Abstract
Introduction
Rural and critical access hospitals rely on the “drip and ship” practice using helicopter emergency medical services (HEMS). But those helicopter flights are an unusual environment with physical factors such as vibration and accelerations that could potentially affect the stability, and pharmacological properties of IV rtPA, an issue that has not been previously addressed.
Materials and Methods
This was a prospective cohort study of consecutive acute ischemic stroke patients receiving IV rtPA through a Comprehensive Stroke Center (CSC) from November 2015 to February 2017 to measure the effects of HEMS on the integrity and activity of rtPA by collecting residual medication left in the vial.
Clinical Trial Registration
Results
A total of 33 patients and rtPA samples were included; 18 patients who presented directly to the CSC emergency department and 15 patients who received rtPA during air ambulance transfer. The median rtPA antigen concentration in the residual medication vial was 3.04 mg/mL (IQR: 1.24–3.87) in the HEMS group and 1.91 mg/mL (IQR: 1.33–2.60) in the controls (p=0.168). There were no significant differences in rtPA activity or specific activity between the HEMS and control groups and there was no association between total HEMS flight time on overall rtPA specific activity.
Conclusions
In summary, this study provides supportive evidence of the lack of a detrimental effect of the HEMS physical environment on the integrity of rtPA, therefore endorsing current drip and ship practices without infusion adjustments.
Introduction
Twenty percent of the US population live in rural areas and rely on the “drip and ship” practice of initiating IV-rtPA before emergent transfer to a tertiary stroke center where mechanical thrombectomy (MT) can be performed if needed. Because acute ischemic stroke is a time-sensitive emergency,1,2 the transfers commonly involve helicopter emergency medical services (HEMS).2–7 This mode of transportation is also used in traffic-congested urban areas. A helicopter flight, however, is an unusual physical environment for a patient receiving rtPA.7 It involves a number of unusual physical factors such as strong low frequency vibrations, accelerations, and rapid changes in barometric pressure, all of which may potentially compromise the pharmacological integrity of IV rtPA.7–10 The impact of HEMS on the stability, and hence pharmacological properties of IV rtPA has never been studied.7 In the era of MT, it is imperative that we optimize the efficacy of “drip and ship” operations by addressing any potential negative factors.7 The primary objective of this study was to assess the potential impact of air ambulance transfer on the integrity and function of rtPA. We hypothesized that the vibration associated with HEMS flight exposure may lead to significant breakdown in the protein structure of rtPA decreasing its specific activity.
Materials and Methods
This was a prospective cohort study of consecutive acute ischemic stroke patients receiving IV rtPA through the University of Iowa Comprehensive Stroke Center from November 2015 to February 2017.
Study Subjects
The experimental group consisted subjects transferred via the “drip and ship” mechanism using the University of Iowa Air Care (AC) HEMS under the direction of a stroke center vascular neurologist. All AC air medical crews (nurses and paramedics) are University of Iowa employees, allowing us to certify them as co-investigators through the University of Iowa Institutional Review Board (IRB). The control group consisted of acute ischemic stroke patients treated with rtPA at the University of Iowa Hospital emergency department (ED) during the same time period without air transfer. The rtPA (Activase®, Genentech USA) was reconstituted through standard procedures using sterile water for Injection to a targeted concentration of 1.0 mg/mL. For all patients, the rtPA infusion was administered directly from the reconstituted vial. Pharmacy and nursing staff at our institution received detailed training on how to accurately prepare rtPA. We also monitored the pharmacy practices in relation to rtPA of the originating “drip and ship” institutions through a survey. The survey was developed to capture acute ischemic stroke practices and rtPA preparation practices at each transferring institution. Patients were excluded if they were transferred through a non-UI employee HEMS service, if no rtPA was delivered during the flight, or if the flight/ground rtPA occurred outside business hours when a clinical pharmacist was available to collect and process the rtPA residual sample in a timely manner.
The study was approved by our local IRB with written informed consent obtained from all patients enrolled. The study protocol was registered on ClinicalTrials.gov protocol NCT02752256.
Primary outcome: measures of rtPA activity
The objective of this study was to measure the integrity and activity of the residual rtPA left in the medication vial after the patient was treated and the infusion was complete. We hypothesized that rtPA administered during flight undergoes significant protein breakdown due to the HEMS-related physical forces. Each vial has a small volume (approximately 0.5 mL) of reconstituted rtPA remaining in each vial after administration. The residual sample was collected from the vial and frozen (−183 Celsius) within 8 hours of preparation of the rtPA.
