Abstract
Motor neuron (MN) development in early onset spasticity is poorly understood. For example, spastic cerebral palsy (sCP), the most common motor disability of childhood, is poorly predicted by brain imaging, yet research remains focused on the brain. By contrast, MNs, via the motor unit and neurotransmitter signaling, are the target of most therapeutic spasticity treatments and are the final common output of motor control. MN development in sCP is a critical knowledge gap, because the late embryonic and postnatal periods are not only when the supposed brain injury occurs but also are critical times for spinal cord neuromotor development. Using an animal model of early onset spasticity [spa mouse (B6.Cg-Glrbspa/J) with a glycine (Gly) receptor mutation], we hypothesized that removal of effective glycinergic neurotransmitter inputs to MNs during development will influence MN pruning (including primary dendrites) and MN size. Spa (Glrb−/−) and wild-type (Glrb+/+) mice, ages 4–9 wk, underwent unilateral retrograde labeling of the tibialis anterior muscle MNs via peroneal nerve dip in tetramethylrhodamine. After 3 days, mice were euthanized and perfused with 4% paraformaldehyde, and the spinal cord was excised and processed for confocal imaging. Spa mice had ~61% fewer lumbar tibialis anterior MNs (P < 0.01), disproportionately affecting larger MNs. Additionally, a ~23% reduction in tibialis anterior MN somal surface area (P < 0.01) and a 12% increase in primary dendrites (P = 0.046) were observed. Thus MN pruning and MN somal surface area are abnormal in early onset spasticity. Fewer and smaller MNs may contribute to the spastic phenotype.
NEW & NOTEWORTHY Motor neuron (MN) development in early onset spasticity is poorly understood. In an animal model of early onset spasticity, spa mice, we found ~61% fewer lumbar tibialis anterior MNs compared with controls. This MN loss disproportionately affected larger MNs. Thus number and heterogeneity of the MN pool are decreased in spa mice, likely contributing to the spastic phenotype.
Keywords: glycine receptor, motor neuron, spasticity, spinal cord
INTRODUCTION
Motor neuron (MN) development in early onset spasticity is poorly understood. For example, the most common motor disability of childhood, spastic cerebral palsy (sCP; Palisano et al. 1997; Rosenbaum et al. 2007; Yeargin-Allsopp et al. 2008), always affects walking and is poorly predicted by brain imaging (Bax et al. 2006; Woodward et al. 2006). Yet, research and clinical interventions have remained focused on the brain being the driver of the motor impairment and spasticity. By contrast, MNs are the final common output of motor control but are poorly understood in the context of sCP, notwithstanding the fact they are the ultimate target of many clinical therapeutic spasticity treatments in sCP (McLaughlin et al. 2002; Quality Standards Subcommittee of the American Academy of Neurology et al. 2010). Indeed, the late embryonic and early postnatal periods are not only when the supposed brain injury occurs in sCP but also are critical time periods for spinal cord neuromotor development (Harris and McCaig 1984; Pittman and Oppenheim 1978; Sheard et al. 1984).
The development of an effective neuromotor system relies on an embryonic and perinatal period of MN pruning, a process of programmed cell death of MNs. Specifically, from the third trimester onward, MNs are rapidly pruned until birth, followed by only a slight decline in the neonatal period (Harris and McCaig 1984; Pittman and Oppenheim 1978; Sheard et al. 1984). This developmental pruning of MNs depends on factors including skeletal muscle activation, MN excitability, and trophic factors (Kanning et al. 2010; Lin et al. 2008; Mantilla and Sieck 2008b). Of these factors, activity appears to be a major driver of MN pruning during this critical time period. For example, conditions in which MN excitability is reduced or skeletal muscle activation is impaired result in reduced MN pruning (i.e., too many MNs; Kanning et al. 2010; Landmesser 1992; Mantilla and Sieck 2008a, 2008b; Oppenheim et al. 2000; Pittman and Oppenheim 1978, 1979). By contrast, experimental perturbations producing increased MN excitability and/or skeletal muscle activity result in increased pruning and MN loss (Banks et al. 2005; Fogarty et al. 2013b, 2015; Oppenheim and Nuñez 1982).
Removing or altering the influence of descending inhibitory pathways may change the activity of embryonic MNs and thereby affect MN pruning. In previous work using a glycine (Gly) receptor mutant mouse model [spa (B6C3Fe-a/a spa/spa) mice, a Gly receptor mutation of the β-subunit (Molon et al. 2006; White and Heller 1982)], it was reported that there is a reduction in the number of MNs in the thoracic spinal cord (Molon et al. 2006). In contradistinction, in mice lacking gephyrin, a Gly-receptor clustering molecule (Feng et al. 1998), and in mice lacking the vesicular inhibitory amino acid transporter (VGAT), thereby deficient in GABA and glycine (Saito et al. 2010; Wojcik et al. 2006), there was a greater number of lumbar MNs following embryonic pruning (Banks et al. 2005; Fogarty et al. 2013b, 2015).
In addition to changes in the number of MNs because of abnormal MN pruning, the size of MNs may also be affected, which will alter motor unit recruitment. Specifically, Hennemen et. al. (1965b) showed that the recruitment order of motor units is related to axonal conduction velocities (i.e., size) of MNs, with smaller MNs (slower axonal conduction velocities) recruited before larger MNs. Altered glycinergic neurotransmission in utero has been shown to influence MN size (Fogarty et al. 2016, 2017a), and altered neurotrophic support can disrupt the heterogeneity of the MN pool (Kanning et al. 2010; McHanwell and Biscoe 1981b; Sabharwal et al. 2011).
The B6.Cg-Glrbspa/J (Gly receptor mutation) spa transgenic mouse has reduced glycinergic neurotransmission to MNs (Graham et al. 2006) and displays symptoms of spasticity and motor impairment by the time of postnatal weaning (Becker et al. 1986; Heller and Hallett 1982; Simon 1997). The symptoms, onset, and smaller size of the spa mouse are homologous to those of humans with sCP. Of clinical interest in relation to humans with sCP, alterations in effective glycine neurotransmission and the resultant changes in lumbar MN pool pruning in the spa mice may relate to the spasticity and physical impairment of this mouse. Thus we hypothesize that removal of effective glycinergic neurotransmitter inputs to MNs during development in B6.Cg-Glrbspa/J spa mice will influence MN pruning and MN size (including the number of primary dendrites).
MATERIALS AND METHODS
Experimental animals and anesthesia.
This study used six wild-type and five homozygous spa knockout mice (B6.Cg-Glrbspa/J), ages 4 to 9 wk, of both sexes. Previous rodent studies have shown that glycinergic neurotransmission is mature in mice at this age (Becker et al. 1992; Singer et al. 1998). The B6.Cg-Glrbspa/J knockout mice have a homozygous insertion of LINE-1 in Glrb, the β-subunit of the glycine receptor gene resulting in a splicing error of this subunit (White and Heller 1982). Mice homozygous for this knockout (spa mice) have symptoms by the third to fifth week of life including abnormal gait, muscle rigidity, myoclonic jerks, exaggerated startle response, smaller size, and spasticity (Becker et al. 1986; Heller and Hallett 1982; Molon et al. 2006). Founder heterozygous mice were obtained from Jackson Laboratories (Bar Harbor, ME) and used in a heterozygote × heterozygote breeding scheme. All mice used in this study were the product of this breeding scheme. All mice were housed in identical conditions. Genotyping was done on tail snips as previously described (Graham et al. 2006). All procedures were approved by the Institutional Animal Care and Use Committee at Mayo Clinic (Protocol #A23215). For survival surgery, animals received carprofen (5 mg/kg) in their water bottle starting at least 48 h before surgery for analgesia. Animals were anesthetized with intraperitoneal injections of diazepam (5 mg/kg) and droperidol (15 mg/kg) for survival surgery. Animals also received an intraperitoneal injection of fentanyl (0.3 mg/kg) and subcutaneous injection of buprenorphine SR (0.5–1.0 mg/kg) for additional analgesia. For terminal surgery, animals received an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg).
