Abstract
Scope
Cheonggukjang (CGJ) is a soybean‐based quick‐fermented food popular in Korea that contains a variety of biologically active compounds including isoflavones and saponins. Isoflavone bioavailability may be important for the bone health of postmenopausal women; therefore, the aim of this study is to evaluate the influence of fermentation on the isoflavone metabolite nutrikinetic profile after single dose CGJ or unfermented soybean administration in ovariectomized (OVX) and sham mice.
Methods and results
We identify 34 isoflavone metabolites using UPLC–QTOF‐MS and analyze their nutrikinetics at different time points (0.25, 0.5, 1, 2, 4, 8, 16, and 24 h) to understand their fermentation‐ and OVX‐mediated time‐dependent concentration changes. Nutrikinetics analysis shows that genistein, daidzein, genistein 4′‐sulfate, dihydrodaidzein sulfate, equol 4′‐sulfate, and equol‐7‐glucuronide are present at high concentrations in all groups based on area‐under‐the‐curve analysis. OVX mice appear to show lower isoflavone bioavailability than mice in the sham group. CGJ enhances various isoflavone metabolite bioavailability including genistein, 3‐hydroxygenistein, and equol 7‐glucuronide, compared to the unfermented soybean‐treated group. Among these metabolites, intact isoflavones, 3‐hydroxygenistein, genistein 4′‐sulfate, and equol 7‐glucuronide promote osteoblastogenesis and inhibit osteoclast formation.
Conclusions
CGJ has good isoflavone bioavailability and may be beneficial for the bone health of postmenopausal women.
Keywords: bioavailability, Cheonggukjang, isoflavones, nutrikinetics, ovariectomized mice
1. Introduction
Soybeans have long been consumed in Asia for their protein content and healthy fat.1, 2 Among the various traditional soybean products, Cheonggukjang (CGJ) is the most famous in Korea. CGJ is a type of quick‐fermented soybean paste (ready within 3 days) that has various potential health benefits including anti‐obesity, antioxidant, anti‐osteoporotic, and anti‐prediabetic effects.3, 4, 5, 6 CGJ notably contains a high content of isoflavones, which have long attracted interest for their potential health benefits in the prevention of cancer, osteoporosis, and other conditions.2, 7, 8 Isoflavones are present in glycoside forms in their nature state.9 Although aglycone forms in fermented food are known to have higher bioavailability than glycoside forms through rapid absorption owing to the first absorption phase being independent of intestinal microbiota, only few studies have focused on the bioavailability of fermented food,10, 11, 12, 13, 14, 15 and study on the bioavailability of isoflavones in CGJ has not yet been explored. During CGJ fermentation, aglycone contents increase owing to increased β‐glucosidase activity, whereas the contents of glycoside forms decrease.16 To better understand the bioavailability of isoflavones in CGJ for maintaining human health, it may be necessary to evaluate the nutrikinetics of isoflavone‐derived metabolites, which may exert direct effects on biological activities.
After soy intake, isoflavones are biotransformed into various metabolites within the human digestive tract. The glycoside forms of isoflavones are deglycosylated by intestinal glucosidases, leading to the release of aglycones that are absorbed and further metabolized into many specific metabolites including phase I‐, phase II‐, and gut‐mediated metabolites.17, 18 To date, more than 20 isoflavone metabolites have been identified in animals and humans following single compound administration or dietary supplementation.19, 20, 21 Among these metabolites, equol is a daidzein‐derived metabolite that may be related to clinical effectiveness.19, 22, 23 However, many more isoflavone metabolites are absorbed and distributed to target organs and tissues.
Nutrikinetics is an expanded concept to describe the pharmacokinetics of food compounds in order to understand the bioactivity of various compounds within the human superorganism.24 To date, most pharmacokinetic studies of foods have been studied in terms of single compounds. These results have limitations for understanding the bioactivity of these individual metabolites in the body. Although the excellent functional benefits of soybeans may be determined by the bioavailability of isoflavones, there is little evidence in the literature of enhancement of isoflavone bioavailability by fermentation that results in bioconversion of isoflavone itself and different effects on disease.
Isoflavones are a class of phytoestrogenic compounds with similar structure to estrogen, the deficiency of which constitutes an important factor in osteopenia. Accordingly, the absorption and distribution of isoflavones may be effective in preventing hormone imbalance‐related disease such as postmenopausal syndrome.25, 26 Although estrogen deprivation has been reported to reduce the absorption of calcium,27 little is known regarding whether the condition affects the bioavailability of phytochemicals. Therefore, it is necessary to study how the bioavailability of isoflavones changes in estrogen‐deficient animal models, or how the bioavailability of isoflavones can be improved upon ingestion of certain types of foods. In addition, it is not well understood whether physical ovariectomy (OVX) affects isoflavone bioavailability and studies addressing the effect of fermented foods on isoflavone bioavailability in OVX mice have been limited. In the present study, we analyzed the nutrikinetic profiles of isoflavone‐derived metabolites in the serum of mice that had undergone sham or OVX surgeries after oral administration of CGJ or unfermented soybeans to determine how fermentation and OVX affect isoflavone bioavailability.
2. Experimental Section
2.1. Reagents and Chemicals
Genistein, daidzein, glycitein, methylgenistein, formononetin, hippuric acid, caffeic acid, gallic acid, resorcinol, and 3‐(4,5‐dime thylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) were purchased from Sigma–Aldrich (St Louis, MO, USA). Genistein 4′‐glucuronide, genistein 7‐glucuronide, genistein 4′‐sulfate, genistein 7‐sulfate, daidzein 7‐glucuronide, daidzein 4′‐sulfate, equol 7‐glucuronide, equol 4′‐sulfate, dehydrogenistein, dihydrogenistein sulfate, dihydrodaidzein, 3‐hydroxygenistein, and 6‐hydroxydaidzein were purchased from Toronto Research Chemicals (Toronto, Ontario, Canada). Alpha minimum essential medium (αMEM), DMEM, fetal bovine serum (FBS), and PBS were obtained from Gibco BRL (Grand Island, NY, USA).
2.2. Preparation of CGJ
CGJ was purchased from the Hamssine Food Company (Jeonju‐si, Jeollabuk‐do, South Korea). Briefly, soybeans were sorted, washed, soaked in water at ambient temperature for 12 h, drained, and boiled for 7 h in water. Boiled soybeans (BS) were cooled to 40 °C and fermented for 3 days using natural microflora such as Bacillus subtilis, in a room maintained at 40 °C. After fermentation, CGJ was stored at −80 °C until use.
