Abstract
Maintaining proteostasis is a key mechanism for preserving cell function. Exercise-stimulated proteostasis is regulated, in part, by redox-sensitive signaling. Several studies suggest that supplementation with exogenous antioxidants blunts exercise-induced cellular adaptations, although this conclusion lacks consensus. Our group uses a fundamentally different approach to maintain redox balance by treatment with bioactive phytochemicals to activate the transcription factor nuclear factor (erythroid-derived 2)-like 2 and downstream endogenous antioxidant pathways. We hypothesized that vitamin C (VitC) would interfere with redox-sensitive proteostatic mechanisms in skeletal muscle, whereas phytochemical treatment would permit proteostatic maintenance. We measured protein and DNA synthesis in skeletal muscle from high-volume voluntary wheel-running rats. Whereas phytochemical treatment permitted mitochondrial and other proteostatic adaptations to exercise, VitC treatment did not. During an in vitro oxidative challenge, phytochemical treatment helped maintain proteostasis, including the mitochondrial fraction while VitC did not. Our findings support the conclusion that VitC can blunt some of the beneficial adaptations to exercise. We propose that regulation of endogenous antioxidants represents a novel approach to maintain redox balance while still permitting redox-sensitive proteostatic adaptations.
NEW & NOTEWORTHY Whether vitamin C blocks aerobic exercise adaptions lacks consensus, perhaps because of approaches that only assess markers of mitochondrial biogenesis. By directly measuring mitochondrial biogenesis, we demonstrate that vitamin C blunts exercise-induced adaptations. Furthermore, we show that treatment with Protandim, a purported nuclear factor (erythroid-derived 2)-like 2 activator that upregulates endogenous antioxidants, permits mitochondrial biogenesis. We confirm that vitamin C blunts aerobic exercise adaptions, whereas Protandim does not, suggesting targeting the endogenous antioxidant network facilitates adaptations to exercise.
Keywords: deuterium oxide, mitochondrial biogenesis, proteostasis, redox
INTRODUCTION
The proteostatic network is a complex and dynamic system of interrelated activities that determine proteome fidelity and contribute to overall cellular and organismal function (3). Protein and cellular turnover are important components of maintaining proteostasis. When cells replicate, DNA is replicated, and protein mass is doubled so that new cells have the full complement of both nuclear material and proteins (15). Thus, cell replication increases the rates of protein and DNA synthesis. Existing cells require newly synthesized proteins to replace proteins that have been degraded because they are damaged/misfolded or not needed. Therefore, it is important to consider protein synthesis in the context of cell proliferation to gain a clear picture of the role of protein turnover in maintaining proteostasis (16, 29).
Exercise is a physiological stress that triggers a host of adaptations. In response to aerobic exercise, skeletal muscle protein turnover increases (43) and new mitochondrial proteins are synthesized (mitochondrial biogenesis) (22, 58). The combination of mitochondrial biogenesis and degradation of damaged mitochondrial proteins maintains proteostasis (26) and presumably improves mitochondrial function. Beneficial skeletal muscle adaptations in response to exercise are likely regulated at least in part by redox-sensitive signaling events (44). Indeed, skeletal muscle contractions result in production of hydrogen peroxide (H2O2) and other reactive oxygen species (ROS) (40, 50) that can in turn activate redox-regulated pathways leading to mitochondrial biogenic signaling (19, 51) and maintenance of mitochondrial proteostasis.
Although ROS are necessary for redox-regulated cell signaling, excess and unremitting production of ROS can damage cellular macromolecules, activate cell death signaling cascades, and potentially contribute to the pathology of chronic diseases (2, 7). In addition, excess oxidative stress during exercise could impede force production in skeletal muscle (1) and interfere with exercise adaptation. Supplementation with exogenous antioxidants such as vitamin C (VitC) and vitamin E emerged as a strategy to counteract the potential detrimental effects of ROS production during exercise. However, the efficacy of this strategy lacks consensus. A 2012 meta-analysis conducted on 11 primary research studies encompassing both human and rodent models and various exercise modalities concluded that supplementation with vitamins C and E for 3 wk or longer had discordant outcomes, with two studies yielding a negative effect, six yielding no effect, and two reporting ergogenic effects on mitochondrial, metabolic, and performance outcomes (37). Additional studies suggest that exogenous antioxidant supplementation may blunt beneficial exercise-induced adaptations (41, 53) although this conclusion lacks consensus (49, 57). In part, these conflicting results are due to methodological approaches, particularly those used to assess mitochondrial biogenesis. More often than not, the experimental approach to assess mitochondrial biogenesis involves measuring expression of signaling molecules and/or mitochondrial protein content. However, none of these approaches is a direct measurement of new mitochondrial proteins synthesized, and they frequently fail to capture important protein turnover events that can occur without a net change in protein content (30). Therefore, definitively identifying if supplementation with exogenous antioxidants like VitC blunts exercise-induced mitochondrial biogenesis and synthesis of other skeletal muscle proteins requires assessing the dynamics of protein turnover.
An alternative approach to supplementing with exogenous antioxidants to mitigate oxidative damage is to increase endogenous antioxidant networks via activation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), the master regulator of cellular antioxidant defenses (12, 55). Nrf2 is a member of the basic leucine zipper transcription factor family and controls both basal and inducible expression of >200 genes (25) by binding to the 5′-upstream cis-acting regulatory sequence known as the antioxidant response element (27). Nrf2 induces the transcription of genes with functions that favor cell survival, including mitochondrial biogenesis (42), phase II antioxidant and detoxification (55), and anti-inflammation (6). Our group has previously shown that treatment of cultured cardiac myocytes and coronary artery endothelial cells with a well-defined phytochemical combination marketed as Protandim (Pro) robustly activated Nrf2 and protects cells against oxidant-induced cell death (8, 46). Furthermore, in older individuals, Pro treatment with concurrent protein supplementation showed trends toward increasing skeletal muscle protein synthesis (23). Finally, as part of the National Institute on Aging-Interventions Testing Program, treatment with Pro resulted in an increased mean lifespan of male mice (54). Although treatment with Nrf2 activators such as Pro shows promise for providing protection against deleterious effects of ROS, and may be a viable approach for improving healthspan-related outcomes, it is not known if treatment with Pro interferes with redox-sensitive adaptations to exercise.
The aim of this study was to determine the effect of treatment with VitC or the phytochemical Nrf2 activator Pro on exercise- or H2O2-induced skeletal muscle mitochondrial biogenesis and proteostasis. We used a deuterium oxide (D2O) tracer to assess in vivo rates of protein and DNA synthesis, as well as protein and DNA synthesis and breakdown in vitro. We hypothesized that, compared with sedentary conditions, exercise would result in beneficial adaptations as evidenced by increased rates of mitochondrial protein synthesis and activation of proteostatic mechanisms in skeletal muscle. Furthermore, we hypothesized that, compared with exercise alone, cotreatment with VitC would blunt the exercise-induced beneficial adaptations, but cotreatment with Pro would permit the adaptations. Additionally, we used a novel in vitro approach to directly measure the effect of ROS exposure and VitC or Pro treatment not only on rates of protein and DNA synthesis but also on rates of protein and DNA degradation. This novel and complementary approach provides a complete assessment of protein and cell turnover.
MATERIALS AND METHODS
Animal Care
All procedures meet or exceed the standards for facilities housing animals as described in the Animal Welfare Act regulations, the Guide for the Care and Use of Laboratory Animals, and the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching and were approved by the Colorado State University Animal Care and Use Committee. Rats were euthanized by American Veterinary Medical Association-consistent injection of ketamine and xylazine. Tissues were excised and immediately flash-frozen in liquid nitrogen for subsequent assessments.
