Abstract
Small RNAs (sRNAs) have been recently recognized as key genetic and epigenetic regulators in various organisms, ranging from the modification of DNA and histone methylations to the modulation of the abundance of coding or non-coding RNAs. In plants, major regulatory sRNAs are classified as respective microRNA (miRNA) and small interfering RNA (siRNA) species, with the former primarily engaging in posttranscriptional regulation while the latter in transcriptional one. Many of these characterized sRNAs are involved in regulation of diverse biological programs, processes, and pathways in response to developmental cues, environmental signals/stresses, pathogen infection, and pest attacks. Recently, sRNAs-mediated regulations have also been extensively investigated in horticultural plants, with many novel mechanisms unveiled, which display far more mechanistic complexity and unique regulatory features compared to those studied in model species. Here, we review the recent progress of sRNA research in horticultural plants, with emphasis on mechanistic aspects as well as their relevance to trait regulation. Given that major and pioneered sRNA research has been carried out in the model and other plants, we also discuss ongoing sRNA research on these plants. Because miRNAs and phased siRNAs (phasiRNAs) are the most studied sRNA regulators, this review focuses on their biogenesis, conservation, function, and targeted genes and traits as well as the mechanistic relation between them, aiming at providing readers comprehensive information instrumental for future sRNA research in horticulture crops.
Genetics: Tiny keys to important traits
Studying small RNAs in horticultural plants may reveal the genetic mechanisms underlying many unique and valuable traits, and help improve plant breeding programs. Small RNAs, short RNA molecules only 20–24 bases long, switch genes on or off to control many aspects of plant growth, development, and reproduction. They are well-studied in model plants, but are only just being investigated in horticultural species. In a review of small RNA research in horticultural plants, Rui Xia at South China Agricultural University and co-workers report that horticultural plants contain many novel sRNAs that hold the keys to important traits such as juvenile-to-adult transition in fruit trees, fruit size, fruit quality, and disease resistance. Better understanding of these miRNAs may help in engineering varieties with earlier production of tastier fruit, as well as enhanced resistance to disease.
Since the first plant small RNA (sRNA) was excavated in Arabidopsis in 20021, numerous sRNAs have been found to orchestrate diverse biological processes critical for plant growth, development, and stress responses. Plant sRNAs are a class of short regulatory RNAs of 20–24 nucleotides in length2,3. According to their biogenesis and function mechanism, sRNAs are classified into two general types, microRNAs (miRNAs) and short interfering RNAs (siRNAs)4,5. Although much of our knowledge regarding sRNAs comes from model plants like Arabidopsis, ongoing research progressively extends into non-model systems, including a group of economically important plants—horticultural plants. These studies greatly expand our understanding of biogenesis, metabolism, and function of sRNA in crops. Here we aim to summarize these sRNA research progress to provide an overview of sRNA-involved regulatory networks vital for the development of critical economic traits in horticultural plants. We focus our review on miRNA and phasiRNA (a class of siRNAs), as they are the most widely studied classes of sRNAs in the recent decades.
Overview of sRNA research in plants
Biogenesis of miRNA and phasiRNA
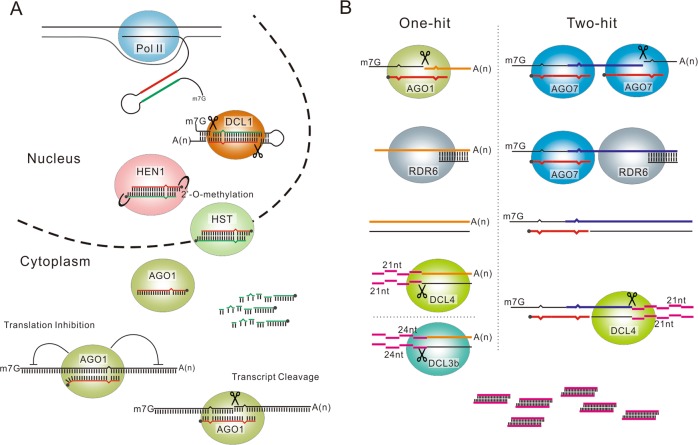
miRNAs are the most functionally important and most studied class of sRNAs in plants, and their biogenesis is an intricate process, widely conserved in plants (Fig. 1a). In brief, a miRNA gene (MIR) is firstly transcribed by RNA POLYMERASE II (Pol II) and produces a 5′ capped and 3′ polyadenylated primary transcript (pri-miRNA) with a self-complementary foldback structure (Fig. 1a)2,6–8. The pri-miRNA is sequentially sliced by the RNase III endoribonuclease DICER-LIKE1 (DCL1) to yield a miRNA/miRNA* duplex with two-nucleotide 3′ overhangs2,6–8. The duplex is then 2-O-methylated at the 3′ terminal residues by HUA ENHANCER1 (HEN1)9 and transported from nucleus to cytoplasm by HASTY(HST)2,6–8. In the cytoplasm, the duplex is separated with the miRNA* rapidly degraded and the mature miRNA incorporated into the ARGONAUTE 1 (AGO1) protein to form an active RNA-INDUCED SILENCING COMPLEX (RISC)2,6–8. After that, the miRNA, requiring almost perfect sequence complementary, guides the RISC complex to regulate its target genes through either transcript cleavage or translation inhibition (Fig. 1a)2,6–8.
Fig. 1. Biogenesis of miRNA and phasiRNA.
a General process of miRNA biogenesis. A primary miRNA is transcribed from a MIRNA locus by pol II, generating a precursor MIRNA with a hairpin structure. DCL1 processes the hairpin into a duplex of miRNA (red) and miRNA* (green) with 2-nt 3′-terminal overhangs. HEN1 methylates the 3′-terminal ribonucleotide of the duplex. Subsequently, HST transports the duplex from nucleus to cytoplasm. miRNA* is rapidly degraded while miRNA is loaded into AGO1 in a RISC complex to perform its function, via translation inhibition or transcript cleavage. b Two typical modes of phasiRNA biogenesis. In one-hit mode, dsRNA synthesized by RDR6, after the miRNA-mediated cleavage on the single site of target transcript, are processed into 21- or 24-nt phasiRNAs by DCL4 or DCL5 from 5′ to 3′. In two-hit mode, a transcript containing two miRNA target sites is cleaved by miRNA-AGO7 complex at the 3′-terminal target site. The upstream fragment is converted into dsRNA by RDR6 for the generation of phasiRNAs through sequential cleavages by DCL4 from 3′ to 5′
Phased siRNAs (phasiRNAs) are a special class of siRNAs, which is only found in plants, to date. Its biogenesis relies on the cleavage mediated by sRNAs (mostly miRNA). There are two modes of phasiRNA biogenesis reported so far according to the number of sRNA target sites on the target gene, one-hit and two-hit. In the one-hit mode, a 22-nucleotide (nt) miRNA cleaves its target transcript into two fragments on a single site. The cleaved fragment downstream to the target site is converted into a double-strand RNA (dsRNA) by RNA-DEPENDENT RNA POLYMERASE 6 (RDR6)6, and then the dsRNA was chopped by a Dicer protein (DCL4 or DCL5 in grasses) from 5′ to 3′ in a continuous head-to-tail manner, producing dozens of phasiRNAs of certain length (21-nt for DCL4 and 24-nt for DCL5)10. In the two-hit mode, a target transcript possesses two target sites, as typified in TAS3 genes, which have two target sites of miR390 with only the 3′-terminal target site usually sliced11. In contrast to the one-hit mode, the fragment upstream to the 3′ target site of miR390 is copied into dsRNA and processed by DCL4 from 3′ to 5′ into 21-nt phasiRNAs11,12. These phasiRNAs can function like miRNAs to regulate their target genes in trans (tasiRNAs) or in cis (casiRNAs).
Major miRNA pathways
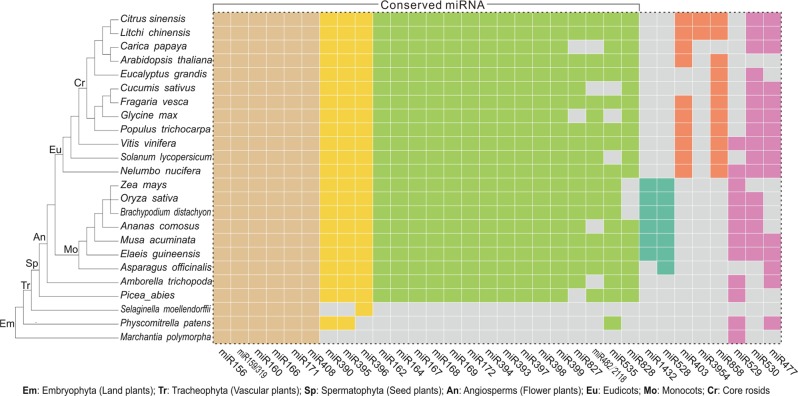
In general, plant miRNAs are classified into conserved miRNAs (present in angiosperms), less-conserved miRNAs (present in a lineage or group of plants), and species-specific miRNAs (present in a single species). A couple of miRNAs families are highly conserved while the majority are lineage-restricted or species-specific3. Here we collected 32 miRNA families to illustrate their conservation, including all the highly conserved ones in plants and a few families of horticultural importance.
