Abstract
Previous research has suggested that behavioral comorbidity is the rule rather than the exception in autism. The present study aimed to trace the respective origins of autistic and general psychopathologic traits—and their association—to infancy. Measurements of autistic traits and early liability for general psychopathology were assessed in 314 twins at 18 months, ascertained from the general population using birth records. 222 twins were re-evaluated at 36 months. Standardized ratings of variation in social communication at 18 months were highly heritable and strongly predicted autistic trait scores at 36 months. These early indices of autistic liability were independent from contemporaneous ratings of behavior problems on the Brief Infant-Toddler Social and Emotional Assessment (which were substantially environmentally-influenced), and did not meaningfully predict internalizing or externalizing scores on the Achenbach Scales of Empirically Based Assessment at 36 months. In this general population infant twin study, variation in social communication was independent from variation in other domains of general psychopathology, and exhibited a distinct genetic structure. The commonly-observed comorbidity of specific psychiatric syndromes with autism may arise from subsequent interactions between autistic liability and independent susceptibilities to other psychopathologic traits, suggesting opportunities for preventive amelioration of outcomes of these interactions over the course of development.
Electronic supplementary material
The online version of this article (10.1007/s10802-018-0410-1) contains supplementary material, which is available to authorized users.
Keywords: Autism, Psychopathology, Twins, Trait overlap, Development
Ever since it was established that the characterizing features of the autistic syndrome were continuously distributed in the human population (Constantino and Todd 2003a; Ronald and Hoekstra 2011), and that the genetic factors which influence population variation in autistic traits overlapped with that of autism itself (Ronald et al. 2006; Constantino 2014), the exploration of how these traits influence general human social and behavioral development—specifically, the development of internalizing and externalizing behaviors, which are present in or characterize a wide array of psychiatric syndromes—has risen to high priority in behavioral neuroscience. The link between autistic traits and psychopathologic abnormalities of human behavior is reinforced by clinical studies indicating that a) within individuals, autism spectrum disorders frequently co-occur with behavioral disability (Dworzynski et al. 2009); b) autistic symptom severity is correlated with the severity of non-autistic behavioral impairments (Constantino and Frazier 2013); and c) within families, some of the genetic influences on the causation of ASD may be non-specific and therefore overlap with genetic influences on other psychiatric conditions of childhood (Constantino 2017; Mous et al. 2017).
The latter became apparent in genetic epidemiologic studies of school-aged children in which significant phenotypic associations were observed between traits that characterized autism and those that characterized attention-deficit/hyperactivity disorder (ADHD; Constantino et al. 2003b; Reiersen et al. 2007; Ronald et al. 2008; Ronald et al. 2010) and internalizing disorders (Hallett et al. 2012; Hallett et al. 2010; Duvekot et al. 2017). Additionally, within individual subjects, clinical studies have observed high comorbidity between autism spectrum disorder (ASD) and general psychopathology (Lundström et al. 2015; Simonoff et al. 2008; Hallett et al. 2013), with 70–96% of ASD diagnoses complicated by at least one co-occurring disorder (Simonoff et al. 2008; Lundström et al. 2015).
Studies of rare, highly-penetrant mutations associated with neuropsychiatric syndromes commonly reveal the phenomenon of pleiotropy, in which a given disease-causing variant can result in different neurobehavioral syndromes depending on the genetic background on which it is superimposed (Geschwind 2011; Moreno-De-Luca et al. 2013). Extending these findings, recent research suggests that common variants can also exert pleiotropic influences, constituting nonspecific etiologic factors that increase risk for symptoms of disparate psychopathologies (Lahey et al. 2017). Thus, phenotypic and genetic overlap between two inherited neuropsychiatric conditions is theoretically attributable to a) genetic influence shared by the conditions, and/or b) interactions over the course of development that result in one condition exacerbating the severity or manifestations of the other when (and only when) symptoms of both are present (Angold et al. 1999). Critically, studies of trait overlap in the general population can inform our understanding of clinical comorbidity if—as is the case for autistic traits—the measured subclinical traits are continuously distributed and causally associated with their more severe manifestations as clinical syndromes (Robinson et al. 2011).
An approach to resolving questions about the mechanisms contributing to overlapping phenotypes is to trace the respective domains to their developmental origins in infancy within a genetically-informative research design. To our knowledge, only two studies have investigated genetic influences on trait overlap between autism and general psychopathology in twins or families prior to school age, and none have investigated genetic influences on trait overlap prior to 2 years of age. Ronald et al. (2010) evaluated associations between ratings on the Child Behavior Checklist (CBCL) Pervasive Developmental Problems Scale (an index of quasi-autistic symptomatology; Achenbach and Rescorla 2000) and ADHD Scale (CBCL ADHD) in a community sample of 2-year-old twins, observing modest phenotypic correlations (r = 0.23–0.26) and shared genetic influences (genetic correlation = 0.27). The utility of the CBCL for ascertaining the characterizing traits and features of autism is somewhat limited, however, because it only captures a fraction of the variance captured by more specific assessment of autistic traits (see Bölte et al. 2008). Micalizzi et al. (2016) used a cross-lagged twin design to evaluate the time course of associations between autistic traits (here again, the CBCL Pervasive Developmental Problems scale was used) and affective problems (CBCL Affective Problems scale) during the third year of life. They observed substantial correlations between autistic traits and affective problems, which were attributable to shared and nonshared environmental influences at age 2 and genetic influences at age 3. In contrast with findings from older children (Hallett et al. 2010), they did not observe reciprocal influences between autistic traits and affective problems over time: autistic traits at age 2 did not influence affective problems at age 3, nor did affective problems at age 2 influence autistic traits at age 3 after controlling for shared genetic and environmental influences.
To better characterize the role of autistic traits in human behavioral development, the present study was designed to prospectively examine relationships among early precursors of social behavioral outcome 1) beginning in infancy (18 months), 2) using specific developmental measures of quantitative autistic traits (QATs; described in Methods, below), and 3) utilizing a comprehensive analytic strategy to evaluate previously uncharacterized associations between QATs and traits of general psychopathology.1 Our primary aim was to evaluate shared genetic and environmental influences on QATs and other early trait-based manifestations of general psychopathology to clarify whether overlap is already present in infancy and, if so, to what degree. The developmental nature of the study design affords potential insights into psychiatric nosology by disentangling independent components of liability that might become confounded (by virtue of their interactions) over the course of development, thereby elucidating domains of overlap and non-overlap among psychopathological constructs (Krueger et al. 2016) and designating potential early intervention targets.
