Abstract
While many of our motor skills are acquired through physical practice, we can also learn how to make movements by observing others. For example, individuals can learn how to reach in novel dynamical environments (‘force fields’, FF) by observing the movements of a tutor. Previous neurophysiology and neuroimaging studies in humans suggest a role for the motor system in motor learning by observing. Here we tested the role of primary motor cortex (M1) in motor learning by observing. We used single-pulse transcranial magnetic stimulation (TMS) to elicit motor evoked potentials (MEPs) in right hand muscles at rest. MEPs were elicited before and after participants observed either a video adapting her reaches to a FF or a control video showing a tutor performing reaches in an unlearnable FF. During MEP acquisition, participants fixated a crosshair while their hand muscles were relaxed. We predicted that observing motor learning would result in greater increases in offline M1 excitability compared to observing movements that did not involve learning. We found that observing FF learning resulted in subsequent increases in MEP amplitudes recorded from right first dorsal interosseous (FDI) and right abductor pollicis brevis (APB) muscles at rest. There were no changes in MEP amplitudes after control participants observed a tutor performing reaches in an unlearnable, randomly varying FF. The observed MEP changes can thus be specifically linked to observing motor learning. These results are consistent with the idea that observing motor learning produces functional changes in M1, or corticospinal networks or both.
Keywords: human, motor learning, action observation, primary motor cortex, corticospinal excitability, transcranial magnetic stimulation
Introduction
Action observation activates brain areas involved in movement production. For example, in the macaque, so-called mirror neurons in area F5 of the premotor cortex are active both while the monkey performs goal-directed actions and while observing others performing similar actions (Di Pellegrino et al. 1992; Gallese et al. 1996; Rizzolatti et al. 1996). In humans, neuroimaging and neurophysiological studies have similarly shown that action observation engages the motor system (Strafella and Paus 2000a; Buccino et al. 2001; Watkins et al. 2003). The majority of this research has investigated the potential role of observation-related brain activity in higher cognitive functions such as action recognition, understanding others’ intentions, imitation, autism, theory of mind, empathy, etc. (Gallese 1998; Prinz 2006). For an opposing view see (Hickok 2009; Turella et al. 2009). However, a growing body of work has demonstrated that action observation can also facilitate motor learning.
Observation-related gains in motor performance have been reported using various experimental paradigms. For example, individuals can learn implicit button press sequences from observing others performing a serial reaction time task (Heyes and Foster 2002), learn novel dance sequences using observation-based training (Cross et al. 2009), and learn about object weights from observing others’ lifts (Alaerts et al. 2010; Buckingham et al. 2014). Of most relevance to the current study is the finding that participants can learn about how to reach in novel force environments from observing the movements of others (Mattar and Gribble 2005). In this study, participants observed a video of another individual (‘a tutor’) adapting his reaching movements to a force field (‘FF’) which was applied by a robotic arm. Participants who later performed reaches in the same FF as that which they had observed in the video showed a benefit, performing straighter movements in the FF compared to non-observing control participants. Conversely, participants who later performed reaches in the opposite FF to what they had observed showed a detriment, performing more curved movements in the FF compared to non-observing control participants. This study therefore demonstrated that participants were able to learn about how to reach in novel FF environments through observing the movements of others. Mattar and Gribble (2005) further found that performing an unrelated bilateral arm movement task during the video reduced the extent to which participants learned from observation, while performing a cognitive distractor task during observation did not. This finding suggested that motor learning by observing depends on the engagement of the observer’s motor system.
Recent neuroimaging and repetitive transcranial magnetic stimulation (rTMS) studies suggest a role for the motor system in motor learning by observing. Using resting-state fMRI, we have previously shown that observing FF learning changes functional connectivity between visual area V5/MT, the cerebellum, S1, and M1. Observation-related functional connectivity changes within this network were correlated with subsequent behavioral measures of motor learning by observing (McGregor and Gribble 2015). This study suggested that observing motor learning results in functional changes among visual and sensory-motor brain areas. Brown and colleagues (2009) investigated the role of M1 in motor learning by observing using low frequency repetitive transcranial magnetic stimulation (rTMS). In this study, participants observed a video of a tutor adapting to a FF and then received rTMS over left M1 following observation in order to reduce M1 excitability. Reducing M1 excitability following observation disrupted motor learning by observing. Participants who received rTMS over M1 and were later tested in the same FF to what was observed showed reduced behavioral gains in the behavioral assessment. Participants who received rTMS over M1 and were then tested in the opposite FF to what was observed showed reduced interference in the behavioral assessment (Brown et al. 2009). These results suggest that M1 plays a key role in motor learning by observing.
In the present study, we tested the role of primary motor cortex (M1) in motor learning by observing using TMS to probe for changes in offline corticospinal excitability following the observation of learning. Our previous fMRI study showed that observing FF learning resulted in functional changes within the hand representation of left M1 and S1 (McGregor and Gribble 2015). Here, we followed up on this finding by using single-pulse TMS to probe corticospinal excitability associated with hand muscles. Given our previous findings, we predicted that corticospinal excitability associated with hand muscles would increase following the observation of learning. We chose first dorsal interosseous (FDI) and the abductor pollicis brevis (APB) as hand muscles for the current study because these muscles have often been used in TMS & action observation studies. Choosing these muscles allowed for comparison with other action observation in the literature recording from FDI, APB (e.g., Stefan et al., 2005) or both (e.g., Fadiga et al., 1995).