In Vivo Study Design and Use of Labeled Water
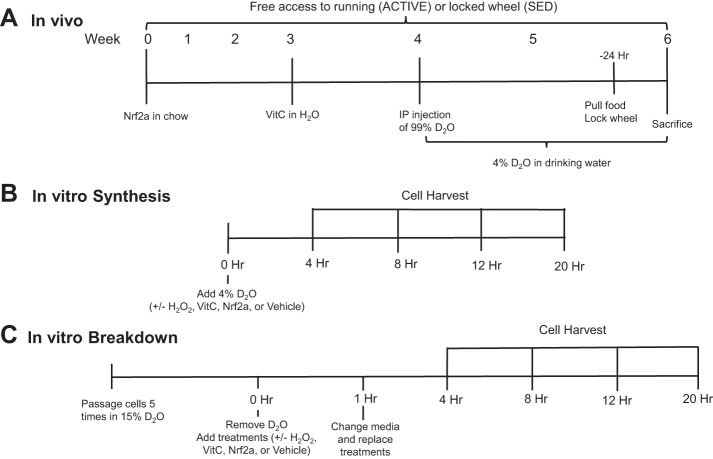
Male Sprague Dawley rats (age 86 ± 17 days) were identified as high- or low-volume wheel runners by monitoring habitual running for a minimum of 4 wk. High-volume wheel runners were given access to running wheels (Mini-Mitter Respironics; Vital View Software; STARR Life Sciences, Oakmont, PA) and allowed to voluntarily run for the 6-wk study duration (ACTIVE). Low-volume runners had locked wheels and served as sedentary controls (SED). ACTIVE rats were randomized to receive VitC, Pro, or no supplementation (CON) for 4–6 wk. All rats received a single intraperitoneal injection of 99% enriched D2O (Sigma-Aldrich, St. Louis, MO) calculated to enrich the body water pool (assumed 60% of body wt) to 5% and given ad libitum access to drinking water enriched to 4% for the remainder of the study (10, 13, 32, 33). The VitC cohort was provided with VitC-supplemented drinking water (l-ascorbic acid; Sigma-Aldrich) at 500 mg/kg for the last 3 wk of the protocol; dosing and timing were based on previous literature surrounding this controversy (14, 17, 21). To minimize potential for oxidation, the VitC drinking water was protected from light and made fresh daily. Rats in the Pro group received a diet (Dyets, Bethlehem, PA) supplemented with Pro (600 ppm; LifeVantage, Sandy, UT) (27, 28) for 6 wk to allow time for the accumulation of downstream effects of Nrf2 activation. The food was completely refreshed at least weekly. The stability of Pro supplement is bioassayed by LifeVantage (18). The night before sacrifice, all running wheels were locked, and the food was removed. The in vivo experimental design is summarized in Fig. 1A.
Fig. 1.
Experimental design for in vivo, in vitro synthesis, and in vitro breakdown experiments. A: male Sprague Dawley rats were given free access to a running wheel (ACTIVE) for 6 wk. Sedentary (SED) rats were given a locked wheel. Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) activator [Protandim (Pro), 600 ppm] was supplemented in the chow for 6 wk while a separate cohort of rats received vitamin C (VitC, 500 mg/kg) in their drinking water for 3 wk. Protein and DNA synthesis was assessed during the last 2 wk of the active wheel-running period. B: for synthesis experiments, myoblasts were seeded on 100-mm culture dishes. Four percent deuterium oxide (D2O)-enriched media were added during initiation of H2O2 stimulus (50 µM). VitC (50 µM), Pro (100 mg/ml), or ethanol (EtOH) vehicle control were also added at time 0. Cells were harvested after 4, 8, 12, and 20 h. C: for breakdown experiments, myoblasts were cultured in 15% D2O-enriched media for 5 passages where the 5th passage was to 100-mm culture dishes. At time 0, the D2O-enriched media were removed, and nonenriched media with treatments (H2O2, VitC, Pro, EtOH) were added. After 1 h, the media were changed to prevent reincorporation of the label. Cells were harvested after 4, 8, 12, and 20 h.
In Vitro Study Design and Cells
Proliferating C2C12 myoblasts (CRL-1772; ATCC, Manassas, VA) were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich) supplemented with 1% penicillin/streptomycin (ATCC) and 10% fetal bovine serum (ATCC) in a 5% CO2 humidified atmosphere at 37°C. The enriched media were made by adding sterilized 99% D2O to supplemented DMEM to yield final percentages of 4% D2O-enriched media for synthesis experiments and 15% D2O-enriched media for breakdown experiments. Cells were harvested in 1.2 ml isolation buffer 1 (in mM: 100 KCl, 40 Tris·HCl, 10 Tris base, 5 MgCl2, 1 EDTA, and 1 ATP, pH 7.5) with phosphatase and protease inhibitors. The total volume was separated into a 1-ml aliquot for protein analysis and a 200-μl aliquot for DNA analysis. Immediately before cell harvesting, 1-ml samples of media were taken to measure the media D2O enrichment.
To mimic in vivo ROS exposure in an in vitro model, we treated C2C12 myoblasts with hydrogen peroxide (H2O2; Sigma-Aldrich) diluted in DMEM to a concentration of 50 µM. This concentration was chosen based on preliminary experiments showing acute stimulation of mitochondrial protein synthesis with H2O2 treatment [Supplemental Fig. S1 (Supplemental data for this article are available on the journal website.)]. VitC (l-ascorbic acid; Sigma-Aldrich) was diluted in DMEM to a final concentration of 50 µM to match physiological concentrations of plasma VitC recorded in humans after a 100-mg supplement (24). The Pro was extracted in 100% ethanol to a final concentration of 100 µg/ml previously shown to activate Nrf2 (27, 28). Control cells were treated with ethanol (EtOH) vehicle.
For protein and DNA synthesis experiments, cells were seeded in non-D2O-enriched media. When cells reached 60–70% confluence, nonenriched media were removed, cells were washed with sterile PBS, and 4% D2O-enriched media (±H2O2, VitC, Pro, or EtOH) were added. Myoblasts were harvested at time points ranging from 4 to 20 h (n = 3/time point). The in vitro synthesis experimental design is summarized in Fig. 1B.
We designed the protein and DNA breakdown experiments as a “pulse-chase” experiment using D2O. For the pulse, cells were cultured in 15% D2O-enriched media for five passages. This prolonged labeling period ensured that we labeled both rapidly and slowly synthesizing proteins, which is a key consideration when determining the pulse (52). When cells reached 60–70% confluence, we removed enriched media, washed cells with sterile PBS, and “chased” with nonenriched media (±H2O2, VitC, Pro, or EtOH). In this case, the unlabeled H2O and alanine in the media act as an effective chase. As an extra insurance against reincorporation of label from rapidly degraded proteins, we removed media after 1 h, cells were washed, and fresh nonenriched mediun with treatments was added. Myoblasts were harvested at time points from 4 to 20 h (n = 3/time point). In a preliminary study, we compared media enrichments after 4 h with or without a media change at 1 h. From this experiment, we validated that replacing the nonenriched media after 1 h removed deuterium label released in the culture media from the degradation of short-lived proteins (data not shown). The in vitro breakdown experimental design is summarized in Fig. 1C.
Tissue and Cell Preparation and Determination of Enrichment
Protein.
