100. (B) Immunostaining with RelA (p65) showed nuclear positivity (brown colour) IHC × 100. (C) NF-κB p50 showed extensive cytoplasmic positivity IHC × 100. (D) Low grade papillary urothelial carcinoma with tumor infiltration H&E × 40. (E) Immunostaining with RelA (p65) showed nuclear positivity (brown colour) IHC ×200. (F) NF-κB p50 showed moderate cytoplasmic positivity IHC × 200. (G) Normal urothelium H&E × 200. (H) Immunostaining with RelA (p65) showed faint to moderate cytoplasmic positivity (brown colour) IHC × 200. Note: Lymphocytes (in-built control) have taken up stain indicated by arrows. (I) NF-κB p50 showed moderate cytoplasmic positivity indicated by arrow IHC × 200. Scale bar indicates 50 µM.
Discussion
The role of NF-κB pathway proteins in the development and progression of UC is still unexplored. Constitutively active NF-κB tends to protect the cells from apoptosis and cross-talks with other pathways that are capable of retaining proliferating cells viable during the process of carcinogenesis (Kim et al., 2013; Mukherjee et al., 2017). Although NF-κB heterodimers act together for gene activation, homodimers of p50 repress transcription by binding to DNA. Furthermore, there are other binding partners for both the subunits to elicit their function. The degree of transcriptional activation which denotes UC progression can be understood from the variations observed in their nuclear/cytoplasmic expression. NF-κB nuclear localization indicates increased transcriptional activity and cytoplasmic localization indicates the inactive protein repository that can be utilized readily by a malignant cell. Differentiating these neoplasms is crucial as they can influence treatment outcomes (Hodges et al., 2010). This study analyzing the nuclear and cytoplasmic localization of the heterodimers in low and high grade UC unravels the disease progression.
In silico analysis was performed to assess the expression of NF-κB family of proteins and their target proteins using the cytoscape app PINA4MS. The app virtually assessed the tissue specific protein expression utilizing the data deposited in six different databases. A list of NF-κB family of proteins with their target proteins used as inputs showed their expression in normal and UC. Our results revealed suppression of NF-κB1 (p50 precursor), NF-κB2 (p52 precursor) and RelB in urothelial tumor tissue (Fig. 2). We also found a reduction in the expression of CHUK (IKKα) and similar regulatory proteins of NF-κB in the tumor cells in comparison to normal (Fig. 3). NF-κBIE (IκBε) and IκBKB (IKKβ) are the upstream molecules that showed overexpression in tumor tissue when compared to normal urothelial tissue. These results convey that the dysregulated upstream molecules of the NF-κB contribute to the constitutive activation of the NF-κB pathway resulting in an over expression of NF-κB downstream target proteins to enrich the tumor microenvironment convincing earlier studies of (Slattery et al., 2018). Accordingly, most of the NF-κB direct target proteins like XIAP, BCL3, TRAF2, BIRC3, RelB, SOD2, CFLAR or the indirect target proteins like MYC, BAD, PTGS2/COX2, CxCL2, VEGFA, VCAM1, ICAM1 found to be expressed in normal urothelium where seen selectively expressed or suppressed in the case of UC (Fig. 2). Our study revealed over expression of pro-survival or pro-proliferative factors (TRAF-1, TRAF-2, CFLAR, XIAP, BIRC2, BIRC3, VEGF-A, BAFF) and suppression of NF-κB inhibitory factors (NF-κB1E, NF-κB1, NF-κB1A, NF-κB1B) in UC.
PINA4MS app in cytoscape revealed involvement of NF-κB direct or indirect target proteins that contribute to UC carcinogenesis by modulating the steps of carcinogenesis (oncogenic control, cellular transformation, proliferation, invasion, angiogenesis and metastasis) as illustrated in Fig. 1. The expression of NF-κB target proteins remained unhindered whereas over expression of RelA and suppression of NF-κB1 (p50 precursor) was observed in UC which was further analyzed by immunohistochemistry.
