Abstract
Aponeuroses are connective tissues found on the surface of pennate muscles and are in close association with muscle fascicles. In addition to transmitting muscle forces to the external tendon, aponeurosis has been hypothesized to influence the direction of muscle shape change during a contraction. Muscle shape changes affect muscle contractile force and velocity because they influence the gear ratio with which muscle fascicles transmit force and velocity to the tendon. If aponeurosis modulates muscle shape changes, altering the aponeurosis’ radial integrity with incisions should alter gearing. We tested the hypothesis that incising the aponeurosis would lead to decreased gearing across force conditions with an in situ preparation of the turkey lateral gastrocnemius muscle. We found that multiple full-length incisions in the aponeurosis altered the relationship between gearing and force relative to the intact aponeurosis condition. Specifically, after multiple aponeurosis incisions, gear ratio decreased by 19% in the high-force contractions compared with the intact condition. These results suggest that aponeuroses influence muscle shape change and can alter muscle contractile force and speed through their effect on muscle gearing.
NEW & NOTEWORTHY Muscle gearing is determined by muscle shape change during a contraction and varies with the force of contraction. Variable gearing influences muscle force and speed, but how gearing is modulated is not well understood. Incising the aponeurosis before and after contractions demonstrates that aponeurosis plays a role in modulating gearing.
Keywords: aponeurosis, architectural gear ratio, connective tissue, muscle gearing, muscle shape change
INTRODUCTION
Aponeuroses are connective tissue sheaths found on the surface of pennate muscles. They are continuous with external tendons and serve as insertion sites for pennate muscle fascicles that do not extend from muscle origin to insertion (14). Like the external tendon, the aponeurosis transmits forces and length changes from the muscle fascicles to the skeleton. In addition to transmitting forces, it has been hypothesized that aponeuroses play a role in modulating the direction of muscle shape changes (1, 2, 29), but this has not been directly tested.
Because muscle is isovolumetric, muscle fibers must bulge radially when they shorten (5). In a unipennate muscle, this radial expansion can be accommodated by bulging in either the thickness dimension, perpendicular to the aponeurosis; or in the width dimension, parallel to the aponeurosis; or by bulging in both dimensions (Fig. 1, B–D). These differences in bulging are important for the mechanical output of the muscle because the pattern of shape changes influences muscle fascicle rotation and therefore gearing. When fibers rotate to higher angles of pennation, muscle shortening is achieved through a combination of fiber shortening and pennation angle change. This amplification of fiber length change is measured by a muscle’s architectural gear ratio (AGR), which is calculated as the ratio of muscle velocity to fascicle velocity. Muscles studied in turkeys, rats, frogs, and humans demonstrate that a muscle’s gear ratio varies with contractile force (1, 3, 11, 18). Muscles operate with a high gear ratio during low-force contractions, indicating fascicle rotations to higher angles of pennation and resulting in faster muscle speeds (for a given fascicle speed). During high-force contractions, reduced fascicle rotation results in muscles operating at a low gear ratio (Fig. 1D). The amount of fascicle rotation is geometrically related to muscle shape change. The more muscle bulging is accommodated by increases in width, rather than thickness, the less fibers will rotate to higher pennation angles. Thus a muscle’s AGR is variable and load dependent.
Fig. 1.
A: aponeuroses are extensions of external tendons on the surface of pennate muscles that function as insertion sites for muscle fascicles and may play a role in modulating fascicle rotation and dynamic gearing during muscle contractions. Because muscle is isovolumetric, it must bulge when it contracts to maintain a constant volume. This schematic illustrates the influence of bulging direction on fiber rotation. B: muscles can bulge in the thickness dimension, perpendicular to the aponeurosis; or in the width dimension, parallel to the aponeurosis; or in both dimensions. C: an increase in thickness could accommodate the greater fascicle rotations that occur during low-force, high-speed contractions. Fascicle rotations to higher pennation angles result in a high gear ratio where muscle velocity is high relative to fascicle velocity. D: during high-force, low-speed contractions, reduced fascicle rotations and a low gear ratio would result from the muscle bulging primarily in the width dimension.
The force-velocity relationship dictates that muscle shortening velocity declines with increasing muscle force when activation level is constant, so that high-force contractions occur at low shortening velocities while low-force contractions occur at relatively high shortening velocities (17). While this force-velocity relationship constrains muscle performance, dynamic changes in muscle gearing allow pennate muscles to partially overcome these constraints (1). Azizi et al. (1) showed that pennate muscles enhance shortening velocity by operating at high gear ratios during low-force contractions and enhance force production by operating at low gear ratios during high-force contractions. Thus dynamic gearing allows muscles to broaden the range of contractions over which they can attain high speeds and high forces.
