Abstract
The endoplasmic reticulum (ER) produces the vast majority of all proteins secreted into the extracellular space, including hormones and cytokines, as well as cell surface receptors and other proteins which interact with the environment. Accordingly, this organelle controls essentially all vital links to a cell’s external milieu, responding to systemic metabolic, inflammatory, endocrine, and mechanical stimuli. The central role the ER plays in meeting protein synthetic and quality control requirements in the face of such demands is matched by an extensive and versatile ER stress response signaling network. ROS mediate several critical aspects of this response. Nox4, an ER resident capable of producing ROS, acts as a proximal signaling intermediate to transduce ER stress-related conditions to the unfolded protein response, a homeostatic corrective mechanism. However, chronic ER stress caused by unrelenting internal or external demands produces a secondary rise in ROS, generally resulting in cell death. Sorting out the involvement of ROS at different levels of the ER stress response in specific cell types is key to understanding the molecular basis for chronic diseases such as atherosclerosis, hypertension, and diabetes. Here, we provide an overview of ER stress signaling with an emphasis on the role of ROS.
Keywords: Oxidative stress, Nox4, autophagy, Ras, atherosclerosis, hypertension
Introduction
Even at repose, the human body is constantly subjected to a variety of biochemical and biomechanical stresses. Thus, a complex and interlocked series of cellular response mechanisms has evolved to cope with the daily stresses of life. Not surprisingly, the molecular basis for a number of diseases such as atherosclerosis, diabetes, and neurodegeneration can be traced to the failure of these response pathways to rectify such stresses. Interestingly, the ER, best known as a protein synthesis organelle, is now recognized as a principal organizer of cellular stress response pathways, and is consequently the focus of much work in these chronic diseases. This functional arrangement in part stems from the sensitivity of de novo protein translation, folding, and post-translational modifications to optimal intracellular conditions; therefore, the ER reacts to any perturbations which compromise the integrity of this process. It has also become clear that the different arms of the ER stress pathways can differentially respond to specific cellular stressors independent of protein misfolding, increasing the biological versatility of ER-based pathways in stress response.
A unique characteristic of the ER is its luminal redox tension. Since the vast majority of proteins translated within the ER lumen are destined for secretion or to span the plasma membrane and extend into the extracellular space, the ER must post-translationally modify proteins to function outside of the cell. Thus, the ER interior mimics the relatively oxidized extracellular environment and promotes crosslinking of sulfhydryl groups to form disulfides that would not survive the reduced cytosol. Accurate titration and strict compartmentalization of the oxidized intraluminal environment renders the ER sensitive to perturbations in overall cellular redox status, so the ER responds rapidly to both energetic and redox stresses. Perhaps related to this, the production of ROS has become an essential part of the ER stress response. While seemingly counterproductive, such spatiotemporally-directed ROS production is now recognized as a specific signal transduction mechanism for many cellular functions. This response raises several key questions such as how do we, and in fact, how does the ER, distinguish such ROS signaling from oxidative stress, and how does it distinguish each of these from normal oxidation reactions within the ER? More importantly, which ER mechanisms go awry in cardiovascular and metabolic diseases, and how do ROS affect disease progression?
The unfolded protein response
ER stress is generally defined as the accumulation of misfolded client proteins within the ER lumen, which itself is difficult to measure and quantify. ER stress is therefore most commonly inferred by activation of the unfolded protein response (UPR). At the outset, it should be recognized that using the UPR as a surrogate measure of ER stress has significant limitations, especially when various arms of the UPR are asymmetrically activated or disabled. In addition, interventions which attenuate the UPR do not necessarily reduce ER stress, and specific pathways associated with ER stress can be coopted by other stress-related stimuli. Thus, different arms of the UPR may move in parallel to or independent of ER stress itself. However, a basic understanding of the UPR is required to study ER stress and its role in human disease.
Under normal conditions, the intraluminal domains of the three canonical UPR sensors PERK (PKR-like eukaryotic initiating factor α kinase), ATF6 (activating transcription factor-6), and IRE1 (inositol requiring enzyme 1) are capped by the chaperone BiP/GRP78 and rendered inactive. Only properly folded proteins are allowed to enter ER exit vesicles and leave the ER; consequently, when excessive synthetic demands or suboptimal processing conditions prevail, misfolded client proteins accumulate in the ER and titrate BiP away from these sensors, signaling ER stress. The dissociation of BiP allows oligomerization of PERK and IRE1 and translocation of ATF6 to the Golgi, collectively initiating the classical tripartite UPR (Figure 1). Mammals express ten known ER stress sensors: IRE1α, IRE1β, PERK, ATF6α, ATF6β, and five other ATF6-like ER membrane-bound transcription factor proproteins (OASIS, BBF2H7, Luman, CREBH, and Tisp40). The large number of stress sensors reflects the evolutionary importance of ER stress and complexity of the cellular response.
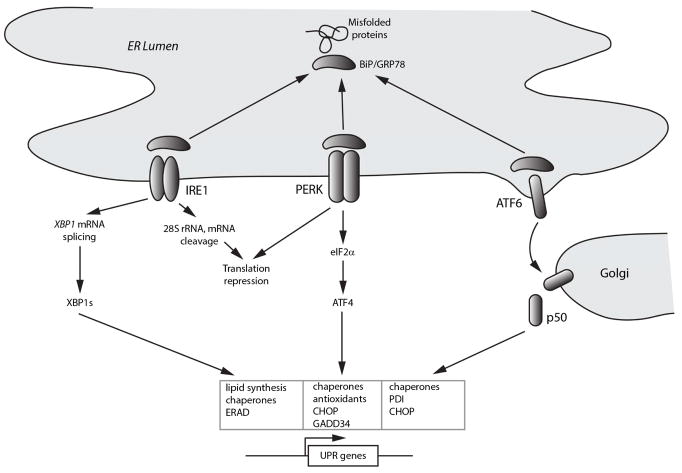
Figure 1. Overview of the unfolded protein response.
Schematic shows titration of the chaperone BiP/Grp78 from the three main ER sensors IRE1 (inositol requiring enzyme 1), PERK (PKR-like eukaryotic initiating factor α kinase), and ATF6 (activating transcription factor 6) by misfolded proteins. The IRE1 pathway, through production of the alternatively translated product XBP1s, induces genes involved in lipid biosynthesis, ER-associated protein degradation (ERAD), and chaperones, to promote ER biogenesis, protein refolding, and disposal of damaged proteins. The PERK pathway, largely through induction of the transcription factor ATF4, additionally induces antioxidant-related genes and the phosphatase cofactor GADD34, which dephosphorylates eIF2α and deactivates the unfolded protein response (UPR). Release of ATF6 from BiP exposes Golgi localization signals, and ATF6 subsequently undergoes regulated intramembrane proteolysis to release a 50 kDa active transcription factor that targets genes involved in ER biogenesis and protein refolding.
The oldest branch of the UPR is IRE1, a serine/threonine kinase conserved from yeast to humans. Upon release of BiP, IRE1 homodimerizes and transphosphorylates, revealing its C-terminal endoribonuclease domain. Yeast Ire1 appears to be capable of directly binding misfolded proteins as well as BiP (Kimata et al. 2004), and its autophosphorylation is dispensable for mRNA splicing but may delay signal termination (Chawla et al. 2011). The IRE1 endoribonuclease domain specifically recognizes and cleaves a 26-base fragment from the XBP1 mRNA transcript (HAC1 in yeast), resulting in an altered translational product, XBP1s. XBP1s in turn acts as a transcription factor which recognizes ER stress-response elements in the promoter regions of genes encoding chaperones such as BiP and proteins involved in ER biogenesis, secretory function, and the degradation of damaged proteins. The latter function primarily entails the exportation of misfolded proteins from the ER into the cytosol, recognition and ubiquitination of these proteins, and proteasomal degradation, in total the process of ERAD (ER-associated protein degradation). IRE1 is thought to cleave additional mRNAs targeted to the ER as well as the 28S ribosomal subunit, thus decreasing global protein translation (Iwawaki et al. 2001, Hollien and Weissman 2006). Interestingly, TRAF2 is also recruited to IRE1 through JIK (JNK inhibitory kinase), triggering activation of the ASK1/JNK pathway. The highly conserved IRE1 sensor thus links ER stress with other stress response pathways, a recurrent paradigm in ER biology.
Like IRE1, PERK is an ER resident type I transmembrane serine/threonine kinase that homodimerizes and autophosphorylates upon release of intraluminal BiP. Phosphorylation of its key substrate, the translation initiation factor eIF2α, converts it into a competitor of eIF2B which broadly suppresses translation to alleviate protein folding and processing demands within the ER. Simultaneously, eIF2α phosphorylation also selectively increases translation of the transcription factor ATF4, which transactivates amino acid transporter and redox control genes (Harding et al. 2000). Because of its latter activity, the PERK pathway is most closely tied to oxidative stress and signaling, as discussed below. ATF4 also upregulates GADD34, a stress response phosphatase cofactor that promotes dephosphorylation eIF2α and translational recovery (Figure 1).
ATF6 represents the third family of ER stress sensors. While mammals express seven ATF6 paralogs, ATF6α and ATF6β are best studied. Dissociation of BiP from ATF6 exposes two Golgi localization signals, causing ATF6 translocation to the Golgi. Notably, reduction of disulfides on the luminal surface of ATF6, normally held in an oxidatively cross-linked state, is also required for Golgi translocation (Nadanaka et al. 2007). Thus ATF6 can directly respond to abnormally reducing conditions within the ER, which is thought to be a unifying biochemical feature of ER stress (Merksamer et al. 2008). Following Golgi translocation, ATF6 is cleaved by regulated intramembrane proteolysis (RIP) by the Golgi resident proteases S1P and S2P to release its 50 kDa cytosolic domain as a functioning bZIP transcription factor. The targets of this factor include genes which promote ER maintenance, protein folding, and post-translational modification such as the chaperones BiP and Grp94, and cysteine cross-linker PDI (protein disulfide-isomerase) (Figure 1). Of the three pathways, ATF6 target genes are perhaps the most focused on maintenance of ER homeostasis.
Intersection of the UPR with other stress pathways
An important concept uncovered in recent years has been the appreciation that different sensors and effector pathways of the UPR respond differentially to a variety of perturbations and cellular stresses which do not directly compromise protein quality; thus, the constituents of the “unfolded protein response” are not necessarily restricted to responding to unfolded proteins. The fact that other stresses coopt UPR pathways generalizes the importance of ER signaling and establishes the ER as an important organelle that integrates a number of other cellular stress responses. The apparent complexity and redundancy of mammalian UPR pathways may in fact reflect the evolution of an efficient and versatile stress response system. Notwithstanding, the activation of certain UPR proteins cannot always be taken as evidence of ER stress, an important concept to consider when studying ER stress signaling.