2.3. Identification of Isoflavones from CGJ
LC–MS/MS analyses were performed with a 1200 Agilent HPLC (Agilent Technologies, Palo Alto, CA, USA) interfaced to a 4000 QTRAP (AB SCIEX, Foster City, CA, USA) using an Acquity BEH C18 column (50 mm, 2.1 mm id, 1.7 mm particle size; Milford, MA, USA), which was maintained at 4 °C. The mobile phase consisted of formic acid 0.1% (A) and acetonitrile (B). Chromatographic gradient elution was as follows: constant flow of 0.2 mL min–1; 5% phase B maintained for 1 mL min–1, then increased up to 100% B in 10 min and successively declined to 5% B in 1 min, and re‐equilibrated for 8 min. The mass spectrometer was operated with a turbo spray ion source in the positive mode (ESI+) with multiple reaction monitoring. The following parameters were used for MS: curtain gas 40 psi, ion spray voltage (V) –4500, temperature 300 °C, ion source gas 180 psi, ion source gas 270 psi. Data acquisition and elaboration were performed using Analyst software (version 1.5, AB SCIEX). Standards were prepared by dissolution of each compound in ethanol at the concentration of 1 mg mL–1. Working solutions were prepared in ethanol at seven different concentrations (10–2500 ng mL–1).
2.4. Animal Experiments
Female sham‐operated and OVX mice (C57BL/6, aged 9 weeks) were purchased from SLC Inc. (Hamamatsu, Japan). They were housed in a room (22 ± 2 °C) comprising a 12:12 h light‐dark cycle (07:00–19:00), and were fed with an AIN‐93G diet. Animal studies were approved by the Animal Care and Use Committee of the Korea Food Research Institute (KFRI‐M‐16014). The mice were divided into four groups based on body weights: BS‐treated sham (n = 10), CGJ‐treated sham (n = 10), BS‐treated OVX (n = 10), and CGJ‐treated OVX (n = 10). Sham and OVX mice received 1 g per kg body weight CGJ or BS via gavage. Blood samples were then collected via cardiac puncture under anesthesia (300 mg kg–1 tribromoethanol intraperitoneally, IP) at different time points (0.25, 0.5, 1, 2, 4, 8, 16, and 24 h). The collected samples were immediately centrifuged at 2000 × g for 10 min and the supernatants were collected and stored at −80 °C until analysis.
2.5. Sample Preparation and LC–MS/MS Analysis
Serum extracts were prepared as follows: 700 μL acetonitrile was added to each 300 μL serum sample and immediately vortexed, allowed to rest for 20 min on ice, sonicated for 15 min, and centrifuged at 15 700 × g for 10 min. The supernatants were completely dried using a speed‐vac, and the residues were resuspended in methanol (100 μL), sonicated for 15 min, and centrifuged again for 10 min. The resulting supernatant was filtered and transferred to the LC–MS/MS system.
Analysis was performed using an Acquity UPLC system (Waters, Milford, MA, USA) with an Acquity UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm). A Waters Synapt G2‐Si mass spectrometer (Waters, Manchester, UK) operating in ESI mode was used. The source was set to negative ESI mode with a scan range of 50–1000 m/z. The mobile phase was composed of (A) 10 mm ammonium acetate and 0.1% formic acid in water and (B) 0.1% formic acid in acetonitrile. The flow rate was 0.35 mL min–1. The linear gradient was started at 2% and gradually increased to 95% B over 14 min before returning to 2% B for a 2‐min re‐equilibration step. The injection volume was 5 μL. Argon was used as a collision gas and nitrogen was used as a desolvation gas. The capillary voltage and cone energy voltages were set at 1.0 kV and 40 V, respectively. The desolvation and cone gas flow rates were 800 and 20 L h–1, respectively. The source and desolvation gas temperatures were 110 °C and 350 °C, respectively. All spectrum data were collected in continuum format using MSE acquisition mode. Mass accuracy was calibrated using sodium formate, and leucine encephalin ([M‐H]−: m/z 554.2615) was used as the lock mass. The concentration of leucine encephalin was 2 ng mL–1, and the flow rate was set at 5 μL min–1. Data were collected in the range of 50–1000 m/z.
2.6. Identification of Genistein Metabolites in Serum
The isoflavone metabolites acquired by UPLC–MS‐based metabolomics profiling were processed using UNIFI software (ver. 1.7.1, Waters) and assembled into a data matrix. Peak data were collected using a mass accuracy (ppm < ±5), fragment match tolerance 2.0 mDa, retention time tolerance of 0.1 min MS tolerance was set to 100 Da with a retention time window of 0.2 min. Analytical validation was based on corresponding accurate masses and retention times compared to reference compounds.
2.7. Noncompartmental Serum Kinetic Analysis
Noncompartmental pharmacokinetics analysis was performed for each metabolite including the evaluation of the maximum peak area (P max), time to reach P max (t max), terminal elimination half‐time (t 1/2), and the area under the curve of the metabolite peak area versus time (AUC0–16 h), using PK solution software version 2.0 (Summit Research Services, Montrose, CO, USA).
2.8. Alkaline Phosphatase (ALP) Activity
MC3T3‐E1 subclone four cells (ATCC, Manassas, VA, USA) were cultured in αMEM (without l‐ascorbic acid). The cells were seeded in a 24‐well plate at 2 × 104 cells/well and osteogenic differentiation was induced with differentiation medium containing 50 μg mL–1 l‐ascorbic acid and 10 mm β‐glycerophosphate. After 6‐day incubation, cells were washed twice with PBS and lysed with 0.2% Triton‐X‐100 lysis buffer. ALP activity was measured with the SensoLyte pNPP Alkaline Phosphatase Assay Kit (AnaSpec, Fremont, CA, USA) as directed by the manufacturer.
2.9. Mineralization Assay
After 21‐day incubation, cells were washed, fixed with 4% formalin, and stained with 2% ARS. The stained cells were solubilized with 10% cetyl chloride in 10 mm sodium phosphate and measured at 562 nm on a multiplate reader (Thermo‐Labsystems, Leuven, Belgium).
