Abstract
The sodium-glucose cotransporter SGLT2 inhibitor empagliflozin (plasma protein binding ~88%) may reach its target in the brush border of the early proximal tubule by glomerular filtration and tubular secretion. Here we determined whether empagliflozin is secreted by renal tubules in mice and whether genetic knockout of the basolateral organic anion transporter 3 (Oat3−/−) affects its tubular secretion or glucosuric effect. Renal clearance studies in wild-type (WT) mice showed that tubular secretion accounted for 50–70% of empagliflozin urinary excretion. Immunostaining indicated that SGLT2 and OAT3 localization partially overlapped in proximal tubule S1 and S2 segments. Glucosuria in metabolic cage studies was reduced in Oat3−/− vs. WT mice for acute empagliflozin doses of 1, 3, and 10 mg/kg, whereas 30 mg/kg induced similar maximal glucosuria in both genotypes. Chronic application of empagliflozin (~25 mg·kg−1 ·day−1) in Oat3−/− mice was associated with lower urinary glucose-to-creatinine ratios despite maintaining slightly higher blood glucose levels than WT. On a whole kidney level, renal secretion of empagliflozin was largely unchanged in Oat3−/− mice. However, the absence of OAT3 attenuated the influence of empagliflozin on fractional glucose excretion; higher levels of plasma or filtered empagliflozin were needed to induce similar increases in fractional renal glucose excretion. We conclude that empagliflozin is excreted into the urine to similar extent by glomerular filtration and tubular secretion. The latter can occur largely independent of OAT3. However, OAT3 increases the glucosuric effect of empagliflozin, which may relate to the partial overlap of its localization with SGLT2 and thus OAT3-mediated tubular secretion of empagliflozin in the early proximal tubule.
Keywords: diabetes, organic anion transporter, proximal tubule, sodium glucose cotransport, tubular secretion
INTRODUCTION
The sodium-glucose cotransporter SGLT2 is localized in the brush border of the early proximal tubule (S1 and S2 segments) where it mediates most of the renal glucose reabsorption (15, 19, 21). In mice, SGLT2 accounts for ~97% of all renal glucose reabsorption in euglycemic conditions with the remaining 3% being reabsorbed by SGLT1 expressed in the late proximal tubule (14, 19). Pharmacological inhibition of SGLT2 inhibits the renal reabsorption of filtered glucose thereby lowering blood glucose levels in diabetes (20). SGLT2 inhibitors act from the luminal side of the brush border (7) and can reach this site by glomerular filtration and/or tubular secretion. The latter could facilitate high local concentrations in the unstirred layer of the brush border, i.e., at the site of SGLT2 expression.
Empagliflozin (1-chloro-4-(β-d-glucopyranos-1-yl)-2-[4-((S)-tetrahydrofuran-3-yl-oxy)-benzyl]-benzene) is a selective SGLT2 inhibitor with an IC50 of 3.1 nM for human SGLT2 and 1.9 nM for mouse SGLT2 and is highly selective for SGLT2 over SGLT1 in humans (>2,500-fold) and mice (~5,800-fold), respectively (8). Empagliflozin has a modest plasma protein binding of 88% and a molecular mass of 450.912 g/mol, i.e., the unbound plasma portion is freely filtered. Free plasma concentrations and thus early proximal tubule concentrations of empagliflozin of 1–2 nM, i.e., close to the IC50 for mouse SGLT2, inhibited 30% of renal glucose reabsorption in euglycemic mice; a 10-fold increase of its plasma concentration inhibited 60% of renal glucose reabsorption, consistent with maximum SGLT2 inhibition based on studies in mice lacking SGLT2 (14, 19). These results are consistent with the notion that a significant portion of the inhibitory effect of empagliflozin on glucose reabsorption can be explained by the filtered compound. However, an additional contribution from renal secretion of empagliflozin could not be excluded.
Many endogenous compounds and drugs are eliminated through the kidneys by secretion in the proximal tubule. This is particularly relevant for compounds with high protein binding due to their limited glomerular filtration, but tubular secretion also affects many compounds that have low or no plasma protein binding. Specific transport proteins have been implicated in the uptake of these compounds across the basolateral membrane (BLM) and the secretion into the tubular fluid across the apical brush-border membrane (BBM) of the proximal tubule (13). Among these transport proteins, the organic anion transporters OAT1 (SLC22A6) and OAT3 (SLC22A8) are localized to the BLM of the proximal tubule where they contribute to the cellular uptake and ultimately secretion of a number of therapeutic agents and endogenous organic anions as well as some cations (3–5, 11–13). This includes the cellular uptake and tubular secretion of the prototypic organic anion para-aminohippurate via OAT1 (6) and of estrone-3-sulfate and the cation creatinine via OAT3 (16, 18).
