Abstract
Background
Requirement for hospitalization in ulcerative colitis (UC) is a marker of severity of disease. However, the paradigm of when to escalate therapy in such patients and the benefits of early immunomodulator initiation is less well established.
Aim
To examine the benefits of early therapy escalation in immunosuppression-naïve patients hospitalized with severe ulcerative colitis responsive to steroids.
Methods
We identified hospitalized UC patients who were immunosuppression naïve at index hospitalization and responded to intravenous steroids, not requiring medical or surgical rescue therapy. The ‘therapy escalated’ group comprised of those who were initiated on immunomodulators within 3 months of hospitalization. Need for colectomy at 12 months were compared to the ‘not escalated’ group who remained on non-immunosuppressive therapy.
Results
Among 133 immunosuppressive naïve patients hospitalized for ulcerative colitis, 13 (9.8%) who responded to intravenous steroids and did not require rescue therapy underwent colectomy by 1 year. Among 123 patients who escalated to either immunomodulators (n=46, 37%) or remained on non-immunosuppressive therapy (92% on 5-ASA), there was no difference in the need for colectomy at 1 year (10.8% vs. 7.8%; multivariate OR 1.29, 95% CI 0.35-4.74). There was also no difference in the time to colectomy between the two groups (p=0.55).
Conclusion
Immunosuppression-naïve ASUC patients who respond to intravenous steroids remain at risk for colectomy. Immunomodulator initiation by 3 months did not reduce risk of colectomy at 1 year. There is an important need for need for prospective studies identifying thresholds for therapy escalation in UC.
Keywords: Hospitalization, colectomy, steroids, acute severe ulcerative colitis
INTRODUCTION
Ulcerative colitis (UC) is a chronic idiopathic inflammatory condition of the colon, affecting nearly 1 million individuals in the United States and many more worldwide1. While the disease course may be characterized by mild-to-moderate symptoms and infrequent flares in many, approximately 18%-25% of the patients will develop severe exacerbation requiring hospitalization known as acute severe ulcerative colitis (ASUC), often within the first year of diagnosis2. Intravenous corticosteroids are the mainstay of treatment of ASUC, and are effective in improving symptoms in nearly two-thirds of patients3. Approximately one-third will not respond to systemic steroids and will require either medical rescue therapy with infliximab or cyclosporine or will undergo surgery with approximately 20-30% of patients undergoing the latter4–6. Therapeutic options for the management of UC include 5-aminosalicylate derivatives, immunomodulators such as thiopurines (azathioprine, 6-mercaptopurine), anti-tumor necrosis factor α biologics (anti-TNF; infliximab, adalimumab, golimumab), and an anti-integrin (vedolizumab). Each of these agents have demonstrated efficacy in inducing or maintaining remission in UC and are widely used7–10. Few direct head-to-head comparisons exist; a single randomized controlled trial showed infliximab to be superior to azathioprine in moderate-to-severe ulcerative colitis11. However, systemic immunosuppression is also associated with rare but serious adverse effects including infections and therapy-related malignancies12–14.
The optimal therapeutic algorithm for the management of UC remains to be robustly established with a strong evidence base. Crohn’s disease (CD) is widely recognized as resulting in progressive bowel damage over time15,16. Landmark clinical trials in CD have demonstrated that early initiation of effective therapy, particularly anti-TNF biologics, are associated with superior rates of clinical remission, mucosal healing, and improved long-term outcome17,18. Consequently, and recognizing the lack of efficacy of non-immunosuppressive therapies like amino-salicylates in this disease, many recommend early effective immunosuppression to prevent long-term bowel damage. In contrast, the concepts of cumulative irreversible bowel damage and the benefit of early effective immunosuppressive therapy remain to be robustly established in UC. Additionally, the established efficacy of a class of non-immunosuppressive therapies, namely the 5-aminosalicylates, in UC raises a genuine debate regarding the appropriate timing for ‘stepping-up’ and initiating immunosuppression in UC. Prior studies have demonstrated that hospitalization represents a marker of disease severity and poor prognosis in UC19,20. We hypothesized that immunosuppression-naïve patients hospitalized for severe ulcerative colitis are at high risk for poor outcomes and would benefit from early initiation of immunomodulator therapy.
