Abstract
Nucleophilic addition to thioalkynes was investigated under various catalytic conditions with gold(I) complexes being identified as the optimal catalysts. Structural evaluation of the product revealed an unexpected cis-addition, arising from a gold-associated thioketene intermediate. Based on this interesting mechanistic insight, a gold(I)-catalyzed thioether addition to thioalkynes was developed as a novel approach to prepare ketene dithioacetals with good yields and high efficiency.
Keywords: gold, ketene dithioacetal, sulfur, thioalkyne, thioketene
Sulfur-containing molecules are among the most important compounds in chemical, material and bio-medicinal research. Organosulfur compounds such as thiols and sulfides, in which sulfur atoms possess a low oxidation state, are synthetically versatile reactants as nucleophiles,[1] radical promoters,[2] and reductants.[3] Despite the vast application of sulfides and thiols in synthesis, utilizing these compounds in transition-metal-catalyzed transformations remains a challenging task, which is largely due to the strong coordination of sulfur to the metal center.[4]
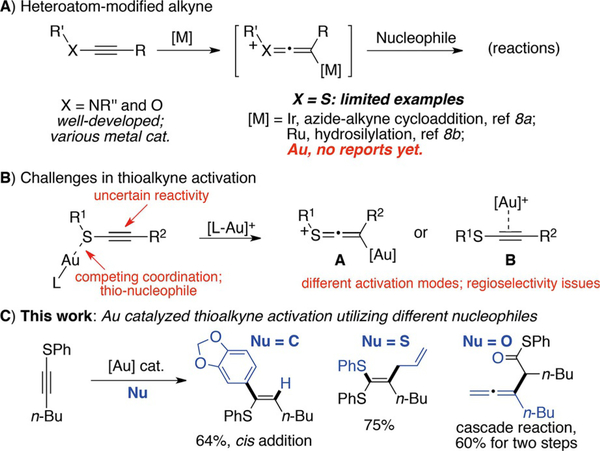
The intrinsic reactivity of C–C triple bonds allows alkynes to occupy a privileged position in organic synthesis.[5] Functionalized alkynes bearing a heteroatom, such as N, O, P, and S, connected directly to the triple bond are especially intriguing, since these electron-rich heteroatoms will not only make the triple bond more reactive by donating electron density to the alkyne π-bond, but also provide valuable functional handles in the resulting products.[6] Interestingly, compared with the well-developed transformation of N/O-substituted alkynes, reactions of thioalkynes are significantly less developed despite the simple preparation of the thioalkyne substrates. This dearth in reaction development is proposed to arise from undesired sulfur coordination to the metal center, and the diminished capability of sulfur’s 3p electrons to effectively conjugate with the carbon 2p orbital of the alkyne system. Thus, studies of thioalkyne towards A) elucidation of its reactivity and B) development of effective transformations from this highly functional building block are of great interest for general chemical and biomedicinal research. Herein, we report our investigations on gold-catalyzed thioalkyne activation. An interesting cis-addition was identified, which suggested the formation of a thioketene intermediate in the overall process. Inspired by this mechanistic discovery, a general synthetic strategy was developed for the synthesis of ketene dithioacetals under mild conditions (Scheme 1C).
Scheme 1.
Gold-catalyzed thioalkyne activation.
The carbophilic nature of the Au cation makes it an excellent catalyst for the activation of alkenes, allenes and, most commonly, alkynes.[7] During the past decade, studies on gold-catalyzed transformations involving ynamides (N-alkynes) have grown tremendously due to their increased reactivity in comparison with regular alkynes (Scheme 1A).[8] According to the literature, only a few successful examples have been reported that involve gold cation and substrates bearing sulfur-containing moieties.[9,10] Particularly, the activation of thioalkynes using gold cations has not been reported. Some key challenges of gold-catalyzed thioalkyne activation include the: A) sulfur coordination to gold due to its nucleophilicity, preventing the desired electrophilic activation of the alkyne; B) unknown reactivity of the C–C triple bond due to the conjugation of sulfur’s 3p electrons to the alkyne p-system, resulting in different activation modes (Scheme 1B). Thus, investigations into the gold-catalyzed activation of thioalkynes are not only practical for the preparation of sulfur-containing compounds, but also relevant towards providing mechanistic insight into the reactivity of thioalkynes.
