Abstract
Copper (Cu) is an essential micronutrient but excess Cu is potentially toxic. Its important propensity to cycle between two oxidation states accounts for its frequent presence as a cofactor in many physiological processes through Cu-containing enzymes, including mitochondrial energy production (via cytochrome c-oxidase), protection against oxidative stress (via superoxide dismutase), and extracellular matrix stability (via lysyl oxidase). Since free Cu is potentially toxic, the bioavailability of intracellular Cu is tightly controlled by Cu transporters and Cu chaperones. Recent evidence reveals that these Cu transport systems play an essential role in the physiological responses of cardiovascular cells, including cell growth, migration, angiogenesis and wound repair. In response to growth factors, cytokines, and hypoxia, their expression, subcellular localization, and function are tightly regulated. Cu transport systems and their regulators have also been linked to various cardiovascular pathophysiologies such as hypertension, inflammation, atherosclerosis, diabetes, cardiac hypertrophy, and cardiomyopathy. A greater appreciation of the central importance of Cu transporters and Cu chaperones in cell signaling and gene expression in cardiovascular biology offers the possibility of identifying new therapeutic targets for cardiovascular disease.
Keywords: cardiovascular diseases, copper chaperones, copper homeostasis, copper transporters, vascular physiology
INTRODUCTION
In recent years it has become increasingly evident that the essential metal micronutrients play critical roles in a wide range of physiological processes. These metals include iron, zinc, manganese, and copper, and in the case of copper (Cu), its importance is evident throughout human physiology.1 The special properties of Cu, particularly its ready conversion between two oxidation states, Cu(I) and Cu(II), have led to its utilization as a cofactor in many oxido-reductive enzymatic processes. However, its propensity to produce reactive oxygen species (ROS) with potentially toxic consequences, together with its promiscuous reactivity with a number of amino acid side-chains of proteins as well as lipids, has necessitated the development of finely regulated homeostatic mechanisms. Examples of enzymes of special relevance to cardiovascular physiology that require Cu as a cofactor include Cu, Zn-superoxide dismutases (SOD) (SOD1 and SOD3), lysyl oxidase, mitochondrial cytochrome c-oxidase, and many others. Accumulating evidence suggests that cellular functions of Cu transporters and chaperones are not limited to Cu trafficking and may include unexpected roles involved in (patho)physiological responses (Fig. 1). In this short review, we will focus on the cellular processes that are especially important to cardiovascular physiology and some of the pathophysiological consequences observed in the cardiovascular system when Cu acquisition and its utilization fail to be appropriately regulated.
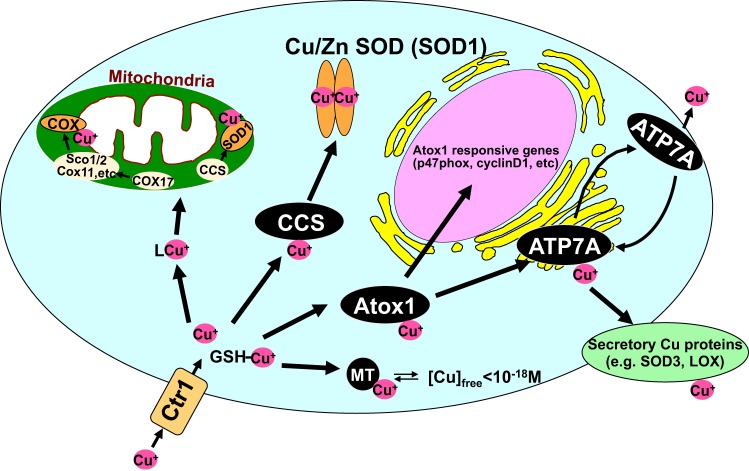
Fig. 1.
Copper trafficking pathways in mammalian cells. Once transported by Cu uptake transporter CTR1, soluble cytosolic Cu carrier proteins termed “Cu chaperones” may obtain the Cu directly from the uptake transporter or from GSH and are required for trafficking Cu to specific Cu-containing enzymes through direct protein-protein interactions. Three main copper chaperone pathways have been characterized thus far: 1) various Cu chaperones (cytochrome c-oxidases Cox1, Cox2, Cox11, and Cox17 and synthesis of cytochrome c-oxidases Sco1 and Sco2) and unknown Cu ligands (L), which deliver Cu to cytochrome c-oxidase in the mitochondria; 2) Cu chaperone for SOD1 (CCS), which delivers copper to SOD1 in the cytosol and mitochondrial intermembrane space (IMS); 3) antioxidant-1 (Atox1), which delivers copper to the secretory copper enzymes such as extracellular superoxide dismutase (ecSOD, SOD3) and lysyl oxidase (LOX) via the copper transporter ATP7A (Menkes disease copper ATPase, MNK) or ATP7B (Wilson disease copper ATPase, WND) in the trans-Golgi network. In addition to its chaperone function, Atox1 also function as a Cu-dependent transcription factor for Atox1-responsive genes such as ecSOD and cyclin D1. Cytosolic concentrations of free Cu are typically maintained at exquisitely low levels (10−18 M) by metal scavenging systems, including metallothionein (MT).
The recommended dietary allowance for Cu in adult men and women is 0.9 mg/day and the total body Cu is around 100 mg (1, 108, 181, 190). Although these are small amounts, the principles that govern cellular Cu homeostasis are the same as those that govern the major metal cations such as Na, K, or Ca. Cellular Cu levels are regulated by dedicated uptake systems balanced by the actions of ion-activated P-type ATPases that are responsible for its removal. These uptake and efflux transporters play additional roles, which are vital to appropriate Cu homeostasis. Under physiological conditions, there are vanishingly low levels of free Cu in cells (157). To protect the cell from the damaging effects of free Cu, a network of proteins, the intracellular metallochaperones, have evolved, which obtain Cu from the protein(s) mediating uptake and distribute it to target sites in the cell via direct interaction with their target proteins (Fig. 1). In this way, the essential acquisition of cofactor Cu is achieved while avoiding the harmful consequences of appreciable levels of free Cu.
There is little systematic knowledge of the chronic occurrence of dietary Cu toxicity and Cu deficiency or its potential effects in human physiology and cardiovascular disease (88). The occurrence of several human genetic diseases has stimulated interest in the field. The most familiar human diseases of Cu homeostasis are Menkes disease and Wilson disease, which result from mutations in the P-type ATPases, ATP7A and ATP7B, respectively. The basis of the different manifestation of these diseases, as systemic Cu deficiency or Cu toxicity, respectively, despite the high sequence identity of the proteins and similarity of causative mutations, can be found in the different locations of the proteins. ATP7B is predominantly expressed in the liver and ATP7A ubiquitously, but particularly in intestinal and neuronal cells (see below).
TRANSMEMBRANE Cu TRANSPORT
Cu Uptake Mediated by CTR1
The human high-affinity Cu transporter hCTR1 (SLC31A1) was first identified by complementation in yeast, in 1997 (195) and the mouse protein, which is 95% identical, three years later (104) (Fig. 1). Gene deletion of the mouse Cu transporter is lethal in midgestation CTR1−/− embryos (96, 105). hCTR1 is a highly conserved high-affinity (42) Cu uptake protein with a Km of about 3 μM, which is highly specific for Cu+ and the only known alternate substrate is Ag+ (for recent review, see Ref. 83). hCTR1 is a relatively small polypeptide of 190 amino acid residues. Its membrane topology has been determined. It has an extracellular amino-terminus, an intracellular carboxy-terminus, and three transmembrane segments (42, 90, 153). It associates into a stable homotrimer in the plasma membrane, forming a central conical pore (7) with an extracellular access of about 8 Å diameter and an intracellular access diameter of about 22 Å (40). The pore is lined at its extracellular side by a ring of six methionine residues (M150, M154, from each monomer) that are believed to provide an initial coordinating site for the permeating Cu (183). The intracellular carboxy terminus of about 14 amino acids ends with an HCH trimer that is completely conserved among mammalian species. This site provides a putative Cu-binding site at the exit of the pore in the cytosol. The lack of measurable free Cu in the serum or in cells leaves many aspects of the energetics of Cu uptake unresolved. It has been proposed that the binding of Cu to the COOH-terminal HCH triad provides a Cu coordinating site prior to Cu exit from the pore to the cytosol. The change in free energy, as Cu is bound to this site of relatively higher affinity than the initial Met ring coordination, was suggested to provide energy necessary for Cu permeation (40). This, however, is probably not the case, as it has subsequently been shown that the COOH-terminal triad, HCH, is not only not essential for CTR1-mediated Cu transport, but its deletion, or replacement by AAA, results in an increase in the maximal rate, the Vmax of Cu transport (125). Thus, the COOH-terminal Cu-binding motif plays an important role in lowering the rate of transport through the pore. It seems likely that conformational changes on Cu loading of CTR1 occur, but their role in transport remains uncharacterized (42). It is apparent that the essential and relatively low requirement that cells have for Cu is achieved by expressing hCTR1 in low copy numbers and by ensuring that the transporter has an intrinsically low turnover number for Cu ions transported per trimer per second (about 5–10) (125).
There are many unresolved questions that relate to the detailed mechanism of Cu permeation through CTR1. The initial step, that is, the transfer to CTR1 from a serum Cu-carrier, remains a mystery, as the identity of these protein(s) or small molecule(s) is still undetermined. Recent work suggests that His residues in the extracellular amino-terminal tail are important for the transfer to hCTR1 (63). In a similar fashion (see below) the precise mechanism of the transfer process, from the transporter to an intracellular chaperone, is the subject of current investigation. It is clear, however, that the entity transported by CTR1 is Cu+. The site of reduction of Cu2+, presumably in the extracellular medium, is not known with certainty, but there is some indication that the plasma membrane-bound STEAP proteins, which have been associated with Cu reductase activity (139), may play a role or perhaps other unidentified reductants in the serum.
The presence of posttranslational glycosylation of hCTR1 has been well characterized. Both N-linked (42) and O-linked modifications are present. The extensive N-linked glycosylation, at Asn15, results in a shift in SDS gels to an apparent mass of about 35 kDa. It seems likely that the N-linked glycosylation at N15 does not greatly influence transport. However, an O-linked modification containing several sugars attached to Thr27 serves to protect hCTR1 against intracellular cleavage by a cellular protease (127). The cleaved product is localized in late endosomal compartments containing Rab9 (128). The shortened transporter, lacking much of the extracellular tail, is a significantly (50%) less effective Cu transporter.
Cu-Dependent Relocalization of CTR1
An additional role of CTR1 that has recently been reported involves a Cu-dependent relocalization of the transporter. It has been appreciated for many years that the P-type ATPases (ATP7A, -7B) responsible for Cu efflux and for Cu delivery into the Golgi and the secretory pathway (see below) are sensitive to cellular Cu levels and relocate to the plasma membrane from the Golgi when cellular Cu is high. This is an important protection against elevated intracellular Cu levels. In a similar fashion, when extracellular Cu is elevated, the uptake transporter, hCTR1, is removed from the plasma membrane and internalized (132). This prevents the cell from acquiring elevated intracellular Cu. Initial studies demonstrating internalization of CTR1 in the face of elevated extracellular Cu claimed that the endocytosed transporter was degraded (148) (60). More recent studies have shown that when extracellular Cu is lowered, hCTR1 recycles back to the plasma membrane, to again facilitate Cu influx. Such “activity-regulated transport” has recently been suggested, in a computational analysis, to play a major role in efficient homeostatic systems (164). The mechanism of hCTR1 internalization and recycling has recently been described. The internalization is clathrin- and dynamin-dependent and takes place via compartments containing Rab5 and EEA1, but not late endosomal or lysosomal pathways, and the recycling occurs via a Rab11-mediated pathway (35). It is also significant that deletion of the putative Cu-binding COOH-terminal triad, 188HCH, or its substitution causes a double phenotype. The internalization of hCTR1 at elevated extracellular Cu no longer occurs and the Vmax for transport (as discussed above) is also increased (125). It seems likely that Cu binding at this site may cause slowing of the transport cycle and facilitate interaction with the internalization machinery.
Other Cu Entry Pathways
There are three other entry pathways in addition to hCTR1 that have received attention as potential mediators of Cu uptake across the plasma membrane in human cells. These are 1) hCTR2, a related protein; 2) DMT1, an unrelated divalent metal ion transporter; and 3) Cu(II) transport in an anionic complex, by an anion exchange transporter. We will briefly discuss the current status of each.
The first of these, CTR2, originally identified via its homology to CTR1 (195), but lacking a major part of the extracellular amino-terminal domain, has been suggested to be an alternate, lower-affinity, CTR1-like transport protein (18). However, recent studies suggest an interesting, alternate, and unexpected role for CTR2 in Cu homeostasis. In a thorough examination and comparison of metazoan CTR1 and CTR2 evolution and function it has been suggested that CTR2 serves as an intracellular vesicular transporter and also plays a significant role in stimulating the degradation of CTR1 (see Ref. 111 and references therein).
The second alternate Cu transporter, DMT1, the epithelial divalent metal ion transporter, was suggested to transport Cu(II) ions (11) following overexpression in HEK cells. However, recent studies bring into question the direct involvement of DMT1 in Cu transport (167).
