Abstract
The pursuit of a physiological indicator of noxious stimulation is desirable as it has the potential to provide mechanistic information regarding acute pain and may ultimately improve pain management strategies. Currently, there are no specific neurophysiological markers of pain to evaluate treatments. Recent attempts to identify neural correlates of pain have focused on different neuroimaging modalities. The purpose of this review is to discuss common neuroimaging techniques and findings thus far.
Keywords: biomarker, neuroimaging, nociception, pain
INTRODUCTION
Our current knowledge of mechanisms underlying pain has been largely derived from animal studies. Scientists have utilized advanced cellular, molecular, and genetic techniques to provide new insights into the generation, conduction, and processing of noxious stimuli. To translate this knowledge to humans, researchers have applied several noninvasive neuroimaging techniques [i.e., electroencephalography (EEG), magnetic resonance imaging (MRI), magnetoencephalography (MEG)]. Results from these investigations show potential in identifying physiological measures/patterns of noxious stimulation that may complement self-reported pain ratings. Practically, these developments hold promise in providing biological markers to evaluate treatment efficacy of current and novel pain management strategies. The goal of this review is to summarize seminal findings of recent research in this area.
EEG: GAMMA BAND OSCILLATIONS
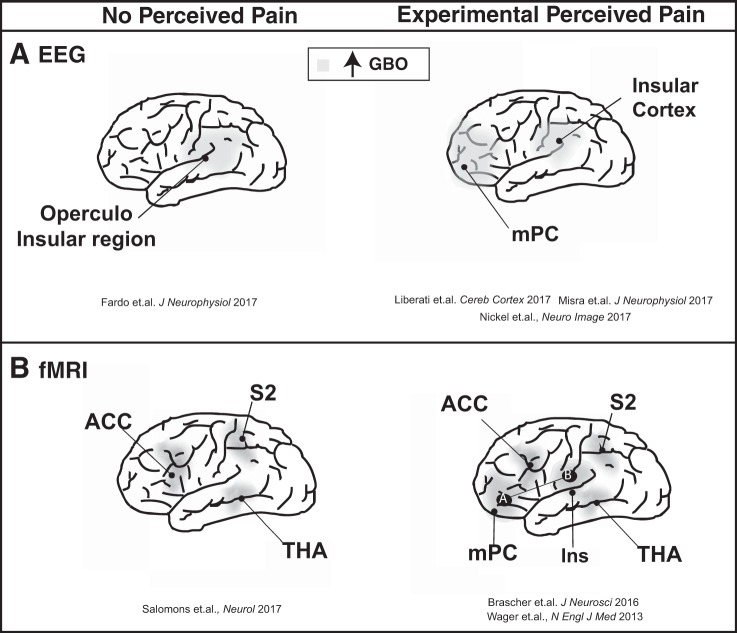
At more than 150 yr old, EEG (a noninvasive electrophysiological technique) represents one of the most extensively applied methods of assessing areas of the brain suspected to be involved with pain. Through source estimation of electrical activity, this method can pinpoint active brain regions during pain processing. Recent and novel lines of investigations related to pain have largely focused on gamma band oscillations (GBO). Based on the physics of oscillatory phenomena, low- and high-frequency neural oscillations can be linked to neural mechanisms occurring at distinct temporal and spatial scales (Fardo et al. 2017). Low-frequency brain oscillations (i.e., delta, theta, and alpha) reflect long-range communication between brain areas and are crucial in functional integration. Contrasting, high-frequency brain oscillations (i.e., gamma) are more rapid and focal (Fardo et al. 2017). Notable for their application of a long duration noxious thermal stimuli (tonic heat), Nickel and colleagues (Nickel et al. 2017) reported higher pain intensity ratings [using the 0–10 numeric rating scale (NRS) where 0 represents no pain and 10 represents the worst pain possible] corresponding with stronger GBO in the medial prefrontal cortex (mPC) for both right/left hand stimulation (Fig. 1A). In contrast to pain intensity, stimulus intensity was associated with a decrease of alpha and beta oscillations in the sensorimotor cortex contralateral to the stimulated hand. These observations are ground breaking as they suggest a distinction between the cortical representation of pain, which is spatially independent (as cortical representation is the same for right/left hand stimulation), and stimuli intensity, which is spatially dependent (cortical representation is contralateral to the stimulated hand). These results display cortical representation for a key symptom of chronic pain conditions (i.e., dissociation between perceived pain and presented pain) (Nickel et al. 2017). Pain perception from shorter bouts of noxious thermal impulses (4 s) has further been shown to be associated with an increase in GBO in the mPC (Misra et al. 2017). Adopting a novel machine learning approach, these authors revealed that differences in gamma and theta power in the mPC and decreases in beta oscillations in the contralateral sensorimotor cortex were a key EEG feature distinguishing low and high pain responders (~90% accuracy for classification).