2.10. Bone Resorption Activity
RAW 264.7 cells (ATCC, Manassas, VA, USA) were cultured in DMEM. The cells were seeded in a 96‐well plate and the medium was replaced with differentiation medium containing 50 ng mL–1 receptor activator of nuclear factor kappa‐B ligand (RANKL). After 5 days, bone resorption activity was determined using a bone resorption assay kit (Cosmo Bio. Co. Ltd., Tokyo, Japan) according to the manufacturer's instructions.
2.11. Statistical Analyses
Descriptive statistics were calculated for P max, t max, t 1/2, and AUC between sham and OVX groups. The results were analyzed by two‐way ANOVA followed by Bonferroni post‐hoc test (GraphPad Software Inc.), with a significance level of p < 0.05. Wilcoxon signed‐rank test was calculated using GraphPad Software to compare the nutrikinetic profiles among the groups.
3. Results
3.1. Changes in Isoflavone Contents During Fermentation
We measured the change in soy isoflavones by fermentation time. A total of 15 isoflavones were identified, and glycosides (genistin and daidzin) were mostly converted into aglycones (genistein and daidzein) during fermentation (Table 1). The isoflavone glycosides accounted for 85% of the total isoflavones in the BS (day 0). On the third day of fermentation, isoflavone glycosides, acetylglycosides, malonylglycosides, and aglycones comprised 8.3%, 0.66%, 0.1%, and 90.8%, respectively, suggesting that most isoflavones are converted into aglycones such as genistein and daidzein. Notably, total isoflavones had decreased by around 26% after final fermentation. These structural and total isoflavone content changes may affect the bioavailability and bioactivity of isoflavones after ingestion.
Table 1.
Changes in isoflavone compositions of Cheonggukjang (CGJ) during fermentation.
| Isoflavones (μg/g, w/w) | Fermentation time (day) | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| Glycosides | Genistin | 144.53 ± 3.85 | 100.67 ± 10.91 | 27.21 ± 5.82 | 11.95 ± 0.44 |
| Daidzin | 463.29 ± 16.31 | 343.16 ± 59.92 | 57.08 ± 12.25 | 29.54 ± 2.34 | |
| Glycitin | 87.95 ± 1.48 | 69.42 ± 7.33 | 13.89 ± 3.191 | 8.66 ± 0.24 | |
| Acetyl‐ glucosides | Acetylgenistin | 48.66 ± 0.84 | 41.75 ± 6.55 | 11.97 ± 3.00 | 3.50 ± 0.22 |
| Acetyldaidzin | 22.24 ± 0.68 | 17.70 ± 2.80 | 2.76 ± 0.58 | 0.20 ± 0.06 | |
| Acetylglycitin | 5.00 ± 0.19 | 4.30 ± 0.62 | 0.94 ± 0.19 | 0.31 ± 0.00 | |
| Malonyl ‐glucosides | Malonylgenistin | 3.19 ± 0.12 | 2.31 ± 0.29 | 0.71 ± 0.20 | 0.40 ± 0.01 |
| Malonyldaidzin | 0.91 ± 0.03 | 0.71 ± 0.09 | 0.26 ± 0.04 | 0.12 ± 0.01 | |
| Malonylglycitin | 0.25 ± 0.02 | 0.22 ± 0.02 | 0.09 ± 0.01 | 0.05 ± 0.00 | |
| Aglycones | Genistein | 20.55 ± 0.83 | 103.71 ± 16.05 | 318.465 ± 64.73 | 291.41 ± 7.60 |
| Daidzein | 11.90 ± 1.27 | 70.47 ± 7.64 | 193.28 ± 37.71 | 187.57 ± 8.39 | |
| Glycitein | 6.54 ± 0.35 | 27.92 ± 4.37 | 73.46 ± 14.20 | 69.01 ± 2.53 | |
| Others | Formononetin | 0.07 ± 0.00 | 0.13 ± 0.02 | 0.25 ± 0.05 | 0.26 ± 0.00 |
| Dihydrodaidzein | trace | 0..02 ± 0.00 | 0.16 ± 0.03 | 0.17 ± 0.00 | |
| Coumestrol | 0.10 ± 0.01 | 0.14 ± 0.04 | 0.12 ± 0.03 | 0.19 ± 0.03 | |
| Total | 815.18 ± 23.71 | 782.61 ± 113.87 | 700.63 ± 141.20 | 603.36 ± 16.15 | |
3.2. Identification of Isoflavone‐Derived Metabolites in Serum After BS or CGJ Administration
Knowing that aglycone forms enhance bioavailability compared with glycoside forms, CGJ may enhance the bioavailability of isoflavones because fermentation increases the aglycone content. Therefore, we orally administered the whole foods BS and CGJ to understand how these differences affect the bioavailability of isoflavones. We first identified isoflavone metabolites by pooling multiple samples collected from sham and OVX groups using UPLC–QTOF‐MS. We analyzed the metabolites compared to the control diet (nontreated samples) and identified the isoflavone‐derived metabolites except the metabolites from the control diet group. A total of 34 metabolites were detected using specific mass fragments and coelution with authenticated standards (Table 2). Natural isoflavone glycosides such as genistin, daidzin, and glycitin were not detected, whereas three aglycones, genistein, daidzein, and glycitein, derived from glycosides were detected in pooled serum samples. We detected two phase I, eight phase II, and 21 putative gut‐mediated metabolites in pooled samples. Representative gut‐mediated metabolites of isoflavones, such as daidzein and genistein, are converted to equol. The majority of circulating equol is present as equol 7‐glucuronide, equol 4ʹ‐sulfate, and 5‐hydroxy equol. In vivo metabolic pathways of identified isoflavone metabolites of BS and CGJ are summarized in Figure 1.
Table 2.