Methods
Participants
Three hundred and fourteen twins and their families participated in the Early Quantitative Characterization of Reciprocal Social Behavior study (ERSB), a longitudinal study characterizing the development of QATs from infancy through the toddler years. Autistic trait data related to this study have been published previously (cf. Marrus et al. 2015), but not as they pertain to developmental overlap between traits of autism and psychopathology. Of these 314 twins, 222 were retained for the duration of the study.2 The twins were epidemiologically ascertained from a record of all twin births that occurred in the state of Missouri between 2011 and 2013 (cf. Constantino et al. 2017). Recruitment protocol and participant characteristics are summarized in Online Resources 1 and 2.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments. Specifically, the WUSM Human Research Protection Office (HRPO; 201208010) and the State of Missouri Department of Health and Senior Services Institutional Review Board (State IRB Approval #1296) approved all study procedures. Informed consent was obtained from parents of twins who participated in the study.
Measures
Measures were completed by the consenting individual. Descriptive statistics for all measures are reported in Table 1, and information regarding their established reliability and validity are reported in Online Resource 3.
Table 1.
Descriptive statistics (mean, standard deviation, range, borderline clinical cut-offs and associated sample characteristics) for study measures
| Mean (SD) | Range | Borderline clinical cut-off (%ile) | Borderline clinical cut-off (score) | # ≥ cut-off | % ≥ cut-off | |
|---|---|---|---|---|---|---|
| vrRSB | ||||||
| SCI | 20.1 (8.1) | 4–65 | 84 | 28 | 45 | 14.3 |
| RRB | 1.7 (2.8) | 0–19 | 84 | 5 | 30 | 9.6 |
| RSB | 21.8 (9.8) | 6–82 | 84 | 31 | 43 | 13.7 |
| BITSEA | ||||||
| Problem | 7.6 (5.0) | 0–34 | 75 | 13 girls, 15 boys | 38 | 12.1 |
| Competence* | 16.9 (2.9) | 4–22 | 85 | 14 | 52 | 16.6 |
| SRS | ||||||
| SCI | 24 (14.7) | 0–115 | 84 | 58 | 4 | 1.8 |
| RRB | 3.2(3.9) | 0–32 | 84 | 10 | 14 | 6.3 |
| RSB | 27.2 (17.9) | 0–147 | 84 | 67 | 6 | 2.7 |
| CBCL | ||||||
| Internalizing | 4.7 (4.6) | 29–71 | 84 | 14 | 6 | 2.7 |
| Externalizing | 7.5 (6.8) | 28–76 | 84 | 21 | 7 | 3.2 |
*Lower scores are of greater clinical concern
Video-Referenced Rating of Reciprocal Social Behavior
A video-referenced rating of Reciprocal Social Behavior (vrRSB; Marrus et al. 2015) was used to ascertain autistic traits at baseline. The vrRSB is described in detail by Marrus et al. (2015). Briefly, it is a 44-item quantitative autistic trait scale suitable for children 12 through 24 months of age. To help parents make nuanced evaluations of variations in early social communication, the vrRSB provides a 3-min video to serve as a ‘scoring anchor.’ In this video, a 19-month old child interacts with several adults. Throughout the video, critical early social behaviors (e.g., turn-taking, motivation to engage, responsiveness to social cues) are portrayed. The scoring anchor is designed to provide a naturalistic benchmark against which to evaluate early childhood behavior, thereby standardizing informants’ responses to items. Following the video, parents compare their child’s behavior to behavior portrayed in the video scoring anchor for 13 “video-referenced” items probing aspects of social communication and interaction. The remaining 31 “non-video-referenced” items also assess behaviors related to DSM5 clinically important domains of ASD, specifically, social communication and interaction (SCI) and restricted interests and repetitive behavior (RRB; Marrus et al. 2015). The vrRSB Total score quantifies reciprocal social behavior (RSB; hereafter, vrRSB Total will be referred to as RSB) and consists of SCI and RRB subscales. Higher RSB, SCI, and RRB scores indicate greater impairment. Importantly, RSB and SCI scores are continuously distributed, capturing trait variation in the general population (Marrus et al. 2015).
Brief Infant-Toddler Social and Emotional Assessment
The Brief Infant-Toddler Social and Emotional Assessment (BITSEA; Briggs-Gowan and Carter 2006) is a 42-item quantitative trait scale assessing social-emotional and behavioral milestones and delays in children one through three years of age. It is a well-established screening tool (Briggs-Gowan and Carter 2006; Briggs-Gowan et al. 2013), can be administered to parents as a questionnaire or as part of a structured interview, and takes roughly seven minutes to complete. The BITSEA generates two main scores: 1) a Behavior Problem score consisting of Externalizing, Internalizing, and Dysregulation subscales, and 2) a Competence score measuring key features of social adaptation, including social-emotional skills and aspects of social relatedness. Specifically, the BITSEA Competence score is comprised of 11 items assessing rule-abiding behavior, play behavior, attention, empathy, motivation to master new skills, and quality of peer interactions. Qualitatively, these items exhibit substantial overlap with the vrRSB SCI scale; however, SCI includes more items assessing communicative competency and social engagement.
Social Responsiveness Scale, Second Edition, Preschool Version
The Social Responsiveness Scale, second edition (SRS-2; Constantino and Gruber 2012) is a 65-item quantitative trait scale measuring quantitative autistic traits (QATs) from preschool through adulthood and requiring about 15–20 min to complete. The SRS-2 Total score quantifies QATs and encompasses both DSM5 criterion domains of ASD (SCI and RRB). Higher Total scores indicate greater impairment. In the present study, SRS-2 Total served as the primary outcome measure in prospective longitudinal predictions of QATs.
Child Behavior Checklist
The Achenbach Scales of Empirically Based Assessment (i.e., the Child Behavior Checklist, CBCL; Achenbach and Rescorla 2000) is an extensively validated parent- and/or teacher-report measure of behavior problems during preschool and childhood (Verhulst and Van der Ende 1992). The CBCL preschool forms (ages 1.5–5 years) yield seven syndrome scores (Emotionally Reactive, Anxious/Depressed, Somatic Complaints, Withdrawn, Sleep Problems, Attention Problems, and Aggressive Behavior) and five DSM-oriented scores (Depressive, Anxiety, Autism Spectrum, Attention Deficit/Hyperactivity, and Oppositional Defiant Problems). Emotionally Reactive, Anxious/Depressed, Somatic Complaints, and Withdrawn syndrome scores can be combined to create an Internalizing composite, and Aggressive Behavior and Attention Problems syndrome scores can be combined to create an Externalizing composite. In the present study, Internalizing and Externalizing composites served as the primary outcome measures in prospective longitudinal predictions of psychopathology. Although there is evidence to support their use as autism screeners, Withdrawn and Autism Spectrum Problems scores were not included in the present analyses due to low discriminative validity (area under the curve = 0.68; Havdahl et al. 2016) and lower sensitivity and specificity for autism relative to the SRS (Hampton and Strand 2015).
