Abstract
The aim of this study was to examine the independent contributions of joint range of motion (ROM), muscle fascicle length (MFL), and joint angular velocity on mechanoreceptor-mediated central cardiovascular dynamics using passive leg movement (PLM) in humans. Twelve healthy men (age: 23 ± 2 yr, body mass index: 23.7 kg/m2) performed continuous PLM at various randomized joint angle ROMs (0°–50° vs. 50°–100° vs. 0°–100°) and joint angular velocities (“fast”: 200°/s vs. “slow”: 100°/s). Measures of heart rate (HR), cardiac output (CO), and mean arterial pressure (MAP) were recorded during baseline and during 60 s of PLM. MFL was calculated from muscle architectural measurements of fascicle pennation angle and tissue thickness (Doppler ultrasound). Percent change in MFL increased across the transition of PLM from 0° to 50° (15 ± 3%; P < 0.05) and from 0° to 100° knee flexion (27 ± 4%; P < 0.05). The average peak percent change in HR (increased, approx. +5 ± 2%; P < 0.05), CO (increased, approx. +5 ± 3%; P < 0.05), and MAP (decreased, approx. −2 ± 2%; P < 0.05) were similar between fast versus slow angular velocities when compared against shorter absolute joint ROMs (i.e., 0°–50° and 50°–100°). However, the condition that exhibited the greatest angular velocity in combination with ROM (0°–100° at 200°/s) elicited the greatest increases in HR (+13 ± 2%; P < 0.05) and CO (+12 ± 2%; P < 0.05) compared with all conditions. Additionally, there was a significant relationship between MFL and HR within 0°–100° at 200°/s condition (r2 = 0.59; P < 0.05). These findings suggest that increasing MFL and joint ROM in combination with increased angular velocity via PLM are important components that activate mechanoreflex-mediated cardioacceleration and increased CO.
NEW & NOTEWORTHY The mechanoreflex is an important autonomic feedback mechanism that serves to optimize skeletal muscle perfusion during exercise. The present study sought to explore the mechanistic contributions that initiate the mechanoreflex using passive leg movement (PLM). The novel findings show that progressively increasing joint angle range of motion and muscle fascicle length via PLM, in combination with increased angular velocity, are important components that activate mechanoreflex-mediated cardioacceleration and increase cardiac output in humans.
Keywords: angular velocity, hemodynamics, fascicle length, mechanoreflex, passive leg movement
INTRODUCTION
During exercise, there are three primary mechanisms mediated by the autonomic nervous system that are instrumental at maintaining and increasing skeletal muscle perfusion to meet metabolic demand of the working muscle: central command, the arterial baroreflex, and the exercise pressor reflex (EPR) (25, 29). Of particular interest is the EPR, which is a feedback mechanism resulting from stimulation of both thinly myelinated (group III; mechanical) and unmyelinated (group IV; metabolic) nerve fibers located within skeletal muscle that project to various sites within the brainstem to evoke increases in efferent sympathetic nervous activity, which serve to increase blood pressure (BP) and heart rate (HR) (1, 36, 41). These autonomic central cardiovascular responses that represent the EPR are the muscle mechanoreflex and metaboreflex, respectively (36, 41).
For many years, the EPR and contributions of the metaboreflex and its mechanisms on cardiovascular affects have been extensively studied in animals and humans; however, little evidence to date has examined the influence of the mechanoreflex and associated cardiovascular contributions in humans (for a synopsis of the available evidence the reader is referred to reviews (4, 7, 9, 11, 28). This is because of the relative difficulty to separate metaboreflex contributions from the mechanoreflex noninvasively in humans. Recently, isolated muscle mechanoreflex activation of type III muscle afferents has been studied in humans using noninvasive experimental reductionist models, either via static stretching (6, 10, 13–15, 20) or dynamic passive leg movement (PLM; 17, 18, 26, 31, 37, 42), as it is believed that these modalities result in negligible muscle activity (16, 35) and therefore, muscle metabolic perturbations. More specifically, PLM, whereby the leg is passively moved through dynamic and cyclical ranges of motion (ROM; typically, 0° extension 90° flexion), has gained considerable recognition as a new experimental paradigm to isolate central command and the contribution from type III (mechanoreflex) and type IV (metaboreflex) afferents in mediating cardioacceleration (i.e., transient rise in HR) and cardiac output (CO) in humans (12). Indeed, cardioacceleration with PLM is abolished with intrathecal administration of the μ-opioid agonist fentanyl, thus highlighting an important role of mechanosensitive afferents in facilitating the hemodynamic response to PLM exercise in humans (37).
Although ample evidence has shown that the mechanoreflex is an important mediator to cardiovascular regulation during exercise in humans (7, 9, 11), the basic understanding regarding the extent to which mechanoreflex activation modulates cardiovascular responses in humans remain relatively unknown. As such, one hypothesis states that the mechanical tension associated with muscle lengthening/stretching of the musculature activates the mechanoreflex and induces cardioacceleration (9) and that a critical stretch/tension threshold is required to initiate this response (19, 20). In addition, very slow PLM (1°/sec via 90° ROM), which does not excessively stretch the musculature produces no cardioacceleration (27), whereas a faster movement velocity of 1 Hz (via 90° ROM) elicits robust cardioacceleration (18, 26). Collectively, these findings raise an intriguing hypothesis: that movement (angular) velocity independent of, or in combination to, muscle lengthening plays a fundamental role in how mechanoreceptors are stimulated. Nevertheless, no evidence to date, to our knowledge, has explicitly tested whether factors of angular velocity and muscle length coexist or act independently of one another to initiate mechanoreflex-mediated alterations in central hemodynamics in humans.
PLM is widely used in clinical and rehabilitation settings, and at-risk individuals make up a large portion of this population. However, at-risk individuals exhibit a heightened EPR, putting undue strain on the heart and resulting in inadequate tissue perfusion (40). Given the limited mechanistic information related to the mechanoreflex, and because of recent studies highlighting PLM as a simple noninvasive model to assess vascular function (12), it would be particularly useful that a comprehensive evaluation be accomplished regarding different joint angular velocities in concert to changing joint ROM, which may help bolster mechanistic knowledge within both the research and clinical setting.