We elicited motor evoked potentials (MEPs) from FDI and APB in the right hand before and after participants observed either a video depicting a tutor adapting her reaches to a FF or a control video showing a tutor performing reaches in an unlearnable FF. During MEP acquisition, participants fixated a crosshair while their hand muscles were relaxed. If M1 is indeed involved in motor learning by observing, then observing motor learning should result in greater increases in offline M1 excitability than observing movements that did not involve learning. As predicted, we found that observing FF learning resulted in subsequent increases in MEP amplitudes. No changes in MEP amplitudes were seen for participants who had observed a tutor performing reaches in an unlearnable FF. The offline MEP changes reported here are thus not due action observation in general or observation of movement errors, but rather can be specifically linked to observation of motor learning. These results provide further support for the idea that observing motor learning involves functional changes in M1 or corticospinal networks, or both.
Methods
Participants
A total of 32 healthy volunteers participated in this study: 16 participants observed a video depicting a tutor learning to reach in a clockwise force field (5 males, mean age 21.6 year ± 0.71 SEM), and 16 participants observed a control video that depicted the same kinds of curved movements but did not depict learning (4 males, mean age 21.3 year ± 0.76 SEM). All participants were right handed, had normal or corrected-to-normal vision, and were naïve to force fields. None of the participants had neurological disorders, musculoskeletal disorders, or any contraindications to TMS (Keel, 2001). Participants provided written informed consent to experimental procedures approved by the Research Ethics Board at the University of Western Ontario.
Reaching Task
Participants were seated in front of a custom tabletop and grasped the handle of a two degree-of-freedom robotic arm (IMT2, Interactive Motion Technologies) with the right hand (as shown in Figure 1A). The participant’s upper arm was abducted approximately 90° from the trunk. The right arm was supported against gravity by an arm sled secured beneath the upper arm. An LCD TV projected visual feedback onto a semi-silvered mirror mounted horizontally above the robotic arm during the reaching task.
Figure 1.
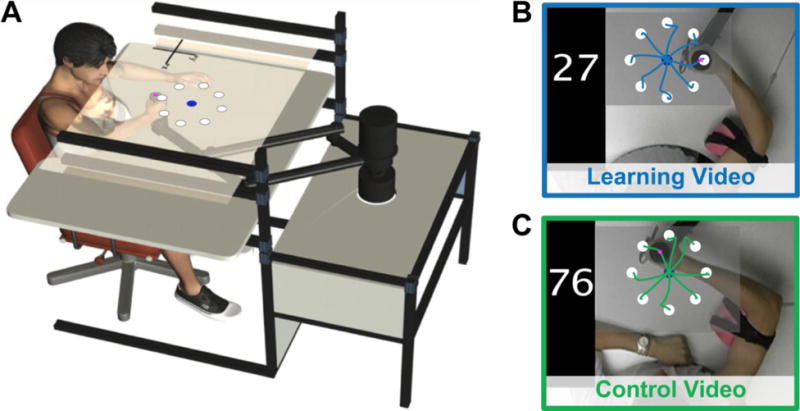
Task. A) Reaching task. Participants grasped the handle at the end of a robotic arm and performed straight reaches from a central start position (blue circle) to 8 targets (white circles). B) Screenshot of the learning video. The learning video showed a tutor adapting his reaches to a clockwise force field (CW FF). Superimposed trajectories are for demonstrative purposes only. C) Screenshot of the control video. The control video showed a tutor performing reaches in an unlearnable FF in which the direction of the applied force varied randomly from trial-to-trial. Superimposed trajectories are for demonstrative purposes only. FF, force field.
Participants were instructed to guide the robot handle from a central start position to a visual target, and were told to move the hand in a straight line. Eight targets were spaced equally around the circumference of a circle (see Figure 1A). Each target was a white circle (24 mm in diameter) located 10 cm from the start position (represented by blue circle that was 20 mm in diameter). A 5-mm pink circular cursor represented the position of the hand. To keep movement speed consistent from trial-to-trial, participants were provided with movement timing feedback at the end of each trial. The target turned blue if the movement duration was within the desired time range of 375 ± 100 ms, turned red if the movement was too fast, or turned green if the movement was too slow. Following movement timing feedback, the start position (blue circle) reappeared to signal that the participant should move the robot handle back to the start position to begin the next trial.
The robotic arm applied a velocity-dependent force field (FF) during the reaching task according to the following equation:
| (1) |
in which x and y are lateral and sagittal directions, Fx and Fy are the robot forces applied at the hand, vx and vy are hand velocities, k=15 Ns/m, and d=+1 (CW FF), −1 (CCW FF) or 0 (null field).
