Abstract
Optimal vision requires an ocular surface with a stable tear film whose many critical tasks include providing >70% of the eye’s refractive power. However, for millions, tear film instability produces uncomfortable sight-impairing dry eye. Despite the multitude of etiologies for dry eye, a universal hallmark is hyperosmolarity of the tear film. Presently, knowledge of how the ocular surface responds to hyperosmolarity remains incomplete with little understood about the role of ion channels. This bioelectric analysis focused on conjunctival goblet cells whose release of tear-stabilizing mucin is a key adaptive response to dry eye. In freshly excised rat conjunctiva, perforated-patch recordings demonstrated that a ≥10% rise in osmolarity triggers goblet cells to rapidly generate a ~15-mV hyperpolarization due to the oxidant-dependent activation of ATP-sensitive K+ (KATP) channels. High-resolution membrane capacitance measurements used to monitor exocytosis revealed that this hyperpolarization results in an approximately fourfold boost in exocytotic activity evoked by cholinergic input, which in vivo occurs via a neural reflex and depends chiefly on calcium influxing down its electro-gradient. We discovered that this adaptive response is transient. During 30–80 min of hyperosmolarity, development of a depolarizing nonspecific cation conductance fully counterbalances the KATP-driven hyperpolarization and thereby eliminates the exocytotic boost. We conclude that hyperosmotic-induced hyperpolarization is a previously unappreciated mechanism by which goblet cells respond to transient ocular dryness. Loss of this voltage increase during long-term dryness/hyperosmolarity may account for the clinical conundrum that goblet cells in chronically dry eyes can remain filled with mucin even though the tear film is hyperosmotic and mucin-deficient.
Keywords: exocytosis, hyperosmolarity, KATP channels
INTRODUCTION
The process of visual perception begins with light passing through the tear film. This biochemically complex ~3-µm thick layer of fluid not only provides nutrition, hydration, lubrication, and protection (10, 46), but is the initial refractory surface and accounts for more than 70% of the refractive power of the eye (6). Consequently, maintenance of tear film homeostasis is a critical prerequisite for optimal visual acuity. With intense evolutionary pressure to maintain excellent vision, the ocular surface system is highly adapted to respond to disruption of the tear film. Yet, despite endogenous protective mechanisms, tear film instability and resulting ocular dryness commonly cause transient and, not infrequently, chronic visual impairment (46). In addition, due to the high density of sensory neurons innervating the ocular surface, tear film dyshomeostasis causes significant discomfort.
Challenges to tear film homeostasis include commonly occurring environmental factors, such as rapid air movement (wind) and low humidity, as well as intrinsic pathological conditions, e.g., inadequate tear production and lid/eye gland dysfunction. Even though there are a multitude of specific causes of ocular dryness (4, 38), a universal finding with dry eye is hyperosmolarity of the tear film (46). A rise in tear osmolarity, which in normal subjects is reported to be ~300 mosM (46), triggers adaptive responses that include neural reflexes to increase the rate of blinking, the production of tears, and the release of tear-stabilizing mucin. For transient environmental causes of accelerated evaporation, the adaptive response of the ocular surface system is usually effective. However, with the prolonged hyperosmolarity associated with intrinsic pathological disorders, the activation of various stress pathways, including those induced by oxidants, initiates a “vicious cycle” of inflammation and cellular damage that often progresses to irreversible severe dry eye (4).
Although tear film hyperosmolarity is the hallmark of dry eye, knowledge of how the ocular surface responds to a rise in osmolarity remains incomplete. For example, even though ion channels are critically involved in the functioning of virtually all types of cells, little is known about their role in the adaptive response of the ocular surface to hyperosmolarity. In this study, we focused on the bioelectric impact of hyperosmolarity on conjunctival goblet cells. These cells are of interest because their exocytotic release of mucin-filled granules slows the evaporation of tears thereby limiting a rise in tear film osmolarity (4). Of pathobiological relevance, a deficiency of mucin in the tear film is an oft-found feature of clinical dry eye (46).
Our bioelectric analysis of conjunctival specimens freshly isolated from the adult rat demonstrated that in response to a ≥10% rise in extracellular osmolarity, goblet cells rapidly generate a 15-mV voltage increase. Electrophysiological recordings further demonstrated that this hyperpolarization is due to the oxidant-dependent activation of ATP-sensitive K+ (KATP) channels. High-temporal resolution assays of membrane capacitance used to monitor exocytotic activity in individual goblet cells revealed that the KATP-driven hyperpolarization boosts evoked exocytosis by more than fourfold. We also discovered that hyperosmotic-induced hyperpolarization is transient. Namely, during 30 to 80 min of sustained hyperosmolarity, the development of a potent depolarizing conductance fully counterbalances the KATP-induced voltage increase and thereby eliminates the exocytotic boost. Thus, despite persistent hyperosmolarity, the ability of conjunctival goblet cells to release a bolus of tear-stabilizing mucin becomes markedly compromised. This bioelectric mechanism may account for the clinical paradox that conjunctival goblet cells in chronically dry eyes may remain filled with mucin-containing granules even though the tear film is hyperosmotic and mucin-deficient (35).
MATERIALS AND METHODS
Experimental protocols for animal use were approved by the Institutional Animal Care and Use Committee of the University of Michigan and were consistent with the guidelines of the American Physiological Society’s Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training. Long-Evans rats obtained from Charles River (Cambridge, MA) were used to establish in-house breeding colonies. At all times, animals were kept on a 12-h alternating light-dark cycle and received food and water ad libitum. In this study, the number of males and females was approximately equal.
Conjunctival specimens.
Immediately after a rising concentration of carbon dioxide resulted in death, 6- to 26-wk-old rats were positioned on the stage of a stereo-microscope. Sequentially, each eye was viewed at ×6.4 to ×16 as micro-Vannas scissors (Fine Science Tools, Foster City, CA) were used to create a ~5-mm superior conjunctival peritomy at the limbus, as well as ~7-mm radial conjunctival incisions extending from each end of the peritomy. While the limbal edge of the surgically created conjunctival flap was grasped with fine forceps (Dumont no. 5, Fine Surgical Tools), micro-Vannas scissors were used to dissect the superficial layer of the flap from subconjunctival tissue, i.e., Tenon’s and sub-Tenon’s capsule. The conjunctival specimen was excised by cutting the flap’s posterior attachment. Using fine forceps, the excised specimen was placed in a custom-made glass-bottom recording chamber (0.75-ml volume), which was initially solution-free. Gently, but quickly, fine forceps were used to orient the specimen so that its exterior surface faced upwards and it was spread maximally onto the glass bottom. Subsequently, careful attention was directed to using a fine forceps to gently remove from the conjunctival surface a gelatinous-like material whose presence we found prevents formation of seals between a recording pipette and a goblet cell. A harp-shaped tissue anchor (SHD26GH/10; Warner Instruments, Hamden, CT) was then placed so that its nylon strings gently touched the specimen thereby providing stabilization. To wash away any residual floating debris whose propensity to stick to the pipette tip may prevent pipette/cell seals, five successive 1-ml aliquots of a 300 mosM solution consisting of 105 mM NaCl, 15 mM NaHCO3, 10 Na-HEPES, 20 mM KCl, 0.5 mM CaCl2, 0.5 mM MgCl2, 3 mM glucose at pH 7.5 and 300 mosM were repetitively ejected onto the specimen and briskly extracted from the recording chamber. Subsequently, unless noted otherwise, the chamber was filled with this 300 mosM solution whose relatively high potassium concentration and pH reflect values measured in the tears of normal and dry eye subjects (41, 46). Prior to an electrophysiological recording session, a specimen-containing chamber was maintained at 100% humidity and 22–23°C in a Billups-Rothenberg modular chamber (Billups-Rothenberg, Del Mar, CA).
Electrophysiology.
Between 20 min to 4 h after conjunctival excision, electrophysiological experiments were performed at 22–23°C. A recording chamber containing a conjunctival specimen was positioned onto the stage of an upright microscope equipped with differential interference contrast/infrared optics and a ×40 water immersion objective that allowed viewing at ×400. The recording chamber could be perfused at ~2 ml/min with solutions from a gravity-fed system using multiple reservoirs. Perfusates included the non-sodium chloride components of the 300 mosM solution described above plus an appropriate NaCl concentration to create solutions having specific osmolarities between 290 to 380 mosM. The osmolarity of each solution was measured by a vapor pressure osmometer (Vapro 5600, Wescor, Logan, UT). In one series of experiments, the perfusate consisted of the 360 mosM solution lacking added calcium. In some experiments, 0.5 µM glibenclamide, 10 µM carbachol, 200 µM N-acetyl cysteine (NAC) or 30 µM H2O2 were added to the 300 mosM and 360 mosM solutions. In experiments using NAC, conjunctival specimens were preincubated for ~2.5 h in the 300 mosM solution supplemented with 200 µM NAC.
