Abstract
Sex hormones appear to play a role in the regulation of hypothalamic-pituitary-adrenal (HPA) axis activity. The objective was to isolate the effects of estradiol (E2) on central activation of the HPA axis. We hypothesized that the HPA axis response to corticotropin-releasing hormone (CRH) under dexamethasone (Dex) suppression would be exaggerated in response to chronic ovarian hormone suppression and that physiologic E2 add-back would mitigate this response. Thirty premenopausal women underwent 20 wk of gonadotropin-releasing hormone agonist therapy (GnRHAG) and transdermal E2 (0.075 mg per day, GnRHAG + E2, n = 15) or placebo (PL) patch (GnRHAG + PL, n = 15). Women in the GnRHAG + PL and GnRHAG + E2 groups were of similar age (38 (SD 5) yr vs. 36 (SD 7) yr) and body mass index (27 (SD 6) kg/m2 vs. 27 (SD 6) kg/m2). Serum E2 changed differently between the groups (P = 0.01); it decreased in response to GnRHAG + PL (77.9 ± 17.4 to 23.2 ± 2.6 pg/ml; P = 0.008) and did not change in response to GnRHAG + E2 (70.6 ± 12.4 to 105 ± 30.4 pg/ml; P = 0.36). The incremental area under the curve (AUCINC) responses to CRH were different between the groups for total cortisol (P = 0.03) and cortisone (P = 0.04) but not serum adrenocorticotropic hormone (ACTH) (P = 0.28). When examining within-group changes, GnRHAG + PL did not alter the HPA axis response to Dex/CRH, but GnRHAG + E2 decreased the AUCINC for ACTH (AUCINC, 1,623 ± 257 to 1,211 ± 236 pg/ml·min, P = 0.004), cortisone (1,795 ± 367 to 1,090 ± 281 ng/ml·min, P = 0.009), and total cortisol (7,008 ± 1,387 to 3,893 ± 1,090 ng/ml·min, P = 0.02). Suppression of ovarian hormones by GnRHAG therapy for 20 wk did not exaggerate the HPA axis response to CRH, but physiologic E2 add-back reduced HPA axis activity compared with preintervention levels.
Keywords: central adiposity, cortisol, estradiol, gonadotropin-releasing hormone agonist, menopause
INTRODUCTION
Hypothalamic-pituitary-adrenal (HPA) axis activity differs by sex and changes with age (33). Aging appears to increase the cortisol response to pharmacological and psychological stressors in both sexes, but this effect is about three times greater in women than in men (33). The menopause-related decrease in sex hormones has been postulated to be the cause for this age-related sex difference. Indeed, exaggerated dynamic HPA axis response to a variety of stressors (i.e., cognitive, psychosocial, behavioral, or pharmacologic) was reported in some (21, 40) but not all (21, 27) studies that compared postmenopausal women with premenopausal women. Furthermore, estradiol (E2) administration appears to reduce the HPA axis response in postmenopausal women (21, 27), although this is not a uniform finding (44). Teasing out the effects of age per se from the specific decline in ovarian hormones is challenging, however, because of the relatively long and variable duration of the natural menopause transition. Determination of the role of sex hormones in HPA axis regulation is of particular importance because many chronic diseases that become more prevalent with age, including diabetes, metabolic syndrome, and hypertension, have been associated with exaggerated cortisol responses to physiological, psychological, or pharmacological challenges (33).
The menopausal transition is characterized by the shift toward preferential accumulation of abdominal visceral adiposity, as evidenced in cross-sectional (45) and longitudinal studies (28). Visceral adiposity, which is thought to be a more potent determinant of metabolic dysfunction and disease risk than subcutaneous abdominal fat (7), not only increases cardiometabolic disease risk (6) but is also linked with dysregulation of HPA axis activity (30, 34). Morning cortisol levels are normal (even low normal) in individuals with abdominal obesity, and feedback inhibition appears to be normal. However, there is an exaggerated central HPA axis response to stimulation (30, 34). Although alternative hypotheses exist (19), there is evidence that 17β-E2 deficiency specifically influences intra-abdominal fat accrual (43). Accordingly, elevated HPA axis activity in postmenopausal women could be the result of their increased abdominal adiposity and/or decreased E2.
The aim of this study was to perform a controlled investigation of the effect of physiologic E2 levels on central activation of HPA axis activity. Our hypothesis was that the HPA axis response to corticotropin-releasing hormone (CRH) stimulation under dexamethasone (Dex) suppression would be exaggerated in premenopausal women after 20 wk of ovarian hormone suppression [gonadotropin-releasing hormone agonist (GnRHAG)], and that this exaggeration would be mitigated by physiologic E2 therapy to mimic levels in the follicular phase.
MATERIALS AND METHODS
Institutional approval.
This randomized, double-blinded, placebo-controlled study was conducted at the University of Colorado Anschutz Medical Campus (CU AMC). The study was approved by the Colorado Multiple Institutional Review Board and the Scientific Advisory and Review Board of the Clinical and Translation Research Center (CTRC) at CU AMC. All volunteers provided written informed consent to participate.
Study participants.
Women included in this analysis were part of a larger parent project investigating the role of the loss of circulating E2 in changes in body composition and bone mineral density. Specific details about inclusion/exclusion criteria and screening procedures and some parent study results have been published previously (31, 43). Briefly, healthy premenopausal women (20 to 49 yr) with normal menstrual cycle function and a body mass index (BMI) ≤39 kg/m2 were included. Premenopausal status was verified during screening by presence of regular menses (no missed cycles in previous year, cycle length 28 ± 5 days) based on self-report and cycle tracking over 2 mo with confirmation of ovulatory status (ClearPlan Easy, Unipath Diagnostics, Waltham, MA). Exclusion criteria included smoking; use of hormonal contraception, oral glucocorticoids, or diabetes medications; lactation, pregnancy, or intent to become pregnant; hypersensitivity to study drugs; symptoms of depression (score >16 on Center for Epidemiologic Studies Depression scale); or proximal femur or lumbar spine bone mineral density T score <−2.0 (dual-energy X-ray absorptiometry scan for bone density was completed as part of prescreening).
Experimental design and study procedures.
Participants underwent preintervention assessments of the HPA axis (described below) during days two to six of the menstrual cycle. At the beginning of their next cycle, all participants began 20 wk of GnRHAG therapy (injections of leuprolide acetate 3.75 mg every 4 wk, Lupron, TAP Pharmaceutical Products, Inc., Lake Forest, IL) to suppress ovarian function chronically (15, 41). Absence of pregnancy was confirmed before each dose by a urine pregnancy test. Participants were also randomized to transdermal placebo (PL) or E2 patch (0.075 mg per day, Bayer HealthCare Pharmaceuticals, Berkeley, CA), which was initiated with the first dose of GnRHAG. The E2 regimen was expected to maintain serum E2 concentrations in the midfollicular phase range (10). Women randomized to E2 received micronized progesterone 200 mg per day for 12 days in weeks 9–11; women in the PL group received PL progesterone. The timing of this was to minimize the effects of progesterone on postintervention testing, which occurred in weeks 18–20 of the intervention (>6 wk after the completion of the progesterone exposure). A second cycle of progesterone was administered after the completion of postintervention testing (i.e., after week 20).
The randomization code was generated in SAS (SAS Institute, Cary, NC) and managed by the research pharmacist. All participants and study personnel except for the research pharmacist and nurse remained blinded to the treatment assignment until their completion in the study was over (for participants) or all data were collected and analyzed (for the study team). It was likely that participants were aware of their treatment assignment because of the nature of the intervention and its side effects (e.g., hot flashes, amenorrhea, etc.). Because all women were receiving active GnRHAG therapy, they were told during the consenting process that these side effects could happen in either intervention group.
Menopausal symptoms.
To assess menopausal symptoms, women completed the Menopausal Symptom List survey before and after 20 wk of intervention (36). The Menopausal Symptom List is a 25-item rating scale of symptoms commonly associated with menopause. A Likert scale is used to rate both the frequency (ranging from 0 = never to 5 = almost always) and severity (ranging from 0 = not applicable to 5 = extreme) of each symptom. We asked women to consider only the previous three months in answering the pre- and postintervention surveys. As detailed previously (36), a summary score for both frequency and severity of the entire 25-symptom list can be calculated, as well as composite scores for three different classes of symptoms: 1) psychological, 2) general somatic, and 3) vaso-somatic. The footnote of Table 4 contains a list of all 25 symptoms and the class categorization of the symptoms.