Oya et al. (2003) state that affirming NF-κB activity with only RelA expression would be insufficient to conclude a finding since the binding of target genes is brought about by both subunits. However, many cancer studies have assessed the involvement of the RelA subunit alone or in combination with other subunits like RelB, NF-κB2, c-Rel, etc. Nonetheless, outcome of the studies that methodically examined RelA and p50 have reported altered expression with an impact on recurrence and survival (Jenkins et al., 2007; Annunziata et al., 2010; Wu et al., 2015). Our study has analyzed the expression of NF-κB subunits, RelA/p50 using immunohistochemistry hypothesizing a difference in their expression as supported by the preliminary in silico results. The clinicopathological parameters such as age, gender, tumor size, stage and grade were assessed with the localization of the RelA/p50 heterodimers. Geriatric males had the highest incidence of UC which can be attributed to an occupational hazard and/or habit and/or lifestyle which was not assessed in this study. The association of tumor size with nuclear localization had significance with RelA (p < 0.05) but not with p50 subunit (p = 0.655).
Our observation on the expression of RelA subunit in UC was similar to the pattern reported by Levidou & Saetta (2008) while the clinical significance of our study was the expression of NF-κB p50 subunit. The heterodimers are retained in the cytoplasm in their inactive form which upon activation translocates to the nucleus for gene transcription. At a given instance, not all the inactive heterodimers are capable of getting activated upon stimulation for translocation since this step is tightly regulated as shown by (King et al., 2011). However, the amount of inactive heterodimers in the cytoplasm shows the capacity of the cell to keep the signaling cascade active. This reflects the potential of over expressed NF-κB to manipulate UC aggressiveness and or progression, hence considering its expression seemed rational. Accordingly, we observed moderate to extensive cytoplasmic localization of RelA and p50 in all low and high grades of UC (Ta, T1, T2 and T3) investigated (Fig. 4). RelA was seen localized in the nucleus of all high grade stages of UC but only a few cases localized p50 (specifically T1, T2) which portrays the differential expression of NF-κB heterodimers in UC. A similar pattern was observed in renal cell carcinoma (Sweeney et al., 2004) where the expression of p50 in the nucleus was seen decreased. The nuclear expression of RelA and p50 in low grade Ta was positive and their expression was comparable though the p50 expression was not extensive like RelA (Tables 1 and 2). RelA was seen localized in the nucleus of all low grade, stages (Ta, T1) of UC conversely NF-κB p50 subunit was seen localized only in Ta stage UC (Fig. 5). Our study revealed that those tissues with muscle invasion and squamous differentiation had altered expression of p50. In intraepithelial neoplasia, a precancerous condition of the prostate also showed increased p50 subunit with other binding partners like RelB and p52 over the classic heterodimer expression supporting disease progression (Lessard et al., 2006; Degoricija et al., 2014). In our study, we have not assessed the expression of RelB or p52 but the pattern of RelA/p50 heterodimer expression studied was similar to Lessard et al. (2006) as reported in prostate cancer. The increased nuclear localization seen in the low grade UC than high-grade UC is suggestive of the contribution of NF-κB heterodimer in the initial stages of UC pathogenesis.
Nuclear expression of p50 homodimers can be independent of RelA since they can form complexes with other proteins like CBP, Bcl-3, histone deacetylase 1 (HDAC-1) and p300 for DNA binding to initiate or repress gene transcription based on the binding partner (Karst et al., 2009). Hence the involvement of p50 in UC is perhaps independent of the I κB regulation and or in combination with other transcriptional activators for gene transcription. In normal conditions, the homodimers of p50 are reported to be repressors and they are up-regulated for inhibiting the NF-κB signaling (Annunziata et al., 2010). In UC, we speculate that this inhibitory mechanism is dysregulated. In our study lymphocytes served as in-built controls of RelA/p50 staining (Figs. 4C, 4H). Tumor-associated macrophages are known to show nuclear p50 positivity attributing to anti-tumor response suppression (Saccani et al., 2006; Annunziata et al., 2010) and many investigations state the presence of tumor infiltrative macrophages with poor prognosis (Szebeni et al., 2017). The significance of lymphocytes showing NF-κB heterodimers positive staining in UC is unknown. Muscle invasion and squamous differentiation are indicators of poor prognosis with adverse outcome in UC (Amin, 2009; Lee et al., 2014). Selective nuclear localization of p50 in certain regions of UC tissues in our study warrants further investigation on the actual mechanism of action in UC pathogenesis. Annunziata et al. (2010) have associated NF-κB p50 with poor survival in ovarian cancer patients post-treatment. Similarly, this study on UC that demonstrates differential p50 expression is suggestive of its participation in disease progression, and hence targeting it would help patient’s early diagnosis and as a novel biomarker.
