Abstract
Although, culture is considered the gold standard for poliovirus detection from stool samples, real‐time PCR has emerged as a faster and more sensitive alternative. Detection of poliovirus from the stool of recently vaccinated children by culture, single and multiplex real‐time PCR was compared. Of the 80 samples tested, 55 (68.75%) were positive by culture compared to 61 (76.25%) and 60 (75%) samples by the single and one step multiplex real‐time PCR assays respectively. Real‐time PCR (singleplex and multiplex) is more sensitive than culture for poliovirus detection in stool, although the difference was not statistically significant.
Keywords: cell culture, poliovirus, shedding
1. INTRODUCTION
Identification of poliovirus (Family Picornaviridae, genus Enterovirus, species Poliovirus) in clinical and environmental samples is a crucial component of the global polio eradication program led by the World Health Organization (WHO).1 Earlier studies aimed at detection of poliovirus in stool used culture in several cell lines including Vero, Hep‐2, and MK cells.2 Since 2004, the WHO has recommended L20B and RD cell lines for poliovirus isolation,3 with samples cultured for 14 days before being reported as negative. Identification of the three poliovirus serotypes in culture positive samples needs intratypic differentiation (ITD) by neutralization, enzyme‐linked immunosorbent assay (ELISA), or polymerase chain reaction (PCR)4 extending the time taken to generate a report. Because of these limitations, conventional and real‐time PCR have emerged as alternatives to culture for the early and more sensitive detection of poliovirus from clinical samples.2, 5
In this study, we evaluated the performance of culture in the L20B cell line and two real‐time PCR assays (for single poliovirus types and a one step multiplex real‐time PCR) in the detection of Sabin poliovirus from stool samples of recently vaccinated Indian children.
2. MATERIALS AND METHODS
2.1. Study design and sample collection
A random set of 80 stool samples from infants vaccinated with trivalent oral poliovirus vaccine (tOPV) at 6 weeks of age was selected for the study. The samples were collected as part of a clinical trial (CTRI/2012/05/002677) evaluating supplementation with zinc and/or probiotics to enhance the immune response of oral rotavirus vaccine and tOPV in Indian infants which was conducted at Chinnallapuram, Vellore between July 2012, and February 2013. A total of 3275 stool samples from 570 infants were collected on days 0, 4, and 7 after administration of tOPV and oral rotavirus vaccine (Rotarix) at 6and 10 weeks of age. The study was approved by the Institutional Review Board (IRB) of Christian Medical College (CMC), Vellore and informed consent was obtained from the parents/legal guardians of all the study participants.
2.2. Culture
Stool culture was performed in the L20B cell line according to WHO guidelines.3 A 20% stool suspension was filtered through a 0.22 micron filter and the filtrate used for culture. The inoculated and control flasks were incubated at 37°C (5% CO2). All observations were recorded for 1 week or till culture positive. For negative samples, a blind passage was performed and the flasks observed for another 7 days. If the cultures showed no cytopathic effect (CPE) by 14 days, the sample was reported as negative for poliovirus.
2.3. RNA extraction and cDNA conversion
Before extraction, a 20% stool suspension of each sample was spiked with 1 μL of MS2 phage as an extraction and amplification control, which was calibrated to yield a cycle threshold (Ct) value of 25‐26 cycles by the multiplex real‐time PCR. Positive and negative controls (nuclease free water) were included with each set of extraction. RNA was extracted using Vx reagents on a QIAxtractor (Qiagen, GmbH, Hilden, Germany). Complementary DNA (cDNA) was generated from the eluted RNA by reverse transcription using random primer oligonucleotides (Invitrogen, Carlsbad, CA) and 200 U/μL of Moloney murine leukemia virus reverse transcriptase (Invitrogen).
2.4. Real‐time PCR for Sabin viruses 1, 2, and 3
The serotype specific primers and probes targeting the VP1 region of the Sabin poliovirus genome were adopted from Kilpatrick et al.5 Plasmids constructed by ligation of poliovirus serotypes 1, 2, and 3 region of the VP1 PCR fragment in TOPO‐TA 2.1 vector propagated in Escherichia coli DH5α cells were used as plasmid DNA standards for calibration of assay interpretation (supplementary information). The detection limit was determined by standard curves using 10‐fold serial dilutions of Sabin plasmids ranging from 3 × 107 to 3 copies/μL. The detection limit was three copies corresponding to a Ct value of <40 for all three Sabin poliovirus types. The TaqMan based real‐time PCR assays used Sabin 1 (S1), Sabin 2 (S2), and Sabin 3 (S3) specific forward and reverse primers (Sigma Aldrich, Bengaluru, India) and probes labeled with FAM and black hole quencher 1 (BHQ‐1) (Sigma Aldrich), according to Kilpatrick et al.5
The serotype specific real‐time PCR assays included the Platinum Quantitative PCR UDG Super Mix (Invitrogen) in a 25 μL total volume containing 12.5 μL of the PCR mix, 0.5 μL each of 20 μM primers, 0.25 μL of 10 μM probe, 0.025 μL of ROX dye, 6.225 μL of nuclease free water (NFW), and 5 μL of the template cDNA. The cycling conditions were 2 min at 50°C, 2 min at 95°C, and 40 cycles of 15 s at 95°C, and 1 min at 45°C on the ABI 7500 Fast real‐time PCR detection system (Applied Biosystems, Foster city, CA). Serotype specific positive control, negative control, and a no template control (NTC) with only the master mix were included in each run.
2.5. One step multiplex real‐time PCR for detection of Sabin serotypes
The multiplex one step reverse transcription real‐time PCR used to detect tOPV shedding for all three Sabin types simultaneously were performed according to Taniuchi et al.6 The S1 and S2 probes were minor‐groove binding TaqMan probes (Applied Biosystems) with the VIC and FAM fluorophores, respectively. The S3 probe was labeled with Texas Red and BHQ2 (Integrated DNA technologies, Coralville, IA) while the MS2 probe was labeled with Quasar 670 and BHQ2 (Biosearch Technologies, Novato, CA). Initially, a primer‐probe mix was prepared by adding 4 μL each of 100μM forward and reverse primers, and 2 μL of each 100 μM probes. The PCR included the 2X qScript XLT One‐Step RT‐qPCR Toughmix (Quanta Biosciences, Gaithersburg, MD) in a 20 μL total volume containing 10 μL of the PCR buffer, 0.4 μL of the primer‐probe mix, 5.6 μL NFW, and 4 μL of template RNA. The cycling conditions were 15 min at 50°C, 1 min at 95°C, and 40 cycles of 10 s at 95°C, and 45 s at 60°C on the Quantstudio 12K Flex real‐time PCR detection system (Applied Biosystems). A cutoff Ct value of 36 for S1 and 37 for S2 and S3 were applied.6
Prior to testing the samples, the positive controls for Sabin 1, 2, and 3, were run on both the real‐time PCR machines (ABI 7500 Fast and the Quantstudio 12 K Flex real‐time PCR detection system), and the results were reproducible (Ct value range of 28‐30 for all three Sabin types). The Sabin plasmid dilutions ranging from 3 × 107 to 3 copies/μL were also run on both the real‐time PCR machine in three separate runs and the results were found to be reproducible between the two equipment (Supplementary Information).
2.6. Statistical analysis
All the statistical analyses were performed by using SPSS Statistical Software 17.0 (SPSS Inc, Chicago, IL), Microsoft Excel, and GraphPad Prism Version 5.0 for Windows (San Diego, CA). A P‐value of <0.05 was considered statistically significant. All P‐values were two tailed. Inter assay agreement statistics (kappa values) were used to compare the detection of Sabin polioviruses by the single and multiplex real‐time PCR assays.
3. RESULTS
Of the 80 stool samples tested, 55 (68.75%) were positive by for polioviruses by culture. In contrast, 61 (76.25%) and 60 (75%) samples were positive by the single and one step multiplex real‐time PCR assays respectively (Table 1). The increased sensitivity for detection by the two real‐time PCR assays, compared to culture, was, however, not statistically significant (P = 0.28 for singleplex, and P = 0.38 for multiplex real‐time PCR). Overall, 51 samples (63.75%) were positive and 14 samples (17.5%) were negative by all three methods. All the stool samples were positive for MS2 by multiplex real‐time PCR, thus, ruling out any PCR inhibitors in the samples.
Table 1.
Comparison of Sabin poliovirus detection by culture, single and multiplex real‐time PCR in stool samples of children recently vaccinated with trivalent oral poliovirus vaccine
| Culture No. of samples (%) | Singleplex real‐time PCR No. of samples (%) | Multiplex real‐time PCR No. of samples (%) | |
|---|---|---|---|
| Positive for Sabin poliovirus | 55 (68.75%) | 61 (76.25%) | 60 (75%) |
| Negative for Sabin poliovirus | 25 (31.25%) | 19 (23.75%) | 20 (25%) |
| S1 | – | 6 (7.5%) | 8 (10%) |
| S2 | – | 12 (15%) | 11 (13.75%) |
| S3 | – | 4 (5%) | 4 (5%) |
| S1 + S2 | – | 9 (11.25%) | 12 (15%) |
| S1 + S3 | – | 2 (2.5%) | 4 (5%) |
| S2 + S3 | – | 6 (7.5%) | 3 (3.75%) |
| S1 +S2 + S3 | – | 22 (27.5%) | 18 (22.5%) |
S1, Sabin 1; S2, Sabin 2; S3, Sabin 3.
If culture in L20B cell line is considered the gold standard for poliovirus detection, the sensitivity of singleplex and multiplex real‐time PCR were 94.5% and 92.7%, respectively, while the specificity was 64% for both the PCR assays. If the PCR methods are considered the gold standard for detection, the sensitivity of culture was 85% and the specificity varied from 84.2% to 80%.
The quantity of virus as estimated by Ct values differed between culture positive and negative samples. Culture positive samples had significantly lower Ct values (singleplex PCR: median [27.6], range [16.4‐39.8]; multiplex PCR: median [26.1], range [15.0‐37.0]) than samples that were negative in culture (singleplex PCR: median [35.6], range [26.7‐39.1]; multiplex PCR: median [33.8], range [27.3‐36.2]), (P < 0.05) (Fig. 1).
Figure 1.
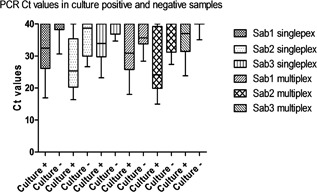
Comparison of culture positive and negative samples with Ct values in single and multiplex real‐time PCR
Figure 2 illustrates the very high correlation (R 2 = 0.931) between the single and multiplex real‐time PCR assays. The two real‐time assays also showed very good agreement for the detection of Sabin polioviruses with a kappa of 0.831 (95%CI, 0.687‐0.974). The median Ct values of the three poliovirus serotypes detected by single and multiplex real‐time PCR were not significantly different ([S1: singleplex {29.3}, multiplex {28.8}; P = 0.6,S2: singleplex {24.8}, multiplex {21.5}; P = 0.2,S3: singleplex {31.8}, multiplex {31.9}; P = 0.5]).
Figure 2.
Correlation between the single and one step multiplex real‐time PCR assays for detection of poliruses in the stool of children given trivalent oral poliovirus vaccine
4. DISCUSSION
The use of two real‐time PCR assays for detection of single or multiple Sabin polioviruses in stool samples from vaccinated children showed that the assays were more sensitive than culture, although the difference did not achieve statistical significance. This could be due to the low sample size (n = 80) in this study, and hence, similar studies in future involving larger sample sizes are necessary. The lower specificity (64%) seen in both the real‐time PCR assays is because these assays were able to detect additional Sabin poliovirus positive samples which were negative by culture. The higher sensitivity of both real‐time PCR assays could be because the PCR also detects virus that may be defective or bound to antibody making it non‐infectious, and thus, non‐detectable by culture.
The PCR assays were easy to use and the results were available in a much shorter time than culture. The turn‐around time for culture was 4‐7 days for positive samples and 14 days for negative samples. In contrast, the turn‐around time was 10‐12 h for singleplex real‐time PCR and only 6‐7 h for the one‐step multiplex real‐time PCR. The substantial amount of time saved using real‐time PCR assays could be crucial in the earlier detection of outbreaks in the post‐eradication era, thus, giving sufficient time to prevent widespread transmission of polioviruses.7
The standard method of evaluating mucosal immunity to poliovirus is to challenge individuals with OPV and measure shedding by culture, but few studies have used PCR.2, 8 A Mexican study, which used a novel real‐time PCR assay, detected low levels of OPV excretion after vaccination in stool and sewage samples and also differentiated between revertant and non‐revertant serotypes.9 A recent study from India evaluating mucosal immunity to a dose of inactivated poliovirus vaccine (IPV) in children previously primed with OPV found the positive predictive value of real‐time PCR for culture in the detection of a challenge dose of Sabin polioviruses in stool to be 87% for serotype 1 and 89% for serotype 3, respectively.10 Some studies have reported the use of PCR for environmental surveillance though culture has been extensively used for such purposes.11 A study from Cuba found PCR to be as sensitive as culture in L20B cell line for poliovirus detection from waste water and stool samples.11
Along with serotype specific singleplex real‐time PCR assays for poliovirus detection, multiplex PCR assays have been developed in the last few years to simultaneously detect all three poliovirus serotypes, thus, reducing turn‐around‐time.6, 12 A study by Buonagurio et al,13 comparing a multiplex reverse transcription PCR (RT‐PCR) method to culture for the direct detection of Sabin polioviruses in stool collected from first‐dose OPV recipients showed that the PCR detected poliovirus types 1, 2, and 3 in 67.2, 82.6%, and 53.8% specimens respectively compared to 55.4%, 64.1%, and 27.7% by culture. The RT‐PCR assay showed an increase of 12%, 18%, and 50% in the detection rates of poliovirus types 1, 2, and 3 compared to culture.13 Another recent study found the sensitivity and specificity of a multiplex one‐step real‐time quantitative PCR assay for the detection of Sabin poliovirus in stool samples to be 89% and 91%, respectively, compared to culture.6 Similar to our findings, the study also showed a significantly higher quantity of the vaccine virus detected by the multiplex PCR in culture positive samples compared to samples which were culture negative.6 Another study by Laassri et al, also found a quantitative multiplex real‐time PCR assay to be highly sensitive and specific compared to culture for Sabin poliovirus detection in clinical samples.7
Despite the present setbacks in eradicating poliomyelitis worldwide, it is expected that the circulation of wild polio viruses (presently WPV1 only) will stop in the near future.7 However, for maintaining a polio free world, continuous surveillance for vaccine virus is necessary as circulation of OPV strains could lead to more outbreaks of vaccine derived polioviruses.7 Real‐time PCR assays can play a crucial role in the surveillance and early detection of vaccine virus circulation globally.7
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information S1.
CONFLICTS OF INTEREST
None.
Giri S, Rajan AK, Kumar N, et al. Comparison of culture, single and multiplex real‐time PCR for detection of Sabin poliovirus shedding in recently vaccinated Indian children. J Med Virol. 2017; 89: 1485–1488. 10.1002/jmv.24793
Institution where work was performed: Christian Medical College, Vellore, India.
REFERENCES
- 1. Sutter RW, Platt L, Mach O, Jafari H, Aylward RB. The new polio eradication end game: rationale and supporting evidence. J Infect Dis. 2014; 210:S434–S438. [DOI] [PubMed] [Google Scholar]
- 2. Tebbens RJD, Pallansch MA, Chumakov KM, et al. Expert review on poliovirus immunity and transmission. Risk Analysis. 2013; 33:544–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Polio Laboratory Manual. WHO/IVB/04.10. Geneva; 2004.
- 4. van der Avoort HGAM, Hull BP, Hovi T, et al. Comparative study of five methods for intratypic differentiation of polioviruses. J Clin Microbiol. 1995; 33:2562–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kilpatrick DR, Yang CF, Ching K, et al. Rapid group‐, serotype‐, and vaccine strain‐specific identification of poliovirus isolates by real‐time reverse transcription‐PCR using degenerate primers and probes containing deoxyinosine residues. J Clin Microbiol. 2009; 47:1939–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taniuchi M, Begum S, Uddin MJ, et al. Kinetics of poliovirus shedding following oral vaccination as measured by quantitative reverse transcription‐PCR versus culture. J Clin Microbiol. 2015; 53:206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laassri M, DiPiazza A, Bidzhieva B, Zagorodnyaya T, Chumakov K. Quantitative one‐step RT‐PCR assay for rapid and sensitive identification and titration of polioviruses in clinical specimens. J Virol Methods. 2013; 189:7–14. [DOI] [PubMed] [Google Scholar]
- 8. Laassri M, Lottenbach K, Belshe R, et al. Effect of different vaccination schedules on excretion of oral poliovirus vaccine strains. J Infect Dis. 2005; 192:2092–2098. [DOI] [PubMed] [Google Scholar]
- 9. Troy SB, Ferreyra‐Reyes L, Huang CH, et al. Use of a novel real‐time PCR assay to detect oral polio vaccine shedding and reversion in stool and sewage samples after a Mexican national immunization day. J Clin Microbiol. 2011; 49:1777–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. John J, Giri S, Karthikeyan AS, et al. Effect of a single inactivated poliovirus vaccine dose on intestinal immunity against poliovirus in children previously given oral vaccine: an open‐label, randomised controlled trial. Lancet. 2014; 9953:1505–1512. [DOI] [PubMed] [Google Scholar]
- 11. Lago PM, Gary HE Jr, Perez LS, et al. Poliovirus detection in waste water and stools following an immunization campaign in Havana, Cuba. Int J Epidemiol. 2003; 32:772–777. [DOI] [PubMed] [Google Scholar]
- 12. Elnifro EM, Ashshi AM, Cooper RJ, Klapper PE. Multiplex PCR: optimization and application in diagnostic virology. Clin Microbiol Rev. 2000; 13:559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buonagurio DA, Coleman JW, Patibandla SA, Prabhakar BS, Tatem JM. Direct detection of Sabin poliovirus vaccine strains in stool specimens of first‐dose vaccinees by a sensitive reverse transcription‐PCR method. J Clin Microbiol. 1999; 37:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information S1.


