Abstract
Autosomal-dominant polycystic kidney disease (ADPKD) is a very common genetic disease leading to renal failure. Numerous aberrantly regulated signaling pathways have been identified as promising molecular drug targets for ADPKD therapy. In rodent models, many small-molecule drugs against such targets have proven effective in reducing renal cyst growth. For example, mammalian target of rapamycin (mTOR) inhibition with rapamycin greatly ameliorates renal cystic disease in several rodent models. However, clinical trials with mTOR inhibitors were disappointing largely due to the intolerable extrarenal side effects during long-term treatment with these drugs. Most other potential drug targets in ADPKD are also widely expressed in extrarenal tissues, which makes it likely that untargeted therapies with small-molecule inhibitors against such targets will lead to systemic adverse effects during the necessary long-term treatment of years and decades in ADPKD patients. To overcome this problem, we previously demonstrated that folate-conjugated rapamycin (FC-rapa) targets polycystic kidneys due to the high expression of the folate receptor (FRα) and that treatment of a nonortholgous PKD mouse model leads to inhibition of renal cyst growth. Here we show, in a head-to-head comparison with unconjugated rapamycin, that FCrapa inhibits renal cyst growth, mTOR activation, cell cycling, and fibrosis in an orthologous Pkd1 mouse model. Both unconjugated rapamycin and FC-rapa are similarly effective on polycystic kidneys in this model. However, FC-rapa lacks the extrarenal effects of unconjugated rapamycin, in particular immunosuppressive effects. We conclude that folate-conjugation is a promising avenue for increasing the tissue specificity of small-molecule compounds to facilitate very long-term treatment in ADPKD.
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is a very common inherited disease characterized by bilateral renal cyst growth due to aberrant proliferation of tubule epithelial cells leading to tissue destruction over time (1, 16). The disease commonly presents clinically in the third decade of life and eventually results in kidney failure requiring long-term dialysis and kidney transplantation. Estimates of the number of people affected by ADPKD in the US range from 200,000 to over 600,000. Currently, the only pharmacological compound that is approved for ADPKD treatment in some countries, although not in the US, is the vasopressin receptor V2 antagonist tolvaptan (37, 38). The utility of this drug is limited by its profile of adverse effects such as extreme polyuria, its potential liver toxicity, and its high cost (3, 43).
The causative mutations in ADPKD occur in either the PKD1 or PKD2 gene in 85 and 15% of cases, respectively. The exact molecular mechanisms underlying the pathogenesis of ADPKD have yet to be ascertained, but numerous altered cellular signaling pathways have been identified (17). This has led to successes in preclinical studies in which several drugs that inhibit these pathways have shown efficacy in slowing disease progression in PKD rodent models (16). A prime example is the mammalian target of rapamycin (mTOR) signaling pathway, which is aberrantly activated in cyst-lining cells in ADPKD and rodent models (29, 30, 36). Treatment of rodent models with mTOR inhibitors, such as rapamycin, profoundly inhibits renal cyst growth and slows disease progression. These promising results quickly led to clinical trials, which were, however, disappointing and did not establish beneficial effects in ADPKD patients (28, 42, 44). In hindsight, the failure of mTOR inhibitors in clinical trials was likely due to the fact that rodents can be treated with much higher doses of mTOR inhibitors than humans and the short duration of treatment of days or weeks in mice and rats. In contrast, ADPKD clinical trials involve drug treatment for a year or more, and mTOR inhibitors have significant, unwanted extrarenal effects, even at low doses, including immunosuppression. Therefore, simply increasing the treatment doses of mTOR inhibitors is not a viable option for ADPKD therapy.
Due to the slowly progressive nature of this chronic disease it is likely that ADPKD patients will require continuous treatment with any pharmacological agent for very extended periods of time, likely for decades. This puts an extremely high burden on the requirement for specificity of any potential pharmacological agent. Most of the potential molecular drug targets identified in ADPKD exhibit relatively ubiquitous tissue expression patterns, such as mTOR, the epidermal growth factor receptor (EGFR), c-src, and many others. Therefore, inhibitors of these targets may be tolerated in the short-term, e.g., for cancer therapy, but are often not tolerated in the long term due to their adverse, systemic effects.
An approach to circumvent this problem would be to target any pharmacological agent specifically to polycystic kidneys. If successful, this may enable effective inhibition of the intended molecule in the target tissue without affecting extrarenal tissues and organs, thereby reducing or eliminating systemic effects. Our group has previously explored strategies for ADPKD-specific kidney targeting for both antibodies and for small molecule compounds. In the case of antibody targeting, we have shown that antibodies in dimeric IgA format can be targeted to renal cyst lumens by exploiting the high levels of expression of the polymeric immunoglobulin receptor in cyst-lining cells (15).
To target small molecule drugs to polycystic kidneys, we have made use of the folate receptor-α (FRα), which is highly expressed in cyst-lining cells and has the ability to take up folate-conjugated (FC) compounds by receptor-mediated endocytosis (29). Folate conjugates of nearly any small molecule drug can be engineered with a cleavable linker that allows the intracellular release of the original drug payload after internalization. This technology was originally designed for cancer therapy by exploiting the fact that many tumors overexpress the FRα (41). We previously showed that the FRα is also expressed in polycystic kidneys, both in human ADPKD and mouse models, and used folate-conjugated rapamycin (FC-rapa) for proof-of-concept studies in the bpk polycystic kidney mouse model (29). Treatment of bpk mice with FC-rapa was similarly effective as treatment with unconjugated rapamycin with regard to inhibition of cyst and kidney growth and led to similar preservation of renal function. In contrast to unconjugated rapamycin, however, FC-rapa exhibited mTOR inhibition preferentially in polycystic kidneys compared with extrarenal tissues (29).
The polycystic kidney mouse model that we previously used to test the efficacy and specificity of FC-rapa was the nonorthologous bpk model in which a gene unrelated to the PKD1 and PKD2 genes is affected (29). This model shares many features with the fast-progressing autosomal-recessive form of PKD (ARPKD). In the present study, to further validate and expand this work, we utilized an orthologous mouse model in which the Pkd1 gene is affected, the same gene that is affected in 85% of the cases of human ADPKD. In this model, the Pkd1 gene is conditionally inactivated by Cre recombinase driven by the nestin promotor, which results in a mosaic null phenotype in the kidneys to mimic the mechanism of loss of heterozygosity in human ADPKD (31). This model has previously been extensively characterized and shown to replicate characteristic features of human ADPKD including aberrant mTOR activation, epithelial proliferation and apoptosis, progressive fibrosis, and cysts derived predominantly from collecting ducts/distal tubules (31). Using a novel fluorescently labeled folate conjugate and visualization by fluorescence microscopy, we show here that the folate receptor is accessible to folate-conjugated compounds on cyst-lining cells and can internalize those compounds into cyst-lining cells. We further show that FC-rapa is effective in inhibiting renal cyst growth in this model. At effective doses, mTOR inhibition is not observed in extrarenal tissues consistent with tissue-specific targeting to polycystic kidneys. Collectively, these results support the concept of using FC-drugs for tissue targeting in the treatment of ADPKD with the goal to reduce extrarenal adverse effects that may allow effective, long-term therapy.
MATERIALS AND METHODS
Animal studies.
