Abstract
Objective
This study investigates whether 99mTc pyrophosphate (PYP) imaging provides a quantitative non-invasive assessment of the extent of electroporation injury, and of the effect of poloxamer in vivo on electroporated skeletal muscle.
Methods
High-voltage electrical shock was used to produce electroporation injury in an anesthetized rat’s hind limb. In each experiment, the injured limb was treated intravenously by either poloxamer-188, dextran, or saline, and subsequently imaged with 99mTc PYP. The radiotracer’s temporal behavior among the experimental groups was compared using curve fitting of time–activity curves from the dynamic image data.
Results
The washout kinetics of 99mTc PYP changed in proportion to the electric current magnitude that produced electroporation. Also, 99mTc PYP washout from electroporated muscle differed between poloxamer-188 treatment and saline treatment. Finally, 10-kDa dextran treatment of electroporated muscle altered 99mTc PYP washout less than poloxamer-188 treatment.
Conclusions
Behavior of 99mTc PYP in electroporated muscle appears to be an indicator of the amount of electroporation injury. Compared to saline, intravenous polaxamer-188 treatment reduced the amount of 99mTc PYP uptake. Coupled to results showing poloxamer-188 seals ruptured cellular membranes, lessens the extent of electroporation injury and improves cell viability, 99mTc PYP imaging appears to be a useful in vivo monitoring tool for the extent of electroporation injury.
Keywords: Tc-99m pyrophosphate, Electrical injury, Electroporation, Poloxamer-188, Small animal imaging
1. Introduction
Since the development of electrical devices and electrical power distribution systems, electrical injury has been a serious problem [1]. Massive trauma can result from high-voltage shock by power–frequency electrical currents [2]. In biological tissues, the severity of response to electrical injury is related to the current involved. Responses range from muscle twitch and mild pain at small current to cardiac defibrillation at high currents [3]. Nerves and muscles are particularly susceptible to damage from electric shock, in part because of the extreme voltage gradients that can occur along these long cells. Burns and cellular disruption result directly from heat generated by an electric arc and the passage of electric current over and through the body, as well as from hot or burning debris [1]. Neuropsychological disorders can result both from physical trauma and emotional reaction to the injury [4,5]. A variety of approaches are used for the treatment of electrical injury, including fluid resuscitation, metabolic and respiratory support, surgical debridement, and skin grafting [6–8]. New therapeutic methods, such as immunologic therapy and artificial skin materials, may provide significant benefit. Basic research into the mechanisms of electrical injury is providing new insight into cellular-level approaches for limiting and repairing damage.
Historically, the damage from high-voltage injury was thought to be a direct result of heat production as current passes through tissue. Heating causes macromolecules to deform and become unable to perform their cellular functions; this can disrupt cellular metabolism and compromise structural integrity, resulting in cell death. Nerve cells typically have low resistivity, decreasing the likelihood of heating, but nerve cells are particularly susceptible to electrical injury. It is not uncommon for electrical shock victims to present with deep muscle and nerve damage, simultaneous with minimal signs of thermal injury at the site of contact [9,10]. This suggests the presence of damage mechanisms in addition to thermal damage [11].
Electroporation has been shown to be another cause of damage in high-voltage electrical injury [12,13]. One postulated mechanism is that field gradients during electric shock force polar water molecules into molecular-scale defects in the cellular membrane; the result is enlargement of the defects to form pores [14]. In electroporated tissues, cellular membranes are perforated, interfering with normal metabolic function. If the membrane damage can be patched, the cell may be able to resume functioning and survive the injury. Poloxamer, a type of surfactant, appears to form a suitable patch [15].
Poloxamers are non-toxic nonionic surface-active triblock copolymers [16]. Poloxamer’s structure, with a hydrophobic region bracketed by hydrophilic regions, has similarities to the phospholipid bilayer that comprises cellular membranes. Research indicates poloxamers associate themselves with a damaged membrane, coating exposed hydrophobic regions of the membrane and plugging water-enlarged pores [15,17]. Shielded from degradatory influences, the cell would be provided the opportunity to recover, repair the membrane damage, and resume normal function.
The potential therapeutic effects of poloxamer-188 have been reported by a variety of researchers for a variety of types of tissue injury. Poloxamer-188 has been shown to limit the damage from electroporation to membranes [15,18] and to improve tissue viability [15,19]. Poloxamer-188 increases capillary circulation in several types of injuries [19,20]. Poloxamer-188 has also been reported to limit damage and improve recovery from mechanical, chemical, thermal, and radiation damage as well as ischemia-reperfusion injury in a variety of tissue types that include neurons, muscles, and heart [21–27]. The bulk of studies have investigated the effects of poloxamer-188 in vitro or in animal models. The literature includes some human case studies, as well as evident interest in using poloxamers in conjunction with electroporation as a tool for transmembrane drug delivery.
1.1. Radiotracer imaging of electrical injury
A number of trauma types can damage skeletal muscle and other soft tissues. Besides electrical injury, frostbite, ischemia and infections result in soft tissue injury. A variety of diagnostic methods are used commonly to evaluate soft tissue injury. These include visual assessment, histological and blood chemistry analysis, and radiological imaging.
Magnetic resonance imaging (MRI) is a valuable diagnostic tool because it provides exceptional delineation of soft tissues compared to other types of radiological imaging. The utility of MRI for electrical injury has been reported in several instances [28–32]. The detail visible from MRI can help to guide the surgeon in removal of tissue; MRI also visualizes the extent of deep tissue damage without the need for invasive exploration.
Functional imaging is also useful for assessing soft tissue injury. Compared to the anatomical visualization provided by MRI and CT scans, functional images show the physiological behavior of an organ system. With appropriate techniques, factors such as tissue perfusion, inflammation, metabolism and necrosis can be visualized. While techniques such as functional MRI have not yet been reported for assessing soft tissue damage, radiotracer imaging has been used extensively for assessment of soft tissue injury [33–36]. A number of tracers, particularly 99mTc-labeled phosphonates and 99mTc pyrophosphate (PYP), accumulate in damaged soft tissue [37–39]. The degree of tracer accumulation is often a good index of the extent of tissue damage [40–42].
Over the past two decades, radiotracer imaging has been used to assess the extent of damage in electrical injury victims [43–48]. Radiotracer imaging can delineate necrotic regions from viable tissue by visualizing the altered metabolism or function in these regions. In many cases, areas of abnormal tracer uptake may not appear visually necrotic until hours or days after the injury. The results of radiotracer imaging can assist in planning the surgical and therapeutic management of an injury [49]. A variety of radiopharmaceuticals are or could prove useful for clinical imaging of electrical injury of soft tissues, including 99mTc PYP, 99mTc hexakis-2-methoxyisobutylisonitrile (MIBI), 133Xe, and 18F-fluorodeoxyglucose (FDG).
Our study reports on 99mTc PYP imaging of electroporation injury in an in vivo rat model. Alterations in 99mTc PYP kinetics caused by differing amounts of electroporation injury and different therapeutic agents were assessed by region of interest (ROI) analysis of dynamic radiotracer images and curve fitting. The purpose of our study was to determine if 99mTc PYP imaging provides a quantitative non-invasive assessment of the extent of electroporation injury and of the effect of poloxamer treatment on electroporated skeletal muscle.
2. Materials and methods
Fig. 1 shows the experimental equipment used in this work. The components are described in the following sections.
Fig. 1.