Table 4.
Results of Menopausal Symptom List Survey before and after 20 wk of gonadotropin-releasing hormone agonist plus add-back of PL or E2
| GnRHAG + PL, n = 15 |
GnRHAG + E2, n = 15 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Before intervention | After intervention | Within-group change (95% CI) | Before intervention | After intervention | Within-group change (95% CI) | Between-group difference in change* (95% CI) | P value |
| MSL summary score | ||||||||
| Symptom frequency | 32.5 ± 4.9 | 39.6 ± 5.7 | 7.1 (−5.7, 20.0) | 31.7 ± 4.1 | 27.8 ± 5.2 | −3.9 (−9.8, 2.1) | 5.6 (−1.0, 12.2) | 0.09 |
| Symptom severity | 34.3 ± 5.2 | 38.1 ± 5.2 | 3.9 (−5.9, 13.6) | 34.7 ± 4.4 | 27.8 ± 5.6 | −6.9 (−14.8, 0.9) | 5.3 (−0.5, 11.2) | 0.07 |
| Psychological symptoms | ||||||||
| Frequency | 13.3 ± 1.9 | 13.6 ± 2.2 | 0.3 (−3.9, 4.6) | 12.9 ± 2.1 | 11.7 ± 2.5 | −1.3 (−5.3, 2.8) | 0.8 (−1.9, 3.6) | 0.53 |
| Severity | 15.3 ± 2.1 | 14.5 ± 2.3 | −0.9 (−4.9, 3.2) | 14.5 ± 2.3 | 11.4 ± 2.6 | −3.1 (−8.4, 2.1) | 1.3 (−1.7, 4.3) | 0.39 |
| General somatic symptoms | ||||||||
| Frequency | 11.5 ± 2.0 | 12.9 ± 1.9 | 1.3 (−3.4, 6.1) | 8.8 ± 2.0 | 6.9 ± 1.7 | −1.9 (−3.9, 0.0) | 2.3 (0.1, 4.4) | 0.04 |
| Severity | 11.3 ± 2.0 | 11.5 ± 1.7 | 0.3 (−3.4, 3.9) | 9.7 ± 1.9 | 7.7 ± 1.8 | −2.0 (−4.4, 0.4) | 1.4 (−0.5, 3.3) | 0.13 |
| Vaso-somatic symptoms | ||||||||
| Frequency | 7.7 ± 1.5 | 13.1 ± 2.5 | 5.47 (0.9, 10.1) | 9.9 ± 1.5 | 9.3 ± 1.9 | −0.7 (−3.8, 2.5) | 2.9 (0.1, 5.6) | 0.04 |
| Severity | 7.7 ± 1.6 | 12.1 ± 1.9 | 4.5 (1.0, 8.0) | 10.5 ± 1.8 | 8.7 ± 2.0 | −1.8 (−5.0, 1.4) | 2.8 (0.5, 5.0) | 0.02 |
| Hot flashes | ||||||||
| Frequency | 0.1 ± 0.1 | 3.1 ± 0.5 | 2.9 (1.8, 4.1) | 0.5 ± 0.3 | 1.2 ± 0.4 | 0.7 (0.1, 1.2) | 1.1 (0.45, 1.74) | <0.01 |
| Severity | 0.2 ± 0.1 | 2.7 ± 0.4 | 2.5 (1.5, 3.6) | 0.6 ± 0.3 | 1.0 ± 0.4 | 0.4 (−0.2, 1.0) | 1.0 (0.38, 1.55) | <0.01 |
Values are means ± SE or mean (95% CI). n = no. of participants. CI, confidence interval; E2, estradiol; GnRHAG, gonadotropin-releasing hormone agonist; MSL, Menopausal Symptom List; PL, placebo. Symptom classes: Psychological: tense feelings2, excitable2, depressed feelings2, moodiness2, irritability2, pressure or tightness in head or body2, crying spells, worrying needlessly; Vaso-somatic: palpitations2, shortness of breath2, numbness and tingling2, loss of feeling in hands or feet, dry eyes, cold hands and feet, headaches, involuntary sweating, hot flashes; General somatic: weight gain2, sleeplessness2, loss of sexual interest2, poor appetite, dyspareunia, poor concentration, constipation, early morning awakenings.
Weighted two times in the summary score and symptom class calculations.
Difference in change is group difference in change controlling for before intervention value.
Body composition.
Body composition (total mass, fat mass, fat-free mass) was measured by dual-energy X-ray absorptiometry before the intervention and during week 18 of the intervention using a Hologic Discovery-W instrument (software v11.2, Waltham, MA). Changes in body composition (and bone mineral density) in the full cohort were reported previously (43). Axial computed tomography images, collected at baseline and after 18 to 20 wk of the intervention, were obtained through the center of the L2-L3 and L4-L5 intervertebral disk spaces for measurement of abdominal fat areas as previously described (43).
Diurnal cortisol index.
The diurnal index of cortisol was measured noninvasively with salivary-free cortisol levels to determine whether circadian rhythmicity was altered by E2 status both before the intervention and again in week 20 of the intervention. Three evening samples were obtained at 2130, 2200, and 2230, and three morning samples were obtained at 30-min intervals, beginning upon awakening. Salivary cortisol diurnal index was calculated as the peak morning value minus the nadir evening value.
Dex/CRH stimulation test.
The Dex/CRH test was used to determine the pituitary [serum adrenocorticotropic hormone (ACTH)] and adrenal (serum cortisol) responses to a controlled stimulus before and during week 20 of the intervention at 0800, after overnight suppression of HPA axis activity by Dex (1 mg, 2300). Conducting the CRH test during Dex suppression is a sensitive determinant of abnormal central HPA axis activity in patients with depression (22, 23) and has been used to evaluate HPA axis activity in healthy adults (21, 47). It is important to note that previous investigations using the Dex-suppressed CRH test involved a 1.5-mg dose of Dex (21–23, 47), whereas we used 1 mg. An advantage of the Dex/CRH test over the CRH-only test is that Dex minimizes endogenous CRH, ACTH, and cortisol secretion, thereby facilitating the measurement of HPA axis response to exogenous CRH administration. This enabled determination of whether pituitary activation of the HPA axis by CRH was specifically altered by E2 levels.
On the evening before the Dex/CRH test, participants took 1 mg Dex at 2300. The next morning, they reported to the CU AMC CTRC at 0700 after an overnight fast. An intravenous catheter was placed, and subjects rested for 30 min. A baseline blood sample was obtained (T-5) followed by a bolus intravenous injection of CRH (Acthrel, corticorelin ovine triflutate for injection, Ferring Pharmaceuticals, Inc., Tarrytown, NY) at a dose of 1 µg/kg body wt (maximum dose, 100 µg). Blood samples were obtained 5 min before dosing and 30, 60, 90, 120, 150, and 180 min after dosing for the measurement of serum ACTH, total cortisol, and cortisone. ACTH, total cortisol, and cortisone responses to Dex/CRH were assessed as incremental and total areas under the curve and calculated by the trapezoidal method.
Estrone (E1), E2, progesterone, total testosterone, sex hormone-binding globulin (SHBG), and corticosteroid-binding globulin (CBG) were measured from a morning blood sample taken the day before the CRH test. Serum-free cortisol was calculated from total cortisol and CBG using an algorithm that accounts for the concentration-dependent binding characteristics of cortisol (11).
Sample analysis.
Serum total cortisol and cortisone were measured by liquid chromatography tandem mass spectrometry by the Proteomics and Metabolomics Facility Colorado State University, as previously described (8). Other assays were performed by the CTRC Core laboratory or by the investigators. For hormone and gonadotropin assays, all samples were batched for an individual to minimize variability.