An example of a highly specified function for ER signaling occurs during the development of sheath cells in Medaka fish, which produce collagen II. As opposed to type VIII collagen, which has recurring flexible hinge regions, collagen II is assembled in the ER as a rigid 300–400 nm rod unable to fit into the standard 60–80 nm COPII vesicle. Specific activation of the ER sensors ATF6 and its paralog BBF2H7 induces a complete set of genes required to enlarge COPII vesicles and permit its exportation (Ishikawa et al. 2017). In this case, a subset of the UPR is employed to handle a large but native, properly folded protein.
A more general example of the versatility of UPR pathways is seen in the various roles of eIF2α in different cellular stresses (Figure 2). Four kinases can phosphorylate eIF2α at Ser51, only one of which, PERK, is an ER resident responding to unfolded proteins, while the other three kinases primarily respond to environmental stresses. PKR (protein kinase R) is activated by double-stranded RNA and therefore has a prominent role in innate immunity. Notably, by activating eIF2α, PKR inhibits viral replication by suppressing global mRNA translation, and in addition activates inflammatory signaling (Williams 2001). Thus, the cellular objectives to rectify ER stress and viral infection overlap extensively and in fact are triggered through the common use of eIF2α. Similarly, GCN2 (general control non-derepressible 2) is an eIF2α kinase that responds to amino acid deprivation, and thus uses eIF2α to limit protein synthesis and adapt to a nutrient-deficient environment (Dalet et al. 2015). Finally, HRI (heme-regulated inhibitor) is a kinase activated under heme-deficient conditions, and limits protein synthesis such as globin in red cells, through eIF2α. Its function is more broad as reflected in yeast, which respond to environmental stresses such as heat shock and arsenic through HRI/eIF2α to induce chaperone production (Zhan et al. 2002). Together, these four kinases mediate an “integrated stress response” through the common use of eIF2α (Harding et al. 2003), responding to stresses which may or may not include misfolded proteins. Of relevance to this discussion, the integrated stress response is also activated in the presence of oxidative stress, as discussed below (Figure 2).
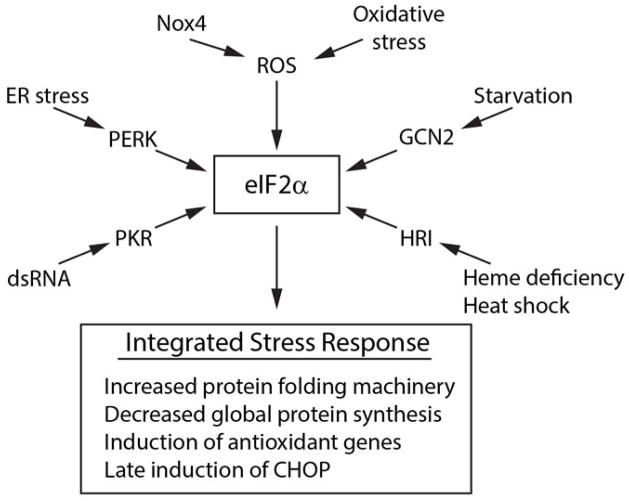
Figure 2. The integrated stress response.
Schematic shows the four main eIF2α kinases PERK, GCN2 (general control non-derepressible 2), PKR, and HRI (heme-regulated inhibitor) which respond to different stimuli to activate events downstream of eIF2α. eIF2α also responds to ROS either through Nox4 in response to ER stress, or to oxidative stress through undefined mechanisms.
In part because the different arms of the UPR serve different cellular functions, its downstream pathways are also intricately linked to broad processes such as inflammation. ER stress is known to increase the synthesis and release of inflammatory cytokines, in large part through the activation of NF-κB and JNK. The principal ER sensor responsible for inflammatory signaling appears to be IRE1α, which assembles a JIK/TRAF2 complex on its cytosolic face to recruit and activate JNK and IKKβ (Urano et al. 2000, Hu et al. 2006). IRE1α also is required for TLR2 and TLR4 signaling in macrophages. In these cells, deletion of XBP1 blocks the production of specific cytokines, including IL-6, TNFα and IFNβ, induced by receptor activation or infection with M. tuberculosis, L. monocytogenes, or F. tularensis (Martinon et al. 2010). Additionally, the PERK-eIF2α arm may contribute to NF-κB activation through noncanonical IκB degradation (Deng et al. 2004). This pathway also serves to activate NF-κB in response to non-ER stressors. Amino acid starvation of MEFs, for instance, initiates phosphorylation of eIF2α through GCN2, causing release of IκB and activation of an NF-κB-dependent survival program (Jiang et al. 2003). Additionally, exposure of macrophages to double stranded RNA or infection with E. coli or S. typhimurium activates PKR, which directly associates with several inflammasomes including the NLR family pyrin domain-containing 3 (NLRP3) inflammasome to express and release HMGB1, IL-1β, and IL-18 (Lu et al. 2012). Finally, the ATF6-like ER sensor CREBH, expressed in hepatocytes, responds to inflammatory signals by releasing its transcriptionally active cytosolic domain to upregulate C-reactive protein and other acute phase proteins (Zhang et al. 2006). Thus, all three arms of the UPR mediate inflammatory signaling to some extent.
ROS signaling versus oxidative stress in the ER
The overlap between physiologic protein sulfhydryl oxidation, ER stress, inflammation, and oxidative stress can make the involvement of ROS as ER-specific signaling agents difficult to recognize. In part, this difficulty stems from the fact that oxidative stress itself is poorly defined in biochemical terms. The presence or detection of ROS or oxidized byproducts does not necessarily imply oxidative stress, as these chemicals can be detected in normal states and can increase in response to other stresses. A more useful working definition of stress may be a force that imposes a biochemical or biophysical perturbation which requires homeostatic correction to preserve physiologic function. Consequently, all vital functions of the cell such as energy management, chromatin integrity, protein synthesis, and osmotic regulation are governed by signaling pathways which respond to stress-induced perturbations by rectifying deviations from the normal state. ROS produced as part of such a response to perturbations, acting primarily to restore homeostasis, can be considered part of cellular stress signaling.
As we discuss in the context of specific diseases, however, a recurring theme in the study of stress response biology is that the onset of cellular stress starts a poorly understood timer of sorts (Figure 3). Persistent, inescapable stress heralds a defective or rogue cell and triggers a delayed apoptotic response to protect the organism at the cell’s expense, although this often ultimately worsens disease. Genomic damage, for instance, induces p53-dependent cell cycle arrest to permit DNA repair; however, prolonged damage leads to p53-dependent apoptosis, shifting p53 from homeostatic stress responder to executioner. In the ER, mutant rhodopsin in retinal epithelium or unrelenting demands for insulin in pancreatic β cells lead to unmitigated ER stress and cell death in retinitis pigmentosa and type II diabetes, respectively (Walter and Ron 2011). Thus, mechanisms that evolved to serve adaptive purposes, including the activation of specific ROS signaling pathways, can sometimes be shown to facilitate downstream cell death and organ dysfunction in the face of chronic ER stress, leading to disease states. The timing, location, and biochemical consequences of early ROS-dependent signaling and late ROS-dependent execution should therefore be distinguished.
Figure 3. Dual role of ROS in ER stress signaling.
The left side of the panel depicts homeostatic signaling which involves ROS as signaling intermediates that report ER stress to the UPR, which in turn mitigates ER stress. Nox4 is shown as an important source of ROS in this capacity. In the event that ER stress is not relieved over time (depicted by the stop watch), delayed expression of proteins such as CHOP initiate a secondary rise in ROS (right side of the panel). This secondary increase in ROS appears to arise in part from induction of ER oxidase 1 (ERO1) and in part from calcium transfer across specialized ER-mitochondria contact sites with release of ROS, together contributing to cell death.
More specifically, a number of studies indicate that in response to ER stress, ROS are also produced downstream of and as a consequence of the UPR, leading to cell death (Figure 3). This secondary increase in ROS is conserved in yeast, worms, and mammals (Harding et al. 2003, Haynes et al. 2004, Marciniak et al. 2004). Deletion of erv29, a gene required for ERAD, causes persistent ER stress and ROS-dependent cell death in S. cerevisiae, whereas suppression of the UPR by co-deletion of erv29 and ire1 or hac1 (the yeast XBP1 gene) blocks both ROS accumulation and cell death despite ongoing ER stress (Haynes et al. 2004). Mammalian cells respond to ER stress induced by tunicamycin, thapsigargin, or amino acid starvation by activation of the PERK/eIF2α pathway, not present in yeast, which mitigates subsequent oxidative stress through upregulation of antioxidant genes. Of note, deletion of Perk or Atf4 in mouse embryonic fibroblasts leads to a sustained induction of ROS following treatment with ER stressors, leading to cell death (Harding et al. 2003). The PERK/eIF2α arm of the UPR may therefore have evolved in part to anticipate and mitigate this late oxidant increase.
The enzymatic source of this secondary rise in ROS is unclear, though most studies implicate either the ER itself or mitochondria. ERO1 (ER oxidase 1) is induced following persistent ER stress and has been implicated in the generation of ROS within the ER. ERO1 is a flavoprotein that uses O2 as an electron acceptor to oxidize PDI (protein disulfide isomerase), a thioredoxin-like intermediary that shuttles electrons from reduced protein sulfhydryls to ERO1. The yeast ortholog Ero1p contains seven conserved cysteine residues which, along with its flavin cofactor, are thought to participate in electron transfer by acting as a capacitor (Tu and Weissman 2004). Each FAD-bound Ero1p molecule can therefore support multiple rounds of PDI oxidation, permitting either two or four-electron reduction of O2 to either H2O2 or H2O, respectively. Purified Ero1p, in the presence of O2 and reduced bacterial thioredoxin, produces near stoichiometric amounts of H2O2 in vitro, demonstrating its ability to generate ROS under specific reducing conditions (Gross et al. 2006). However, in a more physiologic reaction, purified Ero1p coupled with PDI caused oxidation of target RNaseA sulfhydryl groups that produced only ~1 molecule of H2O2 per 20 disulfides formed, indicating its ability to perform oxidative reactions without quantitative H2O2 production (Tu and Weissman 2002).
To monitor actual H2O2 levels within intact ER in live cells, the ratiometric fluorochrome HyPer has been used. This probe contains a redox active sulfhydryl pair buried within a narrow cleft accessible only to H2O2 (Belousov et al. 2006). Surprisingly, HyPer targeted to different subcellular compartments of HeLa cells revealed H2O2 levels highest in the ER, with fluorescence ratios twice that seen in mitochondria (Enyedi et al. 2010). Knockdown of ERO1-Lα (one of two human isoforms) or expression of a dominant negative ERO1-C394S significantly reduced ER luminal HyPer ratios, indicating a high basal level of H2O2 within the ER of HeLa cells. In contrast, primary human endothelial cells report low HyPer ratios in the ER lumen, only modestly higher than that of the cytoplasm and significantly lower than mitochondria under unstressed conditions (Wu et al. 2010). The reason for this difference in measured ER H2O2 levels in these two studies is unclear, but may relate to a difference between normal and transformed cancer cells such as HeLa, as the latter possess constitutively deregulated metabolic and proliferative requirements that chronically stress the ER.