The aims of our study were as follows: (1) to examine the long-term outcomes of immunosuppression-naïve patients hospitalized for severe ulcerative colitis who respond to intravenous corticosteroid therapy and avoid need for medical or surgical rescue while in-hospital; and (2) to determine if initiation of immunomodulator therapy following hospitalization is associated with superior outcomes and reduction in need for colectomy at one year.
METHODS
Study Population
This was a retrospective observational cohort study of patients hospitalized for a diagnosis of ASUC at Massachusetts General Hospital (MGH), a tertiary referral center for IBD serving the greater Boston metropolitan area and surrounding New England. We searched the Partners Research Patient Data Registry (RPDR) for all patients hospitalized with a diagnosis of UC using the International classification of Diseases, 9th edition, clinical modification (ICD-9-CM) code 556.x. The RPDR is a comprehensive data warehouse of all patients receiving inpatient or outpatient care at MGH or any of the other Partners healthcare affiliated hospitals. It is continually populated with information from administrative sources including billing, radiology, endoscopy, in-patient stays and procedures21. Manual chart review of all patients by two study investigators (AV and LX) was performed to identify patients who met the following criteria: (1) first hospitalization for an acute exacerbation of UC at our hospital; (2) received intravenous corticosteroid therapy; and (3) were immunosuppression-naïve (no prior immunomodulator or biologic use) at hospitalization. Patients who were refractory to intravenous corticosteroids and initiated medical rescue therapy with cyclosporine or an anti-TNF biologic or underwent surgery during the index hospitalization were excluded (Figure 1).
Figure 1.
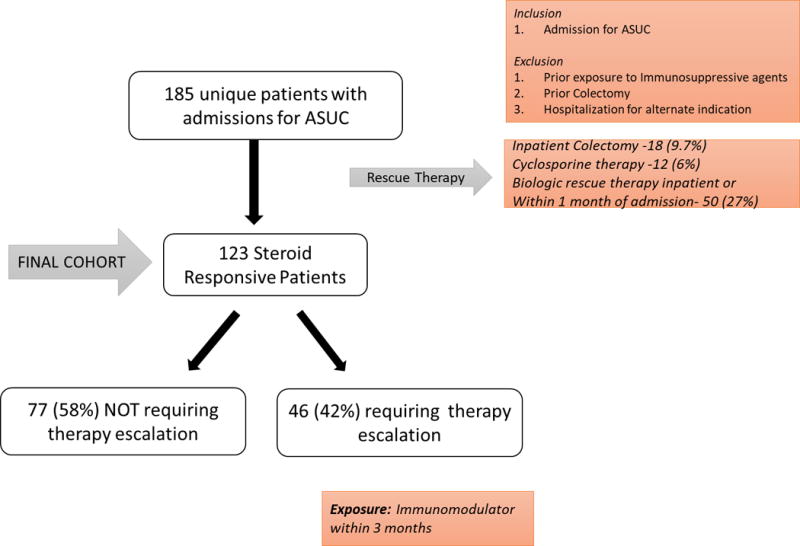
Selection of Study Population
Exposure, variables and outcomes
Several relevant covariates were extracted upon record review including age, gender, disease extent per the Montreal classification and disease duration. Information on past maintenance therapy for IBD was obtained including prior use of oral or topical 5-aminosalicylates (mesalamine, balsalazide, olsalazine, sulfasalazine), and steroids (prednisone, budesonide). As a measure of severity of the episode, data were extracted about baseline laboratory values within 24 hours of admission including hemoglobin, albumin, white blood cell (WBC) count, platelet count, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) when available. For patients who underwent a sigmoidoscopy, severity of disease was noted and classified as severe in the presence of ulcers, bleeding and spontaneous friability.