After collection, samples were stored at −80°C until analysis. E1 was measured by radioimmunoassay (Diagnostic Systems Laboratory, Webster, TX) with intra- and interassay coefficients of variation of 8.7% and 8.6%. E2 (4.3% and 8.2%), progesterone (4.4% and 7.9%), SHBG (3.6% and 5.7%), and total testosterone (2.1% and 5.1%) were analyzed by chemiluminescence immunoassay (Access 2 Immunoassay System Analyzer, Beckman Coulter Inc., Fullerton, CA). The sensitivity of the E2 assay was 10 pg/ml, expanded from 20 pg/ml by the manufacturer. The CU CTRC Core laboratory validates the low end of the assay every 6 mo by measurement of serially diluted control samples. Coefficients of variation for measurements made in the 10–30 pg/ml range are 20%. ACTH was determined by chemiluminescence (Immulite 1000, Siemens Medical Solutions USA, Inc., Hoffman Estates, IL; 4% and 5.3%), serum CBG by sandwich ELISA (BioVendor, LLC, Asheville, NC; 3.4% and 9.2%), and salivary cortisol by enzyme immunoassay (Salimetrics, State College, PA; 8.5% and 9.7%).
Statistical analysis.
The statistical analysis plan was generated before the study was initiated. The primary analysis was the comparison of within-group changes in the E2 and PL groups for ACTH, total cortisol, and cortisone area under the curve (AUC) in response to Dex/CRH and between-group differences in the changes. Preintervention differences in all continuous variables between the GnRHAG + E2 and GnRHAG + PL groups were evaluated using two-group t-tests; race and ethnicity were evaluated using a chi-square test for equal proportions. Within-group changes and between-group differences in changes were evaluated by linear contrast using an analysis of covariance model with adjustment for preintervention values of outcomes. Because of the large BMI range (19–39 kg/m2) for this small sample and the potential alteration in HPA axis activity in people with obesity, BMI was added to the model. All analyses were completed using SAS 9.2 (SAS Institute). Data are reported as means ± SD unless otherwise specified.
RESULTS
As previously reported (43), 79 women were randomized to the 2 drug groups, and 9 participants were lost to follow-up (personal reasons, 4; lack of time, 3; side effects of GnRHAG, 1; and hypertension, 1). Of the 70 women who completed the intervention, 35 were randomized to GnRHAG + E2 and 35 to GnRHAG + PL. Because of budgetary constraints, only 50 of these women were asked to undergo the CRH test. Out of those 50, 2 women did not complete the tests because of scheduling conflicts and 9 women were lost to follow-up. Of the 20 women in the GnRHAG + PL group and 19 women in the GnRHAG + E2 group who underwent the pre- and postintervention Dex/CRH tests, 4 women had abnormal responses to the overnight Dex suppression test (DST) at one of the two testing time points (i.e., first morning cortisol levels >18 ng/ml; n = 2 in each E2 and PL add-back groups). These abnormally elevated morning cortisol levels after Dex suppression made it difficult to interpret the CRH-related AUC results and were therefore excluded from the analysis. In addition, because of scheduling constraints, five women underwent baseline testing in the luteal phase (n = 2 and n = 3 in E2 and PL add-back groups, respectively). Because of the potential effects of menstrual cycle phase and elevated progesterone levels on HPA axis activity (3, 20, 39), the five women tested in the luteal phase were excluded from the analysis. Thus, complete HPA axis data for the current analysis were obtained on 15 women in each the GnRHAG + PL and GnRHAG + E2 groups. There were no between-group differences in subject characteristics at baseline (Table 1).
Table 1.
Preintervention characteristics
| Characteristics | GnRHAG + Placebo | GnRHAG + Estradiol |
|---|---|---|
| n = 15 | n = 15 | |
| Ethnicity | ||
| Hispanic or Latino | 1 (7) | 3 (20) |
| Not Hispanic or Latino | 13 (87) | 11 (73) |
| Unknown | 1 (7) | 1 (7) |
| Race | ||
| Asian | 1 (7) | 0 (0) |
| Black or African American | 0 (0) | 2 (13) |
| More than one race | 2 (13) | 0 (0) |
| Refused to answer | 0 (0) | 2 (13) |
| White | 12 (80) | 11 (73) |
| Age, yr | 38 (SD 5) | 36 (SD 7) |
| BMI, kg/m2 | 27 (SD 6.1) | 27 (SD 6.1) |
| Weight, kg | 74.7 (SD 18.5) | 75.3 (SD 20.7) |
| Fat-free mass, kg | 47.4 (SD 7.0) | 48.6 (SD 7.8) |
| Fat mass, kg | 27.2 (SD 12.6) | 26.6 (SD 13.9) |
| Abdominal fat area, cm2 | 358 (SD 202) | 341 (SD 199) |
| Abdominal SAT area, cm2 | 285 (SD 144) | 280 (SD 172) |
| Abdominal VAT area, cm2 | 72.4 (SD 65.1) | 61.0 (SD 36.2) |
| Estradiol, pg/ml | 77.9 (SD 67.5) | 70.6 (SD 46.3) |
| Estrone, pg/ml | 51.2 (SD 19.5) | 55.0 (SD 14.8) |
| Progesterone, ng/ml | 0.52 (SD 0.44) | 0.59 (SD 0.33) |
| Testosterone, ng/dl | 29.9 (SD 19.8) | 32.7 (SD 10.1) |
| SHBG, nmol/l | 54.7 (SD 27.8) | 40.9 (SD 19.7) |
Values are presented as n (%) or means (SD). n = no. of participants. BMI, body mass index; GnRHAG, gonadotropin-releasing hormone agonist; SAT, subcutaneous adipose tissue; SHBG, sex hormone-binding globulin; VAT, visceral adipose tissue. SI unit conversions: estradiol pg/ml × 3.671 = pmol/l, estrone pg/ml × 3.699 = pmol/l, progesterone ng/ml × 3.18 = nmol/l, testosterone ng/dl × 0.0347 nmol/l.
Circulating sex hormones and body composition.
The GnRHAG intervention was successful in creating two groups that differed by estrogen level. There were significant between-group differences in the changes in E2 and E1 but not testosterone, progesterone, or SHBG.
Analysis of within-group changes demonstrated that GnRHAG + PL therapy significantly decreased E2 and testosterone, with no changes in E1, progesterone, and SHBG (Table 2). GnRHAG + E2 caused a significant decrease in progesterone, but no changes in testosterone, E2, E1, or SHBG.
Table 2.
Circulating factors before and after 20 wk of gonadotropin-releasing hormone agonist plus PL or E2 add-back therapy
| GnRHAG + PL, n = 15 |
GnRHAG + E2, n = 15 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Before intervention | After intervention | Within-group change (95% CI) | Before intervention | After intervention | Within-group change (95% CI) | Between-group difference in change* (95% CI) | P value |
| E2, pg/ml | 77.9 ± 17.4 | 23.2 ± 2.6 | −54.7 (−92.9, −16.4) | 70.6 ± 12.4 | 105 ± 30.4 | 34.1 (−42.8, 110.9) | −80.1 (−141, −19) | 0.01 |
| Estrone, pg/ml | 51.2 ± 5.0 | 39.9 ± 8.0 | −11.3 (−24.5, 1.8) | 55.0 ± 4.0 | 65.2 ± 7.6 | 10.2 (−8.7, 29.1) | −23.0 (−44.7, −1.3) | 0.04 |
| Progesterone, ng/ml | 0.52 ± 0.11 | 0.31 ± 0.05 | −0.21 (−0.45, 0.04) | 0.59 ± 0.09 | 0.33 ± 0.05 | −0.26 (−0.46, −0.06) | −0.01 (−0.16, 0.15) | 0.92 |
| Testosterone, ng/dl | 29.9 ± 5.1 | 25.6 ± 5.5 | −4.5 (−8.9, −0.1) | 32.7 ± 2.8 | 30.1 ± 3.2 | −3.8 (−7.9, 0.3) | −0.9 (−6.8, 5.0) | 0.75 |
| SHBG, nmol/l | 54.7 ± 7.2 | 46.3 ± 6.5 | −8.4 (−18.2, 1.4) | 40.9 ± 4.6 | 41.9 ± 5.8 | 0.9 (−4.3, 6.1) | −6.5 (−17.4, 4.3) | 0.23 |
| CBG, µg/ml | 25.5 ± 1.0 | 26.2 ± 1.0 | 0.7 (−0.7, 2.0) | 25.4 ± 0.9 | 25.4 ± 1.0 | −0.0 (−1.7, 1.7) | 0.7 (−1.3, 2.7) | 0.49 |
| Free cortisol, ng/ml | 3.3 ± 1.2 | 2.3 ± 0.6 | −1.1 (−4.0, 1.9) | 1.8 ± 0.6 | 2.4 ± 0.7 | 0.6 (−1.1, 2.2) | −0.2 (−2.2, 1.9) | 0.86 |
Values are means ± SE or mean (95% CI). n = no. of participants. CBG, corticosteroid-binding globulin; CI, confidence interval; E2, estradiol; GnRHAG, gonadotropin-releasing hormone agonist; PL, placebo; SHBG, sex hormone-binding globulin. SI unit conversions: estradiol pg/ml × 3.671 = pmol/l, estrone pg/ml × 3.699 = pmol/l, progesterone ng/ml × 3.18 = nmol/l, testosterone ng/dl × 0.0347 nmol/l, cortisol ng/ml × 2.76 = nmol/l, CBG µg/ml × 17.18 nmol/l.
