Abstract
Dopamine modulation of retinal signaling has been shown to be an important part of retinal adaptation to increased background light levels, but the role of dopamine modulation of retinal inhibition is not clear. We previously showed that light adaptation causes a large reduction in inhibition to rod bipolar cells, potentially to match the decrease in excitation after rod saturation. In this study, we determined how dopamine D1 receptors in the inner retina contribute to this modulation. We found that D1 receptor activation significantly decreased the magnitude of inhibitory light responses from rod bipolar cells, whereas D1 receptor blockade during light adaptation partially prevented this decline. To determine what mechanisms were involved in the modulation of inhibitory light responses, we measured the effect of D1 receptor activation on spontaneous currents and currents evoked from electrically stimulating amacrine cell inputs to rod bipolar cells. D1 receptor activation decreased the frequency of spontaneous inhibition with no change in event amplitudes, suggesting a presynaptic change in amacrine cell activity in agreement with previous reports that rod bipolar cells lack D1 receptors. Additionally, we found that D1 receptor activation reduced the amplitude of electrically evoked responses, showing that D1 receptors can modulate amacrine cells directly. Our results suggest that D1 receptor activation can replicate a large portion but not all of the effects of light adaptation, likely by modulating release from amacrine cells onto rod bipolar cells.
NEW & NOTEWORTHY We demonstrated a new aspect of dopaminergic signaling that is involved in mediating light adaptation of retinal inhibition. This D1 receptor-dependent mechanism likely acts through receptors located directly on amacrine cells, in addition to its potential role in modulating the strength of serial inhibition between amacrine cells. Our results also suggest that another D2/D4 receptor-dependent or dopamine-independent mechanism must also be involved in light adaptation of inhibition to rod bipolar cells.
Keywords: amacrine cell, dopamine, light inhibition, adaptation, rod bipolar cell
INTRODUCTION
In natural environments, our eyes are exposed to light levels that can vary by 10–12 orders of magnitude, from a moonless night to noontime on a bright sunny day (Shapley and Enroth-Cugell 1984). In contrast to this large range of stimuli, retinal neurons can only vary their firing rate by a factor of ~100–1,000 (Sakmann and Creutzfeldt 1969). Thus, to avoid signal saturation, our retinas must continuously adjust their sensitivity to light based on ambient conditions. The various mechanisms that contribute to this dynamic gain control are collectively referred to as light adaptation.
Part of this adjustment to luminance is attributable to the existence of two kinds of photoreceptors, rods and cones. Rods are activated by dimmer and cones by brighter light levels, with significant response overlap at intermediate intensities. However, rods and cones do not signal through separate circuitry; instead rod signals are inserted into cone pathways through gap junctional connections between AII amacrine cells and cone bipolar cells (Strettoi et al. 1992), as well as between rod and cone photoreceptors (Asteriti et al. 2014). Rods can also contact some types of OFF bipolar cells in the mouse and other mammalian species (Pang et al. 2012). As light levels increase and cone vision becomes dominant over rod vision, there is a need to effectively shut down the rod pathway so that the fidelity of higher acuity cone signaling can be maintained. One mechanism that can contribute to this is lateral inhibition from amacrine cells excited by cone pathways (see Fig. 1).
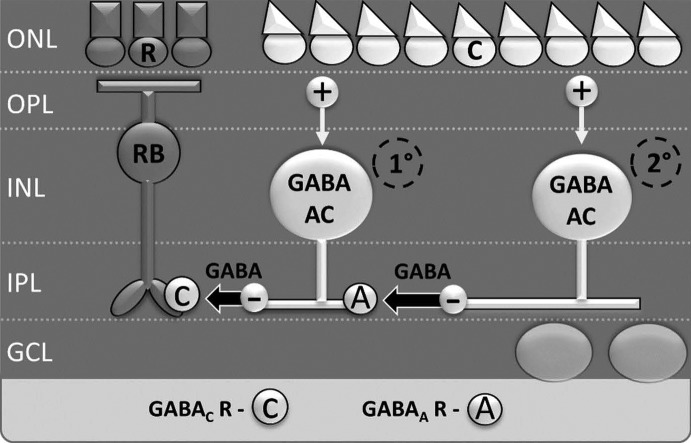
Fig. 1.
Lateral inhibition onto rod bipolar cells is modulated by serial inhibition between amacrine cells. Lateral inhibition to rod bipolar cells is largely mediated by primary wide-field GABAergic amacrine cells (1°) through GABA release onto GABAC receptors. These primary amacrine cells are in turn inhibited by secondary (2°) amacrine cells, through GABA release onto GABAA receptors. When a full-field light stimulus activates both primary and secondary amacrine cells, primary amacrine cells are inhibited by secondary amacrine cells, resulting in reduced GABA release onto rod bipolar cells. This mechanism is known as serial inhibition. ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; R, rod photoreceptor; C, cone photoreceptor; RB, rod bipolar cell; AC, amacrine cell.
Accordingly, light-evoked inhibition to rod bipolar cells reaches its apex at cone-dominant light intensities under dark-adapted conditions (Eggers et al. 2013b; Pang et al. 2004). Interestingly, a previous study from our laboratory found that, when retinal slices were exposed to a rod-saturating background adapting light for 5 min that significantly reduced rod signaling, evoked inhibition at similar intensities was almost completely absent (Eggers et al. 2013b). This suggests that, under bright enough ambient light conditions, the retina may decrease both excitatory and inhibitory drive to rod bipolar cells, perhaps to conserve energy. Because light-evoked inhibitory postsynaptic currents (L-IPSCs) in rod bipolar cells are almost exclusively mediated by amacrine cells (Euler and Masland 2000; Hare and Owen 1996), this change in inhibitory inputs suggests a modification to circuitry of the inner retina. However, the signaling mechanisms that lead to this change have not yet been fully elucidated.
A likely effector of this change is dopamine, a neuromodulator implicated in multiple different aspects of light adaptation (Witkovsky 2004). In the retina, dopamine release by dopaminergic amacrine cells steadily increases in concert with background luminance (Mills et al. 2007). This dopamine mediates its effects in a paracrine manner, diffusing throughout the retinal space and binding to dopamine type 1 receptors (D1Rs) located on horizontal cells, some amacrine cells, some types of bipolar cells, and ganglion cells (Farshi et al. 2016; Veruki and Wässle 1996), as well as type 4 receptors (D4Rs) located on photoreceptors (Cohen et al. 1992) and type 2 receptors on dopaminergic amacrine cells (Derouiche and Asan 1999; Li et al. 2013; Veruki 1997). There is also evidence that D4Rs are expressed to a lesser degree in ganglion cells and inner nuclear neurons as well (Cohen et al. 1992; Li et al. 2013). It is known that dopamine can suppress rod signaling under photopic conditions by decreasing rod-cone photoreceptor coupling (Li et al. 2013; Ribelayga et al. 2008; Ribelayga and Mangel 2010) and can also reduce cone bipolar cell excitability through the D1R-dependent desensitization of voltage-gated sodium channels (Ichinose and Lukasiewicz 2007; Smith et al. 2015a). However, the effects of dopamine on inhibitory signaling to rod bipolar cells have not yet been fully explored. Because D4Rs are primarily located in the outer retina and their expression is negatively correlated with increased light levels (Li et al. 2013), it seems likely that changes in amacrine cell-mediated inhibition are due to the action of D1Rs. The goal of this study was to determine the contribution of D1R activation to light adaptation-induced changes in rod bipolar cell inhibition.
METHODS
Retinal slice preparation.
Animal protocols conformed with the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research and were approved by the University of Arizona Institutional Animal Care and Use Committee. As previously described (Eggers et al. 2013b), C57BL/6J male mice (Jackson Laboratories, Bar Harbor, ME) 35–60 days of age were euthanized using carbon dioxide. The eyes were enucleated, the cornea and lens removed, and the eyecup was incubated in cold extracellular solution (see Solutions and drugs) with 800 U/ml of hyaluronidase for 20 min. The eyecup was washed with cold extracellular solution, and the retina was removed. The retina was trimmed into an approximate rectangle and mounted onto 0.45-µm nitrocellulose filter paper (Millipore, Billerica, MA). The filter paper containing the retina was then transferred to a hand chopper. The filter paper was sliced into 250-µm-thick slices, rotated 90°, and mounted onto glass coverslips using vacuum grease. All dissections and light response recording procedures were performed under infrared illumination to preserve the light sensitivity of our preparations.
Solutions and drugs.
Extracellular solution used as a control bath for dissection and whole cell recordings was bubbled with a mixture of 95% O2-5% CO2, which set the pH to ~7.4 and contained the following (in mM): 125.00 NaCl, 2.50 KCl, 1.00 MgCl2, 1.25 NaH2PO4, 20.00 glucose, 26.00 NaHCO3, and 2.00 CaCl2. The intracellular solution in the recording pipette used for monitoring inhibitory rod bipolar cell currents contained the following (in mM): 120.00 CsOH, 120.00 gluconic acid, 1.00 MgCl2, 10.00 HEPES, 10.00 EGTA, 10.00 tetraethylammonium-Cl, 10.00 phosphocreatine-Na2, 4.00 Mg-ATP, 0.50 Na-GTP, and 50.00 µM Alexa Fluor 488 (Invitrogen, Carlsbad, CA) and was adjusted to pH 7.2 with CsOH. With these concentrations, the driving force for Cl− was calculated as 60 mV in all solutions.
A D1R agonist and antagonist were used to mimic/inhibit the effects of light adaptation. SKF-38393 (SKF, 20 µM; Tocris, Bristol, United Kingdom) was used to selectively activate D1 receptors, and SCH-23390 (SCH, 50 µM; Tocris) was used to block D1Rs. The GABAA receptor (R) antagonist SR-95531 (SR, 20 µM) and glycineR antagonist strychnine (1 µM) were used to isolate GABACR currents. Each was diluted in extracellular solution to the given concentration and applied to the bath during the recordings by a gravity-driven superfusion system (Cell Microcontrols, Norfolk, VA) at a rate of ~1 ml/min. Chemicals were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise indicated.