Reaching Video Stimuli
Two videos were used in the study, and each one showed a tutor performing the reaching task described above from a top-down perspective. Both of the tutors were naïve to force fields. One video depicted a tutor adapting his reaches to a clockwise force field (CW FF) over 96 trials. The video depicted highly curved movements that gradually straightened as the tutor adapted to the CW FF. Participants observed this video twice (12 minutes total, 192 reaches observed in total). A screenshot of the video is shown in Figure 1B. Note that superimposed trajectories have been included for demonstrative purposes but were not shown to participants. Participants in a control group were shown a video that depicted a tutor performing 96 reaches in an unlearnable FF. In the unlearnable FF, the direction of the force field varied randomly from trial-to-trial between a CCW FF, CW FF or null field. The control video therefore showed the tutor performing both high and low curvature movements, but lacked the progressive decrease in movement curvature that was depicted in the learning video. Participants assigned to the control group observed the control video twice (12 minutes total, 192 reaches observed in total). A screenshot of the control video is shown in Figure 1C. Note that superimposed trajectories were not shown to participants.
Experimental Design
The experimental design is shown in Figure 2A. Participants were first familiarized with the robotic arm and the reaching task by performing approximately 40 practice reaches in a null field. Then, in a baseline condition, participants performed 96 reaches in a null field (12 reaches to each of the 8 targets). This was done to assess the participant’s baseline movement curvature prior to observation.
Figure 2.
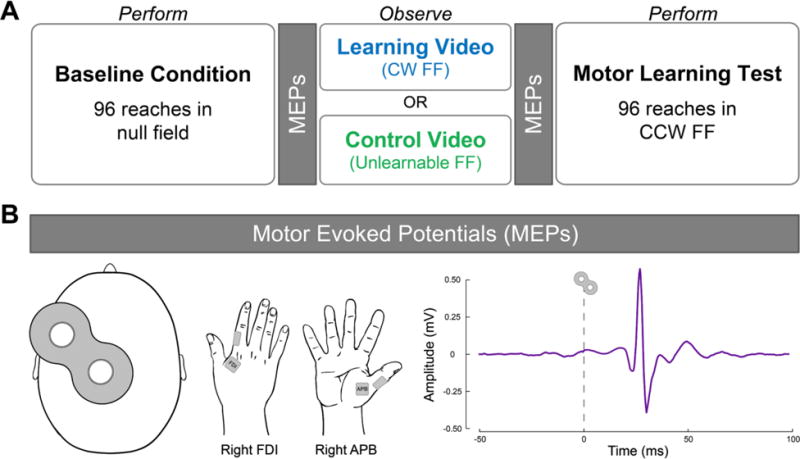
A) Experimental design. In the baseline condition, participants performed reaches in a null field in which the robotic arm applied no force. Fifteen pre-observation MEPs were acquired from the right FDI and APB muscles at rest. Participants then observed either a learning video or a control video. The learning video showed a tutor adapting his reaches to a clockwise force field (CW FF). The control video showed a tutor performing reaches in an unlearnable FF in which the direction of the applied force varied randomly from trial-to-trial. Fifteen post-observation MEPs were then acquired from the right FDI and APB muscles at rest. In the motor learning test, all participants performed reaches in a counterclockwise force field (CCW FF). B) MEPs. Motor evoked potentials (MEPs) were elicited by applying single-pulse TMS over the hand muscle representation of left M1 (shown at far left). MEPs were recorded from the relaxed first dorsal interosseous (FDI) and the abductor pollicis brevis (APB) muscles in the right hand (shown in middle). A sample MEP from one participant is shown on the far right. MEPs were measured peak-to-peak amplitude within the time window from 10-50 ms after TMS. MEPs, motor evoked potentials; FDI, first dorsal interosseous; APB, abductor pollicis brevis; FF, force field; M1, primary motor cortex.
Corticospinal excitability was assessed before and after observation using single-pulse TMS. We recorded motor evoked potentials (MEPs) from muscles in the right hand both before and after observation of either the learning video or the control video. We chose to examine MEP changes involving hand muscles for two reasons: first, we have found in previous work that observing motor learning changes functional connectivity between the the hand area of M1, primary somatosensory cortex, the cerebellum and visual area V5/MT (McGregor and Gribble 2015). Second, MEPs are more easily evoked from distal muscles compared to more proximal muscles (Palmer and Ashby 1992). Fifteen pre-observation MEPs were acquired from the relaxed first dorsal interosseus (FDI) and abductor pollicis brevis (APB) muscles in the right hand (see below for details). During MEP acquisition, participants fixated a crosshair presented in their line of view while resting their forearms on a tabletop. Subjects were instructed to relax their right hand muscles and the experimenters monitored pre-stimulation EMG activity throughout MEP acquisitions. Note that MEPs were not acquired during action observation, but rather while participants were at rest. Our measures therefore reflect offline corticospinal excitability.
Next, one group of participants (n=16) observed the learning video showing a tutor adapting to a CW FF. A control group (n=16) observed the control video showing a tutor performing reaches in an unlearnable FF. In both cases, participants were not told about FFs in the videos. Participants were asked to count the number of times the tutor in the video performed a correctly-timed reach indicated by the target turning blue following a reach. This was done to verify that the participant paid attention to the videos. Following the videos, 15 post-observation MEPs were acquired from the right FDI and APB using the same stimulation site and intensity as in the pre-observation MEP acquisition.
As a behavioral test of motor learning by observing, all participants then performed 96 reaches in a CCW FF. Note that participants were tested in a FF opposite to the one that was observed in the learning video. The idea is that the more participants learned about the CW FF during observation, the more interference this would cause in the CCW FF. Therefore, greater motor learning by observing was indicated by greater movement curvature in the test CCW FF. As we have frequently done in past work (Mattar and Gribble 2005; Cothros et al. 2006; Brown et al. 2009; McGregor and Gribble 2015, 2017; McGregor et al. 2016), we chose to use an interference paradigm because this tends to yield a more sensitive measure of motor learning by observing.
