Abstract
Despite the life-long importance for posture and locomotion, neuromuscular properties of the hamstrings muscle have not been explored with adult aging. The purpose of this study was to assess and compare age-related effects on contractile function, spinal motor neuron output expressed as motor unit (MU) discharge rates in the hamstrings of 11 young (26 ± 4 yr) and 10 old (80 ± 5 yr) men. Maximal voluntary isometric contractions (MVC), stimulated contractile properties, and surface and intramuscular electromyography (EMG) from submaximal to MVC were recorded in the biceps femoris (BF) and semimembranosus-semitendinosus (SS) muscles. MVC torque was ~50% less in the old with both age groups attaining ≥93% mean voluntary activation. Evoked twitches in the old were ~50% lower in amplitude and >150% longer in duration compared with those in the young. At successive voluntary contractions of 25, 50, and 100% MVC, MU discharge rates were up to 45% lower in old, with no differences in relative submaximal surface EMG between age groups. Furthermore, the old had significantly lower MU discharge rates in the SS at all contraction intensities compared with the BF muscle. Men in their 8th to 10th decades of life demonstrate substantially lower strength and MU discharge rates in this functionally important large lower limb muscle group, with greater age-related effect on discharge rates in the medial hamstrings. These findings, compared with those in other muscles studied, highlight that the neuromuscular properties of limb muscles, and indeed within functionally similar portions of a muscle group, are not all affected equally by the aging process.
NEW & NOTEWORTHY In the hamstrings, we found that both contractile function and motor unit discharge rates across the range of voluntary intensities were lower in the old. The differences in discharge rates due to age were greater in the medial hamstrings muscle group compared with the lateral hamstrings. Compared with previous studies, these results highlight that not all muscles are affected equally by aging and there may be compartmental differences within functionally similar muscles.
Keywords: aging, flexor, motor neuron, motor unit, muscle
INTRODUCTION
Neuromuscular factors implicated in aging involve changes in motor unit (MU) structure and function (Alway et al. 2017; Hepple and Rice 2016). During adult aging, the motor neuron population declines (Tomlinson and Irving 1977), leading to orphaned muscle fibers becoming atrophied or collaterally reinnervated by surviving motor axons (Luff 1998). This MU remodeling is regarded as an inevitable compensatory process, in which over decades remaining viable MUs become larger and slower contracting (Doherty et al. 1993; Hepple and Rice 2016). With the notable exception of the hamstrings (posterior thigh muscles), the effects of aging on the neuromuscular system are described for major lower limb muscles, which have essential function in posture and locomotion (Christie and Kamen 2010; Connelly et al. 1999; Dalton et al. 2008, 2009, 2014; Kamen and Knight 2004; Kirk et al. 2016; McNeil et al. 2005; Power et al. 2016; Roos et al. 1999).
Three of the four muscles that comprise the hamstrings (long head of the biceps femoris, semimembranosus, and semitendinosus) are two-joint muscles that act to extend the hip and flex the knee, whereas the short head of the biceps femoris acts to flex the knee only (Moses et al. 2005). With aging of the hamstrings muscle, there is reduced cross-sectional area and muscle volume with increased intramuscular fat infiltration and passive stiffness (Overend et al. 1992a; Palmer and Thompson 2017; Yoshiko et al. 2017). However, these morphological changes do not fully explain the lower strength reported for the hamstrings, and thus other factors are likely involved (Overend et al. 1992b). During the gait cycle, older adults walk slower than young adults due to reduced step length, which may be a result of diminished hamstring function (Lim et al. 2017; Winter et al. 1990). Furthermore, despite this reduced step length, there is relatively greater muscle activation of the hamstrings (Schmitz et al. 2009) and overall greater coactivation of lower limb musculature compared with young adults (Hortobágyi and DeVita 2000). It is unknown how these differences in strength and recovery from fatigue in the old (Thompson et al. 2015) are related to changes at the MU level.
Once MUs are recruited, the rate at which they discharge is an essential component of muscle force generation and control, especially at moderate to maximal contraction intensities (Enoka and Duchateau 2017). In the lower limb muscles, age-related weakness does not appear to be substantially related to voluntary activation, and at moderate to maximal contraction intensities, differences in MU discharge rates are variable. Specifically, MU discharge rates were not lower in the vastus medialis and gastrocnemii muscles in old compared with young men (Kirk et al. 2016; Roos et al. 1999), and in the soleus, rates were lower only at contractile intensities of <50% MVC (Dalton et al. 2009). In contrast, the tibialis anterior and vastus lateralis had lower MU discharge rates at all contraction intensities in old compared with young adults (Christie and Kamen 2010; Connelly et al. 1999; Kamen and Knight 2004). Furthermore, within large functionally similar muscles such as the quadriceps, the vastus lateralis and vastus medialis showed disparate age-related differences in MU discharge rates (Kamen and Knight 2004; Roos et al. 1999), whereas neither portion (medial or lateral head) of the gastrocnemii displayed different discharge rates between old and young men (Kirk et al. 2016). Given these results, age-related MU adaptations for muscles may be dependent on function, MU organization, and anatomical location.
The present study sought to investigate contractile function and MU discharge rates in the hamstrings in a group of men ~80 yr in age and to compare age-related differences with results from a study in young men (Kirk and Rice 2017). Neuromuscular properties were determined with electrical stimulation at varying frequencies and during brief voluntary isometric contractions (at 25, 50, and 100% MVC) with surface and intramuscular EMG recordings. Despite diminished functional capacity consistent for all limb muscles, reports have shown minimal (Dalton et al. 2009) and no (Kirk et al. 2016) age-related effect on MU discharge rates for posterior leg muscles. We hypothesized that the hamstrings in a group of men in their 8th to 10th decades of life will have lower contractile capacity but no difference in MU discharge rates compared with those in young adult men.
MATERIALS AND METHODS
Participants.
Twenty-one male participants volunteered for this investigation (Table 1). Young participants (20–33 yr) were recruited from the University population and were involved in regular recreational activities. A substantial portion of the data from the young men for comparison has been published (Kirk and Rice 2017), but three of these subjects were tested further to acquire a larger sample of MU discharge rates in the young. Healthy older participants (72–92 yr) were recruited from the University community and were involved in a recreational exercise programs three times a week and all living independently. Exclusion criteria included known neuromuscular, orthopedic, and metabolic diseases, alcoholism, and recreational drug use. Participants were instructed to refrain from intense exercise and caffeine consumption within 48 h before testing, and all participants provided oral and written informed consent. The study required each participant to visit the neuromuscular laboratory for 2–4 testing sessions, each separated by 2–7 days. This study conformed to the local University’s research ethics board for human experimentation and the latest revision of the Declaration of Helsinki.
Table 1.
