Abstract
This prospective, parallel‐group, randomized, double‐blind, multicenter study compared the efficacy and safety of FV‐100 with valacyclovir for reducing pain associated with acute herpes zoster (HZ). Patients, ≥50 years of age, diagnosed with HZ within 72 h of lesion appearance who had HZ‐associated pain, were randomized 1:1:1 to a 7‐day course of either FV‐100 200 mg QD (n = 117), FV‐100 400 mg QD (n = 116), or valacyclovir 1000 mg TID (n =117). Efficacy was evaluated on the basis of the burden of illness (BOI; Zoster Brief Pain Inventory scores); incidence and duration of clinically significant pain (CSP); pain scores; incidence and severity of post‐herpetic neuralgia (PHN); and times to full lesion crusting and to lesion healing. Safety was evaluated on the basis of adverse event (AE)/SAE profiles, changes in laboratory and vital signs values, and results of electrocardiograms. The burden of illness scores for pain through 30 days were 114.5, 110.3, and 118.0 for FV‐100 200 mg, FV‐100 400 mg, and valacyclovir 3000 mg, respectively. The incidences of PHN at 90 days for FV‐100 200 mg, FV‐100 400 mg, and valacyclovir 3000 mg were 17.8%, 12.4%, and 20.2%, respectively. Adverse event and SAE profiles of the two FV‐100 and the valacyclovir groups were similar and no untoward signals or trends were evident. These results demonstrate a potential for FV‐100 as an antiviral for the treatment of shingles that could both reduce the pain burden of the acute episode and reduce the incidence of PHN compared with available treatments.
Keywords: antiviral agents, herpes simplex virus, herpes virus, reinfection
1. BACKGROUND
Varicella zoster virus (VZV) is a human alpha herpes virus, which causes varicella (chicken pox) and herpes zoster (HZ). Approximately one out of three people in the United States aged 60 years or older will develop shingles.1
Herpes zoster results from the reactivation of latent VZV and its spread from the ganglion to the corresponding cutaneous dermatome.2, 3 The characteristic vesicular rash (shingles) of HZ pustulates and crusts within 7‐10 days but may take up to a month to heal; however, pain is an important feature of the disease that is often the most debilitating for patients.
Zoster‐associated pain is experienced by approximately 90% of patients. The chronic neuropathic pain phase of the disease, known as postherpetic neuralgia (PHN), is defined as pain that persists 90 days or more after rash onset.4, 5
Postherpetic neuralgia causes the largest HZ‐related burden of illness.6, 7, 8, 9 Risk factors for PHN include greater acute pain severity, presence of a prodrome, greater rash severity, and older patient age at onset.10 Current interventions for PHN, such as lidocaine patches, opioids, tricyclic antidepressants, and anti‐epileptics, are palliative and fail to treat the underlying disease. A substantial proportion of patients fail to obtain satisfactory pain relief using the available medications.8 This pain burden is associated with negative effects on their quality of life such as impaired physical functioning, higher levels of emotional stress, and decreases in social functioning.11
Although the widespread use of the herpes zoster vaccine has reduced the incidence of HZ and PHN,12 a large percentage (∼90% in the US) of the at‐risk population does not receive the vaccine.13 About half of people, 60 years of age or older who have had the shingles vaccine, fail to respond (eg, primary failure meaning they failed to seroconvert after vaccination) and may go on to develop HZ.12, 14 In addition, the efficacy of the vaccine has been shown to decline over time (eg, secondary vaccine failure).15, 16
Several drugs, including acyclovir, valacyclovir, and famciclovir, have demonstrated minimal efficacy in terms of pain control for patients with HZ.17 Additionally, a significant proportion of these patients (∼20‐40% and sometimes more) go on to develop PHN.3, 18 These drugs require multiple doses of medication each day.19, 20, 21 Furthermore, doses of these antiviral agents must be modified for patients with renal impairment. It is evident that a drug with greater antiviral activity, better pain relief, and a more simplified dosing regimen represents a significant unmet medical need. Brivudine is also available in some countries for treating shingles.22
FV‐100 is a prodrug for the bicyclic nucleoside analogue CF‐1743.23 CF‐1743 demonstrates high potency against clinical VZV isolates (EC50 ∼440 pM). The inhibitory activity of FV‐100 and CF‐1743 is highly specific for VZV. Renal clearance for FV‐100 and CF‐1743 was demonstrated to be low for all doses tested (100, 200, 400, and 800 mg QD), indicating that renal elimination is not likely to be an important pathway for either compound. In light of these results, a Phase 2 clinical trial was undertaken in patients with HZ in order to evaluate the efficacy of once‐daily (QD) FV‐100 at both the 200 mg and 400 mg doses, compared with valacyclovir 3000 mg administered three times daily (1000 mg TID) in reducing the acute herpes zoster burden of illness (BOI) and reducing the incidence of PHN.
2. STUDY DESIGN
This was a multicenter, randomized, parallel‐group, comparative study of FV‐100 versus valacyclovir in patients with HZ conducted at 90 centers in the United States between May 2009 and December 2010. Patients who presented within 72 h of zoster rash and met all eligibility criteria were randomized 1:1:1 to one of three treatment groups: 1) FV‐100 200 mg QD; 2) FV‐100 400 mg QD; or 3) valacyclovir 3000 mg (1000 mg TID). Patients were centrally randomized by an interactive web response system and stratified by 1) age category (<65 vs. ≥65); and 2) Visit 1/Day 1 ZBPI worst pain in the last 24 h score (<6 vs. >6). Sites were blinded to all ZBPI subset pain scores throughout the study.
