Abstract
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a canonical regulator of cytoprotective gene expression, but evidence of its cross talk with other pathways, including metabolic ones, is ever increasing. Pharmacologic or systemic genetic activation of the Nrf2 pathway partially protects from obesity in mice and ameliorates fasting hyperglycemia in mice and humans. However, systemic Nrf2 deletion also protects from diet-induced obesity and insulin resistance in mice. To further investigate the effect of the disruption of Nrf2 on obesity in a tissue-specific manner, we focused on adipocytes and hepatocytes with targeted deletion of Nrf2. To this end, mice with cell-specific deletion of Nrf2 in adipocytes (ANKO) or hepatocytes (HeNKO) were fed a high-fat diet (HFD) for 6 mo and showed similar increases in body weight and body fat content. ANKO mice showed a partially deteriorated glucose tolerance, higher fasting glucose levels, and higher levels of cholesterol and nonesterified fatty acids compared with their Control counterparts. The HeNKO mice, though, had lower insulin levels and trended toward improved insulin sensitivity without having any difference in liver triglyceride accumulation. This study compared for the first time two conditional Nrf2 knockout models in adipocytes and in hepatocytes during HFD-induced obesity. None of these models could completely recapitulate the unexpected protection against obesity observed in the whole body Nrf2 knockout mice, but this study points out the differential roles that Nrf2 may play, beyond cytoprotection, in different target tissues and rather suggests systemic activation of the Nrf2 pathway as an effective means of prevention and treatment of obesity and type 2 diabetes.
Keywords: diabetes, fat, Keap1, liver, Nrf2
INTRODUCTION
Obesity and type 2 diabetes are on the rise in Westernized societies (24), and research on effective interventions to prevent them or treat their complications is warranted. Because increased oxidative stress in tissues is associated with obesity and decreased insulin sensitivity in humans and rodents (10, 35, 38), it was deemed reasonable to investigate the manipulation of cytoprotective/antioxidant pathways as an effective means to mitigate insulin resistance and its detrimental effects. In this context, the Kelch-like ECH-associated protein-1 (Keap1)-nuclear factor erythroid 2-related factor 2 (Nrf2) pathway, which regulates the expression of a series of antioxidant and cytoprotective genes (15), has been investigated as a potential target to combat obesity and type 2 diabetes by genetic and pharmacologic manipulation.
Nrf2 is a transcription factor of the cap’n’collar family and under basal nonstressed conditions is bound mainly by Keap1 in the cytoplasm, where it is marked for proteasomal degradation by polyubiquitination. Keap1 is a cysteine-rich protein, and upon exposure to oxidative or electrophilic stress some of the reactive Keap1 cysteines are modified (43) and the function of the Cul3-ubiquitin ligase-Keap1-Nrf2 complex is disrupted. Thus, nascent Nrf2 avoids proteasomal degradation, accumulates in the nucleus, and binds to antioxidant response element sequences in the regulatory regions of its target genes. Manipulation of the Keap1-Nrf2 pathway in mice has been achieved by deleting the Nrf2 or the Keap1 genes. Whereas Nrf2 knockout mice are viable (13) and have been used in numerous disease model systems, Keap1 knockout mice die postnatally because of esophageal hyperkeratosis and consequent malnutrition (44). However, knockdown mice that express less Keap1 (Keap1 hypomorphic) were developed (37) and are a viable and useful genetic model of whole body activation of the Nrf2 pathway.
The use of Keap1 hypomorphic mice in the setting of high-fat diet (HFD)-induced obesity or in genetic models of obesity (db/db mice) has clearly shown that activation of the Nrf2 pathway can partially rescue mice from the development of obesity and type 2 diabetes (39). Some of the mechanisms that underlie this protective effect of Nrf2 against obesity and insulin resistance include protection of pancreatic insulin-producing β-cells (47), repression of hepatic gluconeogenesis and lipogenesis (34), and reduction of muscle glycogen levels (40). It is then evident that this protective role of Nrf2 can be attributed not only to its direct cytoprotective effects but also to its regulation of other potential antioxidant response element genes that regulate metabolic pathways. Moreover, pharmacologic activation of Nrf2 via agents, such as triterpenoids or isothiocyanates, that modify the reactive cysteines of Keap1 have also been shown to be protective against HFD-induced obesity in mice (22, 30) and to reduce hepatic glucose production in humans (1). However, unexpectedly, the exposure of whole body Nrf2 knockout mice to HFD regimens also led to an ameliorated diabetic phenotype compared with wild-type counterparts (5, 19) even though they are more prone to steatohepatitis, pointing to potential cross talk of Nrf2 with other pathways that regulate metabolism [reviewed by Chartoumpekis and Kensler (4)].
Although the activation of the Nrf2 pathway appears to be an appealing strategy to prevent HFD-induced obesity, the effect of genetic deletion of Nrf2 in obesity and diabetes requires further investigation as it gives rise to an ameliorated metabolic phenotype in the case of the whole body Nrf2 knockout mouse. This highlights the need to understand the specific effect of Nrf2 in individual metabolic organs. To this end, a genetic approach with conditional deletion of Nrf2 in the tissue of interest is necessary to pinpoint the role of Nrf2 in specific cell types during HFD-induced obesity. Such an approach requires the use of Nrf2flox/flox mice combined with a mouse that expresses Cre recombinase under the control of a cell-specific gene promoter that is active in the tissue of interest. An initial study showed that deleting Nrf2 in adipocytes using fatty acid-binding protein-4 (Fabp4)-promoter Cre in the genetic background of ob/ob mice led to a worse metabolic phenotype (46). The Fabp4-promoter cassette, however, is not exclusive to adipocytes as it has been shown to influence other cell types including macrophages and muscle (21). Nrf2 has also been shown to affect adipocyte differentiation in vitro (32), and whole body deletion of Nrf2 can affect adipose tissue expansion (27) and enhance the expression of lipogenic and gluconeogenic genes in the liver (19). In the present study, a model of long-term (6 mo) HFD-induced obesity was employed wherein we focused on the effect of deletion of Nrf2 in adipocytes and in hepatocytes using the highly specific promoters of adiponectin (Adipoq) and albumin (Alb) for targeting adipocytes and hepatocytes, respectively. We found that Nrf2 disruption in adipocytes slightly worsened the glycemia and dyslipidemia whereas disruption in hepatocytes decreased hyperinsulinemia. Nevertheless, neither model completely phenocopied the systemically Nrf2-disrupted mice in the HFD-induced obesity setting.
MATERIALS AND METHODS
Mice and genotyping.
All mouse experiments were performed at the University of Pittsburgh and were approved by the Institutional Animal Care and Use Committee. The mice were housed at 22°C and 50% humidity with a 12:12-h light-dark cycle with ad libitum access to water and food. All the mice were in the albino C57BL/6J background [B6(Cg)-Tyrc-2J/J]. Nrf2flox/flox mice were described previously (16). The Adipoq-Cre mice (8) [B6;FVB-Tg(Adipoq-cre)1Evdr/J; stock no. 010803] and the Albumin-Cre mice (28) [B6.Cg-Tg(Alb-cre)21Mgn/J; stock no. 003574] were purchased from the Jackson Laboratory (Bar Harbor, ME). Nrf2flox/flox::Adipoq-Cre (ANKO) and Nrf2flox/flox::Albumin-Cre (HeNKO) mice were developed by consecutive mating of Nrf2flox/+ with Nrf2flox/+::Adipoq-Cre and Nrf2flox/+::Albumin-Cre, respectively. The Cre-negative mice (Nrf2flox/flox) were employed as the control genotype. The HeNKO mice have already been validated in our previous work (25), where we have shown that deletion of Nrf2 in hepatocytes led to decreased Nrf2-dependent gene expression and increased toxicity after acetaminophen administration. For purposes of validating the ANKO model, the adipose tissue genome conversion in the Nrf2 locus can be seen in Fig. 1, A and B, and the decrease in Nrf2 mRNA levels in epididymal white adipose tissue (eWAT) of ANKO mice and in livers of HeNKO mice can be seen in Fig. 1, C and D, respectively. The primers used for genotyping are listed in Table 1. The cycling conditions for primers N2, N3, and Ex-V-32 were 1) 95°C for 180 s, 2) 95°C for 30 s, 3) 67°C for 30 s, and 4) 72°C for 20 s; repeat conditions 2–4 thirty-four times. The cycling conditions for primers Cre1 and Cre2 were 1) 94°C for 60 s, 2) 94°C for 30 s, 3) 60°C for 30 s, and 4) 72°C for 30 s; repeat conditions 2–4 thirty times. The ExTaq DNA polymerase (product no. RR-01A; Takara Bio USA, Mountain View, CA) was used for these genotyping PCRs.
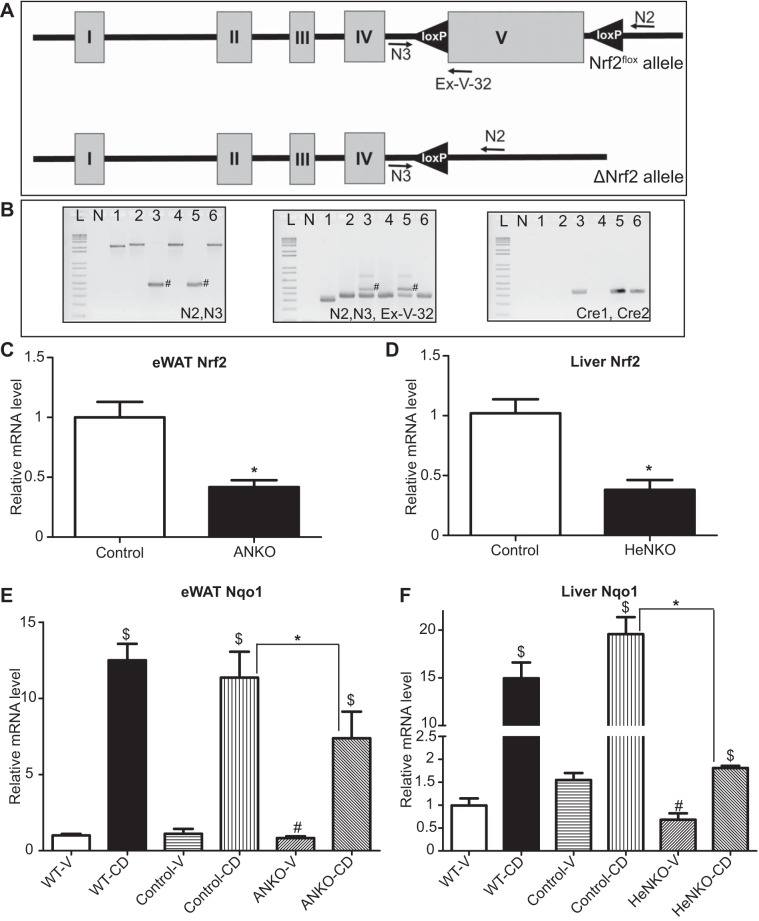
Fig. 1.