The initial analysis examined long-term outcomes of all immunosuppression naïve hospitalized UC patients. We then specifically compared outcomes of patients who were initiated on immunomodulator therapy (azathioprine, 6-mercaptopurine, methotrexate) within 3 months of hospitalization to those who were not escalate. We selected the above time window to ensure that therapy initiation was based on the index hospitalization and did not reflect subsequent relapses. The main study outcome was colectomy within one year of admission. Secondary outcomes include late colectomy defined as those occurring between 90 and 365 days after the index hospitalization, and re-hospitalization within one year. For this analysis, we excluded patients who were initiated directly on biologic therapy following discharge as there were too few patients to robustly examine the impact of this treatment on the rate of colectomy.
Statistical Analysis
All data were analyzed using STATA 13.1 (Stata Corp, College Station, Tx, USA). Continuous variables were summarized using means and standard deviations and compared using a t-test or Wilcoxon Rank-sum test where appropriate. Categorical variables were expressed as proportions and compared using the Chi-squared test or fisher exact test where appropriate. Univariate logistic regression models were used to identify factors associated with colectomy at 1 year. Subsequent multivariable logistic regression models using stepwise backward elimination were used to identify the independent association between therapy escalation and colectomy. Variables significant on univariate analysis at p < 0.1 or those that had published data supporting an association with colectomy were selected for inclusion in the multivariable model. A two-sided p-value < 0.05 indicated independent statistical significance.
As decision to escalate therapy was non-random and at the discretion of treating physicians, propensity score adjustment was performed to minimize the effect of such treatment group allocation. A propensity score was estimated using logistic regression analyses with escalation to immunomodulator therapy as the outcome of interest, and disease extent, serum albumin, hemoglobin, prior 5-ASA exposure, and endoscopic severity as relevant covariates. CRP was not included for calculation in the propensity score given missing admission values in a subset of the cohort. Similar propensity score adjustment was conducted for our secondary outcomes. Kaplan Meier curves were constructed to compare differences in time to colectomy between the two groups and the log rank test was used to estimate statistical significance of such a difference. Institutional Review Board approval was obtained from the Partners Healthcare Human Subjects Research Committee.
RESULTS
Cohort characteristics
A total of 185 immunosuppression-naïve patients with ASUC who received intravenous steroids were hospitalized during the study period. Among this 52 (28%) patients failed intravenous steroid therapy and required medical or surgical rescue therapy during the index hospitalization and were excluded. Among the remaining 133 patients, 13 underwent colectomy during the 1 year following hospitalization (9.8%). Ten patients who were initiated on biologic therapy immediately following hospitalization were then excluded, leaving 123 patients in our cohort to compare the outcomes of those who were initiated on immunomodulator therapy to those who were not. Of these, 46 patients were initiated therapy with an immunomodulator within 3 months and comprised the escalated group. The remaining 77 patients who did not escalate therapy (“not escalated”) comprised the control population.
Table 1 compares the characteristics of the two groups. The median age, gender distribution, and duration of UC was similar between the two groups. Just over half the study population were women, and nearly half had been diagnosed within the past year. A similar proportion of patients in each group had pancolitis which was the most common extent of disease. Among patients who underwent an endoscopic examination of the colon, nearly half in each group were considered to have severe disease characterized by ulceration and spontaneous bleeding. A total of 37 patients (80%) in the escalated group and 45 patients (60%) in the not escalated group were currently or had previously been on a 5-ASA agent prior to the index hospitalization. There was no difference in the mean hemoglobin, albumin, WBC count or ESR between the two groups. Patients who were escalated had higher baseline CRP at hospitalization (91.7 mg/dL) compared to those who were not escalated (37.8mg/dL, p=0.02). Nearly all (92%) of patients in the not escalated group were maintained on 5-ASA therapy (2.4 – 4.8g/day of mesalamine or equivalent) following the hospitalization. A small minority used alternative non-pharmacologic therapy or were on no treatment following the taper of the prednisone.
Table 1.
