Abstract
Carotid artery (CCA) dilation occurs in healthy subjects during cold pressor test (CPT), while the magnitude of dilation relates to cardiovascular risk. To further explore this phenomenon and mechanism, we examined carotid artery responses to different sympathetic tests, with and without α1-receptor blockade and assessed similarity to these responses between carotid and coronary arteries. In randomized order, 10 healthy participants (25 ± 3 yr) underwent sympathetic stimulation using the CPT (3-min left-hand immersion in ice-slush) and lower-body negative pressure (LBNP). Before and during sympathetic tests, CCA diameter and velocity (Doppler ultrasound) and left anterior descending (LAD) coronary artery velocity (echocardiography) were recorded across 3 min. Measures were repeated 90 min following selective α1-receptor blockade via oral prazosin (0.05 mg/kg body wt). CPT significantly increased CCA diameter, LAD maximal velocity, and velocity-time integral area-under-the-curve (all P < 0.05). In contrast, LBNP resulted in a decrease in CCA diameter, LAD maximal velocity, and velocity time integral (VTI; all P < 0.05). Following α1-receptor blockade, CCA and LAD velocity responses to CPT were diminished. In contrast, during LBNP (−30 mmHg), α1-receptor blockade did not alter CCA or LAD responses. Finally, changes in CCA diameter and LAD VTI responses to sympathetic stimulation were positively correlated (r = 0.66, P < 0.01). We found distinct carotid artery responses to different tests of sympathetic stimulation, where α1 receptors partly contribute to CPT-induced responses. Finally, we found agreement between carotid and coronary artery responses. These data indicate similarity between carotid and coronary responses to sympathetic tests and the role of α1 receptors that is dependent on the nature of the sympathetic challenge.
NEW & NOTEWORTHY We showed distinct carotid artery responses to cold pressor test (CPT; i.e., dilation) and lower-body negative pressure (LBNP; i.e., constriction). Blockade of α1-receptors significantly attenuated dilator responses in carotid and coronary arteries during CPT, while no changes were found during LBNP. Our findings indicate strong similarity between carotid and coronary artery responses to distinct sympathetic stimuli, and for the role of α-receptors.
Keywords: α1-adrenoceptors, cardiovascular disease, carotid artery, coronary artery endothelial function, sympathetic nervous system
INTRODUCTION
Activation of the sympathetic nervous system (SNS) is an important and clinically relevant prognostic stimulus to examine artery function (12, 36). During the cold pressor test (CPT), a potent sympathetic stimulus, the coronary arteries can respond in a vasoconstrictor (via α1-receptors) or vasodilatory way (via the α2-, and β-receptors) (2). Vasodilator pathways prevail in healthy volunteers (10, 27), whereas experimental studies in patients with coronary artery disease demonstrate vasoconstriction during SNS activation (30, 47, 49). Coronary artery responses to CPT independently predict future cardiovascular events in patients at risk for cardiovascular disease (31, 32, 36), which highlights the clinical relevance of this response. However, the invasive nature of angiography makes these tests impractical for large-scale clinical use. Interestingly, the carotid artery shows vasodilation during SNS activation in healthy subjects, similar to coronary artery responses. This carotid dilation is abolished or even reversed to vasoconstriction in those with (increased risk for) cardiovascular disease (34, 44). To date, relatively little is known about the underlying mechanisms for the carotid artery reactivity to SNS activation.
Previous studies in peripheral conduit arteries have reported divergent responses to different tests of SNS activation (11, 15, 25, 27, 41). To date, no previous study has compared vasomotor responses of the carotid artery to distinct SNS stimuli. In line with peripheral arteries (i.e., the brachial and superficial femoral artery), we expect that distinct SNS stimuli [i.e., CPT and lower body negative pressure (LBNP)] lead to distinct carotid and coronary artery responses, as these tests mediate sympathetic activation through different pathways. More specifically, CPT evokes sympathetic activation via cold stress. The LBNP test gradually decreases central blood volume, which results in progressive increases in muscle sympathetic nerve activity (8, 9), which can directly lead to constriction of the carotid diameter.
No previous study examined the potential underlying mechanisms mediating carotid artery vasomotion during SNS activation. Work in both animal and human coronary arteries revealed a central role for α1-receptors to mediate vasomotor responses during SNS activation (17, 23, 26). In line with this previous work, we expect that α1-receptors, at least in part, contribute to the carotid artery responses to CPT and LBNP. Therefore, our first aim is to examine the impact of activation of the SNS, either through the CPT (i.e., elevates SNS activity and blood pressure) (18, 46) or LBNP (i.e., elevates SNS activity, with preserved blood pressure) (21, 45) on carotid artery diameter. Our second aim was to assess the role of α1-adrenoreceptors to these carotid artery responses by using an oral, selective α1-adrenoreceptor blocker (i.e., prazosin).
A recent study found good agreement between carotid and coronary responses to the CPT in healthy young and older subjects (44). To further explore this relationship, we aimed to compare the responses between the carotid artery diameter and left anterior descending coronary artery velocity (LAD velocity) during different SNS stimuli, with and without α1-receptor blockade. On the basis of previous work (34, 44), we anticipated that there would be similarity in the magnitude and direction of the vascular responses between both the carotid artery diameter and LAD velocity and that these responses would be partly mediated via α1-receptors.
METHODS
Ethical Approval
This study was approved by the Human Ethics Committee of the University of British Columbia and conformed to the standards set by the Declaration of Helsinki. All volunteers provided written informed consent.
Participants
We recruited 10 healthy male participants (mean age 25 ± 3 yr, height 1.78 ± 0.1 m, and weight 76 ± 9 kg). Exclusion criteria were a history of cardiovascular disease (i.e., angina pectoris, myocardial infarction, heart failure), lung disease (i.e., chronic obstructive pulmonary disease and lung cancer), brain disease (i.e., stroke and dementia), presence of Raynaudʼs phenomenon, scleroderma, chronic pain, and/or open wounds on the upper extremities, obesity (body mass index >30 kg/m2), diabetes mellitus Type 1 or 2, history of smoking, or elevated blood pressure (systolic >130 mmHg; diastolic >85 mmHg).
Experimental Design
All participants reported to our laboratory for a single visit. They were asked to abstain from strenuous exercise for 24 h and abstain from dietary products known to affect endothelial function for ≥18 h before the testing session (i.e., vitamin C, caffeine, and alcohol). Moreover, participants were asked to fast for ≥2 h, adapted from existing guidelines to assess peripheral vascular function (38). Participants rested in the supine position for >15 min on a bed in a temperature-controlled room (23 ± 1°C). Subsequently, participants underwent LBNP and two CPT, in a randomly assigned order, with 45-min rest between tests. All tests involved simultaneous assessment of common carotid artery (CCA) diameter and velocity (ultrasound) and left anterior descending (LAD) coronary artery velocity (echocardiography) before (across a 1-min baseline) and during sympathetic stimulation. The protocol was repeated 90 min after oral administration of prazosin (i.e., α1-adrenergic receptor antagonist that effectively blocks 80% of α1-receptor activity, 0.05 mg/kg body wt) (1, 22).
Experimental Measures
Common carotid artery diameter and velocity.
Left carotid artery diameter and red blood cell velocity were recorded simultaneously and continuously during baseline (1-min CPT) and sympathetic stimuli (i.e., 3-min CPT and ~18-min LBNP). Carotid artery image acquisition was performed using a 10-MHz multifrenquency linear array hand-held probe attached to a high-resolution ultrasound machine (15L4; Terason T3200, Burlington, MA). When an optimal image was found, 2–3 cm proximal from the bifurcation, the probe was held stable, and the ultrasound parameters were set to optimize the longitudinal, B-mode image of the lumen-arterial wall interface. Continuous pulsed-wave Doppler velocity assessments were also obtained and were collected at the lowest possible insonation angle (always <60°). Assessment was performed by an experienced sonographer (A. C. C. M. van Mil), who achieved an hour-to-hour reproducibility (i.e., coefficient of variation) of CCA baseline diameter of 0.8% and a reproducibility of 0.8% for the peak CCA diameter, in line with previous findings (44).