The springy connective tissue elements within and around muscle have been hypothesized to dictate the direction of muscle bulging and hence the muscle’s AGR (1, 2, 29). By forming a sheath overlying a large portion of the muscle belly, aponeurosis has the potential to control the magnitude of muscle bulging in the thickness or width direction. The goal of this study was to examine the role of aponeurosis in modulating muscle shape changes and gearing across contractions of different force. We incised the aponeurosis in an in situ preparation of the turkey lateral gastrocnemius muscle to alter the radial integrity of the aponeurosis and its ability to resist changes in muscle shape. We measured gearing before and after aponeurosis incisions to test the prediction that altering the structural integrity of the aponeurosis with incisions would alter the relationship between gearing and force compared with the intact aponeurosis.
MATERIALS AND METHODS
Seven adult Eastern wild turkeys (Meleagris gallopavo; mass: 4.25 ± 0.21 kg) were obtained from a licensed breeder. Turkeys were housed in the Animal Care Facilities at Brown University and fed a commercial poultry diet and water ad libitum. All experimental procedures were approved by Brown’s Institutional Animal Care and Use Committee.
In situ experiment.
An in situ preparation of the turkey lateral gastrocnemius (LG) muscle was used to measure muscle fascicle gearing during isotonic contractions following Azizi et al. (1). Throughout the experiment, the bird was deeply anesthetized with inhaled isoflurane. Feathers were removed from the limb to be instrumented and an incision was made in the skin overlying the LG muscle. Three 2-mm sonomicrometry crystals (Sonometrics, Ontario, Canada) were implanted in the LG muscle and were used to track fascicle length and pennation angle, while a muscle servomotor (Aurora Instruments 310B-LR; Aurora Scientific, London, ON Canada) measured muscle length and controlled muscle force (Fig. 2A). Through small incisions made in the muscle surface, a pair of crystals was implanted along an LG fascicle that inserted in the superficial aponeurosis and this crystal pair was used to measure fascicle length and velocity. The small muscle incisions for each crystal were closed with 6–0 silk suture.
Fig. 2.

A: schematic of the in situ preparation of the turkey lateral gastrocnemius (LG) muscle. The branch of the sciatic nerve innervating the LG muscle was isolated and stimulated with a nerve cuff. A pair of sonomicrometry crystals was inserted along a muscle fascicle to measure fascicle length and velocity. A 3rd crystal was inserted deep to the distal fascicle crystal to measure pennation angle. The LG tendon was dissected from its insertion and clamped to a servomotor that controlled muscle-tendon unit (MTU) force and measured muscle length and velocity. B: the aponeurosis was incised to examine the effect of reduced radial integrity on architectural gearing. Isotonic contractions were performed in the intact condition and then repeated after incising the aponeurosis with a single partial incision (1 × 25%Lapo), a full-length incision (1 × 100%Lapo), and 3 full-length incisions (3 × 100%Lapo).
To measure pennation angle with a triad, a third crystal was inserted deep to the distal fascicle crystal through an incision in the deep surface of the muscle or with a hypodermic needle through the superficial aponeurosis (Fig. 2A). An accurate measurement of pennation angle required proper alignment of the crystals, which was achieved by inserting the deep crystal so it formed a plane with the fascicle crystals that parallels the direction of fascicle shortening. Pennation angle was calculated using the measured distances between crystal pairs and the law of cosines. Postmortem analysis of crystal alignment indicated that the distal fascicle crystal and deep muscle crystal were not aligned perpendicular to the muscle line of action in many turkeys. While the method of calculating pennation angle does not assume the crystals form a right triangle, this misalignment precluded these crystals from accurately measuring muscle thickness. The deep crystal was in the plane of fascicle shortening with the fascicle crystals in four of seven turkeys, indicating that measures of pennation angle may be inaccurate in some turkeys.
For muscle force and speed measurements, the LG tendon was detached just distal to its calcified portion, and the calcified portion was clamped to the lever of a servomotor (Fig. 2A). The muscle origin was held fixed with two custom-made clamps securing the femur to an external aluminum frame. Servomotor length and force were recorded at 1,000 Hz with a 16-bit A/D converter (NI USB-6251; National Instruments) using a custom-written program in IgorPro (Wavemetrics, OR).
Muscle stimulation was achieved by placing a nerve cuff on a branch of the sciatic nerve. A Grass S48 stimulator generated 0.2-ms pulses. Each muscle’s supramaximal stimulation voltage was determined using a series of muscle twitches at increasing stimulus voltage. Twitches were used to construct a length-tension curve to estimate the muscle’s optimal fascicle length (Lo). For each isotonic contraction, initial muscle length was set so that muscles shortened through Lo.