Mitochondria represent an alternative source for ROS during prolonged ER stress, in large part due to altered calcium homeostasis. Release of calcium from ER stores occurs during ER stress, which is thought to be efficiently taken up by mitochondria at focal points of contact between the two organelles. As much as 20% of the mitochondrial surface is in direct contact with the ER through specific protein tethers, and release of calcium from the stressed ER can result in focal intramitochondrial calcium spikes of 10 μM or more by uptake of calcium through mitochondrial outer membrane microdomains enriched with VDAC1 (voltage-dependent anion selective channel protein 1) and across the inner membrane through MCU (mitochondrial calcium uniporter) (de Brito and Scorrano 2010). Resultant mitochondrial depolarization disrupts electron transport function and increases ROS (Figure 3).
In the stressed ER, available data suggest that both the ER and mitochondria may contribute to a rise in ROS levels. Late in ER stress, the transcription factor CHOP is induced, leading to increased expression of ERO1a. However, knockdown of ERO1α or expression of interfering alleles in mammalian cells does not alter ER redox conditions during ER stress (Marciniak et al. 2004). Further, ρ0 fibroblasts deficient in mitochondrial respiration show decreased ROS production and cell death in response to tunicamycin, implicating mitochondria as a significant source of ROS (Cullinan and Diehl 2004). In contrast, knockdown of the sole C. elegans Ero1 gene improves late survival and decreases ROS production stimulated by tunicamycin (Harding et al. 2003, Marciniak et al. 2004). ROS production in this model is significantly reduced by knockdown of ero-1 as well as interference with mitochondrial respiration (Harding et al. 2003). Overexpression of Ero1p in yeast causes increases in ROS levels, but mitochondria-defective ρ0 cells have decreased ROS under conditions of ER stress caused by the expression of mutant misfolded proteins, again suggesting dual sources for ROS late in ER stress (Haynes et al. 2004)(Figure 3).
Putting these studies together, it seems probable that in normal cells under unstressed conditions, ER intraluminal redox status is kept in an oxidized state through PDI-dependent sulfhydryl oxidation with low basal H2O2 generation. Under these conditions, physiologic protein folding and disulfide formation would be driven largely by oxidized PDI and not by the less specific actions of H2O2. Indeed, the addition of catalase in vitro to the Ero1p-PDI redox pair has no effect on target protein sulfhydryl oxidation (Tu and Weissman 2002). In contrast, a pathologically stressed ER features a hyper-reduced environment with high, induced levels of ERO1, potentially shifting redox coupling from four to two-electron reduction of O2 by ERO1 to create significant amounts of H2O2 instead of H2O. While as yet unproven, this scenario would explain divergent results seen in normal versus pathologic conditions.
The converse scenario in which oxidative stress commonly causes ER stress (as opposed to UPR activation) is widely cited but less well supported. A detailed study of ER stress employed eroGFP, an ER-targeted mutant GFP with a highly exposed cysteine pair, to monitor oxidation state within the ER lumen of yeast (Merksamer et al. 2008). This probe does not report ROS, but rather the redox environment as reflected in reduced-to-oxidized sulfhydryl ratios. Under unstressed conditions, eroGFP was completely oxidized, reflecting the highly oxidized ER interior. As expected, the sulfhydryl reducing agent DTT (dithiothreitol), known to induce ER stress through disruption of cysteine cross links, caused reduction of the ER interior and activation of the UPR. Surprisingly, the glycosylation inhibitor tunicamycin, also known to induce severe ER stress but not directly through redox alterations, also caused a reduced ER interior in association with UPR activation. Milder, physiologic ER stressors such as inositol deprivation and forced expression of mutant secretory proteins caused UPR activation with preserved ER oxidation states; however, genetic disabling of the UPR unmasked ER hyper-reduction in response to mutant misfolded proteins, reflecting the role of the UPR in maintaining an oxidized ER interior (Merksamer et al. 2008). Importantly, diverse ER stressors therefore result in a reduced rather than oxidized ER environment. While the yeast UPR is restricted to the IRE1 pathway, mammalian ATF6 requires reduction of its lumen-facing cysteines for Golgi transport and proteolytic activation (Nadanaka et al. 2007), consistent with reductive stress as a final common pathway for ER stress.
Interestingly, inositol deprivation of ire1-deleted yeast reveals two distinct subpopulations of yeast based on ER redox state. Yeast continue to divide at a low rate despite starvation, and lineage tracing demonstrates that mother cells display a strongly reduced ER lumen in response to ER stress, whereas daughter cells are able to maintain an oxidized ER potential, reflecting a loss of compensatory mechanisms with age (Merksamer et al. 2008). Similarly, the worm C. elegans, when forced to express various aggregation-prone proteins, develops a reduced ER interior and activates the UPR (Kirstein et al. 2015); however, even in the absence of this proteotoxic stress, as worms age and naturally accumulate protein aggregates, the ER becomes more reduced while the cytosol becomes more oxidized. These findings reflect the loss of ability to maintain strict redox compartmentalization within the aging cell (Kirstein et al. 2015). Together, these studies suggest a complex relationship between ER and redox homeostasis, but consistently link ER intraluminal reductive rather than oxidative stress with the early phases of ER stress.
While oxidative stress may not commonly act upstream of ER stress, it clearly can activate parts of the UPR. Of the three canonical branches, the PERK/eIF2α pathway is most strongly linked to oxidative stress. Exogenous H2O2 or organic oxidants such as t-butyl hydroperoxide, menadione, or diamide cause phosphorylation of eIF2α, inducing ATF4 and the integrated stress response (Brostrom et al. 1996, Liu et al. 2008). Interestingly, it is not clear whether PERK or any other upstream kinases are involved in this phosphorylation event. Downstream targets of PERK/eIF2α and ATF4 include antioxidant-related genes, and PERK activation also leads to dissociation of the antioxidant transcription factor Nrf2 from its inhibitor Keap1, increasing cytosolic glutathione levels (Cullinan and Diehl 2004). Tunicamycin treatment of Perk−/− MEFs causes an increase in ROS after 12 h of ER stress compared to wild type cells treated with tunicamycin (Harding et al. 2003). Thus, the eIF2α pathway, mediating the integrated stress response, anticipates UPR-dependent late oxidative stress in the context of ER stress, but is also co-opted for the induction of antioxidant genes in order to respond to a primary oxidative attack from exogenous sources (Figure 2). Overall, these studies indicate that ROS clearly lie upstream of specific parts of the UPR but not necessarily upstream of ER stress. This scenario would suggest that ROS may also act in a physiologic capacity between ER stress and the UPR, signaling at an early stage to rectify ER dysfunction by activating the UPR (Figure 3). What specific mechanisms are involved in this signaling pathway?
Nox4 and ER signaling
Clear evidence exists for the regulated production of ROS as part of the ER stress response. This effect is best seen with Nox4, an ER resident and member of the NADPH oxidase family. Like other Nox family members, Nox4 specifically produces ROS as its major enzymatic product, reflecting its adaptive function as an ROS signaling protein. The human Nox proteins are part of a highly conserved family of NADPH oxidases, thought to mediate the response to environmental stresses such as nutrient deprivation in some of the simplest organisms such as filamentous fungi and the social amoeba Dictyostelium (Terada 2006). The earliest Nox protein, Yno1p (yeast NADPH oxidase 1p) was recently identified in the unicellular eukaryote S. cerevisiae (Rinnerthaler et al. 2012). Interestingly, Yno1p is found exclusively associated with the ER, where it responds to mitochondrial respiratory dysfunction (Leadsham et al. 2013). Thus, Nox-dependent ROS signaling may have its evolutionary origins responding to stress from its location on the ER.
Mammalian models of Nox4 deletion have been well studied, and all mouse models show normal development and physiology with no known phenotype unless the animal is stressed (Zhang et al. 2010, Schroder et al. 2012, Schurmann et al. 2015, Gray et al. 2016). In addition, the majority of studies indicate a deterioration of function with loss of Nox4, consistent with its primary role in homeostatic stress response. Several studies have shed light on the mechanism of Nox4’s involvement in ER signaling. In vascular endothelial cells, both tagged and endogenous Nox4 appear to be largely associated with the ER using biochemical fractionation, live cell imaging, and immunoelectron microscopy (Chen et al. 2008, Wu et al. 2010). Nox4-derived ROS oxidatively inactivates another ER resident, the tyrosine phosphatase PTP1B, which is thought to be a late regulator of EGFR signaling following receptor endocytosis and delivery to a dephosphorylation site within the ER (Chen et al. 2008). Using isoform specific knockdowns, Nox4 but not Nox2 was shown to prolong EGFR-dependent ERK signaling through PTP1B inactivation (Chen et al. 2008).
By monitoring HyPer targeted to the ER, Nox4 has been shown to mediate ER H2O2 production in response to tunicamycin and the ER stressor HIV1 Tat, but not to thapsigargin or DTT, reflecting an interesting ability to distinguish between different ER stressors (Wu et al. 2010). Here, ER-directed HyPer clearly shows elevations in H2O2 restricted to the ER lumen, at times displaying focal increases in ROS at the ER endomembrane where Nox4 resides (Figure 4). Again, selective knockdown studies implicate Nox4 but not Nox2 in the ER stress response, with Nox2 instead accumulating on plasma membrane and endosomal sites (Wu et al. 2010). Nox4 is required for phosphorylation of eIF2α as well as IRE1-dependent XBP1 splicing, thus initiating at least two of the three arms of the UPR (Wu et al. 2010). In cardiac cells, tunicamycin-induced ER stress increases Nox4, which associates with GADD34 and its cofactor, the serine/threonine phosphatase PP1 (Santos et al. 2016). Nox4-derived H2O2 oxidizes the catalytic metal center and inactivates PP1, promoting eIF2α phosphorylation. While this study highlights Nox4’s ability to participate in specific ER stress-dependent signaling, it also suggests a potential mechanism for sensing generalized oxidative stress through direct actions of ROS on eIF2α phosphorylation that then activates the integrated stress response (Figure 2). This mechanism may also account for the ability of Nox4 to selectively activate the eIF2α pathway in response to glucose deprivation in vitro or myocardial ischemia in vivo (Sciarretta et al. 2013, Santos et al. 2016).