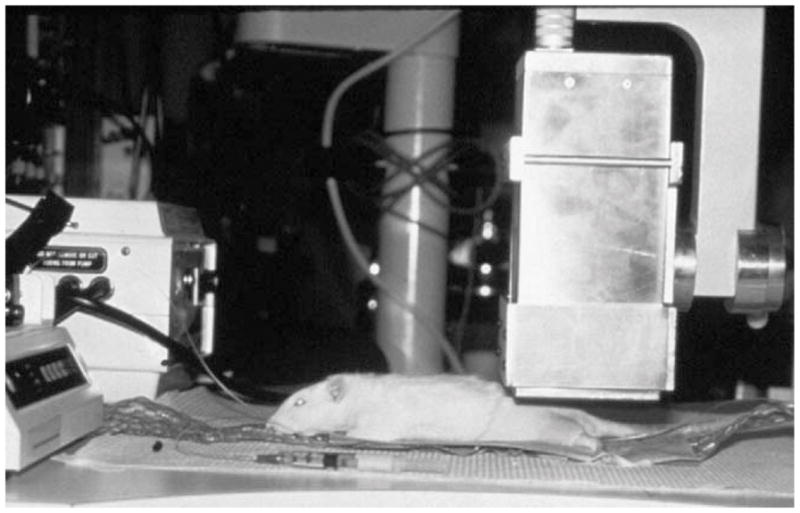
Photograph of the experimental setup, showing the anesthetized animal and the small gamma camera. The anesthetic infusion pump is seen at left and a syringe coupled to the jugular catheter is in the foreground; a pumped-water heating pad maintains the animal’s body temperature.
2.1. In vivo model of electroporation
This research utilized the in vivo model of electroporation developed by Block et al. [50]. This model provides a means to study electroporation in the absence of thermal damage, in the skeletal muscle of the hind limb of an anesthetized rat; our study focuses on visualizing damage to the biceps femoris muscle. Briefly, the centerpiece of the model is a female Sprague–Dawley rat kept fully anesthetized by continuous intraperitoneal infusion of a mixture of ketamine and xylazine. The animals used in this work weighed 280–350 g and all were non-pregnant and free of disease. A jugular cannula introduced radiotracer, saline, and therapeutic agents directly into the bloodstream of the animal. All experiments were not survived; euthanasia at the end of the experiment was accomplished with an overdose of xylazine administered either through the jugular cannula or directly into the heart.
As in Block’s model, electroporation damage was produced throughout the muscle of one hind limb by applying a sequence of high-voltage current-regulated dc electrical pulses from the ankle to the base of the tail [50]. Electric field strength of approximately 150 V/cm was delivered at a constant current between the ankle and base of the tail. The maximum available current was 1.85 A. The pulse duration was 4 ms, equivalent to the length of time that a 60 Hz sine wave exceeds its root-mean-square (rms) amplitude in each half-cycle. Twelve pulses were applied from the base of the tail to the ankle over a 2-min span; the 10-s interval between pulses minimizes heat accumulation in the limb.
2.2. High-resolution small-field-of-view gamma camera
The imaging system used in this work was a high-resolution small-field-of-view gamma camera [51]. The camera provides a 64 × 64 pixel, 4.8 cm × 4.8 cm field of view, sufficient to image one hind limb of the rats used in this study; a second camera was used to image the contralateral (unshocked) limb in some experiments. With its high-resolution collimator, the imaging resolution is 5.4 mm full-width-at-half-maximum at a 5 cm depth.
The camera software provides automated acquisition of dynamic image data; each image is 2 min in duration, with a 2.4 min interval between the start of successive images. (The 0.4-min lag was the length of time required by the operator to manually save each image to disk in a pre-automated version of the software; the lag was maintained to facilitate comparison with earlier imaging studies.) Region of interest (ROI) analysis and curve-fitting of the dynamic image data was performed off-line using analysis programs written in the Interactive Data Language (IDL) software (Research Systems, Boulder, CO).
2.3. Selection of radiotracer
Four radiotracers were considered for use in this research. These were 99mTc PYP, 99mTc diethylenetriaminepentaacetate (DTPA), 99mTc hexakis-2-methoxyisobutylisonitrile (MIBI), and 201Tl thallous chloride. Each tracer has biological characteristics that make it potentially useful for imaging electroporation injury [52]. Technetium-99m PYP was selected as the best candidate radiotracer for imaging electroporation in the in vivo rat model.
Technetium-99m PYP is well known as a tracer for soft tissue injury [37–40]. It accumulates in damaged soft tissue, clears moderately quickly from undamaged soft tissue, and accumulates over time in bone. The biggest drawback to using 99mTc PYP is that the mechanism of its accumulation in damaged tissue is not well understood; it is believed to follow calcium in cellular function [53–55]. The exact structure of 99mTc PYP is not known; PYP forms a complex with technetium in the presence of a tin catalyst, but the number of PYP molecules per technetium atom has never been determined conclusively. Estimates range from one to five PYP molecules per technetium atom; it appears possible that the number is variable.
2.4. Therapeutic agents
The two therapeutic agents investigated in this work were poloxamer and dextran; saline was used as a control. Poloxamer-188 (Pluronic F68, BASF Corp., Florham Park, NJ) was examined at 17 and 68 mg dosages. The dextran experiments used 10 or 40 kDa neutral dextran (Sigma Chemicals, St. Louis, MO) at a 17 mg dosage. Ten kilodaltons dextran is similar in molecular weight to poloxamer-188; 40 kDa dextran is used clinically as a plasma expander in electrical injury victims. Saline was used in control experiments. Saline-treated unshocked muscle provided a normal baseline for comparison. In all cases, a solution volume of 0.4 ml (e.g., 68 mg of poloxamer-188 was dissolved in saline to produce a volume of 0.4 ml) was injected through the jugular cannula. Table 1 summarizes the parameters of the experimental protocol, including the numbers of animals in each experimental group.
Table 1.
Summary of experimental parameters
| Common parameters | ||||
|---|---|---|---|---|
| Pulse sequence | ||||
| 12 constant-current pulses | ||||
| 4 ms duration each | ||||
| Field strength of ~150 V/cm in hind limb | ||||
| 10 s interval between pulses | ||||
| Injection times | ||||
| Therapeutic agent: 10 min post-shock | ||||
| Radiotracer: 30 min post-shock | ||||
| Radiotracer | ||||
| 99mTc pyrophosphate | ||||
| Nominal injected activity: 37 ± 7.4 MBq (1.0 ± 0.2 mCi) | ||||
| Image acquisition | ||||
| 2 min duration for each image | ||||
| New image starting every 2.4 min | ||||
| ~3.5 h (88–90 images) | ||||
| Experimental group namea | No. of rats | Shocked? (current) | Therapeutic agent | |
|
| ||||
| Type | Amountb (blood concc) | |||
|
| ||||
| 0 A saline | 4 | No | Saline | – |
| 0 A 17 mg poloxamer-188 | 3 | No | Poloxamer-188 | 17 mg (1 mg/ml) |
| 0.5 A saline | 2 | Yes (0.5 A) | Saline | – |
| 1.0 A saline | 4 | Yes (1.0 A) | Saline | – |
| 1.85 A saline | 8 | Yes (1.85 A) | Saline | – |
| 1.85 A 17 mg poloxamer-188 | 10 | Yes (1.85 A) | Poloxamer-188 | 17 mg (1 mg/ml) |
| 1.85 A 68 mg poloxamer-188 | 4 | Yes (1.85 A) | Poloxamer-188 | 68 mg (4 mg/ml) |
| 1.85 A 17 mg 10 kDa dextran | 10 | Yes (1.85 A) | 10 kDa dextran | 17 mg (1 mg/ml) |
| 1.85 A 17 mg 40 kDa dextran | 14 | Yes (1.85 A) | 40 kDa dextran | 17 mg (1 mg/ml) |
Designated by applied current and therapeutic agent.
Dissolved in 0.4 ml of saline.
Assuming an average blood volume of 17 ml.