All tetanic contractions were elicited by supramaximal stimulations at 100 pulses/s. After finding the muscle’s maximum tetanic tension and confirming Lo with a series of tetani at fascicle lengths around the twitch-determined value for Lo, the muscle was subjected to a series of isotonic contractions at 10, 20, 60, 80, and 100% maximum isometric force (Po) (except for 1 turkey where contractions were elicited at 10, 30, 60, 90, and 100% Po). In each isotonic contraction, the muscle was stimulated and allowed to shorten until muscle force reached the preset value of the servomotor, after which force was maintained at a constant level as the muscle shortened at a near-constant velocity. During the force rise period of these isotonic contractions, muscle shortening was delayed relative to the fascicle shortening because the fascicles must first shorten against stretch of series elastic elements before fascicle shortening resulted in muscle shortening.
Aponeurosis incisions.
After isotonic contractions were obtained in the intact, no incision condition, the aponeurosis was incised to examine the influence of reduced aponeurosis radial integrity on AGR (Fig. 2B). The aponeurosis was incised superficially with a sharp scalpel to avoid damage to the underlying muscle. Before the incisions, the total length of the aponeurosis was measured with calipers and marked with a surgical pen into quadrants. The partial incision was made through the third most proximal quadrant for 25% of the aponeurosis’ maximum proximal-to-distal length (1 × 25%Lapo; Fig. 2B). Next, this partial incision was extended to a full-length incision made through 100% of the length of the aponeurosis (1 × 100%Lapo). Finally, three full-length incisions were made through 100% of the length of the aponeurosis (3 × 100%Lapo). After each incision condition, the isotonic protocol was repeated and AGR calculated at the same variable force levels. To ensure that the decreased gearing at high forces in the multiple full-length incisions condition was not due to the fatigue or damage to the underlying muscle, a tetanic contraction was performed after the isotonic contractions in each incision condition and maximum isometric force was measured.
Data analysis and statistics.
After a low-pass filter (cutoff frequency: 20–40 Hz) was implemented on muscle and fascicle lengths, muscle and fascicle velocities were calculated during the period of constant force production and used to compute AGR with the following equation:
| (1) |
where VMTU is muscle velocity [muscle-tendon unit (MTU)] and Vf is fascicle velocity. In all contractions before and after aponeurosis incisions, AGR was calculated at a similar range of fascicle lengths. Across turkeys, analyzed ranges fell within 77–103% Lo. AGR measurements were taken only during the constant-force portion of contractions, to isolate the contribution of muscle fascicle shortening, rather than series elasticity, on muscle shortening. By excluding series elasticity, VMTU is equivalent to muscle belly velocity. Fiber rotation was measured over the same range of fascicle lengths as AGR and calculated as the change in pennation angle normalized by the amount of fascicle shortening in degrees per millimeter shortening.
To examine the effect of incision condition on the relationship between gearing and force, a linear mixed model fit by maximum likelihood was implemented using R statistical software (v3.2.3; The R Foundation for Statistical Computing, Vienna, Austria). In the model, fixed effects included relative force (normalized to the muscle’s maximum tetanic tension) and incision condition while individual was included as a random effect, and relative force × incision condition was included as an interaction term. When a significant relative force × incision condition interaction term was detected, indicating a difference in slope in the relationship between gearing and force, we examined whether there was an effect of incision condition on gear ratio and fiber rotation at low-force contractions (10% Po) and high-force contractions (80–90% Po). In one turkey, the high-force contraction was performed at 90% Po and not 80% Po. These high- and low-force contractions were used in a mixed model with incision condition as a fixed effect and individual as the random effect. Pairwise comparisons of least squares means were made with P values adjusted using the Tukey method using the “lsmeans” R package (19). All results were considered significant at a level of P < 0.05 and are reported as means ± SD.
RESULTS
Representative isotonic contractions at 60% Po before and after aponeurosis incisions illustrate that the shortening velocity of the MTU can exceed the shortening velocity of the fascicle (Fig. 3, B and E), resulting in a gear ratio greater than 1 (Fig. 3, C and F). MTU and fascicle velocities are measured when the preset force level is reached and is constant (Fig. 3, A and D). The delay in muscle velocity relative to fascicle velocity is due to stretch of the series elastic element during the period of force rise. In these contractions at 60% Po for this individual, the gear ratio with the intact aponeurosis was 1.57, while the gear ratio at this force level decreased to 1.29 after multiple full-length incisions in the aponeurosis (Fig. 3, C and F).