Fig. 1.
Pain-related and unrelated brain responses using different neuroimaging: EEG (A) and functional MRI (B). ACC, anterior cingulate cortex; fMRI, functional MRI; GBO, gamma band oscillations; Ins, insula (B); mPC, medial prefrontal cortex (A); S2, secondary somatosensory cortex; THA, thalamus.
Importantly, the fact that engagement of a brain area is associated with the perception of pain does not imply that the observed cortical activity is exclusively involved in the perception of pain and not other sensory processes. A notable limitation of both studies is the lack of a control condition, whereby changes in gamma power are examined in response to nonnoxious yet still salient stimuli. The notion that certain neural responses are attributed to stimulation per se rather than specific to the unique qualities of pain cannot be ruled out (Davis et al. 2017). The importance of confirming pain specificity was recently highlighted by an EEG study investigating the effects of pain on motor preparation in the brain. Pain was associated with reduced brain activity related to movement preparation. However, the application of a nonpainful task revealed that painful and nonpainful stimuli could similarly influence motor preparation in the human brain (Postorino et al. 2017).
Others have examined GBO in responses to brief pulses (50 ms) of noxious laser stimuli. In a recent and seminal example of such a study, GBO were recorded from intracerebral electrodes implanted in patients with intractable epilepsy (Liberati et al. 2017). Compared with surface electrodes, intracerebral recording provides an unusual opportunity to measure local field potentials in neurons immediately adjacent to the electrode with high temporal resolution. In addition, the implanted electrodes overcome the common limitation of spatial resolution present when using surface electrodes. In response to laser stimulation, the analysis revealed enhanced GBO in the insular cortex. This increase was markedly greater when compared with nonnociceptive yet equally intense stimuli (i.e., vibrotactile, auditory, and visual), suggesting that GBO in the human insula reflect cortical activity that is preferentially involved in nociception. The comparison with innocuous stimuli is important as it aims to assess the specificity of the physiological response. Nevertheless, it remains unknown whether GBO are specific to painful experiences or simply the thermal information. To address this, Fardo and colleagues (2017) examined central processing of cold thermosensation, employing a nonnoxious cold stimulus while recording MEG and EEG. A main finding of the study was that innocuous cold-related activity is associated with increased GBO in bilateral operculo-insular regions (Fardo et al. 2017). This may suggest that GBO play a key role in mediation of local sensory and attentional processing of cold-related signals—challenging the specificity of GBO in the insular cortex in relation to pain perception exclusively.
The term “pain matrix” has been coined to describe brain regions known to be involved in pain experiences. As such, this term implies that the activity in these brain regions is related to the reporting of pain and does not occur in the absence of pain. However, the involvement of these areas in nonpainful stimuli processing has been demonstrated and thus the term pain matrix has become redundant. Although numerous correlates of pain have been proposed, none have proven to be specific and sensitive enough to be used in treatment efficacy evaluation and/or as biomarkers for clinical trials. A recent placebo-controlled clinical trial tested the effects of analgesics during cold-tonic pain. Relying on the self-report rating, the results found a 65% and 55% decrease in pain with oxycodone (opioid), and venlafaxine [serotonin and norepinephrine reuptake inhibitor (SNRI)], respectively, as opposed to a 20% decrease with placebo (Lelic et al. 2017). In healthy subjects treated with oxycodone, time-frequency EEG analysis revealed decreases in delta and theta frequency activation in several brain structures (i.e., insula, inferior/middle frontal-gyrus, and superior temporal-gyrus). Venlafaxine treatment was found to be associated with alpha activity in the same brain areas (Lelic et al. 2017). Based on their findings, the authors state that “a co-administration of an opioid and an SNRI in the clinic to treat pain could be justifiable” (Lelic et al. 2017, p. 463). Considering that this was the first time these localized frequency bands (and changes therein) were linked to pain and its relief, their validity as an objective neural marker is yet to be determined. Are these changes specific to pain relief or an overall change in perception? Are these changes also observed in chronic pain patients? Identification of brain-based markers (i.e., localized frequency bands as a marker) for pain require technological advancements, large-scale data acquisition across diverse groups and strict application of standard evidence (Davis et al. 2017).
