Abstract
This study examined self-paced, high-intensity exercise during mild hypothermia and whether hyperoxia might offset any potential impairment. Twelve trained males each completed 15-km time trials in three environmental conditions: Neutral (23°C, 0.21), Cold (0°C, 0.21), and Cold+Hyper (0°C, 0.40). Cold and Cold+Hyper trials occurred after a 0.5°C drop in rectal temperature. Rectal temperature was higher (P ≤ 0.016) throughout Neutral compared with Cold and Cold+Hyper; Cold had a higher (P ≤ 0.035) rectal temperature than Cold+Hyper from 2.5 to 7.5 km, and hyperoxia did not alter thermal sensation or comfort. Oxyhemoglobin saturation decreased from ~98% to ~94% with Neutral and Cold, but was maintained at ~99% in Cold+Hyper (P < 0.01). Cerebral tissue oxygenation index (TOI) was higher in Neutral than in Cold throughout the time trial (TT) (P ≤ 0.001), whereas Cold+Hyper were unchanged (P ≥ 0.567) from Neutral by 2.5 km. Muscle TOI was maintained in Cold+Hyper compared with Neutral and was higher (P ≤ 0.046) than Cold throughout the entire TT. Power output during Cold (246 ± 41 W) was lower than Neutral (260 ± 38 W) at all 2.5-km intervals (P ≤ 0.012) except at 12.5 km. Power output during Cold+Hyper (256 ± 42 W) was unchanged (P ≥ 0.161) from Neutral throughout the TT, and was higher than Cold from 7.5 km onward. Average cadence was higher in Neutral (93 ± 8 rpm) than in either Cold or Cold+Hyper (Cold: 89 ± 7 and Cold+Hyper: 90 ± 8 rpm, P = 0.031). In conclusion, mild hypothermia reduced self-paced exercise performance; hyperoxia during mild hypothermia restored performance to thermoneutral levels, likely due to maintenance of oxygen availability rather than any thermogenic benefit.
NEW & NOTEWORTHY We examined self-paced, high-intensity exercise with 0.5°C rectal temperature decreases in a 0°C ambient environment, along with whether hyperoxia could offset any potential impairment. During a 15-km time trial, power output was lower with hypothermia than with thermoneutral. However, with hypothermia, hyperoxia of = 0.40 restored power output despite there being no thermophysiological improvement. Hypothermia impairs exercise performance, whereas hyperoxia likely restored performance due to maintenance of oxygen availability rather than any thermogenic benefit.
Keywords: cold stress, hyperoxia, NIRS, pacing, voluntary exercise
INTRODUCTION
When humans are exposed to cold environments, cooling of the body can result from a combination of low ambient temperatures and inadequate clothing, coupled with insufficient heat production to offset the increased heat loss. Fatigue while exercising during mild hypothermia may be multifactorial, including changes to neuromuscular capacity, fuel utilization, and hormonal changes (8). Cold exposure rapidly elicits strong peripheral vasoconstriction to reduce convective heat loss from the core to the peripheral shell (9). This has been proposed as a potential impairment to exercise capacity, as both cerebral and muscle blood flow have been shown to decrease in the cold (20), which will likely decrease the amount of oxygen (O2) delivered to these tissues. Surprisingly, the effect of mild hypothermia on aerobic exercise is relatively unexplored and equivocal. With acute exposure, Galloway and Maughan (16) reported, there was no difference between 4°C and 21°C ambient temperature in tolerance time to 70% maximal aerobic capacity cycling, whereas Parkin et al. (25) reported a ~40% improvement in time to exhaustion at 3°C compared with 20°C with an identical cycling protocol. However, cold stress in these studies was mild, with no significant reduction in core temperature. With the combination of prolonged cold exposure leading to actual decreases in core temperature, peak aerobic capacity during combined arm/leg ergometry linearly decreased (4). The O2 demands of shivering increases the overall metabolic cost of exercise, with a 0.5°C decrease in core temperature and consequent shivering resulting in a slower treadmill speed required to elicit the same overall O2 uptake (15).
Hyperoxia (Hyper), breathing gas with an inspired oxygen () above 0.21, has been used extensively in thermoneutral environments to improve dynamic exercise performance (19, 32). A primary proposed mechanism of hyperoxic benefit is through an increased O2 availability that may restore the reduction in arterial O2 saturation during intense exercise (2, 32). Although highly individual, arterial O2 saturation at rest may be ~95% and can fall to less than 90% during intense normoxic exercise but restored back to ≥95% with an ≥ 0.40 (23). Hyperoxia also reverses the cerebral deoxygenation that normally occurs during intense exercise in normoxia, and this has been proposed as a mechanism for enhancing central motor drive and preventing central fatigue (22, 24). Furthermore, the raising or lowering of arterial content of O2 () elicited parallel changes in aerobic exercise performance (2). Richardson et al. (27) performed dynamic knee extensions until exhaustion at of 0.12, 0.21, and 1.0, finding that hyperoxia increased along with facilitating greater maximal leg O2 consumption and work rate compared with hypoxia and normoxia. At the same time, no changes to muscle blood flow or venous O2 concentration were observed at maximal exercise, indicating that the increased work was due to increased O2 delivery. Thus, increasing the availability of O2 during intense endurance exercise attenuates the reduction in arterial O2 saturation and permits greater exercise capacity or performance.
The effects of mild hypothermia on self-paced and intense exercise performance is unknown, along with the underlying mechanism(s) for any potential impairment. Mild hypothermia impairs peak aerobic capacity and places greater demands on O2 availability to offset peripheral vasoconstriction and the increased O2 demands from shivering thermogenesis (8, 15). Thus, hyperoxia during mild hypothermia was studied to see whether it could maintain oxygen availability despite reduced muscle blood flow. Therefore, this study had two hypotheses: 1) cycling time trial performance would be impaired with mild hypothermia of 0.5°C, and 2) hyperoxia (: 0.40) would restore hypothermic performance to normoxic levels, and this would primarily be due to increased oxygen availability.
MATERIALS AND METHODS
Participants.