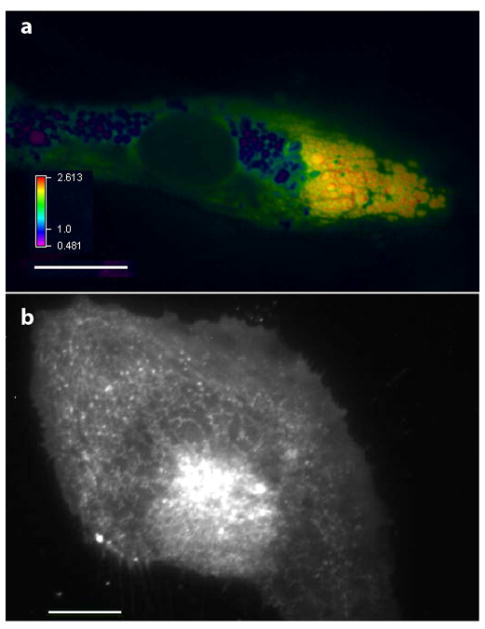
Figure 4. ROS in the stressed ER.
a. Human umbilical vein endothelial cell expressing the H2O2 sensor HyPer targeted to the ER, after treatment with tunicamycin for 16 h. This photomicrograph shows a ratiometric image of HyPer demonstrating H2O2 elevations within swollen, dysmorphic ER vesicles with focal H2O2 accumulations particularly at the periphery of ER endomembranes. b. Nox4-GFP in live unfixed cell localizes to the reticular ER. Scale bars are 20 μm.
The effect of Nox4 on ER signaling is consistent with most subcellular fractionation, immunofluorescence, immunoelectron microscopy, and tagged fusion protein studies, which localize this oxidase on the ER endomembrane. It should be noted, however, that Nox4 has also been detected in mitochondria and the nucleus of endothelial, vascular smooth muscle, and cardiomyocyte cells (Kuroda et al. 2005, Matsushima et al. 2013). Interestingly, immunoreactive nuclear-localized Nox4 was noted to represent a 28 kDa splice variant, Nox4D, which lacks transmembrane domains (Anilkumar et al. 2013). Overexpression of Nox4D causes DNA damage, although its physiologic effects remain unknown.
Unlike Nox2, Nox4 does not require Rac1 or Rac2 as part of its active complex. However, the Rho and Ras GTPase families are nonetheless intimately involved in Nox4 signaling. In response to HIV1 Tat, RhoA is activated upstream of Nox4 (Wu et al. 2007). RhoA is activated on the ER surface and mediates, through unclear mechanisms, local Nox4 activation (Wu et al. 2010). Notably, the worm Nox ortholog BLI-3 also requires Rho1/RhoA for its activation, indicating a conserved functional association of Nox proteins with Rho (Ewald et al. 2017). Downstream of Nox4, K-Ras is activated focally on the ER and mediates broad activation of the UPR through ERK (Wu et al. 2010). ER stress-induced Ras activation involves oxidative inactivation of the ER calcium transporter SERCA by Nox4-dependent H2O2, which increases cytoplasmic calcium and triggers calcium-calmodulin activation of RasGRF1 and RasGRF2 (Wu et al. 2017). These ER resident proteins specifically act as Ras guanyl exchange factors, prompting GTP loading and thus activation of Ras focally on the ER (Wu et al. 2017). Therefore, Nox4 is central to a signaling circuit localized to the ER cytoplasmic surface which integrates RhoA, Ras, ROS, and calcium signaling in the ER stress response (Figure 5).
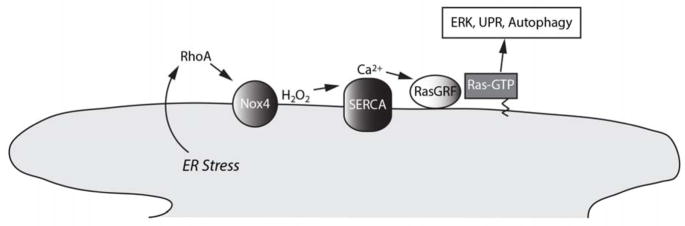
Figure 5. Nox4 signals ER stress.
Schematic showing the role of Nox4 in relaying a local ER stress signaling pathway downstream of RhoA. Nox4-derived H2O2 oxidizes sulfhydryls of the sarcoendoplasmic reticulum calcium ATPase (SERCA), leading to an increase in cytosolic calcium, activation of the calcium sensitive ER guanyl exchange factor RasGRF, and GTP loading of Ras on-site on the ER surface. ER-localized Ras leads to homeostatic correction through the UPR, ERK activation, and autophagy.
In summary, Nox4 is an important signaling oxidase which is part of the ER stress detection and response system. Subsequent activation of the UPR initiates corrective mechanisms but also, in the face of prolonged and intractable ER stress, apoptotic pathways which also prominently feature ROS production, likely generated by mitochondria and/or ERO1. With this in mind, we examine the interplay between ROS and ER stress in specific disease states.
Atherosclerosis
Abundant data link different pathways of the UPR with atherosclerosis, supporting a role for ER stress in this disease (Table 1). In part, this may stem from the ability of the ER to control many key lipogenic pathways. XBP1s, for example, has a role in phosphatidylcholine synthesis and membrane expansion. Given the immense surface area of the ER endomembrane, derangements in lipid metabolism or composition may impose a significant stress on ER biogenesis or function. An important target cell for ER stress in this regard is the macrophage, which is recruited to assist in the removal of excessive cholesterol from vessel walls. BiP and CHOP expression is increased in thin-cap atheromas from human coronary arteries, and BiP and CHOP induction and PERK phosphorylation occur in macrophages in the early fatty streak lesions of Apoe−/− mice (Zhou et al. 2005, Myoishi et al. 2007). Cholesterol transport into the ER membrane by the lipid chaperone aP2 triggers the UPR and CHOP-dependent activation of inflammatory pathways (Makowski et al. 2001, Li et al. 2005, Furuhashi et al. 2007, Thorp et al. 2009). Notably, Nox4 has not been shown to signal ER stress in macrophages, which express low levels of this isoform; in contrast, macrophages express high levels of Nox2, which promotes atherogenic rather than protective events, emphasizing the diverse signaling roles Nox-dependent ROS serve (Li et al. 2010).
Table 1.
Summary of ER stress and Nox4 involvement in cardiovascular disease
| Summary of Evidence | |
|---|---|
| Atherosclerosis and Ischemic Heart Disease | |
| Involvement of ER stress | UPR markers increased in human atheromas, ApoE−/− mice, pig models, shear stressed endothelium; UPR is activated in ischemia reperfusion, ATF6 is protective; CHOP induction after 50 min of ischemia increases myocardial injury |
| Protective role of Nox4 | Nox4 deletion and DN-Nox4 increases atherogenesis in mouse models; Endothelial-specific Nox4 overexpression protects ApoE−/− mice; Nox4 mediates protective autophagy in VSMC treated with oxidized 7-ketocholesterol; Myocardial Nox4 deletion increases infarct size after no-reflow ischemia |
| Hypertension | |
| Involvement of ER stress | UPR activation in arteries, myocardium, and SFO by AT-II infusion; Normalization of systemic and pulmonary BP by TUDCA and PBA; TUDCA or BiP transduction in SFO blocks hypertensive response to AT-II |
| Protection by endothelial Nox4 | Endothelium-specific Nox4 overexpression decreases AT-II hypertension, increases endothelium-dependent vasorelaxation |
| Worsening by VSMC Nox4 | Nox4 increased in pulmonary arteries of humans and mice with pulmonary hypertension; ATF6 mediates VSMC survival and proliferation, worsens pulmonary artery resistance |
| Hypertensive Heart Failure | |
| Involvement of ER stress | UPR activated in failing human myocardium; Mice with abdominal aortic banding develop cardiomyocyte apoptosis that is decreased by Chop deletion |
| Worsening by cardiomyocyte Nox4 | Cariomyocyte deletion of Nox4 decreases myocardial hypertrophy and improves cardiac function after abdominal aortic banding or phenylephrine |
| Protection by vascular effects of Nox4 | Global deletion of Nox4 increases myocardial hypertrophy and worsens cardiac function by decreasing VEGF and decreasing myocardial capillary density; Nox4 deletion decreases hind limb angiogenesis, whereas endothelial Nox4 overexpression increases compensatory angiogenesis after femoral artery ligation |
| Diabetes mellitus | |
| Involvement of ER stress |
Perk deletions cause Wolcott-Rallison syndrome; eIF2α and IRE1/XBP1 activated in obesity in mice; Deletion of Chop reverses hyperglycemia, oxidant stress, and pancreatic β-cell death in multiple models of mouse obesity |
| Nox4 and insulin signaling | Nox4 promotes insulin signaling through PTP1B inactivation and tyrosine phosphorylation of insulin receptor; Fibroblasts from leprechaunism patients have impaired insulin and other growth factor signaling from downregulation of Nox4 |
ER stress also appears to occur in vascular wall cells, particularly the vascular endothelium. Prior to the development of atherosclerosis, atheroprone regions of pig aorta that are subjected to disturbed shear show gene expression profiles consistent with the UPR, with activation of the IRE1α and ATF6 pathways in aortic endothelium (Civelek et al. 2009). Likewise, BiP is induced in atheroprone regions of mouse aorta and in human endothelial cells exposed to disturbed shear (Feaver et al. 2008). In mature human atherosclerotic lesions, ATF4 is strongly induced in the endothelium over inflammatory areas and in regional foam cell macrophages, in areas rich with oxidized lipids (Gargalovic et al. 2006).
Nox4, highly expressed by the endothelium, exerts primarily atheroprotective effects, likely by promoting homeostatic UPR responses. Nox4 expression is reduced in diseased human and mouse atherosclerotic plaques, and deletion of Nox4 in multiple atherosclerosis mouse models accelerates atherogenesis (Schurmann et al. 2015, Gray et al. 2016). Endothelial-specific overexpression of Nox4 reduces T effector cell recruitment into and development of atherosclerotic lesions in Apoe−/− mice; conversely, endothelial-specific expression of a dominant negative mutant Nox4 worsens atherosclerosis in this same model (Craige et al. 2015, Hu et al. 2017). Deletion or chemical inhibition of endothelial Nox4 increases leukocyte adhesion, and overexpression/knockdown studies of Nox4 in human endothelium also support its role in repressing inflammatory and profibrotic factors (Schurmann et al. 2015, Gray et al. 2016).