Flash-frozen plantaris and soleus were fractionated according to our previously published procedures (5, 10, 32, 47). Tissue (25–60 mg) was homogenized 1:10 in isolation buffer 1 with phosphatase and protease inhibitors using a bead homogenizer and then centrifuged at 800 g for 10 min at 4°C. The resulting pellet [myofibrillar (Myo) fraction] was washed in EtOH and H2O. The supernatant was centrifuged at 10,000 g for 30 min at 4°C to pellet the mitochondrial (Mito) fraction. From the supernatant, 400 μl were removed to yield the cytosolic (Cyto) fraction, and equal volume of 14% sulfosalicylic acid was added. The tube was vortexed and incubated on ice for 1 h. The Mito pellet was washed with 200 μl buffer 2 (100 mM KCl, 10 mM Tris·HCl, 10 mM Tris base, 1 mM MgSO4, 0.1 mM EDTA, 0.02 mM ATP, and 1.5% BSA, pH 7.4) and then centrifuged at 8,000 g for 10 min at 4°C. The supernatant was removed, and the pellet was washed a second time with 100 μl buffer 2 and then centrifuged at 6,000 g for 10 min. After the 1-h incubation, the Cyto fraction was centrifuged at 16,000 g for 10 min at 4°C to pellet the Cyto protein fraction. The Cyto pellet was washed in EtOH and H2O. After the wash steps, the Myo, Mito, and Cyto pellets were solubilized by incubation in 250 μl 1 N NaOH at 56°C and 500 revoutions/min for 15 min. The 1-ml aliquots of C2C12 myoblast lysates were centrifuged at 800 g for 10 min at 4°C and then fractionated and solubilized according to the same protocol as the tissue.
Cell Mito and Cyto protein pellets were hydrolyzed by incubation in 1.5 ml 6 N HCl for 24 h at 120°C. Tissue protein pellets were hydrolyzed by incubation in 1.5 (Mito), 3 (Cyto), and 6 (Myo) ml 6 N HCl at 120°C for 24 h. The hydrolysates were ion exchanged, dried under vacuum, and then suspended in 1 ml molecular biology grade H2O. Approximately 500 μl of suspended sample were derivatized by adding 500 μl acetonitrile, 50 μl 1 M K2HPO4 (pH = 11), and 20 μl pentafluorobenzyl bromide, and the sealed mixture was incubated at 100°C for 1 h. Derivatives were extracted in ethyl acetate, and the organic layer was removed and dried by N2. Tissue samples were reconstituted in 1 ml ethyl acetate while cell fractions were reconstituted in 750 μl ethyl acetate. Using negative chemical ionization, derivatized amino acids were analyzed on an Agilent 7890 GC and 7010 triple quad mass spec using an Agilent DB-5MS GC column (30 m × 0.25 mm × 0.25 μm). Samples (1 μl) were injected using splitless mode (inlet temperature 220°C). Helium was the carrier and methane the reagent gas. The mass-to-charge ratios (m/z) of 448, 449, and 450 were monitored for the pentafluorobenzyl-N,N-di(pentafluorobenzyl)alaninate derivative. In all cases, these mass-to-charge ratios represented the primary daughter ions that included all of the original hydrocarbon bonds from the given amino acid.
DNA.
Whole tissue DNA was isolated according to our previously described methods (10, 32, 48). DNA for the precursor pool was measured from bone marrow by extracting ~300 mg from the tibial bone marrow suspension, followed by a 10-min centrifugation at 2,000 g. Whole cell DNA was extracted from 200 μl of the initial cell lysates using the QIAmp DNA mini kit (Qiagen, Valencia, CA) following the manufacturer’s instructions. DNA was suspended in nuclease-free water and hydrolyzed to free deoxyribonucleic acids by incubation with nuclease S1 and potato acid phosphatase overnight at 37°C. Hydrolysates were reacted with pentafluorobenzyl hydroxylamine and acetic acid and then acetylated with acetic anhydride and 1-methylimidazole. Methylene chloride was added, and the organic layer was removed to a GC vial. The DNA extracts were dried, resuspended in ethyl acetate, and analyzed by GC-MS on a DB-17 column with negative chemical ionization, using He as carrier and methane as the reagent gas. The in vitro DNA extracts were analyzed on an Agilent 7890B GC coupled to an Agilent 5977A MS using an Agilent DB-17 GC column. The fractional molar isotope abundances at m/z 435 (M + 0 mass isotopomer) and 436 (M + 1) of the pentafluorobenzyl triacetyl derivative of purine dR were quantified using MassHunter software (Agilent Technologies, Santa Clara, CA) previously described (10, 32, 48).
Body water.
For the water enrichment analysis, 125 μl of plasma or cell culture media were placed in the inner well of an o-ring screw on cap and placed inverted on a heating block overnight at 80°C. Next, 2 μl 10 M NaOH and 20 μl acetone were added to all samples and 20 μl 0–20% D2O standards and capped immediately. Samples were vortexed at low speed and kept overnight at room temperature. Samples were extracted with 200 μl hexane, and the organic layer was transferred through anhydrous Na2SO4 into GC vials and analyzed via EI mode using a DB-17MS column.
Calculations
In vivo and in vitro protein synthesis was calculated using the true precursor enrichment using plasma or media D2O enrichment and Mass Isotopomer Distribution Analysis (MIDA) adjustment (4). Protein synthesis rates were divided by time and expressed as fractional synthesis rates (FSR: %/day in vivo or %/h in vitro) (4, 34). For DNA synthesis, the fraction new was calculated by dividing DNA enrichment by a fully turned over cell population (bone marrow) from the same animals, or from media enrichment and MIDA adjustment, and divided by time (FSR, %/h). Protein and DNA breakdown rates were calculated by dividing the decrease in enrichment from the starting enrichment of passaged cells by the starting enrichment. The average enrichment of three plates at time 0 (protein) or 4 h (DNA) was used as the starting enrichment. The fractional loss of enrichment was divided by time to determine fractional breakdown rates (FBR, %/h). The ratios of protein synthesis to DNA synthesis (protein/DNA) and protein breakdown to DNA breakdown were calculated as indicators of proteostatic mechanisms (9, 16, 23).
Plasma Malondialdehyde
Malondialdehyde (MDA) is a naturally occurring product of lipid peroxidation and is frequently used as a marker of overall oxidant damage. Plasma MDA was assessed using a commercially available kit for thiobarbituric acid reactive substances (Cayman Chemical, Ann Arbor, MI) following the manufacturer’s instructions.
Statistical Analyses
In vivo data were assessed by one-way ANOVA with comparison to SED and correction with Dunnett’s multiple-comparison test. In vitro data were analyzed by one-way ANOVA with planned contrasts. Analyses were performed using Graph Pad Prism. Data were log transformed where they did not meet the assumptions for homoscedasticity. Significance was set a priori at P < 0.05. Data are presented as means ± SE.
RESULTS
Identification of High-Volume Voluntary Wheel Runners
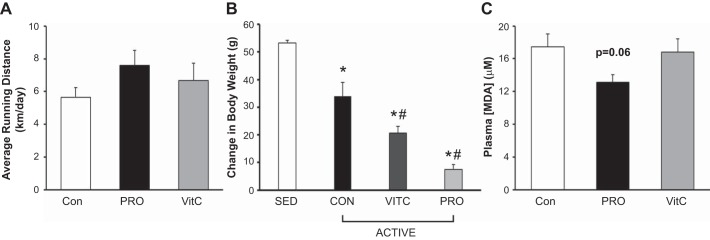
Wheel-running activity was monitored for 4 wk preceding study initiation. Rats identified as high-volume voluntary runners were given continued access to activity wheels while sedentary rats were given locked activity wheels. ACTIVE rats ran 6 km/day, with no significant differences between the three treatment groups (Fig. 2A). All groups gained weight during the study protocol; however, the active rats (CON, VitC, and Pro) all gained significantly less weight than the SED animals (Fig. 2B).
Fig. 2.