Goldsmith Child Zygosity Questionnaire
The Goldsmith Child Zygosity Questionnaire (Goldsmith 1991) is a 27-item parent-report measure developed to assess the degree of physical similarity between twins, from which determinations about zygosity can be made. Agreement with biological indicators of zygosity has been shown to exceed 93% (Price et al. 2000). In the present study, the Goldsmith Child Zygosity Questionnaire was administered over the phone to all families of same-sex twin pairs. Questionnaire-based zygosity determinations were genetically confirmed in 24 randomly-selected families; correspondence between questionnaire and genetic determinations was observed in all instances. Twins pairs were excluded from analyses (ntwin pairs = 6) if zygosity could not be determined by questionnaire.
Data Analysis
All analyses were conducted in R (R Studio, 2016). First, Pearson product-moment correlations were computed to explore bivariate associations among BITSEA (Behavior Problem, Competence, Internalizing, Externalizing, Dysregulation) and vrRSB (RSB, SCI, RRB) subscales. Non-independence of twin observations was mitigated by randomly selecting one twin per family for inclusion in the primary set of analyses, with co-twins used for the purpose of quasi-replication. Next, we implemented exploratory factor analysis (EFA) as an initial step in empirically-deriving independent and overlapping domains of behavioral variation from assessment data collected at baseline. Subscales rather than individual items were entered into factor analyses to increase the ratio of number of observations to number of items. These subscales included Externalizing, Internalizing, Dysregulation, Competence, SCI (social communication and interaction), and RRB (restricted interests and repetitive behavior) indices. Composite indices (i.e., Behavior Problem and RSB) were omitted to avoid item redundancy. As above, dependencies in twin data were accounted for by randomly selecting one twin per family to be included in analyses. Observations were evaluated via principal component and minimum residual factoring methods, followed by oblimin rotation. Tucker Lewis Index (TLI) values 0.90–0.92 were judged adequate fit, 0.92–0.95 good fit, and > 0.95 excellent fit (Marsh et al. 2004); root mean square error of approximation (RMSEA) values < 0.10 were judged adequate fit and < 0.08 good fit (Kline 1998). Differences between models were considered significant if a reduction in the Bayesian information criterion (BIC) greater than 5 points was observed (Kass and Raftery 1995).
Falconer’s formula (Falconer 1960) was used to provide estimates of broad heritability for early developmental domains (SCI, Competence, Behavior Problem): Heritability = 2(rMZ– rDZ). Within domains, heritability was estimated separately for male/male and female/female twin pairs, as well as for the entire sample. Although Falconer’s formula is commonly used to estimate heritability (Rice 2008), it overestimates genetic contributions to behavior in the context of nonadditive variance (Lykken et al. 1993). Thus, estimates of heritability were capped at monozygotic twin correlations in the present study.
Finally, hierarchical linear modeling (HLM) was used to 1) investigate domains of overlap during early development and 2) predict QATs, internalizing, and externalizing behaviors at 36 months. To account for twin dependency, family was modeled as a random effect.
Results
Correlation Analyses
To provide an indication of interrelationships among early developmental traits, Pearson product-moment correlations were calculated examining subscale-level associations at baseline between quantitative measures of general psychopathology (i.e., Competence and Behavior Problem indices, wherein the Behavior Problem index consisted of Externalizing, Internalizing, and Dysregulation subscales) and autistic traits (i.e., total vrRSB score, consisting of SCI and RRB subscales). As expected, Competence was not significantly correlated with Behavior Problem subscales, and both the total vrRSB score and SCI exhibited only weak correlations with Behavior Problem subscales (Table 2; see Online Resource 4 for co-twin analyses). Meanwhile, Competence, SCI, and total vrRSB score exhibited strong correlations with each other, and Behavior Problem subscales exhibited modest correlations with each other. RRB was significantly, albeit modestly, correlated with all scales. Importantly, this overall pattern of results suggests that Competence, SCI, and the total vrRSB score are highly similar to each other and highly distinct from other subscales.
Table 2.
Pearson product-moment correlations (ntwins = 154) among measures of general psychopathology and QATs at 18 months
| Extl. | Intl. | Dysreg. | Behav. Probs | Comp. | SCI | RRB | RSB | |
|---|---|---|---|---|---|---|---|---|
| Externalizing | 1 | |||||||
| Internalizing | 0.20* | 1 | ||||||
| Dysregulation | 0.36*** | 0.41*** | 1 | |||||
| Behavior Problems | 0.70*** | 0.65*** | 0.79*** | 1 | ||||
| Competence | 0.03 | 0.05 | −0.04 | 0.05 | 1 | |||
| SCI | 0.08 | 0.27*** | 0.18* | 0.25** | 0.70*** | 1 | ||
| RRB | 0.24** | 0.23** | 0.37*** | 0.46*** | 0.26** | 0.46*** | 1 | |
| RSB | 0.13 | 0.29*** | 0.26** | 0.34*** | 0.66*** | 0.97*** | 0.67*** | 1 |
*** p < 0.001, ** p < 0.01, * p < 0.05; Ext. = externalizing, Intl. = internalizing, Dysreg. = dysregulation, Behav. Probs = Behavior Problems, Comp. = Competence
Factor Structure at Baseline
To evaluate whether correlational associations reflected an underlying factor structure, vrRSB and BITSEA subscales were submitted to exploratory factor analyses (EFA). Results of EFA identified a two-factor solution wherein SCI and Competence comprised a “social adaptation” factor and Externalizing, Internalizing, Dysregulation, and RRB comprised a “problem behaviors” factor (Online Resources 5 & 6; see Online Resources 7 & 8 for co-twin analyses). Whereas SCI loaded strongly onto social adaptation (twin loading = 0.99, co-twins loading = 1.00) and weakly onto problem behaviors (twin loading = 0.05, co-twin loading = 0.02), factor loadings for RRB were equivocal (twin loadings = 0.36, 0.42 and co-twin loadings = 0.38, 0.37 for social adaptation and problem behaviors, respectively). In this sense, RRB diverged from SCI despite strong correlations between the two traits within individuals. In conjunction with the limited number of (n = 11) and variation in (SD = 2.8) RRB items in typically-developing children, this divergence informed our decision to use SCI as the primary index of autistic traits. The heritability of RRB is provided in Online Resource 9, and we note that its factor structure warrants exploration in future studies.
