Abstract
This study in α-chloralose-anesthetized cats aimed at investigating the bladder responses to saphenous nerve stimulation (SNS). A urethral catheter was used to infuse the bladder with saline and to record changes in bladder pressure. With the bladder fully distended, SNS at 1-Hz frequency and an intensity slightly below the threshold (T) for inducing an observable motor response of the hindlimb muscles induced large amplitude (40–150 cmH2O) bladder contractions. Application of SNS (1 Hz, 2–4T) during cystometrograms (CMGs), when the bladder was slowly (1–3 ml/min) infused with saline, significantly (P < 0.05) increased the duration of the micturition contraction to >200% of the control without changing bladder capacity or contraction amplitude. Repeated application (1–8 times) of intense (4–8T intensity) 30-min tibial nerve stimulation (TNS) produced prolonged post-TNS inhibition that significantly (P < 0.01) increased bladder capacity to 135.9 ± 7.6% and decreased the contraction amplitude to 44.1 ± 16.5% of the pre-TNS control level. During the period of post-TNS inhibition, SNS (1 Hz, 2–4T) applied during CMGs completely restored the bladder capacity and the contraction amplitude to the pre-TNS control level and almost doubled the duration of the micturition contraction. These results indicate that SNS at 1 Hz can facilitate the normal micturition reflex and normalize the reflex when it is suppressed during post-TNS inhibition. This study provides an opportunity to develop a novel neuromodulation therapy for underactive bladder using SNS.
Keywords: bladder, cat, saphenous, stimulation, underactivity
INTRODUCTION
Underactive bladder (UAB) is a symptom complex characterized by prolonged urination time with or without a sensation of incomplete bladder emptying, usually with hesitancy, reduced sensation on filling, and a slow stream (2, 16). UAB is a chronic debilitating disorder that significantly impacts quality of life. Currently, there are no medications to treat UAB (6, 16). Therefore, when urinary retention occurs, intermittent self-catheterization or indwelling catheters are used (2, 7). Sacral neuromodulation, which requires surgical implantation of a neurostimulator, is another Food and Drug Administration-approved therapy to treat selected patients with nonobstructive urinary retention (7). This method is particularly effective in reversing a type of neurogenic urinary retention in women with Fowler’s syndrome (7, 12). Despite the significant clinical need for UAB treatment, relatively few basic science studies have focused on UAB in comparison to the large number of studies of overactive bladder syndrome (OAB).
Our recent study in cats (19) discovered that electrical stimulation of cutaneous afferent axons in the superficial peroneal nerve at a very low frequency (1 Hz) can trigger an excitatory reflex to bladder and produce a large bladder contraction. This discovery prompted us to conduct the current study to determine if 1-Hz stimulation of afferent axons in another cutaneous nerve (the saphenous nerve) can excite the bladder and also reverse bladder dysfunction in an animal model of UAB produced by prolonged tibial nerve stimulation (TNS). In cats, TNS of a 30-min duration elicits a poststimulation inhibitory effect evident as an increased bladder capacity that persists for >2 h (5, 17). Persistent effects of TNS on bladder function lasting for several weeks also occur clinically when stimulation is applied at weekly intervals for 12 wk to treat OAB symptoms (13). Although the mechanism of this sustained inhibition is not known, it is likely to be mediated by mechanisms in the central nervous system and therefore serves as a useful model for testing the efficacy of cutaneous nerve neuromodulation in reversing neurogenic underactive bladder dysfunction. The saphenous nerve can be activated noninvasively by large skin surface electrodes without necessarily causing leg muscle twitch or disrupting locomotion. Therefore, the saphenous nerve is a very good potential target to develop neuromodulation therapy for UAB.
MATERIALS AND METHODS
The experimental protocol and animal use in this study were approved by the Animal Care and Use Committee at the University of Pittsburgh.
Surgical procedures.
A total of six cats (3 males and 3 females, 3–4.3 kg; Liberty Research, Waverly, NY) were used in this study. The animals were anesthetized with isoflurane (2–5% in oxygen) during surgery and then switched to α-chloralose anesthesia (initial 65 mg/kg iv and supplemented as needed) during data collection. The left cephalic vein was catheterized for administration of anesthetics and fluid. A tracheotomy was performed, and a tube was inserted to keep the airway patent. A catheter was inserted into the right carotid artery to monitor systemic blood pressure. Heart rate and blood oxygen were monitored by a pulse oximeter (9847V; NONIN Medical, Plymouth, MN) attached to the tongue. Through an abdominal incision, the ureters were isolated, tied, and cut for external drainage. A double lumen catheter was inserted into the bladder via a small cut on the proximal urethra and secured by a ligature around the urethra. One lumen was connected to a pump to slowly (1–3 ml/min) infuse saline for bladder distention. The other lumen was attached to a pressure transducer to measure bladder pressure. The saphenous nerve on the right side was exposed via a skin incision on the medial thigh slightly above the knee. The tibial nerve on the left side was dissected via skin incision at the ankle. Tripolar cuff electrodes (NC223pt; MicroProbe, Gaithersburg, MD) were implanted on these nerves, and then the electrodes were connected to a dual channel electrical stimulator (S88; Grass Medical Instruments, Quincy, MA) via constant voltage stimulus isolators (SIU5; Grass Medical Instruments). After the surgery, the skin and muscle layers were closed by sutures.
Stimulation protocol.