The concentration of rtPA antigen was measured in samples using a commercially available ELISA (ab108914, Abcam®). All of the samples were diluted 2.5x105 fold with the diluent buffer prior to the assay. Diluted samples and standards were added to 96 well plates coated with rtPA specific antibody and further incubated with rtPA specific biotinylated detection antibody for 1 hour at room temperature, followed by addition of streptavidin-peroxidase conjugate. The substrate TMB was added to monitor enzymatic activity that generates a blue color product. The reaction was stopped by adding an acidic stop solution that changes product to yellow color. The density of yellow coloration was measured using a plate reader at 450 nm.
The rtPA activity in the samples was determined using a commercially available assay kit (ab108905, Abcam®). All the samples were first diluted 1x109 times in a diluent provided in the assay kit. Plasminogen and plasmin substrate were incubated with respective samples or standards for 5 hours at 37°C. The amount of plasmin produced was quantified using a highly specific plasmin substrate releasing a yellow para-nitroaniline (pNA) chromophore which was measured using a plate reader at 405 nm.
Quantification of flight exposure
The amount of HEMS flight time was documented for each patient by the air medical crews. All flights were conducted in Eurocopter helicopters (EC-130 B4 and ASTAR 350-B2, both single pilot and single engine aircraft) based in two different locations of the state. The vibration signature of the helicopter and the frequencies involved was previously recorded by our group using accelerometers.
Clinical Co-variables
Baseline patient characteristics and outcome variables were abstracted from the electronic medical record. Characteristics of interest included; age, weight (estimated or actual), initial National Institutes of Health Stroke Scale (NIHSS), and HEMS flight duration (minutes).
Statistical Analysis
The primary outcome was rtPA specific activity, with secondary outcomes of rtPA antigen concentration and activity. We characterized each outcome through descriptive statistics (mean, median, and interquartile range) for each treatment arm. Differences between each group were evaluated using the Wilcoxon rank sum test with exact p-values. Our hypothesis was that if rtPA undergoes significant agitation or vibration there is risk for protein breakdown. Based on this hypothesis we would expect a significant effect on rtPA activity. Using this assumption, we calculated a sample size of 22 patients would be required (α = 0.05, power = 80%) to detect a mean difference of 30% in rtPA activity between the transfer and control groups. All analyses were completed using SAS software, version 9.3 of the SAS System for Microsoft (SAS Institute Inc, Cary, NC).
Results
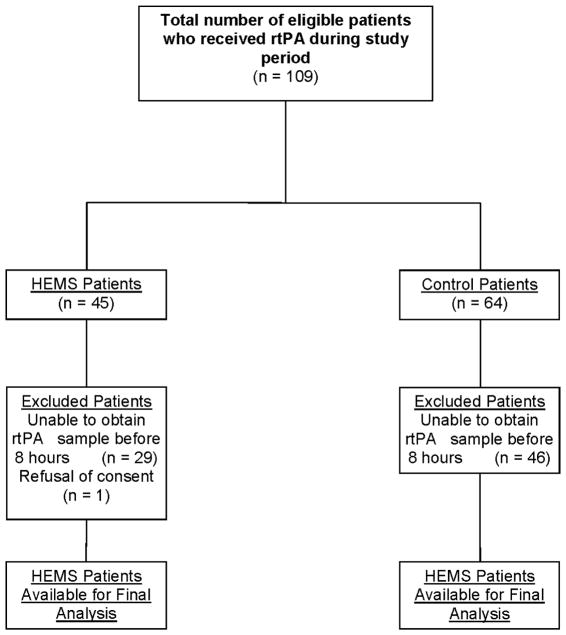
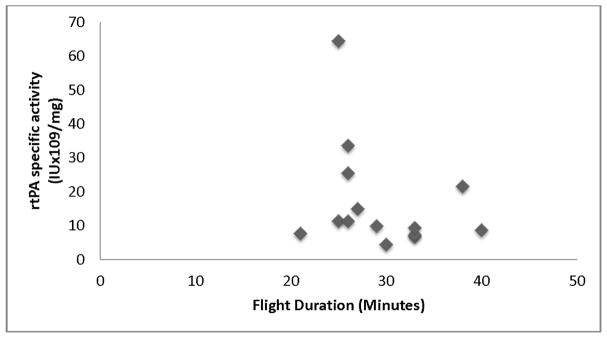
A total of 33 patients were included in our final population; 18 patients who presented directly to the CSC ED and 15 patients who received rtPA during HEMS transfer. The mean NIHSS score was 6±4 in the control group compared to 12±8 in the transfer group. The primary reason for exclusion was unavailability of the pharmacist to collect and process the rtPA sample within 8 hours (Figure 1). Of the 15 transfer patients, 11 rtPA preparation surveys were available for evaluation. Nine (81%) of the patients had their weight estimated by the ED provider compared to 0% of the control group who were physically weighed. Additionally, 3 (27%) of the transferring institutions reported shaking/agitating the rtPA during reconstitution, which goes against proper preparation technique outlined in the rtPA package insert.11 The median rtPA antigen concentration was 3.04 mg/mL (IQR: 1.24–3.87) in the HEMS group and 1.91 mg/mL (IQR: 1.33–2.60) in the controls (p=0.168). There were no significant differences in rtPA activity between the HEMS and control groups (Table 1). Figure 2 shows the lack of association between total HEMS flight time on overall rtPA specific activity.
