Abstract
Background:
It has recently been shown that magnetic resonance (MR) “native T1” mapping is capable of characterizing abnormal microcirculation in patients with obstructive coronary artery disease (CAD). In studies involving women with signs and symptoms of ischemia and no obstructive CAD (INOCA), however, the potential role of native T1 as an imaging marker and its association with indices of diastolic function or vasodilator-induced myocardial ischemia have not been explored. We investigated whether native T1 in INOCA is associated with reduced myocardial perfusion reserve index (MPRI) or with diastolic dysfunction.
Methods:
Twenty-two female patients with INOCA and twelve female reference controls with matching age and body-mass index were studied. The patients had evidence of vasodilator-induced ischemia without obstructive CAD or any prior infarction. All 34 subjects underwent stress/rest MR including native T1 mapping (MOLLI 5(3)3) at 1.5-Tesla.
Results:
Compared with controls, patients had similar morphology/function. As expected, MPRI was significantly reduced in patients compared to controls (1.78 ± 0.39 vs. 2.49 ± 0.41, p <0.0001). Native T1 was significantly elevated in patients (1040.1 ± 29.3 ms vs. 1003.8 ± 18.5 ms, p < 0.001) and the increased T1 showed a significant inverse correlation with MPRI (r = −0.481, p = 0.004), but was not correlated with reduced diastolic strain rate.
Conclusions:
Symptomatic women with INOCA have elevated native T1 compared to matched reference controls and there is a significant association between elevated native T1 and impaired MPRI, considered a surrogate measure of ischemia severity in this cohort. Future studies in a larger cohort are needed to elucidate the mechanism underlying this inverse relationship.
Keywords: coronary microvascular dysfunction, nonobstructive coronary artery disease, microvascular angina, myocardial T1, myocardial perfusion, coronary microcirculation
INTRODUCTION
Cardiovascular disease is the leading cause of mortality in women in the United States with approximately 400,000 deaths occurring annually, and a majority of these deaths are due to ischemic heart disease.1 However, more than 50% of women who present with symptoms of ischemic heart disease have no obstructive coronary artery disease (CAD).2 As a result, many of these symptomatic women are inappropriately deemed to be low-risk patients according to traditional stenosis-based management strategies due to the lack of obstructive CAD and often-preserved left ventricular (LV) ejection fraction (EF). In fact, women with signs and symptoms of ischemia and no obstructive CAD (INOCA) often have coronary microvascular dysfunction (CMD),3,4 demonstrated by the National Heart, Lung and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) clinical studies.5,6 Defined by an abnormal response to gold-standard invasive coronary reactivity testing of microvascular pathways, CMD carries a higher risk of major adverse cardiac events compared to healthy or asymptomatic women, despite the absence of obstructive CAD.2,7,8 Elevated rates of heart failure hospitalization — predominantly with preserved EF — are also observed in these women,8 leading to the hypothesis that CMD and microvascular angina9 may be mechanistically linked to the subsequent development of heart failure with preserved EF (HFpEF).10
Native or pre-contrast myocardial T1 mapping using cardiac magnetic resonance (MR) imaging has emerged as a powerful non-invasive marker of myocardial disease, providing novel insight into myocardial tissue characteristics. Native T1 is sensitive to water content in the intracellular and extracellular myocardial compartments as well as the intravascular space (in form of intramyocardial blood). Diffusely elevated native T1 values are known to be suggestive of expanded extracellular matrix in the presence of diffuse myocardial fibrosis11,12 and/or increased intramyocardial blood, e.g., in patients with aortic stenosis.13
Recently, Liu et al.14 have shown that, in patients with obstructive CAD, native myocardial T1 “reactivity” can detect the myocardial territory affected by significant epicardial stenosis. This feature in obstructive CAD is primarily driven by elevated native T1 at rest.15 Patients with INOCA often have nonobstructive CAD in form of diffuse coronary atherosclerosis as has been observed in intravascular ultrasound studies of WISE16 and another similar cohort.17 In this pilot study of WISE women, we aimed to determine if there is abnormally elevated native T1 in women with INOCA compared to matched normal controls, and to evaluate the relationship between native T1 and MR-derived indices of perfusion reserve and diastolic function in this cohort.
METHODS
Study Population
Women who were previously evaluated at Cedars-Sinai Medical Center for stable and persistent signs and symptoms of ischemia in the absence of obstructive CAD and who had objective evidence of vasodilator-induced ischemia (described below) were studied. All subjects were enrolled in the Women’s Ischemia Syndrome Evaluation Coronary Vascular Dysfunction (WISE-CVD) study with approval from the institutional review board. Reference control subjects (matched to the patient group) were recruited as described below. All subjects provided informed consent and their treating physicians approved their participation.
INOCA Subjects.
