Abstract
Attentional blink (AB) refers to the situation where correctly identifying a target impairs the processing of a subsequent probe in a sequence of stimuli. Although the AB often coincides with a modulation of scalp-recorded cognitive event-related potentials (ERPs), the neural sources of this effect remain unclear. In two separate experiments, we used classical LORETA analysis recursively applied (CLARA) to estimate the neural sources of ERPs elicited by an auditory probe when it immediately followed an auditory target (i.e., AB condition), when no auditory target was present (i.e., no-AB condition), and when the probe followed an auditory target but occurred outside of the AB time window (i.e., no-AB condition). We observed a processing deficit when the probe immediately followed the target, and this auditory AB was accompanied by reduced P3b amplitude. Contrasting brain electrical source activity from the AB and no-AB conditions revealed reduced source activity in the medial temporal region as well as in the temporoparietal junction (extending into inferior parietal lobe), ventromedial prefrontal cortex, left anterior thalamic nuclei, mammillary body, and left cerebellum. The results indicate that successful probe identification following a target relies on a widely distributed brain network and further support the suggestion that the auditory AB reflects the failure of the probe to reach short-term consolidation.
NEW & NOTEWORTHY Within a rapid succession of auditory stimuli, the perception of a predefined target sound often impedes listeners’ ability to detect another target sound that is presented close in succession. This attentional blink may be related to activity in brain areas supporting attention and memory. We show that the auditory attentional blink is associated with brain activity changes in a network including the medial temporal lobe, parietal cortex, and prefrontal cortex. This study suggests that a problem in the interaction between attention and memory underlies the auditory attentional blink.
Keywords: auditory attention, CLARA, distributed source model, event-related potential, short-term consolidation
INTRODUCTION
The attentional blink (AB) refers to the phenomenon in which identifying a target in a sequence of stimuli causes a processing deficit for a subsequently presented probe (Raymond et al. 1992). This AB may last for several hundred milliseconds after the target has been presented. It has been observed for visual (Broadbent and Broadbent 1987; Chun and Potter 1995; Giesbrecht and Di Lollo 1998; Jolicoeur and Dell’Acqua 1998) and auditory stimuli (Horváth and Burgyán 2011; Shen and Mondor 2006; 2008; Tremblay et al. 2005), as well as in cross-modal situations that combine auditory and visual stimuli (Arnell and Jolicoeur 1999). The AB paradigm provides a means to investigate how attention is allocated to rapidly changing elements within an auditory or visual scene.
Evidence from behavioral and electrophysiological studies suggests that the AB may reflect limitations in short-term consolidation (i.e., the stage of encoding information into short-term memory). The probe-processing deficit is often paralleled with reduced P3b amplitude generated by an auditory (Shen and Alain 2010, 2011, 2012; Shen et al. 2016) or visual probe (Dell’Acqua et al. 2003; Vogel and Luck 2002; Vogel et al. 1998). The P3b wave, a positive deflection in the scalp-recorded event-related potential (ERP) that peaks between 300 and 600 ms over parietal regions, reflects conscious information processing of task-relevant events. It has been commonly assumed to reflect the updating of working memory (Donchin 1981) and may represent the transfer of information to consciousness (Picton 1992). The suppression of the P3b wave during the AB has been suggested as evidence that the probe does not reach the short-term consolidation stage (Vogel et al. 1998).
Functional magnetic resonance imaging (fMRI) studies suggests that the visual AB is associated with activity in the right intraparietal sulcus and lateral prefrontal cortex (Kranczioch et al. 2005; Marois et al. 2000). Clinical studies have revealed an increase in the magnitude of the visual AB in stroke patients with damages in frontal (Richer and Lepage 1996) and parietal cortices (Husain et al. 1997). Evidence from magnetoelectroencephalography (MEG) also suggests that the visual AB is associated with source activity in left frontal and right parietal cortices (Gross et al. 2004). Similarly, source modeling of neuroelectric activity revealed changes in cortical activity during the visual AB in left inferior frontal, left temporal, lateral prefrontal, anterior cingulate, and parietal cortices (Sergent et al. 2005).
To date, studies aiming to identify the brain regions implicated in the AB have focused exclusively on the visual AB, making it difficult to determine whether the same brain regions would also be associated with the auditory AB. The processing of a probe immediately following the target is often spared in visual AB domain (Chun and Potter 1995), whereas it is impaired in the auditory domain (Horváth and Burgyán 2011; Shen and Alain 2010; Shen and Mondor 2006; 2008). This suggests that different processes may underlie the visual and auditory AB. Moreover, prior studies did not take into consideration the role of medial temporal lobe (MTL) in the visual AB, which has been shown to play an important role in both short-term consolidation (Diana et al. 2008; Kuhl et al. 2012; McClelland et al. 1995; Rempel-Clower et al. 1996; Scoville and Milner 1957; Squire 1992; Squire et al. 2004; Squire and Zola-Morgan 1991; Teyler and DiScenna 1986; Zola-Morgan et al. 1986) and P3b generation (Halgren et al. 1980; Smith et al. 1986).
Intracerebral recordings in epileptic patients implanted with depth electrodes for presurgical investigation suggest neuronal sources for the auditory P3b in the MTL (Halgren et al. 1995b, 1980) as well as in parietal (Alain et al. 1989; Halgren et al. 1995a) and prefrontal cortices (Alain et al. 1989; Baudena et al. 1995). Findings from lesion studies provide converging evidence for auditory P3b generators located in prefrontal cortex, temporoparietal, and medial temporal lobe (Knight et al. 1989). Moreover, distributed source analysis techniques such as low-resolution electromagnetic tomography (LORETA; Pascual-Marqui et al. 1994) have revealed cortical sources of the auditory P3b in bilateral frontal, parietal, limbic, cingulate, and temporo-occipital regions (Volpe et al. 2007; Wronka et al. 2012). Given that the P3b has been proposed as a neural correlate of auditory AB, one would expect to observe changes in MTL, frontal, and parietal regions during the auditory AB.
The present study compares neuroelectric brain source activity in experimental trials that promote auditory AB and in trials that do not promote auditory AB, using the classical LORETA analysis recursively applied (CLARA). This distributed source model is an iterative application of the LORETA, with a reduction of the source space in each iteration. It presents more focal localizations of the brain activity and can separate closely neighboring sources (Beniczky et al. 2016; Dimitrijevic et al. 2013; Oliveira et al. 2014; Rusiniak et al. 2013). In two separate experiments, we examined effects of the target presence and probe positions on the auditory AB. Participants were presented with sequences of 16 tones in a rapid serial presentation paradigm. There were four trial types that occurred with equal frequency, which were defined by having the target either present or absent and by having the probe either present or absent within the sequence. Participants indicated whether the target and probe were present by sequentially pressing two different buttons. It was predicted that the presence of the target would cause a processing deficit for the probe in trials in which the target was immediately followed by the probe (AB trials). Moreover, it was anticipated that the cortical sources of the auditory AB would include the MTL, as well as frontal and parietal sites, and that the P3b amplitude to the probe would be reduced during the auditory AB, reflecting a deficit of short-term consolidation of the probe.
METHODS
Experiment 1
Participants.
Twenty-four young adults (age: 18–30 yr, 12 men) participated in this study. All showed normal hearing, defined by pure tone thresholds ≤20 dB normal hearing level between 250 and 8,000 Hz. Ethics approval and written informed consent were obtained according to the guidelines set out by the Research Ethics Board of Baycrest Hospital and the University of Toronto.
Stimuli and procedure.
Auditory stimuli included 21 pure tones, with frequencies equally spaced on a logarithmic scale between 529 and 1,330 Hz (f = 529, 554, 580, 607, 636, 666, 697, 730, 764, 800, 838, 877, 918, 961, 1,006, 1,056, 1,106, 1,158, 1,213, 1,270, and 1,330 Hz). Each sound had a duration of 30 ms, including linear onset and offset slopes of 2 ms. The auditory target was composed of six 5-ms pulses with any one frequency from this set. The auditory probe was a frequency-modulated glide that changed smoothly from 636 to 1,006 Hz. Distractor stimuli other than the target and probe were pure tones, randomly selected from the 21 frequencies.
All stimuli were synthesized at a sampling rate of 44,100 Hz using Adobe Audition 1.5. Binaural stimuli were presented at 75 dB sound pressure level (SPL) using Etymotic ER-3A insert earphones (Etymotic Research, Elk Grove, IL). Stimulus presentation was controlled using the E-Prime software system (version 1.1; Psychology Software Tools) on a Dell Precision T3400 computer.
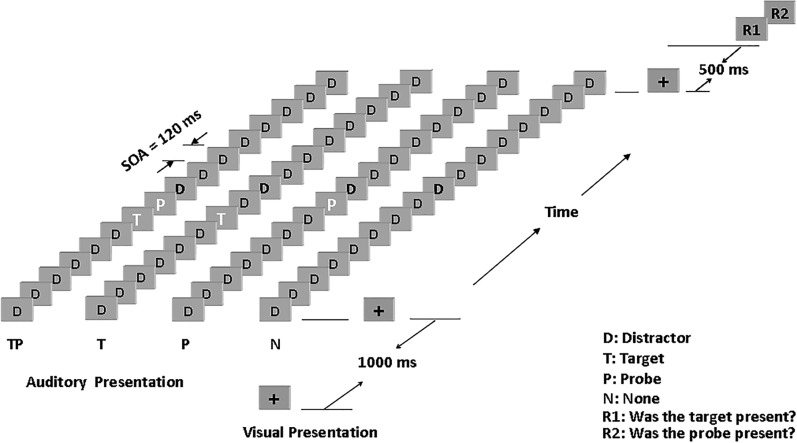
Participants were presented with sequences of 16 sounds that could include the target, the probe, or both (see Fig. 1). The stimulus onset asynchrony (SOA) was fixed at 120 ms. The target, if presented, was always the seventh sound in the sequence. The probe, if presented, was always one position after the target (i.e., +1 position) as the eighth sound in the sequence. The other sounds were considered distractors.
Fig. 1.
Time course of a sequence of stimulus presentation. Auditory stimuli were 16 sounds presented at a stimulus onset asynchrony (SOA) of 120 ms. The target position was the 7th sound, and the probe could be presented at the 1st position following the target. TP, target, probe, and distractors were presented; T, target and distractors were presented; P, probe and distractors were presented; N, only distractors were presented; D, distractor. A fixation cross were presented on a computer screen. Two responses (R1 and R2) were made.
Prior research revealed that the auditory AB was largest at the +1 position (Horváth and Burgyán 2011; Shen and Alain 2010; Shen and Mondor 2006, 2008). This is different from the visual AB, where the target and probe at the +1 position could be processed together during the consolidation stage (Chun and Potter 1995). The largest auditory AB for the probe at the +1 position could be accounted for by the high temporal resolution of the auditory system, which allows processing of the target and the probe separately. Additionally, the issue of whether the largest auditory AB at the +1 position is caused by forward masking was addressed in prior research (Shen and Mondor 2008). The results showed that the auditory AB was eliminated when the target was ignored, and participants only needed to indicate whether the probe was present or not. Such results are difficult to reconcile with forward masking and indicate that the probe was noticeable in the sequence despite the presence of a nearby target. Hence, it appears that forward masking does not meaningfully affect the auditory AB.