Fig. 3.
Representative isotonic contractions in a turkey lateral gastrocnemius (LG) muscle with its aponeurosis intact (A–C) and the same muscle with 3 full-length incisions (D–F). Muscles were maximally stimulated and allowed to generate force to a preset level, which was 60% Po in these contractions. While force was held constant, the muscle fascicle (red) and muscle-tendon unit (black) shortened at a constant velocity. Gear ratio was only calculated over the period of constant force and evaluated over a similar range of fascicle lengths for each contraction (shown in gray).
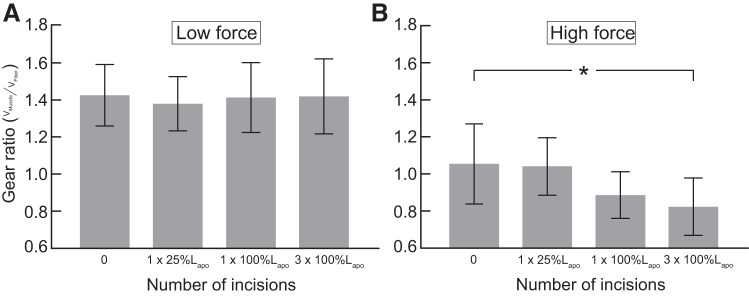
Gear ratio was lower during high-force contractions compared with low-force contractions for all conditions (Fig. 4). The relationship between gearing and force was altered when the aponeurosis was incised compared with the intact condition. The slope of the line describing the relationship between gearing and force significantly decreased with multiple full-length aponeurosis incisions (Fig. 4; P = 0.02) and nearly significantly decreased with a single full-length aponeurosis incision compared with the intact condition (Fig. 4; P = 0.10).
Fig. 4.

Architectural gear ratio in a series of isotonic contractions at varying force levels in the intact condition (filled square), with a single partial aponeurosis incision (1 × 25%Lapo; square with a partial hash mark), a full-length incision (1×100%Lapo; square with a single hash mark), and 3 full-length incisions (3×100%Lapo; square with 3 hash marks). The effect of force on gearing depends on the aponeurosis incision condition. The slope describing the relationship between gearing and force in the intact condition was significantly greater than the slope for the 3×100%Lapo condition (*P = 0.02).
A decrease in the slope of the relationship between gearing and force in these incision conditions suggests that there was either an increase in gearing in low-force contractions or a decrease in gearing during high-force contractions. To assess what drove the decrease in slope, gear ratio was compared across incision conditions in low-force contractions (10% Po) and high-force contractions (80–90% Po). Gearing was not affected by aponeurosis incisions for the low-force contractions (Fig. 5A). For high-force contractions (Fig. 5B), gear ratio was significantly reduced by multiple full-length aponeurosis incisions compared with the intact condition (0.82 ± 0.15 vs. 1.05 ± 0.22; P = 0.02). On average across turkeys, gearing was reduced by 19.1% during high-force contractions after multiple full-length incisions compared with the intact condition. In the high-force contractions, multiple full-length incisions resulted in a trend toward reduced fiber rotation compared with the intact condition (1.16 ± 1.43 vs. 1.75 ± 1.50°/mm), but this difference was not statistically significant (Fig. 6; P = 0.10).
Fig. 5.
Average gear ratio during low-force (A) and high-force (B) contractions in each incision condition. A: the gear ratios during low-force contractions did not change when the aponeurosis was incised. B: however, in high-force contractions, gearing was significantly reduced after multiple full-length incisions (*P = 0.02).
Fig. 6.

Average fiber rotation per millimeter of fiber shortening during high-force contractions in each incision condition. The reduction in fiber rotation after 3 full-length incisions compared with the intact condition was not statistically significant (P = 0.10).
Tetanic contractions performed after the isotonic contractions for the multiple full-length incision condition indicated that maximum isometric force decreased by <20% for all turkeys (average decrease across individuals: 9.2%) and <10% for four of seven turkeys from the intact condition. To examine whether a decrease in maximum isometric force (Po) was driving the decrease in gear ratio at high forces, muscle force values (as %Po) were adjusted as percentages of the new maximum isometric force obtained in each incision condition and the mixed model was run again (Fig. 7). Results of this test confirmed our findings that multiple full-length incisions altered the relationship between gearing and force even after forces were adjusted to the new Po (P = 0.01), suggesting that this result is not due to decreased muscle force production after incisions.
Fig. 7.
