Abstract
Angiotensin converting enzyme 2 (ACE2) and neprilysin (NEP) are metalloproteases that are highly expressed in the renal proximal tubules. ACE2 and NEP generate renoprotective angiotensin (1–7) from angiotensin II and angiotensin I, respectively, and therefore could have a major role in chronic kidney disease (CKD). Recent data demonstrated increased urinary ACE2 in patients with diabetes with CKD and kidney transplants. We tested the hypothesis that urinary ACE2, NEP, and a disintegrin and metalloproteinase 17 (ADAM17) are increased and could be risk predictors of CKD in patients with diabetes. ACE2, NEP, and ADAM17 were investigated in 20 nondiabetics (ND) and 40 patients with diabetes with normoalbuminuria (Dnormo), microalbuminuria (Dmicro), and macroalbuminuria (Dmacro) using ELISA, Western blot, and fluorogenic and mass spectrometric-based enzyme assays. Logistic regression model was applied to predict the risk prediction. Receiver operating characteristic curves were drawn, and prediction accuracies were calculated to explore the effectiveness of ACE2 and NEP in predicting diabetes and CKD. Results demonstrated that there is no evidence of urinary ACE2 and ADAM17 in ND subjects, but both enzymes were increased in patients with diabetes, including Dnormo. Although there was no detectable plasma ACE2 activity, there was evidence of urinary and plasma NEP in all the subjects, and urinary NEP was significantly increased in Dmicro patients. NEP and ACE2 showed significant correlations with metabolic and renal characteristics. In summary, urinary ACE2, NEP, and ADAM17 are increased in patients with diabetes and could be used as early biomarkers to predict the incidence or progression of CKD at early stages among individuals with type 2 diabetes.
Keywords: ACE2, ADAM17, chronic kidney disease, diabetic nephropathy, NEP, type 2 diabetes
INTRODUCTION
Chronic kidney disease (CKD) is characterized by a progressive decline in glomerular filtration rate (GFR) over more than 3 months and is often accompanied by albuminuria. The mechanisms of progression of diabetic kidney disease to end stage renal disease are poorly understood. Most patients suffering from kidney diseases do not have symptoms or markers until later in the course of the disease when it becomes chronic (9). Giving the limitation of the existing clinical biomarkers, there is an urgent need to develop new molecular markers for the early detection and management of CKD.
Currently, the most commonly used noninvasive methods for the early recognition of pending kidney disease in patients with diabetes is the assessment of urine albumin to creatinine ratio (UACR), GFR, serum creatinine, and cystatin C levels. According to the National Kidney Foundation, the prognosis of CKD is characterized in terms of both GFR and albuminuria (18). However, there is some debate about the value of assessing UACR, its sensitivity, and its specificity for the prediction of pending CKD (4). For instance, studies in normotensive, nondiabetic healthy subjects have revealed the occurrence of microalbuminuria without the evidence of kidney injury (35). In contrast, patients with diabetes with progressive renal impairment showed normoalbuminuria or unchanged albumin excretion (37). In addition, a recent follow-up study of the Joslin Kidney Studies indicated that early renal decline precedes the onset of microalbuminuria in patients with type 1 diabetes (22). Therefore, microalbuminuria may not be a reliable and robust marker for assessing the onset and progression of CKD in patients with diabetes, stipulating the need for better noninvasive biomarkers.
The renin angiotensin system (RAS) plays a fundamental role in the pathophysiology of diabetes and its complications (1). RAS blockade slows the progression of renal diseases through inhibition of angiotensin II (Ang II) signaling pathways (40). Angiotensin converting enzyme 2 (ACE2), a type 1 membrane glycoprotein localized in the apical brush border of the proximal tubular epithelium and glomeruli in the kidney, is a recently discovered peptidase with the capability to degrade the vasoconstrictor Ang II to Angiotensin (1–7) [Ang- (1–7)], a bioactive peptide with vasodilatory activity. Therefore, a possible renoprotective role of ACE2 has been suggested (47, 48, 57). It is well established that ACE2 in the human kidney shares similar structural and functional properties to that of rodents (25). Previous data showed that urinary ACE2 is an independent risk factor for microalbuminuria and is associated with the severity of type 2 diabetes in humans (34). Recently, it has been shown that urinary ACE2 shedding is increased in addition to increased albuminuria in patients with type 2 diabetes with CKD (28, 32). Our laboratory previously reported that administration of the peroxisome proliferator activating receptors γ agonist, rosiglitazone, normalized hyperglycemia, attenuated albuminuria, and urinary ACE2 excretion in type 2 diabetic mice (8). We and others support the notion that shedding of ACE2 is mediated by a disintegrin and metalloproteinase-17 (ADAM17) (16, 24, 36, 53). Indeed, treatment of Akita type 1 diabetic mice with insulin normalized hyperglycemia, attenuated urinary ACE2 shedding, albuminuria, and renal ADAM17 expression (41). We have also reported that physical exercise training and/or metformin treatment of type 2 diabetic mice attenuated renal ADAM17 expression and shedding of urinary ACE2 (44). Recently, it was demonstrated that Ang II type 1 receptors promote ADAM17 mediated ACE2 shedding in hypertensive patients (54). In addition, in vitro studies showed that the release of ACE2 into the culture media of human proximal tubular cells was inhibited by treatment with the ADAM17 inhibitor TNF-α protease inhibitor-1 (41). Because of its vital role, there is a need for investigating ACE2 as an early marker for CKD and understanding the role of ADAM17 in mediating urinary ACE2 shedding.
Neprilysin (NEP), another prominent Ang- (1–7) forming metalloprotease, was first identified and isolated from the renal epithelial brush border of proximal tubule (12). NEP is involved in the degradation of many bioactive peptides, including Ang I, Ang II, natriuretic peptides, amyloid-beta, and bradykinin, which gained clinical importance in many diseases, such as neurodegenerative (17) and cardiovascular diseases (12). The role of NEP as a major source for the production of renal Ang- (1–7) in murine and the human kidney was revealed in a recent study (10). In early stages of diabetes, attenuation of renal NEP activity was observed in hypertensive rats, suggesting that the loss of renoprotective enzymes may contribute to disease progression (55). Pajenda and colleagues showed increased urinary NEP shedding in patients with acute kidney disease and its use as a potential urinary marker for proximal tubular cell injury (33). In addition, elevated plasma and adipose tissue NEP levels were seen in obese patients associated with insulin resistance, suggesting a biomarker function for NEP in diabetes (46). Also, NEP deficiency protects against glucose-induced insulin secretion in obese diabetic mice fed high fatty acids (58).
In 2014, management of chronic heart failure through NEP inhibition received more attention based on the positive cardiovascular effects seen in the PARADIGM-HF trial, in which NEP inhibition was combined with Ang II type 1 receptor blockade (31). Aside from its role in chronic heart failure, emerging evidence suggests that NEP could be a novel marker for cancer (30). However, the role of renal NEP in patients with diabetes and its presence in urine or plasma of CKD patients have not yet been elucidated.
In this study, we examined the association of increased urinary ACE2, NEP, and ADAM17 with CKD in patients with type 2 diabetes and their use as potential early biomarkers before the onset of microalbuminuria. We also applied the prediction model to explore whether they could predict decreased estimated glomerular filtration rate (eGFR) (<60 ml/min/1.73 m2) and diabetes [glycated hemoglobin (HbA1C) > 6.5] in this population.
RESEARCH DESIGN AND METHODS
Study protocol.
This cross-sectional study was performed at the outpatient clinic of Dayton Veteran Medical Center from 2008 to 2011. A total of 60 patients with a history of microalbuminuria (30–300 mg albumin/g creatinine) and macroalbuminuria (>300 mg albumin/g creatinine) were considered. Based on their urinary UACR at previous visits, the patients were classified into four groups: 1) nondiabetics (ND), 2) Diabetic patients with normoalbuminuria (Dnormo), 3) Diabetic patients with microalbuminuria (Dmicro), and 4) Diabetic patients with macroalbuminuria (Dmacro). Patients with a history of microalbuminuria whose urine albumin to creatinine ratio (UACR) turned to normal were considered as Dnormos. This study was approved by Wright State University and Dayton Veterans Affairs Institutional Review Board Committee. Written informed consent was obtained from every subject. Patients with liver dysfunction, heart failure, malignancy, pregnancy, and glomerulonephritis were excluded from the study. eGFR was done by the Modification of Diet in Renal Disease formula [= 186 × SerumCr (mg/dl)−1.154 × age−0.203 × 0.742 (if female)]. Metabolic and renal parameters were measured by standard methods at the Dayton Veterans Affairs Medical Center.
Sample collection.
A cocktail of protease inhibitors was prepared by dissolving one cOmplete, mini, EDTA-free tablet in 10 ml of lysis-M, EDTA-free reagent containing 2.5 mM phenylmethylsulfonylfluoride to inhibit a wide spectrum of serine and cysteine proteases in urine samples (Cat # 04719964001, Sigma-Aldrich, St. Louis, MO). Morning spot urine samples (15–20 ml) were collected by the patients into a sterile container containing 50 μl of the protease inhibitor cocktail. Urine samples were aliquoted and stored at −80°C until final analysis. Venous blood samples (10 ml) were collected into heparinized tubes, placed immediately on ice, and centrifuged at 3,000 × g for 10 min. Plasma was transferred to sterile tubes containing 50 μl of the same protease inhibitor cocktail described above, aliquoted, and stored at −80°C. Medical history and anthropometric measurements were also recorded the same day.