All animal studies adhered to the rules and regulations of the National Institutes of Health with approval of the Institutional Animal Care and Use Committee of the University of California, Santa Barbara. Mice were treated between postnatal days 7 and 21 with indicated doses of either folate or unconjugated rapamycin by intraperitoneal injection. Both drugs were reconstituted in DMSO and diluted in PBS to a final concentration of 2% DMSO and 2% ethanol with a standard injection volume of 10 μl/g mouse body wt. The bpk and PKD1fl/fl:NesCre models have been described previously (31). However, in this study, PKD1fl/−:NesCre animals were used. The knockout allele was generated by the inherent germline activity of the Nestin-Cre system (47) and identified by PCR using primers F4 and R2 described previously (18). PKD1fl/wt:NesCre+ animals served as controls. There were no observed sex-related differences in the study. Animal weights were recorded daily and reported as 3-day moving averages over the course of the trial.
Flow cytometry.
To assess receptor necessity for drug activity, folate receptor-α (FRα)-positive KB cells or FRα-negative A549 cells were treated with FC-rapa and stained for phospho-ribosomal protein S6 (P-S6), a surrogate marker of mTORC1 activity. Cells were plated in 12-well plates at 5 × 106 cells per well in folate-deficient RPMI media containing 10% FBS and were allowed to attach for 18 h at 37°C in a 95% O2/5% CO2 cell culture incubator. Folate-conjugated rapamycin (EC0371, or FC-rapa) was diluted in culture medium to a final concentration of 1, 10, or 100 nM. Another series of drug dilutions was prepared containing 10 µM of excess folic acid for competition. The cells were pulsed with drug for 2 h at 37°C, which was followed by two washes with medium. Fresh medium was added to the cells, and they were placed in a cell culture incubator for 20 h. The following day, the cells were detached from the plates using trypsin and resuspended in PBS, pH 7.4 + 1% BSA. After fixation with Leucoperm A reagent (AbD Serotec), the cells were incubated with an Alexa Fluor 647-conjugated antibody to phospho-ribosomal protein S6 (Ser235/236) (rabbit monoclonal D57.2.2E; Cell Signaling Technology) at a dilution of 1:100 in Leucoperm B solution for 30 min at room temperature. Cells were washed and resuspended in PBS/1% BSA for analysis on a Beckman Coulter Gallios flow cytometer.
Histology and immunostaining.
Formalin-fixed, paraffin-embedded (FFPE) tissues were sectioned (5μm) and processed for hematoxylin and eosin, immunofluorescence, or immunohistochemistry staining as described previously (10). Cystic index was measured from these tissue sections as a percentage of cyst area relative to the total renal area using Adobe Photoshop. Standard 5-μm FFPE tissue sections were stained with Sirius Red/Fast Green to assess interstitial fibrosis. For this, sections were treated with 0.1% Direct Red and 0.1% Fast Green FCF (Sigma-Aldrich) for 4 h. Sections were deparaffinized in xylenes and rehydrated. This was followed by antigen retrieval in citrate buffer, pH 6, using a pressure cooker. Samples were blocked in 10% goat serum and 0.5% fish skin gelatin, in Tris-buffered saline plus 0.1% Tween-20. Primary antibodies used to stain include folate receptor (PU19; Endocyte), P-S6 (Ser240/244) (Cell Signaling), and Ki-67 (Millipore). Image acquisition and processing were done equivalently, and cell counts were done on three images per thymus. Immunohistochemistry was performed on 5-μm FFPE kidney sections using an antibody against the FRα as described previously (29).
Receptor accessibiltiy and functional binding.
To assess receptor accessibility and functional binding, 12-day-old wild-type, bpk, or PKD1fl/−:NesCre mice were intraperitoneally injected with 0.3 μmol/kg FC-reporter compound or PBS vehicle. Tissue was collected 4 h postinjection, and kidneys were bisected and then fixed for 24 h in 4% formalin, followed by processing for paraffin embedding. Blocks were then sectioned (5 μm), deparaffinized in xylenes, rehydrated, and stained. Staining was done with anti-FITC primary antibody (Jackson ImmunoResearch) overnight and secondary for 1 h (ThermoFisher). Image acquisition and processing were done equivalently.
Immunoblotting.
Primary antibodies for this study included P-S6 (Ser240/244), total S6, phosphorylated 4e-binding protein 1 (P-4E-BP1; Thr37/46), and total 4E-BP1 (Cell Signaling), and actin (Sigma-Aldrich). Tissue preparation and loading has been described previously (10). Briefly, tissues were snap frozen in liquid nitrogen at the time of collection and then homogenized in a Dounce homogenizer in RIPA lysis buffer. Protein concentrations were estimated using the Promega BCA kit and loaded serially. Each lane represents a single mouse lysate, and lanes between tissues are the same animal for comparison. Bands were quantified using National Institutes of Health ImageJ Software.
RESULTS
Effectiveness of FC-rapa depends on expression of FRα.
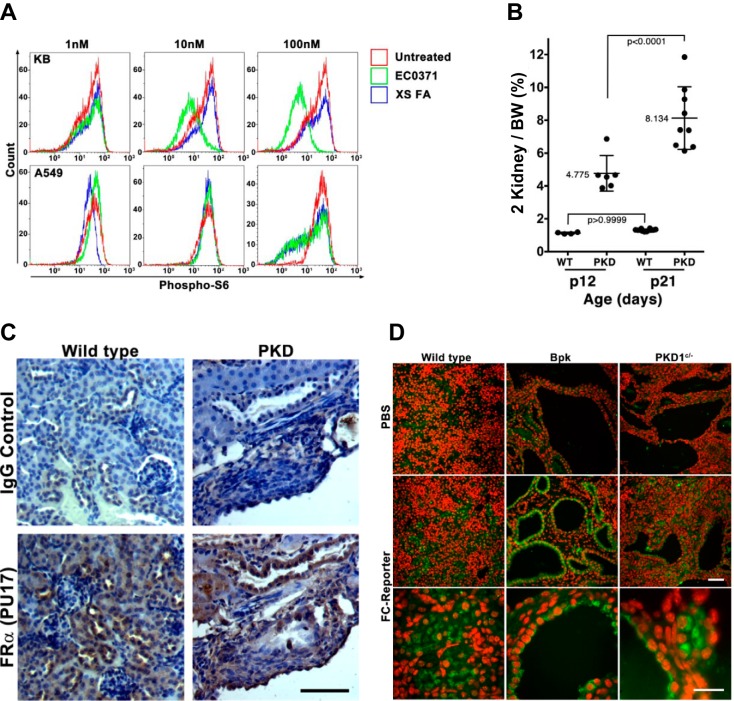
As an example to test the utility of FRα-driven tissue targeting to polycystic kidneys in an orthologous PKD mouse model, we studied the previously described compound folate conjugated rapamycin (EC0371), termed FC-rapa (29). As previously described, this compound is synthesized by conjugating rapamycin to folate using a hydrophilic linker and a biologically cleavable linker that is designed to release rapamycin after successful internalization into cells (29). To confirm that the ability of cells to internalize FC-rapa is dependent on expression of the FRα, we utilized previously characterized, FRα-positive KB cells in comparison to FRα-negative A549 cells (24). These cells were treated with increasing concentrations of FC-rapa overnight, fixed, and stained for phosphorylation of the downstream target of mTORC1 activation, ribosomal protein S6. As shown in Fig. 1A, 10 nM FC-rapa strongly inhibited S6 phosphorylation in almost the entire population of KB cells but had no effect on A549 cells. Even a 10-fold higher concentration of FC-rapa only partially inhibited mTORC1 activity in A549 cells. Furthermore, mTORC1 inhibition by FC-rapa was suppressed in KB cells when competed with excess amounts of free folate (XS). These results demonstrate that FC-rapa is only efficiently internalized into cells that express the FRα and that internalization leads to intracellular cleavage of FC-rapa, release of rapamycin into the cytoplasm, and effective inhibition of mTORC1.
Fig. 1.