At the beginning of each experiment, uniphasic rectangular pulses (1-Hz frequency) were used to determine the intensity threshold (T) for saphenous nerve stimulation (SNS) to induce observable muscle twitches on the posterior thigh, hip, or toe. It is presumed that T represents the threshold for activating the largest diameter (Aβ) afferent axons in the nerve because these afferents elicit flexor reflexes in chloralose anesthetized cats (4, 15). At the threshold intensity determined by 1-Hz SNS, the muscle twitches disappeared when SNS frequency was increased above 2 Hz. Based on our previous studies (5, 17), the intensity threshold (T) for tibial nerve stimulation (TNS) to induce observable toe twitches was determined at the 5-Hz frequency. A pulse width of 0.2 ms was used for both SNS and TNS.
Initially, multiple cystometrograms (CMGs) were performed by slowly infusing the bladder with saline to determine the bladder capacity that was defined as the volume threshold to induce a micturition reflex contraction of large amplitude (>30 cmH2O) and long duration (>20 s). Once the control bladder capacity was determined, additional two CMGs were performed during SNS (1 Hz, 0.2 ms, 2–4T intensity, n = 6 cats). For the first CMG, SNS was applied intermittently (60 s off and 30 s on) starting at the beginning of the CMG and ending with the onset of the micturition reflex at which time the stimulation was switched to continuous stimulation that continued for the duration of the micturition contraction. This stimulation pattern (intermittent-continuous) is termed as SNSi-c in this study (see Fig. 1). For the second CMG, continuous SNS (SNSc) was applied at the beginning and maintained until the end of the CMG. The purpose of testing SNSi-c was to determine if prolonged SNSc during the storage phase produces fatigue and therefore has less effect on bladder capacity than SNSi-c, which might be expected to produce less fatigue. SNS was terminated in some experiments before the reflex contraction ended to prevent bladder overdistension when the contraction duration reached three times the control contraction duration. Following the two CMGs with SNS, another control CMG was performed without SNS to determine any poststimulation effect.
Fig. 1.
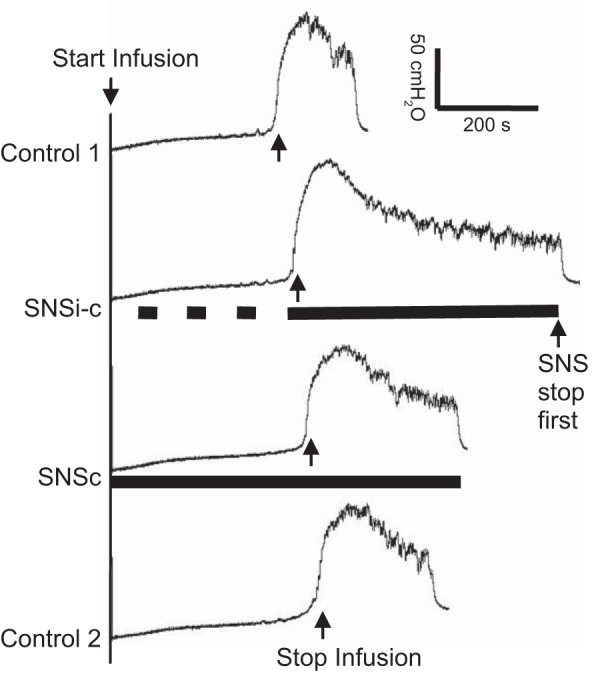
Saphenous nerve stimulation (SNS) applied during a cystometrogram (CMG) significantly increased the duration of the micturition contraction. SNSi-c, SNS applied intermittently during the storage phase but applied continuously once a micturition contraction occurred; SNSc, SNS applied continuously from the beginning of the CMG; T, threshold intensity for SNS to induce muscle twitching on the back of the thigh. The black bar under bladder pressure trace indicates the duration of stimulation. SNS: 1 Hz, 0.2 ms, 2T = 7 V. Infusion rate: 3 ml/min.
Then, the experiments were continued to determine if SNS could facilitate the micturition reflex during the long-lasting poststimulation inhibition induced by TNS. Multiple control CMGs were performed again. At the end of the last control CMG, TNS (5 Hz, 0.2 ms, 4–8T intensity, 30-min duration) was applied one to eight times in different animals to significantly increase bladder capacity and reduce (>40%) the amplitude of the micturition contraction. The prolonged post-TNS inhibition induced by 30-min TNS can last for at least 2 h as shown in our previous studies (5, 17). After the repeated 30-min TNS, four CMGs were performed 1) control CMG without stimulation; 2) CMG with SNSi-c (60 s off and 30 s on during storage phase but continuous during the micturition contraction); 3) CMG with SNSc (continuous SNS during both the storage phase and micturition contraction); and 4) control CMG again to determine any poststimulation effect. During the repeated CMG testing, the bladder was emptied after each CMG followed by a 2- to 3-min resting period to allow the bladder to recover.
At the end of the experiment with the bladder fully distended at the bladder capacity and contracting rhythmically (n = 5 cats), SNS (10 V, 0.2 ms, 30-s duration) at different frequencies (0.5–30 Hz) was repeatedly applied to determine the frequency response. Then, SNS (1 Hz, 0.2 ms, 30-s duration) at different intensities (0.25–10 V) was repeatedly applied to determine the intensity response. During the frequency and intensity tests, a rest period (>60 s) followed each 30-s SNS.
Data analysis.
Repeated measurements (2–3 CMGs) of control bladder capacity before testing SNS effects were averaged in the same animal. Then, the bladder capacity was measured during every CMG and normalized to the averaged control capacity in each cat. The amplitude and duration of bladder contractions were also measured in each CMG and normalized to the averaged values obtained during control CMGs in each cat. After repeated 30-min TNS, some animals (3 out of 6 cats) exhibited complete urinary retention without a micturition reflex contraction even when the baseline bladder pressure reached 40 cmH2O during the CMGs. For those cats with complete retention, the bladder infusion was stopped when the baseline bladder pressure reached 40 cmH2O. The infused volume was used as bladder capacity, and the amplitude and duration of the bladder contraction was measured as zero. For the frequency and intensity tests, the area under the contraction curve was measured and normalized to the maximal response in each cat. The data from different animals are presented as means ± SE. Statistical significance (P < 0.05) was determined by repeated-measures one-way ANOVA followed by Dunnett’s multiple comparison.
