Abstract
Objective
The nonalcoholic fatty liver disease fibrosis score (NFS) is comprised of unique metabolic risk indicators that may accurately predict residual cardiovascular (CV) risk in patients with established coronary disease and metabolic dysfunction.
Methods
We applied the NFS prospectively to 14,819 post-ACS patients randomized to ezetimibe/simvastatin (E/S) or placebo/simvastatin (P/S), in the IMPROVE-IT trial, using validated NFS cutoffs. The primary endpoint included CV death, myocardial infarction, unstable angina, revascularization or stroke. Outcomes were compared between NFS categories and treatment arms using frequency of events, KM rates and adjusted Cox proportional hazard models. The ability of the NFS to predict recurrent CV events was independently validated in 5,395 placebo-treated patients enrolled in the SOLID-TIMI 52 trial.
Results
Among 14,819 patients enrolled in IMPROVE-IT, 14.2% (N=2106) were high-risk (NFS>0.67). The high-risk group had a 30% increased risk of recurrent major CV events, compared to the low-risk NFS group (HR 1.30 [1.19–1.43]; p<0.001). Among high-risk patients, ezetimibe/simvastatin conferred a 3.7% absolute reduction in risk of recurrent CV events, compared to placebo/simvastatin (HR 0.85 [0.74–0.98], translating to a number-needed-to-treat of 27. Similar benefit was not found in the low-risk group (HR ezetimibe/simvastatin vs. placebo/simvastatin, 1.01 [0.91–1.12]; p-interaction=0.053). The relationship between NFS category and recurrent CV events was independently validated in patients enrolled in SOLID-TIMI 52 (HR for NFS>0.67 vs. NFS<−1.455=1.55 [1.32–1.81]; p<0.001).
Conclusion
Stratification of cardiovascular risk by NFS identifies an independent population of patients who are at highest risk of recurrent events, and most likely to benefit from dual lipid-lowering therapy.
Keywords: NAFLD, ezetimibe, statin, acute coronary syndrome, cardiovascular disease, metabolic syndrome, low-density lipoprotein cholesterol, fatty liver
Introduction
Patients with established coronary artery disease demonstrate a range of residual risk for recurrent cardiovascular (CV) events, and may vary in their response to therapies for secondary prevention1, 2. Recently, the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE IT) demonstrated that the addition of ezetimibe to simvastatin significantly reduced recurrent CV events, in 18,144 patients stabilized after acute coronary syndrome (ACS) (HR 0.936; p=0.016)3. While well-validated tools are in place to predict individual risk for initial CV events4, there are fewer validated instruments to guide long-term prognostication and therapeutic decision-making for post-ACS populations5–7. Still fewer strategies exist for estimating risk in the growing population with comorbid metabolic disease, related to diabetes, obesity and the metabolic syndrome. Existing tools for the post-ACS population derive their estimates from established coronary risk indicators, including advanced age, diabetes, smoking, severity of underlying vascular disease, arrhythmias, congestive heart failure (CHF), and adherence to guideline-based statin and anti-platelet regimens5, 6. Such an approach may underestimate the contribution of metabolic factors to individual CV risk.
The nonalcoholic fatty liver disease (NAFLD) Fibrosis Score (NFS) is a simple, serum-based index, originally developed for the diagnosis of advanced hepatic fibrosis in patients with NAFLD8–11. Although created for individuals with liver disease, its constituent factors –age, body mass index (BMI), alanine aminotransferase (ALT), aspartate aminotransferase (AST), platelets, albumin and diabetes – may reflect a high-risk state of systemic metabolic and inflammatory disarray. Such metabolic dysfunction may contribute to recurrent cardiovascular disease risk. Among patients with NAFLD, the NFS accurately predicts overall and cardiovascular mortality11, and in the general population, the components of the NFS are each associated with increased CV event risk12–15. Recently, the NFS was found to predict mortality among hospitalized patients with heart failure16. However, it remains unknown whether the NFS accurately predicts residual cardiovascular risk in a post-ACS population.
Using the IMPROVE-IT trial population, we tested the hypothesis that the NFS accurately identifies patients at highest risk of recurrent CV events, and those most likely to experience secondary CV risk reduction with dual lipid-lowering therapy. We then externally validated the prognostic utility of the NFS in an independent, prospective population with established IHD, enrolled in the placebo arm of the SOLID-TIMI 52 trial.
Methods
The design of IMPROVE-IT has been described previously17, and is detailed in the Supplementary Appendix. Briefly, IMPROVE-IT was an international, multicenter randomized controlled trial, designed to assess the impact of ezetimibe on CV morbidity and mortality, in patients admitted to the hospital with recent ACS. Eligible patients with stabilized ACS underwent randomization a median of 5 days [IQR 3, 8] from their index ACS event, and completed a full laboratory evaluation at this time. For the present study, we defined baseline as the date of randomization. We included all randomized IMPROVE-IT patients with baseline AST and ALT ≤ 2× ULN, with sufficient baseline laboratory data with which to calculate the NFS (Figure S1). Exclusion of those with higher aminotransferase levels was performed in accordance with previous statin trials18, 19, and to prevent confounding by patients with acute liver injury.
The NFS is a non-invasive serum index, with cut-off scores that have been validated against hepatic histology for the identification or exclusion of clinically significant, advanced NAFLD fibrosis8, 10, 20, 21. The NFS is calculated with the following formula8: NFS = −1.675 + 0.037 × age (years) + 0.094 × body mass index (kg/m2) + 1.13 × (impaired fasting glycemia or diabetes [yes=1, no=0]) + 0.99 × (AST/ALT ratio) − 0.013 × platelets (×109/L) − 0.66 × albumin (g/dL). Using validated cut-off scores8, we identified comparison groups for the analysis: high-risk NFS > 0.67 (N=2106), indeterminate (between 1.455 and 0.67), and low-risk NFS < −1.455 (N=5440).
Clinical endpoints
The composite primary efficacy endpoint in IMPROVE-IT3 included CV death, myocardial infarction (MI), unstable angina (UA) requiring hospitalization, coronary revascularization after 30 days, or stroke. The three secondary efficacy endpoints included: 1) a composite of death from any cause, major coronary event, or nonfatal stroke; 2) a composite of death from coronary heart disease, nonfatal MI, or urgent coronary revascularization ≥30 days after randomization; and 3) a composite of death from CV causes, nonfatal MI, hospitalization for UA, revascularization ≥30 days after randomization, or nonfatal stroke. All endpoints were adjudicated by a blinded committee, as previously described3.
Statistical analyses
Baseline characteristics, according to high- vs. low-risk NFS category and treatment group were compared using non-parametric Wilcoxon tests for continuous variables, and Chi-square tests for categorical variables. Differences over time in laboratory values were compared using non-parametric Wilcoxon tests within and between treatment groups.