Identified isoflavone metabolites from the pooled serum.
| No. | Group of metabolites | Metabolite | RT (min) | Exact mass (m/z) | Actual mass (m/z) | Mass error (mDa) | Annotation levela) |
|---|---|---|---|---|---|---|---|
| 1 | Intact isoflavone | Genistein | 8.81 | 269.045 | 269.0457 | 0.73 | 1 |
| 2 | Daidzein | 7.44 | 253.0501 | 253.0507 | 0.6 | 1 | |
| 3 | Glycitein | 7.75 | 283.0606 | 283.0606 | 0.03 | 1 | |
| 4 | Phase I metabolites (Liver) | 3‐Hydroxygenistein | 5.02 | 285.0399 | 285.0399 | 0 | 1 |
| 5 | 6‐Hydroxydaidzein | 6.73 | 269.0450 | 269.043 | −2 | 1 | |
| 6 | Phase II metabolites (Liver) | Genistein 4′‐glucuronide | 5.89 | 445.0771 | 445.0771 | 0.02 | 1 |
| 7 | Genistein 7‐glucuronide | 5.34 | 445.0771 | 445.0772 | 0.07 | 1 | |
| 8 | Genistein 4′‐sulfate | 7.19 | 349.0018 | 349.0022 | 0.41 | 1 | |
| 9 | Genistein 7‐sulfate | 5.84 | 349.0018 | 349.002 | 0.2 | 1 | |
| 10 | Daidzein 7‐glucuronide | 4.36 | 429.0822 | 492.0827 | 0.49 | 1 | |
| 11 | Daidzein 4′‐sulfate | 6.12 | 333.0069 | 349.0072 | 0.32 | 1 | |
| 12 | Methylgenistein | 6.25 | 283.0606 | 283.0612 | 0.64 | 1 | |
| 13 | Formononetin | 10.3 | 267.0657 | 267.0667 | 0.99 | 1 | |
| 14 | Gut‐mediated metabolites | Dihydrogenistein | 7.34 | 271.0612 | 271.0606 | −0.60 | 1 |
| 15 | Dihydrogenistein sulfate | 6.99 | 351.0175 | 351.0165 | −0.97 | 1 | |
| 16 | Dihydrodaidzein | 7.5 | 255.0657 | 255.0643 | −1.4 | 1 | |
| 17 | Dihydrodaidzein sulfate | 6.21 | 335.0225 | 335.0227 | 0.17 | 1 | |
| 18 | Equol 7‐glucuronide | 6.16 | 417.1186 | 417.1181 | −0.47 | 1 | |
| 19 | Equol 4′‐sulfate | 2.31 | 321.0426 | 324.0438 | 1.21 | 1 | |
| 20 | 5‐Hydroxy equol | 7.4 | 257.0814 | 257.0800 | −1.4 | 2 | |
| 21 | Hippuric acid | 1.65 | 178.0532 | 178.0513 | −1.90 | 1 | |
| 22 | 4‐Hydroxybenzoic acid | 3.08 | 137.0239 | 137.0252 | 1.26 | 1 | |
| 23 | 2,6‐Dimethoxy benzoic acid | 5.15 | 181.0501 | 181.0517 | 1.56 | 1 | |
| 24 | Caffeic acid | 3.77 | 179.0344 | 179.0356 | 1.16 | 1 | |
| 25 | Methyl caffeic acid | 6.16 | 193.0501 | 193.0515 | 1.41 | 1 | |
| 26 | Fumaric acid | 0.75 | 115.0031 | 115.0046 | 1.53 | 1 | |
| 27 | 4‐Ethylphenol | 9.46 | 121.0653 | 121.0659 | 0.56 | 1 | |
| 28 | Glutaric acid | 2.29 | 131.0344 | 131.0359 | 2.29 | 1 | |
| 29 | 2‐Phenylpropanoic acid | 8.05 | 149.0603 | 149.062 | 1.65 | 1 | |
| 30 | p‐Coumaric acid | 4.92 | 163.0395 | 163.0408 | 1.31 | 1 | |
| 31 | Gallic acid | 1.89 | 169.0137 | 169.0148 | 1.15 | 1 | |
| 32 | Ferulic acid | 6.03 | 193.0501 | 193.0508 | 0.66 | 1 | |
| 33 | Resorcinol | 2.9 | 109.029 | 109.0302 | 1.24 | 1 | |
| 34 | Resorcinol sulfate | 2.87 | 188.9858 | 188.9866 | 0.8 | 1 |
RT, retention time (min).
a)Annotation level according to the Metabolomics Society Initiative.
Annotation level: 1, unambiguously identified metabolites, checked with standard; 2, putatively annotated, based on accurate mass and MS/MS fragments; and 3, putatively characterized.
Figure 1.
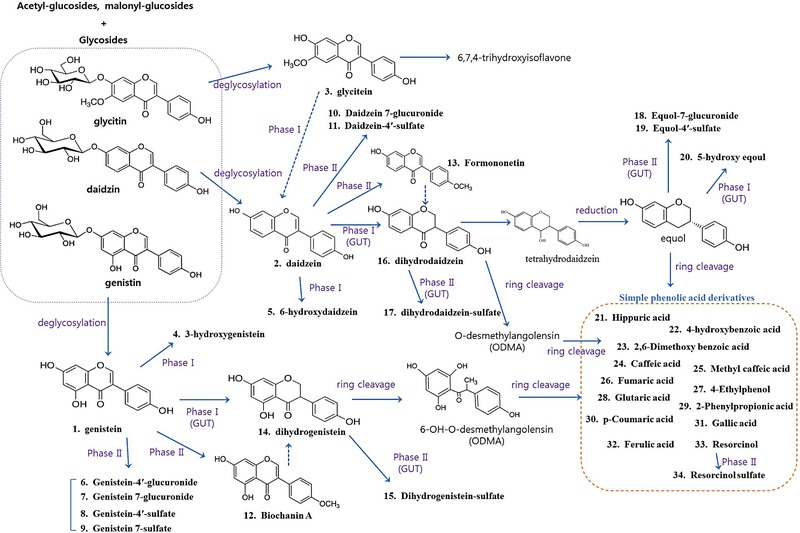
Metabolic pathway of isoflavone metabolites detected in serum after the ingestion of boiled soybean (BS) or Cheonggukjang (CGJ).
3.3. Nutrikinetic Analysis of Isoflavone Metabolites in Serum After BS or CGJ Administration
To compare the bioavailability of isoflavones between BS and CGJ in estrogen deficiency, we analyzed the nutrikinetics of the identified isoflavone metabolites. Nutrikinetic analysis included evaluation of P max, t max, AUC0–t, and t 1/2. The t max and t 1/2 values for all metabolites in both sham and OVX groups after BS or CGJ oral administration are given Figure 2. The t max and t 1/2 values of most metabolites showed a clear difference between BS and CGJ groups. The t max of intact isoflavones are within 1 h and the t max of phase I and Phase II except 3‐hydroxygenistein was within 2 h, whereas the t max of gut‐mediated metabolites was relatively long. CGJ was relatively absorbed rapidly compared to BS in both sham and OVX groups. In addition, t 1/2 of gut‐mediated metabolites was relatively long. In particular, 3‐hydroxygenistein showed the longest time for the t max and the t 1/2. These results indicate that fermentation of soybeans affects the absorption rate of isoflavone metabolites and OVX‐induced hormonal imbalance also affects the absorption rate.