Participants in the present study were presented with four types of equiprobable trials. These are referred to as TP (target and the probe were both present), T (target only), P (probe only), or N (neither the target nor the probe was presented). In N trials, a distractor sound was presented at the same position as the missing target or probe. At the end of each sequence, participants were first asked, “Was the target presented?” and then “Was the probe presented?” They indicated their responses by pressing “1” for “yes” or “0” for “no” on a computer keyboard. A fixation cross was presented on the computer screen starting 1,000 ms before the sequence and ending 500 ms after the sequence. Participants were instructed to keep their eyes open and to maintain their gaze on the fixation cross to minimize eye movements. Participants’ behavior and compliance with task instructions were monitored using a video camera.
The auditory stimulation and EEG recording took place in a double-walled sound-attenuating booth. At the beginning of each session, participants completed six repetitions of all trial types to familiarize themselves with the task. All participants met a criterion of at least 60% correct judgments on the target and the probe in the practice blocks. The four trial types were presented 120 times each in randomized order within 2 blocks of 240 trials. Each trial lasted ~5–6 s, including a self-paced response from the participant. It took ~40–50 min to complete all trials. The duration of the test session was about 2 h, including reading and signing the consent form, EEG preparation, hearing assessment, familiarization with the task, the experiment proper, and removal of electrodes.
Electroencephalogram recording.
Neuroelectric brain activity was recorded from 64 electrodes with a bandpass filter of 0.05–100 Hz and a sampling rate of 250 Hz using NeuroScan SynAmps 2 (Compumedics, El Paso, TX). Electrodes were placed at the outer canthi and the superior and inferior orbit to monitor vertical and horizontal eye movements. The midline central electrode (i.e., Cz) was used as reference during recording. For data analysis, the electroencephalogram (EEG) data were re-referenced to an averaged reference (i.e., average of all electrodes) and the electrode Cz was reinstated. All offline analyses were performed using Brain Electrical Source Analysis software (BESA Research 6.1; MEGIS, Gräfelfing, Germany).
The analysis epoch included 200 ms of prestimulus activity and 1,000 ms of poststimulus activity time-locked to the onset of the probe. ERPs were averaged separately for each trial type (TP, T, P, N) and electrode site. For each participant, a set of ocular movements was obtained before and after the experiment (Picton et al. 2000). From this, averaged eye movements were calculated for both lateral and vertical eye movements as well as for eye blinks. A principal component analysis of these averaged recordings provided a set of components that best explained the eye movements. The scalp projections of these components were then subtracted from the experimental ERPs to minimize ocular contamination such as blinks, saccades, and lateral eye movements for each individual average. After the correction, we also used the artifact scan tool in BESA Research 6.1 to identify bad trials (e.g., muscle movement). Thresholds were set at 100 and 75 µV for amplitude and gradient, respectively. On average, 12.7% (SE 1.2%) trials were excluded from further analysis. The group mean number of trials for each condition was as follows: TP, 104.5 (SE 1.5); T, 103.5 (SE 1.5); P, 104.3 (SE 1.7); and N, 106.5 (SE 1.4). ERPs were digitally low-pass filtered to attenuate frequencies above 20 Hz. Each average was baseline-corrected with respect to the prestimulus interval (i.e., mean amplitude over the 200 ms before probe onset).
CLARA (BESA version 6.1) was employed to estimate source activity. The voxel size in Talairach space was 7 mm; this default setting is appropriate for the distributed images in most situations. The regularization parameters that account for the noise in the data were set at 0.01% singular value decomposition.
Data analysis.
For the behavioral data, we performed a within-subject analysis of variance (ANOVA) on the probe discrimination accuracy with the factors “target presence” and “probe presence,” with each factor having two levels. Note that a conditional probability of correct probe discrimination given correct target discrimination was used to make sure that participants paid attention to the target.
For the ERP data, we computed difference waves to isolate neural activity specific to the probe. We subtracted ERPs elicited in target-only (T) trials from ERPs elicited when both target and probed (TP) were present. We also subtracted ERPs generated by distracters in the absence of a target or probe (N) from those elicited by the probe only (P). Because the P3b wave is task related, only the target and probe would evoke the P3b wave. This approach allowed us to separate the P3b wave elicited by the probe from that of the target. For each participant, the source analysis was performed at each time point from the ERPs elicited by the probe for AB (TP–T) and no-AB (P–N) conditions. We then used a cluster analysis procedure and permutation-based statistics (1,000 permutations) to test for the effect of target presence on the probe ERP amplitudes and cortical sources (BESA Statistics 2.0). The statistical significance level for the cluster analysis was set at α = 0.01. The results for cortical sources are displayed on a standard structural MRI image from BESA Statistics 2.0.
Experiment 2
Experiment 2 aimed to replicate and extend the findings from experiment 1 by conducting a source analysis on the AB and the no-AB conditions in a different sample of participants using a different paradigm from experiment 1. In experiment 2, the probe was presented at either the +1 (i.e., inside the AB window) or +7 (i.e., outside the AB window) position following the target.
Participants.
Twenty-four participants (age: 18–30 yr old; 10 men) had normal hearing as measured by pure tone thresholds. None took part in experiment 1. Ethics approval and written informed consent were obtained according to the guidelines set by the Research Ethics Board of Baycrest Hospital and the University of Toronto.
Stimuli and procedure.
The computer, sound system, and sounds were identical to those used in experiment 1. The sequences and tasks were the same as those used in experiment 1 except that half of the targets were the fifth sound and the other half were the seventh sound of the sequences. Moreover, for each target position, the probe could be presented in the first (+1) or the seventh (+7) temporal position following the target. As in experiment 1, there were four types of trials for each of the two probe positions, namely, TP, T, P, and N. Each type of trial occurred with equal frequency.
All trial types were presented 80 times each in randomized order within 2 blocks of 320 trials. There were two types of SOAs between two items: 90 and 120 ms. Half the participants were tested with 90-ms SOAs, and the other half were tested with 120-ms SOAs. Each trial took ~5–6 s, including a self-paced response. Participants took ~50–60 min to complete all the trials. Each participant completed two blocks of practice trials (32 trials for each). In the first practice block, feedback was given to the participants after they made their responses. No feedback was given in the second practice block, or for the rest of the experiment. Each participant was required to meet a criterion accuracy of 60% correct on judging both the target and the probe in the practice blocks before beginning the experiment.
EEG recording and data analysis.
The electrophysiological recording system, analysis software, and methods were the same as those used in experiment 1. An ANOVA with the factors “target presence,” “probe presence,” and “probe position” was employed, with each factor having two levels. Note that different target positions (at the fifth or seventh position of the sequence) and different SOAs (90 or 120 ms) were used to reduce the effect of perceptual set. They were not the focus of the present study, and the effects of SOA on the auditory AB and its neural correlates have been reported previously by Shen and Alain (2010).
An average of 7.5% (SE 1.4%) trials were excluded from the analyses after corrections of eye movements and scanning for other artifacts. The group mean number of trials per condition was as follows: TP1, 69.1 (SE 2.5); TP7, 73.3 (SE 1.5); T1, 72.1 (SE 1.8); T7, 72.3 (SE 1.5); P1, 75.4 (SE 0.8); P7, 75.6 (SE 0.9); N1, 77.5 (SE 0.7); and N7, 76.5 (SE 0.7).
RESULTS
Experiment 1
Behavioral results.
Target discrimination was very high (0.972, SD ±0.005), indicating that participants indeed paid attention to the target.
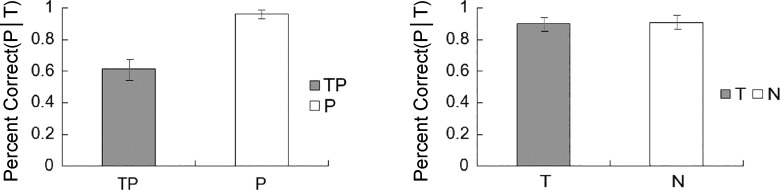
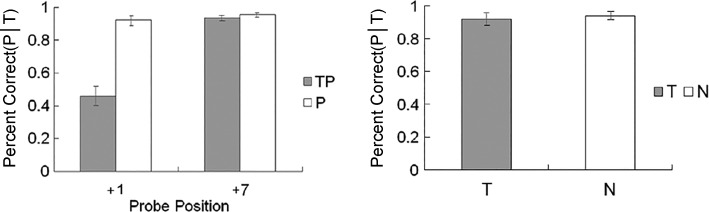
Figure 2 shows the group mean accuracy for probe discrimination. The results revealed main effects of target presence [F(1, 23) = 32.708, P < 0.001, = 0.587] and of probe presence [F(1, 23) = 12.441, P = 0.002, = 0.351]. The interaction between the two was significant [F(1, 23) = 28.057, P < 0.001, = 0.550]. On probe-present trials (i.e., TP and P conditions), the analysis revealed a main effect of target presence [F(1, 23) = 33.260, P < 0.001, = 0.591]; that is, the presence of the target caused a probe-processing deficit. On probe-absent trials (i.e., T and N conditions), there was no such difference [F(1, 23) = 0.507, P = 0.484, = 0.022].
Fig. 2.
Probe discrimination accuracy as a function of target presence in experiment 1. Values are means, error bars are SE. TP, target and probe; T, target only; P, probe only; N, none (i.e., distractors only); P|T, probe discrimination accuracy when the correct target discrimination was given.
Electrophysiological data.