Anthropometric, voluntary, and stimulated contractile properties of the hamstrings
| Parameter | Young | Old |
|---|---|---|
| n | 11 | 10 |
| Age, yr | 26 ± 4 | 80 ± 5* |
| Height, m | 1.82 ± 0.04 | 1.72 ± 0.03* |
| Body mass, kg | 82.9 ± 9.3 | 76.4 ± 9.2 |
| MVC (prone position), Nm | 86.3 ± 16.5 | 43.0 ± 9.6* |
| MVC (seated position), Nm | 172.3 ± 37.3 | 84.7 ± 15.8* |
| Voluntary activation, % | 98.4 ± 0.9 | 92.9 ± 3.2* |
| Evoked peak twitch, Nm | 24.7 ± 8.0 | 11.7 ± 4.6* |
| Time to peak tension (ms) | 95.8 ± 41.6 | 159 ± 53.3* |
| Rate of torque development, Nm/s | 350.9 ± 317.1 | 84.0 ± 48.7* |
| One-half relaxation time, ms | 85.6 ± 50.8 | 167.7 ± 63.1* |
| Negative peak relaxation rate, Nm/s | −446.1 ± 376.1 | −97.9 ± 88.1* |
| Contraction duration, ms | 181.5 ± 64.6 | 327.1 ± 75.8* |
| Potentiated peak twitch, Nm | 28.1 ± 6.6 | 15.1 ± 5.2* |
Values are means ± SD. MVC, maximal voluntary isometric contraction; n, sample size.
P < 0.05 for interactions between age groups.
Experimental setup A.
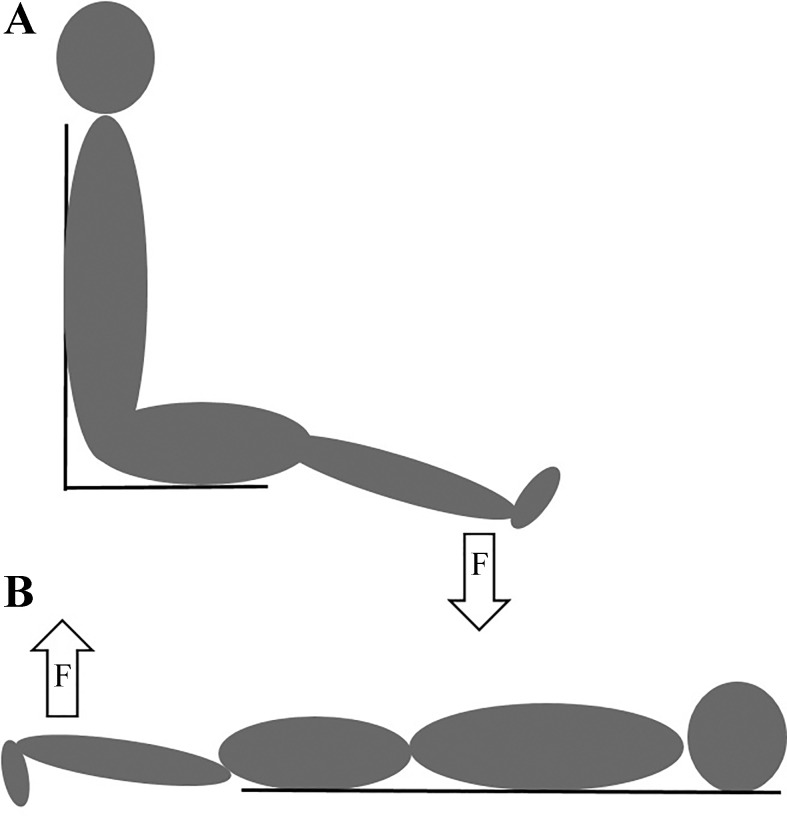
For the first session, participants were seated upright in a dynamometer (Cybex HUMAC NORM; CSMi Medical Solutions, Stoughton, MA) with the nondominant leg fixed to an adaptor arm located on the anterior tibia surface just proximal to the malleoli (Fig. 1A). The hip was extended 10° from the neutral sitting position of 90°; the ankle was positioned at 90°, and the lateral femoral condyle was aligned to the dynamometers axis of rotation. The knee joint was positioned at 110° extension (with 180° representing full extension) to optimize voluntary torque of the hamstrings in a more lengthened position. Participants were firmly positioned in the dynamometer chair by seatbelts at the shoulder and hip, and a Velcro strap secured the thigh, just proximal to the knee. Torque was recorded from the dynamometer and sampled at 500 Hz. Real-time torque production was displayed on a computer screen for visual feedback.
Fig. 1.
Illustrations of experimental setups A and B. A: maximal voluntary isometric contraction and stimulated contractile properties. B: voluntary isometric contractions at submaximal to maximal intensities during recording of surface and intramuscular electromyography.
Muscle stimulation.
Because of the deep anatomical location of the sciatic nerve, we were unable to perform direct nerve stimulation. Therefore, large stimulation electrodes (5 × 15 cm, aluminum foil) covered in electrostimulation gel, were wrapped in paper towel and saturated in saline water (Edwards et al. 1977). The electrodes were securely taped transversely over the proximal aspect of the posterior thigh inferior to the gluteal fold and slightly medial to avoid activation of the vastus lateralis. The second electrode was placed distally over the posterior thigh muscles superior to the popliteal fossa. Stimulation (stimulator model DS7AH; Digitimer, Welwyn Garden City, UK) was applied through the electrodes at 400 V with a pulse duration of 50 μs for the tetanic stimulation protocol and 200 μs for the modified twitch stimulation protocol. Stimulation current intensities for all participants ranged between 250 and 450 mA. For all stimulation events, the current intensity was incrementally increased to a level that activated as much of the hamstring muscles as possible (as measured by torque output) with minimal activation of muscles in the anterior or medial thigh compartments, assessed by visual inspection and manual palpation.
Voluntary activation, evoked twitch, and postactivation evoked twitch.
To assess MVC and electrically stimulated twitch properties of the hamstrings, the hamstrings were placed in a passive muscle-lengthened position (experimental setup A). This was found to be the optimal position to record stimulated contractile responses (Kirk and Rice 2017). The twitch interpolation technique (Hales and Gandevia 1988; Todd et al. 2004) was used to test the ability of participants to maximally activate the hamstring muscles during MVC. For this test, electrical impulses (doublets; 2 pulses at 100 Hz) were applied to the muscle at rest ~1 s before contraction started, during the peak plateau of the 5- to7-s MVC, and ~1 s following the MVC when at rest. From experimental testing, doublet stimulation at 200 μs was most tolerable, eliciting the highest peak torque from participants (Kirk and Rice 2017). Stimulated twitches were maximized for each participant; the current intensity was incrementally increased for the optimal twitch torque response. Visual feedback of torque and strong verbal encouragement were given during the MVCs. Two to four separate MVC trials were performed with a 5-min rest between contractions to avoid fatigue, with the highest MVC value being recorded as maximum strength.
Force-frequency stimulation.
Following the method used for the quadriceps (Edwards et al. 1977), the hamstring muscle was stimulated at 1, 5, 8, 10, 12, 15, 20, 30, 40, 50, 80, and 100 Hz, each for a duration of 1 s with the muscle at rest. A 30-s rest interval was given between each level of stimulation. The stimulus intensity at 100 Hz was adjusted for each subject to achieve a contractile response that was ~30% of MVC and was used for all subsequent stimulation frequencies for this force-frequency protocol. Because some participants were not able to tolerate the stimulus intensity required for ~30% MVC torque output at 100-Hz stimulation, the force-frequency relation was assessed in a subset of participants from each age group (n = 5). The order of stimulation frequencies was randomized.
Experimental setup B.