The aim of the present study was to examine the independent contribution between joint angle ROM, muscle fascicle length (MFL), and angular velocity on mechanoreflex-mediated central cardiovascular dynamics during PLM in young healthy men. We tested the hypothesis that progressively increasing joint ROM and/or MFL will elicit a greater mechanoreflex-driven increase in central hemodynamics (i.e., HR and CO). We further hypothesized that, for a given joint angle of ROM, the condition(s) that exhibited the greatest increase in movement velocity, would result in the greatest mechanoreflex-driven increase in HR and CO.
METHODS
Subjects
Twelve young recreationally active men participated in the study (age: 23 ± 2 yr, body mass index: 23.7 kg/m2). All subjects were normotensive, nonsmokers, free from any form of cardiovascular, metabolic, orthopedic, or musculoskeletal disease, and none were currently on any medication, as determined by a medical health questionnaire and history form. Studies were performed in the morning after an overnight fast, and subjects refrained from exercise, alcohol, and caffeine for at least 24 h before reporting to the laboratory. After receiving a complete description of the protocol and potential risks, written informed consent was obtained from each participant on a form approved by the Institutional Review Board committee at the University of Iowa.
Experimental Overview
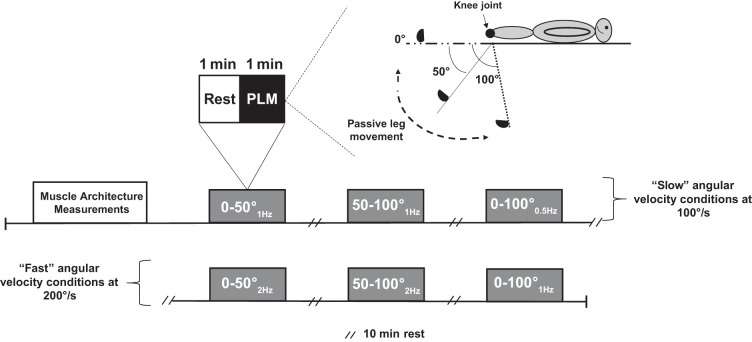
Figure 1 is an overview of the experimental testing session within the study. Subjects were studied while in the supine position. The supine position was selected (as opposed to up-right seated position), which was based on the premise that those individuals lying down would be in a more relaxed position, thus limiting the potential for excessive movements and muscle contractions from confounding hemodynamics during PLM. The protocol was a randomized-counterbalanced design, which consisted of 60 s of resting baseline followed by 60 s of dynamic PLM with at least 10 min of recovery between each trial (Fig. 1). The right leg of each subject was always returned back to baseline (0°) after each trial. Prior to PLM, a measurement of each subject’s vastus lateralis muscle architecture properties were measured at 0° (baseline), 50°, and 100° of static knee flexion. Continuous PLM was then performed through three randomized joint angle ROMs (0°–50° vs. 50°–100° vs. 0°–100°) of knee flexion-extension and two randomized joint angular velocities of either 200°/s (at 2 or 1 Hz) or 100°/s (at 1 or 0.5 Hz; Fig. 1). Central hemodynamic measures of HR, CO, and mean arterial pressure (MAP) were continuously recorded during baseline and during each PLM sequence.
Fig. 1.
Overview of the experimental design representing “slow” (100°/s) and “fast” (200°/s) passive leg movement (PLM) conditions using three different joint angles of range of motion: 1) 0°–50°, 2) 50°–100°, and 3) 0°–100°. The design was randomized and counterbalanced.
Passive Leg Movement Protocol
Prior to PLM trials, the subject’s right leg was equipped with a knee brace (Bledsoe G3 Extender Plus Knee Brace), which prevented mediolateral movement during PLM and to allow movement precision by a self-adjustable hinge that locked each joint angle into place. Subsequently, a practice trial was accomplished to familiarize the subject to the protocol. In the 60 s of baseline before PLM, the leg was supported by a member of the research team for each given joint angle. Continuous PLM consisted of dynamic, back and forth movements (beginning from full extension ~0° knee joint angle) moving the subject’s right lower leg through three randomized joint angles of ROM (0–50° vs. 50–100° vs. 0–100°) at two randomized joint angular velocities of either 200°/s (fast) or 100°/s (slow) (Fig. 1). During this time, a metronome was used to ensure a consistent ROM and maintain the cadence, which was manually performed by a well-trained member of the research team. During PLM, subjects were instructed not to take any aberrant breaths and to maintain their usual normal resting breathing rhythm. Furthermore, to avoid a startle reflex and to ensure no muscle contraction occurred to the passive movement as well as an anticipatory response, subjects were not informed exactly when PLM would be initiated but were made aware at a random time point within a ~30 s window, before the onset of PLM, which is in accordance with recently published evidence (12, 38).
To address specifically whether increasing joint ROM influences mechanoreflex mediated changes in central hemodynamics we employed PLM of the leg at a 0°–50°, 50–100°, or 0°–100° of knee flexion, as represented in Fig. 1. However, because angular velocity is altered using the same cadence (e.g., 1 Hz) during 0°–50°, 50–100°, and 0°–100° knee flexion/extension, we adjusted the cadence using either a joint angular velocity of 200°/s or 100°/s for a given shorter joint angle ROM (slower cadence, i.e., 1 Hz vs. faster cadence, i.e., 2 Hz) as represented in Fig. 1. Thus, by keeping angular velocity constant using the same absolute ROM, this would allow us to determine whether angular velocity independent of muscle lengthening influences central hemodynamics.
Central Hemodynamic Measurements
HR was monitored by three-lead ECG. Brachial artery pressure was measured in duplicate using an automated cuff (Cardiocap/5, Datex-Ohmeda, Louisville, CO) following 20 min of quiet rest, before beginning the experimental testing session. If BP measurements deviated ± 5 mmHg, an additional measurement(s) was taken to meet this criterion. During each PLM experimental sequence, systemic arterial BP was measured noninvasively on a beat-by-beat basis using finger plethsymography (Nexfin, Edward Lifesciences, Irvine, CA) via a finger cuff capable of measuring volume/pressure changes, placed around the middle phalanx of the third finger on the left hand.