Running volume, body weights, and plasma malondialdehyde in sedentary and wheel-running rats. A: control, Protandim (Pro), and vitamin C (VitC) active rats all engaged in voluntary wheel running, averaging 6 km/day. No significant differences between treatment groups were observed. Data were assessed by 1-way ANOVA. B: all three ACTIVE groups gained significantly less body weight than the sedentary (SED) cohort throughout the last 3 wk of the study protocol. In addition, VitC and Pro gained less weight than CON-fed rats. Delta body weights were calculated over the last 3 wk of the study protocol, and delta values were compared by 1-way ANOVA. *Significantly different from SED; #significantly different from control (CON) ACTIVE. C: plasma malondialdehyde (MDA), a marker of lipid peroxidation, was lower in rats receiving Pro supplemented in their chow (P = 0.06) compared with control and VitC supplementation. Data were assessed by 1-way ANOVA with Tukey’s post hoc and expressed as means ± SE. For all cohorts, n = 4/group.
Systemic Oxidative Stress
We measured plasma MDA as a marker of lipid peroxidation to determine if Pro and/or VitC treatment attenuated a marker of oxidative stress. We assessed MDA in a group of sedentary animals to avoid the impact of chronic activity on systemic oxidant damage. Treatment with Pro attenuated lipid peroxidation compared with CON (P = 0.06) while VitC did not improve oxidative damage (Fig. 2C).
Protein and DNA Synthesis in Voluntary Wheel-Running Rats
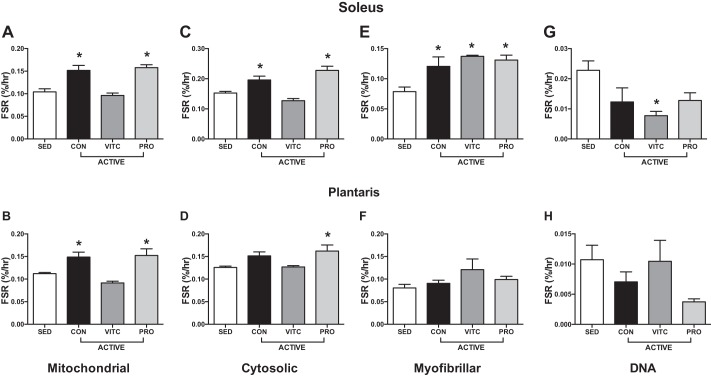
Exercise increased mitochondrial protein synthesis in both the soleus and plantaris. Compared with exercise alone, treatment with VitC blunted this response while treatment with Pro permitted this increase in exercise-induced mitochondrial biogenesis (Fig. 3, A and B). Similar patterns were observed in the Cyto fraction, with exercise stimulating Cyto protein synthesis in the plantaris, an effect blunted by VitC and permitted by Pro (Fig. 3, C and D). Interestingly, Myo protein synthesis was stimulated by exercise in the soleus in all three treatment groups but not in the plantaris (Fig. 3, E and F). DNA synthesis was generally unchanged by exercise or treatment (Fig. 3, G and H).
Fig. 3.
Skeletal muscle protein and DNA synthesis in voluntary wheel-running rats. Six weeks of active voluntary wheel running (ACTIVE) significantly stimulated mitochondrial protein synthesis in the soleus (A) and plantaris (B) compared with sedentary (SED) rats. Vitamin C (VitC) attenuated this adaptation while Protandim (Pro) permitted mitochondrial protein synthesis. Cytosolic fractional synthesis rate (FSR) was stimulated by exercise, blunted by VitC, and permitted by Pro in the soleus (C) while only Pro increased cytosolic FSR in the plantaris (D). Myofibrillar FSR was increased in all three treatment groups in response to exercise in the soleus (E), but the plantaris (F) was not responsive to voluntary while running. VitC attenuated DNA synthesis with exercise in the soleus (G) with no effect in the plantaris in any group (H). Data were analyzed by 1-way ANOVA with comparison to SED and correction with Dunnett’s multiple-comparison test. Data are expressed as means ± SE. *P < 0.05 compared with SED; n = 4/group.
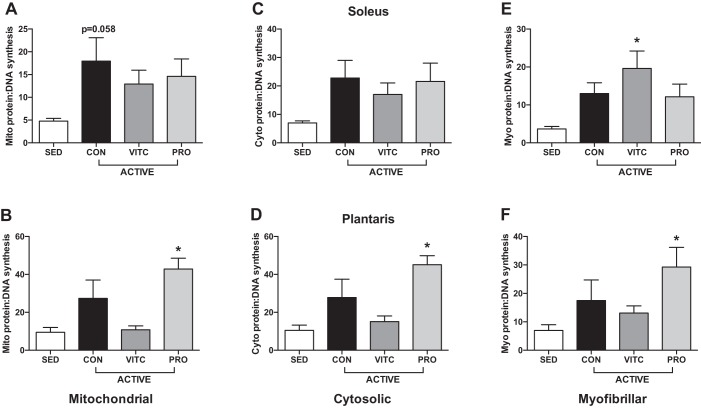
Analysis of the ratio of protein to DNA synthesis rates is indicative of what proportion of newly synthesized proteins are allocated to new cells vs. existing cells, thus providing a measure of proteostatic maintenance. When compared with SED, Mito proteostasis was improved in CON in the soleus and Pro treatment in the plantaris but not improved by VitC in either muscle (Fig. 4, A and B). Pro also improved Cyto and Myo proteostasis in the plantaris (Fig. 4, D and F). Although muscle- and fraction-specific differences exist, overall these data support an improvement of proteostasis with exercise, an effect blunted by VitC.
Fig. 4.
Skeletal muscle proteostasis in voluntary wheel-running rats. A: exercise stimulates mitochondrial proteostasis in the soleus [control (CON)], with no change with Vitamin C (VitC) or Protandim (Pro). B: mitochondrial proteostasis was upregulated by exercise only in Pro. Cytosolic proteostasis was unchanged in the soleus (C) but stimulated by Pro in the plantaris (D). Myofibrillar proteostasis was only improved by VitC in the soleus (E) and Pro in the plantaris (F). Protein-DNA synthesis was calculated as a ratio of protein to DNA fractional synthesis rate (FSR). Data were analyzed by 1-way ANOVA compared with sedentary (SED) and correction with Dunnett’s multiple-comparison test. Data are expressed as means ± SE. *P < 0.05 compared with SED; n = 4/group.