A univariate Cox proportional hazards regression model was used to calculate the hazard ratio (HR), 95% confidence interval (CI) and p-value of the primary clinical endpoint, comparing the effect of ezetimibe/simvastatin versus placebo/simvastatin in the high-risk vs. low-risk NFS groups. For treatment group × NFS category interactions, heterogeneity p-values were based on Wald’s test from a Cox proportional hazards analysis including treatment group, NFS category and the corresponding interaction term. Multivariate (MV) Cox regression models accounted for established predictors of cardiovascular disease, including sex, race, hypertension, current smoking, history of prior PCI and CHF. As the algorithm for the NFS contains age, diabetes and BMI, the adjusted MV model did not include those factors to prevent over-correction and collinearity. The absolute (ARR) and relative risk reduction (RRR) observed with randomization to ezetimibe/simvastatin was then compared between high vs. low NFS groups.
To determine whether the excess observed CVD risk in the high-risk NFS group was related to unmeasured CVD risk factors, we conducted stratified analyses using the validated TIMI Risk Score for Secondary Prevention5, which assigns 1 point for each of the following: CHF, hypertension, age≥75 years, diabetes, current smoking, prior stroke, prior coronary artery bypass graft (CABG), peripheral arterial disease, and estimated glomerular filtration rate (eGFR)<60. The score was validated in IMPROVE-IT, for identifying high-risk patients most likely to benefit from ezetimibe22. We compared the RRR with ezetimibe/simvastatin to placebo/simvastatin, between patients in high-risk and low-risk NFS categories, stratified by TIMI Risk Score for Secondary Prevention category (high vs. intermediate vs. low)22, and tested the p-interaction for each model.
Sensitivity analyses
Three sensitivity analyses were performed. We excluded patients with diabetes and obesity (BMI≥30), respectively, to evaluate whether either diabetes or obesity modified the observed relationship between NFS category and CV events. In the third analysis, we included the 1,377 patients with baseline aminotransferases >2× ULN, who had originally been excluded from the primary analysis.
External Validation
External validation was performed by applying the NFS to a second, independent clinical trial population of 5,935 patients with recent, stabilized ACS randomized to the placebo treatment arm of the SOLID-TIMI 52 Trial (Stabilization of Plaque Using Darapladib-Thrombolysis in Myocardial Infarction 52; Figure S4)23, 24. Within this external validation cohort, multivariate Cox proportional hazards regression models were used to calculate the HR and 95% CI of the primary clinical endpoint according to NFS risk category.
All p-values are two-sided, and a p<0.05 was regarded as statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Table 1 summarizes the characteristics of the 14,819 eligible IMPROVE-IT patients at randomization, by NFS category. Among them, 2,106 (14.2%) were high risk, while 5,440 (36.7%) were low risk (Figure S2). Compared to the low-risk group, high-risk patients were more likely to be older, female, with higher BMI, and to have hypertension and diabetes. High-risk patients had a higher rate of prior lipid-lowering therapy use (41.7% vs. 29.6%; p<0.0001), with a correspondingly lower median LDL-C at the index ACS event. Per protocol, all patients received simvastatin and 96.7% received aspirin after randomization. Median baseline NFS did not differ significantly between groups.
Table 1.
Demographic and clinical characteristics of IMPROVE-IT subjects (N=14,819) according to baseline NAFLD Fibrosis Score (NFS)1 categories
|
Low NFS NFS < −1.455 (N=5440) |
Indeterminate NFS −1.455 ≤ NFS < 0.67 (N=7273) |
High NFS NFS > 0.67 (N=2106) |
P-value Low vs. High NFS2 |
Ptrend3 | |
|---|---|---|---|---|---|
|
| |||||
| Age, years | 59.4 (54.1, 65.8) | 65.4 (59.0, 72.8) | 69.8 (62.3, 76.5) | <0.001 | <0.001 |
|
| |||||
| Male | 4143, 76.2% | 5522, 75.9% | 1489, 70.7% | <0.001 | <0.001 |
|
| |||||
| Caucasian race | 4513, 83.0% | 6229, 85.6% | 1834, 87.1% | <0.001 | <0.001 |
|
| |||||
| Body Mass Index, kg/m2‡ | 26.2 (23.8, 28.9) | 28.1 (25.4, 31.5) | 30.1 (26.7, 35.0) | <0.001 | <0.001 |
|
| |||||
| Smoking (current) | 2336, 42.9% | 2080, 28.6% | 449, 21.3% | <0.001 | <0.001 |
|
| |||||
| Metabolic syndrome* | 2048, 37.7% | 2400, 33.0% | 415, 19.7% | <0.001 | <0.001 |
|
| |||||
| Hypertension | 2819, 51.8% | 4752, 65.3% | 1559, 74.0% | <0.001 | <0.001 |
|
| |||||
| Hyperlipidemia | 3896, 71.6% | 5354. 73.6% | 1537, 73.0% | 0.236 | 0.064 |
|
| |||||
| Diabetes | 490, 9.0% | 2318, 31.9% | 1292, 61.3% | <0.001 | <0.001 |
|
| |||||
| Ezetimibe/Simvastatin group | 2759, 50.7% | 3561, 49.0% | 1060, 50.3% | 0.764 | 0.349 |