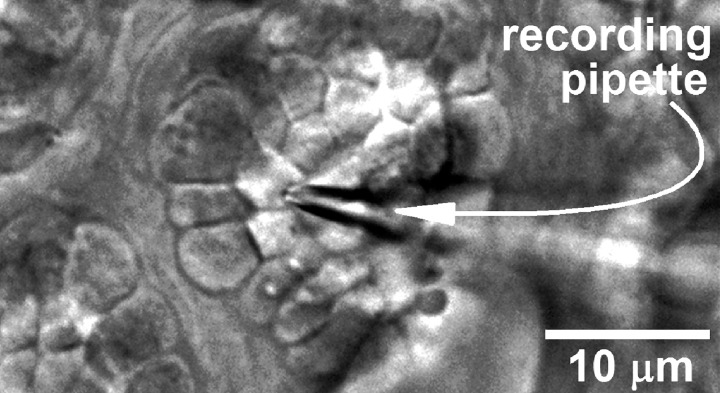
Electrophysiological recordings were obtained via 5 to 10 MΩ perforated-patch pipettes fabricated from thin-walled inner filament-containing borosilicate glass (TW150F-4, World Precision Instruments, Sarasota, FL), filled with a solution consisting of 50 mM KCl, 65 mM K2SO4, 6 mM MgCl2, 10 mM K-HEPES, 60 μg/ml amphotericin B and 60 μg/ml nystatin at pH 7.35 and 280 mosM, and mounted in the holder of either an Axopatch 200B (Molecular Devices, San Jose, CA) or an EPC-9 (HEKA Elekronik, Lambrecht, Germany) patch-clamp amplifier. A remotely controlled micromanipulator (MP-225, Sutter Instruments, Novato, CA) facilitated positioning of a recording pipette tip onto a targeted goblet cell located within one of the numerous goblet cell clusters, which are easily identified (Fig. 1) and a well-characterized feature of the rat conjunctiva (20, 42). Because only the apical surface of a goblet cell is exposed in a conjunctival specimen, this was the surface onto which perforated-patch pipettes were sealed.
Fig. 1.
Differential interference contrast/infrared image of a freshly isolated rat conjunctival specimen in which a perforated-patch recording pipette is sealed onto a goblet cell located within a complex of these mucin-releasing cells.
During positioning of a recording pipette and the subsequent recording session, the specimen was viewed on the monitor of a computer equipped with software (NIS 4.0, Nikon, Tokyo, Japan) to display images obtained by a digital camera (Photometrics Cool Snap EZ, Tucson, AZ) using 50- to 90-ms exposures. A ≥10 GΩ pipette/cell seal was achieved by applying gentle suction to the back end of the pipette. This study used recordings in which the access resistance became <25 MΩ within 5 min after gigaohm seal formation. Throughout a recording, the access resistance was monitored; a >10% change led to termination of the recording. Currents were filtered with a four-pole Bessel filter. In the case of the Axon 200B amplifier, recordings were sampled digitally using a DigiData 1440A acquisition system (Molecular Devices) and pClamp software (version 10, Molecular Devices). For the EPC-9 amplifier, Patchmaster software (HEKA) controlled data sampling and acquisition. Data analysis was aided by graphics software (Origin 2017, OriginLab, Northampton, MA).
Current-voltage (I–V) plots were generated by recording currents evoked by steps of voltage controlled by pClamp or Patchmaster software. As described previously (34, 39), reported membrane potentials were the zero-current voltages, which were chiefly determined from I–V plots, although sometimes obtained by empirically adjusting the holding voltage to briefly “zero out” the basal current. Adjustment was made off-line for the junction potential, which was −8 mV for all solutions used in this study. Because measurement of the resting membrane potential of cells having membrane resistances (Rm) of ≥10 GΩ is prone to imprecision, the 16% of sampled goblet cells with Rm in this range were not further analyzed. To calculate the conductance of an I–V plot, the change in current per 10-mV change in voltage was calculated for each of the thirteen 10-mV intervals sampled from −108 mV to +22 mV during the generation of the I–V curve; these values were then averaged. Of note, for the bathing and pipette solutions used in this study, the calculated Nernst potential (equilibrium potential) was −58 mV for a purely K+-selective current and −1 mV for a current nonspecific for cations. Of note, indicative that the space-clamp of the perforated-patch recordings was fully adequate, the observed reversal potential for the glibenclamide-sensitive current was very close to the calculated equilibrium potential for K+ (Figs. 4 and 5).
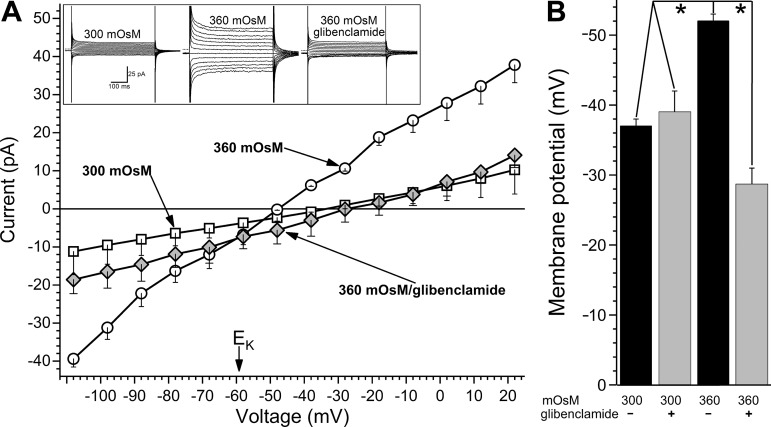
Fig. 4.
Role of ATP-sensitive K+ (KATP) channels in the voltage increase generated by conjunctival goblet cells in response to hyperosmolarity. A: averaged current-voltage (I–V) relations of goblet cells (n = 5) monitored in the 300 mosM solution, >6 min after switching to the 360 mosM solution and >3 min after addition of 0.5 µM glibenclamide to the hyperosmotic solution. EK, equilibrium potential for K+. Inset: current traces obtained from a goblet cell during the three experimental conditions. Dotted lines to the left of the traces show zero-current levels. B: membrane potentials of goblet cells recorded in 300 or 360 mosM solutions without (−) or with (+) glibenclamide. Elimination of the hyperosmotic-induced hyperpolarization by this KATP channel blocker supports the hyperpolarizing role of these ion channels. Of note, the possibility of a small depolarizing component being concomitantly activated is suggested by the finding that the goblet cell voltage was lower (P = 0.0439) in the 360 mosM/glibenclamide solution than in the 300 mosM/glibenclamide solution. Sample sizes were as follows: 300 mosM/no glibenclamide, 21 (data shown in Fig. 2); 360 mosM/no glibenclamide, 28 (data shown in Fig. 2); other experimental groups, 6 each. *P < 0.0001.
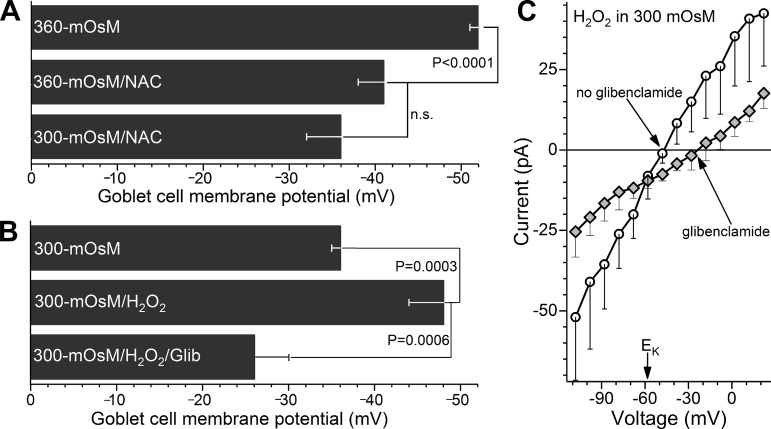
Fig. 5.
Role of oxidants in hyperosmotic-induced hyperpolarization. A: effect of the antioxidant N-acetyl cysteine (NAC, 200 µM) on the hyperosmotic-induced hyperpolarization of goblet cells. Voltages were measured during a 10- to 30-min exposure to NAC-containing 360 (n = 6) or 300 mosM (n = 4) perfusates (details in materials and methods). To facilitate comparison, the goblet cell voltage in the NAC-free 360 mosM solution is shown (n = 28, data in Fig. 2). B: effect of 30 µM H2O2 on goblet cell voltage under normosmotic conditions. A 0.5- to 2-h exposure to H2O2 resulted in significant hyperpolarization, which was eliminated by glibenclamide (Glib). Noteworthy is that goblet cells in the 300 mosM/H2O2/glibenclamide group were more depolarized (P = 0.0197) than in 300 mosM/glibenclamide (data in Fig. 4B); thus, H2O2 appears to concomitantly activate a depolarizing current, as well as the glibenclamide-sensitive ATP-sensitive K+ (KATP) channel conductance. Sample sizes: 300 mosM, 12; 300 mosM/H2O2, 6; 300 mosM/H2O2/glibenclamide, 5. C: current-voltage (I–V) plots generated in the 300 mosM solution supplemented with 30 µM H2O2 in the absence (n = 6) or the presence of 0.5 µM glibenclamide (n = 6). This KATP blocker decreased (P = 0.0003) the conductance by inhibiting a current whose reversal potential was near equilibrium potential for K+ (EK).