Besides mediating homeostatic correction of ER stress through the UPR, the protective effects of Nox4 may also require induction of autophagy as an alternate method for disposing of both damaged proteins as well as dysfunctional ER and mitochondria. Autophagy in many if not most instances arises from a cradle of ER-derived lipids and thus is commonly activated in response to ER stress (Ogata et al. 2006, Yorimitsu et al. 2006, Axe et al. 2008). While it is difficult to detect and monitor autophagy in humans, levels of LC3-II, which correlate with the formation of autophagosomes, are increased in human atherosclerotic plaques (Martinet and De Meyer 2009), and deletion of Atg7 promotes neointima formation in a mouse model of injury/diet-induced atherosclerosis (Grootaert et al. 2015). Nox4-dependent activation of Ras, restricted to the ER cytosolic surface as noted above, is required for the initiation of autophagy following ER stress (Wu et al. 2010). This activity appears to require the ability of ER-localized Ras to differentially activate ERK, which promotes autophagy, but not the PI3K/mTOR pathway, which potently inhibits autophagy. Autophagy in this context is protective, as chemical inhibition of autophagy or Atg5 knockdown promotes ER stress-induced endothelial cell death (Wu et al. 2010). In addition, knockdown of Nox4 blocks autophagy and aggravates cell death in VSMC (vascular smooth muscle cells) treated with the oxidized lipid 7-ketocholesterol, whereas activation of autophagy by rapamycin decreases ER stress, vascular cell apoptosis, and atherosclerosis in Apoe−/− mice (He et al. 2013). In summary, by directing the UPR and subsequent autophagic activity, Nox4 protects the vascular endothelium and preserves its function in response to proatherogenic ER stress.
As a consequence of atherosclerotic coronary artery disease, myocardium can be subjected to ischemic or ischemia-reperfusion (IR) injury, which appears to induce ER stress in part because of the loss of integrity of the ER and possibly because of increased demands related to reparative processes. Thus, the UPR is activated in both in vitro and ex vivo models. In these models, ER stress is corrective. In cardiac myocytes, ATF6 knockdown or dominant negative ATF6 blocks induction of the chaperone PiB and worsens myocyte death upon reperfusion (Doroudgar et al. 2009). In isolated hearts, cardiomyocyte-restricted overexpression of ATF6 also protects against reperfusion-induced necrosis, apoptosis, and myocardial dysfunction (Martindale et al. 2006). Fifty minutes of ischemia, however, induces Chop and causes reperfusion-induced inflammation and injury, and Chop deficient hearts have markedly decreased myocardial injury and inflammation (Miyazaki et al. 2011), indicating an injurious role for the UPR after a prolonged ischemic period.
The role of Nox proteins in IR injury may be isoform specific. Myocardial infarction size is decreased in mice with Nox1, Nox2, or Nox1 and Nox2 deleted, but not in Nox4 deleted mice (Braunersreuther et al. 2013). Interestingly, cardiac-specific expression of a dominant negative Nox that antagonizes both Nox2 and Nox4 decreases ROS production and HIF1α levels but increases infarct size, suggesting a protective role for cardiomyocyte Nox4 and/or Nox2 (Matsushima et al. 2013). Indeed, glucose deprivation in vitro activates the UPR and autophagy through Nox4, and cardiac-specific Nox4 deletion increases infarct size after no reflow ischemia (Sciarretta et al. 2013). In summary, existing studies are consistent with a protective role for Nox4 in IR myocardial injury, working upstream of the UPR; however, prolonged ischemia may induce UPR-induced cardiomyocyte death through CHOP.
Hypertension
ER stress has been strongly implicated in the CNS (central nervous system), pulmonary and systemic vasculature, and heart in the pathogenesis of hypertension (Table 1). Infusion of angiotensin II in mice, for instance, induces the UPR proteins BiP and CHOP in aortas, mesenteric arteries, and myocardium in response to hypertension (Kassan et al. 2012). Of note, cotreatment with the chemical chaperones TUDCA (taurine-conjugated ursodeoxycholic acid) or PBA (4-phenylbutyric acid) normalizes blood pressure, reduces cardiomyocyte UPR activity and apoptosis, and restores endothelium-dependent relaxation of muscular arteries, confirming a key pathogenic role for ER stress in hypertension (Kassan et al. 2012). Angiotensin II also causes hypertension through its effects on the CNS, particularly the circumventricular SFO (subfornical organ), a forebrain structure unprotected by the blood-brain barrier. Angiotensin II induces severe morphologic ultrastructural changes in the ER of the SFO with induction of the UPR, and systemic TUDCA or focal adenoviral transduction of BiP in the SFO blocks the hypertensive response to angiotensin II after 10 d, demonstrating a role for ER stress in the CNS as well (Kassan et al. 2012). Focal BiP transduction in the SFO also blocks an increase in ROS seen after 2 weeks of angiotensin II treatment, suggesting the late induction of ROS downstream of the UPR in this model.
The role of Nox4 in responding to hypertensive ER stress appears to be cell-type specific. Endothelium-specific overexpression of Nox4 reduces basal and angiotensin II-dependent increases in blood pressure and increases endothelium-dependent relaxation, whereas antioxidants reverse these effects (Ray et al. 2011). However, global deletion of Nox4 has no effect on the angiotensin II-induced hypertensive response, although Nox4 increases eNOS expression, preserves endothelium-dependent relaxation, and decreases inflammatory signaling during angiotensin II treatment (Schroder et al. 2012). These studies suggest that endothelial Nox4 overall mitigates the vascular response to hypertension, although its link to ER stress in endothelial cells during hypertension is not clear.
In VSMC by contrast, Nox4 and the UPR may worsen hypertension, particularly in the pulmonary circulation. Viral infections and hypoxia, known triggers of ER stress, predispose to pulmonary hypertension, and pulmonary arteries in patients with these conditions have swollen and dysmorphic ER. The chemical chaperones TUDCA and PBA reduce pulmonary artery pressures in mouse models of pulmonary hypertension, indicating a role for ER stress (Dromparis et al. 2013). However, the response to chronic ER stress in VSMC promotes survival rather than death through the UPR. Hypoxia, for instance, induces the ER structural protein Nogo-B through ATF6 in pulmonary artery VSMC (Sutendra et al. 2011). Nogo-B uncouples ER-mitochondria calcium exchange and thus decreases mitochondrial ROS and apoptosis, resulting in VSMC survival and proliferation. In vivo, this proliferative response results in medial expansion, arterial remodeling, narrowing of the vascular lumen, and worsening of pulmonary vascular resistance, all of which are reversed by genetic deletion of Nogo-A/B (Sutendra et al. 2011). Notably, Nox4 protein is increased in the vascular media of humans with idiopathic pulmonary arterial hypertension and mice subjected to chronic hypoxia, whereas knockdown of Nox4 in vitro decreases pulmonary artery VSMC proliferation (Mittal et al. 2007). Together, these studies suggest that in pulmonary artery VSMC, Nox4 may promote homeostatic pathways that allow the cell to survive and adapt to ER stress while simultaneously worsening pulmonary hypertension because of active vascular wall remodeling and expansion.
Nox4 may play a similar role in cardiomyocytes, which are ontogenically related to VSMC. Indeed, Nox4 is required for cardiomyocyte differentiation during cardiogenesis as well as the maintenance of VSMC differentiation after injury (Li et al. 2006, Clempus et al. 2007). Chronic systemic hypertension leads to heart failure, a condition marked by both cardiomyocyte apoptosis and hypertrophy. Biopsies of the failing human heart show elevated levels of the chaperones BiP and calreticulin and the transcription factor CHOP (Okada et al. 2004, Fu et al. 2010), indicating activation of the UPR. Transverse aortic constriction in mice also induces the same proteins in the heart, and deletion of Chop reduces cardiomyocyte apoptosis and cardiac fibrosis and hypertrophy (Okada et al. 2004, Fu et al. 2010). The UPR therefore mediates both cardiomyocyte death as well as hypertrophy associated with chronic hypertension. Notably, global expression of a mutant KDEL receptor which interferes with ER protein quality control primarily affects the mouse heart, which shows increases in CHOP and cell death, but does not affect other organs (Hamada et al. 2004). Similarly, systemic administration of the proteasome inhibitor bortezomib for multiple myeloma impairs ERAD and induces severe cardiomyopathies in 11–12% of patients (Enrico et al. 2007). These studies reveal the relative sensitivity of cardiomyocytes to prolonged ER stress. Interestingly, conditional deletion of Nox4 in cardiomyocytes using αMHC-Cre significantly improved mortality and cardiac hypertrophy and function following abdominal aortic banding, whereas myocardial overexpression of Nox4 exacerbated all indices, indicating a role for myocardial Nox4 in promoting hypertensive cardiomyopathy (Kuroda et al. 2010). In this study, Nox4 appeared to increase rather than attenuate cardiomyocyte apoptosis. Nox4 caused cell death through mitochondrial production of ROS, although it is not clear whether this was from mitochondria-localized Nox4, as proposed by the authors, or from downstream mitochondrial effects distal to ER-localized Nox4. In a separate study, conditional myocardial deletion of Nox4 reduced myocardial hypertrophy and improved cardiac function following chronic treatment with the alpha agonist phenylephrine (Matsushima et al. 2013), suggesting a situation similar to VSMC in which Nox4 may mount an appropriate cellular response to hypertensive stresses by promoting hypertrophy but with consequent detriment to organ function.
An additional effect of Nox4 in the failing heart was revealed by global deletion of Nox4 followed by abdominal aortic banding. When followed out to 9 wks, a longer time period than the prior study, global rather than conditional Nox4 deletion worsened cardiac dilation, hypertrophy, fibrosis, and systolic function, revealing a protective role for Nox4 in hypertensive cardiomyopathy (Zhang et al. 2010). Importantly, global Nox4 deletion decreased myocardial capillary density, indicating a salutary role for Nox4 primarily through compensatory angiogenesis, and not through direct effects on cardiomyocyte viability. Myocardial overexpression of Nox4 stabilized Hif-1α and increased VEGF expression, suggesting an interaction between cardiomyocytes and endothelial cells mediated by Nox4 (Zhang et al. 2010). In a subsequent study, both cardiomyocyte-specific and endothelial-specific deletion of Nox4 caused decreased HIF1α and VEGF levels and worse cardiac dysfunction following transverse aortic constriction (Zhang et al. 2018). An additional function of Nox4 in the overloaded heart appears to be the reprogramming of glucose and fatty acid metabolism. A recent study reveals that Nox4 redirects glucose flux from oxidation to fatty acid oxidation, and effect mediated by an ATF4-dependent increase in O-GlcNAcylation of the fatty acid transporter CD36 (Nabeebaccus et al. 2017). Other studies confirm the role of Nox4 in mediating compensatory angiogenesis, even though it has no demonstrable role in developmental angiogenesis. Nox4 deletion blunts hind limb angiogenesis following femoral artery ligation, an effect thought to be mediated primarily by endothelial Nox4 (Schroder et al. 2012). Consistent with this interpretation, endothelial-specific overexpression of Nox4 increases eNOS expression and accelerates compensatory angiogenesis following femoral artery ligation (Craige et al. 2011). Taken together, these data indicate that Nox4 appears to improve ER stress-dependent effects at the level of the cell, thus preserving cell-specific functions, but may improve or worsen physiologic cardiovascular function depending on the target cell.