Retrograde labeling of tibialis anterior motor neurons.
Tibialis anterior MNs were selected as they are innervated by the lumbar MN pool and are relevant to humans with sCP because walking is most commonly impaired. All procedures were performed using aseptic technique, and insulated heating pads maintained body temperature at 38°C. In anesthetized mice, the peroneal nerve was identified proximal to the tibialis anterior muscle, and the fascia was then incised and dissected to expose as much of the peroneal nerve as possible. The peroneal nerve was then cut, and the proximal end of the nerve was placed in a sterile microdish with 1–3 μl of a 5% solution of tetramethylrhodamine (rhodamine; Molecular Probes, Life Technologies, Grand Island, NY), previously validated for the accurate labeling of MNs (Novikova et al. 1997; Richmond et al. 1994). Petroleum jelly was placed on the surrounding tissues, which prevented their exposure to errant rhodamine solution. The retrograde labeling procedure was performed for 45 min, during which the microdish was checked at 5-min intervals, with rhodamine solution added as required (up to 9 μl total). Following this, the peroneal nerve end was removed from the microdish and the surgical site irrigated, cleansed, and sutured. A postsurgery period of 3 days provides sufficient time for retrograde transport to MNs (Gransee et al. 2013; Mantilla et al. 2009; Novikova et al. 1997; Zhan et al. 1989). All animals exhibited a unilateral hind foot drag/drop consistent with loss of tibialis anterior function.
Immunohistochemistry and confocal microscopy.
At 3 days following the retrograde MN labeling procedure, animals were anesthetized and euthanized by exsanguination. Following transcardial perfusion with phosphate-buffered saline (PBS; pH 7.4) and 4% paraformaldehyde in PBS, the lumbar spinal cord was postfixed in 4% paraformaldehyde overnight and then transferred to 24% sucrose in PBS for 72 h or until sunk, before flash-freezing and cryosectioning. A cryostat (Leica Biosystems, Buffalo Grove, IL) was used to cut the samples in 70- to 100-µm longitudinal (horizontal) sections. Sections were placed on gelatin-coated slides, treated with graded ethanols and xylenes, and coverslipped with DPX mounting medium (Fluka, Sigma-Aldrich, St. Louis, MO) in a manner identical to past reports (Gransee et al. 2013, 2017; Mantilla et al. 2009).
Motor neuron counts, somal size measurements, and primary dendrite counts.
Spinal cord sections were imaged using an Olympus FluoView 1200 laser scanning confocal microscope (Olympus America, Melville, NY) mounted on an upright Olympus BX50WI microscope. Rhodamine-labeled tibialis anterior MNs were imaged with a ×40 oil-immersion lens (numerical aperture 1.45), and three-dimensional image stacks were collected in a 640 × 640 array (pixel dimensions 0.50 µm × 0.50 µm) with a 2.0-µm step size. Laser intensity was 5.0–12.0% with confocal aperture and photomultiplier gain kept fixed across samples. Optical slices containing the nucleus of a rhodamine-labeled tibialis anterior MN were identified and used to quantify the number of tibialis anterior MNs. Tibialis anterior MN somal volumes and surface areas were determined from confocal image stacks using ImageJ (Schneider et al. 2012). We employed a stereological sampling procedure to select MNs for morphometric analysis. In this procedure, MNs were systematically sampled in the rostral-to-caudal direction, and the long and short somal axis from every third tibialis anterior MN was used to calculate both somal volume and surface area measurements, in a manner previously reported for prolate spheroids (Prakash et al. 1993, 1994; Ulfhake and Cullheim 1988). To determine the number of primary dendrites, a subset of tibialis anterior MNs were systematically sampled such that the number of primary dendrites was determined for five to six MNs per tertile per mouse across wild-type and spa genotypes.
Statistical analysis.
All statistical analyses were performed using standard software (Prism 7.0; GraphPad, La Jolla, CA). With respect to continuous variables, differences between groups were examined using unpaired t-tests when data were normally distributed according to D’Agostino and Pearson normality tests. A Mann-Whitney U-test was used when the data were not normally distributed. Kolmogorov-Smirnov tests were used when comparing distributions, and two-way ANOVA was used when comparing two factors, with Bonferroni post hoc tests where appropriate. Statistical significance was established at the P < 0.05 level. All experimental data are means ± SE unless otherwise specified. The investigator performing the counts and morphometric measurements was blinded to the genotype of the mouse.
RESULTS
Mice characteristics.
The wild-type and spa mice were similar with respect to age and sex. However, spa mice body weights (mean 16.8 ± 1.4 g, range 12.0–20.9 g) were 31% lower compared with controls (mean 24.3 ± 1.7 g, range 19.5–28.0 g; Mann-Whitney U-test, P = 0.02). This difference was expected on the basis of previous work with spa mice (Molon et al. 2006). All spa mice displayed a spastic phenotype including exaggerated startle response when handled, abnormal gait, muscle rigidity, and myoclonic jerks.
Number of tibialis anterior motor neurons in spa mice compared with controls.
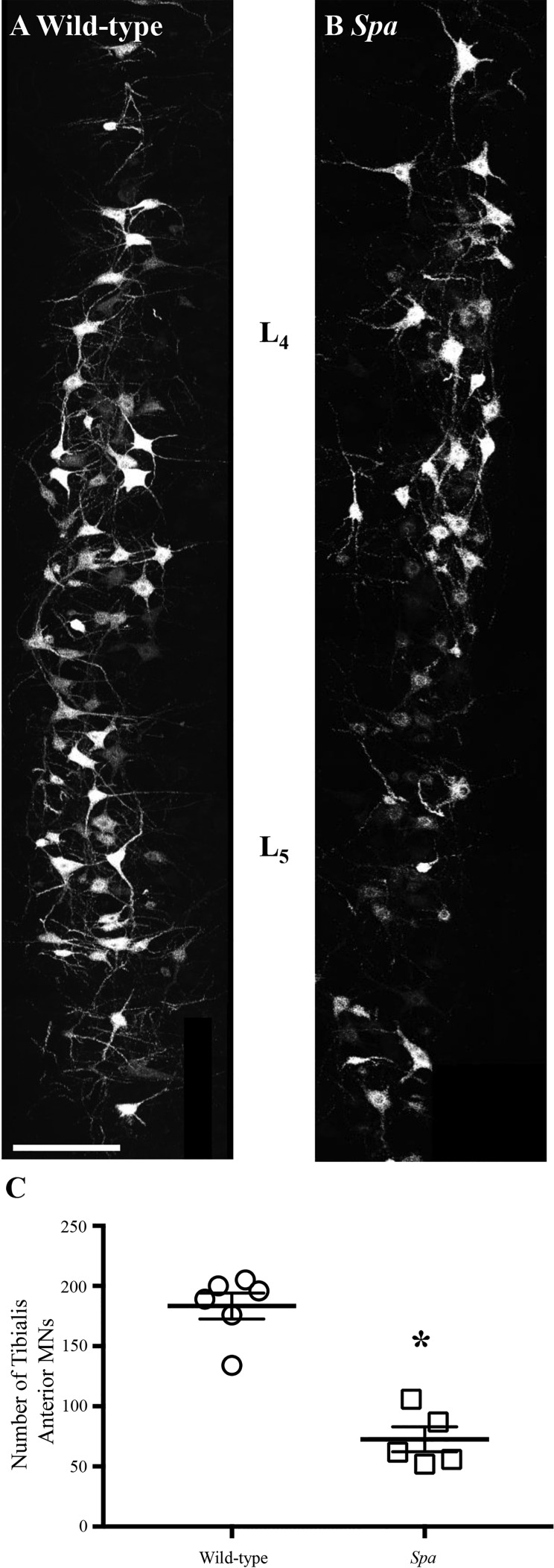
Confocal microscopy of longitudinal sections from the lumbar spinal cord confirmed robust MN staining (Fig. 1) in both wild-type and spa mice. In spa mice there were ~61% fewer tibialis anterior MNs compared with wild-type controls (Mann-Whitney U-test, P = 0.008). Wild-type mice had a mean of 183.3 ± 10.7 (range 134–200) tibialis anterior MNs, and spa mice had a mean of 72.6 ± 10.3 (range 52–106) tibialis anterior MNs (Fig. 1). No changes in the segmental distribution of tibialis anterior MNs were observed between wild-type and spa mice within the spinal cord (Fig. 1). Tibialis anterior MNs were concentrated at the L4 and L5 spinal cord segments, with sparser labeling in the L3 segment, consistent with previous reports (Bácskai et al. 2014; McHanwell and Biscoe 1981a).
