Abstract
Background:
Vascular calcification leads to increased large artery stiffness. Matrix Gla-protein (MGP) is a vitamin K-dependent protein that inhibits arterial calcification. Aldosterone promotes vascular calcification and stiffness, but the relationships between aldosterone, MGP and arterial stiffness are unknown.
Methods:
We studied 199 adults (predominantly older men) with hypertension. We assessed the relationship between levels of dp-ucMGP, aldosterone and carotid-femoral pulse wave velocity (CF-PWV) using standard regression and mediation analyses. Plasma aldosterone was measured in a subgroup of subjects (n=106).
Results:
Aldosterone was strongly associated with dp-ucMGP (Standardized β=0.50, P<0.001), which was independent of potential confounders (β=0.37, P<0.001). Levels of dp-ucMGP were significantly associated with CF-PWV (β=0.30; P<0.001), which persisted after adjustment for potential confounders (β=0.25; P=0.004). Plasma aldosterone was also significantly associated with CF-PWV (Standardized β=0.21; P=0.035). However, in a model that included aldosterone and dp-ucMGP, only the latter was associated with CF-PWV. Mediation analyses demonstrated a significant dp-ucMGP-mediated effect of aldosterone on CF-PWV, without a significant direct (dp-ucMGP-independent) effect.
Conclusions:
our study demonstrates a novel independent association between high aldosterone levels and dp-ucMGP, suggesting that aldosterone may influence the MGP pathway. This relationship appears to underlie the previously documented relationship between aldosterone and increased arterial stiffness.
Keywords: Arterial stiffness, Pulse Wave Velocity, Matrix Gla-Protein, Vitamin K, Hypertension, Aldosterone
Introduction
Increased large artery stiffness is associated with an increased risk of cardiovascular events 1, 2. Large artery stiffening precedes development of hypertension, rather than simply being the end-product of arterial damage from the blood pressure.3–6 Arterial stiffness also increases left ventricular pulsatile hydraulic load,7, 8 as well as pressure and flow pulsatility in microvascular beds, which is relevant for the damage of hypertensive target organs.8, 9 Therefore, the molecular pathways leading to increased arterial stiffness are of great interest.
Matrix Gla-Protein (MGP) is a small secretory protein produced by chondrocytes and vascular smooth muscle cells 10. The inactive form of MGP (dephospho-uncarboxylated MGP, dp-ucMGP) undergoes serial post-translational carboxylation and phosphorylation to form active MGP. The active form of MGP protects against arterial wall calcification by inhibiting bone morphogenetic protein-2 (BMP-2)-induced osteogenic differentiation of the vascular smooth muscle cells. MGP is also a direct calcification inhibitor. Carboxylation of MGP is dependent upon availability of vitamin K and is thus, deficient in vitamin K-depleted states.10 dp-ucMGP is secreted into the circulation and an increase in its levels indicates deficient MGP maturation/activation. 10 Increased dp-ucMGP levels have been associated with increased large artery stiffness.11, 12 However, our understanding of the clinical correlates of dp-ucMGP levels, and its relationship with other pathways associated with arterial stiffness is limited.
Arterial stiffness has been shown to be increased in individuals with primary hyperaldosteronism, when compared to hypertensive controls.13, 14 Moreover, arterial stiffness is significantly reduced after adrenalectomy in patients with adrenal adenoma, suggesting that hyperaldosteronism causally contributes to arterial stiffening.15 In-vitro and animal experiments demonstrate that aldosterone and mineralocorticoid receptors stimulate pro-calcific/pro-stiffening pathways in the arterial wall.16 However, whether aldosterone levels are related to the dp-ucMGP pathway in human hypertension is unknown.
In this study, we aimed to assess the relationship between plasma dp-ucMGP, aldosterone and large artery stiffness in hypertensive adults.
Methods
Study Population
We enrolled a convenience sample of 199 participants with hypertension referred to the cardiovascular imaging studies the Corporal Michael J. Crescenz VA Medical Center. The protocol was approved by the Philadelphia VA Medical Center Institutional Review Board, and a written informed consent was obtained from all participants.
Plasma dp-ucMGP Measurement
Citrate tubes were used for collection of venous blood sample at the time of enrollment. Plasma was prepared and stored at −80°C for batch analysis. A dual-antibody sandwich enzyme linked immunosorbent assay technique (VitaK; Maastricht University; The Netherlands) was used to measure dp-ucMGP.17 Intra-assay coefficients of variation for this assay have previously been reported at 3.1% and 5.4% for lower and upper limit of normal. Inter-assay variation coefficients are 6.9% and 13.6% for lower and upper limits of normal 11.