Evoked changes in the membrane capacitance (Cm) associated with the exocytotic activity of single goblet cells were measured using the “sine + DC” method (31) in which a 30-mV peak sinusoidal signal was applied to a voltage-clamp recording at a frequency 1.8 kHz on a holding potential of either −38 mV or −52 mV. With Patchmaster software (HEKA) emulating a lock-in amplifier, the resulting current response was used to calculate Cm (18). During a Cm assay, the Patchmaster program averaged five successive measurements and stored each of the resulting 2.8-ms sample points. Cm recordings exhibiting >100-fF fluctuations before carbachol exposure were excluded from this study. Consistent with there being minimal electrotonic interaction of a sampled goblet cell with its neighbors, exposure of conjunctival specimens to the gap junction uncoupler, octanol (0.5 mM), did not result in a detected change in Cm (n = 5; data not shown).
Exocytotic activity of a sampled goblet cell was induced by adding 10 µM carbachol to the perfusate. Each specimen was exposed to carbachol only once. Reported carbachol-induced Cm increases are the mean of the maximal Cm changes detected within 10 s after the onset of exposure to this cholinergic agonist. To assure detection of the maximum evoked Cm increase, which typically was at a peak for ~50 ms, Cm sampling at 1.8-kHz was continuous. Although a change in Cm reflects a net measurement of exocytotic and endocytotic activities, the Cm increase detected soon after exposure to carbachol chiefly reflects the initially evoked exocytosis that precedes endocytotic activity (43).
Chemicals.
Unless noted otherwise, chemicals were from Sigma (St. Louis, MO).
Statistics.
Data are given as means ± SE with n being the number of cells sampled. Unless noted otherwise, probability was evaluated by Student’s two-tailed t-test, paired or unpaired with equal or unequal variance, as appropriate. P > 0.05 indicated failure to detect a significant difference. For greater than two groups, an analysis of variance was performed using commercially available software (Origin 2017) with the subsequent application of the Bonferroni correction.
RESULTS
Hyperosmotic-induced hyperpolarization.
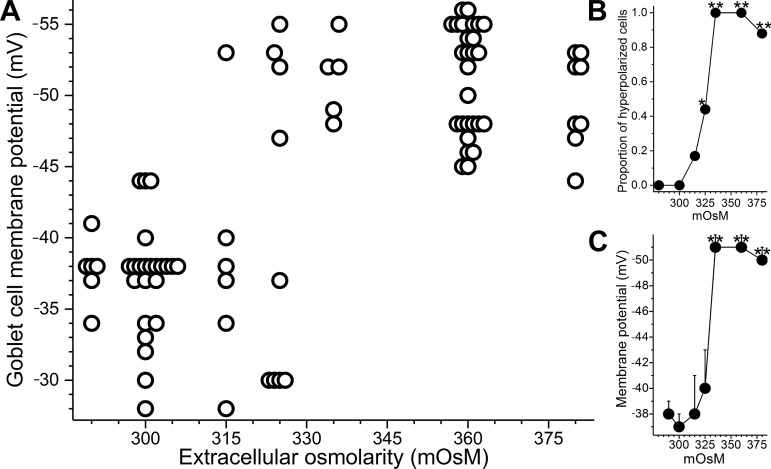
Voltage-clamp recordings using perforated-patch pipettes were obtained from goblet cells located in freshly isolated rat conjunctival specimens bathed for 10 to 30 min in solutions of various osmolarities between 290 to 380 mosM (Fig. 2). This range is of interest since it includes osmolarities (~300 mosM) measured in the normal tear film of humans (46), as well as in rats (33) and other mammals (41), and also includes the higher osmolarities detected in symptomatic dry eye patients (46). As shown in Fig. 2A, the resting membrane potential of goblet cells bathed in solutions of ≤300 mosM was −37 ± 1 mV (n = 27). In this osmolarity range, none of the sampled cells exhibited a voltage more negative than −44 mV. In contrast, at 325 mosM, 44% of the sampled goblet cells were hyperpolarized above −44 mV (P = 0.0078, Fishers exact t-test; Fig. 2B). At ≥335 mosM, 98% of the sampled goblet cells (n = 41) had a membrane potential >−44 mV, and the mean goblet cell voltage was −51 ± 1 mV (Fig. 2C), which is significantly (P < 0.0001) more negative than the membrane potential observed at ≤300 mosM. These observations revealed that conjunctival goblet cells generate a hyperpolarizing voltage in response to hyperosmolarity.
Fig. 2.
Effect of extracellular osmolarity on the membrane potential of conjunctival goblet cells. A: resting membrane potentials of goblet cells located in freshly isolated rat conjunctival specimens exposed for 10 to 30 min to solutions of various osmolarities. To aid visualization, symbols are displaced slightly when multiple goblet cells had identical membrane potentials. B: proportion of sampled goblet cells with resting membrane potentials more negative than −44 mV. C: mean membrane potentials at the 7 osmolarities assayed. *P = 0.0078; **P < 0.0001.
Time course for hyperosmotic-induced hyperpolarization.
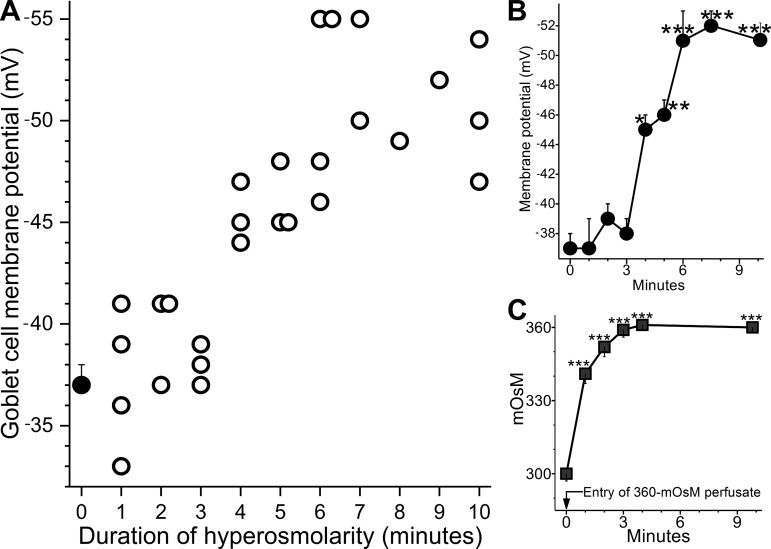
To further characterize the voltage increase generated by goblet cells responding to hyperosmolarity, goblet cell voltages were measured at ~1-min intervals after switching from the 300 mosM perfusate to the 360 mosM perfusate (Fig. 3). As shown in Fig. 3B, hyperpolarization was initially detected (P = 0.0028) 4 min after the entry of the hyperosmotic solution into the recording chamber. Two minutes later, hyperpolarization was maximal. This time course was compared with the time course for the rise in osmolarity of the solution within the recording chamber (Fig. 3C). One minute after entry of the 360 mosM perfusate, the osmolarity in the chamber increased from 300 mosM to 341 ± 4 mosM (P < 0.0001; n = 4). Noteworthy is that, as shown in Fig. 2, this amount of hyperosmolarity is sufficient to cause goblet cells to hyperpolarize. Figure 3C further shows that 3 min after switching to the hyperosmotic perfusate, the osmolarity of the solution within the chamber was ~360 mosM. Comparison of these two time courses indicates that there is a ~3-min lag between the onset of a >10% rise in osmolarity and the detection of a hyperpolarizing response.
Fig. 3.
Time course for the onset of hyperosmotic-induced hyperpolarization. A: duration of hyperpolarization versus goblet cell voltage. Open circles show the resting membrane potentials of sampled goblet cells during the initial 10 min after the entry of the 360 mosM perfusate into the recording chamber. Symbols are plotted to the nearest minute. Closed circle is the mean goblet cell voltage in the 300 mosM solution (n = 21, data from Fig. 2). B: mean membrane potential versus time after entry of the 360 mosM perfusate into the recording chamber. Averages are plotted at the mean sample time; data for 7 and 8 min, as well as 9 and 10 min, were pooled. C: time course of the rise in osmolarity of the solution within the recording chamber after the perfusate was switched from the 300 to the 360 mosM solution (n = 4). *P = 0.0028; **P = 0.0010; ***P < 0.0001.
Ionic basis for the hyperosmotic-induced hyperpolarization.
To help elucidate the mechanism by which conjunctival goblet cells generate a voltage increase in response to hyperosmolarity, current-voltage (I–V) relations were generated during perfusion of the 300 mosM perfusate, after >6-min of perfusion of the 360 mosM solution and finally a few minutes after addition of KATP channel blocker, glibenclamide (0.5 µM), to the hyperosmotic perfusate. As shown in Fig. 4A, these I–V plots revealed that exposure to the 360 mosM solution resulted in a 15-mV hyperpolarization, which was associated with a 3.8-fold increase (P < 0.0001) in cell conductance (calculation details in materials and methods). Of note, since conductance is a measure of the relationship between change in current versus change in voltage (slope of the I–V plot), it reflects the ability of the cell membrane to pass ions, i.e., it is the inverse of membrane resistance. Thus, the activation of ion channels increases conductance.