Fig. 1.
Reduced tibialis anterior motor neurons (MNs) in spa mice compared with wild-type controls. A and B show representative labeling of tibialis anterior MNs of wild-type and spa mice, respectively. C shows plots (means ± SE) comparing numbers of MNs in control and spa mice with circles and squares representing individual animals. There was a ~60% reduction in tibialis anterior MNs of spa mice (squares, n = 5) compared with wild-type (circles, n = 6). *P = 0.008 (Mann-Whitney U-test). Scale bar: 75 µm.
Somal volumes and surface areas of tibialis anterior motor neurons in spa mice compared with controls.
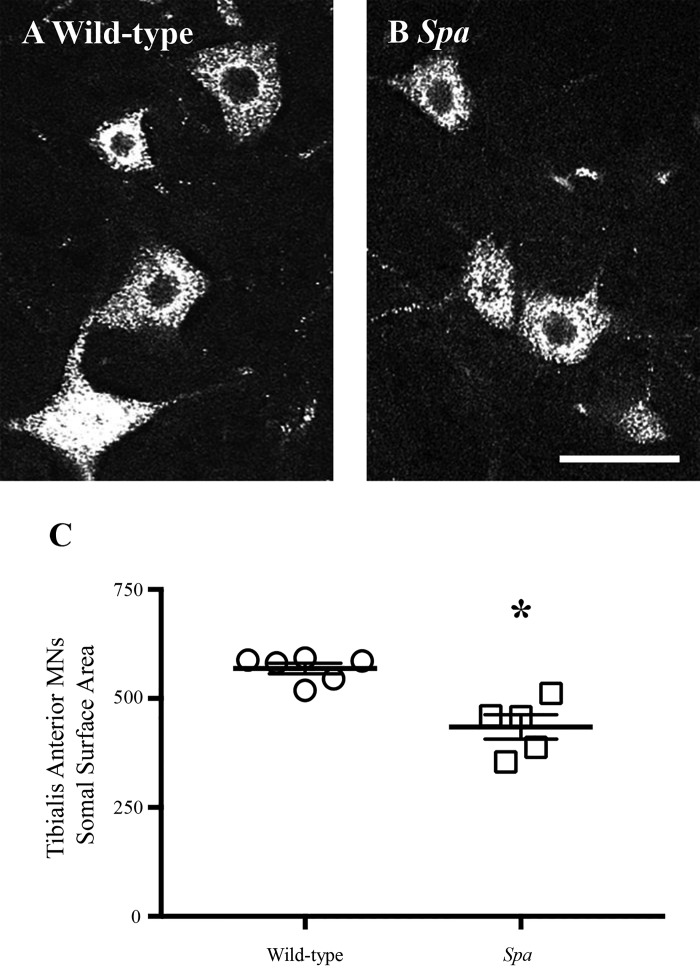
With the use of systematic random sampling (stereological) methodology, tibialis anterior MN somal surface area and volume measurements were obtained from 366 wild-type and 121 spa MNs (24–61 MNs per animal). Mean somal surface area was reduced by ~23% in spa mice compared with wild-type controls (wild-type: 565 ± 14 µm2; spa: 435 ± 28 µm2; Mann-Whitney U-test, P < 0.0001; Fig. 2). Mean somal volume was reduced by ~33% in spa mice compared with wild-type controls (wild-type: 1315 ± 45 µm3; spa: 876 ± 83 µm3; Mann-Whitney U-test, P < 0.0001).
Fig. 2.
Reduced tibialis anterior motor neuron surface area in spa mice compared with wild-type controls. A and B show high-powered representative individual wild-type and spa tibialis anterior motor neurons (MNs), respectively. C shows plots (means ± SE) comparing MN somal surface areas in control and spa mice with circles and squares representing individual animals. There was a ~23% reduction in tibialis anterior MN somal surface areas of spa mice (squares, n = 5) compared with wild-type (circles, n = 6). *P < 0.0001 (Mann-Whitney U-test). Scale bar: 15 µm.
Distribution of somal size in tibialis anterior motor neurons in spa mice compared with controls.
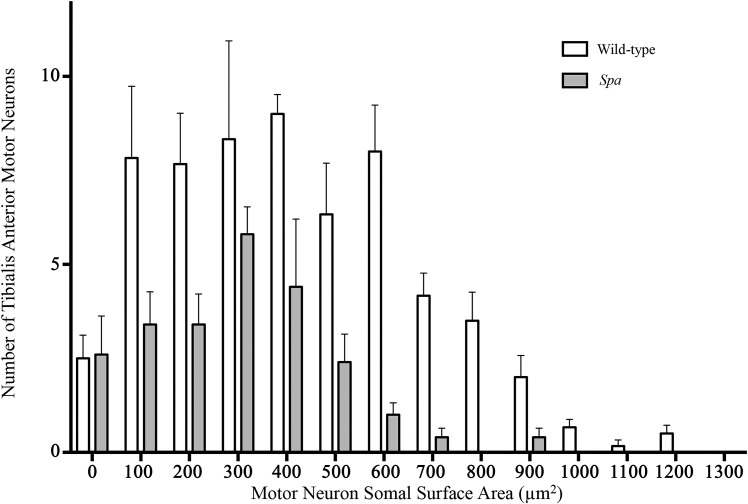
The somal surface areas of tibialis anterior MNs were calculated and plotted as frequency distributions. This frequency distribution was shifted leftward in spa mice compared with wild-type controls, indicating a preponderance toward smaller tibialis anterior MNs in the mutant mice (Kolmogorov-Smirnov test, P < 0.0001; Fig. 3). There was a leftward shift in the frequency distribution of tibialis anterior MN somal volume (data not shown).
Fig. 3.
Altered distribution of tibialis anterior motor neuron (MN) somal surface area in spa mice compared with wild-type controls. Frequency histogram of the number of tibialis anterior MNs (means ± SE) binned with respect to somal surface area shows a difference in the distribution between genotypes (P < 0.0001, Kolmogorov-Smirnov test). Plotted is a systematic random sampling of every third tibialis anterior MN from wild-type (open bars) and spa (shaded bars) mice.
Excessive pruning of larger tibialis anterior motor neurons in spa mice.