The primary endpoint was the reduction in the mean BOI‐30AUC, a standardized score measuring the overall severity of pain experienced by study subjects during the 30 days after the first dose of study drug (see section 2.3). A sample size of at least 333 patients (111 per arm) was calculated to achieve 80% power to detect a 25% reduction in the mean BOI‐30AUC in the FV‐100 treated group compared to the valacyclovir‐treated group. Estimates of BOI‐30AUC and SD (constant SD across treatment arms) were extrapolated from the Zostavax Shingles Prevention Study and the ZBPI validation study.21, 24, 25
All eligible patients were treated for 7 days and followed through Day 30. Patients who continued to have HZ‐associated pain or whose rashes had not healed by Day 30 were followed through Day 90 or until their rash healed and they had two documented Zoster Brief Pain Inventory (ZBPI) worst pain scores of 0, whichever occurred first.
The study was approved by a central institutional review board (IRB) or local IRBs and conducted in accordance with the International Committee on Harmonization Guidelines for Good Clinical Practice and the 1964 Declaration of Helsinki.26, 27 This study was registered on http://clinicaltrials.gov (NCT00900783).
2.1. Patients
Patients were ≥50 years of age, had a clinical diagnosis of HZ as evidenced by a unilateral dermatomal rash present for ≤72 h, and had zoster‐related pain as defined by a ZBPI worst pain score >0.
Patients were excluded for multidermatomal, disseminated, or ophthalmic HZ; impaired renal function, immunosuppression; gastrointestinal dysfunction that could interfere with absorption; chronic pain requiring narcotic analgesics; the use of tricyclic antidepressants, systemic or cutaneous antivirals within 30 days of enrollment, or CYP3A4‐inhibiting protease inhibitors, and strong CYP3A4 inhibitors and inducers.
2.2. Assessments
At screening (Day 1), a complete medical history, physical exam, lesion assessment, vital signs, electrocardiogram (ECG), clinical laboratory specimens (blood for hematology and chemistry, and urine for urinalysis), and a lesion swab for qualitative VZV determination, were obtained. Pain and zoster pain‐related interference in functional categories were evaluated using the ZBPI. When eligibility was confirmed, patients received their first dose of study drug.
Patients completed visits on Days 3, 5, and 7 during the dosing period, and at Days 10, 14, 21, 30, 42, 56, and 90 days post‐dose. Lesion assessments continued until complete healing was demonstrated. Patients continued to complete ZBPIs on Days 42, 56, and 90 unless they achieved two consecutive visits (starting on Day 30 or later) without HZ‐associated pain.
At each visit, the investigators assessed lesions and patients completed the ZBPI. Blood and urine samples for laboratory testing were collected on Days 3, 7, 14, and 30. Vital signs were taken on Days 5 and 7, and ECGs were performed on Days 3, 7, and 30. Adverse events and concomitant medications (including pain medications) were recorded throughout the study. The numbers of capsules and caplets remaining in the study drug wallet on Day 7 were recorded to assess study medication compliance.
2.3. Endpoints
The primary efficacy measure was the reduction of the HZ BOI over 30 days (measured by BOI‐30AUC). This endpoint was derived from a patient's reported score on a scale from 0 (no pain) to 10 (pain as bad as you can imagine) on question #3 of the ZBPI, which rated a patient's “worst pain in the last 24 h” over the period from Study Day 1 to Study Day 30. Secondary endpoints included the following: HZ BOI scores through 14 days (BOI‐14AUC) and 90 days (BOI‐90AUC), incidence and severity of PHN at 90 days after lesion appearance, time to full lesion crusting and lesion healing, use of concomitant pain medication, and zoster pain‐related interference in functional categories as assessed by the ZBPI. CSP was defined as a worst pain score >3 as reported on the ZBPI. Safety was assessed using adverse events (AEs), clinical laboratory testing, vital signs, electrocardiograms, and physical exams. DNA samples were extracted from lesion swabs on days 1 and 7 and analyzed for phenotypic resistance.
The main efficacy analyses were conducted on a modified intent‐to‐treat population (MITT2), defined as all randomized patients who received at least one dose of study drug.
The burden of illness (BOI‐AUC) was calculated as follows:
where: p = worst pain score, t = time point [actual day or nominal day], n = maximum time point for AUC [actual day ± visit window, n = 14 ± 2, 30 ± 3, 90 ± 7; nominal day, n = 14, 30, 90].
2.4. Statistical analyses
The acute HZ burden of illness values over 14, 30, and 90 days (measured by BOI‐14AUC, BOI‐30AUC, and BOI‐90AUC) were evaluated using analysis of covariance (ANCOVA) model, controlling for stratified age (50‐69 and ≥70 years of age), sex, and baseline pain.
The main efficacy analyses were conducted on a modified intent‐to‐treat population (MITT2), defined as all randomized patients who received at least one dose of study drug, except for those whose lesion swabs were positive by polymerase chain reaction (PCR) for herpes simplex virus (HSV) and negative by PCR for VZV. The acute HZ burden of illness over 30 days (measured by BOI‐30AUC) was evaluated using an F test from an analysis of covariance model, controlling for age, gender, and baseline level of pain. BOI‐14AUC and BOI‐90AUC were evaluated in the same manner. A sample size of at least 333 patients (111 per arm) was calculated to achieve 80% power to detect a 25% reduction in the mean BOI‐30AUC in the FV‐100 treated group compared to the valacyclovir treated group, with a 2‐sided α of 0.05 and a valacyclovir mean BOI‐30AUC of 91 (SD 60) in the study.