Validation of the tissue-specific nuclear factor erythroid 2-related factor 2 (Nrf2) knockout models. A: schematic representation of the Nrf2flox locus before and after tissue-specific exon V deletion. N2, N3, and Ex-V-32 represent the primers used for genotyping and detection of the recombined Nrf2flox allele. B: PCR-based Nrf2flox genotyping and detection of the recombined Nrf2flox allele using the primer pairs (N2, N3) for detection of the recombined allele and (N2, N3, Ex-V-32) for genotyping. Primer pair (Cre1, Cre2) was used for detection of Cre gene. Two percent agarose gel stained with ethidium bromide. #Band that corresponds to the presence of the recombined Nrf2flox allele. Lanes are as follows: L, 1-kb ladder; N, nontemplate control; 1, wild-type liver; 2, Nrf2flox/flox epididymal white adipose tissue (eWAT); 3, Nrf2flox/flox::Adipoq-Cre eWAT; 4, Nrf2flox/flox liver; 5, Nrf2flox/flox::Albumin-Cre liver; 6, Nrf2flox/flox::Adipoq-Cre liver. C: mRNA levels of Nrf2 in eWAT of Control and Nrf2flox/flox::Adipoq-Cre (ANKO) 2-mo-old male mice on regular diet; n = 4 per genotype. Data are means ± SE. *P < 0.05. D: mRNA levels of Nrf2 in liver of Control and Nrf2flox/flox::Albumin-Cre (HeNKO) 2-mo-old male mice on regular diet; n = 4 per genotype. Data are means ± SE. *P < 0.05. E: mRNA levels of NAD(P)H quinone oxidoreductase 1 (Nqo1) in eWAT of wild-type (WT), Control, and ANKO male mice on regular diet treated with vehicle (V) or 1[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole (CDDO-Im; CD); n = 4–7 per genotype per treatment. Data are means ± SE. *P < 0.05, #P < 0.05 compared with Control-V, $P < 0.05 compared with vehicle treatment of the same genotype. F: mRNA levels of Nqo1 in liver of wild-type, Control, and HeNKO male mice on regular diet treated with vehicle or CDDO-Im; n = 4–7 per genotype per treatment. Data are means ± SE. *P < 0.05, #P < 0.05 compared with Control-V, $P < 0.05 compared with vehicle treatment of the same genotype.
Table 1.
Primers used for genotyping
| Primer | Sequence |
|---|---|
| Cre1 | ACGTTCACCGGCATCAACGT |
| Cre2 | CTGCATTACCGGTCGATGCA |
| N2 | TACAGCAGGCATACCATTGTGG |
| N3 | TGAGAGCTTCCCAGACTCACTT |
| Ex-V-32 | CTGGGCTGGGAACAGCGGTAGTATCAGCCAGC |
For further validation of the mouse models used, mice were treated by gavage with a single dose of 1[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole (CDDO-Im; 30 μmol/kg) or vehicle (80% PBS 1X, 10% DMSO, and 10% Cremophor), a known Nrf2 pathway activator (48), and tissues were collected after 16 h. The mRNA levels of the prototypical Nrf2 target gene NAD(P)H quinone oxidoreductase 1 (Nqo1) were evaluated by quantitative real-time PCR and were found to be profoundly increased in wild-type and Nrf2flox/flox (Control) mice treated with CDDO-Im (cat. no. 4737; Tocris, Minneapolis, MN), and this increase was attenuated in the eWAT and livers of ANKO and HeNKO mice, respectively (Fig. 1, E and F). From the same figure we can conclude that the Nrf2flox/flox mice respond to CDDO-Im treatment similarly to the wild-type mice, and thus we have designated them as Control mice to both the Cre-positive genotypes (HeNKO and ANKO). The fact that induction of Nqo1 can be observed in the liver and adipose tissue of the HeNKO and ANKO mice, respectively, can be attributed to the presence of cell types other than hepatocytes or adipocytes in these tissues that still express normal levels of Nrf2 and can respond to the CDDO-Im treatment.
Two-month-old male mice were fed a standard diet (StD; 10% kcal fat) or HFD (60% kcal fat) for 170 days. Two weeks before the mice reached the age of 2 mo, they were switched from the regular diet of the facility (Prolab Isopro RMH 3000 5P76 irradiated diet; LabDiet, St. Louis, MO) to the StD (Fig. 2). The diets were purchased gamma-irradiated from Research Diets (D-12450B, StD; D-12492, HFD; New Brunswick, NJ). Then, 12 Control, 11 HeNKO, and 6 ANKO mice were put on the StD, and 12 Control, 10 HeNKO, and 9 ANKO mice were fed HFD.
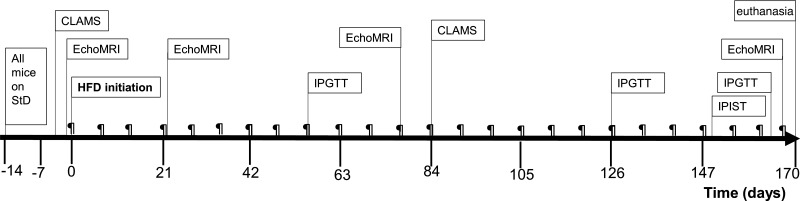
Fig. 2.
Schematic representation of the experimental design. CLAMS, Comprehensive Laboratory Animal Monitoring System for indirect calorimetry; HFD, high-fat diet (60% kcal fat); IPGTT, intraperitoneal glucose tolerance test; IPIST, intraperitoneal insulin sensitivity test; StD; standard diet (10% kcal fat); ¶, body weight measurement.
Body composition analysis and indirect calorimetry.
Body composition (fat mass and lean mass) was assessed using the EchoMRI system (EchoMRI, Houston, TX), which employs an NMR-MRI-based technology. The HFD-fed groups were measured using EchoMRI on day 0 (just before the switch from StD to HFD) and days 24, 77, and 170 on HFD. Metabolic rate and activity were assessed over 48 h using the Comprehensive Laboratory Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH) before switching the mice to HFD and after having been for 3 mo on HFD.
Glucose tolerance test, insulin sensitivity test, and homeostatic model assessment index calculations.
A glucose tolerance test was performed by intraperitoneal injection of a single dose of d-glucose (1 g/kg; Thermo Fisher Scientific, Pittsburgh, PA) after 16-h overnight fasting with free access to water. An insulin sensitivity test was performed by injecting mice intraperitoneally with 0.75 U/kg insulin (Humalog; Eli Lilly, Indianapolis, IN) after 4-h fasting with free access to water. Glucose was measured at the indicated time intervals after glucose or insulin injection, using a Precision Xtra glucose meter (Abbott Laboratories, Chicago, IL) in blood collected from the tail of the mouse.
Homeostatic model assessment (HOMA) is a mathematical model that mainly relies on human experimental data and allows for estimation of insulin resistance and β-cell function based on simultaneous fasting blood glucose and insulin values (45). Although this model has been validated for humans, it should be used with some caution in mice. The HOMA for insulin resistance (HOMA-IR) and HOMA for β-cell function (HOMA-%B) equations used in this paper are the following
where insulin is in microunits per milliliter and glucose is in milligrams per deciliter.
Measurement of serum metabolites.
Serum was prepared by allowing blood to clot at room temperature for 30 min. Then, blood was centrifuged at 2,000 g for 15 min at 4°C. Insulin was measured by enzyme-linked immunosorbent assay (ELISA) using kit 10-1247-01 by Mercodia (Uppsala, Sweden). Adiponectin and fibroblast growth factor 21 (Fgf21) were measured by ELISA using kits MRP-300 and MF-2100, respectively, by R&D Systems (Minneapolis, MN). Cholesterol and triglycerides were assessed by fluorometric (10007640) and colorimetric (10010303) kits, respectively, by Cayman Chemical (Ann Arbor, MI). Nonesterified fatty acids (NEFA) were measured using the NEFA-HR kit (Wako Chemicals, Osaka, Japan).
RNA preparation and quantitative real-time PCR.
Liver and adipose tissues were stored at −20°C in RNAlater (cat. no. AM-7021; Thermo Fisher Scientific). Total RNA was prepared by homogenizing the tissues in TRIzol reagent (cat. no. 15596018; Thermo Fisher Scientific) following the manufacturer’s instructions and by further purification by RNeasy kit (cat. no. 74104; Qiagen, Valencia, CA) including DNase digestion. Quantification of the RNA was performed by photometry at 260 nm using the NanoDrop One (Thermo Fisher Scientific), and the purity was estimated by the following ratios of absorbance: 260 nm/280 nm and 260 nm/230 nm. RNA integrity was assessed by agarose gel electrophoresis. cDNA was synthesized using the qScript system (Quanta BioSciences, Gaithersburg, MD). Real-time PCR was performed on an iCycler-MyiQ (Bio-Rad, Hercules, CA) using iQ SYBR Green Supermix (Bio-Rad) in triplicate 20-μl reactions. The PCR efficiency was calculated by standard curves, and the fold changes were calculated using the Pfaffl method (26). The specificity of the amplified product was ensured by melt curves. The primer sequences are shown in Table 2 and mostly are from PrimerBank unless custom designed (36). Relative quantities were normalized to reference genes; for liver, Gapdh was used as a reference gene, and for white adipose tissue the geometric means of the relative quantities of Actb, Tbp, and Gusb were used. The geNorm algorithm (42) was used to find the most appropriate (most stable) reference genes across our samples.
Table 2.