The enrolled subjects had preserved LV EF, symptoms of ischemia (chest pain and/or dyspnea), and no evidence of obstructive CAD (defined as ≥50% luminal diameter stenosis in ≥1 epicardial coronary artery) based on prior invasive coronary angiograms assessed by the WISE-CVD angiographic core laboratory. Among these subjects, the study was limited to those who had a history of INOCA based on a clinically-indicated abnormal invasive coronary reactivity test or an abnormal myocardial perfusion reserve index (MPRI) from a prior adenosine-stress cardiac MR scan. The invasive coronary reactivity test (within a 5-month period prior to the current study) was performed as previously described18,19 and abnormality was defined as having a coronary flow velocity reserve of ≤ 2.3.7. Abnormal MPRI was defined as a mean value of ≤1.84. This MPRI abnormality threshold was determined to be the best predictor of abnormal coronary reactivity (invasive test) based on receiver-operator characteristic curve analysis in a study of 118 INOCA women from the abovementioned WISE-CVD cohort, conducted prior to the current study.20 The use of invasive/noninvasive measures of coronary/myocardial flow reserve to define INOCA in subjects with symptoms of myocardial ischemia and no obstructive CAD is in accordance with prior inclusion criteria in WISE-related studies20,21 and also is in accordance with the established literature22 including the recently published international standardization of diagnostic criteria for microvascular angina.9 Exclusionary criteria included: prior myocardial infarction, chest pain with known nonischemic etiology, nonischemic cardiomyopathy, acute coronary syndrome, planned or prior percutaneous intervention or coronary bypass surgery, primary valvular disease, pregnancy, noncardiac disease with life expectancy <4 years, and any contraindications to MR including contraindications to gadolinium contrast and vasodilator stress. None of the enrolled patients were suspected of having or had a history of myocarditis or vasculitis, and none had evidence of HFpEF or prior hospitalization for heart failure.
Reference Controls.
Healthy women who were matched in age and body mass index to the INOCA subjects and satisfied all of the following criteria were recruited to serve as reference controls for the imaging study: (1) no symptoms and no cardiac risk factors according to the U.S. National Cholesterol Education Program guidelines; (2) none of the exclusion criteria described above; (3) no evidence of ischemic heart disease based on a normal maximal exercise treadmill stress testing with Bruce protocol.
Imaging Protocol
MR imaging was performed on a 1.5 Tesla clinical scanner (Magnetom Avanto, Siemens Healthcare, Erlangen, Germany) with a vendor-provided 12-channel coil array. All study subjects underwent a standardized cardiac MR protocol including function, stress/rest perfusion, and viability imaging. Subjects were instructed to abstain from caffeine intake and certain medications prior to their scheduled exam as detailed previously.20
Data Acquisition: Function, Perfusion, and Viability.
Cine function images were acquired in 10–12 short-axis slices using a standard steady-state free precession (SSFP) pulse sequence. First-pass perfusion imaging was performed during vasodilator stress followed by a rest scan (after a minimum 10-minute delay) using a gadolinium-based contrast agent (Gadodiamide; Omniscan) with a dose of 0.05 mmol/kg per scan (injection rate: 4 mL/s). Vasodilator stress imaging was performed using adenosine (dose: 140 mcg/kg/min; infusion time ≈ 4 minutes). The pulse sequence for perfusion imaging acquired 3 standard short-axis slices in every heartbeat using a gradient-echo/EPI hybrid sequence with two-fold parallel imaging acceleration. Following the stress/rest perfusion scans, an additional bolus of 0.1 mmol/kg contrast was injected and late gadolinium enhancement (LGE) images were obtained using a standard protocol.20
Data Acquisition: Native T1 Mapping.
In all subjects, native T1 mapping was performed in a mid-ventricular short-axis slice using a previously validated pulse sequence (vendor-provided MOLLI 5(3)3 implemented as “Siemens work-in-progress” package version 780 employing an adiabatic inversion pulse23 optimized forT1 mapping). Native T1 maps were generated “inline” (within the scanner platform) using phase-sensitive inversion recovery fitting with a three-parameter signal model24 following automatic nonrigid motion correction applied to the acquired T1-weighted images using a previously described method.24,25 Careful cardiac shimming was performed to avoid confounding effects of off-resonance on T1 measurements. The MOLLI acquisitions were ECG-triggered and obtained during an 11-heartbeat breath-hold at end-expiration. Typical imaging parameters included flip angle: 35°, repetition/echo time: 2.7/1.1 ms, slice thickness: 8 mm, in-plane resolution: 1.4 × 1.8 mm2, parallel imaging factor: 2.
Data Analysis
LV Function and Viability.
LV function and mass was assessed by manually tracing the epicardial and endocardial borders of short-axis cine images using the CAAS MRV software (Pie Medical Imaging, Maastricht, Netherlands). LV mass index was calculated by normalizing the mass to body surface area (BSA). LV EF was calculated as stroke volume divided by end-diastolic volume. Strain analysis using tissue/feature tracking was performed in short-axis cine images using the “cvi42” software (Circle CVI, Calgary, Canada). LGE images were read by two experienced clinical investigators to identify areas of focal/diffuse enhancement. For INOCA subjects, visual inspection of the cine SSFP images (normalized intensities) was performed in cvi42 (mid slice only; two readers in consensus) to detect T2-weighted intensity heterogeneity as a sign of overt/localized edema according to prior work by Kumar et al.26
Myocardial Perfusion Reserve Index.