The blood volume of a 300 g rat is approximately 17 ml. Thus, the approximate effective blood concentrations in the groups of experimental animals treated with 17 and 68 mg of poloxamer-188 are 1 and 4 mg/ml, respectively. A blood concentration of 1 mg/ml approximates the dosage that might be administered to patients in clinical trials of poloxamer therapy for electroporation. A 4 mg/ml concentration is close to critical micelle concentration for poloxamer-188; above this concentration, poloxamer is expected to form micelles, thereby potentially limiting interactions with ruptured cell membranes.
2.5. Imaging protocol
The experimental protocol for investigating poloxamer calls for the investigative agent to be injected through the jugular cannula at ten minutes after shock. In control experiments, saline was injected at this time. Technetium-99m PYP was injected through the cannula at 30 min post-shock. An activity of 37 × 7.4 MBq (1.0 × 0.2 mCi) in 0.4 ml saline was injected. The small gamma camera acquired serial, posterior view images of the shocked hind limb. Each image was 2 min in duration with a new image starting every 2.4 min. The imaging phase of each experiment extended for 3.5 h post-shock; 88–90 images were usually obtained. During data analysis, the dynamic image data were reviewed. If a cine image loop showed that the limb moved or that radioactive urine leaked into the field of view, the data for that experiment were rejected from further analysis.
Experiments that had excessive vascular damage in the hind limb were excluded also. While the focus of this research is electroporation of skeletal muscle, the electroporation protocol cannot avoid doing some damage to the vasculature of the shocked limb. In some experiments, the amount of tracer uptake in a shocked limb would never rise above the level seen in unshocked limbs. This lack of tracer delivery was due most likely to compromised vasculature. To assess the extent of vascular damage in each experiment, lissamine green was injected through the jugular catheter 10 min before euthanasia. Lissamine green is a dye whose distribution depends on tissue perfusion; poor staining of the muscle indicates compromised vasculature. After euthanasia, the biceps femoralis muscle of the shocked and contralateral limbs would be exposed and visually inspected. If the shocked muscle showed little staining compared to the unshocked limb, the vasculature was deemed compromised and the data rejected. We noted the correlation that experiments where the muscle of the shocked limb showed little staining with lissamine green, the tracer uptake consistently was below unshocked saline-treated levels at all times. Note that the lissamine green observation was always recorded before any image data analysis; rejection of a data set was based on the lissamine green staining, not on the radiotracer image data.
The research plan was to acquire ten experiments at 1.85 A of applied current for each type and concentration of therapeutic agent and five experiments each for the unshocked data sets and the data sets that compared the other levels of applied current. Experiments where compromised vasculature was noted via lissamine green staining, were not counted towards each category’s total and an additional experiment was done in these cases. Instances of limb movement or radioactive urine in the field of view were identified later during the data analysis, and additional experiments were not done to replace these. The final numbers of experiments in each data set are noted in the legends of the figures and in Table 1. Four additional experiments were collected for the 17 mg 40 kDa dextran data set while training a new lab employee; these experiments were included in the analysis because there were no specific procedural issues to justify excluding them from the analysis. Approximately 80 experiments in total were performed to obtain the data reported here.
2.6. ROI analysis
ROI analysis was used to extract time–activity curves (TACs) from the dynamic image data. Using display and analysis code written in IDL, an 8 × 7-pixel (6 mm × 5.25 mm) ROI positioned over the biceps femoralis muscle was used to compute a TAC for each experiment. The TACs were positioned using a composite image produced by summing all images from the dynamic data set. The location of the knee and the outline of soft tissue of the hind limb were clearly visible in the composite image; the ROI was placed visually over the soft tissue below the bones of the lower limb, as shown in Fig. 2. The TACs from individual experiments were averaged to yield a mean curve for each data set. To provide a quantitative characterization of the ROI data for 99mTc PYP, the average TACs were fit by a least-squares algorithm to a simple mathematical model. The fitting function uses an exponential decay on a constant background level, with the form:
| (1) |
to describe the washout of tracer. Tracer uptake was not modeled; uptake occurs rapidly during the first two minutes after radiotracer injection and was not recorded by our imaging protocol.
Fig. 2.
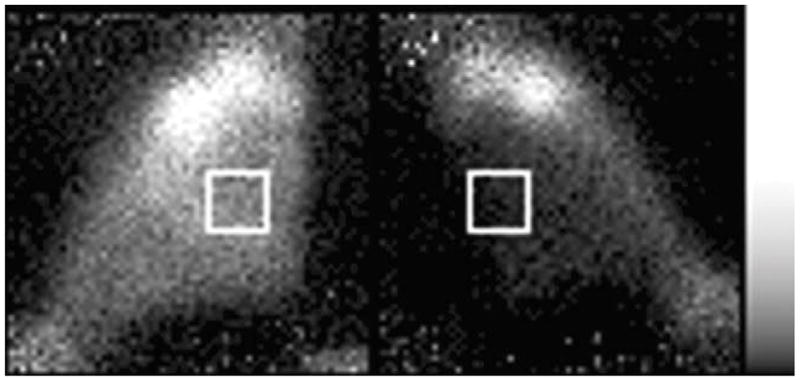
Typical posterior-view image of 99mTc PYP in the hind limbs. The left limb was shocked with 1.85 A pulses and 99mTc PYP was injected at 30 min post-shock. The left limb was imaged for 12 min at 105 min post-shock; the right limb was imaged for 12 min at 120 min post-shock. The muscle of the shocked limb (left) shows substantially more uptake than does the muscle of the unshocked limb (right). The white boxes illustrate the typical placement of ROIs over the muscle.
The fitted parameters for the curves were compared statistically to determine the level of significance of differences in the fitted parameters. Student’s t-value was computed for pairs of corresponding average fitted parameters between the different data sets. A level of p < 0.01 was chosen to indicate the level of significance. This relatively stringent level was chosen because multiple comparisons between data sets are made. Each data set is compared to six other data sets; consequently, the selected p-value corresponds to significance approximately at a 95% confidence level.
3. Results
Technetium-99m PYP is known to accumulate in damaged soft tissue, and the experimental validation of the in vivo electroporation model [50] confirmed that 99mTc PYP accumulates in electroporated tissue. Fig. 3 shows that the TAC magnitudes for 99mTc PYP are proportional to the current magnitude used to produce electroporation and, thus, indicative of the amount of electroporation injury. This result indicates that 99mTc PYP can serve as an index to the extent of electroporation injury. Higher uptake means more damage and lower uptake means less damage. This provides a means for in vivo assessment of the effect of poloxamer-188 on electroporated muscle. If membrane damage is being reduced by poloxamer, the uptake of PYP should be less than in muscle to which poloxamer was not administered.
Fig. 3.
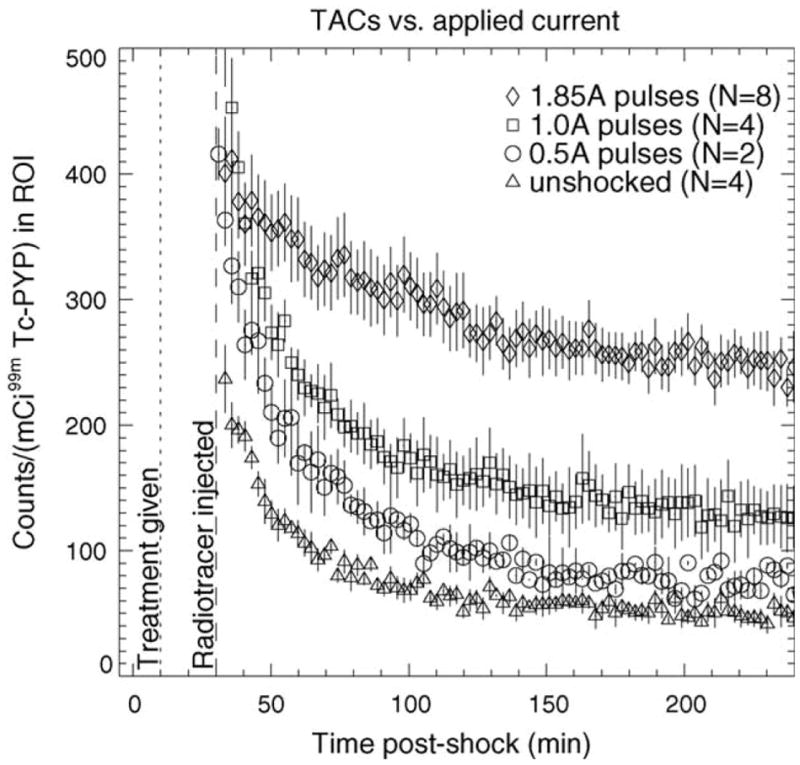
TACs for 99mTc PYP in saline-treated electroporated muscle vs. applied current. Saline was injected 10 min post-shock (dotted line) and radiotracer was injected 30 min post-shock (dashed line). Error bars represent S.E.M.
