Abstract
Sepsis-associated acute kidney injury (S-AKI) independently predicts mortality among critically ill patients. The role of innate immunity in this process is unclear, and there is an unmet need for S-AKI models to delineate the pathophysiological response. Mammals and zebrafish (Danio rerio) share a conserved nephron structure and homologous innate immune systems, making the latter suitable for S-AKI research. We introduced Edwardsiella tarda to the zebrafish. Systemic E. tarda bacteremia resulted in sustained bacterial infection and dose-dependent mortality. A systemic immune reaction was characterized by increased mRNA expressions of il1b, tnfa, tgfb1a, and cxcl8-l1 (P < 0.0001, P < 0.001, P < 0.001, and P < 0.01, respectively). Increase of host stress response genes ccnd1 and tp53 was observed at 24 h postinjection (P < 0.0001 and P < 0.05, respectively). Moderate E. tarda infection induced zebrafish mortality of over 50% in larvae and 20% in adults, accompanied by pericardial edema in larvae and renal dysfunction in both larval and adult zebrafish. Expression of AKI markers insulin-like growth factor-binding protein-7 (IGFBP7), tissue inhibitor of metalloproteinases 2 (TIMP-2), and kidney injury molecule-1 (KIM-1) was found to be significantly increased in the septic animals at the transcription level (P < 0.01, P < 0.05, and P < 0.05) and in nephric tubules compared with noninfected animals. In conclusion, we established a zebrafish model of S-AKI induced by E. tarda injection, with both larval and adult zebrafish showing nephron injury in the setting of infection.
Keywords: acute kidney injury, innate immunity, zebrafish infection
INTRODUCTION
Sepsis is a systemic response triggered by infection. Although accounting for <10% of hospital admissions, sepsis contributes to half of all hospital deaths in the United States (30). Multiple organ dysfunction syndrome is the main cause of death from sepsis, and renal dysfunction has been shown to contribute to this mortality (8). Despite advances in modern medicine, the underlying mechanisms responsible for sepsis-associated acute kidney injury (S-AKI) are still unclear.
The innate immune response is increasingly thought to play a major role in the pathophysiological changes responsible for S-AKI (13). As the first line of defense, innate immunity is activated by host-pathogen interactions, including those between pattern recognition receptors (PRRs) and invading pathogen-associated molecular patterns (PAMPs) originating from exogenous bacteria and/or danger-associated molecular patterns (DAMPs), endogenous “alarmins” released from injured tissue. Although established models in higher vertebrates have successfully recapitulated the major features of human S-AKI (9), there is a continuous and pressing need for additional tools to better understand the signaling networks involved. In addition, the interaction between kidney damage and dysfunction and their association with innate immune activity remain unclear.
The zebrafish (Danio rerio) has an immune system that is similar to that of humans (43). However, larvae possess only innate immunity, whereas the adaptive immune system does not mature until the juvenile stage, at 4 wk postfertilization (27). Given this developmental feature, zebrafish larvae have been used as a model to achieve several breakthroughs in the understanding of host-pathogen interactions that would have been difficult to realize with traditional models (46). In addition, zebrafish kidney develops and functions in almost the same way as that of humans: their key pronephros genes have been identified as homologous to those of humans (14). The larval pronephros starts functioning as early as 48 h postfertilization (hpf; 10). Moreover, nephrotoxic AKI and laser ablation-induced AKI have been successfully developed using zebrafish larvae (17).
We sought to establish a zebrafish model of S-AKI. By challenging zebrafish with a fish-specific pathogen, we tested whether zebrafish develop S-AKI and whether PAMPs invasion and activated innate immunity alone could cause AKI in zebrafish larvae. Successful development of this model will enrich the S-AKI study toolkit, facilitating better understanding of the mechanisms of S-AKI.
MATERIALS AND METHODS
Design.
The study was designed to induce a systemic inflammation (sepsis) with controllable mortality, to identify signs of AKI in both larval and adult zebrafish. All studies of larvae were performed at the age of 78–82 hpf, and adult zebrafish studies were performed at the age of 1 yr. Male and female animals were randomly assigned to study groups.
Animals.
Zebrafish [Pitt AB and the transgenic lines Tg(PT::eGFP) and Tg(cdh17::eGFP)] were raised at 28.5°C under a 14:10-h light-dark cycle in regular tank water at the University of Pittsburgh zebrafish facility (6). Procedures were conducted per the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experimental adult zebrafish and larvae were incubated in E3 medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, and 0.33 mM MgSO4, buffered to pH 7.2 with 10 mM HEPES) and maintained according to The Zebrafish Book (50). Healthy adult zebrafish (Pitt AB, 1 yr old) were used for adult experiments. Larvae were obtained from spawning eggs, incubated in E3 medium-0.01 mg/l methylene blue (Sigma-Aldrich) or 0.003% 1-phenyl-2-thiourea (Sigma-Aldrich) for 78–82 h, and then subject to experiments. To deactivate the translational activity of the target gene, morpholino antisense oligonucleotides (0.50 mM with 0.050% phenol red; Gene Tools) were injected into zebrafish embryos. Morpholino oligonucleotide sequences complementary to igfbp7 and timp2 translation-blocking targets were 5′-GCGAGGACGAGAACACACAGCAT-3′ and 5′-ACAGCTCCTGACGCTCTTCATTTTC-3′, respectively. Standard morpholino control oligonucleotides were injected as controls. Tricaine (MS-222; Sigma-Aldrich) in E3 medium (buffered to pH 7 with 1 M Tris) was used for anesthesia (at 0.168 mg/ml) before and during experimental operations and for euthanasia (at 0.64 mg/ml).
Bacterial infection.
Edwardsiella tarda (Ewing and McWhorter O1483:H1; ATCC 15947; American Type Culture Collection) were microinjected into the larval bloodstream through the duct of Cuvier. After culturing in lysogeny broth medium (Sigma-Aldrich) overnight at 37°C with shaking, the bacteria were centrifuged and resuspended in PBS-0.2% phenol red (Sigma-Aldrich) to the desired concentrations. To induce different severities of infection, an inoculum of E. tarda, 20 µl for adult fish (administered intraperitoneally) and 1 nl for larvae (administered intravenously), at incremental concentrations was injected into zebrafish, and zebrafish were observed for 7 days. Based on mortality rate, inocula of 6 × 106 colony-forming units (CFU) E. tarda, which induced 20% mortality in adult zebrafish, and 300 CFU E. tarda, which induced over 50% mortality in larvae, were selected as moderate infection dosages for the following studies. All infection pathological changes were obtained from moderate infection samples unless otherwise stated. The same volume of vehicle (0.2% phenol red-PBS) was also injected into another group of animals serving as noninfected controls. To determine the bacterial burden, the number of CFUs was quantified in infected zebrafish larvae. Specifically, groups of five larvae were triturated by repeated pipetting in 100 μl of PBS containing 1% Triton X-100 (Sigma-Aldrich). Serial dilutions of this suspension were then cultured on streptomycin-selective agar plates for CFU assessment.