Aldosterone Measurement
Plasma aldosterone levels were measured in a subgroup of patients (n=106). Plasma samples were extracted on SepPak C18 cartridges (Waters, Milford, Massachusetts, USA), and used for aldosterone measurement using an aldosterone ELISA kit (Cayman Chemicals; Cayman Chemical, Ann Arbor, Michigan, USA).
Carotid-Femoral Pulse Wave Velocity Measurement
Carotid femoral pulse wave velocity (CF-PWV), considered the non-invasive reference measure of large artery stiffness,18–20 was measured using the SphygmoCor Px system (Atcor Medical, Sydney, Australia). Briefly, carotid-to-femoral transit time (ΔT) was computed from the foot-to-foot time difference between sequentially acquired carotid and femoral waveforms, using the intersecting tangents method, and the QRS complex of the ECG as a fiducial point. The distance between the sternal notch and the carotid artery was subtracted from the distance between the sternal notch and the femoral artery, in order to estimate the path length (L), and CF-PWV was computed as L/ΔT.18–20
Statistical Analysis
Continuous variables are presented as mean ± standard deviation, whereas categorical variables are presented as proportions (frequencies and percentages). Univariate and multivariate linear regression models were first used to determine the association of clinical and laboratory variables with dp-ucMGP. The relationship between dp-ucMGP and aldosterone was also assessed using linear regression. We also determined the association between dp-ucMGP and CF-PWV, in unadjusted and multivariable models adjusted for potential confounders (including significant correlates of dp-ucMGP). The association of aldosterone and CF-PWV was similarly assessed using unadjusted and multivariable adjusted models.
We also performed formal mediation analyses when appropriate to assess the relationship between plasma dp-ucMGP and aldosterone as predictors of CF-PWV. Mediating variables are “intermediate” factors that act as a link between a predictor variable and an outcome variable. We performed mediation analyses when the following criteria were met: (1) the exposure was significantly correlated to both the hypothesized mediator and the outcome variable, and; (2) the hypothesized mediator was significantly related to the outcome variable. Mediation analyses quantify direct effects and indirect effects that contribute to an observed relation between the predictor variable and an outcome variable (CF-PWV), and examine the role of the mediator.21 Estimates of the total, direct, and indirect effect size (along with 95% confidence intervals) were computed via bootstrapping, with 5000 resamples, using the PROCESS macro for SPSS.21 Significant mediation is established when the indirect effect is significantly different from zero. The percent mediation (PM, interpreted as the percent of the total effect accounted for the indirect effect) was also computed. Standardized regression coefficients and effect sizes are presented for easier comparison of the magnitude of the relationships of different predictors. All probability values are 2-tailed. Statistical significance was defined as a 2-tailed P value<0.05. SPSS software (IBM SPSS for Mac, version 24, Chicago IL) was used for the statistical analyses.
Results
Baseline characteristics of study participants are presented in Table 1. Mean age was 64±10 years and the majority (90%) were males. Most participants were African-American (57%) or Caucasian (40%). The mean aldosterone levels was 0.56±0.25 nmol/L. The mean circulating dp-ucMGP was 763±854 pmol/L and the mean CF-PWV was 10.73±3.5 m/sec.