The study was approved by Brock University’s Bioscience Research Ethics Board (REB no. 16-017) and conformed to the latest version standards of the Declaration of Helsinki. All participants were screened using a modified Physical Activity Readiness Questionnaire (PAR-Q) and were informed of the experimental protocol and associated risks before participating in the experiment. Verbal and written consent was obtained from each participant. Twelve healthy, trained male cyclists were recruited for this experiment. Participants were nonsmokers and were free from cardiovascular, neurological, and skeletal disorders based on the PAR-Q. The means (± SD) for age, height, mass, body fat percentage, and peak power output were 29 ± 8 yr, 178 ± 6 cm, 73.5 ± 7.3 kg, 11.2 ± 4.1%, and 396 ± 42 W, respectively. Based on DePauw et al. (25), participants were classified as at performance level 3 (scale of 1–5).
Experimental design.
Participants performed one familiarization and three counterbalanced experimental sessions. The familiarization session consisted of collecting anthropometric data, completing a graded test to exhaustion on a cycle ergometer, and a 15-km cycling time trial (TT) in thermoneutral and normoxic conditions. The experimental sessions consisted of a 15-km TT in different environmental conditions: Neutral (23°C, : 0.21), Cold (0°C, : 0.21), and Cold+Hyper (0°C, : 0.40). Experimental sessions were separated by a minimum of 1 wk to ensure proper recovery time and reduce the potential effects of cold acclimation.
Experimental protocol.
Participants were instructed to avoid caffeine consumption for 12 h prior to, and alcohol consumption or strenuous exercise for 24 h before, each experimental session. Upon arrival, participants voided their bladders and nude body mass was determined. Urine specific gravity (USG) was measured with a refractometer (PAL-10S; Atago, Tokyo, Japan) as an index of hydration status, with a euhydration threshold of ≤1.020. If this threshold was exceeded, 500 ml of water was ingested, and USG was tested again after 30 min, or else the trial was rescheduled. For all conditions, participants were dressed in short-sleeve cycling jersey, shorts, and shoes and were instrumented with a three-lead electrocardiogram (ECG), skin and core thermistors, pulse oximetry, and near-infrared spectroscopy (NIRS) probes. They then entered an environmental chamber (Can-Trol Environmental Systems, Markham Canada) set at 23°C, ~40% RH, and airflow ~3.0 m/s and remained seated in a mesh chair for a 30-min baseline.
After baseline and depending on the experimental session, the environmental chamber either remained at 23°C, ~40% relative humidity (RH), airflow ~3.0 m/s for Neutral trials or was set to 0°C, ~60% RH, airflow ~3.0 m/s for the Cold or Cold+Hyper trials. During Neutral trials, participants were then set up on the cycle ergometer and fitted with a silicone mask for a 5-min wash-in period, during which they breathed normoxic gas. During Cold and Cold+Hyper trials, participants were assisted with putting on a hat, mittens, and track pants and sat back down to begin the cooling protocol, breathing normoxic air during this phase. These items were deemed necessary during piloting to offset the extreme discomfort to the extremities from the cooling protocol, while the mittens also optimized pulse oximetry signal quality during cooling (31). Cooling was achieved once participant core temperature had decreased by 0.5°C from their baseline core temperature, which took between 40 and 120 min. After cooling, participants were assisted in removing their track pants and were set up on the cycle ergometer and fitted with a silicone mask for a 5-min gas (normoxic or hyperoxic) wash-in period. After wash-in, participants completed a 15-km TT. For all conditions, dry gas (normoxic or hyperoxic) was supplied to the participants during the wash-in period and TT from gas cylinders and Douglas bags located outside the chamber, such that the specific experimental condition was double-blinded to both the participant and the experimenter working with the participant. Clothing during the TT (cycling jersey, shorts, socks, and shoes) were identical for all conditions. During the TT, participants could freely choose their cadence, and the only feedback participants received was distance completed at 2.5-km intervals (e.g., “2.5,” “5,” etc.).
Cycling performance measurements.
The cycle ergometer (Velotron, RacerMate, Seattle, WA) was set up to replicate the participant’s normal bike position. The ergometer was controlled by software (CompuTrainer 3D, RacerMate) recording power output, cadence, and speed every second. Key variables of interest included: TT time, power, and cadence across the entire 15-km as well as at each 2.5-km segment.
Temperature measurements.
Rectal temperature (Tre) was measured with a flexible thermistor (Mon-A-Therm Core; Mallinkrodt Medical, St. Louis, MO) inserted 15 cm beyond the anal sphincter. Type-T thermocouples (PVC-T-24-190; Omega Environmental, Laval, QC, Canada) were taped (TransporeTM; 3-M, St. Paul, MN) to the chest, thigh, upper arm, and calf to calculate mean skin temperature (T̄sk), using the weighting scheme proposed by Ramanathan (26):
All temperature data were continuously sampled at 1 Hz.
Near-infrared spectroscopy.
Cerebral oxygenation and muscle oxygenation was measured with a three-wave-length, (775, 810, 850 nm) high-temporal-resolution, near-infrared spectroscopy (NIRS) device (NIRO-200; Hamamatsu Photonics, Hamamatsu, Japan). The theory, limitations, and reliability of measurements obtained with NIRS have been previously detailed (28, 30). The NIRS unit consists of two detector photodiodes and three laser-emitting diodes held 4 cm apart by rubberized shell casings. The probes were attached to the left forehead, 3 cm from the midline and just above the supraorbital ridge (21) and the right vastus lateralis along the vertical axis of the thigh 10–14 cm from the knee joint (13). These measurements were recorded for each participant during the familiarization trial to facilitate accurate replacement for each participant. After instrumentation, the NIRS probes were set for optimal measurement conditions by commencing the initialization function on the NIRO-200 once baseline had started. The NIRS system was then zeroed to reset oxygenated hemoglobin (O2Hb), deoxygenated hemoglobin (HHb), and total hemoglobin (tHb) values to an arbitrary zero value. Tissue oxygenation index (TOI) values are not affected by this procedure, as TOI is measured in absolute values instead of a change from the arbitrary initial zero value (17). The intensities of incident and transmitted light were recorded continuously at 10 Hz and, along with the specific extinction coefficients and optical path length, used for online estimation and display of concentration changes (Dlmol/l) in O2Hb, HHb, and tHb according to the Modified-Beer-Lambert law. TOI was measured with the spatial resolved spectroscopy method. Probes were affixed using adhesive tape (Ref 71443-02, Hypafix Germany) to prevent movement and signal contamination from external light sources. TOI is the percentage of O2Hb/tHb, which indicates the amount of tissue oxygenation.