On a separate day, participants were positioned prone on the same dynamometer with the same leg fixed to the adaptor arm as described above (Fig. 1B). The hip was flexed 10° with the ankle fixed at 90°. The lateral femoral condyle was aligned to the axis of rotation, and the knee joint was positioned at 110° extension. All participants were firmly fastened by large Velcro straps located on the lower back and posterior distal thigh to prevent extraneous movements.
Surface electromyography.
To assess compound neuromuscular activation, surface EMG in a bipolar configuration for the medial and lateral hamstring muscle groups was used. The interelectrode distance was 2 cm center-to-center with a common ground located on the patella. Electrode pairs were placed over the long head of the biceps femoris (BF) and medial midthigh over the semimembranosus and semitendinosus (SS) muscles half the distance from the head of the fibula to the greater trochanter. Voltage signals were recorded using self-adhering cloth Ag-AgCl electrodes (H59P monitoring electrodes; Kendall, Mansfield, MA). Electrode placement sites were swabbed vigorously with 70% ethanol before placement. All voltage signals were amplified (×1,000), wide-band filtered between 10 Hz and 1 kHz (Neurolog NL844; Digitimer), and sampled at 2 kHz (Power 1401; Cambridge Electronic Design, Cambridge, UK).
Intramuscular electromyography.
To assess MU discharge rates during voluntary contractions, intramuscular tungsten microelectrodes (Bellemare et al. 1983) were inserted into BF and SS half the distance from the head of the fibula to the greater trochanter, 1–3 cm from the surface electrodes. The electrodes were insulated tungsten wire needles (123 µm in diameter and 45 mm in length; Freddy Haer, Bowdoin, ME). Because of their close anatomical association, we did not systematically attempt to differentiate intramuscular EMG between the two portions of the SS group. Before insertion, the skin was cleansed with 70% ethanol over the muscle bellies and the intramuscular electrodes were sterilized by autoclave. The intramuscular electrodes were then connected to separate channels, and an operator manipulated each indwelling needle electrode independently. Intramuscular EMG signals were amplified (×100–1,000), wide-band filtered between 10 Hz and 10 kHz (Neurolog NL844; Digitimer), and sampled at 20 kHz per channel (Power 1401; Cambridge Electronic Design). Reference electrodes for the intramuscular electrodes were positioned over the patella. Audio and visual feedback of intramuscular EMG were provided to each needle operator independently.
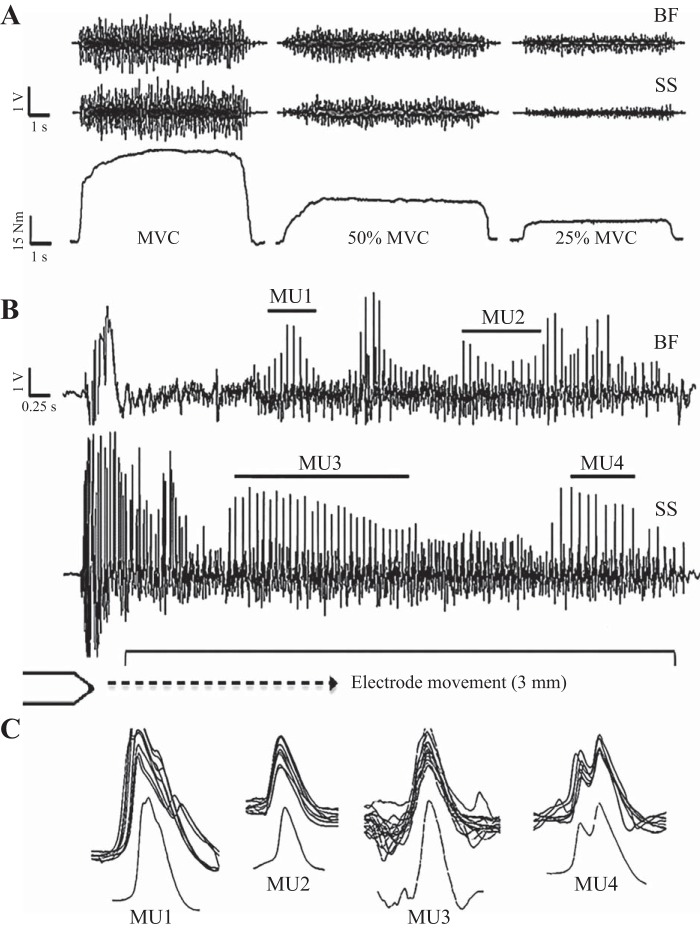
Voluntary isometric contractions were held for 5–10 s at 25 and 50% of MVC and for ~5 s at MVC, with rest periods of 3–5 min between contractions to mitigate fatigue. The order was pseudorandomized with MU trains being sampled during the steady-state torque plateau portion at each contraction intensity (Fig. 2). Visual feedback of torque and strong verbal encouragement were provided to each participant during the different contractions. To sample from as many discrete MUs as possible, each intramuscular electrode was independently manipulated and advanced slowly through the muscle at a rate of ~3–5 mm per contraction (Fig. 2). Intramuscular electrodes were repositioned and reinserted into the BF and SS to achieve a representative sample from each muscle, thus building an overall MU profile range at each contraction intensity (Rich et al. 1998). To collect from many different MUs, 5–10 contractions were made at each intensity until the participant’s MVC level was observed to be ≥5% lower than their initial MVC. To acquire many MU trains without the influence of fatigue, some participants returned to the laboratory to repeat the protocol on separate days.
Fig. 2.
Unprocessed recordings from an old participant (78 yr) with the fastest observed discharge rates. A: raw surface electromyography (EMG) of the biceps femoris (BF) and semitendinosus-semimembranosus (SS) muscles (top) and torque tracings at 100, 50, and 25% of maximal voluntary contraction (bottom). B: an expanded view of raw intramuscular EMG collected through tungsten electrodes individually inserted into the BF (top channel) and SS (bottom channel) during a maximal voluntary contraction. C: examples of action potential shape and overlay of all action potentials from 4 identified motor unit (MU) trains. MU1 is an overlay of 7 action potentials with a coefficient of variation of 12.8, discharging at 21.6 Hz from the BF. MU2 is an overlay of 10 action potentials with a coefficient of variation of 9.4, discharging at 17.8 Hz from the BF. MU3 is an overlay of 25 action potentials with a coefficient of variation of 9.1, discharging at 19.3 Hz from the SS. MU4 is an overlay of 8 action potentials with a coefficient of variation of 12.6, discharging at 14.9 Hz from the SS.
Data acquisition and analyses.
Analyses were performed offline with Spike2 software (Cambridge Electronic Design) as described previously (Dalton et al. 2010). For contractile properties of the evoked twitch, the following measures were made: peak twitch amplitude, twitch time to peak tension, rate of torque development, one-half relaxation time, negative peak relaxation rate, contraction duration (which is the sum of twitch time to peak tension and one-half relaxation time), and potentiated peak twitch amplitude (Morat et al. 2016; Roos et al. 1999). Voluntary activation was calculated from the interpolated twitch technique as described previously (Todd et al. 2004). The force-frequency relation was normalized to the 100-Hz stimulation torque.
To assess surface EMG, 1-s epochs were measured during the torque steady-state portion of voluntary contractions. The electromyographic root-mean-square (EMG-RMS) amplitude during MVC torque of the BF and SS was used to normalize surface EMG of each individual muscle for the 25 and 50% contraction intensities.