Diabetes mellitus
Nutrient management requires protein synthesis and processing through the ER; consequently, excessive nutrients encountered in obesity and type II diabetes mellitus place the ER under chronic stress. Cells responsible for cholesterol metabolism, insulin production, and fat storage endure severe ER stress, damaging the liver, endocrine pancreas, and adipose tissue. Adipose tissues from obese humans show activation of the UPR (Boden et al. 2008, Sharma et al. 2008), and chemical chaperones such as PBA reverse lipid infusion-dependent insulin insensitivity in humans and normalize blood glucose, fatty liver, and insulin sensitivity in obese mice (Ozcan et al. 2006, Xiao et al. 2011). An absolute requirement for an intact UPR response to metabolic stresses was first suggested by the identification of mutations in PERK as the cause of early onset diabetes in the Wolcott-Rallison syndrome (Delepine et al. 2000), and subsequent mouse models have confirmed the involvement of both eIF2α and IRE1/XBP1 arms of the UPR in responding to ER stress related to obesity and even to normal fluctuations in glucose availability (Scheuner et al. 2001, Ozcan et al. 2004, Back et al. 2009).
As might be expected, oxidative stress is found in obese and diabetic states, and is thought to participate in the development of insulin resistance. In part, this may correspond to the prominent activation of ER stress-dependent IKK/NF-κB and JNK and consequent inflammasome formation. In this way, ER stress and the attendant UPR represent a critical link between metabolism and inflammation (Hotamisligil 2010). In addition, however, ROS production and oxidative stress have been shown to occur directly from the UPR itself. In multiple mouse models of obesity, deletion of Chop reduces Ero1p expression, reverses hyperglycemia, and reduces biochemical markers of oxidant stress and pancreatic β-cell death, highlighting the involvement of ROS in a late apoptotic response to unremitting ER stress (Song et al. 2008). While the source of ROS in this circumstance is unclear, catalase targeted to mitochondria afford protection from insulin resistance, consistent with this organelle being an important source of ROS as is the case with other causes of prolonged ER stress (Houstis et al. 2006).
The specific role of Nox4 in responding to ER stress in diabetes has not been well studied. However, Nox4 appears to play an unequivocal role in insulin receptor signaling. In adipocyte-like 3T3-L1 cells, Nox4 is required to inactivate the phosphatase PTP1B upon insulin stimulation, promoting insulin signaling through tyrosine phosphorylation of the insulin receptor (Mahadev et al. 2004). In hepatocytes, knockdown studies also show a major requirement for Nox4 in mediating intact insulin signals (Wu and Williams 2012). Interestingly, fibroblasts from patients with leprechaunism, an insulin resistant condition associated with mutations in the insulin receptor, also show broad defects in receptor tyrosine kinase signaling for other growth factors such as EGF and PDGF, due to downregulation of Nox4 (Park et al. 2005). The reason for impaired Nox4 protein expression in this condition is not clear.
Nox4 may also be required for inflammatory signaling by adipocytes in diabetes. While consistent with a role in the inflammatory response linked to ER stress, this association has not been closely examined. Induction of the inflammatory factors MCP-1 and Saa3 by high glucose in 3T3-L1 cells in vitro, for instance, requires Nox4, and conditional deletion of Nox4 in adipocytes decreases adipose tissue inflammation and delays the onset of diet-induced insulin resistance (Han et al. 2012, Den Hartigh et al. 2017). However, global deletion of Nox4 has the opposite effect, worsening diet-induced insulin resistance, obesity, and inflammatory cytokine production (Li et al. 2012). It is known that Nox4 is required for the differentiation of mature adipocytes from preadipocytes (Schroder et al. 2009); hence, it has been proposed that global deletion of Nox4 may impair adipocyte maturation, leading to hypertrophy of existing adipocytes and inflammation when these mice are fed a high-fat diet (Den Hartigh et al. 2017). Again, however, a specific role for Nox4 in the response to ER stress from excess nutrient intake, while likely, has not been closely studied. In addition, autophagy is now known to be highly active in obese and diabetic states, but the role of Nox4 and Ras in triggering autophagy under these conditions is also not presently understood.
Conclusions
The ER responds to a surprisingly broad range of stresses, including increases in free cholesterol, energetic and physical cardiovascular demands, and nutrient management. The critical function of the ER in responding to these varied stresses highlights its role in the development of atherosclerosis, hypertension, and the metabolic syndrome. However, the complexities of how ROS are formed and contribute to both homeostatic signaling as well as cell demise raise a number of challenges in translating recent findings into clinical application. As an example, attempts to use dietary antioxidants to combat oxidative stress in these and other human diseases have been consistently unsuccessful, as might be predicted from the protective effects of Nox4 and other ROS sources. Large population studies have repeatedly demonstrated that antioxidant supplements such as vitamins A, C, D, and E have no effect on cardiovascular (Abdulkarim et al. 2017) or cancer risk (Devore et al. 2013, Fortmann et al. 2013). Vitamins C and E also abrogate the beneficial effect of exercise on insulin sensitivity in humans, and antioxidants block the life extension produced by caloric restriction or sir-2.1 expression in worms (Schulz et al. 2007, Ristow et al. 2009, Schmeisser et al. 2013). The proposed use of Nox4 inhibitors to treat specific diseases also raises the theoretical danger that early, protective responses may be hindered in addition to affecting targeted downstream pathologic effects.
An alternate pharmacologic strategy currently on the horizon employs compounds that alleviate ER stress. Sephin1 is a recently developed compound that augments the UPR by disrupting the PP1-PPP1R15A phosphatase complex that dephosphorylates eIF2α (Crespillo-Casado et al. 2017). This compound induces remarkable improvement in genetic mouse models of two neurologic diseases due to protein misfolding, Charcot-Marie-Tooth 1B and familial Amyotrophic Lateral Sclerosis (Das et al. 2015). The antihypertensive drug guanabenz has similar effects on the PP1-PPP1R15A phosphatase complex, and has been reported to relieve ER stress in cardiomyocytes (Neuber et al. 2014). However, guanabenz fed to rodents leads to glucose intolerance, an effect that appears to be due to eIF2α-induced CHOP induction and pancreatic β-cell death (Abdulkarim et al. 2017). In addition, conflicting studies report either amelioration or acceleration of disease following guanabenz treatment in mouse models of familial ALS (Jiang et al. 2014, Vieira et al. 2015). Although these studies sound a note of caution, they provide proof of concept that ER stress can be modulated in disease states, and highlight the need for further studies in this area.
Acknowledgments
This work was supported by the NIH (R01-CA208620 and T32-HL098040) and the Cancer Prevention Research Institute of Texas (RP160307).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdulkarim B, Hernangomez M, Igoillo-Esteve M, Cunha DA, Marselli L, Marchetti P, Ladriere L, Cnop M. Guanabenz Sensitizes Pancreatic beta Cells to Lipotoxic Endoplasmic Reticulum Stress and Apoptosis. Endocrinology. 2017;158(6):1659–1670. doi: 10.1210/en.2016-1773. [DOI] [PubMed] [Google Scholar]
- Anilkumar N, San Jose G, Sawyer I, Santos CX, Sand C, Brewer AC, Warren D, Shah AM. A 28-kDa splice variant of NADPH oxidase-4 is nuclear-localized and involved in redox signaling in vascular cells. Arterioscler Thromb Vasc Biol. 2013;33(4):e104–112. doi: 10.1161/ATVBAHA.112.300956. [DOI] [PubMed] [Google Scholar]
- Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol. 2008;182(4):685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, Gildersleeve RD, Pennathur S, Kaufman RJ. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10(1):13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods. 2006;3(4):281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, Cheung P, Merali S. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57(9):2438–2444. doi: 10.2337/db08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunersreuther V, Montecucco F, Asrih M, Pelli G, Galan K, Frias M, Burger F, Quindere AL, Montessuit C, Krause KH, Mach F, Jaquet V. Role of NADPH oxidase isoforms NOX1, NOX2 and NOX4 in myocardial ischemia/reperfusion injury. J Mol Cell Cardiol. 2013;64:99–107. doi: 10.1016/j.yjmcc.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Brostrom CO, Prostko CR, Kaufman RJ, Brostrom MA. Inhibition of translational initiation by activators of the glucose-regulated stress protein and heat shock protein stress response systems. Role of the interferon-inducible double-stranded RNA-activated eukaryotic initiation factor 2alpha kinase. J Biol Chem. 1996;271(40):24995–25002. doi: 10.1074/jbc.271.40.24995. [DOI] [PubMed] [Google Scholar]
- Chawla A, Chakrabarti S, Ghosh G, Niwa M. Attenuation of yeast UPR is essential for survival and is mediated by IRE1 kinase. J Cell Biol. 2011;193(1):41–50. doi: 10.1083/jcb.201008071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181(7):1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelek M, Manduchi E, Riley RJ, Stoeckert CJ, Jr, Davies PF. Chronic endoplasmic reticulum stress activates unfolded protein response in arterial endothelium in regions of susceptibility to atherosclerosis. Circ Res. 2009;105(5):453–461. doi: 10.1161/CIRCRESAHA.109.203711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27(1):42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, Shibata R, Sato K, Walsh K, Keaney JF., Jr NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124(6):731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craige SM, Kant S, Reif M, Chen K, Pei Y, Angoff R, Sugamura K, Fitzgibbons T, Keaney JF., Jr Endothelial NADPH oxidase 4 protects ApoE−/− mice from atherosclerotic lesions. Free Radic Biol Med. 2015;89:1–7. doi: 10.1016/j.freeradbiomed.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespillo-Casado A, Chambers JE, Fischer PM, Marciniak SJ, Ron D. PPP1R15A-mediated dephosphorylation of eIF2alpha is unaffected by Sephin1 or Guanabenz. Elife. 2017:6. doi: 10.7554/eLife.26109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem. 2004;279(19):20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- Dalet A, Gatti E, Pierre P. Integration of PKR-dependent translation inhibition with innate immunity is required for a coordinated anti-viral response. FEBS Lett. 2015;589(14):1539–1545. doi: 10.1016/j.febslet.