Physiological measurements.
Heart Rate was continuously obtained from R–R intervals using a 3-lead electrocardiogram (Bio Amp, ADInstruments, Colorado Springs CO). Oxyhemoglobin saturation (SpO2) was measured with pulse oximetry (Bio Amp, ADInstruments) on the left middle finger of each participant.
To analyze blood lactate concentration (YSI 23L Lactate Analyzer; YSI Scientific, Yellow Springs, OH), a capillary blood sample was obtained from the earlobe at baseline, preexercise, and immediately after the end of exercise.
Perceptual scales.
The perceptual measurementss of rating perceived exertion (RPE), thermal comfort (TC), and thermal sensation (TS) were recorded at baseline, preexercise, and every 2.5 km of the TT. RPE was assessed using a 6–20 scale (5). TC was assessed on a 1 (comfortable) to 4 (very uncomfortable) scale, while TS was reported on a 1 (very cold) to 7 (very hot) scale (14).
Statistical analysis.
Results are presented as means ± SD, and the α level was set to P < 0.05. All data were confirmed for normal distribution before subsequent analysis using Mauchly’s test of sphericity. A paired-samples t-test was performed to compare differences between cooling times for Cold and Cold+Hyper. All variables repeatedly collected over time were analyzed with a two-way repeated-measures ANOVA condition (Neutral, Cold, Cold+Hyper) × time point (Baseline, Cooling, Preexercise, and 2.5, 5.0, 7.5, 10, 12.5, and 15 km), and Bonferroni post hoc corrections were performed for multiple comparisons. These statistical analyses were performed with GraphPad Prism (v. 7; GraphPad Software, La Jolla, CA). Cohen’s d (11) was used to calculate effect sizes for cycling power output and TT data using ESCI-delta (Geoff Cumming, La Trobe University, Australia), and descriptors for magnitudes used was based upon those of Sawilowsky (29): Very small 0.01, Small 0.2, Medium 0.5, Large 0.8, Very Large 1.2, Huge 2.00.
RESULTS
Thermal manipulation.
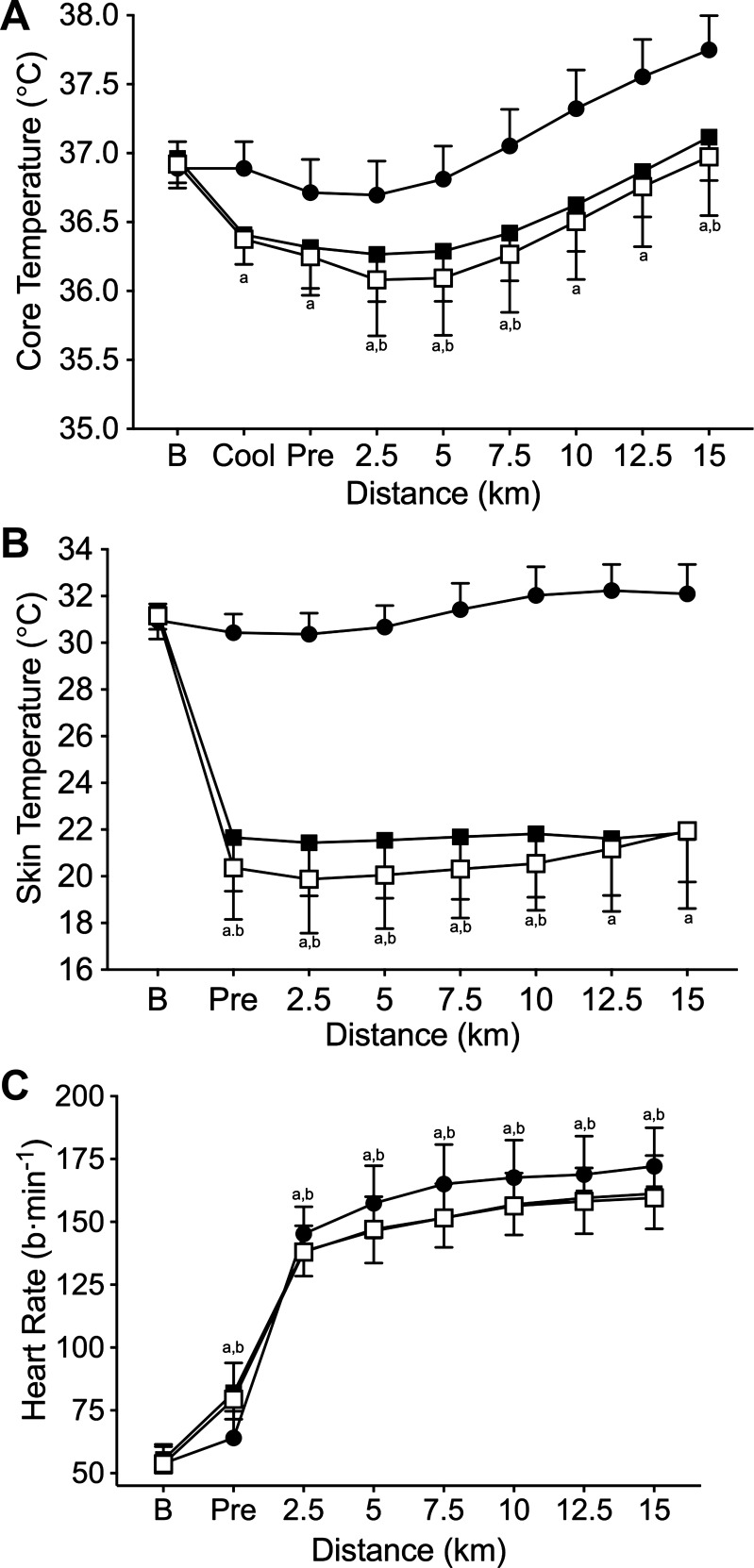
The cooling protocol was successful in eliciting the desired 0.5°C decrease in Tre for the two cold conditions (Fig. 1A). Cooling times were not statistically different between Cold and Cold+Hyper (P = 0.734), taking 86.6 ± 23.9 and 92.0 ± 19.1 min, respectively. Tre was higher (P ≤ 0.016) in Neutral at all time points compared with Cold and Cold+Hyper. Hyperoxia had no thermogenic influence in the cold; Cold had a higher (P ≤ 0.035) Tre than Cold+Hyper from 2.5 to 7.5 km, and Cold had a higher (P ≤ 0.020) T̄sk than Cold+Hyper from 2.5 to 10 km.