Primers used for quantitative real-time PCR
| Gene | Primer 1 | Primer 2 |
|---|---|---|
| Acacb | CCTTTGGCAACAAGCAAGGTA | AGTCGTACACATAGGTGGTCC |
| Acly | AAGAAGGAGGGGAAGCTGAT | TCGCATGTCTGGGTTGTTTA |
| Actb | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
| Arg1 | CTCCAAGCCAAAGTCCTTAGA | AGGAGCTGTCATTAGGGACATC |
| Cd68 | TGTCTGATCTTGCTAGGACCG | GAGAGTAACGGCCTTTTTGTGA |
| Csf1r | TGTCATCGAGCCTAGTGGC | CGGGAGATTCAGGGTCCAAG |
| Dgat2 | GCGCTACTTCCGAGACTACTT | GGGCCTTATGCCAGGAAACT |
| Emr1 | TGACTCACCTTGTGGTCCTAA | CTTCCCAGAATCCAGTCTTTCC |
| Fasn | GGAGGTGGTGATAGCCGGTAT | TGGGTAATCCATAGAGCCCAG |
| Fgf21 | CAAGACACTGAAGCCCACCT | CACCCAGGATTTGAATGACC |
| G6pase | CGACTCGCTATCTCCAAGTGA | GTTGAACCAGTCTCCGACCA |
| Gapdh | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
| Glut1 | CAGTTCGGCTATAACACTGGTG | GCCCCCGACAGAGAAGATG |
| Glut4 | GTGACTGGAACACTGGTCCTA | CCAGCCACGTTGCATTGTAG |
| Gpat1 | ACAGTTGGCACAATAGACGTTT | CCTTCCATTTCAGTGTTGCAGA |
| Gsta4 | TGATTGCCGTGGCTCCATTTA | CAACGAGAAAAGCCTCTCCGT |
| Gusb | GTGGTATGAACGGGAAGCAAT | AACTGCATAATAATGGGCACTGT |
| Il6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| Jun | CCTTCTACGACGATGCCCTC | GGTTCAAGGTCATGCTCTGTTT |
| Lipe | GATTTACGCACGATGACACAGT | ACCTGCAAAGACATTAGACAGC |
| Nqo1 | GGCATCCTGCGTTTCTGTG | GGTTTCCAGACGTTTCTTCCAT |
| Nrf2 | CCATTTACGGAGACCCACCGCCTG | CTCGTGTGAGATGAGCCTCTAAGCGG |
| P2rx5 | CTGCAGCTCACCATCCTGT | CACTCTGCAGGGAAGTGTCA |
| Pat2 | GTGCCAAGAAGCTGCAGAG | TGTTGCCTTTGACCAGATGA |
| Pepck | CTGCATAACGGTCTGGACTTC | CAGCAACTGCCCGTACTCC |
| Pgc1a | CCCTGCCATTGTTAAGACC | TGCTGCTGTTCCTGTTTTC |
| Plin1 | GGGACCTGTGAGTGCTTCC | GTATTGAAGAGCCGGGATCTTTT |
| Pnpla2 | CAACGCCACTCACATCTACGG | GGACACCTCAATAATGTTGGCAC |
| Prdm16 | CAGCACGGTGAAGCCATTC | GCGTGCATCCGCTTGTG |
| Tbp | AAGAGAGCCACGGACAACTG | TACTGAACTGCTGGTGGGTC |
| Tnfa | CATCTTCTCAAAATTATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC |
| Ucp1 | CACTCAGGATTGGCCTCTACG | GGGGTTTGATCCCATGCAGA |
Analysis of liver glycogen, fatty acid content, and triglycerides.
A fluorometric method was used to assess hepatic glycogen content following the manufacturer’s instructions (cat. no. 700480; Cayman Chemical). The same colorimetric method as in the serum was used to assess the liver triglyceride content following the manufacturer’s protocol and normalizing the triglyceride content to total protein levels. For the mass spectrometry-based analyses the following protocol was employed. Livers obtained upon dissection were snap-frozen and stored at −80°C. Sections of liver tissues (~50 mg) were thawed in ice and homogenized in a bullet blender (Next Advance, Averill Park, NY) for 4 min in 0.5 ml phosphate buffer (50 mM, pH 7.4). To analyze liver fatty acids, an aliquot of the homogenate was further diluted in the same buffer to 1 mg tissue/ml final concentration (500 µl) and spiked with 0.8 μg arachidonic acid-d8 and oleic acid-d17 and 40 μg 14:0-d27, 16:0-d31, and 18:0-d35. Lipids were extracted using the Bligh and Dyer procedure (2) based on a chloroform-methanol-water (2:2.2:2) mixture in the presence of 100 μl 10% butylated hydroxytoluene in methanol. The organic fraction was dried using a RapidVap system, resuspended in 0.85 ml of methanol-dichloromethane (8:1, vol/vol), and hydrolyzed by the addition of 0.15 ml 40% aqueous potassium hydroxide solution at 60°C for 30 min. The mixture was then acidified by addition of 100 μl phosphate buffer (0.5 M, pH 7.4) and 100 μl fuming hydrochloric acid. Extraction of fatty acids was performed by sequentially adding 2.5 ml hexane-isopropanol-formic acid (30:20:2, vol/vol/vol), vortexing, and adding 2 ml hexane. After vortexing and centrifugation, fatty acids were recovered in the upper layer, dried, and derivatized according to Li and Franke (18). Briefly, 200 μl of oxalyl chloride (2 M in dichloromethane) were added to the dried fatty acid extract, and the mixture was incubated at 65°C for 5 min and dried. The residue was resuspended in 150 μl of 1% 3-picolylamine in acetonitrile, incubated for 5 min at room temperature, dried under nitrogen, and resuspended in 1 ml methanol. Samples were further diluted (100X in methanol) and assessed by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry (HPLC-ESI-MS/MS) analysis.
To analyze liver triglycerides, an aliquot of homogenate was further diluted in 0.5 ml phosphate buffer (50 mM, pH 7.4) to 0.5 mg tissue/ml final concentration and spiked with 3.18 μg internal standard glyceryl triheptadecanoate. Triglycerides were extracted using the Bligh and Dyer procedure, as described above. The organic extract was dried, resuspended in 200 μl ethyl acetate, and diluted (40X in ethyl acetate) before HPLC-ESI-MS/MS analysis.
Chromatography.
Analysis of derivatized fatty acids was performed by online HPLC-ESI-MS/MS using an analytical C18 Luna column (2 × 100 mm, 5 μm; Phenomenex) at a 0.65 ml/min flow rate, with a gradient solvent system consisting of water containing 0.1% acetic acid (solvent A) and acetonitrile containing 0.1% acetic acid (solvent B). Samples were chromatographically resolved using a 30–90% solvent B linear gradient over 11 min, column washed at 100% solvent B for 2.5 min, and then reequilibrated for 1.5 min at initial conditions.
Analysis of triglycerides was performed by HPLC-ESI-MS/MS using an analytical C18 Luna column (2 × 150 mm, 5 μm; Phenomenex). The flow rate was set at 0.4 ml/min with a gradient solvent system consisting of acetonitrile (solvent A) and ethyl acetate (solvent B) and a postcolumn injection of 50 μl/min 10 mM ammonium acetate in acetonitrile/water (80:20, vol/vol). Samples were chromatographically resolved using a 35–90% solvent B linear gradient over 11 min, column washed at 90% solvent B for 2.5 min, and then reequilibrated for 1.5 min at initial conditions.
Mass spectrometry.
Derivatized fatty acids and triglycerides were detected using an API-4000 Q-trap triple quadrupole mass spectrometer (Applied Biosystems, San Jose, CA) equipped with an ESI source in positive ion mode. Derivatized fatty acids were analyzed with the following parameters: declustering potential, 60 V; entrance potential, 15 V; collision energy, 45 eV; collision cell exit potential, 7 V; desolvation temperature, 700°C. The following multiple reaction-monitoring (MRM) transitions were used to detect the most abundant derivatized fatty acids: docosahexaenoic acid (22:6, 419.3/109.0), arachidonic acid (20:4, 395.3/109), eicosatrienoic acid (20:3, 397.3/109.0), eicosadienoic acid (20:2, 399.3/109.0), eicosaenoic acid (20:1, 401.3/109.0), linolenic acid (18:3, 369.3/109.0), linoleic acid (18:2, 371.3/109.0), oleic acid (18:1, 373.3/109.0), stearic acid (18:0, 375.3/109.0), palmitic acid (16:0, 347.3/109.0), palmitoleic acid (16:1, 345.3/109.0), myristic acid (14:0, 319.3/109.0), and tetradecenoic acid (14:1, 317.3/109.0). Quantification of derivatized fatty acids was performed by stable isotopic dilution analysis using fatty acid calibration curves in the presence of the following labeled internal standards: arachidonic acid-d8 (MRM 403.3/109.0), oleic acid-d17 (MRM 390.3/109.0), 14:0-d27 (MRM 346.3/109.0), 16:0-d31 (MRM 378.3/109.0), and 18:0-d35 (MRM 410.3/109.0). Blank samples were extracted and background subtracted from samples during postprocessing analysis. Data are expressed as concentrations of fatty acids in hepatic tissue in nanomoles of fatty acid per gram of tissue.
Triglycerides were analyzed with the following parameters: declustering potential, 50 V; entrance potential, 10 V; collision energy, 35 eV; collision cell exit potential, 8 V; desolvation temperature, 500°C. Combinations of triglycerides from C42 to C54, containing 10:0, 12:0, 14:0, 14:1, 16:0, 16:1, 18:0, 18:1, 18:2, 18:3, and 20:0 fatty acids, were screened as ammonium adducts by calculated MRM transitions. The most abundant triglycerides were further characterized to establish the fatty acid composition. The triglyceride relative abundance was calculated by the peak area ratio (analyte/internal standard) normalized for milligrams of liver protein.
Liver and adipose tissue histology.
After dissection, livers and adipose tissues were immersed in 10% buffered formalin (Thermo Fisher Scientific), and after at least overnight incubation they were transferred to 70% ethanol. Hematoxylin-eosin staining, trichrome staining, and the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay (ApopTag peroxidase in situ apoptosis detection kit; Millipore, Billerica, MA) were performed in the Department of Pathology Development Laboratory (Pittsburgh, PA).
Statistical analysis.
Data are expressed as means ± SE unless stated otherwise. Student’s t-test and one-way or two-way ANOVA followed by Tukey’s test were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA). No subjects were excluded from our analyses. P < 0.05 was considered significant.
RESULTS
Hepatocyte- or adipocyte-specific deletion of Nrf2 did not affect gain in body weight, fat mass expansion, energy expenditure, or the respiratory exchange ratio in mice fed HFD.