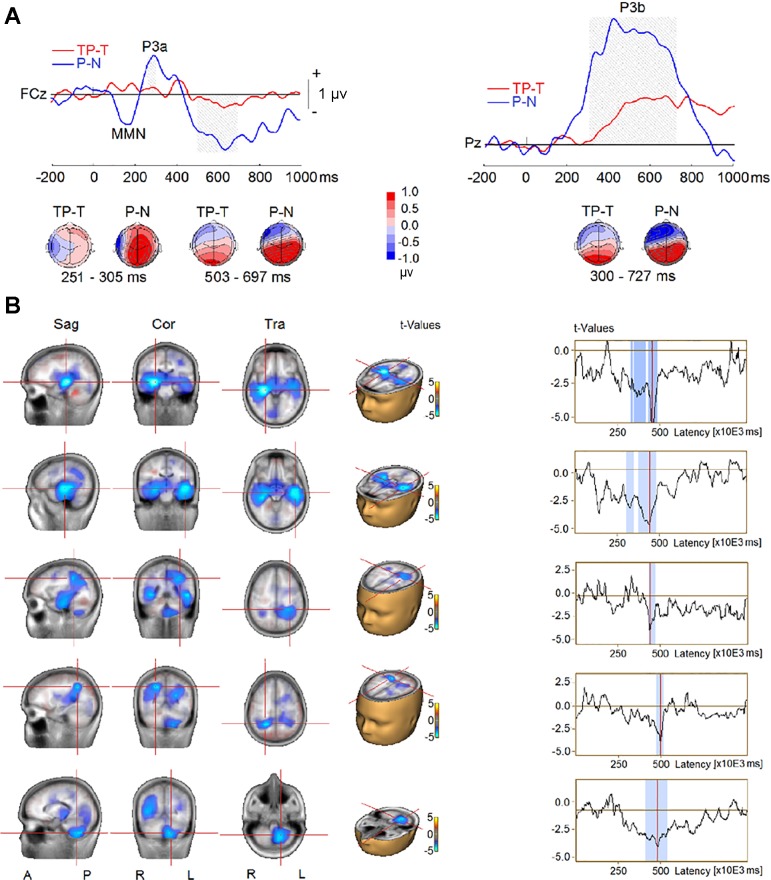
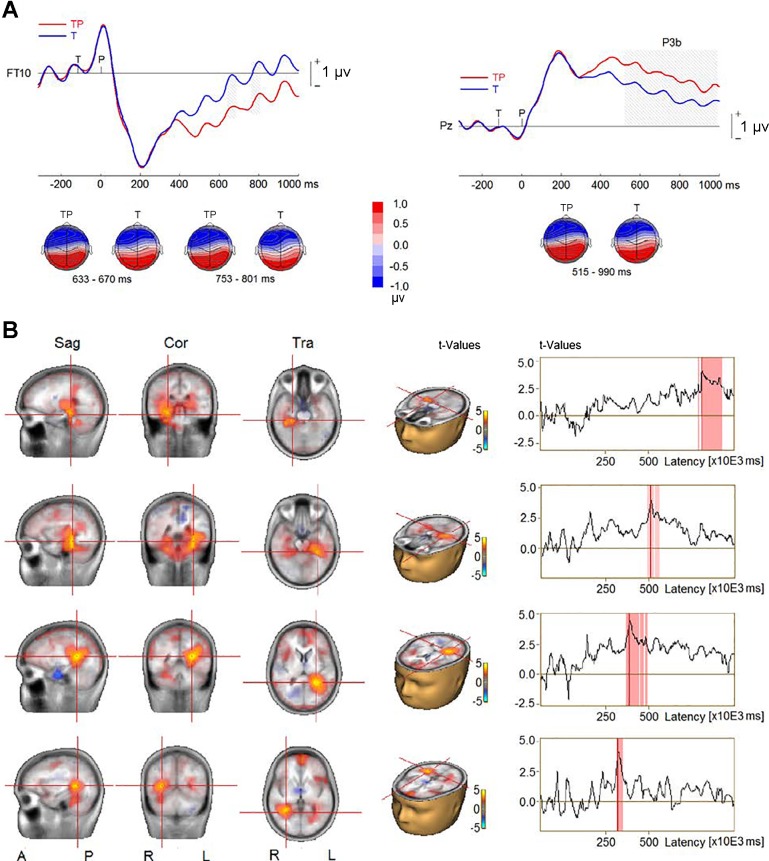
Figure 3 shows the ERPs elicited by the four types of sequences (TP, T, P, and N) from the frontotemporal and parietal scalp sites. The cluster analysis procedure and permutation-based statistics (BESA Statistics 2.0) performed on all time points and electrodes identified two significant clusters (Fig. 4A) when we compared TP–T with P–N trials. The first cluster was related to the P3a elicited by the probe. The P3a was smaller in the AB than in the no-AB condition at frontal and frontocentral scalp electrodes (F1, Fz, F2, FC1, FCz, and FC2; P < 0.001). The P3a reversed polarity at left temporoparietal sites (T7, C5, TP7, CP5, P7, and P5), and the difference between the AB and no-AB conditions at these sites was also significant (P = 0.001). The second cluster was related to the P3b wave elicited by the probe, which was reduced in the AB condition (P < 0.001) at central, parietal, and occipital electrodes (C3, C1, Cz, C2, C4, C6, CP5, CP1, CPz, CP2, CP6, P7, P5, P1, Pz, P2, P4, P6, PO3, POz, PO4, O1, Oz, and O2). The P3b’s corresponding reversal of polarity at frontotemporal sites (F9, F7, F5, F3, F1, Fz, F2, F4, F6, F8, F10, FC5, FC1, FCz, FC2, FC6, FT9, T7, TP9, TP7, and FT10) was significantly reduced in the AB compared with the no-AB condition (P = 0.001).
Fig. 3.
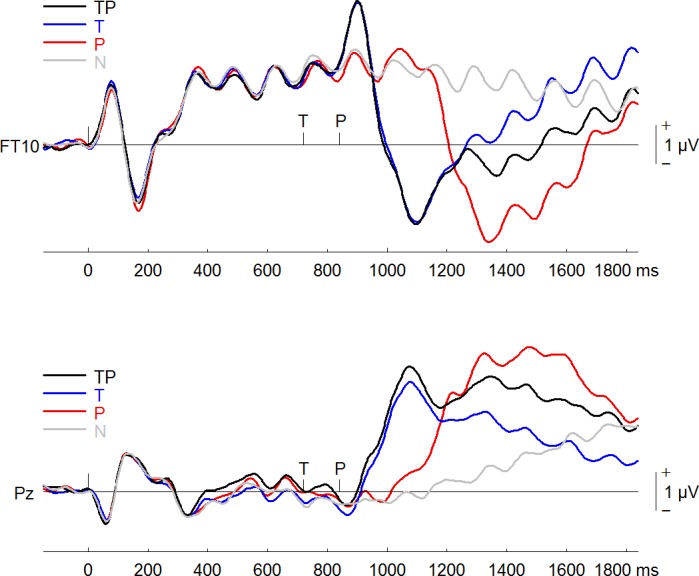
Event-related potentials elicited in all 4 conditions (TP, target and probe; T, target only; P, probe only; and N, distractors only) at a right frontotemporal (FT10) electrode and a midline parietal electrode (Pz).
Fig. 4.
Event-related potential (ERP) difference waves from TP–T (ERP waves elicited by target and probe minus ERPs elicited by target only) and P–N (ERP waves elicited by probe only minus ERPs elicited by distractors only) contrasts in experiment 1. A, top: group-mean difference waves at a midline frontocentral electrode (FCz) and a midline parietal electrode (Pz). Gray shading represents the time intervals of the difference between TP–T and P–N to reach significance. MMN: mismatch negativity. Bottom, topographies of ERP difference during the time intervals. B, left: difference in source activity between TP–T and P–N conditions. Right, time series of difference in source activity, where blue shading represents time intervals of the difference between TP–T and P–N to reach significance. Sag, sagittal; Cor, coronal; Tra, transverse; A, anterior; P, posterior; R, right; L, left.
The comparison of CLARA solutions between the AB (TP–T) and the no-AB condition (P–N) revealed decreases in source activity in the AB condition between 300 and 500 ms after probe onset in bilateral MTL, in bilateral temporoparietal junction (TPJ) extending into inferior parietal lobe (IPL), and in left cerebellum (Fig. 4B).
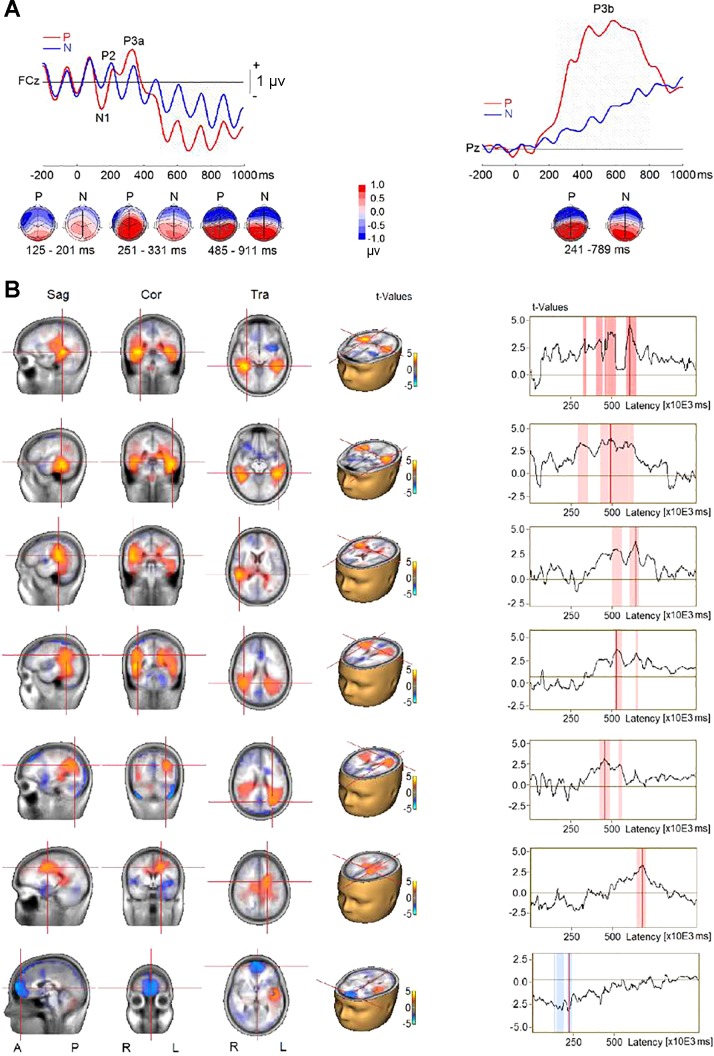
The P3b wave was suppressed during the auditory AB. However, the probe might elicit earlier brain responses similar to those generated by the probe in the no-AB condition. To further examine the source activity associated with processing of the probe, we compared the probe-only (P) with the none (N) condition. The analysis revealed three significant clusters (Fig. 5A). The first cluster was related to increased N1 amplitude and/or mismatch negativity (MMN) responses at frontal, frontocentral, central, temporoparietal, and central-parietal electrodes (F7, F5, F3, F1, Fz, F2, F4, F6, FC5, FC1, FCz, FC2, FC6, T7, C5, C3, C1, Cz, C2, C4, C6, TP7, CP5, and CP1) in the P compared with the N condition (P < 0.001). The second cluster was related to the increased P3a amplitude at anterior frontal, frontal, frontocentral, and central sites (AF4, F3, F1, Fz, F2, F4, F6, FC1, FCz, FC2, C1, Cz, and C2) in the P compared with the N condition (P < 0.001). The P3a’s corresponding reversed polarity appeared at left frontal, temporal, temporoparietal, and parietal sites (F7, F5, T7, C5, C3, TP7, CP5, P7, and P5), and the difference between the two conditions was also significant (P < 0.001). The third cluster was related to increased P3b amplitude (P < 0.001) at central, parietal, and occipital electrodes (Cz, C2, C4, C6, CP5, CP1, CPz, CP2, CP6, P7, P5, P1, Pz, P2, P4, P6, P8, PO3, POz, PO4, O1, Oz, and O2). The P3b’s corresponding reversal of polarity at frontotemporal sites (F9, F7, F5, F3, F1, Fz, F2, F4, F6, F8, F10, FC5, FC1, FCz, FC2, FC6, FT9, T7, C5, C3, C1, TP9, TP10, FT9, and FT10) was also significantly increased when the probe was presented (P < 0.001).
Fig. 5.
Event-related potentials (ERPs) elicited in probe-only (P) and distractor-only (N) conditions in experiment 1. A, top: group-mean ERPs at a midline frontocentral electrode (FCz) and a midline parietal electrode (Pz). Gray shading represents the time intervals of the difference between P and N conditions to reach significance. Bottom, topographies of ERP difference during the time intervals. B, left: difference in source activity between P and N conditions. Right, time series of difference in source activity, where red shading represents time intervals of the difference between P and N to reach significance. Sag, sagittal; Cor, coronal; Tra, transverse; A, anterior; P, posterior; R, right; L, left.
The comparison of CLARA solutions for the P and N conditions revealed two significant clusters of activations (Fig. 5B). One included bilateral MTL, right superior temporal gyrus (STG), bilateral TPJ (extending into IPL), and left cingulate gyrus. The structures exhibited increased source activity when the probe was presented. The increase in source activities ranged from 300 to 700 ms following the probe onset. The second cluster was located in the OFC, which exhibited increased source activity that peaked at ~225 ms when the probe was absent.