To rule out the possibility that any observed change in 99mTc PYP kinetics in poloxamer-treated muscle is caused by direct molecular interaction of poloxamer with PYP, independent of the presence of tissue damage, a set of poloxamer-treated unshocked experiments were conducted. Fig. 4 shows the average TAC for unshocked muscle treated with 17 mg poloxamer-188. The TAC magnitude is marginally higher at all times than that for unshocked muscle treated with saline. This slight increase most likely results from poloxamer-188 acting as a plasma expander [56]. Therefore, any perceived change in tracer behavior includes a small fractional increase due to this effect. In addition, this effect is opposite in magnitude to the observed changes in PYP uptake in poloxamer-treated shocked muscle (see below), giving confidence that the observed decreases in the poloxamer-treated shocked experiments are due primarily to the effects of poloxamer on the damaged tissue, rather than from direct interaction between the 99mTc PYP and the poloxamer. One could remove the effect of this plasma-expansion increase from the other data sets by subtracting away the average unshocked-poloxamer-treated TAC. However, the increase in TAC magnitude is small (relative to the large increase seen in shocked muscle) so we chose not to implement a correction to the other image data for this effect.
Fig. 4.
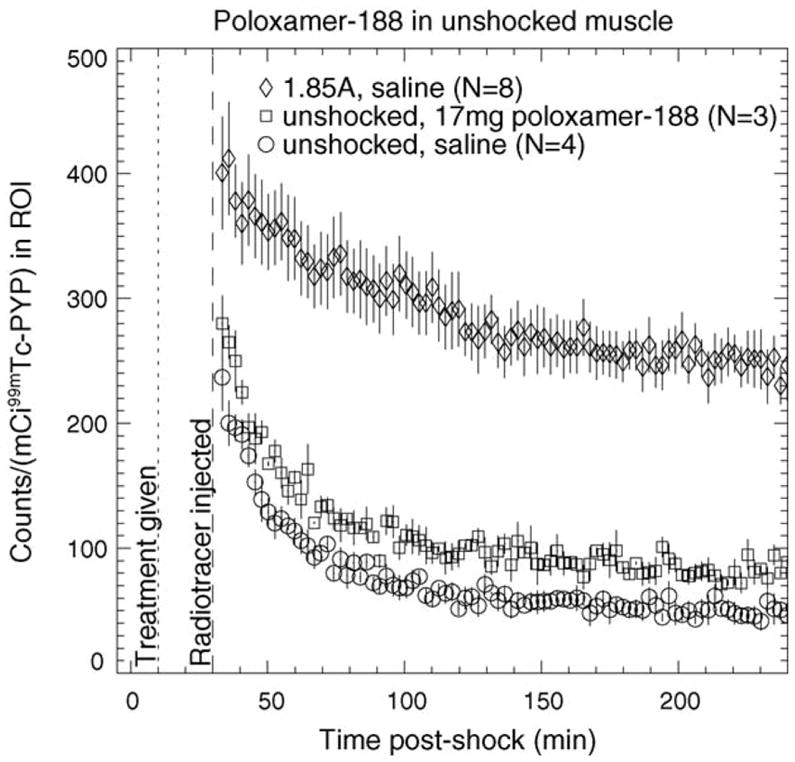
TAC of 99mTc PYP in unshocked muscle treated with 17 mg poloxamer-188. The TACs for unshocked saline-treated and 1.85 A-shocked saline-treated muscle are shown for comparison. Saline or polox-amer-188 was injected 10 min post-shock (dotted line) and radiotracer was injected 30 min post-shock (dashed line). Error bars represent S.E.M.
Shown in Fig. 5 are average TACs for 99mTc PYP in unshocked saline-treated, shocked saline-treated and shocked poloxamer-treated muscle. It is immediately apparent that the magnitude of PYP uptake in electroporated muscle is substantially higher than in normal muscle. Additionally, while the temporal behavior is similar for saline-treated electroporated and saline-treated normal muscle, the behavior appears different for electroporated muscle that has been treated with 17 mg of poloxamer-188. The relatively constant value of the TAC for poloxamer treatment contrasts sharply with the exponential shape of the saline-treated curves. One also sees that treatment with 68 mg of poloxamer-188 results in a TAC that is similar to that for saline-treated muscle. A pertinent question here is what causes this different behavior of PYP in 17 mg poloxamer-188 treated muscle. Ideally, the altered kinetics are due to sealing of ruptured membranes by poloxamer-188, leading to improved long-term cell viability.
Fig. 5.
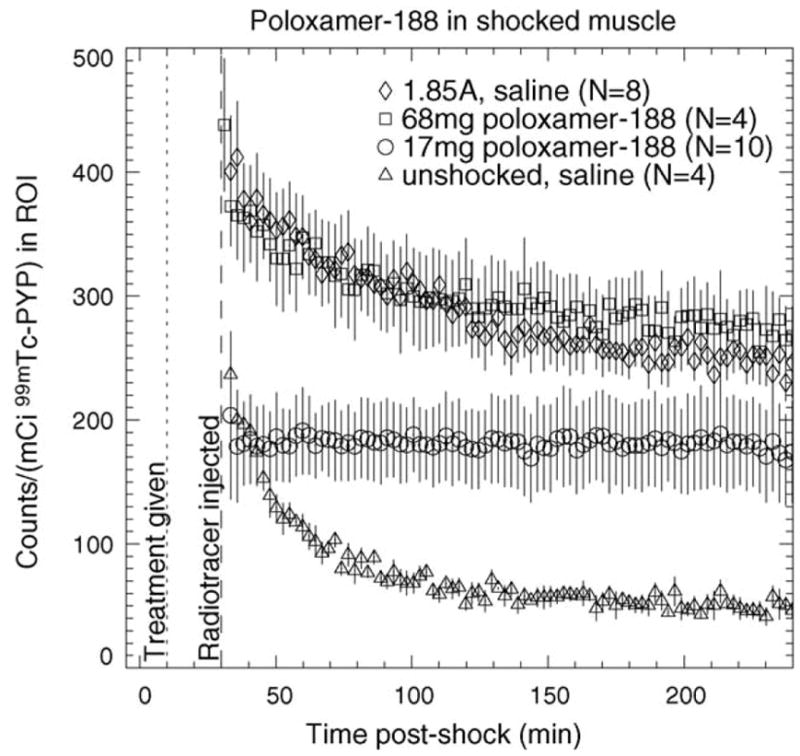
TACs of 99mTc PYP in poloxamer-188-treated electroporated muscle. TACs for unshocked saline-treated muscle and saline-treated muscle shocked at 1.85 A are shown for comparison. Therapeutic agent was injected 10 min post-shock (dotted line); radiotracer was injected 30 min post-shock (dashed line). Error bars represent S.E.M.