First-pass myocardial perfusion images were analyzed to quantify MPRI on a segmental basis using CAAS MRV. MPRI for each segment was calculated as the ratio of normalized stress upslope to normalized rest upslope.20 The average of the segmental MPRI values was used as the “mean MPRI” for each studied subject.
T1 Mapping.
In all subjects, native T1 values were analyzed for the mid-ventricular slice from the inline-generated T1 maps using cvi42, blinded to function and strain. Raw T1 images (before and after inline motion correction) and T1 maps were examined for image quality prior to analysis. Mean “global” native T1 values were measured using conservative endocardial and epicardial segmentation,27 taking care to exclude partial volume effects from the blood-pool and surrounding tissue/fat (representative example shown in Supplementary Figure 1). Six AFIA myocardial segments were automatically delineated using the right-ventricular insertion points as landmarks. In addition to the global native T1 value, the septal native T1 was measured in each case by averaging the T1 values from antero-and infero-septal segments.
Statistical Methods
Statistical analyses were performed using GraphPad Prism (GraphPad, La Jolla, California, United States). Differences between INOCA subjects and reference controls were compared using unpaired Student t-tests. Bivariate correlations were assessed using the Pearson coefficient. All statistical tests were two-tailed with a p value < 0.05 considered statistically significant. Continuous variables are described as mean ± standard deviation.
RESULTS
Baseline Characteristics
One INOCA subject was excluded from the study prior to data analysis due to poor image quality of the generated T1 maps, leaving 22 INOCA subjects. The subject characteristics and clinical information for reference controls (n=12 females) and INOCA subjects (n=22 females) are described in Table 1. By design, the reference control subjects were well matched with INOCA subjects for sex, age, body mass index, and surface area (Table 1).
Table 1. Baseline characteristics.
Baseline clinical and demographic characteristics of the study population.
| Variable | Reference Controls |
INOCA Subjects |
p value |
|---|---|---|---|
| Number | 12 | 22 | |
| Females | 12 (100%) | 22 (100%) | |
| Age (years) | 49.2 ± 11 | 52.6 ± 13 | 0.436 |
| BMI (kg/m2) | 25.5 ± 4.5 | 27.2 ± 5.3 | 0.344 |
| BSA (m2) | 1.78 ± 0.20 | 1.82 ± 0.19 | 0.506 |
| Risk Factors: | |||
| Hypertension | 0 | 11 (50%) | |
| Type II Diabetes | 0 | 3 (14%) | |
| Ever Smoker | 0 | 7 (32%) | |
| Dyslipidemia | 0 | 10 (45%) | |
| Family History of CAD | 0 | 16 (73%) | |
| Medications: | |||
| Beta blockers | 0 | 10 (45%) | |
| Calcium channel blockers | 0 | 4 (18%) | |
| Nitrates | 0 | 6 (27%) | |
| Aspirin | 0 | 14 (64%) | |
| ACE inhibitors | 0 | 3 (14%) | |
| Statins | 0 | 12 (55%) | |
| Symptoms: | |||
| Chest pain | 0 | 11 (50%) | |
| Dyspnea | 0 | 15 (68%) | |
Values are mean ± standard deviation, or number (percentage).
BMI = body mass index; BSA = body surface area; CAD = coronary artery disease; INOCA = ischemia and no obstructive CAD; ACE = Angiotensin-converting enzyme.
Structure and Function
INOCA subjects had preserved LV EF (mean EF: 68.2 ± 6.2%). No significant difference was observed in LV volumes, mass, mass index, or EF between INOCA subjects and reference controls. Only one subject had LV hypertrophy based on > 70 g/m2 abnormality threshold28 for BSA-indexed LV mass in females (all other subjects had mass index < 60 g/m2). Additionally, no difference was observed in peak circumferential systolic strain or systolic strain rate. Compared with controls, the peak circumferential diastolic strain rate was reduced in INOCA subjects compared to reference controls (102.7 ± 29.9 vs. 126.0 ± 34.9 %/s, p = 0.055). Visual inspection of cine SSFP myocardial signal intensities did not detect overt/localized edema in any of the subjects.26
Tissue Characterization: Perfusion and Viability
As expected, mean MPRI was significantly lower in INOCA subjects compared to reference controls (1.78 ± 0.39 vs. 2.49 ± 0.41, p < 0.0001). Positive LGE findings (focal or diffuse/patchy enhancement) were not observed in any of the INOCA subjects or any of the reference control subjects. Moreover, no significant correlation was found between MPRI and diastolic strain rate.