Table 1:
Baseline Characteristics of Study Participants
| All participants (N=199) |
Participants with aldosterone measurement (N=106) |
|
|---|---|---|
| Age, years | 64±10 | 64±10 |
| Male | 179 (90%) | 98 (93%) |
| Ethnicity | ||
| African-American | 113 (57%) | 61 (57%) |
| Caucasian | 80 (40%) | 41 (39%) |
| Other | 6 (3%) | 4 (4%) |
| Body mass index, kg/m2 | 31±6 | 32±6 |
| Systolic Blood Pressure, mmHg | 146.5±17.9 | 148.4±18.2 |
| Diastolic Blood Pressure, mmHg | 84.1±11.5 | 84.5±11.82 |
| Pulse Pressure, mmHg | 62.4±13.87 | 63.9±14.49 |
| Mean arterial pressure, mmHg | 111±17 | 113±17 |
| Current smoker | 46 (23%) | 26 (25%) |
| Diabetes mellitus | 114 (57%) | 67 (63%) |
| Coronary Artery Disease | 61 (31%) | 40 (38%) |
| CVA or TIA | 23 (12%) | 17 (16%) |
| Peripheral Vascular Disease | 17 (9%) | 15 (14%) |
| Number of antihypertensive medications | 2.12±1.07 | 2.19±1.13 |
| ACE inhibitor use | 111 (56%) | 65 (61%) |
| ARB use | 26 (13%) | 13 (12%) |
| Beta-blocker use | 121 (61%) | 66 (62%) |
| Insulin use | 44 (22%) | 33 (31%) |
| Spironolactone use | 8 (4%) | 2 (2%) |
| Statin use | 138 (69%) | 78 (73%) |
| Warfarin use | 18 (9%) | 12 (11%) |
| eGFR, mL/min/1.73 m2 | 77±29 | 79±29 |
| eGFR 30–60 mL/min/1.73 m2 | 51 (25.6%) | 28 (26.4%) |
| eGFR <30 mL/min/1.73 m2 | 5 (2.5%) | 4 (3.8%) |
| Plasma aldosterone, nmol/L | -- | 0.56±0.25 |
| dp-uc-MGP, pmol/L | 763±854 | 751±854 |
| Serum phosphorus, mmol/L | 1.11±0.21 | 1.11±0.20 |
| Serum magnesium, mmol/L | 0.79±0.14 | 0.79±0.15 |
| Serum calcium, mmol/L | 2.26±0.43 | 2.25±0.47 |
| CF-PWV, m/s | 10.7±3.5 | 10.7±3 |
CVA: cerebrovascular accident; TIA: transient ischemic attack; eGFR: estimated glomerular filtration rate; ACE: angiotensin Convertase enzyme; ARB: angiotensin receptor blocker; CF-PWV: carotid-femoral pulse wave velocity.
Clinical Correlates of dp-ucMGP
In univariable analysis, age (β=0.26, P<0.001), coronary artery disease (Standardized β=0.21, P=0.003), and warfarin use (Standardized β=0.39, P<0.001) were associated with higher dp-ucMGP. In contrast, African-American ethnicity (Standardized β=−0.30, P<0.001) and estimated glomerular filtration rate (Standardized β=−0.37, P<0.001) were inversely associated with dp-ucMGP. In multivariable-adjusted analysis, age (Standardized β=0.15, P=0.036), African-American ethnicity (Standardized β=−0.26, P<0.001), a history of stroke or transient ischemic attack (Standardized β=0.10, P=0.013), warfarin use (Standardized β=0.43, P<0.001), estimated glomerular filtration rate (Standardized β=−0.24, P<0.001), and serum calcium (Standardized β=0.22, P=0.04) were significantly associated with dp-ucMGP.
Relationship between Aldosterone and dp-ucMGP
Aldosterone was strongly associated with dp-ucMGP in unadjusted analyses (Standardized β=0.50, P<0.001). Table 2 shows a multivariate model that tested the association of aldosterone with dp-ucMGP, after adjustment for potential confounders. In this model, aldosterone remained independently associated with dp-ucMGP (Standardized β=0.37, P<0.001) after adjustment for age, gender, African-American ethnicity, mean arterial pressure, history of coronary artery disease, stroke or transient ischemic attach, use of various antihypertensive medications, warfarin use, estimated glomerular filtration rate, and serum phosphorus, calcium and magnesium. In this model, African-American ethnicity (Standardized β=−0.19, P=0.027), and warfarin use (Standardized β=0.40, P<0.001), were also independenly associated with dp-ucMGP.
Table 2.
Univariable and Multivariable Associations of Plasma Aldosterone with dp-ucMGP
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| β Coefficient * | P value | 𝛃 Coefficient * | P value | |
| Plasma aldosterone | 0.50 | <0.001 | 0.37 | <0.001 |
| Age | 0.10 | 0.26 | ||
| Male | −0.09 | 0.26 | ||
| African-American ethnicity | −0.19 | 0.027 | ||
| Mean arterial pressure | 0.02 | 0.78 | ||
| Coronary artery disease | −0.02 | 0.80 | ||
| CVA or TIA | −0.01 | 0.99 | ||
| Peripheral Arterial Disease | 0.01 | 0.97 | ||
| ACE inhibitor use | 0.05 | 0.58 | ||
| ARB use | −0.07 | 0.47 | ||
| Insulin use | 0.08 | 0.35 | ||
| Spironolactone use | 0.01 | 0.93 | ||
| Warfarin use | 0.40 | <0.001 | ||
| eGFR | −0.17 | 0.07 | ||
| Serum phosphorus | −0.09 | 0.35 | ||
| Serum magnesium | 0.12 | 0.40 | ||
| Serum calcium | −0.06 | 0.68 | ||
Model 1: unadjusted; Model 2: Multivariable-adjusted model; variables with significant association in the multivariable model are highlighted; CVA: Cerebrovascular accident; TIA: Transient ischemic attack; eGFR: Estimated glomerular filtration rate; ACE: Angiotensin Convertase enzyme; ARB: Angiotensin receptor blocker
Standardized coefficients are presented for easier comparison of various predictors;
dp-ucMGP as a Predictor of CF-PWV
dp-ucMGP was significantly associated with CF-PWV in unadjusted analyses (Standardized β= 0.30; P<0.001; Model 1, Table 3). In multivariate model that adjusted for known correlates of CF-PWV (age, sex, body mass index, heart rate, mean arterial pressure, diabetes mellitus), as well as the variables associated with dp-ucMGP (ethnicity, warfarin use, estimated glomerular filtration rate, serum calcium), dp-ucMGP was independently associated with CF-PWV (Standardized β=0.25; P=0.004; Model 2, Table 3).