The third candidate is on its face somewhat surprising. In the 1980s it was reported that in human red blood cells the uptake of Pb2+, Cd2+, and Cu2+ was mediated by AE1 or Band 3, the prototypical anion exchange transporter (5, 168, 169). The transport was inhibited by DIDS (4,4′-diisothyocyano-stilbene-2,2′-disulfonic acid), a well-characterized inhibitor of anion exchange transporters. These observations were largely lost to the metal transport field. More recently, in studies of Cu uptake across the apical membrane of intestinal monolayers, it was proposed that Cu(II) uptake was mediated via an apical anion exchange system, and the transport was inhibited by DIDS (197). There are several members of this anion exchange family in the apical membrane of intestinal and renal epithelia and many of the observed characteristics of these metal ion fluxes corresponded to the system seen in human erythrocytes. Interestingly, this transport of CuX4 anions would also account for very high fluxes observed in early studies of heterologous overexpression of hCTR1, in the absence or presence of CTR1 (103). It was observed that these “background” fluxes of Cu were greatly diminished in the presence of histidine or serum, effects also seen and discussed in the later studies of Cu uptake in epithelial cells (197). The presence of peptides or Cu-coordinating amino acids in serum make this pathway of little relevance in most physiological situations. However, in the particular environment (pH and salts) in the early intestine (duodenum) this system may play a significant role in dietary acquisition. Interestingly, in a recent study it was proposed that an anion (phosphate) transporter in mitochondria, SLC25A3, also mediated Cu uptake and that, when reconstituted into phospholipid liposomes Cu, uptake was facilitated (25). These findings are in line with the earlier studies, discussed above, in red cells and in epithelia, as well as findings suggesting a role for the mitochondrial phosphate carrier in Cu import in yeast (188). They raise the possibility that Cu entry into mitochondria may also be facilitated via an anionic complex of Cu(II).
Cu Export Mediated by P-type ATPases
The export of Cu from cells is accomplished by the actions of ATP7A and ATP7B, which are Cu-activated ATPases and belong to the P-type ATPase family of ion pumps (74, 117, 143) (Fig. 1). These proteins catalyze the metal cation-activated phosphorylation (from cellular ATP) of an essential aspartate residue and couple conformational equilibria between two phosphoenzyme states to the transmembrane transport of the metal cation. The Cu-ATPases contain many of the usual signature sequences of P-type ATPases and an additional amino-terminal domain with multiple (six) Cu binding domains (118) that serve as sensors of cytosolic Cu levels that presumably trigger the events involved in Cu-dependent relocalization. The two Cu-activated P-type ATPases spend part of their time in the plasma membrane and represent an important aspect of cellular Cu homeostasis (116). Under normal conditions of cellular Cu loads, these proteins are found in the Golgi where they pump Cu from the cytosol (supplied by a Cu chaperone, Atox1, see below) into the lumen of the organelle to be incorporated into secretory cuproenzymes present in the lumen. A recent review comprehensively summarizes the current knowledge of Cu trafficking in the secretory pathway (114) and the role of kinase-dependent phosphorylation in the cellular localization of these proteins. The precise mechanisms whereby the ATPases activate the cuproenzymes in the secretory pathway is the subject of current investigation. Under conditions where cytosolic Cu is elevated, these Cu-ATPases pump excess Cu into vesicles of the Golgi that bud off and fuse with the plasma membrane, thus removing excess Cu and placing the pumps in the plasma membrane where they can continue to lower cell Cu. When Cu is lowered they recycle to the Golgi. This Cu-dependent relocalization is an essential homeostatic mechanism that protects cells against elevated cytosolic Cu (180). The malfunctioning of the pumps, in terms of ATPase activity or Cu-dependent relocalization, will result in elevated cell Cu and also potentially loss of the ability to synthesize and secrete active cuproenzymes. Together, these factors are played out in the symptoms of Wilson and Menkes disease, genetically inherited human diseases (115). Their disease outcomes in vascular biology are a consequence of both functions, and loss of appropriate activity of lysyl oxidase, for example, leads to a lack of integrity of connective tissue and skin, which is a common hallmark of Menkes disease. Many of the neurological effects occur via a loss of cuproenzymes that are important in neurotransmitter and neuropeptide biosynthesis. However, the consequences of loss of function or its disruption are multiple and complex when studied in animal models (114), affecting the expression of many proteins not obviously dependent on, or involved with, Cu balance.
INTRACELLULAR Cu DISTRIBUTION
The Metallochaperones
The demonstration that there is essentially no free Cu available in the intracellular milieu made it clear that intracellular Cu was not captured by collision and binding processes between free Cu ions and the target proteins (157). Experimental data appearing first about 20 years ago pointed to the existence of a new class of intracellular proteins, the metallochaperones, which bind Cu and deliver it to specific proteins (152). Although much of the early work on Cu transport and the roles of metallochaperones was carried out in yeast, for the purposes of this review we will focus on mammalian systems. There are separate and specific chaperones that deliver Cu to their target proteins (Fig. 1): 1) ATOX1 (human antioxidant protein), which delivers Cu to the Cu-activated ATPases, ATP7A, ATP7B, and thus to cuproenzymes in the secretory pathway; 2) CCS (Cu chaperone for superoxide dismutase, SOD), which delivers Cu to Cu, Zn-SOD (71); and 3) various Cu chaperones for mitochondrial cytochrome c-oxidase (COX), which requires Cu for its maturation and function. For the delivery of Cu to COX in the mitochondrion, Cox11 delivers Cu to the Cu(B) site of the oxidase, whereas the delivery of Cu to the Cu(A) site involves a number of associated proteins, including Cox17, Cox19, Cox23 and Sco1, Sco2 (144, 162) (Fig. 1). However, the mechanisms that regulate IMS copper translocation and transfer to Cox17 and CCS at IMS remains elusive (102). The details of overall synthesis of COX and the regulation of the many assembly proteins has been discussed in a recent review (102).
Antioxidant-1
Antioxidant-1 (ATOX1) is a small (68 amino acid residues, 7.4 kDa) cytosolic protein that is highly conserved among mammalian species and is responsible for transferring Cu(I) to the Cu-ATPases for delivery to cuproenzymes in the trans-Golgi system and hence to the secretory pathway (Fig. 1). ATOX1 has a ferredoxin-like βαββαβ-fold. Cu(I) is bound via a MXCXXC motif and is transferred to the ATPase metal binding domain via a ligand transfer mechanism (20). It has recently been suggested that ATOX1 acquires its Cu(I) via direct transfer from the COOH-terminal HCH motif of hCTR1 the uptake protein (47). This proposal has ATOX1 associating with the membrane via coulombic interactions and obtaining Cu via direct interaction with the COOH terminus of the transporter. The lack of free Cu in the cytosol supports the idea that Cu(I) does not dissociate from the transporter, so a direct transfer to an acceptor is an appealing mechanism. However, extensive depletion of ATOX1 (or indeed CCS), or its overexpression had no effect on the rate of Cu uptake (126). The depletion of cellular glutathione (GSH) did, however, lower the rate of Cu uptake (126), suggesting that GSH is more likely to be the initial acceptor of Cu(I) from the transporter. Indeed, the GSH/GSSG pair is proposed to be involved in the metallochaperone function of Atox1, thereby regulating Cu homeostasis (reviewed in Refs. 19, 66). An additional important role for ATOX1 has been reported, which integrates Cu homeostasis with another critical cellular process where ATOX1 can serve as a transcriptional regulator in a Cu-dependent fashion (77). This has led to a series of interesting questions that remain and relate to the mechanisms of trafficking of ATOX1 to the nucleus from the cytosol and the regulation of its return.
Cu chaperone for SOD1
Cu chaperone for SOD1 (CCS) is the Cu chaperone for superoxide dismutase; it delivers Cu(I) to the major cytosolic cuproenzyme SOD1 (152), which is also present in mitochondria (Fig. 1). CCS is a 54-kDa protein composed of three domains. The crystal structure of the homodimeric complex of CCS and the heterodimeric CCS-SOD show the importance of matching protein-protein interactions in the chaperone-SOD metal-loading (97, 98). The precise steps in SOD metallation by CCS have been studied in great detail (see, e.g., Refs. 16, 173, 174). In many eukaryotes, including humans, metallation can also occur through CCS-independent pathways (107) and GSH may play the key Cu delivery role (31). Cellular Cu status regulates CCS levels at the posttranslational level, through effects on the 26S proteasome. When Cu is low, CCS levels are high, and when Cu is elevated, degradation of CCS occurs (17, 149).
Cu TRANSPORT PROTEINS AND CELL GROWTH
Cu stimulates cell proliferation and migration involved in physiological repair processes such as wound healing, angiogenesis, and neurogenesis, as well as in various pathophysiologies including tumor growth, atherosclerosis, and neurodegenerative diseases (82, 84, 115). Cancer cells and cells in atherosclerosis have higher Cu levels and more predominant nuclear localization of Cu in some cells than normal tissues (9, 51). Cu chelation prevents tumor growth and neointimal thickening after vascular injury (27, 58, 65, 119). Furthermore, nuclear accumulation of Cu is strongly associated with proliferation of hepatocytes in ATP7B−/− mice, an animal model of Wilson disease, before the appearance of histopathological alterations (72). Because dysregulation of cell proliferation and migration is frequently associated with a number of diseases, an understanding of the molecular mechanism by which Cu stimulates cell proliferation/migration will potentially provide the targets for new therapeutic strategies.
Cu stimulates, while CTR1 deficiency or Cu chelation inhibit, cell proliferation and migration in endothelial cells (70, 135) and vascular smooth muscle cells (21, 198). Several mechanisms for Cu-dependent cell proliferation and/or migration have been proposed. Insulin or fibroblast growth factor (FGF) induces ERK phosphorylation via interaction of MEK1 with ERK in a Cu-dependent manner in flies and mouse fibroblasts, and mouse cardiac tissues (184). Furthermore, Brady et al. (26) showed that decreasing the levels of CTR1, or introducing mutations in MEK1 that disrupt Cu binding, decrease BRAFV600E-driven signaling and tumorigenesis in mice and human cells. In support of this, the Cu chelator tetrathiomolybdate (TTM) decreased tumor growth of human or murine cells transformed by BRAFV600E (26), and fibroblasts lacking CTR1 showed impaired MAPK signaling (182). Thus, these findings suggest that CTR1 plays an important role in cell proliferation in a number of cell types. It was previously demonstrated that Atox1 also has an unexpected function as a Cu-dependent (and sequence-specific transcription factor) involved in cyclin D1 expression and cell proliferation in vitro (77) and in vivo in a number of cell types and in wounded tissue (32, 39). Importantly, nuclear-targeted Atox1 rescued the impairment of wound healing seen in Atox1−/− mice. This indicates that the nuclear function of Atox1 plays a critical role in cell proliferation and wound healing in vivo. Moreover, it has been proposed that Cu-dependent cell proliferation is mediated through enhanced binding of growth factors to cell surface (13), increased secretion of proangiogenic peptides such as FGF1 and IL-1α (151), or stabilization of the three-dimensional architecture of extracellular matrix such as fibronectin (4). It has also been demonstrated that ATP7A plays an important role in PDGF-induced vascular smooth muscle cell (VSMC) migration (12), as discussed below.
Cu TRANSPORT PROTEINS AND ANTITUMOR DRUGS
An interesting correlation between the cytotoxic actions of the antitumor drug, cisplatin, and cellular expression levels of hCTR1 has been reported and hCTR1-mediated cisplatin uptake has been proposed as its basis (76). Furthermore, a role for the Cu transporters in drug resistance has also been suggested (69). However, the lack of effect of overexpressing or knocking down CTR1 on cellular drug levels does not support the proposal that CTR1 mediates (or directly influences) Pt drug entry (78). This conclusion was recently confirmed and extended in studies of CTR1, CTR2, Atox1 and CCS and drug sensitivity utilizing CRISPR-cas9 genome editing techniques (22). How the proteins of the Cu homeostasis system might influence Pt drug sensitivity in cancer cells awaits characterization.
Cu VASCULAR REMODELING AND ATHEROSCLEROSIS
Cu levels play an important role in atherosclerosis, and inflammation. Several prospective epidemiological studies have shown that high serum Cu levels are associated with an increased future risk of coronary heart disease (CHD) in Finnish (163) and Dutch men (93) and in the US population (48). Moreover, previous studies have shown that high plasma levels of ceruloplasmin (CP), which is the major (60–70%), Cu binding protein in plasma, are associated with CHD risk in prospective studies in both men and women (161). Tissue Cu levels are significantly increased in pathological inflammatory conditions such as atherosclerosis and aortic aneurysm (94, 171). Implanting a Cu cuff promotes neointima thickening in response to vascular injury (189), whereas Cu chelators inhibit vascular inflammation and atherosclerotic lesion development in ApoE−/− mice (14, 147, 171, 191–193) as well as neointimal formation in response to vascular injury (119, 120). Cu also plays an important role in inflammatory responses involved in innate and adaptive immunity (43, 146), which is in part via activating NF-κB (145). Cu deficiency alters intravascular adhesion of leukocytes to the activated endothelial cells (ECs) and expression of adhesion molecules, such as ICAM-1/VCAM-1 (165).
There are several potential mechanisms by which Cu might promote atherosclerosis in vivo. Cu may play an important role in oxidation of LDL. It has been shown that tissue homogenates prepared from atherosclerotic lesions contain detectable levels of catalytically active metal ions (100, 178). Furthermore, the appearance of the marker for protein oxidation (o-tyrosine) induced by Cu has been detected by mass spectrometric analysis in advanced human atherosclerotic lesions (50, 106). Mandinov and colleagues (119, 120) proposed that Cu chelation inhibits neointimal thickening by inhibiting stress-induced release of IL-1α and fibroblast growth factor 1, which have been implicated as regulators of vascular injury in vivo. The polypeptide Cu2+-binding proteins, which lack signal peptides, IL-1α and FGF1, are exported into the extracellular compartment in a stress-dependent manner by using intracellular Cu2+ to facilitate the formation of S100A13 heterotetrameric complexes (101, 121). Furthermore, Cu induces the expression of genes involved in LDL uptake and de novo cholesterol biosynthesis such as 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) synthase and LDL receptor in human macrophages (177). In addition, homocysteine, a risk factor for cardiovascular disease, interacts with Cu to enhance hydrogen peroxide (172). In agreement with this, there seems be a correlation among serum levels of Cu or Cu binding protein ceruloplasmin and homocysteine levels in patients with peripheral vascular disease (122) and ischemic heart disease (80). An optimal Cu level is apparently essential for preventing cardiovascular disease, including inflammatory atherosclerosis. Indeed, Cu deficiency has been proposed as a risk factor for coronary heart disease (88). Moreover, in experimental animal models, it is suggested that optimal dietary Cu intake (1–3 mg/day) in the cholesterol-fed rabbit is associated with a reduced susceptibility to aortic atherosclerosis, whereas Cu deficiency and an excessive daily Cu supplement (20 mg/day) are associated with increased atherogenesis (99). These findings suggest that optimal level of Cu is essential for preventing cardiovascular disease, including inflammatory atherosclerosis.