Whole cell recordings.
All light response recordings were first performed on retinal slices in a dark-adapted state, followed by recordings performed under drug-added and/or light-adapted conditions (see Light Stimuli). Electrical stimulation experiments were performed entirely under light-adapted conditions. Retinal slices on glass coverslips were placed in a custom chamber and heated to 32° by temperature-controlled thin-stage and inline heaters (Cell Microcontrols). For SKF experiments, dark-adapted light responses were measured first, followed by a 5-min incubation period with SKF, after which light responses were again measured in the presence of SKF. For SCH experiments, slices were incubated in SCH-containing extracellular solution for a minimum of 30 min before experimentation, with continuous perfusion of SCH onto the slice after transfer to the bath chamber. Dark-adapted light response measurements were followed by 5 min of light adaptation, before again recording light responses (while maintaining SCH+ perfusion for the entire duration of the experiment.) Whole cell voltage-clamp recordings from rod bipolar cells in retinal slices were made as previously described (Eggers and Lukasiewicz 2006b). L-IPSCs and spontaneous (s)IPSCs were recorded from rod bipolar cells clamped at 0 mV, the reversal potential for currents mediated by nonselective cation channels. At this holding voltage, our average holding current was −4.74 ± 3.86 pA. For all recordings, series resistance was uncompensated. Electrodes were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL) using a P97 Flaming/Brown puller (Sutter Instruments, Novato, CA) and had resistances of 5–7 MΩ. Liquid junction potentials of 20 mV, calculated with Clampex software (Molecular Devices, Sunnyvale, CA), were corrected for before recording. Light responses were sampled at 10 kHz and filtered at 6 kHz with the four-pole 165-Bessel filter on a Multi-Clamp 700B patch-clamp amplifier (Molecular Devices). Electrical stimulation responses were sampled at 10 kHz and filtered with a 5-kHz four-pole low-pass Bessel filter on an Axopatch 200B patch-clamp amplifier (Molecular Devices). Both signals were digitized with a Digidata 1140 data acquisition system (Molecular Devices) and Clampex software. Confirmation of rod bipolar cell morphology (Ghosh et al. 2004) was done at the end of each recording using an Intensilight fluorescence lamp and Digitalsight camera controlled by Elements software (Nikon Instruments, Tokyo, Japan).
Light stimuli.
Full-field light stimuli were evoked with a light-emitting diode (LED; HLMP-3950, λpeak = 525 nm; Agilent, Palo Alto, CA) that was calibrated with an S471 optometer (Gamma Scientific, San Diego, CA) and projected through the camera port of the microscope. The stimulus intensities were chosen to cover both rod-dominant (9.5, 95.0, 950.0 photons·µm−2·s−1) and cone-dominant (9.5·103, 9.5·104, and 9.5·105 photons·µm−2·s−1) response ranges. These intensities were calculated to be equivalent to 4.75, 47.50, 475.00, 4.75·103, 4.75·104, and 4.75·105 R*·rod−1·s−1, respectively (Field and Rieke 2002). Sequential light responses were recorded with a stimulating interval of 30 s. Stimulus intensities, background rod-saturating light (950 photons·µm−2·s−1), and duration (30 ms) were controlled with Clampex software by varying the current through the LED. The background intensity was chosen, as it was shown to maximally activate rods (Wang and Kefalov 2009). A rod-saturating background light was applied for 5 min to light adapt the retina slice and was sustained throughout all light-adapted recordings.
Electrical stimulation.
The stimulating pipette, containing extracellular solution, was placed in the region of the rod bipolar cell axon terminals, in the inner plexiform layer, and a 1-ms, 4- to 20-µA stimulus was applied by an S48 stimulator (Grass, Warwick, RI) with an attached PSIU6 photoelectric isolation unit (Grass). The location of rod bipolar cell terminals was identified by cell filling with the Alexa Fluor included in the recording pipette. The stimulating interval was 60 s.
Data analysis and statistics.
Between two and four traces of both L-IPSCs and electrically evoked (eIPSCs) for each condition were averaged using Clampfit (Molecular Devices) except for cases in which patch seals broke before multiple responses could be recorded. The peak amplitude, charge transfer (Q), time to peak, and decay to 37% of the peak (D37) were determined. The bounds for integration used to calculate Q were marked by the times at which the response began and when it returned to baseline, typically 1–2 s. The time to peak was calculated as the temporal difference between stimulus onset and the response peak amplitude. Because the decay time could not be easily fitted with either a single or double exponential curve, we determined the D37 by computing the time it took for the IPSC to decline from its peak amplitude to 37% of its peak amplitude.
For L-IPSCs, data from each cell were normalized to its dark-adapted response at the maximum light intensity (9.5·105 photons·µm−2·s−1). If there was no discernable response for a given light intensity after filtering and averaging, the peak amplitude was recorded as 0, and it was excluded from our analysis of response kinetics. For eIPSCs, a normalized data value of the percentage of total GABACR-mediated eIPSC that remained after SKF treatment was calculated for each cell. Comparisons between experimental conditions and luminance intensities were made with two-way repeated-measures (RM) ANOVA tests using the Student-Newman-Keuls (SNK) method for pairwise comparisons in SigmaPlot (Systat Software, San Jose, CA). For eIPSCs, paired Student’s t-tests were used to compare the responses before and after SKF treatment. If any data were shown to have a nonnormal distribution or unequal variance, tests were repeated on the log10 values of data.
For spontaneous currents, events were included in the analysis if they occurred after a light response had returned to baseline but before the 1 s preceding the subsequent light stimulus. Frequency, amplitude, interevent interval (IEI), and decay constants were calculated using MiniAnalysis software (Synaptosoft, Fort Lee, NJ). Decay constants were fit with a single exponent. Effects of treatments on sIPSCs were analyzed at the single cell level with Kolmogorov-Smirnov (K-S) tests in Clampfit. Amplitude, decay τ, and IEI histogram distributions were normalized to the number of events. Effects of SKF or light adaptation + SCH on sIPSCs in the same cells were analyzed with paired t-tests, and comparisons between different groups of cells were analyzed with unpaired t-tests, after normalizing each cell to its dark-adapted state. Individual cells were only included in the analysis if they had 10 or more spontaneous events per treatment condition. For all studies, differences were considered significant when P ≤ 0.05. All data are reported as means ± SE.
RESULTS
D1R activation partially mimics changes to rod bipolar cell L-IPSCs that occur with light adaptation.
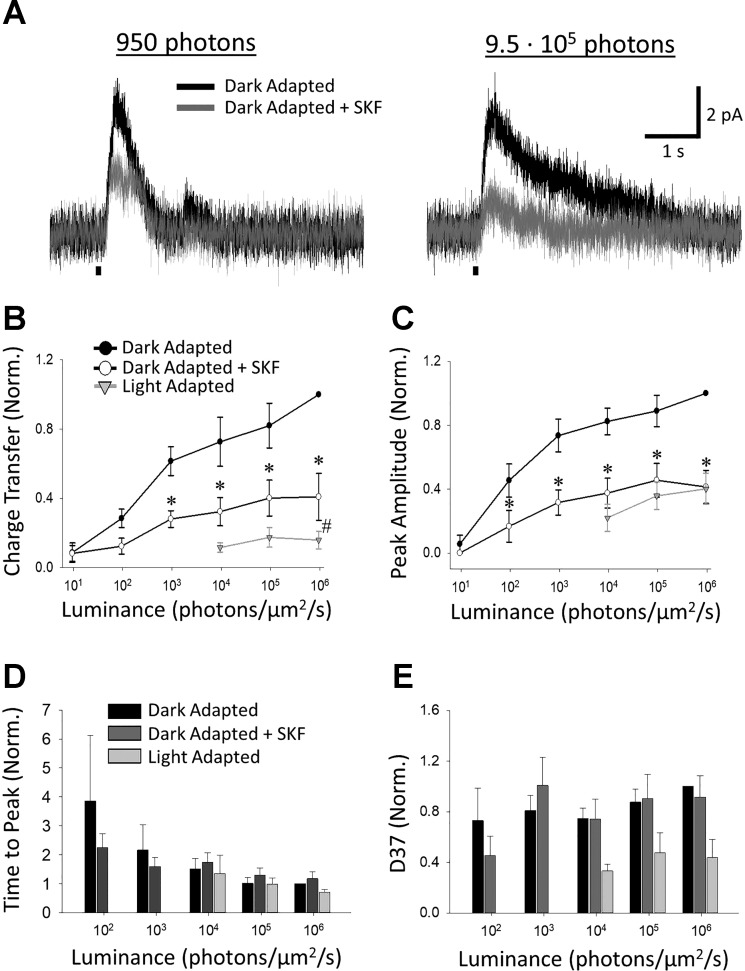
To determine the possible contribution of D1R activation to light adaptation, we measured L-IPSCs of rod bipolar cells in dark-adapted retinal slices both before and after either a light-adapting stimulus or application of the D1R agonist SKF (Fig. 2A). By recording L-IPSCs at multiple light levels of increasing intensity, we were able to examine rod- and cone-driven inputs because rod bipolar cells receive inhibitory amacrine cell inputs that come from both the rod and cone pathways (Eggers and Lukasiewicz 2006a; Pang et al. 2004). For the sake of comparison, control rod bipolar cell light adaptation data (n = 4) were adapted from a previous work from our laboratory (Eggers et al. 2013b) and combined with newly collected control data (n = 4), after ensuring that the dark-adapted responses of the two groups did not significantly differ in unnormalized or normalized (to the response at the maximum light intensity) Q (2-way RM ANOVA, unnormalized: P = 0.721, normalized: P = 0.973), peak amplitude (2-way RM ANOVA, unnormalized: P = 0.139, normalized: P = 0.927), or D37 (2-way RM ANOVA, unnormalized: P = 0.064, normalized: P = 0.947). The unnormalized times to peak were significantly longer for the newly collected data (2-way RM ANOVA, P = 0.010), but this difference was not significant in the normalized responses used for further analysis (2-way RM ANOVA, P = 0.366). Light adaptation caused significant decreases in L-IPSC Q (n = 8; 2-way RM ANOVA, P < 0.001, Fig. 2B) and peak amplitude (n = 8; 2-way RM ANOVA, P < 0.001, Fig. 2C) at all intensities tested above the adapting background, in agreement with previous results (Eggers et al. 2013b). Additionally, after light adaptation, rod bipolar cell L-IPSCs did not show significant changes in time to peak (n = 6; 2-way RM ANOVA, P = 0.972, Fig. 2D) but did have significantly faster 37% decays (D37) (n = 6; 2-way RM ANOVA, P < 0.001, Fig. 2E). After SKF application that mimics dopamine release onto D1Rs during light adaptation, we found that dark-adapted L-IPSCs were significantly smaller. Both Q (n = 6; 2-way RM ANOVA, P = 0.012) and peak amplitude (n = 6; 2-way RM ANOVA, P = 0.003) were reduced by 60 ± 13% (SNK, P < 0.001) and 59 ± 10% (SNK, P < 0.001), respectively, at the highest light intensity (Fig. 2, B and C). Pairwise comparisons revealed that Q was significantly reduced at all cone-dominant intensities (SNK, 9.5·103, P = 0.003; 9.5·104, P = 0.003; 9.5·105, P < 0.001, Fig. 2B) and at the highest rod-dominant intensity (SNK, 950, P = 0.011, Fig. 2B), whereas peak amplitudes were significantly diminished at all light levels (SNK, P < 0.01, Fig. 2C) except for the very dimmest (SNK, 9.5, P = 0.56, Fig. 2C). Unlike light adaptation, SKF did not shorten L-IPSC decays (n = 5; 2-way RM ANOVA, P = 0.783, Fig. 2E). No significant changes in time to peak were found (n = 5; 2-way RM ANOVA, P = 0.154, Fig. 2D).