Figure 1.
Flow diagram of included transfer and control patients for analysis.
Table 1.
rtPA concentrations and activity in the intervention group compared to the control group
| Intervention (n=14) | Control (n=18) | p-value | |
|---|---|---|---|
| Flight time, minutes (mean SD) | 29.4 (5.4) | - | - |
| rtPA antigen, mg/mL (median, IQR) | 3.04 (1.24–3.87) | 1.91 (1.33–2.60) | 0.168 |
| rtPA activity, IUx109/mL (median, IQR) | 27.18 (24.81–38.57) | 29.15 (26.21–33.23) | 0.660 |
| rtPA specific activity, IUx109/mg (median, IQR) | 10.58 (7.72–21.60) | 16.76 (10.53–21.59) | 0.338 |
Figure 2.
Association of flight duration (minutes) and the effect on rtPA specific activity.
Discussion
In the era of MT, HEMS inter-hospital transfer is vital for acute ischemic stroke patients who require specialized care at a CSC. Approximately 1 out of every 6 acute ischemic stroke patients receives rtPA using the drip-and-ship method and this number is expected to significantly increase as MT implementation increases.4,7,12–15 The unique physical factors present during HEMS transfer is complex and has not been studied properly. For example, low-frequency vibration may lead to an increase in blood-brain barrier permeability or the hypobaric environment may worsen the ischemic penumbra.16,17 However, some of the environmental factors could be beneficial for the thrombus.17 The accelerations and low frequency vibrations associated with HEMs transfer may be synergistic with rtPA and enhance the thrombolysis effectiveness of rtPA.18,19 The vibration effects in conjunction with thrombolysis have been shown to increase the rate of recanalization compared to thrombolysis alone.20 On the other hand, vibrations and accelerations could negatively impact the effect of a reconstituted rtPA solution which is not supposed to be shaken per pharmacy practice guidelines.11 In this study we specifically addressed the potential impact of these HEMS forces on rtPA integrity and efficacy. It is important to ensure maximal efficacy of rtPA in transported patients, since any significant deviations from the expected effect may require counteractions, such as dose increase or vibration mitigation in the medication vials. While the values were higher in the HEMS exposed group, we found no evidence of a significant effect of HEMS and practice deviations (shaking the reconstituted vials) in those parameters. As such, our results endorse the efficacy and safety of HEMS in the era of reperfusion without the need for rtPA adjustments or mitigation procedures.
We recognize that we have only addressed a very specific potential mechanism where HEMS transportation could impact “drip and ship” stroke patients. We also recognize the limitations of our study. First, the sample size is small, so it is possible that we were lacking enough power and that this was a false negative study. Second, we were unable to determine if other unmeasured physical environmental factors could have impacted our findings. Third, our study was carried out at a single center utilizing one air ambulance service. While this consistency of exposure is a methodological strength, it raises questions of generalization to different helicopter services with different vibratory signatures.
Summary
In summary, this study provides evidence of rtPA integrity in the HEMS physical environment, therefore endorsing current drip and ship practices.