|
| |||||
| Past cardiovascular history, no. (%) | |||||
|
| |||||
| Myocardial infarction | 972, 17.9% | 1660, 22.8% | 520, 24.7% | <0.001 | <0.001 |
|
| |||||
| Percutaneous coronary intervention | 919, 16.9% | 1585, 21.8% | 492, 23.4% | <0.001 | <0.001 |
|
| |||||
| Coronary artery bypass graft | 326, 6.0% | 784, 10.8% | 317, 15% | <0.001 | <0.001 |
|
| |||||
| Congestive heart failure | 128, 2.4% | 357, 4.9% | 166, 7.9% | <0.001 | <0.001 |
|
| |||||
| Stroke/TIA | 250, 4.6% | 457, 6.3% | 164, 8.5% | <0.001 | <0.001 |
|
| |||||
| Aspirin use | 1957, 36.0% | 3327, 45.8% | 1035, 49.2% | <0.001 | <0.001 |
|
| |||||
| Any lipid-lowering agent use | 1608, 29.6% | 2863, 39.4% | 878, 41.7% | <0.001 | <0.001 |
|
| |||||
| Statin use | 1559, 28.7% | 2785, 38.3% | 849, 40.3% | <0.001 | <0.001 |
|
| |||||
| At index event: no. (%) | |||||
|
| |||||
| Type of event: | |||||
| • MI with ST-segment elevation | 1849, 34.0% | 1780, 24.5% | 550, 26.1% | <0.001 | <0.001 |
| • MI without ST-segment elevation | 2421, 44.5% | 3537, 48.7% | 1063, 50.5% | <0.001 | <0.001 |
| • Unstable angina | 1170, 21.5% | 1952, 26.9% | 493, 23.4% | 0.074 | <0.001 |
|
| |||||
| Diagnostic catheterization | 4904, 90.2% | 6300, 86.6% | 1834, 87.1% | <0.001 | <0.001 |
|
| |||||
| Prerandomization PCI | 3994, 73.4% | 4968, 68.3% | 1522, 72.3% | 0.307 | 0.002 |
|
| |||||
| Laboratory parameters, median (IQR) | |||||
|
| |||||
| Alanine aminotransferase, U/L | 22.0 (15.0, 30.0) | 18.0 (13.0, 26.0) | 16.0 (11.0, 24.0) | <0.001 | <0.001 |
|
| |||||
| Aspartate aminotransferase, U/L | 18.0 (14.0, 23.0) | 19.0 (15.0, 27.0) | 24.0 (16.0, 40.0) | <0.001 | <0.001 |
|
| |||||
| Gamma glutamyltransferase, U/L | 23.0 (15.0, 36.0) | 19.0 (14.0, 31.0) | 16.0 (11.0, 26.0) | <0.001 | <0.001 |
|
| |||||
| C-reactive protein, U/L | 8.2 (3.4, 21.2) | 9.6 (4.0, 25.9) | 16.6 (6.0, 39.1) | <0.001 | <0.001 |
|
| |||||
| Platelets ×1000/mm3 | 281.0 (244.0, 331.0) | 213.0 (185.0, 246.0) | 182.0 (155.0, 211.0) | <0.001 | <0.001 |
|
| |||||
| Triglycerides, mg/dL | 126.5 (98.0, 165.0) | 127.0 (98.0, 167.0) | 126.0 (97.0, 168.0) | 0.844 | 0.974 |
|
| |||||
| Total cholesterol, mg/dL4 | 152.0 (132.0, 171.0) | 148.0 (130.0, 167.0) | 144.0 (127.0, 163.0) | <0.001 | <0.001 |
|
| |||||
| HDL-C, mg/dL | 40.0 (34.0, 48.0) | 40.0 (34.0, 47.0) | 39.0 (33.0, 47.0) | 0.071 | 0.195 |
|
| |||||
| LDL-C, mg/dL¥ | 97.1 (82.0, 112.1) | 93.0 (78.0, 108.6) | 91.0 (75.4, 107.0) | <0.001 | <0.001 |
|
| |||||
| Apolipoprotein B, mg/dL | 94.0 (79.0, 109.0) | 91.0 (77.0, 105.0) | 88.0 (75.0, 101.0) | <0.001 | <0.001 |
|
| |||||
| Hemoglobin A1c¥ | 5.8 (5.4, 6.2) | 6.2 (5.6, 7.5) | 6.7 (5.9, 7.8) | <0.001 | <0.001 |
Categorical variables are shown as no., % and continuous variables as median (IQR).
Abbreviations: BMI, body mass index. HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol. TIA, transient ischemic attack; MI, myocardial infarction; ACS, acute coronary syndrome; PCI, percutaneous coronary intervention; FLI, Fatty Liver Index; IQR, interquartile range.
NAFLD Fibrosis Score (NFS) = −1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × diabetes (yes = 1, no = 0) + 0.99 × (ratio of aspartate aminotransferase : alanine aminotransferase) − 0.013 × platelet (×109/l) − 0.66 × albumin (g/dl)
Chi-square two-way p-value comparing the high-NFS versus low-NFS groups
P-value of the trend across the three NFS categories (low, indeterminate and high NFS)
To convert the values for cholesterol to millimoles per liter, multiple by 0.02586
Values for both low-density lipoprotein cholestreol (LDL-C) and hemoglobin A1c were taken at the time of trial enrollment, rather than at the time of randomization
The body mass index is the weight in kilograms divided by the square of the height inmeters.
Metabolic syndrome was defined as any 3 of the 5 characteristics as determined by ATPIII clinical criteria for the metabolic syndrome: abdominal obesity (waist circumference >102cm in men, or >88cm in women), elevated triglycerides ≥ 150mg/dL, low HDL-C (<40mg/dL in men, or <50mg/dL in women), hypertension (blood pressure ≥ 130/≥85mm Hg), or fasting glucose ≥110mg/dL
NFS and recurrent CV event risk
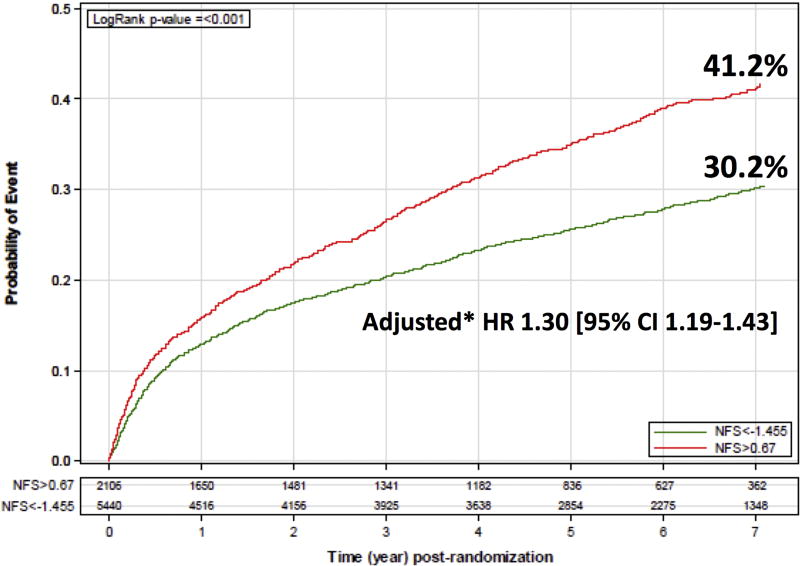
Compared to the low-risk group, the high-risk NFS group had significantly increased 7-year Kaplan-Meier (KM) rates of the primary CV endpoint (41.2% vs. 30.2%, p<0.001; Figure 1). After multivariate adjustment, a high-risk NFS remained associated with 30% increased risk of the primary endpoint (HR 1.30, 95% CI 1.19–1.43; p<0.001; Figure 1). A high-risk NFS was also associated with a 30–55% higher adjusted risk of all pre-specified secondary endpoints, compared to a low-risk NFS (HR for secondary endpoint I, II and III = 1.40 [95% CI 1.29–1.52; p<0.001], 1.55 [1.37–1.75; p<0.001] and 1.31 [1.19–1.43; p<0.001], respectively).