Consistent with the activation of KATP channels causing the hyperosmotic-induced hyperpolarization, glibenclamide eliminated the increase in membrane potential as well as the increase in conductance (Fig. 4). Further, as expected for a KATP current, the glibenclamide-sensitive current exhibited some inward rectification and had a reversal potential near the equilibrium potential for K+ (Fig. 4A). Additional data supporting the role of hyperpolarizing KATP channels are summarized in Fig. 4B. Based on these findings, we concluded that the voltage increase generated by conjunctival goblet cells in response to hyperosmolarity is due to the activation of their KATP channels.
Indicative that goblet cell KATP channels are closed under normosmotic conditions, the membrane potential in the 300 mosM solution was not significantly affected by glibenclamide (Fig. 4B). Suggestive that conjunctival goblet cells express SUR1-containing KATP channels, diazoxide (250 µM), which activates this sulfonylurea receptor (36), caused the goblet cell voltage in the 300 mosM solution to increase to −53 ± 2 mV (n = 8, P < 0.0001, data not illustrated). Further, the KATP-SUR2 activator, pinacidil (5 µM (36), failed to significantly affect goblet cell voltage under normosmotic conditions (pinacidil: −32 ± 3 mV, n = 6, not illustrated; no activator: −37 ± 1 mV, n = 21; Fig. 4B).
Noteworthy is that voltage measurements made while KATP channels were blocked by glibenclamide suggest that a depolarizing conductance may also be activated early in the course of hyperosmolarity. Namely, in the 360 mosM/glibenclamide solution the measured membrane potential was −28 ± 3 mV (n = 6), which was significantly (P = 0.0329) less than the voltage of −38 ± 3 mV (n = 6) recorded in the 300 mosM/glibenclamide solution (Fig. 4B). However, even though a relatively small depolarizing conductance may also be activated during 10 to 30 min of hyperosmolarity, the experimental results in Fig. 4 demonstrate that it is the activation of a potent KATP conductance that causes conjunctival goblet cells to hyperpolarize in response to hyperosmolarity.
Role of oxidation in hyperosmotic-induced hyperpolarization.
We postulated that an oxidant-dependent mechanism may play a role in hyperosmotic-induced hyperpolarization since hyperosmolarity is known to increase oxidant levels in various cell types including ones on the ocular surface (1, 12, 29, 47) and also because KATP channels are redox-sensitive (27, 32). To begin to assess this possibility, freshly isolated conjunctival specimens were exposed to the antioxidant N-acetyl cysteine (NAC, 200 µM; details in materials and methods), which interestingly is sometimes used clinically to lessen preocular mucin (15). Supporting the hypothesis that oxidation does play a role in hyperosmotic-induced hyperpolarization, NAC treatment prevented significant hyperpolarization during 10 to 30 min of hyperosmolarity (Fig. 5A).
In other experiments, conjunctival specimens were exposed under normosmotic conditions to the oxidant, H2O2 (30 µM). As summarized in Fig. 5B, goblet cells became significantly (P = 0.0003) hyperpolarized in the presence of H2O2. Indicative that this oxidant caused KATP channel activation, glibenclamide significantly (P = 0.0047) inhibited the H2O2-induced voltage (Fig. 5B). Furthermore, as expected for a KATP conductance, the reversal potential of the H2O2-activated glibenclamide-inhibited current was close to the equilibrium potential for K+ (Fig. 5C). Noteworthy is that the H2O2-induced hyperpolarization observed under normosmotic conditions (Fig. 5B) was not significantly different from the hyperosmotic-induced voltage increase (Figs. 5A). Demonstration that hyperosmotic-induced hyperpolarization is mimicked by an oxidant and prevented by an antioxidant supports the hypothesis that this bioelectric response of goblet cells is mediated via an oxidant-dependent mechanism.
Functional impact of hyperosmotic-induced hyperpolarization.
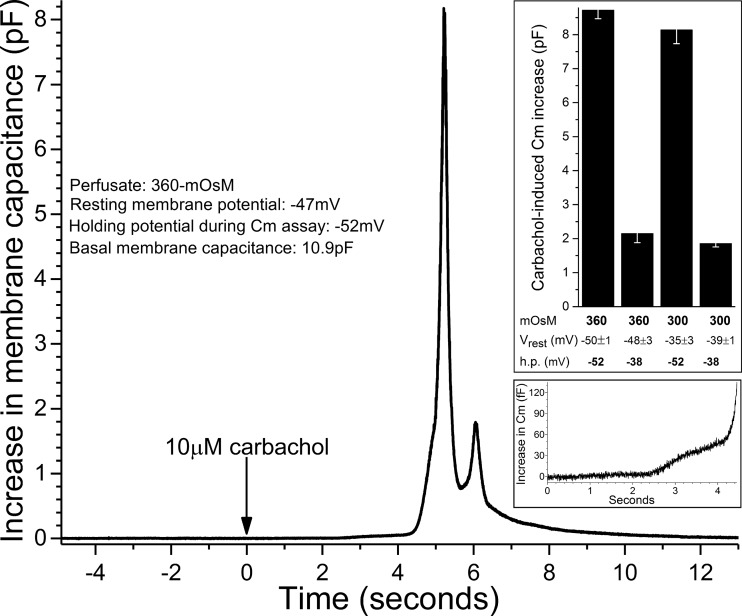
To determine how hyperosmotic-induced hyperpolarization affects goblet cell function, we focused on exocytotic activity because the release of tear-stabilizing mucin by these cells is critical for maintaining the homeostasis of the tear film. To monitor exocytotic activity, we obtained high-temporal resolution measurements of the membrane capacitance (Cm) of single goblet cells located in conjunctival specimens. Because it is well established that the release of mucin-filled granules from conjunctival goblet cells in vivo is potently regulated by a cholinergic parasympathetic reflex (8, 17, 24, 30), we monitored Cm during the exposure of conjunctival specimens to the cholinergic agonist carbachol (10 µM). Figure 6 shows an example of a carbachol-induced increase in Cm monitored in a goblet cell maintained at a holding potential of −52 mV during superfusion of the 360 mosM solution. Similar to each Cm response used in this study (n = 49), the recording in Fig. 6 shows that ~2 s after the onset of carbachol exposure, the Cm began to increase (Fig. 6, bottom inset) then rapidly crescendoed to a peak followed by a return towards the basal level. Of importance to the validity of the Cm assay, exposure to 10 µM carbachol did not significantly affect goblet cell I–V relations generated in the 300 mosM solution (n = 5, data not shown) or in the 360 mosM solution (n = 5, data not shown).
Fig. 6.
Effect of carbachol on goblet cell membrane capacitance (Cm). Recording of the change in Cm of a goblet cell into which the injection of current via the recording pipette established a holding potential (h.p.) of −52 mV, which was close to this cell’s recorded membrane potential. Bottom inset: onset of the carbachol-induced increase in Cm. Top inset: carbachol-induced Cm increases under conditions in which extracellular osmolarity was 360 or 300 mosM and the h.p. was −52 or −38 mV. Sample sizes: 360 mosM/−52 mV, 17; 300 mosM/−38 mV, 12; other groups, 6 each. Mean basal Cm was 15.2 ± 0.6 pF. The two Cm increases assayed at −52 mV were markedly greater (P < 0.0001) than the increases in Cm monitored at −38 mV. There was no significant difference in the two Cm increases assayed at −52 mV or in the two Cm increases assayed at −38 mV. Vrest, resting membrane potential.
In a series of experiments, Cm was monitored at a holding potential of −38 mV during perfusion of the 300 mosM solution and at −52 mV when the perfusate was the 360 mosM solution. Thus, the holding potentials were close to the resting membrane potentials measured at these osmolarities (Fig. 2). As shown in the top inset of Fig. 6, the magnitude of the carbachol-induced Cm increase was approximately fourfold larger (P < 0.0001) at 360 mosM (measured at −52 mV) than at 300 mosM (measured at −38 mV). This finding indicates that a potentially important functional consequence of hyperosmotic-induced hyperpolarization is a marked boost in evoked exocytotic activity.
To help establish the relative importance of hyperpolarization versus hyperosmolarity in causing the observed boost in cholinergic evoked exocytotic activity, we performed additional Cm assays in which current injected via the recording pipette was used to move the voltage away from the resting membrane potential of the sampled goblet cell (Fig. 6, top inset). Specifically, the holding potential of goblet cells in the 360 mosM perfusate was adjusted to −38 mV, i.e., eliminating the hyperosmotic-induced hyperpolarization. Conversely, cells in the 300 mosM solution were assayed at a holding potential of −52 mV, which mimicked the hyperpolarization observed under hyperosmotic conditions. Indicative of the critical importance of voltage, setting the holding potential at −38 mV for goblet cells in the hyperosmotic solution resulted in a markedly smaller (P < 0.0001) carbachol-induced Cm increase than was observed at −52 mV. Similarly, use of a holding potential of −52 mV for goblet cells bathed in the 300 mosM solution boosted (P < 0.0001) the carbachol-induced Cm increase to a level that was not significantly different than the increase observed at −52 mV under hyperosmotic conditions. Of note, the carbachol-induced Cm increase assayed at −38 mV in the 360 mosM solution was not significantly different from the increase monitored at −38 mV in the 300 mosM solution. Taken together, the observations summarized in Fig. 6 support the concept that the exocytotic boost detected under hyperosmotic conditions is virtually totally due to the hyperpolarization of the conjunctival goblet cells.