Coronary artery velocity.
Before and during both CPT and LBNP, the LAD (cm) coronary artery velocity was examined using transthoracic ultrasound. This assessment was performed simultaneously with CCA diameter and velocity responses. All echocardiographic measurements were collected by a trained sonographer (M. Stembridge) on a commercially available ultrasound system (Vivid E9; GE, Fairfield, CT) using a broadband M5S 5 MHz or a 3-V three-dimensional array transducer. In a previous study, the Cronbachʼs α-reliability test revealed α values of 0.81 and 0.89 for both max and mean LAD velocities, respectively, suggesting good consistency between LAD velocity measurements (5). Participants assumed a left lateral position to allow for data collection. The LAD was imaged using a modified parasternal short axis view from the fourth or fifth left intercostal space and was assessed using pulsed-wave Doppler. The transducer was positioned such that a 2- to 3-mm segment of the LAD was imaged along the long axis, taking care to align the pulse-wave cursor with the length of the vessel. With a sample volume (2.0 mm) positioned over the color Doppler signal in the LAD, measurements of the LAD velocity were collected during the sympathetic tests.
Blood pressure and heart rate.
All continuously recorded cardiovascular measurements were acquired at 200 Hz using an analog-to-digital converter (PowerLab/16SP ML 880; ADInstruments, Colorado Springs, CO) interfaced with a personal computer. Before and during CPT and LBNP, systolic and diastolic blood pressure (SBP and DBP in mmHg, respectively), stroke volume (SV, in ml), rate-pressure product (RPP, HR × SBP, a reliable indicator for myocardial oxygen demand) (14), and cardiac output (CO, in l/min) were continuously measured using noninvasive finger photoplethysmography (Finometer Pro, Finapres Medical Systems, Amsterdam, Netherlands). Heart rate (HR, beats per minute) was recorded using three-lead electrocardiography, placed in lead II configuration (Bioamp, ML132; ADInstruments).
Sympathetic Stimuli
Cold pressor test.
The cold pressor tests (CPTs) consisted of a 3-min immersion of the left hand in a bucket of ice slush (~4.0°C) (44). The participant was positioned in supine position on a tilt bed, tilted slightly to the left lateral position (~25–30°), to facilitate arm movement in the bucket of slush without significant movement of the body, and provide adequate coronary assessment. After a 1-min baseline period, the participantʼs hand was immersed up to the wrist in the ice-slush for 3 min. The participant was instructed to remain quiet during the CPT to provide for valid CCA assessment. The partial pressures of end-tidal carbon dioxide () and oxygen () were clamped at baseline values for the entire duration of the protocol to reduce the potential impact of hyperventilation on the vascular responses, upon an end-tidal forcing approach described extensively elsewhere (43). To reduce measurement error, CPT procedures were repeated twice and averaged for analyses (44).
Lower-body negative pressure.
The participant was positioned in the supine position on a tilt bed and strapped into a custom-made air-tight, lower-body suction chamber at the level of the iliac crest (42). The LBNP chamber was then moved from supine position into a left lateral position (~25–30°) to ensure adequate coronary imaging. The lower-body negative pressure test consisted of a 5-min baseline, followed by progressive 2-min stages, using increments of −10 mmHg, to −80 mmHg, or until presyncope. LBNP was terminated when 1) presyncope occurred, defined by a sustained drop in systolic blood pressure <80 mmHg for more than 10 s (24), or 2) upon the participantʼs request due to the onset of subjective symptoms (e.g., feelings of dizziness, nausea, and faintness). During the prazosin condition, participants were unable to last longer than −40 mmHg during the LBNP test. For reliable comparison between the control and drug condition, we chose only to include data until −30 mmHg.
Data Analysis
Carotid artery responses.
Analyses of diameter (cm), blood flow (ml/s), blood velocity (cm/s), and shear (s−1) were performed using custom-designed edge detection and wall-tracking software, which is largely independent of investigator bias, as was extensively described elsewhere (4). Baseline diameter, blood flow, blood velocity, and shear were calculated as the mean of data acquired across a 1-min baseline period (4). For the CPT, data were calculated for 10-s intervals. LBNP data were calculated at 1-min intervals. Subsequently, offline image analysis involves the identification of the region of interest (ROI), to allow for automated calibration on the B-mode image and velocities on the Doppler assessment (40). A ROI is drawn around the optimal B-mode image, in which a pixel-density algorithm automatically identifies the near and far wall. Another ROI is drawn around the Doppler waveform, which is synchronized with the B-mode diameter ROI. Ultimately, this allows for blood flow and shear rate calculations (40). Peak diameter change was calculated relative to baseline diameter.
Coronary artery responses.
All images were exported for offline analysis using commercially available software (EchoPAC version 13.0; GE Medical, Horten, Norway). All echocardiographic values represent an average value of three cardiac cycles representing the clearest of five collected images for each experimental stage. The collected waveforms were analyzed to determine mean diastolic velocity (LAD Vmean, cm/s), peak diastolic velocity (LAD Vmax, cm/s), and the velocity time integral (VTI; cm) (19). Coronary flow velocity reliably reflects changes in absolute coronary blood flow (10, 13, 27), suggesting that an increase in flow velocity reflects coronary artery dilatation. Using observer-independent software, VTI is calculated as the integral of individual velocities across the cardiac cycle. Participants who had at least one suboptimal image were excluded before analyses (5).
Blood pressure and heart rate.
Analyses of systolic and diastolic pressure, heart rate, cardiac output, stroke volume, and the rate-pressure product (RPP) were performed in commercially available software (LabChart V7.1, ADInstruments). Measurements were averaged per 10-s bins for analyses for the CPT and 1-min bins for the LBNP analyses. Baseline CPT was averaged over a 3-min period. Continuous blood pressure measurements were calibrated to automated brachial blood pressure readings during baseline (HEM-775CAN; Omron Healthcare, Bannockburn, IL).
Statistical Analyses
All data were presented as means ± SD unless stated otherwise. Parameters were tested for normality using a Shapiro-Wilk test. Responses of the CCA [i.e., diameter (cm), blood velocity (cm/s), flow (ml/s) and shear (s−1)] and LAD [i.e., mean velocity (cm/s), max velocity (cm/s), and VTI (cm)] were assessed during the sympathetic stimulus with paired Student’s t-tests (in case of nonparametric variables, a Wilcoxon signed-rank test was performed). Changes over time were assessed with two-way, repeated-measures ANOVAs (missing values were only imputed on the basis of previous and consecutive measurements when available). We assessed whether CCA and LAD changes in diameter, velocity, flow, and shear occurred over time (i.e., within-factor time), and whether this differed between conditions (i.e., between-factor Control vs. Prazosin) were examined. In addition to the main effects, the time × condition interaction revealed whether the CCA and LAD changes across time differed between the control condition and prazosin. This was done to assess the potential role of α-receptors in mediating CCA and LAD responses. The two-way, repeated-measures ANOVAs were performed with Sidak correction to account for multiple comparisons. Data were analyzed using SPSS 20.0 software (IBM SPSS; IBM, Armonk, NY). Values of P < 0.05 were assumed to be statistically significant.
RESULTS
Carotid Artery Responses: Different SNS Stimuli
Cold pressor test.