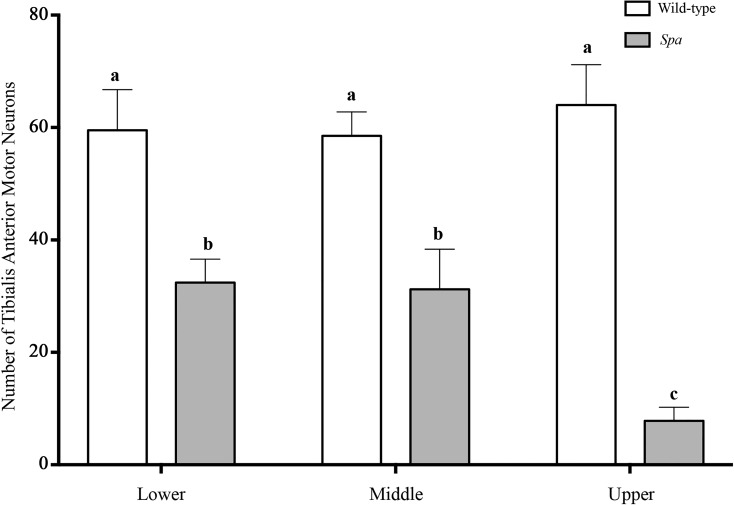
To further assess the interaction between MN pruning and the reduction in MN size in spa mice, tibialis anterior MNs were stratified into tertiles, based on somal surface area values of wild-type controls (Fig. 4), similar to previous reports in phrenic MNs (Fogarty et al. 2018b). Genotype [F(1,9) = 52.28; P < 0.0001] and the interaction between genotype and somal surface area tertile [F(2,18) = 4.2; P = 0.03] both had significant effects on the number of tibialis anterior MNs. As expected, tibialis anterior MN surface area tertile was not a significant factor alone [F(2,18) = 1.8; P = 0.19]. This analysis showed that in spa mice, the numbers of tibialis anterior MNs in the lower, middle, and upper tertiles of somal surface areas were reduced by ~50% (P = 0.01), ~50% (P = 0.009), and ~90% (P < 0.0001), respectively, compared with wild-type controls (Fig. 4). In addition, the number of upper tertile tibialis anterior MNs of spa mice were reduced by ~75% compared with lower (P = 0.03) and middle (P = 0.04) tertile spa tibialis anterior MNs (Fig. 4). In spa mice, there was no difference in the number of tibialis anterior MNs in the lower compared with middle tertiles (P > 0.99; Fig. 4).
Fig. 4.
Disproportionate loss of larger tibialis anterior motor neurons (MNs) during pruning in spa mice compared with wild-type controls. Histogram shows the estimated number of tibialis anterior MNs (means ± SE) within wild-type (open bars) and spa (shaded bars) mice, stratified into lower, middle, and upper tertiles, based on the distribution of tibialis anterior MN somal surface area of wild-type; hence, there are no differences between tertiles in wild-type. In spa mice, all tibialis anterior MN tertiles were significantly reduced compared with wild-type. In spa mice, there was no difference in the number of tibialis anterior MNs from the lower and middle tertiles (P > 0.99). However, in spa mice, the number of upper tertile tibialis anterior MNs was decreased by ~75% compared with lower (P = 0.03) and middle (P = 0.04) tertile spa MNs. Thus, spa mice have a disproportionate loss of the larger, upper tertile tibialis anterior MNs. a,b,cDifferent letters denote significant difference (2-way ANOVA with post hoc Bonferroni testing).
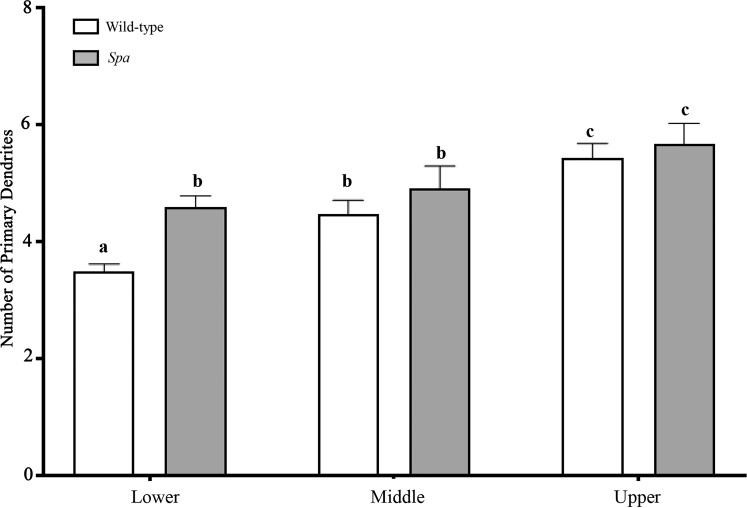
Number of primary dendrites at tibialis anterior motor neurons in spa mice compared with controls.
The mean number of primary dendrites was greater by ~12% in spa mice compared with wild-type controls (wild-type: 4.5 ± 0.1; spa: 5.1 ± 0.2; unpaired t-test, P = 0.046). The number of primary dendrites was stratified according to MN size into lower, middle, and upper tertiles, based on MN somal surface area distributions of wild-type mice (Fig. 5). Genotype [F(1,9) = 5.40; P = 0.045] and the somal surface area tertile [F(2,18) = 22.2; P < 0.0001] both had significant effects on the number of primary dendrites. In wild-type mice, the number of primary dendrites increased with the size of tibialis anterior MNs such that the upper tertile (larger MNs) had 56% more than the lower tertile (P < 0.0001) and 22% more than the middle tertile (P = 0.016), with the middle tertile having 22% more than the lower (smaller MN) tertile (P = 0.016; Fig. 5). In spa mice, there was no difference in the number of primary dendrites between lower and middle tertile tibialis anterior MNs (P > 0.99). However, there was an increase of 23% in the number of primary dendrites of upper tertile spa MNs compared with the lower tertile (P = 0.014; Fig. 5). When wild-type mice were compared with spa mice, the number of primary dendrites of lower tertile MNs was greater by 31% in spa mice (P = 0.017), with no differences between genotypes observed in the middle (P = 0.701) or upper (P > 0.99) tertiles (Fig. 5).
Fig. 5.
Greater number of primary dendrites at smaller tibialis anterior motor neurons (MNs) in spa mice compared with wild-type controls. Histogram of the number of primary of tibialis anterior MNs (means ± SE) in wild-type (open bars) and spa (shaded bars) mice, stratified into lower, middle, and upper tertiles, based on the distribution of tibialis anterior MN somal surface area of wild-type. In wild-type mice, the number of primary dendrites increased with the size of tibialis anterior MNs such that the number of primary dendrites in the lower (smaller MN) tertile was 22% less than in the middle tertile (P = 0.016), and the number of primary dendrites in the upper (larger MN) tertile was 22% greater than in the middle tertile (P = 0.016). The number of primary dendrites in the upper tertile was 56% more than in the lower tertile (P < 0.0001). In spa mice, there was no difference in the number of primary dendrites between lower and middle tertile tibialis anterior MNs (P > 0.99); however, the number of primary dendrites in the upper tertile spa MNs was 23% greater compared with that in the smaller (lower tertile) MNs (P = 0.014). When wild-type and spa mice were compared, the number of primary dendrites in lower tertile MNs was 31% greater in spa mice compared with wild-type mice (P = 0.017), with no differences in the number of primary dendrites for MNs in the middle (P = 0.701) or upper (P > 0.99) tertiles. Thus spa mice have a disproportionate increase in the number of primary dendrites at smaller (lower tertile) tibialis anterior MNs. a,b,cDifferent letters denote significant difference (2-way ANOVA with post hoc Bonferroni testing).
DISCUSSION
In the present study, altered glycinergic neurotransmitter input to tibialis MNs, via a Gly receptor mutation, resulted in increased pruning and fewer MNs in spa mice compared with wild-type littermates. In addition, the size of tibialis anterior MNs was reduced in spa mice. These results support the concept that disruption of glycinergic inputs to MNs during the perinatal period may cause activity- and time-dependent alterations in MN pruning and neurotrophic influence. It has been consistently demonstrated that fetal MN pruning is affected by perturbations of glycinergic and GABAergic neurotransmission (gephyrin mice) that affect MN activity (Banks et al. 2005; Fogarty et al. 2013b, 2015). In prenatal and early postnatal development, glycine is excitatory with a postnatal transition to inhibitory action occurring at ~2 wk after birth, a time when MN pruning is complete (Ben-Ari 2002; Kriegstein and Owens 2001; Wong-Riley and Liu 2008). Therefore, a dual loss of excitatory glycingeric and GABAergic neurotransmitters in the embryonic stage results in a reduction of excitatory input to the MNs. By the late embryonic and early postnatal stage (P0), these mice have less MN pruning (greater number of MNs) in the lumbar spinal cord (Banks et al. 2005; Fogarty et al. 2013b, 2015). Peripheral skeletal muscle activation also influences MN activity and pruning. In the chick embryo, Oppenheim and Nuñez (1982) showed that electrical stimulation of the hindlimb resulted in an increase in MN pruning (fewer number of MNs). Because spa mice have a Gly receptor mutation, it was anticipated that this would result in a loss of net excitatory inputs early in development, less activity, and thus decreased pruning and an increased number of MNs, as was seen with late embryonic gephyrin mice (Banks et al. 2005; Fogarty et al. 2013b, 2015). However, we found the opposite in mature spa mice. Similar to our results, a study using another mouse model with a Gly receptor mutation of the β-subunit (B6C3Fe-a/a-spa/spa) also found a reduction in the number of thoracic MNs (Molon et al. 2006). This suggests that additional MN loss may be occurring in between postnatal development and maturation, possibly after transition of Gly to an inhibitory neurotransmitter, because loss of glycinergic input would then result in less MN inhibition (i.e., excessive excitatory input to MNs).