Fig. 2.
Activation of D1 receptors reduces rod bipolar cell light-evoked inhibitory postsynaptic currents (L-IPSCs). A: example traces of L-IPSCs in the same dark-adapted cell in response to 30-ms stimuli (small black bars below traces) of 950 photons·µm−2·s−1 (left) or 9.5·105 photons·µm−2·s−1 (right), before (black) and after (gray) application of SKF-38393 (SKF). B: charge transfer (Q) before (closed circles) and after (open circles) application of SKF, normalized to response to the highest intensity from the dark-adapted responses. Responses after light adaptation are from separate experiments and are normalized to the dark-adapted values for those cells. SKF caused significant declines in Q at the highest 4 light intensities used [2-way repeated-measures (RM) ANOVA, P = 0.012; Student-Newman-Keuls (SNK) post hoc, P < 0.05], but the SKF response was still significantly larger than its light-adapted equivalent at the brightest intensity (2-way RM ANOVA, P = 0.043; SNK, P = 0.035). C: same as in B except normalized peak amplitudes of responses. SKF significantly reduced peak amplitude values from dark-adapted levels at all light intensities except the very dimmest (2-way RM ANOVA, P = 0.003; SNK, P < 0.01) but was not significantly different from light-adapted amplitudes (2-way RM ANOVA, P = 0.16). D and E: same as B and C but normalized time to peak (D) and 37% decay (D37) (E) values. No significant changes in kinetics were found after SKF application (2-way RM ANOVA; time to peak P = 0.154, D37 P = 0.783) or between SKF and light-adapted responses (2-way RM ANOVA; time to peak P = 0.466, D37 P = 0.129). For B and C, n = 6 SKF and n = 8 light-adapted responses. For D and E, n = 5 SKF and n = 4 light-adapted responses. *SNK P < 0.05 dark-adapted condition vs. SKF, #SNK P < 0.05 SKF vs. light-adapted condition.
Although both SKF and light adaptation decreased L-IPSCs in rod bipolar cells, L-IPSCs in light-adapted rod bipolar cells still had significantly smaller Qs than those treated with SKF (n = 6 SKF, n = 8 light adapted; 2-way RM ANOVA P = 0.043, Fig. 2B). At the highest light intensity (9.5·105 photons·µm−2·s−1), light-adapted L-IPSCs were 15.9 ± 5.1% (SNK, P < 0.001, Fig. 2B) of their dark-adapted Q, whereas those in SKF-treated rod bipolar cells were still roughly 40.9 ± 13.5% (SNK, P < 0.001, Fig. 2B). Light-adapted peak amplitudes were smaller than those in SKF at 9.5·103 and 9.5·104 photons·µm−2·s−1 (Fig. 2C), and light-adapted decays were faster at all intensities tested (Fig. 2E). These differences were not significant (2-way RM ANOVA, peak amplitude P = 0.494; D37 P = 0.129). As expected, because time to peak did not change for either light-adapted or SKF-treated responses, we found no difference in time to peak between the two groups (2-way RM ANOVA, P = 0.288). To ensure that our results were not due to differences in cell behavior between the two groups, we compared their unnormalized dark-adapted responses (data not shown). We found no significant differences (2-way RM ANOVA, Q P = 0.843; peak amplitude P = 0.939; time to peak P = 0.782; D37 P = 0.935). These results show that a significant portion of adaptation-mediated changes in light-evoked inhibition to rod bipolar cells could be attributed to the activation of D1Rs.
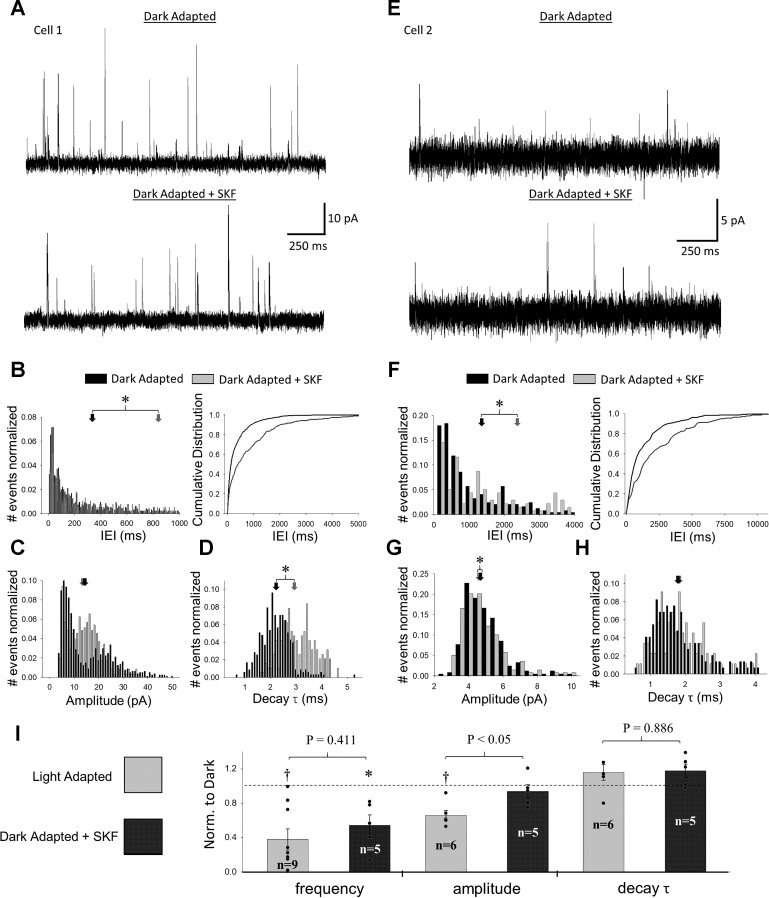
D1R activation decreases the frequency but not amplitude of rod bipolar cell sIPSCs.
Because D1Rs are located at many locations in the retina, the effects on rod bipolar cell L-IPSCs could come from effects on either the rod bipolar cells themselves or presynaptic amacrine cells although previous studies have suggested that rod bipolar cells do not express D1Rs (Farshi et al. 2016; Veruki and Wässle 1996). To determine which cells underlie the effects D1R activation had on rod bipolar cell L-IPSCs, we measured sIPSCs in rod bipolar cells before and after application of SKF (Fig. 3A). When we examined IEI, amplitude, and decay τ histograms for individual cells, we identified some consistent and some inconsistent effects of SKF. For all cells, IEIs shifted from shorter to longer times after SKF application (K-S, P < 0.0001, Fig. 3B), suggesting decreased sIPSC frequency. For three out of five cells, sIPSC amplitudes shifted away from large and small values toward more intermediate ones (K-S, P < 0.0001, Fig. 3C), resulting in significantly different distributions with no concurrent change in average amplitude. These same three cells also exhibited significant shifts in sIPSC decay τ toward larger values (K-S, P < 0.00001, Fig. 3D). However, although the other two cells treated with SKF did exhibit similar shifts in sIPSC IEIs after SKF application (Fig. 3F), they did not adhere to the same trend in amplitude and decay τ (Fig. 3, G and H). After normalizing to dark-adapted conditions, we found that average sIPSC frequency was significantly decreased (n = 5; paired t-test, P = 0.03), but amplitude (n = 5; paired t-test, P = 0.48) and decay τ (n = 5; paired t-test, P = 0.086) were unchanged, following SKF treatment (Fig. 3I).
Fig. 3.