Measurements of Muscle Architecture and Fascicle Excursion
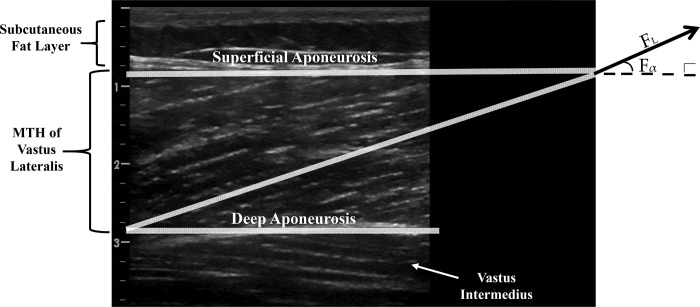
To quantify the magnitude change of MFL for a given absolute joint ROM (0°–50° vs. 0°–100°) a linear array ultrasound probe set in B-mode was used to record sagittal images of the vastus lateralis muscle. The ultrasound transducer was positioned perpendicular to the dermal surface of the vastus lateralis muscle measured midway between the insertions of the lateral condyle of the tibia and the greater trochanter and was configured longitudinally to show the fascicle arrangement from the superficial to the deep aponeurosis (20, 32), as shown in Fig. 2. The probe was coated with a copious amount of water-soluble transmission gel to provide acoustic contact without depressing the dermal surface.
Fig. 2.
Ultrasound assessment of the vastus lateralis muscle fascicle arrangement. On the left muscle tissue thickness (MTH) can be measured as the distance between superficial and deep aponeurosis. On the right, extrapolation technique is used to extend the highlighted fascicle off the ultrasound image to intersect with the superficial aponeurosis. This technique is used to determine fascicle length (FL) and fascicle pennation angle (Fα).
All offline processing and image scans of muscle architecture properties were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD). The following muscle architecture parameters were extracted from the ultrasound images: 1) muscle thickness (mm): perpendicular distance between deep and superficial aponeuroses at midbelly, with a 90° angle from the deep aponeurosis; and 2) fascicle pennation angle (degrees): angle between fascicular and deep aponeurosis direction proximal to the muscle-tendinous junction (Fig. 2). Subsequently, MFL (mm) was measured as the linear distance between the insertions of the fascicle into the lower and upper aponeuroses using the following trigonometric equation (21): MFL (mm) = muscle tissue thickness (mm) × sin [fascicle angle (mm)]−1.
Data analysis and Statistics
Data acquisition.
Throughout the protocol, signals reflecting HR, CO, and MAP underwent analog-to-digital conversion and were simultaneously acquired at 250 Hz, stored on a computer, and analyzed offline with signal processing software (WinDaq, DATAQ Instruments, Akron, OH). Accordingly, MAP (mmHg) was determined from the finger arterial pressure waveforms, and HR (beats/min) was determined from the ECG recordings. Similarly, CO (l/min) was measured using the Nexfin device using an automated pulse contour waveform analysis via a three-element Windkessel model that incorporates the influence of arterial pressure wave form and subject’s characteristics on aortic properties (8). All central hemodynamic measurements for rest (baseline) during each trial were determined by averaging values during the last 30 s before PLM. In addition, beat-by-beat central hemodynamic measurements were collected over 60 s to assure we could analyze 45 consecutive cardiac cycles for any subject that had heart rates under 60 beats/min during PLM. Prior to analysis, hemodynamic data were smoothed using a 3-s rolling average. Given that central hemodynamic responses to PLM are transient and vary between individuals (18, 26, 37, 39), a peak response (confirmed by at least three consecutive cardiac cycles) was determined for all variables on an individual basis. We further chose this method, because baseline HR was similar among the conditions (~61 beats/min), and the cardiodynamic response to PLM is transient, peaking within the first few seconds of PLM before returning back to baseline before 45 s. Peak relative percentage change (%Δ) from baseline was used for statistical analysis of all central hemodynamic variables.
Statistical analysis.
All values are expressed as mean ± standard error (SE) of the mean unless stated otherwise. The main dependent variables are represented as the peak relative %Δ in HR, CO, and MAP during PLM relative to baseline. To assess whether increasing absolute ROM contributes to mechanoreflex-mediated changes in central hemodynamics, two separate one-way analyses of variance (ANOVA) with repeated measures were performed among each cluster of angular velocities, which consisted of either 1) slow (0°1Hz–50°1Hz vs. 50°1Hz–100°1Hz vs. 0°0.5Hz–100°0.5Hz), or 2) fast (0°2Hz–50°2Hz vs. 50°2Hz–100°2Hz vs. 0°1Hz –100°1Hz) conditions. Tukey’s post hoc procedures for multiple comparisons were applied for each ANOVA. To address the hypothesis whether changing angular velocity, independent of absolute ROM influences mechanoreflex-mediated changes in central hemodynamics, three separate dependent t-tests were employed, as based on the following: 1) 0°–50° conditions (2 Hz vs. 1 Hz), 2) 50°–100° conditions (2 Hz vs. 1 Hz), or 3) 0°–100° conditions (1 Hz vs. 0.5 Hz). Lastly the peak relative change in MFL (ΔMFL) was compared with the peak absolute change of cardio-acceleration (ΔHR) using a Pearson product moment correlation applied to the 0°1Hz–100°1Hz condition. All statistical analyses were performed using SigmaPlot software version 11.0 (Systat Software Inc., San Jose, CA). Statistical significance was set a priori at P < 0.05.
RESULTS
Central Hemodynamic Responses to PLM
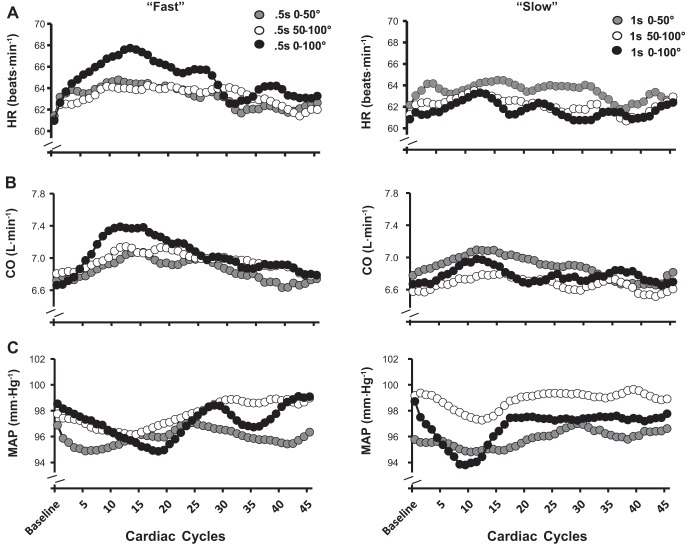
Beat-by-beat central hemodynamic responses across the transition from rest to PLM in each condition are summarized in Fig. 3. Standard error bars were left out for each value in Fig. 3 to provide clarity toward the transition of each response. In general (qualitatively), all fast and slow angular velocity conditions in central hemodynamic variables were transient, peaking within 9–13 cardiac cycles after the onset of PLM and were sustained for 3–27 cardiac cycles. Accordingly, the change (from baseline) in HR and CO were elevated and MAP reduced in each condition (Table 1; P < 0.01) and within each slow and fast 0°–100° velocity, respectively.