Fig. 6 illustrates the results of treating electroporated muscle with 17 mg of 10 or 40 kDa neutral dextran. Forty kilodaltons dextran causes an initial marginal decrease in tracer uptake compared to saline, but little difference is apparent at 4 h post-shock. A larger initial decrease is seen with 10 kDa dextran but the difference is negligible 4 h post-shock. Compared to shocked saline-treated muscle, tracer washout appears slightly faster for 40 kDa dextran and substantially faster for 10 kDa dextran. Fig. 7 shows that treatment with 17 mg of 10 kDa dextran does not alter the kinetics of 99mTc PYP as much as treatment with 17 mg of poloxamer-188 does.
Fig. 6.
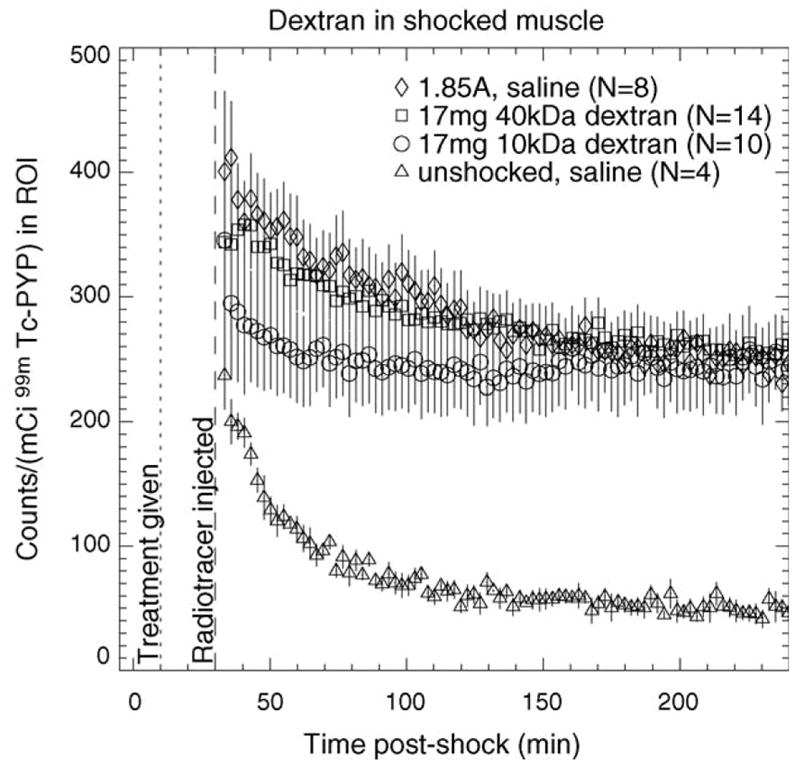
TACs of 99mTc PYP for 1.85 A-shocked electroporated muscle treated with 10 and 40 kDa dextran. TACs for unshocked saline-treated muscle and saline-treated muscle shocked at 1.85 A are shown for comparison. Therapeutic agent was injected 10 min post-shock (dotted line); radiotracer was injected 30 min post-shock (dashed line). Error bars represent S.E.M.
Fig. 7.
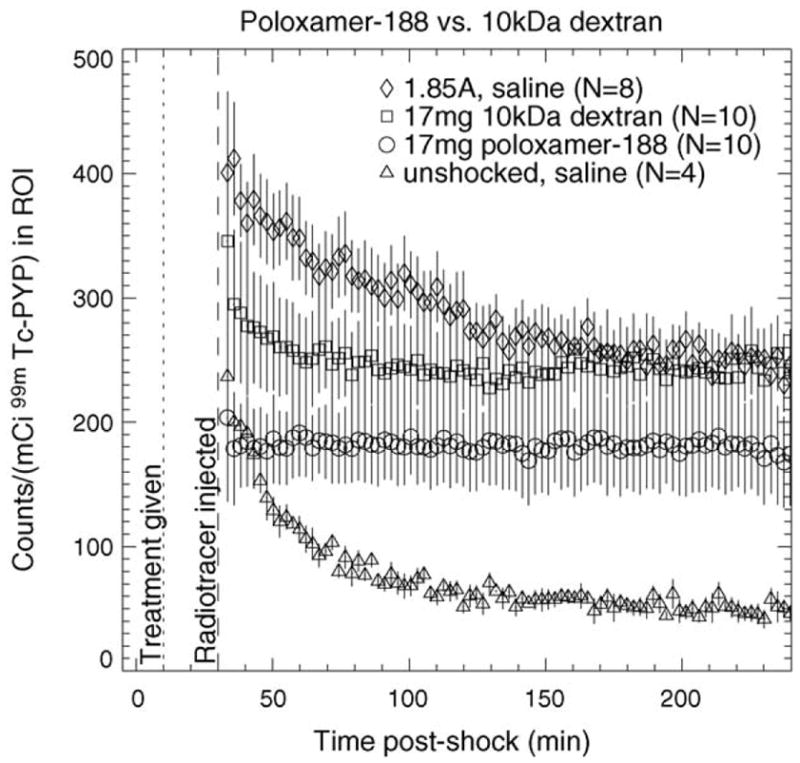
Comparison of TACs for treatment with 17 mg poloxamer-188 and 17 mg 10 kDa dextran. The TACs for 1.85 A-shocked and unshocked muscle treated with saline are shown for comparison. Therapeutic agent was injected 10 min post-shock (dotted line); radiotracer was injected 30 min post-shock (dashed line). Error bars represent S.E.M.
Table 2 lists the fitted values from the average curves; significance levels for pairs of average parameters from individual fitted curves are given in Table 3. Entries with p < 0.01 (corresponding approximately to a 95% confidence level for multiple comparisons between the different experimental groups) are presented in bold typeface. One of the most notable results is that the 17 mg poloxamer-treated data do not show any particular difference in its B-parameter from the other data sets; this indicates that the washout rate is unchanged, so the altered appearance of the curve is due to a change in initial magnitude (A-parameter). Another observation is that the unshocked saline-treated data are not significantly different in any of the fitting parameters from the unshocked poloxamer-treated data. Finally, one notes that the 1.85 A shocked saline-treated and 1.85 A shocked poloxamer-treated data are marginally different at best in the C, or baseline, parameter.
Table 2.
ROI-derived fitted time-activity curve parameters
| Experimental groupa | Fitting parameterb | ||
|---|---|---|---|
|
| |||
| Ac | Bd | Cc | |
| 0 A saline | 538.5 | 0.0359 | 53.0 |
| 1.85 A saline | 251.1 | 0.0146 | 239.3 |
| 1.85 A 17 mg poloxamer-188 | 24.0 | 0.0021 | 162.5 |
| 1.85 A 68 mg poloxamer-188 | 195.2 | 0.0193 | 274.1 |
| 1.85 A 17 mg 10-kDa dextran | 966.2 | 0.0781 | 244.9 |
| 1.85 A 17 mg 40 kDa dextran | 174.6 | 0.0151 | 250.2 |
| 0 A 17 mg poloxamer-188 | 696.7 | 0.0409 | 87.0 |
Designated by applied current and therapeutic agent.
Exponential decay + constant background model: f(t) = A exp(−Bt) + C.
Magnitude; in units of counts in ROI per 2-min image.
Decay constant; in units of min−1.
Table 3.