Activation of D1 receptors decreases frequency and modifies distributions of spontaneous inhibitory postsynaptic currents (sIPSCs) in rod bipolar cells. A: example traces of spontaneous currents in the same cell before (top) and after (bottom) application of SKF-38393 (SKF). For each condition, 2 traces are shown (1 in black and the other in gray). B–D: interevent interval (IEI) (B), amplitude (C), and decay τ (D) distributions of sIPSCs from the same cell as in A before (black) and after (gray) application of SKF. For all distributions, the number of events in each bin was normalized to the total number of events per condition. Arrows represent average dark-adapted (black) and SKF-treated (gray) values for this cell. B: IEI histogram (left) with cumulative distribution function (right) included for clarity. sIPSCs showed significant shifts toward larger IEIs after SKF for 5 out of 5 cells tested (Kolmogorov-Smirnov; K-S, P < 0.05). C: for 3 out of 5 cells tested, SKF caused a significant shift in sIPSC amplitudes away from small and large values toward intermediate ones (K-S, P < 0.05). D: for these same 3 cells, SKF caused an increased shift in decay τ (K-S, P < 0.05). E–H: same as for A–D but for a different cell. In this case, a significant shift in IEI is apparent, but the amplitude distribution changes only marginally. Additionally, no significant shift in decay τ occurs. I: comparison of average sIPSC frequency, amplitude, and decay τ between dark-adapted, SKF, and light-adapted states. All data were normalized to dark-adapted values (represented by dashed line) before averaging. Light adaptation caused significant declines in both frequency and amplitude compared with dark-adapted conditions (paired t-test, P < 0.05), whereas SKF only caused a significant decline in frequency (paired t-test, P = 0.0216). Average values for individual cells within each group are represented by black dots. P values shown represent unpaired t-tests between SKF and light-adapted sIPSCs. *Paired t-test, P < 0.05 dark-adapted condition vs. SKF; †paired t-test, P < 0.05 dark-adapted vs. light-adapted conditions. Cell numbers (n) for t-tests in I are shown.
After light adaptation, sIPSCs in rod bipolar cells also exhibit significant shifts toward smaller amplitudes (5 out of 6 cells, K-S, P < 0.0001), longer IEIs (5 out of 6 cells, K-S, P < 0.01), and longer decay τ (3 out of 6 cells, K-S, P < 0.0005) (Eggers et al. 2013b). However, unlike for the SKF-treated cells, the sIPSC amplitude distributions after light adaptation exhibit stark shifts to smaller, not intermediate, values, suggesting a stronger effect on inhibitory inputs. When we compared normalized sIPSC measurements from SKF-treated rod bipolar cells to those from light-adapted rod bipolar cells, we found no difference in sIPSC frequency or decay τ but did find that SKF sIPSCs had significantly larger normalized amplitudes (Fig. 3I). This suggests that light adaptation mediates an additional change in amacrine cell signaling that supplements D1R activation.
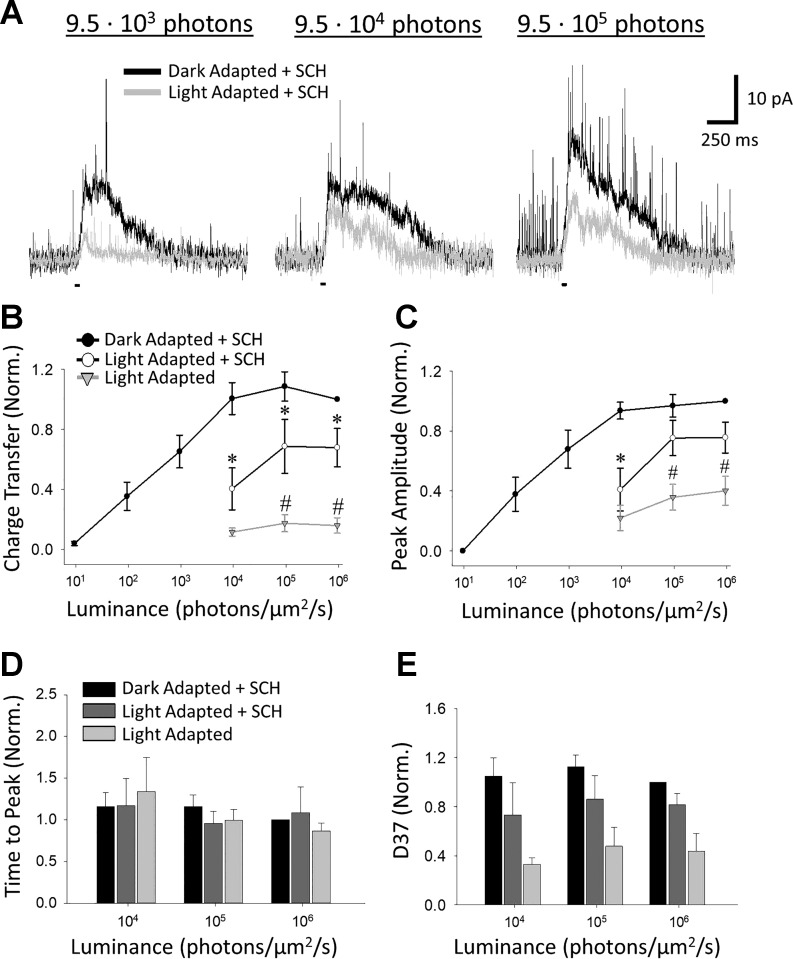
D1R blockade partially prevents changes to rod bipolar cell L-IPSCs induced by light adaptation.
If the changes attributable to light adaptation are primarily due to light-evoked dopamine release activating D1Rs, then antagonizing D1Rs would prevent the changes to evoked inhibition that light adaptation induces. To test this, we recorded L-IPSCs from rod bipolar cells before and after exposure to a rod-saturating background in the presence of SCH (Fig. 4A). To ensure that SCH preincubation did not cause changes in dark-adapted responses, we compared the unnormalized dark-adapted L-IPSCs between SCH and control cells (the control cells exposed to light adaptation in the absence of any drugs from Fig. 2, plus an additional cell in which only dark-adapted responses had been recorded). We found no significant differences in unnormalized responses between SCH and control cells for Q, peak amplitude, or D37 [2-way RM ANOVA (n = 9 control, n = 7 SCH; Q P = 0.254, peak amplitude P = 0.485) (n = 9 control, n = 6 SCH; D37 P = 0.052)]. The unnormalized SCH responses did have significantly longer time to peak values (n = 9 control, n = 6 SCH; 2-way RM ANOVA, P = 0.012), but this difference was not significant in the responses normalized to the maximum response recorded for each cell that were used for further analysis (n = 9 control, n = 6 SCH; 2-way RM ANOVA, P = 0.351).
Fig. 4.
D1 receptor blockade partially prevents light adaptation-induced reduction to rod bipolar cell light-evoked inhibitory postsynaptic currents (L-IPSCs). A: example traces of L-IPSCs in the presence of SCH-23390 (SCH) in the same cell at 9.5·103 (left), 9.5·104 (middle), and 9.5·105 (right) photons·µm−2·s−1 before (black) and after (light gray) exposure to a light-adapting background. Light stimuli (small black bars below traces) were 30 ms in duration. B–E: charge transfer (B), peak amplitude (C), time to peak (D), and 37% decay (D37) (E) of dark-adapted (closed circles) and light-adapted + SCH (open circles) responses, normalized to response to brightest dark-adapted stimulus. Light-adapted values (light gray triangles) included are the same values as those in Fig. 2B. Light adaptation in the presence of SCH still caused significant reductions in Q at all 3 cone intensities (Student-Newman-Keuls, SNK, P < 0.05), but responses were significantly larger than light adaptation without the antagonist at 9.5·104 and 9.5·105 photons·µm−2·s−1 (SNK, P < 0.05). C: peak amplitudes were only significantly diminished from dark-adapted levels at 9.5·103 photons·µm−2·s−1 (SNK, P < 0.001) and were significantly greater than control light-adapted values at the highest 2 intensities (SNK, P < 0.05). D and E: no significant changes in kinetics were found between dark- and light-adapted + SCH responses [2-way repeated-measures (RM) ANOVA; time to peak P = 0.357, D37 P = 0.136] or between light-adapted + SCH and control light-adapted responses (2-way RM ANOVA; time to peak P = 0.590, D37 P = 0.186). For B and C, n = 7 SCH light-adapted responses and n = 8 control light-adapted responses. For D and E, n = 5 SCH light-adapted responses and n = 4 control light-adapted responses. *SNK P < 0.05 dark-adapted responses vs. light-adapted responses + SCH; #SNK P < 0.05 light-adapted responses + SCH vs. control light-adapted responses.
After light adaptation in the presence of SCH, our results showed that Q (n = 7; 2-way RM ANOVA, P = 0.007, Fig. 4B) and peak amplitude (n = 7; 2-way RM ANOVA, P = 0.018, Fig. 4C) were still significantly decreased, with no change in time to peak (n = 4; 2-way RM ANOVA, P = 0.868, Fig. 4D) or D37 (n = 5; 2-way RM ANOVA, P = 0.136, Fig. 4E). However, we found that the light-adapted responses for the SCH group were still much larger than those of our control light-adapted group (n = 7 SCH, n = 8 control; 2-way RM ANOVA, Q P = 0.007, peak amplitude P = 0.035, Fig. 4, B and C). The light-adapted + SCH Q at the maximal light intensity was reduced by only 32.2 ± 12.7% compared with 84.1 ± 5.1% for controls (SNK, P < 0.05), and the light-adapted peak amplitude at the maximal light intensity was similarly preserved (reduced by only 24.3 ± 10.3% vs. 59.8 ± 9.8% for controls, SNK, P < 0.05). No significant differences were found in time to peak (n = 6 control, n = 7 SCH; 2-way RM ANOVA, P = 0.767, Fig. 4D) or D37 (n = 5 control, n = 7 SCH; 2-way RM ANOVA, P = 0.074, Fig. 4E). Our results indicate that D1R blockade substantially impairs light adaptation for rod bipolar cell L-IPSCs although some D1R-independent component of adaptation appears to still occur.