Table 3.
dp-ucMGP as a Predictor of CF-PWV in Various Regression Models
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| β Coefficient * | P value | β Coefficient * | P value | |
| dp-ucMGP | 0.30 | <0.001 | 0.25 | 0.004 |
| Age | 0.41 | <0.001 | ||
| Male | −0.04 | 0.57 | ||
| African-American ethnicity | −0.05 | 0.40 | ||
| Body mass index | 0.05 | 0.39 | ||
| Heart rate | 0.14 | 0.031 | ||
| Mean arterial pressure | 0.24 | <0.001 | ||
| Diabetes mellitus | 0.14 | 0.028 | ||
| eGFR | 0.10 | 0.15 | ||
| Warfarin use | −0.04 | 0.64 | ||
| Serum calcium | −0.12 | 0.06 | ||
Model 1: unadjusted; Model 2: Multivariable-adjusted model; variables with significant association in the multivariable model are highlighted; dp-ucMGP: Dephosphorylated uncarboxylated Matrix Gla-Protein; CF-PWV: carotid-femoral pulse wave velocity; eGFR: Estimated glomerular filtration rate
Standardized coefficients are presented for easier comparison of various predictor
Plasma Aldosterone, CF-PWV, and Mediation analyses
Plasma aldosterone was significantly associated with CF-PWV (Standardized β=0.21; P=0.035). In a multivariable model that adjusted for age, sex, heart rate, mean arterial pressure, and diabetes mellitus, dp-ucMGP (Standardized β=0.19; P=0.047) but not aldosterone (Standardized β=0.01; P=0.92) was associated with CF-PWV. Figure 1 shows the results of formal mediation analyses in which the direct and indirect (dp-ucMGP-mediated) effects of aldosterone on CF-PWV were examined. In this model, aldosterone demonstrated a significant total effect on CF-PWV (β=0.179; 95%CI=0.013 to 0.346; P=0.035). There was a strong effect of aldosterone on dp-ucMGP (β=0.438; 95%CI=0.273 to 0.603; P<0.0001), as well as a significant effect of dp-ucMGP on CF-PWV (β=0.20; 95%CI= 0.012 to 0.398; P=0.038). The direct effect of aldosterone on CF-PWV, however, was non-significant (β=0.09; 95%CI=−0.0985 to 0.274; P=0.34), whereas its indirect (dp-ucMGP-dependent) effect was significant (β=0.102; 95%CI=0.012 to 0.218). This model revealed that dp-ucMGP mediated ~50% of the relationship between aldosterone and CF-PWV (PM=0.5001).
Figure 1: Mediation Model for Examining the Direct and Indirect (dp-ucMGP-mediated) Effects of Aldosterone on Carotid-Femoral Pulse Wave Velocity.
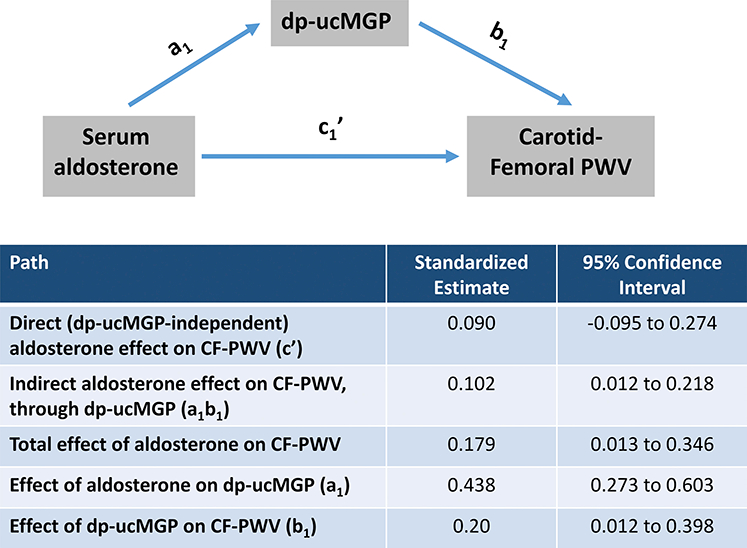
Path a1 represents the effect of aldosterone on dp-ucMGP. Path b1 represents the effect of dp-ucMGP on carotid-femoral pulse wave velocity (CF-PWV). Path c1’ represents the direct effect of aldosterone on CF-PWV. The indirect effect of aldosterone on CF-PWV is also shown, and depends on a1 and b1.