The incidence of PHN at 90 days after lesion appearance was summarized by treatment group and evaluated using the chi‐square test from a logistic regression. The severity of PHN was evaluated using a Poisson model controlling for treatment group, age, sex, and concomitant pain medication. Clinically significant pain (CSP) was defined as a worst pain score >3 as reported on the ZBPI.
For each Study Day, treatment group means and corresponding standard deviations (SDs) were calculated, and the estimates of mean differences (between each active arm individually and the active control arm) are presented with resulting P values from an analysis of covariance (ANCOVA) model, controlling for stratified age (50‐69 and ≥70 years of age), sex and baseline pain.
Comparison of mean time to full lesion crusting and mean time to lesion healing for patients treated with FV‐100 to those treated with valacyclovir were completed through calculation of the Kalplan‐Meier estimators.
The safety population consisted of all randomized patients who received at least one dose of study drug. All safety analyses were descriptive.
3. RESULTS
Of the 466 patients screened, 350 were randomized and received at least one dose of study drug (Fig. 1). Some patients (34) were excluded due to their low creatinine clearance (<50 mL/min/1.73 m2), leaving 329 for the modified ITT efficacy analysis. The mean age of patients was 64.6 years. Most patients (65%) were women. All other baseline demographic characteristics were similar between groups (Table 1). Approximately 15% of patients overall had a history of HZ. The mean number of prior occurrences was 1.5 for the study (range 1‐5). Fewer than 5% of the patients had received the HZ vaccine.
Figure 1.
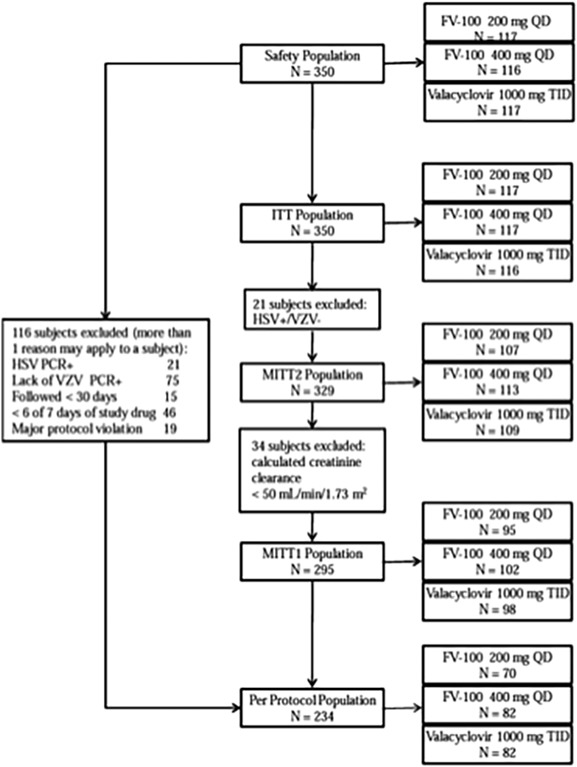
Study flow chart. The chart shows the relationships between all analysis populations. All patients were included in both the safety and ITT population. The differences in Ns of the treatment groups between the safety and ITT population are because of errors in dispensing for two patients that received a treatment different from their randomized assignment. The ITT population consisted of all randomized patients who received at least one dose of study drug. The MITT1 population consisted of all randomized patients who receive at least one dose of study drug except for patients whose lesion swabs were positive by PCR for HSV and negative by PCR for VZV or who had a calculated creatinine clearance <50 mL/min/1.73m2. The MITT2 population consisted of all randomized patients who received at least one dose of study drug except for patients whose lesions swabs were positive by PCR for HSV and negative by PCR for VZV. The per‐protocol population consisted of all patients from the ITT population who had a lesion swab positive for VZV, were followed for at least 30 days, received at least 6 of 7 days of study drug, and had no major protocol violations. HSV, herpes simplex virus; ITT, intent‐to‐treat; MITT, modified intent‐to‐treat; PCR, polymerase chain reaction; QD, once daily; TID, three times daily; VZV, Varicella zoster virus
Table 1.
Demographic characteristics by treatment group
| Characteristic | FV‐100 200 mg QD N = 117 | FV‐100 400 mg QD N = 116 | Valacyclovir 1000 mg TID N = 117 | Total N = 350 |
|---|---|---|---|---|
| Sex | ||||
| Male, n (%) | 40 (34.2) | 42 (36.2) | 38 (32.5) | 120 (34.3) |
| Female, n (%) | 77 (65.8) | 74 (63.8) | 79 (67.5) | 230 (65.7) |
| Age (years) | ||||
| Mean | 63.95 | 64.66 | 64.27 | 64.29 |
| Minimum‐maximum | 50.0‐94.3 | 50.4‐87.9 | 50.4‐90.6 | 50.0‐94.3 |
| Race and ethnicity a | ||||
| Caucasian non‐Hispanic/Latino, n (%) | 88 (75.2) | 97 (83.6) | 97 (82.9) | 282 (80.6) |
| Caucasian Hispanic/Latin, n (%) | 14 (12.0) | 6 (5.2) | 11 (9.4) | 31 (8.9) |
| Black/African descent, n (%) | 13 (11.1) | 9 (7.8) | 9 (7.7) | 31 (8.9) |
| Asian, n (%) | 2 (1.7) | 1 (0.9) | 0 | 3 (0.9) |
| Native North American/Alaskan, n (%) | 0 | 1 (0.9) | 0 | 1 (0.3) |
| Native Hawaiian/Pacific islander, n (%) | 0 | 1 (0.9) | 0 | 1 (0.3) |
| Mixed race Hispanic/Latino, n (%) | 0 | 1 (0.9) | 0 | 1 (0.3) |
| Body Mass Index (kg/m2) | ||||
| Mean | 29.39 | 29.99 | 28.86 | 29.41 |
| Minimum‐maximum | 17.1‐54.9 | 17.9‐52.1 | 19.8‐47.8 | 17.1‐54.9 |
Race and ethnicity (whether or not Hispanic/Latino) were collected as separate variables. All subjects for whom Hispanic/Latino ethnicity was recorded were of Caucasian race except one subject who was of mixed race.