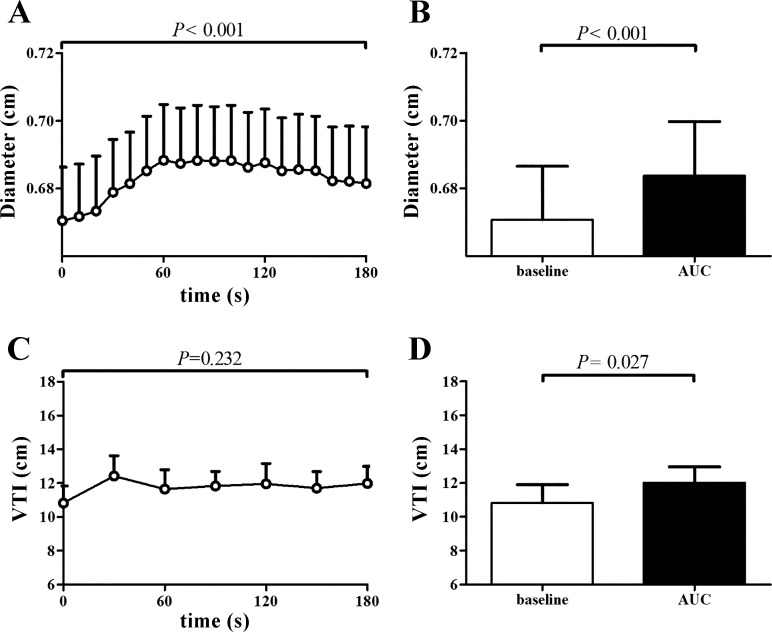
The CPT caused a significant increase in systolic and diastolic blood pressure and the rate-pressure product (RPP), while no change was found in stroke volume, heart rate, and cardiac output (n = 9; Table 1). Although the diameter (cm) of the CCA increased significantly during CPT (P < 0.001; Fig. 1), CCA velocity (cm/s), flow (ml/s), and shear rate (s−1) did not change significantly across time during CPT (P > 0.05, data not shown).
Table 1.
Cold pressor test responses
| 1-min Cold Pressor Test |
2-min Cold Pressor Test |
3-min Cold Pressor Test |
Two-way ANOVA |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cold Pressor Test | Baseline | 30 | 60 | 90 | 120 | 150 | 180 | Time | Trial | Time × Trial |
| Stroke volume, ml | ||||||||||
| Control | 105 ± 15 | 105 ± 17 | 105 ± 20 | 104 ± 20 | 106 ± 21 | 106 ± 20 | 107 ± 19 | 0.450 | 0.296 | 0.450 |
| Prazosin | 112 ± 14 | 114 ± 15 | 111 ± 16 | 111 ± 16 | 110 ± 17 | 111 ± 17 | 112 ± 17 | |||
| Cardiac output, l/min | ||||||||||
| Control | 6.2 ± 1.3 | 6.6 ± 1.4 | 7.0 ± 1.6 | 6.6 ± 1.5 | 6.3 ± 1.4 | 6.3 ± 1.5 | 6.3 ± 1.4 | 0.200 | 0.049 | 0.021 |
| Prazosin | 6.7 ± 0.9 | 7.5 ± 1.3 | 7.5 ± 1.6 | 7.5 ± 1.4 | 7.6 ± 1.2 | 7.6 ± 1.1 | 7.6 ± 1.0 | |||
| Heart Rate, beats/min | ||||||||||
| Control | 59 ± 12 | 64 ± 11 | 67 ± 12 | 63 ± 13 | 61 ± 12 | 61 ± 13 | 59 ± 12 | 0.107 | 0.024 | 0.001 |
| Prazosin | 62 ± 12 | 68 ± 11 | 70 ± 13 | 70 ± 14 | 72 ± 14* | 71 ± 12 | 70 ± 13* | |||
| Diastolic BP, mmHg | ||||||||||
| Control | 79 ± 8 | 84 ± 9 | 91 ± 8* | 92 ± 8 | 92 ± 9* | 90 ± 8* | 89 ± 8* | 0.000 | 0.045 | 0.031 |
| Prazosin | 74 ± 9 | 76 ± 9 | 81 ± 10 | 82 ± 8* | 83 ± 9 | 82 ± 9* | 80 ± 8* | |||
| Systolic BP, mmHg | ||||||||||
| Control | 133 ± 7 | 140 ± 11 | 148 ± 11* | 150 ± 13 | 151 ± 12 | 149 ± 11* | 148 ± 11* | 0.000 | 0.169 | 0.023 |
| Prazosin | 131 ± 7 | 136 ± 7 | 141 ± 9 | 140 ± 6 | 141 ± 9 | 141 ± 9* | 140 ± 7* | |||
| Rate pressure product | ||||||||||
| Control | 7,927 ± 1,754 | 9,028 ± 1,804 | 9,901 ± 2,047 | 9,549 ± 2,097 | 9,212 ± 1,982 | 9,077 ± 2,011 | 8,834 ± 1,927 | 0.008 | 0.307 | 0.014 |
| Prazosin | 8,141 ± 1,534 | 9,284 ± 1,750 | 9,833 ± 2,023 | 9,858 ± 2,024 | 10,217 ± 2,204 | 9,941 ± 1,667* | 9,826 ± 1,709* | |||
| LAD velocity max | ||||||||||
| Control | 0.252 ± 0.03 | 0.325 ± 0.07 | 0.304 ± 0.03 | 0.280 ± 0.05 | 0.261 ± 0.04 | 0.266 ± 0.05 | 0.265 ± 0.05 | 0.007 | 0.769 | 0.594 |
| Prazosin | 0.261 ± 0.04 | 0.312 ± 0.04 | 0.302 ± 0.07 | 0.281 ± 0.06 | 0.278 ± 0.06 | 0.284 ± 0.06 | 0.279 ± 0.05 | |||
| LAD velocity mean | ||||||||||
| Control | 0.201 ± 0.03 | 0.256 ± 0.07 | 0.232 ± 0.03 | 0.224 ± 0.04 | 0.209 ± 0.03 | 0.215 ± 0.03 | 0.207 ± 0.03 | 0.041 | 0.603 | 0.400 |
| Prazosin | 0.199 ± 0.03 | 0.25 ± 0.02 | 0.226 ± 0.03 | 0.228 ± 0.05 | 0.231 ± 0.04 | 0.231 ± 0.04 | 0.228 ± 0.04 | |||
Hemodynamic and coronary responses during Cold pressor test (full Supplemental Table, averaged per 10-s intervals, is available online at the Journal website). P values refer to 2-way repeated-measures ANOVAs for within-participant comparison (time), between-trial comparison, and the interaction time × trial. BP, blood pressure; LAD, left anterior descending (coronary artery).
Significant difference compared with baseline values, P < 0.05. Italicized values indicate significant difference (P < 0.05).
Fig. 1.
Responses of the carotid artery (n = 9) and the lower anterior descending (LAD) coronary artery (n = 6) to CPT. A: diameter change of the carotid artery over time. B: percentage change in carotid diameter at baseline and during the cold pressor test (CPT; area under the curve, AUC). C: velocity time integral (VTI) change of the LAD coronary artery over time. D: percentage change in LAD coronary artery (VTI) at baseline and during CPT (area under the curve, AUC). Data are presented as means ± SE. Open bars represent baseline measurements, whereas solid bars represent peak values.
Lower-body negative pressure.
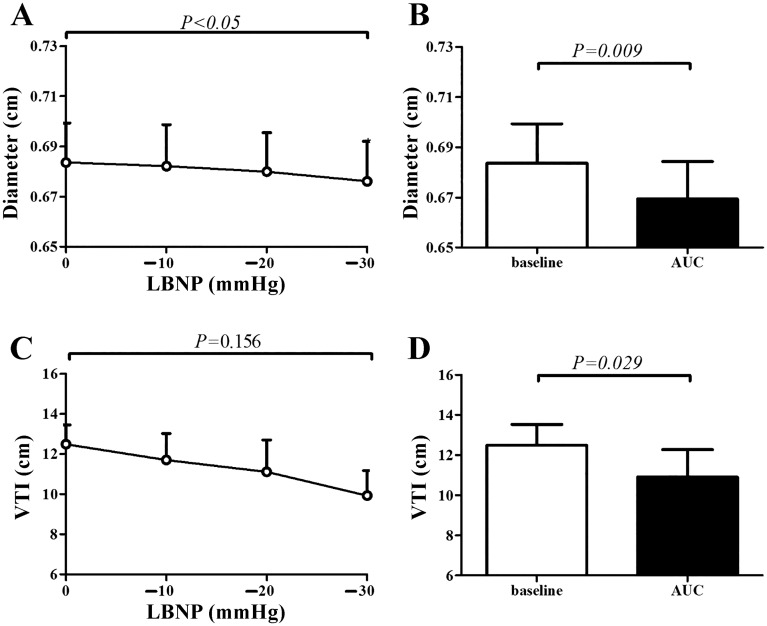
LBNP caused a gradual, but significant increase in heart rate, a decrease in stroke volume, and diastolic blood pressure, while systolic blood pressure and cardiac output were preserved (n = 9; Table 2). LBNP caused a significant decrease in CCA diameter (cm; Fig. 2), whereas no changes were found in CCA velocity (cm/s), flow (ml/s), and shear (s−1) (data not shown).