MU train analysis of the hamstrings was performed using previously described methods (Kirk and Rice 2017). An example of four discrete MU action potential trains extracted during MVC is shown (Fig. 2). For the histogram analysis of the MU distribution, a set point was established at 15 Hz, with the assumption that MUs discharging at <15 Hz represented a greater probability to produce force by recruitment (Enoka and Duchateau 2017; Webber et al. 2009).
Statistics.
The R software program (version 3.2.3) was used for statistical analyses. Anthropometric, voluntary, and stimulated contractile properties were compared between age groups using unpaired one-tailed t-tests. Multiple linear regression analysis was used to determine relationships between voluntary contraction intensity and MU discharge rates for each age and muscle group. For comparisons of regression models between age and muscle, an analysis of covariance (ANCOVA) was used. To assess differences in surface EMG, a 1 × 3 analysis of variance (ANOVA) was used (surface EMG | age × muscle × contraction intensity). To assess differences in MU discharge rates a 1 × 3 ANOVA was used (MU discharge rates | age × muscle × contraction intensity). MU trains were grouped into 3 bins on the basis of torque level: a 25% bin contained 12.5–37.5% of MVC, a 50% bin contained 37.5–62.5% of MVC, and a 100% bin contained 87.5–100% of MVC. When statistical significance was found (P ≤ 0.05), a Tukey’s honestly significant difference post hoc test was used to determine differences within the interactions. Effect sizes (Cohen’s d) were calculated to assess the influences of age and muscle on MU discharge rates.
RESULTS
Voluntary and stimulated contractile properties.
Age-related differences resulted in statistically significant weaker and slower hamstring muscles in the old. Anthropometric, voluntary, and stimulated contractile properties of the hamstrings are presented in Table 1. Torque produced during knee flexion MVC in the seated and prone positions was less by 50% in the old group (Table 1). For voluntary activation of the hamstrings, both groups where able to attain ≥93% mean activation, with the old being 5.5% less than the young (Table 1). In the seated position, resting evoked peak twitch amplitude in the old was 53% less compared with that in the young (Table 1), with the mean evoked twitch torque relative to MVC being similar between both age groups (~13% in the young and ~14% in the old). The time to peak tensions, one-half relaxation time, and contraction duration of the evoked twitch were 165–196% longer in the old (Table 1). When normalized to twitch amplitude, the rate of torque development and relaxation rates were 75 and 79% less in the old, respectively (Table 1). In addition, the postactivation potentiated resting twitch torque was 46% less in the old (Table 1). When the hamstrings were stimulated at incremental frequencies (1 to 100 Hz), the old reached fused tetanus at lower frequencies than the young, with statistically significant age-related differences from 8- to 20-Hz stimulation (Fig. 3). The mean torque output at the 100-Hz stimulation for each age group was 31 ± 4% of MVC for the old and 31 ± 2% of MVC for the young. Furthermore, the average stimulus frequency required for half-maximum tetanic force was 12 Hz for the old and 18 Hz for the young, with both age groups attaining maximum fused tetanus between 40- and 50-Hz stimulation.
Fig. 3.
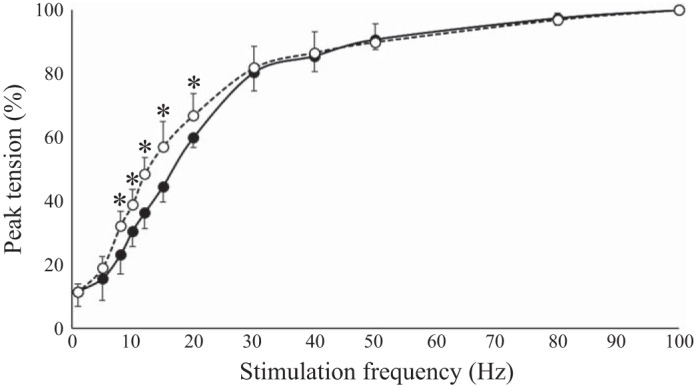
Stimulated force-frequency relationship of the hamstrings in the seated position. Closed circles represent the young (n = 5) and open circles represent the old (n = 5). Peak tetanic tension was normalized to the maximal peak tension of each participant at 100-Hz stimulation. For stimulation at 8, 10, 12, 15, and 20 Hz, there was a significant difference between age groups [t(8) > 1.9, P ≤ 0.05). *P ≤ 0.05 for interactions between age groups.
Intramuscular and surface electromyography.
A total of 2,574 MU action potential trains were identified, with 1,196 from the young and 1,378 from the old (Fig. 4). To compare the relationship between MU discharge rates and contraction intensity influenced by age and muscle group, the data were modeled by linear regression (Fig. 4). For the young, increasing contractile intensity resulted in a significant difference in dependence of the MU discharge rate slope compared with the old in both the BF and SS (P < 0.001). For the distribution of MU discharge rates, the total sample population of MU trains, independent of contraction intensity, was computed, resulting in the distribution having a positive skew for the old compared with young (Fig. 5). In the BF, the percentage of MU trains discharging at <15 Hz was 61.4% in the young and 77.2% in the old, whereas in the SS, the percentage of MU trains discharging at <15 Hz was 35.8% in the young and 85.4% in the old. The effect of age on the distribution of all MU trains discharging at <15 Hz showed that the BF had a 1.3-fold age-related increase, whereas the SS had a 2.4-fold increase.
Fig. 4.
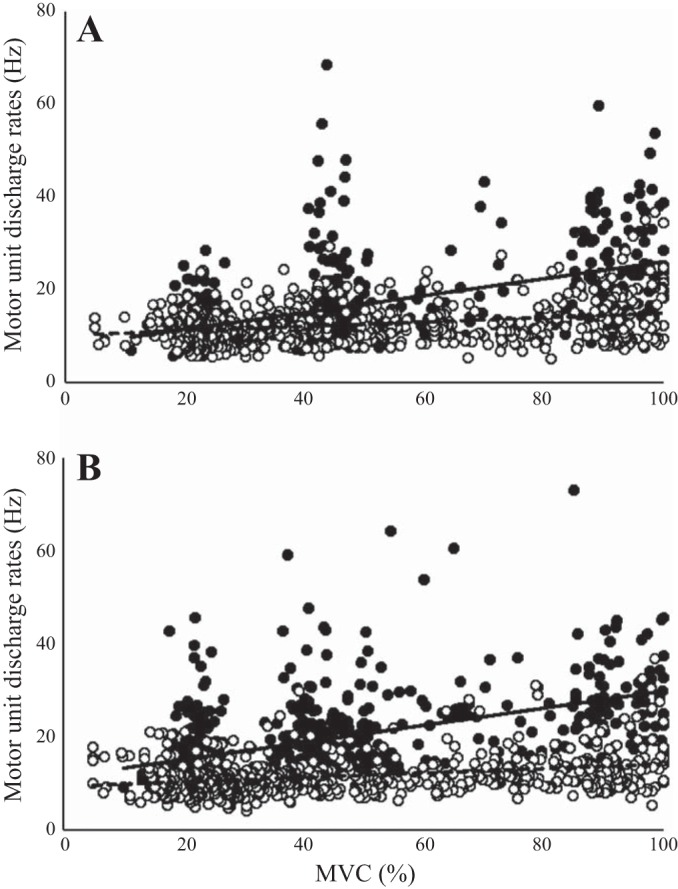
Scatterplots of 2,574 individual motor unit (MU) trains with 1,196 from the young (closed circles) and 1,378 from the old (open circles). A: scatterplot of 1,248 MU trains in the biceps femoris (BF) between young and old. Linear regression equations for young and old are discharge rate (Hz) = 0.18·torque (percent maximal voluntary contraction, MVC%) + 7.76 (solid line) and discharge rate (Hz) = 0.05·torque (MVC%) + 10.28 (dashed line), respectively. B: scatterplot of 1,365 MU trains in the semimembranosus-semitendinosus (SS) between young and old. Linear regression equations for young and old are discharge rate (Hz) = 0.18·torque (MVC%) + 11.48 (solid line) and discharge rate (Hz) = 0.05·torque (MVC%) + 9.34 (dashed line), respectively. For both A and B, there were significant effects for age (P < 0.001) and contraction intensity (P < 0.001) on discharge rates; n = 20.