Urinary albumin and creatinine.
Urine samples were analyzed for albumin using a Human ELISA kit from Bethyl Laboratories (Montogomery, TX). Urinary creatinine assays were performed using a kit purchased from Quidel (San Diego, CA). The absorbance values were measured at 450 nm for urinary albumin and 490 nm for urinary creatinine using a Fusion Packard plate reader (Packard BioScience, Meriden, CT).
Urinary and plasma ACE2 enzyme activity using a fluorogenic enzyme assay.
The ACE2 enzyme activity was measured as described previously, with some modifications (41, 44, 51). Urine samples equivalent to 10 μg of creatinine or 20 μl of plasma were incubated in the assay buffer (50 mM Tris, 5 mM ZnCl2, 150 mM NaCl2, and 10 μM lisinopril) containing the synthetic ACE2 fluorogenic peptide substrate 7-Mca-APK-(Dnp) (Enzo Life Sciences, Farmingdale, NY) for 0.5–2 h. This fluorogenic peptide emits fluorescence, which was measured at excitation (λex) of 328 nm and emission (λem) of 393 nm using a Fusion Packard instrument. The peptide is specific for ACE2 as described previously (8, 41). ACE2 enzyme fluorogenic assay was validated and confirmed by inhibition with 10 μM of specific inhibitors, such as ACE2 inhibitor, MLN-4760 (a gift from the former Millennium Pharmaceuticals, Cambridge, MA), the prolyl endopeptidase/prolyl carboxypeptidase inhibitor Z-prolyl-prolinal (ZPP; Enzo Life Sciences) and NEP inhibitor thiorphan (Sigma-Aldrich).
Urinary and plasma NEP ELISA.
Urine and plasma samples were analyzed for NEP using a human ELISA kit from R&D Systems (Minneapolis, MN). A 96-well plate was coated with a goat anti-human NEP capture antibody overnight. Urine and plasma samples (100 μl) were added directly to the 96-well plate and left at room temperature for a 2-h incubation. After washing, the biotinylated goat anti-human NEP detection antibody was added and incubated for 2 h at room temperature, followed by incubation with streptavidin-horse radish peroxidase (HRP) for 20 min. Unbound streptavidin-HRP was washed off, and 3,3′,5,5′-tetramethylbenzidine substrate was added. The reaction was stopped using 2 N sulfuric acid. The absorbance was measured using a Fusion Packard plate reader (Packard BioScience) at 450 nm. Unknown urinary and plasma NEP concentrations were determined from a standard curve plotted using assay standards in the range of 0.175–8 ng/ml.
ACE2 and NEP activity determination using mass spectrometry.
Urinary ACE2 and NEP activity were measured using matrix-assisted laser desorption/ionization (MALDI) time of flight (TOF) mass spectrometry (MS) as described previously, with some modifications (8, 15). A urine sample equivalent to 25 μg of creatinine was incubated with 0.4 M MES buffer pH 6.75 and 10 μM Ang II or 70 μM Ang I for 2 h at 37°C along with shaking at 800 revolutions/min. For Ang II assays, 0.5 mM 4-aminophosphonobutyric acid and 0.1 mM ZPP were added, and ACE2 activity was tested using 0.1 mM MLN-4760. For Ang I assays, 0.5 mM 4-aminophosphonobutyric acid, 0.1 mM MLN-4760, and 0.1 mM Lisinopril were added, and NEP activity was tested using 0.1 mM Thiorphan. The reaction was stopped by acidification with 1% trifluoroacetic acid (TFA) and 1:20 dilution using 90% acetonitrile containing 0.3% TFA. These samples were spotted on a MALDI target plate together with a MALDI matrix consisting of 10 mg/ml α-cyano-4-hydroxy-cinnamic acid in 60% methanol, 10% acetone, and 0.3% TFA. Mass spectra were obtained using an Autoflex III smartbeam MALDI TOF/TOF instrument (Bruker Daltonics, Billerica, MA) operated with positive polarity in reflectron mode. A total of 3,000 laser shots were acquired randomly for each spot in the range of mass-to-charge ratio (m/z) 500–3,000 at a laser frequency of 100 Hz. Spectra were mass calibrated using a Bruker peptide calibration standard II.
Western blot.
Urine samples (10 µg creatinine) were loaded in 8% SDS-PAGE gel and electro-transferred to an activated PVDF membrane (Millipore, Billerica, MA). Type 2 diabetic db/db mouse (10 wk old) kidney and 24 h urine were collected and used as positive controls. Resulting blots were then blocked and probed with goat anti-mouse ACE2 (1:1,000 dilution, R&D Systems), goat anti-mouse NEP/CD10 (1:1000 dilution, R&D Systems), rabbit anti-human ADAM17 (1:1,000 dilution, Abcam, Cambridge, MA), or goat anti-mouse albumin (1:1,000 dilution, Santa Cruz Biotechnology, Inc., Dallas, TX) primary antibodies followed by incubation with HRP-conjugated rabbit anti-goat secondary antibody (1:2,000, R&D Systems) or HRP conjugated goat anti-rabbit secondary antibody (1:20,000, Jackson, ME). Immobilon western chemiluminescent HRP substrate (Millipore) and autoradiography imaging method by Medical film processor (Konica Minolta Medical & Graphic, Inc., Wayne, NJ) were used to detect signals. The relative intensities of protein bands were quantified by Image J software.
Statistical analysis.
Analysis was performed using Graph Pad Prism 5.01 and R software. One-way ANOVA analysis was carried out to detect the difference in urinary ACE2 and NEP among four subject groups, including ND, Dnormo, Dmicro, and Dmacro. In addition, multiple comparison tests were used to further explore the difference in ACE2/NEP between any given subject groups, and the corresponding P values were corrected by the Bonferroni’s method. A linear regression for Pearson’s population correlation was constructed to identify the association between ACE2 or NEP with selected parameters. A P value of P < 0.05 was considered statistically significant. Backward stepwise selection was used to explore the importance of ACE2 and NEP on kidney disease status. The logistic regression model based on stepwise selection was built to predict the risk of the disease. The receiver operating characteristic (ROC) curves were plotted for the disease status classified by the threshold values of A1C and eGFR, respectively, and the relevant areas under the ROC (AUC) curves were calculated to see the prediction accuracies of ACE2 and NEP on the kidney disease status. To avoid overfitting, cross-validation was applied to calculate the prediction accuracies.
RESULTS
Demographics of the study participants.
Baseline characteristics of the study participants are shown in Table 1. The subjects in Dnormo, Dmicro, and Dmacro were well matched regarding age and body mass index. Mean arterial pressure was significantly higher in Dmacro than in Dmicro patients. HbA1C and blood glucose levels were significantly higher in Dmacro and Dmicro patients compared with ND and Dnormo. Significantly lower eGFR and creatinine clearance values were observed in Dmicro and Dmacro compared with ND and Dnormo. The urinary protein-to-creatinine ratio and ACR exhibited significantly elevated levels in Dmicro and Dmacro compared with ND and Dnormo.
Table 1.
Baseline characteristics of 20 nondiabetic and 40 patients with type 2 diabetes
| Characteristic | ND, n = 20 | Patients with Diabetes | ||
|---|---|---|---|---|
| Normoalbuminuria, n = 12 | Microalbuminuria, n = 8 | Macroalbuminuria, n = 20 | ||
| Age, yr | 51.2 ± 14.9 | 63.2 ± 11.5a | 67 ± 8.4a | 64.3 ± 8.8a |
| Male sex (no. %) | 15 (93.7) | 11 (91.7) | 8 (100) | 20 (100) |
| Race (no. %) | ||||
| White | 19 (93.7) | 7 (58.3) | 5 (62.5) | 19 (95) |
| Black | 1 (6.2) | 5 (41.7) | 3 (37.5) | 1 (5) |
| Body-mass index, lbs/in2 | 30.9 ± 5.6 | 34.3 ± 7.7 | 31.5 ± 6.1 | 36.1 ± 7.8 |
| Mean arterial pressure, mmHg | 92.7 ± 13.5 | 92.58 ± 10.4 | 83.5 ± 10.1 | 99.8 ± 14.1c |
| Glycated hemoglobin, % | 5.7 ± 0.5 | 6.75 ± 0.8 | 8.0 ± 1.8b,f | 6.7 ± 1.4d |
| Serum creatinine, mg/dl | 0.9 ± 0.2 | 1.0 ± 0.2 | 1.2 ± 0.2 | 2.3 ± 1.3a,g |
| Estimated GFR | ||||
| Mean, ml/min/1.73m2 | 97.4 ± 16.8 | 83.1 ± 17.9 | 47.1 ± 23.1b,g | 39.7 ± 20.8b,g |
| Category, no./total no. | ||||
| ≥60 | 20/20 | 12/12 | 1/8 | 5/20 |
| 59.9–45.0 | 3/8 | 2/20 | ||
| 44.9–30.0 | 2/8 | 5/20 | ||
| <30 | 2/8 | 8/20 | ||
| Urinary albumin: creatinine, mg/g | ||||
| Median | 3.65 | 10.25 | 62.7e | 808.5b,d,g |
| Interquartile range | 1.8–5.8 | 3.75–12.08 | 23.3–112.1 | 380.4–3047 |
| Urinary protein: creatinine, mg/g | ||||
| Median | 0.02 | 0.16 | 0.34 | 1.94b,d,g |
| Interquartile range | 0–0.1 | 0.09–0.26 | 0.17–0.51 | 0.5–4.4 |
| Creatinine clearance, ml/min | ||||
| Median | 131.6 | 104.4 | 65.29a | 56a |
| Interquartile range | 114.6–147.8 | 78.5–155.4 | 33.9–94.8 | 38.1–99.0 |
| Glucose, mg/dl | ||||
| Median | 102 | 129 | 130 | 146.5b |
| Interquartile range | 92.7–109.3 | 87.0–160.0 | 128–182 | 125–186.3 |
| Medications, no. (%) | ||||
| ACE inhibitor or Angiotensin II receptor blockers | 4 (20) | 9 (75) | 8 (100) | 17 (85) |
| Diuretics | 4 (20) | 4 (33.4) | 8 (100) | 17 (85) |
| Calcium channel blockers | 1 (5) | 5 (41.7) | 3 (37.5) | 12 (60) |
| Beta blockers | 2 (10) | 8 (66.7) | 8 (100) | 18 (90) |
Variables are expressed as no. (%), mean (SD) or medians and interquartile range. ACE, angiotensin-converting enzyme; GFR, glomerular filtration rate; ND, nondiabetics
P < 0.05 vs. nondiabetic
P < 0.0001 vs. nondiabetic
P < 0.05 vs. microalbuminuria
P < 0.0001 vs. microalbuminuria
P < 0.05 vs. macroalbuminuria
P < 0.0001 vs. macroalbuminuria
P < 0.0001 vs. nondiabetic normoalbumiuria.