Discussion
In sample of hypertensive adults composed predominantly of older men, we demonstrate, for the first time, that dp-ucMGP is strongly associated with high aldosterone levels. Consistent with previous studies, we found that dp-ucMGP is significantly related to CF-PWV. Similarly, we observed a relationship between aldosterone and CF-PWV. However, in a model that included dp-ucMGP, aldosterone and various potential confounders, dp-ucMGP, but not aldosterone, was independently associated with CF-PWV. Formal mediation analyses demonstrated that dp-ucMGP accounts, at least in large part, for the relationship between aldosterone and increased arterial stiffness. We also found that hypertensive African-Americans demonstrated lower levels of dp-ucMGP, compared to Caucasians, even after adjustment for multiple potential confounders, a novel finding that may have implications for our understanding of the role of ethnicity on large artery stiffening in hypertension.
In two recent European community-based cohort analyses, increased circulating dp-ucMGP was independently associated with increased large artery stiffness (measured by CF-PWV).11, 12 Pivin et al. found that the percentage of participants taking antihypertensive agents increased from the lowest (7%) to the highest tertiles (25%) of dp-ucMGP.11 In our cohort of participants with hypertension, we found dp-ucMGP to be an independent predictor of CF-PWV, in line with these previous studies, further supporting a role of the MGP pathway in mediating increased large artery stiffness in hypertension.
Although a relationship between aldosterone and arterial stiffness/calcification has been demonstrated in several studies, the relationship between aldosterone and dp-ucMGP and their role in arterial stiffening in human hypertension has not been reported before. In a classical nephrectomy-aldosterone-salt hypertension animal model, aldosterone leads to increase in arterial stiffness, which is ameliorated by pharmacologic mineralocorticoid receptor antagonism.22 In a cross-sectional analysis, Bernini et al. found elevated CF-PWV in subjects with hyperaldosteronism, compared to hypertensive controls.14 Similarly, aldosterone levels were associated with arterial stiffness in a cross-sectional analysis of overweight and obese participants, irrespective of blood pressure.23 Furthermore, a beneficial effect of mineralocorticoid receptor antagonism on arterial stiffness has been reported.24, 25 Yet, the potential mechanisms by which aldosterone leads to arterial stiffness are poorly understood. Aldosterone increases and spironolactone abrogates the expression of the type III sodium-dependent phosphate transporter (Pit-1), promoting the osteogenic differentiation of vascular smooth muscle cells. 26–28 Aldosterone also promotes tumor necrosis factor-α expression, which may contribute to vascular calcification.25
Vascular calcification leads to increased large artery stiffness. Various extracellular molecular stimuli modulate pro-calcific (BMP-2, osteocalcin) and anti-calcific (MGP, osteopontin) pathways in the vascular smooth muscle cells 29. The inactive form of MGP, dp-ucMGP, undergoes post-translational modifications to become active MGP.10 dp-ucMGP has poor affinity for calcium and is released into the circulation. The determinants of dp-ucMGP synthesis and activation are incompletely understood. We found a strong association between dp-ucMGP with aldosterone levels, which was independent of various confounders. Furthermore, in formal mediation analyses, we found that most of the effect of aldosterone on CF-PWV was mediated by its relationship with dp-ucMGP, whereas its direct (dp-ucMGP-independent) effect was not significant. Our data suggests that circulating dp-ucMGP largely accounts for the association between aldosterone and CF-PWV in hypertension. This might result from aldosterone effects on the maturation of dp-ucMGP, the expression and synthesis of MGP, or through indirect effects mediated by independent biologic pathways.