Architectural gear ratio in a series of isotonic contractions at varying force levels from a representative individual in the intact condition (filled square) and with 3 full-length incisions (3 × 100%Lapo; square with 3 hash marks). This individual had the greatest decrease in maximum isometric force after the incisions (20% decrease after 3 full-length incisions) among individuals. A: forces are normalized to the maximum isometric force (Po) measured before incising the aponeurosis. B: to examine whether the decrease in gear ratio at high-forces was due to a decrease in maximum isometric force after the incisions, forces in the 3 full-length incision condition were normalized to the maximum isometric force measured after the incisions. Results of a mixed model run on the adjusted forces demonstrate that multiple full-length incisions significantly altered the relationship between gearing and force even after forces were adjusted to the new Po.
DISCUSSION
Aponeurosis disruption alters the relationship between gearing and force.
Our results provide evidence that aponeuroses influence muscle shape change and gearing, while demonstrating that other structures must also help determine variable gearing. In this study, we experimentally tested the role of aponeuroses in modulating a muscle’s gear ratio by incising aponeuroses to reduce their radial integrity and found that the relationship between gearing and force was altered when the aponeurosis was incised compared with the intact condition. Under all conditions, muscle gear ratio decreased with contractile force, which is consistent with previous studies (1, 3, 18). Additionally, the results supported our hypothesis that aponeurosis incisions resulted in reduced gearing. We found gearing was reduced only after multiple full-length incisions and only in the high-force, low-velocity contractions. Reduced gearing suggests muscles experience increased width-wise bulging, suggesting that aponeuroses play a central role in providing some resistance to increases in muscle width.
Because higher gear ratios indicate increased fascicle rotation (1, 8, 29), we hypothesized that reduced gearing after multiple full-length incisions would result in reduced fascicle rotation. The decrease in fascicle rotation after multiple full-length incisions in high-force contractions was not significant (P = 0.10). Pennation angle is a noisy measure in this muscle because placement of the deep sonomicrometry crystal is challenging (see materials and methods), and its position relative to the fascicle crystals can only be confirmed postmortem. It is possible that no significant difference in fiber rotation resulted from inaccurate measures of pennation angle.
Connective tissue mechanics are a determinant of gearing.
Our results are consistent with the hypothesis that the interaction of contractile forces and connective tissues determines variable shape change and variable gearing (1, 18). During shortening contractions, a component of force from the contracting fibers oriented in the muscle thickness direction (thickness force), perpendicular to the aponeuroses, acts to pull the superficial and deep aponeuroses together to compress the muscle. Because the muscle must radially expand when it shortens to maintain a constant volume, these contractile forces compressing the muscle in the thickness direction load and stretch connective tissues in the width direction. It is hypothesized that the intramuscular connective tissue (IMCT) and aponeurosis oppose this contractile force and resist muscle thickness compression and muscle width expansion. Pennate muscles compress less in thickness during low-force contractions when thickness force is low because the connective tissues can resist muscle thickness compression and muscle width expansion. At higher contractile forces, the increased thickness force will result in increased tensile forces in the width direction that cannot be resisted by connective tissues, causing the muscle to bulge more in the width direction. Increased thickness compression suggests decreased fiber rotation to greater angles of pennation, resulting in reduced gearing at high-contractile forces. The hypothesized role of the connective tissues in resisting muscle width increases is consistent with our results. We found that disrupting the integrity of the aponeurosis did not alter gearing in low-force contractions. We interpret this to mean that in spite of a reduction in the contribution of aponeuroses to resisting thickness compression, the IMCT and compromised aponeurosis were sufficient to resist muscle thickness compression when the forces tending to compress the muscle were low. Conversely, in the high-force, low-velocity contractions, aponeurosis incisions may have allowed greater muscle width expansion and thickness compression, resulting in lower gear ratios compared with the intact condition.
Our results demonstrate that aponeurosis influences muscle force and speed through its effect on gearing, so changes in aponeurosis stiffness with injury, age, or disease may influence muscle function. For example, connective tissue stiffness is increased in cerebral palsy (31), Parkinson’s disease (21), and following stroke (32), while connective tissue stiffness is decreased in rheumatoid arthritis (9) and osteogenesis imperfecta (15). Both increased (18, 27, 30) and decreased (6, 24, 25, 33) connective tissue stiffness are reported with age. While the effect of altered connective tissue stiffness on passive muscle stiffness is well documented (16, 20, 21, 31), the effects of these changes on active muscle contractile performance have remained ambiguous. Because architectural gearing influences muscle force and speed, the present findings provide a link between muscle connective tissue properties and active muscle performance.