It is estimated that the conserved miRNAs are composed of about 20 miRNA families that share distinct evolutionary routes3. Of them, nine appear to origin from land plants (embryophytes, Fig. 2) except with miR390 and miR395 families that are missing in liverwort (Marchantia polymorpha) and lycopod (Selaginella moellendorffi) and miR396 family that are missing in liverwort and moss (Phycomitrella patens), while the remaining 12 families derived from seed plants (spermatophytes, Fig. 2), except miR827 which was not found in gymnosperms (Picea abies). We also showed that three miRNA families including miR828, miR482/2118, and miR535, were widely present in seed plants but missed in a few lineages or species as evidenced by the absence of miR828 in grasses and miR482/2118 in papaya (Carica papaya) and cucumber (Cucumis sativus), respectively. Hence, miR828, miR482/2118, and miR535 should also be considered as the conserved miRNA families as well. The other eight miRNAs we listed seem to be lineage-restricted. For instance, miR1432 and miR528 are restricted in monocots, while miR403 is specific to eudicots (Fig. 2). Worthy of noting is that miR529 is missing in core rosids, but present in almost all ancient plants including liverwort and moss (Fig. 2).
Fig. 2. Conservation of miRNAs of horticultural importance.
Dendrogram in the left panel denotes the rough evolutionary history of 24 species based on the APG (Angiosperm Phylogeny Group) IV system. Tile plot in the right panel presents the presence/absence of 32 representative miRNA families in the corresponding species. Gray-shaded tiles mean no presence or losing of the miRNA in the corresponding species while tiles filled with other colors denote the presence of the miRNA
The conserved miRNAs share the same target genes across a wide range of plants in general while less-conserved ones show species- or lineage-specific target genes (Table 1). Highly conserved miRNAs often play key roles in the regulation of plant growth and development. For example, the conserved miR156 family is involved in regulation of developmental timing or the vegetative-to-reproductive transition, by down-regulation of SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) genes13. On contrary, lineage-specific miRNAs likely perform functions specific to certain plants or groups. One of the representative examples is miR528 that is monocot-specific and involved in defense against virus infection in rice14. Similarly, miR3954 that triggers phasiRNA biogenesis and potentially regulates flower programming via targeting NAC genes15, is only identified in Sapindale and its close relatives.
Table 1.
Main target genes of miRNA families in Fig. 2
| miRNA | Target | miRNA | Target |
|---|---|---|---|
| miR156 | Squamosa-Promoter Binding Protein-Like gene (SPL) | miR393 | Toll-Like Receptors (TIR) |
| miR159/319 | MYB | miR397 | Laccase (LAC) |
| miR160 | Auxin Response Factor (ARF) | miR398 | Copper Superoxide Dismutase (CSD) |
| miR165/166 | Class III Homeodomain Leucine Zipper transcription factors (Zip III) | miR399 | Phosphate Over accumulator (PHO) |
| miR170/171 | Scarecrow-Like proteins (SCL) | miR828 | MYB, Trans-acting siRNA gene 4 (TAS4) |
| miR408 | Uclacyanin (UCL) | miR482/2118 | Nucleotide Binding Site-Leucine-Rich Repeats (NB-LRR) |
| miR390 | Trans-acting siRNA gene 3 (TAS3) | miR535 | Squamosa-Promoter Binding Protein-Like Gene (SPL) |
| miR395 | Sulfate transporter 2 (SULTR2) | miR827 | Phosphate Transporter 5 (PHT5), Nitrogen Limitation Adaptation (NLA) |
| miR396 | Growth-Regulating factor (GRF) | miR1432 | ABRE-binding factor (BZ-1) |
| miR162 | Dicer-Like Gene (DCL1) | miR528 | Ascorbate Oxidase (AO) |
| miR164 | NAC | miR403 | Argonaute 2 (AGO2) |
| miR167 | Auxin Response Factor (ARF) | miR3954 | NAC |
| miR168 | Argonaute 1 (AGO1) | miR858 | MYB |
| miR169 | Nuclear factor Y (NF-Y) | miR529 | Squamosa-Promoter Binding Protein-Like Gene (SPL) |
| miR172 | APETALA2 (AP2) | miR530 | Argonaute 1 (AGO1) |
| miR394 | F-box gene | miR477 | GRAS domain-containing protein |
Major phasiRNA pathways
Besides miRNA, phasiRNA has been extensively investigated in plants in recent years and a few of phasiRNA pathways are highly conserved as well as conserved miRNAs. PhasiRNAs can be produced from both long noncoding and protein-coding genes (PHAS genes). The first several genes generating phasiRNAs (called TAS genes due to the in trans function of phasiRNAs) identified in Arabidopsis are all noncoding genes, including TAS1–4 genes. TAS1–2 and its trigger miRNA (miR173) are specific to Arabidopsis. The TAS3 targeted by miR390 generates several tasiRNAs that target AUXIN RESPONSIVE FACTOR (ARF) genes11,16. This miR390-TAS3-ARF is prevalently conserved in almost all land plants16. The miR390-TAS3-ARF pathway, an indispensable regulatory component in auxin signaling, is of critical function in the regulation of plant growth and development, including leaf morphology, lateral root growth and developmental timing16. The TAS4 gene likely absent in grasses is targeted by miR828 and produces tasiRNAs targeting MYB genes, which are associated with anthocyanin biosynthesis. With the growth of sRNA research in non-model plants, more and more protein-coding genes have been reported to generate profuse phasiRNAs as well, including those encoding NUCLEOTIDE BINDING LEUCINE-RICH REPEAT PROTEINS (NB-LRR), PENTATRICOPEPTIDE REPEAT PROTEINS (PPR), and MYB TRANSCRIPTION FACTORS (MYB), NAC TRANSCRIPTION FACTORS (NAC), Ca2+ ATPase, F-BOX CONTAINING PROTEIN (FBX)10,17,18. Many of these pathways are present in a wide range of plants. For example, miR482/2118 predominantly targets NB-LRRs (or noncoding transcripts in grasses) and triggers phasiRNA production in almost all seed plants18,19. And the phasiRNA production from PPR genes are universally observed in angiosperms18,20.
Small RNA research in horticultural plants
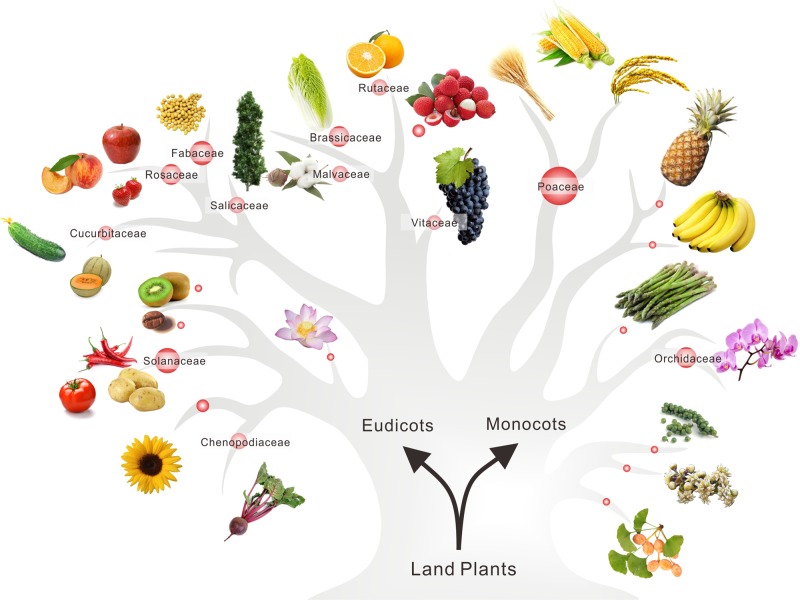
To gain an overview of the scope of sRNA studies in plants, in particular the horticultural plants, we summarized the number of species in every plant families having sRNA deep-sequencing datasets deposited in the public repository NCBI-SRA (National Center for Biotechnology Information-Sequenced Read Archive, https://www.ncbi.nlm.nih.gov/sra). In general, most sRNA studies (with sRNA dataset deposited) focused on the economically important crops as illustrated in Fig. 3. Besides grasses (i.e., Poaceae), Solanaceae is the plant family with the largest number of species of sRNAs studied; it contains 12 species, including tomato21, pepper22, and potato23. The following is Fabaceae containing 11 species, which includes soybean24, Medicago19, and chicken pea25. Rosaceae, Brassicaceae, and Orchidaceae each has eight species in which sRNA population have been explored, while five species in each of Chenopodiaceae, Malvaceae, Rutaceae, and Salicaceae have sRNA datasets reported (Fig. 3).