RESULTS
Facilitation of the normal micturition reflex by 1-Hz SNS.
SNS applied continuously (SNSc) or intermittently (SNSi-c) starting at the beginning of the CMG and continuing until the initiation of the normal micturition reflex did not change bladder capacity (Fig. 1 and Fig. 2A). However, when the SNSc was continued or when the SNSi-c became continuous immediately after the initiation of the micturition reflex, the duration of bladder contraction significantly increased to 236.9 ± 45.7% (P < 0.01) or 211.4 ± 46.3% (P < 0.05) of control duration, respectively (Fig. 1 and Fig. 2C). SNSi-c (3 cats) and SNSc (1 cat) were terminated early to avoid bladder overdistension when the contraction duration reached three times the control duration (see Fig. 1, 2nd trace). These effects of SNSi-c and SNSc on bladder contraction duration were not significantly different (Fig. 2C). On average, the duration of the bladder contractions recovered to the pre-SNS level indicating that there was no statistically significant post-SNS effect (Fig. 2C). The amplitude of the bladder contraction was not significantly changed by either SNSi-c or SNSc (Fig. 1 and Fig. 2B).
Fig. 2.
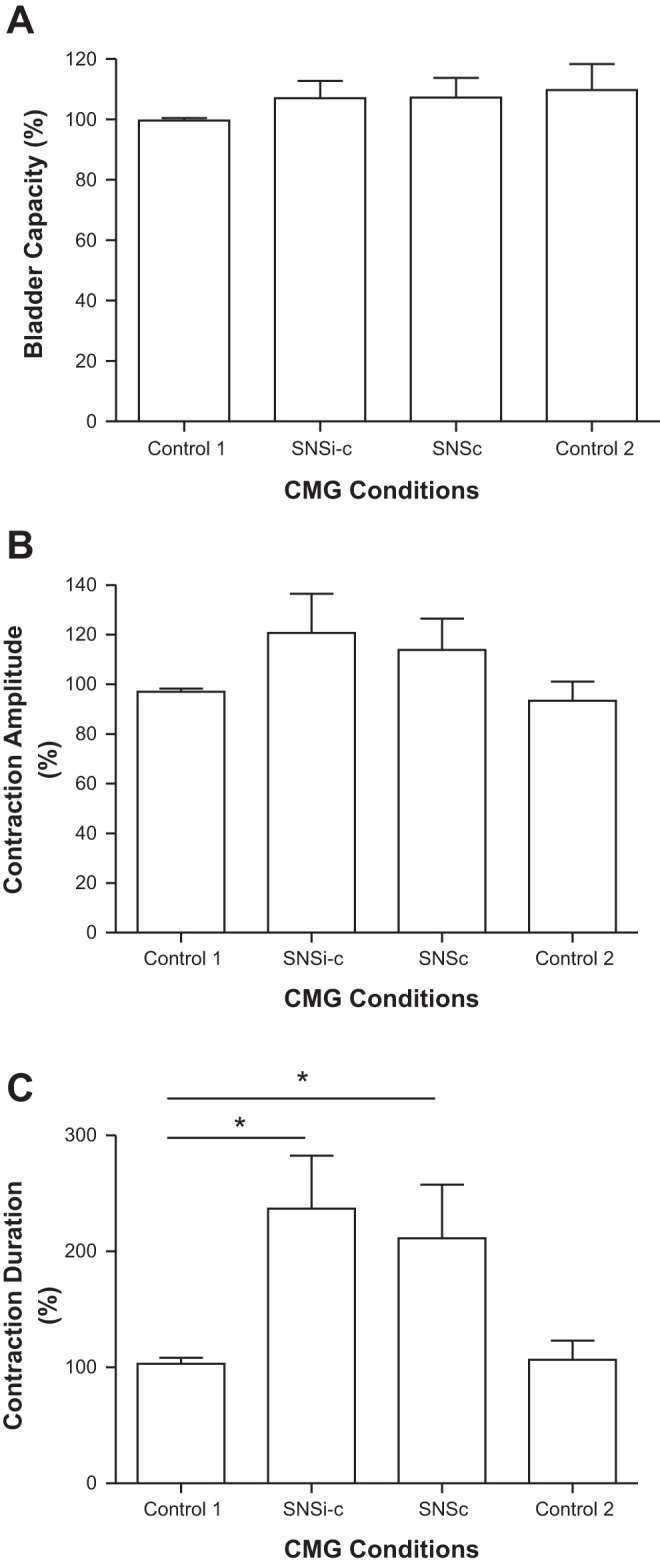
Effects of saphenous nerve stimulation (SNS) on bladder capacity (A), contraction amplitude (B), and contraction duration (C). SNSi-c, SNS applied intermittently during storage phase but applied continuously once a micturition contraction occurred; SNSc, SNS applied continuously from the beginning of the CMG; T, threshold intensity for SNS to induce muscle twitching on the back of the thigh. SNS: 1 Hz, 0.2 ms, 2–4T = 7–10 V. *Significantly (P < 0.05) different (one-way ANOVA); n = 6 cats.
SNS at 1 Hz normalized bladder underactivity during post-TNS inhibition.