Heritability
Having characterized factor structure, we aimed to determine whether the distinction between social adaptation and behavior problems may have emerged, in part, due to differences in patterns of genetic transmission. Thus, the heritabilities of Competence, Behavior Problem, and SCI indices were examined at baseline. Falconer’s estimates of heritability are reported in Table 3. Irrespective of gender, heritability estimates appeared roughly 2.5–3 times larger for SCI and Competence indices compared to the Behavior Problem index. The magnitude of these estimates warrants mention. Across all participants, the estimation of heritability for SCI was 0.90 and the estimation of heritability for Competence was 0.89, indicating that a large majority of variance in these traits can be attributed to genetic factors. In contrast, the estimation of heritability for the Behavior Problem index was 0.30, indicating that environmental factors account for a greater proportion of variance in this trait than genetic factors.
Table 3.
Twin correlations (rMZ and rDZ), associated confidence intervals (5%, 95%), and Falconer’s heritability estimates (H2) at 18 months
| Subscale | Falconer’s heritability | |||
|---|---|---|---|---|
| npairs | r MZ | r DZ | H2 | |
| SCI | ||||
| Male/Male | 58 | 0.89 (0.77, 0.95) | 0.30 (−0.06, 0.59) | 0.89 |
| Male/Female | 36 | NA | 0.15 (−0.19, 0.45) | NA |
| Female/Female | 56 | 0.91 (0.80, 0.96) | 0.48 (0.15, 0.71) | 0.85 |
| All | 150 | 0.90 (0.84, 0.94) | 0.27 (0.08, 0.45) | 0.90 |
| Competence | ||||
| Male/Male | 58 | 0.92 (0.83, 0.96) | 0.29 (−0.07, 0.59) | 0.92 |
| Male/Female | 36 | NA | 0.35 (0.03, 0.61) | NA |
| Female/Female | 56 | 0.78 (0.56, 0.89) | 0.38 (0.03, 0.65) | 0.78 |
| All | 150 | 0.89 (0.81, 0.93) | 0.37 (0.19, 0.53) | 0.89 |
| Behavior Problem | ||||
| Male/Male | 58 | 0.82 (0.65, 0.91) | 0.67 (0.41, 0.83) | 0.31 |
| Male/Female | 36 | NA | 0.54 (0.26, 0.73) | NA |
| Female/Female | 56 | 0.68 (0.41, 0.85) | 0.55 (0.24, 0.76) | 0.27 |
| All | 150 | 0.74 (0.59, 0.84) | 0.59 (0.44, 0.71) | 0.30 |
npairs = number of twin pairs; MZ = monozygotic; DZ = dizygotic; H2 = broad heritability
Construct Overlap
Given the above results, suggesting a dissociation between Behavior Problem indices and SCI and Competence, we next investigated the extent to which quantitative risk factors for autism and general psychopathology are shared in infancy. To this end, we evaluated construct overlap among SCI, Competence, and Behavior Problems at baseline using hierarchical linear modeling (HLM; Online Resource 10). In the first regression, Behavior Problem and Competence indices were used to predict SCI. The Behavior Problem index was entered as the sole predictor in Model 1A, Competence was entered as the sole predictor in Model 1B, and both Behavior Problem and Competence indices were entered as predictors in Model 2. This incremental approach enabled calculation of the proportion of unique variance in SCI accounted for by Behavior Problems (marginal R2 Model 2 – unique R2 Model 1B) and Competence (marginal R2 Model 2 – unique R2 Model 1A). Results indicated that Behavior Problems accounted for 4% of unique variance in SCI, whereas Competence accounted for 48% of unique variance in SCI.
We ran similar incremental analyses with Competence and Behavior Problem indices as our dependent variables (Online Resource 10). Importantly, differences in unique R2 values were smaller when comparing incremental analyses within a given domain (e.g., Model 1B vs. Model 4A, both modeling overlap between Competence & SCI) relative to across domains (e.g., Model 1B vs. Model 2A, modeling overlap between Competence & SCI and Problems & SCI, respectively). This suggested that our estimates of overlap were reliable. To visualize subscale relationships, we created a Venn diagram depicting average estimates of overlap among SCI, Competence, and Behavior Problem indices (Fig. 1). In line with predictions, SCI and Competence exhibited considerable overlap, accounting for almost half of each other’s variance. Meanwhile, SCI and Behavior Problem indices and Competence and Behavior Problem indices exhibited considerably less overlap. These results provide converging evidence for the phenotypic similarity of traits assessed by SCI and Competence.
Fig. 1.
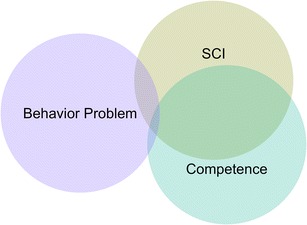
Venn diagram approximately to scale depicting construct overlap among Behavior Problem, SCI, and Competence indices. Overlap between Behavior Problem and SCI (4%) and Behavior Problem and Competence (1%) indices was minimal, whereas overlap between Competence and SCI indices was substantial (48%). These results emphasize the phenotypic similarity between Competence and SCI
Prospective Longitudinal Cross-Trait Prediction at 36 months
Despite relative non-overlap between Behavior Problem indices and SCI and Competence at 18 months, evidence indicates that such traits are inextricably linked in childhood (e.g., Hallett et al. 2010). To clarify the time course of autistic and psychopathologic quantitative trait overlap, we proceeded to investigate the temporal trait and cross-trait stabilities of SCI, Competence, and Behavior Problem indices. Specifically, we were interested whether infant measures of general psychopathology (i.e., the Behavior Problem index) would predict the severity of autistic traits during the toddler years (i.e., QATs; assessed by SRS-2 Total) above and beyond infant measures of social communicative autistic traits (i.e., SCI). Conversely, we were interested whether infant measures of social communicative autistic traits would predict the severity of internalizing and/or externalizing behavior during the toddler years (assessed by the CBCL; Internalizing and Externalizing subscales) above and beyond infant measures of general psychopathology. Finally, we were interested whether Competence would significantly improve predictive capacities above and beyond within-trait predictors. To this end, we ran incremental regressions using HLM. Within-trait predictors were entered into incremental models at Step 1, Competence was entered at Step 2, and cross-trait predictors were entered at Step 3. Independent variables were assessed at 18 months and dependent variables were assessed at 36 months.