Figure 1.
7-year Kaplan-Meier (KM) rates of the primary cardiovascular (CV) endpoint, according to baseline NAFLD Fibrosis Score (NFS) category. The primary CV endpoint was defined as a composite of CV death, non-fatal myocardial infarction (MI), unstable angina, coronary revascularization with percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) ≥ 30 days post-randomization, or non-fatal stroke. To calculate the adjusted hazard ratio of the primary CV endpoint in the high vs. low NFS groups, multivariate cox proportional hazards regression models were used, adjusted for age, race, hypertension, current smoking, prior percutaneous coronary intervention (PCI), and a history of congestive heart failure (CHF).
We explored the relative contribution of each individual component of the NFS to CV event risk. In the multivariable-adjusted model, diabetes, increasing age, and lower albumin were each independently associated with risk of experiencing a primary or secondary CV endpoint (all p<0.0001; Table S1).
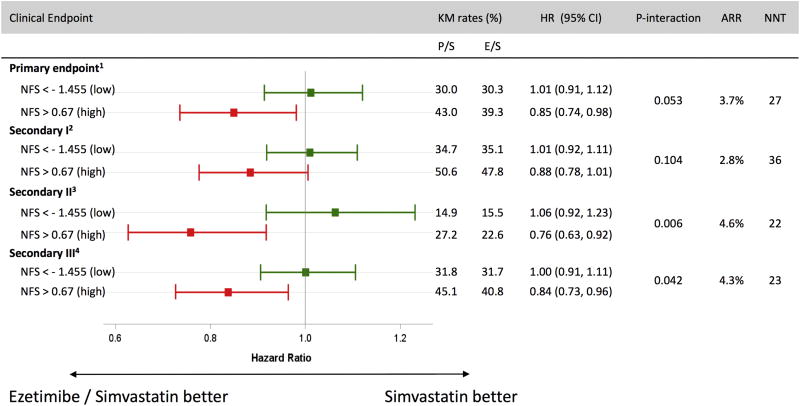
Ezetimibe and CV outcomes (Table 2, Figure 2)
Table 2.
Hazard ratios (HR) of primary, secondary and individual endpoints according to baseline NAFLD Fibrosis Score and treatment group
| Outcome (N, %) | High NAFLD Fibrosis Score (NFS>0.67) | Low NAFLD fibrosis Score (NFS<−1.455) | P-int. | ||||
|---|---|---|---|---|---|---|---|
| Placebo/Simva | EZE/Simva | HR (95% CI) | Placebo/Simva | EZE/Simva | HR (95% CI) | ||
| Primary endpoint: | |||||||
| • Death from CV causes, major coronary event, or nonfatal stroke | 390 (37.3%) | 351 (33.1%) | 0.85 (0.74–0.98) | 725 (27.0%) | 747 (27.1%) | 1.01 (0.91–1.12) | 0.05 |
| Secondary endpoints: | |||||||
| • I: Death from any cause, major coronary event, or nonfatal stroke | 473 (45.2%) | 445 (42.0%) | 0.88 (0.78–1.01) | 846 (31.6%) | 872 (31.6%) | 1.01 (0.92–1.11) | 0.10 |
| • II: Death from coronary heart disease, nonfatal MI, coronary revascularization > 30 days | 238 (22.8%) | 191 (18.0%) | 0.76 (0.63–0.92) | 344 (12.8%) | 370 (13.4%) | 1.06 (0.92–1.23) | 0.006 |
| • III: Death from CV causes, nonfatal MI, hospitalization for UA, all revascularization > 30 days, nonfatal stroke | 410 (39.2%) | 365 (34.4%) | 0.84 (0.73–0.96) | 770 (28.7%) | 785 (28.5%) | 1.00 (0.91–1.11) | 0.04 |
| Tertiary endpoints: | |||||||
| • Death from any cause | 241 (23.0%) | 250 (23.6%) | 1.02 (0.85–1.22) | 254 (9.5%) | 266 (9.6%) | 1.01 (0.85–1.20) | 0.970 |
| • Death from coronary heart disease | 108 (10.3%) | 98 (9.2%) | 0.89 (0.68–1.17) | 80 (3.0%) | 93 (3.4%) | 1.13 (0.84–1.52) | 0.250 |
| • Any MI | 167 (16.0%) | 119 (11.2%) | 0.67 (0.53–0.85) | 279 (10.4%) | 292 (10.6%) | 1.04 (0.88–1.22) | 0.003 |
| • Nonfatal MI | 160 (15.3%) | 113 (10.7%) | 0.66 (0.52–0.85) | 274 (10.2%) | 284 (10.3%) | 1.03 (0.87–1.21) | 0.004 |
| • Any stroke | 54 (5.2%) | 47 (4.4%) | 0.84 (0.57–1.24) | 93 (3.5%) | 70 (2.5%) | 0.73 (0.54–1.00) | 0.579 |
| • Ischemic stroke | 46 (4.4%) | 37 (3.5%) | 0.78 (0.51–1.20) | 77 (2.9%) | 55 (2.0%) | 0.70 (0.49–0.98) | 0.681 |
| • Hemorrhagic stroke | 9 (0.9%) | 10 (0.9%) | 1.07 (0.43–2.64) | 14 (0.5%) | 15 (0.5%) | 1.05 (0.50–2.17) | 0.967 |
| • Urgent coronary revascularization ≥ 30 days | 82 (7.8%) | 58 (5.5%) | 0.67 (0.48–0.94) | 171 (6.4%) | 180 (6.5%) | 1.04 (0.84–1.28) | 0.029 |
| • Hospitalization for unstable angina | 18 (1.7%) | 21 (2.0%) | 1.13 (0.60–2.13) | 43 (1.6%) | 46 (1.7%) | 1.06 (0.70–1.60) | 0.857 |
Abbreviations: NFS, NAFLD Fibrosis Score; HR, hazard ratio; 95% CI, 95% confidence interval; Simva, simvastatin; EZE, ezetimibe; HR, hazard ratio; P-int., P-interaction; CV, cardiovascular; MI, myocardial infarction; UA, unstable angina
Figure 2.
Adjusted, 7-year Kaplan-Meier (KM) rates by treatment group and baseline NAFLD Fibrosis score (NFS). Among those with high baseline NFS, ezetimibe significantly reduced primary cardiovascular (CV) events compared to placebo (HR 0.85; 95% CI 0.74–0.98), corresponding to a 3.7% treatment-related absolute risk reduction (ARR) and number needed to treat (NNT) of 27.