Figure 2.
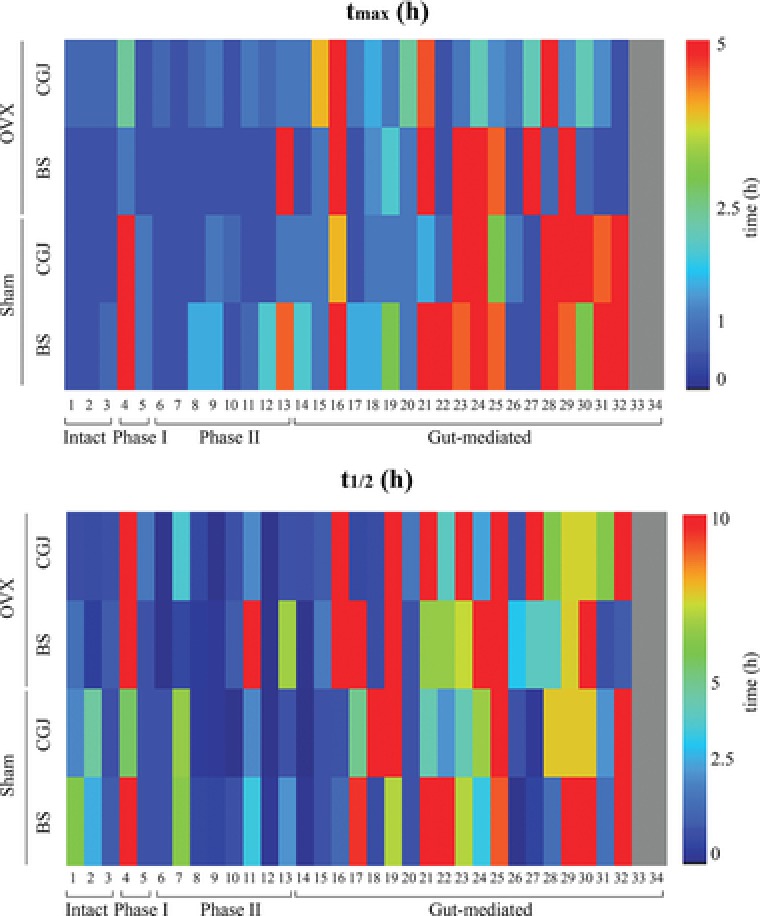
Overview of t max and t 1/2 (h) for 34 isoflavone metabolites of boiled soybean (BS) or Cheonggukjang (CGJ) in sham and ovariectomized (OVX) mouse groups. The numbers correspond to the metabolite numbers in Table 2.
The concentration of each component in the pooled samples from each group is shown in a heatmap (Figure 3). The heatmap shows the change of isoflavone metabolites in the BS and CGJ groups, as influenced by OVX. In particular, genistein, 3‐hydroxygenistein, genistein 4ʹ‐sulfate, dihydrodaidzein sulfate, equol 7‐glucuronide, and dihydrogenistein were present at higher concentrations in CGJ‐treated compared with BS‐treated OVX mice. Although the total content of isoflavone was reduced by fermentation, the concentrations of some isoflavone metabolites in serum were significantly higher than prior to fermentation. A Wilcoxon signed‐rank test was used to compare the nutrikinetic profiles between BS and CGJ treatment in sham and OVX mice. Analysis of P max and AUC(0–24h) showed that some metabolites are present at significantly different concentrations (Table 3). The AUC values for genistein and daidzein were significantly higher after CGJ treatment than BS treatment in the sham group, whereas some phase II and gut‐mediated metabolites decreased in the CGJ group compared to the BS group. In OVX groups, the AUC(0–24h) values of genistein, 3‐hydroxygenistein, and genistein 4ʹ‐sulfate among other components in CGJ administered mice were significantly higher than those of BS administered mice. The AUC is of particular use in estimating bioavailability. These results suggest that CGJ enhances isoflavone bioavailability compared to BS in OVX conditions.
Figure 3.
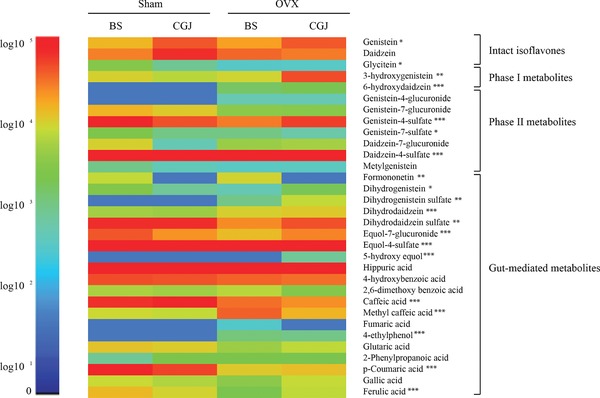
Relative peak area changes of isoflavone metabolites of boiled soybean (BS) and Cheonggukjang (CGJ) in sham and ovariectomized (OVX) mouse groups. The relative peak areas of pooled samples from each group are shown in the heatmap. The relative peak areas are shown with a logarithmic scale. *p < 0.05; **p < 0.01; and ***p < 0.001.
Table 3.
Wilcoxon signed‐rank test comparison of Pmax and area under the curve (AUC) of boiled soybean (BS) and Cheonggukjang (CGJ) treatment in sham and ovariectomized (OVX) mice.