Another novel finding from our study is that dp-ucMGP levels were significantly lower among participants of African-American ethnicity, despite adjusting for multiple potential confounders. Paradoxically, African Americans suffer from higher cardiovascular morbidity and mortality when compared to Caucasians 30 and various cross-sectional studies have reported greater levels of arterial stiffness among African Americans 31. In a recent cross-sectional study, Wei et al. reported lower dp-ucMGP levels in black South African participants when compared to white South African participants 32. This might reflect either 1) decreased expression of MGP in African-Americans, or 2) an increase in dp-ucMGP maturation, leading to lower circulating dp-ucMGP. Further research is required to elucidate the role of specific pathways on ethnic-related differences in arterial stiffness.
In addition to the observations discussed above, we found significant associations of dp-ucMGP with age, estimated glomerular filtration rate, serum calcium, cerebrovascular events and warfarin use. These findings are in line with prior cross-sectional studies and suggest a role for the MGP pathway in mediating arterial stiffness with aging and renal disease.33 We also found a positive association of serum calcium with dp-ucMGP levels. Mendoza et al. found that increased serum calcium led to increased MGP expression in heathy and uremic rats.34 Future studies are warranted to understand the biological basis of the association of serum calcium and MGP. Similarly, warfarin use was associated with increased dp-ucMGP levels in our study, in line with prior reports.10 This finding supports a crucial role for Vitamin K in the post-translational maturation of MGP to its active form. Moreover, our findings raise the concern for a detrimental effect of warfarin, a commonly used anticoagulant in hypertensive subjects, on the arterial wall.35 Importantly, recent data from Knapen et al. suggest that long-term vitamin K2 supplementation ameliorate arterial stiffening.36 The potential efficacy of vitamin K2 for this purpose requires further study.
Our study should be interpreted in context of certain strengths and limitations. To the best of our knowledge, our study is the first to evaluate the association of dp-ucMGP with CF-PWV in a multiethnic cohort of participants with hypertension. Additionally, this is the first study to evaluate the interrelationships between circulating dp-ucMGP, aldosterone, and large artery stiffness. We utilized CF-PWV which is gold-standard test for measurement of large artery stiffness18 and performed formal mediation analyses to clarify whether dp-ucMGP accounts for the observed relationship between aldosterone and CF-PWV.
Our study also has several limitations. We included a relatively small sample of participants through convenience sampling at a Veteran Affairs Medical Center, which may introduce selection bias. Therefore, the majority of our participants were males who were receiving clinical care for hypertension. Participants were receiving a variety of medications, which could affect aldosterone levels and the observed relationships. We did not account for vitamin D levels or supplementation. Measurements of 24-hour urinary aldosterone would have provided more reliable data compared to a single circulating aldosterone level. Only a subset of participants had available aldosterone levels, which could have affected the results. Our study design did not allow us to establish the origin or cause for increased aldosterone levels. Finally, our study was cross-sectional in nature and the associations found do not prove causality. In this regard, it is important to note that the effects deduced from the applied mediation analyses constitute, in a strict sense, a statistical rather than a mechanistic inference, and therefore do not necessarily imply that the MGP pathway participates in the biologic causal pathway between high aldosterone levels and arterial stiffness. Although the proposed effects are biologically plausible, future mechanistic experimental studies are required to assess the molecular basis behind the association between the aldosterone and the MGP pathway.
Conclusion
In summary, among older men with hypertension, we found that dp-ucMGP was independently associated with large artery stiffness, and that the relationship between plasma aldosterone and CF-PWV is largely accounted for by the important relationship between aldosterone and dp-ucMGP. Our data provide novel insights regarding the potential role of the MGP pathway on arterial stiffening in high aldosterone states. Future investigations should assess the mechanisms that may mediate the relationship between aldosterone and arterial stiffness, and whether targeting the MGP pathway may ameliorate the arterial stiffness in hypertension.
Highlights.
We studied the relationship between plasma aldosterone, MGP and arterial stiffness.
Inactive MGP levels were associated with higher large artery stiffness.
Aldosterone were independently associated with inactive MGP levels.
Aldosterone levels were associated with arterial stiffness.
Most of this association was mediated through its association with MGP.
Acknowledgments
Sources of Funding: This study was supported by NIH grants R56HL-124073–01A1 (JAC), R01 HL 121510–01A1 (JAC), 5-R21-AG-043802–02 (JAC) and a VISN-4 research grant from the department of Veterans Affairs (JAC) and the National Institute on Aging, Intramural Research Program (OF and EGL).