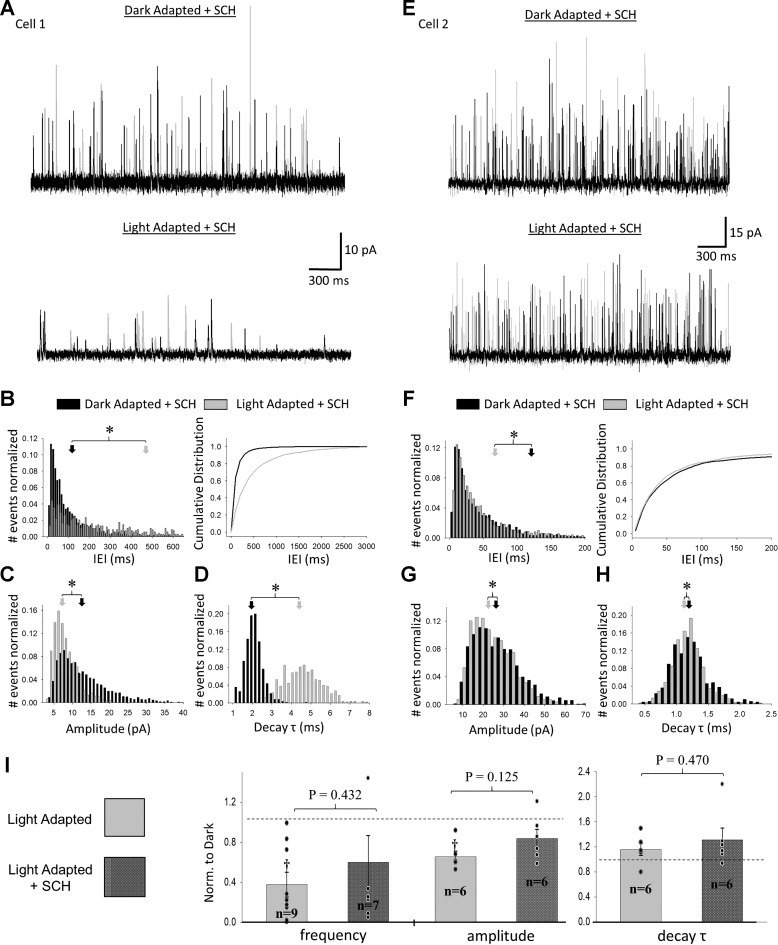
D1R blockade does not completely prevent light adaptation changes to rod bipolar cell sIPSCs.
We measured sIPSCs before and after light adaptation in the presence of SCH to test the necessity of D1R activation for light-induced changes to amacrine cell activity. However, in this case, we failed to identify consistent trends in our cells. For two of six cells, light adaptation caused significant shifts toward longer IEIs, longer decay τ, and smaller amplitudes (Fig. 5, A–D); the rest of the cells did not behave as consistently, exhibiting significant changes in one or two of these parameters but not all (Fig. 5, E–H). Overall, four cells had significantly increased IEIs, four had significantly increased decay τ, and three had significantly decreased amplitudes (K-S, P < 0.05). We examined the number of spontaneous events for each cell to see whether low counts could explain our inconsistent results but found no correlation between baseline frequency and efficacy of SCH. In preliminary experiments, we found that preincubation of slices with SCH for a minimum of 30 min was required to see any antagonistic effects, suggesting that inconsistent tissue penetration of the drug may have contributed to variability in the responses of our cells to this treatment. When we compared average frequency (n = 7; paired t-test, P = 0.186), amplitude (n = 6; paired t-test, P = 0.141), and decay τ (n = 6; paired t-test, P = 0.149) between dark- and light-adapted conditions for our SCH-treated cells (Fig. 5I), we found no significant differences because of a lack of consistent changes in cell responses. When we compared the light-adapted sIPSCs between the control and SCH-treated groups, we found no significant differences in average frequency (unpaired t-test, P = 0.432), amplitude (unpaired t-test, P = 0.125), or decay τ (unpaired t-test, P = 0.470). Thus we found that D1R blockade could limit the effects of light adaptation for some cells but did not completely prevent the effects of light adaptation on sIPSCs.
Fig. 5.
Blockade of D1 receptors limits the effects of light adaptation on spontaneous inhibitory postsynaptic currents (sIPSCs) for some cells but not others. A: example traces of spontaneous currents in the same cell before (top) and after (bottom) light adaptation in the presence of SCH-23390 (SCH). For each condition, 2 traces are shown (one in black and the other in gray). B–D: interevent interval (IEI) (B), amplitude (C), and decay τ (D) distributions of sIPSCs from the same cell as in A before (black) and after (gray) application of SCH. For all distributions, the number of events in each bin was normalized to the total number of events per condition. Arrows represent average dark-adapted (black) and light-adapted + SCH (gray) values. For this cell, SCH did not prevent changes associated with light adaptation. E–H: same as A–D except for a different cell from the SCH group. In this case, SCH largely prevented changes in sIPSC IEI, amplitude, and decay τ distributions although the distributions before and after are still statistically different (Kolmogorov-Smirnov; K-S, P < 0.05). I: comparison of average sIPSC frequency, amplitude, and decay τ between dark-adapted, light-adapted + SCH, and control light-adapted conditions. All data were normalized to dark-adapted values before averaging (dark-adapted level represented by dashed line). Control light adaptation data are same as that in Fig. 3. No significant changes were recorded between dark-adapted and light-adapted + SCH values (paired t-test; frequency P = 0.186, amplitude P = 0.141, decay τ P = 0.149) or between light-adapted + SCH and control light-adapted sIPSCs. Average values for individual cells in each group are represented by black dots. P values shown represent unpaired t-tests between SCH and light-adapted sIPSCs. †Paired t-test, P < 0.05 dark-adapted vs. light-adapted conditions. n values for t-tests in I are shown.
D1R activation decreases GABACR-mediated inputs to rod bipolar cell terminals in the absence of GABAAR-mediated serial inhibition.
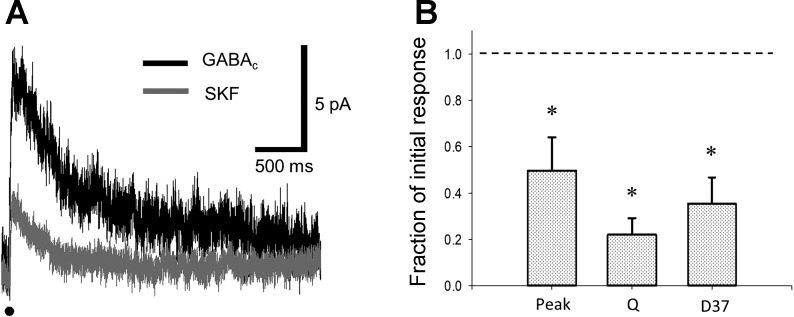
Our data from sIPSCs and previous anatomical data (Greferath et al. 1993; Vaughn et al. 1981; Wong-Riley 1974; Zhang et al. 1997, 2004) suggest that the primary effect of D1R activation on rod bipolar cell inhibition is to limit output from amacrine cells onto rod bipolar cells. Additionally, an ongoing question in the literature is whether light adaptation alters the GABAergic output of amacrine cells onto rod bipolar cells directly or whether it controls the output of these cells by modifying the activity of inhibitory synapses between amacrine cells, i.e., serial inhibition. In this latter interaction, a secondary amacrine cell synapses onto a primary amacrine cell responsible for inhibiting a rod bipolar cell. When both of these amacrine cells are activated, the primary amacrine cell is inhibited by the secondary amacrine cell, and thus its inhibitory output onto the rod bipolar cell is diminished, via inhibition of inhibition (Fig. 1). To determine whether serial inhibition is necessary to see a decrease in inhibition, we measured eIPSCs in rod bipolar cells in response to electrical stimulation before and after the application of SKF (Fig. 6A). Because inhibition between amacrine cells is controlled by GABAA currents (Eggers and Lukasiewicz 2010), we pharmacologically isolated GABAC currents, which are the chief charge-carrying components of rod bipolar cell L-IPSCs (Eggers and Lukasiewicz 2006a, 2006b), to remove the influence of serial inhibition from our experiments. Additionally, electrical stimulation has been shown to selectively activate spiking amacrine cell input to rod bipolar cells, with only minor contributions from A17 reciprocal feedback amacrine cells (Eggers et al. 2013a). This allowed us to more closely examine the predominantly cone-mediated component of rod bipolar cell inhibition. We found that treatment with SKF caused significant declines in the Q, peak amplitude, and D37 (Fig. 6B) of electrically evoked responses (n = 5; paired t-test, P < 0.05), conclusively demonstrating the effect of D1 activation on nonreciprocal amacrine cell-mediated inhibition to rod bipolar cells.
Fig. 6.
D1 receptor activation directly decreases strength of nonreciprocal GABAergic synapses onto rod bipolar cells. A: electrically evoked inhibitory postsynaptic currents from the same rod bipolar cell before (black) and after (dark gray) application of SKF-38393 (SKF). The 1-ms electrical stimulus is represented by the black line below the traces. B: normalized peak amplitude, charge transfer, and 37% decay (D37) were all significantly diminished by SKF. The isolated GABAC-mediated responses in the untreated state are represented by the dashed line. *Significantly different from dark-adapted state by paired t-test (n = 5, P < 0.05).