| No. | Group of metabolites | Metabolite | P max (PA mL–1) | AUC (0–24 h, PA mL–1) | ||
|---|---|---|---|---|---|---|
| Sham | OVX | Sham | OVX | |||
| BS‐CGJ | BS‐CGJ | BS‐CGJ | BS‐CGJ | |||
| 1 | Isoflavone | Genistein | – | – | <0.05 | <0.01 |
| 2 | Daidzein | – | – | <0.001 | – | |
| 3 | Glycitein | – | – | – | – | |
| 4 | Phase I (Liver) | 3‐Hydroxygenistein | – | – | – | <0.05 |
| 5 | 6‐Hydroxydaidzein | – | – | – | <0.05 | |
| 6 | Phase II (Liver) | Genistein 4′‐glucuronide | – | <0.001 | – | – |
| 7 | Genistein 7‐glucuronide | – | – | – | – | |
| 8 | Genistein 4′‐sulfate | <0.05a) | – | <0.05a) | <0.05 | |
| 9 | Genistein 7‐sulfate | – | – | – | – | |
| 10 | Daidzein 7‐glucuronide | – | – | <0.01a) | – | |
| 11 | Daidzein 4′‐sulfate | <0.001a) | – | <0.01a) | – | |
| 12 | Methylgenistein | – | <0.001a) | – | – | |
| 13 | Formononetin | – | – | <0.01a) | <0.001a) | |
| 14 | Gut‐mediated metabolites | Dihydrogenistein | – | – | – | <0.05 |
| 15 | Dihydrogenistein sulfate | – | – | – | <0.05 | |
| 16 | Dihydrodaidzein | – | – | – | <0.05 | |
| 17 | Dihydrodaidzein sulfate | – | – | – | <0.05 | |
| 18 | Equol 7‐glucuronide | – | – | <0.01a) | <0.05 | |
| 19 | Equol 4′‐sulfate | <0.01a) | <0.001a) | <0.05a) | – | |
| 20 | 5‐hydroxy equol | – | – | – | <0.05 | |
| 21 | Hippuric acid | – | – | – | – | |
| 22 | 4‐hydroxybenzoic acid | – | – | <0.01a) | <0.05a) | |
| 23 | 2,6‐Dimethoxy benzoic acid | – | – | – | – | |
| 24 | Caffeic acid | – | – | – | <0.05a) | |
| 25 | Methyl caffeic acid | – | – | – | <0.05a) | |
| 26 | Fumaric acid | – | – | – | – | |
| 27 | 4‐Ethylphenol | – | – | – | – | |
| 28 | Glutaric acid | – | – | – | <0.05 | |
| 29 | 2‐Phenylpropanoic acid | – | – | – | – | |
| 30 | p‐Coumaric acid | – | – | <0.01a) | <0.05 | |
| 31 | Gallic aicd | – | – | – | <0.05 | |
| 32 | Ferulic acid | – | – | – | – | |
| 33 | Resorcinol | – | – | – | – | |
| 34 | Resorcinol sulfate | – | – | – | – | |
a)Metabolites that were significantly increased in the BS‐treated group compared with the CGJ‐treated group.
3.4. Identification of Putative Active Metabolites for Bone Health
Although isoflavones are known to promote bone health, metabolites other than equol have not attracted attention as active isoflavone metabolites that may directly act in various biological activities.28 MC3T3‐E1 cells were treated with various concentrations of isoflavone metabolites (0.1–0 5 μ m) in our study. Among the identified metabolites, 3‐hydroxygenistein, genistein 4′‐sulfate, equol 7‐glucuronide, formononetin, dihydrogenistein, and caffeic acid as well as intact isoflavones stimulated ALP activity during differentiation of MC3T3‐E1 cells (Figure 4 A). Extensive calcium deposition in 3‐hydroxygenistein, genistein 4′‐sulfate, equol 7‐glucuronide, and caffeic acid were also observed by ARS staining (Figure 4B). In particular, 3‐hydroxygenistein attenuated RANKL‐induced bone resorption activity in RAW 264.7 cells (Figure 4C). 3‐Hydroxygenistein was not cytotoxic to RAW 264.7 macrophages at the tested concentrations (Figure 4C).
Figure 4.
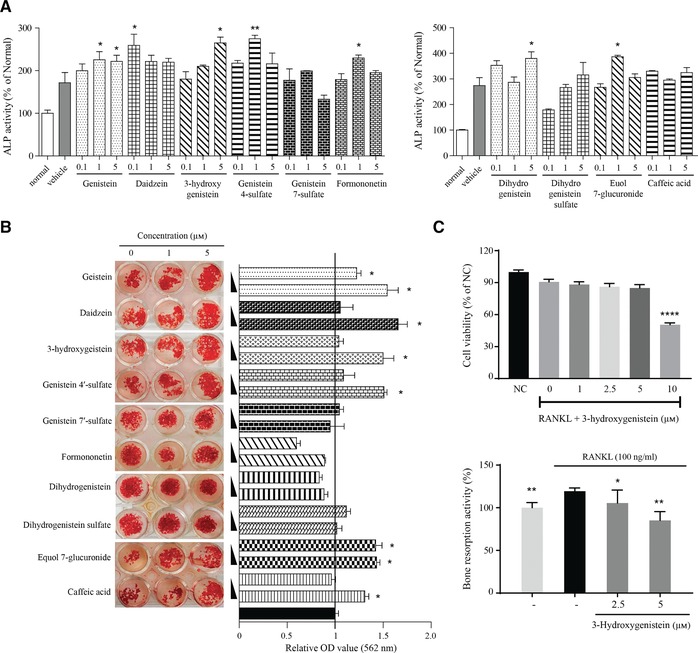
Isoflavone metabolites stimulate alkaline phosphatase (ALP) activity and inhibit bone resorption activity. A) MC3T3‐E1 cells were differentiated for 6 days in medium containing 50 μg mL–1 l‐ascorbic acid, 10 mm β‐glycerophosphate, and 10 nm dexamethasone, and ALP activity was measured. B) Effects of isoflavone metabolites on mineralization of MC3T3‐E1 cells. C) RAW 264.7 cells were treated with 100 ng mL–1 receptor activator of nuclear factor kappa‐B ligand (RANKL) and samples for 5 days without medium change. Bone resorption activity was measured. Values represent the means ± SD (n = 3) of three independent experiments. *p < 0.05; **p < 0.01; and ***p < 0.001 compared to the control group.
4. Discussion
CGJ is a traditional food in Korea, made from soybeans fermented mainly with Bacillus species, and it is well known that isoflavones are the main active compounds in CGJ.29 Although it is expected that CGJ show improved bioavailability of isoflavones owing to the increased aglycone content after fermentation, there has previously been no published evidence of this phenomenon. Our results suggest that ingestion of fermented CGJ may enhance the bioavailability of isoflavones.
The most abundant isoflavones in BS are genistin and daidzin, which in CGJ are mostly converted to genistein and daidzein by β‐glucosidase enzymes that are able to increase the aglycone content during fermentation.16, 30 Several Asian traditional fermented soybean products, such as Japanese natto, Indian kinema, and Thai Thua nao have much higher aglycone contents than unfermented soybeans.31, 32, 33 Most studies have shown that high aglycone contents are better absorbed into the blood than high glycoside content.34 In terms of bioavailability, one of the functional benefits of fermented soy products can be explained by these structural changes to isoflavones.