To further examine the source activity associated with processing of the probe, we compared the target-and-probe (TP) condition with the target-only (T) condition. The epochs for data analysis were from target onset to 1,000 ms following the probe onset, using the 200 ms before the target onset as the baseline. The analysis revealed one significant cluster (Fig. 6A), which coincided with a P3b elicited by the probed in the TP condition (P < 0.001) at central, parietal, and occipital electrodes (CP5, CP1, CPz, CP2, CP6, P5, P3, P1, Pz, P2, P4, P6, PO3, POz, PO4, O1, Oz, and O2). The P3b’s corresponding reversal of polarity at frontotemporal sites (AF3, F5, F3, FC5, F10, FT10, and TP10) was significantly reduced in the AB compared with the no-AB condition (P = 0.014). The analysis revealed decreases in source activity located in bilateral MTL and STG (Fig. 6B). The source activity in STG was reduced between 300 and 500 ms. In MTL, the source activity was reduced between 500 and 900 ms following the probe onset.
Fig. 6.
Event-related potentials (ERPs) elicited in target-and-probe (TP) and target-only (T) conditions in experiment 1. A, top: group-mean ERPs at a right frontotemporal electrode (FT10) and a midline parietal electrode (Pz). Gray shading represents the time intervals of the difference between TP and T conditions to reach significance. Bottom, topographies of ERP difference during the time intervals. B, left: difference in source activity between TP and T conditions. Right, time series of difference in source activity, where red shading represents time intervals of the difference between TP and T to reach significance. Sag, sagittal; Cor, coronal; Tra, transverse; A, anterior; P, posterior; R, right; L, left.
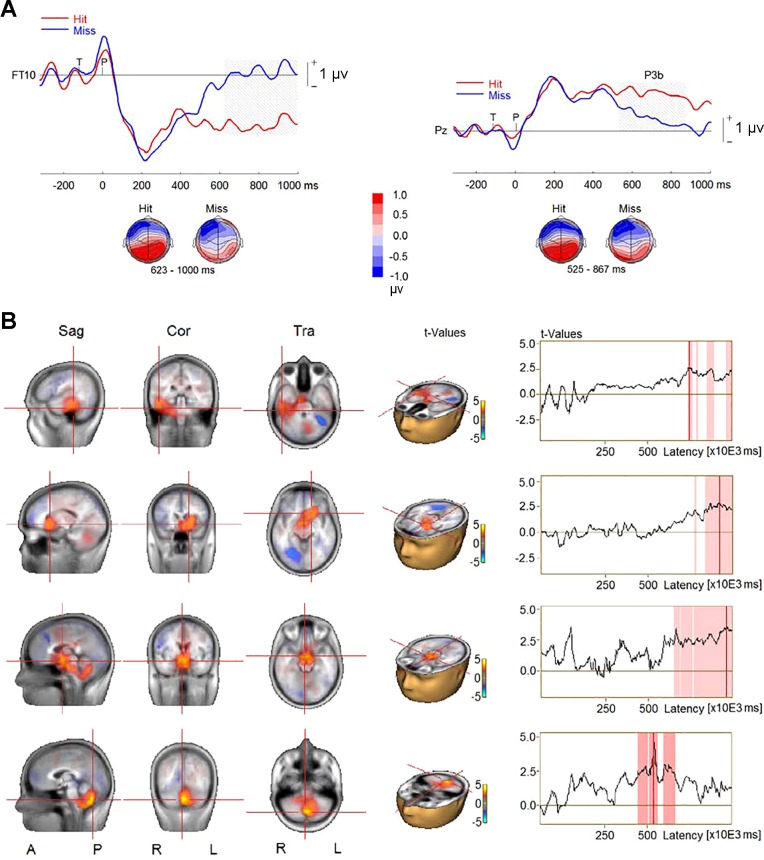
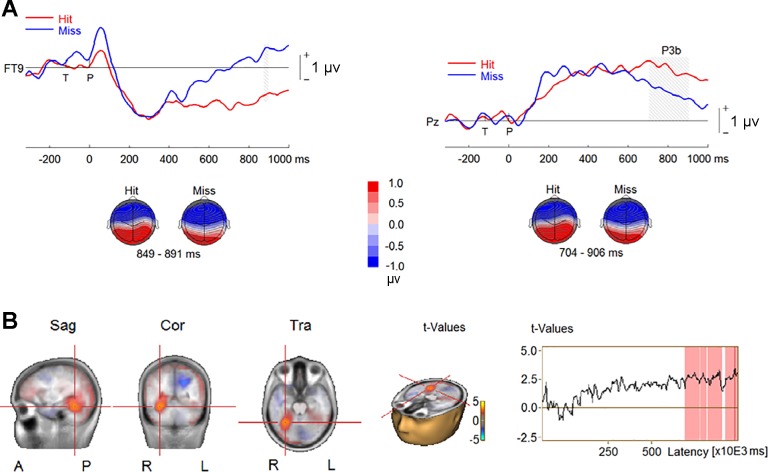
We also examined ERPs and source activity in the auditory AB condition (i.e., the TP condition) when the probe was correctly identified vs. when it was missed. The epochs for data analysis were from target onset to 1,000 ms following the probe onset, using the 200 ms before the target onset as the baseline. For this analysis, we excluded six participants who had fewer than 10 hits or misses. This analysis revealed larger P3b amplitude for hits than misses (P < 0.001; Fig. 7A) at central and parietal electrodes (CP1, CPz, CP2, P3, P1, Pz, P2, P4, PO3, and PO4). The P3b’s corresponding reversed polarity appeared at frontal, temporal, temporoparietal, and parietal sites, and the difference between the two conditions reached significance at right frontal and temporal sites (AF8, F8, F6, F4, FC6, CP6, TP8, T8, and FT10; P = 0.015). The comparison of CLARA solutions revealed greater source activity for hits than misses between 400 and 700 ms in left cerebellum followed by enhanced activity in right MTL, left thalamus, and mammillary body between 700 and 1,000 ms following the probe onset (Fig. 7B).
Fig. 7.
Event-related potentials (ERPs) elicited by hits and misses in the target-and-probe (TP) condition in experiment 1. A, top: group-mean ERPs at a right frontotemporal electrode (FT10) and a midline parietal electrode (Pz). Gray shading represents the time intervals of the difference between hits and misses to reach significance. Bottom, topographies of ERP difference during the time intervals. B, left: difference in source activity between hits and misses. Right, time series of difference in source activity, where red shading represents time intervals of the difference between hits and misses to reach significance. Sag, sagittal; Cor, coronal; Tra, transverse; A, anterior; P, posterior; R, right; L, left.
Experiment 2
Behavioral results.
Target discrimination was high (0.912, SD 0.021), indicating that participants paid attention to the target. Figure 8 shows probe discrimination accuracy for probe-present trials. A two-way ANOVA (target presence × probe position) revealed main effects of target presence [F(1, 23) = 82.190, P < 0.001, = 0.781) and probe position [F(1, 23) = 66.391, P < 0.001, = 0.743]; that is, participants were more accurate in the target-absent trials and when the probe was presented at the +7 position.
Fig. 8.
Probe discrimination accuracy as a function of the target presence and of the probe position in experiment 2. Values are means, error bars are SE. TP, target and probe; T, target only; P, probe only; N, none (i.e., distractor only); P|T: probe discrimination accuracy when the correct target discrimination was given.
The two-way interaction between target presence and probe position was significant [F(1, 23) = 67.595, P < 0.001, = 0.746]. When the probe was at the +1 position, the accuracy was higher in the target-absent (P1) than in the target-present (TP1) trials [F(1, 35) = 80.094, P < 0.001, = 0.777]. However, when the probe was at the +7 position, there was no significant difference between the target-absent (P7) and target-present (TP7) trials [F(1, 23) = 1.868, P = 0.185, = 0.075]. In the probe-absent trials, there was no significant difference between target-present (T) and target-absent (N) trials [F(1, 23) = 1.612, P = 0.217, = 0.066].
Electrophysiological data.
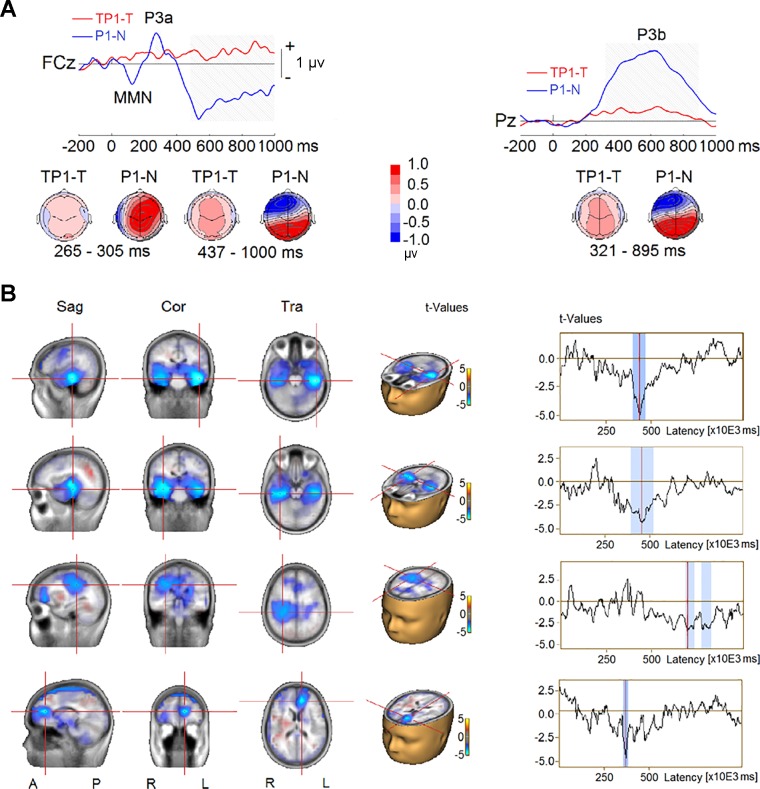
To investigate how the target presence affected the neural activity specific to probe, we compared difference waves of target-present trials (TP1–T) with difference waves of target-absent trials (P1–N) when the probe was at the +1 position. This analysis revealed two significant clusters (Fig. 9A). As in experiment 1, the first cluster showed reduced P3a amplitude to the probe in the TP1–T condition relative to the P1–N condition at frontal, frontocentral, and central scalp electrodes (F1, Fz, F2, FC1, FCz, FC2, and FC6; P < 0.001). The second cluster showed reduced P3b amplitudes to the probe when the target was present (TP1–T) at central, parietal, and occipital scalp regions (TP7, C5, C3, C1, Cz, C2, C4, C6, TP8, CP5, CP1, CPz, CP2, CP6, P7, P5, P1, Pz, P2, P4, P6, PO3, POz, PO4, O1, Oz, and O2; P < 0.001). The P3b’s corresponding reversal of polarity at frontotemporal, frontal, and central scalp sites (F9, F7, F5, F3, F1, Fz, F2, F4, F6, F8, F10, FC5, FC1, FCz, FC2, FC6, FT9, T7, C5, and FT10) was also significantly reduced in amplitude when the target was present (P < 0.001).
Fig. 9.
Event-related potential (ERP) difference waves from TP1–T (ERP waves elicited by sequences containing target and probe at +1 position minus ERPs elicited by sequences with target only) and P1–N (ERP waves elicited by sequences containing probe at +1 position minus EPRs elicited by sequences with only distractors) contrasts in experiment 2. A, top: group-mean difference waves at a midline frontocentral electrode (FCz) and a midline parietal electrode (Pz). MMN: mismatch negativity. Gray shading represents the time intervals of the difference between TP1–T and P1–N to reach significance. Bottom, topographies of ERP difference during the time intervals. B, left: difference in source activity. Right, time series of difference in source activity, where blue shading represents time intervals of the difference between TP1–T and P1–N to reach significance. Sag, sagittal; Cor, coronal; Tra, transverse; A, anterior; P, posterior; R, right; L, left.