DISCUSSION
A hallmark of retinal light adaptation is the switch from rod- to cone-mediated vision, a change that results in greatly diminished excitatory and inhibitory currents to rod bipolar cells. Although the role of dopamine in light adaptation has long been recognized, the mechanisms through which it modulates retinal function still require further elucidation. Here, we demonstrated the efficacy of D1R activation in mediating a portion of these changes to rod bipolar cell inhibition, causing marked reductions in the magnitude of L-IPSCs and decreasing the frequency of sIPSCs. We identified a synaptic mechanism for this dopamine-mediated reduction in rod bipolar cell inhibition by showing that D1R activation directly diminished the inhibitory output of nonreciprocal amacrine cells onto rod bipolar cells, in the absence of serial inhibition. Finally, we showed that the activation of D1Rs represents one component of light adaptation because a D1R antagonist partially prevented light-induced changes to IPSCs.
Inhibition to rod bipolar cells is controlled by D1R-mediated modulation of retinal circuits and direct lateral inhibition.
In this study, we found that the activation of D1Rs led to significant decreases in evoked inhibition to rod bipolar cells under dark-adapted conditions, at both rod-dominant and cone-dominant intensities. The smaller evoked inhibition at rod-dominant intensities (Fig. 2B) surprised us because around 70% of light-evoked inhibition at the rod-saturating intensity (950 photons·µm−2·s−1) is attributable to reciprocal feedback from A17 amacrine cells (Eggers et al. 2013b), whose activity is largely dependent on rod bipolar cell output. Because neither rod bipolar cells nor A17 amacrine cells seem to express D1Rs (Farshi et al. 2016), this change in evoked inhibition must be due to a reduction in excitatory input to rod bipolar cells from the rods or to a change in activity of the lateral inhibitory network. The reduction in light-evoked inhibition at cone-dominant intensities suggests a decline in cone activation, cone-mediated output to the lateral inhibitory network, or activity of the amacrine cells in this network. Because both cone and rod photoreceptors lack D1Rs, direct modulation of their light responses by SKF is highly unlikely.
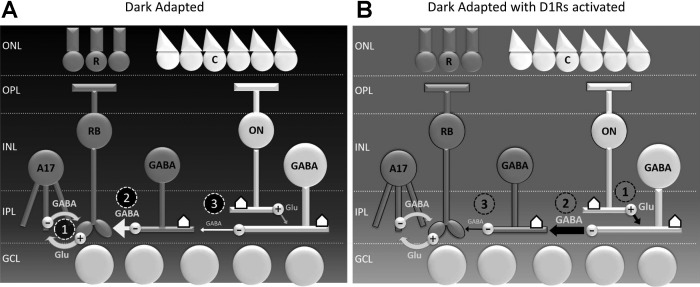
What is perhaps a more likely site of dopaminergic modulation is the lateral inhibitory feedback network composed of amacrine cells. In the rodent retina, light-evoked inhibition to rod bipolar cells arises from two primary sources, rod-activated reciprocal feedback from A17 amacrine cells (Chávez and Diamond 2008; Eggers and Lukasiewicz 2010; Hartveit 1999) and mixed rod-cone lateral feedback from narrow field (glycinergic) and wide-field (GABAergic) amacrine cells (Chávez and Diamond 2008; Chávez et al. 2010; Eggers and Lukasiewicz 2010). In mouse, it has been shown that the magnitudes of L-IPSCs are limited by the activity of serial inhibitory GABAA synapses between amacrine cells (see Fig. 1) and that these connections are strongly activated by full-field light stimuli (Eggers and Lukasiewicz 2010). In a previous study from our laboratory, we found that, upon blocking serial inhibition with the GABAA antagonist SR-95531, reductions in L-IPSCs that usually occur with light adaptation were completely prevented (Eggers et al. 2013b). This implies that, during light adaptation, dopamine acts through D1Rs located on amacrine cells to increase the strength of serial inhibition (see Fig. 7), thus diminishing rod bipolar cell L-IPSCs. In support of this idea, both dopamine and the D1R agonist SKF have been shown to potentiate GABAA currents in retinal amacrine cells in rats (Feigenspan and Bormann 1994). Alternatively, or perhaps in concert, dopamine could potentiate the release of GABA onto serial inhibitory synapses, but no studies to date have conclusively demonstrated that this occurs.
Fig. 7.
Sources of inhibition to rod bipolar cells under dark-adapted conditions and upon D1 receptor activation. A: in dim light, rod bipolar cells receive reciprocal inhibition from A17 amacrine cells (1) and lateral inhibition from wide-field GABAergic amacrine cells (2), as well as narrow-field glycinergic amacrine cells (not shown). When light stimuli of sufficient size are used, the strength of lateral inhibition to rod bipolar cells (2) is diminished by serial inhibition (3). B: when D1 receptors are activated, this circuit is modified at several sites: ON bipolar cell voltage-gated sodium (NaV) channels are inactivated (1), potentially affecting glutamate release onto downstream targets. The strength of serial inhibition is increased (2), either through greater release of GABA by wide-field amacrine cells onto other amacrine cells or the potentiation of GABAA currents in cells that are targets of serial inhibition. GABA release by amacrine cells that mediate lateral inhibition (3) is decreased independent of serial inhibitory influences, possibly through D1-dependent modulation of NaVs or voltage-gated calcium (CaVs). ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; R, rod photoreceptor; C, cone photoreceptor; RB, rod bipolar cell; Glu, glutamate; ⌂, D1 receptor.
In addition to modifying the strength of retinal circuits controlling inhibition, dopamine likely modifies the strength of lateral inhibition to rod bipolar cells directly. A recent study utilizing rod-dominant murine electroretinograms (ERGs) in vivo found that b-wave amplitudes were decreased by a D1R agonist modulating GABAC receptor input when serial inhibitory connections were blocked (Smith et al. 2015b). The authors interpreted this decline in b-wave amplitude as D1R activation decreasing amacrine cell GABA release onto rod bipolar cells because a previous study showed that tonic GABAC receptor-mediated currents can increase the peak amplitude of the scotopic ERG b-wave (Herrmann et al. 2011). However, a more recent study by Travis et al. (2018) contradicts this conclusion of Smith et al. (2015b), suggesting that D1R activation only acts to decrease the activity of serial inhibitory connections between amacrine cells and does not directly modulate release by amacrine cells responsible for lateral inhibition. In our present study, we demonstrated a direct effect of SKF on these amacrine cells by electrically stimulating GABAC receptor-mediated inputs onto rod bipolar cell terminals. The significant decline in eIPSCs that we witnessed is most likely due to dopaminergic effects on the amacrine cells that mediate lateral feedback; a postsynaptic modification seems a less plausible explanation, as rod bipolar cells lack D1Rs (Farshi et al. 2016; Herrmann et al. 2011; Veruki and Wässle 1996). These findings support those of Smith et al. (2015b) over those of Travis et al. (2018).
Likely candidates for the mechanism of this reduction in lateral amacrine cell inhibition to rod bipolar cells are the modulation of voltage-gated sodium (NaV) and calcium (CaV) channels. Studies performed in salamander (Shields and Lukasiewicz 2003), mouse (Eggers et al. 2013a), and rat (Chávez et al. 2010) have demonstrated that IPSCs in bipolar cells are significantly diminished in the presence of tetrodotoxin, which suggests a strong reliance of this network upon spike-mediated signal transmission. Thus it is possible that D1R activation could decrease amacrine cell output by inactivating these NaV currents, as has been demonstrated for cone bipolar cells (Ichinose and Lukasiewicz 2007) and ganglion cells (Hayashida et al. 2009). Dopamine has also been shown to modulate the calcium currents of horizontal cells through the action of D1Rs (Liu et al. 2016; Liu and Lasater 1994; Pfeiffer-Linn and Lasater 1996). If calcium currents in amacrine cells are similarly affected by dopamine, then a reduction in calcium-induced GABA release could also explain the diminished inhibition that we witnessed.
Horizontal cells, which do express D1Rs (Farshi et al. 2016; Veruki and Wässle 1996), could also potentially be mediating a reduction in rod and cone output to downstream bipolar cells.
Previously it has been shown that horizontal cells play a larger role in generating surround inhibition under photopic than mesopic conditions in the tiger salamander retina (Ichinose and Lukasiewicz 2005) although its contributions under mesopic illumination cannot be discounted. It is possible that, in the presence of a saturating D1R treatment (20 µM SKF), feedback inhibition to photoreceptors could be increased in response to a full-field light stimulus, thereby decreasing light-evoked inhibition at either rod- or cone-dominant flash intensities. Some previous results support the idea of photoreceptor modulation, as D1 agonists selectively suppressed rod and enhanced cone inputs onto horizontal cells in the Xenopus retina (Witkovsky et al. 1988) while enhancing rod inputs and decreasing cone responses in the rabbit retina (Pflug et al. 2008). However, in rats, a D1 agonist had no effect on horizontal cell feedback onto rod photoreceptors (Liu et al. 2013). However, asymmetrical modulation of rod and cone output by D1 activation cannot fully explain our results, as we saw diminished responses at both rod- and cone-dominated light intensities. Alternatively, there is some evidence for GABAergic feedforward inhibition from horizontal cells onto bipolar cell dendrites (Du and Yang 2000; Shields et al. 2000; Thoreson and Mangel 2012). In isolated mouse horizontal cells, the D1R agonist SKF decreases calcium currents (Liu et al. 2016), which could result in diminished GABA release and thus decreased inhibition onto rod bipolar cells. However, in rodent rod bipolar cells, sIPSCs and IPSCs evoked with a mesopic light stimulus have been demonstrated to arise primarily from axonal inputs, not dendritic ones (Euler and Masland 2000; Schubert et al. 2008). This suggests that horizontal cells may not be the main mediators of the changes we measured in this study.
D1R activation decreases the rate of vesicle release by amacrine cells onto rod bipolar cells.