We initiated our study by treating thioalkyne 1a with the electron-rich carbon nucleophile 1,3-benzodioxzole 2a under various metal-catalyzed conditions.[11] As shown in Table 1, screening of reaction conditions (see detailed in Supporting Information) revealed the important role of group XI metal cations (Cu, Ag and Au) in promoting alkyne activation. Treating 1a and 2a with HOTf or other metal catalysts, such as Pd, Ru, Rh, Ir, and Pt, gave almost no conversion of thioalkyne 1a, suggesting that the productive activation of the thioalkyne towards carbo-nucleophilic addition did not occur under these conditions. Conversely, the group XI transition-metal catalysts were able to effectively activate the thioalkyne at elevated temperatures, as evidenced by the high conversion of 1a when Cu, Ag or Au catalysts were employed.
Table 1.
Gold-catalyzed C-nucleophile addition to thioalkyne.
 | |||||
| Conditions | Convn. of 1a [%][a] |
Yield 3a (cis:trans) [%] |
Conditions | Convn. of 1a [%] |
Yield 3a (cis:trans) [%] |
| XPhosAuNTf2 (5%) |
HOTf (10 %), 80 °C, 16 h |
<5 | <5 | ||
| RT or 40°C, 16 h |
<5 | n.d. | AgOTf (5 %), 80 °C, 6 h |
93 | 47 (3:1) |
| 80 °C, 10h | 70 | 64 (7:1) | Cu(OTf)2 (5 %), 80 °C, 16 h |
100 | <5 |
| 80 °C, 16h | 75 | 45 (7:1) | other [M], Ir, Ru, Rh, Pt, etc. |
<10 | <5 |
Conversion and yield were determined by 1H NMR using dimethylsul- fone as internal standard.
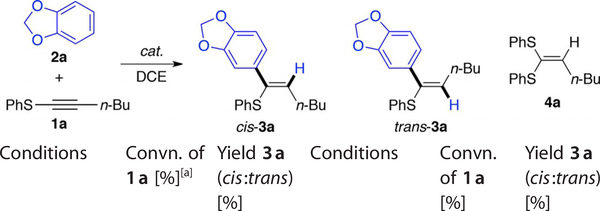
Interestingly, although 1a was converted completely (100%, total consumption) when using Cu(OTf)2 as catalyst, the desired C-nucleophilic addition product 3a was not observed. Using 5% AgOTf as catalyst afforded the desired product 3a along with significant 1a decomposition (93% convn. and 47% yield). Finally, utilizing 5% of the gold catalyst XPhosAuNTf2 furnished the desired 3a in 64% yield. Extending the reaction time from 10 h to 16 h resulted in diminished yields (45%) due to significant decomposition of the product 3a under the reaction conditions. In comparison with copper and silver, gold catalysts yielded the optimal results due to better alkyne activation while minimizing substrate/product decomposition. Solvent screening revealed DCE (1,2-dichloroethane) as the optimal solvent for this transformation.
Typically, nucleophilic addition towards gold-activated alkynes gives trans products. This arises due to formation of the gold–alkyne π-complex and a subsequent outer-sphere nucleophile trans addition. However, for the gold-catalyzed thioalkyne activation, a mixture of cis and trans isomers were observed, with cis-3a obtained as the major isomer (confirmed by X-ray). In comparison with the silver-catalyzed reaction, which afforded a cis/trans ratio of 3:1, the gold-catalyzed conditions exhibited better cis/trans selectivity (up to 7:1). This result suggested the formation of the sulfonium cation A (Scheme 1B).[12] Reactions of other C-nucleophiles with thioalkynes are shown in Table 2.