Figure 9B shows the results from the contrast in source activity between ERPs elicited by the probe without the target (P1–N) and ERPs elicited by the probe when it followed the target (TP1–T). The analysis revealed decreased source activity located in bilateral MTL (~400–600 ms), right TPJ (extending into IPL, ~750–850 ms), and left ventromedial prefrontal cortex (vmPFC; ~350 ms) for the AB (TP1–T) condition.
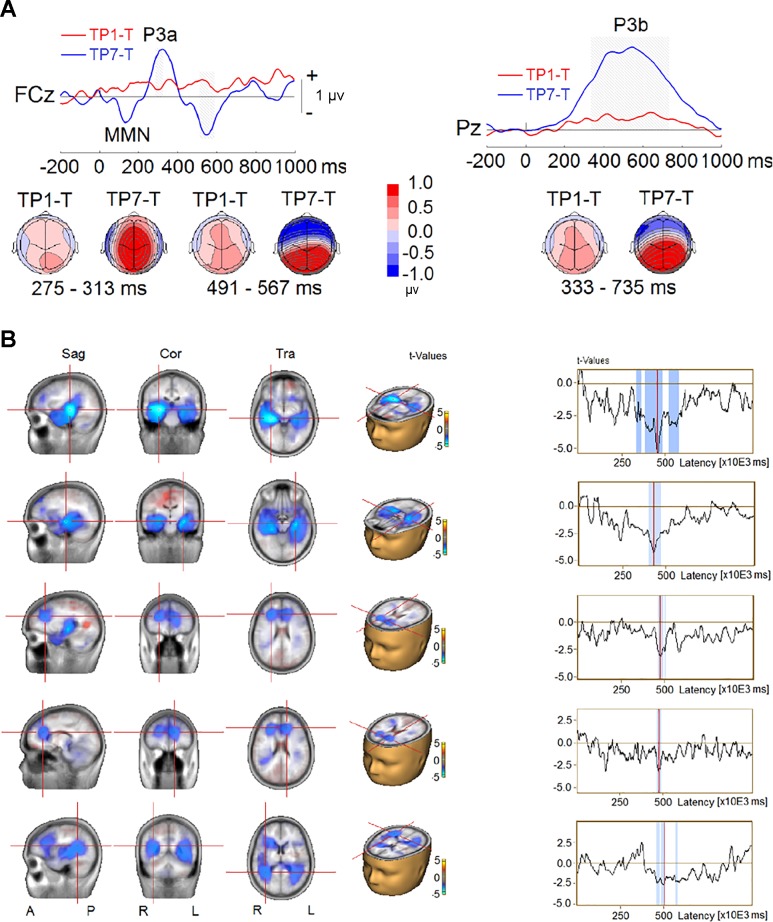
The effect of probe position was examined by comparing the AB (i.e., TP1–T) and no-AB (i.e., TP7–T) conditions when both target and probe were present. This analysis yielded two significant clusters (Fig. 10A). The first cluster showed smaller P3a amplitude to the probe at frontocentral scalp sites (FC1, FCz, and FC2) when the probe was at the +1 position (P < 0.001). The second cluster indicated a reduced P3b amplitudes at central, central-parietal, parietal, and parieto-occipital scalp regions (C3, C1, Cz, C2, C4, CP5, CP1, CPz, CP2, CP6, P7, P5, P1, Pz, P2, P4, P6, PO3, POz, PO4, O1, Oz, and O2) when the probe was at the +1 position (P < 0.001). The P3b’s corresponding reversal of polarity at frontotemporal sites (F9, F7, F5, F3, F1, Fz, F2, F4, F6, F8, F10, FC5, FC1, FCz, FC2, FC6, FT9, T8, TP8, C6, and FT10) was also reduced in amplitude when the probe immediately followed the target (P < 0.001). The reduced P3b amplitude coincided with reduced source activity in bilateral MTL, bilateral vmPFC, and TPJ (extending into IPL) when the probe was at the +1 position (Fig. 10B). It appears that both P3a and P3b amplitudes and source activity were affected by the probe position when the target was present.
Fig. 10.
Event-related potential (ERP) difference waves from TP1–T (ERP waves elicited by sequences containing target and probe at +1 position minus ERPs elicited by sequences with target only) and TP7–T (ERP waves elicited by sequences containing target and probe at +7 position minus ERPs elicited by sequences with target alone) contrasts in experiment 2. A, top: group-mean difference waves at a midline frontocentral electrode (FCz) and a midline parietal electrode (Pz). Gray shading represents the time intervals of the difference between TP1–T and TP7–T to reach significance. Bottom, topographies of ERP difference during the time intervals. MMN: mismatch negativity. B, left: difference in source activity. Right, time series of difference in source activity, where blue shading represents time intervals of the difference between TP1–T and TP7–T to reach significance. Sag, sagittal; Cor, coronal; Tra, transverse; A, anterior; P, posterior; R, right; L, left.
We also examined the difference between the two probe-only conditions (i.e., P1–N and P7–N) and between two conditions outside of the AB time window (i.e., TP7–T and P7–N), but no reliable differences were observed in P3b amplitude or source activity. These findings are consistent with the behavioral results reported above.
Similar to experiment 1, the contrast between probe only at the +1 position (P1) and none (N) trials revealed two significant clusters (Fig. 11A). The first cluster coincided with a P3a deflection at central frontal region (Fz, F2, F4, F6, FC1, FCz, FC2, C1, Cz, C2, C4, and C6) in the P1 compared with the N trials (P < 0.001). The P3a’s corresponding reversed polarity appeared at left temporoparietal sites (F7, F5, T7, C5, FT9, FC5, TP7, CP5, P7, and P5), and the difference between the two conditions was also significant (P = 0.001). The second cluster was associated with the P3b component (P < 0.001) at central, parietal, and parieto-occipital regions (C3, C1, Cz, C2, C4, C6, TP7, CP5, CP1, CPz, CP2, CP6, P7, P5, P1, Pz, P2, P4, P6, PO3, POz, PO4, O1, Oz, and O2) in the P1 compared with the N trials. The P3b’s corresponding reversed polarity at frontotemporal sites (F9, F7, F5, F3, F1, Fz, F2, F4, F6, F8, F10, FC5, FC1, FCz, FC2, FC6, FT9, T7, C5, FT9, and FT10) was also significantly increased when the probe was presented (P = 0.001). Note that in experiment 1, in addition to these two clusters, there was another significant cluster that was related to increased N1 amplitude and/or MMN responses to the probe. In experiment 2, this N1/MMN response to the probe did not reach significance. The lack of significant N1/MMN in experiment 2 could be related to the variability in probe position and SOA, which make it more difficult to extract and encode sequence regularity. The comparison of CLARA solutions for P1 and N revealed greater source activity in bilateral MTL (300–700 ms), right STG (300–700 ms), and left cingulate gyrus (500–800 ms) when the probe was presented (Fig. 11B).
Fig. 11.
Event-related potentials (ERPs) elicited in the P1 (ERP waves elicited by sequences containing probe at +1 position) and N (ERP waves elicited by sequences with only distractors) conditions in experiment 2. A, top: group-mean ERPs at a midline frontocentral electrode (FCz) and a midline parietal electrode (Pz). Gray shading represents the time intervals of the difference between P1 and N to reach significance. Bottom, topographies of ERP difference during the time intervals. B, left: difference in source activity between P1 and N conditions. Right, time series of difference in source activity, where red shading represents time intervals of the difference between TP1–T and TP7–T to reach significance. Sag, sagittal; Cor, coronal; Tra, transverse; A, anterior; P, posterior; R, right; L, left.
As in experiment 1, we compared ERPs and source activities for hits and misses in the TP condition. Seven participants were excluded because they had fewer than 10 trials in either condition (i.e., hits or misses). The analysis revealed larger P3b amplitude for hits than misses (P < 0.01; Fig. 12A) at central and parietal electrodes (Cz, C2, C4, C6, CP1, CPz, CP2, CP6, TP8, P3, P1, Pz, P2, P4, P6, P8, PO3, and PO4). The P3b’s corresponding reversed polarity appeared at frontal, temporal, temporoparietal, and parietal sites, and the difference between the two conditions reached significance at right frontal and temporal sites (F9, F7, F5, F3, FC5, C5, T7, and FT9; P = 0.015). The enhanced P3b for hits coincided with increases in source activity in right MTL between 700 and 1,000 ms following the probe onset (Fig. 12B).
Fig. 12.
Event-related potentials (ERPs) elicited by hits and misses in the TP1 (ERP waves elicited by sequences containing target and probe at +1 position) condition in experiment 2. A, top: group-mean ERPs at a frontotemporal electrode (FT10) and a midline parietal electrode (Pz). Gray shading represents the time intervals of the difference between hits and misses to reach significance. Bottom, topographies of ERP difference during the time intervals. B, left: difference in source activity between hits and misses. Right, time series of difference in source activity, where red shading represents time intervals of the difference between hits and misses to reach significance. Sag, sagittal; Cor, coronal; Tra, transverse; A, anterior; P, posterior; R, right; L, left.
Brain-behavior correlations.
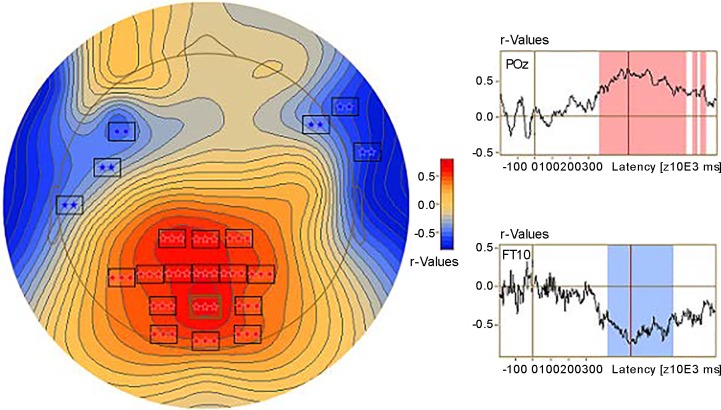
We combined the probe discrimination accuracy data and probe-elicited ERPs in the AB condition from both experiments and then conducted a correlation analysis between the probe discrimination accuracy and ERP amplitude (BESA Statistics 2.0). The correlation between ERP and probe discrimination accuracy revealed two significant clusters (Fig. 13). The first cluster was related to the P3b and appeared at central, central-parietal, parietal, and parieto-occipital scalp regions (C1, Cz, C2, C4, CP1, CPz, CP2,, P7, P5, P3, P1, Pz, P2, P4, P6, PO3, POz, PO4, O1, Oz, and O2). Activity in this cluster was positively correlated with probe discrimination accuracy (P < 0.001). The second cluster was related to the P3b’s reversal of polarity at frontotemporal sites (F5, F3, F1, Fz, F2, F4, F6, F8, F10, FC5, T7, C5, TP8, FT10, and TP10) and was negatively correlated with the probe discrimination accuracy (P = 0.002).
Fig. 13.
Correlation between event-related potentials elicited by the probe and probe discrimination accuracy in the attentional blink (AB) condition. Left, scalp distribution of the correlation. Squares represent electrodes to reach significance: **P < 0.01; ***P < 0.001. Right, time series of correlations at a parietal-occipital electrode (POz) and a frontotemporal electrode (FT10). Red and blue shading represents time intervals of the correlations to reach significance, where red indicates positive correlation and blue indicates negative correlation.