High-risk patients who received ezetimibe/simvastatin displayed significantly greater relative and absolute reductions in risk of the primary endpoint, compared to recipients of ezetimibe/simvastatin in the low-risk group (p-interaction for RRR = 0.053). Specifically, In the high-risk NFS group, ezetimibe/simvastatin conferred a significant 15% RRR and 3.7% ARR in the primary CV endpoint, compared to placebo/simvastatin (adjusted HR 0.85; 95% CI 0.74–0.98), translating to a number needed to treat (NNT) of 27. In contrast, no treatment-related risk reduction was found in low-risk patients (adjusted HR 1.01; 95% CI 0.91–1.12; p-interaction =0.053).
This pattern of enhanced treatment benefit with ezetimibe/simvastatin in patients with a high-risk NFS was also observed with secondary CV endpoints II and III (p-interaction=0.006 and 0.042, respectively), and for individual CV endpoints (Table 2). In the high-risk NFS group, ezetimibe/simvastatin conferred a 4.3% ARR in the composite secondary endpoint (HR 0.76; 95% CI 0.63–0.92; p-interaction=0.006), corresponding to a NNT of 23. These findings were driven largely by significant reductions in individual CV events, including 34% RRR in recurrent MI (HR 0.67, 95% CI 0.53–0.85; p-interaction=0.003), and in coronary revascularization (HR 0.67, 95% CI 0.48–0.94; p-interaction=0.029). In contrast, there was no observed treatment-related reduction in secondary CV endpoints, when low-risk NFS patients were treated with ezetimibe/simvastatin, compared to placebo/simvastatin (HR [95% CI] for secondary endpoint II and MI =1.06 [0.92–1.23], and 1.04 [0.88–1.22], respectively).
Ezetimibe and markers of liver inflammation (Table S2)
Table S2 summarizes treatment-related changes in NFS and its component variables, over time. Ezetimibe/simvastatin use was associated with significantly less AST:ALT elevation (difference in median % change = −1.69, p=0.037), as well as significantly reduced GGT (mean change [SD]= −1.15 (22.75) vs. 3.38 [42.28]; p=0.014) and triglycerides (mean change [SD]= −13.47 [77.64] vs. 0.64 [73.58]; p<0.001), compared to placebo/simvastatin.
Changes in lipid and lipoprotein levels (Table S3)
Among high risk patients treated with ezetimibe/simvastatin, 1,441 (68%) achieved LDL-C <70mg/dL, one month post-randomization. At one year, a significantly greater mean reduction in LDL-C was observed with ezetimibe/simvastatin, compared to placebo/simvastatin (−27.4mg/dL [SD 25.3] vs. −11.3mg/dL [SD 26.7]; p<0.001). There were no treatment-related differences in LDL-C reduction when high- vs. low-risk NFS groups were compared at 72 months (p-interaction=0.81).
Safety (Table S4)
When the ezetimibe/simvastatin and placebo/simvastatin groups were compared, no significant differences were found in the incidence of elevated liver enzymes, drug discontinuation due to elevated liver enzymes, or in liver-related adverse events, regardless of NFS category.
Comparison of the NFS to the TIMI Risk Score for Secondary Prevention (Table S5, S6)
To test whether the association between the NFS and CV risk related to unaccounted CVD risk factors, we stratified patients according to the validated TIMI Risk Score for Secondary Prevention. Among the high-risk NFS group, no effect modification was found by TIMI risk category (all p-interactions>0.05). Similarly, among the high-risk TIMI group, no effect modification by NFS category was observed (all p-interactions>0.05; Table S6).
Sensitivity analyses
In the first sensitivity analysis we excluded diabetics, to assess whether the relationship between NFS and CV risk was dependent upon diabetes. Among non-diabetics, there was no difference in risk of the primary CV endpoint in high- vs. low-risk NFS groups (adjusted HR=1.11 [95% CI 0.97–1.28]). However, non-diabetics with a high-risk NFS had a significantly increased risk for secondary endpoint I, (adjusted HR = 1.23 [95% CI 1.09, 1.40]) and secondary endpoint II (adjusted HR=1.23 [95% CI 1.01, 1.49]). Importantly, the treatment benefit from ezetimibe/simvastatin on the primary endpoint remained significantly greater among high-risk non-diabetic patients (HR=0.77 [95% CI 0.60, 0.99]), compared to low-risk non-diabetic patients (HR=1.02 [95% CI 0.91, 1.14]; p-interaction=0.044).
To test whether the relationship between the NFS and CV outcomes is dependent upon obesity, we excluded patients with obesity. Non-obese patients with a high-risk NFS had significantly increased risk of the primary CV endpoint, compared to the low-risk group (adjusted HR=1.40 [95% CI 1.24–1.57]; p<0.001). Similar associations were found for secondary endpoints I, II and III (HR=1.55 [95% CI 1.39–1.72], p<0.001; HR=1.69 [95% CI 1.44–1.97], p<0.001; HR 1.40 [95% CI 1.25–1.57], p<0.001, respectively).
Finally, we included 1,377 patients who had aminotransferases >2× ULN (N=16,196) at randomization, and had been excluded from our primary analysis, and our estimated effects were unchanged (adjusted HR of the primary CV endpoint in high- vs. low-risk NFS groups = 1.35 [95% CI 1.24–1.47]). Similarly, the observed treatment effect on both primary and secondary CV endpoints was not materially altered (Figure S3; Table S7).
External Validation of the NFS
Table S8 summarizes the baseline characteristics of included patients enrolled in the placebo arm of the external validation cohort (SOLID-TIMI 52; N=5,935); among them, 4,421 (74.5%) were low risk (NFS<−1.455), while 1,231 (20.7%) were high risk (NFS>0.67). Compared to the low-risk NFS group, the high-risk NFS category had significantly increased 3-year rates of the primary CV endpoint (22.1% vs. 13.0%, log-rank p-value<0.001; Figure S5). In the adjusted model, a high-risk NFS remained associated with a 1.6-fold increased risk of the primary endpoint (adjusted HR 1.64 [95% CI 1.32–2.03], p<0.001; Table S9). A high-risk NFS was also associated with a 2.1-fold increased risk of coronary heart disease-related death (adjusted HR 2.11 [95% CI 1.37–3.24], p<0.001) and a 1.7-fold increased risk of recurrent MI, compared to low-risk NFS (adjusted HR 1.67 [95% CI 1.29–2.18], p<0.001).
Discussion
In two large, independent and prospective populations, we demonstrate that the NFS accurately identifies a unique group of post-ACS patients with significantly increased residual cardiovascular risk, who are most likely to derive benefit from early initiation of dual lipid-lowering therapy for secondary risk reduction. Specifically, we observed a strong gradient of increased CV risk across increasing NFS categories, and found that a high-risk NFS score is associated with a significantly increased risk of recurrent major cardiovascular events. Importantly, these effects were consistent for numerous clinically-relevant endpoints, and were robust to multivariable analysis accounting for the severity of underlying cardiovascular disease, as well as a series of sensitivity analyses.