Conclusion
Differential nuclear expression of the NF-κB subunits RelA/p50 poses important questions on their functions in UC. RelA must dimerize for DNA binding activity as they lack the DNA binding domain. From this study, it becomes evident that RelA has a partner that allows it to transcribe the target genes. The lack of extensive nuclear p50 suggests that RelA translocates with another binding partner instead of p50. Among the NF-κB family of proteins, identifying the RelA preferred binding partner during UC progression will shed some light on the role of RelA in the progression of UC. In addition, preventing the migration of RelA by blocking the interaction of its preferred nuclear partner may abrogate the progression of UC. Investigating the significant cytoplasmic expression of p50 similar to RelA across UC tissues when it is not the preferred binding partner will also add some interesting facts to the NF-κB mystery in UC. Altogether, this phenomenon could be contemplated as the capacity of the neoplastic cell to activate the over expressed cytoplasmic NF-κB constitutively for tumor promotion, addressing these critical questions would help in early diagnosis, aid in better treatment and management of UC.
Supplemental Information
(A) High grade non invasive urothelial carcinoma H&E × 200.
(B) Immunostaining with RelA (p65) showed nuclear positivity (Brown colour) indicated by arrow IHC × 200.
(C) NF-κB p50 showed moderate cytoplasmic positivity IHC × 200. Note: adjacent lymphocytes (in-built control) have taken up nuclear staining indicated by arrows.
(D) High grade papillary invasive urothelial carcinoma with squamous differentiation H&E × 200.
(E) Immunostaining with RelA (p65) showed nuclear positivity (Brown colour) as indicated by arrows IHC × 200.
(F) NF-κBp50 showed few nuclear and cytoplasmic positivity in squamous regions which are indicated by arrows IHC × 200.
(G) High grade invasive urothelial carcinoma H&E × 100.
(H) Immunostaining with RelA (p65) showed nuclear positivity (Brown colour) as indicated by arrows IHC × 200.
(I) NF-κBp50 showed extensive cytoplasmic positivity as indicated by arrows IHC × 200.
(A) Low grade papillary urothelial carcinoma H&E × 100.
(B) Immunostaining with RelA (p65) showed nuclear positivity (Brown colour) IHC × 100.
(C) NF-κBp50 showed extensive cytoplasmic positivity IHC × 100.
(D) Low grade papillary urothelial carcinoma with tumor infiltration H&E × 40.
(E) Immunostaining with RelA (p65) showed nuclear positivity (Brown colour) IHC × 200.
(F) NF-κBp50 showed moderate cytoplasmic positivity IHC × 200.
(G) Normal urothelium H&E × 200.
(H) Immunostaining with RelA (p65) showed faint to moderate cytoplasmic positivity (Brown colour) IHC × 200. Note: Lymphocytes (in-built control) have taken up stain indicated by arrows.
(I) NF-κB p50 showed moderate cytoplasmic positivity indicated by arrow IHC × 200.
A, RelA subunit staining is a Negative control (Reagent)—No primary antibody was applied on sections
B, p50 subunit staining is a Negative control (Reagent)—No primary antibody was applied on sections
Acknowledgments
The authors thank Dr. Caven S. Mcloughlin, Fulbright Specialist, Kent State University, USA for his suggestions. The authors acknowledge the assistance in statistics provided by T Gayathiri, Senior Lecturer, Department of Community Medicine, Sri Ramachandra Medical College and Research Institute (Deemed to be University), India.