Footnotes
Conflict of Interest: JAC has received consulting honoraria from Bristol-Myers Squibb, OPKO Healthcare, Fukuda Denshi, Microsoft, Sanifit, Pfizer, Vital Labs and Merck. He received research grants from National Institutes of Health, American College of Radiology Network, Fukuda Denshi, Bristol-Myers Squibb, Microsoft and CVRx Inc., and device loans from AtCor Medical. JAC is named as inventor in a University of Pennsylvania patent application for the use of inorganic nitrates/nitrites for the treatment of Heart Failure and Preserved Ejection Fraction. NEAD and CV are employed by VitaK. Other authors have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D and Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR and Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao D, Arnett DK, Tyroler HA, Riley WA, Chambless LE, Szklo M and Heiss G. Arterial stiffness and the development of hypertension. The ARIC study. Hypertension. 1999;34:201–6. [DOI] [PubMed] [Google Scholar]
- 4.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS and Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. Jama. 2012;308:875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dernellis J and Panaretou M. Aortic stiffness is an independent predictor of progression to hypertension in nonhypertensive subjects. Hypertension. 2005;45:426–31. [DOI] [PubMed] [Google Scholar]
- 6.Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, Spurgeon HP, Ferrucci L and Lakatta EG. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51:1377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chirinos JA and Segers P. Noninvasive evaluation of left ventricular afterload: part 2: arterial pressure-flow and pressure-volume relations in humans. Hypertension. 2010;56:563–70. [DOI] [PubMed] [Google Scholar]
- 8.Nichols WW ORM, Vlachopoulos C. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles, 6th Edition. Hodder Arnold; 2011. [Google Scholar]
- 9.O’Rourke MF and Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–4. [DOI] [PubMed] [Google Scholar]
- 10.Schurgers LJ, Uitto J and Reutelingsperger CP. Vitamin K-dependent carboxylation of matrix Gla-protein: a crucial switch to control ectopic mineralization. Trends Mol Med. 2013;19:217–26. [DOI] [PubMed] [Google Scholar]
- 11.Pivin E, Ponte B, Pruijm M, Ackermann D, Guessous I, Ehret G, Liu YP, Drummen NE, Knapen MH, Pechere-Bertschi A, Paccaud F, Mohaupt M, Vermeer C, Staessen JA, Vogt B, Martin PY, Burnier M and Bochud M. Inactive Matrix Gla-Protein Is Associated With Arterial Stiffness in an Adult Population-Based Study. Hypertension. 2015;66:85–92. [DOI] [PubMed] [Google Scholar]
- 12.Mayer O Jr., Seidlerova J, Wohlfahrt P, Filipovsky J, Vanek J, Cifkova R, Windrichova J, Topolcan O, Knapen MH, Drummen NE and Vermeer C Desphospho-uncarboxylated matrix Gla protein is associated with increased aortic stiffness in a general population. J Hum Hypertens. 2016;30:418–23. [DOI] [PubMed] [Google Scholar]
- 13.Rosa J, Somloova Z, Petrak O, Strauch B, Indra T, Senitko M, Zelinka T, Holaj R and Widimsky J Jr. Peripheral arterial stiffness in primary aldosteronism. Physiol Res. 2012;61:461–8. [DOI] [PubMed] [Google Scholar]
- 14.Bernini G, Galetta F, Franzoni F, Bardini M, Taurino C, Bernardini M, Ghiadoni L, Bernini M, Santoro G and Salvetti A. Arterial stiffness, intima-media thickness and carotid artery fibrosis in patients with primary aldosteronism. J Hypertens. 2008;26:2399–405. [DOI] [PubMed] [Google Scholar]
- 15.Lin YH, Lin LY, Chen A, Wu XM, Lee JK, Su TC, Wu VC, Chueh SC, Lin WC, Lo MT, Wang PC, Ho YL, Wu KD and Group TS. Adrenalectomy improves increased carotid intima-media thickness and arterial stiffness in patients with aldosterone producing adenoma. Atherosclerosis. 2012;221:154–9. [DOI] [PubMed] [Google Scholar]
- 16.Lang F, Ritz E, Alesutan I and Voelkl J. Impact of aldosterone on osteoinductive signaling and vascular calcification. Nephron Physiol. 2014;128:40–5. [DOI] [PubMed] [Google Scholar]
- 17.Cranenburg EC, Koos R, Schurgers LJ, Magdeleyns EJ, Schoonbrood TH, Landewe RB, Brandenburg VM, Bekers O and Vermeer C. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb Haemost. 2010;104:811–22. [DOI] [PubMed] [Google Scholar]