Table 2.
Lower-body negative pressure responses
| Two-Way ANOVA |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower-Body Negative Pressure | Baseline | −10 | −20 | −30 | Time | Trial | Time × Trial | |||
| Stroke volume, ml | ||||||||||
| Control | 106 ± 13 | 102 ± 12 | 100 ± 12 | 97 ± 12 | 97 ± 13 | 93 ± 14 | 90 ± 14 | 0.000 | 0.687 | 0.086 |
| Prazosin | 106 ± 12 | 103 ± 14 | 99 ± 16 | 96 ± 15 | 93 ± 16 | 91 ± 16 | 85 ± 18* | |||
| Cardiac output, l/min | ||||||||||
| Control | 6.4 ± 1.2 | 6.1 ± 1.1 | 6.1 ± 1.1 | 5.9 ± 1.1 | 6.0 ± 1.0 | 5.8 ± 1.0 | 5.9 ± 1.0 | 0.642 | 0.002 | 0.162 |
| Prazosin | 7.1 ± 1.4 | 7.1 ± 1.2 | 7.2 ± 1.4 | 7.2 ± 1.0 | 7.4 ± 0.9 | 7.5 ± 0.8 | 7.3 ± 0.9 | |||
| Heart rate, beats/min | ||||||||||
| Control | 61 ± 12 | 60 ± 12 | 61 ± 13 | 62 ± 13 | 63 ± 13 | 64 ± 14 | 67 ± 14 | 0.000 | 0.002 | 0.009 |
| Prazosin | 68 ± 16 | 70 ± 16 | 75 ± 20 | 77 ± 17* | 82 ± 17* | 84 ± 17* | 89 ± 17* | |||
| Diastolic BP, mmHg | ||||||||||
| Control | 77 ± 9 | 77 ± 9 | 77 ± 9 | 78 ± 9 | 78 ± 9 | 79 ± 9 | 79 ± 10 | 0.177 | 0.160 | 0.060 |
| Prazosin | 73 ± 8 | 74 ± 8 | 72 ± 9 | 74 ± 8 | 73 ± 8 | 74 ± 9 | 73 ± 9 | |||
| Systolic BP, mmHg | ||||||||||
| Control | 132 ± 7 | 131 ± 6 | 129 ± 7 | 130 ± 7 | 131 ± 6 | 130 ± 7 | 131 ± 7 | 0.152 | 0.388 | 0.005 |
| Prazosin | 131 ± 9 | 131 ± 9 | 128 ± 9 | 129 ± 9 | 127 ± 10 | 127 ± 10 | 124 ± 11 | |||
| Rate pressure product | ||||||||||
| Control | 8,048 ± 1,746 | 7,923 ± 1,745 | 7,929 ± 1,831 | 8,024 ± 1,766 | 8,192 ± 1,741 | 8,348 ± 1,895 | 8,708 ± 1,916 | <0.001 | 0.027 | 0.025 |
| Prazosin | 8,954 ± 2,618 | 9,253 ± 2,537 | 9,646 ± 2,963 | 9,969 ± 2,491* | 10,479 ± 2,585* | 10,751 ± 2,568* | 10,960 ± 2,319* | |||
| LAD VTI | ||||||||||
| Control | 12.1 ± 2.7 | 11.1 ± 3.6 | 9.9 ± 3.4 | 8.7 ± 1.3 | 0.019 | 0.09 | 0.547 | |||
| Prazosin | 9.5 ± 0.9 | 9.8 ± 1.9 | 8.1 ± 2.2 | 7.8 ± 1.8 | ||||||
| LAD velocity max | ||||||||||
| Control | 0.277 ± 0.05 | 0.26 ± 0.06 | 0.243 ± 0.05 | 0.226 ± 0.03 | 0.029 | 0.52 | 0.621 | |||
| Prazosin | 0.25 ± 0.06 | 0.249 ± 0.03 | 0.226 ± 0.04 | 0.225 ± 0.04 | ||||||
| LAD velocity mean | ||||||||||
| Control | 0.21 ± 0.03 | 0.19 ± 0.03 | 0.203 ± 0.04 | 0.187 ± 0.03 | 0.496 | 0.973 | 0.177 | |||
| Prazosin | 0.197 ± 0.03 | 0.207 ± 0.02 | 0.188 ± 0.03 | 0.20 ± 0.03 | ||||||
Hemodynamic and coronary responses during lower-body negative pressure test (averaged per 2-min stages). P values refer to two-way repeated-measures ANOVAs, for within-participant comparison (time), between-trial comparison, and the interaction time × trial. BP, blood pressure; LAD, left anterior descending (coronary artery).
Significant difference compared with baseline values, P < 0.05. Italicized values indicate significant difference (P < 0.05).
Fig. 2.
Responses of the carotid artery (n = 9) and the lower anterior descending (LAD) coronary artery (n = 5) to lower-body negative pressure (LBNP). A: diameter change of the carotid artery over time. B: percentage change in carotid diameter at baseline and during LBNP. C: velocity time integral (VTI) change of the LAD coronary artery over time. D: percentage change in LAD coronary artery VTI at baseline and during LBNP. Data are presented as means ± SE. Open bars represent baseline measurements, whereas solid bars represent peak values.
Carotid Artery Response to Sympathetic Activation: Role of α1-Receptors
Cold pressor test.
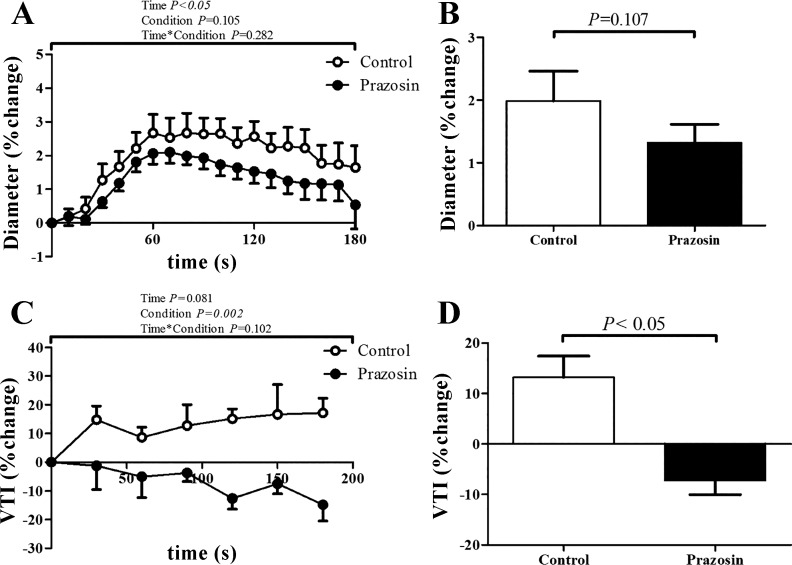
Prazosin increased baseline CCA diameter and decreased shear (0.671 ± 0.05 to 0.703 ± 0.05 cm, and 185.9 ± 50 to 159.1 ± 40 s−1 respectively, all P < 0.05), while no changes were found in carotid blood flow and blood velocity (10.9 ± 1.8 to 10.8 ± 1.5 ml/s; P = 0.405, and 30.7 ± 6.6 to 27.7 ± 5.2 cm/s; P = 0.051). Prazosin caused an abolished CPT-induced increase in diameter (Fig. 3). Prazosin attenuated the increase in blood pressure during CPT and resulted in a larger increase in cardiac output, heart rate, and RPP during the CPT (n = 9; Table 2). We found no change in stroke volume (Table 2), while we also found no change in CCA flow, shear, and velocity (data not shown).
Fig. 3.