Motor Evoked Potential (MEP) Acquisition
We probed corticospinal excitability by using single-pulse TMS to elicit motor evoked potentials (MEPs) before and after observation. Single monophasic TMS pulses were delivered using a custom 50-mm diameter figure-of-eight branding iron coil connected to a Magstim 200 mono pulse stimulator (Magstim, Whitland, UK). The TMS coil was placed on the scalp over the hand representation of left M1 and positioned 45° relative to the sagittal plane to induce a current in the posterior-to-anterior direction. Single TMS pulses were delivered at a frequency of 0.25 Hz. MEPs were recorded using Ag-AgCl surface electrodes placed in a belly-tendon montage over the first dorsal interosseous (FDI) and the abductor pollicis brevis (APB) muscles in the right hand (see Figure 2B). Electromyographic (EMG) signals were amplified (1000x), bandpass filtered online (2 Hz - 2.5 kHz; Intronix Technologies Model 2024F, Bolton, Ontario, Canada), and digitized at 5 kHz by an analog-to-digital interface (Micro 1401, Cambridge Electronic Design, Cambridge, UK), and stored on a computer for offline analysis.
Prior to MEP acquisition, we identified the optimum location of stimulation of each participant’s hand muscle representation. We began by delivering TMS stimulations over site C3 on the scalp. The location of C3 was determined according to the International 10–20 system of electrode placement. This involved measuring the subject’s head from nasion to inion and then from left preauricural point to right preauricural point. We then determined the location of the vertex (Cz) located 50% of the distance between nasion and inion and 50% of the distance between preauricular points. C3 is located between Cz and the left preauricaular point by an amount corresponding to 20% of the distance between the left and right preauricular points (see Klem et al. 1999 for further details). Between stimulations, we moved the coil around C3 (by approx. 0.5 cm increments) and converged on the optimum location of stimulation. We defined the optimum location of stimulation as the location at which we could elicit MEPs of at least 50 mV in (peak-to-peak) amplitude from both the FDI and APB muscles in the relaxed right hand in at least 5 of 10 trials with the lowest stimulator intensity. After identifying the optimum location of stimulation, we adjusted the stimulation intensity such that TMS pulses would elicit MEPs of approximately 1 mV (peak-to-peak amplitude) from both the relaxed FDI and APB muscles. The same stimulation site was used for both the FDI and APB muscles. The same same stimulation site and stimulation intensity were used before and after observation within a given experimental session. We used a frameless stereotaxic neuronavigation system (Brainsight, Rogue Research, Montreal, QC, Canada) to ensure consistency in the TMS coil position.
Behavioral Analysis
Behavioral data were collected at a sampling rate of 600 Hz. Data were lowpass filtered offline at 40 Hz. Each movement trajectory was rotated to a common axis such that it was aligned with the 0° target (straight ahead of the home position). We quantified the curvature of each movement by computing the maximum perpendicular deviation (PD) of the hand path. The PD of a movement was defined as the maximum point of lateral deviation of the hand path relative to a straight line connecting the home and (0°) target. Since movements were aligned to a common axis (aligned to the 0° target), reaches in the CCW FF were curved to the left, reflected as negative PD values. We then computed a measure of initial PD in the CCW FF for each participant. Initial PD in the CCW FF was computed as the average PD of the participant’s first 24 reaches (first 3 reaches to each of the 8 targets) minus the average PD of the last 48 reaches in the null field. This allowed us to assess the extent to which observation interfered with the participant’s initial performance in the CCW FF relative to his or her baseline movement curvature in the null field. As we have demonstrated previously (Mattar and Gribble 2005; Cothros et al. 2006; Brown et al. 2009; McGregor and Gribble 2015, 2017; McGregor et al. 2016), learning about a FF from observation results in more curved movements in the opposite FF. Therefore, we predicted that greater motor learning by observing would result in greater movement curvature in the CCW FF, and hence larger negative PD values. We compared motor learning by observing scores between groups using a one-tailed independent samples t test. We used a one-tailed t test because our behavioural measures would indicate motor learning by observing had occurred only if the learning group exhibited larger negative PD values in the left FF (i.e., greater interference) relative to the control group.
MEP Data Analysis
EMG data were bandpass filtered offline between 20-500 Hz and a notch filter was applied (58-62 Hz). For each trial, we computed the peak-to-peak amplitude of the MEP occurring between 10 and 50 ms following stimulation. Trials in which pre-stimulation activity exceeded +/−5 mV were excluded from analyses. A 2×2×2 split-plot analysis of variance (ANOVA) was carried out using group (learning, control) as a between-subjects factor, and muscle (FDI, APB) and time (pre-observation, post-observation) as within-subject factors.