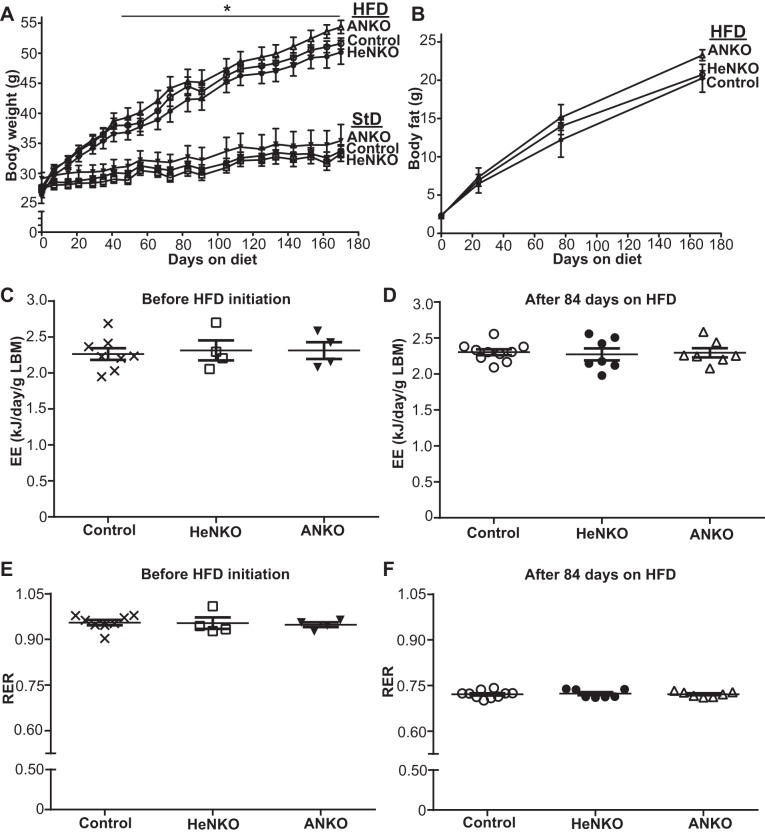
Mice with specific deletion of Nrf2 in adipocytes (Nrf2flox/flox::Adipoq-Cre, designated as ANKO; adipocyte-specific Nrf2 knockout) or in hepatocytes (Nrf2flox/flox::Albumin-Cre, designated as HeNKO; hepatocyte-specific Nrf2 knockout) were fed HFD following the timeline that is schematically described in Fig. 2. HeNKO and ANKO mice did not show differences on StD with respect to body weight (Fig. 3A), body fat composition (Fig. 3B, day 0), energy expenditure (Fig. 3C), or respiratory exchange ratio (Fig. 3E) compared with the Nrf2flox/flox (designated as Control) mice. HFD feeding led to a significant increase in the body weights in all three genotypes compared with the StD, but no difference was observed among the genotypes on HFD (Fig. 3A). Similarly, body fat mass significantly increased in all three genotypes with HFD feeding, but all showed similar increases in their fat mass (Fig. 3B). Additionally, indirect calorimetry after 3 mo on HFD did not show any difference in the energy expenditure (Fig. 3D) or respiratory exchange ratio (Fig. 3F).
Fig. 3.
Body weights, body fat content, and energy expenditure in the tissue-specific nuclear factor erythroid 2-related factor 2 (Nrf2) knockout mice. Α: body weights of Control (Nrf2flox/flox), HeNKO (Nrf2flox/flox::Albumin-Cre), and ANKO (Nrf2flox/flox::Adipoq-Cre) mice fed either a standard (StD) or a high-fat diet (HFD) are plotted against time; n = 6–12 mice per diet type per genotype. Symbols: ×, Control on StD; □, HeNKO on StD; ▼, ANKO on StD; ○, Control on HFD; ●, HeNKO on HFD; △, ANKO on HFD. *P < 0.05 HFD-fed mice compared with the StD-fed mice after day 41 onward. B: body fat content assessed by EchoMRI of Control, HeNKO, and ANKO mice fed HFD is plotted against time; n = 9–12 mice per genotype. C and D: lean body mass (LBM)-normalized energy expenditure (EE) values in Control, HeNKO, and ANKO mice before HFD initiation and after 84 days on HFD; n = 8 for Control, n = 4 for HeNKO, and n = 4 for ANKO before HFD and n = 10 for Control, n = 7 for HeNKO, and n = 7 for ANKO after 84 days on HFD. E and F: respiratory exchange ratio (RER) in Control, HeNKO, and ANKO mice before HFD initiation and after 84 days on HFD; n = 8 for Control, n = 4 for HeNKO, and n = 4 for ANKO before HFD and n = 10 for Control, n = 7 for HeNKO, and n = 7 for ANKO after 84 days on HFD. Data are means ± SE.
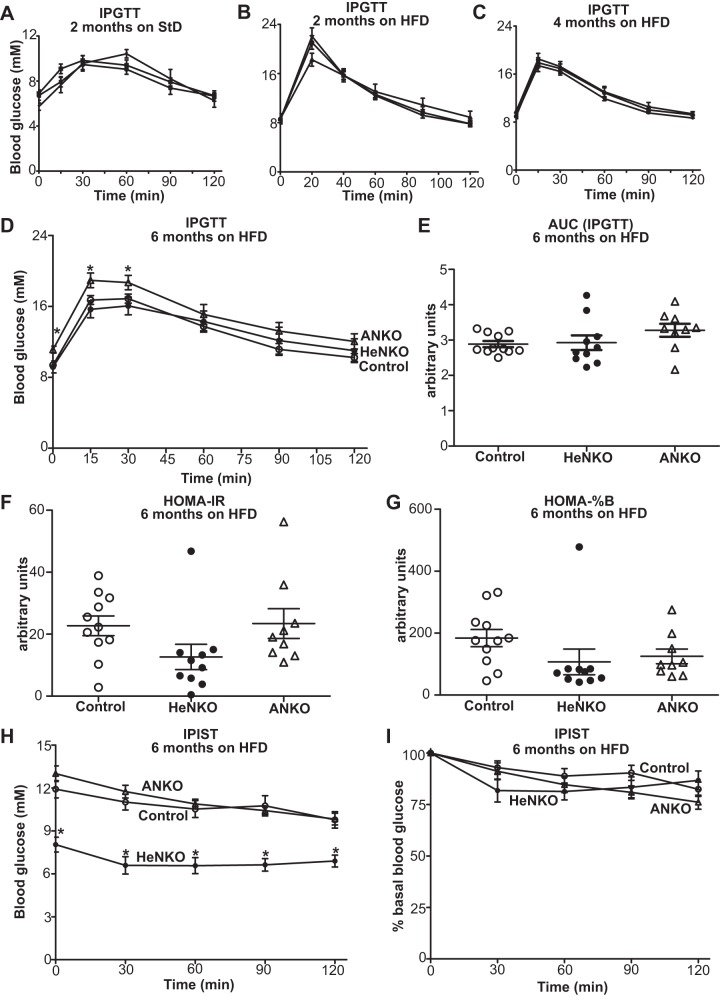
Deletion of Nrf2 in adipocytes deteriorated glucose tolerance after a 6-mo HFD regimen whereas hepatocyte-specific Nrf2 deletion led to lower blood glucose levels after 4-h fasting and tended to ameliorate insulin sensitivity.
There was no difference in the overnight fasting blood glucose levels and glucose tolerance among the Control, HeNKO, and ANKO mice on StD (Fig. 4A) and 2 mo (Fig. 4B) and 4 mo on HFD (Fig. 4C). Only after 6 mo on HFD did the ANKO mice show increased starvation glucose levels (Fig. 4D, time 0) and tend to have a more impaired glucose tolerance (Fig. 4, D and E). HOMA-IR (Fig. 4F) and HOMA-%B (Fig. 4G) displayed a clear trend for the HeNKO mice to have improved insulin sensitivity and lack of requirement for β-cell overfunction to maintain their fasting glucose levels. Interestingly, glucose levels at the beginning of the insulin sensitivity test after the 4-h fast were lower in the HeNKO mice and their response to insulin was better as seen by the reduction in absolute glucose values (Fig. 4H). The most pronounced rate of decline was observed in the first 30 min after insulin injection in the percentage of basal glucose reduction (Fig. 4I).
Fig. 4.
Glucose tolerance and insulin sensitivity in the tissue-specific nuclear factor erythroid 2-related factor 2 (Nrf2) knockout mice. A–C: intraperitoneal glucose tolerance test (IPGTT) in Control, Nrf2flox/flox::Albumin-Cre (HeNKO), and Nrf2flox/flox::Adipoq-Cre (ANKO) mice after 2 mo on standard diet (StD, A), 2 mo on high-fat diet (HFD, B), and 4 mo on HFD (C); n = 6–12 mice per genotype. D: IPGTT in Control, HeNKO, and ANKO mice after 6 mo on HFD; n = 9–11 per genotype. *P < 0.05 ANKO vs. Control. E: area under the curve (AUC) calculations for the IPGTT shown in D. F: homeostatic model assessment (HOMA) index for insulin resistance (HOMA-IR) in Control, HeNKO, and ANKO mice after 6 mo on HFD; n = 9–11 per genotype. G: HOMA index for β-cell function (HOMA-%B) in Control, HeNKO, and ANKO mice after 6 mo on HFD; n = 9–11 per genotype. H: intraperitoneal insulin sensitivity test (IPIST) in Control, HeNKO, and ANKO mice after 6 mo on HFD; n = 9–11 per genotype. *P < 0.05 HeNKO vs. Control. I: IPIST as in H with the glucose values plotted as percentage of basal (time 0) glucose; n = 9–11 per genotype. Data are means ± SE.
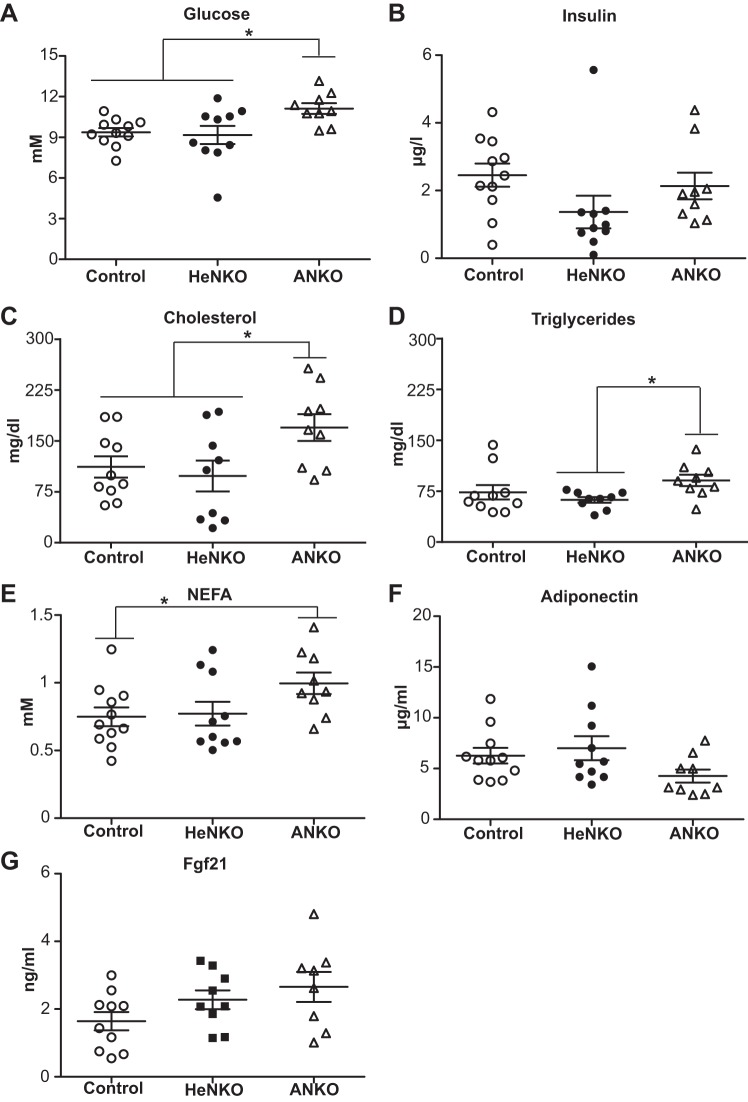
ANKO mice showed increased serum glucose levels and a deteriorated lipid profile whereas HeNKO mice had lower insulin levels consistent with their improved insulin sensitivity after 6 mo on HFD.