Results are detailed in Online Resource 11. As expected, Step 1 (within-trait prediction) was uniformly significant, with SCI accounting for 26% of the variance in QATs and the Behavior Problem index accounting for 15% and 9% of the variances in Internalizing and Externalizing behavior, respectively. In Step 2, Competence accounted for a small albeit significant proportion of additional variance in QATs (unique R2 = 3%); Competence did not account for significant additional variance in either Internalizing or Externalizing behavior. Finally, in Step 3 (cross-trait prediction), the Behavior Problem index accounted for a small albeit significant proportion of additional variance in QATs (unique R2 = 2%); SCI did not account for significant additional variance in either Internalizing or Externalizing behavior. Given Behavior Problem and RRB indices clustered together in factor analyses, it remained unclear to what extent the Behavior Problem index improved prediction of QATs above and beyond comprehensive measures of autistic traits indexing both social communication and restricted, repetitive behaviors. To this end, a fourth incremental regression was run substituting RSB, a composite scale derived from SCI and RRB, for SCI. Under these conditions, Behavior Problems ceased to explain significant additional variance in QATs (χ2 = 3.11, p = 0.08).
Considering the above factor analyses, heritability analyses, and patterns of cross-trait overlap, these findings suggest that half of the causal influence on Competence is shared with that of SCI (Fig. 1), and that the construct jointly indexed by SCI and Competence accounts for about one third of the variation in autistic trait burden at 36 months (Fig. 2). Meanwhile, Behavior Problem indices exhibited separable mechanisms of causation relative to Competence and SCI and significantly predicted internalizing and externalizing traits at 36 months. Of note, correlations between autistic traits and internalizing (r = 0.52, p < 0.001) and externalizing (r = 0.30, p < 0.001) traits were roughly two times larger at 36 compared to 18 months, suggesting a strengthening relationship among these constructs over the course of early development.
Fig. 2.
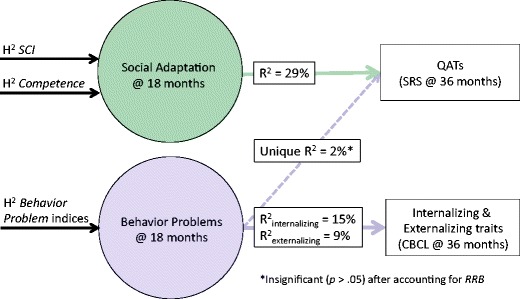
Prospective longitudinal prediction of QATs and general psychopathologic traits at 36 months from social adaptation (Competence, SCI) and behavior problems at 18 months. R2 = proportion of variance explained; unique R2 = proportion of incremental variance explained
Discussion
To our knowledge, the present study is the first to assess standardized ratings of both early autistic traits and general psychopathologic traits 1) during the first two years of life and 2) in a prospective, genetically-informative sample, epidemiologically ascertained from the general population through birth records. Doing so allowed us to investigate the extent to which autistic and general psychopathologic traits—for which extreme variation constitutes clinical abnormality—overlap early in development. We observed a general pattern of independence of these constructs, which manifested highly disparate genetic and environmental structures. Furthermore, we observed that Competence, a well-known index of social adaptation measuring early developmental changes in social-emotional skills and social relatedness (e.g., empathy, play behavior, mastery motivation), exhibited substantial factorial and genetic overlap with variation in SCI (social communication and interaction), which represents a core DSM5 criterion domain for autism. SCI accounted for almost half of variance in Competence at 18 months, and SCI and Competence demonstrated strong heritability. Importantly, we can be confident that rater bias was minimal given observations of high heritability, which are predicated on a single parent’s ability to provide discrepant data regarding non-identical siblings (see Zeeuw et al. 2017 for a discussion of rater bias in parent report measures), and the fact that study measures that were implemented have strong evidence for experimental validity against independent observational assessments (e.g., Briggs-Gowan and Carter 2008; Constantino and Gruber 2005; Keenan and Wakschlag 2000).
In contrast, early developmental precursors of internalizing and externalizing behaviors (Behavior Problem indices) were predominantly influenced by environmental rather than genetic factors in infancy and were only weakly correlated with SCI. The superordinate construct comprised of SCI and Competence explained roughly one-third of the variation in quantitative autistic traits at age 3 but did not improve explanatory power for internalizing or externalizing traits above and beyond Behavior Problem indices, reflecting its role as a major parameter of adaptation that is distinct and dissociable from general psychopathology. Interestingly, RRB (restricted interests and repetitive behavior) was more strongly correlated with Behavior Problem than Competence and SCI indices and segregated with general psychopathologic traits in exploratory factor analyses. Although such results are consistent with a theory of fractionation of autistic symptomatology (e.g., Happé et al. 2006), we defer interpretation of these findings about the role of RRB in development, given the instability of our factor analyses with respect to RRB (see Online Resources 7 & 8) and aforementioned psychometric limitations (e.g., limited number of and variation in RRB items) associated with the measurement of RRBs in the present normative sample. Further work with more comprehensive, RRB-specific measures—which may prove most informative in a sample enriched for children with elevations in autistic trait burden—is warranted to determine whether and how such behaviors influence the developmental course of trait overlap between risk factors for autism and general psychopathology.
Recasting the early psychological construct of Competence as one that arises from the same heritable influences that—at the extremes of trait aggregation within an individual—constitute social communicative deficits in autism represents a substantial shift in prevailing notions of psychological Competence. Specifically, it suggests that understanding the genetic origins of social communicative autistic trait variation is critical to understanding early behavioral adaptation in the general population. Extending recent advances from the field of autism genetics, which indicate that common genetic variants operating in an additive fashion incur heritable influences associated with autism spectrum disorder (Constantino et al. 2013; Gaugler et al. 2014), the present study supports the notion that such common variants represent heritable influences associated with social adaptation for all children. With respect to treatment implications, this raises the possibility that existing social communication interventions for autism may help resolve social and emotional impairments that often co-occur with psychopathology in the absence of categorical autism. However, further research in clinical populations is needed to evaluate the utility of such treatments.