RNA extraction and quantitative PCR.
After being injected with E. tarda, larvae were euthanized and snap-frozen at 24 h post-bacterial injection. To obtain sufficient amounts of RNA, samples of 50 larvae were pooled and subjected to TRIzol (Invitrogen) and chloroform extraction. Samples was then precipitated with isopropanol, washed with 75% ethanol, and suspended in diethyl pyrocarbonate-treated water. After purification with RNeasy Mini Kit (Qiagen), RNA concentration was measured using the NanoDrop system (Thermo Fisher Scientific). Sample DNA was degraded using DNase I (Thermo Fisher Scientific), and cDNA was subsequently prepared with a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Quantitative PCR was performed on a Stratagene Mx3000P instrument (Agilent Technologies) using SYBR Select Master Mix (Thermo Fisher Scientific). The primers were used on the basis of previous publications (28, 36, 52) or designed using Primer3 (http://bioinfo.ut.ee/primer3-0.4.0/primer3/; Table 1). Fold changes in the expression of each target gene normalized to that of gapdh were determined as follows: fold change = 2−Δ(ΔCt), where change in threshold cycle (ΔCt) = Cttarget – Ctgapdh and Δ(ΔCt) = ΔCtinfected – ΔCtcontrol. Each sample was processed in duplicate for quantification of expression.
Table 1.
Primers for quantitative real-time PCR
| Gene | Primer | Sequence 5′–3′ | Reference |
|---|---|---|---|
| cxcl8-l1 | Forward | TGTTTTCCTGGCATTTCTGACC | (52) |
| Reverse | TTTACAGTGTGGGCTTGGAGGG | ||
| il1b | Forward | TGCGGGCAATATGAAGTCA | (52) |
| Reverse | TTCGCCATGAGCATGTCC | ||
| tnfa | Forward | CACTGATAGAACAACCCAGCAAACT | (52) |
| Reverse | GCAACTCTCCTTCTTCAACATCCAA | ||
| tgfb1a | Forward | CGACTGTAAAGCAAACCAGCAGAGCACG | (36) |
| Reverse | GTGTCCTCCCATTGAGATGTTATGTATGTCC | ||
| tp53 | Forward | ACCACTGGGACCAAACGTAG | (36) |
| Reverse | CAGAGTCGCTTCTTCCTTCG | ||
| ccnd1 | Forward | CGCGACGTGGATGCTCGAGGTCTGTGAAGA | (36) |
| Reverse | GGAAGTTGGTGAGGTTCTGGGATGAGAGGC | ||
| col1a1a | Forward | TCATGTCCACTGAGGCCTCCCAGAACATTAC | (36) |
| Reverse | GTTTCGCTCTTTCATTGTCCTTCCTCAGTGG | ||
| timp2a | Forward | CATTGACGTGTCTTTACTGCGCCCTCATC | (36) |
| Reverse | GGGGGGCAGAAAGTGCTCTCGTTTTAAAGG | ||
| gapdh | Forward | ACTTTGTCATCGTTGAAGGT | (28) |
| Reverse | TGTCAGATCCACAACAGAGA | ||
| igfbp7 | Forward | GACGGACGGAACTACAACAGC | |
| Reverse | TGCACCGCCAGATTATCTTTATCTC | ||
| kim1 | Forward | GTTCTCCTGTTACTGTTGGCTTTGA | |
| Reverse | TAATGCCACTGTTCGTATTCGCTTT |
Genes: ccnd1, cyclin D1; col1a1, collagen, type I, α1a; cxcl8-l1, zebrafish homolog of mammalian chemokine (C-X-C motif) ligand 8; igfbp7, insulin-like growth factor-binding protein-7; il1b, interleukin-1β; kim1, kidney injury molecule-1; tgfb1a, transforming growth factor-β1a; timp2a, tissue inhibitor of metalloproteinases 2a; tnfa, tumor necrosis factor-α; tp53, tumor protein p53.
Live imaging.
Live imaging was used to assess kidney excretion function in larvae (17, 33). Specifically, anesthetized larvae were injected into the duct of Cuvier with 40-kDa FITC-dextran (Sigma-Aldrich) at a concentration of 10 mg/ml 2 days after injection of bacteria. To observe changes over time, larvae were anesthetized, transferred to a depression slide, oriented, immobilized in 2% methylcellulose (Sigma-Aldrich) in E3 medium, and imaged under a ZX10 stereomicroscope (Olympus). Bright-field images were obtained 3, 24, 48, and 72 h after injection of bacteria for morphological analysis of edema-like formations. Intensities of in vivo fluorescence in larval pericardia were measured 3 and 24 h after injection of FITC-dextran to evaluate kidney function. Larvae were anesthetized for no longer than 15 min to ensure their vitality and were returned to fresh E3 medium to recover. Fluorescence intensities were quantified using ImageJ (https://imagej.nih.gov/ij/; National Institutes of Health) by measuring average grayscale values in a fixed region of interest of equal size across images and processed with Photoshop (Adobe Systems). Cardiac rates were also recorded by visual inspection. The baseline was arbitrarily set at 150 beats/min, and the heart rate index was acquired from the ratio of actual rate to baseline rate. Normalized renal clearance index was expressed as the total excreted fluorescence normalized by the heart rate index, as follows: pronephric filtration capacity = (FITC intensity3h – FITC intensity24h)/heart rate index.
Renal tubular endocytosis.
Tubular endocytosis was evaluated for tubular epithelial cell injury and dysfunction. Similar to mammalian proximal tubular epithelial cells in the kidney, zebrafish nephrons express an endocytic receptor, megalin/LDL receptor-related protein-2 (LRP2), which plays a crucial role in renal endocytic machinery. Anzenberger et al. reported that 70-kDa dextran was taken up through megalin/LRP2 endocytic receptor in the zebrafish nephron after injection (1). The reabsorbed dextran penetrates the intercellular spaces and tight junctions between tubular cells from the peritubular capillaries; the latter are permeable to all dextran fractions (4). Briefly, 70-kDa tetrametylrhodamine isothiocyanate (TRITC)-dextran (Sigma-Aldrich; 20-µl volume at a concentration of 25 mg/ml in 0.05% phenol red-PBS) was injected into anesthetized adult zebrafish at 1 day postinfection. Uptake of dextran by the nephron tubule segment was examined 18–24 h post-dextran injection. Zebrafish were euthanized, and the abdomen was immediately opened by making a ventral incision from the head to the base of the caudal fin. After removing the internal organs, the nephron was exposed, detached from the dorsal wall, and washed three times in PBS. The zebrafish nephron was then flat mounted in PBS on slides covered by a glass coverslip with modeling clay on each corner and subject to immediate examination under a ZX10 stereomicroscope (Olympus).