Responses of the carotid artery (n = 9) and the lower anterior descending (LAD) coronary artery (n = 6) to cold pressor test (CPT), Control vs. Prazosin condition. A: diameter change of the carotid artery over time. B: percentage change in diameter. C: velocity time integral (VTI) change of the LAD coronary artery over time. D: percentage change in LAD coronary artery VTI at baseline and during the CPT. Data are expressed as means ± SE. Open bars represent the control condition, whereas solid bars represent the prazosin condition.
Lower-body negative pressure.
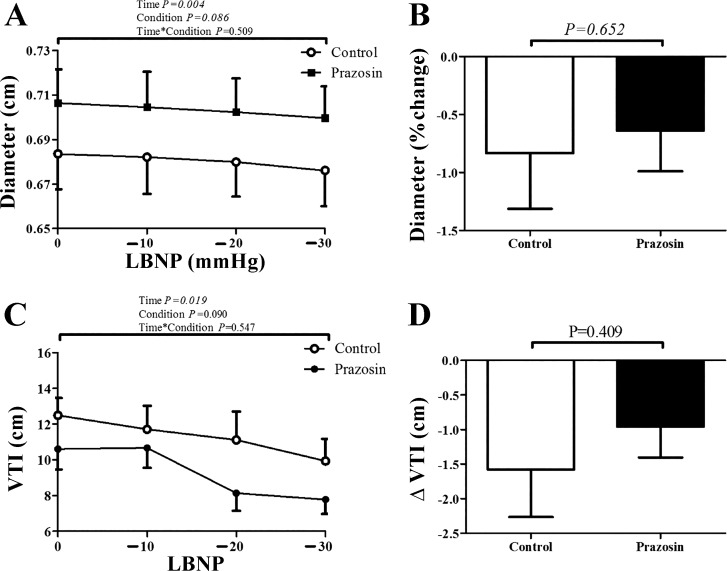
Baseline CCA diameter, flow, and velocity were significantly larger following Prazosin adminstration (0.684 ± 0.05 to 0.706 ± 0.05 cm, 10.2 ± 1.6 to 12.0 ± 2.3 ml/s, and 27.6 ± 5.0 to 30.0 ± 5.5 cm/s, respectively; all P < 0.05). All subjects reached presyncope at −30 or −40 mmHg. Therefore, we compared data between both sessions up to −30 mmHg. Prazosin did not alter CCA diameter responses during LBNP (Table 2, Fig. 4). Prazosin exaggerated the increase in heart rate and RPP during LBNP, while blood pressure decreased during the prazosin trial (n = 9; Table 2).
Fig. 4.
Responses of the carotid artery (n = 9) and the lower anterior descending (LAD) coronary artery (n = 5) to lower-body negative pressure (LBNP), Control vs. Prazosin condition. A: diameter change of the carotid artery over time. B: percentage change in carotid diameter at baseline and during the LBNP. C: velocity time integral (VTI) change of the LAD coronary artery over time. D: percentage change in LAD coronary artery VTI at baseline and during the LBNP. Data are expressed as means ± SE. Open bars represent the control condition, whereas solid bars represent the prazosin condition.
Carotid Artery Responses Versus Coronary Artery Responses
SNS stimulation.
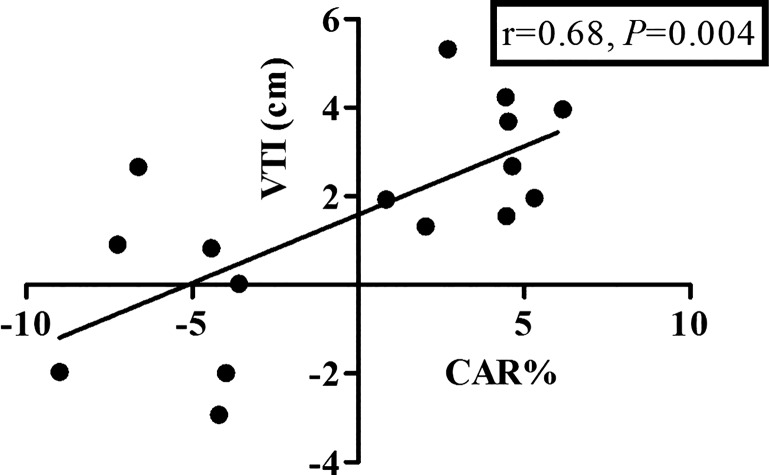
Similar to CCA responses, CPT caused a significant increase in LAD maximum velocity (n = 6; baseline 0.25 ± 0.03 to peak 0.34 ± 0.02 cm/s; P < 0.05) and VTI (P < 0.05; Fig. 1). Because of the suction of the LBNP box, movement of the participants prevented assessment in five participants. Again, in agreement with CCA responses, LBNP caused a reduction in LAD maximum velocity (n = 5; Table 2) and peak VTI (cm, Fig. 2). When pooled, a significant correlation was found between changes in CCA diameter and LAD peak VTI (n = 20; r = 0.65; P < 0.01).
α1-Receptor blockade.
Following prazosin administration, LAD VTI was elevated, and prazosin abolished the increases in CCA diameter and LAD peak VTI (Fig. 3, Table 2). During LBNP, prazosin did not alter CCA diameter (cm), LAD peak VTI, or LAD peak velocity responses (cm/s, up to −30 mmHg; Table 2, Fig. 4).
DISCUSSION
We present the following findings. First, activation of the SNS using the CPT significantly increased CCA diameter, while SNS activation using LBNP mediated a decrease in CCA diameter. Second, systemic blockade of the α1-receptors significantly attenuated the dilator response of the carotid during the CPT, while these changes were unaltered during LBNP. This latter finding suggests the presence of distinct carotid artery responses to different types of SNS activation, with a distinct contribution of α1-receptors mediating these responses. Furthermore, we found good agreement between the direction and magnitude of the coronary and carotid artery responses when comparing the different tests of sympathetic stimulation, but also regarding the contribution of α1-receptors. Taken together, we found divergent responses to distinct tests of SNS activation and the role of α1-receptors mediating these responses, while similarity was found between carotid and coronary arteries in the magnitude and direction of vascular responses to sympathetic stimulation and blockade of α1-receptors.
Carotid Artery Responses to Sympathetic Stimulation
The CPT resulted in a characteristic dilation in the CCA of our healthy subjects, a finding observed previously in our laboratory (44) and others (34). Interestingly, these dilator responses of the carotid artery contrasts with peripheral artery responses, since brachial or superficial femoral arteries demonstrate negligible diameter changes during CPT (11, 25). Central, elastic arteries (such as the carotid artery) may, thus, respond differently to SNS activation using the CPT compared with muscular, peripheral arteries. This notion is further supported by observations of abdominal aorta dilation during the CPT (6). In contrast, LBNP mediated a decrease in CCA diameter. The presence of distinct artery responses to different tests of sympathetic activation has also been reported in peripheral conduit arteries (11). Both CPT and LBNP mediate sympathetic activation through different pathways, leading to distinct vascular responses in peripheral and central arteries. The CPT causes an immediate stressor response (11, 27), leading to rapid catecholamine release and blood pressure elevation, which induces β-receptor-mediated vasodilation and sympathetic blood pressure-mediated constriction, respectively. The result of these responses is an increase in CCA diameter, due to the dominating effect of β-receptor-mediated vasodilation. In contrast, the LBNP mediates a gradual, arterial baroreflex-mediated activation of the sympathetic nervous system and, thus, can directly decrease carotid diameter. Both sympathetic tests demonstrate distinct time-dependent changes in circulating catecholamines, with an immediate elevation after CPT, and a slower (time- and intensity-dependent) elevation during LBNP (11, 21, 27, 33). These data indicate that distinct tests of stimulation of the sympathetic nervous system lead to different carotid artery responses.