Fig. 1.
Core temperature (A), mean skin temperature (B), and heart rate responses (C). Neutral (●), Cold (■), and Cold+Hyper (□). aCold significantly different from Neutral; bNeutral significantly different from Cold+Hyper.
Cycling performance variables.
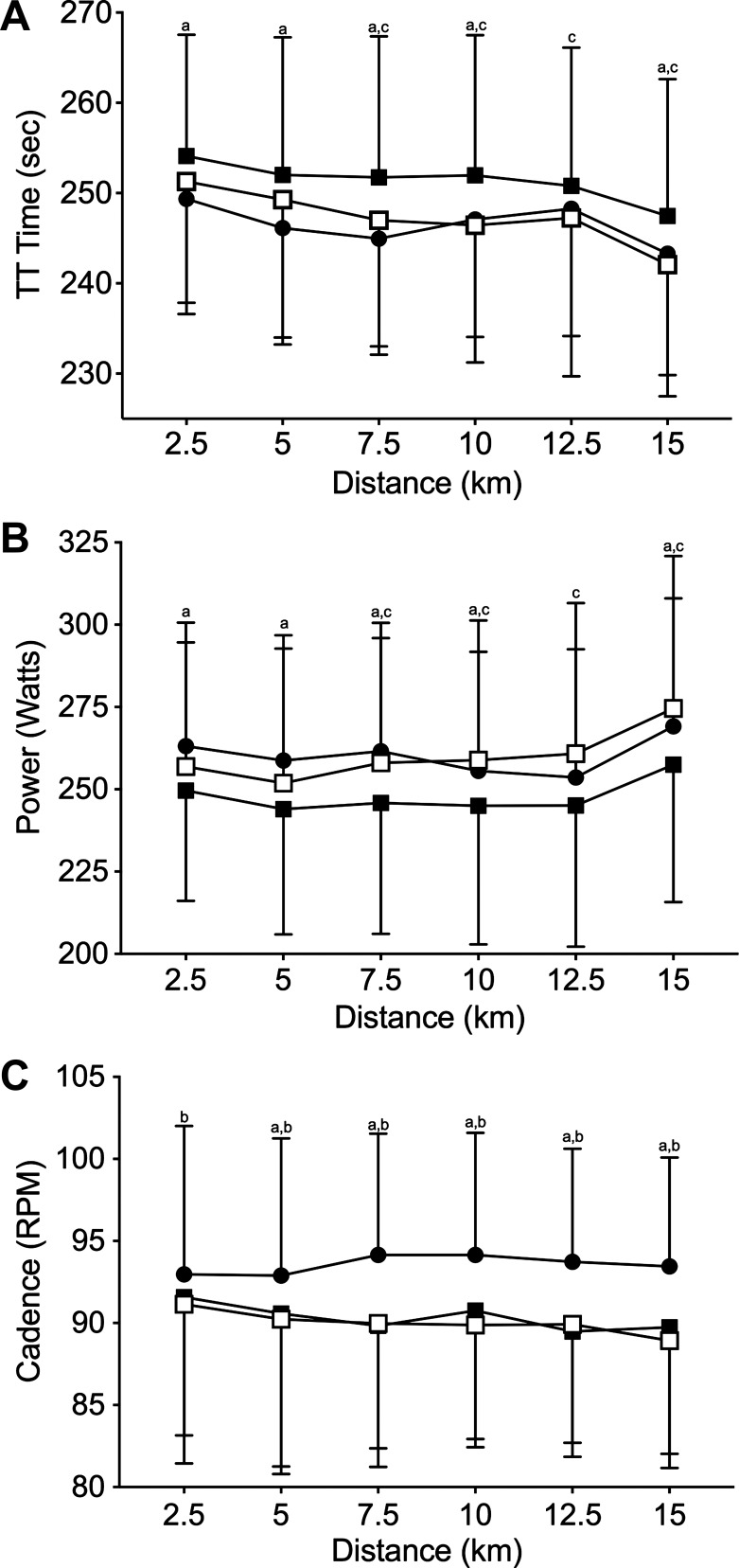
Mean TT time (Fig. 2A; Neutral: 1,479 ± 75 s, Cold: 1,509 ± 88 s, Cold+Hyper: 1,482 ± 85 s) was faster in Neutral vs. Cold (P = 0.005, d = 0.4) and in Cold+Hyper vs. Cold (P = 0.006, d = 0.3), whereas there was no difference in Neutral vs. Cold+Hyper (P = 0.999, d = 0.04). TT time during Neutral was faster than during Cold (P ≤ 0.005) at every time point except 12.5 km. TT time during Cold+Hyper was faster than during Cold (P ≤ 0.001) from 7.5 to 15 km and was not different from Neutral (P ≥ 0.356) at any time point.
Fig. 2.
Time trial (TT) time (A), power output (B), and cadence (C). Neutral (●), Cold (■), Cold+Hyper (□). aNeutral significantly different from Cold; bNeutral significantly different from Cold+Hyper; cCold+Hyper significantly different from Cold.
Mean power output (Neutral: 260 ± 38 W, Cold: 246 ± 41 W, and Cold+Hyper: 256 ± 42 W) was higher in Neutral vs. Cold (P = 0.001, d = 0.5) and Cold+Hyper vs. Cold (P = 0.044, d = 0.4), whereas there was no difference in Neutral vs. Cold+Hyper (P > 0.683, d < 0.01; Fig. 2B). Power output during Neutral was higher than during Cold at all time points except 12.5 km (P ≤ 0.012). Neutral and Cold+Hyper were not different from each other at any time point during the TT (P ≥ 0.161). Power output during Cold was lower than during Cold+Hyper from 7.5 to 15 km (P ≤ 0.003).
Mean cadence (Neutral: 93 ± 8, Cold: 89 ± 7, Cold+Hyper: 90 ± 8 rpm) was faster in Neutral than in both Cold conditions (P = 0.031; Fig. 2C). Cadence during Neutral was faster at all intervals from 5 km to the end of the TT compared with Cold and Cold+Hyper (P ≤ 0.002). Cadence during Cold was not different at any time point compared with Cold+Hyper (P ≥ 0.567).
Oxygenation variables.
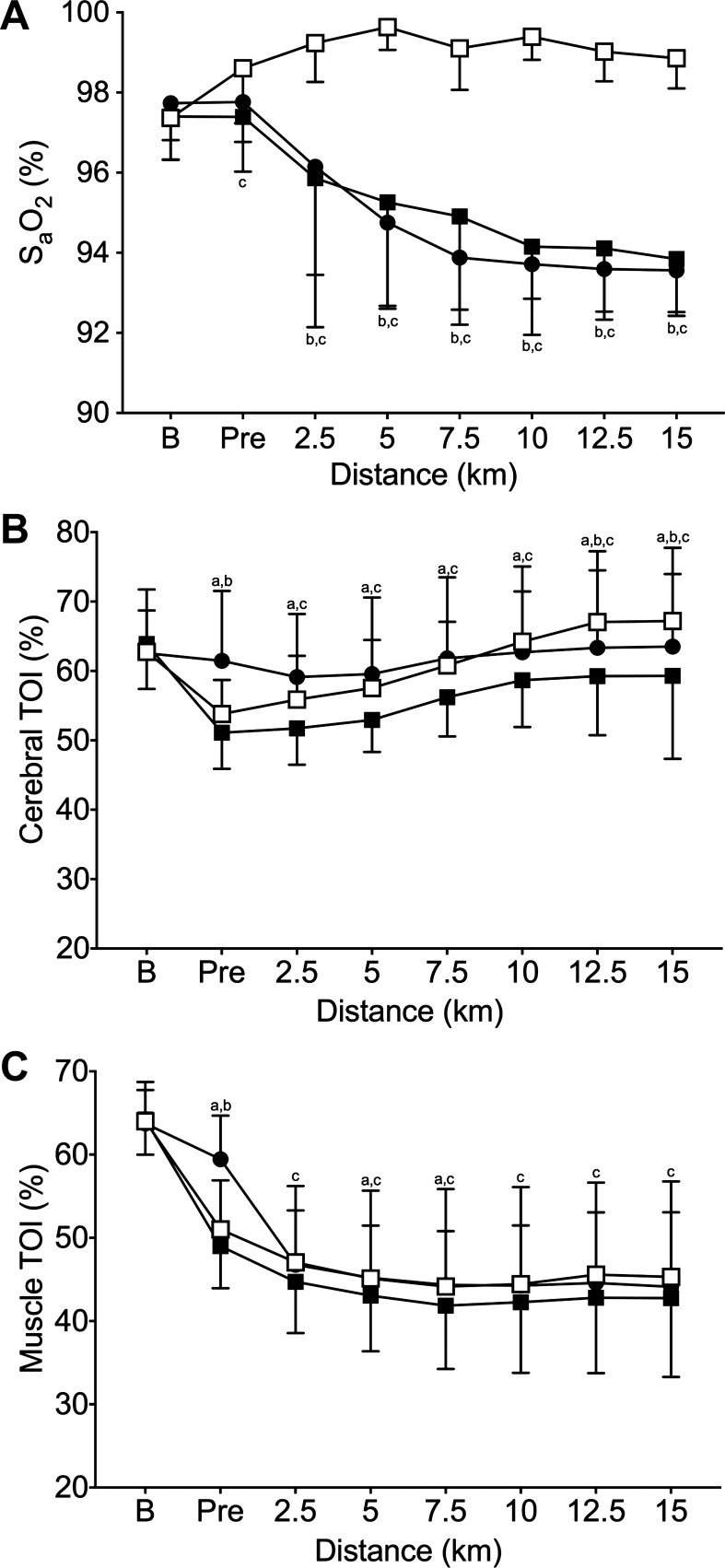
Oxygen saturation data are presented in Fig. 3A. Notably, SpO2 decreased over the course of the TT in both normoxic conditions (Neutral and Cold). In contrast, SpO2 during the hyperoxic condition (Cold+Hyper) was sustained at baseline levels of ~98% or higher throughout the TT.
Fig. 3.
Arterial oxygen saturation, (SpO2; A) and cerebral (B) and muscle tissue (C) oxygenation index responses. Neutral (●), Cold (■), Cold+Hyper (□). aNeutral significantly different from Cold; bNeutral significantly different from Cold+Hyper; cCold+Hyper significantly different from Cold.
Cerebral and muscle TOI values are presented in Fig. 3, B and C, respectively. TOIs at both sites were not different at baseline in any of the conditions (P ≥ 0.999). Cold reduced cerebral TOI compared with Neutral at every time point after baseline (P ≤ 0.011). However, in Cold+Hyper, cerebral TOI was higher than with Cold from 2.5 km onward, returning to Neutral values or higher. Cold exposure reduced muscle TOI pre-TT (P < 0.001). However, Cold+Hyper elicited higher muscle TOI than Cold from 2.5 km to the end of the TT (P ≤ 0.046).
Physiological measurements.
Heart rate was not different between any of the conditions at baseline (P > 0.999; Fig. 1C). Preexercise heart rate was higher in Cold and Cold+Hyper than in Neutral (P < 0.001); Cold and Cold+Hyper were not different preexercise (P = 0.544). Heart rate was higher in Neutral across all time points after preexercise compared with Cold and Cold+Hyper (P ≤ 0.002). Cold was not different from Cold+Hyper at any point during the TT (P > 0.999).
Perceptual scales.