Statistical significance of fitted TAC parameters
| For experimental group | Compared to | p-Valuea,b | ||
|---|---|---|---|---|
|
| ||||
| A | B | C | ||
| 1.85 A saline | 1.85 A 17 mg poloxamer-188 | 0.035 | 0.856 | 0.023 |
| 1.85 A 68 mg poloxamer-188 | 0.271 | 0.569 | 0.912 | |
| 1.85 A 17 mg 10 kDa dextran | 0.217 | 0.360 | 0.845 | |
| 1.85 A 17 mg 40 kDa dextran | 0.775 | 0.335 | 0.905 | |
| 0 A 17 mg poloxamer-188 | 0.125 | 0.244 | 0.003 | |
| 0 A saline | 0.132 | 0.177 | 0.0003 | |
| 0 A saline | 1.85 A 17 mg poloxamer-188 | 0.0003 | 0.544 | 0.0004 |
| 1.85 A 68 mg poloxamer-188 | 0.006 | 0.036 | 0.0001 | |
| 1.85 A 17 mg 10 kDa dextran | 0.419 | 0.774 | 0.006 | |
| 1.85 A 17 mg 40 kDa dextran | 0.038 | 0.016 | 0.0002 | |
| 0 A 17 mg poloxamer-188 | 0.798 | 0.979 | 0.020 | |
| 1.85 A 17 mg poloxamer-188 | 1.85 A 68 mg poloxamer-188 | 0.027 | 0.642 | 0.007 |
| 1.85 A 17 mg 10 kDa dextran | 0.241 | 0.521 | 0.136 | |
| 1.85 A 17 mg 40 kDa dextran | 0.019 | 0.394 | 0.029 | |
| 0 A 17 mg poloxamer-188 | 0.0001 | 0.610 | 0.007 | |
| 1.85 A 68 mg poloxamer-188 | 1.85 A 17 mg 10 kDa dextran | 0.325 | 0.388 | 0.810 |
| 1.85 A 17 mg 40 kDa dextran | 0.274 | 0.847 | 0.833 | |
| 0 A 17 mg poloxamer-188 | 0.002 | 0.065 | 0.0009 | |
| 1.85 A 17 mg 10 kDa dextran | 1.85 A 17 mg 40 kDa dextran | 0.111 | 0.129 | 0.902 |
| 0-A 17-mg poloxamer-188 | 0.496 | 0.803 | 0.033 | |
| 1.85 A 17 mg 40 kDa dextran | 0 A 17 mg poloxamer-188 | 0.036 | 0.033 | 0.003 |
p-Value for Student’s t-statistic computed between the corresponding pair of fitted parameter values for the two datasets indicated.
Bold values indicate the corresponding pair of fitted parameters is significantly different at the p < 0.01 level.
4. Discussion
Our study demonstrates that the in vivo electroporation model is a useful tool for the study of the basic pathophysiologic process of electroporation. Radiotracer imaging of the in vivo model provides a means for quantifying the extent of electroporation injury and the effects of poloxamer-188 and other potential therapeutic agents on electroporated muscle.
Because of its history as a tracer for soft-tissue injury, 99mTc PYP was expected to be a good choice for use in the electroporation research. The curves in Fig. 5 show that the PYP TAC is substantially different in electroporated muscle treated with 17 mg poloxamer-188 than in saline-treated electroporated muscle. In particular, PYP reaches a steady-state level for the poloxamer case that is noticeably less than the plateau level for saline treatment. However, one notes that at the ~95% confidence level chosen for comparison, the A and C parameters for these data sets are not significantly different. Based on the apparent substantial difference in the TACs, one expects that with the addition of more experiments to these data sets, the errors bars will decrease in magnitude and the level of statistical significance will improve.
In unshocked muscle, PYP kinetics are not significantly different if the muscle is treated with 17 mg poloxamer-188 than if it is treated with saline. The baseline parameters for the unshocked data sets are significantly different from all the shocked data sets, as one expects visually from the TACs in Figs. 4–7.
For all data sets, the rate parameters for the TACs are not significantly different between any of the data sets. For the exponential-plus-constant-background fitting function, the B-parameter of the fitted TACs is not significantly different between either unshocked saline-treated and shocked saline-treated experiments or shocked saline-treated and shocked poloxamer-treated experiments. This indicates that the washout mechanism of PYP is unaltered by shocking or by treatment with poloxamer-188, while the mechanisms driving uptake and the equilibrium distribution are affected.
4.1. Radiotracers for future investigation
Technetium-99m PYP was chosen for this work primarily because of its extensive history as a tracer for soft tissue injury. However, 99mTc PYP was not the only possible choice. In addition to other tracers discussed in Ref. [52], 133Xe and 18F-FDG could be useful for clinical and research imaging of electroporation, but these were not considered practical for the current research. FDG has been reported for clinically assessing skeletal muscle viability [57]. Although, the shielding and collimation of the small gamma camera used in this study were not designed for 511 keV imaging, FDG imaging of the in vivo model of electroporation seems an interesting prospect, given the current availability of small animal PET scanners (e.g., microPET, CTI Molecular Imaging, Knoxville, TN). Xenon-133 was rejected as impractical for this investigation because of its long half-life and difficulties in achieving a specific activity of aqueous 133Xe that was high enough to calibrate the small gamma camera system.
Technetium-99m-labeled dextran and 99mTc-labeled poloxamer potentially would be ideal tracers for research and clinical investigations. Technetium-99m poloxamer would be especially attractive for investigating the interaction of poloxamer with electroporated membranes. While dextran has been successfully radiolabeled with 99mTc [58], the feasibility of developing 99mTc-labeled poloxamer for in vivo imaging is an open question; 18F-labeled poloxamer for PET imaging may be more reasonable.
5. Conclusion
In summary, the in vivo electroporation model [50] showed that electroporation without significant Joule heating can occur during high-voltage electrical shock. The model is clinically realistic and provides a means for studying the basic pathophysiology of electroporation injury. Our study used the in vivo model to study the effect of poloxamer on electroporated muscle. Intravenous injection of poloxamer-188 significantly lessens the amount of 99mTc PYP retained by electroporated skeletal muscle. Taken in conjunction with in vitro research reported in the literature [17,18,59], as well as in vivo studies that indicated poloxamer-188 minimizes damage and improves viability [15,19,23,25], the results from our work indicate that poloxamer may have a beneficial therapeutic effect on electroporated cells. Radiotracer imaging of electroporated skeletal muscle appears to be a viable non-invasive method for judging the extent of injury and tissue viability. Further work on modeling the kinetics of 99mTc PYP in poloxamer-treated electroporated tissues may provide the ability to quantify extent of injury from dynamic image data.
Acknowledgments
Support for this work was provided by the Department of Energy through grant no. DE-FG02-86ER60418 and by the Electrical Power Research Institute. Jeffrey Yap, PhD, provided advice on performing the statistical analysis on the image data. Grigory Abramov assisted with animal preparation for some of the experiments; the nuclear medicine technologists in the Department of Radiology prepared all the radiopharmaceuticals used in this work. Finally, the authors thank Thomas Block for his efforts in developing the in vivo model of electroporation. This work is dedicated to the memory of Malcolm Cooper, MD, our teacher, colleague and friend.
Footnotes
This work was conducted in accordance with institutional animal care and use policies and radiation protection practices.