Recent studies implicated a potential role of OAT3 in the renal transport and secretion of empagliflozin. Pharmacokinetic studies in healthy subjects showed that coadministration of empagliflozin with drugs that can inhibit OAT3, like gemfibrozil or probenecid, enhanced the exposure to empagliflozin [area under the plasma concentratiion plasma concentratiion curve to infinity (AUC0-∞) and maximum plasma concentration (Cmax)], indicating that OAT3 may contribute to the renal excretion of empagliflozin in humans (10). In accordance, transfection and uptake studies in Xenopus oocytes showed that empagliflozin is taken up by mouse and rat OAT3 and this uptake was sensitive to inhibition by the OAT3 inhibitor probenecid. The studies further indicated that empagliflozin is a substrate of mouse and rat OATPA1 (17). OATPA1 is an organic anion transporter that is expressed on the BBM of proximal tubule S3 segments, where it has been implicated as a multispecific anion transporter in substrate uptake (9). OATPA1 is expressed in the kidney at much higher rates in male than in female mice and rats; however, the rate and extent of empagliflozin uptake into rat and mouse kidney slices were similar in male and female animals (17), potentially reflecting the dominant role of basolateral uptake. In contrast, empagliflozin uptake was not increased by transfection in Xenopus oocytes of mouse or rat OAT1 or the organic cation transporter OCT1 and OCT2 (17).
This prompted us to investigate in the mouse model 1) whether the SGLT2 inhibitor empagliflozin is secreted by the kidney, 2) whether the localization of SGLT2 and OAT1 or OAT3 overlap along the proximal tubule, and 3) whether genetic deletion of OAT3 or OAT1 affects the renal pharmacokinetics or glucosuric effect of empagliflozin.
METHODS
Animals.
All animal experimentation was conducted in accordance with the Guide for Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD) and was approved by the local Institutional Animal Care and Use Committee of the Veterans Affairs San Diego Healthcare System. Studies were performed in C57BL/6J mice (Jackson Laboratories) as well as in separate sets of C57BL/6J mice lacking Oat1 (Slc22a6) or Oat3 (Slc22a8) and their littermate wild types (WTs). The generation of knockout mice for Oat1 and Oat3 (Oat1−/−, Oat3−/−) has been described (6, 16). Both lines were backcrossed to C57BL/6J mice for 10 generations. Heterozygous mice from the final backcross of each line were bred to each other to generate knockout (−/−) and WT mice, from which all the animals used in the experiments described were descended. All studies were performed in adult male mice. All mice that entered the study also completed the studies, such that no mice were lost. Treatments were not blinded. Four series of studies were performed as described in the following sections.
Series 1: assessment of renal empagliflozin secretion in renal clearance studies.
Renal handling of empagliflozin was assessed in C57BL/6J mice following empagliflozin application (~30–35 mg·kg−1·day−1 via diet) for 3 wk ± acute application of empagliflozin (10 mg/kg ip in sterile saline, 2 µl/g body wt) or vehicle 1 h before the studies. Empagliflozin was provided by Boehringer Ingelheim Pharma (Biberach, Germany). Inulin clearance studies were performed to determine glomerular filtration rate (GFR) and the filtration and tubular secretion rates of empagliflozin under terminal anesthesia. Briefly, mice were anesthetized with thiobutabarbital (100 mg/kg ip, 2 μl/g body wt; Sigma-Aldrich, St. Louis, MO) and ketamine (100 mg/kg im, 2 μl/g body wt; Butler, Dublin, OH). The jugular vein was cannulated for continuous infusion of 2.25% bovine serum albumin and 10 mM glucose in 0.85% NaCl at a rate of 0.4 ml h−1 30 g body wt−1. For assessment of two-kidney GFR by inulin clearance, [3H]inulin was added to the infusion to deliver 5 μCi·h−1·30 g body wt−1. Urinary excretion of [3H]inulin and empagliflozin was assessed by quantitative urine collection via a bladder catheter in 40–45 min periods. To determine plasma concentrations of [3H]inulin and empagliflozin, blood samples were collected at the beginning and end of the urine collection period from an arterial catheter, which was also used to monitor blood pressure and heart rate.