DISCUSSION
The present study aimed to characterize the changes in source activity associated with the auditory AB. In two separate experiments, we showed that the presence of the target caused a probe-processing deficit when the probe was presented at the +1 position following the target, and this auditory AB was paralleled with a reduced P3a at frontal regions and a reduced P3b wave at parietal scalp regions. The probe-processing deficit and the suppression of P3b during the auditory AB is consistent with previous studies (Dell’Acqua et al. 2003; Shen and Alain 2010, 2011, 2012; Shen et al. 2016; Vogel and Luck 2002; Vogel et al. 1998) and is thought to reflect a failure of the probe to reach short-term consolidation.
Brain Cortices with Reduced Activity During the Auditory AB
Converging evidence from fMRI (Kranczioch et al. 2005; Marois et al. 2000) and MEG (Gross et al. 2004) studies suggests that the visual AB is associated with changes in a distributed neural network that include frontal and parietal cortices. By using EEG, Sergent et al. (2005) revealed that the cortical sources for the visual AB were located in left temporal, left inferior frontal, lateral prefrontal, anterior cingulate cortices, and posterior regions.
In the present study we also observed changes in a widely distributed neural network during the auditory AB. In experiment 1, the EEG distributed source modeling showed reduced source activity in bilateral MTL, bilateral TPJ (extending into IPL), and left cerebellum for the TP–T condition compared with the P–N condition. In experiment 2, we found reduced source activity in bilateral MTL, right TPJ (extending into IPL), and left vmPFC for the TP1–T condition compared with the P1–N condition, and reduced source activity in bilateral MTL, TPJ (extending into IPL), and vmPFC, for the TP1–T condition compared with the TP7–T condition.
Medial Temporal Lobe
Both experiments showed the prominent reduction of source activity in the MTL. The association of the auditory AB with MTL is consistent with previous studies of short-term consolidation. Studies of animal and human participants with brain lesion or surgery have revealed that the MTL plays an important role in short-term consolidation (McClelland et al. 1995; Rempel-Clower et al. 1996; Scoville and Milner 1957; Squire 1992; Squire et al. 2004; Squire and Zola-Morgan 1991; Teyler and DiScenna 1986; Zola-Morgan et al. 1986). This location of source activity also agrees well with those of fMRI studies investigating the role of MTL in memory encoding (Diana et al. 2008; Kuhl et al. 2012).
Temporoparietal Junction and Inferior Parietal Lobe
In both experiments, activity in the TPJ (extending into IPL) was reduced during the AB. This result is consistent with prior finding that IPL plays a role in working memory updating (Borst and Anderson 2013; Salmon et al. 1996). Furthermore, TPJ has been shown to play an important role in P3b generation (Comerchero and Polich 1999; Knight et al. 1989; Polich 2007; Polich and Criado 2006; Yamaguchi and Knight 1992), and P3b is an index of working memory updating (Donchin 1981).
Ventromedial Prefrontal Cortex (vmPFC)
Prior research has revealed that vmPFC is involved in short-term consolidation, as well (Akirav and Maroun 2006; Nieuwenhuis and Takashima 2011; Takashima et al. 2007; Takashima et al. 2006). In the present study, vmPFC was associated with the AB in experiment 2, but not in experiment 1. This differential recruitment of the vmPFC could be due to the fact that in experiment 2, participants could not easily predict when the probe would occur because it was randomly presented at one of two positions within the sequence of stimuli. The vmPFC has been associated with inhibiting inappropriate responses as well as in making the decision when to release that inhibition (Bechara et al. 2000; Nieuwenhuis and Takashima 2011; Salmi et al. 2009). The uncertainty of probe position required a decision about the time to release inhibition, resulting in the activation of the vmPFC.
Left Cerebellum
The auditory AB was associated with activity in the left cerebellum in experiment 1, but not in experiment 2. Prior research has shown that the cerebellum plays a role in the monitoring of expected events and is modulated by the timing of stimuli within a sequence (Ben-Yehudah et al. 2007; Ivry 2000; Molinari et al. 2008; Stoodley et al. 2012). In experiment 1, the probe, if present, always occurred at the 8th position within the sequence (i.e., the +1 position following the target), thereby creating a high expectation to hear the probe at that position. However, in experiment 2, the position of the probe was varied randomly, making it difficult for participants to know when it would occur, if at all. This difference in expectation between the two experiments may partly explain the difference in cerebellar activity during the auditory AB.
Additional Source Activity Elicited by the Probe
During the auditory AB, we observed reduced source activity primarily in MTL, with additional sources in IPL, vmPFC, and left cerebellum. However, the probe might elicit earlier brain responses similar to those generated by the probe in the no-AB condition. When we compared the P with the N trials, the results showed that the probe elicited additional increased source activity in right STG, left cingulate gyrus, and decreased activity in OFC besides those observed when we compared the AB with the no-AB conditions (see Figs. 5B and 11B).
Right Superior Temporal Gyrus
Prior research has suggested that the right STG is responsible for pitch processing of complex tones requiring cognitive computation (Johnsrude et al. 2000; Schönwiesner et al. 2007; Zatorre 1988; Zatorre and Samson 1991). The probe in the present study was a frequency-modulated glide that changed smoothly from 636 to 1,006 Hz, but distractors were pure tones. Thus the difference in source activity in right STG could be related to this difference in sound complexity between the AB and no-AB conditions. The right STG may be important for maintaining pitch for a brief time period after initial processing in primary auditory cortex but before further processing (Zatorre and Samson, 1991). Together, these results are consistent with the notion that the AB reflects the failure of processing of the probe to reach short-term consolidation.
Left Cingulate Cortex
Cingulate cortex has been associated with executive functions; especially when conflicting response alternatives are presented, it would be activated to engage strategic processes to reduce response error (Carter et al. 2000; Paus 2001; van Veen et al. 2001). In the P trials of the present study, participants needed to make different responses for the target (“no”) and probe (“yes”) and to press different buttons (“1” for “yes” and “0” for “no”). To avoid response error, strategic processes might be engaged, and thus cingulate cortex was activated.
Orbitofrontal Cortex
In addition, when we compared the P trials with the N trials, there was increased activity in OFC for the N trials in experiment 1, but not in experiment 2. Prior research has revealed that OFC is activated when stimuli deviate from prior expectations during attention tasks (Freedman et al. 1998; Nobre et al. 1999). In experiment 1 of the present study, the probe was always presented at the +1 position. Thus, for the N trials, participants did not perceive the probe, and the OFC was activated. In contrast, in experiment 2, the probe could be presented at +1 or +7 positions, and participants could not anticipate where the probe could be and whether or not the probe would be presented. Thus there was no significant change in OFC activation when we compared probe-only with none conditions.
Hit vs. Miss Trials in AB (TP and TP1) Condition
In experiment 1, we observed increased P3b for hits compared with misses. This increase in P3b amplitude coincided with increased source activity in the right MTL, left cerebellum, left anterior thalamic nuclei, and mammillary body. As in experiment 1, we observed increased P3b for the hits compared with misses in experiment 2, and this coincided with source activity in the right MTL. However, in experiment 2, we did not observe a difference in source activity within the thalamic nuclei and mammillary body. The discrepancy between the two experiments may be related to the timing of the stimuli. An important function of the mammillary body is to relay input from hippocampus to anterior thalamic nuclei, and the hippocampus-mammillary-thalamic network is thought to play an important role in episodic memory (Aggleton and Brown 1999; Aggleton et al. 1995, 2010; Aggleton and Sahgal 1993). The current result can be accounted for by the fact that the probe was always presented at the same sequential position in experiment 1. In comparison, the positions of the target and probe were varied in experiment 2. Hence, timing information in memory could not help in identifying the probe, and thus there was no recruitment of the left anterior thalamic nuclei and mammillary body for the detected trials. This could also account for the difference in cerebellar activity between experiments 1 and 2, because the cerebellum is related to timing. Moreover, there was no activity change in STG when we compared the hit trials with miss trials, providing further evidence that during the auditory AB, the probe sound was perceived but not reported.
Time Courses of Source Activities
In both experiments, we observed source activity change in MTL at ~300–500 ms following probe onset when comparing TP–T (AB) with P–N (no-AB) conditions. This change in source activity peaks earlier than that observed when we compared P with N conditions or compared the hit trials with miss trials in TP conditions. This is consistent with the delay of the P3b to the probe during the AB (Shen and Alain 2010, 2011, 2012; Vogel and Luck 2002). When TP–T and P–N conditions were compared, the P3b to the probe peaked earlier in the P–N condition than in the TP–T condition, and thus the source activity change reached significance earlier. In contrast, when the hit and miss trials in TP conditions were compared, the P3b elicited by the probe was delayed for the hit trials (note that there was no P3b elicited by the probe for miss trials), and then the source activity change appeared later. Accordingly, when the TP and T conditions were compared, the time course of source activity change in right MTL also fell in this time interval. These results provide converging evidence for P3b generators located in MTL. The results also support the roles of P3b and MTL in short-term consolidation.
The increased in source activity in right STG peaked at ~400 ms following the probe onset. It preceded the peak activation in MTL and suggests a progression from early encoding of pitch information in STG to short-term consolidation in MTL. However, we also observed relatively late source activity in STG, which could index sensitization or habituation processes associated with sensory memory, which can last several seconds (Sams et al. 1993).
The time course of source activity changes in TPJ (extending into IPL), vmPFC, and left cerebellum all fell 300–800 ms following the probe onset. This coincided with the P3b latency and further demonstrates that those structures are related to short-term consolidation. Moreover, the time course of source activity in left anterior thalamic nuclei and mammillary body was ~700–1,000 ms following probe onset when the hit and miss trials in the TP condition were compared. This is similar to the time course of source activity in MTL. These results suggest that auditory short-term consolidation engages a widely distributed neural network beyond the auditory cortex.
The time course of source activity in left cingulate cortex peaked at ~500 ms following probe onset when P and N conditions were compared. This activity may index conflict resolution between alternative responses as well as the preparation for the response. Additionally, the changes in source activity in orbitofrontal cortex peaked at ~225 ms following probe onset when P and N conditions were compared. This activity may index expectation processing for the probe.
MMN, P3a, and P3b Waves
Notably, the MMN and P3a wave elicited by the probe were suppressed during the AB. The MMN is thought to index an automatic discrimination process because it can occur even when a participant does not pay attention to stimuli (Alain and Woods 1997; Näätänen and Alho 1997; Näätänen et al. 1993; Schröger 1997; Woldorff et al. 1991). The P3a has been associated with brain activity related to the engagement of involuntary attention and the processing of novelty (Comerchero and Polich 1998; Courchesne et al. 1975; Knight 1984; Squires et al. 1975). Unlike the P3b, the MMN and P3a are not task related. The suppression of MMN and P3a could be attributed to the temporal window of integration required for MMN generation (Sussman et al. 1999; Yabe et al. 2001). When two deviants are presented successively, two MMNs are elicited only when the SOA between them is long enough. In the study of Shen and Alain (2010), when the SOA was 150 ms, the auditory AB was much reduced and a large P3b wave was elicited for the probe when the probe was at the +1 position, but the MMN and P3a were suppressed in that condition. In the present study, the SOA between the two deviants (target and probe) was either 90 or 120 ms, and it appeared that only one MMN and P3a for the target (first deviant) was elicited. Thus, although P3b, MMN and P3a were all suppressed during the auditory AB in the present study, separate mechanisms might be underlain.