Perceptual responses to the preexposure and at the end of the TT are presented in Table 1. RPE was not different in any of the conditions during any 2.5 km interval during the cycling TT (P = 0.182). TS was not different in any condition at baseline (P ≥ 0.308). TS during Neutral was warmer at every time point from pre-exercise onwards compared with Cold and Cold+Hyper (P < 0.001); TS between Cold and Cold+Hyper was not different at any time point (P > 0.999). TC was not different in any condition at baseline (P ≥ 0.852). TC during Neutral was better at each time point after baseline until 10 km (P ≤ 0.004). TC during Neutral was not different than Cold at 10 km (P = 0.157), but it was different compared with Cold+Hyper (P = 0.011). TC was not different between any condition from 12.5 km to 15 km (P ≥ 0.157).
Table 1.
Lactate and perceptual values
| Pre-TT |
TT |
|||||
|---|---|---|---|---|---|---|
| Neutral | Cold | Cold+Hyper | Neutral | Cold | Cold+Hyper | |
| Lactate, mmol/l | 1.5 ± 0.5 | 1.7 ± 0.5 | 1.3 ± 0.4 | 9.1 ± 3.0*† | 7.2 ± 2.4‡ | 6.0 ± 2.4 |
| Rating of perceived exertion | 6 ± 0 | 6 ± 0 | 6 ± 0 | 15 ± 1 | 15 ± 2 | 15 ± 2 |
| Thermal sensation | 3 ± 1*† | 1 ± 0 | 1 ± 0 | 5 ± 1*† | 3 ± 1 | 2 ± 1 |
| Thermal comfort | 1 ± 0*† | 4 ± 1 | 4 ± 1 | 2 ± 1*† | 3 ± 1 | 3 ± 1 |
Pretime trial (TT) values were taken immediately before the start of the TT. TT values represent mean values over the course of the TT (perceptual scales) and immediately upon TT completion (lactate).
Neutral significantly different from Cold;
Neutral significantly different from Cold+Hyper;
Cold+Hyper significantly different from Cold.
DISCUSSION
Passive cooling in 0°C to decrease Tre by 0.5°C reduced self-paced high-intensity exercise, with longer completion times and lower power output for a 15-km TT in Cold compared with Neutral conditions. The administration of hyperoxia (: 0.40) in Cold+Hyper restored completion times and power output to that of Neutral. Thus, both of our primary research hypotheses were accepted. Cadence was lower with mild hypothermia regardless of gas condition, suggesting an altered, centrally derived neural rhythm in cooled individuals. However, since cadence was similar between Cold and Cold+Hyper, the higher power output during Cold+Hyper came through greater torque, suggesting that overall neuromuscular capacity was maintained despite cooling. Tre and T̄sk in Cold+Hyper were actually lower compared with Cold from 2.5 to 7.5 km, suggesting that hyperoxia had no thermogenic effect. Rather, the maintenance of SpO2 near 100% throughout Cold+Hyper suggests that improvements came about through an increased oxygen availability from hyperoxia.
The reduction in exercise performance in the cold may come partially through the increased metabolic and muscular demands from moderate shivering. Using a similar protocol of passive cooling to −0.5°C from baseline, Gagnon et al. (15) demonstrated that treadmill speed at a constant 50 and 70% peak aerobic capacity was lower, implying that extra metabolic demand was required to sustain shivering. In our TT protocol, where self-pacing was possible, power output was decreased for Cold throughout the entire TT compared with Neutral. This may reflect the prior report of a linear decrease in peak aerobic capacity with lowered esophageal temperature (4), such that the actual relative demands of exercise were similar between Neutral and Cold. Despite the addition of hyperoxia in the Cold+Hyper trials, Tre and T̄sk were reduced compared with Cold from 2.5 to 7.5 km of the TT, yet power output was higher than in Cold and similar to Neutral levels throughout the Cold+Hyper TT. In addition, thermal sensation and comfort did not differ between the two Cold conditions at any time point. This combination implies that hyperoxia did not restore exercise capacity in the Cold through a thermogenic benefit such as a reduction in shivering demands or thermal discomfort.
The primary proposed mechanism for the benefit of hyperoxia in thermoneutral environments is through improved O2 availability (19), but whether this occurs despite the systemic vasoconstriction from mild cooling is unknown. Hyperoxia was able to maintain SpO2 in Cold+Hyper at ~99% throughout the TT, whereas Neutral and Cold were reduced by ~4–5%, and the difference in performance between Cold and Cold+Hyper is consistent with data when SpO2 is directly manipulated to similar levels in thermoneutral environments (19). The maintenance of SpO2 at baseline levels during Cold+Hyper implies that any reductions in blood flow, either as a result of vasoconstriction from the cold or from elevated muscle oxygen tension, were compensated for by maintained O2 availability in the blood for extraction. Stellingwerff et al. (33) showed that hyperoxia in a thermoneutral environment reduced lactate concentrations as a result of improved aerobic metabolism, which could explain our results. Reduced rates of various metabolites are of great importance, since group III and IV muscle afferents innervate free nerve endings distributed widely throughout the muscle (2). Metabolic by-products of muscular contractions such as H+ and Pi have been shown to increase the spontaneous discharge of both group III and IV afferents, therefore sending inhibitory feedback to the central nervous system and reducing central motor drive (1). Thus, the reduced lactate concentrations found in the present study during Cold+Hyper compared with Neutral and Cold suggest decreased biochemical and physiological disturbances to homeostasis and possibly an increased reliance on aerobic metabolism.
Near infrared spectroscopy is proposed as a reliable index of muscle blood flow and O2 uptake in normothermia (18) and following local or whole body cold exposure (7, 10), and it was used to explore how cerebral and muscle oxygenation responses during exercise are affected by cooling and/or hyperoxia. Both cerebral and muscle oxygenation were elevated in Cold+Hyper compared with Cold. Amann et al. (3) showed that, at physical fatigue during acute exposure to severe hypoxia (: 0.10), a rapid switch to hyperoxia (: 0.60) improved cerebral oxygenation and prolonged time to exhaustion in the absence of a critical level of peripheral muscle fatigue. Similarly, Subudhi et al. (34) showed that hyperoxia (: 0.60) administered at the point of maximal exertion achieved during acute hypobaric hypoxia (PIO2 = 86 Torr) increased cerebral oxygenation above resting values and increased pedal cadence during a graded exercise test, allowing a similar maximal work rate to be achieved compared with normoxia. Ultimately, while the increased oxygenation response with hyperoxia is intriguing, as we did not manipulate local oxygenation nor measure blood flow, whether cerebral or muscle oxygenation responses directly affected exercise performance in mild hypothermia or with hyperoxia remains unclear.