Acknowledgments
Grant Support: Funding for this project was supported by the National Institute of Neurological Disorders and Stroke (NINDS) StrokeNet Fellowship U10MS086521-01 (Faine) and American Heart Association grant 13SDG14050002 (Dayal) and National Institutes of Health grants RO1AG049784 (Dayal).
We are thankful to the flight nurses and paramedics from UIHC air ambulance crew for their generous effort in collecting rtPA samples for transfer patients. We are also thankful for Priyanka Vakkalanka for her assistance with the statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ecass, atlantis, ninds, and epithet trials. Lancet. 2010;375:1695–703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309:2480–8. doi: 10.1001/jama.2013.6959. [DOI] [PubMed] [Google Scholar]
- 3.Sheth KN, Smith EE, Grau-Sepulveda MV, et al. Drip and ship thrombolytic therapy for acute ischemic stroke: Use, temporal trends, and outcomes. Stroke. 2015;46:732–9. doi: 10.1161/STROKEAHA.114.007506. [DOI] [PubMed] [Google Scholar]
- 4.Tekle WG, Chaudhry SA, Hassan AE, et al. Drip-and-ship thrombolytic treatment paradigm among acute ischemic stroke patients in the united states. Stroke. 2012;43:1971–4. doi: 10.1161/STROKEAHA.112.657817. [DOI] [PubMed] [Google Scholar]
- 5.Silliman SL, Quinn B, Huggett V, et al. Use of a field-to-stroke center helicopter transport program to extend thrombolytic therapy to rural residents. Stroke. 2003;34:729–33. doi: 10.1161/01.STR.0000056529.29515.B2. [DOI] [PubMed] [Google Scholar]
- 6.Leira EC, Hess DC, Torner JC, et al. Rural-urban differences in acute stroke management practices: A modifiable disparity. Arch Neurol. 2008;65:887–91. doi: 10.1001/archneur.65.7.887. [DOI] [PubMed] [Google Scholar]
- 7.Leira EC, Stilley JD, Schnell T, et al. Helicopter transportation in the era of thrombectomy: The next frontier for acute stroke treatment and research. European Stroke Journal. 2016;1:171–79. doi: 10.1177/2396987316658994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silbergleit R, Dedrick DK, Pape J, et al. Forces acting during air and ground transport on patients stabilized by standard immobilization techniques. Ann Emerg Med. 1991;20:875–7. doi: 10.1016/s0196-0644(05)81429-5. [DOI] [PubMed] [Google Scholar]
- 9.Carchietti E, Cecchi A, Valent F, et al. Flight vibrations and bleeding in helicoptered patients with pelvic fracture. Air Med J. 2013;32:80–3. doi: 10.1016/j.amj.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Knotts D, Arthur AO, Holder P, et al. Pneumothorax volume expansion in helicopter emergency medical services transport. Air Med J. 2013;32:138–43. doi: 10.1016/j.amj.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Activase (alteplase) [package insert] Genentech; San francisco, ca: 2018. [Google Scholar]
- 12.Powers WJ, Derdeyn CP, Biller J, et al. 2015 american heart association/american stroke association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2015;46:3020–35. doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 13.Lukovits TG, Von Iderstine SL, Brozen R, et al. Interhospital helicopter transport for stroke. Air Med J. 2013;32:36–9. doi: 10.1016/j.amj.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 15.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–18. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iakubovich TG, Getsel' Kh A. the effect of vibration on the permeability of the blood-brain barrier. Fiziol Zh SSSR Im I M Sechenova. 1972;58:845–50. [PubMed] [Google Scholar]
- 17.Leira EC, Zaheer A, Schnell T, et al. Effect of helicopter transport on neurological outcomes in a mouse model of embolic stroke with reperfusion: Air-mice pilot study. Int J Stroke. 2015;10(Suppl A100):119–24. doi: 10.1111/ijs.12619. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann A, Gill H. Externally applied vibration at 50 hz facilitates dissolution of blood clots in-vitro. 2012 [Google Scholar]
- 19.Smikahl J, Yeung D, Wang S, et al. Alteplase stability and bioactivity after low-power ultrasonic energy delivery with the omnisonics resolution system. J Vasc Interv Radiol. 2005;16:385–9. doi: 10.1097/01.RVI.0000147066.97599.87. [DOI] [PubMed] [Google Scholar]
- 20.Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–8. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]