The population identified by the high-risk NFS category appear most likely to benefit from the addition of ezetimibe to statin therapy. Within this group, ezetimibe/simvastatin conferred a 3.7% absolute and 15% relative risk reduction in recurrent CV events, as well as a 33% relative reduction in recurrent MI and in coronary revascularization, compared to simvastatin monotherapy. These findings were consistent across the pre-specified primary and secondary trial endpoints, and remained significant after adjustment for cardiometabolic risk factors. Importantly, this association did not depend upon the severity of underlying CVD, and it was also independent of a validated risk score for recurrent atherothrombotic events. Although our statistical power was low to detect an interaction between outcomes and stratification by the two risk scores, we nonetheless observed that an increasing NFS continued to predict increased residual CV risk, even after the NFS was stratified by the TIMI Risk Score for Secondary Prevention5. Together, these findings indicate that the NFS contributes novel and independent information to secondary risk stratification.
The NFS was originally validated for identifying advanced hepatic fibrosis in NAFLD, but it is comprised of metabolic factors whose impact extend beyond the liver. The components of the NFS reflect downstream complications of systemic insulin resistance, obesity and the metabolic syndrome, each of which have been independently linked to excess cardiovascular risk12–15. Thus, it is biologically plausible that the NFS could serve as a helpful tool for risk stratification in a broader population of patients with elevated metabolic risk. Recently, it was found that the NFS predicts all-cause mortality in a hospitalized heart failure population16. Similarly, we found that the NFS accurately identifies individuals at highest risk for recurrent CV events. Together, these findings support the adoption of the NFS as a practical strategy for identifying residual risk and for personalizing secondary preventive therapy.
Notably, while the cardioprotective benefits of ezetimibe were most pronounced in the high-risk NFS group, overall LDL-C reduction was similar across NFS categories, suggesting that observed differences in clinical effects may derive from non-lipid factors. Evidence suggests that intensive LDL-C reduction may provide unique anti-inflammatory benefits in patients with underlying metabolic disease, which could translate to cardiovascular risk reduction18, 25. The expansion of visceral adipose tissue and accumulation of intrahepatic lipids activate toll-like receptor (TLR)-4 pathways and increase transforming growth factor (TGF)-B126, which promote lipotoxicity and mitochondrial dysfunction, stimulating production of reactive oxygen species and promoting vascular inflammation as well as hepatic fibrosis27, 28. It is plausible that among patients with the metabolic syndrome, more aggressive cholesterol reduction also mitigates systemic inflammatory activation, thus yielding improved clinical outcomes.
Several lines of evidence suggest that ezetimibe may also confer direct anti-inflammatory benefits. Among patients with established cardiovascular disease, the addition of ezetimibe to statins reduces high-sensitivity C-reactive protein (hsCRP)29. Similarly, in patients with NAFLD and the metabolic syndrome, ezetimibe was associated with reductions in numerous circulating inflammatory cytokines30–34. More recently, the MOZART trial randomized 50 patients with nonalcoholic steatohepatitis (NASH) to 24 weeks of therapy with ezetimibe or placebo35. Ezetimibe was associated with significant reductions in circulating inflammatory markers35, and in the Framingham Risk Score over time (mean 4.4±6.2 to 2.9±4.8, p=0.038)36. Although future prospective studies will be needed to confirm this hypothesis, these data suggest that the cardioprotective benefits of ezetimibe in patients with metabolic disease may derive in part from anti-inflammatory effects.
Given the growing proportion of patients with a history of prior ACS, validated risk stratification tools are needed to determine which patients stand to derive the greatest clinical benefit from the early addition of ezetimibe to a statin-based regimen, after an episode of ACS. It has been recommended that ezetimibe be added to statin therapy in high-risk patients with atherosclerotic cardiovascular disease (ASCVD) and on-treatment LDL-C levels ≥ 70 mg/dL37. Our findings extend these recommendations to individuals with evidence of metabolic disease, as estimated by the NFS. Such patients may be under-recognized by existing risk stratification tools, which account for symptoms and traditional cardiovascular risk factors, but may not comprehensively assess metabolic, nutritional or hepatic parameters. In contrast, the NFS offers clinicians an accessible tool for identifying this high-risk population.
Our findings support application of the NFS for risk stratification in patients with established CVD and high metabolic risk. However, we recognize several important limitations. First, without radiographic or histological liver biopsy data, it is impossible to know what proportion of patients in our two cohorts had underlying NAFLD. Moreover, patients in the low-NFS group had higher GGT levels at randomization, compared to those in the high-NFS group. GGT is a biomarker associated with prevalent NAFLD38, as well as with traditional cardiovascular risk factors, including the metabolic syndrome39, diabetes, hypertension and coronary artery disease40. Future prospective studies will be needed to disentangle whether the excess observed CVD risk is attributable specifically to NAFLD or to the unique risk factors captured by the NFS. Future prospective studies will be needed to disentangle whether the excess CVD risk is related specifically to NAFLD or to the unique risk factors captured by the NFS. Second, despite accessibility and ease-of-use, the NFS does not fully account for all validated indicators of residual risk in patients with coronary disease, including genetic risk factors, imaging or angiographic factors. We eagerly await future studies that will refine our methods for risk stratification, including explorations of other NFS cutpoints, to define the optimal NFS thresholds for prognostic prediction. Nonetheless, our findings demonstrate that the NFS has significant utility for revealing excess residual CV risk, and identifying differential benefit between secondary preventive strategies. Third, statins reduce ALT, AST and GGT activity, and due to protocol-mandated uptitration to 80mg/day of simvastatin in IMPROVE-IT patients with repeat LDL-C> 79mg/dL3, it is possible that higher simvastatin doses administered in the placebo arm influenced our results. However, given the observed similarity in rates of aminotransferase elevations in the trial3, this is unlikely. If anything, higher simvastatin doses in the placebo group likely diminished differences in LDL-C, thus attenuating the observed benefits of ezetimibe.
Finally, the two patient cohorts in this study derive from clinical trials with different study designs, that included populations with different baseline characteristics, who met strict inclusion criteria for clinical trial entry, which could limit generalizability. However, the NFS nonetheless robustly predicted major CV outcomes in both populations, which supports the validity of our results and suggests that these findings may be broadly applicable. We eagerly anticipate future population-based studies that will confirm the generalizability of our findings, and test the utility of the NFS for guiding therapeutic decision-making within the context of other lipid-lowering strategies.
Conclusions
In patients stabilized after admission with a high-risk ACS, the NFS accurately identifies patients at very high risk for recurrent cardiovascular events, including cardiac death, and those most likely to benefit from dual lipid-lowering therapy. Adoption of the NFS may help guide therapeutic decision-marking for secondary prevention, particularly in patients with underlying metabolic disease.