Characteristics of study population, stratified by escalation of therapy following hospitalization
| Characteristic |
Therapy Escalated N =46 |
Therapy Not Escalated N=77 |
p- Value |
|---|---|---|---|
| Female Sex (n, %) | 25 (54.4) | 42 (54.4) | 0.98 |
| Median Age (years, Range) | 37 (16 - 86) | 29 (16 - 88) | 0.37 |
| Median length of Stay (days, Range) | 6 (2 - 18) | 6 (1- 48) | 0.65 |
| UC duration (n, %) | |||
| <1 year | 22 (47.8) | 48 (62.3) | 0.27 |
| 1-5 years | 12 (26.1) | 16 (20.8) | |
| > 5 years | 12 (26.1) | 13 (16.9) | |
| Inpatient Procedure (n, %) | |||
| Colonoscopy | 17 (37.0) | 35 (45.5) | 0.36 |
| Flexible Sigmoidoscopy | 19 (41.3) | 15 (19.5) | 0.01 |
| Upper Endoscopy | 7 (15.2) | 16 (20.8) | 0.44 |
| Endoscopic Severity (n, %) [N] | [39] | [54] | |
| Mild | 1 (2.6) | 1 (1.9) | 0.88 |
| Moderate | 14 (36) | 21 (38.9) | |
| Severe (Ulceration and spontaneous bleeding) | 23 (59) | 29 (53.7) | |
| Extent of Inflammation (n, %) | |||
| Pancolitis | 29 (63) | 47 (61) | 0.48 |
| Left sided | 17 (37) | 26(33.8) | |
| Rectum or Sigmoid | - | 2 (2.6) | |
| ASA Exposure at Admission (n, %) | |||
| Never on ASA | 9 (19.6) | 31 (40.3) | 0.03 |
| At Admission | 31(67.4) | 42 (54.6) | |
| Past Exposure | 6 (13) | 3 (5.2) | |
| Post-discharge 5-ASA maintenance therapy (n, %) | 46 (86.8) | 21 (91.3) | 0.58 |
| Hemoglobin (g/dL) – (mean SD) | 12.1 ± 2.4 | 12.1±2.2 | 0.97 |
| White Blood Cell Count (/mm3) | 12.1±3.6 | 11.3±4.2 | 0.29 |
| Platelet Count (/mm3) | 434.7±168.4 | 419.3±182.1 | 0.64 |
| ESR (in mm) α | 44.1±28.9 | 41.3±24.1 | 0.65 |
| C-reactive protein (mg/L) ¥ | 91.7±100 | 37.8±41.2 | 0.02 |
| Albumin (g/dL) | 3.3±0.6 | 3.3±0.7 | 0.59 |
Available in 83 patients
Available in 50 patients
Among the 123 patients in this cohort, 11 underwent colectomy at 1 year (8.9%). There was no statistically significant difference in the risk of colectomy among those who escalated therapy (10.8%) compared to those who did not (7.8%, p=0.56). On multivariable analysis adjusting for disease duration, pancolitis and prior 5-ASA exposure, therapy escalation was not associated with a reduction in the risk of colectomy by 1 year (OR 1.29, 95% CI 0.35-4.74, p=0.71). We repeated our multivariable analysis including an adjustment for a propensity score estimating likelihood of therapy escalation based on characteristics at admission and prior to hospitalization. In this analysis, inclusion of the propensity score in our multivariable model did not alter our results and therapy escalation was not associated with a significant reduction in risk of colectomy (OR 1.12 95% CI 0.22 - 5.53, p=0.68). Patients who were escalated had a higher risk of re-hospitalization within one year (OR 2.18 95% CI 1.17 – 4.08, p=0.01) but no difference in risk of late colectomy between 90 and 365 days following discharge (OR 0.53, 95% CI 0.15-4.29, p=0.93). Kaplan Meier survival curves demonstrated similar time to colectomy between both the escalated and not escalated groups (Figure 2) (log-rank test p-value =0.55)
Figure 2.