Immunofluorescence staining on whole mount larvae and cryosections.
For whole mount immunofluorescence staining, euthanized larvae were dissected in ice-cold PBS, removing as much unwanted abdominal tissue as possible. Samples were then fixed in an ice-cold solution of 4% (wt/vol) paraformaldehyde and 0.1% DMSO in PBS overnight at 4°C, before being treated with a series of sucrose solutions (10, 20, and 30% in PBS). After incubating overnight in 30% sucrose in PBS for permeabilization, samples were exposed to 5 µg/ml proteinase K in PBS containing 1% Triton X-100 (PBST) for 3 min for antigen retrieval and blocked with 10% FCS and 0.2% sodium azide in PBST for 2 h at room temperature. Primary and secondary antibodies in 2% FCS and 0.02% sodium azide in PBST were incubated sequentially with samples by gentle rotation at 4°C for 2–4 days. Typically, samples were washed three to five times with 0.2% sodium azide in PBST between each step to thoroughly remove residual material. Stained samples were equilibrated in 100% glycerol for 48 h until examined.
For immunofluorescence staining on larval cryosections, larvae were euthanized, fixed with acetone at −20°C for 15 min, embedded with tissue-freezing medium (Tedpella) in cryomolds (Tedpella), orientated to the proper position, frozen on dry ice for ~30 min, and stored at −80°C until sectioned. Samples were then sectioned (10 μm thick) through the entire larva and then air-dried completely for 30 min, rehydrated with 0.1% Tween 20-PBS (PTw) three times for 5 min, and then blocked with 10% sheep serum (Sigma-Aldrich) in PTw for 1 h at room temperature. Section samples were incubated with primary antibodies at 4°C overnight and then secondary antibody at room temperature for 1.5 h. PTw washes were performed three times between each step. The slides were overlaid with mounting medium with DAPI (Vector Laboratories), covered with coverslips, and stored at −20°C until observation under a FluoView FV1000 confocal microscope (Olympus). Images were processed using ImageJ and Photoshop.
Primary antibodies were used at a dilution of 1:100: rat anti-mouse kidney injury molecule-1 (KIM-1; Novus Biologicals), rabbit anti-human insulin-like growth factor-binding protein-7 (IGFBP7; Santa Cruz Biotechnology), and mouse anti-human tissue inhibitor of metalloproteinases 2 (TIMP-2; Abcam). Fluor-conjugated anti-rat, anti-rabbit, and anti-mouse secondary antibodies were used at a dilution of 1:1,000 (Jackson ImmunoResearch Laboratories).
Pathway analysis.
The associated functions were generated from the seven significant genes documented in this paper. Downstream effects (26) were predicted through the use of IPA (QIAGEN, https://www.qiagenbioinformatics.com/products/ingenuitypathway-analysis).
Statistical analysis.
Graphing and statistical tests to compare groups were performed using GraphPad Prism 7 (GraphPad Software). Kaplan-Meier curves are presented as percentages of surviving animals over 7 days, and the significance of difference was assessed by log-rank test. Numeric results are presented as means ± SE, and group differences were tested using two-tailed t-tests. P < 0.05 was considered an indication of significant difference.
Ethics approval.
Procedures involving adult and larval zebrafish were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
RESULTS
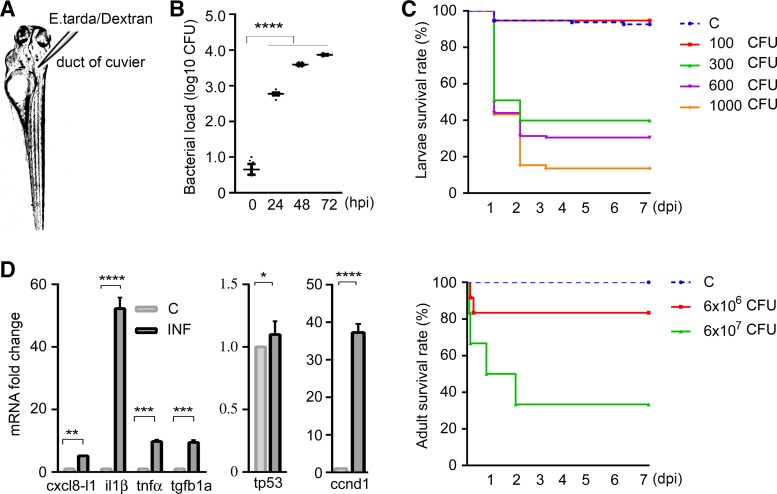
To establish an infection model in zebrafish, we injected E. tarda into 1-yr-old adult zebrafish or larvae at 78–82 hpf at the duct of Cuvier (Fig. 1). Bacterial load increased significantly in infected larvae [starting from 300 CFU and increasing to 600 and 7,333 CFU] from 24 to 72 h post-bacterial injection (Fig. 1B). Microinjection with 300 CFU or more caused 60–90% of larvae to die in a dose-dependent manner (P < 0.0001), whereas infection with 100 CFU did not result in significantly increased mortality compared with the PBS-injected controls. Adult zebrafish injected with 6 × 107 CFU E. tarda exhibited significantly increased mortality compared with controls (Fig. 1C). The appearance of sepsis was characterized by systemic increased immune mediators. Figure 1D shows mRNA fold increase for inflammatory cytokines. Expression of chemokine (C-X-C motif) ligand 8 (cxcl8-l1), interleukin-1β (il1b), tumor necrosis factor-α (tnfa), and transforming growth factor-β1a (tgfb1a) in E. tarda septic larvae was significantly increased compared with control animals (P < 0.01, P < 0.0001, P < 0.001, and P < 0.001, respectively). Transcriptional upregulation of stress response genes cyclin D1 (ccnd1) and tumor protein p53 (tp53) in septic larvae was significantly higher (P < 0.001 and P < 0.05, respectively) compared with control animals at 24 h post-bacterial injection (Fig. 1D).
Fig. 1.