Folate receptor-α (FRα) is necessary for drug uptake, expressed, and accessible in cyst lining epithelial cells. A: KB and A549 cells, representing FRα-positive and -negative cells, respectively, were treated with increasing doses of folate-conjugated rapamycin (FC-rapa) (EC0371) in the absence or presence of excess soluble folic acid (XS FA). The expression of phospho-ribosomal protein S6 (P-S6) was analyzed by immunofluorescence staining and flow cytometry. B: 2-kidney weights relative to whole body weights (BW) demonstrating progression of disease throughout treatment time course. PKD, polycystic kidney disease. C: FRα expression by immunohistochemistry on kidney sections from PKD1fl/−:NesCre mice versus PKD1fl/wt:NesCre+ control mice [wild-type (WT)] showing expression in normal tubules and in cyst lining cells. D: renal immunofluorescence staining for folate-conjugated reporter (FC-reporter) 4 h after intraperitoneal injection into wild-type (left), bpk (middle), or PKD1fl/−:NesCre (right) mice. Animals were injected on postnatal day 12 with either PBS or FC-reporter. Scale bars = 50 μm; red is nuclear stain (DAPI), and green is FC-reporter.
Expression of FRα in polycystic kidneys of an orthologous Pkd1 mouse model.
We previously demonstrated that FRα is expressed in ADPKD kidney tissue and the kidneys of nonorthologous models of PKD (29). To investigate an orthologous mouse model, we generated and used here Pkd1fl/−:NesCre animals. We have previously used Pkd1fl/fl:NesCre mice containing two floxed Pkd1 alleles (10, 15, 31, 34). To reduce animal-to-animal variation, we introduced a null allele to generate Pkd1fl/−:NesCre animals. This model exhibits relatively consistent renal cystic disease with an approximate doubling of the two-kidney weight-to-body weight ratios between postnatal days 12 and 21 (Fig. 1B).
To confirm FRα expression in kidneys from Pkd1fl/−:NesCre animals, renal sections were stained by immunohistochemistry with an FR-specific antibody (PU19) and analyzed. Staining for FRα expression shows specificity to the kidney tubules and cyst-lining epithelial cells (Fig. 1C) suggesting that cysts in this orthologous model should be able to take up FC-rapa.
Use of a novel FC-reporter to visualize uptake in cyst-lining cells in vivo.
To test whether the expression of FRα in polycystic kidneys leads to uptake of parenterally administered folate-conjugated compounds into cyst-lining cells in vivo, we designed a novel small molecule reporter compound to allow direct visualization of the fate of such compounds. This FC-reporter consists of folate conjugated to a hapten that is recognized by readily available commercial antibodies. A chemical linker is utilized that is designed to allow chemical aldehyde cross linking using standard histochemical protocols. A detailed article describing the chemical synthesis of the FC-reporter, its pharmacological properties, and its general utility as an in vivo reporter of the targeting of folate-conjugated compounds is in preparation.
The FC-reporter was administered to the orthologous Pkd1 mouse model (PKD1fl/−:NesCre); the previously studied, nonorthologous bpk mice; and age-matched control mice by intraperitoneal injection. After 4 h, animals were euthanized, and kidneys were removed, fixed using formalin, embedded in paraffin, and sectioned using standard histological procedures. The presence of the FC-reporter in tissue was visualized by immunofluorescence staining and fluorescence microscopy. As shown in Fig. 1D, kidneys of wild-type mice exhibit specific internalization of the FC-reporter in renal tubules. In both PKD mouse models, the FC-reporter can be prominently detected in cyst-lining cells (Fig. 1D). This result indicates that the FRα on cyst-lining cells in the orthologous Pkd1fl/−:NesCre mouse model is accessible to circulating folate-conjugated compounds.
Efficacy of FC-rapa versus unconjugated rapamycin.
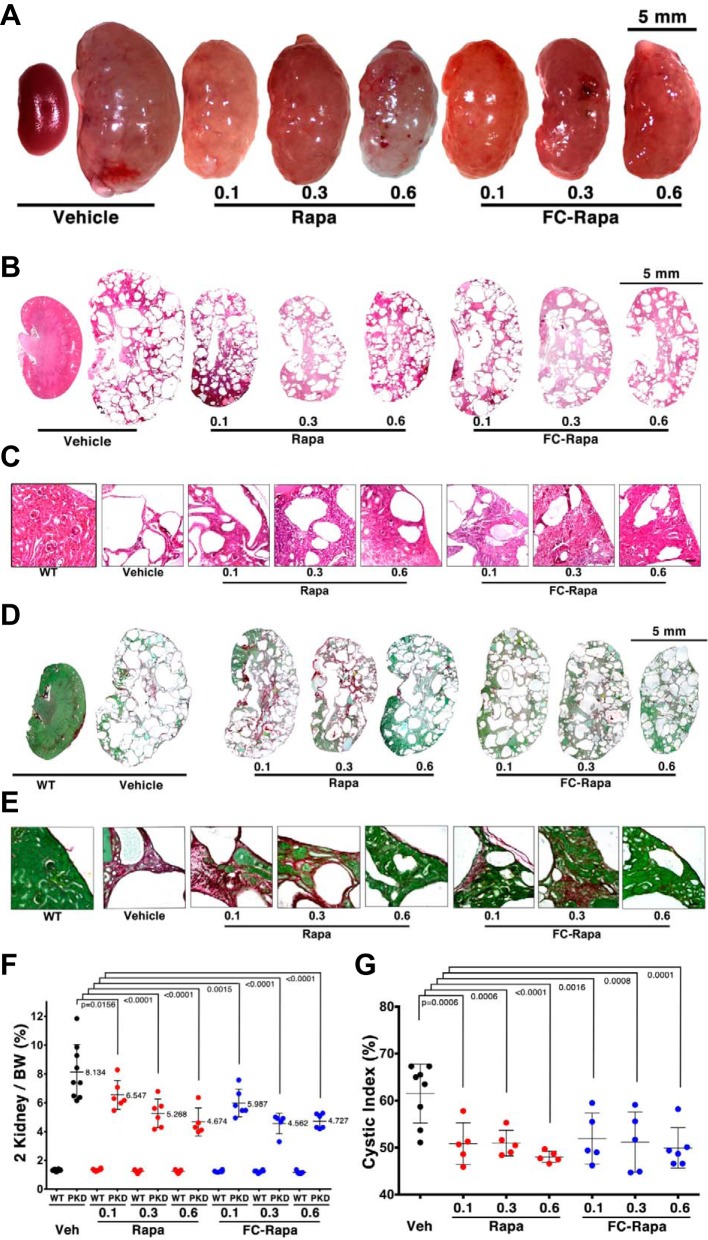
To assess the efficacy of FC-rapa in our orthologous Pkd1 mouse model, Pkd1fl/−:NesCre mice or PKD1fl/+:NesCre controls were treated with a series of doses of FC-rapa (0.1, 0.3, and 0.6 μmol·kg−1·day−1 ip) from postnatal days 7–21. For comparison, matching molar doses of unconjugated rapamycin were administered to another cohort of animals. Both rapamycin and FC-rapa were similarly effective in inhibiting renal cyst growth as is evident from gross kidney images (Fig. 2A) and histological sections (Fig. 2, B and C). The two-kidney weight-to-body weight (2KTBW) ratios were significantly reduced in treated animals, and higher doses of either compound achieved better efficacy (Fig. 2F). Treatment with either compound led to partial preservation of normal renal parenchyma (Fig. 2C) and overall reduction of the cystic index (Fig. 2G). Collagen staining with sirius red revealed that both rapamycin and FC-rapa reduced the development of renal fibrosis (Fig. 2, D and E). Renal function as assessed by blood urea nitrogen did not deteriorate during the study window and, therefore, remained unaffected by either rapamycin or FC-rapa (data not shown). These results indicate that FC-rapa is similarly effective to unconjugated rapamycin in a head-to-head comparison. Both compounds similarly inhibit renal cyst growth and fibrosis in an orthologous mouse model of PKD.
Fig. 2.
Head-to-head comparison of efficacy of folate-conjugated rapamycin (FC-rapa) versus rapamycin in an orthologous model of autosomal-dominant polycystic kidney disease (ADPKD). A: Representative gross kidney images of PKD1fl/−:NesCre mice treated with or without of FC-rapa or unconjugated rapamycin at 0.1, 0.3, and 0.6 µmol·kg−1·day−1 between postnatal days 7 and 21. B and C: hematoxylin and eosin (H&E)-stained kidney sections from treated and untreated mice showing morphology as full-kidney composites (B) or higher magnification images (C). D and E: Sirius Red/Fast Green-stained kidney sections from treated and untreated mice showing interstitial fibrosis (red) as full-kidney composites (D) or higher magnification images (E). F: 2-kidney weights relative to whole body weights (BW) from different treatment groups. PKD, polycystic kidney disease. G: renal cystic index (percent cystic area) from different treatment groups. Statistical significance was determined by one-way ANOVA.
FC-rapa exhibits reduced extrarenal effects.
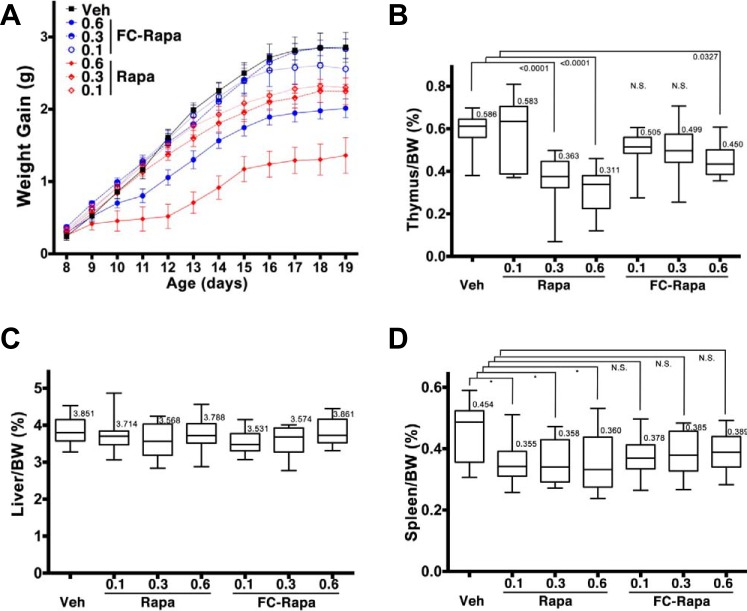
Of critical interest in this study was the extrarenal response to the targeted treatment relative to unconjugated rapamycin. Rapamycin inhibits body growth by a general inhibitory effect on cell growth and proliferation (9). Consequently, we observed a dose-dependent inhibition of overall body weight gain in mice treated with rapamycin during the treatment window between postnatal days 7 and 21 (Fig. 3A). The observed slowed body growth is apparent even at the lowest dose of rapamycin (0.1 μmol·kg−1·day−1). In contrast, body weight gain is unaffected by both the low and intermediate doses of FC-rapa (0.1 and 0.3 μmol·kg−1·day−1, respectively) leading to nearly indistinguishable growth curves compared with vehicle-treated mice over the course of the study (Fig. 3A) and providing evidence that FC-rapa exhibits significantly less systemic toxicity.
Fig. 3.
Off-target effects folate-conjugated rapamycin (FC-rapa) versus unconjugated rapamycin on body weight (BW) gain and thymus weights. A: 3-day moving averages of drug dose dependent changes in animal growth. B–D: thymus weights (B); liver weights (C); D: spleen weights (D) relative to total body weight by treatment group. Statistical significance was determined by one-way ANOVA.
Rapamycin is used clinically as an immunosuppressant and has been shown to affect adaptive and innate immune responses (27). To monitor any gross immunosuppressive effects of rapamycin versus FC-rapa, we assessed their effects on thymus weight as an important organ of the immune system and one in which we have previously shown that high-dose FC-rapa significantly reduces its size (29). Both the intermediate and high doses of unconjugated rapamycin resulted in a significant decrease in the relative thymus weight, while only the highest dose of FC-rapa had a statistically significant effect and even that effect was less severe than the high doses of its unconjugated analog (Fig. 3B). Other extrarenal tissues, including liver (Fig. 3C) and spleen (Fig. 3D), did not exhibit significant growth retardation beyond the effect on total body weight when treated with FC-rapa. Altogether, these results indicate that at doses that significantly inhibit renal cyst growth, FC-rapa exhibits reduced extrarenal effects including effects on the immune system.
Renal specificity of mTOR inhibition with FC-rapa.
To assess the efficacy of rapamycin versus FC-rapa treatment, we monitored the levels of phosphorylation of ribosomal protein S6 (P-S6) as a downstream surrogate marker of mTORC1 activity. Based on total kidney lysates, both rapamycin and FC-rapa result in a dose-dependent inhibition of mTORC1 activity (Fig. 4A). Rapamycin treatment also results in dose-dependent mTORC1 inhibition in the spleen (Fig. 4B). In contrast, treatment with FC-rapa does not lead to dose-dependent mTORC1 inhibition in the spleen (Fig. 4B) suggesting that this tissue does not effectively take up folate conjugated compounds.
Fig. 4.
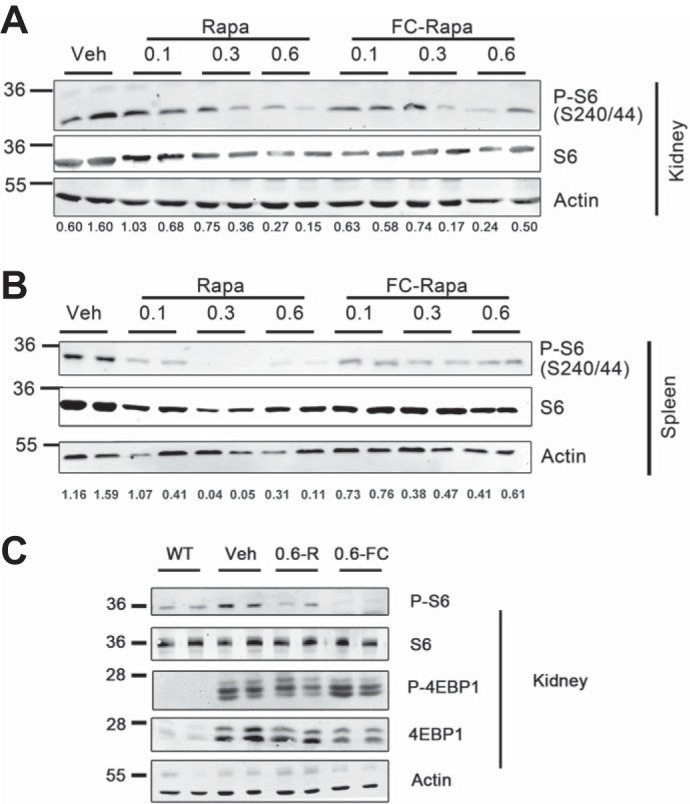
Effects of folate-conjugated rapamycin (FC-rapa) versus unconjugated rapamycin on mammalian target of rapamycin (mTOR) signaling in kidney and spleen. A: immunoblot analysis of total kidney tissue lysates for phospho-ribosomal protein S6 (P-S6; Ser240/244), total S6, and β-actin as a loading control. B: immunoblot analysis of total spleen tissue lysates from treated mice for P-S6 (Ser240/244), total S6, and β-actin. For A and B, each lane represents a single animal that corresponds to all of the tissues shown, with 2 representative mice per treatment group (n = 5 for each treatment group) shown. Immunoblot results were quantified with the values representing the ratios of the P-S6 signals relative to the β-actin signals. C: immunoblot analysis of kidney tissue lysates from wild-type (WT) and treated animals for P-S6 (Ser240/244), total S6, phosphorylated 4e-binding protein 1 (P-4E-BP1; Thr37/46), total 4E-BP1, and β-actin.