Tissue Characterization: Native Myocardial T1 Mapping
Six out of 204 myocardial segments in the 34 subjects (3% of all segments) were excluded due to presence of artifacts in the T1 maps — caused by imperfect motion correction (during the 11-heartbeat breath-hold) or susceptibility effects. INOCA subjects had both significantly higher mean global and septal native T1 values compared with reference controls as shown in Figure 1 (global T1: 1040.1 ±29.3 ms vs. 1003.8 ± 18.5 ms, p < 0.001; septal T1: 1054.7 ± 30.2 ms vs. 1019.5 ± 20.8 ms, p = 0.001). Table 2 summarizes the imaging results.
Figure 1:

Native myocardial T1 in subjects with ischemia and no obstructive coronary artery disease (INOCA) is compared with matched reference controls. Left: comparison of global T1; mean value for reference controls: 1003.8 ± 18.5 ms vs. 1040.1 ± 29.3 ms for INOCA subjects, p < 0.001. Right: comparison of septal T1; mean value for reference controls: 1019.5 ± 20.8 ms vs. 1054.7 ± 30.2 ms for INOCA subjects, p = 0.001.
Table 2. Imaging data for patients and controls.
Cardiac magnetic resonance measures for the reference controls (n=12 females) and INOCA subjects (n=22 females).
| Reference Controls |
INOCA Subjects |
p value | |
|---|---|---|---|
| MPRI | 2.49 ± 0.41 | 1.78 ± 0.39 | < 0.0001* |
| Presence of LGE | 0 | 0 | -- |
| LV EF (%) | 67.7 ± 1.7 | 68.2 ± 6.2 | 0.787 |
| LV ED volume (ml) | 127.5 ± 18.3 | 127.1 ± 25.4 | 0.964 |
| LV ES volume (ml) | 41.2 ± 6.32 | 41.2 ± 15.5 | 0.998 |
| LV stroke volume (ml) | 84.0 ± 12.4 | 85.9 ± 14.1 | 0.690 |
| LV mass (g) | 74.4 ± 17.3 | 85.5 ± 21.7 | 0.136 |
| LV mass index (g/m2) | 41.7 ± 7.0 | 47.1 ± 12.1 | 0.169 |
| Presence of LV hypertrophy | 0 | 1 (5 %) | -- |
| Cardiac output | 5.32 ± 1.21 | 5.86 ± 1.58 | 0.332 |
| Systolic circumferential | −19.7 ± 2.24 | −18.3 ± 3.33 | 0.235 |
| strain (%) | |||
| Systolic circumferential | −94.1 ± 13.0 | −94.8 ± 19.3 | 0.919 |
| strain rate (%/s) | |||
| Diastolic circumferential | 126.0 ± 34.9 | 102.7 ± 29.9 | 0.055 |
| strain rate (%/s) | |||
| Global native | 1003.8 ± 18.5 | 1040.1 ± 29.3 | < 0.001* |
| myocardial T1 (ms) | |||
| Septal native | 1019.5 ± 20.8 | 1054.7 ± 30.2 | 0.001* |
| myocardial T1 (ms) | |||
| Resting systolic BP (mmHg) | 121.7 ± 14.3 | 128.6 ± 18.9 | 0.210 |
| Resting HR (beats per minute) | 60.9 ± 10.9 | 66.9 ± 8.3 | 0.286 |
| Stress HR (beats per minute) | 96.7 ± 19.1 | 92.6 ± 12.7 | 0.474 |
| Resting rate-pressure product | 7409±1493 | 8623±1711 | 0.129 |
| Stress rate-pressure product | 11425±2467 | 11813± 1965 | 0.629 |
indicates statistical significance.
Values are mean ± standard deviation or number (percentage).
INOCA = ischemia and no obstructive coronary artery disease; ED = end diastolic; ES = end systolic; EF = ejection fraction; LGE = late gadolinium enhancement; LV = left ventricular; MPRI = myocardial perfusion reserve index; BP = blood pressure; HR = heart rate.
Examining the Partial-volume Effect on Native T1
INOCA subjects had a slightly higher mean wall thickness than controls but this difference was insignificant (controls: 8.6 ± 1.4 mm vs. INOCA: 9.8 ± 2.6 mm, p = 0.15). To examine if the partial-volume effect contributed to the differences in native T1, subjects with the highest and lowest quartiles of septal wall thickness were analyzed separately. Among all studied subjects, there was not a significant difference between the native T1 of those with the thickest wall vs. those with the thinnest wall (thickest quartile: 1045 ± 27 ms vs. thinnest quartile: 1036 ± 25 ms, p = 0.62). Additionally, wall thickness was not correlated to global or septal native T1 (p = 0.20 and p = 0.74, respectively).