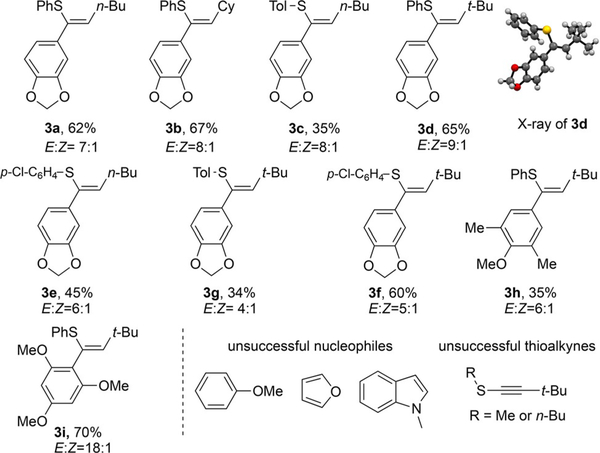
Table 2.
Reaction conditions: 5mol% catalyst was added to a DCE solution (Ί.2 mL) of thioalkyne (0.2 mmol) and C-nucleophile (3 equiv), and reaction was kept at 80°C for Ί0 h.
Isolated yield.
With electron-rich arenes, the desired addition products 3 were formed in moderate yields, and cis-isomers were the major products in all cases. Less electron-rich substrates such as anisol resulted in no addition products under the optimal conditions. Furan and indole gave messy reactions. Alkyl-substituted thioalkynes (R=Me or nBu) also provided very low conversion. Although the reaction scope is limited, this work is the first successful example of carbon nucleophile addition to thioalkynes under metal catalytic conditions. Moreover, it provides a good platform to further explore the general reactivity associated with the unique thioalkyne building block.
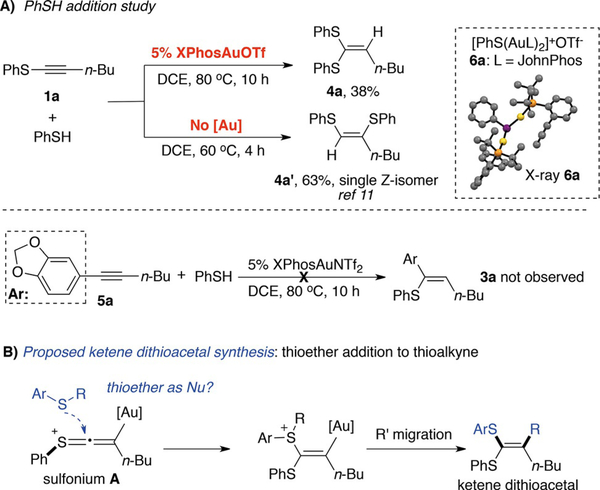
To better understand the reaction mechanism, the decomposition byproducts from the reactions were analyzed. Interestingly, ketene dithioacetal 4a was identified as the major sulfur-containing by-product. To explore the origin of compound 4a, reactions of thiophenol addition to thioalkyne 1a were performed as shown in Scheme 2A.
Scheme 2.
Mechanistic study and proposed thioether addition.