As was evident from the glucose tolerance test, ANKO mice had higher overnight fasting glucose levels (Fig. 5A) while their insulin levels were comparable to those of Control mice. The HeNKO mice had a clear trend for lower insulin levels (Fig. 5B), which is indicative of greater insulin sensitivity compared with Control and ANKO mice. The serum lipid profile of ANKO mice appeared to be worse as evident by a statistically significant increase in cholesterol (Fig. 5C) and NEFA (Fig. 5E) as well as a trend for higher triglycerides (Fig. 5D). Circulating adiponectin (Fig. 5F) and Fgf21 (Fig. 5G) levels were similar among all three genotypes.
Fig. 5.
A–G: serum metabolic parameters of Control, Nrf2flox/flox::Albumin-Cre (HeNKO), and Nrf2flox/flox::Adipoq-Cre (ANKO) mice after 6 mo on high-fat diet; n = 9–11 per genotype. Data are means ± SE. Glucose levels in A represent the glucose levels at time 0 in Fig. 4D. Fgf21, fibroblast growth factor 21; NEFA, nonesterified fatty acids. *P < 0.05.
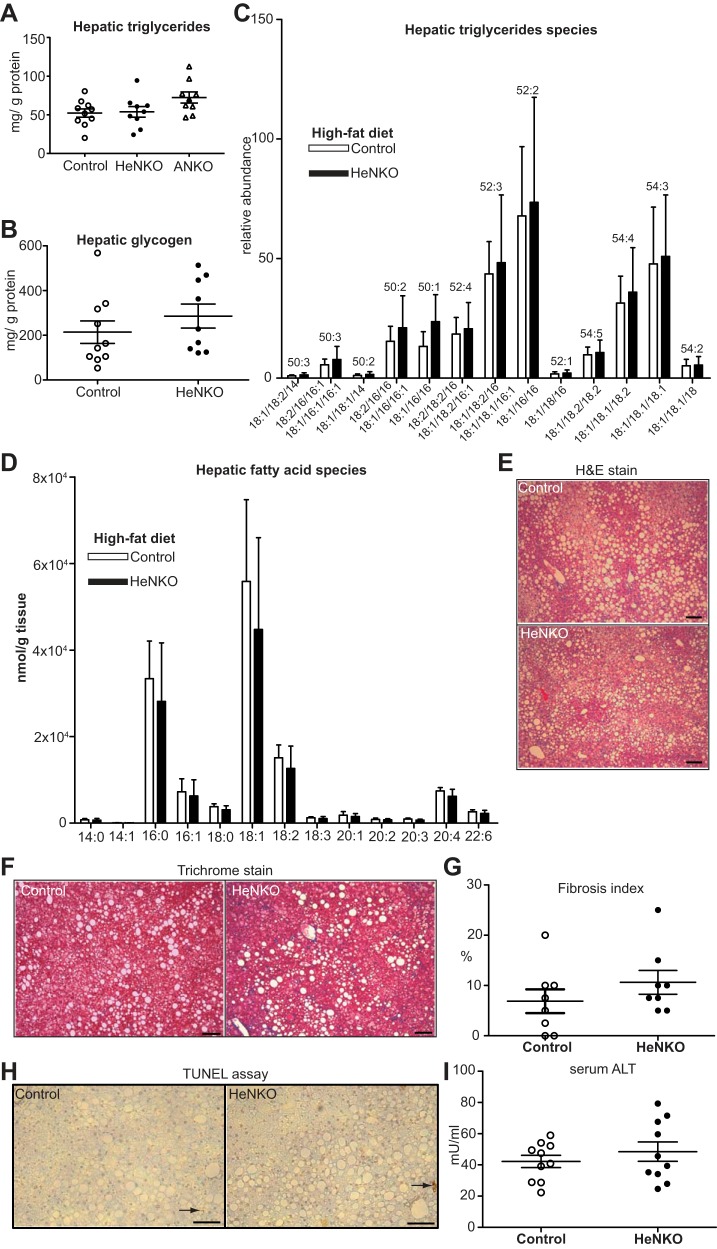
HeNKO mice showed levels of hepatic glycogen and liver steatosis similar to those of Control mice and tended to have increased liver fibrosis, but they showed no difference in apoptosis or serum alanine transaminase levels.
Driven by previous observations that whole body Nrf2 knockout mice fed HFD (19) showed increased liver steatosis and fibrosis, the livers of HeNKO mice were analyzed accordingly. No difference was detected in the total amount of liver triglycerides with the ANKO mice showing a trend toward increased levels (Fig. 6A). The hepatic glycogen levels showed no difference between Control and HeNKO mice either (Fig. 6B). HPLC-MS-based analysis of hepatic triglycerides and fatty acid species showed no difference between the Control and HeNKO mice (Fig. 6, C and D). Hematoxylin-eosin-stained liver sections revealed the presence of macrovesicular and microvesicular steatosis in both genotypes but with no major differences between them (Fig. 6E). To better assess the presence of liver fibrosis, the liver sections were stained with trichrome stain (Fig. 6F). A pathological evaluation did not show a significant difference in the degree of liver fibrosis between the Control and the HeNKO mice after HFD (Fig. 6G), but there was a modest trend for increased liver fibrosis in the HeNKO mice. The TUNEL assay in the liver sections did not show a difference in apoptosis levels between the two genotypes (Fig. 6H). Serum alanine transaminase levels, a marker of liver damage, also did not differ significantly (Fig. 6I).
Fig. 6.
Liver glycogen, triglyceride contents, and histology in the hepatocyte-specific nuclear factor erythroid 2-related factor 2 (Nrf2) knockout mice on high-fat diet (HFD). A: hepatic triglyceride levels normalized to total protein levels in Control, Nrf2flox/flox::Albumin-Cre (HeNKO), and Nrf2flox/flox::Adipoq-Cre (ANKO) mice after 6 mo on HFD; n = 9–10 per genotype. Data are means ± SE. B: liver glycogen content normalized to total protein levels in Control and HeNKO mice; n = 9–10 per genotype. Data are means ± SE. C: hepatic triglyceride species measured by HPLC-mass spectrometry in Control and HeNKO mice after 6 mo on HFD; n = 8 per genotype. Data are means (SD). D: changes of fatty acid content in liver of Control and HeNKO mice after 6 mo on HFD determined by HPLC-electrospray ionization-tandem mass spectrometry; n = 8 per genotype. Data are means (SD). E: representative hematoxylin-eosin (H&E) stain of liver sections of Control and HeNKO mice after 6 mo on HFD. Scale bar: 100 μm. F: representative trichrome stain of liver sections of Control and HeNKO mice after 6 mo on HFD. Scale bar: 100 μm. G: relative fibrosis index (%) as pathologically assessed by trichrome-stained liver sections in Control and HeNKO mice after 6 mo on HFD; n = 8 per genotype. Data are means ± SE. H: representative images of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay on liver sections of Control and HeNKO mice after 6 mo on HFD. Scale bar: 100 μm. Arrows indicate positive staining. I: serum alanine transaminase (ALT) concentration in serum of Control and HeNKO mice after 6 mo on HFD; n = 8 per genotype. Data are means ± SE.
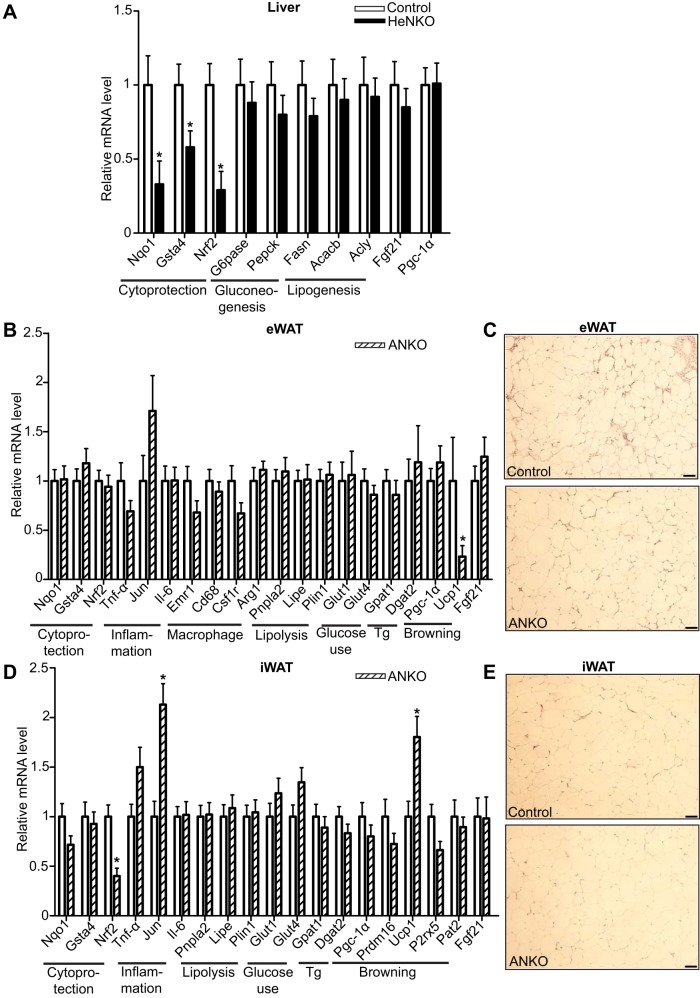
Targeted gene expression profiling in liver and white adipose tissue in HeNKO and ANKO mice, respectively, revealed the expected trend for reduced antioxidant response compared with Control mice on HFD but not a significant difference in inflammation and glucose and lipid metabolism-related markers.
Nqo1 transcript levels were reduced almost threefold in the livers of HeNKO mice (Fig. 7A) as expected since Nqo1 is considered to be a prototypical Nrf2 target gene (23). Similarly, transcript levels of glutathione S-transferase-α4 (Gsta4), a Nrf2-regulated Gst (3), were ~50% lower in the HFD-fed HeNKO mice compared with Control mice fed the same diet (Fig. 7A). As expected, Nrf2 mRNA levels were also decreased threefold in the livers of HeNKO mice that lack Nrf2 specifically in hepatocytes. However, there was no observed difference in hepatic mRNA expression of the key gluconeogenic enzymes phosphoenolpyruvate carboxykinase (Pepck) and glucose-6-phosphatase (G6pase; Fig. 7A). Lipogenic enzymes, such as fatty acid synthase (Fasn), acetyl-CoA carboxylase-β (Acacb), and ATP citrate lyase (Acly), showed similar levels of expression in Control and HeNKO mice (Fig. 7A). Fgf21 and peroxisome proliferator-activated receptor-γ (Pparγ) coactivator-1α (Pgc-1α), which have been shown to be expressed at higher levels in Nrf2 knockout mice (5), showed no difference in the mRNA levels in the livers of HeNKO mice.
Fig. 7.
Gene expression analysis of liver and white adipose tissue in hepatocyte- and adipocyte-specific nuclear factor erythroid 2-related factor 2 (Nrf2) knockout mice, respectively. A, B, and D: mRNA levels of genes assessed by quantitative real-time PCR in livers of Control and Nrf2flox/flox::Albumin-Cre (HeNKO) mice (A) and in epididymal white adipose tissue (eWAT, B) and inguinal white adipose tissue (iWAT, D) in Control and Nrf2flox/flox::Adipoq-Cre (ANKO) mice after 6 mo on HFD; n = 11 for Control, n = 10 for HeNKO, and n = 9 for ANKO. Data are means ± SE. *P < 0.05. Tg, triglyceride synthesis. C and E: representative hematoxylin-eosin-stained sections (from a total of 6 checked per genotype) of eWAT (C) and iWAT (E) of Control and ANKO mice after 6 mo on HFD. Scale bar: 50 μm.
In the epididymal and inguinal white adipose tissues (eWAT and iWAT, respectively) of ANKO mice on HFD, it was not possible to detect a significant decrease in Nqo1 or Gsta4 transcript levels (Fig. 7, B and D), although there was a trend for lower Nqo1 levels in iWAT of ANKO mice. Nrf2 mRNA levels were decreased almost 50% in the iWAT of ANKO mice (Fig. 7D) compared with Control mice whereas no such difference was detectable in the eWAT of HFD-fed mice (Fig. 7B). Inflammation markers tumor necrosis factor-α (Tnf-α) and interleukin-6 (Il-6) did not differ between the two genotypes in either of the white adipose tissue depots, but Jun showed significantly increased levels in iWAT (Fig. 7, B and D). The macrophage markers Egf-like module-containing, mucin-like, hormone receptor-like 1 (Emr1), Cd68, and colony-stimulating factor 1 receptor (Csf1r) showed similar expression in eWAT of ANKO mice (Fig. 7B) indicating no significant difference in macrophage infiltration of eWAT. Arginase 1 (Arg1), a marker of the M2 anti-inflammatory subset of macrophages, was also expressed similarly in the eWAT of ANKO and Control mice (Fig. 7B). Hematoxylin-eosin-stained sections of eWAT and iWAT of both genotypes did not reveal any difference in adipocyte size (Fig. 7, C and E). The recently described, highly specific beige and brown adipocyte markers purinergic receptor P2X ligand-gated ion channel 5 (P2rx5) and proton assistant amino acid transporter-2 (Pat2; 41) were not differentially expressed in iWAT of ANKO versus Control mice (Fig. 7D). Similarly, potential regulators of the browning of white adipose tissue such as Pgc-1α and PR/SET domain 16 (Prdm16) showed similar expression levels. Interestingly, uncoupling protein-1 (Ucp1) expression increased significantly in iWAT of ANKO mice (roughly 75% higher compared with Control, Fig. 7D) while it decreased ~4-fold in eWAT, where its basal levels were borderline detectable. Finally, expression of genes involved in lipolysis, glucose uptake, triglyceride synthesis, and Fgf21 mRNA levels showed no difference both in eWAT and iWAT (Fig. 7, B and D).
DISCUSSION
In this study, we demonstrated that deletion of Nrf2 in adipocytes led to a worsened diabetic phenotype whereas deletion of Nrf2 in hepatocytes modestly reduced insulinemia after HFD-induced obesity even though hepatic lipid accumulation remained unaffected. The ameliorated glucose tolerance and the increased insulin sensitivity that have been reported and reproduced multiple times in whole body Nrf2 knockout mice after long-term HFD feeding could not be completely recapitulated by either the adipocyte- or hepatocyte-specific Nrf2 knockout mouse models. Feeding diets that provided 39.7% (50), 40% (27), 45% (19), or 60% (5, 20) kcal fat to the whole body Nrf2 knockout mice rendered them less obese and more glucose tolerant than their wild-type counterparts. These results, although consistent across studies, were somewhat surprising because the absence of Nrf2 was initially expected to exacerbate the diabetic phenotype given the largely protective role played by Nrf2 in various diseases, such as obesity, characterized by chronic oxidative stress. The hypothesized mechanism for this outcome was thought to be driven by the lowered expression of antioxidant and cytoprotective enzymes that would lead to enhancement of the detrimental effects of HFD-induced oxidative stress in peripheral tissues (muscle, adipose tissue, liver, and pancreas) as well as the brain. However, these observations led investigators to question the original hypothesis and assess the possibility of cross talk of Nrf2 with other pathways that may result in this differential metabolic response.
Pparγ was described to be regulated by Nrf2 in adipocytes (27). This action could affect the increase in adipose tissue mass during HFD-induced obesity. Nevertheless, the regulation of adipogenesis by Nrf2 appears to be more complicated as Nrf2 can induce aryl hydrocarbon receptor, which can negatively regulate Pparγ expression and adipocyte differentiation (32). Fgf21, which is a peptide hormone that regulates energy homeostasis (9), was expressed at higher levels in liver and adipose tissue of HFD-fed Nrf2 knockout mice and had increased circulating levels in HFD-fed global Nrf2 knockout mice. It was speculated that Fgf21 could be partially responsible for the ameliorated diabetic phenotype of these mice (5). This increase in Fgf21 levels was also observed in another study (11), in which it was shown that genetic or pharmacologic Nrf2 pathway activation can lead to such an effect, making the regulation of Fgf21 by Nrf2 multifaceted. These results, along with cell culture-based studies that showed that Nrf2 can regulate CCAAT/enhancer-binding protein-β (12) and that Nrf2 transcriptional activity is diminished during adipocyte differentiation (6), instigated interest in the specific role of Nrf2 in adipocytes in vivo.
In our study, the Adipoq-Cre mouse model was used to target specifically the adipocytes in mice of the Nrf2flox/flox genotype. The Adipoq-Cre mouse has been proven to be the most specific adipocyte Cre model (21) compared with not only the more popular Fabp4-Cre model but also the Resistin-Cre model. The Fabp4-Cre model, which has been used in combination with other strains of Nrf2flox/flox mice in two studies (46, 49), significantly targets brain, muscle, and macrophages besides targeting the adipose tissues (21). By using the Nrf2flox/flox::Adipoq-Cre mice there was no detectable difference in adipose tissue expansion with the StD or at any time point during HFD feeding (Fig. 3B). Thus, the reduced fat mass compared with wild-type mice that the HFD-fed whole body Nrf2 knockout mice exhibited in previous studies (19, 27) cannot be attributed to an adipocyte-specific effect of Nrf2 but is likely related to a systemic effect such as increased energy expenditure (19) or increased circulating Fgf21 levels (5). In the present study, by conditionally deleting Nrf2 in adipocytes, energy expenditure (Fig. 3, C and D) and Fgf21 serum (Fig. 5G) and mRNA levels in adipocytes (Fig. 7, B and D) were not altered.
Nevertheless, it should be noted that Adipoq expression can be detected very late in the gestational period and mostly at postnatal day 1. Hence, the adipocyte-specific Nrf2 deletion driven by the Adipoq promoter can only occur during these days such that we may miss the effect of Nrf2 deletion in earlier developmental stages of adipose tissue (29).
The adipocyte-specific deletion of Nrf2 in ob/ob mice, a genetic model of obesity, employing the Fabp4 promoter (Fabp4-Cre) led to a worse diabetic phenotype as evidenced by decreased glucose tolerance and higher serum triglyceride levels (46). Even though this mouse model lacks Nrf2 not only in adipocytes but also in other tissues (macrophages and brain) as described above, the resulting phenotype appears to be similar to that seen in our study with the ANKO mice on HFD. Whereas a decrease in the weight of white adipose tissue in the ob/ob mice lacking Nrf2 in adipocytes was observed by Xue et al. (46), no difference in fat mass was detected with EchoMRI in our study. However, this difference between these two models may be due to the fact that weighing of adipose tissues is not a very sensitive and precise method to assess total fat mass compared with EchoMRI. Also, the mechanisms of obesity are different in these two models (ob/ob mice, hyperphagia; HFD-induced obesity, increased fat content in the diet). The converging point, though, of that study (46) and our present work is that the deletion of Nrf2 in adipocytes led to a worsened systemic metabolic phenotype as evidenced by increased circulating glucose (Fig. 5A), cholesterol (Fig. 5C), and NEFA levels (Fig. 5E) and the trend for higher serum triglycerides (Fig. 5D). The exact mechanism underlying these effects is not clear.
To identify potential mediators of the worsened metabolic phenotype, quantitative PCR was employed to assess the expression of genes in white adipose tissue of ANKO mice. The expression of genes involved in cytoprotection trended lower in the ANKO mice on HFD; Nrf2 levels were reduced by >50%, as expected in the iWAT, but this reduction could not be detected in the eWAT (Fig. 7, B and D). As evident in Fig. 1C, Nrf2 mRNA levels were indeed lower in the eWAT of ANKO mice fed a regular diet. However, upon feeding HFD, this difference between the two genotypes was abrogated, potentially because of the induction of Nrf2 mRNA in eWAT by the long-term HFD feeding, not only in adipocytes but also in other cell types (macrophages and endothelial cells) of adipose tissue as has been described previously (5). Moreover, proportions of cell populations in eWAT change after HFD as immune cells are recruited. This can be reflected in the gene expression profile in eWAT, where Jun expression (mainly coming from immune cells) tends to be increased whereas Ucp1 expression (mainly coming from adipocytes) was decreased. Nqo1 transcript levels in eWAT tended to be lower in the ANKO mice at basal state on StD (Fig. 1E) and after HFD only in iWAT (Fig. 7D). Nrf2 has recently been described to negatively regulate Il-6 levels (14) in macrophages, and thus it could be hypothesized that lack of Nrf2 could lead to increased Il-6 levels. This hypothesis was not verified in our study as expression of inflammation markers, with the exception of Jun, did not increase in adipose tissues (Fig. 7, B and D).