Upon D1R activation, spontaneous currents in rod bipolar cells decreased in frequency and experienced significant shifts in amplitude distributions. These spontaneous currents arise from both the spontaneous fusion of neurotransmitter-containing vesicles in presynaptic amacrine cells, as well as the calcium-dependent fusion of vesicles that occurs in the absence of a light stimulus. The latter processes in amacrine cells are mediated by a diversity of CaVs and other calcium-permeable channels, the selective blocking of which leads to varying degrees of diminished release (Chávez et al. 2010). By modifying the properties of some or all of these CaVs, dopamine may diminish the rate of vesicle release and probability of simultaneous vesicle fusion, explaining our declines in sIPSC frequency and amplitude.
We also found evidence that D1Rs may not modify the activity of all amacrine cell populations equally. In rod bipolar cells, sIPSCs under dark-adapted conditions are mediated exclusively by GABAARs and glycineRs (Eggers and Lukasiewicz 2006b; Eggers et al. 2007). GlycineRs are thought to predominantly mediate inhibitory input from narrow-field amacrine cells, whereas GABAARs mediate inhibition from the reciprocal feedback A17 amacrine cell, as well as one or more populations of wide-field amacrine cells. At rod bipolar cell terminals, isolated glycine sIPSCs have decay τ distributions that occupy slightly larger values than those of isolated GABAA sIPSCs (Eggers and Lukasiewicz 2006b; Eggers et al. 2007). The shifts in decay τ we measured in some cells upon D1R activation (Fig. 3D) could therefore reflect a diminished contribution of GABAAR-mediated events, which we would not expect to see if the activity of glycinergic amacrine cells was decreased to the same extent as that of GABAergic ones. This could suggest a stronger effect of D1R activation on GABAergic than glycinergic amacrine cells, most likely on nonreciprocal ones because A17 amacrine cells lack D1Rs (Farshi et al. 2016; Veruki and Wässle 1996). This potential differential modulation would be an interesting question for future study, as glycinergic and GABAergic amacrine cells are thought to subserve different functional roles in the retina.
Activation of D1Rs cannot completely account for light-mediated changes to rod bipolar cell inhibition.
Although SKF reduced the magnitude of L-IPSCs, it did not diminish them to the same extent as light adaptation. We have previously shown that rod bipolar cell output to A17 cells is diminished to ~20% of its dark-adapted levels after light adaptation (Eggers et al. 2013b), which results in less reciprocal inhibition. This reduction in rod pathway excitation is due in large part to rod saturation by increased background light levels. Potentially, because rods express D4Rs (Cohen et al. 1992), dopamine release attributable to increased light levels could also contribute to a sustained reduction in rod glutamate transmission (Akopian and Witkovsky 1996; Firsov and Astakhova 2016; Thoreson et al. 2002), resulting in decreased rod bipolar cell responses to light (Snellman et al. 2008). In the case of a dark-adapted retina treated with a D1R agonist, rod output to rod bipolar cells is likely preserved, resulting in greater L-IPSC contributions from the A17 pathway compared with light-adapted conditions. An additional component of this discrepancy in L-IPSC magnitudes could be due to activation of excitatory amino acid transporter glutamate transporter-coupled Cl− channels (Ichinose and Lukasiewicz 2012; Veruki et al. 2006), which is more likely to play a role under dark-adapted conditions.
Similar to our light-evoked results, D1 activation could not fully replicate light-adapted changes to spontaneous currents. Unlike our rod bipolar cells treated with SKF, for most rod bipolar cells that were light adapted, stark shifts in sIPSC amplitudes toward smaller values occurred, resulting in significant declines in average amplitudes as well. Thus it seems as though light adaptation mediates additional changes to amacrine cell signaling not induced by D1R activation.
The results presented here are consistent with dopamine-induced reductions in evoked and spontaneous inhibition to rod bipolar cells as part of the changes associated with light adaptation. We have shown that the activation of D1Rs likely leads to a decrease in amacrine cell release, partially explaining the changes in signaling witnessed upon light adaptation. It is probable that reductions in rod signaling and activation of other dopaminergic mechanisms, such as D4 receptors, also play a role in the modulation of rod bipolar cell inhibition with light adaptation, but further research is needed to demonstrate this directly. Because of the expression of D1Rs on a variety of cell types within the retina, it is difficult to rule out the contribution of horizontal and cone bipolar cells to this process. Further studies capable of activating D1Rs on individual cell types are needed to clarify the changes in circuit dynamics wrought by dopamine.
GRANTS
This work was supported by NIH National Eye Institute RO1-EY026027 (E. Eggers), International Retinal Research Foundation (IRRF) grant (E. Eggers), NSF CAREER award no. 1552184, and the University of Arizona NIH Interdisciplinary Training in Cardiovascular Research grant 4T32HL007249-40 (M. Flood).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.D.F., J.M.M.-D., and E.D.E. conceived and designed research; M.D.F., J.M.M.-D., and E.D.E. performed experiments; M.D.F., J.M.M.-D., and E.D.E. analyzed data; M.D.F., J.M.M.-D., and E.D.E. interpreted results of experiments; M.D.F., J.M.M.-D., and E.D.E. prepared figures; M.D.F., J.M.M.-D., and E.D.E. drafted manuscript; M.D.F., J.M.M.-D., and E.D.E. edited and revised manuscript; M.D.F., J.M.M.-D., and E.D.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Gabrielle Grinslade for technical assistance and Dr. Teresia Carrion, Andrea Wellington, and Timothy Maley for helpful comments on the manuscript.
REFERENCES
- Akopian A, Witkovsky P. D2 dopamine receptor-mediated inhibition of a hyperpolarization-activated current in rod photoreceptors. J Neurophysiol 76: 1828–1835, 1996. doi: 10.1152/jn.1996.76.3.1828. [DOI] [PubMed] [Google Scholar]
- Asteriti S, Gargini C, Cangiano L. Mouse rods signal through gap junctions with cones. eLife 3: e01386, 2014. doi: 10.7554/eLife.01386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez AE, Diamond JS. Diverse mechanisms underlie glycinergic feedback transmission onto rod bipolar cells in rat retina. J Neurosci 28: 7919–7928, 2008. doi: 10.1523/JNEUROSCI.0784-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez AE, Grimes WN, Diamond JS. Mechanisms underlying lateral GABAergic feedback onto rod bipolar cells in rat retina. J Neurosci 30: 2330–2339, 2010. doi: 10.1523/JNEUROSCI.5574-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AI, Todd RD, Harmon S, O’Malley KL. Photoreceptors of mouse retinas possess D4 receptors coupled to adenylate cyclase. Proc Natl Acad Sci USA 89: 12093–12097, 1992. doi: 10.1073/pnas.89.24.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouiche A, Asan E. The dopamine D2 receptor subfamily in rat retina: ultrastructural immunogold and in situ hybridization studies. Eur J Neurosci 11: 1391–1402, 1999. doi: 10.1046/j.1460-9568.1999.00557.x. [DOI] [PubMed] [Google Scholar]
- Du JL, Yang XL. Subcellular localization and complements of GABA(A) and GABA(C) receptors on bullfrog retinal bipolar cells. J Neurophysiol 84: 666–676, 2000. doi: 10.1152/jn.2000.84.2.666. [DOI] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. GABA(A), GABA(C) and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. J Physiol 572: 215–225, 2006a. doi: 10.1113/jphysiol.2005.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Receptor and transmitter release properties set the time course of retinal inhibition. J Neurosci 26: 9413–9425, 2006b. doi: 10.1523/JNEUROSCI.2591-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Interneuron circuits tune inhibition in retinal bipolar cells. J Neurophysiol 103: 25–37, 2010. doi: 10.1152/jn.00458.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, McCall MA, Lukasiewicz PD. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol 582: 569–582, 2007. doi: 10.1113/jphysiol.2007.131763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Klein JS, Moore-Dotson JM. Slow changes in Ca2+ cause prolonged release from GABAergic retinal amacrine cells. J Neurophysiol 110: 709–719, 2013a. doi: 10.1152/jn.00913.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Mazade RE, Klein JS. Inhibition to retinal rod bipolar cells is regulated by light levels. J Neurophysiol 110: 153–161, 2013b. doi: 10.1152/jn.00872.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Masland RH. Light-evoked responses of bipolar cells in a mammalian retina. J Neurophysiol 83: 1817–1829, 2000. doi: 10.1152/jn.2000.83.4.1817. [DOI] [PubMed] [Google Scholar]
- Farshi P, Fyk-Kolodziej B, Krolewski DM, Walker PD, Ichinose T. Dopamine D1 receptor expression is bipolar cell type-specific in the mouse retina. J Comp Neurol 524: 2059–2079, 2016. doi: 10.1002/cne.23932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Bormann J. Facilitation of GABAergic signaling in the retina by receptors stimulating adenylate cyclase. Proc Natl Acad Sci USA 91: 10893–10897, 1994. doi: 10.1073/pnas.91.23.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field GD, Rieke F. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron 34: 773–785, 2002. doi: 10.1016/S0896-6273(02)00700-6. [DOI] [PubMed] [Google Scholar]