The tubular secretion rate of empagliflozin was calculated as the difference between the absolute urinary excretion rate and the glomerular filtration per time of empagliflozin. Glomerular filtration of empagliflozin is given as the product of GFR and free plasma concentrations of empagliflozin. Fractional renal excretion (of empagliflozin or glucose) was calculated as the absolute urinary excretion rate divided by the glomerular filtration per time of the given compound.
Series 2: tissue preparation and immunolocalization of OAT1, OAT3, and SGLT2 in the kidney of WT mice.
Mice were euthanized by cervical dislocation, the abdominal cavity was opened, and the kidneys were removed and rinsed in ice-cold PBS. The ~1-mm-thick sagittal slices from the organ middle were removed from each kidney and fixed in 4% p-formaldehyde overnight in a refrigerator. Thereafter, tissues samples were extensively rinsed in PBS and stored in PBS with 0.02% NaN3 at 4°C until use. Other steps, including cutting of frozen sections, antigen retrieval, and immunostaining have been described in detail previously (1, 15). Immunostaining was performed using well-characterized noncommercial antibodies (Ab) for mouse OAT1 (1), OAT3 (1), and SGLT2 (15, 19), and commercial secondary antibodies Cy3 (GAR-Cy3)- and FITC (GAR-FITC)-conjugated goat anti-rabbit IgG (both from Jackson ImmunoResearch Laboratories). Since the primary antibodies for mouse OAT1, OAT3, and SGLT2 were polyclonal, in double-staining mode we had to optimize the incubation protocol with secondary antibodies. Briefly, frozen sections were incubated with the 1st primary antibody [OAT1-Ab (1:10,000) or OAT3-Ab (1:300)] at 4°C overnight and then with the first secondary antibody (GAR-Cy3; 1:800) at room temperature for 4 h (2 changes for 2 h each) to saturate the primary antibody-related binding sites. Thereafter, the sections were incubated with SGLT2-Ab (1:1000) at 4°C overnight and then with the second secondary antibody (GAR-FITC; 1:100) at room temperature for 15 min to prevent its binding to the first primary antibody. Other steps and fluorescence microscopy have been described in detail before (1, 15).
Series 3: acute glucosuric effect and renal excretion of empagliflozin in metabolic cage studies in mice lacking Oat1 or Oat3.
Empagliflozin (0.3–30 mg/kg in water) or vehicle was applied by oral gavage together with a water load (30 μl/g body wt) to facilitate subsequent quantitative urine collection over 3 h in metabolic cages (no access to fluid or food) and determine the glucosuric response and urinary excretion of empagliflozin.
Series 4, part 1: effect of chronic application of empagliflozin on blood glucose levels and urinary glucose-to-creatinine ratios in mice lacking Oat1 or Oat3.
Mice were treated with empagliflozin (~25 mg·kg−1·day−1) for 15 days in diet while body weight, urine glucose-to-creatinine ratios, blood glucose levels, and food and fluid intake were monitored. Food and fluid intake was determined while the mice were maintained in their regular cages. Urine was obtained at the same time of the day by picking up the mice to elicit reflex urination and holding them over a clean Petri dish for sample collection. For paired glucose measurements, blood was collected by tail snip immediately after urine collection in awake mice.
Series 4, part 2: assessment of renal empagliflozin secretion and glucose excretion in renal clearance studies in mice lacking Oat1 or Oat3.
Following application of empagliflozin (~25 mg·kg−1·day−1) for 15 days in the diet (see above), inulin clearance studies were performed to determine renal handling of empagliflozin in Oat1−/− and Oat3−/− mice and their littermate WT mice as described for series 1. In addition, we measured plasma and urine concentrations of glucose to determine the absolute and fractional renal excretion of glucose.
Blood and urine analysis.