Fig. 5.
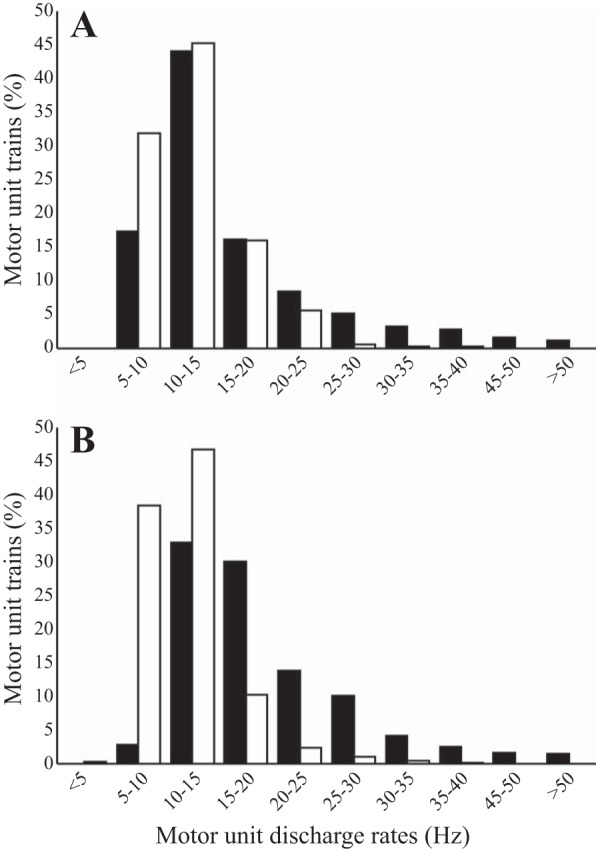
Histograms of the distribution of motor unit (MU) discharge rates from all voluntary contraction levels in young and old in the biceps femoris (A) and semimembranosus-semitendinosus (B) muscle groups. Filled bars represent the young and open bars represent the old. Percentages of total MU trains are sorted into 5-Hz bins; n = 20.
By age group, the mean MU discharge rates were 17.6 Hz for the young and 11.9 Hz for the old, resulting in the old being 32% lower in mean rates than the young. When MU discharge rates were categorized on the basis of 25, 50, and 100% MVC (Table 2), there were significant main effects of contraction intensity (F2,2,317 = 467.9, P < 0.001), age (F1,2,317 = 714, P < 0.001), and muscle group (i.e., SS vs. BF; F1,2,317 = 22.7, P < 0.001). There was a statistically significant interaction effect of age and contraction intensity on MU discharge rate frequency (F2,2,317 = 219.8, P < 0.001). Furthermore, there was no significant interaction effect for muscle group and contraction intensity on MU discharge rates [F2,2,317 = 0.514, P = not significant (N.S.)].
Table 2.
Surface and intramuscular EMG of the BF and SS at 25, 50, and 100% voluntary activation
| Parameter | Young | Old | ||||
|---|---|---|---|---|---|---|
| Targeted MVC, % | 25 | 50 | 100 | 25 | 50 | 100 |
| MVC, % | 23.0 ± 3.9 | 45.9 ± 5.15 | 93.2 ± 4.2 | 25.5 ± 5.9 | 46.9 ± 7.2 | 94.0 ± 3.7 |
| BF | ||||||
| EMG-RMS, % | 24.9 ± 10.8 | 45.6 ± 13.3 | 100 ± 0 | 24.1 ± 9.6 | 51.2 ± 12.9 | 100 ± 0 |
| Discharge rate, Hz | 12.0 ± 3.7 | 16.1 ± 8.6 | 26.1 ± 10.1 | 11.5 ± 3.3 | 12.8 ± 4.0* | 15.6 ± 6.4* |
| No. of MU trains | 226 | 208 | 96 | 299 | 151 | 131 |
| No. of ISIs | 10.3 ± 8.4 | 9.3 ± 6.4 | 7.8 ± 5.8 | 9.5 ± 6.8 | 8.0 ± 4.6 | 10.1 ± 7.6 |
| Coefficient of variation, % | 11.7 ± 5.3 | 14.1 ± 5.9 | 15.7 ± 5.5 | 11.4 ± 4.9 | 13.1 ± 6.1 | 11.9 ± 5.3 |
| SS | ||||||
| EMG-RMS, % | 21.7 ± 5.7 | 40.8 ± 10.3 | 100 ± 0 | 29.4 ± 7.9 | 50.0 ± 11.9 | 100 ± 0 |
| Discharge rate, Hz | 15.6 ± 6.3† | 20.3 ± 7.8† | 27.9 ± 7.8 | 10.5 ± 3.2* | 11.2 ± 3.3* | 15.3 ± 5.9* |
| No. of MU trains | 308 | 200 | 87 | 334 | 156 | 122 |
| No. of ISIs | 10.3 ± 8.7 | 10.1 ± 7.0 | 9.3 ± 7.1 | 9.7 ± 7.1 | 9.4 ± 6.6 | 10.1 ± 9.6 |
| Coefficient of variation, % | 12.9 ± 5.8 | 14.0 ± 6.2 | 15.4 ± 5.1 | 11.8 ± 5.6 | 12.2 ± 5.2 | 11.5 ± 4.3 |
Values are means ± SD from electromyography (EMG) of the biceps femoris (BF) and semimembranosus-semitendinosus (SS) muscles in the prone position of young (n = 10) and old men (n = 10). MVC, maximal voluntary isometric contraction; EMG-RMS, electromyographic root-mean-square; ISI, interspike-intervals; MU, motor unit. For between age group interactions,
P < 0.001 for interactions between age groups.
P < 0.001 for interactions between muscle groups within the same age group.