Urinary ACE2 activity is increased in patients with diabetes.
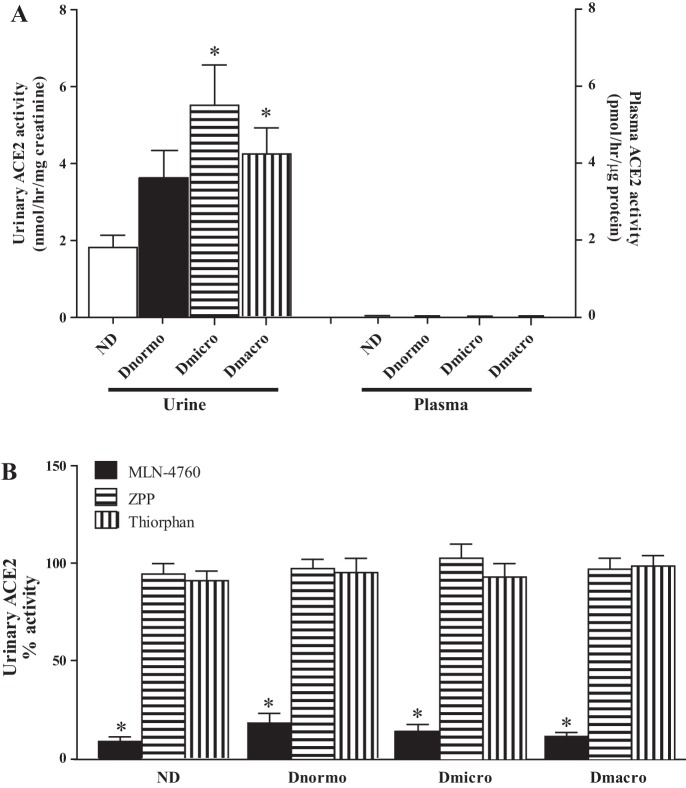
Urinary ACE2 activity was assessed using the fluorogenic substrate Mca-APK (Dnp). Figure 1A shows an increased urinary ACE2 in Dnormo [median 3.1, interquartile range (IQR) 1.17–5.52], Dmicro (median 5.8, IQR 2.65–7.8 nmol·hr−1·mg−1 creatinine), and Dmacro (median 3.85, IQR 2.0–6.75) compared with ND (median 1.2, IQR 0.9–3.0 nmol·hr-1·mg-1 creatinine) (P < 0.01). There was no detectable ACE2 activity found in plasma (Fig. 1A). To further confirm the specificity of the ACE2 fluorogenic assay, different enzyme inhibitors were used. Results show that MLN-4760 significantly inhibited urinary ACE2 activity, while the NEP inhibitor thiorphan and ZPP, an inhibitor of prolyl carboxypeptidase and prolyl endopeptidase, had no effect (Fig. 1B).
Fig. 1.
Urinary and plasma ACE2 activity in ND, Dnormo, Dmicro, and Dmacro subjects using a fluorogenic enzyme assay. A: urinary and plasma ACE2 activity was measured in 10–20μl of samples, *P < 0.01 vs ND B: ACE2 activity assay was validated using MLN-4760 (ACE2 inhibitor), ZPP (Prolyl endopeptidase/prolyl carboxypeptidase inhibitor), and thiorphan (NEP inhibitor). Urinary ACE2 activity without addition of inhibitors (control) was set at 100%. *P < 0.0001 vs. control. Each bar represents mean ± SE. ACE2, angiotensin converting enzyme 2; Dmacro, Type 2 diabetic patients with macroalbuminuria; Dmicro, Type-2 diabetic patients with microalbuminuria; Dnormo, Type 2 diabetic patients with normoalbuminuria; ND, nondiabetics; NEP, neprilysin; ZPP, Z-prolyl-prolinal.
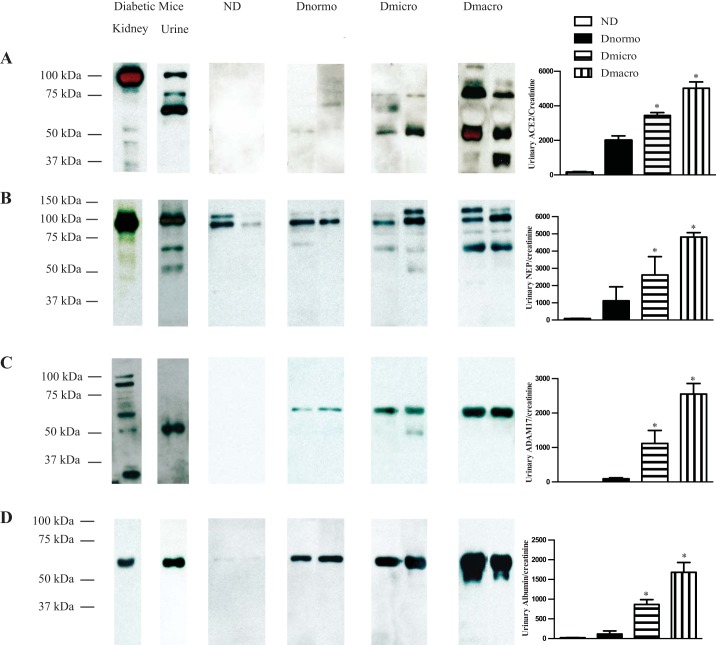
Immunoblotting revealed increased urinary ACE2 and albumin in patients with diabetes.
Figure 2A shows that Western blot analysis of urine equivalent to 10 μg creatinine detected immunoreactive bands for ACE2 at 50 kDa, 65 kDa, 75 kDa, 100 kDa, and 120 kDa. Intensity of the bands varied among patient groups. Dmacro depicted full length (120 kDa) and several fragmented ACE2 immunoreactive bands (50 kDa and 75 kDa) with higher intensity compared with the rest of the groups. Kidney and urine obtained from type 2 diabetic db/db mice (10 wk old) were used as a positive control and showed full-length immunoreactive bands for ACE2 at 100 kDa in kidney and urine as well as two fragmented immunoreactive bands at 75 kDa and 65 kDa in urine. There was no evidence of immunoreactive bands for ACE2 in ND subjects. Increased intensity of the two ACE2 immunoreactive bands at 50 kDa and 75 kDa was observed in Dmicro and Dmacro patients compared with Dnormo patients. For albumin, the presence of an immunoreactive band at 66 kDa was observed in the urine of all subjects with diabetes (Fig. 2D). The intensity of the albumin band varied among the groups, with Dmacro showing the highest intensity. The absence of albumin bands in ND subjects implies the specificity of the albumin antibody. A specific albumin antibody was used to detect albumin in urine and kidneys of diabetic mice.
Fig. 2.
