Abstract
Renal ammonia metabolism has a major role in the maintenance of acid-base homeostasis. Sex differences are well recognized as an important biological variable in many aspects of renal function, including fluid and electrolyte metabolism. However, sex differences in renal ammonia metabolism have not been previously reported. Therefore, the purpose of the current study was to investigate sex differences in renal ammonia metabolism. We studied 4-mo-old wild-type C57BL/6 mice fed a normal diet. Despite similar levels of food intake, and, thus, protein intake, which is the primary determinant of endogenous acid production, female mice excreted greater amounts of ammonia, but not titratable acids, than did male mice. This difference in ammonia metabolism was associated with fundamental structural differences between the female and male kidney. In the female mouse kidney, proximal tubules account for a lower percentage of the renal cortical parenchyma compared with the male kidney, whereas collecting ducts account for a greater percentage of the renal parenchyma than in male kidneys. To further investigate the mechanism(s) behind the greater ammonia excretion in female mice, we examined differences in the expression of proteins involved in renal ammonia metabolism and transport. Greater basal ammonia excretion in females was associated with greater expression of PEPCK, glutamine synthetase, NKCC2, Rhbg, and Rhcg than was observed in male mice. We conclude that there are sex differences in basal ammonia metabolism that involve both renal structural differences and differences in expression of proteins involved in ammonia metabolism.
Keywords: acid-base, ammonia, collecting duct, proximal tubule, sex differences
INTRODUCTION
Acid-base homeostasis is necessary for maintaining normal health. Abnormal acid-base homeostasis causes many disorders, including worsened control of hyperglycemia, increased risk of osteoporosis and skeletal bone disease, increased risk of cardiac arrhythmias, and increased risk of progressive chronic kidney disease, leading to end-stage renal disease (13, 18, 43). Moreover, abnormal acid-base homeostasis, either metabolic acidosis or metabolic alkalosis, is strongly correlated with increased mortality (31, 46, 50). Renal ammonia metabolism has a major role in the maintenance of acid-base homeostasis. Renal ammonia excretion is the predominant component of basal net acid excretion, and increased ammonia excretion is the predominant component of increased net acid excretion in response to a variety of disorders, such as metabolic acidosis and hypokalemia (1, 53, 55, 64, 66, 68).
Sex differences are present in many aspects of mammalian biology. There is sexual dimorphism in structure and/or function in nearly every tissue and organ, including brain, bones, cardiovascular structure, and kidney (25, 26, 52). Sex differences have been reported in the occurrence and development of various diseases, such as hypertension, chronic kidney disease, and acute or chronic renal ischemia (14, 15, 27, 47, 54, 56). However, sex differences in renal ammonia metabolism have not been previously reported.
The purpose of the current study was to investigate sex differences in renal ammonia metabolism. First, to evaluate for sex differences in renal ammonia excretion, we studied normal mice fed a normal diet. Then, we examined whether there were structural differences between the female and male kidney. Finally, to investigate the mechanism(s) behind the increase in ammonia excretion in female mice, we examined changes in the expression of key proteins involved in intrarenal ammonia metabolism and transport.
METHODS
Animals.
Four-month-old C57BL/6 mice for these experiments were obtained from the Jackson Laboratory (Bar Harbor, ME), and from the University of Florida College of Medicine Cancer and Genetics Transgenic Animal Core Facility. The University of Florida College of Medicine and the North Florida/South Georgia Veterans Health System Institutional Animal Care and Use Committees approved all animal protocols.
Metabolic cage studies.
Mice were placed in metabolic cages for 3 days and fed a normal diet (18% protein; Harlan Teklad). Daily food consumption was measured, and urine samples were collected. Urine samples were collected in tubes containing water-equilibrated mineral oil to prevent evaporation. Daily urine volume and pH were recorded. Urine samples were stored at −20°C until analyzed further.
Plasma analyses.
Blood was obtained by cannulation of the aorta, drawn into a heparinized syringe and immediately analyzed for Na+, K+ and bicarbonate concentrations using a Siemens Microanalytic Blood Gas Analyzer (RAPIDLab 348 analyzer; Siemens, Munich, Germany).
Urinary analysis.
Urine ammonia was measured using a modification of a commercially available kit (A7553; Pointe Scientific, Canton, MI), as described previously (5, 36). Urine pH was measured using a micro-pH electrode (ROSS semi-micro pH; Orion 8115BN; Thermo Scientific, Waltham, MA). Titratable acid was measured using standard techniques described previously (36).
Antibodies.
Affinity-purified antibodies to Rhesus B glycoprotein (Rhbg) and Rhesus C glycoprotein (Rhcg) generated in our laboratory have been previously characterized (5, 29, 37, 39, 60, 63). Antibodies to phosphoenolpyruvate carboxykinase (PEPCK) were obtained from Cayman Chemical (Ann Arbor, MI; cat no. 10004943). Antibodies to glutamine synthetase (GS) were obtained from Abcam (Cambridge, MA; cat. no. Ab73593). Antibodies to NBCe1 were obtained from Proteintech (Rosemont, IL; cat. no. 885-1-AP). Antibodies to NHE3 were obtained from StressMarq Biosciences (Victoria BC, Canada; cat. no. SPC-400D). H. Moo Kwon (Ulsan National Institute of Science and Technology, Ulsan, South Korea) graciously provided antibodies to NKCC2.
Protein preparation.
Animals were anesthetized with inhalant isoflurane, and kidneys were rinsed by in vivo cardiac perfusion with PBS (pH 7.4) containing 90,000 units/l Na-heparin and 60 mg/l lidocaine. The right kidney was removed rapidly, and the cortex, outer medulla, and inner medulla were isolated on a cold stage under a dissecting microscope. Samples were snap-frozen in liquid nitrogen and stored frozen at –80°C until used. Tissues were homogenized in T-PER tissue protein extraction reagent (Pierce Biotechnology, Rockford, IL) using microtube pestles (USA Scientific, Ocala, FL), and protein was extracted according to manufacturer’s recommendations. An aliquot was used for total protein quantification using a BCA assay, and the remainder was stored frozen at −80°C until use.
Immunoblot procedure.
Fifteen micrograms of renal protein were electrophoresed on 10% PAGE ReadyGel (Bio-Rad, Hercules, CA). Gels were then transferred electrophoretically to nitrocellulose membranes, blocked with 5 g/dl nonfat dry milk diluted in Blotto buffer (50 mM Tris, 150 mM NaCl, 5 mM Na2EDTA, and 0.05% Tween 20; pH 7.6), and incubated at 4°C overnight with the primary antibody in nonfat dry milk. Loading and transfer equivalence were assessed with Ponceau S staining. After being washed, membranes were exposed to secondary antibody (goat anti-rabbit IgG; Cell Signaling Technology, Beverly, MA) conjugated to horseradish peroxidase at a dilution of 1:5,000. Sites of antibody-antigen reaction were visualized using enhanced chemiluminescence (SuperSignal West Pico Substrate; Pierce) and a Kodak Image Station 440CF digital imaging system. In selected experiments, blots were stripped. Band density was quantified using Kodak ID (version 5.0) software (Kodak Scientific Imaging, New Haven, CT). Band density normalized such that mean density in a region (cortex or outer medulla) in male tissue was 100. The absence of saturation was confirmed by examining pixel intensity distribution in all immunoblots.
Tissue preparation for immunohistochemistry.
Mice were anesthetized with inhalant isoflurane. The kidneys were preserved by in vivo cardiac perfusion with PBS (pH 7.4) containing 6,000 unit/l of Na-heparin and 120 mg/l lidocaine followed by periodate-lysine-2% paraformaldehyde (PLP) and then cut transversely into several 2- to 3-mm-thick slices and immersed for 24–30 h at 4°C in the same fixative. Kidney samples from each animal were embedded in polyester wax using polyethylene glycol 400 distearate (Polysciences, Warrington, PA) with 10% 1-hexadecanol, and 2-μm-thick sections were cut and mounted on gelatin-coated glass slides.