Reaction of thioalkyne 1a and PhSH with Au-catalyst gave ketene dithioacetal 4a in 38% yield. This result is interesting since the reaction between only thiophenol and thioalkyne 1a gave 1,2-disulfide 4a’ (based on our experiment and previous literature reports).[13] Monitoring the reaction with 31P NMR revealed the formation of a new complex while treating PhSH with various LAuOTf permutations. The structure of this complex was confirmed by X-ray as a sulfur-bridged bis-gold complex 6, [(PhS)(AuL)2]+OTf–. Using complex 6 as catalyst to promote the reaction between 1a and 2a gave almost no conversion, implying that complex 6 was likely the gold decomposition product instead of the effective catalyst in this reaction. Additionally, the reaction between thiophenol (PhSH) and internal alkyne 5a gave almost no conversion of both starting materials under gold catalyzed conditions, which ruled out PhSH addition to alkyne as potential reaction path for the formation of 3a. Based on these results, it is reasonable to rationalize that this reaction proceeded through sulfonium intermediate A. The major decomposition path of thioalkyne was through the dissociation of PhS–, which poisoned the gold cation and gave rise to byproduct 4a likely by adding to the sulfonium A.
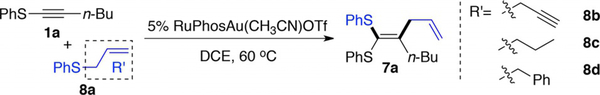
The employment of gold for the activation of thioalkynes is important, as gold catalyst clearly altered the reactivity of the thioalkyne to facilitate the formation of the ketene dithioacetal 4a instead of the regioisomeric 1,2-disulfide alkene 4a’. To reduce the impact of thiophenol-mediated gold decomposition, we postulated that thiol ether (RSR’) could be used as a suitable nucleophile towards sulfonium A.[14] With an appropriate migrating group as R’, ketene dithioacetal 7 may be prepared in a single step. Notably, compound 7 represents a class of sulfur-containing compounds that have been reported to react as enol equivalents in aldol reactions,[15] and as Michael acceptors in radical cyclizations.[16] Furthermore, similar to dithianes, the resulting dithioacetal exhibits potential synthetic utility as an umpulong synthon.[17] Currently, there are few synthetic strategies for the synthesis of these compounds, especially the un-symmetrical substrates (two different RS groups).[18] The proposed synthesis shown in Scheme 2B, if successful, will provide a new strategy to access these compounds with high efficiency. To test this reaction design, several thioethers bearing alkyl, allyl, propargyl and benzyl at the R’ position were prepared. These compounds were treated with thioalkyne 1a under gold catalytic conditions. Gratifyingly, the reaction of allylic thioether 8a and 1a gave the desired ketene dithioacetal 7a in good yield. Other thioethers (8b, 8c, and 8d) gave very low conversion with no desired product detected.[19] After a comprehensive screening of reaction conditions (see details in Supporting Information), RuPhosAu(CH3CN)OTf (5%) was revealed as the optimal catalyst to afford 7a in 75% isolated yield. Results from alternative conditions are summarized in Table 3.
Table 3.
Conditions of Au catalyzed thioether addition to thioalkyne.[a]
 | ||
| Alternation from above conditions | Convn. of 1a [%][b] | Yield 7a [%] |
| none | >95 | 75 |
| 5% Cu(OTf)2 instead of [Au] | >95 | 39 |
| 5% AgOTf instead of [Au] | >95 | 49 |
| [Au]=IPrAuNTf2 | >95 | 60 |
| 8b, 8c, 8d | <5 | <5 |
| Other catalysts: Pd, Ir, Ru, Rh, Pt, HOTf | <20 | <5 |
Reaction conditions: 5 mol% catalyst was added to a DCE solution (0.6 mL) of thioalkyne (0.1 mmol) and thioether (1.2 equiv), and reaction was kept at 608C for 6 h.
Conversion and yield were determined by 1H NMR spectroscopy using dimethylsulfone as internal standard.