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Das I, Krzyzosiak A, Schneider K, Wrabetz L, D’Antonio M, Barry N, Sigurdardottir A, Bertolotti A. Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science. 2015;348(6231):239–242. doi: 10.1126/science.aaa4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. An intimate liaison: spatial organization of the endoplasmic reticulum-mitochondria relationship. Embo j. 2010;29(16):2715–2723. doi: 10.1038/emboj.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25(4):406–409. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- Den Hartigh LJ, Omer M, Goodspeed L, Wang S, Wietecha T, O’Brien KD, Han CY. Adipocyte-Specific Deficiency of NADPH Oxidase 4 Delays the Onset of Insulin Resistance and Attenuates Adipose Tissue Inflammation in Obesity. Arterioscler Thromb Vasc Biol. 2017;37(3):466–475. doi: 10.1161/ATVBAHA.116.308749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24(23):10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devore EE, Kang JH, Stampfer MJ, Grodstein F. The association of antioxidants and cognition in the Nurses’ Health Study. Am J Epidemiol. 2013;177(1):33–41. doi: 10.1093/aje/kws202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroudgar S, Thuerauf DJ, Marcinko MC, Belmont PJ, Glembotski CC. Ischemia activates the ATF6 branch of the endoplasmic reticulum stress response. J Biol Chem. 2009;284(43):29735–29745. doi: 10.1074/jbc.M109.018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromparis P, Paulin R, Stenson TH, Haromy A, Sutendra G, Michelakis ED. Attenuating endoplasmic reticulum stress as a novel therapeutic strategy in pulmonary hypertension. Circulation. 2013;127(1):115–125. doi: 10.1161/CIRCULATIONAHA.112.133413. [DOI] [PubMed] [Google Scholar]
- Enrico O, Gabriele B, Nadia C, Sara G, Daniele V, Giulia C, Antonio S, Mario P. Unexpected cardiotoxicity in haematological bortezomib treated patients. Br J Haematol. 2007;138(3):396–397. doi: 10.1111/j.1365-2141.2007.06659.x. [DOI] [PubMed] [Google Scholar]
- Enyedi B, Varnai P, Geiszt M. Redox state of the endoplasmic reticulum is controlled by Ero1L-alpha and intraluminal calcium. Antioxid Redox Signal. 2010;13(6):721–729. doi: 10.1089/ars.2009.2880. [DOI] [PubMed] [Google Scholar]
- Ewald CY, Hourihan JM, Bland MS, Obieglo C, Katic I, Moronetti Mazzeo LE, Alcedo J, Blackwell TK, Hynes NE. NADPH oxidase-mediated redox signaling promotes oxidative stress resistance and longevity through memo-1 in C. elegans. Elife. 2017:6. doi: 10.7554/eLife.19493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feaver RE, Hastings NE, Pryor A, Blackman BR. GRP78 upregulation by atheroprone shear stress via p38-, alpha2beta1-dependent mechanism in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28(8):1534–1541. doi: 10.1161/ATVBAHA.108.167999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortmann SP, Burda BU, Senger CA, Lin JS, Whitlock EP. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: An updated systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159(12):824–834. doi: 10.7326/0003-4819-159-12-201312170-00729. [DOI] [PubMed] [Google Scholar]
- Fu HY, Okada K, Liao Y, Tsukamoto O, Isomura T, Asai M, Sawada T, Okuda K, Asano Y, Sanada S, Asanuma H, Asakura M, Takashima S, Komuro I, Kitakaze M, Minamino T. Ablation of C/EBP homologous protein attenuates endoplasmic reticulum-mediated apoptosis and cardiac dysfunction induced by pressure overload. Circulation. 2010;122(4):361–369. doi: 10.1161/CIRCULATIONAHA.109.917914. [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Tuncman G, Gorgun CZ, Makowski L, Atsumi G, Vaillancourt E, Kono K, Babaev VR, Fazio S, Linton MF, Sulsky R, Robl JA, Parker RA, Hotamisligil GS. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447(7147):959–965. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargalovic PS, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, Truong A, Baruch-Oren T, Berliner JA, Kirchgessner TG, Lusis AJ. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26(11):2490–2496. doi: 10.1161/01.ATV.0000242903.41158.a1. [DOI] [PubMed] [Google Scholar]
- Gray SP, Di Marco E, Kennedy K, Chew P, Okabe J, El-Osta A, Calkin AC, Biessen EA, Touyz RM, Cooper ME, Schmidt HH, Jandeleit-Dahm KA. Reactive Oxygen Species Can Provide Atheroprotection via NOX4-Dependent Inhibition of Inflammation and Vascular Remodeling. Arterioscler Thromb Vasc Biol. 2016;36(2):295–307. doi: 10.1161/ATVBAHA.115.307012. [DOI] [PubMed] [Google Scholar]
- Grootaert MO, da Costa Martins PA, Bitsch N, Pintelon I, De Meyer GR, Martinet W, Schrijvers DM. Defective autophagy in vascular smooth muscle cells accelerates senescence and promotes neointima formation and atherogenesis. Autophagy. 2015;11(11):2014–2032. doi: 10.1080/15548627.2015.1096485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E, Sevier CS, Heldman N, Vitu E, Bentzur M, Kaiser CA, Thorpe C, Fass D. Generating disulfides enzymatically: reaction products and electron acceptors of the endoplasmic reticulum thiol oxidase Ero1p. Proc Natl Acad Sci U S A. 2006;103(2):299–304. doi: 10.1073/pnas.0506448103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H, Suzuki M, Yuasa S, Mimura N, Shinozuka N, Takada Y, Suzuki M, Nishino T, Nakaya H, Koseki H, Aoe T. Dilated cardiomyopathy caused by aberrant endoplasmic reticulum quality control in mutant KDEL receptor transgenic mice. Mol Cell Biol. 2004;24(18):8007–8017. doi: 10.1128/MCB.24.18.8007-8017.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CY, Umemoto T, Omer M, Den Hartigh LJ, Chiba T, LeBoeuf R, Buller CL, Sweet IR, Pennathur S, Abel ED, Chait A. NADPH oxidase-derived reactive oxygen species increases expression of monocyte chemotactic factor genes in cultured adipocytes. J Biol Chem. 2012;287(13):10379–10393. doi: 10.1074/jbc.M111.304998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11(3):619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Haynes CM, Titus EA, Cooper AA. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol Cell. 2004;15(5):767–776. doi: 10.1016/j.molcel.2004.08.025. [DOI] [PubMed] [Google Scholar]
- He C, Zhu H, Zhang W, Okon I, Wang Q, Li H, Le YZ, Xie Z. 7-Ketocholesterol induces autophagy in vascular smooth muscle cells through Nox4 and Atg4B. Am J Pathol. 2013;183(2):626–637. doi: 10.1016/j.ajpath.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313(5783):104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140(6):900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26(8):3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Wu X, Khandelwal AR, Yu W, Xu Z, Chen L, Yang J, Weisbrod RM, Lee KSS, Seta F, Hammock BD, Cohen RA, Zeng C, Tong X. Endothelial Nox4-based NADPH oxidase regulates atherosclerosis via soluble epoxide hydrolase. Biochim Biophys Acta. 2017;1863(6):1382–1391. doi: 10.1016/j.bbadis.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Toyama T, Nakamura Y, Tamada K, Shimizu H, Ninagawa S, Okada T, Kamei Y, Ishikawa-Fujiwara T, Todo T, Aoyama E, Takigawa M, Harada A, Mori K. UPR transducer BBF2H7 allows export of type II collagen in a cargo- and developmental stage-specific manner. J Cell Biol. 2017;216(6):1761–1774. doi: 10.1083/jcb.201609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwawaki T, Hosoda A, Okuda T, Kamigori Y, Nomura-Furuwatari C, Kimata Y, Tsuru A, Kohno K. Translational control by the ER transmembrane kinase/ribonuclease IRE1 under ER stress. Nat Cell Biol. 2001;3(2):158–164. doi: 10.1038/35055065. [DOI] [PubMed] [Google Scholar]
- Jiang HQ, Ren M, Jiang HZ, Wang J, Zhang J, Yin X, Wang SY, Qi Y, Wang XD, Feng HL. Guanabenz delays the onset of disease symptoms, extends lifespan, improves motor performance and attenuates motor neuron loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neuroscience. 2014;277:132–138. doi: 10.1016/j.neuroscience.2014.03.047. [DOI] [PubMed] [Google Scholar]
- Jiang HY, Wek SA, McGrath BC, Scheuner D, Kaufman RJ, Cavener DR, Wek RC. Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol Cell Biol. 2003;23(16):5651–5663. doi: 10.1128/MCB.23.16.5651-5663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassan M, Galan M, Partyka M, Saifudeen Z, Henrion D, Trebak M, Matrougui K. Endoplasmic reticulum stress is involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice. Arterioscler Thromb Vasc Biol. 2012;32(7):1652–1661. doi: 10.1161/ATVBAHA.112.249318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata Y, Oikawa D, Shimizu Y, Ishiwata-Kimata Y, Kohno K. A role for BiP as an adjustor for the endoplasmic reticulum stress-sensing protein Ire1. J Cell Biol. 2004;167(3):445–456. doi: 10.1083/jcb.200405153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstein J, Morito D, Kakihana T, Sugihara M, Minnen A, Hipp MS, Nussbaum-Krammer C, Kasturi P, Hartl FU, Nagata K, Morimoto RI. Proteotoxic stress and ageing triggers the loss of redox homeostasis across cellular compartments. Embo j. 2015;34(18):2334–2349. doi: 10.15252/embj.201591711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A. 2010;107(35):15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda J, Nakagawa K, Yamasaki T, Nakamura K, Takeya R, Kuribayashi F, Imajoh-Ohmi S, Igarashi K, Shibata Y, Sueishi K, Sumimoto H. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells. 2005;10(12):1139–1151. doi: 10.1111/j.1365-2443.2005.00907.x. [DOI] [PubMed] [Google Scholar]
- Leadsham JE, Sanders G, Giannaki S, Bastow EL, Hutton R, Naeimi WR, Breitenbach M, Gourlay CW. Loss of cytochrome c oxidase promotes RAS-dependent ROS production from the ER resident NADPH oxidase, Yno1p, in yeast. Cell Metab. 2013;18(2):279–286. doi: 10.1016/j.cmet.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Li G, Scull C, Ozcan L, Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J Cell Biol. 2010;191(6):1113–1125. doi: 10.1083/jcb.201006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Stouffs M, Serrander L, Banfi B, Bettiol E, Charnay Y, Steger K, Krause KH, Jaconi ME. The NADPH oxidase NOX4 drives cardiac differentiation: Role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell. 2006;17(9):3978–3988. doi: 10.1091/mbc.E05-06-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Mouche S, Sajic T, Veyrat-Durebex C, Supale R, Pierroz D, Ferrari S, Negro F, Hasler U, Feraille E, Moll S, Meda P, Deffert C, Montet X, Krause KH, Szanto I. Deficiency in the NADPH oxidase 4 predisposes towards diet-induced obesity. Int J Obes (Lond) 2012;36(12):1503–1513. doi: 10.1038/ijo.2011.279. [DOI] [PubMed] [Google Scholar]
- Li Y, Schwabe RF, DeVries-Seimon T, Yao PM, Gerbod-Giannone MC, Tall AR, Davis RJ, Flavell R, Brenner DA, Tabas I. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: model of NF-kappaB- and map kinase-dependent inflammation in advanced atherosclerosis. J Biol Chem. 2005;280(23):21763–21772. doi: 10.1074/jbc.M501759200. [DOI] [PubMed] [Google Scholar]