The voltage dependence of the boost in evoked exocytotic activity was not unexpected since hyperpolarization increases the electro-gradient for calcium influx, which is the chief driver of mucin release from conjunctival goblet cells during cholinergic activation (17, 24, 30). To begin to assess the role of influxing calcium in mediating the exocytotic boost observed during hyperosmotic-induced hyperpolarization, Cm assays were performed using a 360 mosM solution that lacked added calcium. In contrast to observations made in the 360 mosM solution that contained 0.5 mM calcium, Cm assays made in the absence of added calcium showed that the carbachol-induced increase assayed at −52 mV was not significantly (P = 0.28) different from the Cm increase measured at −38 mV. Specifically, the evoked Cm increases were 340 ± 160 fF (n = 4) at −38 mV and 510 ± 205 fF (n = 4) at −52 mV (data not illustrated). Thus, it is very likely that the hyperpolarization-induced boost in evoked exocytotic activity is dependent on extracellular calcium. We conclude from these experiments that by increasing the electro-gradient for calcium influx, hyperpolarization enhances cholinergic evoked exocytosis.
Loss of hyperpolarization during prolonged hyperosmolarity.
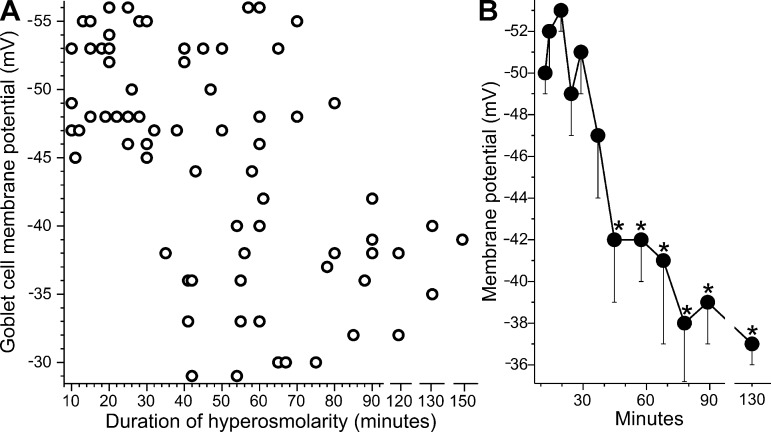
While goblet cell hyperpolarization was consistently observed during 10 to 30 min of hyperosmolarity (Figs. 2–6), the question arose as to whether this bioelectric response persists during more prolonged hyperosmolarity. The long-term response is of interest since persistent tear film hyperosmolarity is the hallmark of clinical dry eye. To address this question, goblet cell voltages were measured during up to 150 min of hyperosmolarity (Fig. 7). These recordings revealed that the membrane potential of these cells begins to decrease after ~30 min in the 360 mosM solution. After 75 min, goblet cell voltage was −38 ± 1 (n = 15), which is not significantly different than the membrane potential measured at ≤300 mosM (Figs. 2 and 5B). Thus, hyperosmotic-induced hyperpolarization is a transient response.
Fig. 7.
Duration of hyperosmolarity versus goblet cell voltage. A: resting membrane potentials of goblet cells located in freshly isolated rat conjunctival specimens exposed to the 360 mosM solution for 10 to 150 min. B: averages of 7 ± 1 sequential data points plotted at their mean sampling time. After 75 min in hyperosmotic solution, the mean membrane potential was not significantly different from the goblet cell voltage recorded in the 300 mosM solution (data in Fig. 2). *P ≤ 0.0001.
Ionic basis for the loss of hyperosmotic-induced hyperpolarization.
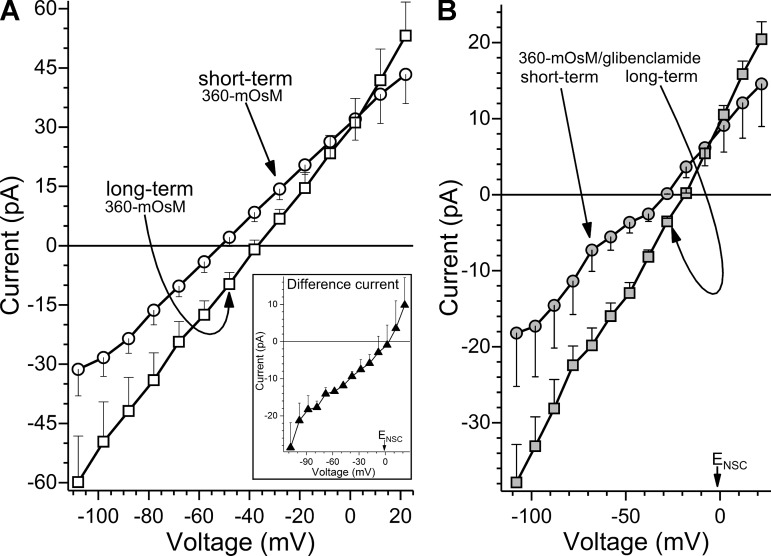
What accounts for the loss of hyperpolarization during prolonged hyperosmolarity? To address this question, I–V relations were generated during short-term (10 to 30 min) and long-term (>80 min) exposures to the 360 mosM solution (Fig. 8A). These I–V plots revealed that the decrease in membrane potential observed in the long-term group was associated with the development of a depolarizing conductance whose reversal potential was near the nonspecific cation (NSC) equilibrium potential (ENSC = −1 mV). Experiments using glibenclamide to block KATP channels (Fig. 8B) provided additional evidence that the decrease in membrane potential during long-term hyperosmolarity is associated with the development of a potent depolarizing current.
Fig. 8.
Effect of long-term hyperosmolarity on the current-voltage (I–V) relations of conjunctival goblet cells. A: averaged I–V plots obtained during “short-term” (10–30 min; n = 15) or “long-term” (>80 min; n = 6) exposure to 360 mosM. With long-term hyperosmolarity, conductance significantly (P < 0.0001) increased, and membrane potential decreased. Inset: difference between the I–V plots shown in main panel. The depolarizing conductance that developed with long-term hyperosmolarity had a reversal potential (Vr) near the equilibrium potential nonspecific for cations (ENSC = −1 mV). B: averaged I–V plots during short-term (n = 7) and long-term (n = 5) hyperosmolarity in the presence of ATP-sensitive K+ channel blocker glibenclamide (0.5 µM). With long-term hyperosmolarity, there was a significant (P = 0.0014) increase in a glibenclamide-insensitive conductance whose Vr was near ENSC.
To evaluate the possibility that the loss of hyperosmotic-induced hyperpolarization may perhaps involve a decrease in KATP channel activity as well as the development of a depolarizing current, the KATP conductances during short- and long-term hyperosmolarity were calculated by determining the difference between the I–V plots generated in the absence (Fig. 8A) and presence of glibenclamide (Fig. 8B). We found that the glibenclamide-sensitive conductances during short- and long-term hyperosmolarity were 310 ± 28 pS and 420 ± 44 pS (P = 0.0462), respectively. Thus, the data in Fig. 8 indicate that the KATP current activated soon after the onset of hyperosmolarity persists and may even increase during the long term. Taken together, our experiments show that during long-term hyperosmolarity, it is the development of a potently depolarizing NSC conductance, rather than a decrease in the KATP current, that causes the loss of hyperosmotic-induced hyperpolarization.
What is the mechanism by which a depolarizing conductance develops during sustained hyperosmolarity? One possibility is that irreversible damage due to sustained hyperosmolarity may cause the goblet cell membrane to become nonspecifically leaky. To assess this possibility, we performed experiments using conjunctival specimens (n = 5) that had been exposed to the 360 mosM solution for 80 to 130 min and were documented to have goblet cells with voltages of ≤−44 mV (mean membrane potential: −36 ± 2 mV, n = 6, not illustrated). These specimens were then bathed for 20 to 60 min (mean duration: 44 ± 6 min) in the 300 mosM solution before subsequently being reexposed to the 360 mosM perfusate. Indicative that hyperosmotic-induced hyperpolarization is a reversible phenomenon, we found that 10 to 30 min after returning to the hyperosmotic solution, goblet cells were hyperpolarized to −48 ± 2 mV (P = 0.0017; n = 6, data not illustrated). Furthermore, because recordings made in the presence of glibenclamide detected (P = 0.0439) goblet cell depolarization during 10 to 30 min at 360 mosM (Fig. 4B), the activation of a depolarizing current is not exclusively a phenomenon of long-term hyperosmolarity, but appears to begin relatively rapidly. Thus, it seem unlikely that the development of a depolarizing conductance is a nonspecific toxic effect of hyperosmolarity.