Abbreviations
- BAD
Bcl-2 associated agonist of cell death
- Bcl-2
Apoptosis regulator Bcl-2
- BCL3
B-cell lymphoma 3 protein
- BCR
B-cell receptor
- BioGRID
Biological General Repository for Interaction Datasets
- BIRC3/cIAP
Baculoviral IAP (inhibitors of apoptosis) repeat-containing protein 3
- CBP
CAMP Response Element Binding (CREB) - protein
- CCL4
C-C motif chemokine 4
- CCND1/Cyclin D1
G1/S-specific cyclin-D1
- CFLAR/c-FLIP
CASP8 and FADD-like apoptosis regulator
- CHUK/IKKα
Conserved helix-loop-helix ubiquitous kinase/inhibitor of nuclear factor-κB kinase α
- CSF-1
Macrophage colony-stimulating factor 1
- DIP
Database of Interacting Proteins
- FFPE
Formalin fixed paraffin embedded
- GOstats
Gene Ontology Statistics
- H&E
Haematoxylin and Eosin staining
- HIFα
Hypoxia inducing factor α
- HPRD
Human Protein Reference Database
- HRP
Horse raddish peroxidase
- ICAM1
Intercellular adhesion molecule 1
- IL-8
Interleukin-8
- IntAct
Molecular interaction database
- IκBβ
Inhibitor of nuclear factor kappa B β
- IKKβ
Inhibitor of nuclear factor κB kinase β
- MINT
Molecular INTeraction database
- MIPS-MPact
Munich Information Center for Protein Sequences- MIPS Mammalian Protein–Protein Interaction Database
- MMP9
Matrix metalloproteinase 9
- MYC
Myelocytomatosis virus oncogene cellular homolog
- NEMO
NF-κB Essential Modulator
- NF-κB
Nuclear factor-kappa B
- NF-κB1
Precursor p105 and processed p50
- NF-κB2
Precursor p100 and processed p52
- NIK
NF-κB inducing kinase
- PINA4MS
Protein Interaction Network Analysis For Multiple Sets
- PTGS2/COX2
Prostaglandin G/H synthase 2
- pTIS
Carcinoma in situ
- RelA
V-Rel reticuloendotheliosis viral oncogene homolog A
- RelB
V-Rel reticuloendotheliosis viral oncogene homolog B
- SGE
Sun Grid Engine
- SOD2
Superoxide dismutase 2
- TNF
Tumor necrosis factor
- TP53
Cellular tumor antigen p53
- TLRs
Toll like receptors
- TCR
T-cell receptor
- TRAF2
TNF receptor-associated factor 2
- VCAM
Vascular cell adhesion protein
- VEGF-A
Vascular endothelial growth factor A
- XIAP
E3 ubiquitin-protein ligase.
Funding Statement
The study utilized institutional research funds, GATE awarded to Dr. Charles Emmanuel Jebaraj Walter and Dr. Dicky John Davis G. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Sankari Durairajan, Mary Divya Samuel, Dicky John Davis G and George Priya Doss C performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Charles Emmanuel Jebaraj Walter conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Dinesh Palani performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Sneha Pasupati performed the experiments, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Thanka Johnson conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The Institutional Ethical Committee of Sri Ramachandra Medical College and Research Institute (Deemed to be University), Chennai, India reviewed and accepted the study, approving collection of archival samples deposited at Sri Ramachandra Medical Centre from the year 2008 for this retrospective study (Ref: CSP/14/FEB/33/25).
Data Availability
The following information was supplied regarding data availability:
For Figs. 4 and 5, native photomicrographs for each stain and controls are available as Supplemental Files.