Evidence of systemic infection in zebrafish. A: larvae were injected in the duct of Cuvier at 3 days postfertilization. B: bacterial loads of zebrafish larvae over time. The scatterplot represents means ± SE. Numbers of colony-forming units (CFU) were calculated using homogenates of 5 larvae per sample, n = 6 samples per group. Significant differences were detected between controls and septic larvae at 24, 48, and 72 h postinfection. C: Kaplan-Meier curve of 7 days postinjection revealed dose-dependent mortality of both larval and adult zebrafish in response to Edwardsiella tarda injections. Sample size for larval groups: control, n = 281; 100 CFU, n = 118; 300 CFU, n = 330; 600 CFU, n = 312; 1,000 CFU, n = 202; for adult groups: control, n = 10; 6 × 106 CFU, n = 12; 6 × 107 CFU, n = 12. Compared with control animals, 100-CFU injections to larvae and 6 × 106-CFU injections to adults had no significant impact on mortality, whereas larvae injected with ≥300 CFU and adults injected with 6 × 107 CFU E. tarda exhibited significantly increased mortality. D: fold changes in mRNA expressions (presented as means ± SE) of inflammatory cytokines and stress response genes were estimated using samples containing homogenates of 50 larvae per sample, n = 4 samples per group. Significant differences were found between control and infected zebrafish larvae injected with 300 CFU E. tarda 1 day postinjection. C, control; ccnd1, cyclin D1; cxcl8-l1, zebrafish homolog of mammalian C-X-C motif chemokine ligand 8; dpi, days postinjection; hpi, hours postinjection; il1β, interleukin-1β; INF, infected; tgfb1a, transforming growth factor-β1a; tnfα, tumor necrosis factor-α; tp53, tumor protein p53. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
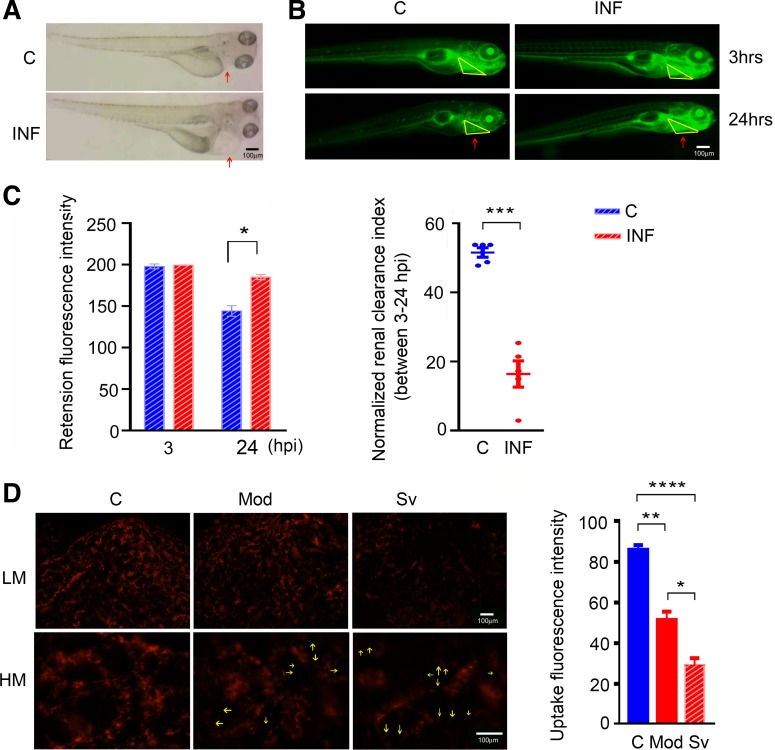
Infected larvae displayed visible edema compared with controls, which suggests fluid retention due to kidney dysfunction (Fig. 2A). To confirm this kidney dysfunction in septic zebrafish, we administered fluorescent dextran to the animals and compared small-molecular-mass dextran intensity (40-kDa FITC-dextran) in circulation for glomerular filtration rate (GFR; Fig. 2B) and middle-molecular-mass dextran intensity (70-kDa TRITC-dextran) in nephron tubules for tubular epithelial cell injury (Fig. 2D). Significantly higher circulating fluorescence intensity was measured in septic larval pericardium at 24 h post-dextran injection compared with control animals (P < 0.05), suggesting reduced renal clearance capacity (P < 0.001; Fig. 2C). Decreased glomerular perfusion pressure, interstitial edema, and tubular obstruction are believed to be among the main pathophysiological mechanisms for decreased GFR in both human and rodent models of S-AKI (2), and any pathological changes in septic animal nephrons could cause a GFR impairment. To test the functional integrity of tubular epithelial cells, TRITC-dextran (70 kDa) was injected into adult zebrafish. Adult zebrafish were used to confirm translatability of S-AKI from larvae to adults and to visualize cellular uptake in a multinephron system (28). TRITC-dextran (70 kDa) is filtered slowly through the glomerular barrier (16) and is therefore useful to visually measure renal tubular cell uptake. As a result, moderately and severely infected adult zebrafish (injected with 6 × 106 CFU and 6 × 107 CFU E. tarda, respectively) showed impaired tubular epithelial cell function compared with controls: low-magnification images show significantly lower nephron fluorescence intensities in the septic animals compared with controls (severe vs. moderate and control: P < 0.05 and P < 0.0001, respectively; moderate vs. control: P < 0.01), whereas higher magnification shows defective uptake of cells in the septic animal nephron (Fig. 2D). Collectively, these data point to nephron damage in the septic animals. Although extrarenal factors (i.e., hemodynamic changes) may also affect GFR readings, such changes would still be indicative of septic AKI.
Fig. 2.
Manifestations of nephron dysfunction during systemic infection in zebrafish. A: representative images of septic larvae showing larval edema in the pericardial area (red arrows) at 2 days after 300-CFU Edwardsiella tarda injection. B: after injection with 300 CFU E. tarda for 2 days, septic vs. control larvae were injected with FITC-dextran (40 kDa) and subjected to fluorescence intensity measurements in the pericardium under the stereomicroscope. Dextran excretions at 24 h post-dextran injection were compared with intensities at 3 h after the injections in the same animal. Representative images of fluorescence accumulation around the zebrafish pericardium (red arrows pointing to yellow triangular area) at the two time points are shown. C: bar graph shows quantified fluorescence intensities (means ± SE) at the two time points; n = 5 larvae per group. The scatterplot (presented as means ± SE) displays nephron clearance index (FITC intensity3h – FITC intensity24h) normalized by the larval heart rate. D: impaired endocytosis of dextran by the adult zebrafish nephron tubule depending on severity of infection. Representative images show lower nephron fluorescence accumulations in the infected animal nephrons compared with control, with the least seen in the severely infected animal. High-magnification images show endocytosis-defective cells in the infected animal nephrons (yellow arrows). The corresponding quantified fluorescence intensities are shown in the bar graph (means ± SE). Statistical analysis suggests significant differences among groups. n = 3 animals per group. C, control; HM, high magnification; hpi, hours postinjection; INF, infected; LM, low magnification; Mod, moderate; Sv, severe. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
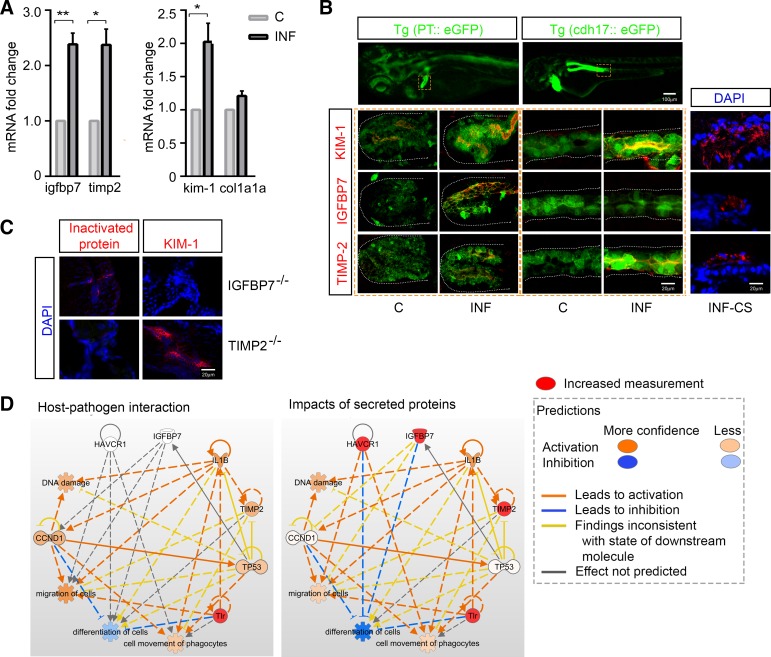