Prior research provides converging evidence for MMN generators located along the STG and prefrontal cortex (Alain et al. 1998, 1999; Alho 1995), whereas the P3a generators are located in the frontal lobe and hippocampal (Comerchero and Polich 1999; Knight 1984, 1996; Knight et al. 1995; Polich 2007; Polich and Criado 2006). In comparison, P3b generators involve a more posterior and distributed neural network that includes prefrontal, cingulate, hippocampal, temporoparietal junction, and parietal structures (Baudena et al. 1995; Comerchero and Polich 1999; Halgren et al. 1980, 1995a, 1995b, 1998; Polich 2007; Polich and Criado 2006; Volpe et al. 2007; Wronka et al. 2012). Thus it seems that the reductions of source activity during the auditory AB were more likely associated with the P3b given that the reductions were more posterior and distributed.
Limitations of Source Analysis
In the present study, we used two methods to analyze source activity during the auditory AB. One method used difference waves to isolate neural activity specific to the probe. By comparing the source activity elicited by the probe in the AB condition (e.g., TP–T) to activity elicited by the probe in the no-AB condition (e.g., P–N), we were able to estimate changes in source activity associated with the auditory AB. However, these contrasts provide only an indirect measure of AB because the ERPs used in the AB condition comprised both hit and miss trials. Moreover, the LORETA approach is an estimation of cortical activation. A subtraction of ERPs (i.e., difference waves) requires additional calculation and might further decrease the interpretability of the estimated locations of neuronal activations.
Our second method compared ERPs elicited when the probe was accurately detected (hit) and when it was missed during the TP (target-probe) condition. This approach revealed source activity in MTL, which was reduced during the AB and thereby provided converging evidence supporting the role of MTL in P3b generation and short-term memory consolidation. However, it should be noted that using such an approach reduced the number of participants due to lack of sufficient hit or miss trials to carry out the ERP data analysis, thereby reducing statistical power. Future research using source modeling to understand the changes in neural activity associated with AB should consider the use of both approaches.
Concluding Remarks
The present study shows in two separate experiments that the auditory AB is associated with the reductions of source activity primarily in MTL, with additional sources in IPL, vmPFC, left anterior thalamic nuclei, mammillary body, and left cerebellum. We also observed decreased source activity during the AB that was specific to each experiment (e.g., reductions of activity in the vmPFC in experiment 2, and decreased activity in left anterior thalamic nuclei, mammillary body, and left cerebellum in experiment 1). These differences in neural activity are likely related to methodological differences between the two experiments. For instance, in experiment 1 the fixed position of the probe likely influenced participants’ expectation of hearing the probe, and this could explain the source activity observed in left anterior thalamic nuclei, mammillary body, and left cerebellum. Accordingly, the uncertainty of the probe position in experiment 2 increased attentional demands, which may have resulted in the recruitment of the vmPFC. Moreover, we did not observe an increased activity in right STG when comparing the hit and miss trials in the TP condition. This suggests that during the auditory AB, the probe was processed in the cortex and generated comparable source activity despite the presence of a nearby target stimulus. These results provide further evidence that the auditory AB reflects the failure of the probe to reach consciousness (through short-term consolidation) to be reported despite the probe being perceived.
GRANTS
This work was supported by the Canadian Institute of Health Research (C. Alain) and the Natural Sciences and Engineering Research Council of Canada (C. Alain).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.S., D.T.V., and C.A. conceived and designed research; D.S. and D.T.V. performed experiments; D.S. analyzed data; D.S. and C.A. interpreted results of experiments; D.S. prepared figures; D.S. drafted manuscript; D.S., D.T.V., and C.A. edited and revised manuscript; D.S., D.T.V., and C.A. approved final version of manuscript.
REFERENCES
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci 22: 425–444, 1999. doi: 10.1017/S0140525X99002034. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Neave N, Nagle S, Hunt PR. A comparison of the effects of anterior thalamic, mamillary body and fornix lesions on reinforced spatial alternation. Behav Brain Res 68: 91–101, 1995. doi: 10.1016/0166-4328(94)00163-A. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, O’Mara SM, Vann SD, Wright NF, Tsanov M, Erichsen JT. Hippocampal-anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. Eur J Neurosci 31: 2292–2307, 2010. doi: 10.1111/j.1460-9568.2010.07251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Sahgal A. The contribution of the anterior thalamic nuclei to anterograde amnesia. Neuropsychologia 31: 1001–1019, 1993. doi: 10.1016/0028-3932(93)90029-Y. [DOI] [PubMed] [Google Scholar]
- Akirav I, Maroun M. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cereb Cortex 16: 1759–1765, 2006. doi: 10.1093/cercor/bhj114. [DOI] [PubMed] [Google Scholar]
- Alain C, Cortese F, Picton TW. Event-related brain activity associated with auditory pattern processing. Neuroreport 10: 2429–2434, 1999. doi: 10.1097/00001756-199908020-00038. [DOI] [PubMed] [Google Scholar]
- Alain C, Richer F, Achim A, Saint Hilaire JM. Human intracerebral potentials associated with target, novel, and omitted auditory stimuli. Brain Topogr 1: 237–245, 1989. doi: 10.1007/BF01129601. [DOI] [PubMed] [Google Scholar]
- Alain C, Woods DL. Attention modulates auditory pattern memory as indexed by event-related brain potentials. Psychophysiology 34: 534–546, 1997. doi: 10.1111/j.1469-8986.1997.tb01740.x. [DOI] [PubMed] [Google Scholar]
- Alain C, Woods DL, Knight RT. A distributed cortical network for auditory sensory memory in humans. Brain Res 812: 23–37, 1998. doi: 10.1016/S0006-8993(98)00851-8. [DOI] [PubMed] [Google Scholar]
- Alho K. Cerebral generators of mismatch negativity (MMN) and its magnetic counterpart (MMNm) elicited by sound changes. Ear Hear 16: 38–51, 1995. doi: 10.1097/00003446-199502000-00004. [DOI] [PubMed] [Google Scholar]
- Arnell KM, Jolicoeur P. The attentional blink across stimulus modalities: Evidence for central processing limitations. J Exp Psychol Hum Percept Perform 25: 630–648, 1999. doi: 10.1037/0096-1523.25.3.630. [DOI] [Google Scholar]
- Baudena P, Halgren E, Heit G, Clarke JM. Intracerebral potentials to rare target and distractor auditory and visual stimuli. III. Frontal cortex. Electroencephalogr Clin Neurophysiol 94: 251–264, 1995. doi: 10.1016/0013-4694(95)98476-O. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain 123: 2189–2202, 2000. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Ben-Yehudah G, Guediche S, Fiez JA. Cerebellar contributions to verbal working memory: beyond cognitive theory. Cerebellum 6: 193–201, 2007. doi: 10.1080/14734220701286195. [DOI] [PubMed] [Google Scholar]
- Beniczky S, Rosenzweig I, Scherg M, Jordanov T, Lanfer B, Lantz G, Larsson PG. Ictal EEG source imaging in presurgical evaluation: High agreement between analysis methods. Seizure 43: 1–5, 2016. doi: 10.1016/j.seizure.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst JP, Anderson JR. Using model-based functional MRI to locate working memory updates and declarative memory retrievals in the fronto-parietal network. Proc Natl Acad Sci USA 110: 1628–1633, 2013. doi: 10.1073/pnas.1221572110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent DE, Broadbent MH. From detection to identification: response to multiple targets in rapid serial visual presentation. Percept Psychophys 42: 105–113, 1987. doi: 10.3758/BF03210498. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci USA 97: 1944–1948, 2000. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM, Potter MC. A two-stage model for multiple target detection in rapid serial visual presentation. J Exp Psychol Hum Percept Perform 21: 109–127, 1995. doi: 10.1037/0096-1523.21.1.109. [DOI] [PubMed] [Google Scholar]
- Comerchero MD, Polich J. P3a, perceptual distinctiveness, and stimulus modality. Brain Res Cogn Brain Res 7: 41–48, 1998. doi: 10.1016/S0926-6410(98)00009-3. [DOI] [PubMed] [Google Scholar]
- Comerchero MD, Polich J. P3a and P3b from typical auditory and visual stimuli. Clin Neurophysiol 110: 24–30, 1999. doi: 10.1016/S0168-5597(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalogr Clin Neurophysiol 39: 131–143, 1975. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Dell’Acqua R, Jolicoeur P, Pesciarelli F, Job CR, Palomba D. Electrophysiological evidence of visual encoding deficits in a cross-modal attentional blink paradigm. Psychophysiology 40: 629–639, 2003. doi: 10.1111/1469-8986.00064. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. High-resolution multi-voxel pattern analysis of category selectivity in the medial temporal lobes. Hippocampus 18: 536–541, 2008. doi: 10.1002/hipo.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrijevic A, Pratt H, Starr A. Auditory cortical activity in normal hearing subjects to consonant vowels presented in quiet and in noise. Clin Neurophysiol 124: 1204–1215, 2013. doi: 10.1016/j.clinph.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Donchin E. Presidential address, 1980. Surprise!... Surprise? Psychophysiology 18: 493–513, 1981. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Freedman M, Black S, Ebert P, Binns M. Orbitofrontal function, object alternation and perseveration. Cereb Cortex 8: 18–27, 1998. doi: 10.1093/cercor/8.1.18. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Di Lollo V. Beyond the attentional blink: visual masking by object substitution. J Exp Psychol Hum Percept Perform 24: 1454–1466, 1998. doi: 10.1037/0096-1523.24.5.1454. [DOI] [PubMed] [Google Scholar]
- Gross J, Schmitz F, Schnitzler I, Kessler K, Shapiro K, Hommel B, Schnitzler A. Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proc Natl Acad Sci USA 101: 13050–13055, 2004. doi: 10.1073/pnas.0404944101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Liégeois C, Chauvel P, Musolino A. Intracerebral potentials to rare target and distractor auditory and visual stimuli. I. Superior temporal plane and parietal lobe. Electroencephalogr Clin Neurophysiol 94: 191–220, 1995a. doi: 10.1016/0013-4694(94)00259-N. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Marinkovic K, Devaux B, Vignal JP, Biraben A. Intracerebral potentials to rare target and distractor auditory and visual stimuli. II. Medial, lateral and posterior temporal lobe. Electroencephalogr Clin Neurophysiol 94: 229–250, 1995b. doi: 10.1016/0013-4694(95)98475-N. [DOI] [PubMed] [Google Scholar]
- Halgren E, Marinkovic K, Chauvel P. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalogr Clin Neurophysiol 106: 156–164, 1998. doi: 10.1016/S0013-4694(97)00119-3. [DOI] [PubMed] [Google Scholar]
- Halgren E, Squires NK, Wilson CL, Rohrbaugh JW, Babb TL, Crandall PH. Endogenous potentials generated in the human hippocampal formation and amygdala by infrequent events. Science 210: 803–805, 1980. doi: 10.1126/science.7434000. [DOI] [PubMed] [Google Scholar]
- Horváth J, Burgyán A. Distraction and the auditory attentional blink. Atten Percept Psychophys 73: 695–701, 2011. doi: 10.3758/s13414-010-0077-3. [DOI] [PubMed] [Google Scholar]