Immunoblot analysis of urinary ACE2, NEP, ADAM17, and albumin in ND, Dnormo, Dmicro, and Dmacro subjects. Immunoblots were depicted using two patients, and the semiquantitative analysis is performed using four to six patients per group. A: urinary ACE2 expression was determined in ND and patients with diabetes with various degrees of albuminuria. Kidney lysate (2 μl) and urine (2 μl) from diabetic mice were used as positive controls. In mice, full length of ACE2 (100 kDa) was observed both in kidney and urine. In addition, fragmented ACE2 bands (75 kDa and 65 kDa) were seen in urine obtained from mice, whereas in humans, a 120-kDa immunoreactive band, as well as fragmented ACE2 immunoreactive bands at 75 kDa, ~70 kDa, and ~50 kDa were seen only in patients with diabetes. Fragmented urinary ACE2 bands (50 kDa) were quantified, and significant increase in urinary ACE2 expression was observed in patients with diabetes compared with ND (*P < 0.0001). B: urinary NEP expression was determined in ND and patients with diabetes using the volume equivalent to 10 μg creatinine. Kidney lysate (2 μl) and urine (2 μl) from diabetic mice were used as positive controls. In mice, full length of NEP (94 kDa) was observed in both kidney and urine. In addition, fragmented NEP immunoreactive bands at 70 kDa and 50 kDa were seen in urine obtained from mice, whereas in humans, full-length as well as fragmented NEP immunoreactive bands were seen at ~110 kDa, ~70 kDa, and ~50 kDa with increased intensity in patients with diabetes. Fragmented NEP immunoreactive bands were not observed in ND patients. Fragmented urinary NEP bands (70 kDa) were quantified, and significant increase in urinary NEP expression was observed in patients with diabetes compared with ND (*P < 0.02). C: urinary ADAM17 expression in ND and patients with diabetes according to volume equivalent to 10 μg creatinine. Kidney lysate (2 μl) and urine (2 μl) from diabetic mice were used as positive controls. The diabetic mouse kidney shows several immunoreactive bands for ADAM17 (~93 kDa, ~65 kDa, and ~55 kDa). A 55-kDa immunoreactive band was observed in urine obtained from diabetic mice. In humans, a 70-kDa immunoreactive band for urinary ADAM17 was only seen in patients with diabetes with increased intensity in patients with diabetes compared with ND subjects (*P < 0.0001). D: urinary albumin was determined in volume equivalent to 10 μg creatinine. A predominant band for albumin at 66 kDa was detected in kidney and urine obtained from diabetic mice and patients with diabetes. Significant increase of urinary albumin expression was observed in patients with diabetes compared with ND (*P < 0.002). ACE2, angiotensin converting enzyme 2; ADAM17, a disintegrin and metalloproteinase 17; Dmacro, patients with type 2 diabetes with macroalbuminuria; Dmicro, patients with type 2 diabetes with microalbuminuria; Dnormo, patients with type 2 diabetes with normoalbuminuria; ND, nondiabetics; NEP, neprilysin.
Immunoblotting shows increased urinary NEP in patients with diabetes.
Immunoreactive bands for NEP were detected in human urine, equivalent to 10 μg of creatinine at 50 kDa, 70 kDa, 94 kDa, and 110 kDa. Diabetic mouse kidney and urine were used as positive controls and showed similar sizes for NEP immunoreactive bands. The 94-kDa and 110-kDa bands were detected in all patients. Dmicro and Dmacro subjects, as well as selected Dnormo samples, also showed a 70 kDa band. Dmicro and Dmacro subjects exhibited an overall increased intensity compared with ND and Dnormo subjects.
Immunoblotting exhibited increased urinary ADAM17 in patients with diabetes.
Figure 2C manifests the presence of urinary ADAM17 in patients with diabetes, whereas there was no evidence of ADAM17 in ND subjects. Immunoreactive bands at 70 kDa were detected for urinary ADAM17 in all patients with diabetes, while a 55-kDa immunoreactive band was only observed in Dmicro subjects. As the albuminuria increased, urinary ADAM17 shedding was increased in Dmicro and Dmacro patients.
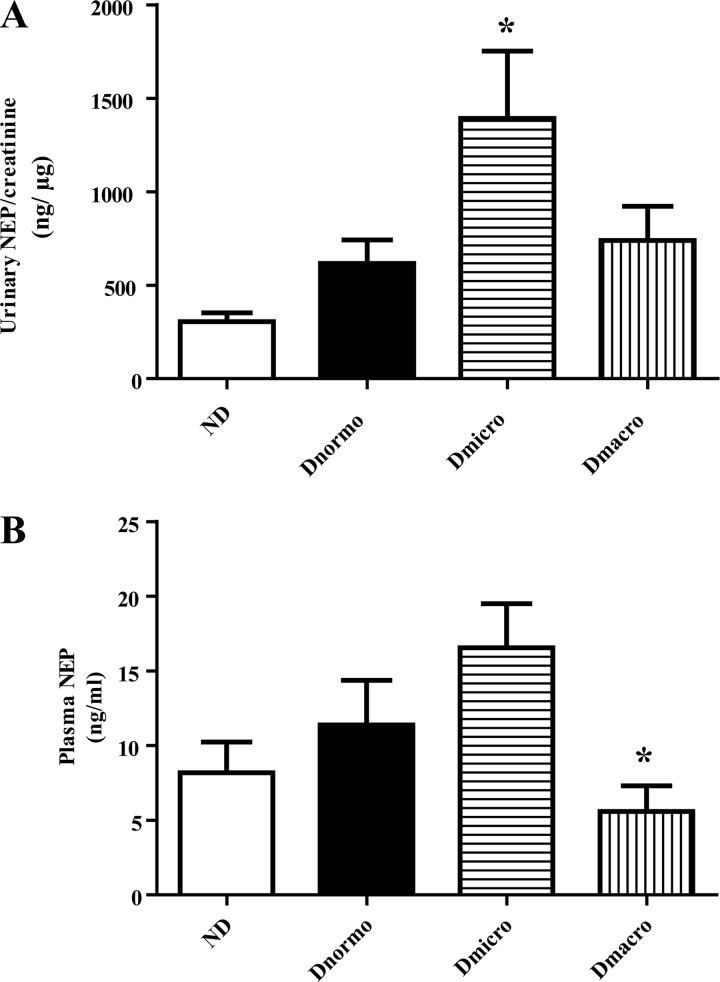
Urinary NEP protein concentration was increased in patients with diabetes.
Figure 3A shows that urinary NEP levels were increased in patients with diabetes, but a significant increase was only seen in Dmicro (median 1,064.0, IQR 807.6–2,079 ng/µg creatinine). There was no significant difference between plasma NEP levels in Dnormo, Dmicro, and Dmacro subjects compared with ND subjects, although plasma NEP levels were significantly decreased in Dmacro subjects compared with Dmicro subjects (P < 0.05) (Fig. 3B).
Fig. 3.
Urinary and plasma NEP ELISA in ND, Dnormo, Dmicro, and Dmacro subjects. A: urinary NEP concentration in ND and patients with diabetes. *P < 0.03 vs. ND. B: plasma NEP concentration in ND and patients with diabetes. *P < 0.05 vs Dmicro. Values are the mean ± SE. Dmacro, Type 2 diabetic patients with macroalbuminuria; Dmicro, Type 2 diabetic patients with microalbuminuria; Dnormo, Type 2 diabetic patients with normoalbuminuria; ND, nondiabetics; NEP, neprilysin.
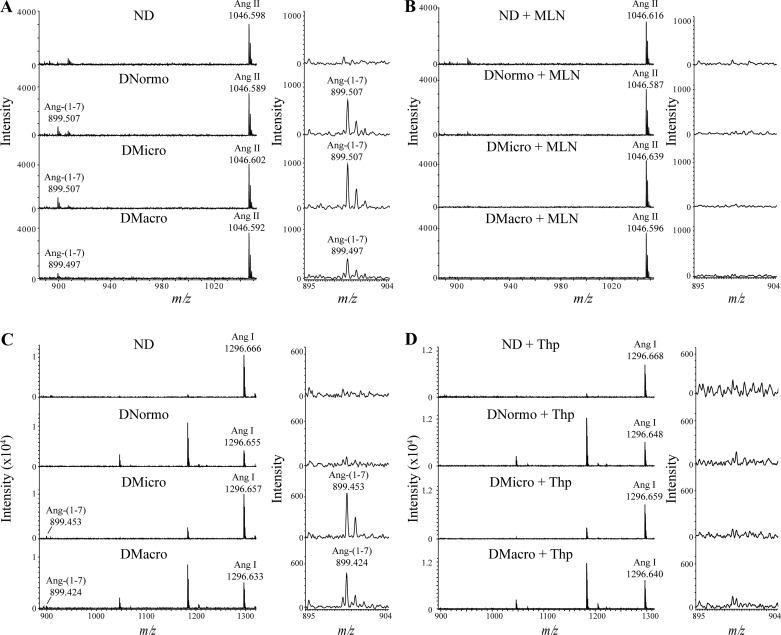
Mass spectrometric analysis of urinary ACE2 and NEP.
MALDI-TOF-MS was used as an additional tool to monitor urinary ACE2 and NEP activities and confirm selectivity of their inhibitors. The formation of the product Ang- (1–7) at m/z 899 was followed by incubations of urine with the ACE2 substrate Ang II at m/z 1,046 and the NEP substrate Ang I at m/z 1,296 (Fig. 4). For Ang II incubations, Ang- (1–7) formation was observed in all diabetic urine samples but not ND. MALDI-MS data generated from one subject from each group are shown in Fig. 4A. Results show that Ang- (1–7) formation from Ang II was completely abolished in the presence of the selective ACE2 inhibitor, MLN-4760 (Fig. 4B). This indicates the presence of ACE2 in the urine. For Ang I incubations, Ang- (1–7) formation was only observed in Dmicro and Dmacro urine samples (Fig. 4C). Ang- (1–7) formation from Ang I was not detected in the presence of the NEP inhibitor thiorphan (Fig. 4D), which indicates the presence of NEP in the urine.
Fig. 4.