Supporting the possibility of MN loss due to excessive excitatory input, loss of inhibitory Gly neurotransmitter input is thought to contribute to MN loss via excitotoxicity in mature SOD1 mice (Chang and Martin 2011; Martin and Chang 2012). The spinal cord, compared with the brain, may be particularly susceptible to injury from excitotoxicity via a loss of Gly neurotransmitter input due to greater prevalence of Gly receptors in the spinal cord (Betz 1991; Betz et al. 1991; van den Pol and Gorcs 1988; Werman et al. 1967, 1968). The mechanism of MN excitotoxicity is likely the presence of persistent inward calcium and sodium currents (PICs), which remain active due to reduced descending inhibitory neurotransmitters (i.e., loss of Gly; Heckmann et al. 2005). Furthermore, altered neurotransmitter inputs may result in abnormal skeletal muscle activation, thereby altering neurotrophic factors, such as brain-derived neurotrophic factor (BDNF)/TrkB, which have been shown to have a role in preserving MN numbers in the mature spinal cord (Escandón et al. 1994; Funakoshi et al. 1993; Henderson et al. 1993; McKay et al. 1996; Pitts et al. 2006). Thus altered neurotransmitter signaling via loss of inhibition and reduced trophic input to MNs in spa mice may contribute to fewer MNs at maturation.
In addition to increased pruning of tibialis anterior MNs, we found that MNs in spa mice were smaller. Specifically, the distribution of tibialis anterior MN sizes showed disproportionately fewer large MNs in spa mice. Generally, larger MNs innervate more fatigable fast-twitch motor units (type FInt and FF motor units), comprising type IIx and/or IIb muscle fibers (Fournier and Sieck 1988; Greising et al. 2015; Mantilla et al. 2013; Mantilla and Sieck 2008a; Miyata et al. 1995). Type IIx and IIb muscle fibers are the predominant fiber type in mouse tibialis anterior muscle (Hämäläinen and Pette 1993; Kammoun et al. 2014). These FInt and FF motor units develop only after ~2–3 wk in rodents (Mantilla et al. 2008) with the appearance of type IIx and/or IIb muscle fibers. Our results suggest that there is a selective vulnerability of FInt and FF motor units related to neurotransmitter imbalance during fetal and early postnatal development.
The reduced somal surface area of tibialis anterior MNs in spa mice may be consistent with increased intrinsic MN excitability, which corresponds with the spastic phenotype. According to the Henneman size principle, MNs with smaller cell bodies are more excitable and are recruited before MNs with larger cell bodies (Henneman 1957; Henneman et al. 1965a, 1965b). Thus, in spa mice, smaller MN somal size, less variation in somal size (as observed by a smaller frequency distribution), and reduced inhibitory neurotransmitter input (i.e., loss of Gly) onto the MNs are all plausible contributors to the spastic symptoms.
In the present study, we observed that tibialis anterior MNs in spa mice have a greater number of primary dendrites. The greater number of primary dendrites was mostly confined to smaller tibialis anterior MNs (>30% compared with wild-type controls). The retrograde rhodamine-labeling technique employed in the present study only allows reliable identification of dendrites to the 2nd dendritic branch (Fogarty et al. 2018b; Issa et al. 2010; Prakash et al. 2000). Therefore, this technique, though advantageous for determining the number of tibialis anterior MNs, precluded a detailed assessment of the full dendritic tree of MNs, which often extends to the 8th order (Fogarty et al. 2017b; Leroy et al. 2014). This is unfortunate, because more distal dendrites are often a major site of synaptic inputs that affect MN excitability (Bae et al. 1999; Brännström 1993; Fogarty et al. 2013a; Ornung et al. 1998; Shigenaga et al. 2005; Starr and Wolpaw 1994). Past studies of inhibitory neurotransmission defects (including glycine receptor mutations) in MNs support the concept of dendritic alterations in response to an imbalance of excitation/inhibition inputs (Fogarty et al. 2016, 2017a). In the absence of any further alterations to the excitation-inhibition balance of spa MNs, more extensive dendritic branching in surviving MNs would increase their overall capacitance and reduce their excitability (Fogarty et al. 2018a; Henneman 1957; Henneman et al. 1965a, 1965b; Mantilla et al. 2010; Sieck and Fournier 1989), which is inconsistent with the spa mouse phenotype. Future studies could make use of Golgi-Cox staining (Fogarty et al. 2017b) or intracellular labeling (Kanjhan et al. 2016; Obregon et al. 2009; Pace et al. 2002) techniques for more detailed morphometric analysis of the dendritic tree (Fogarty et al. 2013a; Klenowski et al. 2017).
Although loss of a single neurotransmitter input is not a known etiology for sCP, spa mice display a well-described spastic phenotype with abnormal locomotion, muscle rigidity, myoclonic jerks, and an exaggerated startle response (Becker et al. 1986; Heller and Hallett 1982). We have also found that spa mice are significantly smaller than wild-type mice. Thus the phenotype of spa mice has striking similarities to individuals with sCP, suggesting that the spa mouse is a good model of sCP. Mutant spa mice have precedence for modeling sCP in studies exploring effect of botulinum toxin on muscle and muscle contractures (Cosgrove and Graham 1994; Ziv et al. 1984). Furthermore, because spinal cord development and maturation lags that of the brain (Eyre et al. 2000), any injury to a developing brain will influence the developing spinal cord, and specifically MNs. Thus spasticity in humans with sCP may be due not only to the early brain injury causing an imbalance of excitatory and inhibitory neurotransmitters but also to the subsequent developmental disruption of MN pruning, resulting in abnormal MN properties Specifically, disruption of Gly, which is abundantly expressed in the spinal cord (van den Pol and Gorcs 1988; Werman et al. 1967, 1968), appears to have a key role in the abnormal MN pruning and spasticity development.
Summary.
This study provides evidence that altered input to MNs, specifically loss of glycinergic input during development and into adulthood in the spa mouse, results in reduced number of MNs in the lumbar spinal cord as manifest by fewer tibialis anterior MNs. Furthermore, this loss of Gly input results in MNs with a smaller somal size and disproportionate loss of larger MNs. Additionally, these MNs display a greater number of primary dendrites. This reduction in MN numbers and size (and increased excitability of passive membrane properties) along with a greater number of primary dendrites likely alters neuromotor control and contributes to the spastic phenotype (impaired locomotion, smaller mice, exaggerated startled response, and muscle rigidity) of this model. It will be important to determine the timing of MN loss and the impact of abnormal MN numbers on motor unit development.