Consistent with Micalizzi et al. (2016), results of the present study indicate that social communicative autistic impairment and general psychopathologic impairment are separable developmental domains with distinct genetic etiologies. Additionally, our results suggest that associations between autistic and psychopathologic traits strengthen over the course of early development (i.e., 18 to 36 months), bridging research in older samples that demonstrated strong correlations between degree of autistic impairment and degree of internalizing (Hallett et al. 2012) and externalizing (Ronald et al. 2010; Taylor et al. 2013) behaviors. Since studies in older children have identified shared genetic variance for autistic impairment and symptoms of general psychopathology (Hallett et al. 2012; Taylor et al. 2013; Ronald et al. 2008; Ronald et al. 2010), one way to reconcile the apparent discrepancy in causal overlap between studies of infants and studies of children is to infer that non-specific (general) psychopathologic symptoms can affect the severity of autistic syndromes (and vice versa) over the course of development—this would reflect the same phenomenon of phenotypic pleiotropy that was recently observed in a sibling study of autism recurrence (Mous et al. 2017) in which symptoms of ADHD and motor impairment exacerbated autistic severity in the context of inherited familial susceptibility to autism.
Importantly, whereas studies of trait overlap in older samples conflate shared genetic influences (i.e., genetic factor A directly influences both traits of autism and internalizing disorder traits) with longitudinal, interactive effects (i.e., a mediation model; genetic factor A influences traits of autism, which in turn influence internalizing disorder traits), studies in infant samples are well-positioned to disentangle these competing hypotheses. Integrating the present findings with extant literature, a pattern emerges wherein shared genetic variance is commonly observed during childhood but not infancy, suggesting a mediation model wherein genetic influences on autistic traits may exacerbate symptoms of general psychopathology over the course of development. Consistent with this notion, abnormalities in Competence during the toddler years predict psychiatric symptoms in elementary school (Briggs-Gowan and Carter 2008). If supported by subsequent research, this carries the implication that adverse interactions between autistic and psychopathologic liabilities might be preventable if recognized in infancy or early childhood. Alternatively, it is possible that genetic influences on autistic and psychopathologic traits take effect at different times.
Although the size of our twin sample was substantial and more than adequate to detect the associations described in this report, the derivation of more precise estimates of heritability using structural equation modeling was not possible at this sample size. Future studies of larger samples would be expected to replicate the heritability estimates obtained by Falconer’s formula. Sample size limitations also precluded item-level factor analyses on Competence and SCI subscales. Such analyses could provide more fine-grained information about the specific elements of SCI and Competence that contribute most strongly to their correlation, thereby designating effective targets for behavioral intervention. It bears mention, however, that there is heterogeneity in autistic syndromes. While this work supports measurable trait-level stability, heterogeneity implies the potential for different developmental mechanisms underlying autistic traits. For example, there are some autistic syndromes whose emergence is preceded by relatively normative development through the age of two years followed by severe regression. In such circumstances, the observed relationships may not apply. Finally, it remains unknown at what age interactive effects between social communicative autistic traits and traits of general psychopathology emerge. Future studies examining the relationship between these constructs over the course of early development, spanning infancy to early school age, are needed to fully characterize the time course and behavioral corollaries of trait overlap. This knowledge, in turn, may pinpoint critical periods of intervention during which negative interactions between autistic traits and traits of general psychopathology can be mitigated.
Conclusions
The present study is the first to trace causal overlap between quantitative, social communicative autistic traits—assessed using a newly-validated measure of early reciprocal social behavior—and specific aspects of general psychopathologic traits to their developmental origins in infancy. At this early juncture, two established predictors of general psychopathology (social competence and problem behaviors) were independent and exhibited separable mechanisms of causation (the former highly heritable, the latter at this age strongly influenced by common environmental factors). Given that autistic trait variation in the general population is related to the genetic causes of autism itself (Ronald et al. 2006; Robinson et al. 2011; Constantino 2014), characterizing the early developmental time course of associations between autistic and psychiatric traits is essential to understanding a) the emergence of their well-documented trait overlap (in typically-developing populations), b) autism comorbidity (in clinical populations), and c) the factors that modulate enduring features of social behavioral adaptation in all children. We note that it would be consistent with these findings that the superimposition of independently-inherited psychopathologic trait liabilities upon a critical level of autistic trait liability might actually contribute to the causation of autism itself (as a categorical clinical condition), particularly in cases in which symptomatic features of joint (comorbid) liability remain appreciable over the course of later development. At a minimum, results of the present study indicate that the clinical comorbidity of specific psychiatric syndromes with autism may arise from interactions between autistic liability and independent susceptibilities to other psychopathologic traits. This suggests opportunities for preventive amelioration of outcomes of these interactions in infancy, perhaps via interventions to target developmentally-independent, behaviorally-modifiable traits (e.g., emotional dysregulation or attention problems—see Mous et al. 2017) that may conspire to exacerbate autistic severity over the course of development.
Electronic supplementary material
(PDF 60.1 kb)
(PDF 70.3 kb)
(PDF 96.6 kb)
(PDF 56.9 kb)
(PDF 60.7 kb)
(PDF 71.2 kb)
(PDF 67.1 kb)
(PDF 74.6 kb)
(PDF 66.4 kb)
(PDF 84.2 kb)
(PDF 96.9 kb)
Acknowledgments
We thank Yi Zhang and Julie Grant for their statistical guidance and feedback on earlier drafts of the manuscript. We also thank all of the families who participated in the Early Quantitative Characterization of Reciprocal Social Behavior study for their generous investment of time and effort.
Funding
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Numbers R01 HD068479 and U54 HD087011 (the Intellectual and Developmental Disabilities Research Center at Washington University). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
JNC receives royalties from Western Psychological Services for the commercial distribution of the Social Responsiveness Scale, a quantitative measure of autistic traits for ages 30 months through adulthood. The remaining authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments. Specifically, the WUSM Human Research Protection Office (HRPO; 201208010) and the State of Missouri Department of Health and Senior Services Institutional Review Board (State IRB Approval #1296) approved all study procedures.
Informed Consent
Informed consent was obtained from parents of twins who participated in the study.
Footnotes
Much like QATs, these general psychopathologic traits are influenced by the same underlying liability as their pathological counterparts (Spatola et al. 2010; Oord et al. 2003), providing an important vantage point from which to study early precursors of childhood and adolescent psychopathology. Although functional impairment from a singular pathologic trait typically only occurs at extreme levels of aggregation (e.g. 2 SD from the population mean), significant impairment can also arise from less extreme aggregation of two or more pathological traits within an individual.
Minor variations in sample size across analyses due to missing data are reported in Tables and Online Resources.
References
- Achenbach, T. M., & Rescorla, L. A. (2000). Child behavior checklist for ages 1 1/2-5. ASEBA. Burlington: University of Vermont, Dept. of Psychiatry.