Repeated (1–8 times) application of 30-min TNS at intensities four to eight times the threshold to induce observable toe twitches significantly increased bladder capacity and reduced (>40%) the amplitude of the micturition reflex contraction in five of the six cats. In three of these animals, a micturition reflex contraction did not occur even when the baseline bladder pressure reached 40 cmH2O during the CMG, indicating complete urinary retention. On average, repeated application of 30-min TNS significantly (P < 0.01) increased bladder capacity to 135.9 ± 7.6% (see Fig. 4A) and decreased amplitude of the micturition contraction to 44.1 ± 16.5% (Fig. 4B) of pre-TNS control, producing a type of bladder underactivity characterized by a large bladder capacity with a reduced bladder contraction amplitude. However, the post-TNS inhibition did not significantly (P > 0.05) change the duration of the bladder contractions (Fig. 4C). During the post-TNS inhibition, SNSi-c and SNSc applied during CMGs normalized the bladder underactivity by significantly reducing the bladder capacity (Figs. 3 and 4) to 100.2 ± 7.0% (P < 0.01) and 113.9 ± 7.2% (P < 0.05) of control and increasing the amplitude of the contractions (Fig. 3 and Fig. 4B) to 111.3 ± 17.0% (P < 0.01) and 91.0 ± 12.5% (P < 0.05) of control, respectively. In addition, SNSi-c and SNSc also increased the duration of the contractions (Fig. 4C) to 201.5 ± 61.3% (P < 0.01) and 185.5 ± 55.6% (P < 0.05) of control, respectively. These effects of SNSi-c and SNSc were not significantly different. Within 20 min after termination of the stimulation the bladder capacity as well as the amplitude and duration of the bladder contractions returned to the pre-SNS levels indicating that there was no post-SNS effect (Figs. 3 and 4).
Fig. 4.
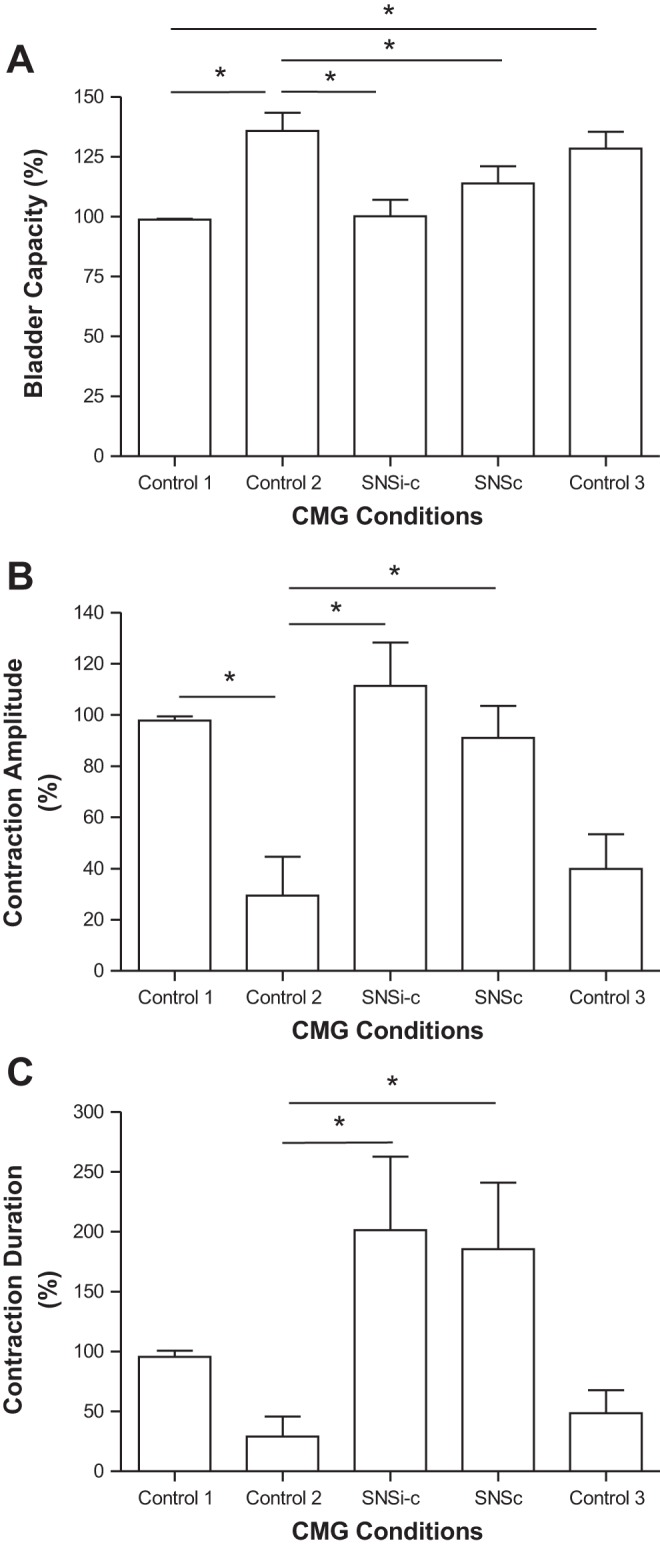
Effects of saphenous nerve stimulation (SNS) on bladder capacity (A), contraction amplitude (B), and contraction duration (C) during poststimulation inhibition induced by tibial nerve stimulation (TNS). SNSi-c, SNS applied intermittently during storage phase but applied continuously once a micturition contraction occurred; SNSc, SNS applied continuously from the beginning of the CMG; T, threshold intensity to induce observable muscle twitch on the back of the thigh. SNS: 1 Hz, 0.2 ms, 2–4T = 7–10 V. TNS: 5 Hz, 0.2 ms, 4–8T = 1.6–4 V, 30 min applied for 1–8 times. *Significantly (P < 0.05) different (one-way ANOVA); n = 6 cats.