Concentrations of [3H]inulin in plasma and urine were measured by liquid scintillation counting. Blood glucose in awake mice was determined using the Ascensia Elite XL glucometer (Bayer, Mishawaka, IN). Urine glucose was determined by the hexokinase/glucose-6-phosphate dehydrogenase method (Infinity; Thermo Electron, Louisville, CO). For the clearance studies, both plasma and urine glucose were determined by the latter assay. Urine and plasma concentrations of empagliflozin were determined by liquid chromatography-tandem mass spectrometrY. 14C-empagliflozin binding to mouse plasma proteins was determined in vitro by equilibrium dialysis for 6 h at 37°C. Teflon dialysis cells and dialysis membranes (Spectra/Por) with a 12,000–14,000 molecular weight cut off were used. We found that mean fractional protein binding of empagliflozin in mouse plasma is constant over a wide range (up to 50 µM) at 88.1 ± 0.5%. We used the latter information to calculate free plasma concentrations. Since albumin in the tubular fluid may also bind empagliflozin and affect its pharmacodynamic effect, urinary albumin (Mouse Albumin ELISA Quantitation Set, Bethyl Laboratories, Montgomery, TX)-to-creatinine (Infinity Creatinine Liquid Stable Reagent; Thermo Fisher Scientific, Middletown, VA) ratios were determined in spontaneous urine samples, and found not to be significantly different between genotypes: Oat1−/− and littermate WT: 22.9 ± 2.4 vs 20.7 ± 1.8 µg/mg (n = 9–10, NS); and Oat3−/− and littermate WT: 22.3 ± 4.0 vs. 22.3 ± 2.9 µg/mg (n = 9–10, NS).
Statistical analysis.
Data are shown as means ± SE. Unpaired Student's t-test was performed to analyze for statistical differences between two groups. To analyze the effect of different empagliflozin doses on glucose excretion or empagliflozin excretion in metabolic cage studies, two-way ANOVA with post-hoc Tukey test for multiple comparisons was performed. P < 0.05 was considered statistically significant.
RESULTS
Series 1: renal tubular secretion of empagliflozin.
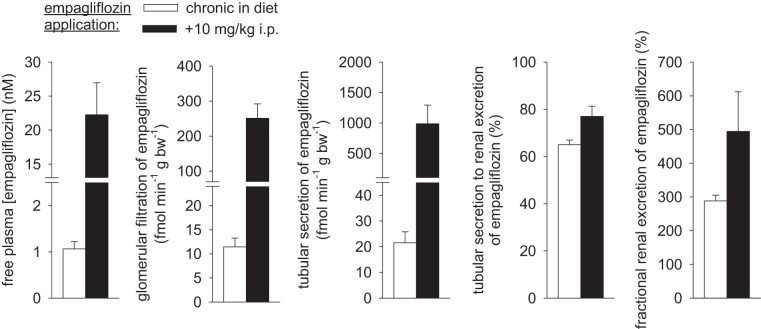
Renal clearance studies revealed tubular secretion of empagliflozin in the tested range of free plasma concentrations of 1–2 nM (close to IC50 for mouse SGLT2; drug in diet only) to ~20 nM (drug in diet plus bolus before study). Tubular secretion contributed to ~65–75% of renal empagliflozin excretion (Fig. 1).
Fig. 1.
Renal tubular secretion of empagliflozin. Renal handling of empagliflozin was assessed in renal clearance studies in mice following empagliflozin application (~30–35 mg·kg−1·day−1) for 3 wk ± acute application of empagliflozin (10 mg/kg ip) 1 h before studies. Tubular secretion of empagliflozin is evident for free plasma concentrations in the range of 1–2 nM (close to IC50 for the mouse sodium-glucose cotransporter SGLT2) to ~20 nM. Tubular secretion contributed 65–75% of renal empagliflozin excretion; n = 6–8/group.
Series 2: partial overlap in the localization of SGLT2 with OAT3 and OAT1 in the proximal convoluted tubule.
Immunostaining confirmed the expected polarized expression pattern with SGLT2 detected in the BBM (Fig. 2, A and B, cortex, SGLT2, green fluorescence) of the proximal convoluted tubule, and OAT1 (Fig. 2A, cortex, OAT1, red fluorescence) and OAT3 (Fig. 2B, cortex, OAT3, red fluorescence) localized to the BLM. In the S1 segments, which is identified by its association with the glomerulus and with similar staining intensity in the surrounding tubule profiles, OAT1 was negative (Fig. 2A) and OAT3 was weakly stained (Fig. 2B) or negative (not shown), whereas SGLT2 was strongly stained. The immunohistochemical analyses indicated opposite expression gradients along the S1 and S2 segments. The immunostaining for SGLT2 was stronger in S1 than in S2 segments whereas staining for OAT1 and OAT3 was, in accordance with our previous detailed study (1), most intense in S2 segments. This pattern of staining for all three transporters, described above for the cortical nephrons, was retained in the nephron segments originating from juxtamedullary glomeruli (not shown). However, S3 segments in the outer stripe were not stained for any of the three transporters (Fig. 2, A and B, Outer stripe). The immunostaining indicated partial overlap between OAT3 and SGLT2 in S1 segments and a full overlap between OAT1, OAT3, and SGLT2 in S2 segments (Fig. 2). Similar expression patterns were observed in mice treated with empagliflozin for 7 days (data not shown).