Difference in change is group difference in change controlling for before intervention value.
There were no significant changes in body composition in either treatment group in this subset of women (Table 3). In the parent trial, whole body fat-free mass decreased, and abdominal subcutaneous and visceral adipose tissue area increased after GnRHAG + PL, and these changes were attenuated in the E2 add-back group (43). The smaller sample size of this subset likely decreased our ability to detect significant changes in these parameters in the present analysis.
Table 3.
Body composition before and after 20 wk of gonadotropin-releasing hormone agonist plus add-back of PL or E2
| GnRHAG + PL, n = 15 |
GnRHAG + E2, n = 15 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Before intervention | After intervention | Within-group change (95% CI) | Before intervention | After intervention | Within-group change (95% CI) | Between-group difference in change* (95% CI) | P value |
| BMI, kg/m2 | 27 ± 1.6 | 26 ± 1.6 | −0.2 (−0.7, 0.2) | 27 ± 1.6 | 27 ± 1.6 | −0.1 (−0.9, 0.7) | −0.1 (−1.0, 0.8) | 0.81 |
| Total mass, kg | 74.7 ± 4.8 | 74.0 ± 4.9 | −0.7 (−1.9, 0.5) | 75.3 ± 5.3 | 74.7 ± 5.1 | −0.5 (−2.9, 1.8) | −0.2 (−2.7, 2.4) | 0.88 |
| Fat-free mass, kg | 47.4 ± 1.8 | 47.1 ± 1.7 | −0.3 (−0.9, 0.3) | 48.6 ± 2.0 | 48.8 ± 1.8 | 0.1 (−0.7, 1.0) | −0.5 (−1.4, 0.3) | 0.20 |
| Fat mass, kg | 27.2 ± 3.3 | 26.8 ± 3.4 | −0.4 (−1.6, 0.8) | 26.6 ± 3.6 | 26.0 ± 3.7 | −0.7 (−2.6, 1.3) | 0.3 (−2.0, 2.5) | 0.80 |
| Abdominal fat area, cm2 | 358 ± 52.1 | 368 ± 55.0 | 10.1 (−8.5, 28.7) | 341 ± 51.4 | 338 ± 55.2 | −3.4 (−29.0, 22.3) | 12.7 (−17.4, 42.8) | 0.39 |
| Abdominal SAT area, cm2 | 285 ± 37.3 | 293 ± 39.9 | 8.0 (−7.5, 23.6) | 280 ± 44.4 | 278 ± 47.9 | −2.1 (−23.6, 19.4) | 9.9 (−15.1, 34.8) | 0.43 |
| Abdominal VAT area, cm2 | 72.4 ± 16.8 | 74.4 ± 17.3 | 2.1 (−3.3, 7.5) | 61.0 ± 9.3 | 59.8 ± 9.6 | −1.2 (−6.6, 4.1) | 3.2 (−4.3, 10.6) | 0.39 |
Values are means ± SE or mean (95% CI). n = no. of participants. BMI, body mass index; CI, confidence interval; E2, estradiol; GnRHAG, gonadotropin-releasing hormone agonist; PL, placebo; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Difference in change is group difference in change controlling for before intervention value.
Menopausal symptoms.
As expected, women in the GnRHAG + PL and GnRHAG + E2 groups reported different frequency and severity of some menopausal symptoms, although the overall menopausal symptom summary score was similar between the groups (Table 4). Hot flashes and the composite vaso-somatic class of symptoms increased in both frequency and severity in the GnRHAG + PL group, and there was no change in the E2 add-back group. The frequency, but not severity, of general somatic symptoms also changed differently between the groups, driven primarily by fewer reported general somatic symptoms in the GnRHAG + E2 group. Neither the frequency nor the severity of psychological symptoms changed within or between groups.
Dex suppression/CRH stimulation test.
The morning total cortisol response to an overnight DST was not altered by 20 wk of sex hormone suppression. Women included in this study responded normally to the DST with morning mean cortisol levels <18 ng/ml before and after the intervention (Table 5).
Table 5.
HPA axis response to Dexamethasone and CRH before and after 20 wk of gonadotropin-releasing hormone agonist plus add-back of PL or E2
| GnRHAG + PL, n = 15 |
GnRHAG + E2, n = 15 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Before intervention | After intervention | Within-group change (95% CI) | Before intervention | After intervention | Within-group change (95% CI) | Between-group difference in change* (95% CI) | P value |
| Dex suppressed morning values | ||||||||
| ACTH†, pg/ml | 9.6 ± 2.2 | 9.5 ± 2.1 | 0.1 (−0.4, 0.5) | 8.3 ± 0.6 | 8.7 ± 0.6 | 0.5 (−0.3, 1.3) | −0.9 (−3.2, 1.3) | 0.41 |
| Total cortisol†, ng/ml | 8.0 ± 0.9 | 8.9 ± 1.0 | 0.9 (−1.0, 2.8) | 8.6 ± 0.8 | 10.1 ± 0.7 | 1.5 (−0.4, 3.5) | −0.4 (−1.3, 0.5) | 0.35 |
| Cortisone†, ng/ml | 1.7 ± 0.3 | 2.1 ± 0.6 | 0.4 (−0.7, 1.5) | 1.6 ± 0.3 | 1.7 ± 0.4 | 0.0 (−0.7, 0.8) | 0.4 (−0.9, 1.6) | 0.57 |
| AUCINC response to CRH | ||||||||
| ACTH, pg/ml × min | 1,327 ± 238 | 1,291 ± 237 | −59 (−534, 416) | 1,623 ± 257 | 1,211 ± 236 | −413 (−666, −159) | 270 (−232, 772) | 0.28 |
| Total cortisol, ng/ml × min | 7,918 ± 1969 | 8,106 ± 2,055 | 189 (−2,481, 2,859) | 7,008 ± 1,387 | 3,893 ± 1,090 | −3,115 (−5,322, −907) | 3,538 (351, 6,725) | 0.03 |
| Cortisone, ng/ml × min | 1,879 ± 406 | 1,742 ± 345 | −138 (−559, 284) | 1,795 ± 367 | 1,090 ± 281 | −705 (−1,273, −137) | 597 (24, 1,169) | 0.04 |
Values are means ± SE or mean (95% CI). n = no. of participants. ACTH, adrenocorticotropic hormone; AUCINC, incremental area under the curve; CI, confidence interval; CRH, corticotropin-releasing hormone; Dex, Dexamethasone; E2, estradiol; GnRHAG, gonadotropin-releasing hormone agonist; HPA, hypothalamic-pituitary-adrenal; PL, placebo. SI unit conversions: ACTH pg/ml × 0.22 = pmol/l, cortisol ng/ml × 2.76 = nmol/l.
Difference in change is group difference in change controlling for before intervention value.
Sample drawn at 0800 under Dex suppression (1 mg at 2300), T-5.
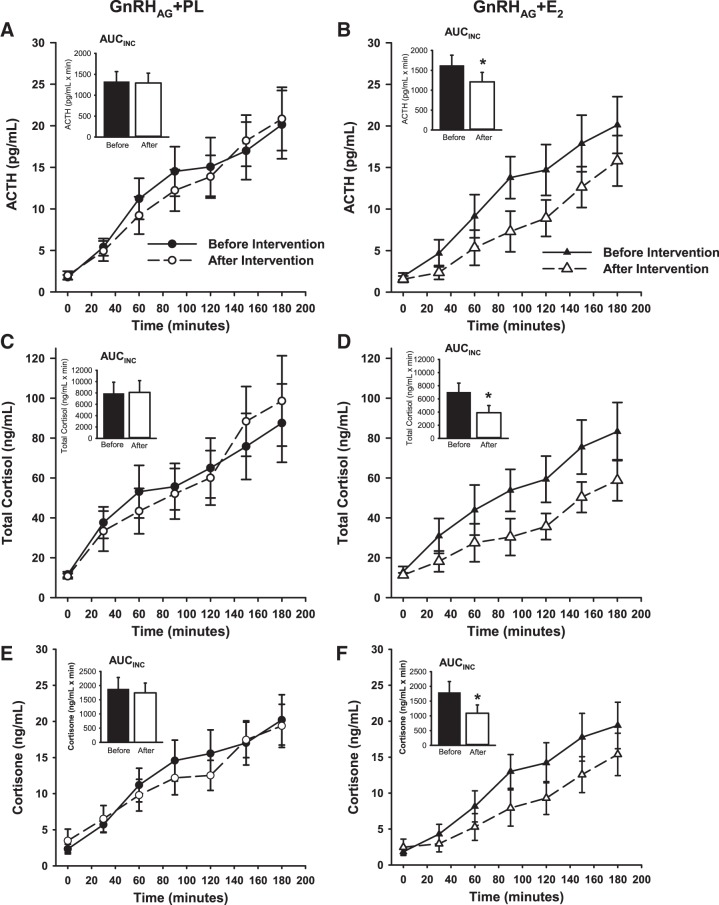
Significant differences in the between-group changes, adjusted for preintervention values, were observed for total cortisol and cortisone but not ACTH [GnRHAG + PL minus GnRHAG + E2, mean (95% confidence interval); Table 5]. However, within-group analyses demonstrated that the GnRHAG + PL group did not have an exaggerated HPA responsiveness to the CRH test (Fig. 1, A, C, and E; Table 5). Rather, GnRHAG + E2 resulted in decreased incremental area under the curve responses for all three measures (Fig. 1, B, D, and F; Table 5). Similar results were observed in the whole cohort (data not shown).
Fig. 1.
ACTH, total cortisol, and cortisone response to a Dex/CRH stimulation test before and after 20 wk of GnRHAG with placebo (PL) or estradiol (E2) add-back therapy in premenopausal women. Mean response to CRH stimulation (1 µg/kg body wt; Acthrel, corticorelin ovine triflutate for injection) under Dex suppression (1 mg at 2300). Testing occurred at 0800 with baseline blood drawn at T-5 before intravenous administration of CRH. ACTH (A and B), total cortisol (C and D), and cortisone (E and F) were measured at each time point before (solid line and closed symbol) and after (dashed line and open symbol) 20 wk of GnRHAG therapy with add-back of PL (A, C, and E) or transdermal E2 (B, D, and F). Inset graphs represent incremental area under the curve (AUCINC) as calculated by the trapezoidal method with baseline set at T-5 for each data set. *P ≤ 0.02 vs. baseline. n = 15 participants per group. Data presented as means ± SE. ACTH, adrenocorticotropic hormone; CRH, corticotropin-releasing hormone; Dex, Dexamethasone; GnRHAG, gonadotropin-releasing hormone agonist; SE, standard error.
Other HPA axis measurements.
Evening and first morning salivary cortisol measurements were made on two consecutive days different from the Dex/CRH test. GnRHAG + PL or GnRHAG + E2 did not alter the evening nadir or morning peak salivary cortisol levels (Fig. 2). Accordingly, the diurnal cortisol index did not change in response to GnRHAG + PL [0.48 ± 0.05 µg/dl pre- vs. 0.54 ± 0.11 µg/dl postintervention; change 0.06 (95% confidence interval; −0.15, 0.27)] µg/dl or GnRHAG + E2 [0.44 ± 0.08 µg/dl pre- vs. 0.45 ± 0.05 µg/dl postintervention; change 0.01 (−0.14, 0.16)] µg/dl. There were no between-group differences in any diurnal cortisol measurements.
Fig. 2.
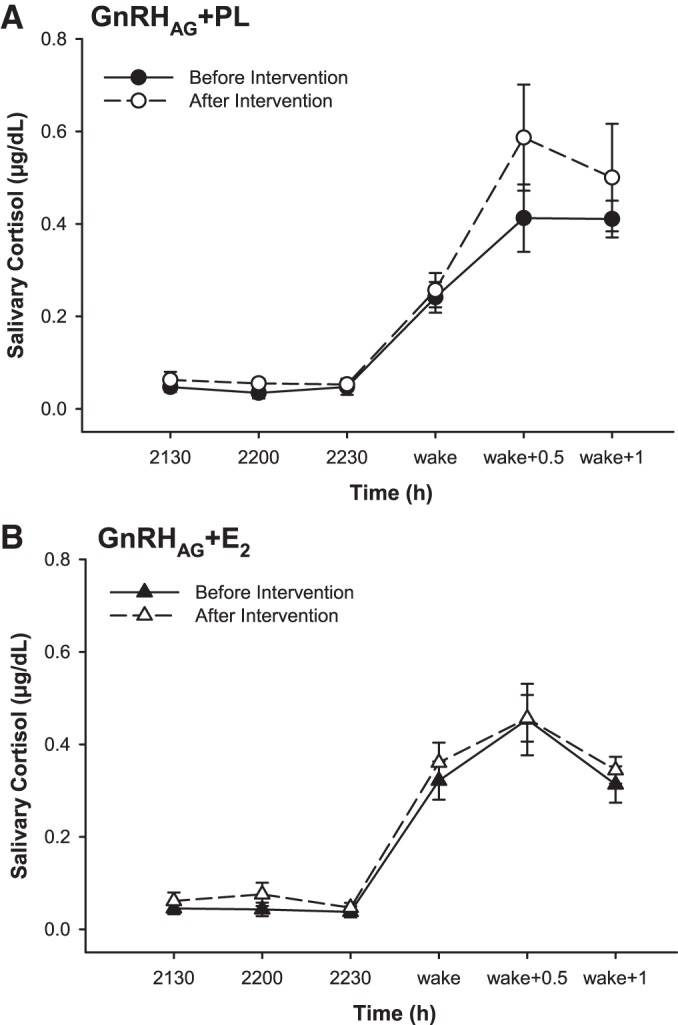
Diurnal cortisol index as measured by salivary cortisol before and after 20 wk of gonadotropin-releasing hormone agonist (GnRHAG) + PL (A) or GnRHAG + E2 (B) add-back therapy in premenopausal women. Saliva samples were collected at three time points in the evening before bed (2130, 2200, and 2230) and at three time points upon awakening in the morning [immediately after awakening (wake), 30 min after awakening (wake + 0.5), and 1 h after awakening (wake + 1)]. n = 15 participants per group. Data presented as means ± standard error (SE). E2, estradiol; PL, placebo.
Ovarian hormone suppression with or without E2 add-back did not change circulating CBG or calculated serum-free cortisol (Table 2), as measured on a separate day from the DST (i.e., not under Dex suppression).
DISCUSSION
The goal of this study was to isolate the effects of E2 on HPA axis activity. Our experimental model included healthy premenopausal women who underwent 20 wk of ovarian hormone suppression with concurrent transdermal PL or E2 therapy. We measured indicators of HPA axis activity in the basal state and in response to a Dex/CRH stimulation test before and after the GnRHAG intervention. The major findings included between-group differences in the changes in cortisol and cortisone responses to CRH and between-group differences in the changes in E2 and E1 but not testosterone, progesterone, or SHBG. However, contrary to our hypothesis, the analysis of within-group changes revealed that GnRHAG + PL did not alter basal HPA axis activity or result in an exaggerated HPA axis response to the CRH test. Rather, the observed between-group differences in responses of the HPA axis to Dex/CRH were driven by attenuated responses in the GnRHAG + E2 group.
Hormonal intervention.
Although E2 levels in the GnRHAG + PL group did not reach castrate levels, there was a significant decrease from the preintervention level. In addition, transdermal E2 add-back therapy prevented a decrease in serum E2 concentration. Thus, the intervention approach was successful in generating two groups that had significantly different circulating E2 levels, enabling us to investigate the effects of E2 on the HPA axis. Whether the responses observed in the GnRHAG + PL group were representative of what would be observed in the postmenopausal state, in which circulating E2 levels would be even lower, cannot be assessed from this intervention.
Although unexpected, SHBG was not altered in response to the intervention in either group. This was likely due to the relatively small sample size in this study. Previously published results from the larger cohort of this intervention study reported significantly different responses of SHBG between the intervention groups (31, 43).