Both groups were equally capable of targeting various MVC intensities (Table 2). For surface EMG at 25 and 50% MVC, there was a significant effect of contraction intensity (F1, 79 = 76.8, P < 0.001) with no significant interaction effect of age and contraction intensity (F1,79 = 0.626, P = N.S.) or muscle and contraction intensity (F1,79 = 0.638, P = N.S.). When interactions between age, muscle group and contraction intensity were compared, surface EMG was not different between muscle or age groups (Table 2). In the BF and SS, mean MU discharge rates in the old were lower at 50 and 100% MVC than in the young, but only the SS group had lower mean discharge rates at 25% MVC (Table 2). To explore whether age-related differences in discharge rates were larger for the BF or SS, effect sizes were calculated for each contraction intensity. The effect size was greater in the SS at every contraction intensity (Cohen’s d = 1.01, 1.52, and 1.82 at 25, 50, and 100% MVC, respectively) compared with that in the BF (Cohen’s d = 0.14, 0.49, and 1.24 at 25, 50 and 100% MVC, respectively).
DISCUSSION
Neuromuscular properties of the human hamstrings, which included measures of voluntary strength, evoked contractility, and MU discharge rates, were diminished in old compared with young adult men (Tables 1 and 2). Within the concept of age-related MU remodeling, no difference in relative surface EMG concurrent with significantly lower MU discharge rates may indicate that graded force generation in the hamstrings of old men is more dependent on MU recruitment. In addition, the slower contractile properties of the old men are appropriate for lower MU rates of excitation. Moreover, MU discharge rates of the medial muscles (SS) had a greater age-related effect compared with the those of the lateral muscles (BF). These results contribute an important addition to the characterization of neuromuscular properties in lower limb muscles and when integrated with results described previously from other muscle groups, highlight that neuromuscular properties of lower limb muscles are affected differently by the aging process. Furthermore, these differences can vary within muscular portions of the same functional group.
Voluntary strength of the hamstrings at MVC was ~50% less in the old compared with the young (Table 1). This age-related deficit in strength is likely due to less excitable muscle mass (Vandervoort and McComas 1986), as demonstrated from other studies that have shown decreased skeletal muscle mass and infiltration of noncontractile tissue in aged hamstrings (Overend et al. 1992a; Yoshiko et al. 2017). However, hamstring voluntary activation at MVC was ~5% less in the old group compared with the young (Table 1), indicating that other factors contributed to the 50% strength difference. This is not a unique finding, and based on results from other limb muscles, a ~5% difference in voluntary activation between age groups may be expected due to greater variability in the old (Jakobi and Rice 2002; Rozand et al. 2017). There are many possible spinal and supraspinal factors that could explain age-related effects on voluntary activation, and the physiological significance of a ~5% difference with the use of this technique is unclear (Rozand et al. 2017). Regardless of these factors, strength loss with age seems mainly a result of diminished contractile capacity at the muscle with secondary contributions due to activation impairment.
Evoked twitch tension in the hamstrings of the old was lower in amplitude and slower to generate tension compared with that in the young (Table 1), which may be explained partly by muscle mass differences. When rates of torque development and relaxation were compared between age groups, these differences showed that the old were reduced by ~80% (Table 1). There are numerous muscle specific adaptations related to aging, including MU remodeling, muscle fiber membrane dysfunction, decreased sarcoplasmic reticulum function, altered calcium ion kinetics, and changes in connective tissue elements, implicated in the explanation of diminished evoked twitch properties (Hepple and Rice 2016; Narici et al. 2008). From in vitro animal models, the contractile speed of muscle fibers matches MU discharge rates (Kernell 1979); however, this matching was not found with muscles composed of different fiber type distributions in humans (Bellemare et al. 1983). In the hamstrings, we report slowed contractile speed concomitant with lower discharge rates in old compared with young men; however this relationship is not observed in all human muscles (Dalton et al. 2009, 2010; Kirk et al. 2016; Roos et al. 1999). In the thigh, both the anterior (Roos et al. 1999) and posterior compartments (Table 1) have significant reductions in stimulated contractile speed in old compared with young men. Although fiber-type distributions are not importantly different between the vastus medialis and hamstrings with ~50% type I fiber distribution in both (Garrett et al. 1984; Johnson et al. 1973), the hamstrings have significantly lower MU discharge rates in old men, which are opposing findings to the vastus medialis portion of the quadriceps (Roos et al. 1999). Unlike muscles of the thigh, the tibialis anterior is ~70% type I (Johnson et al. 1973) and is the only muscle tested in the leg to have shown age-related reductions in MU discharge rates from moderate to high contraction intensities (Connelly et al. 1999). In the leg, the soleus has ~80% type I and gastrocnemii have ~50% type I fibers (Johnson et al. 1973) and have not demonstrated age-related reductions in MU discharge rates at moderate to maximal force intensities (Dalton et al. 2009; Kirk et al. 2016). On the basis of these investigations and now with the addition of the hamstrings, it becomes evident that other factors in addition to fiber composition and contraction speed are important in determining age-related alterations in force generation.
The hamstring muscles exhibit prominent age-related reductions in MU discharge rates (Fig. 4) indicative of altered MU facilitation and age-related remodeling (Hepple and Rice 2016; Webber et al. 2009). Although there were lower discharge rates at the submaximal intensities of 25 and 50% MVC, the relative surface EMG signal was not different between age groups (Table 2). Within the concept of age-related MU remodeling, no difference in relative surface EMG concurrent with significantly lower MU discharge rates may indicate that graded force generation in the hamstrings of old men is more dependent on MU recruitment rather than rate coding compared with that in young men. Therefore, it is reasonable that the hamstring MU pool of the old is composed of a greater percentage of slower MUs with expanded innervation (McNeil et al. 2005), and slower contractile properties in conjunction with lower MU discharge rates indicate that MU recruitment may be of greater importance than rate coding for force gradation (Erim et al. 1999). This concept is illustrated by the positive skew of the MU distributions in the old compared with the young (Fig. 3). Furthermore, the intrinsic slowed contractile properties of the old men result in a shift to the left of the normalized force-frequency relationship with significant differences during the important steep portion of the curve between 8 and 20 Hz (Table 1 and Fig. 3). For graded force generation, this may be functionally beneficial in relation to the lower MU discharge rates in the old.
Age-related neuromuscular adaptations appear to be muscle dependent, potentially influenced by muscle function, MU structure, and anatomical location. In muscles of the lower limb, the hamstrings support findings from another flexor, the tibialis anterior, that include age-related reductions in MU discharge rates in association with reduced contractile properties and voluntary strength (Christie and Kamen 2010; Connelly et al. 1999). Mechanistic insight from animal and in vitro preparations comparing limb flexors and extensors suggest that in aging, the loss of proprioceptive sensory neurons and afferent innervation occurs at different rates between flexors and extensors (Vaughan et al. 2017). Furthermore, extensor motor neurons are more plastic to activity-based interventions following injury (Chopek et al. 2015), and there is enhanced presynaptic modulation in monosynaptic reflexes of flexors compared with extensors (Chopek et al. 2013). Structurally, the MU territory in the semitendinosus and BF contain muscle fibers arranged in longitudinal arrays with transverse bands of endplates across the middle of the parallel fibers likely to reduce the time of whole muscle excitation (Manzano and McComas 1988). In humans, hamstring MU activity is influenced by muscle length and compartment (Kirk and Rice 2017), and possible additional influences of MU organization, afferent activity to the motor neuron (Macefield et al. 1993), and flexor muscle characteristics may further impact age-related changes.