To test the expression of AKI biomarkers during sepsis in zebrafish and localize expression in the renal tubule, transcriptional levels of AKI biomarkers, namely, KIM-1, TIMP-2, and IGFBP7, were measured at 24 h after injection of E. tarda in larvae. Fold changes of kim1, timp2, and igfbp7 mRNA were significantly increased (compared with control animals: P < 0.05, P < 0.05, and P < 0.01, respectively) in septic animals, in contrast to no significant changes in the chronic fibrosis-associated gene collagen, type I, α1a (col1α1a) levels (compared with control animals: P > 0.05; Fig. 3A). To identify the spatial expression of these markers, we colocalized staining of the above-mentioned proteins with the transgenic zebrafish lines Tg(PT::eGFP) (7) or Tg(cdh17::eGFP) (37), which fluorescently label cells in the proximal pronephric tubule or the whole length of the pronephric tubule, respectively (Fig. 3B). Our results showed increased expression of TIMP-2 and IGFBP7 in the pronephros at 48 h post-bacterial injection, with TIMP-2 predominantly found in the distal tubule and IGFBP7 in the proximal tubule. Increased KIM-1 expression was detected in the whole length of the pronephros tubule in the septic larvae (Fig. 3B). We thus confirmed the expression of tubule injury markers in septic larvae at both protein and mRNA levels, which was consistent with the decline in pronephric filtration function. To dissect the possible associations among injury markers, igfbp7 and timp2 translations were inactivated by corresponding morpholino injections. Immunofluorescence staining showed that KIM-1 protein expression in infected larvae remained present in TIMP-2−/− animals whereas absent in IGFBP7−/− animals (Fig. 3C). These data suggest that all three markers, IGFBP7, TIMP-2, and KIM-1, were upregulated in response to infection and had increased expression at the protein level in the septic kidney tubule; expression of KIM-1 is affected by IGFBP7 but not by TIMP-2.
Fig. 3.
Expressions of kidney injury markers during systemic infection in zebrafish. A: fold changes in mRNA expressions (mean ± SE) of tubule injury marker genes in 300 CFU-infected vs. control larvae 24 h postinjection. Each sample contains 50 larvae, with n = ~3–4 samples per group. Significant differences were detected for igfbp7, timp2, and kim1 expression, as opposed to no changes of the chronic fibrosis marker gene col1a1a. B: expressions of kidney injury markers in septic animal (300 CFU) nephrons. Protein staining was colocalized with expression of enhanced green fluorescent protein (eGFP) in the transgenic zebrafish lines Tg(PT::eGFP) and Tg(cdh17::eGFP), which fluorescently label cells in the proximal and distal tubule, respectively. Whole mount and cross-section immunofluorescent staining identified the expression of kidney injury markers kidney injury molecule-1 (KIM-1), insulin-like growth factor-binding protein-7 (IGFBP7), and tissue inhibitor of metalloproteinases 2 (TIMP-2) (red) in the pronephric tubule (green) 48 h postinfection. Increased KIM-1 and IGFBP7 expressions were observed in the proximal tubule and increased KIM-1 and TIMP-2 expressions were observed in the distal tubule of septic zebrafish larvae. C: immunofluorescence staining of igfbp7−/− or timp2−/− larvae showing absent target proteins at 2 days post-Edwardsiella tarda (300 CFU) injections. As IGFBP7 and TIMP-2 protein expressions were inactivated, KIM-1 protein expression remained existent in TIMP-2−/− animals whereas absent in IGFBP7−/− animals during infection. D: pathway analysis showing the possible roles of upregulated kidney injury markers in addition to activated Toll-like receptor (TLR)-based host-pathogen cascades. Three major downstream functions were chosen, namely, migration of cells, differentiation of cells, and movement of phagocytes. Increased TIMP-2 was involved in TLR signaling, and KIM-1 [hepatitis A virus cellular receptor 1 (HAVCR1)] and IGFBP7 expressions had a combined inhibitory effect on cellular differentiation per the analysis. C, control; CCND1, cyclin D1; col1a1a, collagen, type I, α1a; CS, cross section; DAPI, fluorochrome 4′,6-diamidino-2-phenylindole; IL1B, interleukin-1β; INF, infected; Tg(cdh17::eGFP), transgenic zebrafish line that fluorescently labels cells in the pronephros whole length tubule; Tg(PT::eGFP), transgenic zebrafish line that fluorescently labels cells in the pronephros proximal tubule; TP53, tumor protein p53. *P < 0.05, **P < 0.01.
To better understand possible functions and generate hypotheses for increased expression of markers during AKI, we performed pathway analyses. Interaction between the pathogen and Toll-like receptors (TLRs), which are classic PRRs, upregulated il1b, tp53, and ccnd1; associated with events of DNA damage, cell migration, movement of phagocytes, and inhibited cell differentiation. Notably, TIMP-2 expression could increased as a consequence of TLR pathway activation. The analysis also suggested that KIM-1 [hepatitis A virus cellular receptor 1 (HAVCR1)] may promote cell migration and phagocyte movement, whereas TIMP-2 and IGFBP7 appear to inhibit these processes. KIM-1 and IGFBP7, but not TIMP-2, inhibited cell differentiation (individual results not shown). The overall consequence of activating KIM-1, IGFBP7, and TIMP-2 in addition to TLR activation likely was promotion of cell migration and phagocyte movement and inhibition of cell differentiation (Fig. 3D).
In summary, a systemic zebrafish infection was induced by injection of the bacteria E. tarda in both larval and adult animals. Similar to adult zebrafish nephron injuries, septic zebrafish larvae with only innate immunity resulted in characteristic features of AKI. Pathway analysis suggested that the effects of KIM-1 are in opposition to those of IGFBP7 and TIMP-2 on cell migration and phagocyte movement whereas both IGFBP7 and KIM-1 have inhibitory effects on cell differentiation after injury.