Menopausal symptoms.
The appearance of a number of physiological and psychological symptoms characterizes the menopausal transition. Some of these menopausal symptoms, such as hot flashes, anxiety, and depression, have been associated with alterations in HPA axis activity (9, 16, 17). Indeed, women with altered HPA axis activity (e.g., elevated hair cortisol levels and flattened diurnal index) report increased frequency and severity of hot flashes (16). However, it remains uncertain if altered HPA axis activity is a cause or a consequence of exaggerated menopausal symptoms. Alterations to thermoregulation or the autonomic nervous system as a result of altered HPA axis activity could lead to increased sensitivity to, or frequency of, hot flashes. Yet, experiencing frequent and disruptive menopausal symptoms could also result in altered cortisol secretion. We found that suppression of ovarian hormones with PL add-back resulted in more frequent and severe vaso-somatic menopausal symptoms, including hot flashes, compared with the E2 add-back group. If the burden of experiencing more menopausal symptoms influenced our measurements, we would have expected exaggerated HPA axis responses in the GnRHAG + PL group. However, there was no evidence of altered HPA axis activity in the PL add-back group. These findings suggest that elevated HPA axis activity is not causally linked with the initiation of menopausal symptoms.
Basal HPA axis activity.
Previous research has led to conflicting conclusions concerning the role of E2 in the regulation of HPA axis activity (21, 27, 44). Our results suggest that basal HPA axis activity is not perturbed by a decrease in circulating ovarian hormones over a 20-wk period, because morning free cortisol concentrations and salivary cortisol diurnal index did not change in either treatment group.
The dissociation of age from menopausal status is problematic in many studies of the HPA axis in pre- versus postmenopausal women. For instance, lower first morning salivary cortisol and cortisol awakening response (32) or higher first morning serum ACTH and cortisol (26) have been reported in postmenopausal compared with premenopausal women, but age differences between the groups prohibits conclusions regarding the impact of ovarian steroids compared with that of age itself. Evidence arguing against sex hormones playing a primary role in alterations in the HPA axis, at least acutely, is presented in studies of premenopausal women before and 8 days after ovariectomy (12), or 12 wk of transdermal E2 therapy in previously estrogen-deficient postmenopausal women (44). These studies found no change in first morning serum ACTH or cortisol measures with acute changes in circulating estrogen status, consistent with our findings.
Abundant evidence exists that aging, per se, has a greater progressive influence on basal HPA axis activity. Cortisol awakening response is dampened (18, 32) and diurnal index is flattened (13, 18, 42, 46) in older compared with younger adults. With these previous findings in mind, the present study suggests that alterations in unstimulated HPA axis activity in older women are likely the result of aging and not the loss of circulating ovarian hormones. An interaction between aging and ovarian steroids in the regulation of basal HPA axis activity cannot be excluded because our intervention was less than 6 mo (24 wk) in duration, and circulating E2 was not suppressed to postmenopausal levels.
CBG appears to be similar between nonobese pre- and postmenopausal women (21, 26), which is consistent with our finding that it was not altered with GnRHAG therapy. Additionally, substantial evidence from the current study, and work by others (21, 25, 37, 39), indicates that transdermal, as opposed to oral, estrogen treatment (37) at these levels does not increase CBG levels.
Dynamic HPA axis activity.
The use of the overnight DST combined with the CRH stimulation test enabled us to determine that, in our model of ovarian hormone suppression, neither pituitary activation of the HPA axis nor its sensitivity to Dex were altered by E2. These results were similar to previous findings of no difference in morning serum cortisol after Dex suppression between pre- and postmenopausal women (26) or after 12 wk of transdermal E2 therapy in postmenopausal women (44).
Conducting a CRH test during Dex suppression has been found to be a sensitive determinant of abnormal central HPA axis activity in patients with depression (22, 23) and has also been used to evaluate HPA axis activity in healthy adults (21, 47). An advantage of experimentally stimulating the HPA axis during Dex suppression is that pre- and poststimulation cortisol feedback inhibition is minimized, thereby better isolating the effects of E2 on central HPA axis activation. Utilizing this model, we found cortisone and cortisol responses to CRH differed between the PL and E2 add-back groups. In opposition to our hypothesis, the GnRHAG + PL intervention did not result in exaggerated HPA axis responses to CRH. This observation suggests that the elevated dynamic HPA axis response to Dex/CRH in older women (21) may be a consequence of aging and not the loss of ovarian hormones.
Although the response in the GnRHAG + PL group was unchanged, the GnRHAG + E2 group had attenuated ACTH, cortisol, and cortisone responses to CRH when compared with preintervention. The consistent pattern of decreased ACTH, cortisol, and cortisone in the GnRHAG + E2 group suggests, but does not confirm, that estrogen levels are involved in the regulation of dynamic HPA axis activity. Nonetheless, the interpretation of these findings is complicated by the fact that E2 levels in the GnRHAG + E2 group did not change statistically over the intervention. We cautiously interpret the increase in E2 of 34 pg/ml as being of potential clinical relevance despite being statistically nonsignificant, thereby suggesting that elevated E2 levels may have a role in lowering cortisol responses to CRH. Importantly, in support of this interpretation, previous studies also found that GnRHAG + E2 for 5 wk or transdermal E2 alone for 2 wk (both utilizing 0.1 mg transdermal E2 daily) resulted in lower cortisol responses to Dex/CRH compared with GnRHAG alone (25) or the estrogen-deficient postmenopausal state (21), respectively. Kudielka et al. (21) concluded that the effect of E2 treatment to lower HPA axis response suggests that E2 could affect HPA feedback sensitivity.
Finally, because the decreased HPA axis responses to Dex/CRH in the GnRHAG + E2 group were a within-group change, it is possible this was associated with changes in factors other than estrogens.
Other considerations and limitations.
It is possible that ovarian suppression did not result in the hypothesized increase in HPA axis responses to CRH because GnRHAG therapy causes decreases in luteinizing hormone and follicle stimulating hormone rather than the increases that occur during menopause. A direct correlation between basal HPA axis activity and luteinizing hormone level has been observed in both premenopausal (ovulatory phase) and postmenopausal women (2, 24, 48).
There were some limitations to this study. First, the pharmacological model of ovarian hormone suppression does not simulate all aspects of the natural menopause (e.g., abrupt vs. gradual hormone withdrawal; suppression vs. elevation of gonadotropins; decline in circulating E2 did not reach postmenopausal levels). Therefore, the relevance of the results to the menopausal transition is limited until further studies in that population are conducted. In addition, high interindividual variability of responses to HPA axis stressors is a limitation that is commonly reported in the literature (38, 39). We used a weight-adjusted CRH dose to standardize the test across subjects and sessions. However, interindividual variability in HPA axis responses may have limited our ability to detect significant between-group differences in the changes in response to the intervention. The CRH stimulation test was performed in the morning, when endogenous HPA axis activity is high. However, the test was conducted under Dex suppression to minimize the influence of the normal circadian morning increase in cortisol. It is possible that the extent of Dex suppression differed between the groups, because sex steroids can alter the absorption or clearance of Dex (14, 29). This seems unlikely because, in a similar GnRHAG intervention in premenopausal women with or without transdermal E2 replacement, plasma Dex level at the time of CRH testing was not influenced by hormone status (25). Lastly, to address safety concerns regarding endometrial hyperplasia, women in the GnRHAG + E2 group received 12 days of micronized progesterone starting in week 12 of the intervention. The timing of this administration should not have had residual effects on the final outcome measures, which were assessed more than 6 wk after progesterone exposure. Nonetheless, the progesterone exposure was different between the E2 and PL add-back groups.
To facilitate recruitment, women with a wide range of BMI were enrolled (19–39 kg/m2). Obesity is associated with alterations in HPA axis activity and can alter the response to a DST (35). However, not everyone with obesity has abnormal cortisol regulation (1). Indeed, 3 of the 4 women that were excluded because of abnormal DST responses at one of their two visits had BMI >30, but an additional 10 obese women enrolled had normal DST responses at both visits.
Finally, adipose tissue and liver are important sites for glucocorticoid metabolism that may be altered by age and/or ovarian hormone status (4, 5). It is possible that changes in peripheral glucocorticoid metabolism influenced our results, but conducting these measurements was beyond the scope of this investigation. Future studies are needed for a more complete understanding of the influence of E2 on cortisol metabolism.