Correlates of Native Myocardial T1
LV EF was not correlated with septal or global native T1. No significant correlation was observed between native T1 (septal or global) and circumferential diastolic strain rate. LV mass index showed a slight correlation to native T1 that was not statistically significant (global: r = 0.305, p = 0.079; septal: r = 0.295, p = 0.091). A significant inverse correlation was observed between native myocardial T1 (both global and septal) and MPRI (global: r = −0.481, p = 0.004; septal: r = −0.381, p = 0.026). Figure 2 shows the correlation result for global native T1 against MPRI.
Figure 2:
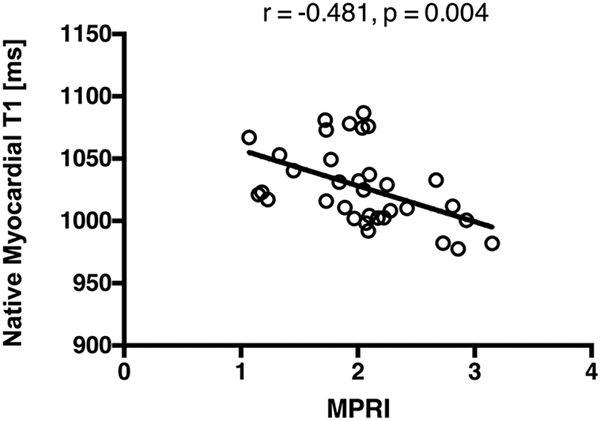
Evaluation of the linear association between elevated native myocardial T1 and reduced perfusion reserve quantified by myocardial perfusion reserve index (MPRI). A statistically significant inverse correlation is observed between global native myocardial T1 and MPRI (r = −0.481, p = 0.004).
DISCUSSION
The present pilot study applied native myocardial T1 mapping in a distinct population of women with signs and symptoms of ischemia and no obstructive CAD (INOCA) in comparison to matched reference controls to: (a) evaluate whether native T1 values are abnormally elevated in INOCA subjects; and (b) interrogate the link between elevated native T1 and impaired myocardial perfusion reserve index (MPRI), a surrogate measure of vasodilator-induced ischemia, and MR-derived markers of diastolic function. Our results showed elevated native myocardial T1 values in the INOCA group compared to the reference controls. Furthermore, in addition to significantly reduced MPRI in INOCA subjects versus controls, in this study we observed a significant inverse association between elevated native T1 and MPRI. On the other hand, elevated native T1 was not found to be related to diastolic dysfunction (reduced circumferential diastolic strain rate). We also did not find a significant correlation between impaired MPRI (a surrogate marker of “microvascular ischemia”) and diastolic dysfunction, which is consistent with our prior study in a similar population that used tagged cine MR for measurement of diastolic strain rate.21
The list of risk factors for the patient group in our study—specifically the high prevalence of well-controlled hypertension and low prevalence of diabetes—is consistent with prior WISE studies7,8,20,21,29 and other large studies involving male/female patients with INOCA;30 and, along with aging, are considered to be the common risk factors driving the pathophysiology in such cohorts.22,31 Of note, it has been shown that arterial hypertension is associated with diffuse remodeling of intramyocardial microvasculature,22, 32 which can occur in hypertensive patients with microvascular angina in the absence of LV hypertrophy.33 Although our patient cohort was nearly free of LV hypertrophy (Table 2), the slightly higher LV mass for INOCA subjects compared to reference controls has also been observed in previous WISE studies,20,21 and is consistent with the association between systemic vasomotor dysfunction and subclinical LV hypertrophy in apparently healthy adults.34 Similarly to our results that showed no significant correlation between MPRI and LV mass index, a larger WISE study did not observe a correlation between invasively measured coronary flow velocity reserve and indices of LV mass and LV concentric remodeling.7 Outside the scope of WISE-related investigations, a recent study of women with suspected CMD as part of the iPOWER study did not observe a relationship between myocardial perfusion reserve and native T1.35 Aside from notable differences in methodology (e.g., perfusion reserve in iPOWER was measured 1–8 months after the MRI study using PET), this lack of association may be attributed to the major differences in patient characteristics/demographics and risk-factors, which include the following compared to our WISE patient population: the iPOWER subjects were ~10 years older, did not all have symptoms of ischemia (72% vs. 100% in our study), had a notably higher systolic blood pressure (mean of 146.5 mmHg vs. 128.6 mmHg in our study) and had a 2-fold higher prevalence for smoking (63% vs. 32% in our study).
The primary imaging marker for noninvasive assessment of INOCA or suspected CMD is myocardial perfusion reserve, which has been established based on PET studies22, 36, 37 and MR-measured MPRI.20 However, MR-based quantification of perfusion reserve requires administration of gadolinium-based contrast agents, which have recently been under increased scrutiny in the imaging community38 and may pose limitations especially for serial assessment in therapeutic studies given the large cumulative gadolinium dose needed for serial imaging. In such scenarios and if the association between impaired MPRI and elevated native T1 observed in our pilot study is confirmed in a larger INOCA study, native myocardial T1 may evolve as a gadolinium-free surrogate imaging marker of disease severity/progression for evaluating the effectiveness of novel therapies29,39 in this cohort.