Protein and DNA Turnover In Vitro
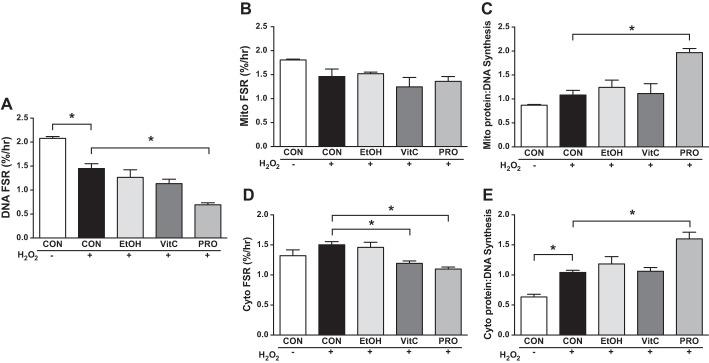
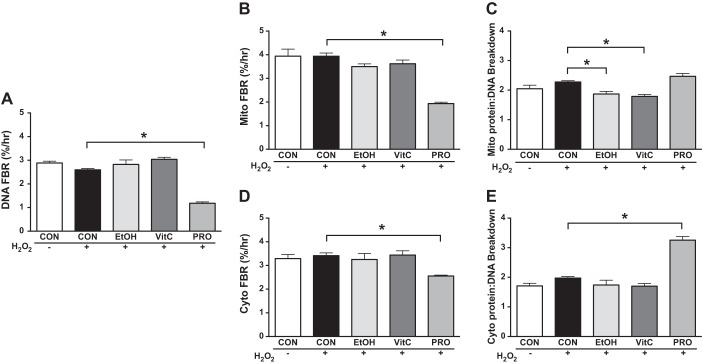
To mechanistically investigate the redox-sensitive mitochondrial adaptations to exercise, we exposed myoblasts to H2O2 in culture to mimic exercise-induced increases in ROS production. Analyses demonstrated similar outcomes at the 8- and 12-h time points; therefore, only results from the 12-h time point are presented. At 12 h of H2O2 treatment, there were no differences in Mito or Cyto protein synthesis or breakdown (Figs. 5, B, and D, and 6, B, and D). However, compared with CON + H2O2, H2O2 + VitC decreased Cyto FSR. Compared with CON + H2O2, H2O2 + Pro had lower rates of Cyto protein synthesis and lower rates of Mito and Cyto FBR. By examining DNA FSR and FBR, we gain novel insight into how the treatments affect the apparent protein synthesis rates. DNA FSR was slower during CON + H2O2 compared with CON (Fig. 4A). DNA FSR during H2O2 + VitC was not different from CON + H2O2. However, DNA FSR and FBR were slower in H2O2 + Pro compared with CON + H2O2 (Figs. 5A and 6A). Therefore, when considering the ratio protein/DNA synthesis, H2O2 + Pro increased the Mito and Cyto ratio (Fig. 5, C and E) compared with CON + H2O2 while H2O2 + VitC did not. Furthermore, H2O2 + VitC decreased protein/DNA Mito breakdown and maintained the Cyto ratio while H2O2 + Pro maintained the ratio of protein to DNA Mito breakdown and had a greater Cyto ratio compared with CON + H2O2 (Fig. 6, C and E). We noted similar responses for these treatments at 4 and 8 h, and by 20 h of treatment, most of the differences compared with CON + H2O2 were attenuated, suggesting a transient impact of Pro on mitochondrial proteostasis (Supplemental Figs. S2 and S3).
Fig. 5.
Protein and DNA synthesis in cultured myoblasts during an H2O2 challenge. A: control (CON) + H2O2 decreased DNA fractional synthesis rate (FSR) compared with CON, with H2O2 + Protandim (Pro) lower than CON + H2O2. B: mitochondrial FSR was not different between treatments. C: compared with CON + H2O2, H2O2 + Pro increased the ratio of mitochondrial (Mito) protein to DNA synthesis, indicating improved proteostatic maintenance. D: vitamin C (VitC) and Pro in combination with H2O2 decreased cytosolic FSR compared with CON + H2O2. E: CON + H2O2 increased the ratio of cytosolic (Cyto) protein to DNA synthesis while H2O2 + Pro was greater than CON + H2O2. Myoblasts were treated with 50 µM H2O2 alone (+) or in combination with ethanol (EtOH), 50 µM VitC, 100 mg/ml Pro, or left untreated (−) for 12 h in 4% deuterium oxide (D2O)-enriched media. Data were assessed by 1-way ANOVA with planned contrasts and presented as means ± SE. *P < 0.05; n = 3/group, except CON + H2O2 Mito FSR and Mito protein-to-DNA ratio; n = 2/condition.
Fig. 6.
Protein and DNA breakdown in cultured myoblasts during an H2O2 challenge. A: Compared with CON + H2O2, H2O2+ Protandim (Pro) lowered the DNA fractional breakdown rate (FBR, A), B) mitochondrial (Mito) FBR (B), and Cyto FBR (D). C: compared with CON + H2O2, H2O2 + VitC lowered the ratio of Mito protein to DNA breakdown, as did H2O2 + EtOH, indicating reduced proteostatic maintenance. E: compared with CON + H2O2, H2O2 + Pro increased the ratio of cytosolic (Cyto) protein to DNA FBR. Data were assessed by 1-way ANOVA with planned contrasts and presented as means ± SE. *P < 0.05; n = 3/condition.
DISCUSSION
Maintained mitochondrial proteostasis is among the beneficial adaptations to exercise that contribute to maintenance of skeletal muscle function and protect against chronic disease. Redox-sensitive signaling promotes mitochondrial biogenesis and potentially proteostasis. Previous studies testing whether VitC blunts mitochondrial adaptations are conflicting, but none of these studies included a direct measurement of mitochondrial protein synthesis such as stable isotopic tracers. Our results show that VitC blunts exercise-induced increases in protein synthesis (including mitochondrial) and activation of proteostatic mechanisms in skeletal muscle, whereas Pro did not. Furthermore, in vitro VitC treatment during an oxidative challenge blunted mitochondrial proteostatic mechanisms while Pro promoted these outcomes. Collectively, our data suggest that VitC treatment blunts positive protein adaptations to exercise and stress while Pro treatment is permissive of these outcomes.
In Vivo Skeletal Muscle Mitochondrial Proteostasis
The notion that supplementation with exogenous antioxidants such as VitC blunts exercise adaptations lacks consensus (14, 35, 53, 57, 59). Previous studies used mRNA and protein content of signaling proteins or markers of mitochondrial content (14, 57), rather than direct measures of mitochondrial biogenesis, which likely contributes to the differing results. As previously discussed, because of posttranscriptional regulation, mRNA is often not indicative of protein outcomes, and protein content masks changes in turnover (30, 31). To circumvent these issues, we used stable isotopes to make direct measurements of protein and DNA synthesis and breakdown to assess exercise- or H2O2-induced adaptation of skeletal muscle cells and tissue. By considering both the rates of protein turnover and DNA turnover, it was possible to account for how much of total protein turnover can be accounted for by cell turnover (cell replication and cell loss) vs. how much is directed toward maintaining the quality of proteins in the absence of cell turnover (29).
In the current study, wheel-running exercise increased mitochondrial protein synthesis in both the plantaris and soleus muscle. In addition, the soleus appeared to be more responsive than the plantaris to exercise in that protein synthesis in the myofibrillar and cytosolic fractions also increased, whereas this was not the case for plantaris. These data support the important role of aerobic exercise to maintain skeletal muscle protein quality, even if this was not to result in skeletal muscle hypertrophy (36). VitC supplementation during exercise clearly blunted the exercise-induced increases in mitochondrial biogenesis in both soleus and plantaris muscles. In addition, VitC blunted cytosolic FSR in both muscles. DNA synthesis did not change in either muscle, indicating that VitC treatment did not affect DNA replication in skeletal muscle tissue. Because DNA synthesis was not altered with VitC treatment, the ratio of protein to DNA synthesis simply reflected protein synthesis rates. These data provide compelling evidence that exogenous antioxidants are detrimental to exercise-induced skeletal muscle adaptation.
To our knowledge, this study is the first report of Pro during chronic exercise training in rats. In the current study, Pro treatment did not blunt exercise-induced increases in mitochondrial protein synthesis in either the soleus or plantaris. In addition, Pro treatment did not blunt the positive effects of exercise on Cyto and Myo protein synthesis in the soleus. When examining the ratio of protein to DNA synthesis, there were clear additional benefits of Pro on proteostatic processes in all three protein fractions of the plantaris, whereas these were not apparent in the soleus. Previous studies in mice indicate that Nrf2 activation may activate signaling involved with mitochondrial biogenesis (28), providing a potential mechanism by which Pro was permissive to mitochondrial adaptations.