In conclusion, the major finding was that changes in dynamic HPA axis activity were different in women on GnRHAG + E2 than in those on GnRHAG + PL. This points to a role of E2 in regulating the HPA axis response to CRH. Notably, ovarian hormone suppression alone did not alter basal or dynamic HPA axis activity in premenopausal women. This suggests that aging, as opposed to the loss of ovarian hormones, contributes to increased HPA axis activity in older women. Additional studies are necessary to more thoroughly understand the complex relations among the HPA axis, aging, and the loss of ovarian hormones.
GRANTS
This work was supported by NIH grants R01 AG-018198, P-50 HD-073063, P-30 DK-048520, T-32 AG-000279, F-32 AG-046957, K-01 DK-109053, and UL-1 TR-001082. K. Gavin, R. Schwartz, and W. Kohrt were supported by the Veterans Affairs Eastern Colorado Health Care System Geriatric Research, Education and Clinical Center.
DISCLAIMERS
The contents of this paper do not represent the views of the US Department of Veterans Affairs or the United States Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.S.S., M.E.W., and W.M.K. conceived and designed research; K.L.S. and E.G. performed experiments; K.M.G. and P.W. analyzed data; K.M.G., P.W., M.E.W., and W.M.K. interpreted results of experiments; K.M.G. prepared figures; K.M.G., P.W., and W.M.K. drafted manuscript; K.M.G., K.L.S., E.G., P.W., R.S.S., M.E.W., and W.M.K. edited and revised manuscript; K.M.G., K.L.S., E.G., P.W., R.S.S., M.E.W., and W.M.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to the nursing, bionutrition, core laboratory, information systems, and administrative staff of the Clinical and Translational Research Center and the Energy Balance Core of the Nutrition and Obesity Research Center for their support of the study. We also acknowledge the members of our research group who helped with the initiation of the study and carried out day-to-day activities for the project, specifically Wendolyn S. Gozansky, MD. Finally, we thank the women who volunteered to participate in the study for their time and effort.
Clinical Trials Registration Number: NCT00687739.
REFERENCES
- 1.Abraham SB, Rubino D, Sinaii N, Ramsey S, Nieman LK. Cortisol, obesity, and the metabolic syndrome: a cross-sectional study of obese subjects and review of the literature. Obesity (Silver Spring) 21: E105–E117, 2013. doi: 10.1002/oby.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alevizaki M, Saltiki K, Mantzou E, Anastasiou E, Huhtaniemi I. The adrenal gland may be a target of LH action in postmenopausal women. Eur J Endocrinol 154: 875–881, 2006. doi: 10.1530/eje.1.02165. [DOI] [PubMed] [Google Scholar]
- 3.Altemus M, Roca C, Galliven E, Romanos C, Deuster P. Increased vasopressin and adrenocorticotropin responses to stress in the midluteal phase of the menstrual cycle. J Clin Endocrinol Metab 86: 2525–2530, 2001. doi: 10.1210/jcem.86.6.7596. [DOI] [PubMed] [Google Scholar]
- 4.Andersson T, Simonyte K, Andrew R, Strand M, Burén J, Walker BR, Mattsson C, Olsson T. Tissue-specific increases in 11beta-hydroxysteroid dehydrogenase type 1 in normal weight postmenopausal women. PLoS One 4: e8475, 2009. doi: 10.1371/journal.pone.0008475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson T, Söderström I, Simonyté K, Olsson T. Estrogen reduces 11beta-hydroxysteroid dehydrogenase type 1 in liver and visceral, but not subcutaneous, adipose tissue in rats. Obesity (Silver Spring) 18: 470–475, 2010. doi: 10.1038/oby.2009.294. [DOI] [PubMed] [Google Scholar]
- 6.Björntorp P. Body fat distribution, insulin resistance, and metabolic diseases. Nutrition 13: 795–803, 1997. doi: 10.1016/S0899-9007(97)00191-3. [DOI] [PubMed] [Google Scholar]
- 7.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab 89: 2583–2589, 2004. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 8.Broccardo CJ, Schauer KL, Kohrt WM, Schwartz RS, Murphy JP, Prenni JE. Multiplexed analysis of steroid hormones in human serum using novel microflow tile technology and LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 934: 16–21, 2013. doi: 10.1016/j.jchromb.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cagnacci A, Cannoletta M, Caretto S, Zanin R, Xholli A, Volpe A. Increased cortisol level: a possible link between climacteric symptoms and cardiovascular risk factors. Menopause 18: 273–278, 2011. doi: 10.1097/gme.0b013e3181f31947. [DOI] [PubMed] [Google Scholar]
- 10.Chetkowski RJ, Meldrum DR, Steingold KA, Randle D, Lu JK, Eggena P, Hershman JM, Alkjaersig NK, Fletcher AP, Judd HL. Biologic effects of transdermal estradiol. N Engl J Med 314: 1615–1620, 1986. doi: 10.1056/NEJM198606193142505. [DOI] [PubMed] [Google Scholar]
- 11.Coolens JL, Van Baelen H, Heyns W. Clinical use of unbound plasma cortisol as calculated from total cortisol and corticosteroid-binding globulin. J Steroid Biochem 26: 197–202, 1987. doi: 10.1016/0022-4731(87)90071-9. [DOI] [PubMed] [Google Scholar]
- 12.De Leo V, la Marca A, Talluri B, D’Antona D, Morgante G. Hypothalamo-pituitary-adrenal axis and adrenal function before and after ovariectomy in premenopausal women. Eur J Endocrinol 138: 430–435, 1998. doi: 10.1530/eje.0.1380430. [DOI] [PubMed] [Google Scholar]
- 13.Deuschle M, Gotthardt U, Schweiger U, Weber B, Körner A, Schmider J, Standhardt H, Lammers CH, Heuser I. With aging in humans the activity of the hypothalamus-pituitary-adrenal system increases and its diurnal amplitude flattens. Life Sci 61: 2239–2246, 1997. doi: 10.1016/S0024-3205(97)00926-0. [DOI] [PubMed] [Google Scholar]
- 14.Finken MJ, Andrews RC, Andrew R, Walker BR. Cortisol metabolism in healthy young adults: sexual dimorphism in activities of A-ring reductases, but not 11beta-hydroxysteroid dehydrogenases. J Clin Endocrinol Metab 84: 3316–3321, 1999. doi: 10.1210/jcem.84.9.6009. [DOI] [PubMed] [Google Scholar]
- 15.Gerhard I, Schindler AE, Bühler K, Winkler U, Meinen K, Mancarella D, Hoffmann G, Schüssler B, Kimmig R, Kranzfelder D. Treatment of endometriosis with leuprorelin acetate depot: a German multicentre study. Clin Ther 14, Suppl A: 3–16, 1992. [PubMed] [Google Scholar]
- 16.Gibson CJ, Thurston RC, Matthews KA. Cortisol dysregulation is associated with daily diary-reported hot flashes among midlife women. Clin Endocrinol (Oxf) 85: 645–651, 2016. doi: 10.1111/cen.13076. [DOI] [PubMed] [Google Scholar]
- 17.Gordon JL, Eisenlohr-Moul TA, Rubinow DR, Schrubbe L, Girdler SS. Naturally occurring changes in estradiol concentrations in the menopause transition predict morning cortisol and negative mood in perimenopausal depression. Clin Psychol Sci 4: 919–935, 2016. doi: 10.1177/2167702616647924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heaney JL, Phillips AC, Carroll D. Ageing, depression, anxiety, social support and the diurnal rhythm and awakening response of salivary cortisol. Int J Psychophysiol 78: 201–208, 2010. doi: 10.1016/j.ijpsycho.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Janssen I, Powell LH, Kazlauskaite R, Dugan SA. Testosterone and visceral fat in midlife women: the Study of Women’s Health Across the Nation (SWAN) fat patterning study. Obesity (Silver Spring) 18: 604–610, 2010. doi: 10.1038/oby.2009.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med 61: 154–162, 1999. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Kudielka BM, Schmidt-Reinwald AK, Hellhammer DH, Kirschbaum C. Psychological and endocrine responses to psychosocial stress and dexamethasone/corticotropin-releasing hormone in healthy postmenopausal women and young controls: the impact of age and a two-week estradiol treatment. Neuroendocrinology 70: 422–430, 1999. doi: 10.1159/000054504. [DOI] [PubMed] [Google Scholar]