Fig. 2.
The organic anion transporter OAT1 and OAT3 expression partially overlap with SGLT2 in the proximal convoluted tubules. All three transport proteins were localized in the cortical proximal tubule segments; OAT1 (A) and OAT3 (B) in the basolateral membrane and SGLT2 (A and B) in the brush border. S3 segments in the outer stripe were negative for all 3 transporters. A: in the S1 segments [a tubule attached to the glomerulus (G) and several surrounding unstained tubule profiles (arrowheads)], OAT1 was negative, whereas SGLT2 was stained strongly in S1 segments (arrowheads) and weakly in S2 segments. The segments stained weakly for OAT1 and relatively strongly for SGLT2 probably represent the transition between S1 and S2 (arrow). B: OAT3 was stained weakly in some (S1) but not all (not shown) S1 segments, and strongly in S2 segments, whereas SGLT2 was stained strongly in S1 and weaker in S2 segments. Similar staining pattern was observed in the nephron segments located in both outer (A and B) and deep cortex (not shown). The analysis indicates opposite expression gradients along S1/S2 segments for SGLT2 (S1 > S2) vs. OAT1/OAT3 (S1 < S2), some overlap between OAT3 and SGLT2 in S1, and a full overlap of all 3 transporters in S2 segments, possibly including S1 to S2 segment transitions. The images represent similar findings in the kidneys from 5 wild-type animals. Bar (for all images in the respective panel) = 20 µm.
Series 3: rightward shift in the acute glucosuric response in mice lacking Oat3 or Oat1.
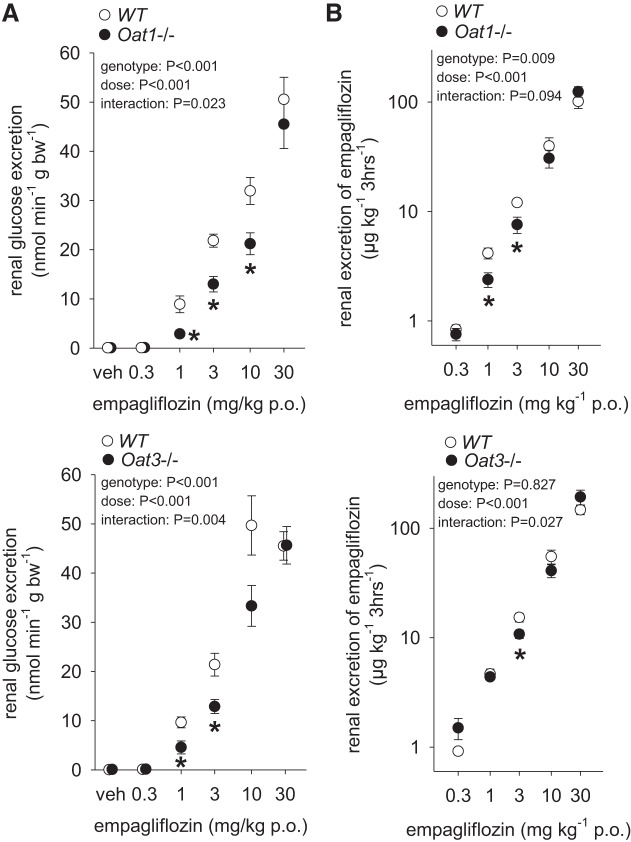
Metabolic cage studies showed the expected empagliflozin-induced dose-dependent increase in glucosuria and urinary empagliflozin excretion in WT mice (Fig. 3). Mice lacking Oat1 or Oat3 showed a small and similar rightward shift in the glucosuria response curve to empagliflozin when compared with their WT littermates with lower glucosuria observed for doses of 1 and 3 mg/kg, while the glucosuric effect of the highest tested dose (30 mg/kg) was not different between genotypes. There was a small reduction in cumulative excretion of empagliflozin over the 3-h urine collection period for the 1 and 3 mg/kg doses in Oat1−/− and for the 3 mg/kg dose in Oat3−/− compared with WT littermates, whereas cumulative urinary empagliflozin excretion was not different between genotypes for other doses of empagliflozin. These findings may be indicative of overall similar systemic empagliflozin dosing and exposure rather than differences in renal secretion, as it would be expected that impaired tubular secretion is at least in part compensated by enhanced glomerular filtration over the 3-h urine collection period.