References
- 1.Bernstein T. Electrical injury: Electrical engineer’s perspective and an historical review. In: Lee RC, Capelli-Schellpfeffer M, Kelly KM, editors. Ann NY Acad Sci. Vol. 720. 1994. pp. 1–10. Electrical injury: a multidisciplinary approach to therapy, prevention, and rehabilitation. [DOI] [PubMed] [Google Scholar]
- 2.Lee RC, Cravalho EG, Burke JF. Electrical trauma: the pathophysiology, manifestations, and clinical management. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- 3.Reilly JP. Scales of reaction to electric shock. In: Lee RC, Capelli-Schellpfeffer M, Kelly KM, editors. Ann NY Acad Sci. Vol. 720. 1994. pp. 21–37. Electrical injury: a multidisciplinary approach to therapy, prevention, and rehabilitation. [DOI] [PubMed] [Google Scholar]
- 4.Kelley KM, Pliskin N, Meyer G, Lee RC. Neuropsychiatric aspects of electrical injury: The nature of psychiatric disturbance. In: Lee RC, Capelli-Schellpfeffer M, Kelly KM, editors. Ann NY Acad Sci. Vol. 720. 1994. pp. 213–8. Electrical injury: a multi-disciplinary approach to therapy, prevention, and rehabilitation. [DOI] [PubMed] [Google Scholar]
- 5.Duff K, McCaffrey RJ. Electrical injury and lightning injury: a review of their mechanisms and neuropsychological, psychiatric, and neurological sequalae. Neuropsychol Rev. 2001;11:101–16. doi: 10.1023/a:1016623318049. [DOI] [PubMed] [Google Scholar]
- 6.Waymack JP, Rutan RL. Recent advances in burn care. In: Lee RC, Capelli-Schellpfeffer M, Kelly KM, editors. Ann NY Acad Sci. Vol. 720. 1994. pp. 230–8. Electrical injury: a multi-disciplinary approach to therapy, prevention, and rehabilitation. [DOI] [PubMed] [Google Scholar]
- 7.Hammond JS, Ward CG. High-voltage electrical injuries: management and outcome of 60 cases. South Med J. 1988;81:1351–2. doi: 10.1097/00007611-198811000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Lee RC, Dougherty W. Electrical injury: mechanisms, manifestations, and therapy. IEEE Trans Dielectr Electr Insul. 2003;10:810–9. [Google Scholar]
- 9.Lee RC, Kolodney MS. Electrical injury mechanisms: dynamics of the thermal response. Plast Reconstr Surg. 1987;80:663–71. [PubMed] [Google Scholar]
- 10.Smith MA, Muehlberger T, Dellon AL. Peripheral nerve compression associated with low-voltage electrical injury without associated significant cutaneous burn. Plastic Reconstruct Surg. 2002;109:137–44. doi: 10.1097/00006534-200201000-00023. [DOI] [PubMed] [Google Scholar]
- 11.Lee RC, Gaylor DC, Bhatt D, Israel DA. Role of cell membrane rupture in the pathogenesis of electrical trauma. J Surg Res. 1988;44:709–19. doi: 10.1016/0022-4804(88)90105-9. [DOI] [PubMed] [Google Scholar]
- 12.Bhatt DL, Gaylor DC, Lee RC. Rhabdomyolysis due to pulsed electric fields. Plastics Reconstruct Surg. 1990;86:24–34. doi: 10.1097/00006534-199007000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Lee RC, Kolodney MS. Electrical injury mechanisms: electrical breakdown of cell membranes. Plastics Reconstruct Surg. 1987;80:672–9. doi: 10.1097/00006534-198711000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Abidor IG, Arakelyan VB, Chernomordik LV, Chizmadzhev YA, Pastushenko VF, Tarasevick MR. Electric breakdown of bilayer lipid membranes. I. The main experimental facts and their qualitative discussion. Bioelectrochem Bioenerget. 1979;6:37–52. [Google Scholar]
- 15.Lee RC, River P, Pan F-S, Ji L, Wollmann RL. Surfactant-induced sealing of electropermeabilized skeletal muscle membranes in vivo. Proc Natl Acad Sci USA. 1992;89:4524–8. doi: 10.1073/pnas.89.10.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmolka IR. Artificial skin. I. Preparation and properties of pluronic F-127 gels for treatment of burns. J Biomed Mater Res. 1972;6:571–82. doi: 10.1002/jbm.820060609. [DOI] [PubMed] [Google Scholar]
- 17.Maskarinec SA, Hannig J, Lee RC, Lee KYC. Direct observation of poloxamer 188 insertion into lipid monolayers. Biophys J. 2002;82:1453–9. doi: 10.1016/S0006-3495(02)75499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma V, Stebe K, Murphy JC, Tung L. Poloxamer 188 decreases susceptibility of artificial lipid membranes to electroporation. Biophys J. 1996;71:3229–41. doi: 10.1016/S0006-3495(96)79516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basakaran H, Toner M, Yarmush ML, Berthiaume F. Poloxamer-188 improves capillary blood flow and tissue viability in a cutaneous burn wound. J Surg Res. 2001;101:56–61. doi: 10.1006/jsre.2001.6262. [DOI] [PubMed] [Google Scholar]
- 20.Hsu LL, Liu XW, Pierangeli S, et al. Microcirculatory effects of poloxamer 188 in transgenic sickle cell mice. Blood. 2002;100:664A. [Google Scholar]
- 21.Mezrow CK, Mazzoni M, Wolfe D, Shiang HH, Litwak RS, Griepp RB. Poloxamer-188 improves neurologic outcome after hypothermic circulatory arrest. J Thoracic Cardiovasc Surg. 1992;103:1143–6. [PubMed] [Google Scholar]
- 22.Merchant FA, Holmes WH, Capelli-Schellpfeffer M, Lee RC, Toner M. Poloxamer 188 enhances functional recovery of lethally heat-shocked fibroblasts. J Surg Res. 1998;74:131–40. doi: 10.1006/jsre.1997.5252. [DOI] [PubMed] [Google Scholar]
- 23.Kelly RF, Hursey TL, Patel RB, Parrillo JE, Schaer GL. Effect of poloxamer 188 on collateral blood flow, myocardial infarct size, and left ventricular function in a canine model of prolonged (3-h) coronary occlusion and reperfusion. J Thrombosis Thrombolysis. 1998;5:239–47. doi: 10.1023/A:1008848026759. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe M, Okada T. Lysophosphatidylcholine-induced myocardial damage is inhibited by pretreatment with poloxamer 188 in isolated rat heart. Mol Cell Biochem. 2003;248:209–15. doi: 10.1023/a:1024165125139. [DOI] [PubMed] [Google Scholar]
- 25.Curry DJ, Wright DA, Lee RC, Kang UJ, Frim DM. Surfactant poloxamer 188-related decreases in inflammation and tissue damage after experimental brain injury in rats. J Neurosurg. 2004;101:91–6. doi: 10.3171/ped.2004.101.2.0091. [DOI] [PubMed] [Google Scholar]
- 26.Greenebaum B, Blossfield K, Hannig J, et al. Poloxamer 188 prevents acute necrosis of adult skeletal muscle cells following high-dose irradiation. Burns. 2004;30:539–47. doi: 10.1016/j.burns.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Serbest G, Horwitz J, Barbee K. The effect of poloxamer-188 on neuronal cell recovery from mechanical injury. J Neurotrauma. 2005;22:119–32. doi: 10.1089/neu.2005.22.119. [DOI] [PubMed] [Google Scholar]
- 28.Ohashi M, Koizumi J, Hosoda Y, Fujishiro Y, Tuyuki A, Kikuchi K. Correlation between magnetic resonance imaging and histopathology of an amputated forearm after electrical injury. Burns. 1988;24:362–8. doi: 10.1016/s0305-4179(98)00025-4. [DOI] [PubMed] [Google Scholar]
- 29.Weissenburger J, Clement PF. MRI correlates of electrical injury. Arch Clin Neuropsychol. 1998;13:134. [Google Scholar]