Fig. 3.
Average beat-by-beat heart rate (HR, A), cardiac output (CO, B), and mean arterial pressure (MAP, C) for each joint range of motion condition during passive leg movement (PLM) at 200°/s and 100°/s.
Table 1.
Baseline and passive leg movement hemodynamics
| 200°/s |
100°/s |
|||
|---|---|---|---|---|
| Baseline | Peak | Baseline | Peak | |
| HR | ||||
| 0°–50° | 61 ± 3 | 65 ± 3* | 61 ± 3 | 65 ± 3* |
| 50°–100° | 61 ± 4 | 64 ± 3* | 61 ± 4 | 64 ± 3* |
| 0°–100° | 61 ± 3 | 68 ± 3*† | 61 ± 3 | 68 ± 3*† |
| CO | ||||
| 0°–50° | 6.7 ± 0.4 | 7.1 ± 0.3* | 6.7 ± 0.4 | 7.1 ± 0.3* |
| 50°–100° | 6.6 ± 0.3 | 6.9 ± 0.3* | 6.6 ± 0.3 | 6.9 ± 0.3* |
| 0°–100° | 6.7 ± 0.3 | 7.4 ± 0.4*† | 6.7 ± 0.3 | 7.4 ± 0.4*† |
| MAP | ||||
| 0°–50° | 97 ± 3 | 95 ± 3* | 97 ± 3 | 95 ± 3* |
| 50°–100° | 98 ± 3 | 96 ± 2* | 98 ± 3 | 96 ± 2* |
| 0°–100° | 99 ± 3 | 94 ± 2* | 99 ± 3 | 94 ± 2* |
Data are means ± SE. Baseline and peak absolute values in HR, CO, and MAP among fast and slow conditions. CO, cardiac output; fast condition, 200°/s; HR, heart rate; MAP, mean arterial pressure; slow condition, 100°/s.
Significantly different (P < 0.05) compared with baseline.
Significantly different (P < 0.05) than 0°–50° and 50°–100° within 200°/s velocity.
Central hemodynamic responses, expressed as the peak %∆ across each condition are summarized in Figs. 4, 5, and 6. Specifically, one-way ANOVA within slow angular velocities (0°1Hz–50°1Hz vs. 50°1Hz–100°1Hz vs. 0°0.5Hz–100°0.5Hz) were similar for HR and CO, whereas MAP was significantly reduced (P < 0.05). However, one-way ANOVA within fast (0°2Hz–50°2Hz vs. 50°2Hz–100°2Hz vs. 0°1Hz–100°1Hz) velocities revealed that 0°1Hz–100°1Hz elicited a significantly greater increase in HR and CO and decrease in MAP compared with both 0°2Hz–50°2Hz and 50°2Hz–100°2Hz conditions.
Fig. 4.
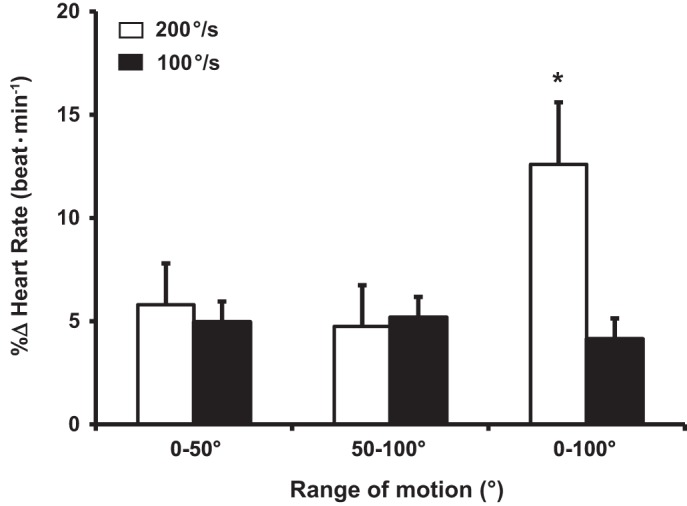
Percent change (%Δ) of peak heart rate during passive leg movement (PLM) at 200°/s and 100°/s. *Significantly different (P < 0.05) within conditions (0°–100° at 100°/s) and between 0°–50° and 50°–100° conditions. Data are means ± SE
Fig. 5.
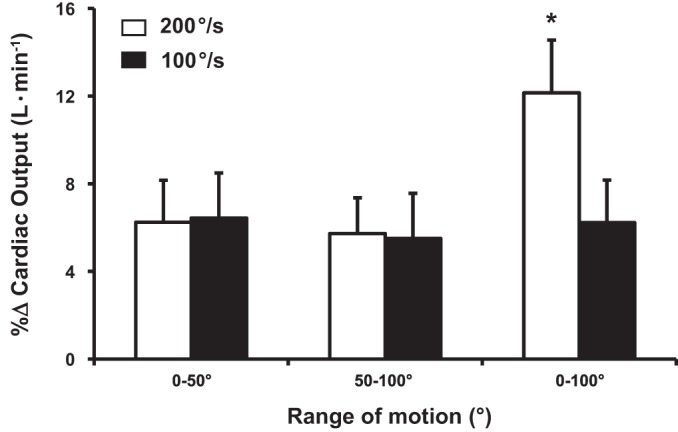
Percent change (%Δ) of peak cardiac output during passive leg movement (PLM) at 200°/s and 100°/s. *Significantly different (P < 0.05) within conditions (00.5Hz–1000.5Hz) and between 0°–50° and 50–100° conditions. Data are means ± SE
Fig. 6.