In Vitro Protein Turnover and Proteostatic Mechanisms
The combination of protein synthesis and protein breakdown provides a comprehensive picture of proteostatic processes regulating protein content. The degradation of proteins is necessary for removing damaged or dysfunctional proteins, including those in the mitochondria (11), to maintain proteostasis. The measurement of protein breakdown in vivo, especially long-term protein breakdown, is technically challenging. For this study, to further explore mechanisms of ROS-induced proteostatic processes, we developed a novel approach to simultaneously assess protein breakdown and DNA breakdown using D2O. As far as we are aware, this is the first study to simultaneously measure protein and DNA breakdown in vitro. By using this approach in combination with measuring protein synthesis and DNA synthesis, we provide a comprehensive picture of the dynamic nature of protein turnover with H2O2 treatment with or without Pro and VitC.
The importance of assessing DNA turnover is especially evident in our in vitro studies. At 12 h of treatment, it appeared that H2O2 alone or in combination with Pro or VitC had no effect on mitochondrial protein synthesis. In addition, when we treated cells with H2O2 + Pro or H2O2 + VitC, there appeared to be a decrease in the rate of cytosolic protein synthesis. However, H2O2 and H2O2 + Pro both significantly decreased cell proliferation, as evident by decreased DNA synthesis. Therefore, when accounting for changes in proliferation by using the protein-to-DNA synthesis ratio, H2O2 + Pro actually increased the ratios of mitochondrial and cytosolic protein to DNA while H2O2 + VitC did not. These findings support that Pro is permissive or even enhances cellular adaptations by decreasing cellular proliferation and increasing proteostatic processes. In contrast, VitC did not have these positive effects.
As mentioned, for this project we developed a novel approach to simultaneously assess protein and DNA breakdown. At 12 h of treatment there was a significantly slower rate of protein breakdown in myoblasts treated with H2O2 and Pro compared with H2O2 alone. However, as was the case with DNA synthesis, H2O2 + Pro resulted in a significantly slower rate of DNA breakdown compared with H2O2 alone. Although the ratio of protein to DNA breakdown was not significantly different at 12 h compared with CON + H2O2, this ratio was significantly greater by 20 h. In addition, the ratio H2O2 + Pro was significantly greater in the cytosolic fraction at both 12 and 20 h compared with CON + H2O2. Conversely, the ratio protein/DNA breakdown with H2O2 + VitC was significantly lower compared with CON + H2O2 at 12 h, with no change at 20 h. When considering these findings in summary, it appears that, with H2O2 + Pro there is a cytoprotective effect in vitro. This effect is evident by the finding that cells are lost at a lower rate, and there is maintained or improved protein turnover.
It is important to reiterate the insight gained by using the protein-to-DNA ratio, especially in vitro. In the CON + H2O2 condition, the protein-to-DNA synthesis ratio approximated one. A ratio close to one means that cell proliferation essentially accounted for all the measured protein synthesis. Therefore, when using a treatment that changes the rate of proliferation, cell cycle arrest, or cell death, it is difficult to interpret changes in protein turnover because cell turnover masks those changes. For example, many lifespan-extending treatments slow growth (e.g., rapamycin treatment or Snell dwarf mice) (9, 10) and also appear to decrease protein synthesis (9, 10, 20, 45). As we have indicated previously, the finding that slowed aging treatments decrease protein synthesis is likely simply due to the fact that most studies do not account for the influence of cell turnover on measured protein synthesis. When DNA synthesis is measured, it becomes clear that protein turnover is maintained or even increased (9, 29). Therefore, we advocate for this approach to determine the differences in protein synthesis for maintaining proteostasis vs. protein synthesis for growth, especially in vitro.
Study Limitations
The current study examines the effects of an exogenous antioxidant, VitC, and a purported Nrf2 activator, Pro, but assessments of antioxidant activity and oxidant stress are lacking. VitC treatments were based on previously published studies (14, 57). Pro treatment was based off in vivo recommended doses and our previous studies in vitro demonstrating Nrf2 activation and cytoprotection (8, 46). Nrf2 activation in vivo is notoriously difficult to assess since at the time of animal sacrifice there is a high likelihood of missing transient (<15 min) Nrf2 activation. In addition, in an otherwise healthy young animal, Nrf2 activation likely occurs on an “as needed” basis for stress resistance, thus accentuating the transient nature. The same general argument has been put forth for exogenous antioxidant supplements, with some groups proposing that antioxidant deficiency is required for exogenous antioxidants to be therapeutically beneficial (39, 56). In fact, in humans supplemented with VitC, those with low baseline circulating concentrations showed improvements in physical performance, whereas those with high levels did not (38). Finally, these experiments should be repeated in human subjects to confirm our in vitro and in vivo animal studies. It is worth noting that we have previously shown that supplementing human subjects with Pro does not blunt basal mitochondrial biogenesis (23) although what impact exercise has on skeletal muscle adaptations and redox-sensitive mechanisms remains to be tested.
Conclusions and Future Directions
Despite efforts by multiple research groups over the past decade, consensus on the impact of VitC and vitamin E on exercise adaptation has remained elusive. As discussed above, we believe this is in part because of the failure to directly measure mitochondrial protein synthesis and the failure to account for changes in cell proliferation. When using these direct measures, it is clear that VitC supplementation decreased skeletal muscle adaptations to exercise in vivo. Our in vitro studies suggest that the decreased adaptations may be a result of attenuated ROS-induced stimulation of proteostatic mechanisms; however, further assessment of in vivo redox signaling is required to verify these findings. In contrast, Pro did not blunt these adaptations. It is not yet entirely clear why treatment with phytochemicals that presumably activate Nrf2 and upregulate endogenous antioxidants is permissive to these adaptations. However, we speculate that parallel signaling pathways are activated such as activation of nuclear respiratory factor 1 (42), or there are temporal patterns of ROS bursts that stimulate adaptation while prolonged oxidative stress is mitigated. Future work could elucidate the impact of key biological variables, including exercise intensity and modality, age and disease status, and other variables known to impact proteostasis in response to exercise.
In conclusion, our data support the notion that VitC supplementation blunts exercise-induced increases in mitochondrial biogenesis and improvements in proteostatic mechanisms. However, treatment with Pro, a phytochemical-based treatment previously shown to activate Nrf2, permits mitochondrial adaptations and proteostatic maintenance. This insight was gained through the direct measure of protein and DNA synthesis and through a novel approach to assess protein and DNA synthesis and breakdown in vitro. Thus, we propose that targeting the endogenous antioxidant network is a better approach than the use of exogenous antioxidants to allow beneficial adaptations to exercise training.
GRANTS
During the execution of the studies described in this manuscript, the laboratory was supported the by the Defense Advanced Research Projects Agency (N66001–10-C-2134) and National Institute on Aging Grant 1R01-AG-042569.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.R.B., S.E.E., S.K., B.F.M., and K.L.H. conceived and designed research; D.R.B., S.E.E., L.M.B., and F.F.P. performed experiments; D.R.B., S.E.E., L.M.B., F.F.P., B.F.M., and K.L.H. analyzed data; D.R.B., S.E.E., B.F.M., and K.L.H. interpreted results of experiments; D.R.B. and S.E.E. prepared figures; D.R.B. and S.E.E. drafted manuscript; D.R.B., S.E.E., B.F.M., and K.L.H. edited and revised manuscript; D.R.B., S.E.E., S.K., L.M.B., F.F.P., B.F.M., and K.L.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Joe McCord for support and consultation on this and related projects. We also thank LifeVantage for donating the Protandim supplement and for support of the project.