Hypothesis on the Link Between Elevated Native T1 and Impaired Vasodilator Reserve
The multi-factorial nature of native T1 as an imaging marker and the small sample size in the presented pilot study do not allow us to elucidate the mechanism for the observed increase in native T1 in INOCA subjects, e.g., to differentiate the potential contribution of diffuse fibrosis versus intramyocardial blood volume to the elevated native T1. However, the lack of association between impaired MPRI and diastolic dysfunction combined with the significant correlation between impaired MPRI and native T1 leads us to hypothesize that the increased native T1 observed in this relatively younger cohort of INOCA subjects without LV hypertrophy may be secondary to elevated intramyocardial water content (intramyocardial blood volume and/or intracellular water content) at rest. Additional support for this hypothesis is that the difference in native T1 in our patient cohort versus controls is similar to the difference seen at 1.5 Tesla in resting native T1 of obstructive CAD patients versus controls by Liu et al.,14 which is also explained by microcirculatory autoregulation,15 and is notably lower than the difference seen in cohorts with known diffuse myocardial fibrosis.40 Other factors such as myocardial edema may contribute to elevated native T1 - although, in the current study, we did not observe any overt localized edema. Future studies involving multi-parametric tissue characterization — including T2 mapping for detection of diffuse/subclinical edema — and stress/rest native T1 mapping14 using heart-rate-independent techniques41 may be able to provide more insight into the underlying mechanisms contributing to elevated native T1 and its inverse association with MPRI in this cohort.
Study Limitations
Our imaging protocol used a clinically-available perfusion MR technique that did not include the acquisition of an unsaturated arterial input function to reliably enable the quantification of absolute myocardial blood flow. Therefore, our analysis, similarly to previous WISE studies,20, 29 was limited to a semi-quantitative measure of perfusion reserve (i.e., MPRI) — which has been shown to be non-inferior to fully-quantitative analysis in detection of obstructive CAD42 and a potential prognostic marker in INOCA.43 Nevertheless, future work using absolute quantification of myocardial blood flow44,45 at peak hyperemia vs. rest can provide additional insight into the mechanism for impaired perfusion reserve and its association with elevated native T1. The mean normal native T1 value determined in this study is within the range of previously reported normal values at 1.5 Tesla.46–48 Also, the difference between global and septal T1 in our study (≈20 ms) is likely due to known off-resonance effects in MOLLI sequences, and is consistent with previous studies at 1.5 Tesla.49 The current study was limited to T1 mapping in a mid-ventricular slice with the underlying assumption that the mid slice is representative of the diffuse/global pathophysiology in INOCA.
CONCLUSIONS
Women with signs and symptoms of ischemia (chest pain and/or shortness of breath) in the absence of obstructive CAD show an elevated native myocardial T1 compared to matched reference controls. Our findings in this cohort show an inverse association between elevated native myocardial T1 and MR-derived myocardial perfusion reserve index, which is considered a surrogate measure of ischemia severity in this cohort. The underlying mechanism for this association remains to be investigated in future studies. If this inverse relationship is confirmed in a larger study, it would represent a step towards demonstrating the utility of native T1 mapping in assessment of patients with INOCA as it may lead to native T1 evolving as a non-contrast surrogate marker of disease severity/progression in this cohort with implications for serial imaging-based evaluation of therapies without using Gadolinium-based contrast agents.
Supplementary Material
Supplementary Fig. 1: Representative contours for native myocardial T1 maps. The corresponding epicardial and endocardial contours (divided into 6 myocardial segments) are shown. Native T1 values were measured using conservative endocardial and epicardial segmentation, taking care to exclude partial volume effects from the blood-pool and surrounding tissue/fat.
Highlights.
Women with signs and symptoms of myocardial ischemia and no obstructive coronary artery disease (INOCA) often have coronary microvascular dysfunction.
In this pilot study, native T1 was significantly elevated in INOCA patients compared to matched reference controls.
The increased native T1 showed a significant inverse correlation with myocardial perfusion reserve index.
Native myocardial T1 may evolve as a useful imaging marker in serial studies of INOCA patients.
Acknowledgments
Funding Acknowledgements: This work was supported in part by the following: National Institutes of Health (NIH) grants: R00 HL124323 (PI: B. Sharif) and R01 HL090957 (PI: N. Bairey Merz); American Heart Association predoctoral fellowship 15-PRE21590006; the Barbra Streisand Women’s Cardiovascular Research and Education Program, the Linda Joy Pollin Women’s Heart Health Program, and the Erika Glazer Women’s Heart Health Project, Cedars-Sinai Medical Center.
List of Abbreviations:
- INOCA
ischemia and no obstructive coronary artery disease
- CAD
coronary artery disease
- CMD
coronary microvascular dysfunction
- WISE
Women’s Ischemia Syndrome Evaluation
- MR
magnetic resonance
- MPRI
myocardial perfusion reserve index
- EF
ejection fraction.