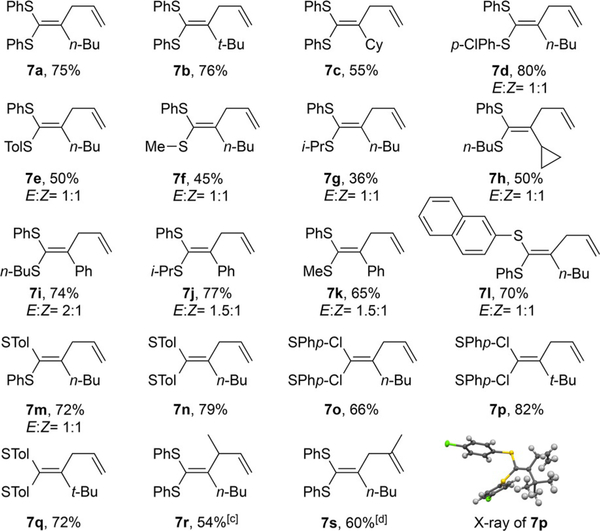
As shown in Table 4, aryl sulfide derived aliphatic thioalkyne in general provided good yield (7a–7e, 7l–7q), whereas the alkyl sulfide derived aliphatic thioalkyne gave lower yield (7 f– 7h). We believe the 1:1 E/Z regioselectivity is due to the thermal equilibration of the product under gold catalytic conditions. As a result, using the bulkier alkyl derived aromatic thioalkynes increased the regioselectivity to 1.5:1, favoring the thermodynamically more stable trans-alkene (7i–7k). More sterically hindered allyl sulfides could also participate in this reaction, as demonstrated in product 7r and 7s. Notably, the formation of 7s was observed when treating the thioalkyne with crotyl phenyl sulfide, suggesting that this reaction underwent an SN2’ type rearrangement.
Table 4.
Reaction conditions: 5mol% catalyst was added to a DCE solution (1.2 mL) of thioalkyne (0.2 mmol) and allyl sulfide (1.2 equiv), and reaction was kept at 60 °C for 6 h. [b] Isolated yield. [c] Crotyl phenyl sulfide was applied. [d] 2-Methylpropenyl phenyl sulfide was applied.
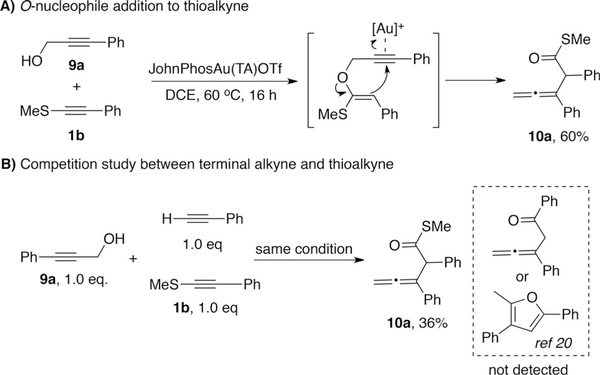
We recently achieved the intermolecular addition of propargyl alcohol to terminal alkyne utilizing triazole–gold catalyst (TA–Au).[20] This result inspired us to investigate the performance of thioalkyne in an analogous system. As shown in Scheme 3A, the desire allene product 10a was obtained in 60% yield with triaozle–gold catalyst. We then compared the reactivity of thioalkyne 1b and phenylacetylene by treating them with equal amount of propargyl alcohol 9a. Only product 10a was detected, with no indication of products derived from phenylacetylene. This result suggested that by conjugating with sulfur, the triple bond in thioalkyne became more reactive than phenylacetylene towards nucleophilic attack.
Scheme 3.
Propargyl alcohol addition to thioalkyne.
In summary, for the first time we successfully disclosed the possibility of gold-catalyzed intermolecular functionalization of thioalkynes. With the proper choice of gold catalysts, different nucleophiles could be used to afford trisubstituted alkenyl sulfides, ketene dithioacetal and allenyl thioesters efficiently. These newly synthesized products are a range of highly functionalized sulfur-containing compounds, the previous syntheses of which are not straightforward. Sulfur is both advantageous and deleterious in homogeneous gold-catalyzed functionalization of thioalkynes. Although the sulfur-containing substrate can poison the gold catalyst by forming stable deactivated complexes, it enhances the reactivity of the triple bond and promotes formation of sulfonium intermediate A. Thus, this reported transformations represent novel methodologies to functionalize thioalkynes, and serve as the foundation for the development of other gold-catalyzed reactions involving sulfur.
Supplementary Material
Acknowledgements
We are grateful to the NSF (CHE-1619590), NIH (1R01GM120240–01) and NSFC (21629201) for financial support.
Footnotes
Supporting information for this article can be found under https://doi.org/10.1002/chem.201702710.
References
- [1].Peach ME, in The Thiol Group Volume 2 (Chapter 16), John Wiley & Sons, Bristol: 1974, 721–784. [Google Scholar]
- [2].Majumdar KC, Debnath P, Tetrahedron 2008, 64, 9799–9820. [Google Scholar]
- [3].Sturala J, Bohacova S, Chudoba J, Metelcova R, Cibulka R, J. Org. Chem 2015, 80, 2676–2699. [DOI] [PubMed] [Google Scholar]
- [4].a) Li GY, Angew. Chem. Int. Ed 2001, 40, 1513; Angew. Chem. 2001, 113, 1561. [DOI] [PubMed] [Google Scholar]; b) Beletskaya IP, Ananikov VP, Chem. Rev 2011, 111, 1596. [DOI] [PubMed] [Google Scholar]; c) Eichman CC, Stambuli JP, Molecules 2011, 16, 590. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Modha SG, Mehta VP, Van der Eycken EV, Chem. Soc. Rev 2013, 42, 5042. [DOI] [PubMed] [Google Scholar]; e) Desnoyer AN, Love JA, Chem. Soc. Rev 2017, 46, 197. [DOI] [PubMed] [Google Scholar]; f) Liu YJ, Liu SS, Xiao Y, Beilstein J. Org. Chem 2017, 13, 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) Schore NE, Chem. Rev 1988, 88, 1081–1119. [Google Scholar]; b) Dorel R, Echavarren AM, Chem. Rev 2015, 115, 9028–9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].a) For selected examples, see: DeKorver KA, Li HY, Lohse AG, Hayashi R, Lu ZJ, Zhang Y, Hsung RP, Chem. Rev 2010, 110, 5064. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Evano G, Coste A, Jouvin K, Angew. Chem. Int. Ed 2010, 49, 2840 Angew. Chem. 2010, 122, 2902. [DOI] [PubMed] [Google Scholar]; c) Zhang X, Liu BQ, Shu X, Gao Y, Lv HP, Zhu J, J. Org. Chem 2012, 77, 501. [DOI] [PubMed] [Google Scholar]; d) Zhao WX, Wang ZB, Sun JW, Angew. Chem. Int. Ed 2012, 51, 6209; Angew. Chem. 2012, 124, 6313. [DOI] [PubMed] [Google Scholar]; e) Montavon TJ, Turkmen YE, Shamsi NA, Miller C, Sumaria CS, Rawal VH, Kozmin SA, Angew. Chem. Int. Ed 2013, 52, 13576; Angew. Chem. 2013, 125, 13821. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Qian H, Zhao WX, Sun JW, Chem. Rec 2014, 14, 1070. [DOI] [PubMed] [Google Scholar]; g) Wang XN, Yeom HS, Fang LC, He SH, Ma ZX, Kedrowski BL, Hsung RP, Acc. Chem. Res 2014, 47, 560. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Evano G, Blanchard N, Compain G, Coste A, Demmer CS, Gati W, Guissart C, Heimburger J, Henry N, Jouvin K, Karthikeyan G, Laouiti A, Lecomte M, Martin-Mingot A, Metayer B, Michelet B, Nitelet A, Theunissen C, Thibaudeau S, Wang JJ, Zarca M, Zhang CY, Chem. Lett 2016, 45, 574. [Google Scholar]
- [7].a) Hashmi ASK, Hutchings GJ, Angew. Chem. Int. Ed 2006, 45, 7896; Angew. Chem. 2006, 118, 8064. [DOI] [PubMed] [Google Scholar]; b) Widenhoefer RA, Han XQ, Eur. J. Org. Chem 2006, 4555. [Google Scholar]; c) Ferstner A, Davies PW, Angew. Chem. Int. Ed 2007, 46, 3410; Angew. Chem. 2007, 119, 3478. [DOI] [PubMed] [Google Scholar]; d) Gorin DJ, Toste FD, Nature 2007, 446, 395. [DOI] [PubMed] [Google Scholar]; e) Arcadi A, Chem. Rev 2008, 108, 3266. [DOI] [PubMed] [Google Scholar]; f) Gorin DJ, Sherry BD, Toste FD, Chem. Rev 2008, 108, 3351. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Ferstner A, Chem. Soc. Rev 2009, 38, 3208.19847352 [Google Scholar]; h) Garcia P, Malacria M, Aubert C, Gandon V, Fensterbank L, ChemCatChem 2010, 2, 493. [Google Scholar]; i) Hashmi ASK, Angew. Chem. Int. Ed 2010, 49, 5232; Angew. Chem. 2010, 122, 5360. [DOI] [PubMed] [Google Scholar]; j) Sengupta S, Shi XD, ChemCatChem 2010, 2, 609. [Google Scholar]; k) Nolan SP, Acc. Chem. Res 2011, 44, 91. [DOI] [PubMed] [Google Scholar]; l) Xiao J, Li XW, Angew. Chem. Int. Ed 2011, 50, 7226; Angew. Chem. 2011, 123, 7364. [DOI] [PubMed] [Google Scholar]; m) Liu LP, Hammond GB, Chem. Soc. Rev 2012, 41, 3129. [DOI] [PubMed] [Google Scholar]; n) Obradors C, Echavarren AM, Acc. Chem. Res 2014, 47, 902. [DOI] [PMC free article] [PubMed] [Google Scholar]; o) Wang YM, Lackner AD, Toste FD, Acc. Chem. Res 2014, 47, 889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].a) For reviews, see Couty S, Meyer C, Cossy J, Tetrahedron 2009, 65, 1809. [Google Scholar]; b) Pan F, Shu C, Ye LW, Org. Biomol. Chem 2016, 14, 9456. [DOI] [PubMed] [Google Scholar]; c) For selected examples, see: Couty S, Meyer C, Cossy J, Angew. Chem. Int. Ed 2006, 45, 6726; Angew. Chem. 2006, 118, 6878. [DOI] [PubMed] [Google Scholar]; d) Dateer RB, Pati K, Liu RS, Chem. Commun 2012, 48, 7200. [DOI] [PubMed] [Google Scholar]; e) Shu C, Wang YH, Zhou B, Li XL, Ping YF, Lu X, Ye LW, J. Am. Chem. Soc 2015, 137, 9567. [DOI] [PubMed] [Google Scholar]; f) Zhou AH, He Q, Shu C, Yu YF, Liu S, Zhao T, Zhang W, Lu X, Ye LW, Chem. Sci 2015, 6, 1265. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Zhu L, Yu YH, Mao ZF, Huang XL, Org. Lett 2015, 17, 30. [DOI] [PubMed] [Google Scholar]
- [9].a) Ding S, Jia G, Sun J, Angew. Chem. Int. Ed 2014, 53,1877–1880. [DOI] [PubMed] [Google Scholar]; b) Ding D, Song L, Wang Y, Zhang X, Wa C, Wu Y, Sun J, Angew. Chem. Int. Ed 2015, 54, 5632–5635; Angew. Chem. 2015, 127, 5724– 5727. [DOI] [PubMed] [Google Scholar]; c) Song L, Ding S, Wang Y, Zhang X, Wu Y, Sun J, J. Org. Chem 2016, 81, 6157–6164. [DOI] [PubMed] [Google Scholar]