REFERENCES
- 1.Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol 509: 565–575, 1998. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aruoma OI, Kaur H, Halliwell B. Oxygen free radicals and human diseases. J R Soc Health 111: 172–177, 1991. doi: 10.1177/146642409111100506. [DOI] [PubMed] [Google Scholar]
- 3.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science 319: 916–919, 2008. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 4.Busch R, Kim YK, Neese RA, Schade-Serin V, Collins M, Awada M, Gardner JL, Beysen C, Marino ME, Misell LM, Hellerstein MK. Measurement of protein turnover rates by heavy water labeling of nonessential amino acids. Biochim Biophys Acta 1760: 730–744, 2006. doi: 10.1016/j.bbagen.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 5.Butz CE, McClelland GB, Brooks GA. MCT1 confirmed in rat striated muscle mitochondria. J Appl Physiol (1985) 97: 1059–1066, 2004. doi: 10.1152/japplphysiol.00009.2004. [DOI] [PubMed] [Google Scholar]
- 6.Chen X-L, Kunsch C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases [no date]. Curr Pharm Des 10: 879–891, 2004. doi: 10.2174/1381612043452901. [DOI] [PubMed] [Google Scholar]
- 7.Davies KJ. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life 50: 279–289, 2000. doi: 10.1080/15216540051081010. [DOI] [PubMed] [Google Scholar]
- 8.Donovan EL, McCord JM, Reuland DJ, Miller BF, Hamilton KL. Phytochemical activation of Nrf2 protects human coronary artery endothelial cells against an oxidative challenge. Oxid Med Cell Longev 2012: 132931, 2012. doi: 10.1155/2012/132931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drake JC, Bruns DR, Peelor FF III, Biela LM, Miller RA, Miller BF, Hamilton KL. Long-lived Snell dwarf mice display increased proteostatic mechanisms that are not dependent on decreased mTORC1 activity. Aging Cell 14: 474–482, 2015. doi: 10.1111/acel.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drake JC, Peelor FF III, Biela LM, Watkins MK, Miller RA, Hamilton KL, Miller BF. Assessment of mitochondrial biogenesis and mTORC1 signaling during chronic rapamycin feeding in male and female mice. J Gerontol A Biol Sci Med Sci 68: 1493–1501, 2013. doi: 10.1093/gerona/glt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drake JC, Yan Z. Mitophagy in maintaining skeletal muscle mitochondrial proteostasis and metabolic health with ageing. J Physiol 595: 6391–6399, 2017. doi: 10.1113/JP274337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eggler AL, Gay KA, Mesecar AD. Molecular mechanisms of natural products in chemoprevention: induction of cytoprotective enzymes by Nrf2. Mol Nutr Food Res 52, Suppl 1: S84–S94, 2008. doi: 10.1002/mnfr.200700249. [DOI] [PubMed] [Google Scholar]
- 13.Estrada AL, Hudson WM, Kim PY, Stewart CM, Peelor FF, Wei Y, Wang D, Hamilton KL, Miller BF, Pagliassotti MJ. Short-term changes in diet composition do not affect in vivo hepatic protein synthesis in rats. Am J Physiol Endocrinol Metab 314: E241–E250, 2018. doi: 10.1152/ajpendo.00209.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Viña J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr 87: 142–149, 2008. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 15.Grebien F, Dolznig H, Beug H, Müllner EW. Cell size control: new evidence for a general mechanism. Cell Cycle 4: 418–421, 2005. doi: 10.4161/cc.4.3.1523. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton KL, Miller BF. Mitochondrial proteostasis as a shared characteristic of slowed aging: the importance of considering cell proliferation. J Physiol 595: 6401–6407, 2017. doi: 10.1113/JP274335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higashida K, Kim SH, Higuchi M, Holloszy JO, Han DH. Normal adaptations to exercise despite protection against oxidative stress. Am J Physiol Endocrinol Metab 301: E779–E784, 2011. doi: 10.1152/ajpendo.00655.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med 32: 234–246, 2011. doi: 10.1016/j.mam.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Kang C, O’Moore KM, Dickman JR, Ji LL. Exercise activation of muscle peroxisome proliferator-activated receptor-γ coactivator-1α signaling is redox sensitive. Free Radic Biol Med 47: 1394–1400, 2009. doi: 10.1016/j.freeradbiomed.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Karunadharma PP, Basisty N, Dai D-F, Chiao YA, Quarles EK, Hsieh EJ, Crispin D, Bielas JH, Ericson NG, Beyer RP, MacKay VL, MacCoss MJ, Rabinovitch PS. Subacute calorie restriction and rapamycin discordantly alter mouse liver proteome homeostasis and reverse aging effects. Aging Cell 14: 547–557, 2015. doi: 10.1111/acel.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JC, Park GD, Kim SH. Inhibition of oxidative stress by antioxidant supplementation does not limit muscle mitochondrial biogenesis or endurance capacity in rats. J Nutr Sci Vitaminol (Tokyo) 63: 277–283, 2017. doi: 10.3177/jnsv.63.277. [DOI] [PubMed] [Google Scholar]
- 22.Konopka AR, Castor WM, Wolff CA, Musci RV, Reid JJ, Laurin JL, Valenti ZJ, Hamilton KL, Miller BF. Skeletal muscle mitochondrial protein synthesis and respiration in response to the energetic stress of an ultra-endurance race. J Appl Physiol (1985) 126: 1516–1524, 2017. doi: 10.1152/japplphysiol.00457.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konopka AR, Laurin JL, Musci RV, Wolff CA, Reid JJ, Biela LM, Zhang Q, Peelor FF III, Melby CL, Hamilton KL, Miller BF. Influence of Nrf2 activators on subcellular skeletal muscle protein and DNA synthesis rates after 6 weeks of milk protein feeding in older adults. Geroscience 39: 175–186, 2017. doi: 10.1007/s11357-017-9968-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA 93: 3704–3709, 1996. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Ichikawa T, Janicki JS, Cui T. Targeting the Nrf2 pathway against cardiovascular disease. Expert Opin Ther Targets 13: 785–794, 2009. doi: 10.1517/14728220903025762. [DOI] [PubMed] [Google Scholar]
- 26.Lionaki E, Tavernarakis N. Oxidative stress and mitochondrial protein quality control in aging. J Proteomics 92: 181–194, 2013. doi: 10.1016/j.jprot.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 27.Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, De Galarreta CMR, Cuadrado A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J Biol Chem 279: 8919–8929, 2004. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- 28.Merry TL, Ristow M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice. J Physiol 594: 5195–5207, 2016. doi: 10.1113/JP271957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller BF, Drake JC, Naylor B, Price JC, Hamilton KL. The measurement of protein synthesis for assessing proteostasis in studies of slowed aging. Ageing Res Rev 18: 106–111, 2014. doi: 10.1016/j.arr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller BF, Hamilton KL. A perspective on the determination of mitochondrial biogenesis. Am J Physiol Endocrinol Metab 302: E496–E499, 2012. doi: 10.1152/ajpendo.00578.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller BF, Konopka AR, Hamilton KL. The rigorous study of exercise adaptations: why mRNA might not be enough. J Appl Physiol (1985) 121: 594–596, 2016. doi: 10.1152/japplphysiol.00137.2016. [DOI] [PubMed] [Google Scholar]