While these findings add novel insight into the effects of hyperoxia in mildly cooled individuals, it is important to acknowledge several limitations and place the findings into context. Importantly, while hyperoxia has been studied extensively as an ergogenic aid for athletic performance (19), our goal was not to propose the practical use of hyperoxia as an ergogenic aid during either thermoneutral or environmentally stressful exercise. This is due to both the impracticality of athletes carrying air cylinders during exercise and the well-known risks of oxygen toxicity with prolonged increases in oxygen partial pressure (6). Rather, our primary goals were to study the effects of mild hypothermia on self-paced exercise, along with whether hyperoxia would offset any impairment through a thermal or oxygen availability mechanism. Placing the NIRS probes on the left frontal lobe and right vastus lateralis to determine cerebral and muscle oxygenation may not be reflective of global oxygenation changes occurring in the entire body, as more active regions of the brain or muscle may receive a greater proportion of blood flow (12). Future studies should examine oxygenation at multiple sites, along with measuring cerebral and muscle blood flow, to gain a clear understanding of the integrated hemodynamic response to hyperoxia administered in mildly cooled individuals. Additionally, examining components of central and peripheral fatigue in depth could help determine the neuromuscular mechanism behind the influence of hyperoxia on mildly cooled individuals.
Conclusion.
In summary, this study demonstrated that mild hypothermia significantly impaired power output and cadence during self-paced, high-intensity exercise in cold environments. Hypothermia likely did not affect overall muscular capacity, as hyperoxia enabled greater torque application to the pedals despite a reduction in cadence while hypothermic. Hyperoxia had no thermogenic effects during exercise in the cold, nor did it influence thermal perception. Hypothermic impairment was likely due to reduced oxygen availability to the active musculature, as hyperoxia (: 0.40) restored TT times and voluntary power output to Neutral levels while maintaining higher levels of both arterial saturation and cerebral and muscle oxygenation.
GRANTS
This study was supported by the Natural Science and Engineering Research Council of Canada through a Discovery grant (227912-12, to S. S. Cheung). S. S. Cheung was also supported by a Canada Research Chair.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.A.F., N.D.E., B.D.R., and S.S.C. conceived and designed research; S.A.F., G.J.H., and S.S.C. performed experiments; S.A.F., G.J.H., and S.S.C. analyzed data; S.A.F., N.D.E., B.D.R., G.J.H., and S.S.C. interpreted results of experiments; S.A.F. prepared figures; S.A.F. and S.S.C. drafted manuscript; S.A.F., N.D.E., B.D.R., G.J.H., and S.S.C. edited and revised manuscript; S.A.F., N.D.E., B.D.R., G.J.H., and S.S.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We express our thanks to our participants for their effort and enthusiasm.
REFERENCES
- 1.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J Physiol 589: 5299–5309, 2011. doi: 10.1113/jphysiol.2011.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann M, Eldridge MW, Lovering AT, Stickland MK, Pegelow DF, Dempsey JA. Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J Physiol 575: 937–952, 2006. doi: 10.1113/jphysiol.2006.113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann M, Romer LM, Subudhi AW, Pegelow DF, Dempsey JA. Severity of arterial hypoxaemia affects the relative contributions of peripheral muscle fatigue to exercise performance in healthy humans. J Physiol 581: 389–403, 2007. doi: 10.1113/jphysiol.2007.129700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergh U, Ekblom B. Physical performance and peak aerobic power at different body temperatures. J Appl Physiol Respir Environ Exerc Physiol 46: 885–889, 1979. [DOI] [PubMed] [Google Scholar]
- 5.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Brubakk AO, Ross JAS, Thom SR. Saturation diving; physiology and pathophysiology. Compr Physiol 4: 1229–1272, 2014. doi: 10.1002/cphy.c130048. [DOI] [PubMed] [Google Scholar]
- 7.Budidha K, Abay TY, Kyriacou PA. Investigation of photoplethysmography, laser doppler flowmetry and near infrared spectroscopy during induced thermal stress. Conf Proc IEEE Eng Med Biol Soc 2015: 6417–6420, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Castellani JW, Tipton MJ. Cold stress effects on exposure tolerance and exercise performance. Compr Physiol 6: 443–469, 2015. doi: 10.1002/cphy.c140081. [DOI] [PubMed] [Google Scholar]
- 9.Castellani JW, Young AJ. Human physiological responses to cold exposure: Acute responses and acclimatization to prolonged exposure. Auton Neurosci 196: 63–74, 2016. doi: 10.1016/j.autneu.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Choo HC, Nosaka K, Peiffer JJ, Ihsan M, Yeo CC, Abbiss CR. Peripheral blood flow changes in response to postexercise cold water immersion. Clin Physiol Funct Imaging 38: 46–55, 2018. doi: 10.1111/cpf.12380. [DOI] [PubMed] [Google Scholar]
- 11.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum, 1998. [Google Scholar]