Most patients (78.4%) reported baseline prodromal pain. At onset of treatment the mean (SD) worst pain score was 6.3 (2.5). However, 30% of baseline average pain scores were rated at three or below by patients, limiting the ability of this study to analyze the impact of the treatment on pain.
3.1. Burden of illness
The LS mean BOI‐30AUC values (SD) for FV‐100 200 mg, FV‐100 400 mg, and valacyclovir 3000 mg were 114.5 (6.2), 110.3 (6.1), and 118.0 (6.3), respectively (Table 2). This Phase 2 study was not powered to show differences in the BOI‐30AUC between groups of less than 25%, however, the results suggested a dose response for FV‐100. Notably, the differences between the mean BOIs for FV‐400 mg and valacyclovir 3000 mg became more pronounced with time.
Table 2.
Burden of illness area under the curve by treatment group
| Endpoint measure statistic | FV‐100 200 mg QD n = 107 | FV‐100 400 mg QD n = 113 | Valacyclovir 1000 mg TID n = 109 |
|---|---|---|---|
| Primary efficacy endpoint BOI‐30AUC | |||
| LS mean (SE) | 114.485 (6.2396) | 110.309 (6.0823) | 117.956 (6.2529) |
| LS mean difference from valacyclovir 1000 mg TID | −3.471 | −7.647 | |
| 95%CI | −22.1017, 15.1595 | −26.0468, 10.7527 | |
| P value | 0.883 | 0.553 | |
| Secondary efficacy endpoint BOI‐14AUC | |||
| LS mean (SE) | 65.354 (2.4385) | 63.078 (2.3770) | 63.781 (2.4437) |
| LS mean difference from valacyclovir 1000 mg TID | 1.573 | −0.703 | |
| 95%CI | −5.7076, 8.8543 | −7.8935, 6.4881 | |
| P value | 0.846 | 0.966 | |
| Secondary efficacy endpoint BOI‐90AUC a | |||
| LS mean (SE) | 221.526 (19.5052) | 196.942 (19.0133) | 229.587 (19.5467) |
| LS mean difference from valacyclovir 1000 mg TID | −8.061 | −32.645 | |
| 95%CI | −66.3008, 50.1789 | −90.1631, 24.8732 | |
| P value | 0.933 | 0.346 | |
Patients reporting no pain after Study Day 30 on two consecutive visits were relieved of subsequent visits (Study Days 56 and/or 90) and presumed to have a “worst pain in the last 24 h” score of 0 on those days.
CI, confidence interval; LS mean, least squares mean; SD, standard deviation; SE, standard error.
3.2. Post‐herpetic neuralgia
A numerically lower incidence of PHN was observed for the patients receiving the 200 and 400 mg doses of FV‐100 compared with those receiving 3000 mg of valacyclovir (17.8% and 12.4% vs. 20.2%, respectively) (Table 3). Even though this study was not powered to demonstrate a difference, there was also a numerical trend for patients treated with FV‐100 at either 200 mg or 400 mg to have a lower severity of pain than those treated with valacyclovir. Both the incidence and severity of PHN results suggest the presence of a dose response in the efficacy of FV‐100.
Table 3.
Postherpetic neuralgia (PHN) incidence and severity at 90 days
| Endpoint measure statistic | FV‐100 200 mg QD N = 107 | FV‐100 400 mg QD N = 113 | Valacyclovir 1000 mg TID N = 109 |
|---|---|---|---|
| PHN incidence | |||
| n (%) | 19 (17.8) | 14 (12.4) | 22 (20.2) |
| Odds ratio vs. valacyclovir 1000 mg TID | 0.9 | 0.6 | |
| 95%CI | 0.4, 1.8 | 0.3, 1.2 | |
| P value | 0.703 | 0.158 | |
| PHN severity | |||
| Number of patients with PHN | 19 | 14 | 22 |
| LS mean (SE) | 5.4 (0.47) | 5.3 (0.52) | 5.7 (0.46) |
| LS mean ratio vs. valacyclovir 1000 mg TID | 0.9 | 0.9 | |
| 95%CI | 0.8, 1.2 | 0.7, 1.2 | |
| P value | 0.547 | 0.491 |
Modified intent‐to‐treat population. Missing values were imputed using the last observation carried forward approach. CI, confidence interval; LS mean, least squares mean; SD, standard deviation; SE, standard error.
3.3. Pain endpoints
Trends in faster resolution of pain in both the FV‐100 groups compared to valacyclovir were noted for other a priori endpoints, with potential dose effects observed between the two groups (Table 4). The mean duration of CSP was 31.3 and 27.3 days in the FV‐100 200 and 400 mg groups respectively, and 32.3 days in the valacyclovir group. These results suggest a dose response effect with a quicker elimination of pain at the higher dose of FV‐100. Likewise, the median duration until the first resolution of CSP was 26.7 and 25.1 days in the FV‐100 200 and 400 mg groups, respectively, versus 28.8 days in the valacyclovir group. A higher proportion of patients achieved a permanent resolution of CSP in the FV‐100 groups (77.6 % for the 200 mg dose and 80.5% for 400 mg dose) compared with the patients on valacyclovir (75.2%). A difference in mean worst pain scores between valacyclovir and the two FV‐100 doses and a potential dose effect between the two FV‐100 doses was observed from Study Day 14 onward, with the same consistent pattern occurring with least pain, average pain, and pain right now scores favoring the FV‐100 treatment arms.