Fig. 3. A draft tree representing main horticultural plants with sRNA studied.
Pictures of representative species in plant families are posted on the tree drawn based on the APG (Angiosperm Phylogeny Group) IV system, with the size of red bubbles denoting the quantity of species. The bigger the bubble is, the more members of the plant family with sRNA datasets
For only a few plants of horticultural importance, their sRNA repertoire were relatively well profiled, including vegetables (tomato, potato, and cucumber26), fruits (grape27, citrus15,28,29, apple30, peach31,32, and strawberry33), and ornamental plants (petunia34 and orchid35). For fruit trees and ornamental plants, researchers focus their sRNA studies on processes related flowering time, fruit color pattern and fruit size15,34,36–40. In contrast, for vegetables, studies were mainly concerned about resistance to abiotic or biotic stresses that are directly associated with plant growth condition41–44. Here, we discuss several miRNA/phasiRNA-mediated pathways that are directly relevant to horticultural trait performance.
Conserved miRNA or phasiRNA pathways and their regulation of horticultural traits
In plants, conserved miRNAs usually play fundamental regulatory roles in plant growth and development. For horticultural plants, leaf (vegetables), flower (ornamental plants), and fruit (fruit trees) are usually the final products for harvest. Accordingly, as illustrated in Fig. 4, we classified the miRNA pathways in three major categories, leaf development, flower development, and fruit development, based on the main biological functions of miRNAs reported in the model plant Arabidopsis and a few other well-studied plants. In addition, we also added the category of disease resistance, as it is an indispensable part for healthy trait development of horticultural plants.
Fig. 4. Main miRNA/phasiRNA pathways involved in the development of horticultural traits.
Main miRNA/phasiRNA pathways are classified into four categories, including leaf development, flower development, fruit development, and disease resistance, which are illustrated in a cartoon apple tree. Pathways with phasiRNA integration are indicated in bold, and those lineage- or species-specific pathways are marked in cyan
miR390, miR319, and miR396 are involved in the leaf development, including morphogenesis, growth polarity via regulating their target genes TAS3-ARF, TCP, and GRF, respectively. Many more miRNAs are associated with the development of flower and fruit45–47. For instance, miR165/166, miR172, and miR319 are essential for the flower organ development, and miR156, miR159, miR172, miR393, and many others are involved in the process of flowering time regulation48–55 (Fig. 4). In the fruit development, many conserved miRNAs, including miR160, miR167, miR172, miR390, miR393, miR828, miR858, participate in diverse part of fruit development, like fruit initiation, fruit size formation, fruit coloration, fruit ripening, etc.39,56–62 (Fig. 4). Regarding the disease resistance, the miR482/2118 superfamily are of vital roles via targeting a large number of NB-LRR resistance genes, which are a critical component of the effector-triggered immunity in plants63,64. Among these miRNA-involved regulatory pathways important for horticultural trait development, many are integrated with the generation of secondary phasiRNA (or tasiRNAs), which are believed to reinforce or broaden the downstream silencing effect of target genes, for instance, the miR390-TAS3-ARF, miR393-TIR1/AFB2, miR828-MYB, and miR482/2118-NB-LRR16,19,30,65.
A few miRNA pathways have a broad function, playing multiple roles in a few biological processes, which are different but interconnected. For instance, the miR390-TAS3-ARF is a critical regulatory circuit in the signaling pathway of a vital phytohormone auxin, thus the pathway is important for the development of all leaf, flower, and fruit; miR172 not only regulates the flower organ determination but also helps the fruit size formation. Therefore, miRNA-mediated regulations are sophisticated and interlinked; they can be associated with a wide range of processes important for the development of diverse traits. In the following discussion, we delineated a few major miRNA/phasiRNA-involved pathways, which have been studied relatively well in horticultural plants, to demonstrate their functional importance.
miRNAs-phase transition
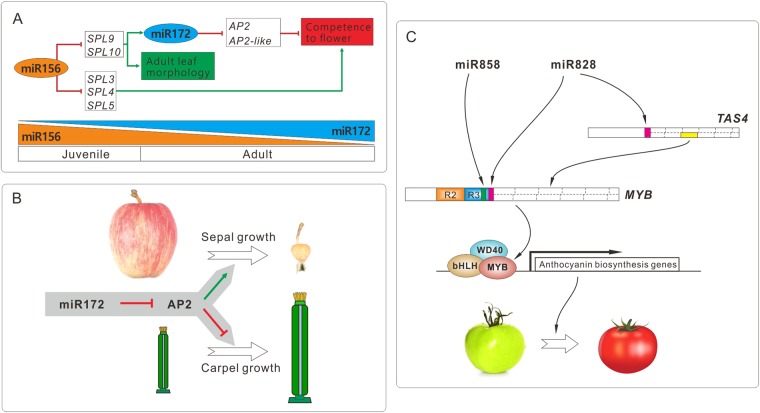
MiR172 is conserved in plants and plays vital roles in plant development. It has been shown that miR172 repressed the expression level of APETALA2 (AP2) or AP2-like genes by inhibiting translation or initiating degradation of the target mRNA66–68. MiR172 is important for the floral transition in many plants, including tomato, apple and so on69–74. MiR172, in collaboration with miR156, participates in the regulation of juvenile-to-adult phase transition in plants75. These two miRNAs play antagonistic roles in flowering induction; high level of miR156 extends juvenile phase and delays flowering, while miR172 accumulation leads to early flowering. In Arabidopsis, miR156 is highly abundant in young seedling and decreases with the phase transition, while miR172 has an opposite expression pattern, as miR156 represses the expression of the MIR172b gene via its targeted SPL9 and SPL10 genes (Fig. 5a). The other group of miR156-targeted SPL genes (SPL3/4/5) promotes the floral meristem identity transition to induce flowering13 (Fig. 5a). This complex regulatory cascade consisting of miR156, miR172, and their target genes is conserved in both annual and perennial plants, and miR156 and miR172 are closely correlated with the juvenile and adult phases of woody species76,77. Overexpression of miR156 in transgenic Populus x canadensis reduces the expression of miR156-targeted SPL genes and miR172, and dramatically prolongs the juvenile phase76. Long juvenile phase is a common issue for perennial fruit trees, in which it usually takes 4–8 years to finish the juvenile-to-adult transition. miR156-SPL and miR172-AP2 modules are important regulatory hubs in the control of this transition, therefore, they have been considered as potential elements to be engineered biotechnologically to shorten the juvenile phase of fruit trees78. On the other hand, once proceeded to the adult phase, perennial fruit trees normally flower one time a year. It always needs a balance between vegetative growth and reproduction to achieve stable flowering annually. Whether the miR156 and miR172 are coordinated similarly to regulate flowering during a yearly developmental cycle of fruit trees needs further investigation.
Besides miR156 and miR172, there are many other miRNAs involved in the control of plant flowering time as elucidated in the model plant Arabidopsis (as reviewed in ref. 79). As the majority of them have not been studied in horticultural plants, whether their function or regulatory pathways are similar or how conserved their function remains elusive.
miRNAs-fruit development
miR172-fruit size
In addition to its pivotal role in phase change, miR172 makes a great contribution in the process of fruit growth. Its target gene AP2 is a negative regulator of fruit ripening with evidence that knock-down of SlAP2a leads to orange color, split open and bumpy surface of fruits in tomato80. Moreover, recent studies found that the miR172-AP2 pathway affects fruit size in different species depending on fruit type57 (Fig. 5a). In Arabidopsis, the fruit (silique) is derived from carpel tissues; its growth is negatively regulated by AP2. MiR172 inhibits the expression of AP2 that limits cell division and expansion, therefore miR172 overexpression in Arabidopsis gives rise to bigger siliques56. In contrast, apple fruit is mostly derived from the hypanthium contributed most by sepal tissues which is positively regulated by AP2 and over-accumulation of miR172 leads to the silencing of AP2, then leading to the dramatic reduction of fruit size and weight (Fig. 5b)39. In addition, as an ovary-derived flesh fruit, tomato is found to develop parthenocarpic seedless fruit with smaller fruit size when miR172 is overexpressed57. Therefore, fine-tuning the expression of miR172 might be a good strategy to produce the fruit of desirable size. But given the vital role of miR172 in flower development, it might be much complicated to modulate the miR172 expression only in fruit without the disturbance of flowering time and flower organ development.