Besides the S6K/S6 branch of the mTORC1 signaling pathway, mTOR also affects another branch by phosphorylation of eukaryotic translation initiation factor 4e-binding protein 1 (4E-BP1). It has previously been reported that rapamycin inhibits primarily the phosphorylation of S6K but much less so the phosphorylation of 4E-BP1 (35). To assess the relative effect of rapamycin and FC-rapa treatment on these two branches of the mTOR signaling pathway, we compared, head-to-head, the effect of treatment with the highest doses of rapamycin versus FC-rapa on total kidney lysates. As shown in Fig. 4C, polycystic kidneys not only exhibit a very strong increase in P-4E-BP1 but also a strong increase in the total expression of 4E-BP1. The activation of the 4E-BP1 branch of the mTOR signaling pathway appears to far exceed the effect on the S6 branch of the pathway. To our knowledge, this differential activation downstream of mTOR has not previously been reported in polycystic kidney disease. As expected, while both rapamycin and FC-rapa reduce the level of S6 phosphorylation in kidneys of treated animals, these drugs have little, if any, effect on either the total expression of 4E-BP1 or its phosphorylation (Fig. 4C). These results may suggest that compounds that affect the 4E-BP1 branch of mTOR signaling may be advantageous for PKD therapy.
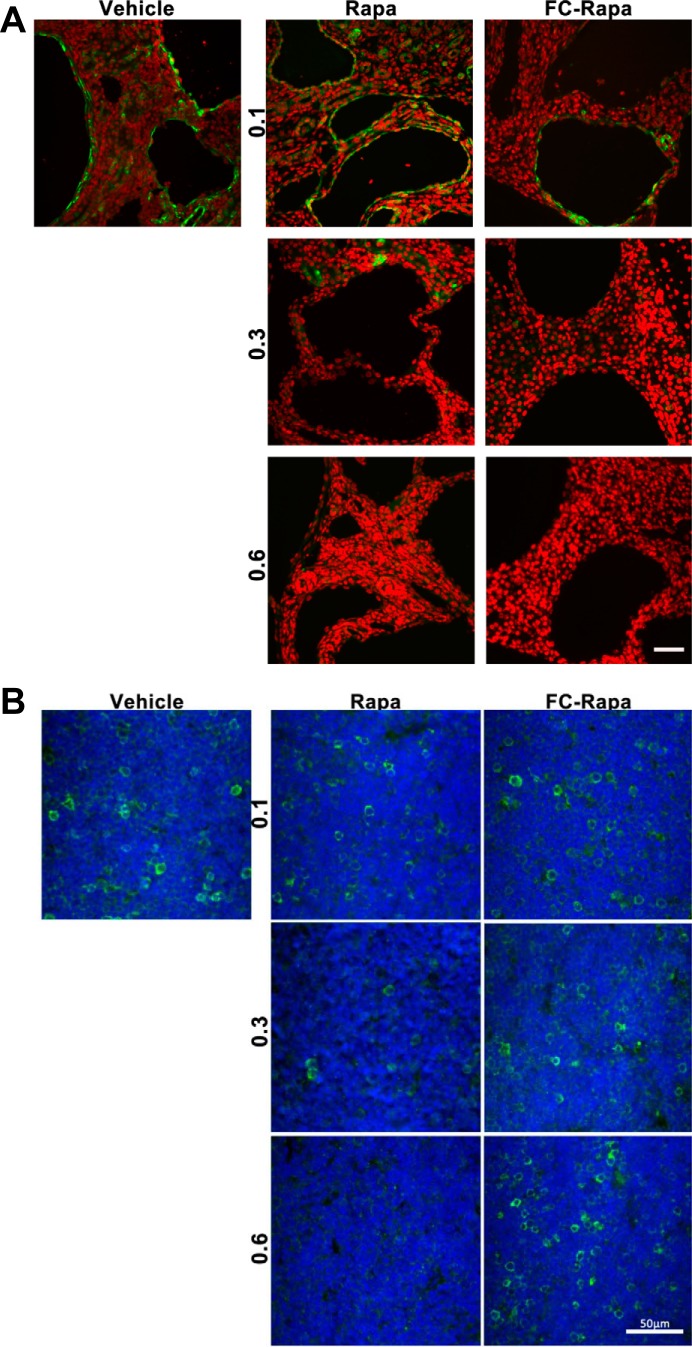
Because the analysis of total tissue lysates does not distinguish between effects on different cell types present in the tissue, we next investigated P-S6 levels by immunofluorescence microscopy. Consistent with previous reports, polycystic kidneys exhibit high levels of P-S6 in the cell lining in many, but not all, cysts (Fig. 5A). In addition, some interstitial cells are positive for P-S6. Treatment with both rapamycin and FC-rapa reduces, dose dependently, the levels of P-S6 in both cyst-lining and interstitial cells (Fig. 5A). We have recently reported that folate-conjugated drugs are taken up by macrophages (13). Activated, pericystic macrophages are abundant in polycystic kidneys and were found to promote cyst growth (8). These results suggest that many of the P-S6-positive interstitial cells we see in PKD kidneys may be activated macrophages and that inhibition of mTOR in these macrophages may contribute to the observed suppression of cyst growth.
Fig. 5.
Effect of folate-conjugated rapamycin (FC-rapa) versus unconjugated rapamycin on mammalian target of rapamycin (mTOR) signaling in kidney and thymus. A: images are representative of immunofluorescence staining for phospho-ribosomal protein S6 (P-S6; Ser240/244) in polycystic kidneys of different treatment groups. B: matched thymus staining representative of immunofluorescence staining for P-S6 (Ser240/244) in thymus sections of different treatment groups. The scale bars = 50 μm; green is P-S6 (Ser240/244), and red (A) or blue (B) is nuclear staining (DAPI).
To further evaluate the relative renal specificity of FC-rapa, we investigated effects on mTOR inhibition in the thymus. Many cells in the thymus of vehicle-treated animals exhibit strong P-S6 signals, which presumably include proliferating/maturing T cells. In a dose-dependent manner, treatment with rapamycin leads to strong suppression of mTOR activity in these cells (Fig. 5B). In contrast, treatment with FC-rapa had no effect on these cells. Together with the observed lack of effect of FC-rapa on the spleen (Fig. 4B), these results suggest that FC-rapa has little immunosuppressive activity on the immune-related organs examined.