Cu Transport Proteins, Vascular Remodeling, and Atherosclerosis
The Cu exporter ATP7A and its chaperone Atox1 are highly expressed and colocalized in the intimal lesions of atherosclerotic vessels or in neointimal vascular smooth muscle cells (VSMCs) where Cu is highly accumulated in wire-injured vessels (12, 79, 92, 155). Atox1−/− mice show inhibition of neointimal formation and extracellular matrix (ECM) expansion in response to vascular injury, which is associated with decreased VSMC accumulation within neointima and lowered LOX activity (92) (Fig. 2). In cultured VSMC, depletion of Atox1 or ATP7A inhibits VSMC migration in response to PDGF or wound scratch in a CTR1/Cu-dependent manner (12, 92). PDGF stimulation promoted ATP7A translocation from the TGN to lipid rafts at the leading edge, where it colocalized with PDGF receptor and Rac1 in migrating VSMCs. Knockdown of ATP7A or CTR1 prevented PDGF-induced Rac1 translocation to the leading edge, thereby inhibiting lamellipodia formation. Atox1 depletion prevents PDGF-induced translocation of ATP7A, causes decrease in secretory Cu enzyme precursor prolysyl oxidase (Pro-LOX) in lipid rafts as well as lowering LOX activity. Atox1−/− mice show decreased perivascular macrophage infiltration in wire-injured vessels as well as thioglycollate-induced peritoneal macrophage recruitment. These findings strongly suggest that ATP7A and Atox1 play an important role in PDGF-stimulated VSMC migration via recruiting Rac1 to lipid rafts at the leading edge as well as regulating LOX activity. Furthermore, Atox1 is involved in neointimal formation after vascular injury through promoting VSMC migration and inflammatory cell recruitment in injured vessels (Fig. 2). Qin et al. (156) reported that ATP7A downregulation attenuates cell-mediated oxidation of LDL in THP-1 macrophages, which is associated with decreased expression and enzymatic activity of cPLA2 (a key intracellular enzyme involved in cell-mediated LDL oxidation). Moreover, they showed that ATP7A downregulation reduces macrophage infiltration into dermal wounds (86). The role of ATP7A in inflammatory response/immune defenses is further emphasized by the increased susceptibility to infection in patients with Menkes disease who lack a functional ATP7A Cu transporter (59, 185). In in vitro systems, White et al. reported that proinflammatory agents increased expression of CTR1, Cu uptake, and Cu-stimulated trafficking of ATP7A from the Golgi to cytoplasmic vesicles that overlap with the phagosomal compartment (68, 194). Not surprisingly, silencing of ATP7A impaired bactericidal activity, suggesting that Cu transport via ATP7A is required for bacterial killing. The significant role of macrophage ATP7A in inflammatory disease is in need of more detailed investigation
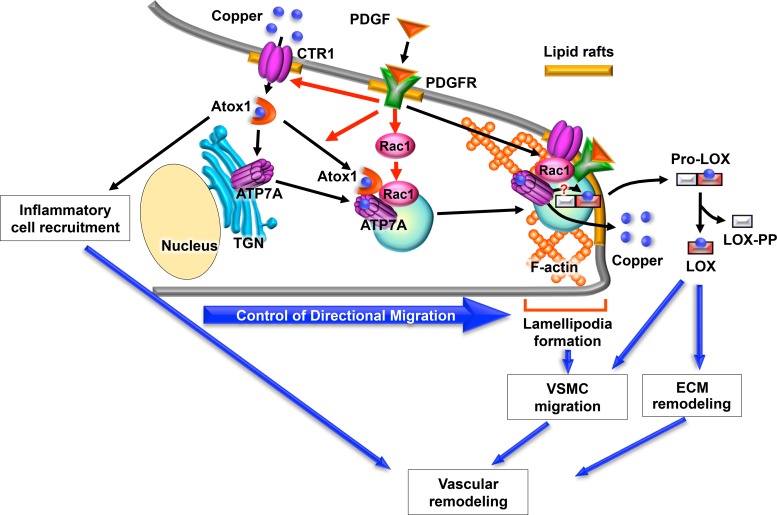
Fig. 2.
Role of Cu transporters and Cu chaperones in platelet-derived growth factor (PDGF)-induced vascular smooth muscle cell (VSMC) migration and vascular remodeling. PDGF promotes interaction of ATP7A with antioxidant-1 (Atox1) and translocation from the trans-Golgi network (TGN) to the lipid rafts at the leading edge. This stimulates lamellipodia formation via recruiting Rac1, which in turn promotes directional VSMC migration. This is associated with a decrease in the cellular copper level and secretory copper enzyme prolysyl oxidase (Pro-LOX) at the lipid rafts, which is processed following secretion and activated by proteolysis to a mature lysyl oxidase (LOX) and a propeptide (LOX-PP), which may promote extracellular matrix (ECM) remodeling. Secreted copper may also contribute to PDGF-induced cell migration. Atox1 also regulates inflammatory cell recruitment. Thus, Cu transporters and Cu chaperones may play an important role in vascular remodeling by regulating inflammatory cell recruitment, VSMC migration, and ECM remodeling. CTR1, copper transporter 1; PDGFR, PDGF receptor.
HYPERTENSION
Cu Transport Proteins and Hypertension
The importance of Cu homeostasis in hypertension has been reported (88). Tissue levels of Cu are altered in hypertensive rats, which are associated with the severity of the hypertension (34, 55). Serum Cu concentration is increased in hypertensive patients (3, 140) and in rodents of various hypertension models (34). A Cu-deficient diet changes blood pressure in an age-dependent manner (89, 131). Several lines of evidence support a role for vascular Cu transport proteins, which regulate not only activation of Cu-containing enzymes such as SOD1 and SOD3, but also total intracellular Cu levels. In angiotensin II (ANG II)-induced hypertension, it was demonstrated that Atox1 functions to prevent ANG II-induced endothelial dysfunction and hypercontraction in resistant vessels, as well as in hypertension, in vivo by reducing extracellular O2− levels via increasing vascular SOD3 expression and activity (142). Furthermore, ANG II infusion into mice significantly decreases vascular Cu levels, which is inhibited in Atox1−/− mice. This may be due to ANG II-induced increase in secretion of Cu-loaded SOD3 to the circulation. This is consistent with the decrease in levels of Cu in some tissues such as liver and kidney in hypertensive rodents (34).
Full activation of SOD3 requires not only Cu chaperone/transcription factor Atox1 but also the Cu transporter ATP7A, which localizes at the trans-Golgi network where ATP7A delivers Cu to SOD3 (53). Indeed, it was reported that ATP7A plays an important role in modulating ANG II-induced hypertension and endothelial function by regulating SOD3 activity and vascular superoxide anion production (154). In contrast, norepinephrine-induced hypertension, which is not associated with an increase in vascular O2·− production (158), is not affected in ATP7Amut mice with reduced Cu transport, which is consistent with findings in SOD3 knockout mice (57). Basal and ANG II infusion-induced increase in vascular SOD3-specific activity is significantly inhibited in ATP7Amut mice. ANG II stimulation promotes association of ATP7A with SOD3 in cultured vascular smooth muscle cells and in mouse aortas, which may contribute to a SOD3-specific activity by increasing Cu delivery to SOD3 through ATP7A (154).
It is also possible that Cu transport proteins in the kidney and central nervous system regulate blood pressure. Atox1 expression is observed in several regions of the brain including the choroid plexus that belongs to the circumventricular organ (CVO), where ATP7A is also expressed (137). It has been reported that deletion of SOD3 in the CVO increases blood pressure in the basal state and after ANG II infusion, in part by modulating sympathetic outflow (109). In particular, O2·− production in the brain is required for the genesis of hypertension (109, 110). It is conceivable that Atox1 expressed in brain may regulate blood pressure either by regulating SOD3 activity or other secretory Cu enzymes via its Cu chaperone function in the brain. Both Atox1 and ATP7A are also highly expressed in the kidney (115, 133). Gene transfer of SOD3 reduces renal sodium retention in hypertensive rats (33). Thus, Atox1 and ATP7A may play an important role in hypertension, in part, by regulating renal and brain function (196).
Cu Transport Proteins and Pulmonary Hypertension
Pulmonary vascular remodeling and increased arterial wall stiffness are two major causes of pulmonary hypertension (PH). Zimnicka et al. (198) demonstrated that hypoxia increases Cu fluxes by upregulating CTR1 and ATP7A in pulmonary arteries and pulmonary arterial smooth muscle cells, which is associated with an increase in LOX activity and cross-linking of ECM, eventually leading to the increase in pulmonary arterial stiffness and development of pulmonary hypertension. Consistent with this, Nave et al. (136) showed that LOX is dysregulated in clinical and experimental PH. LOX plays a causal role in experimental PH and represents a candidate therapeutic target, suggesting that modulation of lung matrix cross-linking can affect pulmonary vascular remodeling associated with PH. Furthermore, Bogaard et al. (21) showed that a Cu-depleted diet prevented, and Cu chelation with TTM reversed, the development of PH and inhibited the proliferation of human pulmonary microvascular endothelial cells isolated from PH patients. The Cu chelation-induced reopening of obstructed vessels is caused by caspase-independent apoptosis, reduced vessel wall cell proliferation, and a normalization of vessel wall structure, while neither SOD1 inhibition nor LOX-1 inhibition prevents cell proliferation.
DIABETES
Tissue Cu levels are increased in streptozotocin (STZ)-induced diabetes, which is rescued by insulin treatment (44). Furthermore, Cu chelation therapy has been shown to mitigate various pathogenic states of diabetes, such as left ventricular hypertrophy in diabetic patients (37), diabetic neuropathy (30), and diabetic nephropathy (56).
Cu Transport Proteins and Diabetes
We recently found that ATP7A protein expression, but not Atox1, CCS, or COX17 (Cu chaperone for cytochrome c-oxidase), is selectively and significantly decreased in vessels of type 1 diabetes mellitus (T1DM) mice including STZ-induced and genetically induced (i.e., Ins2Akita) mice (176) as well as those of type T2DM mice including high-fat diet (HFD)-induced and genetically induced (i.e., db/db) mice (Fig. 3). These data imply that Cu transport systems coupled to distinct Cu enzymes can be differentially regulated in response to diabetes, as reported for hypoxia (194). Given that ATP7A is required for activation of extracellular SOD (SOD3), but not that of SOD1 (53), the ATP7A downregulation in diabetic vessels contributes to the decrease in specific activity of SOD3, but not that of SOD1, which may result in increased superoxide production and endothelial dysfunction in both T1DM and T2DM mice (175, 176). We found that ATP7A protein expression is significantly reduced in blood vessels from T1DM mice, in part due to the insulin deficiency, but not high glucose, and that insulin treatment of VSMCs increases ATP7A protein expression without regulating its transcription (176). Consistent with this, insulin-induced ATP7A expression is also observed in human placental Jeg-3 cells (64). It is reported that ATP7A protein downregulation in T2DM mice vessels with hyperinsulinemia and insulin resistance is due to impaired insulin/Akt2 pathway in VSMCs (175). Akt2, activated by insulin in VSMCs, promotes ATP7A stabilization by preventing its ubiquitination/degradation that contributes to activation of SOD3 that in turn protects against T2DM-induced endothelial dysfunction (Fig. 3). Downregulation of ATP7A in T2DM vessels is restored by constitutively active Akt or in PTP1B−/− (protein-tyrosine phosphatase 1B-deficient) T2DM mice, which enhances insulin-Akt signaling. Immunoprecipitation, in vitro kinase assays, and mass spectrometry analysis reveal that insulin stimulates Akt2 binding to ATP7A to induce its phosphorylation at Ser1424/1463/1466. Furthermore, SOD3 activity is reduced in Akt2−/− or T1DM or T2DM vessels, which is rescued by ATP7A overexpression. Endogenous roles for SOD3 and ATP7A are demonstrated by enhanced endothelial dysfunction and vascular O2− production in the SOD3−/− or ATP7A mutant DM mice. These findings suggest a protective role for the endogenous insulin-Akt2/ATP7A/SOD3 pathway in vascular dysfunction in diabetes (Fig. 3).
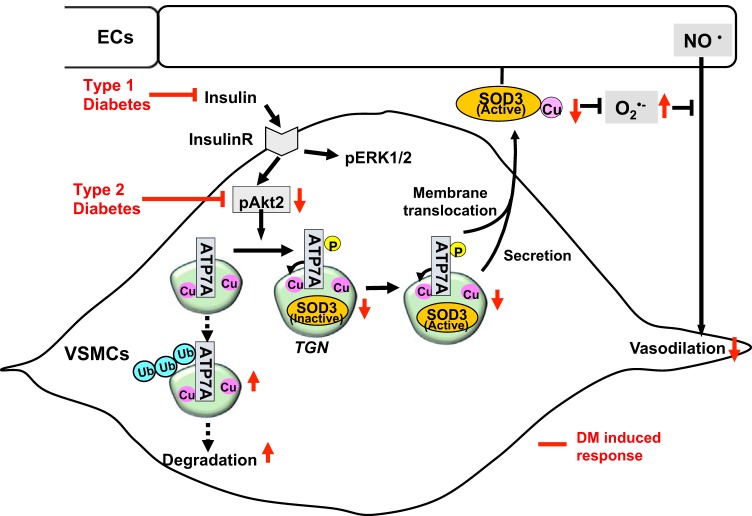
Fig. 3.
Protective role of the insulin-Akt2/ATP7A/superoxide dismutase 3 (SOD3) pathway against diabetes mellitus (DM)-induced endothelial dysfunction. Decreased ATP7A expression in diabetic vessels with either hypoinsulinemia (type1 DM) or selective impairment of insulin/Akt signaling (type 2 DM) contributes to decreased SOD3 activity, resulting in increased O2·− production and endothelial dysfunction. Mechanistically, insulin increases Akt2 binding to ATP7A to induce ATP7A phosphorylation, which may increase ATP7A protein expression via preventing proteasomal degradation as well as ATP7A translocation to the plasma membrane, which contributes to full activation of SOD3 in vascular smooth muscle cells (VSMCs) and preserves endothelial function. ECs, endothelial cells; TGN, trans-Golgi network.