miR828/miR858-fruit coloration
As mentioned above, miR828 targets TAS4 to generate tasiRNAs regulating MYB genes (Fig. 5c). In addition, miR828 and miR858 work together to co-regulate a large number of MYB genes by directly targeting at the region encoding the conserved R3 domain of MYB proteins, and miR828 triggers the production of secondary phasiRNAs from targeted MYB genes to reinforce its silencing effects30 (Fig. 5c). Most of these regulated MYBs belong to the R2R3 class, a main component of the MYB-bHLH-WD40 protein complex, which is associated with diverse biological processes81, especially the biosynthesis of anthocyanin, one of the main pigments in plants. In Arabidopsis, overexpression of miR828 reduces anthocyanin accumulation by repressing genes encoding MYB transcription factors82. In tomato, miR858 plays a negative role in anthocyanin biosynthesis, and blockage of MIR858 leads to increased anthocyanin accumulation by modulating the expression of SlMYB7 and SlMYB4861, while another report demonstrates that miR858a represses the translation of MYBL2 in Arabidopsis seedlings, as a positive regulator of anthocyanin biosynthesis62. However, in Rosaceae plants, the apple MYB10 and its homologs in close species, which have central roles in fruit coloration83, do not have a good target site for miR828 and miR858, indicating that these two miRNAs likely play versatile or indirect roles in anthocyanin biosynthesis. As this pathway of miR828/miR858 targeting MYB genes is in Gymnosperm18, how this pathway is evolved with anthocyanin biogenesis thereafter is interesting to study. Conceivably, the miR8282/miR858 pathway is evolved with broader function with the expansion of MYB genes, as evidenced by their roles in fiber development84 and cyst nematode parasitism85.
miR397/miR399-fruit quality
Plant laccases, a large family of oxidases, are involved in lignin polymerization. Recently it was found that miR397 regulated fruit cell lignification in pear fruits by inhibiting expression of laccase gene86. A single nucleotide polymorphism (SNP) identified in the promoter of PbrMIR397 gene is associated with low levels of fruit lignin86. This SNP may serve as a good genetic marker for the breeding selection of pear trees bearing fruits with low lignin content. In strawberry, fruits of high content of soluble solids are preferred by customer. Researchers found that the high content of soluble solids is positively correlated with high level of Pi content among different strawberry cultivars87. Phosphorus nutrition is a process under the regulation of miRNAs. The Pi-starvation responsive miR399 guides the cleavage of PHO2 RNA, which encodes an E2 ubiquitin conjugase-related protein that negatively affects Pi content and remobilization88. Overexpression of miR399 can significantly improve fruit quality by increasing the Pi content and thereby the soluble solid content in strawberry fruit89. Higher soluble solids content is a common desirable trait for fruits. Whether this positive correlation of miR399 expression with soluble solid content is present in other types of fruits is worthy of an investigation.
As reported, miRNAs likely participate in every aspect of fruit development, from fruit set to fruit ripening, and from fruit size determination, fruit shape formation to fruit coloration. Although the function of conserved miRNAs is well-maintained among different plants, they are likely to have different effects on fruit quality, because “fruits” (eventual product for harvest) of many horticultural plants come from different organs, which are likely under a different regulation.
miRNAs-auxin signaling
Another important role of miRNAs and phasiRNAs is that they are involved in the signaling pathway of auxin, a key plant hormone regulating plant growth and development, through regulating the AUXIN RESPONSIVE FACTORS (ARFs). ARFs, a class of transcription factors critical in auxin signaling, work together with Aux/IAAs in auxin-mediated growth and developmental processes by binding to the AUXIN RESPONSE ELEMENT (AuxRE) site in the promoter region of early auxin response genes. Many ARFs are regulated by miRNAs to trigger miRNA-mediated regulation of auxin responses in plant development. For example, there are 22 ARFs in tomato, falling into three clusters. There are a few members in each cluster regulated by miRNAs90 (Fig. 6). ARF6/8 have been shown to be negatively regulated by miR16791, and ARF10/16/17 are post-transcriptionally regulated by miR16092–94. Furthermore, as mentioned above, miR390 triggers the production of tasiRNA (tasiARF) from TAS3 genes to target ARF2/3/46,95,96. These three miRNA-mediated regulatory pathways are highly conserved in diverse plants11,97.
Fig. 5. Representative miRNA/phasiRNA pathways functionally important in horticultural plants.
a miR156 and miR172 cooperatively regulate the juvenile-to-adult phase transition in plants. MiR172, in collaboration with miR156, participates in the regulation of juvenile-to-adult phase transition in plants. These two miRNAs play antagonistic roles in flowering induction; high level of miR156 extends juvenile phase and delays flowering, while miR172 accumulation leads to early flowering75. b miR172 affects fruit size differently in apple and Arabidopsis depending on fruit type. Apple fruit is mostly derived from the hypanthium contributed most by sepal tissues which is positively regulated by AP2 and over-accumulation of miR172 leads to the silencing of AP2, then leading to the dramatic reduction of fruit size and weight39. In contrast, the Arabidopsis fruit (silique) is derived from carpel tissues, in which AP2 limits cell division and expansion; therefore miR172 overexpression (inhibiting the AP2 expression) in Arabidopsis gives rise to bigger siliques56. c miR828 and miR858 function together to regulate the expression of MYB genes, which are involved in the pathway of anthocyanin biosynthesis, affecting fruit coloration30
miR160-ARF10/16/17
MiR160 is involved in many biotic processes in plants, including flower identity specification, leaf development, fruit formation and etc.7. Upon sly-miR160 down-regulation using a short tandem target mimic (STTM160), its target genes ARF10/16/17 all up-regulate, and tomato fruits show elongated, pear-shaped morphology compared to control tomatoes due to the pre-anthesis shape alteration98. In addition, sly-miR160 down-regulation also alters the phenotype of vegetative lateral organs and inhibits the abscission of petal, anther, and fruit in tomato98. Ectopic expression of miR160-insensitive SlARF10A (mSlARF10A) results in narrow leaflet blades, sepals and petals, and abnormally shaped fruit; notably, transgenic fruits have a clear cone shape and are almost seedless with abnormal seeds that could not germinate99. Overexpression of the miR160-targeted ARFs SlARF10A, SlARF10B, or SlARF17, leads to reduced lamina and increased leaf complexity, and suppresses auxin response in tomato in young leaves100.
miR167-ARF6/8
MiR167 plays vital roles in the development of flower and fruit as well as root. MiR167 targeted ARF6/8 regulate flower organ development91. Down-regulation of ARF6 and ARF8 by miR167 results in shorter petals, stamens, and leaves size, with the largest defects in floral development and female sterility101. Transgenic introduction of aberrant ARF8 transcripts affects fruit initiation, leading to parthenocarpic fruit formation in both Arabidopsis and tomato102,103. MiR167 has also been implicated in plant immunity. MiR167 is down-regulated in response to fungal infection in Arabidopsis104 and during bacterial stress, miR167 alters the expression of genes of the host auxin signaling pathway, including ARF6/8105.
miR390-TAS3-ARF2/3/4
The miR390-TAS3-ARF pathway is mainly involved in the regulation of leaf and flower development, especially the leaf morphogenesis. The tasiRNA-mediated regulation of ARF3 and ARF4 is required for normal leaf morphogenesis; it stabilizes abaxial organ identity in Arabidopsis thaliana, tomato, and tobacco45,95,106,107. When tasiARFs fail to accumulate in tomato, misexpression of ARF3 and/or ARF4 leads to needle-like leaves in a species-specific manner, while reducing the activity of both ARF3 and ARF4 can rescue the wiry leaf lamina108. A collection of mutant plants of AGO7, a dispensable Argonaute partner of miR390 activity, including Arabidopsis, maize, tomato, Medicago, and monkey flower, show severe leaf and flower defects, for example, wiry leaves in tomato, and lobed and elongated leaves and abnormal flowers with defected organs in Medicago45,109–111. Overexpression of SlARF2 in tomato results in pleiotropic morphological and developmental phenotypes, such as increased lateral root formation and flower organ senescence112.
In summary, these three miRNA-(tasiRNA)-ARF regulatory modules tend to have distinct main functions, i.e., the miR160-ARF10/16/17 module is important for the development of leaf and fruit, miR167-ARF6/8 essential for flower and fruit, and miR390-TAS3-ARF2/3/4 for leaf and flower (Fig. 6). On the other hand, these three modules are intertwined with each other to have a common function. For instance, they all function in root development; AtARF6/8 (targeted by miR167) and AtARF17 (targeted by miR160) control adventitious rooting in Arabidopsis113. miR390 is regulating lateral root elongation by suppressing the expression of ARF4 to allow the outgrowth of the emerging lateral root114,115. Auxin is a chemical widely used in almost all the aspects of the horticultural industry. miR160, miR167, and miR390 comprise three major regulatory hubs, adding more plasticity to the auxin signaling pathway. A good understanding of the roles of these hubs is of great significance for more effective and efficient application of auxin in the industry. In addition to auxin, miRNAs are involved in the metabolism or signaling of almost all other phytohormones, including ethylene, gibberellin, cytokinin, and abscisic acid (as reviewed in ref.116). But so far, studies regarding miRNA-involved phytohormone homeostasis in horticultural plants are very few.