We considered the possibility that oxidation may have a role in the development of the depolarizing NSC conductance because some types of NSC channels are redox-sensitive (16, 23, 44) and, as noted, hyperosmolarity can induce oxidative stress. To begin to evaluate this idea, conjunctival specimens were exposed to the 300 mosM solution supplemented with 30 µM H2O2. To eliminate confounding effects caused by the activation of KATP channels, glibenclamide (0.5 µM) was also added to the 300 mosM solution. We found that during a 0.5- to 2-h exposure to the 300 mosM/glibenclamide/H2O2 solution, the goblet cell voltage was −26 ± 3 mV (n = 5; data in Fig. 5B), which was significantly (P = 0.0197) less negative than the −36 ± 1 mV (n = 6; Fig. 5B) recorded in the H2O2-free 300 mosM/glibenclamide solution. A mechanistic role for oxidation was also suggested by experiments using the antioxidant NAC. Namely, when the 360 mosM solution contained 200 µM NAC (details in materials and methods) plus 0.5 µM glibenclamide, no significant voltage decrease was detected between 10 and 30 min (short-term) and 80 and 120 min (long-term). Specifically, short- and long-term voltages were −41 ± 2 mV (n = 6) and −38 ± 3 mV (n = 6; P = 0.43), respectively (data not illustrated). In contrast, during long-term exposure to a 360 mosM/glibenclamide solution lacking NAC, goblet cells became 10 ± 3 mV (P = 0.0029) more depolarized (Fig. 8B). Taken together, these observations support the hypothesis that an oxidant-dependent mechanism mediates the development of the depolarizing NSC conductance during hyperosmolarity. Thus, under hyperosmotic conditions, oxidation appears to play a role not only in the activation of KATP channels, but also in NSC channel activation. Future investigation is needed to establish the relative importance of oxidant-induced alterations in cellular metabolism versus direct oxidant effects on channel activity in mediating these bioelectric responses of conjunctival goblet cells to hyperosmolarity.
Functional impact of the loss of hyperpolarization.
Based on finding that goblet cell exocytosis is highly sensitive to voltage (Fig. 6), we postulated that the loss of hyperosmotic-induced hyperpolarization during long-term hyperosmolarity (Fig. 7) would attenuate the exocytotic response to cholinergic input. To assess this possibility, we compared carbachol-induced Cm increases measured during short- (Fig. 6) and long-term hyperosmolarity. For the latter group, conjunctival specimens were exposed to the 360 mosM solution for ≥40 min (mean duration: 55 ± 3 min), and the sampled goblet cells were documented to have membrane potentials (Vrest) of ≤−44 mV (mean voltage: −34 ± 2 mV). We found that when Cm in the long-term group was monitored at a holding potential of −38 mV, i.e., close to their Vrest (Fig. 7), carbachol triggered a 2.62 ± 0.23 pF (n = 6, data not illustrated) increase in Cm. In contrast, as shown in Fig. 6, when goblet cells in the short-term group were assayed at −52 mV, which is near their Vrest (Fig. 2), the carbachol-induced Cm increase was a markedly larger (P < 0.0001) 8.72 ± 0.25 pF (Fig. 6). Thus, when measured close to the observed Vrest, the size of the evoked exocytotic activity becomes significantly less during long-term hyperosmolarity.
Of mechanistic importance, our Cm data indicate that the voltage sensitivity of evoked exocytosis is not lost during long-term hyperosmolarity. Specifically, increasing the holding potential from −38 mV to −52 mV in the long-term hyperosmotic group boosted the carbachol-evoked Cm increase by 248 ± 29% (n = 6; P = 0.0066) to 6.50 ± 0.78 pF (not illustrated). In addition, our findings indicate that sustained hyperosmolarity did not cause a detectable change in the size of the pool of releasable granules since the observed 6.50-pF increase recorded at −52 mV was not significantly different than the evoked Cm increase observed at the holding potential in the short-term group (Fig. 6). Overall, our experimental results reveal that during a sustained rise in osmolarity, the release of tear-stabilizing mucin from conjunctival goblet cells becomes severely compromised due to the loss of hyperosmotic-induced hyperpolarization.
DISCUSSION
This study provides new insights into bioelectric mechanisms by which the ocular surface responds to extracellular hyperosmolarity (Fig. 9), which is the hallmark of the oft-occurring, pain-causing, and sight-impairing condition of dry eye. Perforated-patch recordings from goblet cells located in excised conjunctival specimens showed that a ≥10% rise in osmolarity causes these mucin-releasing cells to rapidly generate a ~15-mV hyperpolarization due to the oxidant-dependent activation of their KATP channels. High-resolution Cm assays used to monitor exocytotic activity of single goblet cells revealed that hyperosmotic-induced hyperpolarization markedly boosts the exocytotic activity evoked by cholinergic input, which in vivo triggers mucin release by a mechanism chiefly dependent on calcium influx. However, when hyperosmolarity is sustained for more than 30 min, the development of a depolarizing nonspecific cation conductance fully counterbalances the KATP-driven hyperpolarization thereby eliminating the exocytotic boost. Loss of the hyperpolarization-induced boost in evoked exocytosis is a previously unappreciated mechanism by which goblet cells in chronically dry eyes can remain filled with mucin even though the tear film is hyperosmotic and mucin-deficient (35).
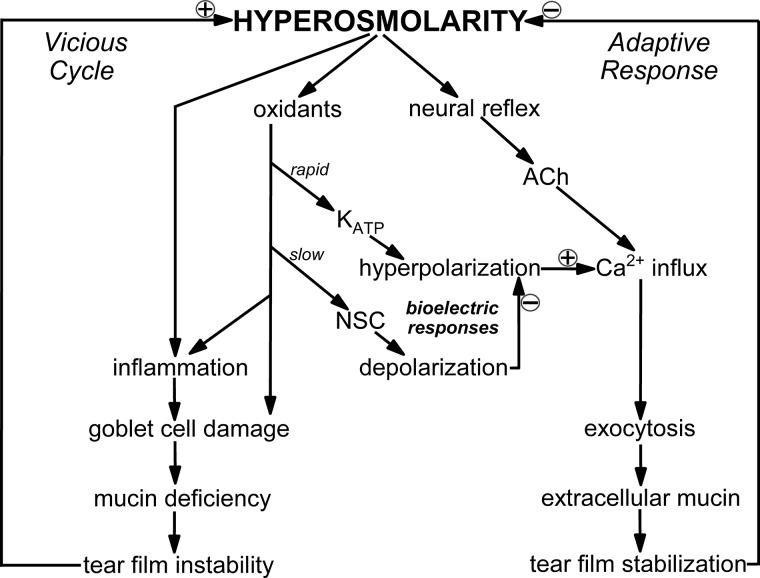
Fig. 9.
Model of bioelectric mechanisms by which conjunctival goblet cells respond to hyperosmolarity. A few minutes after a ≥10% rise in extracellular osmolarity, hyperpolarizing ATP-sensitive K+ (KATP) channels are activated via an oxidant-dependent mechanism. The resulting increase in voltage enhances the electro-gradient for Ca2+ influx and thereby boosts the exocytosis of tear-stabilizing mucin evoked by cholinergic input, which in vivo is triggered by a neural reflex and is chiefly driven by influxing Ca2+. The hyperpolarization-induced boost in the ability of goblet cells to release a bolus of tear-stabilizing mucin is a previously unappreciated mechanism by which the ocular surface effectively adapts to dryness/hyperosmolarity. However, during prolonged hyperosmolarity, development of a depolarizing nonspecific cation (NSC) conductance eventually fully counterbalances the KATP-driven hyperpolarization and thereby eliminates the boost in evoked exocytosis. Thus, the ability of goblet cells to release a bolus of mucin in response to cholinergic input becomes severely compromised during persistent hyperosmolarity. As illustrated in a highly simplified manner, unremitting hyperosmolarity activates stress pathways that if unchecked, activate a vicious cycle involving inflammation and ocular surface damage that can result in chronic dry eye.
The transient duration of hyperosmotic-induced hyperpolarization indicates that this bioelectric response is not well suited to ameliorate the chronic dry eye associated with various intrinsic pathological ocular conditions. On the other hand, optimal visual function is more commonly threatened by relatively transient episodes of ocular dryness/hyperosmolarity caused by external environmental conditions, such as low humidity and/or increased wind velocity. Because of intense evolutionary pressure to maintain excellent visual acuity, it is not surprising that the ocular surface system is highly adapted to respond to “everyday” causes of transient dry eye. This adaptive response chiefly involves neural reflexes to increase blinking, tear production, and mucin release. Our study revealed that by the previously unsuspected bioelectric mechanism of hyperosmotic-induced hyperpolarization, the ability of conjunctival goblet cells to the release a bolus of tear-stabilizing mucin is boosted during short-term dryness/hyperosmolarity.
Does the loss of KATP-driven hyperpolarization during prolonged hyperosmolarity provide an adaptive advantage? Since intrinsic causes of chronic dry eye occur chiefly in older individuals, there has been minimal evolutionary pressure to effectively adapt to persistent tear film hyperosmolarity. However, because we have found that the vulnerability of nonconjunctival ocular cells to oxidative, as well as other, stresses is markedly increased when hyperpolarization is sustained (16, 37), it may be that the decrease in voltage observed during prolonged hyperosmolarity serves to enhance goblet cell survival. Additional research, including identification of the specific type(s) of depolarizing NSC channels, will be needed to assess this putative scenario.