- Liu L, Wise DR, Diehl JA, Simon MC. Hypoxic reactive oxygen species regulate the integrated stress response and cell survival. J Biol Chem. 2008;283(45):31153–31162. doi: 10.1074/jbc.M805056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundback P, Valdes-Ferrer SI, Olofsson PS, Kalb T, Roth J, Zou Y, Erlandsson-Harris H, Yang H, Ting JP, Wang H, Andersson U, Antoine DJ, Chavan SS, Hotamisligil GS, Tracey KJ. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488(7413):670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24(5):1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, Parker RA, Suttles J, Fazio S, Hotamisligil GS, Linton MF. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7(6):699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18(24):3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale JJ, Fernandez R, Thuerauf D, Whittaker R, Gude N, Sussman MA, Glembotski CC. Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ Res. 2006;98(9):1186–1193. doi: 10.1161/01.RES.0000220643.65941.8d. [DOI] [PubMed] [Google Scholar]
- Martinet W, De Meyer GR. Autophagy in atherosclerosis: a cell survival and death phenomenon with therapeutic potential. Circ Res. 2009;104(3):304–317. doi: 10.1161/CIRCRESAHA.108.188318. [DOI] [PubMed] [Google Scholar]
- Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11(5):411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima S, Kuroda J, Ago T, Zhai P, Ikeda Y, Oka S, Fong GH, Tian R, Sadoshima J. Broad suppression of NADPH oxidase activity exacerbates ischemia/reperfusion injury through inadvertent downregulation of hypoxia-inducible factor-1alpha and upregulation of peroxisome proliferator-activated receptor-alpha. Circ Res. 2013;112(8):1135–1149. doi: 10.1161/CIRCRESAHA.111.300171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima S, Kuroda J, Ago T, Zhai P, Park JY, Xie LH, Tian B, Sadoshima J. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circ Res. 2013;112(4):651–663. doi: 10.1161/CIRCRESAHA.112.279760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merksamer PI, Trusina A, Papa FR. Real-time redox measurements during endoplasmic reticulum stress reveal interlinked protein folding functions. Cell. 2008;135(5):933–947. doi: 10.1016/j.cell.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res. 2007;101(3):258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Kaikita K, Endo M, Horio E, Miura M, Tsujita K, Hokimoto S, Yamamuro M, Iwawaki T, Gotoh T, Ogawa H, Oike Y. C/EBP homologous protein deficiency attenuates myocardial reperfusion injury by inhibiting myocardial apoptosis and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):1124–1132. doi: 10.1161/ATVBAHA.111.224519. [DOI] [PubMed] [Google Scholar]
- Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, Asada Y, Okada K, Ishibashi-Ueda H, Gabbiani G, Bochaton-Piallat ML, Mochizuki N, Kitakaze M. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116(11):1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- Nabeebaccus AA, Zoccarato A, Hafstad AD, Santos CX, Aasum E, Brewer AC, Zhang M, Beretta M, Yin X, West JA, Schroder K, Griffin JL, Eykyn TR, Abel ED, Mayr M, Shah AM. Nox4 reprograms cardiac substrate metabolism via protein O-GlcNAcylation to enhance stress adaptation. JCI Insight. 2017;2(24) doi: 10.1172/jci.insight.96184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadanaka S, Okada T, Yoshida H, Mori K. Role of disulfide bridges formed in the luminal domain of ATF6 in sensing endoplasmic reticulum stress. Mol Cell Biol. 2007;27(3):1027–1043. doi: 10.1128/MCB.00408-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuber C, Uebeler J, Schulze T, Sotoud H, El-Armouche A, Eschenhagen T. Guanabenz interferes with ER stress and exerts protective effects in cardiac myocytes. PLoS One. 2014;9(6):e98893. doi: 10.1371/journal.pone.0098893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26(24):9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, Kitakaze M. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004;110(6):705–712. doi: 10.1161/01.CIR.0000137836.95625.D4. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Jin DK, Shin SM, Jang MK, Longo N, Park JW, Bae DS, Bae YS. Impaired generation of reactive oxygen species in leprechaunism through downregulation of Nox4. Diabetes. 2005;54(11):3175–3181. doi: 10.2337/diabetes.54.11.3175. [DOI] [PubMed] [Google Scholar]
- Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, Shah AM. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol. 2011;31(6):1368–1376. doi: 10.1161/ATVBAHA.110.219238. [DOI] [PubMed] [Google Scholar]
- Rinnerthaler M, Buttner S, Laun P, Heeren G, Felder TK, Klinger H, Weinberger M, Stolze K, Grousl T, Hasek J, Benada O, Frydlova I, Klocker A, Simon-Nobbe B, Jansko B, Breitenbach-Koller H, Eisenberg T, Gourlay CW, Madeo F, Burhans WC, Breitenbach M. Yno1p/Aim14p, a NADPH-oxidase ortholog, controls extramitochondrial reactive oxygen species generation, apoptosis, and actin cable formation in yeast. Proc Natl Acad Sci U S A. 2012;109(22):8658–8663. doi: 10.1073/pnas.1201629109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106(21):8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos CX, Hafstad AD, Beretta M, Zhang M, Molenaar C, Kopec J, Fotinou D, Murray TV, Cobb AM, Martin D, Zeh Silva M, Anilkumar N, Schroder K, Shanahan CM, Brewer AC, Brandes RP, Blanc E, Parsons M, Belousov V, Cammack R, Hider RC, Steiner RA, Shah AM. Targeted redox inhibition of protein phosphatase 1 by Nox4 regulates eIF2alpha-mediated stress signaling. Embo j. 2016;35(3):319–334. doi: 10.15252/embj.201592394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7(6):1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- Schmeisser K, Mansfeld J, Kuhlow D, Weimer S, Priebe S, Heiland I, Birringer M, Groth M, Segref A, Kanfi Y, Price NL, Schmeisser S, Schuster S, Pfeiffer AF, Guthke R, Platzer M, Hoppe T, Cohen HY, Zarse K, Sinclair DA, Ristow M. Role of sirtuins in lifespan regulation is linked to methylation of nicotinamide. Nat Chem Biol. 2013;9(11):693–700. doi: 10.1038/nchembio.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Wandzioch K, Helmcke I, Brandes RP. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler Thromb Vasc Biol. 2009;29(2):239–245. doi: 10.1161/ATVBAHA.108.174219. [DOI] [PubMed] [Google Scholar]
- Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res. 2012;110(9):1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6(4):280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Schurmann C, Rezende F, Kruse C, Yasar Y, Lowe O, Fork C, van de Sluis B, Bremer R, Weissmann N, Shah AM, Jo H, Brandes RP, Schroder K. The NADPH oxidase Nox4 has anti-atherosclerotic functions. Eur Heart J. 2015;36(48):3447–3456. doi: 10.1093/eurheartj/ehv460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciarretta S, Zhai P, Shao D, Zablocki D, Nagarajan N, Terada LS, Volpe M, Sadoshima J. Activation of NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2alpha/activating transcription factor 4 pathway. Circ Res. 2013;113(11):1253–1264. doi: 10.1161/CIRCRESAHA.113.301787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma NK, Das SK, Mondal AK, Hackney OG, Chu WS, Kern PA, Rasouli N, Spencer HJ, Yao-Borengasser A, Elbein SC. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J Clin Endocrinol Metab. 2008;93(11):4532–4541. doi: 10.1210/jc.2008-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118(10):3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutendra G, Dromparis P, Wright P, Bonnet S, Haromy A, Hao Z, McMurtry MS, Michalak M, Vance JE, Sessa WC, Michelakis ED. The role of Nogo and the mitochondria-endoplasmic reticulum unit in pulmonary hypertension. Sci Transl Med. 2011;3(88):88ra55. doi: 10.1126/scitranslmed.3002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada LS. Specificity in reactive oxidant signaling: think globally, act locally. J Cell Biol. 2006;174(5):615–623. doi: 10.1083/jcb.200605036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 2009;9(5):474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu BP, Weissman JS. The FAD- and O(2)-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol Cell. 2002;10(5):983–994. doi: 10.1016/s1097-2765(02)00696-2. [DOI] [PubMed] [Google Scholar]
- Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. 2004;164(3):341–346. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287(5453):664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- Vieira FG, Ping Q, Moreno AJ, Kidd JD, Thompson K, Jiang B, Lincecum JM, Wang MZ, De Zutter GS, Tassinari VR, Levine B, Hatzipetros T, Gill A, Perrin S. Guanabenz Treatment Accelerates Disease in a Mutant SOD1 Mouse Model of ALS. PLoS One. 2015;10(8):e0135570. doi: 10.1371/journal.pone.0135570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Williams BR. Signal integration via PKR. Sci STKE. 2001;2001(89):re2. doi: 10.1126/stke.2001.89.re2. [DOI] [PubMed] [Google Scholar]
- Wu RF, Liao C, Hatoum H, Fu G, Ochoa CD, Terada LS. RasGRF Couples Nox4-Dependent Endoplasmic Reticulum Signaling to Ras. Arterioscler Thromb Vasc Biol. 2017;37(1):98–107. doi: 10.1161/ATVBAHA.116.307922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RF, Ma Z, Liu Z, Terada LS. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol Cell Biol. 2010;30(14):3553–3568. doi: 10.1128/MCB.01445-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RF, Ma Z, Myers DP, Terada LS. HIV-1 Tat activates dual Nox pathways leading to independent activation of ERK and JNK MAP kinases. J Biol Chem. 2007;282(52):37412–37419. doi: 10.1074/jbc.M704481200. [DOI] [PubMed] [Google Scholar]
- Wu X, Williams KJ. NOX4 pathway as a source of selective insulin resistance and responsiveness. Arterioscler Thromb Vasc Biol. 2012;32(5):1236–1245. doi: 10.1161/ATVBAHA.111.244525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Giacca A, Lewis GF. Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and beta-cell dysfunction in humans. Diabetes. 2011;60(3):918–924. doi: 10.2337/db10-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem. 2006;281(40):30299–30304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan K, Vattem KM, Bauer BN, Dever TE, Chen JJ, Wek RC. Phosphorylation of eukaryotic initiation factor 2 by heme-regulated inhibitor kinase-related protein kinases in Schizosaccharomyces pombe is important for fesistance to environmental stresses. Mol Cell Biol. 2002;22(20):7134–7146. doi: 10.1128/MCB.22.20.7134-7146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, Back SH, Kaufman RJ. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124(3):587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A. 2010;107(42):18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Mongue-Din H, Martin D, Catibog N, Smyrnias I, Zhang X, Yu B, Wang M, Brandes RP, Schroder K, Shah AM. Both cardiomyocyte and endothelial cell Nox4 mediate protection against hemodynamic overload-induced remodelling. Cardiovasc Res. 2018;114(3):401–408. doi: 10.1093/cvr/cvx204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Lhotak S, Hilditch BA, Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2005;111(14):1814–1821. doi: 10.1161/01.CIR.0000160864.31351.C1. [DOI] [PubMed] [Google Scholar]