In order to accurately measure the bioavailability of isoflavones in fermented soy products, various metabolites derived from isoflavones should be identified. A single isoflavone can be converted into various metabolites in vivo. Recent developments in LC–MS equipment and identification programs have identified phase I‐ and gut‐mediated metabolites in addition to phase II metabolites. Although the aglycone form increases the bioavailability compared with that of glycosides, the bioavailability of individual metabolites should be measured with diverse metabolite identification. Bioavailability studies of individual metabolites could then be used as key markers to determine the functional properties of foods.
In this study, we identified 34 isoflavone‐derived metabolites from both BS and CGJ and performed nutrikinetics analyses of individual metabolites. The isoflavones deglycosylated by the digestive tract are further metabolized by intestinal and liver enzymes. The major bioconversion of isoflavones generally begins with the hydrogenation of the double bond between C‐2 and C‐3 of the C‐ring, such as during the formation of dihydrodaidzein and dihydrogenistein.35 This hydrogenation is performed by intestinal bacteria and the resulting compounds are subjected to further microbial transformation by the colonic microflora. A representative gut‐mediated metabolite of isoflavones is equol, and we found that the majority of circulating equol was present as equol 7‐glucuronide, equol 4ʹ‐sulfate, and 5‐hydroxy equol in our study. When CGJ was orally administered to the OVX mice, equol 7‐glucuronide and 5‐hydroxy equol showed higher bioavailability in CGJ‐treated compared to BS‐treated OVX mice.
Numerous factors affect the bioavailability of ingested dietary ingredients. Estrogen deficiency also results in various environmental change such as alteration of gut microbiota36 and OVX can affect the bioavailability of functional ingredients owing to intestinal microbial changes as well37; changes in the intestinal microbial environment in turn will affect the metabolism and absorption of dietary ingredients. Our results showed that bioavailability of isoflavone metabolites differed significantly between sham and OVX groups even when administered the same sample, suggesting that OVX affects the bioavailability of isoflavones. A previous study showed that there was no difference in isoflavone bioavailability between fermented and nonfermented versions of the same foods.13 However, most studies have reported that aglycone‐rich fermented foods increase the bioavailability of isoflavones.15, 38 It is reasonable to assume that aglycone is superior to glycoside as the first absorption step is independent of the intestinal microbiota. In the present study, a high concentration of intact isoflavones, such as genistein and daidzein, was detected and was significantly higher in the group fed with CGJ compared with BS in OVX mice. The concentrations of phase I metabolites of isoflavone, including 3‐hydroxygenistein and 6‐hydroxydaidzein, in serum were much higher after CGJ than BS treatment. However, nutrikinetics analysis also showed that formononetin, 4‐hydroxybenzoic acid, caffeic acid, and methyl caffeic acid were higher in BS‐treated compared with CGJ‐treated OVX mice. These differences are due to various factors such as the food matrix, chemical form, and protein quality changes owing to fermentation.39 In a recent study, fermented food was shown to alter microbiota in the intestinal tract. With respect to isoflavones, these findings indicated that CGJ may be more effective in estrogen deficiency conditions than in normal conditions.40 Also, these results may be related to alterations of the gut microbiota secondary to OVX. In our results, there was also a difference in bioavailability for the same sample between sham and OVX groups. These data suggest that CGJ may increase the bioavailability of isoflavones in conditions of estrogen deficiency owing to menopause.
In addition, as the functionality of the identified metabolites is not clear, further investigation is needed to identify whether the increased content of isoflavone metabolites in CGJ will exert increased or decreased effects on various biological activities. In this study, we investigated the potency of osteoblast activation and osteoclast inhibition by identified isoflavone metabolites. Among these, intact isoflavones, 3‐hydroxygenistein, genistein 4′‐sulfate, equol 7‐glucuronide, and caffeic acid increased the ALP activity and mineralization, suggesting that these metabolites were more effective than other metabolites in osteoblastogenesis. 3‐Hydroxygenistein also inhibited osteoclast activity. Accordingly, significantly elevated isoflavone metabolites in CGJ‐treated compared to BS‐treated mice may contribute to bone health.
In conclusion, CGJ consumption can enhance isoflavone bioavailability owing to isoflavone glycoside conversion to aglycones in OVX mice. It has been reported that ingestion in the form of fermented soybean food increases the bioavailability of isoflavones38; furthermore, CGJ increases the bioavailability of isoflavone in our OVX model. Thus, the results of our study suggest the possibility that CGJ may increase the bioavailability of isoflavones of fermented foods in postmenopausal women. However, further clinical studies need to be carried out to confirm the generality of our findings. In the future, this study will provide a useful basis to interpret the functions of foods during investigations of the manner in which well‐known functional ingredients in foods are metabolized and absorbed in vivo.
Abbreviations
- BS
boiled soybeans
- CGJ
Cheonggukjang
- FBS
fetal bovine serum
- OVX
ovariectomized
- Pmax
the maximum peak area
- tmax
time to reach Pmax
- t1/2
terminal elimination half‐time
- AUC
area under the curve
- ALP
alkaline phosphatase
- αMEM
alpha minimal essential medium
Conflict of Interest
The authors have declared no conflict of interest.
Acknowledgements
D.H.L. and M.J.K. contributed equally to this work. C.H.J. and D.H.L. wrote the manuscript and were responsible for study conception and design. D.H.L., J.A., and Y.J.J. performed the in vivo experiments and analyzed the data. M.J.K., S.H.L., and J.H.K. performed the LC‐MS analyses and interpretation of data. T.Y.H. performed in vitro experiments. All authors approved the final version of the manuscript. This study was supported by Main Research Program E0150101‐03 of the Korea Food Research Institute (http://www.kfri.re.kr) funded by the Ministry of Science, ICT & Future Planning.