ANGIOGENESIS
Cu and Angiogenesis
Cu plays an important role in physiological and pathological angiogenesis (27, 28, 46, 58, 65, 193). Cu or Cu complexes directly stimulate angiogenesis in a number of model systems including the rabbit corneal system (159) and various source of endothelial cells (6, 129). Furthermore, Cu chelators inhibit tumor growth and angiogenic responses (27, 28, 58, 65) in numerous animal and xenograft models (58). Cu metabolism appears to be altered in tumors (8, 75), while angiogenic lesions in cancer have higher Cu levels in cell nuclei than normal tissues and serum Cu levels themselves appear to be elevated in a number of tumor types (36) including breast cancer (61). Thus, systemic removal of Cu may have promise in tumor treatment in an antiangiogenic strategy. Indeed, a number of clinical trials for the treatment of solid tumors by Cu chelation showed efficacy in disease stabilization (29, 112, 160). There are several possible mechanisms for Cu-mediated angiogenesis. It has been shown that expression of VEGF is induced by the treatment of cells with Cu salts, partly by activation of hypoxia-inducible factor-1α (HIF-1α) or other factors (81, 166, 186). FGF1, angiogenic growth factor, is a Cu-binding protein and its secretion requires the Cu-dependent formation of a complex consisting of FGF1, S100A13, and p40 synaptotagmin 1, due to the lack of a signal peptide (101, 121). Moreover, Cu binds to the receptor for FGF, angiogenic growth factor, and enables it to interact with heparin (65, 186). Cu also increases the affinity with which angiogenin, a potent angiogenic molecule, binds to high-affinity endothelial receptors (170). This array of findings suggests multiple other mechanisms whereby Cu may act as an essential cofactor in angiogenesis. Other relevant potential targets for Cu chelation approaches include various Cu containing enzymes such as SOD1, cytochrome c-oxidase (COX), tyrosinase, dopamine β-hydroxylase, LOX, and ceruloplasmin, because their levels are associated with various tumor progressions (65, 112, 186).
Cu Transport Proteins and Angiogenesis
We reported that Atox1 plays an essential role in inflammatory neovascularization via regulating ROS/NF-κB pathway (32) (Fig. 4). Atox1 expression is significantly upregulated in patients and mice with critical limb ischemia. Mice lacking Atox1 exhibit impaired ischemia-induced arteriogenesis/angiogenesis and infiltration of inflammatory cells that produce angiogenic cytokines including VEGF and TNF-α. Bone marrow transplantation experiments reveal that Atox1 in endothelial cells, but not bone marrow cells, is required for post-ischemic revascularization. In cultured ECs, Atox1 functions as a Cu chaperone to promote VEGF-induced angiogenesis via increasing LOX activity in a Cu/ATP7A-dependent manner. In response to TNF-α, but not VEGF, Atox1 translocates to the nucleus, resulting in binding to the p47phox promoter, thereby promoting the ROS/NF-κB-dependent ICAM-1/VCAM-1 expression in ECs, in an ATP7A-independent manner, which in turn recruits inflammatory cells. This study provides a novel linkage between Atox1 and NADPH oxidase as well as positive feedback loops that promote inflammatory neovascularization organized by the Cu chaperone and transcription factor functions of Atox1 (Fig. 4). Furthermore, Finney et al. (45) showed endothelial Cu secretion in FGF2- and VEGF-activated microvascular ECs by using X-ray fluorescent microscopy that allows for the quantitative imaging of numerous metals at the submicron scale. It is possible that Cu exporter ATP7A may be involved in this response; however, its functional significance remains unclear. ATP7A is highly expressed in the vasculature (endothelial cells and VSMCs), macrophages, and fibroblasts (2, 12, 86, 155, 156, 194). The involvement of ATP7A in angiogenesis is implicated by the etiological role of mutant transporters, responsible for the human Menkes syndrome, in the onset of congenital cardiovascular abnormalities (67). Further investigation into the roles of ATP7A and CTR1 in postnatal angiogenesis is needed.
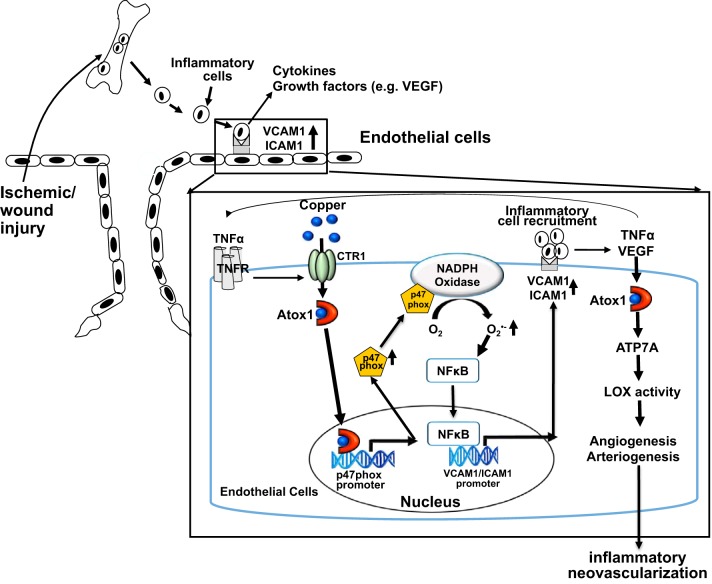
Fig. 4.
Role of antioxidant-1 (Atox1) in inflammatory neovascularization in response to ischemia and wound injury, which is dependent on arteriogenesis/angiogenesis and inflammation. In response to proinflammatory cytokine TNF-α, Atox1 functions as a Cu-dependent transcription factor for NADPH oxidase organizer, p47phox, to increase the reactive oxygen species (ROS)/NF-κB/VCAM-1/ICAM-1 axis in endothelial cells. This in turn promotes recruitment of inflammatory cells that secrete TNF-α and VEGF. In response to VEGF, Atox1 functions as a Cu chaperone for ATP7A, to increase lysyl oxidase (LOX) activity involved in angiogenesis. This process represents a novel positive feedback loop whereby the Cu chaperone protein Atox1 promotes inflammatory neovascularization. CTR1, copper transporter 1; TNFR, TNF receptor.
WOUND HEALING
The earliest recorded application of Cu in wounds can be traced to around 2000 BC in ancient Egypt (24, 41). Indeed, Cu-accelerated wound healing has been demonstrated in various clinical and experimental settings (23, 123, 166), including diabetic ulcers (134). Wound healing proceeds in three overlapping but functionally distinct phases: an inflammatory phase, the cell proliferation phase, and a maturation phase (62, 187). Little information is available regarding the mechanism of how exogenous or endogenous Cu promotes wound angiogenesis and an inflammation response required for tissue repair.
Endothelial Atox1 plays an essential role to sense Cu levels that accelerate wound angiogenesis and healing (39). Using a cutaneous wound healing model, it was shown that the Cu content and nuclear Atox1 are increased after wounding, while wound healing is impaired in global and EC-specific Atox1−/− mice. Expression of Atox1 transcription factor-target proteins, such as p47phox, NADPH oxidase, and cyclin D1, as well as extracellular matrix LOX activity in wound tissues are decreased in Atox1−/− mice. This decrease, in turn, inhibits angiogenesis, macrophage recruitment, and ECM maturation by reducing O2− production in ECs, NF-κB activity, cell proliferation and collagen formation, resulting in impaired wound healing (Fig. 4). This Atox1-mediated wound angiogenesis may occur through several alternate mechanisms. Cu treatment markedly increases Atox1 and VEGF expression in wound tissues, which are abolished in Atox1−/− mice. Consistent with this, earlier studies showed that Cu addition increases VEGF expression in part via activation of HIF-1α (81, 124, 166). We previously reported that Atox1 functions as a Cu-dependent transcription factor (77) and found that HIF-1α promoter has Atox1-responsive elements (GAAAGA), which raises the possibility that the Cu/Atox1/HIF-1α pathway may upregulate VEGF expression, thereby promoting wound angiogenesis (53, 54, 91, 113, 179). We found that Atox1−/− mice exhibit reduced LOX-specific activity and collagen deposition in wounded skin compared with wild-type mice (32). Furthermore, Atox1 also delivers Cu to the extracellular superoxide dismutase (ecSOD), which plays an important role in ischemia- and wounding-induced angiogenesis, thereby promoting wound healing (52, 87, 141). Taken together, these results suggest that in vivo Atox1 is involved in Cu-dependent wound healing via promoting ECM maturation and angiogenesis by regulating VEGF expression, activity of LOX, and ecSOD.
Cu, CARDIAC HYPERTROPHY, AND CARDIOMYOPATHY
Cardiac tissue requires high levels of Cu to meet large amounts of energy demands by sustaining mitochondrial function (130). Animal models with a Cu-deficient diet induce cardiac hypertrophy and severe cardiovascular dysfunction (119, 150). The loss of CTR1 in the intestinal epithelium (genetically imposed Cu deficiency) also resulted in cardiac hypertrophy (138). Furthermore, a cardiac-specific Ctr1 knockout mouse showed a cardiac-specific reduction in Cu levels and cardiac hypertrophy, supporting a cardiac-intrinsic requirement to prevent hypertrophy (85). Interestingly, these cardiac-specific Ctr1 knockout mice showed increased Cu exporter ATP7A expression in both liver and intestinal enterocytes, which is associated with reduced Cu levels in the liver and concomitant increase in circulating serum Cu (85). These results suggest that the reduction in Cu levels in the heart in cardiac-specific Ctr1 knockout mice is signaled to the liver, as a major Cu storage organ, to express elevated levels of ATP7A (or ATP7B) to mobilize Cu into the periphery. Furthermore, the relevance of ATP7A to cardiac development is underscored by the congenital cardiovascular abnormalities shown in Menkes disease (67).
Cu supplementation can reverse hypertrophic cardiomyopathy in some patients. For example, the Cu supplement therapy in patients with mutations of Sco2 with severe hypertrophic cardiomyopathy reverses the hypertrophic cardiomyopathy along with significant improvement in all parameters of heart function and normalization of ECG signs and blood pressure (49). Consistent with this, in rodent models of cardiac dysfunction due to prolonged pressure overload, Cu supplementation can rescue cardiac function and improve electrical conduction (81). This Cu supplementation rescue effect is associated with restoration of VEGF expression and angiogenesis, and inhibited by anti-VEGF antibody (81). Moreover, using cardiac-specific Sco1-deficient mice, it was shown that the mitochondrial metallochaperone SCO1 maintains CTR1 at the plasma membrane to preserve Cu homeostasis in the murine heart (15).
CONCLUSIONS AND FUTURE DIRECTIONS
It is apparent that although much has been learned in recent years about the major players that are important for Cu homeostasis, the involvement of this trace metal in so many fundamental cellular processes ensures that perturbations of this system will have widespread physiological consequences. We have summarized current findings regarding biochemical aspects of Cu transporters and Cu chaperones and their roles in cardiovascular physiology and disease. It is clear that dysregulation of these Cu transport systems is likely a causative or contributing factor in many disease states. Much has been learned from studies on isolated proteins, in vitro cell culture systems, and global knockout or mutant mice. The next significant advances will rely on the development of new methods and approaches, including CRISPR Cas9 technology, cell type-specific and temporal gene targeting for each Cu transporter and Cu chaperone using the Cre-loxp system, and the new optical and physical methods of imaging Cu to find out where it is and what it does when it gets there (10, 38, 46). Understanding the roles of Cu transporters and Cu chaperones in Cu homeostasis, as well as those in redox signaling, inflammatory and immune responses, tumor progression, and cardiovascular and metabolic diseases (73, 95), will doubtlessly provide new targets for emerging therapeutic strategies.
GRANTS
Research carried out in the author’s laboratories was supported by National Institutes of Health National Heart, Lung, and Blood Institute (NHLBI) Grant R01 HL-070187 (to T. Fukai), Department of Veterans Affairs Merit Review Grant 2I01BX001232-05 (to T. Fukai), and NHLBI Grants R01 HL-133613 and R01 HL-116976 (to T. Fukai and M. Ushio-Fukai) and R01 HL-135584 (to M. Ushio-Fukai).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.F., M.U.-F., and J.H.K. prepared figures; T.F., M.U.-F., and J.H.K. drafted manuscript; T.F., M.U.-F., and J.H.K. edited and revised manuscript; T.F., M.U.-F., and J.H.K. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of T. Fukai: Vascular Biology Center, Department of Pharmacology and Toxicology, Medical College of Georgia at Augusta University, Charlie Norwood VA Medical Center, 1459 Laney-Walker Blvd., Augusta, GA 30912 (e-mail: tfukai@augusta.edu).