Fig. 6. MiR160, miR167, and miR390 are involved in the regulation of ARFs.
Left is the unrooted tree of tomato ARFs; the middle is the domain structure of ARFs in tomato with most of the ARFs consisting of three domains, B3 (green), Auxin_resp (yellow), and AUX/IAA (pink). Target sites of miR160, miR167, and tasiARF are marked respectively in their target genes; the right illustrates the biological functions of miR160/miR167/miR390-ARF pathways in plants, with thicker arrow denoting major functions of the corresponding pathway
miRNAs-disease resistance
miRNAs have also been demonstrated to play critical roles in many other aspects, especially stress responses67,104,117–119. miRNAs have been shown to be directly involved in regulation of disease resistance (R) genes19,41,120. Among them includes the miR482/2118 superfamily, which target a large number of NB-LRR genes19,41. In virus- or bacteria-infected tomato, the expression of miR482 is suppressed while some of its disease-resistant NBS-LRR target genes are up-regulated120. miR482/2118 is 22 nt long and has been demonstrated to trigger the production of 21-nt phasiRNAs from their targeted NB-LRR genes. Members of the miR482 family are down-regulated in cotton seedlings infected with a fungal pathogen Verticillium dahliae; they induce the expression of specific NBS-LRR genes in cotton, implying that miR482-mediated silencing of NBS-LRR genes is released in cotton upon fungal pathogen infection to activate disease defense121. miR482/2118-targeted NB-LRR genes comprise one of the largest gene families producing abundant phasiRNAs, a clear understanding of the role of these secondary phasiRNAs is still lacking. One possibility is that phasiRNAs is to maintain the low-level expression of NB-LRR genes in normal condition without pathogenic stresses64. miR528 and miR398 are also involved in resistance to virus or other biotic stresses14,122. They target a group of oxidases, including laccase, ascorbic acid oxidase, superoxide dismutase, which contribute to plant defense through the regulation of the level of reactive oxygen species.
Lineage- or species-specific miRNA or phasiRNA pathways important in horticultural plants
Increasing studies have demonstrated that a few miRNAs restricted in certain plant lineages also play vital roles in various biological processes. Xia et al.17 characterized two clusters of miRNAs, which regulate a large number of F-box (FBX) genes from woodland strawberry (diploid), and one of these miRNAs is able to trigger subsequent phasiRNA production to reinforce the silencing of FBX genes. This miRNA-FBX-phasiRNA circuit targets an array of genes that are possibly involved in regulation of different biological events, including disease resistance and fruit development17. Another specific miRNA found in Fragaria ananassa (Octoploid) targets the ABI5 (ABA-INSENSITIVE 5) gene, which encodes a critical transcription factor in the ABA signaling pathway; this regulation is likely involved in fruit ripening and responses to environmental stresses123. Recently, a study in citrus reported that 22-nt miR3954 targets a NAC transcript and two citrus-specific non-coding transcripts to trigger the biogenesis of phasiRNAs, which might be involved in the induction of early flowering in citrus15. This regulatory pathway has also been found in litchi124, a plant phylogenetically close to citrus, implicating that the miR3954-NAC/lncRNA-phasiRNA is likely a lineage-restricted pathway related to flowering induction. Lineage- or species-specific miRNAs represent a large class of sRNAs in plants. Although their function is not as fundamental or broad as conserved miRNAs, they are believed to be associated with the development of specific feature of certain lineage or species, like unique traits of horticultural plants.
Other interesting studies on sRNAs in horticultural plants
In addition to miRNA and phasiRNAs, other sRNAs have also been reported to contribute to phenotypic diversity of horticultural plants. A study on persimmon (Diospyros lotus), a dioecy plant with heterogametic males (XY), identified a Y-specific sex-determinant candidate (OGI), which produces a sRNA targeting the autosomal MeGI gene, encoding a homeodomain transcription factor regulating anther fertility in a dosage-dependent fashion125. Another study investigating the formation of flower color pattern in snapdragon (Antirrhinum majus) found that an inverted duplication that generates sRNAs which repress a pigment biosynthesis gene, is the cause of population-wide differences in color patterns; the inverted duplication is under selection and is likely an intermediate on the pathway to miRNA evolution126. These sRNAs uncovered in these two exceptional studies do not belong to the miRNA or phasiRNA, or other well-known sRNA classes, demonstrating that the sRNA population and their function in plants are probably much more complicated than what we understand now.
Concluding remarks
In the past one and a half decades, the rapid development of next-generation sequencing technologies stimulates an unprecedented sRNA research progress in plants in general, and horticultural crops in particular, because many of them (e.g., apple, peach, etc.) are not amenable to genetic analysis due to long juvenility and complexity genetics. It becomes apparent that horticultural crops share the conserved miRNA and phasiRNA pathways with other plants, and they also evolve their lineage- or species-specific miRNA/phasiRNA pathways, which have not been found even in other horticultural plants. Conserved miRNA/phasiRNAs often plays fundamental roles in processes important for healthy growth and normal development, for instance, flowering programming, fruit development, and disease resistance. Lineage- or species-specific miRNAs/phasiRNAs are biologically meaningful as well to researchers because they may confer or regulate the traits that other plants or crops lack or have not evolved. Hence, the elaboration of the relation between regulation of certain miRNA/phasiRNAs and expression of specific traits would provide invaluable information for practical breeding programs. Although a great progress has been made in sRNA research in horticultural crops, such progress is yet limited to a few species but have not been achieved in many other horticultural crops. Hence, continuous profiling and analysis of sRNAs, discovery of new miRNAs and unraveling of their regulatory pathways using computation-based approach are necessary. Ideally, these identified miRNAs need to be functionally validated in host plants through down-regulation using RNAi or CRISPR and up-regulation using ecotopical expression approaches. Unfortunately, performing such analyses in many horticultural plants remains challenging because of lack of an effective transformation system. In short, in the past decade we have just opened the door and have a glimpse of sRNAs in horticultural crops; further studies, including both exhaustive bioinformatics data mining and in-depth functional decoding, will be needed to uncover a more complete picture of them in the future.