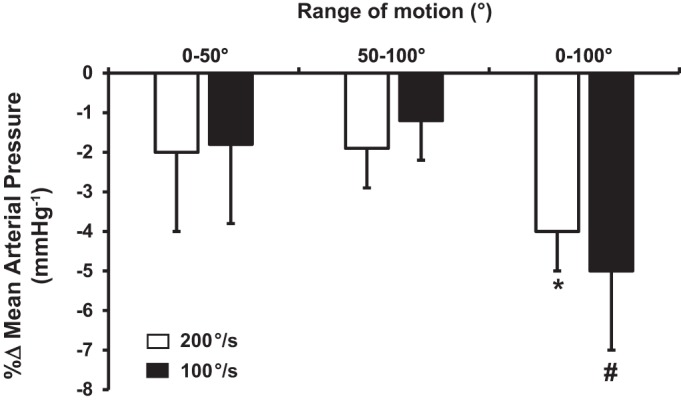
Percent change (%Δ) of mean arterial pressure during passive leg movement (PLM) at 200°/s and 100°/s. *Significantly different (P < 0.05) than 0°–50° and 50°–100° joint range of motion conditions within 100°/s. #Significantly different (P < 0.05) than 0°–50° and 50°–100° joint range of motion conditions within 200°/s. Data are means ± SE
Peak %∆ in HR and CO increased (~5 ± 2%; P < 0.05 and 5% ± 3%; P < 0.05) within fast and slow velocities during 0°–50° and 50–100° joint ROM conditions. Peak %∆ in MAP decreased (~2 ± 2%; P < 0.05) within fast and slow velocities during 0°–50° and 50°–100° joint ROM. When the %∆ in HR, CO, and MAP responses were compared between fast and slow angular velocities (t-tests) relative to their respective joint ROM (Figs. 4, 5 and 6), no changes in any central hemodynamic parameters were detected within 0°–50° and 50°–100° conditions. However, 0°1Hz–100°1Hz elicited a greater (P < 0.05) HR and CO response relative to its slow angular velocity counterpart (i.e., 0°0.5Hz–100°0.5Hz). There was no difference in MAP between 0°1Hz and 100°1Hz vs. 0°0.5Hz–100°0.5Hz, respectively.
Relationship between MFL and HR
MFL (%ΔMFL) increased ~15% across the transition of PLM from 0° to 50° (8.8 ± 2.3 vs. 10.2 ± 3.8 mm; P < 0.05) and ~27% from 0° to 100° knee flexion (8.8 ± 2.3 to 12.3 ± 4.1; P < 0.05). As an exploratory analysis, and because 01Hz–1001Hz condition elicited the greatest change in HR relative to other PLM conditions, we examined the relationship between %ΔMFL and %ΔHR using the Pearson correlation (Fig. 7). Accordingly, Pearson linear regression analyses of the ΔMFL and ΔHR responses for 01Hz–1001Hz condition revealed a moderate significant relationship (y = 1.8266x−4.411, r2 = 0.59; P < 0.05; Fig. 7).
Fig. 7.
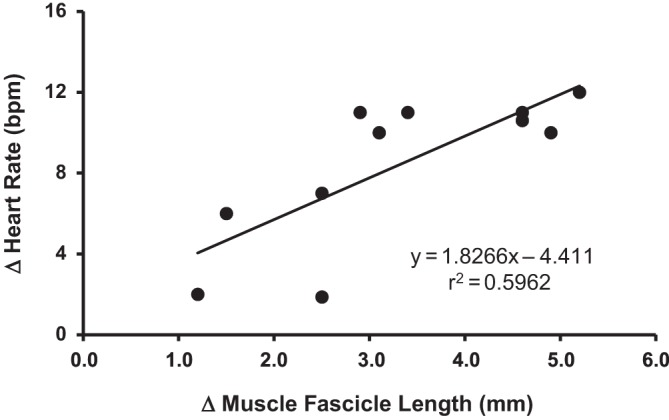
Relationship between the absolute change in (Δ) muscle fascicle length and the absolute Δ heart rate (HR) during passive leg movement (PLM) from 0°–100° during fast (200°/s) angular velocity passive leg movement trial.
DISCUSSION
This is the first study, to our knowledge, to address two important components involved with the mechanoreflex that have been hypothesized but never directly studied in humans. Utilizing the PLM model, we manipulated knee joint angles of ROM concomitant to different angular velocities to test the hypothesis of whether changing joint ROM independent of or in combination with angular velocity activates type III afferents and initiates a mechanoreflex-driven alteration in central hemodynamics in humans. Accordingly, the major novel finding of the study show that fast angular velocities elicit a similar HR and CO response compared with slow angular velocities for a given shorter absolute joint ROM (0°–50° and 50°–100°). However, the condition with the greatest increase in angular velocity in combination to absolute joint ROM (i.e., 0°1Hz–100°1Hz) elicited the greatest cardioacceleration and CO (Figs. 3 and 4). Furthermore, there was a significant relationship between muscle fascicle excursion and HR within the 0°1Hz–100°1Hz condition (Fig. 7). Collectively, our findings suggest that progressively increasing MFL and absolute joint ROM in combination with increased angular velocity via PLM are important mediators that stimulate the type III afferents and activate mechanoreflex mediated cardioacceleration and increase CO in young, healthy men.
Influence of Movement (Angular) Velocity on Mechanoreflex-Mediated Changes in Central Hemodynamics
Our basic understanding of the mechanoreflex activation on central cardiovascular responses is limited by a small amount of mechanistic evidence presented in both animals and humans (7, 9, 11). These data suggest that the mechanoreflex operates exclusively as a result of “mechanical-inducing” changes within muscles (7, 9, 11). Moreover, it has been suggested that selective activation of mechanically sensitive skeletal muscle afferents to the cardiovascular control center via passive limb movement or passive stretching elicits a brief increase in muscle sympathetic nerve activity and a longer-lasting decrease in parasympathetic activity to the heart (9, 23, 30). Recently, it has been postulated that mechanoreceptors are frequency-dependent (as opposed to stretch/tension), and activation may be increased to a greater extent during more rapid passive movement tasks (22, 39). In support of this, McDaniel et al. (27) demonstrated that slow passive movement (1°/s) of the knee extensor/flexor muscles via 90° of ROM evoked no cardioacceleration, whereas other PLM evidence using an angular velocity of 90°/s (i.e., 1 Hz) elicited robust cardioacceleration (18, 26, 37). Nonetheless, these studies only assessed mechanoreflex contributions on central hemodynamic responses to PLM using one absolute ROM in combination with one angular velocity. In this context, it remained unknown which mechanistic contributor(s), muscle lengthening or angular velocity, activates the mechanoreflex and induces a central cardiovascular response. To this end, the results of present study demonstrate that the HR and CO responses remain similar between fast versus slow angular velocities when compared against shorter absolute joint ROMs (i.e., 0°–50° and 50°–100°). Interestingly, when 0°1Hz–100°1Hz was compared with its slower counterpart (0°0.5Hz–100°0.5Hz), the faster angular velocity (0°1Hz–100°1Hz) elicited a greater HR and CO response, whereas MAP did not differ between conditions. Collectively, these findings are interpreted to suggest that the rate of movement (angular velocity) about a joint is instrumental at initiating a mechanoreflex; however, this is only when an augmented ROM (i.e., 0°–100°) during PLM is applied.