Glossary
- ANG II
Angiotensin II
- AE1
Anion exchanger 1
- Atox1
Antioxidant-1
- ApoE
Apolipoprotein E
- BRAF
B-rapidly accelerated fibrosarcoma
- CP
Ceruloplasmin
- CVO
Circumventricular organ
- Cu
Copper
- ATP7A
ATP7B Copper-transporting p-type ATPases
- CHD
Coronary heart disease
- COX
Cytochrome c-oxidase
- CCS
Cu chaperone for SOD1
- CTR1
Copper transporter 1 (SLC31A1)
- CRISPR
Clustered regulatory interspaced short palindromic repeats
- Cas9
CRISPR-associated protein 9
- cPLA2
Calcium-dependent phospholipase A2
- DMT1
Divalent metal transporter 1
- DIDS
4,4′-diisothyocyano-stilbene-2,2′-disulfonic acid
- ERK
Extracellular signal-related kinase
- MEK1
MAPK/ERK kinase
- EEA1
Early endosome antigen 1
- ECG
Electrocardiogram
- ECs
Endothelial cells
- ECM
Extracellular matrix
- FGF
Fibroblast growth factor
- GSH
Glutathione
- HMG CoA
3-Hydroxy-3-methylglutaryl coenzyme A
- HEK
Human embryonic kidney cells
- hCTR1
Human CTR1
- HFD
High-fat diet
- IL-1α
Interleukin-1α
- IMS
Mitochondrial intermembrane space
- MAPK
Mitogen-activated protein kinase
- LOX
Lysyl oxidase
- MT
Metallothionein
- Pt
Platinum
- Pro-LOX
Proenzyme of lysyl oxidase
- LOX-PP
Lysyl oxidase propeptide
- PH
Pulmonary hypertension
- PDGF
Platelet-derived growth factor
- RAF
Rapidly accelerated fibrosarcoma
- RASMs
Rat aortic smooth muscle cells
- Rab
Ras-associated binding proteins
- ROS
Reactive oxygen species
- Sco1
Synthesis of cytochrome c-oxidase 1
- SLC25A3
Solute carrier family 25 member 3
- SOD
Superoxide dismutase
- STEAP
Six-transmembrane epithelial antigen of the prostate
- STZ
Streptozotocin
- TTM
Tetrathiomolybdate
- T1DM
Type 1 diabetes mellitus
- T2DM
Type 2 diabetes mellitus
- TGN
Trans-Golgi network
- VEGF
Vascular endothelial growth factor
- VSMC
Vascular smooth muscle cell
Footnotes
Glossary of abbreviations appears at the end of the article.
REFERENCES
- 1.Adelstein SJ, Vallee BL. Copper metabolism in man. N Engl J Med 265: 892–897, 1961. doi: 10.1056/NEJM196111022651806. [DOI] [PubMed] [Google Scholar]
- 2.Afton SE, Caruso JA, Britigan BE, Qin Z. Copper egress is induced by PMA in human THP-1 monocytic cell line. Biometals 22: 531–539, 2009. doi: 10.1007/s10534-009-9210-y. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed T, Sackner MA. Increased serum copper in primary pulmonary hypertension: a possible pathogenic link? Respiration 47: 243–246, 1985. doi: 10.1159/000194778. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed Z, Idowu BD, Brown RA. Stabilization of fibronectin mats with micromolar concentrations of copper. Biomaterials 20: 201–209, 1999. doi: 10.1016/S0142-9612(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 5.Alda JO, Garay R. Chloride (or bicarbonate)-dependent copper uptake through the anion exchanger in human red blood cells. Am J Physiol Cell Physiol 259: C570–C576, 1990. doi: 10.1152/ajpcell.1990.259.4.C570. [DOI] [PubMed] [Google Scholar]
- 6.Alessandri G, Raju K, Gullino PM. Angiogenesis in vivo and selective mobilization of capillary endothelium in vitro by heparin-copper complex. Microcirc Endothelium Lymphatics 1: 329–346, 1984. [PubMed] [Google Scholar]
- 7.Aller SG, Unger VM. Projection structure of the human copper transporter CTR1 at 6-A resolution reveals a compact trimer with a novel channel-like architecture. Proc Natl Acad Sci USA 103: 3627–3632, 2006. doi: 10.1073/pnas.0509929103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apelgot S, Coppey J, Fromentin A, Guille E, Poupon MF, Roussel A. Altered distribution of copper (64Cu) in tumor-bearing mice and rats. Anticancer Res 6: 159–164, 1986. [PubMed] [Google Scholar]
- 9.Arnold M, Sasse D. Quantitative and histochemical analysis of Cu, Zn, and Fe in spontaneous and induced primary tumors of rats. Cancer Res 21: 761–766, 1961. [PubMed] [Google Scholar]
- 10.Aron AT, Ramos-Torres KM, Cotruvo JA Jr, Chang CJ. Recognition- and reactivity-based fluorescent probes for studying transition metal signaling in living systems. Acc Chem Res 48: 2434–2442, 2015. doi: 10.1021/acs.accounts.5b00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arredondo M, Mendiburo MJ, Flores S, Singleton ST, Garrick MD. Mouse divalent metal transporter 1 is a copper transporter in HEK293 cells. Biometals 27: 115–123, 2014. doi: 10.1007/s10534-013-9691-6. [DOI] [PubMed] [Google Scholar]
- 12.Ashino T, Sudhahar V, Urao N, Oshikawa J, Chen GF, Wang H, Huo Y, Finney L, Vogt S, McKinney RD, Maryon EB, Kaplan JH, Ushio-Fukai M, Fukai T. Unexpected role of the copper transporter ATP7A in PDGF-induced vascular smooth muscle cell migration. Circ Res 107: 787–799, 2010. doi: 10.1161/CIRCRESAHA.110.225334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badet J, Soncin F, Guitton JD, Lamare O, Cartwright T, Barritault D. Specific binding of angiogenin to calf pulmonary artery endothelial cells. Proc Natl Acad Sci USA 86: 8427–8431, 1989. doi: 10.1073/pnas.86.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagheri B, Akbari N, Tabiban S, Habibi V, Mokhberi V. Serum level of copper in patients with coronary artery disease. Niger Med J 56: 39–42, 2015. doi: 10.4103/0300-1652.149169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker ZN, Jett K, Boulet A, Hossain A, Cobine PA, Kim BE, El Zawily AM, Lee L, Tibbits GF, Petris MJ, Leary SC. The mitochondrial metallochaperone SCO1 maintains CTR1 at the plasma membrane to preserve copper homeostasis in the murine heart. Hum Mol Genet 26: 4617–4628, 2017. doi: 10.1093/hmg/ddx344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banci L, Bertini I, Cantini F, Kozyreva T, Massagni C, Palumaa P, Rubino JT, Zovo K. Human superoxide dismutase 1 (hSOD1) maturation through interaction with human copper chaperone for SOD1 (hCCS). Proc Natl Acad Sci USA 109: 13555–13560, 2012. doi: 10.1073/pnas.1207493109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertinato J, L’Abbé MR. Copper modulates the degradation of copper chaperone for Cu,Zn superoxide dismutase by the 26 S proteosome. J Biol Chem 278: 35071–35078, 2003. doi: 10.1074/jbc.M302242200. [DOI] [PubMed] [Google Scholar]
- 18.Bertinato J, Swist E, Plouffe LJ, Brooks SP, L’abbé MR. Ctr2 is partially localized to the plasma membrane and stimulates copper uptake in COS-7 cells. Biochem J 409: 731–740, 2008. doi: 10.1042/BJ20071025. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharjee A, Chakraborty K, Shukla A. Cellular copper homeostasis: current concepts on its interplay with glutathione homeostasis and its implication in physiology and human diseases. Metallomics 9: 1376–1388, 2017. doi: 10.1039/C7MT00066A. [DOI] [PubMed] [Google Scholar]
- 20.Boal AK, Rosenzweig AC. Structural biology of copper trafficking. Chem Rev 109: 4760–4779, 2009. doi: 10.1021/cr900104z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bogaard HJ, Mizuno S, Guignabert C, Al Hussaini AA, Farkas D, Ruiter G, Kraskauskas D, Fadel E, Allegood JC, Humbert M, Vonk Noordegraaf A, Spiegel S, Farkas L, Voelkel NF. Copper dependence of angioproliferation in pulmonary arterial hypertension in rats and humans. Am J Respir Cell Mol Biol 46: 582–591, 2012. doi: 10.1165/rcmb.2011-0296OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bompiani KM, Tsai CY, Achatz FP, Liebig JK, Howell SB. Copper transporters and chaperones CTR1, CTR2, ATOX1, and CCS as determinants of cisplatin sensitivity. Metallomics 8: 951–962, 2016. doi: 10.1039/C6MT00076B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borkow G. Using copper to improve the well-being of the skin. Curr Chem Biol 8: 89–102, 2014. doi: 10.2174/2212796809666150227223857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borkow G, Gabbay J. Copper, an ancient remedy returning to fight microbial, fungal and viral infections. Curr Chem Biol 3: 272–278, 2009. doi: 10.2174/187231309789054887. [DOI] [Google Scholar]
- 25.Boulet A, Vest KE, Maynard MK, Gammon MG, Russell AC, Mathews AT, Cole SE, Zhu X, Phillips CB, Kwong JQ, Dodani SC, Leary SC, Cobine PA. The mammalian phosphate carrier SLC25A3 is a mitochondrial copper transporter required for cytochrome c oxidase biogenesis. J Biol Chem 293: 1887–1896, 2018. doi: 10.1074/jbc.RA117.000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brady DC, Crowe MS, Turski ML, Hobbs GA, Yao X, Chaikuad A, Knapp S, Xiao K, Campbell SL, Thiele DJ, Counter CM. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature 509: 492–496, 2014. doi: 10.1038/nature13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brewer GJ. Anticopper therapy against cancer and diseases of inflammation and fibrosis. Drug Discov Today 10: 1103–1109, 2005. doi: 10.1016/S1359-6446(05)03541-5. [DOI] [PubMed] [Google Scholar]
- 28.Brewer GJ. Tetrathiomolybdate anticopper therapy for Wilson’s disease inhibits angiogenesis, fibrosis and inflammation. J Cell Mol Med 7: 11–20, 2003. doi: 10.1111/j.1582-4934.2003.tb00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brewer GJ, Dick RD, Grover DK, LeClaire V, Tseng M, Wicha M, Pienta K, Redman BG, Jahan T, Sondak VK, Strawderman M, LeCarpentier G, Merajver SD. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: Phase I study. Clin Cancer Res 6: 1–10, 2000. [PubMed] [Google Scholar]
- 30.Cameron NE, Cotter MA. Neurovascular dysfunction in diabetic rats. Potential contribution of autoxidation and free radicals examined using transition metal chelating agents. J Clin Invest 96: 1159–1163, 1995. doi: 10.1172/JCI118104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carroll MC, Girouard JB, Ulloa JL, Subramaniam JR, Wong PC, Valentine JS, Culotta VC. Mechanisms for activating Cu- and Zn-containing superoxide dismutase in the absence of the CCS Cu chaperone. Proc Natl Acad Sci USA 101: 5964–5969, 2004. doi: 10.1073/pnas.0308298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen GF, Sudhahar V, Youn SW, Das A, Cho J, Kamiya T, Urao N, McKinney RD, Surenkhuu B, Hamakubo T, Iwanari H, Li S, Christman JW, Shantikumar S, Angelini GD, Emanueli C, Ushio-Fukai M, Fukai T. Copper transport protein antioxidant-1 promotes inflammatory neovascularization via chaperone and transcription factor function. Sci Rep 5: 14780, 2015. doi: 10.1038/srep14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu Y, Iida S, Lund DD, Weiss RM, DiBona GF, Watanabe Y, Faraci FM, Heistad DD. Gene transfer of extracellular superoxide dismutase reduces arterial pressure in spontaneously hypertensive rats: role of heparin-binding domain. Circ Res 92: 461–468, 2003. doi: 10.1161/01.RES.0000057755.02845.F9. [DOI] [PubMed] [Google Scholar]
- 34.Clegg MS, Ferrell F, Keen CL. Hypertension-induced alterations in copper and zinc metabolism in Dahl rats. Hypertension 9: 624–628, 1987. doi: 10.1161/01.HYP.9.6.624. [DOI] [PubMed] [Google Scholar]
- 35.Clifford RJ, Maryon EB, Kaplan JH. Dynamic internalization and recycling of a metal ion transporter: Cu homeostasis and CTR1, the human Cu+ uptake system. J Cell Sci 129: 1711–1721, 2016. doi: 10.1242/jcs.173351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coates RJ, Weiss NS, Daling JR, Rettmer RL, Warnick GR. Cancer risk in relation to serum copper levels. Cancer Res 49: 4353–4356, 1989. [PubMed] [Google Scholar]
- 37.Cooper GJ, Young AA, Gamble GD, Occleshaw CJ, Dissanayake AM, Cowan BR, Brunton DH, Baker JR, Phillips AR, Frampton CM, Poppitt SD, Doughty RN. A copper(II)-selective chelator ameliorates left-ventricular hypertrophy in type 2 diabetic patients: a randomised placebo-controlled study. Diabetologia 52: 715–722, 2009. doi: 10.1007/s00125-009-1265-3. [DOI] [PubMed] [Google Scholar]
- 38.Cotruvo JA Jr, Aron AT, Ramos-Torres KM, Chang CJ. Synthetic fluorescent probes for studying copper in biological systems. Chem Soc Rev 44: 4400–4414, 2015. doi: 10.1039/C4CS00346B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Das A, Sudhahar V, Chen GF, Kim HW, Youn SW, Finney L, Vogt S, Yang J, Kweon J, Surenkhuu B, Ushio-Fukai M, Fukai T. Endothelial antioxidant-1: a key mediator of copper-dependent wound healing in vivo. Sci Rep 6: 33783, 2016. doi: 10.1038/srep33783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Feo CJ, Aller SG, Siluvai GS, Blackburn NJ, Unger VM. Three-dimensional structure of the human copper transporter hCTR1. Proc Natl Acad Sci USA 106: 4237–4242, 2009. doi: 10.1073/pnas.0810286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dollwet HHA, Sorenson JRJ. History uses of copper compounds in medicine. Trace Elem Med 2: 80–87, 1985. [Google Scholar]
- 42.Eisses JF, Kaplan JH. Molecular characterization of hCTR1, the human copper uptake protein. J Biol Chem 277: 29162–29171, 2002. doi: 10.1074/jbc.M203652200. [DOI] [PubMed] [Google Scholar]
- 43.Failla ML. Trace elements and host defense: recent advances and continuing challenges. J Nutr 133, Suppl 1: 1443S–1447S, 2003. doi: 10.1093/jn/133.5.1443S. [DOI] [PubMed] [Google Scholar]