DISCUSSION
We established a zebrafish model of S-AKI by administration of the fish-specific pathogen, E. tarda bacteria. This model recapitulated several key features of human S-AKI, including cytokine upregulation, host transcriptional response to injury, decreased kidney function, and increased expression of tubular injury biomarkers. Given that sepsis is a major cause of AKI, a zebrafish model of S-AKI is urgently needed and will hopefully serve as a highly useful complement to higher-vertebrate models.
Zebrafish, with a functional pronephros by 2 days postfertilization (10), appear to be an ideal model system for S-AKI research. Their nephron segment pattern and cellular composition are similar to those of mammalian nephrons (14). In addition, transgenic animals are being applied for precise determination of the location, function, and expression profiles of particular renal cells (46). Tg(cdh17::eGFP) and Tg(PT::eGFP) are transgenic lines that express enhanced green fluorescent protein (eGFP) driven by the tubule-specific gene promoters. The zebrafish homolog of mammalian kidney-specific cadherin, cdh17, has been reported to be expressed in the epithelium and ducts of the entire tubule during larval development and adulthood (19, 37). Tg(PT:eGFP) zebrafish express eGFP in only the proximal tubules (5, 7). Several kidney injury models have been established using zebrafish in recent years, successfully recapitulating the features of mammalian AKI, including typical histological changes, reduced renal function, and pericardial edema (6, 17, 23). In the present study, we documented infection-associated kidney dysfunction in both adult and larval zebrafish (Fig. 2). Typical function changes included nephron excretion impairment and tubular endocytosis dysfunction (Fig. 2), accompanied by traditional injury marker expressions in the pronephric tubule (Fig. 3, A and B). The anatomic localization of IGFBP7 (proximal tubule) and TIMP-2 (distal tubule) matches that recently described in humans (11).
To induce zebrafish infection, multiple pathogens have been tested. Zebrafish did not respond well to typical pathogens such as Escherichia coli or lipopolysaccharide, which are commonly used to induce sepsis in mammalian models (data not shown). These findings are consistent with the previous reports stating that E. coli can be completely cleared by the zebrafish immune system (18). In addition, lipopolysaccharide was unable to be recognized by zebrafish paralogs of TLR4, TLR4a, and TLR4b because of structural differences in their extracellular domains (38). E. tarda bacteria are gram-negative aquatic pathogens that can be used to induce reproducible systemic infection in zebrafish (35, 49). E. tarda invades zebrafish tissues by inhibiting host lysozyme activity, resisting the complement system, and surviving inside macrophages (29, 42). E. tarda caused systemic infection and dose-dependent mortality in our zebrafish study (Fig. 1, B and C). The affected animals exhibited a milder manifestation than that seen in Edwardsiella septicemia (35, 42). Our data showed that the induced infection manifested sustained bacterial load and a globally increased proinflammatory transcriptional response involving il1b, cxcl8-l1, tnfa, and tgfb1a. The il1b expression may imply the activation of the inflammasome formation pathway after PRR activation by PAMPs and DAMPs; increased transcription of il1b is also thought to be linked to macrophage activation, which is associated with sepsis-induced tissue injury (15). To further confirm the systemic infection, host response gene expression was also tested. Transcriptional changes of the cell cycle regulatory genes tp53 and ccnd1 were examined postinfection. Activation of tp53 leads to cell cycle arrest; ccnd1 promotes differentiated cells reentering the cell cycle and starts repair processes (45).
This model, for the first time to our knowledge, confirmed the association between the activated innate immune response and kidney injury. Increasing evidence shows that innate immunity might play crucial roles in determining sepsis outcomes (12, 24, 25, 39). In sepsis, innate immune responses are thought to be primarily governed by factors affecting the recognition of PAMPs by PRRs, including the virulence of the invading pathogen, initial pathogen load, and host genetics (12). Hyperinflammatory or persistent inflammatory states could be caused by overreactions following recognition of PAMPs and/or DAMPs by PRRs, potentially leading to multiorgan (44) and kidney injuries (13, 21, 22). However, owing to the intricacy of the immune interactions that occur in sepsis, the effect of innate immunity on kidney injury is often difficult to ascertain in traditional mammalian models (3, 9, 22). The zebrafish innate immune system is homologous to that of mammals and widely used in understanding the role of immunity in human infectious diseases (32, 43, 47, 48). Larvae present innate immunity from 1 day postfertilization, whereas adaptive immunity is not active until 4 wk postfertilization (27, 43), providing a unique window to delineate the role that innate immunity plays in the process of infection and associated organ injury. By analyzing septic larvae, our results indicate that activation of the innate immune response alone could cause S-AKI in this model system. S-AKI is thought to contribute to sepsis mortality not only because of the nephron dysfunction itself but also because of the increased number of failure organs mediated by AKI cross talk with other organs through soluble inflammatory mediators (8, 40, 51).
In summary, we established a zebrafish model of S-AKI and confirmed the crucial role that innate immune activation plays in this condition. Despite significant differences in the physiology of zebrafish compared with mammals (20, 48), we consider the model a significant complement to vertebrate models of S-AKI not only because of similar pathologic features but also because of the unique advantages of the zebrafish. The larval model inherits important benefits from the zebrafish’s fecundity, rapid development, optical transparency, and availability of a rich variety of tools for genetic manipulation. It is also less time consuming and requires less effort compared with classic mammalian models. Future S-AKI studies may seek to include other components of the innate immunity (the complement cascade, dendritic cells, T cells and microphages, morphology/phenotype analyses of the cilia or ciliated cells), podocytes, endothelial cells, slit diaphragm, and mesangial cells, which are all thought to be key elements in the initiation and progression of S-AKI (31, 53). The model could also be directed toward immune effector cell-tubule cell interactions, mechanisms underlying host cell injury and recovery, large-scale drug screens, and other research that would be difficult and/or prohibitively expensive to execute in traditional mammalian models.
GRANTS
This project was supported by the Center for Critical Care Nephrology at the University of Pittsburgh. The fish facility and zebrafish equipment were in part supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants R01-DK-069403 and R01-DK-112652 and O’Brien Kidney Center NIDDK Grant 1P30-DK-079307. Image data collection using Olympus FluoView 1000 I in the Center for Biological Imaging, University of Pittsburgh, was supported by National Center for Research Resources Shared Instrumentation Grant 1S10-RR-028478-01.
DISCLAIMERS
The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies.
DISCLOSURES
J. A. Kellum reports consulting fees, grant support and licensing fees for unrelated technology for Astute Medical. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
X.W., N.A.H., and J.A.K. conceived and designed research; X.W., L.C., S.M., D.M., and D.E. performed experiments; X.W., L.C., and S.W. analyzed data; X.W., N.A.H., and J.A.K. interpreted results of experiments; X.W., L.C., and D.M. prepared figures; X.W. drafted manuscript; L.C., N.A.H., and J.A.K. edited and revised manuscript; X.W., L.C., S.M., D.M., D.E., S.W., N.A.H., and J.A.K. approved final version of manuscript.