Acknowledgements
We thank other members of the Xia lab for helpful discussion and comments on the manuscript. We apologize to authors whose work could not be included in this review, due to space limits. We also thank the anonymous reviewers for their constructive comments, which helped us improve the review. This work was supported with funding from the Chinese Thousand Young Talents Program and the Innovation Team Project of the Department of Education of Guangdong Province (2016KCXTD011).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chengjie Chen, Zaohai Zeng
References
- 1.Llave C, Xie Z, Kasschau KD, Carrington JC. Cleavage of scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science. 2002;297:2053–2056. doi: 10.1126/science.1076311. [DOI] [PubMed] [Google Scholar]
- 2.Voinnet O. Origin, biogenesis, and activity of plant microRNAs. Cell. 2009;136:669–687. doi: 10.1016/j.cell.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 3.Cuperus JT, Fahlgren N, Carrington JC. Evolution and functional diversification of miRNA genes. Plant Cell. 2011;23:431–442. doi: 10.1105/tpc.110.082784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Ario M, Griffiths-Jones S, Kim M. Small RNAs: big impact on plant development. Trends Plant Sci. 2017;22:1056–1068. doi: 10.1016/j.tplants.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Axtell MJ. Classification and comparison of small RNAs from plants. Annu. Rev. Plant Biol. 2013;64:137–159. doi: 10.1146/annurev-arplant-050312-120043. [DOI] [PubMed] [Google Scholar]
- 6.Allen E, Xie Z, Gustafson AM, Carrington JC. MicroRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell. 2005;121:207–221. doi: 10.1016/j.cell.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Jones-Rhoades MW, Bartel DP, Bartel B. MicroRNAs and their regulatory roles in plants. Annu. Rev. Plant Biol. 2006;57:19–53. doi: 10.1146/annurev.arplant.57.032905.105218. [DOI] [PubMed] [Google Scholar]
- 8.Achkar NP, Cambiagno DA, Manavella PA. MiRNA biogenesis: a dynamic pathway. Trends Plant Sci. 2016;21:1034–1044. doi: 10.1016/j.tplants.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Yu B, et al. Methylation as a crucial step in plant microRNA biogenesis. Science. 2005;307:932–935. doi: 10.1126/science.1107130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fei Q, Xia R, Meyers BC. Phased, secondary, small interfering RNAs in posttranscriptional regulatory networks. Plant Cell. 2013;25:2400–2415. doi: 10.1105/tpc.113.114652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Axtell MJ, Jan C, Rajagopalan R, Bartel DP. A two-hit trigger for siRNA biogenesis in plants. Cell. 2006;127:565–577. doi: 10.1016/j.cell.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 12.Borges F, Martienssen RA. The expanding world of small RNAs in plants. Nat. Rev. Mol. Cell Biol. 2015;16:727–741. doi: 10.1038/nrm4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu M, et al. Developmental functions of miR156-regulated SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) genes in Arabidopsis thaliana. PLoS Genet. 2016;12:e1006263. doi: 10.1371/journal.pgen.1006263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J, et al. ROS accumulation and antiviral defence control by microRNA528 in rice. Nat. Plants. 2017;3:16203. doi: 10.1038/nplants.2016.203. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, et al. MiR3954 is a trigger of phasiRNAs that affects flowering time in citrus. Plant J. 2017;92:263–275. doi: 10.1111/tpj.13650. [DOI] [PubMed] [Google Scholar]
- 16.Xia R, Xu J, Meyers BC. The emergence, evolution, and diversification of the miR390-TAS3-ARF pathway in land plants. Plant Cell. 2017;29:1232–1247. doi: 10.1105/tpc.17.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia R, Ye S, Liu Z, Meyers BC, Liu Z. Novel and recently evolved microRNA clusters regulate expansive F-BOX gene networks through phased small interfering RNAs in wild diploid strawberry. Plant Physiol. 2015;169:594–610. doi: 10.1104/pp.15.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia R, Xu J, Arikit S, Meyers BC. Extensive families of miRNAs and PHAS loci in norway spruce demonstrate the origins of complex phasiRNA networks in seed plants. Mol. Biol. Evol. 2015;32:2905–2918. doi: 10.1093/molbev/msv164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhai J, et al. MicroRNAs as master regulators of the plant NB-LRR defense gene family via the production of phased, trans-acting siRNAs. Genes Dev. 2011;25:2540–2553. doi: 10.1101/gad.177527.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia R, et al. MicroRNA superfamilies descended from miR390 and their roles in secondary small interfering RNA biogenesis in eudicots. Plant Cell. 2013;25:1555–1572. doi: 10.1105/tpc.113.110957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moxon S, et al. Deep sequencing of tomato short RNAs identifies microRNAs targeting genes involved in fruit ripening. Genome Res. 2008;18:1602–1609. doi: 10.1101/gr.080127.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang DG, et al. The hot pepper (Capsicum annuum) microRNA transcriptome reveals novel and conserved targets: a foundation for understanding microRNA functional roles in hot pepper. PLoS One. 2013;8:e64238. doi: 10.1371/journal.pone.0064238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang R, Marshall D, Bryan GJ, Hornyik C. Identification and characterization of miRNA transcriptome in potato by high-throughput sequencing. PLoS One. 2013;8:e57233. doi: 10.1371/journal.pone.0057233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arikit S, et al. An atlas of soybean small RNAs identifies phased siRNAs from hundreds of coding genes. Plant Cell. 2014;26:4584–4601. doi: 10.1105/tpc.114.131847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srivastava S, et al. High throughput sequencing of small RNA component of leaves and inflorescence revealed conserved and novel miRNAs as well as phasiRNA loci in chickpea. Plant Sci. 2015;235:46–57. doi: 10.1016/j.plantsci.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Mao W, Li Z, Xia X, Li Y, Yu J. A combined approach of high-throughput sequencing and degradome analysis reveals tissue specific expression of microRNAs and their targets in cucumber. PLoS One. 2012;7:e33040. doi: 10.1371/journal.pone.0033040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pantaleo V, et al. Identification of grapevine microRNAs and their targets using high-throughput sequencing and degradome analysis. Plant J. 2010;62:960–976. doi: 10.1111/j.0960-7412.2010.04208.x. [DOI] [PubMed] [Google Scholar]
- 28.Song C, et al. Deep sequencing discovery of novel and conserved microRNAs in trifoliate orange (Citrus trifoliata) BMC Genomics. 2010;11:431–442. doi: 10.1186/1471-2164-11-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu XM, et al. Genomewide analysis of small RNAs in nonembryogenic and embryogenic tissues of citrus: microRNA- and siRNA-mediated transcript cleavage involved in somatic embryogenesis. Plant Biotechnol. J. 2015;13:383–394. doi: 10.1111/pbi.12317. [DOI] [PubMed] [Google Scholar]
- 30.Xia R, Zhu H, An Y, Beers EP, Liu Z. Apple miRNAs and tasiRNAs with novel regulatory networks. Genome Biol. 2012;13:R47. doi: 10.1186/gb-2012-13-6-r47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eldem V, et al. Genome-wide identification of miRNAs responsive to drought in peach (Prunus persica) by high-throughput deep sequencing. PLoS One. 2012;7:e50298. doi: 10.1371/journal.pone.0050298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu H, et al. Unique expression, processing regulation, and regulatory network of peach (Prunus persica) miRNAs. BMC Plant Biol. 2012;12:149. doi: 10.1186/1471-2229-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ge A, et al. Deep sequencing discovery of novel and conserved microRNAs in strawberry (Fragaria x ananassa) Physiol. Plant. 2013;148:387–396. doi: 10.1111/j.1399-3054.2012.01713.x. [DOI] [PubMed] [Google Scholar]
- 34.De Paoli E, et al. Distinct extremely abundant siRNAs associated with cosuppression in petunia. RNA. 2009;15:1965–1970. doi: 10.1261/rna.1706109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.An FM, Hsiao SR, Chan MT. Sequencing-based approaches reveal low ambient temperature-responsive and tissue-specific microRNAs in phalaenopsis orchid. PLoS One. 2011;6:e18937. doi: 10.1371/journal.pone.0018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solofoharivelo MC, van der Walt AP, Stephan D, Burger JT, Murray SL. MicroRNAs in fruit trees: discovery, diversity and future research directions. Plant Biol. 2014;16:856–865. doi: 10.1111/plb.12153. [DOI] [PubMed] [Google Scholar]
- 37.Pei H, et al. Integrative analysis of miRNA and mRNA profiles in response to ethylene in rose petals during flower opening. PLoS One. 2013;8:e64290. doi: 10.1371/journal.pone.0064290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xing L, et al. Shoot bending promotes flower bud formation by miRNA-mediated regulation in apple (Malus domestica Borkh.) Plant Biotechnol. J. 2016;14:749–770. doi: 10.1111/pbi.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao JL, et al. A microRNA allele that emerged prior to apple domestication may underlie fruit size evolution. Plant J. 2015;84:417–427. doi: 10.1111/tpj.13021. [DOI] [PubMed] [Google Scholar]