Mass spectrometric based enzyme activity assay for urinary ACE2 and NEP in ND, Dnormo, Dmicro, and Dmacro subjects. A: urinary ACE2 activity was measured in volume equivalent to 25 µg of creatinine. Samples were incubated for 2 h at 37°C in 0.4 M MES buffer pH 6.75 containing 0.1 mg/ml Ang II. B: urinary ACE2 activity inhibition in the presence of specific ACE2 inhibitor, MLN-4760 (0.1 mM) C: urinary NEP activity was measured in volume equivalent to 25 µg of creatinine incubated for 2 h at 37°C in 0.4 M MES buffer pH 6.75 containing 1 mg/ml Ang I. D: urinary NEP activity inhibition in the presence of specific NEP inhibitor thiorphan (0.1 mM). ACE2, angiotensin converting enzyme 2; And I, angiotensin I; Ang II, angiotensin II; Dmacro, patients with type 2 diabetes with macroalbuminuria; Dmicro, patients with type 2 diabetes with microalbuminuria; Dnormo, patients with type 2 diabetes with normoalbuminuria; m/z, mass-to-charge ratio; ND, nondiabetics; NEP, neprilysin.
Correlations with metabolic and renal parameters.
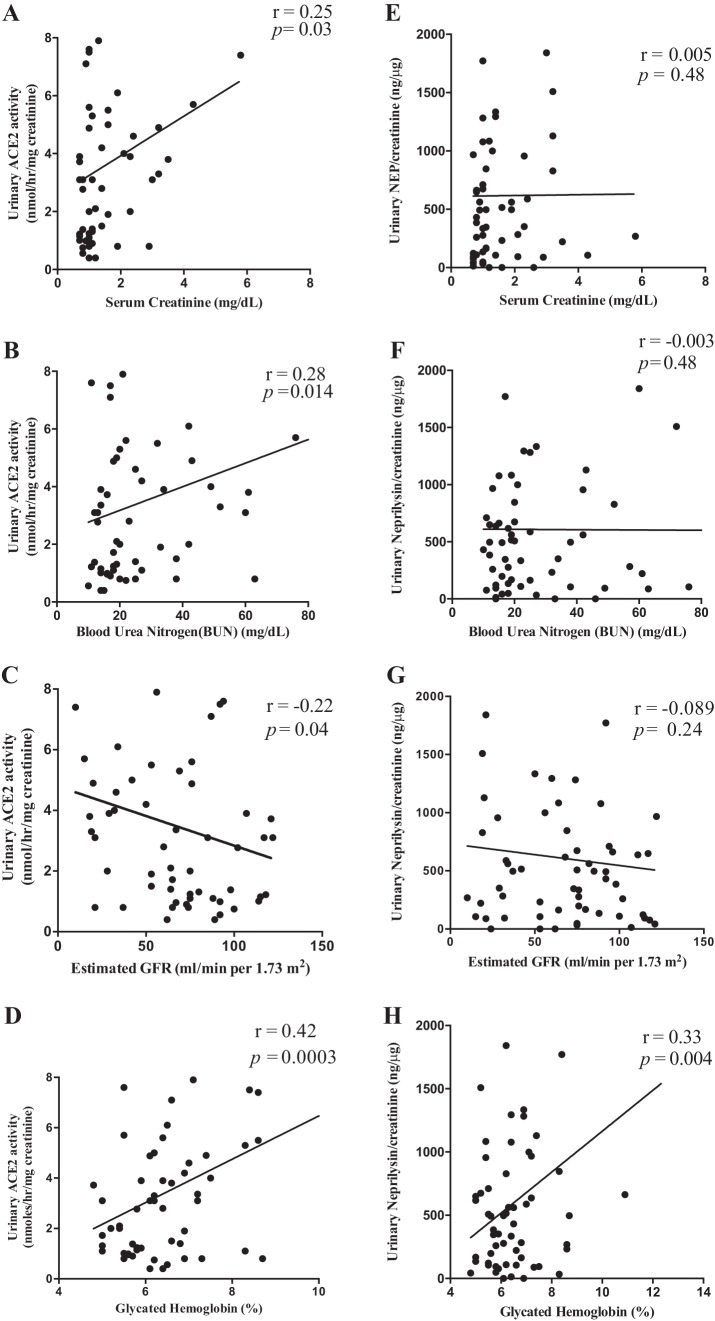
Urinary ACE2 and NEP were correlated with metabolic and renal characteristics (Fig. 5). Important significant correlations with eGFR, HbA1C, blood urea nitrogen (BUN), and serum creatinine are shown in Fig. 5. A significant correlation was observed for urinary ACE2 excretion with eGFR (P < 0.05), HbA1C (P < 0.001), BUN (P < 0.014), and serum creatinine (P < 0.05), while urinary NEP excretion was significantly correlated with HbA1C (P < 0.01) but not eGFR, BUN, or serum creatinine. Notably, urinary ACE2 and NEP did not correlate with albuminuria.
Fig. 5.
Linear regression analysis of urinary ACE2 and NEP with renal functional parameters. (A–D): urinary ACE2 activity was significantly and positively correlated with HbA1C (r = 0.42, **P < 0.0003), BUN (r = 0.28, *P < 0.014), and SCr (r = 0.25, *P < 0.03) but negatively correlated with eGFR (r = −0.22, *P < 0.04). (E–H): a linear regression analysis of urinary NEP activity was only significantly and positively correlated with HbA1C (r = 0.33, *P < 0.01) but no correlation with eGFR, BUN, or SCr. ACE2, angiotensin converting enzyme 2; eGFR, estimated glomerular filtration rate (ml/min/1.73 m2); HbA1C, glycated hemoglobin; BUN, blood urea nitrogen (mg/dl); NEP, neprilysin; SCr, serum creatinine (mg/dl).
Selection of predictors for HbA1C and eGFR and their performance.
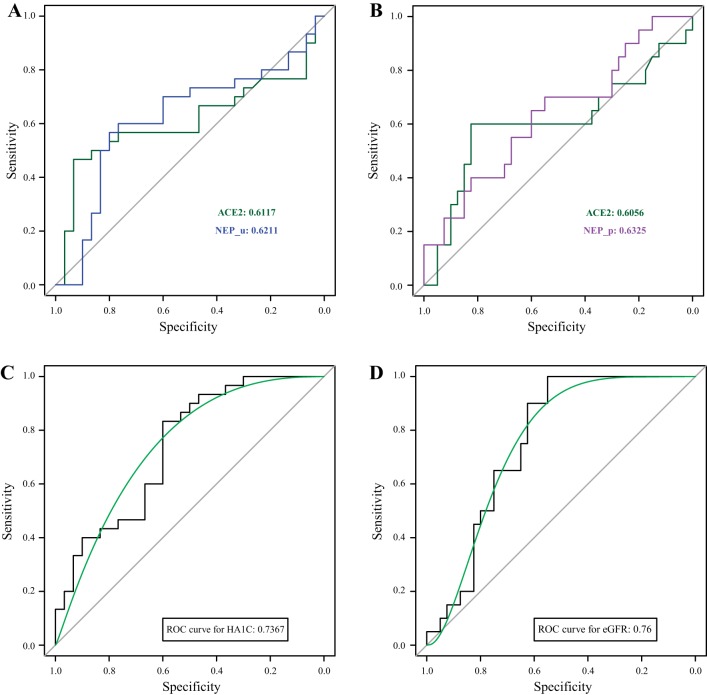
Among all the variables, including age, gender, race, body mass index, urinary ACE2, urinary NEP, plasma NEP, and ADAM17, potential risk predictors were selected based on their level of significance when they were added to predict the progression of type 2 diabetes (HbA1C > 6.5) and CKD (eGFR < 60 ml/min/1.73m2). According to backward stepwise selection, it is clearly seen that in addition to age, urinary ACE2 and urinary NEP were strongly related to the continuous variable HbA1C as shown in Table 2. However, these two factors gradually lost their importance during this transformation of continuous variable to a binary variable. The major reason could be loss of information in a binary variable than that of a quantitative trait during the transformation procedure. Therefore, when a logistic regression model was used to predict diabetes, two factors still needed to be recruited in the final model. Similarly, when we investigated the effect of these factors on eGFR, plasma NEP offered a stronger effect than urinary NEP. Thus, ACE2 and plasma NEP were finally selected for an eGFR prediction model. The prediction accuracies and ROC curves were calculated to evaluate the performance of selected risk predictors for HbA1C and eGFR (Fig. 6). Tenfold cross-validation was applied here to avoid the overfitting, thus the prediction accuracies (AUCs) were calculated from the testing set. The results illustrate that ACE2 and urinary NEP are important factors to help predict the HbA1C (ACE2: 0.6117; urinary NEP: 0.6211). In contrast, urinary ACE2 and plasma NEP predicted eGFR with AUCs (0.6056 and 0.6325), respectively. More importantly, when we combined factor age with ACE2, urinary and plasma NEP, the prediction accuracies were further improved (HbA1C: 0.7367; eGFR: 0.76).
Table 2.