Table 4.
Duration and resolution of clinically significant pain
| Measure statistic | FV‐100 200 mg QD N = 107 | FV‐100 400 mg QD N = 113 | Valacyclovir 1000 mg TID N = 109 |
|---|---|---|---|
| Duration of CSP (days) | |||
| n (%) | 102 (95.3) | 105 (92.9) | 104 (95.4) |
| Mean (SD) | 31.3 (32.29) | 27.3 (28.24) | 32.3 (32.92) |
| Median | 14.0 | 15.0 | 20.5 |
| Range (minimum, maximum) | 1, 114 | 1, 93 | 1, 96 |
| Duration until first resolution of CSP (days) | |||
| n (%) | 102 (95.3) | 105 (92.9) | 104 (95.4) |
| Mean (SD) | 26.7 (30.95) | 25.1 (28.69) | 28.8 (32.43) |
| Median | 12.0 | 14.0 | 13.5 |
| Range (minimum, maximum) | 1, 114 | 1, 93 | 1, 96 |
| Permanent resolution of CSP | |||
| Yes—n (%) | 83 (77.6) | 91 (80.5) | 82 (75.2) |
| Study Day on which permanent resolution of CSP was achieved | |||
| Mean (SD) | 26.0 (24.02) | 26.5 (23.06) | 24.4 (21.08) |
| Median | 15.0 | 21.0 | 17.0 |
| Range (minimum, maximum) | 3, 96 | 3, 97 | 2, 92 |
| No—n (%) | 19 (17.8) | 14 (12.4) | 22 (20.2) |
| CSP not experienced—n (%) | 5 (4.7) | 8 (7.1) | 5 (4.6) |
3.4. Post‐hoc analyses
Post hoc analyses were conducted to gain more insight into the effect of FV‐100 on subacute and chronic pain. BOI‐14‐90AUC and BOI‐30‐90AUC both showed that patients on FV‐100 generally had lower burdens of illness than those on valacyclovir. A dose response was suggested for BOI‐14‐90AUC and a trend toward better pain management from week 2 onward was observed for FV‐100 400 mg. These results corroborated the prospective observations. These post hoc analyses results are shown in the supplemental material.
3.5. Virology
The virology results are described in the supplemental material.
3.6. Safety
Approximately 48% of the total population experienced an AE (FV‐100 200 mg = 47%, FV‐100 400 mg = 54%, and valacyclovir = 42%) (Table 5). Overall, most AEs were mild in severity, and there were no Grade 4 AEs in either of the FV‐100 arms. The only grade four event (fatal congestive heart failure) occurred in one patient in the valacyclovir group. Overall, headache was the most frequently observed AE for both the FV‐100 and valacyclovir arms (7.4% of patients overall, 5% for FV‐100 200 mg and valacyclovir and 13% for FV‐100 400 mg). Nausea was the second‐most frequently observed AE (7.1% overall, approximately 6.0% for FV‐100 200 mg and valacyclovir and 9.5% for FV‐100 400 mg).
Table 5.
Burden of illness area under the curve by treatment group—Study Days 14‐90 and 30‐90
| Measure statistic | FV‐100 200 mg QD N = 107 | FV‐100 400 mg QD N = 113 | Valacyclovir 1000 mg TID N = 109 |
|---|---|---|---|
| BOI‐14‐90AUC | |||
| LS mean (SE) | 155.0, (18.0) | 132.091 (17.6) | 164.739 (18.1) |
| LS mean difference from valacyclovir 1000 mg TID | −9.7 | −32.6 | |
| 95%CI | −63.5, 44.1 | −85.8, 20.5 | |
| P value | 0.890 | 0.292 | |
| BOI‐30‐90AUC | |||
| LS mean (SE) | 106.5 (14.5) | 85.838 (14.2) | 107.794 (14.6) |
| LS mean difference from valacyclovir 1000 mg TID | −1.3 | −22.0 | |
| 95%CI | −44.7, 42.0 | −64.8, 20.9 | |
| P value | 0.997 | 0.415 | |
Only two patients in each treatment arm had an AE that led to study drug discontinuation. No SAEs were observed in the FV‐100 200 mg arm. The SAEs were approximately equal in the FV‐100 400 mg and valacyclovir arms of the study (approximately 4%)(Table 6).
Table 6.
Overall summary of adverse events by treatment group
| FV111‐100 200 mg QD N = 117 n (%) | FV‐100 400 mg QD N = 116 n (%) | Valacyclovir 1000 mg TID N = 117 n (%) | Total N = 350 n (%) | |
|---|---|---|---|---|
| Number of AEs | 117 | 154 | 108 | 379 |
| Number (%) of patients with any AE | 55 (47.0) | 63 (54.3) | 49 (41.9) | 167 (47.7) |
| Severity | ||||
| Grade 1 (mild) | 32 (27.4) | 27 (23.3) | 22 (18.8) | 81 (23.1) |
| Grade 2 (moderate) | 19 (16.2) | 28 (24.1) | 23 (19.7) | 70 (20.0) |
| Grade 3 (severe) | 4 (3.4) | 8 (6.9) | 3 (2.6) | 15 (4.3) |
| Grade 4 (potentially life‐threatening) | 0 | 0 | 1 (0.9) | 1 (0.3) |
| Leading to study drug discontinuation | 2 (1.7) | 2 (1.7) | 2 (1.7) | 6 (1.7) |
| Number of SAEs | 0 | 6 | 5 | 11 |
| Number (%) of patients with any SAE | 0 | 5 (4.3) | 4 (3.4) | 9 (2.6) |
| Any SAE within first 10 days of the study | 0 | 3 (2.6) | 2 (1.7) | 5 (1.4) |
| Drug‐related | 0 | 0 | 2 (1.7) | 2 (0.6) |
| Leading to study drug discontinuation | 0 | 1 (0.9) | 1 (0.9) | 2 (0.6) |
n, number of subjects reporting at least one adverse event; (%), percentage of subjects among treatment group (N).