- [10].a) Brown-Xu SE, Chisholm MH, Durr CB, Spilker TF, J. Am. Chem. Soc 2013, 135, 8254–8259. [DOI] [PubMed] [Google Scholar]; b) Cambeiro XC, Boorman TC, Lu P, Larrosa I, Angew. Chem. Int. Ed 2013, 52, 1781–1784; Angew. Chem. 2013, 125, 1825–1828. [DOI] [PubMed] [Google Scholar]
- [11].a) For examples of gold-catalyzed hydroarylation of alkyne, see: Reetz MT, Sommer K, Eur. J. Org. Chem 2003, 3485. [Google Scholar]; b) Samala S, Mandadapu AK, Saifuddin M, Kundu B, J. Org. Chem 2013, 78. [DOI] [PubMed] [Google Scholar]; c) Pirovano V, Negrato M, Abbiati G, Dell’Acqua M, Rossi E, Org. Lett 2016, 18. [DOI] [PubMed] [Google Scholar]; d) Schiessl J, Rudolph M, Hashmi ASK, Adv. Synth. Catal 2017, 359, 639. [Google Scholar]
- [12].a) The good E/Z selectivity might also be attributed to the isomerization of the initially formed Z-1,1-thio-aryl alkenes. See examples: Zhdanko A, Maier ME, Chem. Eur. J 2014, 20, 1918. [DOI] [PubMed] [Google Scholar]; b) Zhdanko A, Maier ME, Angew. Chem. Int. Ed 2014, 53, 7760; Angew. Chem. 2014, 126, 7894. [DOI] [PubMed] [Google Scholar]
- [13].Ping T, Chin. J. Chem 2011, 29, 765–768. [Google Scholar]
- [14].Nakamura I, Sato T, Terada M, Yamamoto Y, Org. Lett 2008, 10, 2649. [DOI] [PubMed] [Google Scholar]
- [15].Saitoh T, Jimbo N, Ichikawa J, Chem. Lett 2004, 33, 1032–1033. [Google Scholar]
- [16].a) Harrowven DC, Browne R, Tetrahedron Lett. 1994, 35, 5301–5302. [Google Scholar]; b) Ishibashi H, Kameoka C, Iriyama H, Kodama K, Sato T, Ikeda M, J. Org. Chem 1995, 60, 1276–1284. [Google Scholar]
- [17].Smith AB, Adams CM, Acc. Chem. Res 2004, 37, 365–377. [DOI] [PubMed] [Google Scholar]
- [18].a) Cohen T, Gapinski RE, Hutchins RR, J. Org. Chem 1979, 44, 3599– 3601; [Google Scholar]; b) Cristau HJ, Chabaud B, Labaudiniere R, Christol H J. Org. Chem 1986, 51, 875–878. [Google Scholar]; c) Aggarwal VK, Steele RM, Ritmaleni JK Barrell, Grayson I, J. Org. Chem 2003, 68, 4087–4090. [DOI] [PubMed] [Google Scholar]; d) Manvar A, O’Shea DF, Eur. J. Org. Chem 2015, 7259–7263. [Google Scholar]
- [19].a) Hu L, Gui Q, Chen X, Tan Z, Zhu G, J. Org. Chem 2016, 81, 4861. [DOI] [PubMed] [Google Scholar]; b) Kaldre D, Maryasin B, Kaiser D, Gajsek O, Gonz#lez L, Maulide N, Angew. Chem. Int. Ed 2017, 56, 2212; Angew. Chem. 2017, 129, 2248. [DOI] [PubMed] [Google Scholar]
- [20].a) Hosseyni S, Su YJ, Shi XD, Org. Lett 2015, 17, 6010. [DOI] [PubMed] [Google Scholar]; b) Hosseyni S, Ding ST, Su YJ, Akhmedov NG, Shi XD, Chem. Commun 2016, 52, 296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.