This is the first bioelectric analysis of goblet cells (of any tissue) in situ, rather than in dissociated or cultured cells. The only previous electrophysiological study of conjunctival goblet cells consisted of whole cell recordings from two cells dissociated from rabbit conjunctiva and placed in a normosmotic solution (45). Noteworthy is that, over the range of voltages assayed in our study, the I–V relations of these isolated cells closely match those we observed in goblet cells in situ. Ours is the first investigation of the bioelectric impact of hyperosmolarity on goblet cells. Furthermore, although Cm assays have been used as an indicator of mucin release from nonconjunctival primary cultures and cancer cell lines (2, 3, 5, 7, 22), this study is the first use of single-cell Cm measurements to analyze the voltage dependence of exocytotic activity evoked in goblet cells.
How do the results of our high-resolution Cm assays of single goblet cells fit with classical morphological studies (8, 11) showing that stimulated goblet cells near-simultaneously release the bulk of their mucin-filled granules? Comparison of the estimated numbers of stored and released granules supports the concept of bulk exocytosis by conjunctival goblet cells. On the one hand, with granules of these cells being ~1-µm in diameter (20, 42) and having a 1-µF/cm2 specific membrane capacitance (7), calculations indicate that the exocytotic fusion of one granule into the plasma membrane would result in a ~30-fF increase in Cm. Consequently, the ~8-pF Cm increase shown in Fig. 6 is likely to reflect the exocytosis of ~270 granules. On the other hand, with a conjunctival goblet cell having a 2.5-µm radius and a 17-µm length and being approximately two-thirds filled with granules (19, 20, 42), the calculated number of granules per cell is ~250. Taken together, these estimates indicate that an 8-pF Cm increase reflects the secretion of essentially all of the granules stored within a conjunctival goblet cell. Of interest, although traditional histological classification typically named the bulk release of mucin by goblet cells as apocrine (14), newer analytical techniques have further defined apocrine as a nonexocytotic process (14) whereas the classical histological term, merocrine, applies to exocytotic release. Thus, the Cm analysis initiated in this study of conjunctival goblet cells focuses on the exocytotic merocrine secretion that mediates the release of tear-stabilizing mucin.
An experimental advantage of freshly excised conjunctival specimens is that goblet cells can be studied in their in situ environment without the morphological and functional alterations associated with enzyme-facilitated tissue dissociation or with maintenance in long-term culture. However, a caveat with the study of goblet cells in situ is that precise quantification of cell volume changes that may occur under hyperosmotic conditions appears to not be feasible at present. Thus, it remains to be evaluated whether a change in cell volume, as well as an alteration in redox status, is involved in the bioelectric response of conjunctival goblet cells to hyperosmolarity. Yet, it should be noted that volume-regulated changes in ion channel activity typically commence within a few seconds after a change in osmolarity (25), rather than the ~3-min lag between hyperosmolarity and hyperpolarization observed in this study.
Although our long-range goal is to better understand the pathophysiology of the human ocular surface system, goblet cells in the rat conjunctiva were studied because appropriate human specimens are rarely available. It is reassuring that despite goblet cells in the rat conjunctiva being located chiefly in clusters whereas most of those in the human are solitary (19–21, 42), individual goblet cells in the conjunctivas of rats and humans share many fundamental morphological and functional features (19, 26, 42). Yet despite similarities of rodent and human goblet cells, recordings of human tissue will ultimately be needed to establish that bioelectric mechanisms discovered in rat conjunctiva are also operative in humans.
An additional caveat is that confirmation of the conclusions derived from our study of freshly excised conjunctiva awaits technical advances permitting comparable experiments to be performed in vivo. However, an advantage of using excised specimens is that the effect of applied chemicals, such as carbachol, can be assessed in the absence of potentially confounding actions of neurally released secretagogues such as acetylcholine and vasoactive intestinal peptide (8, 30). Because the cholinergic neural reflex is the most intensively studied pathway regulating mucin release from conjunctival goblet cells (13, 26, 28, 30, 40), this study analyzed carbachol-induced exocytosis. By exposing each conjunctival specimen only once to this cholinergic agonist, our study minimized variability caused by alterations in the releasable pool of granules or functional recovery of cholinergic receptors. On the other hand, since goblet cells in vivo are likely to receive repetitive neural inputs during sustained hyperosmolarity, additional research will be needed to explore the effects of multiple exposures to acetylcholine and other secretagogues.
In conclusion, this study revealed previously unappreciated bioelectric mechanisms by which conjunctival goblet cells respond to hyperosmolarity, which is the universal hallmark of dry eye. By rapidly generating a KATP-driven voltage increase, goblet cells enhance their ability to release a bolus of tear-stabilizing mucin in response to cholinergic input. However, with sustained hyperosmolarity, this exocytotic boost is lost as the development of a depolarizing NSC conductance fully counterbalances the hyperpolarization. Continued progress in elucidating the bioelectric mechanisms by which the ocular surface responds to dryness/hyperosmolarity should provide novel strategies for ameliorating the uncomfortable sight-impairing condition of dry eye.
GRANTS
This work was supported by a Research to Prevent Blindness Stein Innovation Award and NIH National Eye Institute Grant EY-007003.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
D.G.P. conceived and designed research; D.G.P. performed experiments; D.G.P. analyzed data; D.G.P. interpreted results of experiments; D.G.P. prepared figures; D.G.P. drafted manuscript; D.G.P. edited and revised manuscript; D.G.P. approved final version of manuscript.
ACKNOWLEDGMENTS
The author thanks Edward Stuenkel for providing equipment and advice and Bret Hughes for helpful discussions.
REFERENCES
- 1.Augustin AJ, Spitznas M, Kaviani N, Meller D, Koch FH, Grus F, Göbbels MJ. Oxidative reactions in the tear fluid of patients suffering from dry eyes. Graefes Arch Clin Exp Ophthalmol 233: 694–698, 1995. doi: 10.1007/BF00164671. [DOI] [PubMed] [Google Scholar]
- 2.Bertrand CA, Durand DM, Saidel GM, Laboisse C, Hopfer U. System for dynamic measurements of membrane capacitance in intact epithelial monolayers. Biophys J 75: 2743–2756, 1998. doi: 10.1016/S0006-3495(98)77718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertrand CA, Laboisse CL, Hopfer U. Purinergic and cholinergic agonists induce exocytosis from the same granule pool in HT29-Cl.16E monolayers. Am J Physiol Cell Physiol 276: C907–C914, 1999. doi: 10.1152/ajpcell.1999.276.4.C907. [DOI] [PubMed] [Google Scholar]
- 4.Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, Knop E, Markoulli M, Ogawa Y, Perez V, Uchino Y, Yokoi N, Zoukhri D, Sullivan DA. TFOS DEWS II pathophysiology report. Ocul Surf 15: 438–510, 2017. doi: 10.1016/j.jtos.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Chen P, Hwang TC, Gillis KD. The relationship between cAMP, Ca2+, and transport of CFTR to the plasma membrane. J Gen Physiol 118: 135–144, 2001. doi: 10.1085/jgp.118.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courville CB, Smolek MK, Klyce SD. Contribution of the ocular surface to visual optics. Exp Eye Res 78: 417–425, 2004. doi: 10.1016/j.exer.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Danahay H, Atherton HC, Jackson AD, Kreindler JL, Poll CT, Bridges RJ. Membrane capacitance and conductance changes parallel mucin secretion in the human airway epithelium. Am J Physiol Lung Cell Mol Physiol 290: L558–L569, 2006. doi: 10.1152/ajplung.00351.2005. [DOI] [PubMed] [Google Scholar]
- 8.Dartt DA. Regulation of mucin and fluid secretion by conjunctival epithelial cells. Prog Retin Eye Res 21: 555–576, 2002. doi: 10.1016/S1350-9462(02)00038-1. [DOI] [PubMed] [Google Scholar]