Supplementary Material
Highlights.
The NFS is a novel tool for predicting residual CV risk in established CAD.
A high-risk NFS group had 30% higher risk of CV events, compared to low-risk NFS.
A high-risk NFS group was significantly more likely to benefit from early ezetimibe.
The positive association between high-risk NFS and CV risk was externally validated.
Acknowledgments
Funding:
K24DK078772 (RTC)
K23DK099422 (KEC)
The IMPROVE-IT trial was funded by Merck.
The SOLID-TIMI 52 trial was funded by GlaxoSmithKline.
List of abbreviations
- NAFLD
nonalcoholic fatty liver disease
- CV
cardiovascular
- CVD
cardiovascular disease
- E/S
Ezetimibe / Simvastatin
- P/S
placebo / Simvastatin
- IMPROVE-IT
Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT)
- ACS
acute coronary syndromes
- NFS
NAFLD Fibrosis Score
- BMI
Body Mass Index
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- ULN
upper limit of normal
- LDL-C
low density lipoprotein cholesterol
- MI
myocardial infarction
- UA
unstable angina
- MV
Multivariate
- CHF
congestive heart failure
- eGFR
estimated glomerular filtration rate
- KM
Kaplan-Meier
- HR
hazard ratio
- CI
confidence interval
- ARR
absolute risk reduction
- RRR
relative risk reduction
- NNT
number needed to treat
- GGT
gamma glutamyltransferase
- TLR
toll-like receptor
- TGF-B
transforming growth factor beta
- hsCRP
high-sensitivity C-reactive protein
- NASH
nonalcoholic steatohepatitis
- MRI
magnetic resonance imaging
- ASCVD
atherosclerotic cardiovascular disease
- CAD
coronary artery disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations
Competing interests: The authors report no conflicts of interest.
Ethics approval and consent to participate:
All participants in the IMPROVE-IT trial provided informed consent. The trial was conducted in accordance with the Declaration of Helsinki and was approved by the institutional review board (IRB) of each participating center.
Consent for publication: No individual patient data is included in this manuscript.
- Tracey G. Simon: none
- Jeong-Gun Park: none
- Kathleen E. Corey: none
- Christopher P. Cannon: has received research grant support from Amgen, Arisaph, Boehringer-Ingelheim (BI), Bristol-Myers Squibb (BMS), Daiichi Sankyo, Janssen, Merck & Co., Inc., and from Takeda. Dr. Cannon has also received consulting fees from Alnylam, Amgen, Arisaph, Astra Zeneca, BI, BMS, GlaxoSmithKline, Kowa, Lipimedix, Merck & Co., Inc., Pfizer, Regeneron, Sanofi, and Takeda.
- Michael Blazing: has received research grant support from Amgen, Bristol Myers Squibb and Merck & Co., Inc., to conduct clinical trials related to lipid therapy. Dr. Blazing has received honoraria for consulting and/or advisory board membership from AstraZeneca, Pfizer and Merck & Co., Inc.
- Michelle L. O’Donoghue: has received grants from GlaxoSmithKline, Eisai, AstraZeneca, Merck and Janssen.
- Raymond T. Chung: has received research grant support from Merck, Gilead, Abbvie, Boehringer-Ingelheim and Janssen.
- Robert Giugliano: member of the TIMI Study Group, which has received research grant support from Amgen, Bristol Myers Squibb, and Merck & Co., Inc., to conduct clinical trials related to lipid therapy. Dr. Giugliano has received honoraria for CME lectures and/or consulting from Amgen, Bristol Myers Squibb, Daiichi Sankyo, Merck & Co., Inc., and Pfizer.
- TGS: literature review, data interpretation, drafting of the article, critical revision
- RTC: interpretation of the data, critical revision
- KEC: interpretation of the data, critical revision
- CPC: interpretation of the data, critical revision
- MB: interpretation of the data, critical revision
- JGP: data analysis and interpretation, critical revision
- MOD: interpretation of the data, critical revision
- RPG: interpretation of the data, critical revision, study supervision and guarantor of this work.
- All authors read and approved the final manuscript.
References
- 1.Eagle KA, Hirsch AT, Califf RM, et al. Cardiovascular ischemic event rates in outpatients with symptomatic atherothrombosis or risk factors in the united states: insights from the REACH Registry. Crit Pathw Cardiol. 2009;8:91–7. doi: 10.1097/HPC.0b013e3181a84613. [DOI] [PubMed] [Google Scholar]
- 2.Morrow DA. Cardiovascular risk prediction in patients with stable and unstable coronary heart disease. Circulation. 2010;121:2681–91. doi: 10.1161/CIRCULATIONAHA.109.852749. [DOI] [PubMed] [Google Scholar]
- 3.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med. 2015;372:2387–97. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 4.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2960–84. doi: 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Bohula EA, Bonaca MP, Braunwald E, et al. Atherothrombotic Risk Stratification and the Efficacy and Safety of Vorapaxar in Patients With Stable Ischemic Heart Disease and Previous Myocardial Infarction. Circulation. 2016;134:304–13. doi: 10.1161/CIRCULATIONAHA.115.019861. [DOI] [PubMed] [Google Scholar]
- 6.Wilson PW, D'Agostino R, Sr, Bhatt DL, et al. An international model to predict recurrent cardiovascular disease. Am J Med. 2012;125:695–703 e1. doi: 10.1016/j.amjmed.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Bohula EA, Morrow DA, Giugliano RP, et al. Atherothrombotic Risk Stratification and Ezetimibe for Secondary Prevention. J Am Coll Cardiol. 2017;69:911–921. doi: 10.1016/j.jacc.2016.11.070. [DOI] [PubMed] [Google Scholar]
- 8.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–54. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 9.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 10.Wong VW, Wong GL, Chim AM, et al. Validation of the NAFLD fibrosis score in a Chinese population with low prevalence of advanced fibrosis. Am J Gastroenterol. 2008;103:1682–8. doi: 10.1111/j.1572-0241.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim D, Kim WR, Kim HJ, et al. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357–65. doi: 10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schindhelm RK, Dekker JM, Nijpels G, et al. Alanine aminotransferase predicts coronary heart disease events: a 10-year follow-up of the Hoorn Study. Atherosclerosis. 2007;191:391–6. doi: 10.1016/j.atherosclerosis.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Schillinger M, Exner M, Mlekusch W, et al. Serum albumin predicts cardiac adverse events in patients with advanced atherosclerosis - interrelation with traditional cardiovascular risk factors. Thromb Haemost. 2004;91:610–8. doi: 10.1160/TH03-08-0504. [DOI] [PubMed] [Google Scholar]