In the iWAT, which has the potential to develop a “beige” phenotype, no change in brown adipocyte markers (41) was detected (Fig. 7D). Interestingly, the levels of Ucp1 were found to be almost 75% higher in the iWAT of ANKO mice and 4 times lower in the eWAT (where its basal levels were barely detectable though). Ucp1 uncouples oxidative phosphorylation from ATP synthesis and drives dissipation of energy as heat. Even though no mitochondrial function measurements were performed in the white adipose tissue of these mice, the ANKO mice showed no change in their energy expenditure (Fig. 3D) meaning that this change in the expression of Ucp1 was not adequate to elicit a systemic metabolic effect. Two independent studies demonstrated that deletion of Nrf2 resulted in increased energy expenditure in two different Nrf2 knockout lines (19, 31) and exhibited higher Ucp1 expression in white adipose tissue in one of those studies (31; in the other, it was not measured). This is interesting in view of the emerging role of Nrf2 in mitochondrial function and homeostasis (7). Our data suggest that this increase in Ucp1 expression in iWAT was not enough to drive a systemic increase in energy expenditure, although other mechanisms and tissues, such as liver, were likely to be responsible for driving the increase observed in the whole body Nrf2 knockout mice.
The hepatocyte-specific deletion of Nrf2 (HeNKO mice) shared a phenotype that was more closely associated with the two independent studies (1 of those was ours) that observed an improved metabolic profile (better glucose tolerance and insulin sensitivity) in whole body Nrf2 knockout mice subjected to HFD (5, 19). The HeNKO mice tended to have lower serum insulin levels (Fig. 5B), and the HOMA-IR was lower than for both the Control and ANKO mice (Fig. 4F). However, no significant difference between the Control and HeNKO mice was observed in the intraperitoneal glucose tolerance test (Fig. 4, D and E) whereas, surprisingly, the HeNKO mice had lower glucose levels at the beginning of the intraperitoneal insulin sensitivity test, after 4 h of fasting (Fig. 4H). It is thus interesting that the glucose levels of the HeNKO mice after 4 h of fasting (start of the intraperitoneal insulin sensitivity test) and after overnight (16 h) fasting (start of the intraperitoneal glucose tolerance test) were almost similar. This observation could potentially be explained by the repressive effect of Nrf2 on gluconeogenesis. It has been shown previously that Nrf2 pathway activation can repress the expression of key gluconeogenic enzymes such as Pepck and G6pase (34, 39) and this can be due to repression of the cAMP response element binding protein-mediated transcriptional activity (39) or due to activation of Ampk signaling (34). Short-term fasting was not sufficient to induce gluconeogenesis, and the increased insulin sensitivity of the HeNKO mice could lead to the observed lower glucose levels. However, after overnight starvation, gluconeogenesis was induced. Thus, the HeNKO mice that lack Nrf2 in their hepatocytes also lacked the repressive effect of Nrf2 on gluconeogenesis. Increased induction of gluconeogenesis could potentially explain the observation that after overnight fasting the HeNKO mice showed glucose levels similar to those of the Control mice. In the present study, no difference was detected in Pepck and G6pase mRNA expression (Fig. 7A). Moreover, no difference in hepatic glycogen content was detected either (Fig. 6B). However, more detailed assessment of gluconeogenesis is required by using more precise methods such as measurement of glucose production during the basal period of the euglycemic-hyperinsulinemic clamp test with the use of tracers (17).
Taking into account that the whole body Nrf2 knockout mice develop fatty liver and steatohepatitis when fed HFD, all while maintaining increased insulin sensitivity (19), we assessed the level of liver steatosis and damage in the HeNKO mice. Levels of hepatic total triglycerides (Fig. 6A), triglyceride species levels (Fig. 6C), and fatty acid species levels (Fig. 6D) did not differ between Control and HeNKO mice. However, the HeNKO mice displayed slightly more fibrotic livers (Fig. 6, F and G), as is observed to a greater degree in the whole body Nrf2 knockout model (19). Liver damage did not differ significantly between Control and HeNKO mice as assessed by enumerating the number of apoptotic cells (TUNEL assay; Fig. 6H) and by serum alanine transaminase levels (Fig. 6I). This is in contrast to what has been shown in the whole body Nrf2 knockout mice on HFD (19). This could mean that although the HeNKO mice lack Nrf2 in their hepatocytes, other liver cell types such as Kupffer cells, Ito cells, and sinusoidal endothelial cells that still express Nrf2 may partially protect the liver from extensive steatohepatitis. A mouse model that overexpresses Nrf2 specifically in hepatocytes placed on a long-term HFD in parallel with the HeNKO mice would be useful to evaluate the hepatocyte-specific role of Nrf2 in fatty liver disease progression.
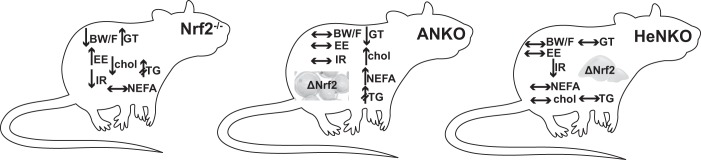
In conclusion, feeding adipocyte- or hepatocyte-specific Nrf2 knockout mice HFD for 6 mo did not lead to the same metabolic phenotype seen in whole body Nrf2 knockout mice as previously reported by us (5) and others (19; Fig. 8), in which they were partially protected from diet-induced obesity and showed improved insulin sensitivity and glucose tolerance. This means that absence of Nrf2 can exert differential metabolic effects in different tissues. Lack of Nrf2 in adipocytes did not lead to an ameliorated metabolic phenotype, but the opposite: increased glucose, cholesterol, and NEFA levels. Absence of Nrf2 in hepatocytes did not lead to a worse diabetic phenotype but, on the contrary, tended to lead to better insulin sensitivity with yet-unclear mechanisms. In a previously published model of Nrf2 deletion in myocytes, HFD feeding led to slightly worsened glucose tolerance (40). Of all these cell-specific Nrf2 knockout models, only the hepatocyte-specific model appears to mimic, at least to a limited degree, the outcomes of whole body Nrf2 knockout mice fed HFD. Moreover, the presence of a congenital intrahepatic shunt that can be seen in two-thirds of Nrf2-disrupted mice (33) is another parameter that should be taken into account when metabolically evaluating the Nrf2 knockout mice as this shunt can affect the gradient of oxygen and protein expression, such as that of Pepck, in the liver and thus have a direct metabolic effect, e.g., in hepatic glucose production or in oxidation of fatty acids.
Fig. 8.
Schematic summary of the main phenotypic changes of nuclear factor erythroid 2-related factor 2 knockout (Nrf2−/−) mice compared with wild-type mice (based on references) and of Nrf2flox/flox::Albumin-Cre (HeNKO) and Nrf2flox/flox::Adipoq-Cre (ANKO) mice versus Control after long-term exposure to high-fat diet. Arrows pointing upward indicate increase, arrows pointing downward indicate decrease, horizontal left-right arrows mean no change, and arrows with two intersecting lines indicate trend. BW/F, body weight and body fat; chol, serum cholesterol; EE, energy expenditure; IR, insulin resistance; GT, glucose tolerance; NEFA, serum nonesterified fatty acids; TG, serum triglycerides.
Consequently, whereas current interventions on the Nrf2 pathway involve foods and drugs that activate this pathway (e.g., sulforaphane in broccoli and CDDO-methyl ester), the murine models of Nrf2 deletion are useful from mechanistic perspectives. By deletion of Nrf2 specifically in a cell type, such as adipocytes or hepatocytes in the present study, useful information can be gleaned regarding the contribution of Nrf2 in a cell type to the resulting diet-induced obese phenotype. However, in some cases the lack of Nrf2 cannot further deteriorate an already extreme phenotype (such as extreme obesity), highlighting that the response to Nrf2 levels (from absence to overexpression) is not necessarily linear and that unexpected phenotypes may be revealed in the upper and lower amplitudes of Nrf2 signaling. Given that recently an Nrf2 pathway activator, sulforaphane, in the form of broccoli sprout extracts was shown to ameliorate hyperglycemia in a clinical trial with type 2 diabetes patients (1), such information from murine studies can be helpful to properly target Nrf2-modulating therapies to the tissue(s) that can be most responsive or to avoid any side effects by subjecting “sensitive” cell types to extreme (low or high) Nrf2 levels.
GRANTS
This work was supported by National Institutes of Health Grants R35-CA-197222 (to T. W. Kensler) and R01-DK-102839 (to R. M. O’Doherty). D. V. Chartoumpekis was supported by Marie Curie PIOF-GA-2012-329442 “ADIPONRF2” (7th European Community Framework Programme).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.V.C. and T.W.K. conceived and designed research; D.V.C., D.L.P., M.F., N.K.H.K., I.S., and Y.Y. performed experiments; D.V.C., M.F., and G.K.M. analyzed data; D.V.C., D.L.P., N.W., N.K.H.K., F.J.S., and R.M.O. interpreted results of experiments; D.V.C. drafted manuscript; D.V.C. and T.W.K. approved final version of manuscript; D.L.P., N.W., N.K.H.K., and T.W.K. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. Shyam Biswal (Johns Hopkins Bloomberg School of Public Health) for providing the Nrf2flox/flox mice and Kimberly Fuhrer (Department of Pathology, University of Pittsburgh Medical Center) for technical support in histology. We also acknowledge the support of Center for Metabolism and Mitochondrial Medicine of University of Pittsburgh.