Relationship Between Muscle Fascicle Excursion and Cardioacceleration
An assumption of this study was that those individuals who were studied under the same absolute joint angle of ROM would exhibit differences in the relative degree of muscle fascicle excursion because of inherent differences in muscle architecture and/or flexibility. This in return would differentially modulate the detection thresholds of the type III afferents and subsequently, the degree of cardioacceleration. Indeed, constant angle (i.e., ROM) stretching elicits a different spatial HR response compared with when a constant torque (varying ROM) is applied in humans, suggesting that a critical stretch tension may be needed to either initiate a mechanoreflex mediated-cardioacceleration or maintain it (20). Therefore, we speculated that individuals who exhibit different degrees of flexibility may not result in the same mechanoreflex mediated cardioacceleration. To circumvent this issue, we measured muscle architecture properties and calculated MFL throughout 0°–100° ROM to determine the relationship between MFL and HR. Accordingly, the present study demonstrated a moderate significant relationship between MFL and HR. That is, individuals who exhibit a greater level of muscle fascicle excursion through 100° of PLM appear to exhibit the greatest cardioacceleration, and vice versa. Collectively, our findings are interpreted to suggest that the relative proportional length change of the muscle fascicles that operate about that joint (33, 34), are influential at initiating mechanoreflex mediated cardioacceleration during PLM in humans.
Experimental Considerations to PLM-Induced Modulation of the Mechanoreflex
Our findings are not without a few experimental considerations. First, the study was conducted in young, healthy men and therefore, it remains unknown whether women, older adults, and/or patient populations with certain pathologies (congestive heart failure, chronic kidney disease, hypertension, etc.) exhibit different cardiovascular responses to changes in MFL and/or movement velocity via PLM. In addition, our findings can only be interpreted in the context of our experimental design. Therefore, it is possible that a higher degree of absolute ROM and/or relative degree of MFL beyond the 100° flexion employed by the present study, regardless of angular velocity, elicits a mechanoreflex.
It is also important to recognize the potential for other factors that may have confounded the interpretation of our results. For example, the cardioacceleration associated with PLM during the 01Hz–1001Hz condition is suggested to be attributed to a baroreflex, as previous evidence has demonstrated a robust hyperemic response to PLM using a similar angular velocity as the present study (3, 26, 37, 39). Furthermore, it may be speculated that faster angular velocities trigger a Golgi tendon or muscle spindle reflex, contributing to the cardioacceleration of the present study. However, it has been shown that type Ia and Ib as well as type II afferents do not contribute significantly to cardiovascular adjustments during rapid movements in humans (24, 25). In addition, intrathecal administration of the μ-opioid agonist, fentanyl, shows no cardioacceleration during PLM (37). Lastly, when the upper thigh is occluded during PLM in humans to prevent a hyperemic response, the level of cardioacceleration remains similar compared with when the same leg is nonoccluded (26). Collectively, these studies clearly highlight an exclusive and important role of mechanosensitive afferents in facilitating HR and CO responses during PLM in humans. Therefore, the findings of the present study provide important mechanistic evidence to suggest that, apart from a critical stretch/tension, movement velocity in combination with a sufficient relative degree of muscle lengthening (stretch/tension stimulus) via PLM are primary requisites, which appear to result in optimal activation of the mechanoreflex and consequently, cardioacceleration in humans.
Perspectives and Significance
As highlighted in a recent review (12), PLM has been identified as a simple, noninvasive, and inexpensive method to assess the mechanoreflex and vascular function in young and older humans. The present study has identified select mechanistic contributions by which the mechanoreflex mediates cardiovascular responses during PLM in humans. Characterizing the central cardiovascular response to mechanoreflex activation via PLM using different angular velocities and joint ROM is practically relevant given that dynamic activities of daily living, such as standing and walking, and during exercise (e.g., running and cycling) are performed at various joint angles of ROM and using varying angular velocities. Furthermore, although PLM has become increasingly popular, it appears that no standardized movement velocity or ROM with PLM has been adopted by the literature in which to study the mechanoreflex in humans. Although much of the literature has employed the experimental protocol using 0°–90° of knee joint ROM at 1 Hz, the premise for using such a protocol has remained uncertain. Thus, the present study has provided a comprehensive examination of the PLM model on mechanoreflex contributions to central hemodynamics by employing two specific joint angles of ROM and joint angular velocities. Therefore, our findings may be experimentally useful for future research when studying the mechanoreflex using PLM in humans.
Our finding of robust cardioacceleration in the 01Hz–1001Hz condition is in line with recent research using PLM over 90° of ROM at the same angular velocity (18, 26, 37, 38). However, our finding of no cardioacceleration within the slower, 0°0.5Hz–100°0.5Hz condition is similar to the findings of a recent study (2) employing the same angular velocity of PLM in congestive heart failure patients compared with healthy controls. Given the present study finding of no cardioacceleration within the slow PLM condition, and the well-known fact that congestive heart failure patients exhibit a heightened mechanoreflex compared with normal healthy individuals (40), it could be presumed that the reason for no cardioacceleration because of passive movement within the previous study design was related to an angular velocity being below the threshold (slow) needed to activate a mechanoreflex. This hypothesis in congestive heart failure patients warrants further study.