Laboratory results were unremarkable, no evidence for renal or hepatic toxicity was noted, and vital signs and electrocardiograms demonstrated no untoward safety signal.
4. DISCUSSION
Postherpetic neuralgia is known to cause the largest burden of illness for patients with HZ.8, 9 A significant proportion of patients with HZ develop PHN despite treatment with the current antiviral agents.6, 7 These antivirals require dosing regimens (3‐5 times daily) that must be modified for patients with renal impairment.19, 20, 21 The current medications used to treat the pain associated with PHN fall short in terms of relief for many patients.8 In addition pain medications are only palliative and do not provide a cure for HZ. Hence, a drug with greater antiviral activity, the ability to prevent PHN, better pain relief, and a more simplified dosing regimen is needed.
Although there was no statistically significant difference among the treatment groups for the primary endpoint of BOI‐30AUC, a difference between the FV‐100 400 mg and valacyclovir groups emerged in the 90‐day data (a 14% reduction), suggesting a potential effect of FV‐100 on subacute and chronic pain. Numerical differences in the incidence and severity of PHN at 90 days favored both doses of FV‐100 over valacyclovir. These results represent a clinically meaningful reduction in pain with FV‐100. Faster resolution of pain in both the FV‐100 groups compared to valacyclovir was consistently noted, with potential dose effects observed between the two FV‐100 groups. The mean duration of CSP was shorter in the FV‐100 groups compared with the valacyclovir group. Likewise, the resolution of CSP was quicker and a higher proportion of patients achieved a permanent resolution of CSP in the FV‐100 groups compared with the patients on valacyclovir. These differences are clinically meaningful since pain following lesion healing is likely a better measure of chronic pain than is pain after study enrollment.
In the post‐hoc analysis, the 400 mg dose of FV‐100 provided a 20% reduction in the burden of illness for pain in both the 14‐90 day periods and 30‐90 day periods when compared to valacyclovir. Patients receiving the 400 mg dose of FV‐100 experienced on average a 37% reduction in the incidence of PHN over valacyclovir. The proportions of patients on valacyclovir 3000 mg experiencing PHN were comparable to those from previous published reports, which lends validity to the overall design of the study.17, 28 Average pain scores demonstrated consistent improvement over the 3 months of the study for the FV‐100 400 mg group. The consistent advantages of FV‐100 over valacyclovir indicate that FV‐100 supports a therapeutic role for this new agent in the reduction of subacute and chronic HZ‐associated pain.
We acknowledge that the assessment of pain is a relatively subjective measure. However, the ZBPI,18 developed from the standard Brief Pain Inventory29 for use in the clinical trials for an anti‐HZ vaccine, is a validated instrument for the measurement of HZ‐associated pain and the effect of that pain on functional activities of daily living.25 This index measures the impact of HZ and PHN across all four domains of health: physical, psychological, social, and functional5 and is a sensitive and reliable measure of the impact of HZ and PHN on patients’ daily lives.25 In addition, the use of worst pain score of ≥3 in the definition of PHN has been validated as part of the development of the ZBPI25 and reflects consensus that this level of pain constitutes clinically meaningful chronic pain.
Clinicians should not underestimate the fact that neuralgic pain can be quite severe.2 Some studies of postherpetic neuralgia suggest the early attenuation of acute pain may prevent the onset of central mechanisms of chronic pain, thus potentially reducing the risk of postherpetic neuralgia.2, 30 Thus, antiviral therapies, such as FV‐100, may act to inhibit these mechanisms of pain transmission.
Several design characteristics likely affected the study's ability to identify statistically significant differences between the two antiviral agents, including the use of BOI as a primary efficacy measure, a pain score of >0 at study entry, and the possibility that 14 days may not have been a sufficient length of time for differences in pain to be assessed. However, this Phase 2 study demonstrated proof of concept for FV‐100 in terms of efficacy and safety in the treatment of HZ and provided valuable data, which guided the subsequent design of a larger potentially pivotal Phase 3 trial.
In spite of the availability of antiviral drugs approved for the treatment of HZ, and the availability of the HZ vaccine, up to 40% of all patients with HZ will go on to develop PHN.6, 7, 12, 31, 32, 33 Pain associated with PHN is often refractory to treatment, and symptomatic relief is obtained in fewer than half of PHN sufferers despite the frequent use of multiple drugs for pain control.34, 35, 36, 37 While the majority of patients experience complete resolution of their pain by one year, long‐term studies indicate that PHN may persist indefinitely.36, 38, 39 Taken as a whole, these findings support the conclusion that PHN represents a significant unmet medical need.
Based on the analyses in this Phase 2 study, a multicenter, randomized, double‐blind, parallel‐group Phase 3 study has been designed, which is powered to assess the safety and efficacy of 400 mg FV‐100 dosed either once or twice daily. The study measures the effect on HZ‐associated subacute and chronic pain, compared with TID valacyclovir 1000 mg (3000 mg daily dose). This Phase 3 study is designed to confirm that FV‐100 will address the need for a more effective medication to prevent and treat HZ.