Fig. 3.
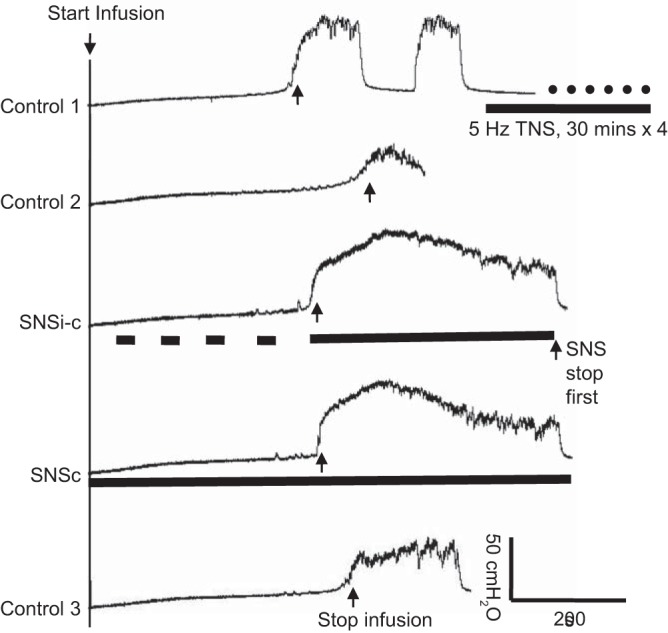
Saphenous nerve stimulation (SNS) eliminated the poststimulation inhibition induced by tibial nerve stimulation (TNS). SNSi-c, SNS applied intermittently during storage phase but applied continuously once a micturition contraction occurred; SNSc, SNS applied continuously from the beginning of the CMG; T, threshold intensity to induce observable muscle twitch on the back of the thigh. The black bar under bladder pressure trace indicates the duration of stimulation. SNS: 1 Hz, 0.2 ms, 2T = 7V. TNS: 5 Hz, 0.2 ms, 4T = 2.8 V, 30 min × 4 times.
Effects of SNS frequency and intensity on micturition reflex.
At the end of the experiment with the bladder fully distended and contracting rhythmically, 30-s duration trains of SNS (10 V, 0.2 ms) at frequencies of 0.5–2 Hz induced significantly (P < 0.05) larger bladder contractions (Fig. 5, A and B) than those induced by 30-Hz stimulation.
Fig. 5.
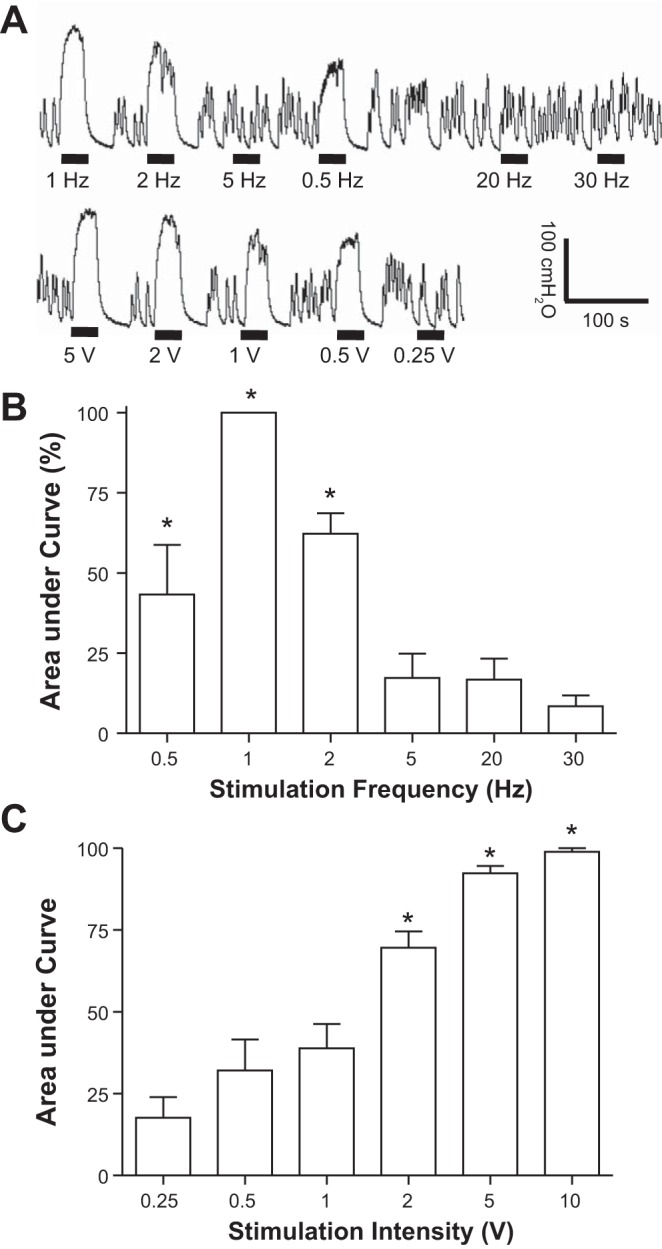
Bladder pressure responses to different frequencies (0.5–30 Hz) and intensities (0.25–10 V) of saphenous nerve stimulation (SNS). A: bladder pressure traces from the same cat. The black bar under the pressure trace indicates the duration (30 s) of SNS. SNS: 10 V, 0.2 ms for top trace; 1 Hz, 0.2 ms for bottom trace. B: normalized area under curve for different frequencies of SNS (10 V, 0.2 ms). C: normalized area under curve for different intensities of SNS (1 Hz, 0.2 ms). *Significantly (P < 0.05) different from the response at 30 Hz in B or at 0.25 V in C (one-way ANOVA); n = 5 cats.