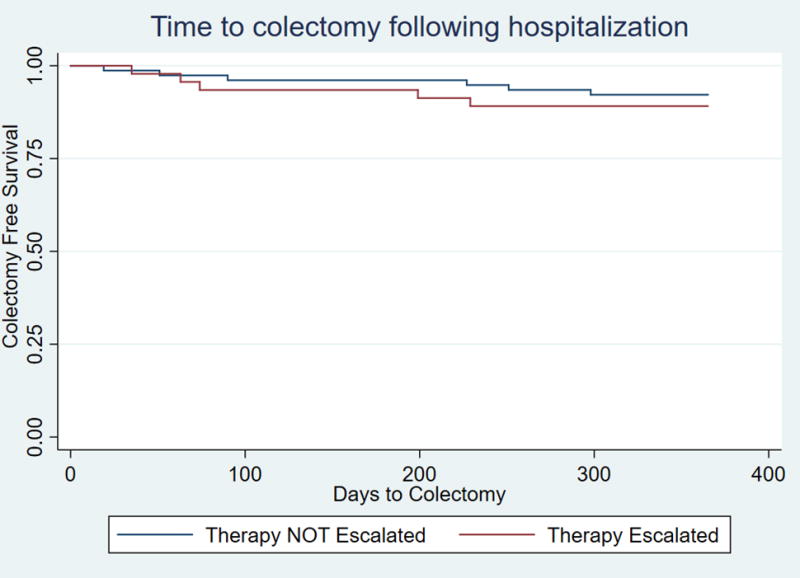
Time to colectomy following hospitalization for ulcerative colitis in immunosuppression naïve patients
DISCUSSION
Robust definition of thresholds for stepping-up treatment of immunosuppressive-naïve patients with UC is an important clinical need. In Crohn’s disease, the concept of permanent irreversible bowel damage is well recognized and the benefit for early biologic therapy has been robustly demonstrated in clinical trials. In contrast, both these questions remain a matter of debate in UC. In a large cohort of immunosuppression-naïve steroid responders with ASUC, we demonstrate there is a significant risk of colectomy (~10%) at 1 year even in patients who were responsive to steroids, supporting the recognition of this population as being at high risk. However, initiation of immunomodulator therapy within 3 months of hospitalization did not reduce the need for colectomy at 1 year. This suggests need for further study to identify clinical and biologic predictors that would optimally inform the threshold for therapy escalation in UC, as well as the need to define optimal therapy in this cohort of high-risk patients.
The first key finding from our study is that even among steroid-responsive patients with UC, there is a one-in-ten likelihood of colectomy at 1 year after hospitalization. Several prior studies, most with smaller sample sizes, have described long-term outcomes following hospitalization for UC. A single center study of 103 hospitalized UC patients suggested that 20% of those requiring hospitalization eventually required colectomy but did not separately examine outcomes in those who were immunosuppression naïve or did not require inpatient colectomy20. Long-term outcome of the Oxford severe colitis cohort demonstrated that 16/32 (50%) complete or incomplete responders to corticosteroid therapy eventually required colectomy at a median of 28 months after hospitalization19. Within 1 year following the index hospitalization, 25% of patients (5% of complete responders and 54% of incomplete responders) required colectomy. Aratari et al. examined the outcome of 52 patients hospitalized for severe ulcerative colitis. Among steroid-responsive patients, 11% eventually required colectomy5. Two studies from Asia identified similar rates of colectomy following ASUC. In a Korean study of 99 hospitalized ASUC patients, among patients who avoided colectomy during the index hospitalization, 16% underwent colectomy during a median follow up of 10 years22. A similar colectomy rate of 26% was noted from a tertiary referral center in India23. While we were unable to separate out complete from partial responders in our cohort, our estimates of long-term morbidity following ASUC hospitalization is similar to published findings and suggests need for continued close follow-up of these patients.
A second finding from our study was that use of immunomodulator therapy within 3 months of hospitalization was not associated with a reduction in risk of colectomy in steroid-responsive immunosuppression naïve ASUC patients. There are several possible explanations why therapy escalation was not associated with lower rates of colectomy in steroid-responsive ASUC patients. First, the evidence behind the efficacy of immunomodulator therapy in UC is less robust than for other therapies such as the biologics. Four small trials have demonstrated azathioprine to be effective in maintaining remission in UC but were limited by small sample sizes with few observational cohorts with long-term follow-up to support its efficacy24–28. In contrast, three randomized controlled trials have not noted a benefit to the use of methotrexate monotherapy in UC29,30. As well, two recent trials in Crohn’s disease have similar failed to demonstrate a benefit for early azathioprine initiation in recently diagnosed patients29,30. Second, patients in the escalated group may not have been optimized for their thiopurine dosing. The study period comprised a wide range of years where therapeutic drug monitoring was not routinely available or was cost prohibitive for many. It is possible that a more proactive dose optimization strategy may have demonstrated a benefit though our findings reflects the prevalent clinical care. Third, biologic determinants such as genetic or genomic factors may determine disease prognosis in UC and less refined clinical determinants such as need for hospitalization may prove imperfect for stratifying risk and allocating to various treatment strategies. Fourth, while the concept of irreversible bowel damage with each relapse potentially causing progressive damage may hold true in Crohn’s disease, this may be less apparent in UC and many may experience may experience prolonged remission even following a hospitalization. Indeed, in the 10-year follow-up of the IBSEN cohort, 55% of patients with UC experienced an initial period of high activity but remained in remission or with only mildly active disease subsequently31, a higher proportion than noted in the parallel Crohn’s disease cohort32. With the high morbidity following an episode of ASUC, there is an important need for prospective clinical trials of strategies to optimize outcomes in this population.