Consistent with our finding that connective tissue properties influence variable gearing, Holt et al. (18) found that altered muscle-associated connective tissue stiffness resulted in an altered relationship between gearing and force in old vs. young rats. Specifically, gearing remained relatively high (~1.3) and constant across force levels in the aged rat medial gastrocnemius muscles that had increased fiber bundles and aponeurosis stiffness compared with the young rat muscles. Constant high gearing with increased force results in lower muscle forces for a given fascicle force. Together, our study and Holt et al. (18) provide evidence that muscle-associated connective tissues influence active muscle contractile properties through their influence on gearing, and thus rehabilitative strategies for patients with age- or disease-related muscle dysfunction should target both muscle and connective tissue.
Implications for musculoskeletal health.
Our results provide insight into the effects of the surgical incision of aponeurosis on muscle contractile function. Common surgical interventions such as aponeurosis lengthening and fasciotomy often involve incising aponeuroses. The effect of these treatments on long-term muscle function is not well understood. Aponeurosis-lengthening procedures are used to treat severe muscle contractures in cerebral palsy patients (4, 26), but many patients experience reduced muscle strength (7, 12). Compartment syndrome occurs when increased pressure within the enclosed compartment encompassing a muscle compromises circulation to the muscle (22). This urgent condition can necessitate a fasciotomy, or the surgical incision of the fascial and aponeurotic sheaths surrounding muscle (10), but this treatment may lead to long-term deficits in muscle strength. Mithoefer et al. (23) showed that nearly half of fasciotomy patients had strength deficits of 50%, on average, in the fasciotomy limb compared with the contralateral limb 5 yr after the procedure. Our results demonstrate that this reduced muscle strength is not likely due to the influence of the incised aponeurosis on muscle shape changes and architectural gearing. After surgical interventions, the reduction in strength is likely due to other factors, such as muscle necrosis during ischemia in the case of compartment syndrome. However, reduced gearing at high-force contractions would result in reduced muscle speeds suggesting that surgical incision of aponeuroses may compromise muscle speed when muscle force is high. Further exploration is necessary to determine the effects of these other injury-related factors on muscle contractile function after surgical incision of the aponeurosis.
Role of aponeuroses in pennate muscles.
Biomechanists have long recognized functional advantages of a pennate muscle architecture. Pennate muscles pack more, shorter fibers in a given volume to increase maximum force production (13, 28). Aponeuroses are necessary components of pennate muscles and serve as an insertion for fascicles terminating within the muscle belly and transmit fascicle forces to the external tendon. Our results suggest aponeuroses also play a role in modulating gearing in pennate muscles. Presumably selection for effective function in both transmitting force and influencing muscle gearing has shaped the evolution of aponeurosis structural and material properties.
Study limitations.
There are limitations to our approach of using incisions to alter aponeurosis properties. Our results indicate that the drop in maximum isometric force in the incised condition was not due to a change in gearing but may indicate many possible effects that the experimental protocol had on the muscle. Incising the aponeurosis may have damaged the underlying muscle, leading to decreased force production. However, a tetanic contraction was performed after the isotonic contractions in each incision condition and maximum isometric force decreased by <20% for all turkeys and <10% for most turkeys after multiple full-length incisions. The muscle may have fatigued after performing multiple sets of isotonic contractions. Azizi et al. (1) showed a similar decrease in maximum isometric force of up to 5% with repeated contractions, and the greater increase in isometric force in our study is consistent with the greater number of repeated contractions used here. While our approach required that the incision conditions were not randomized, our finding that the relationship between gearing and force was altered after the forces were adjusted for the new maximum isometric force in each incision condition suggests that the change in gearing with incisions is not due to a drop in force.
Conclusions.
Architectural gearing varies with the force of contraction, and these task-dependent shape changes of muscle are important for normal muscle function (1, 3). There are likely multiple components that influence the relationship between gearing and force including aponeurosis, IMCT, and contractile tissue, but no study has yet been able to tease apart the contribution of each. The goal of this study was to examine the role of one of these components, the aponeurosis, to begin to answer the question of how variable gearing is modulated. This study confirms that changes in the stiffness and structure of muscle-associated connective tissues affects muscle force and speed via gearing. While our results provide evidence that aponeurosis plays an important role in modulating muscle shape change and gearing, the fact that there was only a 19% decrease in gearing after multiple full-length incisions in the high-force contractions and no change in gearing under low-force conditions suggests that other structures must also help determine variable gearing. Future work exploring the role of the IMCT and contractile tissue in modulating gearing is vital for creating a complete picture of the modulation of variable gearing during muscle contractions.
GRANTS
This research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants F32-AR-067564 (to C. M. Eng) and AR-055295 (to T. J. Roberts).