- 18.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T and American Heart Association Council on H. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension. 2015;66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H and European Network for Non-invasive Investigation of Large A. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–605. [DOI] [PubMed] [Google Scholar]
- 20.Chirinos JA. Arterial stiffness: basic concepts and measurement techniques. J Cardiovasc Transl Res. 2012;5:243–55. [DOI] [PubMed] [Google Scholar]
- 21.Hayes A Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York, NY: Guilford Press; 2013. [Google Scholar]
- 22.Galmiche G, Pizard A, Gueret A, El Moghrabi S, Ouvrard-Pascaud A, Berger S, Challande P, Jaffe IZ, Labat C, Lacolley P and Jaisser F. Smooth muscle cell mineralocorticoid receptors are mandatory for aldosterone-salt to induce vascular stiffness. Hypertension. 2014;63:520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper JN, Tepper P, Barinas-Mitchell E, Woodard GA and Sutton-Tyrrell K. Serum aldosterone is associated with inflammation and aortic stiffness in normotensive overweight and obese young adults. Clin Exp Hypertens. 2012;34:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White WB, Duprez D, St Hillaire R, Krause S, Roniker B, Kuse-Hamilton J and Weber MA. Effects of the selective aldosterone blocker eplerenone versus the calcium antagonist amlodipine in systolic hypertension. Hypertension. 2003;41:1021–6. [DOI] [PubMed] [Google Scholar]
- 25.Edwards NC, Steeds RP, Stewart PM, Ferro CJ and Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol. 2009; 54: 505–12. [DOI] [PubMed] [Google Scholar]
- 26.Lang F, Ritz E, Voelkl J and Alesutan I. Vascular calcification--is aldosterone a culprit? Nephrol Dial Transplant. 2013;28:1080–4. [DOI] [PubMed] [Google Scholar]
- 27.Voelkl J, Alesutan I, Leibrock CB, Quintanilla-Martinez L, Kuhn V, Feger M, Mia S, Ahmed MS, Rosenblatt KP, Kuro OM and Lang F. Spironolactone ameliorates PIT1-dependent vascular osteoinduction in klotho-hypomorphic mice. J Clin Invest. 2013;123:812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu SY, Yu YR, Cai Y, Jia LX, Wang X, Xiao CS, Tang CS and Qi YF. Endogenous aldosterone is involved in vascular calcification in rat. Exp Biol Med (Maywood). 2012;237:31–7. [DOI] [PubMed] [Google Scholar]
- 29.Montezano AC, Zimmerman D, Yusuf H, Burger D, Chignalia AZ, Wadhera V, van Leeuwen FN and Touyz RM. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension. 2010;56:453–62. [DOI] [PubMed] [Google Scholar]
- 30.Writing Group M, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–360. [DOI] [PubMed] [Google Scholar]
- 31.Morris AA, Patel RS, Binongo JN, Poole J, Al Mheid I, Ahmed Y, Stoyanova N, Vaccarino V, Din-Dzietham R, Gibbons GH and Quyyumi A. Racial differences in arterial stiffness and microcirculatory function between Black and White Americans. J Am Heart Assoc. 2013;2:e002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei FF, Drummen NE, Schutte AE, Thijs L, Jacobs L, Petit T, Yang WY, Smith W, Zhang ZY, Gu YM, Kuznetsova T, Verhamme P, Allegaert K, Schutte R, Lerut E, Evenepoel P, Vermeer C and Staessen JA. Vitamin K Dependent Protection of Renal Function in Multi-ethnic Population Studies. EBioMedicine. 2016;4:162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schurgers LJ, Barreto DV, Barreto FC, Liabeuf S, Renard C, Magdeleyns EJ, Vermeer C, Choukroun G and Massy ZA. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clin J Am Soc Nephrol. 2010;5:568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendoza FJ, Martinez-Moreno J, Almaden Y, Rodriguez-Ortiz ME, Lopez I, Estepa JC, Henley C, Rodriguez M and Aguilera-Tejero E. Effect of calcium and the calcimimetic AMG 641 on matrix-Gla protein in vascular smooth muscle cells. Calcif Tissue Int. 2011;88:169–78. [DOI] [PubMed] [Google Scholar]
- 35.Zhang YT and Tang ZY. Research progress of warfarin-associated vascular calcification and its possible therapy. J Cardiovasc Pharmacol. 2014;63:76–82. [DOI] [PubMed] [Google Scholar]
- 36.Knapen MH, Braam LA, Drummen NE, Bekers O, Hoeks AP and Vermeer C. Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. A double-blind randomised clinical trial. Thromb Haemost. 2015;113:1135–44. [DOI] [PubMed] [Google Scholar]