- Angold A, Costello EJ, Erkanli A. Comorbidity. Journal of Child Psychology and Psychiatry. 1999;40(1):57–87. [PubMed] [Google Scholar]
- Bölte S, Poustka F, Constantino JN. Assessing autistic traits: cross-cultural validation of the social responsiveness scale (SRS) Autism Research. 2008;1(6):354–363. doi: 10.1002/aur.49. [DOI] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Carter AS. Brief infant-toddler social and emotional assessment (BITSEA) manual. San Antonio: PsychCorp, Harcourt Assessment; 2006. [Google Scholar]
- Briggs-Gowan MJ, Carter AS. Social-emotional screening status in early childhood predicts elementary school outcomes. Pediatrics. 2008;121(5):957–962. doi: 10.1542/peds.2007-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Carter AS, McCarthy K, Augustyn M, Caronna E, Clark R. Clinical validity of a brief measure of early childhood social–emotional/behavioral problems. Journal of Pediatric Psychology. 2013;38(5):577–587. doi: 10.1093/jpepsy/jst014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN. Recurrence rates in autism spectrum disorders. JAMA. 2014;312(11):1154–1155. doi: 10.1001/jama.2014.9841. [DOI] [PubMed] [Google Scholar]
- Constantino JN. Measurement of autism symptomatology in children with neurodevelopmental impairment. Journal of the American Academy of Child & Adolescent Psychiatry. 2017;56(4):354–355. doi: 10.1016/j.jaac.2017.01.017. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Frazier TW. Commentary: The observed association between autistic severity measured by the social responsiveness scale (SRS) and general psychopathology–a response to Hus et al.() Journal of Child Psychology and Psychiatry. 2013;54(6):695–697. doi: 10.1111/jcpp.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social responsive scale (SRS) manual. Los Angeles: Western Psychological Services; 2005. [Google Scholar]
- Constantino JN, Gruber CP. Social responsiveness scale (SRS-2) Torrance: Western Psychological Services; 2012. [Google Scholar]
- Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Archives of General Psychiatry. 2003;60(5):524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Hudziak JJ, Todd RD. Deficits in reciprocal social behavior in male twins: Evidence for a genetically independent domain of psychopathology. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42(4):458–467. doi: 10.1097/01.CHI.0000046811.95464.21. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todorov A, Hilton C, Law P, Zhang Y, Molloy E, et al. Autism recurrence in half siblings: Strong support for genetic mechanisms of transmission in ASD. Molecular Psychiatry. 2013;18(2):137. doi: 10.1038/mp.2012.9. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Kennon-McGill S, Weichselbaum C, Marrus N, Haider A, Glowinski AL, et al. Infant viewing of social scenes is under genetic control and is atypical in autism. Nature. 2017;547(7663):340–344. doi: 10.1038/nature22999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvekot J, Leontine W, Slappendel G, van der Ende J, Verhulst FC, van der Sijde A, Greaves-Lord K. Design and cohort characteristics of the social spectrum study: a multicenter study of the autism spectrum among clinically referred children. Journal of Autism and Developmental Disorders. 2017;47(1):33–48. doi: 10.1007/s10803-016-2919-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworzynski K, Happé F, Bolton P, Ronald A. Relationship between symptom domains in autism spectrum disorders: a population based twin study. Journal of Autism and Developmental Disorders. 2009;39(8):1197–1210. doi: 10.1007/s10803-009-0736-1. [DOI] [PubMed] [Google Scholar]
- Falconer DS. Introduction to quantitative genetics. Edinburgh: Oliver and Boyd; 1960. [Google Scholar]
- Gaugler T, Klei L, Sanders S, Bodea C, Goldberg A, Lee A, et al. Most genetic risk for autism resides with common variation. Nature Genetics. 2014;46(8):881–885. doi: 10.1038/ng.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH. Genetics of autism spectrum disorders. Trends in Cognitive Sciences. 2011;15(9):409–416. doi: 10.1016/j.tics.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HH. A zygosity questionnaire for young twins: a research note. Behavior Genetics. 1991;21(3):257–269. doi: 10.1007/BF01065819. [DOI] [PubMed] [Google Scholar]
- Hallett V, Ronald A, Rijsdijk F, Happé F. Association of autistic-like and internalizing traits during childhood: a longitudinal twin study. American Journal of Psychiatry. 2010;167(7):809–817. doi: 10.1176/appi.ajp.2009.09070990. [DOI] [PubMed] [Google Scholar]
- Hallett V, Ronald A, Rijsdijk F, Happé F. Disentangling the associations between autistic-like and internalizing traits: a community based twin study. Journal of Abnormal Child Psychology. 2012;40(5):815–827. doi: 10.1007/s10802-011-9596-1. [DOI] [PubMed] [Google Scholar]
- Hallett V, Ronald A, Colvert E, Ames C, Woodhouse E, Lietz S, et al. Exploring anxiety symptoms in a large-scale twin study of children with autism spectrum disorders, their co-twins and controls. Journal of Child Psychology and Psychiatry. 2013;54(11):1176–1185. doi: 10.1111/jcpp.12068. [DOI] [PubMed] [Google Scholar]
- Hampton J, Strand PS. A review of level 2 parent-report instruments used to screen children aged 1.5–5 for autism: a meta-analytic update. Journal of Autism and Developmental Disorders. 2015;45(8):2519–2530. doi: 10.1007/s10803-015-2419-4. [DOI] [PubMed] [Google Scholar]
- Happé F, Angelica R, Plomin R. Time to give up on a single explanation for autism. Nature Neuroscience. 2006;9(10):1218. doi: 10.1038/nn1770. [DOI] [PubMed] [Google Scholar]
- Havdahl KA, von Tetzchner S, Huerta M, Lord C, Bishop SL. Utility of the child behavior checklist as a screener for autism spectrum disorder. Autism Research. 2016;9(1):33–42. doi: 10.1002/aur.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass RE, Raftery AE. Bayes factors. Journal of the American Statistical Association. 1995;90(430):773–795. [Google Scholar]
- Keenan K, Wakschlag LS. More than the terrible twos: the nature and severity of behavior problems in clinic-referred preschool children. Journal of Abnormal Child Psychology. 2000;28(1):33–46. doi: 10.1023/a:1005118000977. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modelling. New York: The Guilford Press; 1998. [Google Scholar]