AUTHOR CONTRIBUTIONS
C.M.E. conceived and designed research; C.M.E. and T.J.R. performed experiments; C.M.E. analyzed data; C.M.E. interpreted results of experiments; C.M.E. prepared figures; C.M.E. drafted manuscript; C.M.E. and T.J.R. edited and revised manuscript; C.M.E. and T.J.R. approved final version of manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ENDNOTE
At the request of the authors, readers are herein alerted to the fact the raw data with IgorPro analysis scripts can be found on the “Aponeurosis incisions and muscle gearing” project page on Open Science Framework (DOI: 10.17605/OSF.IO/5MSV4). These materials are not a part of this manuscript and have not undergone peer review by the American Physiological Society (APS). APS and the journal editors take no responsibility for these materials, for the project page, or for any links to or from it.
ACKNOWLEDGMENTS
We acknowledge Miranda Norlin, Roy Ruttiman, and Greg Mouradian for technical assistance during experiments. We thank Dr. Emanuel Azizi for helpful discussions.
REFERENCES
- 1.Azizi E, Brainerd EL, Roberts TJ. Variable gearing in pennate muscles. Proc Natl Acad Sci USA 105: 1745–1750, 2008. doi: 10.1073/pnas.0709212105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azizi E, Gillis GB, Brainerd EL. Morphology and mechanics of myosepta in a swimming salamander (Siren lacertina). Comp Biochem Physiol A Mol Integr Physiol 133: 967–978, 2002. doi: 10.1016/S1095-6433(02)00223-4. [DOI] [PubMed] [Google Scholar]
- 3.Azizi E, Roberts TJ. Geared up to stretch: pennate muscle behavior during active lengthening. J Exp Biol 217: 376–381, 2014. doi: 10.1242/jeb.094383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker LD. A rational approach to the surgical needs of the cerebral palsy patient. J Bone Joint Surg Am 38-A: 313–323, 1956. doi: 10.2106/00004623-195638020-00007. [DOI] [PubMed] [Google Scholar]
- 5.Baskin RJ, Paolini PJ. Volume change and pressure development in muscle during contraction. Am J Physiol 213: 1025–1030, 1967 10.1152/ajplegacy.1967.213.4.1025. [DOI] [PubMed] [Google Scholar]
- 6.Blevins FT, Hecker AT, Bigler GT, Boland AL, Hayes WC. The effects of donor age and strain rate on the biomechanical properties of bone-patellar tendon-bone allografts. Am J Sports Med 22: 328–333, 1994. doi: 10.1177/036354659402200306. [DOI] [PubMed] [Google Scholar]
- 7.Borton DC, Walker K, Pirpiris M, Nattrass GR, Graham HK. Isolated calf lengthening in cerebral palsy. Outcome analysis of risk factors. J Bone Joint Surg Br 83: 364–370, 2001. doi: 10.1302/0301-620X.83B3.10827. [DOI] [PubMed] [Google Scholar]
- 8.Brainerd EL, Azizi E. Muscle fiber angle, segment bulging and architectural gear ratio in segmented musculature. J Exp Biol 208: 3249–3261, 2005. doi: 10.1242/jeb.01770. [DOI] [PubMed] [Google Scholar]
- 9.Cooney JK, Law R-J, Matschke V, Lemmey AB, Moore JP, Ahmad Y, Jones JG, Maddison P, Thom JM. Benefits of exercise in rheumatoid arthritis. J Aging Res 2011: 681640, 2011. doi: 10.4061/2011/681640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dente CJ, Wyrzykowski AD, Feliciano DV. Fasciotomy. Curr Probl Surg 46: 779–839, 2009. doi: 10.1067/j.cpsurg.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Dick TJ, Wakeling JM. Shifting gears: dynamic muscle shape changes and force-velocity behavior in the medial gastrocnemius. J Appl Physiol (1985) 123: 1433–1442, 2017. doi: 10.1152/japplphysiol.01050.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etnyre B, Chambers CS, Scarborough NH, Cain TE. Preoperative and postoperative assessment of surgical intervention for equinus gait in children with cerebral palsy. J Pediatr Orthop 13: 24–31, 1993. [PubMed] [Google Scholar]
- 13.Gans C. Fiber architecture and muscle function. Exerc Sport Sci Rev 10: 160–207, 1982. [PubMed] [Google Scholar]
- 14.Gans C, de Vree F. Functional bases of fiber length and angulation in muscle. J Morphol 192: 63–85, 1987. doi: 10.1002/jmor.1051920106. [DOI] [PubMed] [Google Scholar]
- 15.Gautieri A, Uzel S, Vesentini S, Redaelli A, Buehler MJ. Molecular and mesoscale mechanisms of osteogenesis imperfecta disease in collagen fibrils. Biophys J 97: 857–865, 2009. doi: 10.1016/j.bpj.2009.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 44: 318–331, 2011. doi: 10.1002/mus.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond B Biol Sci 126: 136–195, 1938. doi: 10.1098/rspb.1938.0050. [DOI] [PubMed] [Google Scholar]
- 18.Holt NC, Danos N, Roberts TJ, Azizi E. Stuck in gear: age-related loss of variable gearing in skeletal muscle. J Exp Biol 219: 998–1003, 2016. doi: 10.1242/jeb.133009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lenth RV. Least-Squares Means: The R Package lsmeans. https://cran.r-project.org/web/packages/lsmeans/lsmeans.pdf [11 May 2018].