Lee D., Kim M. J., Ahn J., Lee S. H., Lee H., Kim J. H., Park S., Jang Y., Ha T., Jung C. H., Mol. Nutr. Food Res. 2017, 61, 1700322 10.1002/mnfr.201700322
References
- 1. Hughes G. J., Ryan D. J., Mukherjea R., Schasteen C. S., J. Agric. Food Chem. 2011, 59, 12707. [DOI] [PubMed] [Google Scholar]
- 2. Messina M., Nutrients 2016, 8, 754. [Google Scholar]
- 3. Choi J. H., Pichiah P. B., Kim M. J., Cha Y. S., J. Clin. Biochem. Nutr. 2016, 59, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kang S. J., Seo J. Y., Cho K. M., Lee C. K., Kim J. H., Kim J. S., Prev. Nutr. Food Sci. 2016, 21, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu W. J., Lee H. Y., Lee G. H., Chae H. J., Ahn B. Y., J. Med. Food 2014, 17, 1298. [DOI] [PubMed] [Google Scholar]
- 6. Shin S. K., Kwon J. H., Jeong Y. J., Jeon S. M., Choi J. Y., Choi M. S., J. Med. Food 2011, 14, 108. [DOI] [PubMed] [Google Scholar]
- 7. Hilakivi‐Clarke L., Andrade J. E., Helferich W., J. Nutr. 2010, 140, 2326s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Q., Ge X., Tian X., Zhang Y., Zhang J., Zhang P., Biomed. Rep. 2013, 1, 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liggins J., Bluck L. J., Coward W. A., Bingham S. A., Anal. Biochem. 1998, 264, 1. [DOI] [PubMed] [Google Scholar]
- 10. Piskula M. K., Yamakoshi J., Iwai Y., FEBS Lett. 1999, 447, 287. [DOI] [PubMed] [Google Scholar]
- 11. Hutchins A. M., Slavin J. L., Lampe J. W., J. Am. Diet. Assoc. 1995, 95, 545. [DOI] [PubMed] [Google Scholar]
- 12. Izumi T., Piskula M. K., Osawa S., Obata A., Tobe K., Saito M., Kataoka S., Kubota Y., Kikuchi M., J. Nutr. 2000, 130, 1695. [DOI] [PubMed] [Google Scholar]
- 13. Tsangalis D., Wilcox G., Shah N. P., Stojanovska L., Br. J. Nutr. 2005, 93, 867. [DOI] [PubMed] [Google Scholar]
- 14. Kano M., Takayanagi T., Harada K., Sawada S., Ishikawa F., J. Nutr. 2006, 136, 2291. [DOI] [PubMed] [Google Scholar]
- 15. Cassidy A., Brown J. E., Hawdon A., Faughnan M. S., King L. J., Millward J., Zimmer‐Nechemias L., Wolfe B., Setchell K. D., J. Nutr. 2006, 136, 45. [DOI] [PubMed] [Google Scholar]
- 16. Cho K. M., Joo O. S., Kor. J. Food Preser. 2015, 22, 119. [Google Scholar]
- 17. Alekel D. L., Van Loan M. D., Koehler K. J., Hanson L. N., Stewart J. W., Hanson K. B., Kurzer M. S., Peterson C. T., Am. J. Clin. Nutr. 2010, 91, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smit S., Szymanska E., Kunz I., Gomez Roldan V., van Tilborg M. W., Weber P., Prudence K., van der Kloet F. M., van Duynhoven J. P., Smilde A. K., de Vos R. C., Bendik I., Mol. Nutr. Food Res. 2014, 58, 2111. [DOI] [PubMed] [Google Scholar]
- 19. Yuan J. P., Wang J. H., Liu X., Mol. Nutr. Food Res 2007, 51, 765. [DOI] [PubMed] [Google Scholar]
- 20. Uehara M., J. Clin. Biochem. Nutr. 2013, 52, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang T. S., Int. J. Mol. Sci. 2014, 15, 5699.24705463 [Google Scholar]
- 22. Sareddy G. R., Vadlamudi R. K., Chin. J. Nat. Med. 2015, 13, 801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Setchell K. D., Clerici C., J. Nutr. 2010, 140, 1355s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Duynhoven J. P. M., van Velzen E. J. J., Westerhuis J. A., Foltz M., Jacobs D. M., Smilde A. K., Trends Food Sci. Technol. 2012, 26, 4. [Google Scholar]
- 25. Abdi F., Alimoradi Z., Haqi P., Mahdizad F., Climacteric 2016, 19, 535. [DOI] [PubMed] [Google Scholar]
- 26. Setchell K. D., Lydeking‐Olsen E., Am. J. Clin. Nutr. 2003, 78, 593s. [DOI] [PubMed] [Google Scholar]
- 27. O'Loughlin P. D., Morris H. W., J. Physiol. 1998, 511, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rafii F., Metabolites 2015, 5, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shin D., Jeong D., Jang. J. Ethnic Foods 2015, 2, 2. [Google Scholar]
- 30. Hati S., Vij S., Singh B. P., Mandal S., J. Sci. Food Agric. 2015, 95, 216. [DOI] [PubMed] [Google Scholar]
- 31. Li‐Jun Y., Li‐Te L., Zai‐Gui L., Tatsumi E., Saito M., Food Chem. 2004, 87, 587. [Google Scholar]
- 32. Yamabe S., Kobayashi‐Hattori K., Kaneko K., Endo H., Takita T., Food Chem. 2007, 100, 369. [Google Scholar]
- 33. Mani V., Ming L. C., in Fermented Foods in Health and Disease Prevention, (Eds: Martinez‐Villaluenga C., Peñas E.), Academic Press, Boston: 2017, pp. 453. [Google Scholar]
- 34. Kumar S., Pandey A. K., Sci. World J. 2013, 2013, 162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maddalena Rossi A. A., Roncaglia L., Leonardi A., Raimondi S., in Isoflavones Biosynthesis, Occurence and Health Effects, (Ed: Thompson M. J.), Nova Science Publishers, Inc. 2010, pp. 137. [Google Scholar]
- 36. Chen K. L., Madak‐Erdogan Z., Trends Endocrinol. Metab. 2016, 27, 752. [DOI] [PubMed] [Google Scholar]
- 37. Cox‐York K. A., Sheflin A. M., Foster M. T., Gentile C. L., Kahl A., Koch L. G., Britton S. L., Weir T. L., Physiol. Rep. 2015, 3, e12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Okabe Y., Shimazu T., Tanimoto H., J. Sci. Food Agricult. 2011, 91, 658. [DOI] [PubMed] [Google Scholar]
- 39. Hotz C., Gibson R. S., J. Nutr. 2007, 137, 1097. [DOI] [PubMed] [Google Scholar]
- 40. Unno T., Choi J. H., Hur H. G., Sadowsky M. J., Ahn Y. T., Huh G. S., Kim G. B., Cha C. J., J. Dairy Sci. 2015, 98, 3568. [DOI] [PubMed] [Google Scholar]