SNS at 1 Hz induced significantly (P < 0.05) larger bladder contractions at intensities of 2–10 V than 0.25 V (Fig. 5, A and C). The range of stimulus intensities for evoking reflex contractions of hindlimb muscles in these experiments (i.e., the motor threshold intensity T) was 2.5–5 V (n = 5 cats). In one cat, muscle twitches were not elicited at SNS intensities ranging up to 80 V and therefore the motor threshold T could not be determined. In this cat, 10 V was used to test SNS effects, which was the upper limit of the 2–4T (7–10 V) used in other cats. This comparison of stimulus intensity thresholds (Fig. 5 C) showed that on average a significantly larger bladder contraction could be induced by 1-Hz SNS at an intensity (2 V) slightly below the motor threshold T (2.5–5 V).
DISCUSSION
This study revealed that electrical stimulation of cutaneous afferent axons in the saphenous nerve at low frequencies (0.5–2 Hz) and low intensities (1–4 times the threshold for inducing reflex contractions of hindlimb muscles) elicited three types of excitatory bladder responses depending upon the state of the bladder. When the bladder was distended beyond the volume necessary to induce a micturition reflex, SNS 1) enhanced the micturition reflex contraction under isovolumetric conditions (Fig. 5); 2) increased the duration of the micturition reflex contraction during a CMG (Figs. 1 and 2); and 3) reversed the sustained inhibition of reflex bladder activity that followed prolonged stimulation of afferent axons in the tibial nerve (Figs. 3 and 4). However, SNS did not elicit excitatory responses when bladder volume was below the threshold for inducing a micturition reflex (Fig. 1 and Fig. 2A). These data suggest that SNS selectively facilitates the micturition reflex pathway but is not capable of eliciting a reflex contraction when the bladder is only partially full and in storage mode. Because SNS was only effective at a bladder volume large enough to induce a micturition reflex, this indicates that a synergistic interaction in the CNS between bladder afferent and saphenous afferent inputs is necessary to unmask the influence of SNS. Although SNS enhanced reflex bladder contractions under both normal and underactive bladder conditions (Fig. 2C and Fig. 4, B and C), it is still uncertain if the enhanced bladder contractions will result in a better voiding efficiency. Because of the closed urethral outlet preparation used in our study, the effect of SNS on voiding efficiency could not be measured.
The reversal of post-TNS inhibition of bladder activity by SNS raises the possibility that SNS enhances the micturition reflex by suppressing a tonic inhibitory mechanism that can be facilitated by prolonged TNS. Although the present experiments do not identify the location of this inhibitory mechanism, it is tempting to speculate that the pontine micturition center (PMC) is the site of interaction between TNS and SNS because pharmacological and brain imaging experiments in animals indicate that neural circuitry in the PMC functions like a switch to control bladder capacity (8, 18). It has been proposed that the PMC switch is subject to tonic inhibition during urine storage and that the inhibition can in turn be suppressed by increased bladder afferent firing during bladder distension eventually leading to the initiation of voiding (3). This switching circuit may be the target of somatic afferents in the tibial and saphenous nerves, the former facilitating the inhibitory circuit to increase bladder capacity and latter suppressing the effect of TNS to normalize bladder capacity. Other possible sites of interaction between these two modulatory mechanisms could be on the afferent limb of the micturition reflex pathway, because changes in afferent input to the PMC would be expected to elicit effects similar to those produced by directly modulating the PMC switching circuit. An effect on the afferent limb could occur at the level of the spinal cord, where bladder primary afferents activate spinal tract neurons that send information to the periaqueductal gray or in the periaqueductal gray, which relays afferent information to the PMC (3).
Because prolonged TNS elicits effects resembling some of the symptoms of UAB, more detailed studies of post-TNS inhibition might provide insights into the mechanisms of UAB. In addition, if it is reasonable to consider post-TNS inhibition as a model of neurogenic UAB, then this raises the possibility that 1-Hz SNS might be developed as a neuromodulation therapy for UAB. If the saphenous nerve were to be used in neuromodulation therapy, an important question is what types of afferent axons have to be stimulated to elicit excitatory bladder reflexes? The saphenous nerve contains group II (Aβ), group III (Aδ), and group IV (C) type afferents. Group II consists of nonnociceptive mechanosensitive afferents, whereas groups III and IV consist in part of nociceptive afferents that would elicit pain if stimulated and therefore would not be appropriate targets for neuromodulation. Previous studies (4, 15) in chloralose-anesthetized cats revealed that electrical stimulation of the lowest threshold, fastest conducting afferents (50–80 m/s) in the saphenous nerve as well as in other cutaneous nerves evoked reflex contractions of flexor muscles. Because we elicited excitatory bladder reflexes at or below the threshold stimuli for eliciting reflex contractions of various hindlimb muscles, we conclude that the afferent limb of the saphenous nerve-to-bladder reflex pathway is mediated by group II cutaneous afferent axons. These afferents respond to nonnoxious stimulation of the skin of the leg below the knee, and therefore, it is possible that the saphenous nerve-to-bladder reflex can also be activated noninvasively using low-intensity electrical stimulation via large skin surface electrodes. It is also worth noting that α-chloralose anesthesia was used in the study. Whether SNS will have the same effects on the bladder in unanesthetized cats is still unknown. Ultimately, the efficacy and applicability of SNS to treat UAB still need to be determined by clinical trials in human subjects.