- 44.Failla ML, Kiser RA. Altered tissue content and cytosol distribution of trace metals in experimental diabetes. J Nutr 111: 1900–1909, 1981. doi: 10.1093/jn/111.11.1900. [DOI] [PubMed] [Google Scholar]
- 45.Finney L, Mandava S, Ursos L, Zhang W, Rodi D, Vogt S, Legnini D, Maser J, Ikpatt F, Olopade OI, Glesne D. X-ray fluorescence microscopy reveals large-scale relocalization and extracellular translocation of cellular copper during angiogenesis. Proc Natl Acad Sci USA 104: 2247–2252, 2007. doi: 10.1073/pnas.0607238104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Finney L, Vogt S, Fukai T, Glesne D. Copper and angiogenesis: unravelling a relationship key to cancer progression. Clin Exp Pharmacol Physiol 36: 88–94, 2009. doi: 10.1111/j.1440-1681.2008.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flores AG, Unger VM. Atox1 contains positive residues that mediate membrane association and aid subsequent copper loading. J Membr Biol 246: 903–913, 2013. doi: 10.1007/s00232-013-9592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ford ES. Serum copper concentration and coronary heart disease among US adults. Am J Epidemiol 151: 1182–1188, 2000. doi: 10.1093/oxfordjournals.aje.a010168. [DOI] [PubMed] [Google Scholar]
- 49.Freisinger P, Horvath R, Macmillan C, Peters J, Jaksch M. Reversion of hypertrophic cardiomyopathy in a patient with deficiency of the mitochondrial copper binding protein Sco2: is there a potential effect of copper? J Inherit Metab Dis 27: 67–79, 2004. doi: 10.1023/B:BOLI.0000016614.47380.2f. [DOI] [PubMed] [Google Scholar]
- 50.Fu S, Davies MJ, Stocker R, Dean RT. Evidence for roles of radicals in protein oxidation in advanced human atherosclerotic plaque. Biochem J 333: 519–525, 1998. doi: 10.1042/bj3330519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuchs AG, de Lustig ES. Localization of tissue copper in mouse mammary tumors. Oncology 46: 183–187, 1989. doi: 10.1159/000226711. [DOI] [PubMed] [Google Scholar]
- 52.Fujiwara T, Duscher D, Rustad KC, Kosaraju R, Rodrigues M, Whittam AJ, Januszyk M, Maan ZN, Gurtner GC. Extracellular superoxide dismutase deficiency impairs wound healing in advanced age by reducing neovascularization and fibroblast function. Exp Dermatol 25: 206–211, 2016. doi: 10.1111/exd.12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 15: 1583–1606, 2011. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fushida-Takemura H, Fukuda M, Maekawa N, Chanoki M, Kobayashi H, Yashiro N, Ishii M, Hamada T, Otani S, Ooshima A. Detection of lysyl oxidase gene expression in rat skin during wound healing. Arch Dermatol Res 288: 7–10, 1996. doi: 10.1007/BF02505035. [DOI] [PubMed] [Google Scholar]
- 55.Garrow TA, Clegg MS, Metzler G, Keen CL. Influence of hypertension and dietary copper on indexes of copper status in rats. Hypertension 17: 793–797, 1991. doi: 10.1161/01.HYP.17.6.793. [DOI] [PubMed] [Google Scholar]
- 56.Gong D, Lu J, Chen X, Reddy S, Crossman DJ, Glyn-Jones S, Choong YS, Kennedy J, Barry B, Zhang S, Chan YK, Ruggiero K, Phillips AR, Cooper GJ. A copper(II)-selective chelator ameliorates diabetes-evoked renal fibrosis and albuminuria, and suppresses pathogenic TGF-beta activation in the kidneys of rats used as a model of diabetes. Diabetologia 51: 1741–1751, 2008. doi: 10.1007/s00125-008-1088-7. [DOI] [PubMed] [Google Scholar]
- 57.Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, Harrison DG. Role of extracellular superoxide dismutase in hypertension. Hypertension 48: 473–481, 2006. doi: 10.1161/01.HYP.0000235682.47673.ab. [DOI] [PubMed] [Google Scholar]
- 58.Goodman VL, Brewer GJ, Merajver SD. Copper deficiency as an anti-cancer strategy. Endocr Relat Cancer 11: 255–263, 2004. doi: 10.1677/erc.0.0110255. [DOI] [PubMed] [Google Scholar]
- 59.Gunn TR, Macfarlane S, Phillips LI. Difficulties in the neonatal diagnosis of Menkes’ kinky hair syndrome–trichopoliodystrophy. Clin Pediatr (Phila) 23: 514–516, 1984. doi: 10.1177/000992288402300915. [DOI] [PubMed] [Google Scholar]
- 60.Guo Y, Smith K, Lee J, Thiele DJ, Petris MJ. Identification of methionine-rich clusters that regulate copper-stimulated endocytosis of the human Ctr1 copper transporter. J Biol Chem 279: 17428–17433, 2004. doi: 10.1074/jbc.M401493200. [DOI] [PubMed] [Google Scholar]
- 61.Gupta SK, Shukla VK, Vaidya MP, Roy SK, Gupta S. Serum trace elements and Cu/Zn ratio in breast cancer patients. J Surg Oncol 46: 178–181, 1991. doi: 10.1002/jso.2930460311. [DOI] [PubMed] [Google Scholar]
- 62.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 453: 314–321, 2008. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 63.Haas KL, Putterman AB, White DR, Thiele DJ, Franz KJ. Model peptides provide new insights into the role of histidine residues as potential ligands in human cellular copper acquisition via Ctr1. J Am Chem Soc 133: 4427–4437, 2011. doi: 10.1021/ja108890c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hardman B, Michalczyk A, Greenough M, Camakaris J, Mercer JF, Ackland ML. Hormonal regulation of the Menkes and Wilson copper-transporting ATPases in human placental Jeg-3 cells. Biochem J 402: 241–250, 2007. doi: 10.1042/BJ20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harris ED. A requirement for copper in angiogenesis. Nutr Rev 62: 60–64, 2004. doi: 10.1111/j.1753-4887.2004.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 66.Hatori Y, Lutsenko S. The role of copper chaperone Atox1 in coupling redox homeostasis to intracellular copper distribution. Antioxidants (Basel) 5: 5, 2016. doi: 10.3390/antiox5030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hicks JD, Donsante A, Pierson TM, Gillespie MJ, Chou DE, Kaler SG. Increased frequency of congenital heart defects in Menkes disease. Clin Dysmorphol 21: 59–63, 2012. doi: 10.1097/MCD.0b013e32834ea52b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hodgkinson V, Petris MJ. Copper homeostasis at the host-pathogen interface. J Biol Chem 287: 13549–13555, 2012. doi: 10.1074/jbc.R111.316406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Howell SB, Safaei R, Larson CA, Sailor MJ. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol Pharmacol 77: 887–894, 2010. doi: 10.1124/mol.109.063172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu GF. Copper stimulates proliferation of human endothelial cells under culture. J Cell Biochem 69: 326–335, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 71.Huffman DL, O’Halloran TV. Function, structure, and mechanism of intracellular copper trafficking proteins. Annu Rev Biochem 70: 677–701, 2001. doi: 10.1146/annurev.biochem.70.1.677. [DOI] [PubMed] [Google Scholar]
- 72.Huster D, Finegold MJ, Morgan CT, Burkhead JL, Nixon R, Vanderwerf SM, Gilliam CT, Lutsenko S. Consequences of copper accumulation in the livers of the Atp7b−/− (Wilson disease gene) knockout mice. Am J Pathol 168: 423–434, 2006. doi: 10.2353/ajpath.2006.050312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huster D, Purnat TD, Burkhead JL, Ralle M, Fiehn O, Stuckert F, Olson NE, Teupser D, Lutsenko S. High copper selectively alters lipid metabolism and cell cycle machinery in the mouse model of Wilson disease. J Biol Chem 282: 8343–8355, 2007. doi: 10.1074/jbc.M607496200. [DOI] [PubMed] [Google Scholar]
- 74.Inesi G, Pilankatta R, Tadini-Buoninsegni F. Biochemical characterization of P-type copper ATPases. Biochem J 463: 167–176, 2014. doi: 10.1042/BJ20140741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ishida S, Andreux P, Poitry-Yamate C, Auwerx J, Hanahan D. Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc Natl Acad Sci USA 110: 19507–19512, 2013. doi: 10.1073/pnas.1318431110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci USA 99: 14298–14302, 2002. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Itoh S, Kim HW, Nakagawa O, Ozumi K, Lessner SM, Aoki H, Akram K, McKinney RD, Ushio-Fukai M, Fukai T. Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell proliferation. J Biol Chem 283: 9157–9167, 2008. doi: 10.1074/jbc.M709463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ivy KD, Kaplan JH. A re-evaluation of the role of hCTR1, the human high-affinity copper transporter, in platinum-drug entry into human cells. Mol Pharmacol 83: 1237–1246, 2013. doi: 10.1124/mol.113.085068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jeney V, Itoh S, Wendt M, Gradek Q, Ushio-Fukai M, Harrison DG, Fukai T. Role of antioxidant-1 in extracellular superoxide dismutase function and expression. Circ Res 96: 723–729, 2005. doi: 10.1161/01.RES.0000162001.57896.66. [DOI] [PubMed] [Google Scholar]
- 80.Jeremy JY, Shukla N, Angelini GD, Day A, Wan IY, Talpahewa SP, Ascione R. Sustained increases of plasma homocysteine, copper, and serum ceruloplasmin after coronary artery bypass grafting. Ann Thorac Surg 74: 1553–1557, 2002. doi: 10.1016/S0003-4975(02)03807-9. [DOI] [PubMed] [Google Scholar]
- 81.Jiang Y, Reynolds C, Xiao C, Feng W, Zhou Z, Rodriguez W, Tyagi SC, Eaton JW, Saari JT, Kang YJ. Dietary copper supplementation reverses hypertrophic cardiomyopathy induced by chronic pressure overload in mice. J Exp Med 204: 657–666, 2007. doi: 10.1084/jem.20061943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaplan JH, Lutsenko S. Copper transport in mammalian cells: special care for a metal with special needs. J Biol Chem 284: 25461–25465, 2009. doi: 10.1074/jbc.R109.031286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaplan JH, Maryon EB. How mammalian cells acquire copper: an essential but potentially toxic metal. Biophys J 110: 7–13, 2016. doi: 10.1016/j.bpj.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol 4: 176–185, 2008. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- 85.Kim BE, Turski ML, Nose Y, Casad M, Rockman HA, Thiele DJ. Cardiac copper deficiency activates a systemic signaling mechanism that communicates with the copper acquisition and storage organs. Cell Metab 11: 353–363, 2010. doi: 10.1016/j.cmet.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim HW, Chan Q, Afton SE, Caruso JA, Lai B, Weintraub NL, Qin Z. Human macrophage ATP7A is localized in the trans-Golgi apparatus, controls intracellular copper levels, and mediates macrophage responses to dermal wounds. Inflammation 35: 167–175, 2012. doi: 10.1007/s10753-011-9302-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim HW, Lin A, Guldberg RE, Ushio-Fukai M, Fukai T. Essential role of extracellular SOD in reparative neovascularization induced by hindlimb ischemia. Circ Res 101: 409–419, 2007. doi: 10.1161/CIRCRESAHA.107.153791. [DOI] [PubMed] [Google Scholar]
- 88.Klevay LM. Cardiovascular disease from copper deficiency–a history. J Nutr 130, Suppl: 489S–492S, 2000. doi: 10.1093/jn/130.2.489S. [DOI] [PubMed] [Google Scholar]
- 89.Klevay LM. Hypertension in rats due to copper deficiency. Nutr Rep Int 35: 999–1005, 1987. [Google Scholar]
- 90.Klomp AE, Juijn JA, van der Gun LT, van den Berg IE, Berger R, Klomp LW. The N-terminus of the human copper transporter 1 (hCTR1) is localized extracellularly, and interacts with itself. Biochem J 370: 881–889, 2003. doi: 10.1042/bj20021128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kobayashi H, Ishii M, Chanoki M, Yashiro N, Fushida H, Fukai K, Kono T, Hamada T, Wakasaki H, Ooshima A. Immunohistochemical localization of lysyl oxidase in normal human skin. Br J Dermatol 131: 325–330, 1994. doi: 10.1111/j.1365-2133.1994.tb08518.x. [DOI] [PubMed] [Google Scholar]
- 92.Kohno T, Urao N, Ashino T, Sudhahar V, McKinney RD, Hamakubo T, Iwanari H, Ushio-Fukai M, Fukai T. Novel role of copper transport protein antioxidant-1 in neointimal formation after vascular injury. Arterioscler Thromb Vasc Biol 33: 805–813, 2013. doi: 10.1161/ATVBAHA.112.300862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kok FJ, Van Duijn CM, Hofman A, Van der Voet GB, De Wolff FA, Paays CH, Valkenburg HA. Serum copper and zinc and the risk of death from cancer and cardiovascular disease. Am J Epidemiol 128: 352–359, 1988. doi: 10.1093/oxfordjournals.aje.a114975. [DOI] [PubMed] [Google Scholar]
- 94.Koksal C, Ercan M, Bozkurt AK, Cortelekoglu T, Konukoglu D. Abdominal aortic aneurysm or aortic occlusive disease: role of trace element imbalance. Angiology 58: 191–195, 2007. doi: 10.1177/0003319707300354. [DOI] [PubMed] [Google Scholar]