- 10.Dartt DA, Willcox MD. Complexity of the tear film: importance in homeostasis and dysfunction during disease. Exp Eye Res 117: 1–3, 2013. doi: 10.1016/j.exer.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol 70: 487–512, 2008. doi: 10.1146/annurev.physiol.70.113006.100638. [DOI] [PubMed] [Google Scholar]
- 12.Deng R, Hua X, Li J, Chi W, Zhang Z, Lu F, Zhang L, Pflugfelder SC, Li DQ. Oxidative stress markers induced by hyperosmolarity in primary human corneal epithelial cells. PLoS One 10: e0126561, 2015. doi: 10.1371/journal.pone.0126561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diebold Y, Ríos JD, Hodges RR, Rawe I, Dartt DA. Presence of nerves and their receptors in mouse and human conjunctival goblet cells. Invest Ophthalmol Vis Sci 42: 2270–2282, 2001. [PubMed] [Google Scholar]
- 14.Farkaš R. Apocrine secretion: new insights into an old phenomenon. Biochim Biophys Acta 1850: 1740–1750, 2015. doi: 10.1016/j.bbagen.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Foulks GN, Forstot SL, Donshik PC, Forstot JZ, Goldstein MH, Lemp MA, Nelson JD, Nichols KK, Pflugfelder SC, Tanzer JM, Asbell P, Hammitt K, Jacobs DS. Clinical guidelines for management of dry eye associated with Sjögren disease. Ocul Surf 13: 118–132, 2015. doi: 10.1016/j.jtos.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Fukumoto M, Nakaizumi A, Zhang T, Lentz SI, Shibata M, Puro DG. Vulnerability of the retinal microvasculature to oxidative stress: ion channel-dependent mechanisms. Am J Physiol Cell Physiol 302: C1413–C1420, 2012. doi: 10.1152/ajpcell.00426.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García-Posadas L, Hodges RR, Li D, Shatos MA, Storr-Paulsen T, Diebold Y, Dartt DA. Interaction of IFN-γ with cholinergic agonists to modulate rat and human goblet cell function. Mucosal Immunol 9: 206–217, 2016. doi: 10.1038/mi.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillis KD. Admittance-based measurement of membrane capacitance using the EPC-9 patch-clamp amplifier. Pflugers Arch 439: 655–664, 2000. doi: 10.1007/s004249900173. [DOI] [PubMed] [Google Scholar]
- 19.Gipson IK. Goblet cells of the conjunctiva: a review of recent findings. Prog Retin Eye Res 54: 49–63, 2016. doi: 10.1016/j.preteyeres.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gipson IK, Tisdale AS. Visualization of conjunctival goblet cell actin cytoskeleton and mucin content in tissue whole mounts. Exp Eye Res 65: 407–415, 1997. doi: 10.1006/exer.1997.0351. [DOI] [PubMed] [Google Scholar]
- 21.Greiner JV, Henriquez AS, Covington HI, Weidman TA, Allansmith MR. Goblet cells of the human conjunctiva. Arch Ophthalmol 99: 2190–2197, 1981. doi: 10.1001/archopht.1981.03930021066016. [DOI] [PubMed] [Google Scholar]
- 22.Guo XW, Merlin D, Laboisse C, Hopfer U. Purinergic agonists, but not cAMP, stimulate coupled granule fusion and Cl− conductance in HT29-Cl.16E. Am J Physiol Cell Physiol 273: C804–C809, 1997. doi: 10.1152/ajpcell.1997.273.3.C804. [DOI] [PubMed] [Google Scholar]
- 23.Hecquet CM, Ahmmed GU, Vogel SM, Malik AB. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ Res 102: 347–355, 2008. doi: 10.1161/CIRCRESAHA.107.160176. [DOI] [PubMed] [Google Scholar]
- 24.Hodges RR, Bair JA, Carozza RB, Li D, Shatos MA, Dartt DA. Signaling pathways used by EGF to stimulate conjunctival goblet cell secretion. Exp Eye Res 103: 99–113, 2012. doi: 10.1016/j.exer.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev 89: 193–277, 2009. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 26.Horikawa Y, Shatos MA, Hodges RR, Zoukhri D, Rios JD, Chang EL, Bernardino CR, Rubin PA, Dartt DA. Activation of mitogen-activated protein kinase by cholinergic agonists and EGF in human compared with rat cultured conjunctival goblet cells. Invest Ophthalmol Vis Sci 44: 2535–2544, 2003. doi: 10.1167/iovs.02-1117. [DOI] [PubMed] [Google Scholar]
- 27.Ishizaki E, Fukumoto M, Puro DG. Functional KATP channels in the rat retinal microvasculature: topographical distribution, redox regulation, spermine modulation and diabetic alteration. J Physiol 587: 2233–2253, 2009. doi: 10.1113/jphysiol.2009.169003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanno H, Horikawa Y, Hodges RR, Zoukhri D, Shatos MA, Rios JD, Dartt DA. Cholinergic agonists transactivate EGFR and stimulate MAPK to induce goblet cell secretion. Am J Physiol Cell Physiol 284: C988–C998, 2003. doi: 10.1152/ajpcell.00582.2001. [DOI] [PubMed] [Google Scholar]
- 29.Kültz D. Hyperosmolality triggers oxidative damage in kidney cells. Proc Natl Acad Sci USA 101: 9177–9178, 2004. doi: 10.1073/pnas.0403241101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li D, Jiao J, Shatos MA, Hodges RR, Dartt DA. Effect of VIP on intracellular [Ca2+], extracellular regulated kinase 1/2, and secretion in cultured rat conjunctival goblet cells. Invest Ophthalmol Vis Sci 54: 2872–2884, 2013. doi: 10.1167/iovs.12-11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindau M, Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch 411: 137–146, 1988. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Gutterman DD. Oxidative stress and potassium channel function. Clin Exp Pharmacol Physiol 29: 305–311, 2002. doi: 10.1046/j.1440-1681.2002.03649.x. [DOI] [PubMed] [Google Scholar]
- 33.Marques DL, Alves M, Modulo CM, Silva LE, Reinach P, Rocha EM. Lacrimal osmolarity and ocular surface in experimental model of dry eye caused by toxicity. Rev Bras Oftalmol 74: 68–72, 2015. doi: 10.5935/0034-7280.20150016. [DOI] [Google Scholar]
- 34.Matsushita K, Puro DG. Topographical heterogeneity of KIR currents in pericyte-containing microvessels of the rat retina: effect of diabetes. J Physiol 573: 483–495, 2006. doi: 10.1113/jphysiol.2006.107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore JE, Vasey GT, Dartt DA, McGilligan VE, Atkinson SD, Grills C, Lamey PJ, Leccisotti A, Frazer DG, Moore TC. Effect of tear hyperosmolarity and signs of clinical ocular surface pathology upon conjunctival goblet cell function in the human ocular surface. Invest Ophthalmol Vis Sci 52: 6174–6180, 2011. doi: 10.1167/iovs.10-7022. [DOI] [PubMed] [Google Scholar]
- 36.Moreau C, Prost AL, Dérand R, Vivaudou M. SUR, ABC proteins targeted by KATP channel openers. J Mol Cell Cardiol 38: 951–963, 2005. doi: 10.1016/j.yjmcc.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 37.Nakaizumi A, Puro DG. Vulnerability of the retinal microvasculature to hypoxia: role of polyamine-regulated KATP channels. Invest Ophthalmol Vis Sci 52: 9345–9352, 2011. doi: 10.1167/iovs.11-8176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pflugfelder SC, de Paiva CS. The pathophysiology of dry eye disease: what we know and future directions for research. Ophthalmology 124, 11S: S4–S13, 2017. doi: 10.1016/j.ophtha.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puro DG, Kohmoto R, Fujita Y, Gardner TW, Padovani-Claudio DA. Bioelectric impact of pathological angiogenesis on vascular function. Proc Natl Acad Sci USA 113: 9934–9939, 2016. doi: 10.1073/pnas.1604757113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ríos JD, Zoukhri D, Rawe IM, Hodges RR, Zieske JD, Dartt DA. Immunolocalization of muscarinic and VIP receptor subtypes and their role in stimulating goblet cell secretion. Invest Ophthalmol Vis Sci 40: 1102–1111, 1999. [PubMed] [Google Scholar]
- 41.Rismondo V, Osgood TB, Leering P, Hattenhauer MG, Ubels JL, Edelhauser HF. Electrolyte composition of lacrimal gland fluid and tears of normal and vitamin A-deficient rabbits. CLAO J 15: 222–228, 1989. [PubMed] [Google Scholar]
- 42.Setzer PY, Nichols BA, Dawson CR. Unusual structure of rat conjunctival epithelium. Light and electron microscopy. Invest Ophthalmol Vis Sci 28: 531–537, 1987. [PubMed] [Google Scholar]
- 43.Smith CB, Betz WJ. Simultaneous independent measurement of endocytosis and exocytosis. Nature 380: 531–534, 1996. doi: 10.1038/380531a0. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi N, Mori Y. TRP channels as sensors and signal integrators of redox status changes. Front Pharmacol 2: 58, 2011. doi: 10.3389/fphar.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watsky MA. Characterization of voltage-gated, whole-cell ionic currents from conjunctival epithelial cells. Invest Ophthalmol Vis Sci 39: 351–357, 1998. [PubMed] [Google Scholar]
- 46.Willcox MDP, Argüeso P, Georgiev GA, Holopainen JM, Laurie GW, Millar TJ, Papas EB, Rolland JP, Schmidt TA, Stahl U, Suarez T, Subbaraman LN, Uçakhan OO, Jones L. TFOS DEWS II Tear Film Report. Ocul Surf 15: 366–403, 2017. doi: 10.1016/j.jtos.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang T, Zhang A, Honeggar M, Kohan DE, Mizel D, Sanders K, Hoidal JR, Briggs JP, Schnermann JB. Hypertonic induction of COX-2 in collecting duct cells by reactive oxygen species of mitochondrial origin. J Biol Chem 280: 34966–34973, 2005. doi: 10.1074/jbc.M502430200. [DOI] [PubMed] [Google Scholar]