- 20.Lieber RL, Runesson E, Einarsson F, Fridén J. Inferior mechanical properties of spastic muscle bundles due to hypertrophic but compromised extracellular matrix material. Muscle Nerve 28: 464–471, 2003. doi: 10.1002/mus.10446. [DOI] [PubMed] [Google Scholar]
- 21.Marusiak J, Jaskólska A, Budrewicz S, Koszewicz M, Jaskólski A. Increased muscle belly and tendon stiffness in patients with Parkinson’s disease, as measured by myotonometry. Mov Disord 26: 2119–2122, 2011. doi: 10.1002/mds.23841. [DOI] [PubMed] [Google Scholar]
- 22.Matsen FA., 3rd Compartmental Syndromes. New York: Grune and Stratton, 1980, p. 162. [Google Scholar]
- 23.Mithoefer K, Lhowe DW, Vrahas MS, Altman DT, Erens V, Altman GT. Functional outcome after acute compartment syndrome of the thigh. J Bone Joint Surg Am 88: 729–737, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Nachemson AL, Evans JH. Some mechanical properties of the third human lumbar interlaminar ligament (ligamentum flavum). J Biomech 1: 211–220, 1968. doi: 10.1016/0021-9290(68)90006-7. [DOI] [PubMed] [Google Scholar]
- 25.Noyes FR, Grood ES. The strength of the anterior cruciate ligament in humans and Rhesus monkeys. J Bone Joint Surg Am 58: 1074–1082, 1976. doi: 10.2106/00004623-197658080-00006. [DOI] [PubMed] [Google Scholar]
- 26.Olney BW, Williams PF, Menelaus MB. Treatment of spastic equinus by aponeurosis lengthening. J Pediatr Orthop 8: 422–425, 1988. doi: 10.1097/01241398-198807000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Plate JF, Wiggins WF, Haubruck P, Scott AT, Smith TL, Saul KR, Mannava S. Normal aging alters in vivo passive biomechanical response of the rat gastrocnemius-Achilles muscle-tendon unit. J Biomech 46: 450–455, 2013. doi: 10.1016/j.jbiomech.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Powell PL, Roy RR, Kanim P, Bello MA, Edgerton VR. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J Appl Physiol Respir Environ Exerc Physiol 57: 1715–1721, 1984. [DOI] [PubMed] [Google Scholar]
- 29.Randhawa A, Jackman ME, Wakeling JM. Muscle gearing during isotonic and isokinetic movements in the ankle plantarflexors. Eur J Appl Physiol 113: 437–447, 2013. doi: 10.1007/s00421-012-2448-z. [DOI] [PubMed] [Google Scholar]
- 30.Shadwick RE. Elastic energy storage in tendons: mechanical differences related to function and age. J Appl Physiol (1985) 68: 1033–1040, 1990. doi: 10.1152/jappl.1990.68.3.1033. [DOI] [PubMed] [Google Scholar]
- 31.Smith LR, Lee KS, Ward SR, Chambers HG, Lieber RL. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J Physiol 589: 2625–2639, 2011. doi: 10.1113/jphysiol.2010.203364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svantesson U, Takahashi H, Carlsson U, Danielsson A, Sunnerhagen KS. Muscle and tendon stiffness in patients with upper motor neuron lesion following a stroke. Eur J Appl Physiol 82: 275–279, 2000. doi: 10.1007/s004210000216. [DOI] [PubMed] [Google Scholar]
- 33.Vogel HG. Influence of maturation and aging on mechanical and biochemical properties of connective tissue in rats. Mech Ageing Dev 14: 283–292, 1980. doi: 10.1016/0047-6374(80)90002-0. [DOI] [PubMed] [Google Scholar]