References
- Abol-Enein (2008).Abol-Enein H. Infection: is it a cause of bladder cancer? Scandinavian Journal of Urology and Nephrology Supplementum. 2008;42:79–84. doi: 10.1080/03008880802325309. [DOI] [PubMed] [Google Scholar]
- Amin (2009).Amin MB. Histological variants of urothelial carcinoma: diagnostic, therapeutic and prognostic implications. Modern Pathology. 2009;22:S96–S118. doi: 10.1038/modpathol.2009.26. [DOI] [PubMed] [Google Scholar]
- Annunziata et al. (2010).Annunziata CM, Stavnes HT, Kleinberg L, Berner A, Hernandez LF, Birrer MJ, Steinberg SM, Davidson B, Kohn EC. Nuclear factor κB transcription factors are co-expressed and convey a poor outcome in ovarian cancer. Cancer. 2010;116:3276–3284. doi: 10.1002/cncr.25190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babjuk et al. (2017).Babjuk M, Böhle A, Burger M, Capoun O, Cohen D, Compérat EM, Hernández V, Kaasinen E, Palou J, Rouprêt M, Van Rhijn BAS, Shariat SF, Soukup V, Sylvester RJ, Zigeuner R. EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder: update 2016. European Urology. 2017;71:447–461. doi: 10.1016/j.eururo.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Bellmunt et al. (2014).Bellmunt J, Orsola A, Leow JJ, Wiegel T, De Santis M, Horwich A. Bladder cancer: ESMO practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2014;25:40–48. doi: 10.1093/annonc/mdu223. [DOI] [PubMed] [Google Scholar]
- Cowley et al. (2012).Cowley MJ, Pinese M, Kassahn KS, Waddell N, Pearson JV, Grimmond SM, Biankin AV, Hautaniemi S, Wu J. PINA v2.0: mining interactome modules. Nucleic Acids Research. 2012;40:862–865. doi: 10.1093/nar/gkr967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degoricija et al. (2014).Degoricija M, Šitum M, Korać J, Miljković A, Matić K, Paradžik M, Terzić IM, Jerončić A, Tomić S, Terzić J. High NF-κB and STAT3 activity in human urothelial carcinoma: a pilot study. World Journal of Urology. 2014;32:1469–1475. doi: 10.1007/s00345-014-1237-1. [DOI] [PubMed] [Google Scholar]
- Gilmore (2006).Gilmore TD. Introduction to NF-κB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- Hodges et al. (2010).Hodges KB, Beltran AL, Davidson DD, Montironi R, Cheng L. Urothelial dysplasia and other flat lesions of the urinary bladder: clinicopathologic and molecular features. Human Pathology. 2010;41:155–162. doi: 10.1016/j.humpath.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Jenkins et al. (2007).Jenkins GJS, Mikhail J, Alhamdani A, Brown TH, Caplin S, Manson JM, Bowden R, Toffazal N, Griffiths AP, Parry JM, Baxter JN. Immunohistochemical study of nuclear factor-κB activity and interleukin-8 abundance in oesophageal adenocarcinoma; a useful strategy for monitoring these biomarkers. Journal of Clinical Pathology. 2007;60:1232–1238. doi: 10.1136/jcp.2006.043976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst et al. (2009).Karst AM, Gao K, Nelson CC, Li G. Nuclear factor kappa B subunit p50 promotes melanoma angiogenesis by upregulating interleukin-6 expression. International Journal of Cancer. 2009;124:494–501. doi: 10.1002/ijc.23973. [DOI] [PubMed] [Google Scholar]
- Kim et al. (2013).Kim H, Park J, Cho Y, Cho C, Kim J, Shin H, Chung DH, Kim SJ, Chun Y. Pathogenic role of HIF-1α in prostate hyperplasia in the presence of chronic inflammation. Biochimica et Biophysica Acta Molecular Basis of Disease. 2013;1832:183–194. doi: 10.1016/j.bbadis.2012.09.002. [DOI] [PubMed] [Google Scholar]