Role of α1-Receptors in Carotid Artery Responses to Sympathetic Stimulation
Under physiological conditions, α1-receptors mediate vasoconstriction in coronary arteries during a sympathetic stimulus (23, 29). Indeed, blockade of α1-receptors resulted in an increase in baseline CCA diameter and velocity, but also LAD velocity. However, in contrast to our hypothesis, α1-blockade attenuated the carotid artery dilator responses during the CPT, while no impact of α1-blockade was found during LBNP. One potential explanation is that the increase in baseline diameter and/or velocity (induced by α1-receptor blockade) prevented a further increase in diameter upon additional SNS stimulation. This explanation is supported by previous work in peripheral arteries, which found that an increase in baseline diameter is associated with a smaller endothelium-(in)dependent vasodilation (37, 39). However, our data do not reveal such a relation between resting carotid diameter and peak responses (CPT control: r = −0.280; prazosin: r = −0.275; LBNP control: r = −0.401; prazosin: r = −0.219, all P > 0.05). Therefore, CCA dilation during α1-blockade may not explain the attenuated vasomotor responses to CPT or preserved response to LBNP.
An alternative explanation for the attenuated dilator response may relate to the pharmacological actions of α1-receptor blockers. In healthy coronary arteries, vasoconstriction upon sympathetic stimulation is largely mediated via α1-receptors, with only a minor role for α2-receptors (3, 48). Previous studies in both animals and humans found that during α1-receptor blockade, SNS activation still mediates coronary constriction through activation of α2-receptors (7, 16, 20). Possibly, α1-receptor blockade in our study yielded stimulation of α2-receptors during activation of the SNS using the CPT. Consequently, the vasodilator responses may be attenuated by the constrictive actions of α2-receptors. This hypothesis needs further exploration. A final reason for the diminished CCA dilation during CPT could reside in the attenuated blood pressure responses. However, it is unclear whether blood pressure represents the principal contributor to the carotid dilation, especially since peak diameter responses precede peak blood pressure values. Moreover, blood pressure rises similarly between individuals who demonstrate carotid artery vasodilation versus vasoconstriction (44). Nonetheless, we cannot exclude a potential role for the blood pressure response to contribute to the carotid dilation.
Carotid Artery Versus Coronary Artery
Our findings provide strong evidence for the similarity between the carotid and coronary arteries regarding the direction and magnitude of the vasomotor response. Indeed, both carotid and coronary arteries demonstrated dilation in response to CPT, but constriction was present in both arteries during LBNP. The presence of coronary dilation to CPT (30, 49), but also coronary constriction to LBNP (27), has been reported in previous studies. This further confirms the presence of distinct artery responses to distinct stimuli to activate the sympathetic nervous system. Furthermore, α1-receptor blockade mediated similar effects between carotid and coronary arteries for the LBNP test. During the CPT, we observed that α1-receptor blockade attenuated the carotid responses, while the coronary responses were reversed. Agreement between arteries was further supported by the presence of a significant and strong correlation between both arteries (Fig. 5), a finding that is in line with previous work (34, 44). A potential limitation of the echocardiographic measurement is the inability to examine blood flow. However, strong agreement is present between changes in coronary artery blood velocity and blood flow in response to sympathetic stimulation (10, 27, 28), suggesting that the increase in LAD velocity can be interpreted as true coronary vasodilation.
Fig. 5.
Correlation between the carotid artery diameter response (CAR%) and coronary LAD response [change in the velocity time integral (VTI in cm)] pooled for the cold pressor test and lower-body negative pressure test (n = 16).
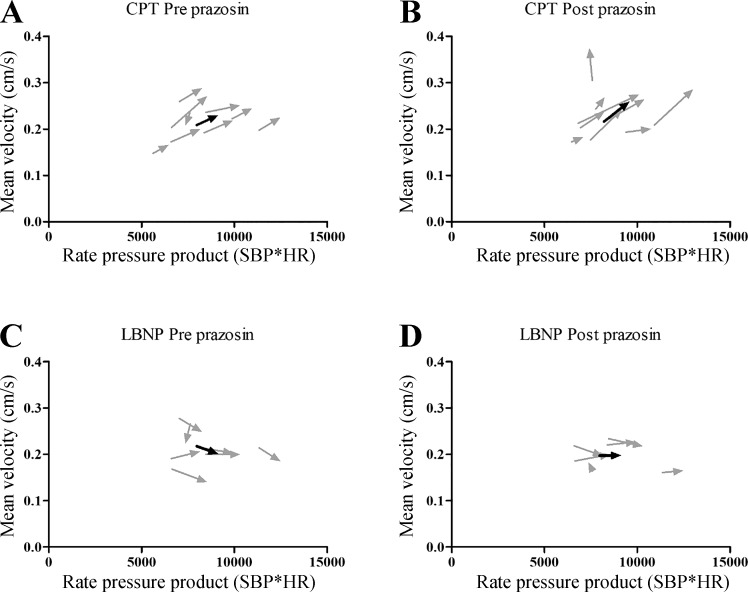
Despite these similarities in magnitude and direction of vascular responsiveness, it is important to emphasize that the mechanisms contributing to vascular control may differ between arteries. For example, coronary artery flow and velocity during sympathetic stimulation are dependent on both local metabolic and vasodilatory mechanisms sensitive to the rate of myocardial oxygen consumption () (27). For this purpose, we have calculated the RPP, a commonly used index for myocardial oxygen consumption (Fig. 6). The increase in RPP during the CPT suggests that the dilation of the coronary artery is, at least partly, related to the increase in myocardial oxygen uptake. Whether similar mechanisms are present in the brain to contribute to carotid artery dilation during the CPT is currently unknown. For the LBNP, we found no important role for RPP to contribute to the vascular responses in our study. When correcting our responses for potential differences for the RPP, correlation between LAD VTI and CAR(%) remained present (r = 0.66; P < 0.05). Future studies are required to better understand the mechanisms contributing to the vascular responses during sympathetic stimulation in both carotid and coronary arteries.
Fig. 6.
Mean coronary velocity expressed versus the rate pressure product (RPP). A: responses of mean coronary velocity vs. RPP during the cold pressor test (CPT). B: responses of mean coronary velocity vs. RPP during CPT with prazosin. C: responses of mean coronary velocity versus RPP during lower-body negative pressure (LBNP). D: responses of mean coronary velocity vs. RPP during LBNP with prazosin. Light gray arrows indicate individual responses, while the black arrows indicate the mean response.
Clinical relevance.
Coronary artery responsiveness to SNS stimulation, including the CPT, has shown a strong predictive ability for future cardiovascular disease and/or events (31, 32, 36). Similarity in vasomotor responsiveness between coronary and carotid arteries suggests that the carotid artery may serve as an alternative measure for coronary vascular responses to SNS stimulation. An important advantage of measuring the carotid artery is its easy accessibility, high reproducibility, and the accuracy of the test. This warrants future studies to further explore the potential clinical use of examining carotid responses to SNS stimulation. To further explore the similarity between the carotid and coronaries, future studies could be performed in a catheterization laboratory, to simultaneously measure both carotid and coronary artery responses during sympathetic stimulation. These studies can be extended by the addition of selective α- and/or β-adrenergic agonist and antagonists, to further resolve the contribution of adrenergic receptors to sympathetically mediated carotid and coronary artery responses.
Methodological considerations.
A strength of our study was that we controlled for end-tidal gases at baseline values, during both CPT and LBNP, and in the α1-receptor blockade condition. Fluctations and alterations in are known to directly influence the diameter of the CCA (35) and LAD VTI (5). Following our α1-receptor blockade, which directly affects mean arterial pressure and ventilatory regulation during sympathetic activation, clamping and to baseline values reduced the possible interference with our carotid and coronary artery responses.
To summarize, our data demonstrate that the carotid artery shows distinct vascular responses to different stimuli to activate the sympathetic nervous system. Additionally, blockade of the α1-receptors significantly attenuated the dilator responses in the carotid artery during the CPT, while no changes were found during LBNP, suggesting a potential role for α-receptors to contribute to vasomotor responses in carotid arteries. Finally, even though α1-blockade resulted in disparate responses during CPT, our findings indicate strong similarity between carotid and coronary artery reactivity in response to distinct sympathetic stimuli.