We readily acknowledge several limitations of our study, Firstly, this is a retrospective observational study where escalation of therapy was non-random at the discretion of the treating gastroenterologist or medical team. However, a propensity score adjustment matching for potential confounders of therapy escalation did not significantly alter our findings. Secondly, clinical severity using validated disease activity indices were not routinely recorded during hospitalization or at follow-up. Third, protocols to initiate and optimize thiopurine therapy was not in place during much of the study period and therapeutic drug monitoring was not widely available. It is possible that a discernible difference may have apparent if the escalated group were pro-actively adjusted early to achieve therapeutic levels of either the thiopurine therapy. The single center design and small number of patients may have limited our ability to demonstrate a statistically significant difference. Future multi-center cohort studies may be able to more robustly examine this association.
In conclusion, we report a one-in-ten risk of colectomy 1 year following hospitalization even in patients who are responsive to intravenous steroids during a hospitalization for ulcerative colitis. We did not identify a beneficial effect of early immunomodulator therapy escalation in this population. Further study to identify which patients with UC benefit from early escalation of therapy are essential to inform our therapeutic algorithms. Specifically, it is important to robustly examine whether use of upfront optimized biologic therapy in this population would be beneficial in modifying natural history of disease and preventing colectomy.
Table 2.
Comparison of outcomes of patients at 1 year based on escalation of therapy following hospitalization for acute severe ulcerative colitis
| Outcome | Therapy Escalated (n=46) [N(%)] |
Therapy Not Escalated (n=77) [N(%)] |
Unadjusted Odds ratio | 95% CI | p-value |
|---|---|---|---|---|---|
| Colectomy within 1 year | 5 (10.8) | 6 (7.8) | 1.39 | 0.45-4.31 | 0.56 |
|
Late colectomy (91 – 365 days following hospitalization) |
2 (4.3) | 4 (5.2) | 0.53 | 0.15-4.29 | 0.93 |
| Rehospitalization in 1 year | 17 (37) | 13 (16.9) | 2.18 | 1.17-4.08 | 0.01 |
Table 3.
Univariate Analysis of predictors of colectomy at 1 year following hospitalization in immunosuppression-naïve acute severe ulcerative colitis patients
| Characteristic | Odds Ratio | 95% Confidence Interval |
|---|---|---|
| Disease duration | ||
| <1 year | 1 | 0.74-10.66 |
| 1-5 years | 2.82 | 0.06-4.87 |
| > 5 years | 0.54 | |
| Extent of Involvement | ||
| Localized | 1 | 0.30-3.94 |
| Pancolitis | 1.09 | |
| Severity on endoscopy | ||
| Mild or Moderate | 1 | 0.18-3.29 |
| Severe | 0.77 | |
| Albumin | 0.82 | 0.34-1.97 |
| CRP | 1.00 | 0.99-1.02 |
| ESR | 1.01 | 0.97-1.03 |
| Prior ASA Exposure | ||
| Never | 1 | 0.47-11.59 |
| Current | 2.33 | 0.17-25.92 |
| Past | 2.11 | |
| Therapy escalation | ||
| No | 1 | 0.41-5.02 |
| Yes | 1.44 |
Acknowledgments
Source of funding: Ananthakrishnan is supported in part by grants from the National Institutes of Health (R03 DK112909) and the Crohn’s and Colitis Foundation.
Footnotes
Conflicts of Interest: Ananthakrishnan has served on scientific advisory boards for Abbvie, Takeda, Gilead, and Merck and has received research support from Pfizer.
Author contributions:
Vedamurthy – study design, data extraction and statistical analysis, drafting of the manuscript, approval of the final manuscript
Xu – data extraction, approval of the final manuscript
Luther, Garber, Colizzo, Khalili – approval of the final manuscript,
Ananthakrishnan – study design and supervision, statistical analysis, drafting of the manuscript, approval of the final manuscript.
References
- 1.Bernstein CN, Wajda A, Svenson L, et al. The Epidemiology of Inflammatory Bowel Disease in Canada: A Population-Based Study. 2006;101 doi: 10.1111/j.1572-0241.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- 2.Dinesen LC, Walsh AJ, Protic MN, et al. The pattern and outcome of acute severe colitis. Journal of Crohn’s and Colitis. 2010;4(4):431–437. doi: 10.1016/j.crohns.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2(4947):1041–1048. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2007;5(1):103–110. doi: 10.1016/j.cgh.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 5.Aratari A, Papi C, Clemente V, et al. Colectomy rate in acute severe ulcerative colitis in the infliximab era. Dig Liver Dis. 2008;40(10):821–826. doi: 10.1016/j.dld.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Ho GT, Chiam P, Drummond H, Loane J, Arnott ID, Satsangi J. The efficacy of corticosteroid therapy in inflammatory bowel disease: analysis of a 5-year UK inception cohort. Aliment Pharmacol Ther. 2006;24(2):319–330. doi: 10.1111/j.1365-2036.2006.02974.x. [DOI] [PubMed] [Google Scholar]
- 7.Lichtiger S, Present DH, Kornbluth A, et al. Cyclosporine in Severe Ulcerative Colitis Refractory to Steroid Therapy. N Engl J Med. 1994;330(26):1841–1845. doi: 10.1056/NEJM199406303302601. [DOI] [PubMed] [Google Scholar]
- 8.Arts J, D’Haens G, Zeegers M, et al. Long-term outcome of treatment with intravenous cyclosporin in patients with severe ulcerative colitis. Inflamm Bowel Dis. 2004;10(2):73–78. doi: 10.1097/00054725-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2005;353(23):2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 10.Adalimumab in the Treatment of Moderate-to-Severe Ulcerative Colitis: ULTRA 2 Trial Results. Gastroenterol Hepatol (N Y) 2013;9(5):317–320. [PMC free article] [PubMed] [Google Scholar]
- 11.Panccione R, Ghosh S, Middleton S, et al. Infliximab, Azathioprine, or Infliximab + Azathioprine for Treatment of Moderate to Severe Ulcerative Colitis: The UC Success Trial. Gastroenterology. 140(5):S-134. [Google Scholar]
- 12.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. The Lancet. 2002;359(9317):1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 13.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab Maintenance Therapy for Fistulizing Crohn’s Disease. N Engl J Med. 2004;350(9):876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 14.Rutgeerts P, D’Haens G, Targan S, et al. Efficacy and safety of retreatment with anti–tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology. 1999;117(4):761–769. doi: 10.1016/s0016-5085(99)70332-x. [DOI] [PubMed] [Google Scholar]
- 15.Sipponen T, Savilahti E, Kärkkäinen P, et al. Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNF-alpha therapy for Crohn’s disease. Inflamm Bowel Dis. 2008;14(10):1392–1398. doi: 10.1002/ibd.20490. [DOI] [PubMed] [Google Scholar]