- King et al. (2011).King CC, Sastri M, Chang P, Pennypacker J, Taylor SS. The rate of NF-κB nuclear translocation is regulated by PKA and A kinase interacting protein 1. PLOS ONE. 2011;6:e18713. doi: 10.1371/journal.pone.0018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi et al. (2014).Kobayashi H, Kikuchi E, Mikami S, Maeda T, Tanaka N, Miyajima A, Nakagawa K, Oya M. Long term follow-up in patients with initially diagnosed low grade Ta non-muscle invasive bladder tumors: tumor recurrence and worsening progression. BMC Urology. 2014;14:5. doi: 10.1186/1471-2490-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2012).Lee S, Lee E, Kim S, Jeong P, Cho Y, Yun SJ, Kim S, Kim G, Choi YH, Cha E, Kim W, Moon S. Identification of pro-inflammatory cytokines associated with muscle invasive bladder cancer; the roles of IL-5, IL-20, and IL-28A. PLOS ONE. 2012;7:e40267. doi: 10.1371/journal.pone.0040267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2014).Lee YJ, Moon KC, Jeong CW, Kwak C, Kim HH, Ku JH. Impact of squamous and glandular differentiation on oncologic outcomes in upper and lower tract urothelial carcinoma. PLOS ONE. 2014;9:e107027. doi: 10.1371/journal.pone.0107027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard et al. (2006).Lessard L, Karakiewicz PI, Bellon-gagnon P, Alam-fahmy M, Ismail HA, Saad F. Nuclear localization of nuclear factor-κB p65 in primary prostate tumors is highly predictive of pelvic lymph node metastases. Clinical Cancer Research. 2006;12:5741–5746. doi: 10.1158/1078-0432.CCR-06-0330. [DOI] [PubMed] [Google Scholar]
- Levidou & Saetta (2008).Levidou G, Saetta AA. Clinical significance of nuclear factor (NF)-κB levels in urothelial carcinoma of the urinary bladder. Virchows Archives. 2008;452:295–304. doi: 10.1007/s00428-007-0560-y. [DOI] [PubMed] [Google Scholar]
- Masilamoni et al. (2006).Masilamoni JG, Jesudason EP, Baben B, Jebaraj CE, Dhandayuthapani S, Jayakumar R. Molecular chaperone α-crystallin prevents detrimental effects of neuroinflammation. Biochimica et Biophysica Acta Molecular Basis of Disease. 2006;1762:284–293. doi: 10.1016/j.bbadis.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Meteoglu et al. (2008).Meteoglu I, Erdogdu IH, Meydan N, Erkus M, Barutca S. NF-KappaB expression correlates with apoptosis and angiogenesis in clear cell renal cell carcinoma tissues. Journal of Experimental & Clinical Cancer Research. 2008;27:53. doi: 10.1186/1756-9966-27-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller et al. (2016).Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA: A Cancer Journal for Clinicians. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- Mukherjee et al. (2017).Mukherjee N, Cardenas E, Bedolla R, Ghosh R. SETD6 regulates NF-κB signaling in urothelial cell survival: implications for bladder cancer. Oncotarget. 2017;8:15114–15125. doi: 10.18632/oncotarget.14750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee et al. (2015).Mukherjee N, Houston TJ, Cardenas E, Ghosh R. To be an ally or an adversary in bladder cancer: the NF-κB story has not unfolded. Carcinogenesis. 2015;36:299–306. doi: 10.1093/carcin/bgu321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oya et al. (2003).Oya M, Takayanagi A, Horiguchi A, Mizuno R, Ohtsubo M, Marumo K, Shimizu N, Murai M. Increased nuclear factor-κB activation is related to the tumor development of renal cell carcinoma. Carcinogenesis. 2003;24:377–384. doi: 10.1093/carcin/24.3.377. [DOI] [PubMed] [Google Scholar]
- Öztürk (2015).Öztürk H. Comparing RECIST with EORTC criteria in metastatic bladder cancer. Journal of Cancer Research and Clinical Oncology. 2015;142:187–194. doi: 10.1007/s00432-015-2022-2. [DOI] [PubMed] [Google Scholar]
- Saccani et al. (2006).Saccani A, Schioppa T, Porta C, Biswas SK, Nebuloni M, Vago L, Bottazzi B, Colombo MP, Mantovani A, Sica A. p50 Nuclear factor-κB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Research. 2006;66:11432–11440. doi: 10.1158/0008-5472.CAN-06-1867. [DOI] [PubMed] [Google Scholar]