FUNCTIONAL MRI: FROM PAIN CENTERS TO PAIN NETWORKS
The noninvasive functional MRI (fMRI) technique takes advantage of the fact that brain activation (i.e., increased neural activity) leads to elevation in blood flow and oxyhemoglobin, which in turn results in increased signal intensity on MRI (BOLD; blood oxygen level dependent). According to previous investigations, stimuli intensity and perceptions of pain have a meaningful positive correlation with the BOLD activation in cortical and subcortical brain areas. Specifically, Bräscher and colleagues (2016) used phasic contact heat stimuli and warm nonnoxious stimuli in two conditions: controllable pain where participants were instructed to maintain their pain perception constant and had full instrumental control of the temperature, and uncontrollable pain where the temperature profiles of the previous controllable trials were replayed unbeknownst to the subject. This was performed to investigate activation in pain-processing brain regions. A positive correlation was evident between increased pain ratings and activity of the anterior cingulate cortex (ACC), insula, and thalamus. For both conditions, painful stimulation was associated with significantly more activation in pain-processing brain regions than warm stimulation. Furthermore, the connectivity between the anterior insula and mPC was increased in subjects with higher pain ratings (Fig. 1B) (Bräscher et al. 2016). The reciprocal assumption that an experience of pain can be solely inferred by observing activity in a set of brain regions is controversially debated. Nevertheless, this assumption, has served as a building block for the development of machine learning techniques to identify dynamic patterns of activity, rather than increased activation in a single area. In the recent years, these techniques have been implemented to analyze whole-brain or regional fMRI data aiming to identify complex patterns of BOLD signal changes that underlie pain in experimental settings. Combining fMRI and machine learning approaches, Wager et al. (2013) identified a pattern of fMRI activity across brain regions that consistently responded to experimental nociceptive pain. A set of structures (i.e., thalamus, insula, somatosensory cortex, ACC) have been related to acute pain intensity perception within and between individuals (Fig. 1B). This pattern of activation [so-called “neurological signature of pain” (NSP)] was found to be highly predictive (accuracy 93%) (Wager et al. 2013) and responsive to changes in the stimulus intensity (Woo et al. 2015). Based on these findings, this study was considered as a significant step toward a neural marker of pain. Thus far, studies investigating NSP have done so mainly in healthy brains, in response to experimental pain, and the activation has been related to perceived pain intensity. Bräscher and colleagues tested the correspondence of painful stimulation with the NSP. Their study revealed that the NSP response increased significantly for painful compared with warm stimulation intensities (Bräscher et al. 2016). Intriguingly, Salomons and colleagues (2016) challenged this relationship by demonstrating intact NSP responses (i.e., comparable to healthy controls) in individuals congenitally unable to experience pain (i.e., genetic mutation resulting in pain insensitivity through an impaired peripheral drive that leaves tactile percepts fully intact). Furthermore, Woo and colleagues (2015) provided evidence that distinct brain networks mediate the subjective changes in pain that result from nociceptive input and individual cognitive modulation. Changes in the noxious stimulus intensity have been found to be moderated by the NSP (Woo et al. 2015). In contrast, individual cognitive modulation effects on pain perception were mediated through functional connections between the nucleus accumbens and ventromedial prefrontal cortex—a pathway known to be involved in emotions associated with pain. Conclusively, these observations reinforce the need for caution in using the NSP as a universal indicator of pain.
As previously discussed, the notion that certain neural responses are attributed to general stimulation rather than qualities unique to pain cannot be ruled out. As Kucyi and Davis (2015) suggested, acute and chronic pain often engages resting state networks (i.e., the default-mode, salience, and somatosensory networks) that are involved in attentional, cognitive emotional, and sensory function. As painful stimuli are inherently salient, they cannot be completely dissociated. Although there is no defined boundary of these networks, they overlap with regions typically included in the NSP. The combination of these resting state networks and the top-down control systems that are engaged when experiencing pain has been termed the “dynamic pain connectome” (Kucyi and Davis 2015). The concept of a dynamic pain connectome suggests that it is necessary to record the cellular activity underlying interactions between groups of neurons and the cerebral cortex, rather than simply recording within isolated brain areas. As neurotransmitters play a crucial role in pain processing, future studies should include multimodal imaging involving metabolic techniques, such as magnetic resonance spectroscopy.
CONCLUSION
To date, a specific physiological measure of cerebral pain processing is lacking. The quest for a biological indicator of acute pain, namely one that could provide insights into neurophysiological responses, is desirable to accurately diagnose patients and objectively evaluate pain management strategies. The neuroimaging techniques evaluated in the present review show some agreement regarding the brain areas being active during pain processing (Fig. 1). However, there are also major differences in these reportedly active areas, and specificity remains uncertain. Limitations within each technique (e.g., poor spatial resolution in EEG, poor temporal resolution in fMRI) or differences in study design (e.g., no proper control group, varying populations) might explain some of the divergent findings. Although none of the neuroimaging techniques available has been found to reliably act as an indicator of nociception, researchers have taken a noteworthy step in the translation of pain mechanisms from animal experiments to humans. Not surprisingly, this introduces greater complexity in the relationship between variables, yet these results have the potential to impact pain management strategies in a clinical setting more directly. Moreover, developing experimental pain models that more closely mimic clinical pain may begin to bridge the gap between pain research and pain management. Furthermore, including appropriate control conditions is integral for future investigations. Future research is needed to examine the reliability and specificity of proposed correlates of pain to determine whether they can be used as biomarkers of acute pain.
GRANTS
J. Archibald is supported by a Masters’ research scholarship of the National Council of Science and Technology (CONACYT) and GSM-NSERC. F. M. Warner is supported by a University of British Columbia 4-Year Research Fellowship Award. C. R. Jutzeler is supported by postdoctoral research fellowships from the Swiss National Science Swiss National Science Foundation (SNSF, P2EZP3_172162) and Craig H. Neilsen Foundation (460378).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.A. conceived and designed research; J.A. and C.R.J. interpreted results of experiments; J.A. and C.R.J. prepared figures; J.A. and C.R.J. drafted manuscript; J.A., F.M.W., O.O., M.T., and C.R.J. edited and revised manuscript; J.A., F.M.W., O.O., M.T., and C.R.J. approved final version of manuscript.
REFERENCES
- Bräscher A-K, Becker S, Hoeppli M-E, Schweinhardt P. Different brain circuitries mediating controllable and uncontrollable pain. J Neurosci 36: 5013–5025, 2016. doi: 10.1523/JNEUROSCI.1954-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Flor H, Greely HT, Iannetti GD, Mackey S, Ploner M, Pustilnik A, Tracey I, Treede RD, Wager TD. Brain imaging tests for chronic pain: medical, legal and ethical issues and recommendations. Nat Rev Neurol 13: 624–638, 2017. doi: 10.1038/nrneurol.2017.122. [DOI] [PubMed] [Google Scholar]
- Fardo F, Vinding MC, Allen M, Jensen TS, Finnerup NB. Delta and gamma oscillations in operculo-insular cortex underlie innocuous cold thermosensation. J Neurophysiol 117: 1959–1968, 2017. doi: 10.1152/jn.00843.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Davis KD. The dynamic pain connectome. Trends Neurosci 38: 86–95, 2015. doi: 10.1016/j.tins.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Lelic D, Hansen TM, Mark EB, Olesen AE, Drewes AM. The effects of analgesics on central processing of tonic pain: a cross-over placebo controlled study. Neuropharmacology 123: 455–464, 2017. doi: 10.1016/j.neuropharm.2017.06.022. [DOI] [PubMed] [Google Scholar]
- Liberati G, Klöcker A, Algoet M, Mulders D, Maia Safronova M, Ferrao Santos S, Ribeiro Vaz J-G, Raftopoulos C, Mouraux A. Gamma-band oscillations preferential for nociception can be recorded in the human insula. Cereb Cortex, 2017. doi: 10.1093/cercor/bhx237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra G, Wang WE, Archer DB, Roy A, Coombes SA. Automated classification of pain perception using high-density electroencephalography data. J Neurophysiol 117: 786–795, 2017. doi: 10.1152/jn.00650.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel MM, May ES, Tiemann L, Schmidt P, Postorino M, Ta Dinh S, Gross J, Ploner M. Brain oscillations differentially encode noxious stimulus intensity and pain intensity. Neuroimage 148: 141–147, 2017. doi: 10.1016/j.neuroimage.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postorino M, May ES, Nickel MM, Tiemann L, Ploner M. Influence of pain on motor preparation in the human brain. J Neurophysiol 118: 2267–2274, 2017. doi: 10.1152/jn.00489.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons TV, Iannetti GD, Liang M, Wood JN. The “pain matrix” in pain-free individuals. JAMA Neurol 73: 755–756, 2016. doi: 10.1001/jamaneurol.2016.0653. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med 368: 1388–1397, 2013. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C, Roy M, Buhle JT, Wager TD. Distinct brain systems mediate the effects of nociceptive input and self-regulation on pain. PLoS Biol 13: e1002036, 2015. doi: 10.1371/journal.pbio.1002036. [DOI] [PMC free article] [PubMed] [Google Scholar]