REFERENCES
- 1.Anzenberger U, Bit-Avragim N, Rohr S, Rudolph F, Dehmel B, Willnow TE, Abdelilah-Seyfried S. Elucidation of megalin/LRP2-dependent endocytic transport processes in the larval zebrafish pronephros. J Cell Sci 119: 2127–2137, 2006. doi: 10.1242/jcs.02954. [DOI] [PubMed] [Google Scholar]
- 2.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol 2: 1303–1353, 2012. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caulfield JP, Farquhar MG. The permeability of glomerular capillaries to graded dextrans. Identification of the basement membrane as the primary filtration barrier. J Cell Biol 63: 883–903, 1974. doi: 10.1083/jcb.63.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiba T, Skrypnyk NI, Skvarca LB, Penchev R, Zhang KX, Rochon ER, Fall JL, Paueksakon P, Yang H, Alford CE, Roman BL, Zhang MZ, Harris R, Hukriede NA, de Caestecker MP. Retinoic acid signaling coordinates macrophage-dependent injury and repair after AKI. J Am Soc Nephrol 27: 495–508, 2016. doi: 10.1681/ASN.2014111108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cianciolo Cosentino C, Roman BL, Drummond IA, Hukriede NA. Intravenous microinjections of zebrafish larvae to study acute kidney injury. J Vis Exp 42: 2079, 2010. doi: 10.3791/2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cianciolo Cosentino C, Skrypnyk NI, Brilli LL, Chiba T, Novitskaya T, Woods C, West J, Korotchenko VN, McDermott L, Day BW, Davidson AJ, Harris RC, de Caestecker MP, Hukriede NA. Histone deacetylase inhibitor enhances recovery after AKI. J Am Soc Nephrol 24: 943–953, 2013. doi: 10.1681/ASN.2012111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doi K. Role of kidney injury in sepsis. J Intensive Care 4: 17, 2016. doi: 10.1186/s40560-016-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest 119: 2868–2878, 2009. doi: 10.1172/JCI39421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummond IA, Majumdar A, Hentschel H, Elger M, Solnica-Krezel L, Schier AF, Neuhauss SC, Stemple DL, Zwartkruis F, Rangini Z, Driever W, Fishman MC. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development 125: 4655–4667, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Emlet DR, Pastor-Soler N, Marciszyn A, Wen X, Gomez H, Humphries WH IV, Morrisroe S, Volpe JK, Kellum JA. Insulin-like growth factor binding protein 7 and tissue inhibitor of metalloproteinases-2: differential expression and secretion in human kidney tubule cells. Am J Physiol Renal Physiol 312: F284–F296, 2017. doi: 10.1152/ajprenal.00271.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gogos C, Kotsaki A, Pelekanou A, Giannikopoulos G, Vaki I, Maravitsa P, Adamis S, Alexiou Z, Andrianopoulos G, Antonopoulou A, Athanassia S, Baziaka F, Charalambous A, Christodoulou S, Dimopoulou I, Floros I, Giannitsioti E, Gkanas P, Ioakeimidou A, Kanellakopoulou K, Karabela N, Karagianni V, Katsarolis I, Kontopithari G, Kopterides P, Koutelidakis I, Koutoukas P, Kranidioti H, Lignos M, Louis K, Lymberopoulou K, Mainas E, Marioli A, Massouras C, Mavrou I, Mpalla M, Michalia M, Mylona H, Mytas V, Papanikolaou I, Papanikolaou K, Patrani M, Perdios I, Plachouras D, Pistiki A, Protopapas K, Rigaki K, Sakka V, Sartzi M, Skouras V, Souli M, Spyridaki A, Strouvalis I, Tsaganos T, Zografos G, Mandragos K, Klouva-Molyvdas P, Maggina N, Giamarellou H, Armaganidis A, Giamarellos-Bourboulis EJ. Early alterations of the innate and adaptive immune statuses in sepsis according to the type of underlying infection. Crit Care 14: R96, 2010. doi: 10.1186/cc9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonçalves GM, Zamboni DS, Câmara NO. The role of innate immunity in septic acute kidney injuries. Shock 34, Suppl 1: 22–26, 2010. doi: 10.1097/SHK.0b013e3181e7e69e. [DOI] [PubMed] [Google Scholar]
- 14.Grunwald DJ, Eisen JS. Headwaters of the zebrafish: emergence of a new model vertebrate. Nat Rev Genet 3: 717–724, 2002. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- 15.Guo H, Callaway JB, Ting JP-Y. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21: 677–687, 2015. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hentschel DM, Mengel M, Boehme L, Liebsch F, Albertin C, Bonventre JV, Haller H, Schiffer M. Rapid screening of glomerular slit diaphragm integrity in larval zebrafish. Am J Physiol Renal Physiol 293: F1746–F1750, 2007. doi: 10.1152/ajprenal.00009.2007. [DOI] [PubMed] [Google Scholar]
- 17.Hentschel DM, Park KM, Cilenti L, Zervos AS, Drummond I, Bonventre JV. Acute renal failure in zebrafish: a novel system to study a complex disease. Am J Physiol Renal Physiol 288: F923–F929, 2005. doi: 10.1152/ajprenal.00386.2004. [DOI] [PubMed] [Google Scholar]
- 18.Herbomel P, Thisse B, Thisse C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development 126: 3735–3745, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Horsfield J, Ramachandran A, Reuter K, LaVallie E, Collins-Racie L, Crosier K, Crosier P. Cadherin-17 is required to maintain pronephric duct integrity during zebrafish development. Mech Dev 115: 15–26, 2002. doi: 10.1016/S0925-4773(02)00094-1. [DOI] [PubMed] [Google Scholar]
- 20. Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assunção JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, . et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496: 498–503, 2013. [Erratum in Nature 505: 248, 2014. doi: 10.1038/nature12813.] doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang HR, Rabb H. The innate immune response in ischemic acute kidney injury. Clin Immunol 130: 41–50, 2009. doi: 10.1016/j.clim.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang HR, Rabb H. Immune cells in experimental acute kidney injury. Nat Rev Nephrol 11: 88–101, 2015. doi: 10.1038/nrneph.2014.180. [DOI] [PubMed] [Google Scholar]
- 23.Johnson CS, Holzemer NF, Wingert RA. Laser ablation of the zebrafish pronephros to study renal epithelial regeneration. J Vis Exp 54: 2845, 2011. doi: 10.3791/2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, Fine J, Krichevsky A, Delude RL, Angus DC; GenIMS Investigators . Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med 167: 1655–1663, 2007. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellum JA, Pike F, Yealy DM, Huang DT, Shapiro NI, Angus DC; Protocol-Based Care for Early Septic Shock Investigators; (ProCESS) Investigators . Relationship between alternative resuscitation strategies, host response and injury biomarkers, and outcome in septic shock. Crit Care Med 45: 438–445, 2017. doi: 10.1097/CCM.0000000000002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krämer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30: 523–530, 2014. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam SH, Chua HL, Gong Z, Lam TJ, Sin YM. Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Dev Comp Immunol 28: 9–28, 2004. doi: 10.1016/S0145-305X(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 28.Levraud JP, Boudinot P, Colin I, Benmansour A, Peyrieras N, Herbomel P, Lutfalla G. Identification of the zebrafish IFN receptor: implications for the origin of the vertebrate IFN system. J Immunol 178: 4385–4394, 2007. doi: 10.4049/jimmunol.178.7.4385. [DOI] [PubMed] [Google Scholar]