In conclusion, the comprehensive results of the burden of illness, PHN, time to clinically significant pain resolution, and pain score analyses demonstrate a potential role for FV‐100 in the reduction of subacute and chronic pain as well as the prevention of PHN. The safety profile of FV‐100 remains favorable both in isolation and when compared to valacyclovir. Current antiviral medications have limited effectiveness in the reduction of subacute and chronic pain, do not satisfactorily prevent or adequately treat PHN, and require dosing modifications in patients with renal insufficiencies. The efficacy results from this study support further investigation of FV‐100 to address these unmet medical needs.
5. CONFLICTS OF INTERESTS
JSB and TM are employees of ContraVir Pharmaceuticals. JSB is a stockholder in ContraVir Pharmaceuticals. No other competing interests.
Tyring SK, Lee P, Hill GT Jr, Silverfield JC, Moore AY, Matkovits T, and Sullivan‐Bolyai J. FV‐100 versus valacyclovir for the prevention of post‐herpetic neuralgia and the treatment of acute herpes zoster‐associated pain: A randomized‐controlled trial. J Med Virol. 2017; 89:1255–1264. 10.1002/jmv.24750
Medical Writing was funded by ContraVir Pharmaceuticals.
Ethical approval was given by Integ Review.
REFERENCES
- 1.Center for Disease Control and Prevention. What you need to know about the shingles vaccine. Available at: http://www.cdc.gov/vaccines/hcp/patient-ed/adults/downloads/fs-shingles.pdf Updated August 2014.
- 2. Gnann JW, Jr , Whitley. Clinical practice. Herpes zoster. N Engl J Med. 2002; 347:340–346. [DOI] [PubMed] [Google Scholar]
- 3. Schmader KE, Dworkin RH. Natural history and treatment of herpes zoster. J Pain. 2008; 9:3–9. [DOI] [PubMed] [Google Scholar]
- 4. Arani RB, Soong SJ, Weiss HL, Wood MJ, Fiddian PA, Gnann JW, Whitley R. Phase specific analysis of herpes zoster associated pain data: A new statistical approach. Stat Med. 2001; 20:2429–2439. [DOI] [PubMed] [Google Scholar]
- 5. Johnson RW, Bouhassira D, Kassianos G, Leplege A, Schmader KE, Weinke T. The impact of herpes zoster and post‐herpetic neuralgia on quality‐of‐life. BMC Med. 2010; 8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bowsher D. The lifetime occurrence of Herpes zoster and prevalence of post‐herpetic neuralgia: A retrospective survey in an elderly population. Eur J Pain. 1999; 3:335–342. [DOI] [PubMed] [Google Scholar]
- 7. Jung BF, Johnson RW, Griffin DR, Dworkin RH. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology. 2004; 62:1545–1551. [DOI] [PubMed] [Google Scholar]
- 8. Tontodonati M, Ursini T, Polilli E, Vadini F, Di Masi F, Volpone D, Parruti G. Post‐herpetic neuralgia. Int J Gen Med. 2012; 5:861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Massengill JS, Kittredge JL. Practical considerations in the pharmacological treatment of postherpetic neuralgia for the primary care provider. J Pain Res. 2014; 7:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Forbes HJ, Thomas SL, Smeeth L, Clayton T, Farmer R, Bhaskaran K, Langan SM. A systematic review and meta‐analysis of risk factors for postherpetic neuralgia. Pain. 2016; 157:30–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katz J, Cooper EM, Walther RR, Sweeney EW, Dworkin RH. Acute pain in herpes zoster and its impact on health‐related quality of life. Clin Infect Dis. 2004; 39:342–348. [DOI] [PubMed] [Google Scholar]
- 12. Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, Weinberg A, Boardman KD, Williams HM, Zhang JH, Peduzzi PN, Beisel CE, Morrison VA, Guatelli JC, Brooks PA, Kauffman CA, Pachucki CT, Neuzil KM, Betts RF, Wright PF, Griffin MR, Brunell P, Soto NE, Marques AR, Keay SK, Goodman RP, Cotton DJ, Gnann JW, Jr , Loutit J, Holodniy M, Keitel WA, Crawford GE, Yeh SS, Lobo Z, Toney JF, Greenberg RN, Keller PM, Harbecke R, Hayward AR, Irwin MR, Kyriakides TC, Chan CY, Chan IS, Wang WW, Annunziato PW, Silber JL, Shingles Prevention Study G. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N England J Med. 2005; 352:2271–2284. [DOI] [PubMed] [Google Scholar]
- 13.National Health Interview Survey (NHIS). Estimated proportion of adults aged >19 years who received selected vaccinations, by age group, high‐risk status, and race/ethnicity. United States; 2009. http://www.cdc.gov/vaccines/imz-managers/coverage/nhis/2009-nhis.html Accessed March 29, 2016.