- Schulz (2006).Schulz WA. Understanding urothelial carcinoma through cancer pathways. International Journal of Cancer. 2006;119:1513–1518. doi: 10.1002/ijc.21852. [DOI] [PubMed] [Google Scholar]
- Shannon et al. (2003).Shannon P, Markiel A, Ozier O, Baliga SN, Wang TJ, Ramage D, Amin N, Schwikowski B, andIdeker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Research. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery et al. (2018).Slattery ML, Mullany LE, Sakoda L, Samowitz WS, Wolff RK, Stevens JR, Herrick JS. The NF-κB signalling pathway in colorectal cancer: associations between dysregulated gene and miRNA expression. Journal of Cancer Research and Clinical Oncology. 2018;144:269–283. doi: 10.1007/s00432-017-2548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney et al. (2004).Sweeney C, Li L, Shanmugam R, Bhat-Nakshatri P, Jayaprakasan V, Baldridge LA, Gardner T, Smith M, Nakshatri H, Cheng L. Nuclear factor-κB is constitutively activated in prostate cancer in vitro and is overexpressed in prostatic intraepithelial neoplasia and adenocarcinoma of the prostate. Clinical Cancer Research. 2004;10:5501–5507. doi: 10.1158/1078-0432.CCR-0571-03. [DOI] [PubMed] [Google Scholar]
- Szebeni et al. (2017).Szebeni GJ, Vizler C, Kitajka K, Puskas LG. Inflammation and cancer: extra- and intracellular determinants of Tumor-Associated Macrophages as tumor promoters. Mediators of Inflammation. 2017;2017:1–13. doi: 10.1155/2017/9294018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (2015).Wu D, Wu P, Zhao L, Huang L, Zhang Z, Zhao S, Huang J. NF-κB expression and outcomes in solid tumors: A systematic review and meta-analysis. Medicine. 2015;94:e1687. doi: 10.1097/MD.0000000000001687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) High grade non invasive urothelial carcinoma H&E × 200.
(B) Immunostaining with RelA (p65) showed nuclear positivity (Brown colour) indicated by arrow IHC × 200.
(C) NF-κB p50 showed moderate cytoplasmic positivity IHC × 200. Note: adjacent lymphocytes (in-built control) have taken up nuclear staining indicated by arrows.
(D) High grade papillary invasive urothelial carcinoma with squamous differentiation H&E × 200.
(E) Immunostaining with RelA (p65) showed nuclear positivity (Brown colour) as indicated by arrows IHC × 200.
(F) NF-κBp50 showed few nuclear and cytoplasmic positivity in squamous regions which are indicated by arrows IHC × 200.
(G) High grade invasive urothelial carcinoma H&E × 100.
(H) Immunostaining with RelA (p65) showed nuclear positivity (Brown colour) as indicated by arrows IHC × 200.
(I) NF-κBp50 showed extensive cytoplasmic positivity as indicated by arrows IHC × 200.
(A) Low grade papillary urothelial carcinoma H&E × 100.
(B) Immunostaining with RelA (p65) showed nuclear positivity (Brown colour) IHC × 100.
(C) NF-κBp50 showed extensive cytoplasmic positivity IHC × 100.
(D) Low grade papillary urothelial carcinoma with tumor infiltration H&E × 40.
(E) Immunostaining with RelA (p65) showed nuclear positivity (Brown colour) IHC × 200.
(F) NF-κBp50 showed moderate cytoplasmic positivity IHC × 200.
(G) Normal urothelium H&E × 200.
(H) Immunostaining with RelA (p65) showed faint to moderate cytoplasmic positivity (Brown colour) IHC × 200. Note: Lymphocytes (in-built control) have taken up stain indicated by arrows.
(I) NF-κB p50 showed moderate cytoplasmic positivity indicated by arrow IHC × 200.
A, RelA subunit staining is a Negative control (Reagent)—No primary antibody was applied on sections
B, p50 subunit staining is a Negative control (Reagent)—No primary antibody was applied on sections
Data Availability Statement
The following information was supplied regarding data availability:
For Figs. 4 and 5, native photomicrographs for each stain and controls are available as Supplemental Files.