- 40.Qian M, et al. Response of miR156-SPL module during the red peel coloration of bagging-treated Chinese sand pear (Pyrus pyrifolia Nakai) Front. Physiol. 2017;8:550. doi: 10.3389/fphys.2017.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li F, et al. MicroRNA regulation of plant innate immune receptors. Proc. Natl. Acad. Sci. U.S.A. 2011;109:1790–1795. doi: 10.1073/pnas.1118282109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin W, Wu F. Characterization of miRNAs associated with Botrytis cinerea infection of tomato leaves. BMC Plant Biol. 2015;15:1. doi: 10.1186/s12870-014-0410-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Li C, Bai L, He C, Yu X. MicroRNA and target gene responses to salt stress in grafted cucumber seedlings. Acta Physiol. Plant. 2016;38:42. doi: 10.1007/s11738-016-2070-5. [DOI] [Google Scholar]
- 44.Yu X, et al. Identification of conserved and novel microRNAs that are responsive to heat stress in Brassica rapa. J. Exp. Bot. 2012;63:1025–1038. doi: 10.1093/jxb/err337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adenot X, et al. DRB4-dependent TAS3 trans-acting siRNAs control leaf morphology through AGO7. Curr. Biol. 2006;16:927–932. doi: 10.1016/j.cub.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 46.Ori N, et al. Regulation of LANCEOLATE by miR319 is required for compound-leaf development in tomato. Nat. Genet. 2007;39:787–791. doi: 10.1038/ng2036. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez RE, et al. Control of cell proliferation in Arabidopsis thaliana by microRNA miR396. Development. 2010;137:103–112. doi: 10.1242/dev.043067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jung JH, Park CM. MIR166/165 genes exhibit dynamic expression patterns in regulating shoot apical meristem and floral development in Arabidopsis. Planta. 2007;225:1327–1338. doi: 10.1007/s00425-006-0439-1. [DOI] [PubMed] [Google Scholar]
- 49.Aukerman MJ, Sakai H. Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell. 2003;15:2730–2741. doi: 10.1105/tpc.016238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nag A, King S, Jack T. miR319a targeting of TCP4 is critical for petal growth and development in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 2009;106:22534–22539. doi: 10.1073/pnas.0908718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang W, et al. The role of miR319a and its target gene TCP4 in the regulation of pistil development in Prunus mume. Genome. 2017;61:43–48. doi: 10.1139/gen-2017-0118. [DOI] [PubMed] [Google Scholar]
- 52.Wei Q, et al. Control of chrysanthemum flowering through integration with an aging pathway. Nat. Commun. 2017;8:829. doi: 10.1038/s41467-017-00812-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang JW, Czech B, Weigel D. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell. 2009;138:738–749. doi: 10.1016/j.cell.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 54.Xia K, et al. OsTIR1 and OsAFB2 downregulation via OsmiR393 overexpression leads to more tillers, early flowering and less tolerance to salt and drought in rice. PLoS One. 2012;7:e30039. doi: 10.1371/journal.pone.0030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen ZH, et al. Regulation of auxin response by miR393-targeted transport inhibitor response protein 1 is involved in normal development in Arabidopsis. Plant Mol. Biol. 2011;77:619–629. doi: 10.1007/s11103-011-9838-1. [DOI] [PubMed] [Google Scholar]
- 56.José Ripoll J, et al. MicroRNA regulation of fruit growth. Nat. Plants. 2015;1:15036. doi: 10.1038/nplants.2015.36. [DOI] [PubMed] [Google Scholar]
- 57.Yao JL, Tomes S, Xu J, Gleave AP. How microRNA172 affects fruit growth in different species is dependent on fruit type. Plant Signal. Behav. 2016;11:e1156833. doi: 10.1080/15592324.2016.1156833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sagar M, et al. SlARF4, an auxin response factor involved in the control of sugar metabolism during tomato fruit development. Plant Physiol. 2013;161:1362–1374. doi: 10.1104/pp.113.213843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu J, et al. New insights into the roles of cucumber TIR1 homologs and miR393 in regulating fruit/seed set development and leaf morphogenesis. BMC Plant Biol. 2017;17:130. doi: 10.1186/s12870-017-1075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jia X, et al. Negative regulation of anthocyanin biosynthesis in tomato by microRNA828 under phosphate deficiency. Sci. Agric. Sin. 2015;48:2911–2924. [Google Scholar]
- 61.Jia X, et al. Small tandem target mimic-mediated blockage of microRNA858 induces anthocyanin accumulation in tomato. Planta. 2015;242:283–293. doi: 10.1007/s00425-015-2305-5. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y, Wang Y, Song Z, Zhang H. Repression of MYBL2 by both microRNA858a and HY5 leads to the activation of anthocyanin biosynthetic pathway in Arabidopsis. Mol. Plant. 2016;9:1395–1405. doi: 10.1016/j.molp.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 63.Ouyang S, et al. MicroRNAs suppress NB domain genes in tomato that confer resistance to Fusarium oxysporum. PLoS. Pathog. 2014;10:e1004464. doi: 10.1371/journal.ppat.1004464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fei Q, Zhang Y, Xia R, Meyers BC. Small RNAs add zing to the zig-zag-zig model of plant defenses. Mol. Plant–Microbe Interact. 2016;29:165–169. doi: 10.1094/MPMI-09-15-0212-FI. [DOI] [PubMed] [Google Scholar]
- 65.Si-Ammour A, et al. miR393 and secondary siRNAs regulate expression of the TIR1/AFB2 auxin receptor clade and auxin-related development of Arabidopsis leaves. Plant Physiol. 2011;157:683–691. doi: 10.1104/pp.111.180083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen X. A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science. 2004;303:2022–2025. doi: 10.1126/science.1088060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu QH, Helliwell CA. Regulation of flowering time and floral patterning by miR172. J. Exp. Bot. 2011;62:487–495. doi: 10.1093/jxb/erq295. [DOI] [PubMed] [Google Scholar]
- 68.Glazińska P, Zienkiewicz A, Wojciechowski W, Kopcewicz J. The putative miR172 target gene InAPETALA2-like is involved in the photoperiodic flower induction of Ipomoea nil. J. Plant Physiol. 2009;166:1801–1813. doi: 10.1016/j.jplph.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 69.Almada R, Cabrera N, Casaretto JA, Ruiz-Lara S, Villanueva EG. VvCO and VvCOL1, two CONSTANS homologous genes, are regulated during flower induction and dormancy in grapevine buds. Plant Cell Rep. 2009;28:1193–1203. doi: 10.1007/s00299-009-0720-4. [DOI] [PubMed] [Google Scholar]
- 70.Tränkner C, et al. Over-expression of an FT-homologous gene of apple induces early xowering in annual and perennial plants. Planta. 2010;232:1309–1324. doi: 10.1007/s00425-010-1254-2. [DOI] [PubMed] [Google Scholar]
- 71.An L, Lei H, Shen X, Li T. Identification and characterization of PpLFL, a homolog of FLORICAULA/LEAFY in peach (Prunus persica) Plant Mol. Biol. Rep. 2012;30:1488–1495. doi: 10.1007/s11105-012-0459-x. [DOI] [Google Scholar]
- 72.Porto DD, et al. Transcription profiling of the chilling requirement for bud break in apples: a putative role for FLC-like genes. J. Exp. Bot. 2015;66:2659–2672. doi: 10.1093/jxb/erv061. [DOI] [PubMed] [Google Scholar]
- 73.Wells CE, Vendramin E, Tarodo SJ, Verde I, Bielenberg DG. A genome-wide analysis of MADS-box genes in peach [Prunus persica (L.) Batsch] BMC Plant Biol. 2015;15:41. doi: 10.1186/s12870-015-0436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ito A, et al. Physiological differences between bud breaking and flowering after dormancy completion revealed by DAM and FT/TFL1 expression in Japanese pear (Pyrus pyrifolia) Tree Physiol. 2015;36:109–120. doi: 10.1093/treephys/tpv115. [DOI] [PubMed] [Google Scholar]
- 75.Wu G, et al. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell. 2009;138:750–759. doi: 10.1016/j.cell.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang JW, et al. MiRNA control of vegetative phase change in trees. PLoS Genet. 2011;7:21–25. doi: 10.1371/journal.pgen.1002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poethig, R. S. Vegetative phase change and shoot maturation in plants. Curr. Top. Dev. Biol.105, 125–152 (2013). [DOI] [PMC free article] [PubMed]
- 78.Jiang Y, Gabriel DW. Breaking citrus juvenility by modulating endogenous miR156 and miR172 levels. J. Citrus Pathol. 2014;1:1. [Google Scholar]
- 79.Teotia S, Tang G. To bloom or not to bloom: role of micrornas in plant flowering. Mol. Plant. 2015;8:359–377. doi: 10.1016/j.molp.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 80.Karlova R, et al. Transcriptome and metabolite profiling show that APETALA2a is a major regulator of tomato fruit ripening. Plant Cell. 2011;23:923–941. doi: 10.1105/tpc.110.081273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramsay NA, Glover BJ. MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 2005;10:63–70. doi: 10.1016/j.tplants.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 82.Yang F, Cai J, Yang Y, Liu Z. Overexpression of microRNA828 reduces anthocyanin accumulation in Arabidopsis. Plant Cell Tissue Organ Cult. 2013;115:159–167. doi: 10.1007/s11240-013-0349-4. [DOI] [Google Scholar]
- 83.Espley RV, et al. Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J. 2007;49:414–427. doi: 10.1111/j.1365-313X.2006.02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guan X, et al. MiR828 and miR858 regulate homoeologous MYB2 gene functions in Arabidopsis trichome and cotton fibre development. Nat. Commun. 2014;5:3050. doi: 10.1038/ncomms4050. [DOI] [PubMed] [Google Scholar]