Fig. 3.
Knockout of Oat3 or Oat1 attenuates acute glucosuric effect of empagliflozin. A: in mice lacking Oat3 or Oat1, glucosuria was reduced vs. wild-type (WT) littermates for empagliflozin doses of 1 and 3 mg/kg, whereas 30 mg/kg induced similar maximal glucosuria in all genotypes. B: urinary empagliflozin excretion was slightly lower for the 1 and 3 mg/kg dose in Oat1−/− and for the 3 mg/kg dose in Oat3−/−, whereas lower or higher doses induced similar excretion rates. *P < 0.05 vs WT, by two-way ANOVA followed by Tukey’s test for multiple comparisons; n = 9–19/group.
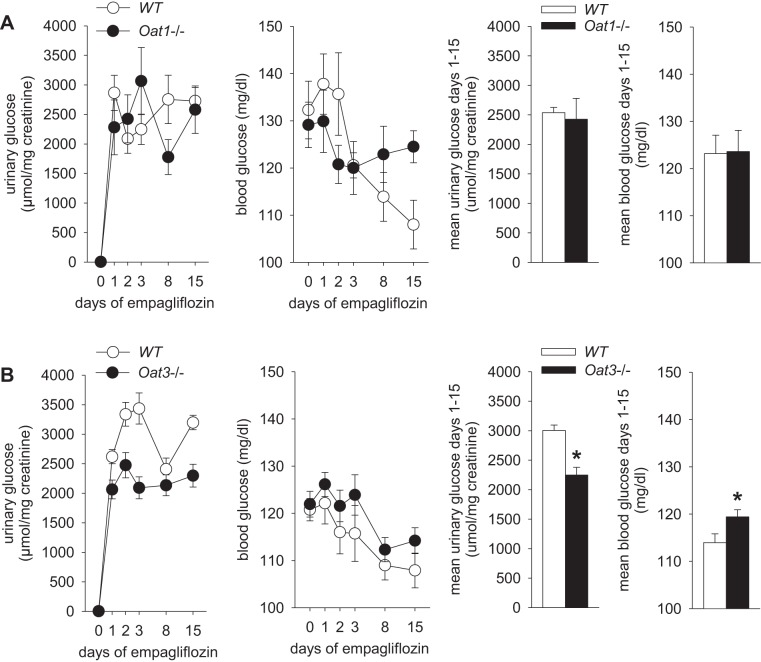
Series 4, part 1: chronic glucosuric effect of empagliflozin is reduced in mice lacking Oat3.
In mice lacking Oat1 and their WT littermates, empagliflozin application in diet over 15 days induced sustained and similar increases in urinary glucose-to-creatinine ratios, associated with similar blood glucose levels (Fig. 4). The increased loss of glucose calories into the urine was associated with a similarly reduced body weight and increased fluid intake in mice lacking Oat1 and their WT littermates (Table 1). In contrast, chronic application of empagliflozin in Oat3−/− mice was associated with lower urinary glucose-to-creatinine ratios despite maintaining higher blood glucose levels than their WT littermates (Fig. 4); this was associated with similar reductions in body weight and increases in fluid intake in both groups. Empagliflozin did not change or tended to increase food intake (Table 1).
Fig. 4.
Knockout of Oat3 attenuates the chronic glucosuric effect of empagliflozin. Empagliflozin (~25 mg·kg−1·day−1) was applied for 15 days and blood glucose and urinary glucose-to-creatinine ratios determined in awake mice. As expected, empagliflozin induced a sustained glucosuria in wild-type (WT) mice. A: no significant differences were observed in mice lacking Oat1 with regard to mean glucosuria or blood glucose levels. B: knockout of Oat3 attenuated the glucosuric effect of empagliflozin despite maintaining higher blood glucose levels.*P < 0.05 vs. WT, by unpaired Student's t-test, n = 9–11/group.
Table 1.