Results
Behavioral Results
Figure 3A shows the evolution of PD over the course of the experiment for the learning and control groups. It can be seen that movements are straight in the baseline null field condition for both groups. Following observation, the learning group’s initial movements in the CCW FF are more curved compared to those of the control group. This difference in initial performance in the CCW FF can also be seen in Figure 3B in which we have shown hand path traces of movements performed in the first block of trials from individual participants, one from the learning group and the other from the control group. Therefore, as predicted, participants who observed the video of the tutor learning to reach in the CW FF experienced interference during initial performance in the CCW FF compared to control participants who did not observe learning. The two groups’ learning curves converge after the first few blocks of movements in the CCW FF. This is expected as participants in both groups begin to actively adapt to the CCW FF. Figure 3C shows initial PD in the CCW FF for both groups. Participants who observed the learning video (blue) exhibited greater movement curvature in the CCW FF and this was reflected as more negative PD values compared to participants who observed the control video (green) [t(30) = 1.85, p = 0.037, one-tailed]. Therefore, as a result of having observed CW FF learning in the video, participants in the learning group performed worse, more curved movements in the CCW FF relative to the control group.
Figure 3.
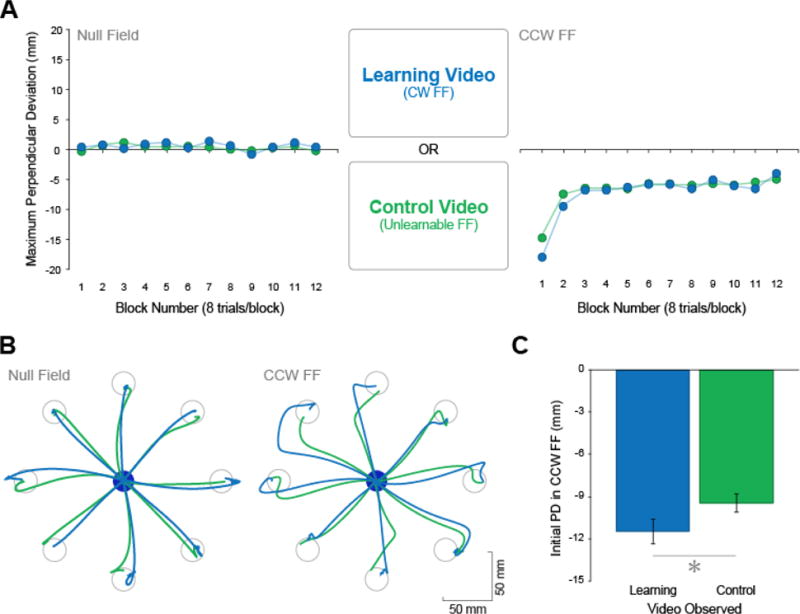
Behavioral Results. A) Reaching behavior. Evolution of movement curvature over the course of the experiment. Positive values along the y-axis indicate rightward curvature, negative values along the y-axis indicate leftward curvature. B) Sample trajectories. Typical hand trajectories in the null field (left) and in the CCW FF (right) from a participant in the learning group (blue) and a participant in the control group (green). C) Initial PD in the CCW FF. Average PD during the first 3 blocks in the CCW FF relative to the participant’s own baseline PD in the null field for the learning group (blue) and control group (green). * indicates p < 0.05. Error bars indicate standard error.
MEP Results
We probed offline corticospinal excitability by using single-pulse TMS to elicit MEPs from hand muscles before and after participants observed either the learning video or the control video. A split-plot ANOVA revealed a statistically significant interaction between group (learning vs control) and time (pre- vs. post-observation; F(1,30) = 8.74, p = 0.006). Post-observation MEP amplitudes measured from both the FDI muscle and APB muscle increased only for the group who had observed the learning video (t(30) = 2.28, p = 0.031 and t(30) = 2.08, p = 0.046, Bonferroni-Holm corrected, respectively). This interaction is shown in Figure 4 which shows pre- and post-observation MEP amplitudes. It can be seen that greater increases in MEP amplitudes from pre-observation to post-observation were measured for the learning group compared to the control group, regardless of the hand muscle from which MEPs were recorded.
Figure 4.
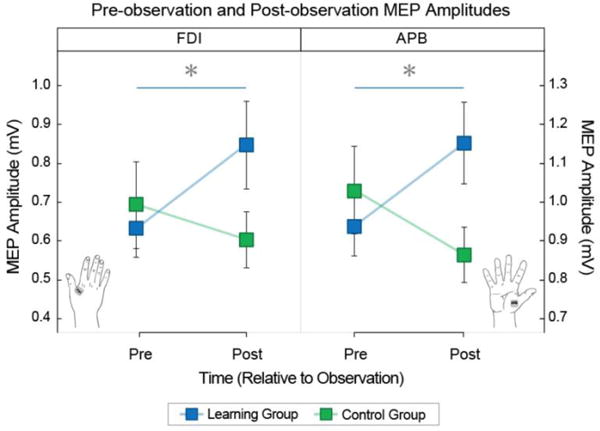
Pre- and post-observation MEP amplitudes. Pre- and post-observation MEP amplitudes collected from FDI (left panel) and from APB (right panel). Data from the learning group and the control group are shown in blue and green, respectively. Error bars indicate standard error.
We further examined if there was a relationship between MEP changes and the extent to which participants learned from observation. However, we found no statistically significant correlations between post-observation changes in MEP amplitudes and motor learning by observing scores for either group. Correlations between behavioural scores and post-observation MEP changes for the Learning group were as follows: r(30) = 0.43, p = 0.09 and r(30) = 0.28, p = 0.32 for FDI and APB MEP changes, respectively. Correlations between behavioural scores and post-observation MEP changes for the Control group were as follows: r(30) = −0.39, p = 0.15 and r(30) = −0.16, p = 0.57 for FDI and APB MEP changes, respectively.