Footnotes
Potential Conflicts of Interest: D. Berman and B. Sharif receive support from Astellas Pharma U.S. The authors report no other relationships that could be construed as a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics−−2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- 2.Gulati M, Cooper-DeHoff RM, McClure C, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Archives of internal medicine. 2009;169:843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camici PG and Crea F. Coronary microvascular dysfunction. The New England journal of medicine. 2007;356:830–840. [DOI] [PubMed] [Google Scholar]
- 4.Crea F, Camici PG and Bairey Merz CN. Coronary microvascular dysfunction: an update. European Heart Journal. 2014;35:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bairey Merz CN, Kelsey SF, Pepine CJ, et al. The Women’s Ischemia Syndrome Evaluation (WISE) Study: protocol design, methodology and feasibility report. Journal of the American College of Cardiology. 1999;33:1453–1461. [DOI] [PubMed] [Google Scholar]
- 6.Bairey Merz CN, Shaw LJ, Reis SE, et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. Journal of the American College of Cardiology. 2006;47:S21–9. [DOI] [PubMed] [Google Scholar]
- 7.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. Journal of the American College of Cardiology. 2010;55:2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakir M, Nelson MD, Jones E, et al. Heart failure hospitalization in women with signs and symptoms of ischemia: A report from the Women’s Ischemia Syndrome Evaluation study. International Journal of Cardiology. 2016;223:936–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ong P, Camici PG, Beltrame JF, et al. International standardization of diagnostic criteria for microvascular angina. International Journal of Cardiology. 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 10.Crea F, Bairey Merz CN, Beltrame JF, et al. The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. European heart journal. 2017;38:473–477. [DOI] [PubMed] [Google Scholar]
- 11.Puntmann VO, Voigt T, Chen Z, et al. Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovascular Imaging. 2013;6:475–484. [DOI] [PubMed] [Google Scholar]
- 12.Captur G, Manisty C and Moon JC. Cardiac MRI evaluation of myocardial disease. Heart. 2016;102:1429–1435. [DOI] [PubMed] [Google Scholar]
- 13.Mahmod M, Piechnik SK, Levelt E, et al. Adenosine stress native T1 mapping in severe aortic stenosis: evidence for a role of the intravascular compartment on myocardial T1 values. Journal of Cardiovascular Magnetic Resonance. 2014;16:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu A, Wijesurendra RS, Francis JM, et al. Adenosine stress and rest T1 mapping can differentiate between ischemic, infarcted, remote, and normal myocardium without the need for gadolinium contrast agents. JACC: Cardiovascular Imaging. 2016;9:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagel E and Puntmann VO. Is Myocardial Native T1 the One Answer for All? JACC: Cardiovascular Imaging. 2016;9:37–39. [DOI] [PubMed] [Google Scholar]
- 16.Khuddus MA, Pepine CJ, Handberg EM, et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE). Journal of interventional cardiology. 2010;23:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee B-K, Lim H-S, Fearon WF, et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei J, Mehta PK, Johnson BD, et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovascular Interventions. 2012;5:646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasdai D, Cannan CR, Mathew V, Holmes DR and Lerman A. Evaluation of patients with minimally obstructive coronary artery disease and angina. International Journal of Cardiology. 1996;53:203–208. [DOI] [PubMed] [Google Scholar]
- 20.Thomson LEJ, Wei J, Agarwal M, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation. Circulation Cardiovascular imaging. 2015;8:e002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson MD, Szczepaniak LS, Wei J, et al. Diastolic dysfunction in women with signs and symptoms of ischemia in the absence of obstructive coronary artery disease: a hypothesis-generating study. Circulation: Cardiovascular Imaging. 2014;7:510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camici PG, d’Amati G and Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nature Reviews Cardiology. 2014;12:48–62. [DOI] [PubMed] [Google Scholar]
- 23.Kellman P, Herzka DA and Hansen MS. Adiabatic inversion pulses for myocardial T1 mapping. Magnetic resonance in medicine. 2014;71:1428–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue H, Greiser A, Zuehlsdorff S, et al. Phase-sensitive inversion recovery for myocardial T1 mapping with motion correction and parametric fitting. Magnetic Resonance in Medicine. 2013;69:1408–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellman P, Wilson JR, Xue H, Ugander M and Arai AE. Extracellular volume fraction mapping in the myocardium, Part 1: evaluation of an automated method. Journal of Cardiovascular Magnetic Resonance. 2012; 14:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A, Beohar N, Arumana JM, et al. CMR Imaging of Edema in Myocardial Infarction Using Cine Balanced Steady-State Free Precession. JACC: Cardiovascular Imaging. 2011;4:1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD and Ugander M. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013; 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maceira A, Prasad S, Khan M and Pennell D. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. Journal of Cardiovascular Magnetic Resonance. 2006;8:417–426. [DOI] [PubMed] [Google Scholar]