- 16.Bouguen G, Levesque BG, Feagan BG, et al. Treat to Target: A Proposed New Paradigm for the Management of Crohn’s Disease. Clin Gastroenterol Hepatol. 13(6):1042–1050.e1042. doi: 10.1016/j.cgh.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 17.D’Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371(9613):660–667. doi: 10.1016/S0140-6736(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 18.Khanna R, Bressler B, Levesque BG, et al. Early combined immunosuppression for the management of Crohn’s disease (REACT): a cluster randomised controlled trial. Lancet. 2015;386(10006):1825–1834. doi: 10.1016/S0140-6736(15)00068-9. [DOI] [PubMed] [Google Scholar]
- 19.Bojic D, Radojicic Z, Nedeljkovic-Protic M, Al-Ali M, Jewell DP, Travis SP. Long-term outcome after admission for acute severe ulcerative colitis in Oxford: the 1992-1993 cohort. Inflamm Bowel Dis. 2009;15(6):823–828. doi: 10.1002/ibd.20843. [DOI] [PubMed] [Google Scholar]
- 20.Ananthakrishnan AN, Issa M, Beaulieu DB, et al. History of medical hospitalization predicts future need for colectomy in patients with ulcerative colitis. Inflammatory bowel diseases. 2009;15(2):176–181. doi: 10.1002/ibd.20639. [DOI] [PubMed] [Google Scholar]
- 21.Nalichowski R, Keogh D, Chueh HC, Murphy SN. Calculating the benefits of a Research Patient Data Repository. AMIA Annual Symposium proceedings AMIA Symposium. 2006:1044. [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HS, Yang SK, Soh JS, et al. Short- and Long-Term Outcomes of Acute Severe Ulcerative Colitis in Korea: The 1999-2005 Cohort. Inflamm Bowel Dis. 2015;21(8):1825–1831. doi: 10.1097/MIB.0000000000000447. [DOI] [PubMed] [Google Scholar]
- 23.Jain S, Kedia S, Sethi T, et al. Predictors of long-term outcomes in patients with acute severe colitis: A Northern Indian cohort study. J Gastroenterol Hepatol. 2017 doi: 10.1111/jgh.13921. [DOI] [PubMed] [Google Scholar]
- 24.Ardizzone S, Maconi G, Russo A, Imbesi V, Colombo E, Bianchi Porro G. Randomised controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut. 2006;55(1):47–53. doi: 10.1136/gut.2005.068809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawthorne AB, Logan RF, Hawkey CJ, et al. Randomised controlled trial of azathioprine withdrawal in ulcerative colitis. BMJ (Clinical research ed) 1992;305(6844):20–22. doi: 10.1136/bmj.305.6844.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jewell DP, Truelove SC. Azathioprine in ulcerative colitis: final report on controlled therapeutic trial. British medical journal. 1974;4(5945):627–630. doi: 10.1136/bmj.4.5945.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sood A, Midha V, Sood N, Avasthi G. Azathioprine versus sulfasalazine in maintenance of remission in severe ulcerative colitis. Indian journal of gastroenterology: official journal of the Indian Society of Gastroenterology. 2003;22(3):79–81. [PubMed] [Google Scholar]
- 28.Steinhart AH, Baker JP, Brzezinski A, Prokipchuk EJ. Azathioprine therapy in chronic ulcerative colitis. Journal of clinical gastroenterology. 1990;12(3):271–275. doi: 10.1097/00004836-199006000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Carbonnel F, Colombel JF, Filippi J, et al. Methotrexate Is Not Superior to Placebo for Inducing Steroid-Free Remission, but Induces Steroid-Free Clinical Remission in a Larger Proportion of Patients With Ulcerative Colitis. Gastroenterology. 2016;150(2):380–388.e384. doi: 10.1053/j.gastro.2015.10.050. [DOI] [PubMed] [Google Scholar]
- 30.Oren R, Arber N, Odes S, et al. Methotrexate in chronic active ulcerative colitis: a double-blind, randomized, Israeli multicenter trial. Gastroenterology. 1996;110(5):1416–1421. doi: 10.1053/gast.1996.v110.pm8613046. [DOI] [PubMed] [Google Scholar]
- 31.Solberg IC, Lygren I, Jahnsen J, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study) Scand J Gastroenterol. 2009;44(4):431–440. doi: 10.1080/00365520802600961. [DOI] [PubMed] [Google Scholar]
- 32.Solberg IC, Vatn MH, Hoie O, et al. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5(12):1430–1438. doi: 10.1016/j.cgh.2007.09.002. [DOI] [PubMed] [Google Scholar]