The selected predictors for HbA1C and eGFR responses using stepwise regression
| HbA1C | eGFR | ||
|---|---|---|---|
| Predictor | Estimate | Predictor | Estimate |
| Intercept | −3.44 | Intercept | −6.834 |
| Age | 0.0488 | Age | 0.099 |
| ACE2 | 0.0213 | ACE2 | 0.058 |
| NEP_u | −0.000224 | NEP_p | −0.048 |
| ACE2: NEP_u | 0.000092 | ACE2: NEP_p | 0.011 |
Backward stepwise selection was used to finalize the predictors for HbA1C (>6.5%) and eGFR (<60 ml/min/1.73m2). Finalized predictors for HbA1C are age (0.0488), ACE2 (0.0213), NEP_u (−0.000224), and ACE2 [NEP_u (0.000092)]. Finalized predictors for eGFR are age (0.099), ACE2 (0.058), NEP_p (−0.048), and ACE2 [NEP_p (0.011)]. eGFR, estimated glomerular filtration rate; HbA1C, glycated hemoglobin; NEP, neprilysin; NEP_p, plasma NEP; NEP_u, urinary NEP.
Fig. 6.
ROC curves to predict HbA1C (>6.5) and eGFR (<60 ml/min/1.73 m2). A: ROC curves for urinary ACE2 (AUC = 0.6117) and urinary NEP (AUC = 0.6211) for the prediction of HbA1C (>6.5%). B: ROC curves for urinary ACE2 (AUC = 0.6117) and plasma NEP (AUC = 0.6211) for the prediction of eGFR (<60 ml/min/1.73 m2). C: the addition of age, urinary ACE2, and urinary NEP to the model for predicting HbA1C (>6.5) in all the patients (AUC = 0.7367). D: inclusion of age, urinary ACE2, and plasma NEP to the model for predicting eGFR (<60ml/min/1.73 m2) in all the patients (AUC = 0.76). ACE2, angiotensin converting enzyme 2; AUC, areas under the ROC; eGFR, estimated glomerular filtration rate; HbA1C, glycated hemoglobin; NEP, neprilysin; ROC, receiver operating characteristic.
DISCUSSION
In this study, we demonstrated increased urinary ACE2 levels and activity in patients with type 2 diabetes, which agrees with recent studies in subjects with diabetic nephropathy (7, 28, 32, 34, 49, 52). It has also been shown that increased urinary ACE2 in patients with type 1 diabetes is positively correlated with HbA1C but not with UACR (5). Previously, we have identified NEP to be a major producer of Ang- (1–7) in both mice and humans (10). In the present study, we have shown for the first time increased urinary shedding of NEP and ADAM17 in patients with type 2 diabetes. Interestingly, based on ROC curves and linear regression models to eGFR < 60 ml/min/1.73m2 and HbA1C > 6.5%, both ACE2 and NEP are good predictors for the progression of CKD and diabetes. Taken together, ACE2 and NEP could be used as early urinary biomarkers for diabetic nephropathy.
Characterization and prognosis of CKD in patients with diabetes are usually determined by albuminuria and eGFR, according to the guidelines proposed by the National Kidney Foundation (18). However, these are associated with some drawbacks. For instance, excretion of high molecular weight albumin was observed because of hyperglycemia, elevated risk of cardiovascular diseases, activated RAS (21), and existence of microalbuminuria in healthy patients because of reduced glomerular size selectivity and charge selectivity (19). Because of the modest prediction ability of albuminuria and eGFR, there is a robust need for inclusion of other urinary biomarkers for the potential risk prediction of CKD in patients with diabetes (11). Yet, there is a gap, and limited research focus was on the risk predictors for kidney disease. Some of the proposed candidates are neutrophil gelatinase-associated lipocalin, angiotensinogen, renin, and NEP (20, 29, 33, 42, 49, 56). In addition, other RAS enzymes, such as urinary ACE and ACE2, have also been investigated as potential new markers for hypertension (6) and diabetes (28). Previously, ACE2 has also been suggested as a biomarker for diabetic nephropathy (28, 32), but its ability to predict risk for CKD has not been investigated. On the other hand, the risk prediction ability of circulatory NEP and urinary NEP has only been determined for cardiovascular outcomes in patients with heart failure (3) and patients with acute kidney injury (33). In our study, we determined the benefit of using urinary ACE2, urinary NEP, and plasma NEP as risk predictors for diabetes and CKD. ROC curves showed that ACE2 and NEP are good risk predictors for diabetes in terms of HbA1C and for CKD in terms of eGFR. In addition, other results demonstrated increased urinary ACE2 shedding in Dnormo patients, indicating renal impairments independent of the degree of urinary albumin excretion. This assumption is further supported by the data showing that albuminuria and proteinuria did not correlate with urinary ACE2 or NEP. In patients with diabetes, HbA1C is one of the factors that causes progression of microalbuminuria to macroalbuminuria (14). Both urinary ACE2 and NEP are strongly correlated with HbA1C. In contrast, only urinary ACE2 significantly correlates with eGFR (P < 0.01) because of the absence of urinary ACE2 in ND individuals. This result is supported by the data generated in diabetic mice, showing that improved kidney function is associated with the attenuation of urinary ACE2 shedding (8).
ACE2 was first observed in the urine of healthy individuals (27). It is possible that elevated shedding of urinary ACE2 and deviation from these baseline levels could be caused by mechanisms involving increased glomerular filtration from plasma or kidney damage. The literature on plasma ACE2 is limited, and there are some reports showing increased levels of circulating ACE2 in patients with type 1 diabetes and vascular complications (45), or in patients with CKD (39). However, we did not detect plasma ACE2 in our patient population using a fluorogenic substrate assay and mass spectrometric analysis, which is in agreement with recent findings in diabetic animal models (2, 41, 50) and with clinical findings in patients with CKD (32). Therefore, our results support involvement of renal pathologies resulting in urinary ACE2 excretion. Discrepancy in findings between these studies may be due to the presence of an endogenous inhibitor (26), the variations in the method used for the detection of ACE2, plasma or serum preparations, species, gender, incubation time, and type of buffer or substrate used in the ACE2 enzyme activity assays.
The present study also identified NEP in urine and plasma of patients with diabetes. Therefore, circulating NEP or renal NEP could both serve as main sources for urinary NEP. However, low abundance of NEP in blood and urinary NEP fragments with a small molecular weight suggests that urinary NEP might reflect its origin and shedding from the kidney (43). A significant decrease of plasma NEP in Dmacro compared with Dmicro may indicate contribution of plasma NEP to urinary NEP in Dmacro patients, albeit insignificant. We detected a small immunoreactive band for NEP at 50-kDa in patients with diabetes, which could possibly be a result of shedding of renal NEP. Recent studies suggest a role of ADAM17 in shedding of soluble, active NEP into the media of endothelial cells (23). Similar to NEP, presence of smaller, soluble, and active fragments of ACE2 in diabetic urine supports a role for active proteolytic shedding of renal enzyme, most likely because of ADAM17 (8, 41, 44).
ADAM17 is abundant in different organs and cleaves several cell surface enzymes, including ACE2 and NEP (8, 16, 23, 36, 38, 41, 54). We detected increased urinary ADAM17 in all patients with diabetes, including Dnormo. One of the major findings in our study is that there is no evidence of an immunoreactive band for ADAM17 in ND patients. This suggests that detection of degradation products of ACE2 and NEP could be important indicators for renal disease before the presence of microalbuminuria. Thus, it is tempting to speculate that increased shedding of urinary ACE2 and NEP is mediated by ADAM17.
Study limitations.
Our study has some limitations, including the cross-sectional nature of the study and the use of a single time point for urinary and plasma measurements in a relatively small number of subjects. Long-term follow-up longitudinal studies in larger groups of patients at early stages of diabetes are needed to establish whether ACE2 and NEP could be used as early biomarkers for CKD. Because of the limited number of patients, it was not feasible to analyze the improved performance of ACE2, NEP, and ADAM17 as independent risk predictors for diabetic nephropathy. The performance of ACE2 and NEP was not compared with that of albuminuria in ROC curves. Further characterization of renal ADAM17 or sequence analysis of urinary ACE2 and NEP fragments could give new insights into the underlying pathologies and open potential new treatment targets for CKD. Lastly, the potential role of medication, including ACE inhibitors and Ang II type 1 receptor blockades, on urinary NEP and ACE2 in patients with diabetes is not well understood and needs further investigation. However, in one study, the alteration of urinary ACE2 was investigated in hypertensive patients taking the calcium channel blockers amlodipine and long-acting nifedipine, the ACE inhibitor enalapril, and the Ang II receptor blockers losartan, candesartan, valsartan, telmisartan, and olmesartan (13). Only olmesartan was found to increase urinary ACE2 concentration, and other antihypertensive drugs have no such effect.
In conclusion, our data showed elevated urinary ACE2 in patients with diabetes with kidney disease and its association with reduced eGFR, which may imply the severity of disease progression at early stages. Our study also provides a unique approach for the investigation of urinary NEP and ACE2 using new mass spectrometric tools. Western blot analysis of urinary ADAM17 suggests a potential mechanistic basis for the degradation of ACE2 and NEP and the shedding of their proteolytic fragments into the urine. The identification of ACE2 and NEP as risk predictors for CKD in patients with type 2 diabetes is a major asset of our study. Taken together, our findings suggest ACE2 and NEP as noninvasive biomarkers to assess kidney damage in patients with diabetes at an early stage.
GRANTS
This work was supported by grants from Wright State University Boonshoft School of Medicine, the American Heart Association (SDG0735112N to K. M. Elased), the NIH (R01HL093567 to K. M. Elased and 1F32DK093226 to N. Grobe), and the Carl W. Gottschalk award from the American Society of Nephrology (to N. Grobe).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.G., N.G., M.K., H.O., M.S., G.L., and K.M.E. conceived and designed research; S.G., N.G., M.K., H.O., and K.M.E. performed experiments; S.G., N.G., M.K., M.S., G.L., and K.M.E. analyzed data; S.G., N.G., M.K., H.O., M.S., G.L., and K.M.E. interpreted results of experiments; S.G., N.G., M.K., G.L., and K.M.E. prepared figures; S.G., N.G., M.K., H.O., M.S., G.L., and K.M.E. drafted manuscript; S.G., N.G., M.K., H.O., M.S., G.L., and K.M.E. edited and revised manuscript; S.G., N.G., M.K., H.O., M.S., G.L., and K.M.E. approved final version of manuscript.
ACKNOWLEDGMENTS
K. M. Elased is the guarantor of this work, and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Anderson S, Jung FF, Ingelfinger JR. Renal renin-angiotensin system in diabetes: functional, immunohistochemical, and molecular biological correlations. Am J Physiol 265: F477–F486, 1993. doi: 10.1152/ajprenal.1993.265.4.F477. [DOI] [PubMed] [Google Scholar]
- 2.Bae EH, Fang F, Williams VR, Konvalinka A, Zhou X, Patel VB, Song X, John R, Oudit GY, Pei Y, Scholey JW. Murine recombinant angiotensin-converting enzyme 2 attenuates kidney injury in experimental Alport syndrome. Kidney Int 91: 1347–1361, 2017. doi: 10.1016/j.kint.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Bayés-Genís A, Barallat J, Galán A, de Antonio M, Domingo M, Zamora E, Urrutia A, Lupón J. Soluble neprilysin is predictive of cardiovascular death and heart failure hospitalization in heart failure patients. J Am Coll Cardiol 65: 657–665, 2015. doi: 10.1016/j.jacc.2014.11.048. [DOI] [PubMed] [Google Scholar]
- 4.Brosius FC, Pennathur S. How to find a prognostic biomarker for progressive diabetic nephropathy. Kidney Int 83: 996–998, 2013. doi: 10.1038/ki.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns KD, Lytvyn Y, Mahmud FH, Daneman D, Deda L, Dunger DB, Deanfield J, Dalton RN, Elia Y, Har R, Van JAD, Bradley TJ, Slorach C, Hui W, Xiao F, Zimpelmann J, Mertens L, Moineddin R, Reich HN, Sochett E, Scholey JW, Cherney DZI. The relationship between urinary renin-angiotensin system markers, renal function, and blood pressure in adolescents with type 1 diabetes. Am J Physiol Renal Physiol 312: F335–F342, 2017. doi: 10.1152/ajprenal.00438.2016. [DOI] [PubMed] [Google Scholar]
- 6.Casarini DE, Plavinik FL, Zanella MT, Marson O, Krieger JE, Hirata IY, Stella RCR. Angiotensin converting enzymes from human urine of mild hypertensive untreated patients resemble the N-terminal fragment of human angiotensin I-converting enzyme. Int J Biochem Cell Biol 33: 75–85, 2001. doi: 10.1016/S1357-2725(00)00072-8. [DOI] [PubMed] [Google Scholar]
- 7.Cherney DZI, Xiao F, Zimpelmann J, Har RLH, Lai V, Scholey JW, Reich HN, Burns KD. Urinary ACE2 in healthy adults and patients with uncomplicated type 1 diabetes. Can J Physiol Pharmacol 92: 703–706, 2014. doi: 10.1139/cjpp-2014-0065. [DOI] [PubMed] [Google Scholar]
- 8.Chodavarapu H, Grobe N, Somineni HK, Salem ESB, Madhu M, Elased KM. Rosiglitazone treatment of type 2 diabetic db/db mice attenuates urinary albumin and angiotensin converting enzyme 2 excretion. PLoS One 8: e62833, 2013. doi: 10.1371/journal.pone.0062833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins AJ, Couser WG, Dirks JH, Kopple JD, Reiser T, Riella MC, Robinson S, Shah SV, Wilson A. World Kidney Day: an idea whose time has come. Natl Med J India 19: 55–57, 2006. [PubMed] [Google Scholar]
- 10.Domenig O, Manzel A, Grobe N, Königshausen E, Kaltenecker CC, Kovarik JJ, Stegbauer J, Gurley SB, van Oyen D, Antlanger M, Bader M, Motta-Santos D, Santos RA, Elased KM, Säemann MD, Linker RA, Poglitsch M. Neprilysin is a mediator of alternative renin-angiotensin-system activation in the murine and human kidney. Sci Rep 6: 33678, 2016. doi: 10.1038/srep33678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunkler D, Gao P, Lee SF, Heinze G, Clase CM, Tobe S, Teo KK, Gerstein H, Mann JFE, Oberbauer R; ONTARGET and ORIGIN Investigators . Risk prediction for early CKD in type 2 diabetes. Clin J Am Soc Nephrol 10: 1371–1379, 2015. doi: 10.2215/CJN.10321014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erdös EG, Skidgel RA . Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J 3: 145–151, 1989. doi: 10.1096/fasebj.3.2.2521610. [DOI] [PubMed] [Google Scholar]
- 13.Furuhashi M, Moniwa N, Mita T, Fuseya T, Ishimura S, Ohno K, Shibata S, Tanaka M, Watanabe Y, Akasaka H, Ohnishi H, Yoshida H, Takizawa H, Saitoh S, Ura N, Shimamoto K, Miura T. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens 28: 15–21, 2015. doi: 10.1093/ajh/hpu086. [DOI] [PubMed] [Google Scholar]
- 14.Giorgino F, Laviola L, Cavallo Perin P, Solnica B, Fuller J, Chaturvedi N. Factors associated with progression to macroalbuminuria in microalbuminuric type 1 diabetic patients: the EURODIAB Prospective Complications Study. Diabetologia 47: 1020–1028, 2004. doi: 10.1007/s00125-004-1413-8. [DOI] [PubMed] [Google Scholar]
- 15.Grobe N, Elased KM, Cool DR, Morris M. Mass spectrometry for the molecular imaging of angiotensin metabolism in kidney. Am J Physiol Endocrinol Metab 302: E1016–E1024, 2012. doi: 10.1152/ajpendo.00515.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grobe N, Di Fulvio M, Kashkari N, Chodavarapu H, Somineni HK, Singh R, Elased KM. Functional and molecular evidence for expression of the renin angiotensin system and ADAM17-mediated ACE2 shedding in COS7 cells. Am J Physiol Cell Physiol 308: C767–C777, 2015. doi: 10.1152/ajpcell.00247.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan H, Liu Y, Daily A, Police S, Kim M-H, Oddo S, LaFerla FM, Pauly JR, Murphy MP, Hersh LB. Peripherally expressed neprilysin reduces brain amyloid burden: a novel approach for treating Alzheimer’s disease. J Neurosci Res 87: 1462–1473, 2009. doi: 10.1002/jnr.21944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 63: 713–735, 2014. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 19.Jensen JS, Borch-Johnsen K, Deckert T, Deckert M, Jensen G, Feldt-Rasmussen B. Reduced glomerular size- and charge-selectivity in clinically healthy individuals with microalbuminuria. Eur J Clin Invest 25: 608–614, 1995. doi: 10.1111/j.1365-2362.1995.tb01753.x. [DOI] [PubMed] [Google Scholar]
- 20.Juretzko A, Steinbach A, Hannemann A, Endlich K, Endlich N, Friedrich N, Lendeckel U, Stracke S, Rettig R. Urinary angiotensinogen and renin excretion are associated with chronic kidney disease. Kidney Blood Press Res 42: 145–155, 2017. doi: 10.1159/000474932. [DOI] [PubMed] [Google Scholar]
- 21.Kamiyama M, Zsombok A, Kobori H. Urinary angiotensinogen as a novel early biomarker of intrarenal renin-angiotensin system activation in experimental type 1 diabetes. J Pharmacol Sci 119: 314–323, 2012. doi: 10.1254/jphs.12076FP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krolewski AS, Niewczas MA, Skupien J, Gohda T, Smiles A, Eckfeldt JH, Doria A, Warram JH. Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria. Diabetes Care 37: 226–234, 2014. doi: 10.2337/dc13-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuruppu S, Rajapakse NW, Minond D, Smith AI. Production of soluble Neprilysin by endothelial cells. Biochem Biophys Res Commun 446: 423–427, 2014. doi: 10.1016/j.bbrc.2014.01.158. [DOI] [PubMed] [Google Scholar]
- 24.Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, Hooper NM, Turner AJ. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem 280: 30113–30119, 2005. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lely AT, Hamming I, van Goor H, Navis GJ. Renal ACE2 expression in human kidney disease. J Pathol 204: 587–593, 2004. doi: 10.1002/path.1670. [DOI] [PubMed] [Google Scholar]
- 26.Lew RA, Warner FJ, Hanchapola I, Yarski MA, Ramchand J, Burrell LM, Smith AI. Angiotensin-converting enzyme 2 catalytic activity in human plasma is masked by an endogenous inhibitor. Exp Physiol 93: 685–693, 2008. doi: 10.1113/expphysiol.2007.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lew RA, Warner FJ, Hanchapola I, Smith AI. Characterization of angiotensin converting enzyme-2 (ACE2) in human urine. Int J Pept Res Ther 12: 283–289, 2006. doi: 10.1007/s10989-006-9031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang Y, Deng H, Bi S, Cui Z, A L, Zheng D, Wang Y. Urinary angiotensin converting enzyme 2 increases in patients with type 2 diabetic mellitus. Kidney Blood Press Res 40: 101–110, 2015. doi: 10.1159/000368486. [DOI] [PubMed] [Google Scholar]
- 29.Liu KD, Yang W, Anderson AH, Feldman HI, Demirjian S, Hamano T, He J, Lash J, Lustigova E, Rosas SE, Simonson MS, Tao K, Hsu CY; Chronic Renal Insufficiency Cohort (CRIC) study investigators . Urine neutrophil gelatinase-associated lipocalin levels do not improve risk prediction of progressive chronic kidney disease. Kidney Int 83: 909–914, 2013. doi: 10.1038/ki.2012.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maguer-Satta V, Besançon R, Bachelard-Cascales E. Concise review: neutral endopeptidase (CD10): a multifaceted environment actor in stem cells, physiological mechanisms, and cancer. Stem Cells 29: 389–396, 2011. doi: 10.1002/stem.592. [DOI] [PubMed] [Google Scholar]
- 31.McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM-HF Investigators and Committees . Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 371: 993–1004, 2014. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 32.Mizuiri S, Aoki T, Hemmi H, Arita M, Sakai K, Aikawa A. Urinary angiotensin-converting enzyme 2 in patients with CKD. Nephrology (Carlton) 16: 567–572, 2011. doi: 10.1111/j.1440-1797.2011.01467.x. [DOI] [PubMed] [Google Scholar]
- 33.Pajenda S, Mechtler K, Wagner L. Urinary neprilysin in the critically ill patient. BMC Nephrol 18: 172, 2017. doi: 10.1186/s12882-017-0587-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park SE, Kim WJ, Park SW, Park JW, Lee N, Park CY, Youn BS. High urinary ACE2 concentrations are associated with severity of glucose intolerance and microalbuminuria. Eur J Endocrinol 168: 203–210, 2013. doi: 10.1530/EJE-12-0782. [DOI] [PubMed] [Google Scholar]
- 35.Parvanova AI, Trevisan R, Iliev IP, Dimitrov BD, Vedovato M, Tiengo A, Remuzzi G, Ruggenenti P. Insulin resistance and microalbuminuria: a cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes 55: 1456–1462, 2006. doi: 10.2337/db05-1484. [DOI] [PubMed] [Google Scholar]
- 36.Patel VB, Clarke N, Wang Z, Fan D, Parajuli N, Basu R, Putko B, Kassiri Z, Turner AJ, Oudit GY. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol 66: 167–176, 2014. doi: 10.1016/j.yjmcc.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 37.Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol 18: 1353–1361, 2007. doi: 10.1681/ASN.2006080872. [DOI] [PubMed] [Google Scholar]
- 38.Riera M, Anguiano L, Clotet S, Roca-Ho H, Rebull M, Pascual J, Soler MJ. Paricalcitol modulates ACE2 shedding and renal ADAM17 in NOD mice beyond proteinuria. Am J Physiol Renal Physiol 310: F534–F546, 2016. doi: 10.1152/ajprenal.00082.2015. [DOI] [PubMed] [Google Scholar]
- 39.Roberts MA, Velkoska E, Ierino FL, Burrell LM. Angiotensin-converting enzyme 2 activity in patients with chronic kidney disease. Nephrol Dial Transplant 28: 2287–2294, 2013. doi: 10.1093/ndt/gft038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruggenenti P, Ruggiero B, Cravedi P, Vivarelli M, Massella L, Marasà M, Chianca A, Rubis N, Ene-Iordache B, Rudnicki M, Pollastro RM, Capasso G, Pisani A, Pennesi M, Emma F, Remuzzi G; Rituximab in Nephrotic Syndrome of Steroid-Dependent or Frequently Relapsing Minimal Change Disease Or Focal Segmental Glomerulosclerosis (NEMO) Study Group . Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol 25: 850–863, 2014. doi: 10.1681/ASN.2013030251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salem ESB, Grobe N, Elased KM. Insulin treatment attenuates renal ADAM17 and ACE2 shedding in diabetic Akita mice. Am J Physiol Renal Physiol 306: F629–F639, 2014. doi: 10.1152/ajprenal.00516.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salih M, Bovee DM, Roksnoer LCW, Casteleijn NF, Bakker SJL, Gansevoort RT, Zietse R, Danser AHJ, Hoorn EJ. Urinary renin-angiotensin markers in polycystic kidney disease. Am J Physiol Renal Physiol 313: F874–F881, 2017. doi: 10.1152/ajprenal.00209.2017. [DOI] [PubMed] [Google Scholar]
- 43.Skidgel RA, Schulz WW, Tam LT, Erdös EG. Human renal angiotensin I converting enzyme and neutral endopeptidase. Kidney Int Suppl 20: S45–S48, 1987. [PubMed] [Google Scholar]
- 44.Somineni HK, Boivin GP, Elased KM. Daily exercise training protects against albuminuria and angiotensin converting enzyme 2 shedding in db/db diabetic mice. J Endocrinol 221: 235–251, 2014. doi: 10.1530/JOE-13-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soro-Paavonen A, Gordin D, Forsblom C, Rosengard-Barlund M, Waden J, Thorn L, Sandholm N, Thomas MC, Groop P-H; FinnDiane Study Group . Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J Hypertens 30: 375–383, 2012. doi: 10.1097/HJH.0b013e32834f04b6. [DOI] [PubMed] [Google Scholar]
- 46.Standeven KF, Hess K, Carter AM, Rice GI, Cordell PA, Balmforth AJ, Lu B, Scott DJ, Turner AJ, Hooper NM, Grant PJ. Neprilysin, obesity and the metabolic syndrome. Int J Obes 35: 1031–1040, 2011. doi: 10.1038/ijo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275: 33238–33243, 2000. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 48.Warner FJ, Lew RA, Smith AI, Lambert DW, Hooper NM, Turner AJ. Angiotensin-converting enzyme 2 (ACE2), but not ACE, is preferentially localized to the apical surface of polarized kidney cells. J Biol Chem 280: 39353–39362, 2005. doi: 10.1074/jbc.M508914200. [DOI] [PubMed] [Google Scholar]
- 49.Wysocki J, Goodling A, Burgaya M, Whitlock K, Ruzinski J, Batlle D, Afkarian M. Urine RAS components in mice and people with type 1 diabetes and chronic kidney disease. Am J Physiol Renal Physiol 313: F487–F494, 2017. doi: 10.1152/ajprenal.00074.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wysocki J, Ye M, Khattab AM, Fogo A, Martin A, David NV, Kanwar Y, Osborn M, Batlle D. Angiotensin-converting enzyme 2 amplification limited to the circulation does not protect mice from development of diabetic nephropathy. Kidney Int, 91: 1336–1346, 2017. doi: 10.1016/j.kint.2016.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wysocki J, Ye M, Soler MJ, Gurley SB, Xiao HD, Bernstein KE, Coffman TM, Chen S, Batlle D. ACE and ACE2 activity in diabetic mice. Diabetes 55: 2132–2139, 2006. doi: 10.2337/db06-0033. [DOI] [PubMed] [Google Scholar]
- 52.Xiao F, Hiremath S, Knoll G, Zimpelmann J, Srivaratharajah K, Jadhav D, Fergusson D, Kennedy CRJ, Burns KD. Increased urinary angiotensin-converting enzyme 2 in renal transplant patients with diabetes. PLoS One 7: e37649, 2012. doi: 10.1371/journal.pone.0037649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao F, Zimpelmann J, Agaybi S, Gurley SB, Puente L, Burns KD. Characterization of angiotensin-converting enzyme 2 ectodomain shedding from mouse proximal tubular cells. PLoS One 9: e85958, 2014. doi: 10.1371/journal.pone.0085958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu J, Sriramula S, Xia H, Moreno-Walton L, Culicchia F, Domenig O, Poglitsch M, Lazartigues E. Clinical relevance and role of neuronal AT1 receptors in ADAM17-Mediated ACE2 shedding in neurogenic hypertension. Circ Res 121: 43–55, 2017. doi: 10.1161/CIRCRESAHA.116.310509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamaleyeva LM, Gilliam-Davis S, Almeida I, Brosnihan KB, Lindsey SH, Chappell MC. Differential regulation of circulating and renal ACE2 and ACE in hypertensive mRen2.Lewis rats with early-onset diabetes. Am J Physiol Renal Physiol 302: F1374–F1384, 2012. doi: 10.1152/ajprenal.00656.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang X, Chen C, Tian J, Zha Y, Xiong Y, Sun Z, Chen P, Li J, Yang T, Ma C, Liu H, Wang X, Hou FF. Urinary angiotensinogen level predicts AKI in acute decompensated heart failure: a prospective, two-stage study. J Am Soc Nephrol 26: 2032–2041, 2015. doi: 10.1681/ASN.2014040408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D. Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol 17: 3067–3075, 2006. doi: 10.1681/ASN.2006050423. [DOI] [PubMed] [Google Scholar]
- 58.Zraika S, Koh DS, Barrow BM, Lu B, Kahn SE, Andrikopoulos S. Neprilysin deficiency protects against fat-induced insulin secretory dysfunction by maintaining calcium influx. Diabetes 62: 1593–1601, 2013. doi: 10.2337/db11-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]