- 32.Miller BF, Robinson MM, Bruss MD, Hellerstein M, Hamilton KL. A comprehensive assessment of mitochondrial protein synthesis and cellular proliferation with age and caloric restriction. Aging Cell 11: 150–161, 2012. doi: 10.1111/j.1474-9726.2011.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller BF, Robinson MM, Reuland DJ, Drake JC, Peelor FF III, Bruss MD, Hellerstein MK, Hamilton KL. Calorie restriction does not increase short-term or long-term protein synthesis. J Gerontol A Biol Sci Med Sci 68: 530–538, 2013. doi: 10.1093/gerona/gls219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller BF, Wolff CA, Peelor FF III, Shipman PD, Hamilton KL. Modeling the contribution of individual proteins to mixed skeletal muscle protein synthetic rates over increasing periods of label incorporation. J Appl Physiol (1985) 118: 655–661, 2015. doi: 10.1152/japplphysiol.00987.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison D, Hughes J, Della Gatta PA, Mason S, Lamon S, Russell AP, Wadley GD. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic Biol Med 89: 852–862, 2015. doi: 10.1016/j.freeradbiomed.2015.10.412. [DOI] [PubMed] [Google Scholar]
- 36.Musci RV, Hamilton KL, Miller BF. Targeting mitochondrial function and proteostasis to mitigate dynapenia. Eur J Appl Physiol 118: 1–9, 2018. doi: 10.1007/s00421-017-3730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nikolaidis MG, Kerksick CM, Lamprecht M, McAnulty SR. Does vitamin C and E supplementation impair the favorable adaptations of regular exercise? Oxid Med Cell Longev 2012: 707941, 2012. doi: 10.1155/2012/707941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paschalis V, Theodorou AA, Kyparos A, Dipla K, Zafeiridis A, Panayiotou G, Vrabas IS, Nikolaidis MG. Low vitamin C values are linked with decreased physical performance and increased oxidative stress: reversal by vitamin C supplementation. Eur J Nutr 55: 45–53, 2016. doi: 10.1007/s00394-014-0821-x. [DOI] [PubMed] [Google Scholar]
- 39.Paschalis V, Theodorou AA, Margaritelis NV, Kyparos A, Nikolaidis MG. N-acetylcysteine supplementation increases exercise performance and reduces oxidative stress only in individuals with low levels of glutathione. Free Radic Biol Med 115: 288–297, 2018. doi: 10.1016/j.freeradbiomed.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 40.Pattwell DM, McArdle A, Morgan JE, Patridge TA, Jackson MJ. Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radic Biol Med 37: 1064–1072, 2004. doi: 10.1016/j.freeradbiomed.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 41.Paulsen G, Cumming KT, Holden G, Hallén J, Rønnestad BR, Sveen O, Skaug A, Paur I, Bastani NE, Østgaard HN, Buer C, Midttun M, Freuchen F, Wiig H, Ulseth ET, Garthe I, Blomhoff R, Benestad HB, Raastad T. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: a double-blind, randomised, controlled trial. J Physiol 592: 1887–1901, 2014. doi: 10.1113/jphysiol.2013.267419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res 103: 1232–1240, 2008. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pikosky MA, Gaine PC, Martin WF, Grabarz KC, Ferrando AA, Wolfe RR, Rodriguez NR. Aerobic exercise training increases skeletal muscle protein turnover in healthy adults at rest. J Nutr 136: 379–383, 2006. doi: 10.1093/jn/136.2.379. [DOI] [PubMed] [Google Scholar]
- 44.Powers SK, Duarte J, Kavazis AN, Talbert EE. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol 95: 1–9, 2010. doi: 10.1113/expphysiol.2009.050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price JC, Khambatta CF, Li KW, Bruss MD, Shankaran M, Dalidd M, Floreani NA, Roberts LS, Turner SM, Holmes WE, Hellerstein MK. The effect of long term calorie restriction on in vivo hepatic proteostatis: a novel combination of dynamic and quantitative proteomics. Mol Cell Proteomics 11: 1801–1814, 2012. doi: 10.1074/mcp.M112.021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reuland DJ, Khademi S, Castle CJ, Irwin DC, McCord JM, Miller BF, Hamilton KL. Upregulation of phase II enzymes through phytochemical activation of Nrf2 protects cardiomyocytes against oxidant stress. Free Radic Biol Med 56: 102–111, 2013. doi: 10.1016/j.freeradbiomed.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 47.Robinson MM, Richards JC, Hickey MS, Moore DR, Phillips SM, Bell C, Miller BF. Acute β-adrenergic stimulation does not alter mitochondrial protein synthesis or markers of mitochondrial biogenesis in adult men. Am J Physiol Regul Integr Comp Physiol 298: R25–R33, 2010. doi: 10.1152/ajpregu.00524.2009. [DOI] [PubMed] [Google Scholar]
- 48.Robinson MM, Turner SM, Hellerstein MK, Hamilton KL, Miller BF. Long-term synthesis rates of skeletal muscle DNA and protein are higher during aerobic training in older humans than in sedentary young subjects but are not altered by protein supplementation. FASEB J 25: 3240–3249, 2011. doi: 10.1096/fj.11-186437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shill DD, Southern WM, Willingham TB, Lansford KA, McCully KK, Jenkins NT. Mitochondria-specific antioxidant supplementation does not influence endurance exercise training-induced adaptations in circulating angiogenic cells, skeletal muscle oxidative capacity or maximal oxygen uptake. J Physiol 594: 7005–7014, 2016. doi: 10.1113/JP272491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silveira LR, Pereira-Da-Silva L, Juel C, Hellsten Y. Formation of hydrogen peroxide and nitric oxide in rat skeletal muscle cells during contractions. Free Radic Biol Med 35: 455–464, 2003. doi: 10.1016/S0891-5849(03)00271-5. [DOI] [PubMed] [Google Scholar]
- 51.Silveira LR, Pilegaard H, Kusuhara K, Curi R, Hellsten Y. The contraction induced increase in gene expression of peroxisome proliferator-activated receptor (PPAR)-γ coactivator 1α (PGC-1α), mitochondrial uncoupling protein 3 (UCP3) and hexokinase II (HKII) in primary rat skeletal muscle cells is dependent on reactive oxygen species. Biochim Biophys Acta 1763: 969–976, 2006. doi: 10.1016/j.bbamcr.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 52.Sin C, Chiarugi D, Valleriani A. Degradation parameters from pulse-chase experiments. PLoS One 11: e0155028, 2016. doi: 10.1371/journal.pone.0155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strobel NA, Peake JM, Matsumoto A, Marsh SA, Coombes JS, Wadley GD. Antioxidant supplementation reduces skeletal muscle mitochondrial biogenesis. Med Sci Sports Exerc 43: 1017–1024, 2011. doi: 10.1249/MSS.0b013e318203afa3. [DOI] [PubMed] [Google Scholar]
- 54.Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, Fernandez E, Flurkey K, Hamilton KL, Lamming DW, Javors MA, de Magalhães JP, Martinez PA, McCord JM, Miller BF, Müller M, Nelson JF, Ndukum J, Rainger GE, Richardson A, Sabatini DM, Salmon AB, Simpkins JW, Steegenga WT, Nadon NL, Harrison DE. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 15: 872–884, 2016. doi: 10.1111/acel.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Surh Y-J, Kundu JK, Na H-K. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med 74: 1526–1539, 2008. doi: 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- 56.Traber MG. Vitamin E inadequacy in humans: causes and consequences. Adv Nutr 5: 503–514, 2014. doi: 10.3945/an.114.006254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wadley GD, McConell GK. High-dose antioxidant vitamin C supplementation does not prevent acute exercise-induced increases in markers of skeletal muscle mitochondrial biogenesis in rats. J Appl Physiol (1985) 108: 1719–1726, 2010. doi: 10.1152/japplphysiol.00127.2010. [DOI] [PubMed] [Google Scholar]
- 58.Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 586: 3701–3717, 2008. doi: 10.1113/jphysiol.2008.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yfanti C, Akerström T, Nielsen S, Nielsen AR, Mounier R, Mortensen OH, Lykkesfeldt J, Rose AJ, Fischer CP, Pedersen BK. Antioxidant supplementation does not alter endurance training adaptation. Med Sci Sports Exerc 42: 1388–1395, 2010. doi: 10.1249/MSS.0b013e3181cd76be. [DOI] [PubMed] [Google Scholar]