- 12.Delp MD, Armstrong RB, Godfrey DA, Laughlin MH, Ross CD, Wilkerson MK. Exercise increases blood flow to locomotor, vestibular, cardiorespiratory and visual regions of the brain in miniature swine. J Physiol 533: 849–859, 2001. doi: 10.1111/j.1469-7793.2001.t01-1-00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farra SD, Cheung SS, Thomas SG, Jacobs I. Rate dependent influence of arterial desaturation on self-selected exercise intensity during cycling. PLoS One 12: e0171119, 2017. doi: 10.1371/journal.pone.0171119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagge AP, Stolwijk JA, Hardy JD. Comfort and thermal sensations and associated physiological responses at various ambient temperatures. Environ Res 1: 1–20, 1967. doi: 10.1016/0013-9351(67)90002-3. [DOI] [PubMed] [Google Scholar]
- 15.Gagnon DD, Rintamäki H, Gagnon SS, Oksa J, Porvari K, Cheung SS, Herzig K-H, Kyröläinen H. Fuel selection during short-term submaximal treadmill exercise in the cold is not affected by pre-exercise low-intensity shivering. Appl Physiol Nutr Metab 39: 282–291, 2014. doi: 10.1139/apnm-2013-0061. [DOI] [PubMed] [Google Scholar]
- 16.Galloway SD, Maughan RJ. Effects of ambient temperature on the capacity to perform prolonged cycle exercise in man. Med Sci Sports Exerc 29: 1240–1249, 1997. doi: 10.1097/00005768-199709000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Ihsan M, Watson G, Lipski M, Abbiss CR. Influence of postexercise cooling on muscle oxygenation and blood volume changes. Med Sci Sports Exerc 45: 876–882, 2013. doi: 10.1249/MSS.0b013e31827e13a2. [DOI] [PubMed] [Google Scholar]
- 18.Lucero AA, Addae G, Lawrence W, Neway B, Credeur DP, Faulkner J, Rowlands D, Stoner L. Reliability of muscle blood flow and oxygen consumption response from exercise using near-infrared spectroscopy. Exp Physiol 103: 90–100, 2018. doi: 10.1113/EP086537. [DOI] [PubMed] [Google Scholar]
- 19.Mallette MM, Stewart DG, Cheung SS. The effects of hyperoxia on sea-level exercise performance, training, and recovery: a meta-analysis. Sports Med 48: 153–175, 2018. doi: 10.1007/s40279-017-0791-2. [DOI] [PubMed] [Google Scholar]
- 20.Minett GM, Duffield R, Billaut F, Cannon J, Portus MR, Marino FE. Cold-water immersion decreases cerebral oxygenation but improves recovery after intermittent-sprint exercise in the heat. Scand J Med Sci Sports 24: 656–666, 2014. doi: 10.1111/sms.12060. [DOI] [PubMed] [Google Scholar]
- 21.Miyazawa T, Horiuchi M, Komine H, Sugawara J, Fadel PJ, Ogoh S. Skin blood flow influences cerebral oxygenation measured by near-infrared spectroscopy during dynamic exercise. Eur J Appl Physiol 113: 2841–2848, 2013. doi: 10.1007/s00421-013-2723-7. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen HB, Boushel R, Madsen P, Secher NH. Cerebral desaturation during exercise reversed by O2 supplementation. Am J Physiol Heart Circ Physiol 277: H1045–H1052, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Nummela A, Hämäläinen I, Rusko H. Effect of hyperoxia on metabolic responses and recovery in intermittent exercise. Scand J Med Sci Sports 12: 309–315, 2002. doi: 10.1034/j.1600-0838.2002.10157.x. [DOI] [PubMed] [Google Scholar]
- 24.Nybo L, Rasmussen P. Inadequate cerebral oxygen delivery and central fatigue during strenuous exercise. Exerc Sport Sci Rev 35: 110–118, 2007. doi: 10.1097/jes.0b013e3180a031ec. [DOI] [PubMed] [Google Scholar]
- 25.Parkin JM, Carey MF, Zhao S, Febbraio MA. Effect of ambient temperature on human skeletal muscle metabolism during fatiguing submaximal exercise. J Appl Physiol (1985) 86: 902–908, 1999. doi: 10.1152/jappl.1999.86.3.902. [DOI] [PubMed] [Google Scholar]
- 26.Ramanathan NL. A new weighting system for mean surface temperature of the human body. J Appl Physiol 19: 531–533, 1964. doi: 10.1152/jappl.1964.19.3.531. [DOI] [PubMed] [Google Scholar]
- 27.Richardson RS, Grassi B, Gavin TP, Haseler LJ, Tagore K, Roca J, Wagner PD. Evidence of O2 supply-dependent V̇o2 max in the exercise-trained human quadriceps. J Appl Physiol (1985) 86: 1048–1053, 1999. doi: 10.1152/jappl.1999.86.3.1048. [DOI] [PubMed] [Google Scholar]
- 28.Rooks CR, Thom NJ, McCully KK, Dishman RK. Effects of incremental exercise on cerebral oxygenation measured by near-infrared spectroscopy: a systematic review. Prog Neurobiol 92: 134–150, 2010. doi: 10.1016/j.pneurobio.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Sawilowsky SS. New effect size rules of thumb. J Mod Appl Stat Methods 8: 597–599, 2009. doi: 10.22237/jmasm/1257035100. [DOI] [Google Scholar]
- 30.Scheeren TWL, Schober P, Schwarte LA. Monitoring tissue oxygenation by near infrared spectroscopy (NIRS): background and current applications. J Clin Monit Comput 26: 279–287, 2012. doi: 10.1007/s10877-012-9348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schramm WM, Bartunek A, Gilly H. Effect of local limb temperature on pulse oximetry and the plethysmographic pulse wave. Int J Clin Monit Comput 14: 17–22, 1997. doi: 10.1007/BF03356574. [DOI] [PubMed] [Google Scholar]
- 32.Sperlich B, Zinner C, Hauser A, Holmberg H-C, Wegrzyk J. The impact of hyperoxia on human performance and recovery. Sports Med 47: 429–438, 2017. doi: 10.1007/s40279-016-0590-1. [DOI] [PubMed] [Google Scholar]
- 33.Stellingwerff T, Leblanc PJ, Hollidge MG, Heigenhauser GJF, Spriet LL. Hyperoxia decreases muscle glycogenolysis, lactate production, and lactate efflux during steady-state exercise. Am J Physiol Endocrinol Metab 290: E1180–E1190, 2006. doi: 10.1152/ajpendo.00499.2005. [DOI] [PubMed] [Google Scholar]
- 34.Subudhi AW, Lorenz MC, Fulco CS, Roach RC. Cerebrovascular responses to incremental exercise during hypobaric hypoxia: effect of oxygenation on maximal performance. Am J Physiol Heart Circ Physiol 294: H164–H171, 2008. doi: 10.1152/ajpheart.01104.2007. [DOI] [PubMed] [Google Scholar]