- 95.Krishnamoorthy L, Cotruvo JA Jr, Chan J, Kaluarachchi H, Muchenditsi A, Pendyala VS, Jia S, Aron AT, Ackerman CM, Wal MN, Guan T, Smaga LP, Farhi SL, New EJ, Lutsenko S, Chang CJ. Copper regulates cyclic-AMP-dependent lipolysis. Nat Chem Biol 12: 586–592, 2016. doi: 10.1038/nchembio.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuo YM, Zhou B, Cosco D, Gitschier J. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc Natl Acad Sci USA 98: 6836–6841, 2001. doi: 10.1073/pnas.111057298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lamb AL, Torres AS, O’Halloran TV, Rosenzweig AC. Heterodimeric structure of superoxide dismutase in complex with its metallochaperone. Nat Struct Biol 8: 751–755, 2001. doi: 10.1038/nsb0901-751. [DOI] [PubMed] [Google Scholar]
- 98.Lamb AL, Wernimont AK, Pufahl RA, Culotta VC, O’Halloran TV, Rosenzweig AC. Crystal structure of the copper chaperone for superoxide dismutase. Nat Struct Biol 6: 724–729, 1999. doi: 10.1038/11489. [DOI] [PubMed] [Google Scholar]
- 99.Lamb DJ, Avades TY, Ferns GA. Biphasic modulation of atherosclerosis induced by graded dietary copper supplementation in the cholesterol-fed rabbit. Int J Exp Pathol 82: 287–294, 2001. doi: 10.1046/j.1365-2613.2001.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lamb DJ, Mitchinson MJ, Leake DS. Transition metal ions within human atherosclerotic lesions can catalyse the oxidation of low density lipoprotein by macrophages. FEBS Lett 374: 12–16, 1995. doi: 10.1016/0014-5793(95)01068-P. [DOI] [PubMed] [Google Scholar]
- 101.Landriscina M, Bagalá C, Mandinova A, Soldi R, Micucci I, Bellum S, Prudovsky I, Maciag T. Copper induces the assembly of a multiprotein aggregate implicated in the release of fibroblast growth factor 1 in response to stress. J Biol Chem 276: 25549–25557, 2001. doi: 10.1074/jbc.M102925200. [DOI] [PubMed] [Google Scholar]
- 102.Leary SC. Redox regulation of SCO protein function: controlling copper at a mitochondrial crossroad. Antioxid Redox Signal 13: 1403–1416, 2010. doi: 10.1089/ars.2010.3116. [DOI] [PubMed] [Google Scholar]
- 103.Lee J, Petris MJ, Thiele DJ. Characterization of mouse embryonic cells deficient in the ctr1 high affinity copper transporter. Identification of a Ctr1-independent copper transport system. J Biol Chem 277: 40253–40259, 2002. doi: 10.1074/jbc.M208002200. [DOI] [PubMed] [Google Scholar]
- 104.Lee J, Prohaska JR, Dagenais SL, Glover TW, Thiele DJ. Isolation of a murine copper transporter gene, tissue specific expression and functional complementation of a yeast copper transport mutant. Gene 254: 87–96, 2000. doi: 10.1016/S0378-1119(00)00287-0. [DOI] [PubMed] [Google Scholar]
- 105.Lee J, Prohaska JR, Thiele DJ. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc Natl Acad Sci USA 98: 6842–6847, 2001. doi: 10.1073/pnas.111058698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leeuwenburgh C, Rasmussen JE, Hsu FF, Mueller DM, Pennathur S, Heinecke JW. Mass spectrometric quantification of markers for protein oxidation by tyrosyl radical, copper, and hydroxyl radical in low density lipoprotein isolated from human atherosclerotic plaques. J Biol Chem 272: 3520–3526, 1997. doi: 10.1074/jbc.272.6.3520. [DOI] [PubMed] [Google Scholar]
- 107.Leitch JM, Yick PJ, Culotta VC. The right to choose: multiple pathways for activating copper,zinc superoxide dismutase. J Biol Chem 284: 24679–24683, 2009. doi: 10.1074/jbc.R109.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Linder MC. Biochemistry of Copper. New York: Plenum Press, 1991. doi: 10.1007/978-1-4757-9432-8. [DOI] [Google Scholar]
- 109.Lob HE, Marvar PJ, Guzik TJ, Sharma S, McCann LA, Weyand C, Gordon FJ, Harrison DG. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension 55: 277–283, 2010. doi: 10.1161/HYPERTENSIONAHA.109.142646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lob HE, Vinh A, Li L, Blinder Y, Offermanns S, Harrison DG. Role of vascular extracellular superoxide dismutase in hypertension. Hypertension 58: 232–239, 2011. doi: 10.1161/HYPERTENSIONAHA.111.172718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Logeman BL, Wood LK, Lee J, Thiele DJ. Gene duplication and neo-functionalization in the evolutionary and functional divergence of the metazoan copper transporters Ctr1 and Ctr2. J Biol Chem 292: 11531–11546, 2017. doi: 10.1074/jbc.M117.793356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lowndes SA, Harris AL. The role of copper in tumour angiogenesis. J Mammary Gland Biol Neoplasia 10: 299–310, 2005. doi: 10.1007/s10911-006-9003-7. [DOI] [PubMed] [Google Scholar]
- 113.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 3: a005058, 2011. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lutsenko S. Copper trafficking to the secretory pathway. Metallomics 8: 840–852, 2016. doi: 10.1039/C6MT00176A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiol Rev 87: 1011–1046, 2007. doi: 10.1152/physrev.00004.2006. [DOI] [PubMed] [Google Scholar]
- 116.Lutsenko S, Gupta A, Burkhead JL, Zuzel V. Cellular multitasking: the dual role of human Cu-ATPases in cofactor delivery and intracellular copper balance. Arch Biochem Biophys 476: 22–32, 2008. doi: 10.1016/j.abb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lutsenko S, Kaplan JH. Organization of P-type ATPases: significance of structural diversity. Biochemistry 34: 15607–15613, 1995. doi: 10.1021/bi00048a001. [DOI] [PubMed] [Google Scholar]
- 118.Lutsenko S, Petrukhin K, Cooper MJ, Gilliam CT, Kaplan JH. N-terminal domains of human copper-transporting adenosine triphosphatases (the Wilson’s and Menkes disease proteins) bind copper selectively in vivo and in vitro with stoichiometry of one copper per metal-binding repeat. J Biol Chem 272: 18939–18944, 1997. doi: 10.1074/jbc.272.30.18939. [DOI] [PubMed] [Google Scholar]
- 119.Mandinov L, Mandinova A, Kyurkchiev S, Kyurkchiev D, Kehayov I, Kolev V, Soldi R, Bagala C, de Muinck ED, Lindner V, Post MJ, Simons M, Bellum S, Prudovsky I, Maciag T. Copper chelation represses the vascular response to injury. Proc Natl Acad Sci USA 100: 6700–6705, 2003. doi: 10.1073/pnas.1231994100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mandinov L, Moodie KL, Mandinova A, Zhuang Z, Redican F, Baklanov D, Lindner V, Maciag T, Simons M, de Muinck ED. Inhibition of in-stent restenosis by oral copper chelation in porcine coronary arteries. Am J Physiol Heart Circ Physiol 291: H2692–H2697, 2006. doi: 10.1152/ajpheart.00148.2006. [DOI] [PubMed] [Google Scholar]
- 121.Mandinova A, Soldi R, Graziani I, Bagala C, Bellum S, Landriscina M, Tarantini F, Prudovsky I, Maciag T. S100A13 mediates the copper-dependent stress-induced release of IL-1alpha from both human U937 and murine NIH 3T3 cells. J Cell Sci 116: 2687–2696, 2003. doi: 10.1242/jcs.00471. [DOI] [PubMed] [Google Scholar]
- 122.Mansoor MA, Bergmark C, Haswell SJ, Savage IF, Evans PH, Berge RK, Svardal AM, Kristensen O. Correlation between plasma total homocysteine and copper in patients with peripheral vascular disease. Clin Chem 46: 385–391, 2000. [PubMed] [Google Scholar]
- 123.Maquart FX, Bellon G, Chaqour B, Wegrowski J, Patt LM, Trachy RE, Monboisse JC, Chastang F, Birembaut P, Gillery P. In vivo stimulation of connective tissue accumulation by the tripeptide-copper complex glycyl-l-histidyl-l-lysine-Cu2+ in rat experimental wounds. J Clin Invest 92: 2368–2376, 1993. doi: 10.1172/JCI116842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Martin F, Linden T, Katschinski DM, Oehme F, Flamme I, Mukhopadhyay CK, Eckhardt K, Tröger J, Barth S, Camenisch G, Wenger RH. Copper-dependent activation of hypoxia-inducible factor (HIF)-1: implications for ceruloplasmin regulation. Blood 105: 4613–4619, 2005. doi: 10.1182/blood-2004-10-3980. [DOI] [PubMed] [Google Scholar]
- 125.Maryon EB, Molloy SA, Ivy K, Yu H, Kaplan JH. Rate and regulation of copper transport by human copper transporter 1 (hCTR1). J Biol Chem 288: 18035–18046, 2013. doi: 10.1074/jbc.M112.442426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maryon EB, Molloy SA, Kaplan JH. Cellular glutathione plays a key role in copper uptake mediated by human copper transporter 1. Am J Physiol Cell Physiol 304: C768–C779, 2013. doi: 10.1152/ajpcell.00417.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maryon EB, Molloy SA, Kaplan JH. O-linked glycosylation at threonine 27 protects the copper transporter hCTR1 from proteolytic cleavage in mammalian cells. J Biol Chem 282: 20376–20387, 2007. doi: 10.1074/jbc.M701806200. [DOI] [PubMed] [Google Scholar]
- 128.Maryon EB, Zhang J, Jellison JW, Kaplan JH. Human copper transporter 1 lacking O-linked glycosylation is proteolytically cleaved in a Rab9-positive endosomal compartment. J Biol Chem 284: 28104–28114, 2009. doi: 10.1074/jbc.M109.044925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McAuslan BR, Reilly WG, Hannan GN, Gole GA. Angiogenic factors and their assay: activity of formyl methionyl leucyl phenylalanine, adenosine diphosphate, heparin, copper, and bovine endothelium stimulating factor. Microvasc Res 26: 323–338, 1983. doi: 10.1016/0026-2862(83)90080-8. [DOI] [PubMed] [Google Scholar]
- 130.Medeiros DM, Davidson J, Jenkins JE. A unified perspective on copper deficiency and cardiomyopathy. Proc Soc Exp Biol Med 203: 262–273, 1993. doi: 10.3181/00379727-203-43599. [DOI] [PubMed] [Google Scholar]
- 131.Medeiros DM, Lin KM, Liu C, Thorne BM. Pre-gestation dietary copper restriction and blood pressure in the Long-Evans rat. Nutr Rep Int 30: 559–564, 1984. [Google Scholar]
- 132.Molloy SA, Kaplan JH. Copper-dependent recycling of hCTR1, the human high affinity copper transporter. J Biol Chem 284: 29704–29713, 2009. doi: 10.1074/jbc.M109.000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moore SD, Helmle KE, Prat LM, Cox DW. Tissue localization of the copper chaperone ATOX1 and its potential role in disease. Mamm Genome 13: 563–568, 2002. doi: 10.1007/s00335-002-2172-9. [DOI] [PubMed] [Google Scholar]
- 134.Mulder GD, Patt LM, Sanders L, Rosenstock J, Altman MI, Hanley ME, Duncan GW. Enhanced healing of ulcers in patients with diabetes by topical treatment with glycyl-l-histidyl-l-lysine copper. Wound Repair Regen 2: 259–269, 1994. doi: 10.1046/j.1524-475X.1994.20406.x. [DOI] [PubMed] [Google Scholar]
- 135.Narayanan G, Bharathidevi SR, Vuyyuru H, Muthuvel B, Konerirajapuram Natrajan S. CTR1 silencing inhibits angiogenesis by limiting copper entry into endothelial cells. PLoS One 8: e71982, 2013. doi: 10.1371/journal.pone.0071982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nave AH, Mižíková I, Niess G, Steenbock H, Reichenberger F, Talavera ML, Veit F, Herold S, Mayer K, Vadász I, Weissmann N, Seeger W, Brinckmann J, Morty RE. Lysyl oxidases play a causal role in vascular remodeling in clinical and experimental pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 34: 1446–1458, 2014. doi: 10.1161/ATVBAHA.114.303534. [DOI] [PubMed] [Google Scholar]
- 137.Nishihara E, Furuyama T, Yamashita S, Mori N. Expression of copper trafficking genes in the mouse brain. Neuroreport 9: 3259–3263, 1998. doi: 10.1097/00001756-199810050-00023. [DOI] [PubMed] [Google Scholar]
- 138.Nose Y, Kim BE, Thiele DJ. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab 4: 235–244, 2006. doi: 10.1016/j.cmet.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 139.Ohgami RS, Campagna DR, McDonald A, Fleming MD. The Steap proteins are metalloreductases. Blood 108: 1388–1394, 2006. doi: 10.1182/blood-2006-02-003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Olatunbosun DA, Bolodeoku JO, Cole TO, Adadevoh BK. Relationship of serum copper and zinc to human hypertension in Nigerians. Bull World Health Organ 53: 134–135, 1976. [PMC free article] [PubMed] [Google Scholar]
- 141.Oshikawa J, Urao N, Kim HW, Kaplan N, Razvi M, McKinney R, Poole LB, Fukai T, Ushio-Fukai M. Extracellular SOD-derived H2O2 promotes VEGF signaling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PLoS One 5: e10189, 2010. doi: 10.1371/journal.pone.0010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ozumi K, Sudhahar V, Kim HW, Chen GF, Kohno T, Finney L, Vogt S, McKinney RD, Ushio-Fukai M, Fukai T. Role of copper transport protein antioxidant 1 in angiotensin II-induced hypertension: a key regulator of extracellular superoxide dismutase. Hypertension 60: 476–486, 2012. doi: 10.1161/HYPERTENSIONAHA.111.189571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Palmgren MG, Nissen P. P-type ATPases. Annu Rev Biophys 40: 243–266, 2011. doi: 10.1146/annurev.biophys.093008.131331. [DOI] [PubMed] [Google Scholar]
- 144.Palumaa P. Copper chaperones. The concept of conformational control in the metabolism of copper. FEBS Lett 587: 1902–1910, 2013. doi: 10.1016/j.febslet.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 145.Pan Q, Bao LW, Merajver SD. Tetrathiomolybdate inhibits angiogenesis and metastasis through suppression of the NFκB signaling cascade. Mol Cancer Res 1: 701–706, 2003. [PubMed] [Google Scholar]
- 146.Percival SS. Copper and immunity. Am J Clin Nutr 67, Suppl: 1064S–1068S, 1998. doi: 10.1093/ajcn/67.5.1064S. [DOI] [PubMed] [Google Scholar]