Within the hamstrings there were greater age-related differences of MU discharge rates in the medial (SS) group. The SS had a greater age-related effect size at each contraction intensity with a greater increase in the count of MU discharge rates <15 Hz (Fig. 5). These results contrast with the anterior muscles of the thigh, in which MU rates of the lateral musculature (vastus lateralis) underwent an age-related reduction (Kamen and Knight 2004), whereas the medial group (vastus medialis) had no age-related difference (Roos et al. 1999). Furthermore, in the posterior leg, there is no difference between MU discharge rates in the medial and lateral muscles of the gastrocnemii (Kirk et al. 2016). Despite both portions of the hamstrings having similar function to extend the hip and flex the knee, it is probable that the type of functional demands placed on a muscle throughout the aging process are important. One example is that the BF exerts more force than the SS during lengthening contractions due to increased muscle displacement (Dolman et al. 2014).
The strengths of this investigation are in comparing multiple parameters of MU properties in two groups of healthy recreationally active men (Booth et al. 2017) separated in age by ~50 yr. A main limitation of the present investigation is that it is cross-generational and not longitudinal in design. Furthermore, because of the deep anatomical location of the sciatic nerve, we were unable to perform direct nerve stimulation.
In summary, age-related differences of the hamstrings demonstrate important decreases in voluntary strength, muscle contractility, and MU discharge rates during a range of voluntary isometric contraction intensities. In comparisons of muscles within the hamstrings, the SS had greater age-related reductions in MU discharge rates than the BF. These findings help advance the understanding of the human neuromuscular system, providing evidence that MU populations with discrete functional regions may be differentially affected by the aging process.
GRANTS
This work is supported by the Natural Sciences and Engineering Research Council of Canada.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.A.K. and C.L.R. conceived and designed research; E.A.K., K.J.G., and C.L.R. performed experiments; E.A.K. analyzed data; E.A.K., K.J.G., and C.L.R. interpreted results of experiments; E.A.K. prepared figures; E.A.K., K.J.G., and C.L.R. drafted manuscript; E.A.K., K.J.G., and C.L.R. edited and revised manuscript; E.A.K., K.J.G., and C.L.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank individuals from the Retirement Research Association and Canadian Centre for Activity and Ageing for their participation and enthusiasm.
REFERENCES
- Alway SE, Mohamed JS, Myers MJ. Mitochondria initiate and regulate sarcopenia. Exerc Sport Sci Rev 45: 58–69, 2017. doi: 10.1249/JES.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellemare F, Woods JJ, Johansson R, Bigland-Ritchie B. Motor-unit discharge rates in maximal voluntary contractions of three human muscles. J Neurophysiol 50: 1380–1392, 1983. doi: 10.1152/jn.1983.50.6.1380. [DOI] [PubMed] [Google Scholar]
- Booth FW, Roberts CK, Thyfault JP, Ruegsegger GN, Toedebusch RG. Role of inactivity in chronic diseases: evolutionary insight and pathophysiological mechanisms. Physiol Rev 97: 1351–1401, 2017. doi: 10.1152/physrev.00019.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopek JW, MacDonell CW, Power KE, Gardiner K, Gardiner PF. Removal of supraspinal input reveals a difference in the flexor and extensor monosynaptic reflex response to quipazine independent of motoneuron excitation. J Neurophysiol 109: 2056–2063, 2013. doi: 10.1152/jn.00405.2012. [DOI] [PubMed] [Google Scholar]
- Chopek JW, Sheppard PC, Gardiner K, Gardiner PF. Serotonin receptor and KCC2 gene expression in lumbar flexor and extensor motoneurons posttransection with and without passive cycling. J Neurophysiol 113: 1369–1376, 2015. doi: 10.1152/jn.00550.2014. [DOI] [PubMed] [Google Scholar]
- Christie A, Kamen G. Short-term training adaptations in maximal motor unit firing rates and afterhyperpolarization duration. Muscle Nerve 41: 651–660, 2010. doi: 10.1002/mus.21539. [DOI] [PubMed] [Google Scholar]
- Connelly DM, Rice CL, Roos MR, Vandervoort AA. Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiol (1985) 87: 843–852, 1999. doi: 10.1152/jappl.1999.87.2.843. [DOI] [PubMed] [Google Scholar]
- Dalton BH, Allen MD, Power GA, Vandervoort AA, Rice CL. The effect of knee joint angle on plantar flexor power in young and old men. Exp Gerontol 52: 70–76, 2014. doi: 10.1016/j.exger.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Dalton BH, Harwood B, Davidson AW, Rice CL. Triceps surae contractile properties and firing rates in the soleus of young and old men. J Appl Physiol (1985) 107: 1781–1788, 2009. doi: 10.1152/japplphysiol.00464.2009. [DOI] [PubMed] [Google Scholar]
- Dalton BH, Jakobi JM, Allman BL, Rice CL. Differential age-related changes in motor unit properties between elbow flexors and extensors. Acta Physiol (Oxf) 200: 45–55, 2010. doi: 10.1111/j.1748-1716.2010.02100.x. [DOI] [PubMed] [Google Scholar]
- Dalton BH, McNeil CJ, Doherty TJ, Rice CL. Age-related reductions in the estimated numbers of motor units are minimal in the human soleus. Muscle Nerve 38: 1108–1115, 2008. doi: 10.1002/mus.20984. [DOI] [PubMed] [Google Scholar]
- Doherty TJ, Vandervoort AA, Brown WF. Effects of ageing on the motor unit: a brief review. Can J Appl Physiol 18: 331–358, 1993. doi: 10.1139/h93-029. [DOI] [PubMed] [Google Scholar]
- Dolman B, Verrall G, Reid I. Physical principles demonstrate that the biceps femoris muscle relative to the other hamstring muscles exerts the most force: implications for hamstring muscle strain injuries. Muscles Ligaments Tendons J 4: 371–377, 2014. [PMC free article] [PubMed] [Google Scholar]
- Edwards RH, Young A, Hosking GP, Jones DA. Human skeletal muscle function: description of tests and normal values. Clin Sci Mol Med 52: 283–290, 1977. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Duchateau J. Rate coding and the control of muscle force. Cold Spring Harb Perspect Med 7: 029702, 2017. doi: 10.1101/cshperspect.a029702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erim Z, Beg MF, Burke DT, De Luca CJ. Effects of aging on motor-unit control properties. J Neurophysiol 82: 2081–2091, 1999. doi: 10.1152/jn.1999.82.5.2081. [DOI] [PubMed] [Google Scholar]
- Garrett WE Jr, Califf JC, Bassett FH 3rd. Histochemical correlates of hamstring injuries. Am J Sports Med 12: 98–103, 1984. doi: 10.1177/036354658401200202. [DOI] [PubMed] [Google Scholar]
- Hales JP, Gandevia SC. Assessment of maximal voluntary contraction with twitch interpolation: an instrument to measure twitch responses. J Neurosci Methods 25: 97–102, 1988. doi: 10.1016/0165-0270(88)90145-8. [DOI] [PubMed] [Google Scholar]