- 29.Li MF, Wang C, Sun L. Edwardsiella tarda MliC, a lysozyme inhibitor that participates in pathogenesis in a manner that parallels Ivy. Infect Immun 83: 583–590, 2015. doi: 10.1128/IAI.02473-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, Iwashyna TJ. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 312: 90–92, 2014. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 31.McCullough JW, Renner B, Thurman JM. The role of the complement system in acute kidney injury. Semin Nephrol 33: 543–556, 2013. doi: 10.1016/j.semnephrol.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meijer AH, Spaink HP. Host-pathogen interactions made transparent with the zebrafish model. Curr Drug Targets 12: 1000–1017, 2011. doi: 10.2174/138945011795677809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitra S, Lukianov S, Ruiz WG, Cianciolo Cosentino C, Sanker S, Traub LM, Hukriede NA, Apodaca G. Requirement for a uroplakin 3a-like protein in the development of zebrafish pronephric tubule epithelial cell function, morphogenesis, and polarity. PLoS One 7: e41816, 2012. doi: 10.1371/journal.pone.0041816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pressley ME, Phelan PE III, Witten PE, Mellon MT, Kim CH. Pathogenesis and inflammatory response to Edwardsiella tarda infection in the zebrafish. Dev Comp Immunol 29: 501–513, 2005. doi: 10.1016/j.dci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Rekha RD, Amali AA, Her GM, Yeh YH, Gong HY, Hu SY, Lin GH, Wu JL. Thioacetamide accelerates steatohepatitis, cirrhosis and HCC by expressing HCV core protein in transgenic zebrafish Danio rerio. Toxicology 243: 11–22, 2008. doi: 10.1016/j.tox.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Sanker S, Cirio MC, Vollmer LL, Goldberg ND, McDermott LA, Hukriede NA, Vogt A. Development of high-content assays for kidney progenitor cell expansion in transgenic zebrafish. J Biomol Screen 18: 1193–1202, 2013. doi: 10.1177/1087057113495296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sepulcre MP, Alcaraz-Pérez F, López-Muñoz A, Roca FJ, Meseguer J, Cayuela ML, Mulero V. Evolution of lipopolysaccharide (LPS) recognition and signaling: fish TLR4 does not recognize LPS and negatively regulates NF-κB activation. J Immunol 182: 1836–1845, 2009. doi: 10.4049/jimmunol.0801755. [DOI] [PubMed] [Google Scholar]
- 39.Shi Z, Wu CH, Ben-Arieh D, Simpson SQ. Mathematical model of innate and adaptive immunity of sepsis: a modeling and simulation study of infectious disease. BioMed Res Int 2015: 504259, 2015. doi: 10.1155/2015/504259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singbartl K, Bishop JV, Wen X, Murugan R, Chandra S, Filippi MD, Kellum JA. Differential effects of kidney-lung cross-talk during acute kidney injury and bacterial pneumonia. Kidney Int 80: 633–644, 2011. doi: 10.1038/ki.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srinivasa Rao PS, Lim TM, Leung KY. Functional genomics approach to the identification of virulence genes involved in Edwardsiella tarda pathogenesis. Infect Immun 71: 1343–1351, 2003. doi: 10.1128/IAI.71.3.1343-1351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stein C, Caccamo M, Laird G, Leptin M. Conservation and divergence of gene families encoding components of innate immune response systems in zebrafish. Genome Biol 8: R251, 2007. doi: 10.1186/gb-2007-8-11-r251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suresh R, Mosser DM. Pattern recognition receptors in innate immunity, host defense, and immunopathology. Adv Physiol Educ 37: 284–291, 2013. doi: 10.1152/advan.00058.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tane S, Kubota M, Okayama H, Ikenishi A, Yoshitome S, Iwamoto N, Satoh Y, Kusakabe A, Ogawa S, Kanai A, Molkentin JD, Nakamura K, Ohbayashi T, Takeuchi T. Repression of cyclin D1 expression is necessary for the maintenance of cell cycle exit in adult mammalian cardiomyocytes. J Biol Chem 289: 18033–18044, 2014. doi: 10.1074/jbc.M113.541953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tobin DM, May RC, Wheeler RT. Zebrafish: a see-through host and a fluorescent toolbox to probe host-pathogen interaction. PLoS Pathog 8: e1002349, 2012. doi: 10.1371/journal.ppat.1002349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torraca V, Masud S, Spaink HP, Meijer AH. Macrophage-pathogen interactions in infectious diseases: new therapeutic insights from the zebrafish host model. Dis Model Mech 7: 785–797, 2014. doi: 10.1242/dmm.015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Vaart M, Spaink HP, Meijer AH. Pathogen recognition and activation of the innate immune response in zebrafish. Adv Hematol 2012: 159807, 2012. doi: 10.1155/2012/159807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Soest JJ, Stockhammer OW, Ordas A, Bloemberg GV, Spaink HP, Meijer AH. Comparison of static immersion and intravenous injection systems for exposure of zebrafish embryos to the natural pathogen Edwardsiella tarda. BMC Immunol 12: 58, 2011. doi: 10.1186/1471-2172-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) (4th ed). Eugene, OR: Univ. of Oregon Press, 2000. [Available at https://zfin.org/zf_info/zfbook/zfbk.html.] [Google Scholar]
- 51.White LE, Chaudhary R, Moore LJ, Moore FA, Hassoun HT. Surgical sepsis and organ crosstalk: the role of the kidney. J Surg Res 167: 306–315, 2011. doi: 10.1016/j.jss.2010.11.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Witte M, Huitema LF, Nieuwenhuis EE, Brugman S. Deficiency in macrophage-stimulating protein results in spontaneous intestinal inflammation and increased susceptibility toward epithelial damage in zebrafish. Zebrafish 11: 542–550, 2014. doi: 10.1089/zeb.2014.1023. [DOI] [PubMed] [Google Scholar]
- 53.Zarjou A, Agarwal A. Sepsis and acute kidney injury. J Am Soc Nephrol 22: 999–1006, 2011. doi: 10.1681/ASN.2010050484. [DOI] [PubMed] [Google Scholar]