- 29.Bairey Merz CN, Handberg EM, Shufelt CL, et al. A randomized, placebo-controlled trial of late Na current inhibition (Ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. European Heart Journal. 2016;37:1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO and Lerman A. Prevalence of Coronary Microvascular Dysfunction Among Patients With Chest Pain and Nonobstructive Coronary Artery Disease. JACC: Cardiovascular Interventions. 2015;8:1445–1453. [DOI] [PubMed] [Google Scholar]
- 31.Bairey MC, Pepine C, Walsh M and Fleg J. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation. 2017; 135:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartzkopff B, Motz W, Frenzel H, Vogt M, Knauer S and Strauer BE. Structural and functional alterations of the intramyocardial coronary arterioles in patients with arterial hypertension. Circulation. 1993; 88. [DOI] [PubMed] [Google Scholar]
- 33.Brush JEJ, Cannon ROI, Schenke WH, et al. Angina Due to Coronary Microvascular Disease in Hypertensive Patients without Left Ventricular Hypertrophy. New England Journal of Medicine. 1988;319:1302–1307. [DOI] [PubMed] [Google Scholar]
- 34.Lazdam M, Lewandowski AJ, Kylintireas I, et al. Impaired Endothelial Responses in Apparently Healthy Young People Associated With Subclinical Variation in Blood Pressure and Cardiovascular Phenotype. American Journal of Hypertension. 2012;25:46–53. [DOI] [PubMed] [Google Scholar]
- 35.Mygind ND, Michelsen MM, Pena A, et al. Coronary microvascular function and myocardial fibrosis in women with angina pectoris and no obstructive coronary artery disease: the iPOWER study. Journal of Cardiovascular Magnetic Resonance. 2016;18:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murthy VL, Naya M, Taqueti VR, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schindler TH, Schelbert HR, Quercioli A and Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovascular imaging. 2010;3:623–640. [DOI] [PubMed] [Google Scholar]
- 38.Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy. Radiology. 2015;276:228–232. [DOI] [PubMed] [Google Scholar]
- 39.Nelson MD, Sharif B, Shaw JL, et al. Myocardial tissue deformation is reduced in subjects with coronary microvascular dysfunction but not rescued by treatment with ranolazine. Clinical Cardiology. 2017;40:300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Treibel TA, Zemrak F, Sado DM, et al. Extracellular volume quantification in isolated hypertension - changes at the detectable limits? Journal of Cardiovascular Magnetic Resonance. 2015; 17:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piechnik SK, Neubauer S and Ferreira VM. State-of-the-art review: stress T1 mapping— technical considerations, pitfalls and emerging clinical applications. Magnetic Resonance Materials in Physics, Biology and Medicine. 2018;31:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Dijk R, van Assen M, Vliegenthart R, de Bock GH, van der Harst P and Oudkerk M. Diagnostic performance of semi-quantitative and quantitative stress CMR perfusion analysis: a meta-analysis. Journal of Cardiovascular Magnetic Resonance. 2017; 19:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doyle M, Weinberg N, Pohost GM, et al. Prognostic value of global MR myocardial perfusion imaging in women with suspected myocardial ischemia and no obstructive coronary disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovascular Imaging. 2010;3:1030–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salerno M, Sharif B, Arheden H, et al. Recent Advances in Cardiovascular Magnetic Resonance: Techniques and Applications. Circulation: Cardiovascular Imaging. 2017;10:e003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen D, Sharif B, Dharmakumar R, et al. Quantification of myocardial blood flow using non-ECG-triggered MR imaging. Magnetic Resonance in Medicine. 2015;74:765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiter U, Reiter G, Dorr K, Greiser A, Maderthaner R and Fuchsjager M. Normal diastolic and systolic myocardial T1 values at 1.5-T MR imaging: correlations and blood normalization. Radiology. 2014;271:365–372. [DOI] [PubMed] [Google Scholar]
- 47. Alam MH, Auger D, Smith GC, et al. T1 at 1.5T and 3T compared with conventional T2* at 1.5T for cardiac siderosis. Journal of Cardiovascular Magnetic Resonance. 2015;17:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kellman P, Wilson JR, Xue H, et al. Extracellular Volume Fraction Mapping in the Myocardium, Part 2: Initial Clinical Experience. Journal of Cardiovascular Magnetic Resonance. 2012;14:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogers T, Dabir D, Mahmoud I, et al. Standardization of T1 measurements with MOLLI in differentiation between health and disease--the ConSept study. Journal of Cardiovascular Magnetic Resonance. 2013;15:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1: Representative contours for native myocardial T1 maps. The corresponding epicardial and endocardial contours (divided into 6 myocardial segments) are shown. Native T1 values were measured using conservative endocardial and epicardial segmentation, taking care to exclude partial volume effects from the blood-pool and surrounding tissue/fat.