GRANTS
This work was supported by National Institutes of Health Grants R01-AG044615 (to G. C. Sieck) and R01-HL96750 (to G. C. Sieck), an Australian National Health & Medical Research Council CJ Martin Early Career Fellowship (to M. J. Fogarty), Mayo Clinic Children’s Research Center Pediatric Team Science Award (to J. E. Brandenburg), and Mayo Clinic Clinical and Translational Science Award UL1 TR000135 (to J. E. Brandenburg).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.E.B., H.M.G., and G.C.S. conceived and designed research; J.E.B. and H.M.G. performed experiments; J.E.B. and M.J.F. analyzed data; J.E.B., M.J.F., and G.C.S. interpreted results of experiments; J.E.B. and M.J.F. prepared figures; J.E.B. and M.J.F. drafted manuscript; J.E.B., H.M.G., M.J.F., and G.C.S. edited and revised manuscript; J.E.B., H.M.G., M.J.F., and G.C.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jeffrey Bailey, Yun-Hua Fang, Rebecca Macken, and Wen-Zhi Zhan, MD, for technical assistance in the completion of this project.
REFERENCES
- Bácskai T, Rusznák Z, Paxinos G, Watson C. Musculotopic organization of the motor neurons supplying the mouse hindlimb muscles: a quantitative study using Fluoro-Gold retrograde tracing. Brain Struct Funct 219: 303–321, 2014. doi: 10.1007/s00429-012-0501-7. [DOI] [PubMed] [Google Scholar]
- Bae YC, Nakamura T, Ihn HJ, Choi MH, Yoshida A, Moritani M, Honma S, Shigenaga Y. Distribution pattern of inhibitory and excitatory synapses in the dendritic tree of single masseter α-motoneurons in the cat. J Comp Neurol 414: 454–468, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- Banks GB, Kanjhan R, Wiese S, Kneussel M, Wong LM, O’Sullivan G, Sendtner M, Bellingham MC, Betz H, Noakes PG. Glycinergic and GABAergic synaptic activity differentially regulate motoneuron survival and skeletal muscle innervation. J Neurosci 25: 1249–1259, 2005. doi: 10.1523/JNEUROSCI.1786-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bax M, Tydeman C, Flodmark O. Clinical and MRI correlates of cerebral palsy: the European Cerebral Palsy Study. JAMA 296: 1602–1608, 2006. doi: 10.1001/jama.296.13.1602. [DOI] [PubMed] [Google Scholar]
- Becker CM, Hermans-Borgmeyer I, Schmitt B, Betz H. The glycine receptor deficiency of the mutant mouse spastic: evidence for normal glycine receptor structure and localization. J Neurosci 6: 1358–1364, 1986. doi: 10.1523/JNEUROSCI.06-05-01358.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CM, Schmieden V, Tarroni P, Strasser U, Betz H. Isoform-selective deficit of glycine receptors in the mouse mutant spastic. Neuron 8: 283–289, 1992. doi: 10.1016/0896-6273(92)90295-O. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci 3: 728–739, 2002. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Betz H. Glycine receptors: heterogeneous and widespread in the mammalian brain. Trends Neurosci 14: 458–461, 1991. doi: 10.1016/0166-2236(91)90045-V. [DOI] [PubMed] [Google Scholar]
- Betz H, Langosch D, Hoch W, Prior P, Pribilla I, Kuhse J, Schmieden V, Malosio ML, Matzenbach B, Holzinger F, Kuryatov A, Schmitt B, Maulet Y, Becker CM. Structure and expression of inhibitory glycine receptors. Adv Exp Med Biol 287: 421–429, 1991. doi: 10.1007/978-1-4684-5907-4_37. [DOI] [PubMed] [Google Scholar]
- Brännström T. Quantitative synaptology of functionally different types of cat medial gastrocnemius alpha-motoneurons. J Comp Neurol 330: 439–454, 1993. doi: 10.1002/cne.903300311. [DOI] [PubMed] [Google Scholar]
- Chang Q, Martin LJ. Glycine receptor channels in spinal motoneurons are abnormal in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci 31: 2815–2827, 2011. doi: 10.1523/JNEUROSCI.2475-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove AP, Graham HK. Botulinum toxin A prevents the development of contractures in the hereditary spastic mouse. Dev Med Child Neurol 36: 379–385, 1994. doi: 10.1111/j.1469-8749.1994.tb11863.x. [DOI] [PubMed] [Google Scholar]
- Escandón E, Soppet D, Rosenthal A, Mendoza-Ramírez JL, Szönyi E, Burton LE, Henderson CE, Parada LF, Nikolics K. Regulation of neurotrophin receptor expression during embryonic and postnatal development. J Neurosci 14: 2054–2068, 1994. doi: 10.1523/JNEUROSCI.14-04-02054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre JA, Miller S, Clowry GJ, Conway EA, Watts C. Functional corticospinal projections are established prenatally in the human foetus permitting involvement in the development of spinal motor centres. Brain 123: 51–64, 2000. doi: 10.1093/brain/123.1.51. [DOI] [PubMed] [Google Scholar]
- Feng G, Tintrup H, Kirsch J, Nichol MC, Kuhse J, Betz H, Sanes JR. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science 282: 1321–1324, 1998. doi: 10.1126/science.282.5392.1321. [DOI] [PubMed] [Google Scholar]
- Fogarty MJ, Hammond LA, Kanjhan R, Bellingham MC, Noakes PG. A method for the three-dimensional reconstruction of Neurobiotin™-filled neurons and the location of their synaptic inputs. Front Neural Circuits 7: 153, 2013a. doi: 10.3389/fncir.2013.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ, Kanjhan R, Bellingham MC, Noakes PG. Glycinergic neurotransmission: a potent regulator of embryonic motor neuron dendritic morphology and synaptic plasticity. J Neurosci 36: 80–87, 2016. doi: 10.1523/JNEUROSCI.1576-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ, Kanjhan R, Yanagawa Y, Noakes PG, Bellingham MC. Alterations in hypoglossal motor neurons due to GAD67 and VGAT deficiency in mice. Exp Neurol 289: 117–127, 2017a. doi: 10.1016/j.expneurol.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Fogarty MJ, Mantilla CB, Sieck GC. Breathing: motor control of diaphragm muscle. Physiology (Bethesda) 33: 113–126, 2018a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ, Mu EW, Lavidis NA, Noakes PG, Bellingham MC. Motor areas show altered dendritic structure in an amyotrophic lateral sclerosis mouse model. Front Neurosci 11: 609, 2017b. doi: 10.3389/fnins.2017.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ, Omar TS, Zhan WZ, Mantilla CB, Sieck GC. Phrenic motor neuron loss in aged rats. J Neurophysiol 119: 1852–1862, 2018b. doi: 10.1152/jn.00868.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ, Smallcombe KL, Yanagawa Y, Obata K, Bellingham MC, Noakes PG. Genetic deficiency of GABA differentially regulates respiratory and non-respiratory motor neuron development. PLoS One 8: e56257, 2013b. doi: 10.1371/journal.pone.0056257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ, Yanagawa Y, Obata K, Bellingham MC, Noakes PG. Genetic absence of the vesicular inhibitory amino acid transporter differentially regulates respiratory and locomotor motor neuron development. Brain Struct Funct 220: 525–540, 2015. doi: 10.1007/s00429-013-0673-9. [DOI] [PubMed] [Google Scholar]