- 14. Pannuti CS, Morello RJ, Moraes JC, Curti SP, Afonso AM, Camargo MC, Souza VA. Identification of primary and secondary measles vaccine failures by measurement of immunoglobulin G avidity in measles cases during the 1997 Sao Paulo epidemic. Clin Diagn Lab Immunol. 2004; 11:119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schmader KE, Oxman MN, Levin MJ, Johnson G, Zhang JH, Betts R, Morrison VA, Gelb L, Guatelli JC, Harbecke R, Pachucki C, Keay S, Menzies B, Griffin MR, Kauffman C, Marques A, Toney J, Keller PM, Li X, Chan IS, Annunziato P, Shingles Prevention Study G. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short‐term persistence substudy. Clin Infect Dis. 2012; 55:1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morrison VA, Johnson GR, Schmader KE, Levin MJ, Zhang JH, Looney DJ, Betts R, Gelb L, Guatelli JC, Harbecke R, Pachucki C, Keay S, Menzies B, Griffin MR, Kauffman CA, Marques A, Toney J, Boardman K, Su SC, Li X, Chan IS, Parrino J, Annunziato P, Oxman MN. Long‐term persistence of zoster vaccine efficacy. Clin Infect Dis. 2015; 60:900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beutner KR, Friedman DJ, Forszpaniak C, Andersen PL, Wood MJ. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995; 39:1546–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Drolet M, Brisson M, Schmader K, Levin M, Johnson R, Oxman M, Patrick D, Camden S, Mansi JA. Predictors of postherpetic neuralgia among patients with herpes zoster: A prospective study. J Pain. 2010; 11:1211–1221. [DOI] [PubMed] [Google Scholar]
- 19.ZOVIRAX® (acyclovir) capsules, tablets, suspension [package insert] Research Triangle Park, NC. GlaxoSmithKline; 2005.
- 20.FAMVIR® (famciclovir) tablets [package insert] Eastt Hanover, NJ. Novartis Pharmaceuticals Corp.; 2013.
- 21.VALTREX® (valacyclovir hydrochloride) caplets [package insert] Research Triangle Park, NC. GlaxoSmithKline; 2008.
- 22. Lilie HM, Wassilew S. The role of antivirals in the management of neuropathic pain in the older patient with herpes zoster. Drugs Aging. 2003; 20:561–570. [DOI] [PubMed] [Google Scholar]
- 23. McGuigan C, Pathirana RN, Migliore M, Adak R, Luoni G, Jones AT, Diez‐Torrubia A, Camarasa MJ, Velazquez S, Henson G, Verbeken E, Sienaert R, Naesens L, Snoeck R, Andrei G, Balzarini J. Preclinical development of bicyclic nucleoside analogues as potent and selective inhibitors of Varicella zoster virus. J Antimicrob Chemother. 2007; 60:1316–1330. [DOI] [PubMed] [Google Scholar]
- 24. Rohan P. 2005. FDA Clinical Briefing Document for Merck & Co., Inc., Zoster Vaccine Live (Oka/Merck) Zostavax®.
- 25. Coplan PM, Schmader K, Nikas A, Chan IS, Choo P, Levin MJ, Johnson G, Bauer M, Williams HM, Kaplan KM, Guess HA, Oxman MN. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: Adaptation of the brief pain inventory. J Pain. 2004; 5:344–356. [DOI] [PubMed] [Google Scholar]
- 26.World Medical Association Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects; 2016. Available at: http://www.wma.net/en/30publications/10policies/b3 [DOI] [PubMed]
- 27.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline: Clinical Safety Data Management: Definitions and Standards For Expedited Reporting E2A. 1994. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2A/Step4/E2A_Guideline.pdf
- 28. Beutner KR. Valacyclovir: A review of its antiviral activity, pharmacokinetic properties, and clinical efficacy. Antiviral Res. 1995; 28:281–290. [DOI] [PubMed] [Google Scholar]
- 29. Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994; 23:129–138. [PubMed] [Google Scholar]
- 30. Dworkin RH, Perkins FM, Nagasako EM. Prospects for the prevention of postherpetic neuralgia in herpes zoster patients. Clin J Pain. 2000; 16:90–100. [DOI] [PubMed] [Google Scholar]
- 31. Chen N, Li Q, Yang J, Zhou M, Zhou D, He L. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database System Rev. 2014; 2:CD006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kawai K, Rampakakis E, Tsai TF, Cheong HJ, Dhitavat J, Covarrubias AO, Yang L, Cashat‐Cruz M, Monsanto H, Johnson K, Sampalis JS, Acosta CJ. Predictors of postherpetic neuralgia in patients with herpes zoster: A pooled analysis of prospective cohort studies from North and Latin America and Asia. Int J Infect Dis. 2015; 34:126–131. [DOI] [PubMed] [Google Scholar]
- 33. Oxman MN, Levin MJ, Shingles Prevention Study G. Vaccination against herpes zoster and postherpetic neuralgia. J Infect Dis. 2008; 197:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Christo PJ, Hobelmann G, Maine DN. Post‐herpetic neuralgia in older adults: Evidence‐based approaches to clinical management. Drugs Aging. 2007; 24:1–19. [DOI] [PubMed] [Google Scholar]
- 35. Johnson RW, McElhaney J. Postherpetic neuralgia in the elderly. Int J Clin Pract. 2009; 63:1386–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oster G, Harding G, Dukes E, Edelsberg J, Cleary PD. Pain, medication use, and health‐related quality of life in older persons with postherpetic neuralgia: Results from a population‐based survey. J Pain. 2005; 6:356–363. [DOI] [PubMed] [Google Scholar]
- 37. Serpell M, Gater A, Carroll S, Abetz‐Webb L, Mannan A, Johnson R. Burden of post‐herpetic neuralgia in a sample of UK residents aged 50 years or older: Findings from the Zoster Quality of Life (ZQOL) study. Health Qual Life Outcomes. 2014; 12:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Helgason S, Petursson G, Gudmundsson S, Sigurdsson JA. Prevalence of postherpetic neuralgia after a first episode of herpes zoster: Prospective study with long term follow up. BMJ. 2000; 321:794–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McKendrick MW, Ogan P, Care CC. A 9 year follow up of post herpetic neuralgia and predisposing factors in elderly patients following herpes zoster. J Infect. 2009; 59:416–420. [DOI] [PubMed] [Google Scholar]