GRANTS
Philip N. Ainslie, and the experimental studies that were conducted in his laboratory, was supported by a Canadian Research Chair in Cerebrovascular Physiology, a Natural Sciences and Engineering Research Council Discovery Grant and Canadian Foundation of Innovation. Grant. M. Tymko is funded by a Natural Sciences and Engineering Research Council Doctoral award. D. J. Green is supported by a National Health and Medical Research Council Principal Research Fellowship (APP1080914). A. C. C. M. van Mil is financially supported by a Top Institute for Food and Nutrition Grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.C.v.M., M.M.T., P.N.A., and D.H.T. conceived and designed research; A.C.v.M., M.M.T., T.P.K., and M.S. performed experiments; A.C.v.M. and D.H.T. analyzed data; A.C.v.M., M.M.T., T.P.K., M.S., D.J.G., P.N.A., and D.H.T. interpreted results of experiments; A.C.v.M. and D.H.T. prepared figures; A.C.v.M. and D.H.T. drafted manuscript; A.C.v.M., M.M.T., T.P.K., M.S., D.J.G., P.N.A., and D.H.T. edited and revised manuscript; A.C.v.M., M.M.T., T.P.K., M.S., D.J.G., P.N.A., and D.H.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Kevin Wildfong for valuable contribution to the experiments and Glen Foster for providing the necessary equipment and laboratory space.
REFERENCES
- 1.Atkinson CL, Lewis NC, Carter HH, Thijssen DH, Ainslie PN, Green DJ. Impact of sympathetic nervous system activity on post-exercise flow-mediated dilatation in humans. J Physiol 593: 5145–5156, 2015. doi: 10.1113/JP270946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbato E. Role of adrenergic receptors in human coronary vasomotion. Heart 95: 603–608, 2009. doi: 10.1136/hrt.2008.150888. [DOI] [PubMed] [Google Scholar]
- 3.Baumgart D, Haude M, Görge G, Liu F, Ge J, Grosse-Eggebrecht C, Erbel R, Heusch G. Augmented α-adrenergic constriction of atherosclerotic human coronary arteries. Circulation 99: 2090–2097, 1999. doi: 10.1161/01.CIR.99.16.2090. [DOI] [PubMed] [Google Scholar]
- 4.Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension 51: 203–210, 2008. doi: 10.1161/HYPERTENSIONAHA.107.101014. [DOI] [PubMed] [Google Scholar]
- 5.Boulet LM, Stembridge M, Tymko MM, Tremblay JC, Foster GE. The effects of graded changes in oxygen and carbon dioxide tension on coronary blood velocity independent of myocardial energy demand. Am J Physiol Heart Circ Physiol 311: H326–H336, 2016. doi: 10.1152/ajpheart.00107.2016. [DOI] [PubMed] [Google Scholar]
- 6.Chandraratna PA, Wijegunaratne K, Farag KF, Nimalasuriya AR, Mathews SJ. Changes in abdominal aortic diameter in response to the cold pressor test and nitroglycerin: a new noninvasive model for the assessment of endothelial-dependent and endothelial-independent vascular relaxation. Echocardiography 26: 1211–1216, 2009. doi: 10.1111/j.1540-8175.2009.00942.x. [DOI] [PubMed] [Google Scholar]
- 7.Chilian WM. Functional distribution of alpha 1- and alpha 2-adrenergic receptors in the coronary microcirculation. Circulation 84: 2108–2122, 1991. doi: 10.1161/01.CIR.84.5.2108. [DOI] [PubMed] [Google Scholar]
- 8.Cooke WH, Rickards CA, Ryan KL, Kuusela TA, Convertino VA. Muscle sympathetic nerve activity during intense lower body negative pressure to presyncope in humans. J Physiol 587: 4987–4999, 2009. doi: 10.1113/jphysiol.2009.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui J, Wilson TE, Crandall CG. Muscle sympathetic nerve activity during lower body negative pressure is accentuated in heat-stressed humans. J Appl Physiol (1985) 96: 2103–2108, 2004. doi: 10.1152/japplphysiol.00717.2003. [DOI] [PubMed] [Google Scholar]
- 10.Doucette JW, Corl PD, Payne HM, Flynn AE, Goto M, Nassi M, Segal J. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation 85: 1899–1911, 1992. doi: 10.1161/01.CIR.85.5.1899. [DOI] [PubMed] [Google Scholar]
- 11.Dyson KS, Shoemaker JK, Hughson RL. Effect of acute sympathetic nervous system activation on flow-mediated dilation of brachial artery. Am J Physiol Heart Circ Physiol 290: H1446–H1453, 2006. doi: 10.1152/ajpheart.00771.2005. [DOI] [PubMed] [Google Scholar]
- 12.Feigl EO. The paradox of adrenergic coronary vasoconstriction. Circulation 76: 737–745, 1987. doi: 10.1161/01.CIR.76.4.737. [DOI] [PubMed] [Google Scholar]
- 13.Gao Z, Wilson TE, Drew RC, Ettinger J, Monahan KD. Altered coronary vascular control during cold stress in healthy older adults. Am J Physiol Heart Circ Physiol 302: H312–H318, 2012. doi: 10.1152/ajpheart.00297.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gobel FL, Norstrom LA, Nelson RR, Jorgensen CR, Wang Y. The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation 57: 549–556, 1978. doi: 10.1161/01.CIR.57.3.549. [DOI] [PubMed] [Google Scholar]
- 15.Harris CW, Edwards JL, Baruch A, Riley WA, Pusser BE, Rejeski WJ, Herrington DM. Effects of mental stress on brachial artery flow-mediated vasodilation in healthy normal individuals. Am Heart J 139: 405–411, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Heusch G, Baumgart D, Camici P, Chilian W, Gregorini L, Hess O, Indolfi C, Rimoldi O. Alpha-adrenergic coronary vasoconstriction and myocardial ischemia in humans. Circulation 101: 689–694, 2000. doi: 10.1161/01.CIR.101.6.689. [DOI] [PubMed] [Google Scholar]
- 17.Heusch G, Deussen A, Schipke J, Thämer V. Alpha 1- and alpha 2-adrenoceptor-mediated vasoconstriction of large and small canine coronary arteries in vivo. J Cardiovasc Pharmacol 6: 961–968, 1984. doi: 10.1097/00005344-198409000-00034. [DOI] [PubMed] [Google Scholar]
- 18.Hines EA Jr, Brown GE. The cold pressor test for measuring the reactibility of the blood pressure: Data concerning 571 normal and hypertensive subjects. Am Heart J 11: 1–9, 1936. doi: 10.1016/S0002-8703(36)90370-8. [DOI] [Google Scholar]
- 19.Hozumi T, Yoshida K, Ogata Y, Akasaka T, Asami Y, Takagi T, Morioka S. Noninvasive assessment of significant left anterior descending coronary artery stenosis by coronary flow velocity reserve with transthoracic color Doppler echocardiography. Circulation 97: 1557–1562, 1998. doi: 10.1161/01.CIR.97.16.1557. [DOI] [PubMed] [Google Scholar]
- 20.Indolfi C, Piscione F, Villari B, Russolillo E, Rendina V, Golino P, Condorelli M, Chiariello M. Role of alpha 2-adrenoceptors in normal and atherosclerotic human coronary circulation. Circulation 86: 1116–1124, 1992. doi: 10.1161/01.CIR.86.4.1116. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs MC, Goldstein DS, Willemsen JJ, Smits P, Thien T, Lenders JW. Differential effects of low- and high-intensity lower-body negative pressure on noradrenaline and adrenaline kinetics in humans. Clin Sci (Lond) 90: 337–343, 1996. doi: 10.1042/cs0900337. [DOI] [PubMed] [Google Scholar]
- 22.Jones H, Lewis NC, Green DJ, Ainslie PN, Lucas SJ, Tzeng YC, Grant EJ, Atkinson G. α1-Adrenoreceptor activity does not explain lower morning endothelial-dependent, flow-mediated dilation in humans. Am J Physiol Regul Integr Comp Physiol 300: R1437–R1442, 2011. doi: 10.1152/ajpregu.00042.2011. [DOI] [PubMed] [Google Scholar]
- 23.Kern MJ, Horowitz JD, Ganz P, Gaspar J, Colucci WS, Lorell BH, Barry WH, Mudge GH Jr. Attenuation of coronary vascular resistance by selective α1-adrenergic blockade in patients with coronary artery disease. J Am Coll Cardiol 5: 840–846, 1985. doi: 10.1016/S0735-1097(85)80421-6. [DOI] [PubMed] [Google Scholar]
- 24.Lewis NC, Bain AR, MacLeod DB, Wildfong KW, Smith KJ, Willie CK, Sanders ML, Numan T, Morrison SA, Foster GE, Stewart JM, Ainslie PN. Impact of hypocapnia and cerebral perfusion on orthostatic tolerance. J Physiol 592: 5203–5219, 2014. doi: 10.1113/jphysiol.2014.280586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lind L, Johansson K, Hall J. The effects of mental stress and the cold pressure test on flow-mediated vasodilation. Blood Press 11: 22–27, 2002. doi: 10.1080/080370502753543927. [DOI] [PubMed] [Google Scholar]
- 26.Mohrman DE, Feigl EO. Competition between sympathetic vasoconstriction and metabolic vasodilation in the canine coronary circulation. Circ Res 42: 79–86, 1978. doi: 10.1161/01.RES.42.1.79. [DOI] [PubMed] [Google Scholar]
- 27.Momen A, Mascarenhas V, Gahremanpour A, Gao Z, Moradkhan R, Kunselman A, Boehmer JP, Sinoway LI, Leuenberger UA. Coronary blood flow responses to physiological stress in humans. Am J Physiol Heart Circ Physiol 296: H854–H861, 2009. doi: 10.1152/ajpheart.01075.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monahan KD, Feehan RP, Sinoway LI, Gao Z. Contribution of sympathetic activation to coronary vasodilatation during the cold pressor test in healthy men: effect of ageing. J Physiol 591: 2937–2947, 2013. doi: 10.1113/jphysiol.2013.251298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mudge GH Jr, Grossman W, Mills RM Jr, Lesch M, Braunwald E. Reflex increase in coronary vascular resistance in patients with ischemic heart disease. N Engl J Med 295: 1333–1337, 1976. doi: 10.1056/NEJM197612092952401. [DOI] [PubMed] [Google Scholar]
- 30.Nabel EG, Ganz P, Gordon JB, Alexander RW, Selwyn AP. Dilation of normal and constriction of atherosclerotic coronary arteries caused by the cold pressor test. Circulation 77: 43–52, 1988. doi: 10.1161/01.CIR.77.1.43. [DOI] [PubMed] [Google Scholar]
- 31.Nitenberg A, Chemla D, Antony I. Epicardial coronary artery constriction to cold pressor test is predictive of cardiovascular events in hypertensive patients with angiographically normal coronary arteries and without other major coronary risk factor. Atherosclerosis 173: 115–123, 2004. doi: 10.1016/j.atherosclerosis.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 32.Nitenberg A, Valensi P, Sachs R, Cosson E, Attali JR, Antony I. Prognostic value of epicardial coronary artery constriction to the cold pressor test in type 2 diabetic patients with angiographically normal coronary arteries and no other major coronary risk factors. Diabetes Care 27: 208–215, 2004. doi: 10.2337/diacare.27.1.208. [DOI] [PubMed] [Google Scholar]
- 33.Robertson D, Johnson GA, Robertson RM, Nies AS, Shand DG, Oates JA. Comparative assessment of stimuli that release neuronal and adrenomedullary catecholamines in man. Circulation 59: 637–643, 1979. doi: 10.1161/01.CIR.59.4.637. [DOI] [PubMed] [Google Scholar]
- 34.Rubenfire M, Rajagopalan S, Mosca L. Carotid artery vasoreactivity in response to sympathetic stress correlates with coronary disease risk and is independent of wall thickness. J Am Coll Cardiol 36: 2192–2197, 2000. doi: 10.1016/S0735-1097(00)01021-4. [DOI] [PubMed] [Google Scholar]
- 35.Sato K, Sadamoto T, Hirasawa A, Oue A, Subudhi AW, Miyazawa T, Ogoh S. Differential blood flow responses to CO2 in human internal and external carotid and vertebral arteries. J Physiol 590: 3277–3290, 2012. doi: 10.1113/jphysiol.2012.230425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schächinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 101: 1899–1906, 2000. doi: 10.1161/01.CIR.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 37.Silber HA, Bluemke DA, Ouyang P, Du YP, Post WS, Lima JA. The relationship between vascular wall shear stress and flow-mediated dilation: endothelial function assessed by phase-contrast magnetic resonance angiography. J Am Coll Cardiol 38: 1859–1865, 2001. doi: 10.1016/S0735-1097(01)01649-7. [DOI] [PubMed] [Google Scholar]
- 38.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thijssen DH, Dawson EA, Black MA, Hopman MT, Cable NT, Green DJ. Heterogeneity in conduit artery function in humans: impact of arterial size. Am J Physiol Heart Circ Physiol 295: H1927–H1934, 2008. doi: 10.1152/ajpheart.00405.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 53: 986–992, 2009. doi: 10.1161/HYPERTENSIONAHA.109.131508. [DOI] [PubMed] [Google Scholar]
- 41.Thijssen DH, de Groot P, Kooijman M, Smits P, Hopman MT. Sympathetic nervous system contributes to the age-related impairment of flow-mediated dilation of the superficial femoral artery. Am J Physiol Heart Circ Physiol 291: H3122–H3129, 2006. doi: 10.1152/ajpheart.00240.2006. [DOI] [PubMed] [Google Scholar]
- 42.Tymko MM. How to build a lower-body differential pressure chamber integrated on a tilt-table: A pedagogy tool to demonstrate the cardiovagal baroreflex. FACETS 1: 225–244, 2017. doi: 10.1139/facets-2016-0012. [DOI] [Google Scholar]
- 43.Tymko MM, Hoiland RL, Kuca T, Boulet LM, Tremblay JC, Pinske BK, Williams AM, Foster GE. Measuring the human ventilatory and cerebral blood flow response to CO2: a technical consideration for the end-tidal-to-arterial gas gradient. J Appl Physiol (1985) 120: 282–296, 2016. doi: 10.1152/japplphysiol.00787.2015. [DOI] [PubMed] [Google Scholar]
- 44.van Mil ACCM, Hartman Y, van Oorschot F, Heemels A, Bax N, Dawson EA, Hopkins N, Hopman MTE, Green DJ, Oxborough DL, Thijssen DHJ. Correlation of carotid artery reactivity with cardiovascular risk factors and coronary artery vasodilator responses in asymptomatic, healthy volunteers. J Hypertens 35: 1026–1034, 2017. doi: 10.1097/HJH.0000000000001274. [DOI] [PubMed] [Google Scholar]
- 45.Victor RG, Leimbach WN Jr. Effects of lower body negative pressure on sympathetic discharge to leg muscles in humans. J Appl Physiol (1985) 63: 2558–2562, 1987. doi: 10.1152/jappl.1987.63.6.2558. [DOI] [PubMed] [Google Scholar]
- 46.Victor RG, Leimbach WN Jr, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension 9: 429–436, 1987. doi: 10.1161/01.HYP.9.5.429. [DOI] [PubMed] [Google Scholar]
- 47.Vita JA, Treasure CB, Yeung AC, Vekshtein VI, Fantasia GM, Fish RD, Ganz P, Selwyn AP. Patients with evidence of coronary endothelial dysfunction as assessed by acetylcholine infusion demonstrate marked increase in sensitivity to constrictor effects of catecholamines. Circulation 85: 1390–1397, 1992. doi: 10.1161/01.CIR.85.4.1390. [DOI] [PubMed] [Google Scholar]
- 48.Young MA, Knight DR, Vatner SF. Autonomic control of large coronary arteries and resistance vessels. Prog Cardiovasc Dis 30: 211–234, 1987. doi: 10.1016/0033-0620(87)90013-2. [DOI] [PubMed] [Google Scholar]
- 49.Zeiher AM, Drexler H, Wollschlaeger H, Saurbier B, Just H. Coronary vasomotion in response to sympathetic stimulation in humans: importance of the functional integrity of the endothelium. J Am Coll Cardiol 14: 1181–1190, 1989. doi: 10.1016/0735-1097(89)90414-2. [DOI] [PubMed] [Google Scholar]