Immunohistochemistry.
Immunolocalization for GS, Rhbg, Rhcg, NKCC2, and NBCe1 was accomplished using previously described immunoperoxidase procedures (4, 35, 38, 59). Briefly, sections were dewaxed in ethanol, rehydrated, heated in Trilogy (Cell Marque, Rocklin, CA) to 88°C for 30 min and then to 96°C for 30 min, cooled for 30 min, and rinsed in PBS. Endogenous peroxidase activity was blocked by incubating the sections in 3% H2O2 in distilled water for 45 min. Sections were blocked for 15 min with serum-free protein block (Dako Cytomation) and then incubated overnight at 4°C with the primary antibody. Sections were washed in PBS and incubated for 30 min with polymer-linked, peroxidase-conjugated horse anti-rabbit IgG (ImmPRESS; Vector Laboratories, Burlingame, CA), washed again with PBS, and then exposed to diaminobenzidine (DAB) for 5 min. Sections were washed in distilled water, dehydrated with xylene, mounted, and observed by light microscopy. Comparisons of labeling were made between sections from the same immunohistochemistry experiment. Sections were examined on a Nikon E600 microscope equipped with DIC optics and photographed using a DXM1200F digital camera and ACT-1 software (Nikon).
Immunolocalization for PEPCK was accomplished using modified immunoperoxidase procedures described previously (21, 22, 33, 34, 38, 48). Briefly, sections were dewaxed in ethanol, rehydrated, heated in Trilogy (Cell Marque, Rocklin CA) to 96°C for 60 min, cooled for 30 min, and rinsed in PBS. Endogenous peroxidase activity was blocked by incubation of the sections in 3% H2O2 in methanol for 45 min. The sections were treated with 0.5% Triton X-100 in PBS for 15 min, The sections then underwent several washes in PBS containing 1% BSA, 0.05% saponin, and 0.2% gelatin, followed by blocking for 15 min with serum-free protein block (DAKO Cytomation) and then incubated overnight at 4°C with the primary antibody. Sections were washed in PBS containing 0.1% BSA, 0.05% saponin, and 0.2% gelatin, followed by PBS and incubated for 60 min with polymer-linked, peroxidase-conjugated goat anti-rabbit IgG (MACH2, Biocare Medical, Concord CA), washed again with PBS, and then exposed to diaminobenzidine (DAB) for 5 min.
Negative controls.
Each immunohistochemistry experiment included a section that was exposed to the immunolabeling procedure without the primary antibody to ensure that the label was due to the primary antibody binding only.
Morphometric analysis.
The volume density of the proximal tubule in the cortex, the collecting duct in the cortex and inner stripe of the outer medulla (OMi), and the thick ascending limb of the loop of Henle (TAL) in the cortex and OMi was determined using standard point-counting techniques (3). We used PEPCK immunolabeling to identify proximal tubule segments, Rhbg immunolabel to identify collecting duct segments, and NKCC2 immunolabel to identify TAL segments. We used high-resolution digital micrographs of PEPCK, Rhbg, or NKCC2 immunolabel overlaid with a standardized grid using Adobe Photoshop C5 software. The volume density of the specific tubule type of interest (proximal tubule, collecting duct, or TAL) in each photomicrograph was calculated by dividing the number of points in the grid overlying the epithelial segment of interest (including the tubule lumen) by the total number of points over all structures in the micrograph, excluding vessels, and then multiplied by 100. At least four photomicrographs were analyzed for each kidney, and data from all micrographs examined for each mouse were averaged to yield a single data point per animal for statistical analysis.
Quantitative immunohistochemistry.
Quantitative immunohistochemistry was performed as we have described and validated previously (28, 34, 59). Proximal tubule segments were studied in sections labeled under identical conditions in the same PEPCK immunolabeling experiment by an observer blinded to the sex of the mouse. The specific proximal tubule segments measured were the initial proximal convoluted tubule (PCT), defined as PCT segments continuous with Bowman’s capsule; the proximal straight tubule (PST) in the medullary ray; and the PST in the outer stripe of the outer medulla (OMo). We used high-resolution digital micrographs taken of randomly selected fields of the renal cortex and OMo using a Nikon E600 microscope equipped with a DXM1200F digital camera and ACT-1 software (Nikon) using no image enhancement techniques. Using ImageJ software (version 1.34j; National Institutes of Health, Bethesda, MD), we measured pixel intensity across a line drawn from the tubule lumen through an individual cell. These data were then analyzed using custom-written software executed in Quattro Pro. Net intensity at each pixel on the line was determined as the difference between absolute intensity and mean background pixel intensity measured outside the cell. Total cellular expression was determined by integrating net pixel intensity across the entire cell. Cell height was determined as the distance in pixels between the apical and basolateral edges of the cells and converted to absolute length using calibrated determination of individual pixel size. A minimum of 15 individual cells from at least four photomicrographs from each kidney was analyzed. Data from all cells examined of a given proximal tubule segment type were averaged to yield a single data point per animal for statistical analysis.
To determine Type A intercalated cell volume and per cell Rhbg immunolabel intensity, individual intercalated cells expressing Rhbg immunolabel in the OMi were identified and circumscribed using ImageJ software (version 1.34j; National Institutes of Health). Net intensity at each pixel was determined as the difference between absolute pixel intensity and mean background intensity. Immunolabel intensity within the cell was determined using custom-written software executed in Microsoft Excel 2010 to quantify integrated net pixel intensity. Cell area was determined as the number of pixels within the outlined region and converted to an area using calibrated measurement of pixels per micrometer.
Statistics.
Results are presented as means ± SE; n refers to the number of animals studied. Statistical analyses were performed using an unpaired Student’s t-test or one-way ANOVA, and P < 0.05 was considered statistically significant.
RESULTS
Physiological parameters.
We began investigating sex differences in renal ammonia metabolism by evaluating physiological parameters in adult male and female mice (Table 1). Female mice weighed significantly less than did male mice (P < 0.01; n = 12 for each group). Thus, for several physiological parameters, we evaluated both absolute and weight-adjusted values. Absolute daily food intake did not differ significantly between the sexes (P = NS; n = 12 for each group). However, weight-adjusted daily food intake was significantly greater in female (F) mice than in male (M) mice (P < 0.01; n = 12 for each group). There was no significant difference in plasma bicarbonate (P = NS; n = 22 F, 29 M) or plasma electrolyte concentrations (P = NS for Na+ and for K+; n = 12 for each group) between female and male mice. Female mice had significantly greater urinary ammonia excretion than male mice, both when considering absolute or weight-adjusted ammonia excretion (P < 0.01; n = 11 F, 9 M). There was no significant difference in titratable acid excretion, either absolute or weight-adjusted, between the sexes (P = NS; n = 11 F, 9 M). This difference in ammonia excretion occurred despite no significant difference in urine pH (P = NS; n = 12 for each group). These findings show that there are sex differences in basal ammonia excretion in female and male mice that are disproportionately greater than the weight-adjusted differences in dietary protein intake, which is the primary determinate of endogenous acid production.
Table 1.
Effect of sex on acid-base parameters
| Parameters | Female | Male | P Value |
|---|---|---|---|
| Food intake, g | 8.9 ± 0.9 (12) | 9.6 ± 1.2 (12) | NS |
| Food intake, g/g body wt | 0.41 ± 0.04 (12) | 0.33 ± 0.04 (12) | <0.01 |
| Body weight, g | 22.2 ± 1.5 (12) | 29.1 ± 1.6 (12) | <0.01 |
| Plasma Na+, mmol/l | 147 ± 3 (12) | 148 ± 3 (12) | NS |
| Plasma K+, mmol/l | 3.9 ± 0.4 (12) | 4.2 ± 0.4 (12) | NS |
| Plasma HCO3−, mmol/l | 20.0 ± 3.1 (22) | 19.6 ± 2.2 (29) | NS |
| Urine ammonia, µmol/day | 72 ± 23 (11) | 46 ± 19 (9) | <0.01 |
| Urine ammonia, µmol/g body wt/day | 3.2 ± 0.9 (11) | 1.6 ± 0.7 (9) | <0.01 |
| Titratable acid, µmol/day | 53 ± 26 (11) | 74 ± 20 (9) | NS |
| Titratable acid, µmol/g body wt/day | 2.40 ± 1.16 (11) | 2.44 ± 0.67 (9) | NS |
| Net acid excretion, µmol/day | 125 ± 35 (11) | 120 ± 28 (9) | NS |
| Net acid excretion, µmol·g−1 body wt·day−1 | 5.63 ± 2.09 | 4.09 ± 0.88 (9) | <0.02 |
| Urine pH | 6.40 ± 0.18 (12) | 6.36 ± 0.14 (12) | NS |
Results are reported as means ± SE. The number of mice is shown in parentheses. NS, not significant.
Sex differences in renal structure.
The sex difference in ammonia excretion could reflect specific differences in ammonia metabolism and transport, or it could reflect generalized differences in the structure of the female and male kidneys. To examine these possibilities, we first determined whether there were structural differences between the female and male kidneys. We studied separately the proximal tubule, TAL, and the collecting duct.
Since ammonia is produced primarily in the proximal tubule, we began by examining whether there were structural differences in the proximal tubule of male and female mice. To do so, we used PEPCK immunolabel as a marker of proximal tubule cells. Immunohistochemistry showed that PEPCK immunolabel was present in and was limited to all proximal tubule epithelial cells. Low-power micrographs of PEPCK immunolabel showed that a smaller proportion of the cortex appeared to exhibit PEPCK immunolabel in the female kidney than in the male kidney (Fig. 1). Quantitative analysis showed that 60 ± 3% of the male cortex was proximal tubules, whereas only 42 ± 3% of the female cortex was proximal tubules, which was significantly less than the male cortex (P < 0.001; Fig. 1; n = 6 for each group). Proximal tubule volume density in the OMi did not differ significantly between male and female mice (P = NS; Fig. 1; n= 6 for each group). Thus, there are sex differences in cortical proximal tubule volume density.
Fig. 1.
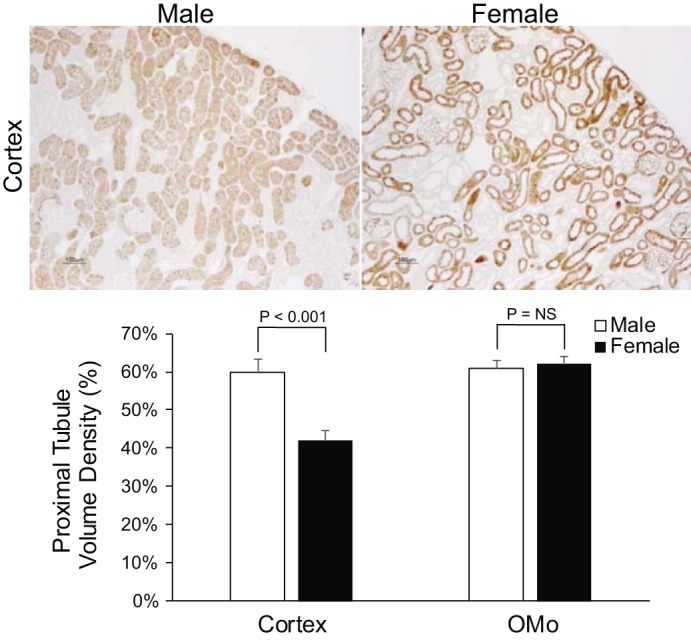
Sex differences in proximal tubule volume density. Top: low-power photomicrographs of phosphoenolpyruvate carboxykinase (PEPCK) immunolabel in the cortex of male (left) and female (right) mice. Proximal tubules appear relatively sparse in the female mouse kidney compared with the male mouse kidney. Bottom: quantitative analysis of cortical and outer stripe of the outer medulla (OMo) proximal tubule volume density. Female mice had significantly lower proximal tubule volume density in the cortex than did male mice. OMo proximal tubule volume density did not differ significantly between male and female mice. n = 6 for each group.
This difference in proximal tubule volume density could be due to a difference in cell size. To examine this possibility, we measured proximal tubule cell height. We separately determined cell height in the proximal convoluted tubule (PCT), cortical proximal straight tubule (cortical PST), and OM PST. Female mice have significantly lower PCT and cortical PST cell height than do male mice (P < 0.01; Fig. 2; n = 6 for each group). The OM PST cell height did not differ significantly between the sexes (P = NS; Fig. 2; n= 6 for each group).
Fig. 2.

Sex differences in proximal tubule cell height. Quantitative analysis of proximal tubule cell height in the proximal convoluted tubule (PCT), cortical proximal straight tubule (PST), and outer medulla (OM) PST. Female mice had significantly lower PCT and cortical PST cell height than did male mice. OM PST cell height did not differ significantly between male and female mice. n = 6 for each group.
These findings demonstrate that there are fundamental structural differences between the male and female kidney. Cortical proximal tubule volume density and cell height are significantly less in the female kidney than in the male kidney. Because both cortical proximal tubule volume density and cell height in the female were ~75% of that in the male kidney, these findings suggest that the sex difference in cortical proximal tubule volume density is caused, at least in part, by a difference in cortical proximal tubule cell size.
Because the TAL is involved in ammonia reabsorption, we next determined whether there were sex differences in TAL structure. We used NKCC2 immunolabel as a marker of the TAL. Low-power micrographs of NKCC2 immunolabel did not show a detectable difference in the proportion of TALs in either the cortex or OMi between the male and female kidney (Fig. 3). Morphometric analysis showed TAL volume density did not differ significantly between male and female kidney in either the cortex or OMi (P = NS; Fig. 3; n = 6 for each group).
Fig. 3.

Thick ascending limb of the loop of Henle (TAL) volume density. Top: low-power photomicrographs of NKCC2 immunolabel in the cortex (top) and the inner stripe of the outer medulla (OMi; bottom) in male (left) and female (right) mice. There was not an apparent difference in the abundance of TAL between male and female mice. Bottom: quantitative analysis of TAL volume density in the cortex and OMi. There was no significant difference in volume density of the TAL in either the cortex or the OMi between male and female mice. n = 6 for each group.
Since the collecting duct secretes the majority of urinary ammonia, we next determined whether there were sex differences in the collecting duct structure. We used Rhbg immunolabel as a marker of the collecting duct. Low-power micrographs of Rhbg immunolabel revealed easily detectable differences between the male and female kidney. A larger proportion of the cortex and OMi appeared to exhibit Rhbg immunolabel in the female kidney compared with the male kidney (Fig. 4). In the cortex, morphometric analysis showed the collecting duct accounted for a significantly greater proportion of volume density in the female kidney than in the male kidney (F: 15.4 ± 2.0%; M: 9.6 ± 1.6%, P < 0.001, n = 6 for each sex). Similar findings were observed in the OMi, where the collecting duct accounted for a significantly greater proportion of volume density in the female kidney than in the male kidney (F: 11.1 ± 1.3%; M: 7.8 ± 0.6%, P < 0.001; Fig. 4; n = 6 for each group). Thus, the collecting duct accounts for a larger proportion of both cortical and OMi volume density in female mice than in male mice.
Fig. 4.
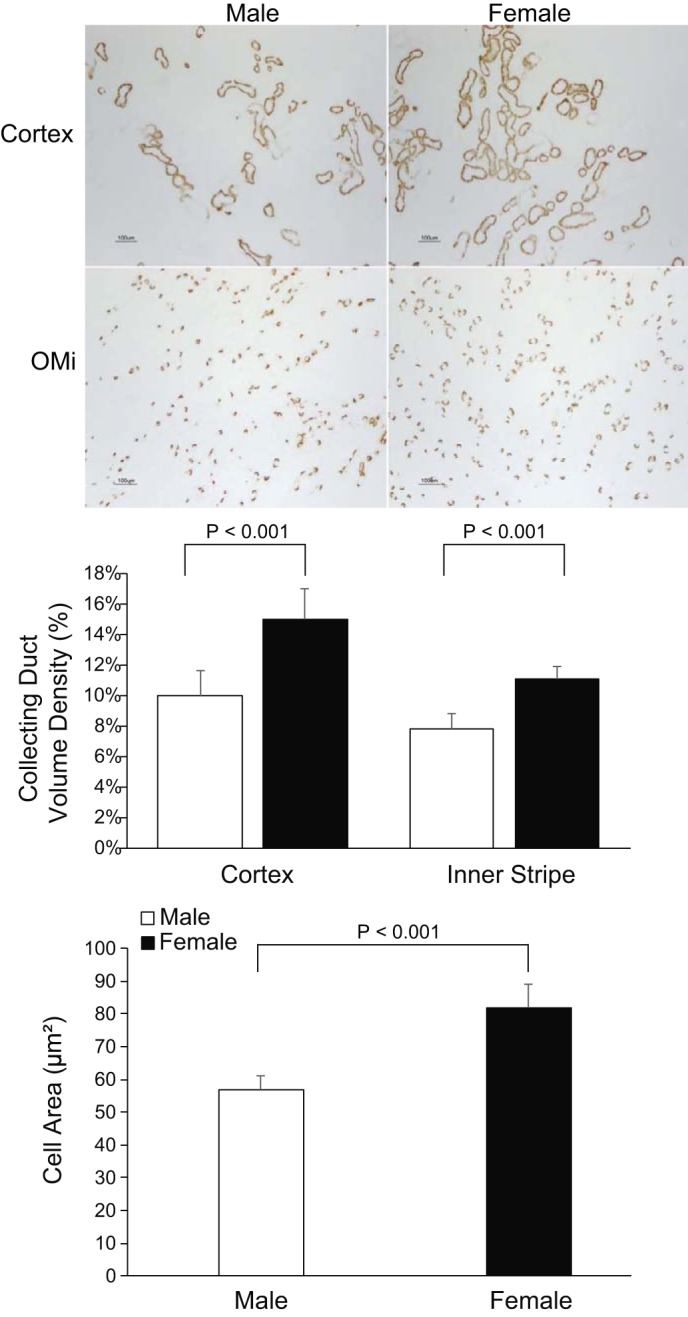
Sex differences in collecting duct volume density. Top: low-power photomicrographs of Rhbg immunolabel in the cortex (top) and OMi (bottom) in male (left) and female (right) mice. Collecting ducts appear more abundant in female mice when compared with male mice in the cortex and OMi. Middle: quantitative analysis of collecting duct volume density in the cortex and OMi. Female mice had significantly greater volume density of collecting duct segments in the cortex and OMi when compared with male mice. Bottom: single-cell digital quantitative analysis in male and female mice. Female mice had significantly greater intercalated cell area of the collecting duct in the OMi when compared with male mice n = 6 for each group.
Because intercalated cells are the primary collecting duct cell involved in ammonia metabolism, we then determined whether sex altered intercalated cell size. Quantitative immunohistochemistry (qIHC) showed OMi intercalated cell size was significantly greater in female mice than in male mice (F: 82 ± 7 µm2; M: 57 ± 4 µm2, P < 0.001; n = 6 for each group, Fig. 4). Thus, both collecting duct volume density and intercalated cell size are greater in the female kidney than in the male kidney.
Sex differences in proteins involved in ammoniagenesis.
Greater urinary ammonia excretion in female mice could also result from differences in mechanisms of ammonia metabolism and transport. To test this possibility, we examined expression of key proteins involved in ammonia generation and recycling. We used PEPCK expression as a marker of ammonia generation and glutamine synthetase expression as a marker of ammonia recycling (12, 66).
Immunoblot analysis showed PEPCK protein was significantly greater in female than in male mice (P < 0.05; Fig. 5A; n = 6 for each group). Because the proximal tubule volume density and proximal tubule cell height are less in female than in male mice, the greater PEPCK protein expression cannot be attributed to increased number or size of proximal tubule segments. Supporting this interpretation is that PEPCK immunohistochemistry qualitatively showed greater PEPCK immunolabel in female mice than observed in male mice throughout the entire proximal tubule (Fig. 5B). To quantify these qualitative observations, we performed qIHC. This showed significantly greater PEPCK immunolabel in each proximal tubule segment, PCT, cortical PST, and OM PST, in female mice than in male mice (P < 0.05; Fig. 5C; n = 6 for each group). Thus, the female mouse kidney, despite having a lesser proximal tubule volume density, has greater total and proximal tubule segment-specific PEPCK expression.
Fig. 5.
Sex differences in PEPCK expression. A: immunoblot analysis of phosphoenolpyruvate carboxykinase (PEPCK) expression with quantification. Female mice express significantly more PEPCK protein than do male mice. B: immunohistochemistry examining PEPCK immunolabel in proximal convoluted tubule (PCTs) (top), cortical proximal straight tubule (PST) (middle), and PST outer medulla (OM; bottom) in male (left) and female (right) mice. PEPCK immunolabel intensity was markedly greater in all proximal tubule segments in female mice than in male mice. C: quantitative immunohistochemistry demonstrated that female mice have significantly greater PEPCK expression in PCT, cortical PST, and OM PST. n = 6 for each group.
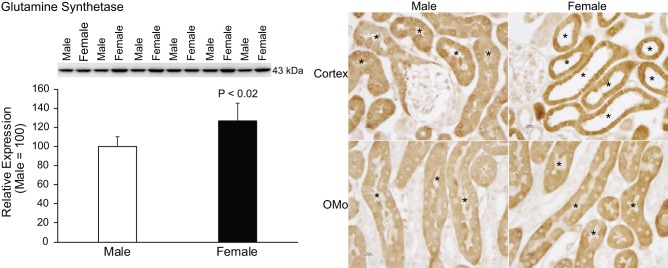
In addition to generating ammonia, the proximal tubule also has an ammonia recycling process. GS recycles ammonia by catalyzing the reaction of NH4+ with glutamate to regenerate glutamine, thereby decreasing net ammoniagenesis (10, 59). Immunoblot analysis showed significantly greater GS protein expression in female cortex than in male cortex (P < 0.02; Fig. 6; n = 6 for each group). Immunohistochemistry demonstrated greater GS immunolabel in females throughout the proximal convoluted and proximal straight tubule (Fig. 6). Thus, because GS decreases net ammoniagenesis, the observed differences in GS expression cannot explain the greater urinary ammonia excretion in female mice.
Fig. 6.
Sex differences in glutamine synthetase (GS) expression. Left: immunoblot analysis of GS protein expression in homogenates of renal cortex with quantification. Female mice express significantly greater GS protein than male mice. Right: immunohistochemistry examining GS immunolabeling the cortex (top) and outer stripe of the outer medulla (OMo; bottom) in male (left) and female (right) mice. GS immunolabel intensity was substantially greater in the female proximal tubule epithelial cells in the cortex and OMo when compared with male mice. n = 6 for each group. The asterisk (*) denotes proximal tubule segments.
Effect of sex on NBCe1.
We recently identified that NBCe1, a basolateral proximal tubule sodium bicarbonate cotransporter, regulates proximal tubule ammonia metabolism (24). NBCe1 protein expression by immunoblot analysis did not differ significantly between male and female mice (P = NS; Fig. 7; n = 6 for each group). Immunohistochemistry demonstrated no detectable difference in NBCe1 immunolabel intensity throughout the proximal convoluted and proximal straight tubule (Fig. 7). Thus, the sex differences in enzymes involved in proximal tubule ammonia metabolism apparently are independent from NBCe1 expression.
Fig. 7.
NBCe1 expression. Left: immunoblot analysis of NBCe1 protein expression with quantification. NBCe1 expression did not differ significantly between the sexes. Right: immunohistochemistry examining NBCe1 immunolabel in the cortex (top) and outer stripe of the outer medulla (OMo; bottom) in male (left) and female (right) mice. There was no detectable difference in NBCe1 immunolabel intensity between the sexes. n = 4 for males and 5 for females.
Sex differences in proteins involved in ammonia transport.
Renal ammonia excretion involves coordinated NH3 and NH4+ transport by specific membrane proteins (61, 62, 65). Therefore, understanding the regulation of renal ammonia excretion requires evaluating the primary ammonia transporters expressed in the proximal tubule, TAL, and collecting duct. Ammonia generated in the proximal tubule undergoes preferential secretion into the luminal fluid, and NHE3 is believed to be the major mechanism of proximal tubule ammonia secretion (61, 64, 66). NHE3 cortical protein expression did not differ significantly between female and male mice (P = NS; Fig. 8; n = 6 for each group). NKCC2 mediates TAL ammonia reabsorption. NKCC2 cortical protein expression, measured using immunoblot analysis, was significantly greater in female mice than male mice (P < 0.01; Fig. 8; n = 6 for each group). Immunohistochemistry demonstrated increased NKCC2 immunolabel intensity in the TAL in the cortex and OMi (Fig. 8). These findings suggest greater NKCC2-mediated TAL ammonia reabsorption may occur in females and contribute to the greater ammonia excretion in females compared with males.
Fig. 8.
Sex differences in ammonia transport proteins. Top, left: immunoblot analysis of NHE3 protein expression with quantification. There was no significant difference in NHE3 protein expression between the sexes. Top, right: immunoblot analysis of NKCC2 protein expression with quantification. Female mice express significantly greater NKCC2 protein than do male mice. Bottom: immunohistochemistry examining NKCC2 immunolabel in the cortex (top) and the inner stripe of the outer medulla (OMi; bottom) in male (left) and female (right) mice. NKCC2 immunolabel intensity was substantially greater in female thick ascending limb of the loop of Henle epithelial cells in the cortex and OMi when compared with male mice. n = 6 for each group.
The collecting duct secretes the majority of urinary ammonia, and the Rhesus glycoproteins Rhbg and Rhcg are the primary collecting duct ammonia-transporting proteins (20, 61, 62). Immunoblot analysis showed significantly greater Rhbg protein expression in female mice than in male mice in both the cortex and OMi (P < 0.01; Fig. 9A; n = 6 for each group). Immunohistochemistry demonstrated substantially greater Rhbg immunolabel intensity in the cortex and OMi in female mice compared with male mice (Fig. 9B). To quantify the qualitative observation of increased Rhbg expression in intercalated cells in the OMi, we performed single-cell quantitative immunohistochemistry. These findings show greater Rhbg immunolabel intensity in intercalated cells in the OMi in female mice than in male mice (P < 0.001; Fig. 9C; n = 6 for each group).
Fig. 9.
Sex differences in Rhbg expression. A: immunoblot analysis of Rhbg protein expression in the cortex and inner stripe of the outer medulla (OMi) with quantification. Female mice express significantly greater Rhbg protein then do male mice in the cortex and OMi. B: immunohistochemistry examining Rhbg immunolabel in cortical collecting ducts (top), and collecting duct in the OMi (bottom) in male (left) and female (right) mice. Increased Rhbg expression was evident in intercalated and principal cells in the cortical collecting and intercalated cells in the collecting duct in the OMi in female mice compared with male mice. C: single-cell digital quantitative analysis of intercalated cells in the OMi. Female mice when compared with male mice have significantly greater Rhbg immunolabel intensity in OMi intercalated cells. n = 6 for each group.
Immunohistochemistry also demonstrated sex-dependent differences in Rhcg expression. Rhcg immunolabel was substantially greater in female than male mice in the cortex and OMi (Fig. 10). The overall increase in these regions was due to increased immunolabel intensity in connecting segment cells and the basolateral membrane of collecting duct intercalated and principal cells in the OMi. These findings suggest that differences in Rhbg- and Rhcg-mediated ammonia secretion may contribute to sex differences in ammonia excretion.
Fig. 10.
Sex differences in Rhcg expression. High-power micrographs of Rhcg immunolabel in the connecting segments (top), and collecting duct in the inner stripe of the outer medulla (bottom) of male (left) and female (right) kidney. Female mice have significantly greater immunolabel intensity in the basolateral region of connecting segment cells and in the basolateral region of intercalated and principal cells in the collecting duct in the OMi. n= 6 for each group. The asterisk (*) denotes connecting segment cells; the white arrows denote intercalated cells; and the black arrows denote principal cells.
DISCUSSION
The current studies provide important new information regarding sex differences in basal acid-base homeostasis. Our results show that female mice have greater ammonia excretion than male mice with no difference in titratable acid excretion. There were significant structural differences between the female and male kidney; in the female mouse kidney, proximal tubules account for a lower proportion of the renal cortical parenchyma than in the male kidney, and the collecting duct accounted for a larger proportion of the renal parenchyma. Finally, female mice have greater expression of multiple key proteins involved in ammonia metabolism and ammonia transport.
The first major finding in these studies is that female mice have greater ammonia excretion than male mice. Metabolism of dietary proteins generates endogenous acid loads, which are balanced under normal conditions by renal net acid excretion. These studies show that there are significant differences in dietary protein intake and net renal acid excretion when taking into account the sex difference in body weight, with female mice having greater food intake and net acid excretion. However, even with this difference in protein intake and, thus, endogenous acid production, female mice had disproportionately greater ammonia excretion. This suggests that there are sex-dependent differences in renal ammonia metabolism that cannot be explained by differences in diet-related endogenous acid production alone.
The current study did not find a difference in urine pH between male and female mice. Thus, the difference in ammonia excretion cannot be attributed to differences in urine acidity. Our observation of no sex difference in urine pH in mice is in contrast to findings in humans, where epidemiologic cohort studies have demonstrated that women have higher urine pH than men of similar age (11, 57). This difference in humans may be partly of gastrointestinal tract origin. A recent study in humans showed that women have a higher urine pH, which was associated with greater gastrointestinal tract anion absorption, than observed in men fed an identical diet (67).
The second major finding in these studies is that there are fundamental structural differences between the female and male mouse kidney. The current study shows that cortical proximal tubule volume density and cell height are significantly less in the female than in the male mouse kidney. This is consistent with the rat kidney (49). However, this earlier study did not quantify the proximal tubule epithelial cell height (49). The current study showed there was no significant sex difference in the TAL volume density in either the cortex or outer medulla. This is also similar to findings in the rat kidney (49). Finally, the current study shows that the collecting duct comprises a larger component of kidney volume in the female and that intercalated cells are larger in the outer medulla in the female than the male mouse kidney. The collecting duct was not evaluated in the previous study of the rat kidney (49). Thus, there are sex-dependent structural differences in both the mouse and rat kidney, with less proximal tubule volume, increased collecting duct volume, and no significant change in the TAL.
The ammonia component of net acid excretion is almost completely due to ammonia generation in the proximal tubule of the kidney. Ammonia generation occurs through a metabolic pathway primarily involving glutamine metabolism that results in equimolar NH4+ and bicarbonate generation (12, 61, 64, 66). The current study shows that there are sex-dependent differences in expression of two major components of proximal tubule ammonia metabolism, PEPCK and GS. These findings suggest that the female mouse kidney has the capacity for greater rates of both ammonia generation, via PEPCK, and ammonia recycling, via GS, than does the male mouse kidney. Since the proximal tubule is, under basal conditions, a net generator, and not a net recycler, of ammonia, the increased PEPCK expression likely leads to quantitatively greater ammoniagenesis. Importantly, structural differences between the male and female mouse kidney do not account for the greater expression of PEPCK and GS in the female mouse proximal tubule, because the greater expression occurs despite a lower proximal tubule volume density in females than in males.
NBCe1 has a critical role in acid-base homeostasis, in proximal tubule-filtered bicarbonate reabsorption and in proximal tubule ammonia metabolism. This role in ammonia metabolism is based on studies showing that NBCe1 deletion, despite causing a metabolic acidosis, decreases ammonia excretion and alters the expression of key proteins involved in proximal tubule ammonia metabolism (16, 24). In the present study, no sex differences in NBCe1 expression were detected. Thus, the sex differences in enzymes involved in proximal tubule ammonia generation do not result from differences in NBCe1 expression.
Ammonia produced in the proximal tubule is secreted preferentially into the luminal fluid. Several studies suggest that apical NHE3 is the primary mechanism of proximal tubule ammonia secretion (32, 44, 45). In the present study, there were no significant sex differences in NHE3 expression. This is in contrast to another study evaluating sex differences in renal transporters, which found that the female mouse kidney had significantly less NHE3 expression than the male mouse kidney (58). The reason for the different results between the current study and the previous study is unknown but could be related to differences in diet or time of kidney tissue collection.
Another mechanism by which female mice may have greater ammonia excretion may involve altered ammonia reabsorption in the thick ascending limb of the loop of Henle. This process establishes the corticomedullary gradient for ammonia concentration necessary for collecting duct ammonia secretion. Ammonia reabsorption in the TAL involves ammonium transport at the K+ binding site of NKCC2 (17, 30). Female mice have greater NKCC2 protein expression than do male mice, which may, through the medullary interstitial shunting pathway, contribute to greater collecting duct ammonia secretion and partially explain the greater ammonia excretion in female mice than in male mice. Furthermore, the greater renal NKCC2 expression in females occurred despite no difference in TAL volume density between male and female mice. The greater renal NKCC2 expression that we observed in female mice is similar to a previous study evaluating sex differences in renal transporters (58).
Finally, the collecting duct secretes 60–80% of urinary ammonia through a mechanism involving parallel H+ and NH3 secretion (19, 20, 62). The Rhesus glycoprotein transporters, Rhbg and Rhcg, are the primary ammonia transporters in the collecting duct contributing to NH3 secretion (6, 7, 23, 29). This is the first study to show sex differences in the expression of Rhbg and Rhcg, in connecting segment cells and collecting duct intercalated and principal cells. Thus, female mice have greater expression of Rhbg and Rhcg, which may lead to increased collecting duct ammonia secretion and, thus, increased total ammonia excretion.
The multiple differences in renal aspects of ammonia metabolism between male and female mice could result from multiple possible mechanisms. Certainly, differences in male and female gonadal hormones, e.g., testosterone, estradiol, and progesterone, are likely candidates (2, 40–42). Another possibility is a sex difference in the expression of sex steroid hormone receptors (2, 42). Finally, we cannot exclude differences in sex chromosomes independent of gonadal hormones (2, 8, 42). Additional studies will be necessary to evaluate these multiple potential mechanisms.
An important consideration when using animal models is whether they accurately reflect what occurs in other species, particularly humans. Unfortunately, there are relatively few published studies directly addressing sex differences in human acid-base homeostasis. One study evaluated serum bicarbonate levels in 9,724 men and women and reported that men had slightly, but significantly, higher serum bicarbonate levels than did women (men: 25.2 ± 2.2; women, 24.5 ± 2.2 mmol/l) (9). This is in contrast to our study in which we did not find a sex difference in plasma bicarbonate concentrations; however, the difference between men and women observed in the human study, 0.7 mmol/l, was small. A report from the Health, Aging, and Body Composition (Health ABC) Study, which evaluated 2,287 adults, aged 70–79 yr old, did not identify sex-specific mean bicarbonate levels but did find that female sex correlated with higher serum bicarbonate levels (50). These findings contrast with data from the NHANES study, which also did not report sex-specific serum bicarbonate but found the opposite correlation, with female sex associated with lower serum bicarbonate levels (51). Finally, one study evaluating normal men and women fed an identical diet observed no difference in urinary ammonia excretion (67). However, this study only evaluated seven men and seven women subjects over a wide age span (24 to 55 yr old) and so may have had a limited power to identify differences in ammonia excretion.
In summary, these studies show that female mice excrete more urinary ammonia than male mice. This correlates with greater PEPCK, NKCC2, Rhbg, and Rhcg expression. Furthermore, these studies have identified fundamental sex differences in renal structure. In contrast, proximal tubules account for a lesser percentage of the cortex in the female mouse kidney than in the male kidney, whereas the opposite is true for the collecting duct. In conclusion, there are significant sex differences in basal ammonia excretion that result from sex differences in the expression of multiple important proteins involved in ammonia metabolism and transport.
GRANTS
These studies were supported by funding from National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-045788 (to I. D. Weiner), RO1-DK-107798 (to I. D. Weiner and J. W. Verlander) and 5T32-DK-104721.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.N.H., J.W.V., and I.D.W. conceived and designed research; A.N.H., H.-W.L., G.O., and K.L.W. performed experiments; A.N.H., H.-W.L., G.O., J.W.V., and I.D.W. analyzed data; A.N.H., H.-W.L., G.O., J.W.V., and I.D.W. interpreted results of experiments; A.N.H. prepared figures; A.N.H. drafted manuscript; A.N.H., H.-W.L., L.F., K.L.W., J.W.V., and I.D.W. edited and revised manuscript; A.N.H., H.-W.L., G.O., L.F., K.L.W., J.W.V., and I.D.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Sharon W. Matthews and Chao Chen of the University of Florida College of Medicine Electron Microscopy Core Laboratory for excellent tissue processing for immunohistochemical studies.
REFERENCES
- 1.Baertl JM, Sancetta SM, Gabuzda GJ. Relation of acute potassium depletion to renal ammonium metabolism in patients with cirrhosis. J Clin Invest 42: 696–706, 1963. doi: 10.1172/JCI104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146: 1650–1673, 2005. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 3.Bellhouse DR. Area estimation by point-counting techniques. Biometrics 37: 303–312, 1981. doi: 10.2307/2530419. [DOI] [Google Scholar]
- 4.Bishop JM, Lee HW, Handlogten ME, Han KH, Verlander JW, Weiner ID. Intercalated cell-specific Rh B glycoprotein deletion diminishes renal ammonia excretion response to hypokalemia. Am J Physiol Renal Physiol 304: F422–F431, 2013. doi: 10.1152/ajprenal.00301.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop JM, Verlander JW, Lee HW, Nelson RD, Weiner AJ, Handlogten ME, Weiner ID. Role of the Rhesus glycoprotein, Rh B glycoprotein, in renal ammonia excretion. Am J Physiol Renal Physiol 299: F1065–F1077, 2010. doi: 10.1152/ajprenal.00277.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, Houillier P, Szpirer J, Szpirer C, Wagner CA, Devuyst O, Marini AM. A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456: 339–343, 2008. doi: 10.1038/nature07518. [DOI] [PubMed] [Google Scholar]
- 7.Bourgeois S, Bounoure L, Christensen EI, Ramakrishnan SK, Houillier P, Devuyst O, Wagner CA. Haploinsufficiency of the ammonia transporter Rhcg predisposes to chronic acidosis: Rhcg is critical for apical and basolateral ammonia transport in the mouse collecting duct. J Biol Chem 288: 5518–5529, 2013. doi: 10.1074/jbc.M112.441782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgoyne PS, Arnold AP. A primer on the use of mouse models for identifying direct sex chromosome effects that cause sex differences in non-gonadal tissues. Biol Sex Differ 7: 68, 2016. doi: 10.1186/s13293-016-0115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Melamed ML, Abramowitz MK. Serum bicarbonate and bone mineral density in US adults. Am J Kidney Dis 65: 240–248, 2015. doi: 10.1053/j.ajkd.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conjard A, Komaty O, Delage H, Boghossian M, Martin M, Ferrier B, Baverel G. Inhibition of glutamine synthetase in the mouse kidney: a novel mechanism of adaptation to metabolic acidosis. J Biol Chem 278: 38159–38166, 2003. doi: 10.1074/jbc.M302885200. [DOI] [PubMed] [Google Scholar]
- 11.Curhan GC, Taylor EN. 24-h uric acid excretion and the risk of kidney stones. Kidney Int 73: 489–496, 2008. doi: 10.1038/sj.ki.5002708. [DOI] [PubMed] [Google Scholar]
- 12.Curthoys NP, Moe OW. Proximal tubule function and response to acidosis. Clin J Am Soc Nephrol 9: 1627–1638, 2014. doi: 10.2215/CJN.10391012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20: 2075–2084, 2009. doi: 10.1681/ASN.2008111205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erdely A, Greenfeld Z, Wagner L, Baylis C. Sexual dimorphism in the aging kidney: Effects on injury and nitric oxide system. Kidney Int 63: 1021–1026, 2003. doi: 10.1046/j.1523-1755.2003.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fekete A, Vannay A, Vér A, Vásárhelyi B, Müller V, Ouyang N, Reusz G, Tulassay T, Szabó AJ. Sex differences in the alterations of Na+, K+-ATPase following ischaemia-reperfusion injury in the rat kidney. J Physiol 555: 471–480, 2004. doi: 10.1113/jphysiol.2003.054825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gawenis LR, Bradford EM, Prasad V, Lorenz JN, Simpson JE, Clarke LL, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/- cotransporter. J Biol Chem 282: 9042–9052, 2007. doi: 10.1074/jbc.M607041200. [DOI] [PubMed] [Google Scholar]
- 17.Good DW. Ammonium transport by the thick ascending limb of Henle’s loop. Annu Rev Physiol 56: 623–647, 1994. doi: 10.1146/annurev.ph.56.030194.003203. [DOI] [PubMed] [Google Scholar]
- 18.Goraya N, Simoni J, Jo CH, Wesson DE. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int 86: 1031–1038, 2014. doi: 10.1038/ki.2014.83. [DOI] [PubMed] [Google Scholar]
- 19.Hamm LL, Nakhoul N, Hering-Smith KS. Acid-base homeostasis. Clin J Am Soc Nephrol 10: 2232–2242, 2015. doi: 10.2215/CJN.07400715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamm LL, Simon EE. Roles and mechanisms of urinary buffer excretion. Am J Physiol Renal Physiol 253: F595–F605, 1987. doi: 10.1152/ajprenal.1987.253.4.F595. [DOI] [PubMed] [Google Scholar]
- 21.Han K-H, Croker BP, Clapp WL, Werner D, Sahni M, Kim J, Kim H-Y, Handlogten ME, Weiner ID. Expression of the ammonia transporter, Rh C, glycoprotein in normal and neoplastic human kidney. J Am Soc Nephrol 17: 2670–2679, 2006. doi: 10.1681/ASN.2006020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han KH, Lee HW, Handlogten ME, Whitehill F, Osis G, Croker BP, Clapp WL, Verlander JW, Weiner ID. Expression of the ammonia transporter family member, Rh B glycoprotein, in the human kidney. Am J Physiol Renal Physiol 304: F972–F981, 2013. doi: 10.1152/ajprenal.00550.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Apical ammonia transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol 289: F347–F358, 2005. doi: 10.1152/ajprenal.00253.2004. [DOI] [PubMed] [Google Scholar]
- 24.Handlogten ME, Osis G, Lee HW, Romero MF, Verlander JW, Weiner ID. NBCe1 expression is required for normal renal ammonia metabolism. Am J Physiol Renal Physiol 309: F658–F666, 2015. doi: 10.1152/ajprenal.00219.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Institute of Medicine Committee on Understanding the Biology of Sex and Gender Differences Exploring the Biological Contributions to Human Health: Does Sex Matter? , edited by Wizemann TM and Pardue ML. Washington, D.C.: National Academies Press; 2001. [PubMed] [Google Scholar]
- 26.Karp NA, Mason J, Beaudet AL, Benjamini Y, Bower L, Braun RE, Brown SDM, Chesler EJ, Dickinson ME, Flenniken AM, Fuchs H, Angelis MH, Gao X, Guo S, Greenaway S, Heller R, Herault Y, Justice MJ, Kurbatova N, Lelliott CJ, Lloyd KCK, Mallon AM, Mank JE, Masuya H, McKerlie C, Meehan TF, Mott RF, Murray SA, Parkinson H, Ramirez-Solis R, Santos L, Seavitt JR, Smedley D, Sorg T, Speak AO, Steel KP, Svenson KL, Wakana S, West D, Wells S, Westerberg H, Yaacoby S, White JK; International Mouse Phenotyping Consortium . Prevalence of sexual dimorphism in mammalian phenotypic traits. Nat Commun 8: 15475, 2017. doi: 10.1038/ncomms15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kher A, Meldrum KK, Wang M, Tsai BM, Pitcher JM, Meldrum DR. Cellular and molecular mechanisms of sex differences in renal ischemia-reperfusion injury. Cardiovasc Res 67: 594–603, 2005. doi: 10.1016/j.cardiores.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Kim HY, Baylis C, Verlander JW, Han KH, Reungjui S, Handlogten ME, Weiner ID. Effect of reduced renal mass on renal ammonia transporter family, Rh C glycoprotein and Rh B glycoprotein, expression. Am J Physiol Renal Physiol 293: F1238–F1247, 2007. doi: 10.1152/ajprenal.00151.2007. [DOI] [PubMed] [Google Scholar]
- 29.Kim HY, Verlander JW, Bishop JM, Cain BD, Han KH, Igarashi P, Lee HW, Handlogten ME, Weiner ID. Basolateral expression of the ammonia transporter family member Rh C glycoprotein in the mouse kidney. Am J Physiol Renal Physiol 296: F543–F555, 2009. doi: 10.1152/ajprenal.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinne R, Kinne-Saffran E, Schütz H, Schölermann B. Ammonium transport in medullary thick ascending limb of rabbit kidney: involvement of the Na+,K+,Cl−-cotransporter. J Membr Biol 94: 279–284, 1986. doi: 10.1007/BF01869723. [DOI] [PubMed] [Google Scholar]
- 31.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol Dial Transplant 24: 1232–1237, 2009. doi: 10.1093/ndt/gfn633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laghmani K, Preisig PA, Moe OW, Yanagisawa M, Alpern RJ. Endothelin-1/endothelin-B receptor-mediated increases in NHE3 activity in chronic metabolic acidosis. J Clin Invest 107: 1563–1569, 2001. doi: 10.1172/JCI11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee HW, Handlogten ME, Osis G, Clapp WL, Wakefield DN, Verlander JW, Weiner ID. Expression of sodium-dependent dicarboxylate transporter 1 (NaDC1/SLC13A2) in normal and neoplastic human kidney. Am J Physiol Renal Physiol 312: F427–F435, 2017. doi: 10.1152/ajprenal.00559.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee HW, Osis G, Handlogten ME, Lamers WH, Chaudhry FA, Verlander JW, Weiner ID. Proximal tubule-specific glutamine synthetase deletion alters basal and acidosis-stimulated ammonia metabolism. Am J Physiol Renal Physiol 310: F1229–F1242, 2016. doi: 10.1152/ajprenal.00547.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HW, Verlander JW, Bishop JM, Handlogten ME, Han KH, Weiner ID. Renal ammonia excretion in response to hypokalemia: effect of collecting duct-specific Rh C glycoprotein deletion. Am J Physiol Renal Physiol 304: F410–F421, 2013. doi: 10.1152/ajprenal.00300.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee HW, Verlander JW, Bishop JM, Igarashi P, Handlogten ME, Weiner ID. Collecting duct-specific Rh C glycoprotein deletion alters basal and acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 296: F1364–F1375, 2009. doi: 10.1152/ajprenal.90667.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee HW, Verlander JW, Bishop JM, Nelson RD, Handlogten ME, Weiner ID. Effect of intercalated cell-specific Rh C glycoprotein deletion on basal and metabolic acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 299: F369–F379, 2010. doi: 10.1152/ajprenal.00120.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee HW, Verlander JW, Handlogten ME, Han KH, Weiner ID. Effect of collecting duct-specific deletion of both Rh B Glycoprotein (Rhbg) and Rh C Glycoprotein (Rhcg) on renal response to metabolic acidosis. Am J Physiol Renal Physiol 306: F389–F400, 2014. doi: 10.1152/ajprenal.00176.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mak DO, Dang B, Weiner ID, Foskett JK, Westhoff CM. Characterization of ammonia transport by the kidney Rh glycoproteins RhBG and RhCG. Am J Physiol Renal Physiol 290: F297–F305, 2006. doi: 10.1152/ajprenal.00147.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. J Neurosci 32: 2241–2247, 2012. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGregor AJ, Hasnain M, Sandberg K, Morrison MF, Berlin M, Trott J. How to study the impact of sex and gender in medical research: a review of resources. Biol Sex Differ 7, Suppl 1: 46, 2016. doi: 10.1186/s13293-016-0099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller VM, Kaplan JR, Schork NJ, Ouyang P, Berga SL, Wenger NK, Shaw LJ, Webb RC, Mallampalli M, Steiner M, Taylor DA, Merz CN, Reckelhoff JF. Strategies and methods to study sex differences in cardiovascular structure and function: a guide for basic scientists. Biol Sex Differ 2: 14, 2011. doi: 10.1186/2042-6410-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitch WE. Metabolic and clinical consequences of metabolic acidosis. J Nephrol 19, Suppl 9: S70–S75, 2006. [PubMed] [Google Scholar]
- 44.Nagami GT. Luminal secretion of ammonia in the mouse proximal tubule perfused in vitro. J Clin Invest 81: 159–164, 1988. doi: 10.1172/JCI113287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagami GT. Role of angiotensin II in the enhancement of ammonia production and secretion by the proximal tubule in metabolic acidosis. Am J Physiol Renal Physiol 294: F874–F880, 2008. doi: 10.1152/ajprenal.00286.2007. [DOI] [PubMed] [Google Scholar]
- 46.Navaneethan SD, Schold JD, Arrigain S, Jolly SE, Wehbe E, Raina R, Simon JF, Srinivas TR, Jain A, Schreiber MJ Jr, Nally JV Jr. Serum bicarbonate and mortality in stage 3 and stage 4 chronic kidney disease. Clin J Am Soc Nephrol 6: 2395–2402, 2011. doi: 10.2215/CJN.03730411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol 11: 319–329, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Osis G, Handlogten ME, Lee HW, Hering-Smith KS, Huang W, Romero MF, Verlander JW, Weiner ID. Effect of NBCe1 deletion on renal citrate and 2-oxoglutarate handling. Physiol Rep 4: e12778, 2016. doi: 10.14814/phy2.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oudar O, Elger M, Bankir L, Ganten D, Ganten U, Kriz W. Differences in rat kidney morphology between males, females and testosterone-treated females. Ren Physiol Biochem 14: 92–102, 1991. [DOI] [PubMed] [Google Scholar]
- 50.Raphael KL, Murphy RA, Shlipak MG, Satterfield S, Huston HK, Sebastian A, Sellmeyer DE, Patel KV, Newman AB, Sarnak MJ, Ix JH, Fried LF; Health ABC Study . Bicarbonate concentration, acid-base status, and mortality in the health, aging, and body composition study. Clin J Am Soc Nephrol 11: 308–316, 2016. doi: 10.2215/CJN.06200615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raphael KL, Zhang Y, Wei G, Greene T, Cheung AK, Beddhu S. Serum bicarbonate and mortality in adults in NHANES III. Nephrol Dial Transplant 28: 1207–1213, 2013. doi: 10.1093/ndt/gfs609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sabolić I, Asif AR, Budach WE, Wanke C, Bahn A, Burckhardt G. Gender differences in kidney function. Pflügers Arch 455: 397–429, 2007. doi: 10.1007/s00424-007-0308-1. [DOI] [PubMed] [Google Scholar]
- 53.Shear L, Gabuzda GJ. Potassium deficiency and endogenous ammonium overload from kidney. Am J Clin Nutr 23: 614–618, 1970. doi: 10.1093/ajcn/23.5.614. [DOI] [PubMed] [Google Scholar]
- 54.Takaoka M, Yuba M, Fujii T, Ohkita M, Matsumura Y. Oestrogen protects against ischaemic acute renal failure in rats by suppressing renal endothelin-1 overproduction. Clin Sci (Lond) 103, Suppl 48: 434S–437S, 2002. doi: 10.1042/CS103S434S. [DOI] [PubMed] [Google Scholar]
- 55.Tannen RL. Relationship of renal ammonia production and potassium homeostasis. Kidney Int 11: 453–465, 1977. doi: 10.1038/ki.1977.63. [DOI] [PubMed] [Google Scholar]
- 56.Tarry-Adkins JL, Ozanne SE, Norden A, Cherif H, Hales CN. Lower antioxidant capacity and elevated p53 and p21 may be a link between gender disparity in renal telomere shortening, albuminuria, and longevity. Am J Physiol Renal Physiol 290: F509–F516, 2006. doi: 10.1152/ajprenal.00215.2005. [DOI] [PubMed] [Google Scholar]
- 57.Taylor EN, Stampfer MJ, Mount DB, Curhan GC. DASH-style diet and 24-hour urine composition. Clin J Am Soc Nephrol 5: 2315–2322, 2010. doi: 10.2215/CJN.04420510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Veiras LC, Girardi ACC, Curry J, Pei L, Ralph DL, Tran A, Castelo-Branco RC, Pastor-Soler N, Arranz CT, Yu ASL, McDonough AA. Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. J Am Soc Nephrol 28: 3504–3517, 2017. doi: 10.1681/ASN.2017030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verlander JW, Chu D, Lee HW, Handlogten ME, Weiner ID. Expression of glutamine synthetase in the mouse kidney: localization in multiple epithelial cell types and differential regulation by hypokalemia. Am J Physiol Renal Physiol 305: F701–F713, 2013. doi: 10.1152/ajprenal.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins RhBG and RhCG in mouse kidney. Am J Physiol Renal Physiol 284: F323–F337, 2003. doi: 10.1152/ajprenal.00050.2002. [DOI] [PubMed] [Google Scholar]
- 61.Weiner ID. Roles of renal ammonia metabolism other than in acid-base homeostasis. Pediatr Nephrol 32: 933–942, 2017. doi: 10.1007/s00467-016-3401-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weiner ID, Hamm LL. Molecular mechanisms of renal ammonia transport. Annu Rev Physiol 69: 317–340, 2007. doi: 10.1146/annurev.physiol.69.040705.142215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiner ID, Miller RT, Verlander JW. Localization of the ammonium transporters, Rh B glycoprotein and Rh C glycoprotein, in the mouse liver. Gastroenterology 124: 1432–1440, 2003. doi: 10.1016/S0016-5085(03)00277-4. [DOI] [PubMed] [Google Scholar]
- 64.Weiner ID, Mitch WE, Sands JM. Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin J Am Soc Nephrol 10: 1444–1458, 2015. doi: 10.2215/CJN.10311013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weiner ID, Verlander JW. Ammonia transport in the kidney by Rhesus glycoproteins. Am J Physiol Renal Physiol 306: F1107–F1120, 2014. doi: 10.1152/ajprenal.00013.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiner ID, Verlander JW. Renal ammonia metabolism and transport. Compr Physiol 3: 201–220, 2013. doi: 10.1002/cphy.c120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Worcester EM, Bergsland KJ, Gillen DL, Coe FL. Mechanism for higher urine pH in normal women compared to men. Am J Physiol Renal Physiol 314: F623–F629, 2018. doi: 10.1152/ajprenal.00494.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wrong O, Davies HE. The excretion of acid in renal disease. Q J Med 28: 259–313, 1959. [PubMed] [Google Scholar]