REFERENCES
- 1.Axelsson AS, Tubbs E, Mecham B, Chacko S, Nenonen HA, Tang Y, Fahey JW, Derry JM, Wollheim CB, Wierup N, Haymond MW, Friend SH, Mulder H, Rosengren AH. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci Transl Med 9: eaah4477, 2017. doi: 10.1126/scitranslmed.aah4477. [DOI] [PubMed] [Google Scholar]
- 2.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- 3.Chanas SA, Jiang Q, McMahon M, McWalter GK, McLellan LI, Elcombe CR, Henderson CJ, Wolf CR, Moffat GJ, Itoh K, Yamamoto M, Hayes JD. Loss of the Nrf2 transcription factor causes a marked reduction in constitutive and inducible expression of the glutathione S-transferase Gsta1, Gsta2, Gstm1, Gstm2, Gstm3 and Gstm4 genes in the livers of male and female mice. Biochem J 365: 405–416, 2002. doi: 10.1042/bj20020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chartoumpekis DV, Kensler TW. New player on an old field; the keap1/Nrf2 pathway as a target for treatment of type 2 diabetes and metabolic syndrome. Curr Diabetes Rev 9: 137–145, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chartoumpekis DV, Ziros PG, Psyrogiannis AI, Papavassiliou AG, Kyriazopoulou VE, Sykiotis GP, Habeos IG. Nrf2 represses FGF21 during long-term high-fat diet-induced obesity in mice. Diabetes 60: 2465–2473, 2011. doi: 10.2337/db11-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chartoumpekis DV, Ziros PG, Sykiotis GP, Zaravinos A, Psyrogiannis AI, Kyriazopoulou VE, Spandidos DA, Habeos IG. Nrf2 activation diminishes during adipocyte differentiation of ST2 cells. Int J Mol Med 28: 823–828, 2011. doi: 10.3892/ijmm.2011.761. [DOI] [PubMed] [Google Scholar]
- 7.Dinkova-Kostova AT, Abramov AY. The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med 88, Part B: 179–188, 2015. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, Dushay J, Estall JL, Klein U, Maratos-Flier E, Rosen ED. Transcriptional control of adipose lipid handling by IRF4. Cell Metab 13: 249–259, 2011. doi: 10.1016/j.cmet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher FM, Maratos-Flier E. Understanding the physiology of FGF21. Annu Rev Physiol 78: 223–241, 2016. doi: 10.1146/annurev-physiol-021115-105339. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114: 1752–1761, 2004. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furusawa Y, Uruno A, Yagishita Y, Higashi C, Yamamoto M. Nrf2 induces fibroblast growth factor 21 in diabetic mice. Genes Cells 19: 864–878, 2014. doi: 10.1111/gtc.12186. [DOI] [PubMed] [Google Scholar]
- 12.Hou Y, Xue P, Bai Y, Liu D, Woods CG, Yarborough K, Fu J, Zhang Q, Sun G, Collins S, Chan JY, Yamamoto M, Andersen ME, Pi J. Nuclear factor erythroid-derived factor 2-related factor 2 regulates transcription of CCAAT/enhancer-binding protein β during adipogenesis. Free Radic Biol Med 52: 462–472, 2012. doi: 10.1016/j.freeradbiomed.2011.10.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236: 313–322, 1997. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, Tanaka N, Moriguchi T, Motohashi H, Nakayama K, Yamamoto M. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun 7: 11624, 2016. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul 46: 113–140, 2006. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Kong X, Thimmulappa R, Craciun F, Harvey C, Singh A, Kombairaju P, Reddy SP, Remick D, Biswal S. Enhancing Nrf2 pathway by disruption of Keap1 in myeloid leukocytes protects against sepsis. Am J Respir Crit Care Med 184: 928–938, 2011. doi: 10.1164/rccm.201102-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kowalski GM, Bruce CR. The regulation of glucose metabolism: implications and considerations for the assessment of glucose homeostasis in rodents. Am J Physiol Endocrinol Metab 307: E859–E871, 2014. doi: 10.1152/ajpendo.00165.2014. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Franke AA. Improved LC-MS method for the determination of fatty acids in red blood cells by LC-orbitrap MS. Anal Chem 83: 3192–3198, 2011. doi: 10.1021/ac103093w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meakin PJ, Chowdhry S, Sharma RS, Ashford FB, Walsh SV, McCrimmon RJ, Dinkova-Kostova AT, Dillon JF, Hayes JD, Ashford ML. Susceptibility of Nrf2-null mice to steatohepatitis and cirrhosis upon consumption of a high-fat diet is associated with oxidative stress, perturbation of the unfolded protein response, and disturbance in the expression of metabolic enzymes but not with insulin resistance. Mol Cell Biol 34: 3305–3320, 2014. doi: 10.1128/MCB.00677-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meher AK, Sharma PR, Lira VA, Yamamoto M, Kensler TW, Yan Z, Leitinger N. Nrf2 deficiency in myeloid cells is not sufficient to protect mice from high-fat diet-induced adipose tissue inflammation and insulin resistance. Free Radic Biol Med 52: 1708–1715, 2012. doi: 10.1016/j.freeradbiomed.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullican SE, Tomaru T, Gaddis CA, Peed LC, Sundaram A, Lazar MA. A novel adipose-specific gene deletion model demonstrates potential pitfalls of existing methods. Mol Endocrinol 27: 127–134, 2013. doi: 10.1210/me.2012-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagata N, Xu L, Kohno S, Ushida Y, Aoki Y, Umeda R, Fuke N, Zhuge F, Ni Y, Nagashimada M, Takahashi C, Suganuma H, Kaneko S, Ota T. Glucoraphanin ameliorates obesity and insulin resistance through adipose tissue browning and reduction of metabolic endotoxemia in mice. Diabetes 66: 1222–1236, 2017. doi: 10.2337/db16-0662. [DOI] [PubMed] [Google Scholar]
- 23.Nioi P, McMahon M, Itoh K, Yamamoto M, Hayes JD. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: reassessment of the ARE consensus sequence. Biochem J 374: 337–348, 2003. doi: 10.1042/bj20030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 311: 806–814, 2014. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palliyaguru DL, Chartoumpekis DV, Wakabayashi N, Skoko JJ, Yagishita Y, Singh SV, Kensler TW. Withaferin A induces Nrf2-dependent protection against liver injury: role of Keap1-independent mechanisms. Free Radic Biol Med 101: 116–128, 2016. doi: 10.1016/j.freeradbiomed.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pi J, Leung L, Xue P, Wang W, Hou Y, Liu D, Yehuda-Shnaidman E, Lee C, Lau J, Kurtz TW, Chan JY. Deficiency in the nuclear factor E2-related factor-2 transcription factor results in impaired adipogenesis and protects against diet-induced obesity. J Biol Chem 285: 9292–9300, 2010. doi: 10.1074/jbc.M109.093955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem 274: 305–315, 1999. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 29.Qiao L, Yoo HS, Madon A, Kinney B, Hay WW Jr, Shao J. Adiponectin enhances mouse fetal fat deposition. Diabetes 61: 3199–3207, 2012. doi: 10.2337/db12-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saha PK, Reddy VT, Konopleva M, Andreeff M, Chan L. The triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic-acid methyl ester has potent anti-diabetic effects in diet-induced diabetic mice and Leprdb/db mice. J Biol Chem 285: 40581–40592, 2010. doi: 10.1074/jbc.M110.176545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider K, Valdez J, Nguyen J, Vawter M, Galke B, Kurtz TW, Chan JY. Increased energy expenditure, Ucp1 expression, and resistance to diet-induced obesity in mice lacking nuclear factor-erythroid-2-related transcription factor-2 (Nrf2). J Biol Chem 291: 7754–7766, 2016. doi: 10.1074/jbc.M115.673756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin S, Wakabayashi N, Misra V, Biswal S, Lee GH, Agoston ES, Yamamoto M, Kensler TW. NRF2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis. Mol Cell Biol 27: 7188–7197, 2007. doi: 10.1128/MCB.00915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skoko JJ, Wakabayashi N, Noda K, Kimura S, Tobita K, Shigemura N, Tsujita T, Yamamoto M, Kensler TW. Loss of Nrf2 in mice evokes a congenital intrahepatic shunt that alters hepatic oxygen and protein expression gradients and toxicity. Toxicol Sci 141: 112–119, 2014. doi: 10.1093/toxsci/kfu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slocum SL, Skoko JJ, Wakabayashi N, Aja S, Yamamoto M, Kensler TW, Chartoumpekis DV. Keap1/Nrf2 pathway activation leads to a repressed hepatic gluconeogenic and lipogenic program in mice on a high-fat diet. Arch Biochem Biophys 591: 57–65, 2016. doi: 10.1016/j.abb.2015.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spahis S, Borys JM, Levy E. Metabolic syndrome as a multifaceted risk factor for oxidative stress. Antioxid Redox Signal 26: 445–461, 2017. doi: 10.1089/ars.2016.6756. [DOI] [PubMed] [Google Scholar]
- 36.Spandidos A, Wang X, Wang H, Dragnev S, Thurber T, Seed B. A comprehensive collection of experimentally validated primers for polymerase chain reaction quantitation of murine transcript abundance. BMC Genomics 9: 633, 2008. doi: 10.1186/1471-2164-9-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taguchi K, Maher JM, Suzuki T, Kawatani Y, Motohashi H, Yamamoto M. Genetic analysis of cytoprotective functions supported by graded expression of Keap1. Mol Cell Biol 30: 3016–3026, 2010. doi: 10.1128/MCB.01591-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urakawa H, Katsuki A, Sumida Y, Gabazza EC, Murashima S, Morioka K, Maruyama N, Kitagawa N, Tanaka T, Hori Y, Nakatani K, Yano Y, Adachi Y. Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab 88: 4673–4676, 2003. doi: 10.1210/jc.2003-030202. [DOI] [PubMed] [Google Scholar]
- 39.Uruno A, Furusawa Y, Yagishita Y, Fukutomi T, Muramatsu H, Negishi T, Sugawara A, Kensler TW, Yamamoto M. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol Cell Biol 33: 2996–3010, 2013. doi: 10.1128/MCB.00225-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uruno A, Yagishita Y, Katsuoka F, Kitajima Y, Nunomiya A, Nagatomi R, Pi J, Biswal SS, Yamamoto M. Nrf2-mediated regulation of skeletal muscle glycogen metabolism. Mol Cell Biol 36: 1655–1672, 2016. doi: 10.1128/MCB.01095-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ussar S, Lee KY, Dankel SN, Boucher J, Haering MF, Kleinridders A, Thomou T, Xue R, Macotela Y, Cypess AM, Tseng YH, Mellgren G, Kahn CR. ASC-1, PAT2, and P2RX5 are cell surface markers for white, beige, and brown adipocytes. Sci Transl Med 6: 247ra103, 2014. doi: 10.1126/scitranslmed.3008490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, Kensler TW, Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci USA 101: 2040–2045, 2004. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, Harada T, Engel JD, Yamamoto M. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet 35: 238–245, 2003. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- 45.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 27: 1487–1495, 2004. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 46.Xue P, Hou Y, Chen Y, Yang B, Fu J, Zheng H, Yarborough K, Woods CG, Liu D, Yamamoto M, Zhang Q, Andersen ME, Pi J. Adipose deficiency of Nrf2 in ob/ob mice results in severe metabolic syndrome. Diabetes 62: 845–854, 2013. doi: 10.2337/db12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yagishita Y, Fukutomi T, Sugawara A, Kawamura H, Takahashi T, Pi J, Uruno A, Yamamoto M. Nrf2 protects pancreatic β-cells from oxidative and nitrosative stress in diabetic model mice. Diabetes 63: 605–618, 2014. doi: 10.2337/db13-0909. [DOI] [PubMed] [Google Scholar]
- 48.Yates MS, Tauchi M, Katsuoka F, Flanders KC, Liby KT, Honda T, Gribble GW, Johnson DA, Johnson JA, Burton NC, Guilarte TR, Yamamoto M, Sporn MB, Kensler TW. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther 6: 154–162, 2007. doi: 10.1158/1535-7163.MCT-06-0516. [DOI] [PubMed] [Google Scholar]
- 49.Zhang L, Dasuri K, Fernandez-Kim SO, Bruce-Keller AJ, Keller JN. Adipose-specific ablation of Nrf2 transiently delayed high-fat diet-induced obesity by altering glucose, lipid and energy metabolism of male mice. Am J Transl Res 8: 5309–5319, 2016. [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang YK, Wu KC, Liu J, Klaassen CD. Nrf2 deficiency improves glucose tolerance in mice fed a high-fat diet. Toxicol Appl Pharmacol 264: 305–314, 2012. doi: 10.1016/j.taap.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]