Body weight and food and water intake under basal conditions and in response to empagliflozin
| Oat1 |
Oat3 |
|||
|---|---|---|---|---|
| WT | −/− | WT | −/− | |
| Body weight, g | ||||
| Basal | 31.8 ± 1.0 | 32.9 ± 1.5 | 28.5 ± 0.9 | 30.7 ± 0.7 |
| Rmpagliflozin | 30.3 ± 0.8* | 31.2 ± 1.1* | 27.5 ± 0.8* | 29.6 ± 0.6* |
| Food intake, g | ||||
| Basal | 5.90 ± 0.66 | 4.97 ± 0.33 | 4.53 ± 0.32 | 4.78 ± 0.17 |
| Empagliflozin | 9.87 ± 3.15 | 7.23 ± 1.13 | 4.49 ± 0.15 | 5.16 ± 0.68 |
| Water intake, g | ||||
| Basal | 3.77 ± 0.08 | 3.84 ± 0.29 | 3.63 ± 0.25 | 3.57 ± 0.16 |
| Empagliflozin | 6.51 ± 0.32* | 6.47 ± 0.45* | 5.98 ± 0.17* | 6.77 ± 0.52* |
Values are means ± SE. Mean data per mouse were calculated. Body weight, n = 8–11 in each group; and food intake and water intake, n = 4 cages in each group with 2–3 mice/cage.
P < 0.05 vs basal.
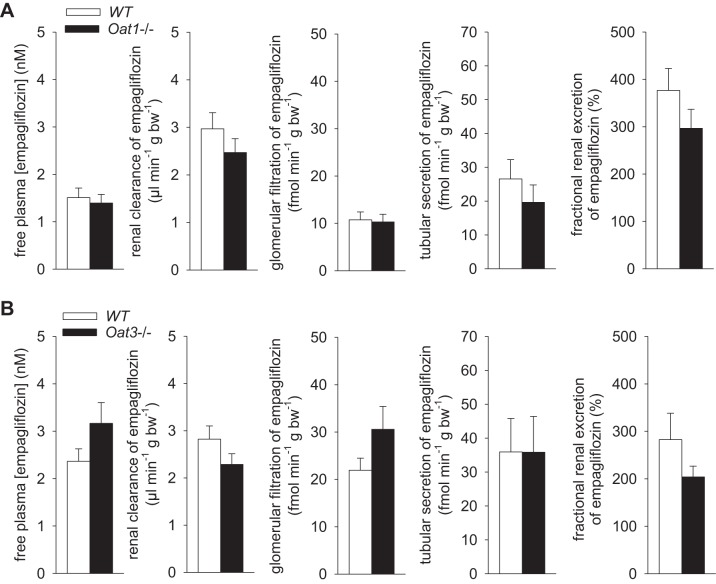
Series 4, part 2: whole kidney tubular secretion of empagliflozin is largely preserved in the absence of Oat3 or Oat1.
Renal clearance studies following empagliflozin application (~25 mg·kg−1·day−1) for 15 days confirmed tubular secretion of empagliflozin, which contributed 45–65% of renal empagliflozin excretion in WT (Fig. 5). Previous studies reported that GFR is not different between Oat1−/− and Oat3−/− mice and their WT littermates in the absence of empagliflozin treatment (6, 18). Following chronic empagliflozin treatment, GFR values were also not different between Oat1−/− and their WT littermates (7.3 ± 0.5 vs. 7.0 ± 0.5 µl·min−1·g body wt−1 body; NS) or between Oat3−/− and their WT littermates (9.7 ± 0.7 vs. 9.6 ± 0.8 µl·min−1·g body wt−1 body; NS). Overall similar results were observed for tubular secretion of empagliflozin in mice lacking Oat1 or Oat3. Comparing data for pooled WT groups vs. pooled Oat1/Oat3 knockout groups indicated a tendency for a reduced renal clearance and a reduced fractional renal excretion of empagliflozin in knockout mice (P = 0.084 and P = 0.078, unpaired Student's t-test).
Fig. 5.
Following chronic drug application the renal clearance and tubular secretion of empagliflozin is largely preserved in the absence of Oat1 or Oat3. Renal clearance studies following empagliflozin application (~25 mg·kg−1·day−1) for 15 days revealed free plasma concentrations of empagliflozin close to IC50 for mouse SGLT2 (1–2 nM), and showed that tubular secretion contributed 45–65% of renal empagliflozin excretion in wild-type (WT) mice. Overall similar results were observed in the absence of Oat1 (A) or Oat3 (B). A comparison of pooled WT vs. Oat1/Oat3 knockout data indicated a tendency for a reduced renal clearance and fractional renal excretion of empagliflozin in knockout mice (P = 0.084 and P = 0.078, by unpaired Student's t-test); n = 9–11/group.