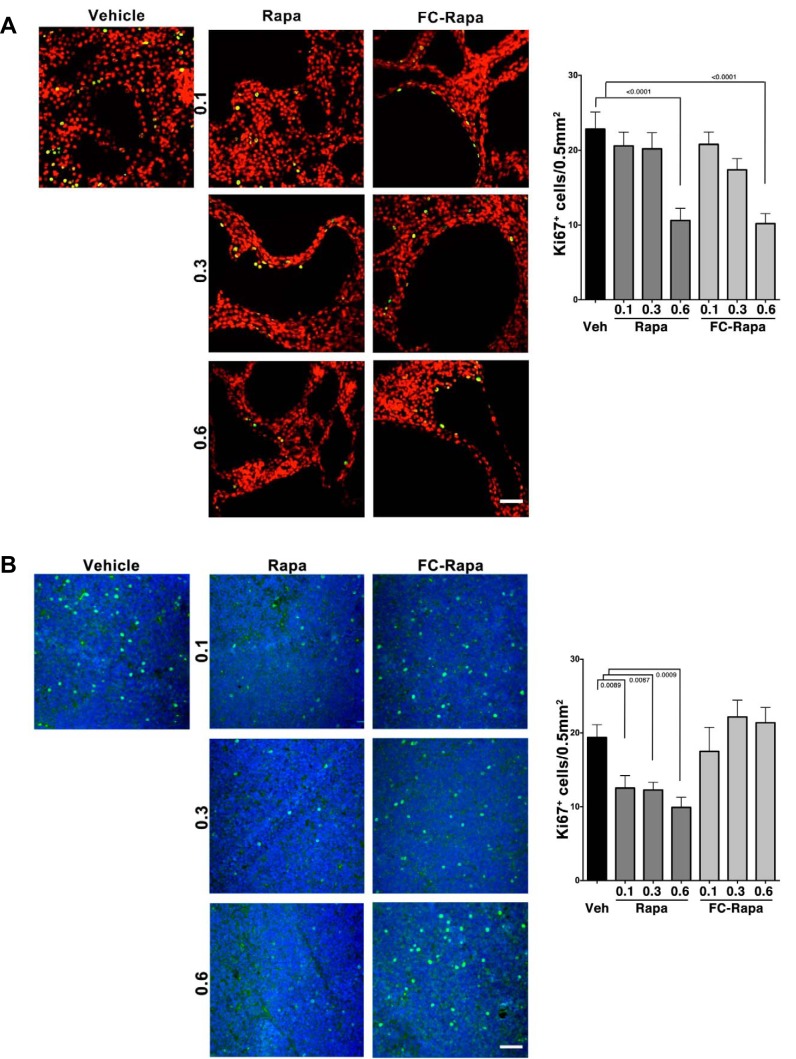
Cell cycle suppression is thought to be a major mechanism by which mTOR inhibition antagonizes renal cyst growth in PKD (32). We assessed the effect of rapamycin and FC-rapa on the cell cycle by monitoring the degree of cellular Ki-67 positivity. Treatment with both rapamycin and FC-rapa led to a comparable effect on the number of Ki-67-positive cyst-lining cells, with a significant decrease by ~50% observed with the highest doses of either drug (Fig. 6A). In contrast, while cycling cells in the thymus are significantly inhibited by rapamycin treatment, they are unaffected by FC-rapa (Fig. 6B). These results again suggest that FC-rapa, in contrast to rapamycin, does not affect extrarenal, immune-related organs.
Fig. 6.
Effect of folate-conjugated rapamycin (FC-rapa) versus unconjugated rapamycin on cell cycling in polycystic kidney and thymus. A: images are representative of immunofluorescence staining for the cell cycle marker Ki-67 in polycystic kidneys of different treatment groups. Green is Ki-67, and red is DAPI. Quantification based on counting of Ki-67-positive cyst-lining cells per 0.5-mm2 field; n = 15 total fields from 5 animals per treatment group. Error bars represent standard error calculated based on ordinary one-way ANOVA statistics. B: matched thymus staining representative of immunofluorescence staining for Ki-67 in thymus sections of different treatment groups. Green is Ki-67, and blue is DAPI. Quantification based on counting of Ki-67-positive cells per 0.5-mm2 field; n = 15 total fields from 5 animals per treatment group. Error bars represent SE, and statistical analysis were calculated using ordinary one-way ANOVA. Scale bars are 50 = μm.
DISCUSSION
We report here that FC-rapa is similarly effective as unconjugated rapamycin in a head-to-head comparison on an orthologous mouse model of PKD. Both drugs affect renal cyst growth, mTOR activity, proliferation, and fibrosis to a similar extent. The minimal comparative difference in drug efficacy on the renal cystic phenotype at the total body doses tested is likely due to several factors, including the specific nature of cellular entry (lipohilic versus endocytic), specific FRα status, and the relative differences in pharmacokinetics between these compounds. However, in contrast to unconjugated rapamycin, FC-rapa does not affect body weight gain, thymus or spleen weight, mTOR activity in thymus and spleen, or cell cycling in the thymus at doses that are effective on the polycystic kidneys. We therefore conclude that extrarenal effects, in particular on organ systems of the immune system, are greatly reduced with FC-rapa compared with unconjugated rapamycin. The efficacy of FC-rapa to ameliorate renal cystic disease and the simultaneous lack of extrarenal adverse effects are similar to what we previously observed using a nonorthologous mouse model of PKD (29). Therefore, the present report increases confidence that FC-rapa, or other folate-conjugated compounds, would exhibit similar specificity toward polycystic kidneys in ADPKD patients.
The concept of exploiting FRα as a cellular, endocytosis-mediated mechanism to deliver therapeutic payloads into cells has been demonstrated and discussed previously (2, 40). The premise of FRα targeting is based on the observation that FRα expression is significantly upregulated in a variety of cancer indications compared with their respective normal tissues (19). While in normal tissues FRα is most highly expressed in kidney epithelial cells, it is also expressed in other normal tissues. For example, in humans, other sites of FRα expression include the choroid plexus, lung, and small intestine (6, 19, 45). However, biodistribution studies with EC20, a folate-targeted, technetium-based imaging agent, in human patients reveals very little uptake by the choroid plexus and no uptake in the lung and it has been proposed that the specific polarized location of FRα in epithelial tissues (i.e., apical or basolateral) may determine whether the receptor would be accessible to an intravenous injection of an FR-targeted conjugate (4, 25). Furthermore, it is important to point out that to date minimal toxicity has been observed with folate-targeted lead small molecule drug conjugates in both animal models (22–24) and human subjects (7, 11, 12, 14). In addition to the polarized nature and relative accessibility of FRα, as discussed above, this may in part be due to the expression of the reduced folate carrier, which is the primary mediator of folate transport in most normal epithelial cells and for which FR-targeted conjugates do not serve as substrates. However, we acknowledge that the majority of studies to date have primarily been focused on various cancer indications in an acute setting so the long-term, chronic effects of, for example, FC-rapa administration in an ADPKD population, including potential accumulation and tissue toxicity in FRα-positive extrarenal tissues over time, have yet to be evaluated and may require monitoring in a clinical setting.
Many promising molecular drug targets have been identified in PKD, and many small molecule drugs have shown efficacy in PKD rodent models (46). Common to almost all of them is that these molecular drug targets are not uniquely expressed in PKD kidneys but have important functions in many extrarenal tissues as well. For example, aberrant activation of the EGFR is well documented in PKD, and a small molecule EGFR inhibitor significantly reduced renal cyst growth in a PKD mouse model (18, 26, 33, 39). However, similar small molecule EGFR inhibitors have proven problematic for long-term cancer therapy due to skin and gastrointestinal toxicities (5, 20), and their use for ADPKD therapy is therefore probably not feasible. Besides rapamycin, folate-conjugation could be a promising strategy to greatly improve tissue specificity of small-molecule drugs against many molecular targets implicated in PKD and may make it feasible to use such compounds for long-term therapy in ADPKD.
GRANTS
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-078043 and R01-DK-109563 and Department of Defense Grant PRMRP W81XWH-07–1-0509 (to T. Weimbs) and gifts from the Lillian Goldman Charitable Trust and the Amy P. Goldman Foundation to University of California, Santa Barbara to support the work of T. Weimbs.