- Husain M, Shapiro K, Martin J, Kennard C. Abnormal temporal dynamics of visual attention in spatial neglect patients. Nature 385: 154–156, 1997. doi: 10.1038/385154a0. [DOI] [PubMed] [Google Scholar]
- Ivry R. Exploring the role of the cerebellum in sensory anticipation and timing: commentary on Tesche and Karhu. Hum Brain Mapp 9: 115–118, 2000. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsrude IS, Penhune VB, Zatorre RJ. Functional specificity in the right human auditory cortex for perceiving pitch direction. Brain 123: 155–163, 2000. doi: 10.1093/brain/123.1.155. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P, Dell’Acqua R. The demonstration of short-term consolidation. Cognit Psychol 36: 138–202, 1998. doi: 10.1006/cogp.1998.0684. [DOI] [PubMed] [Google Scholar]
- Knight R. Contribution of human hippocampal region to novelty detection. Nature 383: 256–259, 1996. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- Knight RT. Decreased response to novel stimuli after prefrontal lesions in man. Electroencephalogr Clin Neurophysiol 59: 9–20, 1984. doi: 10.1016/0168-5597(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Knight RT, Grabowecky MF, Scabini D. Role of human prefrontal cortex in attention control. Adv Neurol 66: 21–36, 1995. [PubMed] [Google Scholar]
- Knight RT, Scabini D, Woods DL, Clayworth CC. Contributions of temporal-parietal junction to the human auditory P3. Brain Res 502: 109–116, 1989. doi: 10.1016/0006-8993(89)90466-6. [DOI] [PubMed] [Google Scholar]
- Kranczioch C, Debener S, Schwarzbach J, Goebel R, Engel AK. Neural correlates of conscious perception in the attentional blink. Neuroimage 24: 704–714, 2005. doi: 10.1016/j.neuroimage.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Kuhl BA, Rissman J, Wagner AD. Multi-voxel patterns of visual category representation during episodic encoding are predictive of subsequent memory. Neuropsychologia 50: 458–469, 2012. doi: 10.1016/j.neuropsychologia.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marois R, Chun MM, Gore JC. Neural correlates of the attentional blink. Neuron 28: 299–308, 2000. doi: 10.1016/S0896-6273(00)00104-5. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev 102: 419–457, 1995. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Molinari M, Chiricozzi FR, Clausi S, Tedesco AM, De Lisa M, Leggio MG. Cerebellum and detection of sequences, from perception to cognition. Cerebellum 7: 611–615, 2008. doi: 10.1007/s12311-008-0060-x. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Alho K. Mismatch negativity--the measure for central sound representation accuracy. Audiol Neurotol 2: 341–353, 1997. doi: 10.1159/000259255. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Paavilainen P, Tiitinen H, Jiang D, Alho K. Attention and mismatch negativity. Psychophysiology 30: 436–450, 1993. doi: 10.1111/j.1469-8986.1993.tb02067.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis IL, Takashima A. The role of the ventromedial prefrontal cortex in memory consolidation. Behav Brain Res 218: 325–334, 2011. doi: 10.1016/j.bbr.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Coull JT, Frith CD, Mesulam MM. Orbitofrontal cortex is activated during breaches of expectation in tasks of visual attention. Nat Neurosci 2: 11–12, 1999. doi: 10.1038/4513. [DOI] [PubMed] [Google Scholar]
- Oliveira FT, Hickey C, McDonald JJ. Proactive and reactive processes in the medial frontal cortex: an electrophysiological study. PLoS One 9: e84351, 2014. doi: 10.1371/journal.pone.0084351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol 18: 49–65, 1994. doi: 10.1016/0167-8760(84)90014-X. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2: 417–424, 2001. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Picton TW. The P300 wave of the human event-related potential. J Clin Neurophysiol 9: 456–479, 1992. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Picton TW, van Roon P, Armilio ML, Berg P, Ille N, Scherg M. The correction of ocular artifacts: a topographic perspective. Clin Neurophysiol 111: 53–65, 2000. doi: 10.1016/S1388-2457(99)00227-8. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol 118: 2128–2148, 2007. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. Int J Psychophysiol 60: 172–185, 2006. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: an attentional blink? J Exp Psychol Hum Percept Perform 18: 849–860, 1992. doi: 10.1037/0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci 16: 5233–5255, 1996. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richer F, Lepage M. Frontal lesions increase post-target interference in rapid stimulus streams. Neuropsychologia 34: 509–514, 1996. doi: 10.1016/0028-3932(95)00132-8. [DOI] [PubMed] [Google Scholar]
- Rusiniak M, Lewandowska M, Wolak T, Pluta A, Milner R, Ganc M, Włodarczyk A, Senderski A, Sliwa L, Skarżyński H. A modified oddball paradigm for investigation of neural correlates of attention: a simultaneous ERP-fMRI study. MAGMA 26: 511–526, 2013. doi: 10.1007/s10334-013-0374-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi J, Rinne T, Koistinen S, Salonen O, Alho K. Brain networks of bottom-up triggered and top-down controlled shifting of auditory attention. Brain Res 1286: 155–164, 2009. doi: 10.1016/j.brainres.2009.06.083. [DOI] [PubMed] [Google Scholar]
- Salmon E, Van der Linden M, Collette F, Delfiore G, Maquet P, Degueldre C, Luxen A, Franck G. Regional brain activity during working memory tasks. Brain 119: 1617–1625, 1996. doi: 10.1093/brain/119.5.1617. [DOI] [PubMed] [Google Scholar]
- Sams M, Hari R, Rif J, Knuutila J. The human auditory sensory memory trace persists about 10 sec: neuromagnetic evidence. J Cogn Neurosci 5: 363–370, 1993. doi: 10.1162/jocn.1993.5.3.363. [DOI] [PubMed] [Google Scholar]
- Schönwiesner M, Novitski N, Pakarinen S, Carlson S, Tervaniemi M, Näätänen R. Heschl’s gyrus, posterior superior temporal gyrus, and mid-ventrolateral prefrontal cortex have different roles in the detection of acoustic changes. J Neurophysiol 97: 2075–2082, 2007. doi: 10.1152/jn.01083.2006. [DOI] [PubMed] [Google Scholar]
- Schröger E. On the detection of auditory deviations: a pre-attentive activation model. Psychophysiology 34: 245–257, 1997. doi: 10.1111/j.1469-8986.1997.tb02395.x. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20: 11–21, 1957. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent C, Baillet S, Dehaene S. Timing of the brain events underlying access to consciousness during the attentional blink. Nat Neurosci 8: 1391–1400, 2005. doi: 10.1038/nn1549. [DOI] [PubMed] [Google Scholar]
- Shen D, Alain C. Neuroelectric correlates of auditory attentional blink. Psychophysiology 47: 184–191, 2010. doi: 10.1111/j.1469-8986.2009.00924.x. [DOI] [PubMed] [Google Scholar]
- Shen D, Alain C. Temporal attention facilitates short-term consolidation during a rapid serial auditory presentation task. Exp Brain Res 215: 285–292, 2011. doi: 10.1007/s00221-011-2897-3. [DOI] [PubMed] [Google Scholar]
- Shen D, Alain C. Implicit temporal expectation attenuates auditory attentional blink. PLoS One 7: e36031, 2012. doi: 10.1371/journal.pone.0036031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Mondor TA. Effect of distractor sounds on the auditory attentional blink. Percept Psychophys 68: 228–243, 2006. doi: 10.3758/BF03193672. [DOI] [PubMed] [Google Scholar]
- Shen D, Mondor TA. Object file continuity and the auditory attentional blink. Percept Psychophys 70: 896–915, 2008. doi: 10.3758/PP.70.5.896. [DOI] [PubMed] [Google Scholar]
- Shen D, Ross B, Alain C. Temporal cuing modulates alpha oscillations during auditory attentional blink. Eur J Neurosci 44: 1833–1845, 2016. doi: 10.1111/ejn.13266. [DOI] [PubMed] [Google Scholar]
- Smith ME, Stapleton JM, Halgren E. Human medial temporal lobe potentials evoked in memory and language tasks. Electroencephalogr Clin Neurophysiol 63: 145–159, 1986. doi: 10.1016/0013-4694(86)90008-8. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev 99: 195–231, 1992. doi: 10.1037/0033-295X.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci 27: 279–306, 2004. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science 253: 1380–1386, 1991. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr Clin Neurophysiol 38: 387–401, 1975. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage 59: 1560–1570, 2012. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman E, Winkler I, Ritter W, Alho K, Näätänen R. Temporal integration of auditory stimulus deviance as reflected by the mismatch negativity. Neurosci Lett 264: 161–164, 1999. doi: 10.1016/S0304-3940(99)00214-1. [DOI] [PubMed] [Google Scholar]
- Takashima A, Nieuwenhuis IL, Rijpkema M, Petersson KM, Jensen O, Fernández G. Memory trace stabilization leads to large-scale changes in the retrieval network: a functional MRI study on associative memory. Learn Mem 14: 472–479, 2007. doi: 10.1101/lm.605607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A, Petersson KM, Rutters F, Tendolkar I, Jensen O, Zwarts MJ, McNaughton BL, Fernández G. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci USA 103: 756–761, 2006. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyler TJ, DiScenna P. The hippocampal memory indexing theory. Behav Neurosci 100: 147–154, 1986. doi: 10.1037/0735-7044.100.2.147. [DOI] [PubMed] [Google Scholar]
- Tremblay S, Vachon F, Jones DM. Attentional and perceptual sources of the auditory attentional blink. Percept Psychophys 67: 195–208, 2005. doi: 10.3758/BF03206484. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage 14: 1302–1308, 2001. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Luck SJ. Delayed working memory consolidation during the attentional blink. Psychon Bull Rev 9: 739–743, 2002. doi: 10.3758/BF03196329. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Luck SJ, Shapiro KL. Electrophysiological evidence for a postperceptual locus of suppression during the attentional blink. J Exp Psychol Hum Percept Perform 24: 1656–1674, 1998. doi: 10.1037/0096-1523.24.6.1656. [DOI] [PubMed] [Google Scholar]
- Volpe U, Mucci A, Bucci P, Merlotti E, Galderisi S, Maj M. The cortical generators of P3a and P3b: a LORETA study. Brain Res Bull 73: 220–230, 2007. doi: 10.1016/j.brainresbull.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Hackley SA, Hillyard SA. The effects of channel-selective attention on the mismatch negativity wave elicited by deviant tones. Psychophysiology 28: 30–42, 1991. doi: 10.1111/j.1469-8986.1991.tb03384.x. [DOI] [PubMed] [Google Scholar]
- Wronka E, Kaiser J, Coenen AM. Neural generators of the auditory evoked potential components P3a and P3b. Acta Neurobiol Exp (Wars) 72: 51–64, 2012. [DOI] [PubMed] [Google Scholar]
- Yabe H, Koyama S, Kakigi R, Gunji A, Tervaniemi M, Sato Y, Kaneko S. Automatic discriminative sensitivity inside temporal window of sensory memory as a function of time. Brain Res Cogn Brain Res 12: 39–48, 2001. doi: 10.1016/S0926-6410(01)00027-1. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Knight RT. Effects of temporal-parietal lesions on the somatosensory P3 to lower limb stimulation. Electroencephalogr Clin Neurophysiol 84: 139–148, 1992. doi: 10.1016/0168-5597(92)90018-7. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ. Pitch perception of complex tones and human temporal-lobe function. J Acoust Soc Am 84: 566–572, 1988. doi: 10.1121/1.396834. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Samson S. Role of the right temporal neocortex in retention of pitch in auditory short-term memory. Brain 114: 2403–2417, 1991. doi: 10.1093/brain/114.6.2403. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci 6: 2950–2967, 1986. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]