- 85.Piya S, Kihm C, Rice JH, Baum TJ, Hewezi T. Cooperative regulatory functions of miR858 and MYB83 during Cyst nematode parasitism. Plant Physiol. 2017;174:1897–1912. doi: 10.1104/pp.17.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xue C, et al. PbrmiR397a regulates lignification during stone cell development in pear fruit. Plant Biotechnol. J. 2018;0:1467–7644. doi: 10.1111/pbi.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cao F, Guan C, Dai H, Li X, Zhang Z. Soluble solids content is positively correlated with phosphorus content in ripening strawberry fruits. Sci. Hortic. 2015;195:183–187. doi: 10.1016/j.scienta.2015.09.018. [DOI] [Google Scholar]
- 88.Bari R, Pant BD, Stitt M, Golm SP. PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol. 2006;141:988–999. doi: 10.1104/pp.106.079707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Y, et al. Improvement in fruit quality by overexpressing miR399a in woodland strawberry. J. Agric. Food Chem. 2017;65:7361–7370. doi: 10.1021/acs.jafc.7b01687. [DOI] [PubMed] [Google Scholar]
- 90.Zouine M, et al. Characterization of the tomato ARF gene family uncovers a multi-levels post-transcriptional regulation including alternative splicing. PLoS One. 2014;9:e84203. doi: 10.1371/journal.pone.0084203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu MF, Tian Q, Reed JW. Arabidopsis microRNA167 controls patterns of ARF6 and ARF8 expression, and regulates both female and male reproduction. Development. 2006;133:4211–4218. doi: 10.1242/dev.02602. [DOI] [PubMed] [Google Scholar]
- 92.Liu PP, et al. Repression of AUXIN RESPONSE FACTOR10 by microRNA160 is critical for seed germination and post-germination stages. Plant J. 2007;52:133–146. doi: 10.1111/j.1365-313X.2007.03218.x. [DOI] [PubMed] [Google Scholar]
- 93.Wang JW, et al. Control of root cap formation by microRNA-targeted auxin response factors in Arabidopsis. Plant Cell. 2005;17:2204–2216. doi: 10.1105/tpc.105.033076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mallory AC, Bartel DP, Bartel B. MicroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes. Development. 2005;17:1360–1375. doi: 10.1105/tpc.105.031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hunter C. Trans-acting siRNA-mediated repression of ETTIN and ARF4 regulates heteroblasty in Arabidopsis. Development. 2006;133:2973–2981. doi: 10.1242/dev.02491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fahlgren N, et al. Regulation of AUXIN RESPONSE FACTOR3 by TAS3 ta-siRNA affects developmental timing and patterning in Arabidopsis. Curr. Biol. 2006;16:939–944. doi: 10.1016/j.cub.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 97.Curaba J, Spriggs A, Taylor J, Li Z, Helliwell C. MiRNA regulation in the early development of barley seed. BMC Plant Biol. 2012;12:120. doi: 10.1186/1471-2229-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Damodharan S, Zhao D, Arazi T. A common miRNA160-based mechanism regulates ovary patterning, floral organ abscission and lamina outgrowth in tomato. Plant J. 2016;86:458–471. doi: 10.1111/tpj.13127. [DOI] [PubMed] [Google Scholar]
- 99.Hendelman A, Buxdorf K, Stav R, Kravchik M, Arazi T. Inhibition of lamina outgrowth following Solanum lycopersicum AUXIN RESPONSE FACTOR 10 (SlARF10) derepression. Plant Mol. Biol. 2012;78:561–576. doi: 10.1007/s11103-012-9883-4. [DOI] [PubMed] [Google Scholar]
- 100.Ben-Gera H, et al. Auxin-mediated lamina growth in tomato leaves is restricted by two parallel mechanisms. Plant J. 2016;86:443–457. doi: 10.1111/tpj.13188. [DOI] [PubMed] [Google Scholar]
- 101.Liu N, et al. Down-regulation of AUXIN RESPONSE FACTORS 6 and 8 by microRNA 167 leads to floral development defects and female sterility in tomato. J. Exp. Bot. 2014;65:2507–2520. doi: 10.1093/jxb/eru141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goetz M. AUXIN RESPONSE FACTOR8 is a negative regulator of fruit initiation in Arabidopsis. Plant Cell. 2006;18:1873–1886. doi: 10.1105/tpc.105.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goetz M, et al. Expression of aberrant forms of AUXIN RESPONSE FACTOR 8 stimulates parthenocarpy in Arabidopsis and tomato. Plant Physiol. 2007;145:351–366. doi: 10.1104/pp.107.104174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khraiwesh B, Zhu JK, Zhu J. Role of miRNAs and siRNAs in biotic and abiotic stress responses of plants. Biochim. Biophys. Acta. 2012;1819:137–148. doi: 10.1016/j.bbagrm.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jodder J, Basak S, Das R, Kundu P. Coherent regulation of miR167a biogenesis and expression of auxin signaling pathway genes during bacterial stress in tomato. Physiol. Mol. Plant Pathol. 2017;100:97–105. doi: 10.1016/j.pmpp.2017.08.001. [DOI] [Google Scholar]
- 106.Pekker I. Auxin response factors mediate Arabidopsis organ asymmetry via modulation of KANADI activity. Plant Cell. 2005;17:2899–2910. doi: 10.1105/tpc.105.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alvarez JP. Endogenous and synthetic microRNAs stimulate simultaneous, efficient, and localized regulation of multiple targets in diverse species. Plant Cell. 2006;18:1134–1151. doi: 10.1105/tpc.105.040725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alvarado V, Scholthof HB. Plant responses against invasive nucleic acids: RNA silencing and its suppression by plant viral pathogens. Semin. Cell Dev. Biol. 2009;20:1032–1040. doi: 10.1016/j.semcdb.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou C, et al. The trans-acting short interfering RNA3 pathway and NO APICAL MERISTEM antagonistically regulate leaf margin development and lateral organ separation, as revealed by analysis of an argonaute7/lobed leaflet1 mutant in Medicago truncatula. Plant Cell. 2013;25:4845–4862. doi: 10.1105/tpc.113.117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yifhar T, et al. Failure of the tomato trans-acting short interfering RNA program to regulate AUXIN RESPONSE FACTOR3 and ARF4 underlies the wiry leaf syndrome. Plant Cell. 2012;24:3575–3589. doi: 10.1105/tpc.112.100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Douglas RN, et al. Ragged seedling2 encodes an ARGONAUTE7-like protein required for mediolateral expansion, but not dorsiventrality, of maize leaves. Plant Cell. 2010;22:1441–1451. doi: 10.1105/tpc.109.071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ren Z, Liu R, Gu W, Dong X. The Solanum lycopersicum auxin response factor SlARF2 participates in regulating lateral root formation and flower organ senescence. Plant Sci. 2017;256:103–111. doi: 10.1016/j.plantsci.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 113.Gutierrez L, et al. Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell. 2012;24:2515–2527. doi: 10.1105/tpc.112.099119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marin E, et al. MiR390, Arabidopsis TAS3 tasiRNAs, and their AUXIN RESPONSE FACTOR targets define an autoregulatory network quantitatively regulating lateral root growth. Plant Cell. 2010;22:1104–1117. doi: 10.1105/tpc.109.072553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yoon EK, et al. Auxin regulation of the microRNA390-dependent transacting small interfering RNA pathway in Arabidopsis lateral root development. Nucleic Acids Res. 2009;38:1382–1391. doi: 10.1093/nar/gkp1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Curaba J, Singh MB, Bhalla PL. MiRNAs in the crosstalk between phytohormone signalling pathways. J. Exp. Bot. 2014;65:1425–1438. doi: 10.1093/jxb/eru002. [DOI] [PubMed] [Google Scholar]
- 117.Ruiz-Ferrer V, Voinnet O. Roles of plant small RNAs in biotic stress responses. Annu. Rev. Plant. Biol. 2009;60:485–510. doi: 10.1146/annurev.arplant.043008.092111. [DOI] [PubMed] [Google Scholar]
- 118.Chen X. Small RNAs and their roles in plant development. Annu. Rev. Cell Dev. Biol. 2009;25:21–44. doi: 10.1146/annurev.cellbio.042308.113417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sunkar R, Li YF, Jagadeeswaran G. Functions of microRNAs in plant stress responses. Trends Plant Sci. 2012;17:196–203. doi: 10.1016/j.tplants.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 120.Shivaprasad PV, et al. A microRNA superfamily regulates nucleotide binding site-leucine-rich repeats and other mRNAs. Plant Cell. 2012;24:859–874. doi: 10.1105/tpc.111.095380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhu QH, et al. MiR482 regulation of NBS-LRR defense genes during fungal pathogen infection in cotton. PLoS One. 2013;8:e84390. doi: 10.1371/journal.pone.0084390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sunkar R. Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell Online. 2006;18:2051–2065. doi: 10.1105/tpc.106.041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li D, et al. Developmental and stress regulation on expression of a novel miRNA, Fan-miR73, and its target ABI5 in strawberry. Sci. Rep. 2016;6:28385. doi: 10.1038/srep28385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ma W, et al. Coupling of microRNA-directed phased small interfering RNA generation from long noncoding genes with alternative splicing and alternative polyadenylation in small RNA-mediated gene silencing. New Phytol. 2017;217:1535–1550. doi: 10.1111/nph.14934. [DOI] [PubMed] [Google Scholar]
- 125.Akagi T, Henry IM, Tao R, Comai LA. Y-chromosome-encoded small RNA acts as a sex determinant in persimmons. Science. 2014;346:646–650. doi: 10.1126/science.1257225. [DOI] [PubMed] [Google Scholar]
- 126.Bradley D, et al. Evolution of flower color pattern through selection on regulatory small RNAs. Science. 2017;358:925–928. doi: 10.1126/science.aao3526. [DOI] [PubMed] [Google Scholar]