We examined whether subjects’ EMG activity prior to TMS stimulation was consistent from pre- to post-observation for both groups. For each trial, we computed the root mean square error of EMG activity within the 100-ms window prior to TMS stimulation (−100 to 0 ms). The results of a 2×2×2 split-plot ANOVA showed that pre-TMS EMG activity did not reliably differ between pre- and post-observation conditions for either muscles across the two groups (F(1,30) = 0.002, 0.96).
Discussion
Here we used single-pulse TMS to test the role of M1 in motor learning by observing. We measured changes in offline corticospinal excitability by eliciting MEPs from the participants’ hand muscles before and after the observation of a FF reaching task. We found that those participants who observed the video of a tutor undergoing FF adaptation showed reliable increases in MEP amplitudes recorded from FDI and APB hand muscles following observation. However, a control group who observed a video showing a tutor performing reaches in an unlearnable FF did not show post-observation changes in MEP amplitudes. These MEP changes can therefore be linked to observation of motor learning, and not to observing movements in general or observing movement errors. This is consistent with learning-dependent, adaptive physiological changes in motor cortex associated with physical practice (Plautz et al. 2000). This result suggests that observation of motor learning involves functional changes within M1 and/or corticospinal networks.
In the current study, we showed that observing motor learning resulted in MEP increases, but this was not the case for observing similar movements that did not depict learning. This finding is perhaps surprising in light of previous work showing that action observation increases corticospinal excitability and/or M1 excitability (Fadiga et al. 1995; Strafella and Paus 2000b; Watkins et al. 2003; Montagna et al. 2005); however, an important distinction is that the studies described above assessed corticospinal activity during action observation whereas here we assessed offline corticospinal excitability while participants rested and fixated a crosshair. It is feasible that the discrepancy between the current and previous findings stems from our use of offline measures of corticospinal excitability as compared to more commonly-used online measures acquired during action observation.
Here we found that observing FF learning, a reaching task primarily involving shoulder and elbow movement, facilitates MEPs acquired from hand muscles. This finding is at odds with previous work showing that changes in M1 excitability are specific to the muscles used for the observed movement (Fadiga et al. 1995; Strafella and Paus 2000b; Watkins et al. 2003; Stefan et al. 2005; Borroni and Baldissera 2008; Alaerts et al. 2010; de Beukelaar et al. 2016). For example, Strafella and Paus (2000) applied single-pulse TMS over M1 while participants observed videos showing an actor performing hand movements (i.e., writing) or arm movements (i.e., flexion, extension, abduction and drawing shapes with the arm while the wrist and hand remained a prone position). MEPs elicited from the observers’ hand muscles (FDI) increased during hand movement observation. MEPs elicited from the observers’ biceps increased during arm movement observation. Results from this study and others have shown that action observation facilitates the observer’s motor system in a muscle-specific manner.
In the current study, we observed changes in MEP amplitudes from hand muscles following the observation of a learning task that largely involves shoulder and elbow movement. One possible reason why we observed changes in MEPs from hand muscles is that participants looked at the tutor’s hand and the robot handle during the video. While the reaching task involved elbow and shoulder movement, the goal of the task was to move the robot handle (and hand) to visual targets along a straight hand path. Looking at the tutor’s hand likely activates the hand area of the observer’s motor cortex, as we have indeed reported previously using similar video stimuli (McGregor and Gribble 2015). Moreover, while the FF reaching task used in the current study primarily involves changes in forces generated at the elbow and shoulder, there is evidence that grip forces also change with motor learning. For example, Flanagan et al. (2003) demonstrated that grip forces show anticipatory adjustments over the course of motor learning. In their experiment, participants gripped an object and were instructed to move it along a straight path. The object was attached to a robotic manipulandum which applied a FF that varied in magnitude with the velocity of movement. Participants learned to scale their grip forces well before they were able to accurately move the object along the desired path (Flanagan et al. 2003). Despite their use of a different grip type (precision vs. power grip) and FF (vertical versus horizontal curl field), this finding may provide insight into why MEP changes were seen in hand muscles following the observation of FF learning.
During piloting for this experiment, we attempted to acquire MEPs from the biceps in addition to hand muscles. However, we encountered ceiling effects such that MEPs from the biceps did not scale with increasing stimulation intensity (even when pilot subjects maintained a slight tonic contraction). Future studies should examine whether the MEP increases observed in the current study also occur in the biceps.
We found that observation of a tutor adapting to a consistent FF increased MEP amplitudes whereas observing a tutor performing reaches in a randomly-varying (and hence unpredictable) FF did not bring about MEP changes. A recent study performed by de Beukelaar and colleagues (2016) showed that being able to predict an upcoming observed movement can elicit anticipatory M1 facilitation. In this study, participants observed videos of an actor lifting an object using either a precision grip or a whole hand grip. Participants were provided with a cue prior to each video, informing them of the type of grip they would see the actor use in the upcoming trial. The video began and the actor’s arm hovered above the object, giving no visual indication of the grip type that would be used. During this pre-movement phase of the video, participants showed increases in MEP amplitudes from muscles that would be used in the predicted grasp. Therefore, being able to predict the upcoming observed movement resulted in anticipatory, muscle-specific M1 facilitation before the actor executed the movement (de Beukelaar et al. 2016). In the context of the current study, it is possible that this anticipatory facilitation may explain why we found MEP increases for the learning group but not for the control group. Recall that the upcoming force direction could only be predicted in the learning video, which depicted a CW FF applied throughout. It is feasible that participants who observed the learning video became able to predict the tutor’s upcoming movement kinematics and therefore showed similar anticipatory M1 facilitation throughout the video. This may be a possible explanation as to why MEP amplitudes increased following the learning video only.