- 147.Persichini T, Percario Z, Mazzon E, Colasanti M, Cuzzocrea S, Musci G. Copper activates the NF-kappaB pathway in vivo. Antioxid Redox Signal 8: 1897–1904, 2006. doi: 10.1089/ars.2006.8.1897. [DOI] [PubMed] [Google Scholar]
- 148.Petris MJ, Smith K, Lee J, Thiele DJ. Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J Biol Chem 278: 9639–9646, 2003. doi: 10.1074/jbc.M209455200. [DOI] [PubMed] [Google Scholar]
- 149.Prohaska JR, Geissler J, Brokate B, Broderius M. Copper, zinc-superoxide dismutase protein but not mRNA is lower in copper-deficient mice and mice lacking the copper chaperone for superoxide dismutase. Exp Biol Med (Maywood) 228: 959–966, 2003. doi: 10.1177/153537020322800812. [DOI] [PubMed] [Google Scholar]
- 150.Prohaska JR, Heller LJ. Mechanical properties of the copper-deficient rat heart. J Nutr 112: 2142–2150, 1982. [DOI] [PubMed] [Google Scholar]
- 151.Prudovsky I, Mandinova A, Soldi R, Bagala C, Graziani I, Landriscina M, Tarantini F, Duarte M, Bellum S, Doherty H, Maciag T. The non-classical export routes: FGF1 and IL-1alpha point the way. J Cell Sci 116: 4871–4881, 2003. doi: 10.1242/jcs.00872. [DOI] [PubMed] [Google Scholar]
- 152.Pufahl RA, Singer CP, Peariso KL, Lin SJ, Schmidt PJ, Fahrni CJ, Culotta VC, Penner-Hahn JE, O’Halloran TV. Metal ion chaperone function of the soluble Cu(I) receptor Atx1. Science 278: 853–856, 1997. doi: 10.1126/science.278.5339.853. [DOI] [PubMed] [Google Scholar]
- 153.Puig S, Lee J, Lau M, Thiele DJ. Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J Biol Chem 277: 26021–26030, 2002. doi: 10.1074/jbc.M202547200. [DOI] [PubMed] [Google Scholar]
- 154.Qin Z, Gongora MC, Ozumi K, Itoh S, Akram K, Ushio-Fukai M, Harrison DG, Fukai T. Role of Menkes ATPase in angiotensin II-induced hypertension: a key modulator for extracellular superoxide dismutase function. Hypertension 52: 945–951, 2008. doi: 10.1161/HYPERTENSIONAHA.108.116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Qin Z, Itoh S, Jeney V, Ushio-Fukai M, Fukai T. Essential role for the Menkes ATPase in activation of extracellular superoxide dismutase: implication for vascular oxidative stress. FASEB J 20: 334–336, 2006. doi: 10.1096/fj.05-4564fje. [DOI] [PubMed] [Google Scholar]
- 156.Qin Z, Konaniah ES, Neltner B, Nemenoff RA, Hui DY, Weintraub NL. Participation of ATP7A in macrophage mediated oxidation of LDL. J Lipid Res 51: 1471–1477, 2010. doi: 10.1194/jlr.M003426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O’Halloran TV. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 284: 805–808, 1999. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 158.Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Raju KS, Alessandri G, Ziche M, Gullino PM. Ceruloplasmin, copper ions, and angiogenesis. J Natl Cancer Inst 69: 1183–1188, 1982. [PubMed] [Google Scholar]
- 160.Redman BG, Esper P, Pan Q, Dunn RL, Hussain HK, Chenevert T, Brewer GJ, Merajver SD. Phase II trial of tetrathiomolybdate in patients with advanced kidney cancer. Clin Cancer Res 9: 1666–1672, 2003. [PubMed] [Google Scholar]
- 161.Reunanen A, Knekt P, Aaran RK. Serum ceruloplasmin level and the risk of myocardial infarction and stroke. Am J Epidemiol 136: 1082–1090, 1992. doi: 10.1093/oxfordjournals.aje.a116573. [DOI] [PubMed] [Google Scholar]
- 162.Robinson NJ, Winge DR. Copper metallochaperones. Annu Rev Biochem 79: 537–562, 2010. doi: 10.1146/annurev-biochem-030409-143539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Salonen JT, Salonen R, Korpela H, Suntioinen S, Tuomilehto J. Serum copper and the risk of acute myocardial infarction: a prospective population study in men in eastern Finland. Am J Epidemiol 134: 268–276, 1991. doi: 10.1093/oxfordjournals.aje.a116080. [DOI] [PubMed] [Google Scholar]
- 164.Savir Y, Martynov A, Springer M. Achieving global perfect homeostasis through transporter regulation. PLOS Comput Biol 13: e1005458, 2017. doi: 10.1371/journal.pcbi.1005458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schuschke DA, Saari JT, Miller FN. Leukocyte-endothelial adhesion is impaired in the cremaster muscle microcirculation of the copper-deficient rat. Immunol Lett 76: 139–144, 2001. doi: 10.1016/S0165-2478(01)00171-7. [DOI] [PubMed] [Google Scholar]
- 166.Sen CK, Khanna S, Venojarvi M, Trikha P, Ellison EC, Hunt TK, Roy S. Copper-induced vascular endothelial growth factor expression and wound healing. Am J Physiol Heart Circ Physiol 282: H1821–H1827, 2002. doi: 10.1152/ajpheart.01015.2001. [DOI] [PubMed] [Google Scholar]
- 167.Shawki A, Anthony SR, Nose Y, Engevik MA, Niespodzany EJ, Barrientos T, Öhrvik H, Worrell RT, Thiele DJ, Mackenzie B. Intestinal DMT1 is critical for iron absorption in the mouse but is not required for the absorption of copper or manganese. Am J Physiol Gastrointest Liver Physiol 309: G635–G647, 2015. doi: 10.1152/ajpgi.00160.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Simons TJ. Passive transport and binding of lead by human red blood cells. J Physiol 378: 267–286, 1986. doi: 10.1113/jphysiol.1986.sp016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Simons TJ. The role of anion transport in the passive movement of lead across the human red cell membrane. J Physiol 378: 287–312, 1986. doi: 10.1113/jphysiol.1986.sp016220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Soncin F, Guitton JD, Cartwright T, Badet J. Interaction of human angiogenin with copper modulates angiogenin binding to endothelial cells. Biochem Biophys Res Commun 236: 604–610, 1997. doi: 10.1006/bbrc.1997.7018. [DOI] [PubMed] [Google Scholar]
- 171.Stadler N, Lindner RA, Davies MJ. Direct detection and quantification of transition metal ions in human atherosclerotic plaques: evidence for the presence of elevated levels of iron and copper. Arterioscler Thromb Vasc Biol 24: 949–954, 2004. doi: 10.1161/01.ATV.0000124892.90999.cb. [DOI] [PubMed] [Google Scholar]
- 172.Starkebaum G, Harlan JM. Endothelial cell injury due to copper-catalyzed hydrogen peroxide generation from homocysteine. J Clin Invest 77: 1370–1376, 1986. doi: 10.1172/JCI112442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Stasser JP, Eisses JF, Barry AN, Kaplan JH, Blackburn NJ. Cysteine-to-serine mutants of the human copper chaperone for superoxide dismutase reveal a copper cluster at a domain III dimer interface. Biochemistry 44: 3143–3152, 2005. doi: 10.1021/bi0478392. [DOI] [PubMed] [Google Scholar]
- 174.Stasser JP, Siluvai GS, Barry AN, Blackburn NJ. A multinuclear copper(I) cluster forms the dimerization interface in copper-loaded human copper chaperone for superoxide dismutase. Biochemistry 46: 11845–11856, 2007. doi: 10.1021/bi700566h. [DOI] [PubMed] [Google Scholar]
- 175.Sudhahar V, Okur MN, Bagi Z, O’Bryan JP, Hay N, Makino A, Patel VS, Phillips SA, Stepp D, Ushio-Fukai M, Fukai T. Akt2 (protein kinase B Beta) stabilizes ATP7A, a Copper transporter for extracellular superoxide dismutase, in vascular smooth muscle: novel mechanism to limit endothelial dysfunction in type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol 38: 529–541, 2018. doi: 10.1161/ATVBAHA.117.309819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sudhahar V, Urao N, Oshikawa J, McKinney RD, Llanos RM, Mercer JF, Ushio-Fukai M, Fukai T. Copper transporter ATP7A protects against endothelial dysfunction in type 1 diabetic mice by regulating extracellular superoxide dismutase. Diabetes 62: 3839–3850, 2013. doi: 10.2337/db12-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Svensson PA, Englund MC, Markström E, Ohlsson BG, Jernås M, Billig H, Torgerson JS, Wiklund O, Carlsson LM, Carlsson B. Copper induces the expression of cholesterogenic genes in human macrophages. Atherosclerosis 169: 71–76, 2003. doi: 10.1016/S0021-9150(03)00145-X. [DOI] [PubMed] [Google Scholar]
- 178.Swain J, Gutteridge JM. Prooxidant iron and copper, with ferroxidase and xanthine oxidase activities in human atherosclerotic material. FEBS Lett 368: 513–515, 1995. doi: 10.1016/0014-5793(95)00726-P. [DOI] [PubMed] [Google Scholar]
- 179.Szauter KM, Cao T, Boyd CD, Csiszar K. Lysyl oxidase in development, aging and pathologies of the skin. Pathol Biol (Paris) 53: 448–456, 2005. doi: 10.1016/j.patbio.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 180.Telianidis J, Hung YH, Materia S, Fontaine SL. Role of the P-Type ATPases, ATP7A and ATP7B in brain copper homeostasis. Front Aging Neurosci 5: 44, 2013. doi: 10.3389/fnagi.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc 101: 294–301, 2001. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- 182.Tsai CY, Finley JC, Ali SS, Patel HH, Howell SB. Copper influx transporter 1 is required for FGF, PDGF and EGF-induced MAPK signaling. Biochem Pharmacol 84: 1007–1013, 2012. doi: 10.1016/j.bcp.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tsigelny IF, Sharikov Y, Greenberg JP, Miller MA, Kouznetsova VL, Larson CA, Howell SB. An all-atom model of the structure of human copper transporter 1. Cell Biochem Biophys 63: 223–234, 2012. doi: 10.1007/s12013-012-9358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Turski ML, Brady DC, Kim HJ, Kim BE, Nose Y, Counter CM, Winge DR, Thiele DJ. A novel role for copper in Ras/mitogen-activated protein kinase signaling. Mol Cell Biol 32: 1284–1295, 2012. doi: 10.1128/MCB.05722-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Uno H, Arya S, Opitz JM, Bernstein J. Neuronal and vascular disorders of the brain and spinal cord in Menkes kinky hair disease. Am J Med Genet Suppl 28: S3: 367–377, 1987. doi: 10.1002/ajmg.1320280542. [DOI] [PubMed] [Google Scholar]
- 186.Urso E, Maffia M. Behind the link between copper and angiogenesis: established mechanisms and an overview on the role of vascular copper transport systems. J Vasc Res 52: 172–196, 2015. doi: 10.1159/000438485. [DOI] [PubMed] [Google Scholar]
- 187.Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res 37: 1528–1542, 2009. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- 188.Vest KE, Leary SC, Winge DR, Cobine PA. Copper import into the mitochondrial matrix in Saccharomyces cerevisiae is mediated by Pic2, a mitochondrial carrier family protein. J Biol Chem 288: 23884–23892, 2013. doi: 10.1074/jbc.M113.470674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Völker W, Dorszewski A, Unruh V, Robenek H, Breithardt G, Buddecke E. Copper-induced inflammatory reactions of rat carotid arteries mimic restenosis/arteriosclerosis-like neointima formation. Atherosclerosis 130: 29–36, 1997. doi: 10.1016/S0021-9150(96)06039-X. [DOI] [PubMed] [Google Scholar]
- 190.Ward EM, Keen CL, McArdle HJ. The Impact of Copper On Human Health. New York: International Copper Association, 2003. [Google Scholar]
- 191.Wei H, Frei B, Beckman JS, Zhang WJ. Copper chelation by tetrathiomolybdate inhibits lipopolysaccharide-induced inflammatory responses in vivo. Am J Physiol Heart Circ Physiol 301: H712–H720, 2011. doi: 10.1152/ajpheart.01299.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wei H, Zhang WJ, Leboeuf R, Frei B. Copper induces–and copper chelation by tetrathiomolybdate inhibits–endothelial activation in vitro. Redox Rep 19: 40–48, 2014. doi: 10.1179/1351000213Y.0000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wei H, Zhang WJ, McMillen TS, Leboeuf RC, Frei B. Copper chelation by tetrathiomolybdate inhibits vascular inflammation and atherosclerotic lesion development in apolipoprotein E-deficient mice. Atherosclerosis 223: 306–313, 2012. doi: 10.1016/j.atherosclerosis.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.White C, Kambe T, Fulcher YG, Sachdev SW, Bush AI, Fritsche K, Lee J, Quinn TP, Petris MJ. Copper transport into the secretory pathway is regulated by oxygen in macrophages. J Cell Sci 122: 1315–1321, 2009. doi: 10.1242/jcs.043216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhou B, Gitschier J. hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci USA 94: 7481–7486, 1997. doi: 10.1073/pnas.94.14.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res 91: 1038–1045, 2002. doi: 10.1161/01.RES.0000043501.47934.FA. [DOI] [PubMed] [Google Scholar]
- 197.Zimnicka AM, Ivy K, Kaplan JH. Acquisition of dietary copper: a role for anion transporters in intestinal apical copper uptake. Am J Physiol Cell Physiol 300: C588–C599, 2011. doi: 10.1152/ajpcell.00054.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zimnicka AM, Tang H, Guo Q, Kuhr FK, Oh MJ, Wan J, Chen J, Smith KA, Fraidenburg DR, Choudhury MS, Levitan I, Machado RF, Kaplan JH, Yuan JX. Upregulated copper transporters in hypoxia-induced pulmonary hypertension. PLoS One 9: e90544, 2014. doi: 10.1371/journal.pone.0090544. [DOI] [PMC free article] [PubMed] [Google Scholar]