The antagonistic interaction between SNS and TNS indicates that different types of somatic afferent axons activate competing bladder reflex mechanisms. Unlike the saphenous nerve that contains only cutaneous afferents, the tibial nerve contains muscle afferents as well as cutaneous afferents. As reported by McPherson (9), stimulation of hindlimb nerves innervating muscles at intensities that activate groups I and II afferents conducting at ≥50 m/s inhibits reflex bladder activity induced by bladder distension. Under the same bladder conditions, stimulation of groups III and IV muscle or cutaneous afferents also produces bladder inhibition (14). Thus a broad range of hindlimb proprioceptive and nociceptive somatic afferents produce bladder inhibition. In our experiments, post-TNS inhibition was elicited by four to eight times the threshold for evoking muscle movement indicating that the TNS inhibition was due to activation of groups II and III afferent axons.
The effects of somatic afferent stimulation on bladder activity are also influenced by the frequency of stimulation. For example, in a previous study we showed that electrical stimulation of low threshold cutaneous afferents in the superficial peroneal nerve was only effective in exciting the bladder using a narrow range of frequencies between 1 and 3 Hz (19). In the present experiments, low-frequency SNS (0.5–2 Hz) was also effective in eliciting excitatory responses, while high frequencies (5–30 Hz) were not effective (Fig. 5). Low-frequency TNS (2 Hz) also evokes excitatory bladder responses (10), while higher frequency TNS (5–20 Hz) evokes inhibitory bladder responses (5, 10, 17). Because stimulation of muscle afferents in the tibial nerve produces only bladder inhibition (14), it is probable that the excitatory effect induced by low-frequency TNS is due to activation of cutaneous afferents, while the inhibitory effects induced by higher frequencies are due to activation of muscle afferents. Thus the selective excitatory effect of low-frequency stimulation on reflex bladder activity appears to be a common characteristic of bladder reflexes evoked by hindlimb cutaneous afferents.
There are some similarities as well as differences between our current study of SNS and our previous study (19) of superficial peroneal nerve stimulation (SPNS). The effective stimulation frequencies are similar for SNS (0.5–2 Hz) and SPNS (1–3 Hz). Both SNS and SPNS enhanced the normal micturition reflex contraction by significantly increasing either the contraction duration (SNS) or the area under the contraction curve (SPNS). However, SPNS significantly reduced the bladder capacity for inducing a normal micturition reflex, but SNS failed to do so (Fig. 2A). Meanwhile, both SNS and SPNS reversed the capacity increase induced by post-TNS inhibition and significantly increased the duration of the micturition contraction during post-TNS inhibition. However, in the current study high-intensity TNS (4–8 T) of 30-min duration applied repeatedly (1–8 times) produced persistent bladder underactivity consisting of a significantly larger bladder capacity and reduced amplitude of the micturition contraction. In our previous study of SPNS, a less intense (4 T) 30-min TNS applied only once produced a larger bladder capacity without a reduction in the amplitude of the micturition contraction. Therefore, in current study we are able to show that SNS reversed both the bladder capacity and contraction amplitude changes during post-TNS inhibition, while in our previous study the ability of SPNS to reverse both changes could not be tested. As our understanding of the prolonged post-TNS inhibitory effect improves, we will certainly reexamine the effect of SPNS on bladder underactivity induced by post-TNS inhibition.
We compared the effects of continuous and intermittent patterns of SNS (30 s on and 60 s off) because we wanted to determine if the excitatory effects of continuous SNS might fatigue during the prolonged period of bladder filling especially during the post-TNS inhibition when the duration of the storage phase of the CMG could range up to 6–10 min (Fig. 3). This comparison was also of interest because a recent study in cats (1) revealed that the efficacy of certain types of bladder neuromodulation can be affected by the stimulus pattern. Bladder excitatory effects elicited by stimulation of pudendal nerve afferents or urethral afferents were more prominent during an intermittent or bursting pattern of stimulation than during continuous stimulation. Therefore, either intermittent or continuous SNS was applied during the storage phase to evaluate the changes in bladder capacity (Fig. 1 and Fig. 3). The experiments showed that intermittent and continuous stimulation were both ineffective in changing bladder capacity in normal bladders but were equally effective in reversing the post-TNS increase in bladder capacity. Thus the pattern of stimulation appeared to have a minimal influence on the response to SNS.
A recent study in rats shows that SNS at 20 Hz can inhibit bladder activity and at 2 Hz has no effect (11). Our study in cats shows that 2-Hz SNS can excite bladder while 20 Hz has no effect (Fig. 5). These differences could be due to the different animal models (rats vs. cats) or due to different anesthesia (urethane vs. chloralose). Another possible cause for these differences might be due to the different states of the bladder reflex. In the rat study the different frequencies of SNS were tested on the normal bladder reflex activity, while in our cat study the different frequencies were tested during prolonged post-TNS inhibition when the bladder reflex was underactive with a larger bladder capacity and a smaller micturition reflex contraction.
In summary, this study discovered a facilitatory effect of SNS on the micturition reflex, which normalized the bladder underactivity produced by intense and prolonged TNS. The results raise the possibility that electrical stimulation of the saphenous nerve using either invasive implantable electrodes or noninvasive skin surface electrodes might be developed as a neuromodulation therapy to treat UAB disorders.
GRANTS
This study is supported by the National Institutes of Diabetes, Digestive, and Kidney Diseases Grants DK-094905, DK-102427, and DK-111382.