- 22.Kunugi H, Urushibara T, Nanko S. Combined DEX/CRH test among Japanese patients with major depression. J Psychiatr Res 38: 123–128, 2004. doi: 10.1016/S0022-3956(03)00103-1. [DOI] [PubMed] [Google Scholar]
- 23.Künzel HE, Binder EB, Nickel T, Ising M, Fuchs B, Majer M, Pfennig A, Ernst G, Kern N, Schmid DA, Uhr M, Holsboer F, Modell S. Pharmacological and nonpharmacological factors influencing hypothalamic-pituitary-adrenocortical axis reactivity in acutely depressed psychiatric in-patients, measured by the Dex-CRH test. Neuropsychopharmacology 28: 2169–2178, 2003. doi: 10.1038/sj.npp.1300280. [DOI] [PubMed] [Google Scholar]
- 24.Lacroix A, Hamet P, Boutin JM. Leuprolide acetate therapy in luteinizing hormone–dependent Cushing’s syndrome. N Engl J Med 341: 1577–1581, 1999. doi: 10.1056/NEJM199911183412104. [DOI] [PubMed] [Google Scholar]
- 25.Lee EE, Nieman LK, Martinez PE, Harsh VL, Rubinow DR, Schmidt PJ. ACTH and cortisol response to Dex/CRH testing in women with and without premenstrual dysphoria during GnRH agonist-induced hypogonadism and ovarian steroid replacement. J Clin Endocrinol Metab 97: 1887–1896, 2012. doi: 10.1210/jc.2011-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li S, Liu W, Wang L, Huang R, Chen Q, Wu Y, Cai Y. Effects of menopause on hepatic 11β-hydroxysteroid dehydrogenase type 1 activity and adrenal sensitivity to adrenocorticotropin in healthy non-obese women. Gynecol Endocrinol 27: 794–799, 2011. doi: 10.3109/09513590.2010.507288. [DOI] [PubMed] [Google Scholar]
- 27.Lindheim SR, Legro RS, Bernstein L, Stanczyk FZ, Vijod MA, Presser SC, Lobo RA. Behavioral stress responses in premenopausal and postmenopausal women and the effects of estrogen. Am J Obstet Gynecol 167: 1831–1836, 1992. doi: 10.1016/0002-9378(92)91783-7. [DOI] [PubMed] [Google Scholar]
- 28.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes 32: 949–958, 2008. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Low SC, Chapman KE, Edwards CR, Seckl JR. ‘Liver-type’ 11 beta-hydroxysteroid dehydrogenase cDNA encodes reductase but not dehydrogenase activity in intact mammalian COS-7 cells. J Mol Endocrinol 13: 167–174, 1994. doi: 10.1677/jme.0.0130167. [DOI] [PubMed] [Google Scholar]
- 30.Mårin P, Darin N, Amemiya T, Andersson B, Jern S, Björntorp P. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism 41: 882–886, 1992. doi: 10.1016/0026-0495(92)90171-6. [DOI] [PubMed] [Google Scholar]
- 31.Melanson EL, Gavin KM, Shea KL, Wolfe P, Wierman ME, Schwartz RS, Kohrt WM. Regulation of energy expenditure by estradiol in premenopausal women. J Appl Physiol (1985) 119: 975–981, 2015. doi: 10.1152/japplphysiol.00473.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olbrich D, Dittmar M. The cortisol awakening response is related with PERIOD1 clock gene expression in older women. Exp Gerontol 47: 527–533, 2012. doi: 10.1016/j.exger.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC. A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology 30: 80–91, 2005. doi: 10.1016/j.psyneuen.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Pasquali R, Cantobelli S, Casimirri F, Capelli M, Bortoluzzi L, Flamia R, Labate AM, Barbara L. The hypothalamic-pituitary-adrenal axis in obese women with different patterns of body fat distribution. J Clin Endocrinol Metab 77: 341–346, 1993. doi: 10.1210/jcem.77.2.8393881. [DOI] [PubMed] [Google Scholar]
- 35.Pasquali R, Vicennati V. Activity of the hypothalamic-pituitary-adrenal axis in different obesity phenotypes. Int J Obes Relat Metab Disord 24, Suppl 2: S47–S49, 2000. doi: 10.1038/sj.ijo.0801277. [DOI] [PubMed] [Google Scholar]
- 36.Perz JM. Development of the menopause symptom list: a factor analytic study of menopause associated symptoms. Women Health 25: 53–69, 1997. doi: 10.1300/J013v25n01_04. [DOI] [PubMed] [Google Scholar]
- 37.Qureshi AC, Bahri A, Breen LA, Barnes SC, Powrie JK, Thomas SM, Carroll PV. The influence of the route of oestrogen administration on serum levels of cortisol-binding globulin and total cortisol. Clin Endocrinol (Oxf) 66: 632–635, 2007. doi: 10.1111/j.1365-2265.2007.02784.x. [DOI] [PubMed] [Google Scholar]
- 38.Richter SD, Schürmeyer TH, Schedlowski M, Hädicke A, Tewes U, Schmidt RE, Wagner TO. Time kinetics of the endocrine response to acute psychological stress. J Clin Endocrinol Metab 81: 1956–1960, 1996. doi: 10.1210/jcem.81.5.8626864. [DOI] [PubMed] [Google Scholar]
- 39.Roca CA, Schmidt PJ, Altemus M, Deuster P, Danaceau MA, Putnam K, Rubinow DR. Differential menstrual cycle regulation of hypothalamic-pituitary-adrenal axis in women with premenstrual syndrome and controls. J Clin Endocrinol Metab 88: 3057–3063, 2003. doi: 10.1210/jc.2002-021570. [DOI] [PubMed] [Google Scholar]
- 40.Seeman TE, Singer B, Wilkinson CW, McEwen B. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology 26: 225–240, 2001. doi: 10.1016/S0306-4530(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 41.Serra GB, Panetta V, Colosimo M, Romanini C, Lafuenti GB, Garcea N, Votano S, Agatensi L. Efficacy of leuprorelin acetate depot in symptomatic fibromatous uteri: the Italian Multicentre Trial. Clin Ther 14, Suppl A: 57–73, 1992. [PubMed] [Google Scholar]
- 42.Sharma M, Palacios-Bois J, Schwartz G, Iskandar H, Thakur M, Quirion R, Nair NP. Circadian rhythms of melatonin and cortisol in aging. Biol Psychiatry 25: 305–319, 1989. doi: 10.1016/0006-3223(89)90178-9. [DOI] [PubMed] [Google Scholar]
- 43.Shea KL, Gavin KM, Melanson EL, Gibbons E, Stavros A, Wolfe P, Kittelson JM, Vondracek SF, Schwartz RS, Wierman ME, Kohrt WM. Body composition and bone mineral density after ovarian hormone suppression with or without estradiol treatment. Menopause 22: 1045–1052, 2015. doi: 10.1097/GME.0000000000000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slayden SM, Crabbe L, Bae S, Potter HD, Azziz R, Parker CR Jr. The effect of 17 beta-estradiol on adrenocortical sensitivity, responsiveness, and steroidogenesis in postmenopausal women. J Clin Endocrinol Metab 83: 519–524, 1998. doi: 10.1210/jcem.83.2.4562. [DOI] [PubMed] [Google Scholar]
- 45.Tchernof A, Desmeules A, Richard C, Laberge P, Daris M, Mailloux J, Rhéaume C, Dupont P. Ovarian hormone status and abdominal visceral adipose tissue metabolism. J Clin Endocrinol Metab 89: 3425–3430, 2004. doi: 10.1210/jc.2003-031561. [DOI] [PubMed] [Google Scholar]
- 46.Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab 81: 2468–2473, 1996. doi: 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- 47.Ward AM, Syddall HE, Wood PJ, Dennison EM, Phillips DI. Central hypothalamic-pituitary-adrenal activity and the metabolic syndrome: Studies using the corticotrophin-releasing hormone test. Metabolism 53: 720–726, 2004. doi: 10.1016/j.metabol.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 48.Wolfram M, Bellingrath S, Kudielka BM. The cortisol awakening response (CAR) across the female menstrual cycle. Psychoneuroendocrinology 36: 905–912, 2011. doi: 10.1016/j.psyneuen.2010.12.006. [DOI] [PubMed] [Google Scholar]