- Krueger, R. F., Tackett, J. L., & MacDonald, A. (2016). Toward validation of a structural approach to conceptualizing psychopathology: a special section of the journal of abnormal psychology. Journal of Abnormal Psychology, 125(8), 1023. [DOI] [PubMed]
- Lahey BB, Krueger RF, Rathouz PJ, Waldman ID, Zald DH. A hierarchical causal taxonomy of psychopathology across the life span. Psychological Bulletin. 2017;143(2):142–186. doi: 10.1037/bul0000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundström S, Reichenberg A, Melke J, Råstam M, Kerekes N, Lichtenstein P, et al. Autism spectrum disorders and coexisting disorders in a nationwide Swedish twin study. Journal of Child Psychology and Psychiatry. 2015;56(6):702–710. doi: 10.1111/jcpp.12329. [DOI] [PubMed] [Google Scholar]
- Lykken DT, Bouchard TJ, Jr, McGue M, Tellegen A. Heritability of interests: a twin study. Journal of Applied Psychology. 1993;78(4):649. doi: 10.1037/0021-9010.78.4.649. [DOI] [PubMed] [Google Scholar]
- Marrus N, Glowinski AL, Jacob T, Klin A, Jones W, Drain CE, et al. Rapid video-referenced ratings of reciprocal social behavior in toddlers: a twin study. Journal of Child Psychology and Psychiatry. 2015;56(12):1338–1346. doi: 10.1111/jcpp.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh HW, Hau KT, Wen Z. In search of golden rules: Comment on hypothesis-testing approaches to setting cutoff values for fit indexes and dangers in overgeneralizing Hu and Bentler's (1999) findings. Structural Equation Modeling. 2004;11(3):320–341. [Google Scholar]
- Micalizzi L, Ronald A, Saudino KJ. A genetically informed cross-lagged analysis of autistic-like traits and affective problems in early childhood. Journal of Abnormal Child Psychology. 2016;44(5):937–947. doi: 10.1007/s10802-015-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-De-Luca A, Myers SM, Challman TD, Moreno-De-Luca D, Evans DW, Ledbetter DH. Developmental brain dysfunction: revival and expansion of old concepts based on new genetic evidence. The Lancet Neurology. 2013;12(4):406–414. doi: 10.1016/S1474-4422(13)70011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mous SE, Jiang A, Agrawal A, Constantino JN. Attention and motor deficits index non-specific background liabilities that predict autism recurrence in siblings. Journal of Neurodevelopmental Disorders. 2017;9(1):32. doi: 10.1186/s11689-017-9212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oord EJ, Pickles A, Waldman ID. Normal variation and abnormality: an empirical study of the liability distributions underlying depression and delinquency. Journal of Child Psychology and Psychiatry. 2003;44(2):180–192. doi: 10.1111/1469-7610.00112. [DOI] [PubMed] [Google Scholar]
- Price TS, Freeman B, Craig I, Petrill SA, Ebersole L, Plomin R. Infant zygosity can be assigned by parental report questionnaire data. Twin Research and Human Genetics. 2000;3(3):129–133. doi: 10.1375/136905200320565391. [DOI] [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Volk HE, Todd RD. Autistic traits in a population-based ADHD twin sample. Journal of Child Psychology and Psychiatry. 2007;48(5):464–472. doi: 10.1111/j.1469-7610.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- Rice TK. Familial resemblance and heritability. Advances in Genetics. 2008;60:35–49. doi: 10.1016/S0065-2660(07)00402-6. [DOI] [PubMed] [Google Scholar]
- Robinson EB, Koenen KC, McCormick MC, Munir K, Hallett V, Happé F, et al. Evidence that autistic traits show the same etiology in the general population and at the quantitative extremes (5%, 2.5%, and 1%) Archives of General Psychiatry. 2011;68(11):1113–1121. doi: 10.1001/archgenpsychiatry.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A, Hoekstra RA. Autism spectrum disorders and autistic traits: a decade of new twin studies. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2011;156(3):255–274. doi: 10.1002/ajmg.b.31159. [DOI] [PubMed] [Google Scholar]
- Ronald A, Happé F, Price TS, Baron-Cohen S, Plomin R. Phenotypic and genetic overlap between autistic traits at the extremes of the general population. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(10):1206–1214. doi: 10.1097/01.chi.0000230165.54117.41. [DOI] [PubMed] [Google Scholar]
- Ronald A, Simonoff E, Kuntsi J, Asherson P, Plomin R. Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. Journal of Child Psychology and Psychiatry. 2008;49(5):535–542. doi: 10.1111/j.1469-7610.2007.01857.x. [DOI] [PubMed] [Google Scholar]
- Ronald A, Edelson LR, Asherson P, Saudino KJ. Exploring the relationship between autistic-like traits and ADHD behaviors in early childhood: findings from a community twin study of 2-year-olds. Journal of Abnormal Child Psychology. 2010;38(2):185–196. doi: 10.1007/s10802-009-9366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudio Team . RStudio: Integrated development for R. Boston: RStudio, Inc.; 2016. [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(8):921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Spatola, C. A., Rende, R., & Battaglia, M. (2010). Genetic and environmental influences upon the CBCL/6-18 DSM-oriented scales: similarities and differences across three different computational approaches and two age ranges. European Child & Adolescent Psychiatry, 19(8), 647-658. [DOI] [PubMed]
- Taylor MJ, Charman T, Robinson EB, Plomin R, Happé F, Asherson P, Ronald A. Developmental associations between traits of autism spectrum disorder and attention deficit hyperactivity disorder: a genetically informative, longitudinal twin study. Psychological Medicine. 2013;43(08):1735–1746. doi: 10.1017/S003329171200253X. [DOI] [PubMed] [Google Scholar]
- Verhulst FC, Van der Ende J. Six-year developmental course of internalizing and externalizing problem behaviors. Journal of the American Academy of Child & Adolescent Psychiatry. 1992;31(5):924–931. doi: 10.1097/00004583-199209000-00022. [DOI] [PubMed] [Google Scholar]
- Zeeuw EL, Beijsterveldt CE, Hoekstra RA, Bartels M, Boomsma DI. The etiology of autistic traits in preschoolers: a population-based twin study. Journal of Child Psychology and Psychiatry. 2017;58(8):893–901. doi: 10.1111/jcpp.12741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 60.1 kb)
(PDF 70.3 kb)
(PDF 96.6 kb)
(PDF 56.9 kb)
(PDF 60.7 kb)
(PDF 71.2 kb)
(PDF 67.1 kb)
(PDF 74.6 kb)
(PDF 66.4 kb)
(PDF 84.2 kb)
(PDF 96.9 kb)