Conclusions
In conclusion, the data acquired from this investigation have enhanced our current understanding regarding mechanoreflex mechanisms that contribute to central hemodynamics in humans. The principle findings reveal that, during PLM, faster angular velocities in combination to the greatest absolute ROM (i.e., 0°–100°) elicit the greatest cardioacceleration and CO compared with slower angular velocities and joint angle ROMs. Furthermore, a relationship exists between MFL and HR within the condition that elicited the greatest increase in angular velocity in combination to the greatest joint ROM, suggesting that the relative proportional length change of the muscle fascicles that operate about that joint are influential at initiating a mechanoreflex. Collectively, our findings are interpreted to suggest that that the primary mechanisms by which a mechanoreflex is activated during PLM in humans is likely dependent on the extent of both the relative level of the tension developed (i.e., MFL) on the musculature in combination to an adequate (faster) movement velocity incurred about the joint.
GRANTS
This research was supported by NIH Grant T32HL-007121 (to N. Kruse).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.T.K. and D.P.C. conceived and designed research; N.T.K. and W.E.H. performed experiments; N.T.K. analyzed data; N.T.K., W.E.H., and D.P.C. interpreted results of experiments; N.T.K. prepared figures; N.T.K. drafted manuscript; N.T.K., W.E.H., and D.P.C. edited and revised manuscript; N.T.K., W.E.H., and D.P.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful for the subjects who volunteered to participate in this study. Extended appreciation goes out to Reginald Hochstedler, Erika Iwamoto, Caley Medinger, and Ryan Tillma for their valuable technical assistance during experimental data collection.
REFERENCES
- 1.Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol (1985) 82: 1811–1817, 1997. doi: 10.1152/jappl.1997.82.6.1811. [DOI] [PubMed] [Google Scholar]
- 2.Antunes-Correa LM, Nobre TS, Groehs RV, Alves MJ, Fernandes T, Couto GK, Rondon MU, Oliveira P, Lima M, Mathias W, Brum PC, Mady C, Almeida DR, Rossoni LV, Oliveira EM, Middlekauff HR, Negrao CE. Molecular basis for the improvement in muscle metaboreflex and mechanoreflex control in exercise-trained humans with chronic heart failure. Am J Physiol Heart Circ Physiol 307: H1655–H1666, 2014. doi: 10.1152/ajpheart.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns KJ, Pollock BS, McDaniel J. The cardiovascular response to passive movement is joint dependent. Physiol Rep 4: e12721, 2016. doi: 10.14814/phy2.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crisafulli A, Marongiu E, Ogoh S. Cardiovascular reflexes activity and their interaction during exercise. BioMed Res Int 2015: 394183, 2015. doi: 10.1155/2015/394183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drew RC, Blaha CA, Herr MD, Cui R, Sinoway LI. Muscle mechanoreflex activation via passive calf stretch causes renal vasoconstriction in healthy humans. Am J Physiol Regul Integr Comp Physiol 312: R956–R964, 2017. doi: 10.1152/ajpregu.00322.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fadel PJ. Reflex control of the circulation during exercise. Scand J Med Sci Sports 25, Suppl 4: 74–82, 2015. doi: 10.1111/sms.12600. [DOI] [PubMed] [Google Scholar]
- 8.Fischer MO, Avram R, Cârjaliu I, Massetti M, Gérard JL, Hanouz JL, Fellahi JL. Non-invasive continuous arterial pressure and cardiac index monitoring with Nexfin after cardiac surgery. Br J Anaesth 109: 514–521, 2012. doi: 10.1093/bja/aes215. [DOI] [PubMed] [Google Scholar]
- 9.Fisher JP. Autonomic control of the heart during exercise in humans: role of skeletal muscle afferents. Exp Physiol 99: 300–305, 2014. doi: 10.1113/expphysiol.2013.074377. [DOI] [PubMed] [Google Scholar]
- 10.Fisher JP, Bell MP, White MJ. Cardiovascular responses to human calf muscle stretch during varying levels of muscle metaboreflex activation. Exp Physiol 90: 773–781, 2005. doi: 10.1113/expphysiol.2005.030577. [DOI] [PubMed] [Google Scholar]
- 11.Fisher JP, Young CN, Fadel PJ. Autonomic adjustments to exercise in humans. Compr Physiol 5: 475–512, 2015. doi: 10.1002/cphy.c140022. [DOI] [PubMed] [Google Scholar]
- 12.Gifford JR, Richardson RS. CORP: Ultrasound assessment of vascular function with the passive leg movement technique. J Appl Physiol (1985) 123: 1708–1720, 2017. doi: 10.1152/japplphysiol.00557.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gladwell VF, Coote JH. Heart rate at the onset of muscle contraction and during passive muscle stretch in humans: a role for mechanoreceptors. J Physiol 540: 1095–1102, 2002. doi: 10.1113/jphysiol.2001.013486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gladwell VF, Fletcher J, Patel N, Elvidge LJ, Lloyd D, Chowdhary S, Coote JH. The influence of small fibre muscle mechanoreceptors on the cardiac vagus in humans. J Physiol 567: 713–721, 2005. doi: 10.1113/jphysiol.2005.089243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayes SG, Kindig AE, Kaufman MP. Comparison between the effect of static contraction and tendon stretch on the discharge of group III and IV muscle afferents. J Appl Physiol (1985) 99: 1891–1896, 2005. doi: 10.1152/japplphysiol.00629.2005. [DOI] [PubMed] [Google Scholar]
- 16.Høier B, Rufener N, Bojsen-Møller J, Bangsbo J, Hellsten Y. The effect of passive movement training on angiogenic factors and capillary growth in human skeletal muscle. J Physiol 588: 3833–3845, 2010. doi: 10.1113/jphysiol.2010.190439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ives SJ, Amann M, Venturelli M, Witman MA, Groot HJ, Wray DW, Morgan DE, Stehlik J, Richardson RS. The mechanoreflex and hemodynamic response to passive leg movement in heart failure. Med Sci Sports Exerc 48: 368–376, 2016. doi: 10.1249/MSS.0000000000000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ives SJ, McDaniel J, Witman MA, Richardson RS. Passive limb movement: evidence of mechanoreflex sex specificity. Am J Physiol Heart Circ Physiol 304: H154–H161, 2013. doi: 10.1152/ajpheart.00532.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruse NT, Scheuermann BW. Cardiovascular responses to skeletal muscle stretching: “stretching” the truth or a new exercise paradigm for cardiovascular medicine? Sports Med 47: 2507–2520, 2017. doi: 10.1007/s40279-017-0768-1. [DOI] [PubMed] [Google Scholar]
- 20.Kruse NT, Silette CR, Scheuermann BW. Influence of passive stretch on muscle blood flow, oxygenation and central cardiovascular responses in healthy young males. Am J Physiol Heart Circ Physiol 310: H1210–H1221, 2016. doi: 10.1152/ajpheart.00732.2015. [DOI] [PubMed] [Google Scholar]
- 21.Kumagai K, Abe T, Brechue WF, Ryushi T, Takano S, Mizuno M. Sprint performance is related to muscle fascicle length in male 100-m sprinters. J Appl Physiol (1985) 88: 811–816, 2000. doi: 10.1152/jappl.2000.88.3.811. [DOI] [PubMed] [Google Scholar]
- 22.Macefield VG. Physiological characteristics of low-threshold mechanoreceptors in joints, muscle and skin in human subjects. Clin Exp Pharmacol Physiol 32: 135–144, 2005. doi: 10.1111/j.1440-1681.2005.04143.x. [DOI] [PubMed] [Google Scholar]
- 23.Matsukawa K, Wall PT, Wilson LB, Mitchell JH. Reflex stimulation of cardiac sympathetic nerve activity during static muscle contraction in cats. Am J Physiol Heart Circ Physiol 267: H821–H827, 1994. doi: 10.1152/ajpheart.1994.267.2.H821. [DOI] [PubMed] [Google Scholar]
- 24.McCloskey DI, Matthews PB, Mitchell JH. Absence of appreciable cardiovascular and respiratory responses to muscle vibration. J Appl Physiol 33: 623–626, 1972. doi: 10.1152/jappl.1972.33.5.623. [DOI] [PubMed] [Google Scholar]
- 25.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDaniel J, Hayman MA, Ives S, Fjeldstad AS, Trinity JD, Wray DW, Richardson RS. Attenuated exercise induced hyperaemia with age: mechanistic insight from passive limb movement. J Physiol 588: 4507–4517, 2010. doi: 10.1113/jphysiol.2010.198770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDaniel J, Ives SJ, Richardson RS. Human muscle length-dependent changes in blood flow. J Appl Physiol (1985) 112: 560–565, 2012. doi: 10.1152/japplphysiol.01223.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell JH. Abnormal cardiovascular response to exercise in hypertension: contribution of neural factors. Am J Physiol Regul Integr Comp Physiol 312: R851–R863, 2017. doi: 10.1152/ajpregu.00042.2017. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- 30.Murata J, Matsukawa K. Cardiac vagal and sympathetic efferent discharges are differentially modified by stretch of skeletal muscle. Am J Physiol Heart Circ Physiol 280: H237–H245, 2001. doi: 10.1152/ajpheart.2001.280.1.H237. [DOI] [PubMed] [Google Scholar]
- 31.Nóbrega AC, Araújo CG. Heart rate transient at the onset of active and passive dynamic exercise. Med Sci Sports Exerc 25: 37–41, 1993. doi: 10.1249/00005768-199301000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Otsuki A, Fujita E, Ikegawa S, Kuno-Mizumura M. Muscle oxygenation and fascicle length during passive muscle stretching in ballet-trained subjects. Int J Sports Med 32: 496–502, 2011. doi: 10.1055/s-0031-1275297. [DOI] [PubMed] [Google Scholar]
- 33.Refshauge KM, Chan R, Taylor JL, McCloskey DI. Detection of movements imposed on human hip, knee, ankle and toe joints. J Physiol 488: 231–241, 1995. doi: 10.1113/jphysiol.1995.sp020961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Refshauge KM, Taylor JL, McCloskey DI, Gianoutsos M, Mathews P, Fitzpatrick RC. Movement detection at the human big toe. J Physiol 513: 307–314, 1998. doi: 10.1111/j.1469-7793.1998.307by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva TM, Aranda LC, Paula-Ribeiro M, Oliveira DM, Medeiros WM, Vianna LC, Nery LE, Silva BM. Hyperadditive ventilatory response arising from interaction between the carotid chemoreflex and the muscle mechanoreflex in healthy humans. J Appl Physiol (1985) 125: 215–225, 2018. doi: 10.1152/japplphysiol.00009.2018. [DOI] [PubMed] [Google Scholar]
- 36.Stebbins CL, Brown B, Levin D, Longhurst JC. Reflex effect of skeletal muscle mechanoreceptor stimulation on the cardiovascular system. J Appl Physiol (1985) 65: 1539–1547, 1988. doi: 10.1152/jappl.1988.65.4.1539. [DOI] [PubMed] [Google Scholar]
- 37.Trinity JD, Amann M, McDaniel J, Fjeldstad AS, Barrett-O’Keefe Z, Runnels S, Morgan DE, Wray DW, Richardson RS. Limb movement-induced hyperemia has a central hemodynamic component: evidence from a neural blockade study. Am J Physiol Heart Circ Physiol 299: H1693–H1700, 2010. doi: 10.1152/ajpheart.00482.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Venturelli M, Amann M, McDaniel J, Trinity JD, Fjeldstad AS, Richardson RS. Central and peripheral hemodynamic responses to passive limb movement: the role of arousal. Am J Physiol Heart Circ Physiol 302: H333–H339, 2012. doi: 10.1152/ajpheart.00851.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venturelli M, Cè E, Limonta E, Bisconti AV, Devoto M, Rampichini S, Esposito F. Central and peripheral responses to static and dynamic stretch of skeletal muscle: mechano- and metaboreflex implications. J Appl Physiol (1985) 122: 112–120, 2017. doi: 10.1152/japplphysiol.00721.2016. [DOI] [PubMed] [Google Scholar]
- 40.Wang H-J, Zucker IH, Wang W. Muscle reflex in heart failure: the role of exercise training. Front Physiol 3: 398, 2012. doi: 10.3389/fphys.2012.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson LB, Wall PT, Pawelczyk JA, Matsukawa K. Cardiorespiratory and phrenic nerve responses to graded muscle stretch in anesthetized cats. Respir Physiol 98: 251–266, 1994. doi: 10.1016/0034-5687(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 42.Witman MA, Ives SJ, Trinity JD, Groot HJ, Stehlik J, Richardson RS. Heart failure and movement-induced hemodynamics: partitioning the impact of central and peripheral dysfunction. Int J Cardiol 178: 232–238, 2015. doi: 10.1016/j.ijcard.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]