DISCLOSURES
N.P., J.M.S. and C.P.L. are or were employees of Endocyte, Inc.
AUTHOR CONTRIBUTIONS
K.R.K., S.L.K., J.M.S., and T.W. conceived and designed research; K.R.K., S.L.K., M.F.S., N.P., and J.M.S. performed experiments; K.R.K., S.L.K., M.F.S., N.P., J.M.S., and T.W. analyzed data; K.R.K., S.L.K., M.F.S., N.P., J.M.S., C.P.L., and T.W. interpreted results of experiments; K.R.K., S.L.K., M.F.S., N.P., J.M.S., and T.W. prepared figures; K.R.K., S.L.K., J.M.S., and T.W. drafted manuscript; K.R.K., S.L.K., J.M.S., C.P.L., and T.W. edited and revised manuscript; T.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Alyssa Sahu for help with experiments.
Present address of J. M. Shillingford: Dept. of Internal Medicine, Division of Nephrology, Univ. of Michigan, Ann Arbor, MI.
REFERENCES
- 1.Antignac C, Calvet JP, Germino GG, Grantham JJ, Guay-Woodford LM, Harris PC, Hildebrandt F, Peters DJ, Somlo S, Torres VE, Walz G, Zhou J, Yu AS. The future of polycystic kidney disease research–as seen by the 12 Kaplan awardees. J Am Soc Nephrol 26: 2081–2095, 2015. doi: 10.1681/ASN.2014121192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assaraf YG, Leamon CP, Reddy JA. The folate receptor as a rational therapeutic target for personalized cancer treatment. Drug Resist Updat 17: 89–95, 2014. doi: 10.1016/j.drup.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Erickson KF, Chertow GM, Goldhaber-Fiebert JD. Cost-effectiveness of tolvaptan in autosomal dominant polycystic kidney disease. Ann Intern Med 159: 382–389, 2013. doi: 10.7326/0003-4819-159-6-201309170-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher RE, Siegel BA, Edell SL, Oyesiku NM, Morgenstern DE, Messmann RA, Amato RJ. Exploratory study of 99mTc-EC20 imaging for identifying patients with folate receptor-positive solid tumors. J Nucl Med 49: 899–906, 2008. doi: 10.2967/jnumed.107.049478. [DOI] [PubMed] [Google Scholar]
- 5.Galimont-Collen AF, Vos LE, Lavrijsen AP, Ouwerkerk J, Gelderblom H. Classification and management of skin, hair, nail and mucosal side-effects of epidermal growth factor receptor (EGFR) inhibitors. Eur J Cancer 43: 845–851, 2007. doi: 10.1016/j.ejca.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Grapp M, Wrede A, Schweizer M, Hüwel S, Galla H-J, Snaidero N, Simons M, Bückers J, Low PS, Urlaub H, Gärtner J, Steinfeld R. Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat Commun 4: 2123, 2013. doi: 10.1038/ncomms3123. [DOI] [PubMed] [Google Scholar]
- 7.Herzog TJ, Kutarska E, Bidzińsk M, Symanowski J, Nguyen B, Rangwala RA, Naumann RW. Adverse event profile by folate receptor status for vintafolide and pegylated liposomal doxorubicin in combination, versus pegylated liposomal doxorubicin alone, in platinum-resistant ovarian cancer: exploratory analysis of the Phase II PRECEDENT Trial. Int J Gynecol Cancer 26: 1580–1585, 2016. doi: 10.1097/IGC.0000000000000806. [DOI] [PubMed] [Google Scholar]
- 8.Karihaloo A, Koraishy F, Huen SC, Lee Y, Merrick D, Caplan MJ, Somlo S, Cantley LG. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 22: 1809–1814, 2011. doi: 10.1681/ASN.2011010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HJ, Edelstein CL. Mammalian target of rapamycin inhibition in polycystic kidney disease: From bench to bedside. Kidney Res Clin Pract 31: 132–138, 2012. doi: 10.1016/j.krcp.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kipp KR, Rezaei M, Lin L, Dewey EC, Weimbs T. A mild reduction of food intake slows disease progression in an orthologous mouse model of polycystic kidney disease. Am J Physiol Renal Physiol 310: F726–F731, 2016. doi: 10.1152/ajprenal.00551.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Sausville EA, Klein PJ, Morgenstern D, Leamon CP, Messmann RA, LoRusso P. Clinical pharmacokinetics and exposure-toxicity relationship of a folate-Vinca alkaloid conjugate EC145 in cancer patients. J Clin Pharmacol 49: 1467–1476, 2009. doi: 10.1177/0091270009339740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorusso PM, Edelman MJ, Bever SL, Forman KM, Pilat M, Quinn MF, Li J, Heath EI, Malburg LM, Klein PJ, Leamon CP, Messmann RA, Sausville EA. Phase I study of folate conjugate EC145 (Vintafolide) in patients with refractory solid tumors. J Clin Oncol 30: 4011–4016, 2012. doi: 10.1200/JCO.2011.41.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Y, Parker N, Kleindl PJ, Cross VA, Wollak K, Westrick E, Stinnette TW, Gehrke MA, Wang K, Santhapuram HK, You F, Hahn SJ, Vaughn JF, Klein PJ, Vlahov IR, Low PS, Leamon CP. Antiinflammatory activity of a novel folic acid targeted conjugate of the mTOR inhibitor everolimus. Mol Med 21: 584–596, 2015. doi: 10.2119/molmed.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naumann RW, Coleman RL, Burger RA, Sausville EA, Kutarska E, Ghamande SA, Gabrail NY, Depasquale SE, Nowara E, Gilbert L, Gersh RH, Teneriello MG, Harb WA, Konstantinopoulos PA, Penson RT, Symanowski JT, Lovejoy CD, Leamon CP, Morgenstern DE, Messmann RA. PRECEDENT: a randomized phase II trial comparing vintafolide (EC145) and pegylated liposomal doxorubicin (PLD) in combination versus PLD alone in patients with platinum-resistant ovarian cancer. J Clin Oncol 31: 4400–4406, 2013. doi: 10.1200/JCO.2013.49.7685. [DOI] [PubMed] [Google Scholar]
- 15.Olsan EE, Matsushita T, Rezaei M, Weimbs T. Exploitation of the polymeric immunoglobulin receptor for antibody targeting to renal cyst lumens in polycystic kidney disease. J Biol Chem 290: 15679–15686, 2015. doi: 10.1074/jbc.M114.607929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong AC, Devuyst O, Knebelmann B, Walz G; ERA-EDTA Working Group for Inherited Kidney Diseases . Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet 385: 1993–2002, 2015. [Erratum in Lancet 385: 2576, 2015]. doi: 10.1016/S0140-6736(15)60907-2. [DOI] [PubMed] [Google Scholar]
- 17.Ong AC, Harris PC. A polycystin-centric view of cyst formation and disease: the polycystins revisited. Kidney Int 88: 699–710, 2015. doi: 10.1038/ki.2015.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orellana SA, Sweeney WE, Neff CD, Avner ED. Epidermal growth factor receptor expression is abnormal in murine polycystic kidney. Kidney Int 47: 490–499, 1995. doi: 10.1038/ki.1995.62. [DOI] [PubMed] [Google Scholar]
- 19.Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem 338: 284–293, 2005. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 20.Pérez-Soler R, Delord JP, Halpern A, Kelly K, Krueger J, Sureda BM, von Pawel J, Temel J, Siena S, Soulières D, Saltz L, Leyden J. HER1/EGFR inhibitor-associated rash: future directions for management and investigation outcomes from the HER1/EGFR inhibitor rash management forum. Oncologist 10: 345–356, 2005. doi: 10.1634/theoncologist.10-5-345. [DOI] [PubMed] [Google Scholar]