- Fournier M, Sieck GC. Mechanical properties of muscle units in the cat diaphragm. J Neurophysiol 59: 1055–1066, 1988. 10.1152/jn.1988.59.3.1055. [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Frisén J, Barbany G, Timmusk T, Zachrisson O, Verge VM, Persson H. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol 123: 455–465, 1993. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BA, Schofield PR, Sah P, Margrie TW, Callister RJ. Distinct physiological mechanisms underlie altered glycinergic synaptic transmission in the murine mutants spastic, spasmodic, and oscillator. J Neurosci 26: 4880–4890, 2006. doi: 10.1523/JNEUROSCI.3991-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gransee HM, Gonzalez Porras MA, Zhan WZ, Sieck GC, Mantilla CB. Motoneuron glutamatergic receptor expression following recovery from cervical spinal hemisection. J Comp Neurol 525: 1192–1205, 2017. doi: 10.1002/cne.24125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gransee HM, Zhan WZ, Sieck GC, Mantilla CB. Targeted delivery of TrkB receptor to phrenic motoneurons enhances functional recovery of rhythmic phrenic activity after cervical spinal hemisection. PLoS One 8: e64755, 2013. doi: 10.1371/journal.pone.0064755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Medina-Martínez JS, Vasdev AK, Sieck GC, Mantilla CB. Analysis of muscle fiber clustering in the diaphragm muscle of sarcopenic mice. Muscle Nerve 52: 76–82, 2015. doi: 10.1002/mus.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen N, Pette D. The histochemical profiles of fast fiber types IIB, IID, and IIA in skeletal muscles of mouse, rat, and rabbit. J Histochem Cytochem 41: 733–743, 1993. doi: 10.1177/41.5.8468455. [DOI] [PubMed] [Google Scholar]
- Harris AJ, McCaig CD. Motoneuron death and motor unit size during embryonic development of the rat. J Neurosci 4: 13–24, 1984. doi: 10.1523/JNEUROSCI.04-01-00013.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve 31: 135–156, 2005. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Heller AH, Hallett M. Electrophysiological studies with the spastic mutant mouse. Brain Res 234: 299–308, 1982. doi: 10.1016/0006-8993(82)90870-8. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Camu W, Mettling C, Gouin A, Poulsen K, Karihaloo M, Rullamas J, Evans T, McMahon SB, Armanini MP, Berkemeier L, Phillips HS, Rosenthal A. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature 363: 266–270, 1993. doi: 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- Henneman E. Relation between size of neurons and their susceptibility to discharge. Science 126: 1345–1347, 1957. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol 28: 599–620, 1965a. doi: 10.1152/jn.1965.28.3.599. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol 28: 560–580, 1965b. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Issa AN, Zhan WZ, Sieck GC, Mantilla CB. Neuregulin-1 at synapses on phrenic motoneurons. J Comp Neurol 518: 4213–4225, 2010. doi: 10.1002/cne.22449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammoun M, Cassar-Malek I, Meunier B, Picard B. A simplified immunohistochemical classification of skeletal muscle fibres in mouse. Eur J Histochem 58: 2254, 2014. doi: 10.4081/ejh.2014.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjhan R, Fogarty MJ, Noakes PG, Bellingham MC. Developmental changes in the morphology of mouse hypoglossal motor neurons. Brain Struct Funct 221: 3755–3786, 2016. doi: 10.1007/s00429-015-1130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanning KC, Kaplan A, Henderson CE. Motor neuron diversity in development and disease. Annu Rev Neurosci 33: 409–440, 2010. doi: 10.1146/annurev.neuro.051508.135722. [DOI] [PubMed] [Google Scholar]
- Klenowski PM, Wright SE, Mu EWH, Noakes PG, Lavidis NA, Bartlett SE, Bellingham MC, Fogarty MJ. Investigating methodological differences in the assessment of dendritic morphology of basolateral amygdala principal neurons—a comparison of Golgi-Cox and neurobiotin electroporation techniques. Brain Sci 7: E165, 2017. doi: 10.3390/brainsci7120165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein AR, Owens DF. GABA may act as a self-limiting trophic factor at developing synapses. Sci STKE 2001: pe1, 2001. [DOI] [PubMed] [Google Scholar]
- Landmesser L. The relationship of intramuscular nerve branching and synaptogenesis to motoneuron survival. J Neurobiol 23: 1131–1139, 1992. doi: 10.1002/neu.480230906. [DOI] [PubMed] [Google Scholar]
- Leroy F, Lamotte d’Incamps B, Imhoff-Manuel RD, Zytnicki D. Early intrinsic hyperexcitability does not contribute to motoneuron degeneration in amyotrophic lateral sclerosis. eLife 3: e04046, 2014. doi: 10.7554/eLife.04046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Landmann L, Ruegg MA, Brenner HR. The role of nerve- versus muscle-derived factors in mammalian neuromuscular junction formation. J Neurosci 28: 3333–3340, 2008. doi: 10.1523/JNEUROSCI.5590-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Greising SM, Zhan WZ, Seven YB, Sieck GC. Prolonged C2 spinal hemisection-induced inactivity reduces diaphragm muscle specific force with modest, selective atrophy of type IIx and/or IIb fibers. J Appl Physiol (1985) 114: 380–386, 2013. doi: 10.1152/japplphysiol.01122.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol 173: 101–106, 2010. doi: 10.1016/j.resp.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Key aspects of phrenic motoneuron and diaphragm muscle development during the perinatal period. J Appl Physiol (1985) 104: 1818–1827, 2008a. doi: 10.1152/japplphysiol.01192.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Trophic factor expression in phrenic motor neurons. Respir Physiol Neurobiol 164: 252–262, 2008b. doi: 10.1016/j.resp.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Sill RV, Aravamudan B, Zhan WZ, Sieck GC. Developmental effects on myonuclear domain size of rat diaphragm fibers. J Appl Physiol (1985) 104: 787–794, 2008. doi: 10.1152/japplphysiol.00347.2007. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Zhan WZ, Sieck GC. Retrograde labeling of phrenic motoneurons by intrapleural injection. J Neurosci Methods 182: 244–249, 2009. doi: 10.1016/j.jneumeth.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Chang Q. Inhibitory synaptic regulation of motoneurons: a new target of disease mechanisms in amyotrophic lateral sclerosis. Mol Neurobiol 45: 30–42, 2012. doi: 10.1007/s12035-011-8217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHanwell S, Biscoe TJ. The localization of motoneurons supplying the hindlimb muscles of the mouse. Philos Trans R Soc Lond B Biol Sci 293: 477–508, 1981a. doi: 10.1098/rstb.1981.0082. [DOI] [PubMed] [Google Scholar]
- McHanwell S, Biscoe TJ. The sizes of motoneurons supplying hindlimb muscles in the mouse. Proc R Soc Lond B Biol Sci 213: 201–216, 1981b. doi: 10.1098/rspb.1981.0062. [DOI] [PubMed] [Google Scholar]
- McKay SE, Garner A, Caldero J, Tucker RP, Large T, Oppenheim RW. The expression of trkB and p75 and the role of BDNF in the developing neuromuscular system of the chick embryo. Development 122: 715–724, 1996. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, Bjornson K, Temkin N, Steinbok P, Wright V, Reiner A, Roberts T, Drake J, O’Donnell M, Rosenbaum P, Barber J, Ferrel A. Selective dorsal rhizotomy: meta-analysis of three randomized controlled trials. Dev Med Child Neurol 44: 17–25, 2002. doi: 10.1017/S0012162201001608. [DOI] [PubMed] [Google Scholar]
- Miyata H, Zhan WZ, Prakash YS, Sieck GC. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol (1985) 79: 1640–1649, 1995. doi: 10.1152/jappl.1995.79.5.1640. [DOI] [PubMed] [Google Scholar]
- Molon A, Di Giovanni S, Hathout Y, Natale J, Hoffman EP. Functional recovery of glycine receptors in spastic murine model of startle disease. Neurobiol Dis 21: 291–304, 2006. doi: 10.1016/j.nbd.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Novikova L, Novikov L, Kellerth JO. Persistent neuronal labeling by retrograde fluorescent tracers: a comparison between Fast Blue, Fluoro-Gold and various dextran conjugates. J Neurosci Methods 74: 9–15, 1997. doi: 10.1016/S0165-0270(97)02227-9. [DOI] [PubMed] [Google Scholar]
- Obregon G, Ermilov LG, Wen-Zhi Z, Sieck GC, Mantilla CB. Modeling dendritic arborization based on 3D-reconstructions of adult rat phrenic motoneurons. Rev Ing Biomed 3: 47–54, 2009. [Google Scholar]