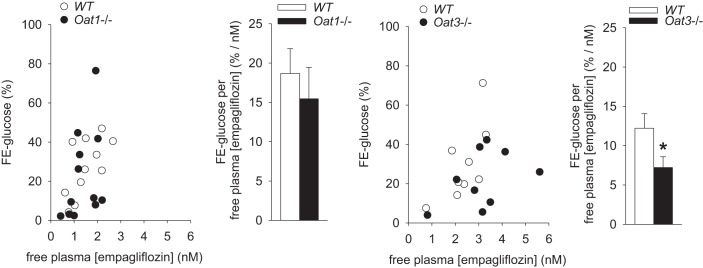
Knockout of Oat3 attenuated the influence of empagliflozin on renal glucose excretion.
Renal clearance studies showed the expected positive relationship between free empagliflozin plasma concentration and the fractional renal glucose excretion in WT mice (Fig. 6). The absence of Oat3 attenuated the influence of empagliflozin on fractional glucose excretion, such that higher concentrations of free empagliflozin, assumed to represent empagliflozin concentrations in the glomerular filtrate, were needed to induce similar increases in fractional renal glucose excretion. In comparison, no significant effect was observed in mice lacking Oat1.
Fig. 6.
Following chronic drug application knockout of Oat3 attenuates the influence of empagliflozin on fractional renal glucose excretion. Renal clearance studies showed that the ratio of fractional renal glucose excretion (FE-glucose) to free empagliflozin plasma concentration is reduced in Oat3 knockout mice. In comparison, no significant effects were observed in mice lacking Oat1. The differences in plasma empagliflozin concentrations observed within a given group reflect the normal variation in achieved empagliflozin levels with chronic administration. *P < 0.05 vs. wild type (WT), by unpaired Student's t-test; n = 9–11/group.
DISCUSSION
The current study shows that in mice the SGLT2 inhibitor empagliflozin is excreted into the urine to a similar extent by glomerular filtration and tubular secretion. The latter occurs in large part independent of OAT3. However, SGLT2 localization partially overlapped with OAT3 in the S1 and S2 segments of the proximal convoluted tubule and studies in knockout mice indicated that the absence of OAT3 attenuated the influence of empagliflozin on glucose excretion. This may implicate that OAT3-mediated tubular empagliflozin secretion in the early proximal tubule, in addition to the filtration of free empagliflozin by the glomeruli, contributes to the pool of empagliflozin that inhibits SGLT2 in the brush border.
Such a role for OAT3 would be consistent with recent transfection and uptake studies in Xenopus oocytes indicating that empagliflozin is taken up by overexpressed mouse and rat OAT3 (17). Moreover, a role for OAT3 in the active renal uptake of empagliflozin has also been proposed in healthy human subjects, based on the findings that coadministration of empagliflozin with drugs that can inhibit OAT3, like gemfibrozil or probenecid, enhanced the exposure to empagliflozin (AUC0-∞ and Cmax). Furthermore, the same study proposed a 50% decrease in the renal clearance of empagliflozin when it was coadministered with probenecid, indicating that inhibition of renal OAT3 might impair renal clearance and increase systemic exposure of empagliflozin (10). The latter study did not measure GFR and thus did not provide insights on the glomerular filtration vs. tubular secretion of empagliflozin. In the current study in mice, the absence of OAT3 protein numerically increased plasma empagliflozin concentrations and reduced the renal clearance and fractional renal empagliflozin excretion following chronic application of the drug, but these changes did not reach statistical significance. It is possible that quantitative differences in the contribution of specific transporters to the overall renal clearance and tubular secretion of empagliflozin differ between mice and humans.
The discussed study in healthy subjects also found that probenecid, which can inhibit OAT3, had no effect on the glucosuria induced by empagliflozin (10). The authors concluded that the fraction of empagliflozin cleared by glomerular filtration was sufficient to induce increased urinary glucose excretion and that glomerular filtration has a predominant role in the pharmacodynamic effect of empagliflozin. However, probenecid increased AUC0-∞ and Cmax of empagliflozin and thus its glomerular filtration (10), which is expected to counterbalance for a reduced glucosuric effect due to less tubular secretion of empagliflozin. In other words, the finding that probenecid enhanced AUC0-∞ and Cmax of empagliflozin without increasing glucosuria would be consistent with a pharmacodynamic effect of empagliflozin due to its tubular secretion that is blunted by probenecid.
In contrast to Oat3 knockout mice, no consistent inhibition of the glucosuric effect of empagliflozin was observed in mice lacking Oat1, especially with chronic application of the drug. In particular, the absence of Oat1 did not significantly affect urinary glucose-to-creatinine ratios when empagliflozin was given in diet for 15 days. