Abstract
A common aspect of individuality is our subjective preferences in evaluation of reward and effort. The neural circuits that evaluate these commodities influence circuits that control our movements, raising the possibility that vigor differences between individuals may also be a trait of individuality, reflecting a willingness to expend effort. In contrast, classic theories in motor control suggest that vigor differences reflect a speed-accuracy trade-off, predicting that those who move fast are sacrificing accuracy for speed. Here we tested these contrasting hypotheses. We measured motion of the eyes, head, and arm in healthy humans during various elementary movements (saccades, head-free gaze shifts, and reaching). For each person we characterized their vigor, i.e., the speed with which they moved a body part (peak velocity) with respect to the population mean. Some moved with low vigor, while others moved with high vigor. Those with high vigor tended to react sooner to a visual stimulus, moving both their eyes and arm with a shorter reaction time. Arm and head vigor were tightly linked: individuals who moved their head with high vigor also moved their arm with high vigor. However, eye vigor did not correspond strongly with arm or head vigor. In all modalities, vigor had no impact on end-point accuracy, demonstrating that differences in vigor were not due to a speed-accuracy trade-off. Our results suggest that movement vigor may be a trait of individuality, not reflecting a willingness to accept inaccuracy but demonstrating a propensity to expend effort.
NEW & NOTEWORTHY A common aspect of individuality is how we evaluate economic variables like reward and effort. This valuation affects not only decision making but also motor control, raising the possibility that vigor may be distinct between individuals but conserved across movements within an individual. Here we report conservation of vigor across elementary skeletal movements, but not eye movements, raising the possibility that the individuality of our movements may be driven by a common neural mechanism of effort evaluation across modalities of skeletal motor control.
Keywords: basal ganglia, effort, movement vigor, reward, speed-accuracy trade-off
INTRODUCTION
Individuality is reflected in many aspects of behavior, including forms of decision making that depend on subjective evaluation of reward and effort. For example, in a task where people were asked to press a key a number of times for a given amount of money, some preferred the low-reward/low-effort option, while others chose the high-reward/high-effort option (Treadway et al. 2009). This suggested that the degree to which people were willing to exert effort varied among healthy individuals. Importantly, these differences were correlated with between-subject differences in the neural circuits that evaluate reward and effort, particularly circuits that regulate dopamine transmission (Treadway et al. 2012). Some of these same circuits are also involved in control of movement, modulating the vigor, i.e., peak velocity as a function of amplitude, with which a movement is performed (da Silva et al. 2018; Kravitz et al. 2010; Pasquereau and Turner 2013). Changes in these circuits due to disease or drugs not only alter patterns of decision making (depression, less willing to exert effort; amphetamine, more willing to exert effort) but also affect patterns of elementary movements: saccades are slower in the case of depression and faster in the case of amphetamine (Shadmehr et al. 2010).
These observations raise the possibility that the neural circuits that evaluate reward/effort and influence the decision of what to do partly overlap with the neural circuits that influence the decision of how fast to move. As a result, similar to the individuality that is present in patterns of decision making, there may be individuality present in patterns of movement vigor.
Indeed, across healthy people there is diversity in the vigor with which elementary movements are produced. For example, during saccadic eye movements, some people move their eyes with velocities that are nearly twice as fast as others (Choi et al. 2014). If we assume that the purpose of a voluntary movement is to acquire a rewarding state, then the motor commands that are produced during a movement are analogous to effort expended to acquire reward and duration of the movement is analogous to a temporal discount of that reward (Berret and Jean 2016; Rigoux and Guigon 2012; Shadmehr et al. 2010; Wang et al. 2016). In this framework, the objective of motor control may be to move in a way that maximizes the rate of reward (reward attained minus effort expended, divided by time) (Niv et al. 2007).
Such a theory predicts that the vigor with which a movement is performed will depend on the subjective evaluation of reward, effort, and time. Indeed, movements that are directed toward more rewarding stimuli are performed with greater vigor (Manohar et al. 2015; Reppert et al. 2015; Summerside et al. 2018; Takikawa et al. 2002; Xu-Wilson et al. 2009b). Given a constant amount of reward, movements that are expected to require greater effort are performed with less vigor (Shadmehr et al. 2016). In this framework, the between-subject differences in vigor of movements are potentially a reflection of differences in how the brain evaluates reward, effort, and time. Consistent with this idea is the observation that individuals who exhibit greater impatience in their decision making, potentially reflecting a greater cost of time, also tend to have greater vigor in their saccades (Choi et al. 2014).
Alternatively, between-subject differences in vigor may reflect differences in speed-accuracy trade-off. That is, the willingness to move faster may not reflect a willingness to exert greater effort but instead a willingness to accept greater inaccuracy. Therefore, the potential link between vigor and inaccuracy, as predicted by earlier models of motor control (Harris and Wolpert 1998), complicates the problem of interpreting vigor differences between individuals.
The between-subject differences in vigor have previously been described for eye movements (Bargary et al. 2017; Choi et al. 2014; Rigas et al. 2016), raising the question of whether vigor differences in one type of movement generalize to other types. Here we measured saccadic eye movements of >300 healthy people, discovering that the diversity in saccade vigor translated into differences in reaction time (low-vigor individuals had longer reaction time) as well as variability of the motor commands (high-vigor individuals had more variable peak velocities). However, saccade vigor had no bearing on end-point accuracy. That is, individuals who moved their eyes with high velocities were not sacrificing accuracy for speed.
We next measured eye, head, and arm movements in a reaching task. Similar to the diversity of vigor that we had noted in motion of the eyes, we found that there were between-subject differences in vigor of head and arm movements. Saccade vigor did not correspond to vigor of arm or head movements. However, individuals who moved their head with high vigor also moved their arm with high vigor. Like saccades, individuals who exhibited high arm vigor also reacted sooner to the visual stimulus, and, like saccades, high arm vigor did not translate into reach inaccuracy. Therefore, in multiple modalities of motor control we found that the individuality expressed in vigor could not be ascribed to a willingness to accept inaccuracy but may be a reflection of a willingness to exert effort.
MATERIALS AND METHODS
Experiment 1.
The purpose of our first experiment was to characterize saccadic eye movements of a large number of healthy individuals in a head-fixed condition and ask whether vigor translated into inaccuracy. We asked n = 335 healthy subjects (157 women) between the ages of 18 and 46 yr to participate (age 22 ± 4 yr, mean ± SD). All were naive to the paradigm and to the purpose of the experiments. This large data set afforded us robust analysis of a multitude of saccade characteristics, including effects of sex on saccade velocity, effect of displacement on reaction time, effect of saccade direction on reaction time, and, critically, the relationship between saccade vigor and end-point variability. Subjects slept 7.0 ± 1.5 h the evening before the study. They signed a written consent form to participate in protocols reviewed and approved by the Texas State University Institutional Review Board.
Subjects sat with head movement restrained by a forehead rest (Fig. 1A). They were seated in front of a ViewSonic 22-in. screen (474 ×297 mm, 1,680 × 1,050 pixels, 60 Hz) that displayed the visual targets and were instructed to “look at the visual stimulus as it appears.” The distance from the subject to the display was 550 mm. We used a head-fixed setup in which the eyes were located ~420 mm from the level of the desk upon which the screen sat. Targets were displayed at 234–532 mm above the level of the desk. We used an EyeLink 1000 system (SR Research; sampling rate = 1,000 Hz) to track the left eye of all subjects. The spatial accuracy of the eye tracker was 0.48 ± 0.17°.
Fig. 1.
Experimental paradigms. A: experimental protocol for recording head-fixed saccades. Gray and black circles denote gaze and target position, respectively. Dashed box denotes space of possible target placements. B: experimental protocol for recording head-unrestrained reaching. Subjects were seated in a custom-built chair with shoulder-fitted seat belts. Eye tracker was placed above the viewing screen with use of a hot mirror to enable simultaneous recording of hand movements. Subjects were asked to make reaching movements and gaze shifts to a series of circular targets (diameter = 1.0°) in the upper horizontal quadrant of the viewing screen.
We measured saccade properties across a range of vertical and horizontal movements. The computer presented a series of 200 targets (diameter = 1°) chosen from a uniform distribution bounded ±15° horizontally and ±9° vertically. The minimum target displacement was 4°. Each trial lasted 1 s.
Experiment 2.
The purpose of our second experiment was to characterize head-unrestrained gaze shifts and arm movements in healthy individuals and ask whether vigor was conserved across modalities of elementary movements. That is, did people who have high vigor in one type of movement also have high vigor in other types of movement? We asked n = 47 healthy subjects (26 women) between the ages of 18 and 63 yr (26 ± 8 yr, mean ± SD) and with no known neurological deficits to participate in this study. All were naive to the paradigm and the purpose of the experiment. The participants signed a written consent form to participate in protocols reviewed and approved by the Johns Hopkins University Institutional Review Board. We required all subjects for experiment 2 to have a minimum of 50 task-relevant gaze shifts and 50 task-relevant arm movements. Of the 47 subjects who started experiment 2, 10 were excluded because of poor eye tracker calibration. For instance, if a subject blinked during the primary gaze shift we could not analyze the saccade and therefore could not record a movement for that trial. Additionally, data from one subject were excluded because this subject’s arm vigor parameter was an outlier (Rousseeuw and Croux 1993). We report data from n = 36 subjects for experiment 2.
Subjects sat in a chair that restrained motion of their shoulder and torso via a harness (Fig. 1B). They were seated in front of a screen (LG model 32LN5300; 700 × 395 mm, 1,920 × 1,080 pixels, light gray background, 60 Hz). The video buffer was refreshed at 120 Hz. An EyeLink 1000 recording system was used to acquire gaze position. To measure head position, we made a mold for the upper and lower teeth of each subject and placed a Trakstar 3D tracking system (Northern Digital) on the dental bite bar. We used the same tracking system to record the position and orientation of the right index finger. The head and arm data were sampled at 200 Hz, whereas eye position was sampled at 500 Hz.
We recorded position of the eye, head, and finger simultaneously as subjects completed a pointing task. Subjects were presented with visual targets (diameter = 1°) and then reached and touched each target with their right index finger. Subjects were instructed to reach and touch the target as quickly and accurately as possible. Target displacement ranged from 10° to 50° in 15 subjects and from 5° to 55° in the remaining 21 subjects. Targets always appeared at roughly the same vertical location but various horizontal locations. In this manner, all gaze shifts and reaching movements were almost entirely horizontal. The intertrial interval was randomly drawn from a uniform distribution between 750 and 1,250 ms for 15 subjects and drawn from a normal distribution for 21 subjects (1,500 ± 200 ms, mean ± SD). Gaze data were collected from the right eye.
The task was a standard point-to-point target overlap task in which the target location for trial n served as the starting location for trial n + 1. At the beginning of each trial, there was a period of overlap during which both the fixation stimulus and the target stimulus were visible. The fixation point was extinguished as soon as the subject began the gaze shift to the target (velocity > 15°/s).
Control studies.
In experiment 2 we measured eye, head, and arm movements simultaneously during reaching and found that vigor was conserved across certain modalities. This might have arisen because the various movements (eye, head, and arm) were performed together, making those who had high arm vigor also produce high head vigor. We wanted to know whether people who had high eye vigor during reaching also had high eye vigor when they made saccades without reaching. We also wanted to know whether people who had high head vigor during reaching also had high head vigor when they changed gaze without reaching. Therefore, we performed three control experiments.
In our first control experiment we asked a subset of the subjects who completed experiment 2 (n = 16) to make head-free gaze shifts to the same reach targets as in experiment 2, but without reaching. These subjects completed eight blocks of 70 trials. Eight of the 16 subjects chose not to move their head during this control experiment. Therefore, we could only assess consistency of head movement vigor with and without arm movements in n = 8 subjects.
In our second control group, we asked a separate subset of the subjects who completed experiment 2 (n = 18) to perform a series of head-fixed gaze shifts. As in experiment 2, subjects sat with their eyes located at a distance of 40 cm from the viewing screen. Gaze position of the right eye was recorded at 1,000 Hz. With these two control experiments (head-free gaze shifts, head-fixed saccades) we attempted to test whether vigor measures we had acquired from a subject for a particular modality (eye, head, arm) were reproducible across other contexts of movement.
In our third control study, we considered the question of reaction time and movement velocity. We had used a velocity threshold to detect onset of a saccade, which then served as our temporal marker to estimate reaction time. We had found that individuals who expressed their movements with greater vigor also tended to have faster reaction times. However, we were concerned that differences in reaction time may have been solely due to the greater time that it took for a low-velocity signal to reach this threshold. Therefore, we wished to quantify how much this method of detecting movement onset biased our estimate of reaction time.
To answer this question, we used a simulation in which precise estimates of movement onset were known. We used a third-order model of the oculomotor system with position, velocity, and torque as states (Shadmehr and Mussa-Ivaldi 2012). The parameters of the eye plant were set so that the resulting system had time constants of 224, 13, and 4 ms. We used an optimal feedback control of the eye that penalized the distance of the target to the fovea, penalized eccentricity of the eye, and minimized the motor commands to eye. We simulated 15° saccades with a wide range of peak velocities and measured how the time to velocity threshold (15°/s) changed as a function of peak velocity. The simulation results provided an estimate of the bias that was introduced in the reaction time measurements as a function of vigor.
Data analysis.
All gaze data were filtered with a third-order Butterworth low-pass filter with cutoff frequency of 90 Hz. All hand and head data were filtered with a cutoff frequency of 20 Hz. Saccades were identified with a velocity threshold of 15°/s and minimum hold time of 20 ms at saccade end (i.e., velocity could not exceed the cutoff for a minimum 20 ms after end point). Hand and head movements were identified with velocity cutoffs of 2 cm/s and 4°/s, respectively. The velocity cutoff values were chosen to avoid false positive identification of movements while maintaining a precise measure of reaction time. We identified saccades as horizontal or vertical depending on the angle of the target displacement: rightward horizontal saccades were defined to have a target displacement angle within 0 ± 45°, and upward vertical saccades were defined to have a target displacement angle within 90 ± 45°.
Head-fixed and head-unrestrained saccades were identified as task relevant on the basis of the following criteria: 1) velocity profile skew < 0.75 (possible values ranged from 0.0 to 1.0); 2) duration < 180 ms; 3) ratio of movement displacement to target jump displacement between 0.6 and 1.4; 4) reaction time between 80 and 350 ms (head fixed) or between 80 and 550 ms (head unrestrained); and 5) peak velocity < 850°/s.
Arm movements were identified as task relevant on the basis of the following criteria: 1) velocity profile skew < 0.75; 2) duration < 1,200 ms; 3) ratio of displacements between 0.6 and 1.4; and 4) reaction time between 80 and 800 ms.
Constraints on movement parameters were generous because our goal was to capture natural variability in vigor across subjects and our intention was to reject as few of the movements as possible. Head-fixed saccades had skew 0.379 ± 0.004 (mean ± SE across subjects), duration 58.7 ± 0.3 ms, and displacement ratio 0.968 ± 0.003. Head-free saccades had skew 0.354 ± 0.011, duration 94.0 ± 3.2 ms, and displacement ratio 0.942 ± 0.009. Arm movements had skew 0.416 ± 0.005, duration 542 ± 22 ms, and displacement ratio 0.98 ± 0.01. Overall, 17 ± 2% of head-fixed saccades, 26 ± 2% of head-unrestrained saccades, and 5 ± 1% of arm movements were removed from analysis.
Head movements were identified with the same criteria as those for arm movements, with the exception that head movements with ratio of movement displacement to target displacement between 0.1 and 1.4 were accepted. The reason for the relaxed head movement displacement criterion was that the task did not enforce head movement displacement; different subjects had vastly different distributions of head movement displacement.
We required subjects for experiment 1 to have a minimum of 50 task-relevant horizontal saccades and 25 task-relevant vertical saccades. Of the 335 subjects who started experiment 1, 14 did not complete the task and 32 had less than the required number of task-relevant saccades. The lack of a sufficient number of task-relevant saccades for these subjects was due to a combination of frequent blinks and low signal-to-noise ratio in the gaze data. The latter occurred when a subject’s eyelid or eyelashes partially occluded viewing of the pupil. We report data from n = 289 subjects for experiment 1.
While displacement contingencies were placed on saccade and arm movements, the subjects were not given instructions regarding movements of the head. Of the 36 subjects that completed experiment 2, n = 9 subjects chose not to move their head. This lack of head movements in such a large number of subjects is likely due to the fact that our experiment design included a critical feature: every movement to one side was followed by another movement to the opposite side. Earlier work has shown that this reduces the propensity to move the head compared with a design in which subjects expect to move twice in the same direction (Monteon et al. 2012; Oommen et al. 2004). Because we could not estimate vigor of head movements in these subjects, we had to exclude them from comparisons that required head vigor data. We report head movement data from n = 27 (of 36) subjects from experiment 2. However, we included all subjects (n = 36) in analyses that required eye and/or arm movement vigor data.
Vigor.
Saccades have a subject-specific velocity-displacement relationship: some people produce fast saccades, whereas others produce slow saccades (Choi et al. 2014; Reppert et al. 2015). Previous work has suggested that velocity-displacement patterns for saccades share the same underlying shape among subjects and that subject-specific velocities are a scaled version of this underlying function (Choi et al. 2014). To characterize vigor of a given movement, we used a maximum likelihood approach.
For horizontal saccades, we began by finding the canonical function that related peak velocity to displacement. We fitted the data across all subjects in experiment 1 to a hyperbolic function that related the displacement x of the saccade and its peak velocity:
| (1) |
Once g(x) was found, we estimated saccade vigor for subject n by assuming that his/her peak velocity v for saccade of displacement x on trial i was described by
| (2) |
In the above expression, εn is zero mean noise and kn is the vigor of horizontal saccades of subject n, where kn > 1 implies that the vigor of saccades for subject n was on average greater than the mean of the population. The parameters that need to be estimated are . The likelihood of any data point is defined as
| (3) |
The log of the likelihood is
| (4) |
The log-likelihood of all the data is
| (5) |
To find parameter kn, we take the derivative of the above equation and find the following:
| (6) |
We repeated this procedure separately for horizontal and vertical saccades, as well as arm movements, finding for each subject his/her vigor parameter kn for each modality of movement.
The head velocity-displacement relationship was approximately linear. Therefore, for head movement we set g(x) = αx. As a result, in every modality we had a measure of vigor that described the velocity-displacement relationship in one subject with respect to the mean relationship across the population.
End-point variability.
To measure end-point variability of saccades in experiment 1 and arm movements in experiment 2, we assessed the relationship between movement end point and target location (relative to movement start point) via vectors Δx and Δxt, respectively. We used the following equation to model this relationship:
| (7) |
In the above equation, the terms β0 and β1 represent systematic error (offset and gain) in movement end point. The vector ε is a two-dimensional noise, assumed to have variance-covariance matrix Q. We report the square root of the trace of the variance-covariance matrix as our proxy of variability in end-point error.
Hypothesis testing.
Our hypothesis was that various variables that we measured in a given subject were generated by a single latent variable: trait vigor of that subject. Therefore, given this single variable we should be able to predict the various variables that we measured in that subject. Using a Bayesian approach, we determined the probability that in each individual the measured data were generated by a single trait. Our approach is an example of causal inference, where we use posterior probabilities to compare one generative model (trait vigor) with another (chance).
Let us define the variable yn as the measured quantities in subject n. For example, in experiment 1, yn is a 4 × 1 vector that includes the average peak velocity of horizontal saccades, the average peak velocity of vertical saccades, as well as average reaction times of these saccades for subject n. For experiment 2, yn is a 6 × 1 vector that includes the average peak velocity of saccades, head movements, and reaching movements as well as the average reaction times of these movements in subject n. The random variable z indicates whether the measured variables were generated from a common source (trait vigor) or by chance.
If z = 1 (data were generated from a common source), we define scalar variable xn as the trait vigor of this individual. The generative model of the data is defined by the following equation:
| (8) |
In the above expression, a and b are parameters that are constant across subjects (each a 4 × 1 vector for experiment 1, a 6 × 1 vector for experiment 2) and ε is zero mean Gaussian noise with variance-covariance matrix R. Let us use θ(1) to describe the parameters of the likelihood function for the hypothesis z = 1: θ(1) = {a,b,R}. The likelihood of the measured variable y in subject n is defined as
| (9) |
For the alternate hypothesis, we have z = 0 (data within each subject were not generated from a common source of vigor). The likelihood of the measured variable is
| (10) |
In the above expression, θ(0) refers to the parameters of the likelihood function for the null hypothesis: θ(0) = {μ,Q}. These parameters are found with a maximum-likelihood approach. Once they are found, we can compute the probability of the hypothesis that a common source of vigor in each subject generated their data:
| (11) |
We assumed equality in the prior probabilities Pr(z = 1) = Pr(z = 0), setting them equal to 0.5. Using the above expression, we computed the probability of a trait vigor in each subject in each experiment.
To compute the posterior probability, one needs to estimate the latent variable xn (i.e., trait vigor) in each subject. One approach is to search for this variable such that it maximizes the joint probability of all the observed data . A more conservative approach is to set xn equal to the mean of a prior belief. We took the more conservative approach. For experiment 1, we set xn for each subject to be equal to the average of the vigor term kn measured for horizontal and vertical saccades. For experiment 2, we set xn for each subject to be equal to the average of the vigor term kn measured for eye and arm movements. We confirmed that the posterior probability for our hypothesis was indeed lower when we did not search the latent vigor space. Therefore, the results we report here are a conservative estimate of the posterior probability that a single latent variable produced the various measurements in each subject.
We tested whether our posterior probability had a distribution that was significantly different from chance. Because the distribution of the posterior was by definition nonnormal, we used Wilcoxon’s signed-rank test and compared the resulting distribution to chance (i.e., posterior probability of 0.5).
In addition, because the two hypotheses relied on an unequal number of model parameters, we compared the two hypotheses with an information theoretic approach, the Akaike information criterion (AIC). For each subject we computed the AIC for the main and the null hypotheses, using the likelihood of the observed data in that subject under each hypothesis (Eq. 9 and Eq. 10). This allowed us to compare the two hypotheses using a within-subject difference in AIC measure via Wilcoxon’s signed-rank test.
RESULTS
In experiment 1, we analyzed saccades of healthy people (n = 289) in a head-fixed condition. We measured eye movements as subjects directed their gaze toward visual stimuli (Fig. 1A). The computer presented a series of 200 targets (diameter = 1°) placed randomly between ±15° horizontally and ±9° vertically. Because the brain stem neural circuitry differs for horizontal and vertical saccades (Leigh and Zee 2015), we began our analysis by asking whether individuals who exhibited high vigor in their horizontal saccades also exhibited high vigor in their vertical saccades.
Experiment 1: vigor of saccades.
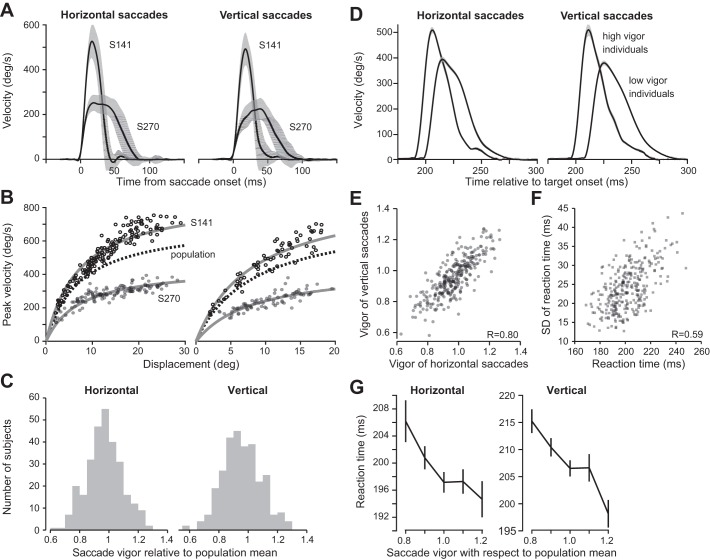
Saccade velocity profiles for two exemplar subjects are displayed in Fig. 2A, and their peak velocity values are plotted as a function of displacement in Fig. 2B. Regardless of displacement, saccades of subject S141 exhibited much greater peak velocity than saccades of subject S270, in both horizontal and vertical directions.
Fig. 2.
Vigor and reaction time of saccades. A: average velocity profile for horizontal and vertical saccades for 2 representative subjects. The displacements are between 10° and 20° in the plotted trials. Error bars are within-subject SD across trials. B: peak velocity vs. amplitude relationship for individual horizontal and vertical saccades for the same 2 subjects. The dashed line represents the average relationship as computed across the population, while the gray solid lines are the scaled relationship from fit to Eq. 1. C: the term “vigor” refers to the relationship between each individual’s amplitude-velocity function with respect to the population mean. Plots show the distribution of horizontal and vertical saccade vigor. D: horizontal and vertical saccades of individuals with high and low vigor. The figure illustrates velocities for 12–14° saccades for a group of low-vigor subjects kn < 0.85 and for a group of high-vigor subjects kn > 1.15. Individuals who had high vigor also reacted sooner to the visual stimulus. Error bars are SE. E: relationship between vigor of vertical and horizontal saccades. Each dot represents a single subject (n = 289, R = 0.80). Individuals with high-vigor horizontal saccades also tended to have high-vigor vertical saccades. F: relationship between mean reaction time and SD of reaction time. Each dot represents a single subject. G: relationship between reaction time and vigor for vertical and horizontal saccades. Error bars are SE. Individuals who had greater vigor with respect to population mean also tended to react earlier to the visual stimulus.
To estimate saccade vigor, we used a maximum likelihood approach. The canonical function g(x) had the following parameters: for vertical saccades α = 831, β = 0.090 and for horizontal saccades α = 714, β = 0.125. For a given subject n, we found vigor parameter kn, resulting in the subject-specific curves shown in Fig. 2B. We quantified goodness of fit via an R2 value for each subject and observed the following distribution: horizontal saccades R2 = 0.80 ± 0.10; vertical saccades R2 = 0.73 ± 0.13 (mean ± SD). Together, these results indicated that the single-parameter model used for describing peak saccade velocity as a function of amplitude (Eq. 2) was a reasonable proxy for the data of each subject.
The parameter kn represented the saccade vigor for individual n. We estimated kn for horizontal and vertical saccades separately. Despite large between-subject differences in saccade vigor (Fig. 2C), we observed a high degree of consistency: across individuals, the vigor of horizontal and vertical saccades were linearly related [Fig. 2E; F(1,287) = 512, P = 8 × 10−66, R = 0.80]. That is, people who exhibited more vigorous horizontal saccades also exhibited more vigorous vertical saccades.
A relationship between reaction time and vigor of saccades.
During the period between presentation of a stimulus and start of a movement, the brain is thought to integrate utility of the current option and then start the movement when the integration reaches threshold. If we represent utility of the movement as a rate (reward minus effort, divided by time), then the time it takes for the integration to reach threshold will depend on the same variables that affect movement vigor: reward and effort. This framework is consistent with the observation that increased reward not only makes the movement more vigorous but also makes the reaction time of that movement shorter (Milstein and Dorris 2007; Opris et al. 2011; Takikawa et al. 2002; Xu-Wilson et al. 2009b). In this framework, individuals who move faster may have a greater utility for their movements, and this translates into a rate that reaches threshold faster, resulting in shorter reaction times. Therefore, we asked whether there was a relationship between reaction time and vigor: did individuals who exhibit greater vigor also react sooner to a visual stimulus?
Figure 2D illustrates velocities for 12–14° saccades for a group of individuals who had low saccade vigor kn < 0.85 and for a group of individuals who had high saccade vigor kn > 1.15. It appeared that those who moved faster also responded earlier to the visual stimulus. To test for this possibility, we computed the average reaction times for each individual for horizontal and vertical saccades and found that across individuals increased vigor corresponded with decreased reaction times [vertical saccades: F(1,287) = 28.3, P = 2.01 × 10−7; horizontal saccades: F(1,287) = 9.6, P = 0.002; Fig. 2G].
If utility of a movement is a rate that fluctuates between trials as a normally distributed random variable, and if during the reaction time period this random variable is integrated to threshold, then the distribution of reaction times will have a variance that increases with the mean rate, implying that people who have a longer reaction time will also have a larger trial-to-trial variability in their reaction times. Our data were consistent with this prediction, demonstrating that people who had a longer mean reaction time also tended to have a larger variability in how long they took to respond to the stimulus [Fig. 2F; F(1,287) = 155, P = 9 × 10−29, R = 0.59].
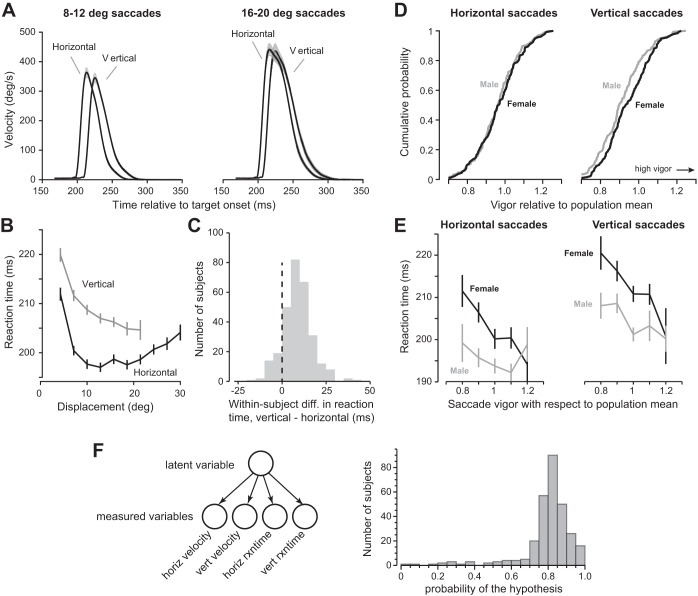
While there was a strong within-individual correspondence between vigor of horizontal and vertical saccades, there were also consistent differences among these saccades. Figure 3A displays saccade velocity profiles averaged across the entire population for two ranges of amplitude. We found that reaction times of horizontal saccades were consistently shorter than vertical saccades. To quantify this, we considered reaction time as a function of amplitude (Fig. 3B) and found that for horizontal saccades reaction times exhibited a U-shaped pattern, exhibiting a minimum around 10° and then increasing with amplitude. For vertical saccades, reaction time decreased with increasing amplitude [repeated-measures ANOVA, F(6,702) = 18.0, P < 0.001]. A within-subject comparison of reaction times between horizontal and vertical saccades (collapsed across all amplitudes) revealed that vertical saccades started ~10 ms later than horizontal saccades (Fig. 3C; Wilcoxon signed-rank test, P = 2.56 × 10−47).
Fig. 3.
Reaction time of vertical and horizontal saccades and evaluation of the hypothesis that the movement characteristics of each subject are related to a traitlike vigor. A: average velocity profiles for horizontal and vertical saccades across all subjects. Saccades were divided into 2 groups based on total displacement: 8–12° and 16–20°. Profiles are time-locked to target onset. Shaded areas represent mean ± 5 SE. Vertical saccades had longer reaction time than horizontal saccades. B: relationship between reaction time and displacement of saccades. Error bars are SE across subjects. C: histogram of difference in reaction time between vertical and horizontal saccades. For each subject, we calculated the mean reaction time separately for vertical and horizontal saccades. We then took the within-subject difference between the 2 values. D: vigor of horizontal and vertical saccades in men and women. Vigor of horizontal saccades was comparable in men and women, but women had greater vigor in their vertical saccades than men. E: reaction times of horizontal and vertical saccades as a function of vigor with respect to population mean. Women on average took a longer time to react to a visual stimulus. Within each group, individuals who had greater vigor also responded earlier to the visual stimulus. Error bars are SE. Saccades were classified as horizontal or vertical if they were within ±45° with respect to the horizontal or vertical axis, respectively. F: the hypothesis that a single latent variable, trait vigor, may account for the various measured variables in each subject. Left: representation of a generative model in which the measured variables are assumed to be conditionally dependent on a single latent variable. For each subject we computed the likelihood of data given this hypothesis and then computed the posterior probability of the hypothesis given the data. Right: the distribution of the posterior probabilities (the null hypothesis, i.e., no trait vigor, has a probability of 0.5).
Our large population sample also allowed us to examine the effects of sex on vigor. For horizontal saccades, vigor was not different between male and female populations [Fig. 3D, left; P = 0.60, t(287) = 0.52, unpaired t-test]. However, there was a significant difference in reaction times: saccades in men were ~10 ms earlier than in women [Fig. 3E, left; P < 0.001, t(287) = 4.40, unpaired t-test]. For vertical saccades, women had significantly greater vigor than men (Fig. 3D, right; P < 0.05, t(287) = 2.25, unpaired t-test]. Despite this greater peak velocity of vertical saccades in women, men had shorter reaction times [Fig. 3E, right; P < 0.001, t(287) = 3.69, unpaired t-test]. Importantly, in each population the individuals who had greater vigor tended to also have shorter reaction times (Fig. 3E; men: R = −0.20, P = 0.01, women: R = −0.34, P < 10−4).
Testing the trait vigor hypothesis.
Our main hypothesis was that the various variables that we had measured in a given subject were generated by a single latent variable, the trait vigor of that subject (Fig. 3F). That is, given this latent variable, we should be able to predict the various measurements in that subject. Using a Bayesian approach, we determined the probability that the measured data (horizontal and vertical peak velocities and horizontal and vertical reaction times) were generated by a single latent variable.
We set the vector variable yn to represent the data in subject n and then, using maximum likelihood, computed the parameters of our main hypothesis (Eq. 9) as well as the parameters of the null hypothesis (Eq. 10). We then used Eq. 11 to estimate the posterior probability of the main hypothesis. The resulting distribution is shown in Fig. 3F. We found that the posterior of the main hypothesis was 0.776 ± 0.0045 (mean ± SE), significantly larger than chance (Wilcoxon signed-rank test, P = 6.3 × 10−43).
Next, we compared our hypothesis with the null hypothesis. using an information theoretic measure that corrected for the disparity in the number of parameters in the two models (AIC). From the measured data, we computed the likelihood of each hypothesis in each subject and then compared the two hypotheses, using the distribution of the two AICs. After correcting for the disparity in the number of parameters in each hypothesis, we confirmed that the main hypothesis was significantly more likely than the null hypothesis (Wilcoxon’s signed-rank test, P = 4.19 × 10−49).
These results could have been driven by a subset of measurements in each subject, for example, peak velocity relationship to vigor but not reaction time. To determine whether all the measured variables in vector yn were significantly related to the vigor trait variable xn, we analyzed elements of the parameter a, a 4 × 1 vector that represented the gain of the relationship between trait vigor and the measured variables yn. We computed the variance-covariance matrix of a and found that for all elements of a the mean was more than two standard deviations (SDs) from zero.
In summary, we found that people who exhibited more vigorous saccades also tended to react sooner in response to a visual stimulus. Vigor was conserved across horizontal and vertical saccades, but vertical saccades tended to have a longer reaction time. Women tended to have greater vigor in their vertical saccades than men. Regardless of sex, the time needed to react to a visual stimulus was shorter in people who moved more vigorously. We conclude that with 77.6% probability (±0.5%) the velocity and reaction times of saccades in each subject were related to a single variable in that subject, his/her trait vigor.
Vigor and end-point accuracy.
We next examined within-subject variability of saccades, asking whether high-vigor individuals suffered from greater end-point variability. To allow for comparison between vertical and horizontal saccades, we included saccades with displacement < 18° (this approximately corresponds to the range for vertical saccades; Fig. 3B). We began our analysis by focusing on the early part of the saccade and measured variability in peak velocity. We binned saccades by displacement and computed the average SD of peak velocity across bins. This gave us, for each subject, a measure of variability in peak velocity for horizontal and vertical saccades. We found that as vigor increased, SD of the peak velocity also increased [Fig. 4A; horizontal: F(1,287) = 14.7, P = 0.00015; vertical: F(1,287) = 24.7, P = 1.1 × 10−6]. (The reported statistics represent each subject as a single data point. In contrast, Fig. 4 plots the results in a binned form for ease of visual inspection.) Therefore, peak velocity was more variable in individuals who had greater vigor.
Fig. 4.
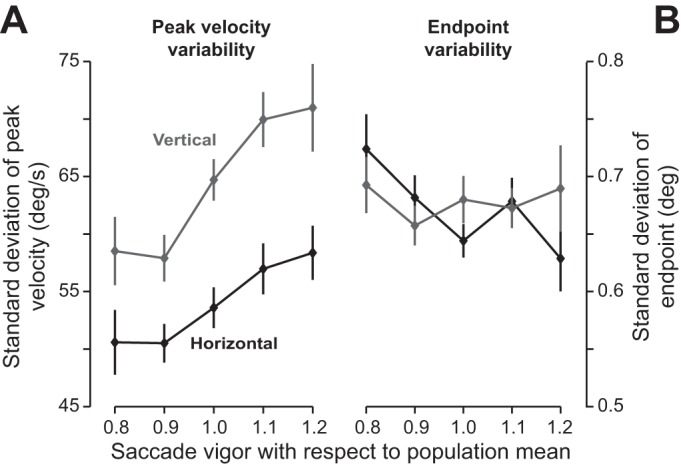
Vigor of saccades and end-point variance. A: SD of peak velocity of saccades vs. vigor of saccades. For each subject, we calculated the SD of peak velocity separately for horizontal and vertical saccades. Across subjects, the SD of peak velocity tended to increase with saccade vigor. Error bars are SE. B: SD of saccade end point vs. vigor of saccades. For each subject, we fitted Eq. 7 to the relationship between saccade displacement and target displacement. We then calculated the variance-covariance matrix of the 2-dimensional saccade displacement residual. The square root of the trace of this matrix was our proxy for end-point variability. Saccade vigor appeared to be unrelated to end-point variability.
Did this variability in peak velocity translate into end-point variability? We fit Eq. 7 to the measured data and then computed the variance-covariance matrix of the vector ε. The trace of this matrix was our proxy for end-point variability, and the square root of this measure was the SD of the proxy. We found no relationship between saccade vigor and SD of end points for vertical [F(1,287) = 0.08, P = 0.78] or horizontal [F(1,287) = 3.5, P = 0.06] saccades (horizontal saccade end-point variability tended to decrease with increased vigor). Therefore, whereas greater peak velocity (i.e., greater vigor) coincided with greater variability in the motor commands that initiated the saccade, this variability did not accumulate during the saccade, producing comparable end-point accuracy between low- and high-vigor subjects.
In summary, we quantified saccade vigor via a function that related amplitude to peak velocity and found a wide distribution across healthy individuals: some people moved their eyes with more than twice the velocity of others. Individuals who had high vigor in their horizontal saccades also had high vigor in their vertical saccades. Individuals with high vigor tended to react sooner to visual stimuli. Reaction times for vertical saccades were on average 10 ms longer than horizontal saccades. Reaction times for horizontal saccades exhibited a U-shaped pattern with respect to amplitude, exhibiting a minimum for 10° saccades and then increasing for larger saccades. Peak velocity was more variable in individuals who exhibited greater vigor, but this variability in the motor commands that initiated the saccade did not translate into greater variability in the movement end point. As a result, individuals who had high vigor were as accurate in placing their eyes as those who had low vigor.
Experiment 2: vigor of eye, head, and arm movements.
If vigor is a traitlike feature of individuality, then one might expect that it may be conserved across modalities of motor control. That is, people who exhibit high vigor in one modality of movement may also exhibit high vigor in another modality. To explore this question, we measured eye, head, and arm movements simultaneously as subjects made reaching movements.
Figure 5A displays data gathered from a typical subject during the reaching task. After presentation of a visual stimulus, the subject moved their eyes to the target via a saccade, followed by gradual movement of the head and the arm, followed by a counterrotation of the eyes. The differences in reaction times of the various body parts across the group are summarized in Fig. 5B. In general, the eyes moved first, then the arm, and finally the head. As target displacement increased, there was an increase in reaction time of the eye [repeated-measures ANOVA, F(4,104) = 39.5, P < 0.001] and the arm [repeated-measures ANOVA, F(4,104) = 27.2, P < 0.001] but a reduction in the reaction time of the head [repeated-measures ANOVA, F(4,56) = 2.77, P < 0.05]. Therefore, in both head-fixed (experiment 1) and head-free (experiment 2) conditions we found that as amplitude of the horizontal movement increased (beyond 10°) there was a general increase in saccade reaction times. In addition, we found an increase in reaction times of arm movements with increasing amplitude. Finally, it should be noted that as gaze amplitude increased, head reaction time decreased whereas eye reaction times increased. This suggests that head latency with respect to gaze onset declined as gaze amplitude increased, a finding that reiterates observations in the nonhuman primate (Freedman and Sparks 1997).
Fig. 5.
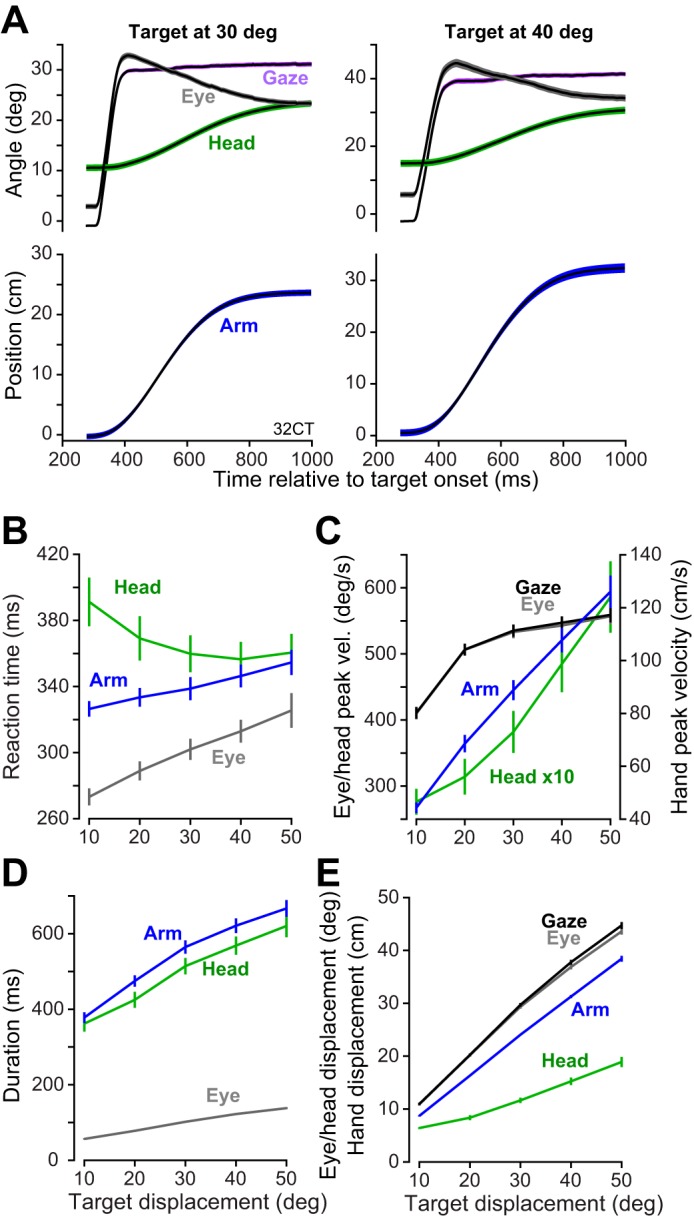
Kinematics of head-unrestrained gaze shifts and arm movements during reaching. A: average displacement profiles of gaze shifts (i.e., eye, head, and gaze) and arm movements for a single subject (32CT), split by target displacement (30° and 40°). Displacement profiles are time-locked to time of target appearance. Shaded areas are SD across trials. B: relationship between reaction time and target displacement. We binned movements based on target displacement for each trial and then computed the average reaction time per bin separately for each subject. Error bars are SE. C: relationship between peak velocity and target displacement. D: relationship between movement duration and target displacement. E: relationship between displacement of the body part and target displacement.
As expected, the increase in target displacement coincided with increases in peak velocity of the eyes [repeated-measures ANOVA, F(4,104) = 145.9, P < 0.001; Fig. 5C], the head [repeated-measures ANOVA, F(4,56) = 43.5, P < 0.001], and the arm [repeated-measures ANOVA, F(4,104) = 305.3, P < 0.001]. Similarly, the increase in target displacement coincided with an increase in saccade duration [repeated-measures ANOVA, F(4,104) = 670.8, P < 0.001; Fig. 5D] and the duration of the head [repeated-measures ANOVA, F(4,56) = 29.2, P < 0.001] and arm [repeated-measures ANOVA, F(4,104) = 556.5, P < 0.001] movements.
Our main question was whether there was a relationship between vigor across various modalities of motor control: did people who expressed high vigor in one kind of movement also have high vigor in other kinds of movement? We found that some subjects exhibited a high vigor in their head movements, and some subjects exhibited a high vigor in their arm movements (Fig. 6A): subject 35PV generated head and arm movements at velocities that were consistently higher than subject 32GT. Was this pattern consistent across individuals?
Fig. 6.

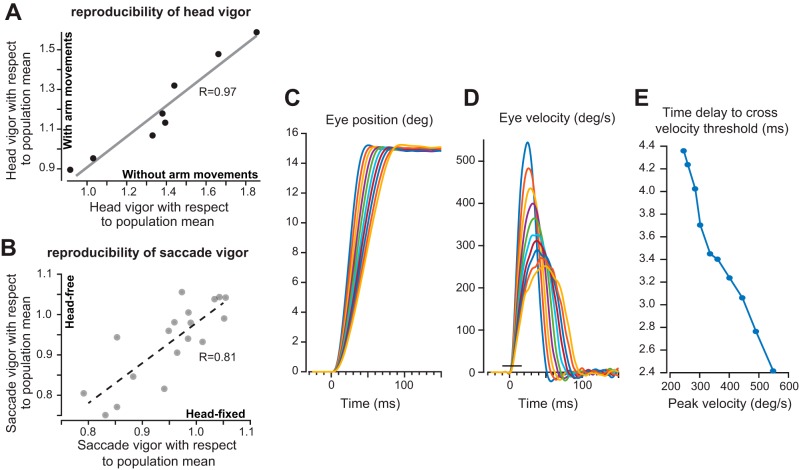
Consistency of vigor across head and arm movements. A: average velocity profile for head and arm movements for two representative subjects. The displacements are between 13–15 deg (head) and 15–18 cm (arm) in the plotted trials. Error bars are within-subject SD across trials. B: peak velocity vs. amplitude relationship of arm movements and head movements for 2 representative subjects. Data for subjects 32GT and 35PV are shown in gray and black, with lines of best fit shown in black and gray. For each subject, we fit a line constrained to pass through the origin. The fit across all participants is shown as a dashed line (population). Each point represents a single movement. C: the term “vigor” refers to the relationship between each individual’s amplitude-velocity function with respect to the population. Plots show the distribution of head (left) and arm (right) vigor. D: relationship between vigor of head movements and arm movements. Each point represents a single subject. Certain subjects (n = 9) did not move their head during the gaze shift, and therefore we could not estimate vigor of head movements for those subjects. Across subjects that we could estimate head vigor (n = 28), we found a strong correspondence between vigor of head and arm movements (R = 0.83, P < 0.001).
To address the question of conservation of vigor across movement modalities, we computed vigor of each individual’s eye, head, and arm movements separately. For saccades, the reference population data were those we had acquired in experiment 1. For arm movements, we fitted Eq. 1 to the velocity-displacement relationship for each subject (n = 37), resulting in the canonical function g(x) with the following parameter values: α = 2.45, β = 2.36. This canonical function is shown by the dashed lines in Fig. 6B. Finally, we scaled this population-level definition to fit each individual subject’s data, providing a one-parameter estimate of vigor of arm movements. We used a similar procedure to estimate vigor of head movements (a constrained linear fit, population-level slope γ = 3.2). This resulted in a one-parameter estimate of vigor for each modality of movement in each individual. The distribution of vigor is illustrated in Fig. 6C.
We compared vigor across modalities of movement within each individual. Figure 6D shows the relationship between vigor of head and arm movements, where each point represents a single individual. We observed a strong positive relationship between vigor of arm and head [F(1,25) = 38.72, P = 1.64 × 10−6, R = +0.83]. That is, individuals who moved their arm faster than average tended to also move their head faster than average. Although in all cases we found a positive relationship between vigor of eye, head, and arm (eye vs. arm R = +0.27, P = 0.11; eye vs. head R = +0.31, P = 0.11), these relationships were not significant. That is, saccade vigor was not a strong predictor of head or arm vigor, but arm vigor was a predictor of head vigor.
We asked whether individuals who exhibited high vigor of arm movements also tended to react sooner to the visual stimulus. Figure 7A shows the arm velocity profile aligned to stimulus onset for individuals with high (kn > 1.15) and low (kn < 0.85) vigor. People who moved their arm with greater vigor also tended to begin their arm movements earlier [Fig. 7B; F(1,34) = 10.6, P = 0.0026, R = −0.488]. The trend between vigor of head movements and head reaction time was also negative, but these parameters were not significantly correlated (R = −0.26, P = 0.19).
Fig. 7.
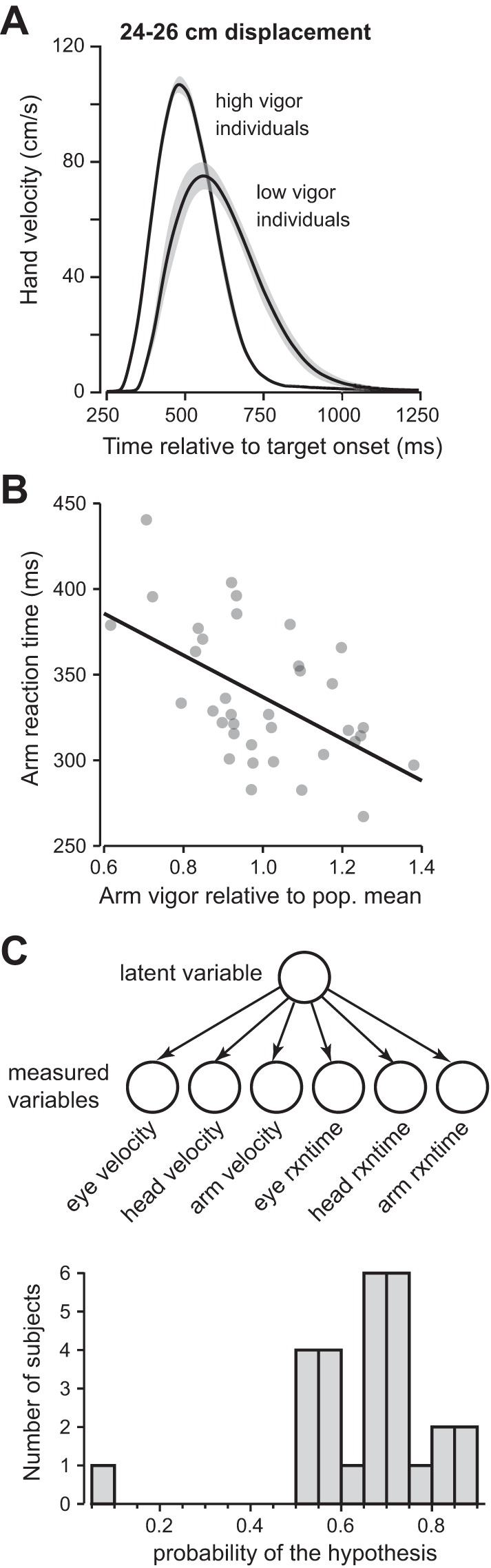
Reaction times and vigor of reaching and evaluation of the hypothesis that the movement characteristics of each subject are related to a traitlike vigor. A: hand velocity profiles for subjects that moved their arm with high or low vigor. Low-vigor individuals appeared to start their movements later with respect to the onset of the visual stimulus. Shaded areas are SE. B: relationship between arm vigor and arm reaction time (R = −0.33, P < 0.05). C: hypothesis that a single latent variable, trait vigor, may account for the various measured variables in each subject. For each subject we computed the likelihood of his/her data given this hypothesis and then computed the posterior probability of the hypothesis given the data. The distribution of the posterior probabilities is shown (the null hypothesis, i.e., no trait vigor, has a probability of 0.5).
Testing the trait vigor hypothesis.
Our hypothesis was that the various variables that we had measured in a given subject (eye, head, and arm velocity and reaction times) were generated by a single latent variable, a trait vigor of that subject (Fig. 7C). We set the vector variable yn to represent the data in subject n and then, using maximum likelihood, computed the parameters of our main hypothesis (Eq. 9) as well as the parameters of the null hypothesis (Eq. 10). We then used Eq. 11 to estimate the posterior probability of the main hypothesis in each subject. The resulting distribution is shown in Fig. 7C. We found that the posterior of the main hypothesis was 0.652 ± 0.012 (mean ± SE), significantly larger than chance (Wilcoxon signed-rank test, P = 1.04 × 10−4). We conclude that with 65% probability (±1.2%), the velocity and reaction times of eye, head, and arm movements in each subject were related to a single variable in that subject, his/her trait vigor.
We next compared our hypothesis with the null hypothesis, using the AIC. From the measured data, we computed the likelihood of each hypothesis in each subject and then compared the two hypotheses, using the distribution of the two AICs. After correcting for the disparity in the number of parameters in each hypothesis, we confirmed that the main hypothesis was significantly more likely than the null hypothesis (Wilcoxon’s signed-rank test, P = 5.93 × 10−6).
These results could have been driven by a subset of measurements in each subject. To determine whether all the measured variables in vector yn were significantly related to the vigor trait variable xn, we analyzed elements of the parameter a, a 6 × 1 vector that represented the gain of the relationship between trait vigor and the measured variables yn. We computed the variance-covariance matrix of a and found that for all but one element of a the mean was more than two SDs from zero. The only nonsignificant element of the model was reaction times of head movements. Finally, we recomputed the posterior probabilities but only for the arm and head data in each subject, excluding their saccade vigor and reaction times. Posterior of the trait vigor hypothesis for this reduced data set was 0.660 ± 0.015 (mean ± SE), essentially identical to when all the data were included (65 ± 1.2% probability that velocity and reaction times of eyes, head, and arm were related to a single trait vigor variable).
Reach vigor and end-point accuracy.
In experiment 1 we had observed that individuals who had more vigorous saccades also had greater variability in their saccade peak velocity (Fig. 4A), but this variability did not translate into end-point variability (Fig. 4B). We asked the same question regarding control of arm movements. For each individual, we binned arm movements according to target displacement and took the average SD of peak velocity across bins to arrive at a single estimate for the individual. Like saccades, we found that individuals who moved their arm with greater vigor also had greater variability in their arm peak velocity [Fig. 8A; F(1,34) = 15.2, P = 0.00044, R = +0.56]. And similar to what we had found with saccades, individuals with greater arm vigor did not suffer from greater end-point variability [Fig. 8B; F(1,34) = 0.75, P = 0.39, R = +0.15]. Therefore, for both saccades and reaching, greater vigor was not associated with greater inaccuracy.
Fig. 8.
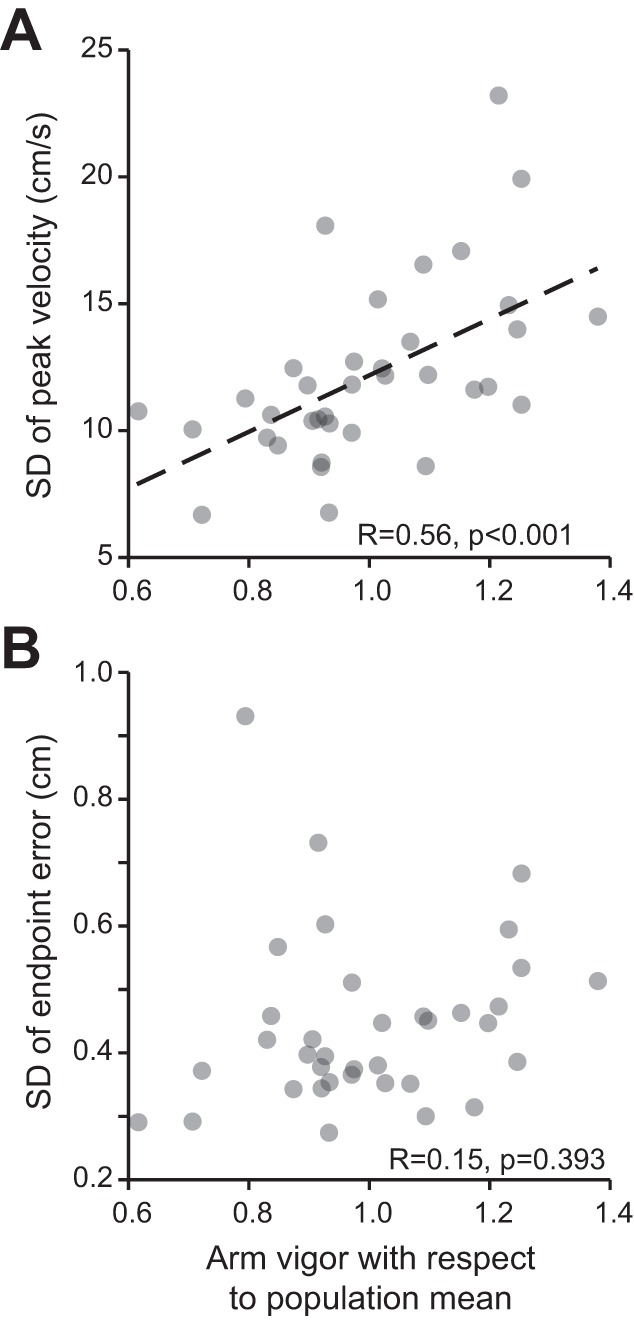
Vigor of reaching and its relationship to variability of peak velocity and end-point accuracy. A: for each subject, we binned reaching movements by target displacement and took the average SD of peak velocity at each bin. We then averaged across bins to attain a single measure of peak velocity variability for each participant. Across subjects, the SD of peak velocity tended to increase with reach vigor (R = +0.72, P < 0.001). B: SD of reach end point vs. reach vigor of the subject. For each subject, we fit Eq. 7 to the relationship between arm displacement and target displacement. We used SD of the residual parameter ε as our proxy for variability. Reach vigor appeared unrelated to end-point variability (R = +0.17, P = 0.32).
We also considered the possibility that individuals who expressed greater variability in their saccades may also express greater variability in their reaching movements. We compared variability of saccade end points with reach end points across subjects and found no evidence for this conjecture (P = 0.72).
Control studies.
In experiment 2 we measured motion of the head and arm during a reaching movement and found that head and arm vigor were highly correlated across individuals. However, this could have arisen because subjects performed the two movements together. To check for this, we performed a head-free version of the same task but without arm movements to test whether individuals who expressed high head vigor did so regardless of whether the head movements were accompanied by arm movements. We found that, in general, head movements had lower velocity when the subjects changed gaze while reaching (Fig. 9A). However, head vigor during arm movements was highly correlated with head vigor without arm movements (R = 0.97, P < 0.001). That is, individuals who had high head vigor in a task that included arm movements also had high head vigor in another task that did not include arm movements.
Fig. 9.
Control studies. A: reproducibility of head vigor: head vigor as measured during a task that included arm movements and a task that did not include arm movements. Each point is a single subject. Individuals who moved their head with high vigor in the context of arm movements also tended to move their head with high vigor without accompanying arm movements (R = +0.97). Vigor data are with respect to the population mean acquired during experiment 2 (head-free gaze shifts with arm movements). B: reproducibility of saccade vigor. Saccade vigor was measured in head-fixed and head-free conditions. Each point is a single subject. Individuals who moved their eyes with high vigor in the head-fixed condition also tended to move their eyes with high vigor in the head-free condition (R = +0.81). Vigor data are with respect to the population mean acquired during experiment 1 (head-fixed saccades). C: the bias produced by using a velocity threshold to estimate the reaction time. Simulated eye position traces for 15° saccades with various peak velocities. D: velocity traces for the simulated saccades. The horizontal line near saccade onset shows the velocity threshold used for detecting movement onset. This is the same threshold that was used in detecting movement onset in our experiments. E: as saccade velocity increases, the time delay from simulated movement onset to detected onset decreases. The magnitude of this effect is ∼2 ms.
The saccades that we had measured in experiment 2 were in a head-unrestrained condition. Did the individuals who moved their eyes with high vigor in a head-free condition also move their eyes with high vigor in a head-fixed condition? We tested n = 18 subjects in both head-free and head-fixed conditions and found that saccade vigor within an individual remained highly consistent (Fig. 9B; R = 0.81, P < 0.001).
In our experiments we observed that individuals who moved with high vigor also tended to have a shorter reaction time. However, because we used a velocity threshold to detect onset of a movement, differences in reaction time may have been solely due to the greater time that it took for a low-velocity signal to reach threshold. How much did this method of detecting reaction time bias our estimates?
To answer this question, we employed a simulation in which precise estimates of movement onset were known. We simulated 15° saccades (Fig. 9C) with a wide range of peak velocities (Fig. 9D). Our simulations suggested that as peak velocity increased the apparent reaction time decreased (Fig. 9E). Therefore, the simulations demonstrated that given a constant amplitude saccade, reductions in peak velocity introduced a bias in the reaction time. However, over a 2.5-fold range of peak saccade velocities, the maximum size of the bias was ∼2.0 ms. In comparison, we found that vigor-dependent between-subject differences in reaction time were ∼10 ms (Fig. 2, D and F). Therefore, the simulation results suggested that although between-subject differences in vigor introduced a bias in our ability to measure reaction time, the actually measured differences in reaction times were generally five times larger than the expected measurement bias.
DISCUSSION
During elementary movements such as saccades and reaching, motion tends to exhibit a characteristic relationship between peak velocity and displacement. However, among healthy individuals this velocity-displacement relationship is variable, with some consistently moving their eyes with velocities that are twice as fast as others (Choi et al. 2014; Rigas et al. 2016). Importantly, this between-subject variability is structured: the function that relates displacement to peak velocity of saccades in an individual is well represented as a scaling of the velocity-displacement function of the population (Choi et al. 2014). We use the term “vigor” to refer to this scaling factor. Here we measured vigor of various elementary movements and investigated the question of whether vigor is a traitlike feature of individuality.
We analyzed saccadic eye movements of ~300 individuals and found that vigor was conserved across horizontal and vertical saccades: individuals who had high vigor in their horizontal saccades also had high vigor in their vertical saccades. While there was considerable diversity in the time it took for individuals to respond to a visual stimulus, saccade vigor correlated with reaction time: individuals who had higher saccade vigor also tended to respond earlier to a visual cue. This pattern was also present for reaching movements: individuals who moved their arm faster during a reach also responded sooner to a visual stimulus. Furthermore, individuals who had greater vigor in their arm movements also had greater vigor in their head movements, but, notably, saccade vigor was not a strong predictor of arm or head vigor.
Did individuals who have greater vigor sacrifice accuracy for speed? We found that greater vigor coincided with greater variability in peak velocity of saccades, as well as peak velocity of reaching, but this variability at movement midpoint did not result in end-point inaccuracy. As a result, individuals who moved their eyes with high vigor, or moved their arms with high vigor, were not sacrificing accuracy for speed.
Using a Bayesian model of inference, we asked whether the various speed and reaction time variables that we had measured in a given subject were related to each other, possibly arising from a common source. The results suggested that with ~65% probability, a single latent variable, termed trait vigor, influenced speed and reaction time of all movements.
Why are there between-subject differences in vigor?
Differing biomechanics will influence how neural commands are transformed into action. For example, rhesus monkeys have saccade velocities that are about twice as fast as humans (Chen-Harris et al. 2008; Straube et al. 1997). Monkeys have eye biomechanics that are somewhat different from humans (Fuchs et al. 1988), but once these differences are accounted for, there remain persistent differences in movement vigor (Shadmehr et al. 2010).
In a number of models of motor control (Berret and Jean 2016; Rigoux and Guigon 2012; Shadmehr et al. 2010, 2016), it is thought that vigor is related to how the brain evaluates utility of the movement. Utility depends on three types of variables: subjective value of reward, effort, and time. Intriguingly, monkeys exhibit a greater temporal discount rate: when making a choice between stimuli that promise juice over a range of tens of seconds, thirsty monkeys (Hwang et al. 2009; Kobayashi and Schultz 2008) exhibit temporal discount rates that are higher than those of thirsty humans (Jimura et al. 2009). In a recent report, we measured saccade vigor in humans and then estimated the subjective value of time in each individual via a decision making task in which they decided how long to wait to improve their odds of success (Choi et al. 2014). We found that individuals who had high saccade vigor tended to have a higher cost of time, as evidenced by the shorter period of time they were willing to tolerate to improve their probability of reward. Therefore, it is possible that between-subject differences in vigor are partly due to individual differences in how the brain evaluates reward, effort, and time.
An alternate view is that vigor differences reflect a speed-accuracy trade-off: people who move rapidly are sacrificing accuracy for the purpose of arriving sooner. Our results appear to reject this hypothesis. We found that in both saccades and reaching greater vigor resulted in movements that near the midpoint exhibited greater variability (as indicated via peak velocity). This is in agreement with the idea that neural commands carry signal-dependent noise (Jones et al. 2002). If left uncompensated, this noise should also produce greater end-point variability. However, we found that the variability in peak velocity did not translate into variability in movement end point. That is, the variability that began the movement was partially corrected as the movement unfolded.
Earlier work had demonstrated that for saccades as peak velocity varied the brain adjusted the duration of the movement, partially compensating for the variability (Barton et al. 2003; Jürgens et al. 1981; Quaia et al. 2000). That is, even for saccades that have duration of 60 ms, the inaccuracies in the neural commands that start the movement are monitored and partially corrected as the movement unfolds (Keller et al. 1996; Soetedjo et al. 2002; Xu-Wilson et al. 2011). For saccades, this online correction appears to be partly dependent on the cerebellum (Xu-Wilson et al. 2009a). For reaching, variability that is present in the onset of the movement is also corrected as the movement unfolds (Smith et al. 2000), although control of reaching benefits from delayed sensory feedback to allow for further noise compensation (Izawa and Shadmehr 2008). These forms of within-movement control may account for our observations here, where we found that although some individuals moved twice as fast as others and produced greater variability near the midpoint of their movements, their elevated vigor did not translate into increased end-point inaccuracy. That is, speed-accuracy trade-off was not the main factor that accounted for the between-subject differences in vigor.
A relationship between vigor and reaction time.
We found that individuals who responded earlier to a visual stimulus also tended to produce the ensuing movement with greater vigor. That is, across individuals, saccade vigor and reaction time, as well as reach vigor and reaction time, were correlated. We were concerned that the differences in reaction time were an artifact of our measurement method (we used a velocity threshold to estimate the time at which the movement initiated). To check for this, we estimated the bias caused by our technique and found that the effect size that we had measured was about five times the bias induced by the measurement technique. Close inspection of the movement traces provided further confidence that the individuals who moved slower actually waited a longer period of time to start their movements (Fig. 2D, Fig. 7A).
Reaction time, i.e., the time it takes to respond to a stimulus and start a movement, is thought to reflect a process in which evidence for that action is accumulated until it reaches threshold (Carpenter 1999; Hanes and Schall 1996; McGill 1961; Ratcliff 1978; Vickers 1970). However, reaction time is affected by many of the same variables that affect vigor: greater reward increases movement vigor (Kobayashi et al. 2006; Reppert et al. 2015; Takikawa et al. 2002; Xu-Wilson et al. 2009b) and also reduces its reaction time (Kawagoe et al. 1998; Summerside et al. 2018). Increased effort reduces movement vigor (Shadmehr et al. 2016) and also reduces its reaction time (Fuller 1996; Ivry 1986; Rosenbaum 1980; Stelmach and Worringham 1988). Increasing the intertrial interval between movements reduces vigor and increases reaction time (Haith et al. 2012). These results suggest that if reaction time involves a process of integration of evidence to threshold, utility of the ensuing action likely influences the “evidence” that is being integrated.
One way to link vigor and reaction time is to consider that the utility of an action can be thought of as the difference between expected reward and effort, divided by time (Niv et al. 2007; Shadmehr et al. 2016). In this formulation, utility is a rate that expresses an average measure of goodness for the planned movement. Reward and effort affect this rate, influencing vigor of the movement. If we view reaction time as a period during which utility is integrated, then all the variables that influence vigor also influence reaction time. In this framework, a correlation between individual differences in vigor and individual differences in reaction time is expected because both measures are a reflection of the individual’s tendencies in evaluation of reward and effort.
An increasing target distance implies increasing effort, which should reduce utility of the action and increase reaction time. Indeed, for reaching we found that reaction time increased with target distance. This result is consistent with the view that during the reaction time period the brain integrates the utility of the upcoming action, and because increased distance implies increased effort and reduced utility, the reaction time increases.
However, for both horizontal and vertical saccades, when the amplitude of the saccade was small, reaction time decreased as saccade magnitude increased. This highlights an inconsistency in the theoretical view expressed above, suggesting that increased effort due to amplitude is not the only important variable that affects reaction time. To consider this, note that to produce a saccade one has to release fixation. Importantly, if the stimulus is visual, in the moments before a saccade two simultaneous neural events take place: neurons in the rostral pole of the superior colliculus (encoding the stimulus that encourages fixation) reduce their firing to baseline (Munoz and Wurtz 1993), whereas neurons in the caudal regions of the colliculus (encoding the stimulus that starts the movement) increase their firing until they produce a burst of spikes (Mays and Sparks 1980). This implies that the act of moving coincides with release from holding (Dorris and Munoz 1995; Shadmehr 2017). That is, reaction time is an interaction between two processes: decline of activity that supports the act of holding and buildup of activity that encourages moving (Dorris et al. 1997). This is consistent with the fact that saccades have a shorter reaction time when the fixation target disappears (gap task) compared with the scenario where the fixation target remains visible (overlap task) (Everling et al. 1999). In contrast, if the stimulus at fixation is not visual, during fixation there is little or no activity in the rostral pole of the superior colliculus (Munoz and Wurtz 1993), and as a result reaction times are shorter. Therefore, the convex function that we found relating saccade reaction time and amplitude may reflect two processes: utility of the stimulus that is at the fovea, encouraging fixation, and utility of the stimulus that is at the periphery, encouraging a movement. Further experiments are needed to test the predictions of this view.
While variables like reward and effort may jointly influence vigor and reaction time, our data also demonstrated fundamental differences between these traits of individuals. We found significant sex-based differences in control of saccades. Women made vertical (but not horizontal) saccades with greater vigor than men. However, for both horizontal and vertical saccades, men reacted sooner to the visual stimulus. Some of these observations are consistent with those reported by Bargary et al. (2017), who measured horizontal saccades in ~1,000 healthy people and also noted that men had shorter reaction times.
Neural basis of vigor.
Vigor appears to be partially under the control of the basal ganglia. For example, in the case of saccades, substantia nigra pars reticulate (SNr), an output nucleus of the basal ganglia, inhibits the superior colliculus, and removal of this inhibition reduces saccade reaction time and increases saccade peak velocity (Hikosaka and Wurtz 1985). More vigorous saccades are associated with a deeper pause in the firing rates of SNr cells (Sato and Hikosaka 2002), and reward modulates the depth of this pause (Handel and Glimcher 1999). Within the basal ganglia, some cells in the caudate nucleus influence the discharge of SNr neurons directly, while other cells do so indirectly via their projections to the external segment of globus pallidus (GPe). Caudate cells receive dopamine projections and generally fire more before a rewarding saccade (Kawagoe et al. 1998). Onset of a stimulus that promises reward results in a burst of dopamine (Matsumoto and Hikosaka 2007), which is followed by a more vigorous saccade (Tachibana and Hikosaka 2012). Indeed, chronic reduction in the concentration of dopamine in the caudate reduces saccade vigor by ∼30% (Kori et al. 1995). GPe cells inhibit SNr and fire more strongly preceding a more vigorous saccade, and bilateral lesion of this region eliminates the ability of the animal to modulate saccade vigor in response to changes in reward (Tachibana and Hikosaka 2012). Taken together, it appears that control of saccade vigor is partly associated with the amount of dopamine in the basal ganglia, modulating activity of caudate, affecting the depth of pause in the SNr.
The neural basis of between-subject differences in vigor is not known. However, between-subject differences in dopamine transmission in the basal ganglia are correlated with between-subject differences in the willingness to exert effort (Treadway et al. 2012). If vigor is viewed as an implicit measure of willingness to exert effort in order to acquire a rewarding state (Mazzoni et al. 2007), our results would predict that between-subject differences in vigor may be a reflection of between-subject differences in dopamine transmission in the basal ganglia.
However, we did not find a strong positive correlation between vigor of all movements within an individual. That is, although head and arm vigor were highly correlated, saccade vigor was not strongly correlated with arm vigor. While vigor of saccades appears to be controlled through the projections of SNr onto the superior colliculus, vigor of skeletal movements is likely controlled through the projections of the internal segment of globus pallidus onto the thalamus. Therefore, different circuits within the basal ganglia likely control vigor of oculomotor and skeletomotor behaviors. Our results indicate that the neural basis of vigor is more closely shared for control of skeletal movements than between eye movements and skeletal movements.
Limitations.
If vigor is a trait of individuality, it should remain stable across repeated measurements. Although here we did not measure movements over repeated sessions, our earlier study (Choi et al. 2014) measured saccades over the course of 4 days and found that the within-subject measure of vigor varied within a range of ~3.5% (this compares to between-subject range of 50%; Fig. 2E). Bargary et al. (2017) measured saccades twice over an interval of 26 days and also found vigor to be invariant within an individual. Here we measured saccades in the head-fixed and head-free conditions and found that the within-subject measures remained stable. For movements of the head, we also measured vigor with and without arm movements and found that the within-subject measures remained stable. While it is known that certain variables like predictability of the spatial location of the reach target modulate reach vigor (Isaias et al. 2011), we currently do not know whether reach vigor is stable over repeated measures. However, it appears that the vigor with which an individual moves a body part varies over a range that is typically much smaller than the between-subject range. If vigor is a trait, then its quantification may be useful in the problem of human recognition (Rigas et al. 2016). Recent work shows that aggregation of information from a number of eye movement-derived features allows machines to identify humans nearly as well as is possible from fingerprints (Friedman et al. 2017).
We focused on vigor traits of individuals but did not measure decision making traits. Therefore, we cannot draw any conclusions regarding a potential link between decision making and movement making. However, our earlier study did measure both features of behavior and found that individuals who exhibited high temporal discounting in their patterns of decision making also tended to move more vigorously (Choi et al. 2014). Future studies will need to measure subjective evaluation of reward and effort during decision making and ask whether these patterns are consistent with individual differences in movement vigor.
Conclusions.
We found that the speed with which people moved their eyes, head, and arm, as well as the amount of time that they needed to react to a visual stimulus to initiate those movements, all were influenced by a single latent variable that we labeled as trait vigor. Individuals who moved their eyes or arm more vigorously (than the population mean) also exhibited shorter reaction times. Those who moved their arm with greater vigor during reaching also moved their head with greater vigor during gaze shifts. These differences in vigor did not translate into differences in end-point accuracy, demonstrating that individual differences in vigor were not a reflection of a speed-accuracy trade-off. Rather, our results are more consistent with the view that differences in vigor may be features of individuality, reflecting potential differences in the willingness to exert effort.
GRANTS
The work was supported by grants from the National Institute of Neurological Disorders and Stroke (5-R01 NS-078311), the Office of Naval Research (N00014-15-1-2312), the National Science Foundation (CNS-1714623 and CNS-1250718), and the National Institute of Standards and Technology (60NANB15D325 and 60NANB16D293).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.R.R., O.K., and R.S. conceived and designed research; T.R.R. and I.R. performed experiments; T.R.R., E.S.-N., and R.S. analyzed data; T.R.R., D.H., O.K., and R.S. interpreted results of experiments; T.R.R., E.S.-N., and R.S. prepared figures; T.R.R. and R.S. drafted manuscript; T.R.R., D.H., O.K., and R.S. edited and revised manuscript; T.R.R., I.R., D.H., E.S.-N., O.K., and R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Amirsaman Sajad for comments on the manuscript.
REFERENCES
- Bargary G, Bosten JM, Goodbourn PT, Lawrance-Owen AJ, Hogg RE, Mollon JD. Individual differences in human eye movements: an oculomotor signature? Vision Res 141: 157–169, 2017. doi: 10.1016/j.visres.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Barton EJ, Nelson JS, Gandhi NJ, Sparks DL. Effects of partial lidocaine inactivation of the paramedian pontine reticular formation on saccades of macaques. J Neurophysiol 90: 372–386, 2003. doi: 10.1152/jn.01041.2002. [DOI] [PubMed] [Google Scholar]
- Berret B, Jean F. Why don’t we move slower? The value of time in the neural control of action. J Neurosci 36: 1056–1070, 2016. doi: 10.1523/JNEUROSCI.1921-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RH. A neural mechanism that randomises behaviour. J Conscious Stud 6: 13–22, 1999. [Google Scholar]
- Chen-Harris H, Joiner WM, Ethier V, Zee DS, Shadmehr R. Adaptive control of saccades via internal feedback. J Neurosci 28: 2804–2813, 2008. doi: 10.1523/JNEUROSCI.5300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JE, Vaswani PA, Shadmehr R. Vigor of movements and the cost of time in decision making. J Neurosci 34: 1212–1223, 2014. doi: 10.1523/JNEUROSCI.2798-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva JA, Tecuapetla F, Paixão V, Costa RM. Dopamine neuron activity before action initiation gates and invigorates future movements. Nature 554: 244–248, 2018. doi: 10.1038/nature25457. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Munoz DP. A neural correlate for the gap effect on saccadic reaction times in monkey. J Neurophysiol 73: 2558–2562, 1995. doi: 10.1152/jn.1995.73.6.2558. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Paré M, Munoz DP. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J Neurosci 17: 8566–8579, 1997. doi: 10.1523/JNEUROSCI.17-21-08566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Dorris MC, Klein RM, Munoz DP. Role of primate superior colliculus in preparation and execution of anti-saccades and pro-saccades. J Neurosci 19: 2740–2754, 1999. doi: 10.1523/JNEUROSCI.19-07-02740.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Eye-head coordination during head-unrestrained gaze shifts in rhesus monkeys. J Neurophysiol 77: 2328–2348, 1997. doi: 10.1152/jn.1997.77.5.2328. [DOI] [PubMed] [Google Scholar]
- Friedman L, Nixon MS, Komogortsev OV. Method to assess the temporal persistence of potential biometric features: application to oculomotor, gait, face and brain structure databases. PLoS One 12: e0178501, 2017. doi: 10.1371/journal.pone.0178501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs AF, Scudder CA, Kaneko CR. Discharge patterns and recruitment order of identified motoneurons and internuclear neurons in the monkey abducens nucleus. J Neurophysiol 60: 1874–1895, 1988. doi: 10.1152/jn.1988.60.6.1874. [DOI] [PubMed] [Google Scholar]
- Fuller JH. Eye position and target amplitude effects on human visual saccadic latencies. Exp Brain Res 109: 457–466, 1996. doi: 10.1007/BF00229630. [DOI] [PubMed] [Google Scholar]
- Haith AM, Reppert TR, Shadmehr R. Evidence for hyperbolic temporal discounting of reward in control of movements. J Neurosci 32: 11727–11736, 2012. doi: 10.1523/JNEUROSCI.0424-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel A, Glimcher PW. Quantitative analysis of substantia nigra pars reticulata activity during a visually guided saccade task. J Neurophysiol 82: 3458–3475, 1999. doi: 10.1152/jn.1999.82.6.3458. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science 274: 427–430, 1996. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature 394: 780–784, 1998. doi: 10.1038/29528. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. II. Effects of muscimol in monkey substantia nigra pars reticulata. J Neurophysiol 53: 292–308, 1985. doi: 10.1152/jn.1985.53.1.292. [DOI] [PubMed] [Google Scholar]
- Hwang J, Kim S, Lee D. Temporal discounting and inter-temporal choice in rhesus monkeys. Front Behav Neurosci 3: 9, 2009. doi: 10.3389/neuro.08.009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaias IU, Moisello C, Marotta G, Schiavella M, Canesi M, Perfetti B, Cavallari P, Pezzoli G, Ghilardi MF. Dopaminergic striatal innervation predicts interlimb transfer of a visuomotor skill. J Neurosci 31: 14458–14462, 2011. doi: 10.1523/JNEUROSCI.3583-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivry RB. Force and timing components of the motor program. J Mot Behav 18: 449–474, 1986. doi: 10.1080/00222895.1986.10735390. [DOI] [PubMed] [Google Scholar]
- Izawa J, Shadmehr R. On-line processing of uncertain information in visuomotor control. J Neurosci 28: 11360–11368, 2008. doi: 10.1523/JNEUROSCI.3063-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, Myerson J, Hilgard J, Braver TS, Green L. Are people really more patient than other animals? Evidence from human discounting of real liquid rewards. Psychon Bull Rev 16: 1071–1075, 2009. doi: 10.3758/PBR.16.6.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KE, Hamilton AF, Wolpert DM. Sources of signal-dependent noise during isometric force production. J Neurophysiol 88: 1533–1544, 2002. doi: 10.1152/jn.2002.88.3.1533. [DOI] [PubMed] [Google Scholar]
- Jürgens R, Becker W, Kornhuber HH. Natural and drug-induced variations of velocity and duration of human saccadic eye movements: evidence for a control of the neural pulse generator by local feedback. Biol Cybern 39: 87–96, 1981. doi: 10.1007/BF00336734. [DOI] [PubMed] [Google Scholar]
- Kawagoe R, Takikawa Y, Hikosaka O. Expectation of reward modulates cognitive signals in the basal ganglia. Nat Neurosci 1: 411–416, 1998. doi: 10.1038/1625. [DOI] [PubMed] [Google Scholar]
- Keller EL, Gandhi NJ, Shieh JM. Endpoint accuracy in saccades interrupted by stimulation in the omnipause region in monkey. Vis Neurosci 13: 1059–1067, 1996. doi: 10.1017/S0952523800007719. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Nomoto K, Watanabe M, Hikosaka O, Schultz W, Sakagami M. Influences of rewarding and aversive outcomes on activity in macaque lateral prefrontal cortex. Neuron 51: 861–870, 2006. doi: 10.1016/j.neuron.2006.08.031. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Schultz W. Influence of reward delays on responses of dopamine neurons. J Neurosci 28: 7837–7846, 2008. doi: 10.1523/JNEUROSCI.1600-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kori A, Miyashita N, Kato M, Hikosaka O, Usui S, Matsumura M. Eye movements in monkeys with local dopamine depletion in the caudate nucleus. II. Deficits in voluntary saccades. J Neurosci 15: 928–941, 1995. doi: 10.1523/JNEUROSCI.15-01-00928.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466: 622–626, 2010. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The Neurology of Eye Movements. Oxford, UK: Oxford Univ. Press, 2015. doi: 10.1093/med/9780199969289.001.0001 [DOI] [Google Scholar]
- Manohar SG, Chong TT, Apps MA, Batla A, Stamelou M, Jarman PR, Bhatia KP, Husain M. Reward pays the cost of noise reduction in motor and cognitive control. Curr Biol 25: 1707–1716, 2015. doi: 10.1016/j.cub.2015.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 447: 1111–1115, 2007. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Mays LE, Sparks DL. Dissociation of visual and saccade-related responses in superior colliculus neurons. J Neurophysiol 43: 207–232, 1980. doi: 10.1152/jn.1980.43.1.207. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Hristova A, Krakauer JW. Why don’t we move faster? Parkinson’s disease, movement vigor, and implicit motivation. J Neurosci 27: 7105–7116, 2007. doi: 10.1523/JNEUROSCI.0264-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill WJ. Loudness and reaction time: a guided tour of the Listener’s private world. Acta Psychol (Amst) 19: 193–199, 1961. doi: 10.1016/S0001-6918(61)80070-X. [DOI] [Google Scholar]
- Milstein DM, Dorris MC. The influence of expected value on saccadic preparation. J Neurosci 27: 4810–4818, 2007. doi: 10.1523/JNEUROSCI.0577-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteon JA, Avillac M, Yan X, Wang H, Crawford JD. Neural mechanisms for predictive head movement strategies during sequential gaze shifts. J Neurophysiol 108: 2689–2707, 2012. doi: 10.1152/jn.00222.2012. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J Neurophysiol 70: 559–575, 1993. doi: 10.1152/jn.1993.70.2.559. [DOI] [PubMed] [Google Scholar]
- Niv Y, Daw ND, Joel D, Dayan P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology (Berl) 191: 507–520, 2007. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- Oommen BS, Smith RM, Stahl JS. The influence of future gaze orientation upon eye-head coupling during saccades. Exp Brain Res 155: 9–18, 2004. doi: 10.1007/s00221-003-1694-z. [DOI] [PubMed] [Google Scholar]
- Opris I, Lebedev M, Nelson RJ. Motor planning under unpredictable reward: modulations of movement vigor and primate striatum activity. Front Neurosci 5: 61, 2011. doi: 10.3389/fnins.2011.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquereau B, Turner RS. Limited encoding of effort by dopamine neurons in a cost-benefit trade-off task. J Neurosci 33: 8288–8300, 2013. doi: 10.1523/JNEUROSCI.4619-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaia C, Paré M, Wurtz RH, Optican LM. Extent of compensation for variations in monkey saccadic eye movements. Exp Brain Res 132: 39–51, 2000. doi: 10.1007/s002219900324. [DOI] [PubMed] [Google Scholar]
- Ratcliff R. A theory of memory retrieval. Psychol Rev 85: 59–108, 1978. doi: 10.1037/0033-295X.85.2.59. 3406246 [DOI] [Google Scholar]
- Reppert TR, Lempert KM, Glimcher PW, Shadmehr R. Modulation of saccade vigor during value-based decision making. J Neurosci 35: 15369–15378, 2015. doi: 10.1523/JNEUROSCI.2621-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas I, Komogortsev O, Shadmehr R. Biometric recognition via eye movements: saccadic vigor and acceleration cues. ACM Trans Appl Percept 13: 6, 2016. doi: 10.1145/2842614. [DOI] [Google Scholar]
- Rigoux L, Guigon E. A model of reward- and effort-based optimal decision making and motor control. PLOS Comput Biol 8: e1002716, 2012. doi: 10.1371/journal.pcbi.1002716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DA. Human movement initiation: specification of arm, direction, and extent. J Exp Psychol Gen 109: 444–474, 1980. doi: 10.1037/0096-3445.109.4.444. [DOI] [PubMed] [Google Scholar]
- Rousseeuw PJ, Croux C. Alternatives to the median absolute deviation. J Am Stat Assoc 88: 1273–1283, 1993. doi: 10.1080/01621459.1993.10476408. [DOI] [Google Scholar]
- Sato M, Hikosaka O. Role of primate substantia nigra pars reticulata in reward-oriented saccadic eye movement. J Neurosci 22: 2363–2373, 2002. doi: 10.1523/JNEUROSCI.22-06-02363.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R. Distinct neural circuits for control of movement vs. holding still. J Neurophysiol 117: 1431–1460, 2017. doi: 10.1152/jn.00840.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Huang HJ, Ahmed AA. A representation of effort in decision-making and motor control. Curr Biol 26: 1929–1934, 2016. doi: 10.1016/j.cub.2016.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi S. Biological Learning and Control: How the Brain Builds Representations, Predicts Events, and Makes Decisions. Cambridge, MA: MIT Press, 2012. doi: 10.7551/mitpress/9780262016964.001.0001. [DOI] [Google Scholar]
- Shadmehr R, Orban de Xivry JJ, Xu-Wilson M, Shih TY. Temporal discounting of reward and the cost of time in motor control. J Neurosci 30: 10507–10516, 2010. doi: 10.1523/JNEUROSCI.1343-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Brandt J, Shadmehr R. Motor dysfunction in Huntington’s disease begins as a dysfunction in error feedback control. Nature 403: 544–549, 2000. doi: 10.1038/35000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetedjo R, Kaneko CR, Fuchs AF. Evidence that the superior colliculus participates in the feedback control of saccadic eye movements. J Neurophysiol 87: 679–695, 2002. doi: 10.1152/jn.00886.2000. [DOI] [PubMed] [Google Scholar]
- Stelmach GE, Worringham CJ. The preparation and production of isometric force in Parkinson’s disease. Neuropsychologia 26: 93–103, 1988. doi: 10.1016/0028-3932(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Straube A, Fuchs AF, Usher S, Robinson FR. Characteristics of saccadic gain adaptation in rhesus macaques. J Neurophysiol 77: 874–895, 1997. doi: 10.1152/jn.1997.77.2.874. [DOI] [PubMed] [Google Scholar]
- Summerside EM, Shadmehr R, Ahmed AA. Vigor of reaching movements: reward discounts the cost of effort. J Neurophysiol 119: 2347–2357, 2018. doi: 10.1152/jn.00872.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana Y, Hikosaka O. The primate ventral pallidum encodes expected reward value and regulates motor action. Neuron 76: 826–837, 2012. doi: 10.1016/j.neuron.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takikawa Y, Kawagoe R, Itoh H, Nakahara H, Hikosaka O. Modulation of saccadic eye movements by predicted reward outcome. Exp Brain Res 142: 284–291, 2002. doi: 10.1007/s00221-001-0928-1. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Kessler RM, Zald DH. Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci 32: 6170–6176, 2012. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the “EEfRT”? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One 4: e6598, 2009. doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers D. Evidence for an accumulator model of psychophysical discrimination. Ergonomics 13: 37–58, 1970. doi: 10.1080/00140137008931117. [DOI] [PubMed] [Google Scholar]
- Wang C, Xiao Y, Burdet E, Gordon J, Schweighofer N. The duration of reaching movement is longer than predicted by minimum variance. J Neurophysiol 116: 2342–2345, 2016. doi: 10.1152/jn.00148.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Wilson M, Chen-Harris H, Zee DS, Shadmehr R. Cerebellar contributions to adaptive control of saccades in humans. J Neurosci 29: 12930–12939, 2009a. doi: 10.1523/JNEUROSCI.3115-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Wilson M, Tian J, Shadmehr R, Zee DS. TMS perturbs saccade trajectories and unmasks an internal feedback controller for saccades. J Neurosci 31: 11537–11546, 2011. doi: 10.1523/JNEUROSCI.1584-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Wilson M, Zee DS, Shadmehr R. The intrinsic value of visual information affects saccade velocities. Exp Brain Res 196: 475–481, 2009b. doi: 10.1007/s00221-009-1879-1. [DOI] [PMC free article] [PubMed] [Google Scholar]