- 14.Chu SG, Becker RC, Berger PB, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:148–56. doi: 10.1111/j.1538-7836.2009.03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu H, Chen WL, Huang CC, et al. Diagnostic performance of mean platelet volume for patients with acute coronary syndrome visiting an emergency department with acute chest pain: the Chinese scenario. Emerg Med J. 2011;28:569–74. doi: 10.1136/emj.2010.093096. [DOI] [PubMed] [Google Scholar]
- 16.Yoshihisa A, Sato Y, Yokokawa T, et al. Liver fibrosis score predicts mortality in heart failure patients with preserved ejection fraction. ESC Heart Fail. 2017 doi: 10.1002/ehf2.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cannon CP, Giugliano RP, Blazing MA, et al. Rationale and design of IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial): comparison of ezetimbe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes in patients with acute coronary syndromes. Am Heart J. 2008;156:826–32. doi: 10.1016/j.ahj.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 18.Athyros VG, Tziomalos K, Gossios TD, et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376:1916–22. doi: 10.1016/S0140-6736(10)61272-X. [DOI] [PubMed] [Google Scholar]
- 19.Tikkanen MJ, Fayyad R, Faergeman O, et al. Effect of intensive lipid lowering with atorvastatin on cardiovascular outcomes in coronary heart disease patients with mild-to-moderate baseline elevations in alanine aminotransferase levels. Int J Cardiol. 2013;168:3846–52. doi: 10.1016/j.ijcard.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 20.McPherson S, Stewart SF, Henderson E, et al. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265–9. doi: 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
- 21.Musso G, Gambino R, Cassader M, et al. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–49. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 22.Bohula EA, Morrow DA, Giugliano RP, et al. Atherothrombotic Risk Stratification and Ezetimibe for Secondary Prevention. Journal of the American College of Cardiology. 2016 doi: 10.1016/j.jacc.2016.11.070. [DOI] [PubMed] [Google Scholar]
- 23.O'Donoghue ML, Braunwald E, White HD, et al. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA. 2014;312:1006–15. doi: 10.1001/jama.2014.11061. [DOI] [PubMed] [Google Scholar]
- 24.O'Donoghue ML, Braunwald E, White HD, et al. Study design and rationale for the Stabilization of pLaques usIng Darapladib-Thrombolysis in Myocardial Infarction (SOLID-TIMI 52) trial in patients after an acute coronary syndrome. Am Heart J. 2011;162:613–619 e1. doi: 10.1016/j.ahj.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Farrell G. Should we lower lipids in nonalcoholic fatty liver disease? Clin Gastroenterol Hepatol. 2014;12:152–5. doi: 10.1016/j.cgh.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 26.Tomita K, Teratani T, Suzuki T, et al. Free cholesterol accumulation in hepatic stellate cells: mechanism of liver fibrosis aggravation in nonalcoholic steatohepatitis in mice. Hepatology. 2014;59:154–69. doi: 10.1002/hep.26604. [DOI] [PubMed] [Google Scholar]
- 27.Gerin I, Clerbaux LA, Haumont O, et al. Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J Biol Chem. 2010;285:33652–61. doi: 10.1074/jbc.M110.152090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musso G, Cassader M, Bo S, et al. Sterol regulatory element-binding factor 2 (SREBF-2) predicts 7-year NAFLD incidence and severity of liver disease and lipoprotein and glucose dysmetabolism. Diabetes. 2013;62:1109–20. doi: 10.2337/db12-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bohula EA, Giugliano RP, Cannon CP, et al. Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE-IT. Circulation. 2015;132:1224–33. doi: 10.1161/CIRCULATIONAHA.115.018381. [DOI] [PubMed] [Google Scholar]
- 30.Hughes EA, Tracey I, Singhal S, et al. Unexpected beneficial effect in the use of ezetimibe in non-alcoholic fatty liver disease. Med Hypotheses. 2006;67:1463–4. doi: 10.1016/j.mehy.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 31.Patel JV, Hughes EA. Efficacy, safety and LDL-C goal attainment of ezetimibe 10 mg-simvastatin 20 mg vs. placebo-simvastatin 20 mg in UK-based adults with coronary heart disease and hypercholesterolaemia. Int J Clin Pract. 2006;60:914–21. doi: 10.1111/j.1742-1241.2006.01023.x. [DOI] [PubMed] [Google Scholar]
- 32.Chan DC, Watts GF, Gan SK, et al. Effect of ezetimibe on hepatic fat, inflammatory markers, and apolipoprotein B-100 kinetics in insulin-resistant obese subjects on a weight loss diet. Diabetes Care. 2010;33:1134–9. doi: 10.2337/dc09-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enjoji M, Machida K, Kohjima M, et al. NPC1L1 inhibitor ezetimibe is a reliable therapeutic agent for non-obese patients with nonalcoholic fatty liver disease. Lipids Health Dis. 2010;9:29. doi: 10.1186/1476-511X-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeshita Y, Takamura T, Honda M, et al. The effects of ezetimibe on non-alcoholic fatty liver disease and glucose metabolism: a randomised controlled trial. Diabetologia. 2014;57:878–90. doi: 10.1007/s00125-013-3149-9. [DOI] [PubMed] [Google Scholar]
- 35.Loomba R, Sirlin CB, Ang B, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial) Hepatology. 2015;61:1239–50. doi: 10.1002/hep.27647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin SC, Ang B, Hernandez C, et al. Cardiovascular risk assessment in the treatment of nonalcoholic steatohepatitis: a secondary analysis of the MOZART trial. Therap Adv Gastroenterol. 2016;9:152–61. doi: 10.1177/1756283X15621232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Writing C, Lloyd-Jones DM, Morris PB, et al. 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2016;68:92–125. doi: 10.1016/j.jacc.2016.03.519. [DOI] [PubMed] [Google Scholar]
- 38.Franzini M, Fornaciari I, Fierabracci V, et al. Accuracy of b-GGT fraction for the diagnosis of non-alcoholic fatty liver disease. Liver Int. 2012;32:629–34. doi: 10.1111/j.1478-3231.2011.02673.x. [DOI] [PubMed] [Google Scholar]
- 39.Franzini M, Scataglini I, Ricchiuti A, et al. Association between plasma gamma-glutamyltransferase fractions and metabolic syndrome among hypertensive patients. Sci Rep. 2017;7:12003. doi: 10.1038/s41598-017-12356-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Onat A, Can G, Ornek E, et al. Serum gamma-glutamyltransferase: independent predictor of risk of diabetes, hypertension, metabolic syndrome, and coronary disease. Obesity (Silver Spring) 2012;20:842–8. doi: 10.1038/oby.2011.136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.