- Oppenheim RW, Núñez R. Electrical stimulation of hindlimb increases neuronal cell death in chick embryo. Nature 295: 57–59, 1982. doi: 10.1038/295057a0. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, Prevette D, D’Costa A, Wang S, Houenou LJ, McIntosh JM. Reduction of neuromuscular activity is required for the rescue of motoneurons from naturally occurring cell death by nicotinic-blocking agents. J Neurosci 20: 6117–6124, 2000. doi: 10.1523/JNEUROSCI.20-16-06117.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornung G, Ottersen OP, Cullheim S, Ulfhake B. Distribution of glutamate-, glycine- and GABA-immunoreactive nerve terminals on dendrites in the cat spinal motor nucleus. Exp Brain Res 118: 517–532, 1998. doi: 10.1007/s002210050308. [DOI] [PubMed] [Google Scholar]
- Pace CJ, Tieman DG, Tieman SB. Intracellular injection in fixed slices: obtaining complete dendritic arbors of large cells. J Neurosci Methods 119: 23–30, 2002. doi: 10.1016/S0165-0270(02)00152-8. [DOI] [PubMed] [Google Scholar]
- Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 39: 214–223, 1997. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- Pittman R, Oppenheim RW. Cell death of motoneurons in the chick embryo spinal cord. IV. Evidence that a functional neuromuscular interaction is involved in the regulation of naturally occurring cell death and the stabilization of synapses. J Comp Neurol 187: 425–446, 1979. doi: 10.1002/cne.901870210. [DOI] [PubMed] [Google Scholar]
- Pittman RH, Oppenheim RW. Neuromuscular blockade increases motoneurone survival during normal cell death in the chick embryo. Nature 271: 364–366, 1978. doi: 10.1038/271364a0. [DOI] [PubMed] [Google Scholar]
- Pitts EV, Potluri S, Hess DM, Balice-Gordon RJ. Neurotrophin and Trk-mediated signaling in the neuromuscular system. Int Anesthesiol Clin 44: 21–76, 2006. doi: 10.1097/00004311-200604420-00004. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Mantilla CB, Zhan WZ, Smithson KG, Sieck GC. Phrenic motoneuron morphology during rapid diaphragm muscle growth. J Appl Physiol (1985) 89: 563–572, 2000. doi: 10.1152/jappl.2000.89.2.563. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Smithson KG, Sieck GC. Measurements of motoneuron somal volumes using laser confocal microscopy: comparisons with shape-based stereological estimations. Neuroimage 1: 95–107, 1993. doi: 10.1006/nimg.1993.1003. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Smithson KG, Sieck GC. Application of the Cavalieri principle in volume estimation using laser confocal microscopy. Neuroimage 1: 325–333, 1994. doi: 10.1006/nimg.1994.1017. [DOI] [PubMed] [Google Scholar]
- Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society; Delgado MR, Hirtz D, Aisen M, Ashwal S, Fehlings DL, McLaughlin J, Morrison LA, Shrader MW, Tilton A, Vargus-Adams J. Practice parameter: pharmacologic treatment of spasticity in children and adolescents with cerebral palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 74: 336–343, 2010. doi: 10.1212/WNL.0b013e3181cbcd2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond FJ, Gladdy R, Creasy JL, Kitamura S, Smits E, Thomson DB. Efficacy of seven retrograde tracers, compared in multiple-labelling studies of feline motoneurones. J Neurosci Methods 53: 35–46, 1994. doi: 10.1016/0165-0270(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B, Jacobsson B. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl 109: 8–14, 2007. [Erratum in Dev Med Child Neurol 49: 480, 2007.] 10.1111/j.1469-8749.2007.tb12610.x. [DOI] [PubMed] [Google Scholar]
- Sabharwal P, Lee C, Park S, Rao M, Sockanathan S. GDE2 regulates subtype-specific motor neuron generation through inhibition of Notch signaling. Neuron 71: 1058–1070, 2011. doi: 10.1016/j.neuron.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Kakizaki T, Hayashi R, Nishimaru H, Furukawa T, Nakazato Y, Takamori S, Ebihara S, Uematsu M, Mishina M, Miyazaki J, Yokoyama M, Konishi S, Inoue K, Fukuda A, Fukumoto M, Nakamura K, Obata K, Yanagawa Y. The physiological roles of vesicular GABA transporter during embryonic development: a study using knockout mice. Mol Brain 3: 40, 2010. doi: 10.1186/1756-6606-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheard P, McCaig CD, Harris AJ. Critical periods in rat motoneuron development. Dev Biol 102: 21–31, 1984. doi: 10.1016/0012-1606(84)90171-4. [DOI] [PubMed] [Google Scholar]
- Shigenaga Y, Moritani M, Oh SJ, Park KP, Paik SK, Bae JY, Kim HN, Ma SK, Park CW, Yoshida A, Ottersen OP, Bae YC. The distribution of inhibitory and excitatory synapses on single, reconstructed jaw-opening motoneurons in the cat. Neuroscience 133: 507–518, 2005. doi: 10.1016/j.neuroscience.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol (1985) 66: 2539–2545, 1989. 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- Simon ES. Phenotypic heterogeneity and disease course in three murine strains with mutations in genes encoding for alpha 1 and beta glycine receptor subunits. Mov Disord 12: 221–228, 1997. doi: 10.1002/mds.870120213. [DOI] [PubMed] [Google Scholar]
- Singer JH, Talley EM, Bayliss DA, Berger AJ. Development of glycinergic synaptic transmission to rat brain stem motoneurons. J Neurophysiol 80: 2608–2620, 1998. doi: 10.1152/jn.1998.80.5.2608. [DOI] [PubMed] [Google Scholar]
- Starr KA, Wolpaw JR. Synaptic terminal coverage of primate triceps surae motoneurons. J Comp Neurol 345: 345–358, 1994. doi: 10.1002/cne.903450303. [DOI] [PubMed] [Google Scholar]
- Ulfhake B, Cullheim S. Postnatal development of cat hind limb motoneurons. III: Changes in size of motoneurons supplying the triceps surae muscle. J Comp Neurol 278: 103–120, 1988. doi: 10.1002/cne.902780107. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Gorcs T. Glycine and glycine receptor immunoreactivity in brain and spinal cord. J Neurosci 8: 472–492, 1988. doi: 10.1523/JNEUROSCI.08-02-00472.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werman R, Davidoff RA, Aprison MH. Inhibition of motoneurones by iontophoresis of glycine. Nature 214: 681–683, 1967. doi: 10.1038/214681a0. [DOI] [PubMed] [Google Scholar]
- Werman R, Davidoff RA, Aprison MH. Inhibitory of glycine on spinal neurons in the cat. J Neurophysiol 31: 81–95, 1968. doi: 10.1152/jn.1968.31.1.81. [DOI] [PubMed] [Google Scholar]
- White WF, Heller AH. Glycine receptor alteration in the mutant mouse spastic. Nature 298: 655–657, 1982. doi: 10.1038/298655a0. [DOI] [PubMed] [Google Scholar]
- Wojcik SM, Katsurabayashi S, Guillemin I, Friauf E, Rosenmund C, Brose N, Rhee JS. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron 50: 575–587, 2006. doi: 10.1016/j.neuron.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, Liu Q. Neurochemical and physiological correlates of a critical period of respiratory development in the rat. Respir Physiol Neurobiol 164: 28–37, 2008. doi: 10.1016/j.resp.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 355: 685–694, 2006. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- Yeargin-Allsopp M, Van Naarden Braun K, Doernberg NS, Benedict RE, Kirby RS, Durkin MS. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaboration. Pediatrics 121: 547–554, 2008. doi: 10.1542/peds.2007-1270. [DOI] [PubMed] [Google Scholar]
- Zhan WZ, Ellenberger HH, Feldman JL. Monoaminergic and GABAergic terminations in phrenic nucleus of rat identified by immunohistochemical labeling. Neuroscience 31: 105–113, 1989. doi: 10.1016/0306-4522(89)90033-X. [DOI] [PubMed] [Google Scholar]
- Ziv I, Blackburn N, Rang M, Koreska J. Muscle growth in normal and spastic mice. Dev Med Child Neurol 26: 94–99, 1984. doi: 10.1111/j.1469-8749.1984.tb04412.x. [DOI] [PubMed] [Google Scholar]