- Firsov ML, Astakhova LA. The role of dopamine in controlling retinal photoreceptor function in vertebrates. Neurosci Behav Physiol 46: 138–145, 2016. doi: 10.1007/s11055-015-0210-9. [DOI] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wässle H. Types of bipolar cells in the mouse retina. J Comp Neurol 469: 70–82, 2004. doi: 10.1002/cne.10985. [DOI] [PubMed] [Google Scholar]
- Greferath U, Müller F, Wässle H, Shivers B, Seeburg P. Localization of GABAA receptors in the rat retina. Vis Neurosci 10: 551–561, 1993. doi: 10.1017/S0952523800004764. [DOI] [PubMed] [Google Scholar]
- Hare WA, Owen WG. Receptive field of the retinal bipolar cell: a pharmacological study in the tiger salamander. J Neurophysiol 76: 2005–2019, 1996. doi: 10.1152/jn.1996.76.3.2005. [DOI] [PubMed] [Google Scholar]
- Hartveit E. Reciprocal synaptic interactions between rod bipolar cells and amacrine cells in the rat retina. J Neurophysiol 81: 2923–2936, 1999. doi: 10.1152/jn.1999.81.6.2923. [DOI] [PubMed] [Google Scholar]
- Hayashida Y, Rodríguez CV, Ogata G, Partida GJ, Oi H, Stradleigh TW, Lee SC, Colado AF, Ishida AT. Inhibition of adult rat retinal ganglion cells by D1-type dopamine receptor activation. J Neurosci 29: 15001–15016, 2009. doi: 10.1523/JNEUROSCI.3827-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann R, Heflin SJ, Hammond T, Lee B, Wang J, Gainetdinov RR, Caron MG, Eggers ED, Frishman LJ, McCall MA, Arshavsky VY. Rod vision is controlled by dopamine-dependent sensitization of rod bipolar cells by GABA. Neuron 72: 101–110, 2011. doi: 10.1016/j.neuron.2011.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose T, Lukasiewicz PD. Inner and outer retinal pathways both contribute to surround inhibition of salamander ganglion cells. J Physiol 565: 517–535, 2005. doi: 10.1113/jphysiol.2005.083436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose T, Lukasiewicz PD. Ambient light regulates sodium channel activity to dynamically control retinal signaling. J Neurosci 27: 4756–4764, 2007. doi: 10.1523/JNEUROSCI.0183-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose T, Lukasiewicz PD. The mode of retinal presynaptic inhibition switches with light intensity. J Neurosci 32: 4360–4371, 2012. doi: 10.1523/JNEUROSCI.5645-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang Z, Blackburn MR, Wang SW, Ribelayga CP, O’Brien J. Adenosine and dopamine receptors coregulate photoreceptor coupling via gap junction phosphorylation in mouse retina. J Neurosci 33: 3135–3150, 2013. doi: 10.1523/JNEUROSCI.2807-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lasater EM. Calcium currents in turtle retinal ganglion cells. I. The properties of T- and L-type currents. J Neurophysiol 71: 733–742, 1994. doi: 10.1152/jn.1994.71.2.733. [DOI] [PubMed] [Google Scholar]
- Liu X, Hirano AA, Sun X, Brecha NC, Barnes S. Calcium channels in rat horizontal cells regulate feedback inhibition of photoreceptors through an unconventional GABA- and pH-sensitive mechanism. J Physiol 591: 3309–3324, 2013. doi: 10.1113/jphysiol.2012.248179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Grove JC, Hirano AA, Brecha NC, Barnes S. Dopamine D1 receptor modulation of calcium channel currents in horizontal cells of mouse retina. J Neurophysiol 116: 686–697, 2016. doi: 10.1152/jn.00990.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills SL, Xia XB, Hoshi H, Firth SI, Rice ME, Frishman LJ, Marshak DW. Dopaminergic modulation of tracer coupling in a ganglion-amacrine cell network. Vis Neurosci 24: 593–608, 2007. doi: 10.1017/S0952523807070575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Light-evoked current responses in rod bipolar cells, cone depolarizing bipolar cells and AII amacrine cells in dark-adapted mouse retina. J Physiol 558: 897–912, 2004. doi: 10.1113/jphysiol.2003.059543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Paul DL, Wu SM. Rod, M-cone and M/S-cone inputs to hyperpolarizing bipolar cells in the mouse retina. J Physiol 590: 845–854, 2012. doi: 10.1113/jphysiol.2011.224113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer-Linn CL, Lasater EM. Dopamine modulates unitary conductance of single PL-type calcium channels in Roccus chrysops retinal horizontal cells. J Physiol 496: 607–616, 1996. doi: 10.1113/jphysiol.1996.sp021712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflug R, Nelson R, Huber S, Reitsamer H. Modulation of horizontal cell function by dopaminergic ligands in mammalian retina. Vision Res 48: 1383–1390, 2008. doi: 10.1016/j.visres.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga C, Mangel SC. Identification of a circadian clock-controlled neural pathway in the rabbit retina. PLoS One 5: e11020, 2010. doi: 10.1371/journal.pone.0011020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga C, Cao Y, Mangel SC. The circadian clock in the retina controls rod-cone coupling. Neuron 59: 790–801, 2008. doi: 10.1016/j.neuron.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B, Creutzfeldt OD. Scotopic and mesopic light adaptation in the cat’s retina. Pflugers Arch 313: 168–185, 1969. doi: 10.1007/BF00586245. [DOI] [PubMed] [Google Scholar]
- Schubert T, Kerschensteiner D, Eggers ED, Misgeld T, Kerschensteiner M, Lichtman JW, Lukasiewicz PD, Wong RO. Development of presynaptic inhibition onto retinal bipolar cell axon terminals is subclass-specific. J Neurophysiol 100: 304–316, 2008. doi: 10.1152/jn.90202.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley RM, Enroth-Cugell C. Visual adaptation and retinal gain controls. Prog Retin Eye Res 3: 263–346, 1984. doi: 10.1016/0278-4327(84)90011-7. [DOI] [Google Scholar]
- Shields CR, Lukasiewicz PD. Spike-dependent GABA inputs to bipolar cell axon terminals contribute to lateral inhibition of retinal ganglion cells. J Neurophysiol 89: 2449–2458, 2003. doi: 10.1152/jn.00916.2002. [DOI] [PubMed] [Google Scholar]
- Shields CR, Tran MN, Wong RO, Lukasiewicz PD. Distinct ionotropic GABA receptors mediate presynaptic and postsynaptic inhibition in retinal bipolar cells. J Neurosci 20: 2673–2682, 2000. doi: 10.1523/JNEUROSCI.20-07-02673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BJ, Côté PD, Tremblay F. D1 dopamine receptors modulate cone ON bipolar cell Nav channels to control daily rhythms in photopic vision. Chronobiol Int 32: 48–58, 2015a. doi: 10.3109/07420528.2014.951054. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Côté PD, Tremblay F. Dopamine modulation of rod pathway signaling by suppression of GABAC feedback to rod-driven depolarizing bipolar cells. Eur J Neurosci 42: 2258–2270, 2015b. doi: 10.1111/ejn.12993. [DOI] [PubMed] [Google Scholar]
- Snellman J, Kaur T, Shen Y, Nawy S. Regulation of ON bipolar cell activity. Prog Retin Eye Res 27: 450–463, 2008. doi: 10.1016/j.preteyeres.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Raviola E, Dacheux RF. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol 325: 152–168, 1992. doi: 10.1002/cne.903250203. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Mangel SC. Lateral interactions in the outer retina. Prog Retin Eye Res 31: 407–441, 2012. doi: 10.1016/j.preteyeres.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Stella SL Jr, Bryson EI, Clements J, Witkovsky P. D2-like dopamine receptors promote interactions between calcium and chloride channels that diminish rod synaptic transfer in the salamander retina. Vis Neurosci 19: 235–247, 2002. doi: 10.1017/S0952523802192017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis AM, Heflin SJ, Hirano AA, Brecha NC, Arshavsky VY. Dopamine-dependent sensitization of rod bipolar cells by GABA is conveyed through wide-field amacrine cells. J Neurosci 38: 723–732, 2018. doi: 10.1523/JNEUROSCI.1994-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn JE, Famiglietti EV Jr, Barber RP, Saito K, Roberts E, Ribak CE. GABAergic amacrine cells in rat retina: immunocytochemical identification and synaptic connectivity. J Comp Neurol 197: 113–127, 1981. doi: 10.1002/cne.901970109. [DOI] [PubMed] [Google Scholar]
- Veruki ML. Dopaminergic neurons in the rat retina express dopamine D2/3 receptors. Eur J Neurosci 9: 1096–1100, 1997. doi: 10.1111/j.1460-9568.1997.tb01461.x. [DOI] [PubMed] [Google Scholar]
- Veruki ML, Wässle H. Immunohistochemical localization of dopamine D1 receptors in rat retina. Eur J Neurosci 8: 2286–2297, 1996. doi: 10.1111/j.1460-9568.1996.tb01192.x. [DOI] [PubMed] [Google Scholar]
- Veruki ML, Mørkve SH, Hartveit E. Activation of a presynaptic glutamate transporter regulates synaptic transmission through electrical signaling. Nat Neurosci 9: 1388–1396, 2006. doi: 10.1038/nn1793. [DOI] [PubMed] [Google Scholar]
- Wang JS, Kefalov VJ. An alternative pathway mediates the mouse and human cone visual cycle. Curr Biol 19: 1665–1669, 2009. doi: 10.1016/j.cub.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Doc Ophthalmol 108: 17–40, 2004. doi: 10.1023/B:DOOP.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Stone S, Besharse JC. Dopamine modifies the balance of rod and cone inputs to horizontal cells of the Xenopus retina. Brain Res 449: 332–336, 1988. doi: 10.1016/0006-8993(88)91048-7. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT. Synaptic organization of the inner plexiform layer in the retina of the tiger salamander. J Neurocytol 3: 1–33, 1974. doi: 10.1007/BF01111929. [DOI] [PubMed] [Google Scholar]
- Zhang J, Jung CS, Slaughter MM. Serial inhibitory synapses in retina. Vis Neurosci 14: 553–563, 1997. doi: 10.1017/S0952523800012219. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang HH, Yang CY. Synaptic organization of GABAergic amacrine cells in the salamander retina. Vis Neurosci 21: 817–825, 2004. doi: 10.1017/S0952523804216029. [DOI] [PubMed] [Google Scholar]