DISCLOSURES
No conflicts of interests, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.L., X.L., K.T., J.B., B.S., J.W., J.R.R., W.C.d.G., and C.T. conceived and designed research; S.L., X.L., K.T., J.B., B.S., J.W., J.R.R., W.C.d.G., and C.T. performed experiments; S.L., X.L., K.T., J.B., B.S., J.W., J.R.R., W.C.d.G., and C.T. analyzed data; S.L., X.L., K.T., J.B., B.S., J.W., J.R.R., W.C.d.G., and C.T. interpreted results of experiments; S.L., X.L., K.T., J.B., B.S., J.W., J.R.R., W.C.d.G., and C.T. prepared figures; S.L., X.L., K.T., J.B., B.S., J.W., J.R.R., W.C.d.G., and C.T. drafted manuscript; S.L., X.L., K.T., J.B., B.S., J.W., J.R.R., W.C.d.G., and C.T. edited and revised manuscript; S.L., X.L., K.T., J.B., B.S., J.W., J.R.R., W.C.d.G., and C.T. approved final version of manuscript.
REFERENCES
- 1.Bruns TM, Bhadra N, Gustafson KJ. Bursting stimulation of proximal urethral afferents improves bladder pressures and voiding. J Neural Eng 6: 066006, 2009. doi: 10.1088/1741-2560/6/6/066006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapple CR, Osman NI, Birder L, van Koeveringe GA, Oelke M, Nitti VW, Drake MJ, Yamaguchi O, Abrams P, Smith PP. The underactive bladder: a new clinical concept? Eur Urol 68: 351–353, 2015. doi: 10.1016/j.eururo.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 3.de Groat WC, Wickens C. Organization of the neural switching circuitry underlying reflex micturition. Acta Physiol (Oxf) 207: 66–84, 2013. doi: 10.1111/apha.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devanandan MS, Eccles RM, Lewis DM, Stenhouse D. Responses of flexor alpha-motoneurones in cats anaesthetised with chloralose. Exp Brain Res 8: 163–176, 1969. [DOI] [PubMed] [Google Scholar]
- 5.Ferroni MC, Slater RC, Shen B, Xiao Z, Wang J, Lee A, Roppolo JR, de Groat WC, Tai C. Role of the brain stem in tibial inhibition of the micturition reflex in cats. Am J Physiol Renal Physiol 309: F242–F250, 2015. doi: 10.1152/ajprenal.00135.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juszczak K, Drewa T. Pharmacotherapy in detrusor underactivity: A new challenge for urologists and pharmacologists (from lab to clinic). Pharmacol Rep 68: 703–706, 2016. doi: 10.1016/j.pharep.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Kessler TM, Fowler CJ. Sacral neuromodulation for urinary retention. Nat Clin Pract Urol 5: 657–666, 2008. doi: 10.1038/ncpuro1251. [DOI] [PubMed] [Google Scholar]
- 8.Mallory BS, Roppolo JR, de Groat WC. Pharmacological modulation of the pontine micturition center. Brain Res 546: 310–320, 1991. doi: 10.1016/0006-8993(91)91495-M. [DOI] [PubMed] [Google Scholar]
- 9.McPherson A. The effects of somatic stimuli on the bladder in the cat. J Physiol 185: 185–196, 1966. doi: 10.1113/jphysiol.1966.sp007980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moazzam Z, Duke AR, Yoo PB. Inhibition and excitation of bladder function by tibial nerve stimulation using a wirelessly powered implant: an acute study in anesthetized cats. J Urol 196: 926–933, 2016. doi: 10.1016/j.juro.2016.04.077. [DOI] [PubMed] [Google Scholar]
- 11.Moazzam Z, Yoo PB. Frequency-dependent inhibition of bladder function by saphenous nerve stimulation in anesthetized rats. Neurourol Urodyn 37: 592–599, 2018. doi: 10.1002/nau.23323. [DOI] [PubMed] [Google Scholar]
- 12.Osman NI, Chapple CR. Fowler’s syndrome–a cause of unexplained urinary retention in young women? Nat Rev Urol 11: 87–98, 2014. doi: 10.1038/nrurol.2013.277. [DOI] [PubMed] [Google Scholar]
- 13.Peters KM, Carrico DJ, Perez-Marrero RA, Khan AU, Wooldridge LS, Davis GL, Macdiarmid SA. Randomized trial of percutaneous tibial nerve stimulation versus Sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol 183: 1438–1443, 2010. doi: 10.1016/j.juro.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 14.Sato A, Sato Y, Schmidt RF. Reflex bladder activity induced by electrical stimulation of hind limb somatic afferents in the cat. J Auton Nerv Syst 1: 229–241, 1980. doi: 10.1016/0165-1838(80)90019-3. [DOI] [PubMed] [Google Scholar]
- 15.Shimamura M, Akert K. Peripheral nervous relations of propriospinal and spino-bulbo-spinal reflex system. Jpn J Physiol 15: 638–647, 1965. doi: 10.2170/jjphysiol.15.638. [DOI] [Google Scholar]
- 16.Smith PP, Birder LA, Abrams P, Wein AJ, Chapple CR. Detrusor underactivity and the underactive bladder: Symptoms, function, cause-what do we mean? ICI-RS think tank 2014. Neurourol Urodyn 35: 312–317, 2016. doi: 10.1002/nau.22807. [DOI] [PubMed] [Google Scholar]
- 17.Tai C, Shen B, Chen M, Wang J, Roppolo JR, de Groat WC. Prolonged poststimulation inhibition of bladder activity induced by tibial nerve stimulation in cats. Am J Physiol Renal Physiol 300: F385–F392, 2011. doi: 10.1152/ajprenal.00526.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tai C, Wang J, Jin T, Wang P, Kim SG, Roppolo JR, de Groat WC. Brain switch for reflex micturition control detected by FMRI in rats. J Neurophysiol 102: 2719–2730, 2009. doi: 10.1152/jn.00700.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu M, Uy J, Jiang X, Li X, Jones C, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. An excitatory reflex from the superficial peroneal nerve to the bladder in cats. Am J Physiol Renal Physiol 313: F1161–F1168, 2017. doi: 10.1152/ajprenal.00265.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]