- Hepple RT, Rice CL. Innervation and neuromuscular control in ageing skeletal muscle. J Physiol 594: 1965–1978, 2016. doi: 10.1113/JP270561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortobágyi T, DeVita P. Muscle pre- and coactivity during downward stepping are associated with leg stiffness in aging. J Electromyogr Kinesiol 10: 117–126, 2000. doi: 10.1016/S1050-6411(99)00026-7. [DOI] [PubMed] [Google Scholar]
- Jakobi JM, Rice CL. Voluntary muscle activation varies with age and muscle group. J Appl Physiol (1985) 93: 457–462, 2002. doi: 10.1152/japplphysiol.00012.2002. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci 18: 111–129, 1973. doi: 10.1016/0022-510X(73)90023-3. [DOI] [PubMed] [Google Scholar]
- Kamen G, Knight CA. Training-related adaptations in motor unit discharge rate in young and older adults. J Gerontol A Biol Sci Med Sci 59: 1334–1338, 2004. doi: 10.1093/gerona/59.12.1334. [DOI] [PubMed] [Google Scholar]
- Kernell D. Rhythmic properties of motoneurones innervating muscle fibres of different speed in m. gastrocnemius medialis of the cat. Brain Res 160: 159–162, 1979. doi: 10.1016/0006-8993(79)90612-7. [DOI] [PubMed] [Google Scholar]
- Kirk EA, Copithorne DB, Dalton BH, Rice CL. Motor unit firing rates of the gastrocnemii during maximal and sub-maximal isometric contractions in young and old men. Neuroscience 330: 376–385, 2016. doi: 10.1016/j.neuroscience.2016.05.059. [DOI] [PubMed] [Google Scholar]
- Kirk EA, Rice CL. Contractile function and motor unit firing rates of the human hamstrings. J Neurophysiol 117: 243–250, 2017. doi: 10.1152/jn.00620.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YP, Lin YC, Pandy MG. Effects of step length and step frequency on lower-limb muscle function in human gait. J Biomech 57: 1–7, 2017. doi: 10.1016/j.jbiomech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Luff AR. Age-associated changes in the innervation of muscle fibers and changes in the mechanical properties of motor units. Ann N Y Acad Sci 854: 92–101, 1998. doi: 10.1111/j.1749-6632.1998.tb09895.x. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Gandevia SC, Bigland-Ritchie B, Gorman RB, Burke D. The firing rates of human motoneurones voluntarily activated in the absence of muscle afferent feedback. J Physiol 471: 429–443, 1993. doi: 10.1113/jphysiol.1993.sp019908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano G, McComas AJ. Longitudinal structure and innervation of two mammalian hindlimb muscles. Muscle Nerve 11: 1115–1122, 1988. doi: 10.1002/mus.880111103. [DOI] [PubMed] [Google Scholar]
- McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old and very old men. Clin Neurophysiol 116: 1342–1347, 2005. doi: 10.1016/j.clinph.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Morat T, Gilmore KJ, Rice CL. Neuromuscular function in different stages of sarcopenia. Exp Gerontol 81: 28–36, 2016. doi: 10.1016/j.exger.2016.04.014. [DOI] [PubMed] [Google Scholar]
- Moses KP, Banks JC, Nava PB, Petersen D. Atlas of Clinical Gross Anatomy. Philadelphia, PA: Elsevier, 2005. [Google Scholar]
- Narici MV, Maffulli N, Maganaris CN. Ageing of human muscles and tendons. Disabil Rehabil 30: 1548–1554, 2008. doi: 10.1080/09638280701831058. [DOI] [PubMed] [Google Scholar]
- Overend TJ, Cunningham DA, Kramer JF, Lefcoe MS, Paterson DH. Knee extensor and knee flexor strength: cross-sectional area ratios in young and elderly men. J Gerontol 47: M204–M210, 1992b. doi: 10.1093/geronj/47.6.M204. [DOI] [PubMed] [Google Scholar]
- Overend TJ, Cunningham DA, Paterson DH, Lefcoe MS. Thigh composition in young and elderly men determined by computed tomography. Clin Physiol 12: 629–640, 1992a. doi: 10.1111/j.1475-097X.1992.tb00366.x. [DOI] [PubMed] [Google Scholar]
- Palmer TB, Thompson BJ. Influence of age on passive stiffness and size, quality, and strength characteristics. Muscle Nerve 55: 305–315, 2017. doi: 10.1002/mus.25231. [DOI] [PubMed] [Google Scholar]
- Power GA, Allen MD, Gilmore KJ, Stashuk DW, Doherty TJ, Hepple RT, Taivassalo T, Rice CL. Motor unit number and transmission stability in octogenarian world class athletes: Can age-related deficits be outrun? J Appl Physiol 121: 1013–1020, 2016. doi: 10.1152/japplphysiol.00149.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich C, O’Brien GL, Cafarelli E. Probabilities associated with counting average motor unit firing rates in active human muscle. Can J Appl Physiol 23: 87–94, 1998. doi: 10.1139/h98-006. [DOI] [PubMed] [Google Scholar]
- Roos MR, Rice CL, Connelly DM, Vandervoort AA. Quadriceps muscle strength, contractile properties, and motor unit firing rates in young and old men. Muscle Nerve 22: 1094–1103, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- Rozand V, Senefeld JW, Hassanlouei H, Hunter SK. Voluntary activation and variability during maximal dynamic contractions with aging. Eur J Appl Physiol 117: 2493–2507, 2017. doi: 10.1007/s00421-017-3737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A, Silder A, Heiderscheit B, Mahoney J, Thelen DG. Differences in lower-extremity muscular activation during walking between healthy older and young adults. J Electromyogr Kinesiol 19: 1085–1091, 2009. doi: 10.1016/j.jelekin.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BJ, Conchola EC, Stock MS. Effects of age and muscle action type on acute strength and power recovery following fatigue of the leg flexors. Age (Dordr) 37: 111, 2015. doi: 10.1007/s11357-015-9845-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G, Gorman RB, Gandevia SC. Measurement and reproducibility of strength and voluntary activation of lower-limb muscles. Muscle Nerve 29: 834–842, 2004. doi: 10.1002/mus.20027. [DOI] [PubMed] [Google Scholar]
- Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci 34: 213–219, 1977. doi: 10.1016/0022-510X(77)90069-7. [DOI] [PubMed] [Google Scholar]
- Vandervoort AA, McComas AJ. Contractile changes in opposing muscles of the human ankle joint with aging. J Appl Physiol (1985) 61: 361–367, 1986. doi: 10.1152/jappl.1986.61.1.361. [DOI] [PubMed] [Google Scholar]
- Vaughan SK, Stanley OL, Valdez G. Impact of aging on proprioceptive sensory neurons and intrafusal muscle fibers in mice. J Gerontol A Biol Sci Med Sci 72: 771–779, 2017. doi: 10.1093/gerona/glw175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber SC, Porter MM, Gardiner PF. Modeling age-related neuromuscular changes in humans. Appl Physiol Nutr Metab 34: 732–744, 2009. doi: 10.1139/H09-052. [DOI] [PubMed] [Google Scholar]
- Winter DA, Patla AE, Frank JS, Walt SE. Biomechanical walking pattern changes in the fit and healthy elderly. Phys Ther 70: 340–347, 1990. doi: 10.1093/ptj/70.6.340. [DOI] [PubMed] [Google Scholar]
- Yoshiko A, Hioki M, Kanehira N, Shimaoka K, Koike T, Sakakibara H, Oshida Y, Akima H. Three-dimensional comparison of intramuscular fat content between young and old adults. BMC Med Imaging 17: 12, 2017. doi: 10.1186/s12880-017-0185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]