- 22.Reddy JA, Bloomfield A, Nelson M, Dorton R, Vetzel M, Leamon CP. Abstract 832: pre-clinical development of EC1456: a potent folate targeted tubulysin SMDC. Cancer Res 74, Suppl. 19: 832–832, 2014. doi: 10.1158/1538-7445.AM2014-832. [DOI] [Google Scholar]
- 23.Reddy JA, Bloomfield A, Taylor C, Hargett K, Nelson M, Leamon C. Abstract 5359: antitumor efficacy of EC1456 in patient derived xenograft models of ovarian, endometrial, NSCLC and TNBC (Abstract). Cancer Res 75, Suppl. 15: 5359–5359, 2015. doi: 10.1158/1538-7445.AM2015-5359. [DOI] [Google Scholar]
- 24.Reddy JA, Westrick E, Santhapuram HK, Howard SJ, Miller ML, Vetzel M, Vlahov I, Chari RVJ, Goldmacher VS, Leamon CP. Folate receptor-specific antitumor activity of EC131, a folate-maytansinoid conjugate. Cancer Res 67: 6376–6382, 2007. doi: 10.1158/0008-5472.CAN-06-3894. [DOI] [PubMed] [Google Scholar]
- 25.Reddy JA, Xu LC, Parker N, Vetzel M, Leamon CP. Preclinical evaluation of (99m)Tc-EC20 for imaging folate receptor-positive tumors. J Nucl Med 45: 857–866, 2004. [PubMed] [Google Scholar]
- 26.Richards WG, Sweeney WE, Yoder BK, Wilkinson JE, Woychik RP, Avner ED. Epidermal growth factor receptor activity mediates renal cyst formation in polycystic kidney disease. J Clin Invest 101: 935–939, 1998. doi: 10.1172/JCI2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitz F, Heit A, Dreher S, Eisenächer K, Mages J, Haas T, Krug A, Janssen KP, Kirschning CJ, Wagner H. Mammalian target of rapamycin (mTOR) orchestrates the defense program of innate immune cells. Eur J Immunol 38: 2981–2992, 2008. doi: 10.1002/eji.200838761. [DOI] [PubMed] [Google Scholar]
- 28.Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, Scheffel H, Weishaupt D, Wüthrich RP. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 363: 820–829, 2010. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 29.Shillingford JM, Leamon CP, Vlahov IR, Weimbs T. Folate-conjugated rapamycin slows progression of polycystic kidney disease. J Am Soc Nephrol 23: 1674–1681, 2012. doi: 10.1681/ASN.2012040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA 103: 5466–5471, 2006. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shillingford JM, Piontek KB, Germino GG, Weimbs T. Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1. J Am Soc Nephrol 21: 489–497, 2010. doi: 10.1681/ASN.2009040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stayner C, Shields J, Slobbe L, Shillingford JM, Weimbs T, Eccles MR. Rapamycin-mediated suppression of renal cyst expansion in del34 Pkd1-/- mutant mouse embryos: an investigation of the feasibility of renal cyst prevention in the foetus. Nephrology (Carlton) 17: 739–747, 2012. doi: 10.1111/j.1440-1797.2012.01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sweeney WE Jr, Hamahira K, Sweeney J, Garcia-Gatrell M, Frost P, Avner ED. Combination treatment of PKD utilizing dual inhibition of EGF-receptor activity and ligand bioavailability. Kidney Int 64: 1310–1319, 2003. doi: 10.1046/j.1523-1755.2003.00232.x. [DOI] [PubMed] [Google Scholar]
- 34.Talbot JJ, Song X, Wang X, Rinschen MM, Doerr N, LaRiviere WB, Schermer B, Pei YP, Torres VE, Weimbs T. The cleaved cytoplasmic tail of polycystin-1 regulates Src-dependent STAT3 activation. J Am Soc Nephrol 25: 1737–1748, 2014. doi: 10.1681/ASN.2013091026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 284: 8023–8032, 2009. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torres VE, Boletta A, Chapman A, Gattone V, Pei Y, Qian Q, Wallace DP, Weimbs T, Wüthrich RP. Prospects for mTOR inhibitor use in patients with polycystic kidney disease and hamartomatous diseases. Clin J Am Soc Nephrol 5: 1312–1329, 2010. doi: 10.2215/CJN.01360210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres VE, Gansevoort RT, Czerwiec FS. Tolvaptan in autosomal dominant polycystic kidney disease. N Engl J Med 368: 1259, 2013. doi: 10.1056/NEJMc1300762. [DOI] [PubMed] [Google Scholar]
- 38.Torres VE, Higashihara E, Devuyst O, Chapman AB, Gansevoort RT, Grantham JJ, Perrone RD, Ouyang J, Blais JD, Czerwiec FS; TEMPO 3:4 Trial Investigators . Effect of tolvaptan in autosomal dominant polycystic kidney disease by CKD stage: results from the TEMPO 3:4 Trial. Clin J Am Soc Nephrol 11: 803–811, 2016. doi: 10.2215/CJN.06300615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torres VE, Sweeney WE Jr, Wang X, Qian Q, Harris PC, Frost P, Avner ED. EGF receptor tyrosine kinase inhibition attenuates the development of PKD in Han:SPRD rats. Kidney Int 64: 1573–1579, 2003. doi: 10.1046/j.1523-1755.2003.00256.X. [DOI] [PubMed] [Google Scholar]
- 40.Vergote I, Leamon CP. Vintafolide: a novel targeted therapy for the treatment of folate receptor expressing tumors. Ther Adv Med Oncol 7: 206–218, 2015. doi: 10.1177/1758834015584763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlahov IR, Leamon CP. Engineering folate-drug conjugates to target cancer: from chemistry to clinic. Bioconjug Chem 23: 1357–1369, 2012. doi: 10.1021/bc2005522. [DOI] [PubMed] [Google Scholar]
- 42.Walz G, Budde K, Mannaa M, Nürnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Hörl WH, Obermüller N, Arns W, Pavenstädt H, Gaedeke J, Büchert M, May C, Gschaidmeier H, Kramer S, Eckardt KU. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 363: 830–840, 2010. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 43.Watkins PB, Lewis JH, Kaplowitz N, Alpers DH, Blais JD, Smotzer DM, Krasa H, Ouyang J, Torres VE, Czerwiec FS, Zimmer CA. Clinical pattern of tolvaptan-associated liver injury in subjects with autosomal dominant polycystic kidney disease: analysis of clinical trials database. Drug Saf 38: 1103–1113, 2015. doi: 10.1007/s40264-015-0327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watnick T, Germino GG. mTOR inhibitors in polycystic kidney disease. N Engl J Med 363: 879–881, 2010. doi: 10.1056/NEJMe1006925. [DOI] [PubMed] [Google Scholar]
- 45.Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, Zurawski VR Jr, Kamen BA. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res 52: 3396–3401, 1992. [PubMed] [Google Scholar]
- 46.Wilson PD. Therapeutic targets for polycystic kidney disease. Expert Opin Ther Targets 20: 35–45, 2016. doi: 10.1517/14728222.2015.1083979. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Dublin P, Griemsmann S, Klein A, Brehm R, Bedner P, Fleischmann BK, Steinhäuser C, Theis M. Germ-line recombination activity of the widely used hGFAP-Cre and nestin-Cre transgenes. PLoS One 8: e82818, 2013. doi: 10.1371/journal.pone.0082818. [DOI] [PMC free article] [PubMed] [Google Scholar]