The reported increases in offline corticospinal excitability following the observation of motor learning may reflect motor memory consolidation involving M1. A previous fMRI study from our group has similarly shown offline changes in functional connectivity involving M1 at rest (McGregor and Gribble, 2015). Furthermore, we have previously shown that dampening offline M1 excitability following observation diminished motor learning by observing (Brown et al. 2009). Together, these studies suggest that observing motor learning changes offline activity involving M1 and that interfering with such post-observation functional changes disrupts motor learning by observing.
Previous TMS studies have demonstrated that M1 excitability increases during action observation (Fadiga et al. 1995; Strafella and Paus 2000b; Watkins et al. 2003; Montagna et al. 2005; Naish et al. 2014). In contrast to this body of work, Caspers and colleagues (2010) recently conducted a meta-analysis using functional neuroimaging data from 104 action observation experiments and showed that M1 is not consistently activated during action observation. The findings of this meta-analysis raise the possibility that MEP increases during action observation reported in TMS studies are driven by brain areas other than M1. Premotor cortex, primary somatosensory cortex, and inferior parietal cortex are consistently activated during action observation (Caspers et al. 2010) and have also been implicated in observational learning (Cross et al. 2009; Bernardi et al. 2013; McGregor and Gribble 2015, 2017; McGregor et al. 2016). It is feasible that projections from these or other brain areas mediate M1 activity during action observation (Koch et al. 2010) and influence the extent to which visual input facilitates motor learning. For example, observing motor learning increases S1 excitability by amounts that correspond to behavioral learning scores. Moreover, interfering with somatosensory processing during observation can disrupt motor learning by observing (McGregor et al. 2016). Further TMS studies (including repetitive, paired-pulse, and dual-site TMS connectivity approaches) combined with EEG and fMRI could determine the influence of premotor cortex and parietal cortex on M1 after observational learning (Vesia and Davare 2011; Hallett 2017).
Here we showed that observing FF learning increases MEP amplitudes recorded from FDI and APB hand muscles. In contrast, control participants who observed a tutor performing reaches in an unlearnable, randomly-varying FF did not show post-observation changes in MEP amplitudes. This suggests that observing motor learning results in rapid functional changes in M1 or corticospinal networks, or both. Future studies should assess stimulus/response curves and use paired-pulse TMS to determine the contribution of cortical inhibitory and excitatory neural circuits to motor learning by observing. This study contributes to growing evidence that motor learning by observing is supported by a broad network of visual, somatosensory and motor brains areas such as premotor cortex, primary motor cortex, primary cortex, superior parietal lobule, visual area V5/MT, and the cerebellum (McGregor and Gribble 2015, 2017).
Acknowledgments
The authors wish to thank Dinant Kistemaker for the figure showing the robotic manipulandum setup. This work was supported by the Natural Sciences and Engineering Research Council of Canada, and by the National Institute of Child Health and Human Development R01 HD075740.
Abbreviations
- FF
Force field
- CW
clockwise
- CCW
counterclockwise
- PD
Maximum perpendicular deviation
- TMS
Transcranial magnetic stimulation
- MEP
Motor evoked potential
Footnotes
The authors report no financial interests or conflicts of interests.