- 30.Kalita J, Jose M, Misra UK. Myelopathy and amnesia following accidental electrical injury. Spinal Cord. 2002;40:253–5. doi: 10.1038/sj.sc.3101275. [DOI] [PubMed] [Google Scholar]
- 31.Sahiner T, Kurt T, Bir LS, et al. Reversible hyperintense T2 MRI lesions of basal ganglia after an electrical injury. Burns. 2002;28:607–8. doi: 10.1016/s0305-4179(02)00054-2. [DOI] [PubMed] [Google Scholar]
- 32.Freeman CB, Goyal M, Bourque PR. MR imaging findings in delayed reversible myelopathy from lightning strike. Emerg Med J. 2004;21:750–1. [PMC free article] [PubMed] [Google Scholar]
- 33.Brill DR. Radionuclide imaging of non-neoplastic soft tissue disorders. Semin Nucl Med. 1981;11:277–88. doi: 10.1016/s0001-2998(81)80025-6. [DOI] [PubMed] [Google Scholar]
- 34.Malki AA, El-Gazzar A, Ashqar T, Owunwanne A, Abdel-Dayem H. New technique for assessing muscle damage after trauma. J R Coll Surg Edinburgh. 1992;37:131–3. [PubMed] [Google Scholar]
- 35.Mehta RC, Wilson MA. Frostbite injury: prediction of tissue viability with triple-phase bone scanning. Radiology. 1989;170:511–4. doi: 10.1148/radiology.170.2.2911677. [DOI] [PubMed] [Google Scholar]
- 36.Labbe R, Lindsay T, Gatley R, et al. Quantitation of postischemic skeletal muscle necrosis: histochemical and radioisotope techniques. J Surg Res. 1988;44:45–53. doi: 10.1016/0022-4804(88)90121-7. [DOI] [PubMed] [Google Scholar]
- 37.Haseman MK, Kriss JP. Selective, symmetric, skeletal muscle uptake of Tc-99m pyrophosphate in rhabdomyolysis. Clin Nucl Med. 1985;10:180–3. doi: 10.1097/00003072-198503000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Delpassand ES, Dhekne RD, Barron BJ, Moore WH. Evaluation of soft tissue injury by Tc-99m bone agent scintigraphy. Clin Nucl Med. 1991;16:309–14. doi: 10.1097/00003072-199105000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Owunwanne A, Malki A, Sadek S, El-Gazzar A, Yacoub T, Abdel-Dayem HM. Investigative study of radiopharmaceuticals useful for imaging skeletal muscle injury in experimental animals. Am J Physiol Imag. 1989;4:62–5. [PubMed] [Google Scholar]
- 40.Siegel BA, Engel WK, Derrer EC. 99mTc-diphosphonate uptake in skeletal muscle: a quantitative index of acute damage. Neurology. 1975;25:1055–8. doi: 10.1212/wnl.25.11.1055. [DOI] [PubMed] [Google Scholar]
- 41.Timmons JH, Hartshorne MF, Peters VJ, Cawthon MA, Bauman JM. Muscle necrosis in the extremities: evaluation with Tc-99m pyrophosphate scanning—a retrospective review. Radiology. 1988;167:173–8. doi: 10.1148/radiology.167.1.2831562. [DOI] [PubMed] [Google Scholar]
- 42.Affleck DG, Edelman L, Morris SE, Saffle JR. Assessment of tissue viability in complex extremity injuries: utility of the pyrophosphate nuclear scan. J Trauma Injury Infect Crit Care. 2001;50:263–9. doi: 10.1097/00005373-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Chang LY, Yang JY. The role of bone scans in electric burns. Burns. 1991;17:250–3. doi: 10.1016/0305-4179(91)90115-w. [DOI] [PubMed] [Google Scholar]
- 44.Lewis SE, Hunt JL, Baxter C, Hickey DC, Parkey RW. Identification of muscle damage in acute electrical burns with technetium-99m pyrophosphate (Tc-99m-PYP) scintigraphy. J Nucl Med. 1979;20:646–7. [Google Scholar]
- 45.Sayman HB, Urgancioglu I, Uslu I, Kapicioglu T. Prediction of muscle viability after electrical burn necrosis. Clin Nucl Med. 1992;17:395–6. doi: 10.1097/00003072-199205000-00012. [DOI] [PubMed] [Google Scholar]
- 46.Spencer RR, Williams AG, Jr, Mettler FA, Jr, Christie JH, Rosenberg RD, Weaver WD. Tc-99m PYP scanning following low voltage electrical injury. Clin Nucl Med. 1983;8:591–3. doi: 10.1097/00003072-198312000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Hunt JL, Lewis S, Parkey R, Baxter C. The use of technetium-99m stannous pyrophosphate scintigraphy to identify muscle damage in acute electric burns. J Trauma. 1979;19:409–13. doi: 10.1097/00005373-197906000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Ino H, Chikamori T, Hatano T, et al. High-tension electrical injury to the heart as assessed by radionuclide imaging. Ann Nucl Med. 2002;16:557–761. doi: 10.1007/BF02988633. [DOI] [PubMed] [Google Scholar]
- 49.Hunt JL, Sato RM, Baxter C. Acute electric burns: current diagnostic and therapeutic approaches to management. Arch Surg. 1980;115:434–8. doi: 10.1001/archsurg.1980.01380040062011. [DOI] [PubMed] [Google Scholar]
- 50.Block TA, Aarsvold JN, Matthews KL, et al. The 1995 Lindberg award: Non-thermally mediated muscle injury and necrosis in electrical trauma. J Burn Care Rehab. 1995;16:581–8. [PubMed] [Google Scholar]
- 51.Aarsvold JN, Mintzer RA, Yasillo NJ, et al. A miniature gamma camera. In: Lee RC, Capelli-Schellpfeffer M, Kelly KM, editors. Ann NY Acad Sci. Vol. 720. 1994. pp. 192–205. Electrical injury: a multidisciplinary approach to therapy, prevention, and rehabilitation. [PubMed] [Google Scholar]
- 52.Matthews KL, II, Aarsvold JN, Mintzer RA, et al. Radiotracers for imaging electroporation. In: Chen C-T, Lee RC, Shih J-X, Zhong M-H, editors. Ann NY Acad Sci. Vol. 888. 1999. pp. 285–99. Occupational electrical injury: an international symposium. [DOI] [PubMed] [Google Scholar]
- 53.Shen AC, Jennings RB. Kinetics of calcium accumulation in acute myocardial ischemic injury. Am J Pathol. 1972;67:441–52. [PMC free article] [PubMed] [Google Scholar]
- 54.Jung A, Bisaz S, Fleisch H. The binding of pyrophosphate and two diphosphonates by hydroxyapatite crystals. Calcif Tissue Res. 1973;11:269–80. doi: 10.1007/BF02547227. [DOI] [PubMed] [Google Scholar]
- 55.Lessem J, Polimeni PI, Page E, Resnekov L, Harper PV, Stark V. Accumulation of technetium-99m pyrophosphate in experimental infarctions in the rat. Cardiovasc Res. 1980;14:352–9. doi: 10.1093/cvr/14.6.352. [DOI] [PubMed] [Google Scholar]
- 56.Hymes AC, Safavian MH, Arbulu A, Baute P. A comparison of Pluronic F-68, low molecular weight dextran, mannitol, and saline as priming agents in the heart-lung apparatus. I. Pluronic F-68: first use as a plasma substitute. J Thorac Cardiovasc Surg. 1968;56:16–22. [PubMed] [Google Scholar]
- 57.Smith GT, Wilson TS, Hunter K, et al. Assessment of skeletal muscle viability by PET. J Nucl Med. 1995;36:1408–14. [PubMed] [Google Scholar]
- 58.Abernathy VJ, Pou NA, Wilson TL, et al. Noninvasive measures of radiolabeled dextran transport in in situ rabbit lung. J Nucl Med. 1995;36:1436–41. [PubMed] [Google Scholar]
- 59.Lee RC, Myerov A, Maloney CP. Promising therapy for cell membrane damage. In: Lee RC, Capelli-Schellpfeffer M, Kelly KM, editors. Ann NY Acad Sci. Vol. 720. 1994. pp. 239–45. Electrical injury: a multidisciplinary approach to therapy, prevention, and rehabilitation. [DOI] [PubMed] [Google Scholar]