References
- Alaerts K, Senot P, Swinnen SP, Craighero L, Wenderoth N, Fadiga L. Force requirements of observed object lifting are encoded by the observer’s motor system: a TMS study. Eur J Neurosci. 2010;31:1144–1153. doi: 10.1111/j.1460-9568.2010.07124.x. [DOI] [PubMed] [Google Scholar]
- Bernardi NF, Darainy M, Bricolo E, Ostry DJ. Observing motor learning produces somatosensory change. J Neurophysiol. 2013;110:1804–1810. doi: 10.1152/jn.01061.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beukelaar TT, Alaerts K, Swinnen SP, Wenderoth N. Motor facilitation during action observation: The role of M1 and PMv in grasp predictions. Cortex. 2016;75:180–192. doi: 10.1016/j.cortex.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Borroni P, Baldissera F. Activation of motor pathways during observation and execution of hand movements. Soc Neurosci. 2008;3:276–288. doi: 10.1080/17470910701515269. [DOI] [PubMed] [Google Scholar]
- Brown LE, Wilson ET, Gribble PL. Repetitive transcranial magnetic stimulation to the primary motor cortex interferes with motor learning by observing. J Cogn Neurosci. 2009;21:1013–1022. doi: 10.1162/jocn.2009.21079. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund H-J. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- Buckingham G, Wong JD, Tang M, Gribble PL, Goodale MA. Observing object lifting errors modulates cortico-spinal excitability and improves object lifting performance. Cortex. 2014;50:115–124. doi: 10.1016/j.cortex.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50:1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cothros N, Köhler S, Dickie EW, Mirsattari SM, Gribble PL. Proactive interference as a result of persisting neural representations of previously learned motor skills in primary motor cortex. J Cogn Neurosci. 2006;18:2167–2176. doi: 10.1162/jocn.2006.18.12.2167. [DOI] [PubMed] [Google Scholar]
- Cross ES, Kraemer DJM, de C Hamilton AF, Kelley WM, Grafton ST. Sensitivity of the action observation network to physical and observational learning. Cereb Cortex. 2009;19:315–326. doi: 10.1093/cercor/bhn083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Exp Brain Res. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol. 1995;73:2608–2611. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Vetter P, Johansson RS, Wolpert DM. Prediction Precedes Control in Motor Learning. Curr Biol. 2003;13:146–150. doi: 10.1016/s0960-9822(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Gallese V. Mirror neurons and the simulation theory of mind-reading. Trends Cogn Sci. 1998;2:493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Hallett M. Smart brain stimulation. Clin Neurophysiol. 2017;128:839–840. doi: 10.1016/j.clinph.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Heyes CM, Foster CL. Motor learning by observation: evidence from a serial reaction time task. Q J Exp Psychol A. 2002;55:593–607. doi: 10.1080/02724980143000389. [DOI] [PubMed] [Google Scholar]
- Hickok G. Eight problems for the mirror neuron theory of action understanding in monkeys and humans. J Cogn Neurosci. 2009;21:1229–1243. doi: 10.1162/jocn.2009.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klem GH, Lüders HO, Jasper HH, Elger C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:3–6. [PubMed] [Google Scholar]
- Koch G, Versace V, Bonnì S, Lupo F, Lo Gerfo E, Oliveri M, Caltagirone C. Resonance of cortico– cortical connections of the motor system with the observation of goal directed grasping movements. Neuropsychologia. 2010;48:3513–3520. doi: 10.1016/j.neuropsychologia.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Mattar AAG, Gribble PL. Motor learning by observing. Neuron. 2005;46:153–160. doi: 10.1016/j.neuron.2005.02.009. [DOI] [PubMed] [Google Scholar]
- McGregor HR, Cashaback JGA, Gribble PL. Functional Plasticity in Somatosensory Cortex Supports Motor Learning by Observing. Curr Biol. 2016;26:921–927. doi: 10.1016/j.cub.2016.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor HR, Gribble PL. Changes in visual and sensory-motor resting-state functional connectivity support motor learning by observing. J Neurophysiol. 2015;114:677–688. doi: 10.1152/jn.00286.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor HR, Gribble PL. Functional connectivity between somatosensory and motor brain areas predicts individual differences in motor learning by observing. J Neurophysiol. 2017;118:1235–1243. doi: 10.1152/jn.00275.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagna M, Cerri G, Borroni P, Baldissera F. Excitability changes in human corticospinal projections to muscles moving hand and fingers while viewing a reaching and grasping action. Eur J Neurosci. 2005;22:1513–1520. doi: 10.1111/j.1460-9568.2005.04336.x. [DOI] [PubMed] [Google Scholar]
- Naish KR, Houston-Price C, Bremner AJ, Holmes NP. Effects of action observation on corticospinal excitability: Muscle specificity, direction, and timing of the mirror response. Neuropsychologia. 2014;64:331–348. doi: 10.1016/j.neuropsychologia.2014.09.034. [DOI] [PubMed] [Google Scholar]
- Palmer E, Ashby P. Corticospinal projections to upper limb motoneurones in humans. J Physiol. 1992;448:397–412. doi: 10.1113/jphysiol.1992.sp019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem. 2000;74:27–55. doi: 10.1006/nlme.1999.3934. [DOI] [PubMed] [Google Scholar]
- Prinz W. What Re-Enactment Earns Us. Cortex. 2006;42:515–517. doi: 10.1016/s0010-9452(08)70389-7. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Cognitive Brain Research. 1996;3:131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Stefan K, Cohen LG, Duque J, Mazzocchio R, Celnik P, Sawaki L, Ungerleider L, Classen J. Formation of a motor memory by action observation. J Neurosci. 2005;25:9339–9346. doi: 10.1523/JNEUROSCI.2282-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T. Modulation of cortical excitability during action observation. Neuroreport. 2000a;11:2289–2292. doi: 10.1097/00001756-200007140-00044. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Paus T. Modulation of cortical excitability during action observation: a transcranial magnetic stimulation study. Neuroreport. 2000b;11:2289–2292. doi: 10.1097/00001756-200007140-00044. [DOI] [PubMed] [Google Scholar]
- Turella L, Pierno A, Tubaldi F, Castiello U. Mirror neurons in humans: Consisting or confounding evidence? Brain Lang. 2009;108:10–21. doi: 10.1016/j.bandl.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Vesia M, Davare M. Decoding action intentions in parietofrontal circuits. J Neurosci. 2011;31:16491–16493. doi: 10.1523/JNEUROSCI.4408-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Strafella AP, Paus T. Seeing and hearing speech excites the motor system involved in speech production. Neuropsychologia. 2003;41:989–994. doi: 10.1016/s0028-3932(02)00316-0. [DOI] [PubMed] [Google Scholar]


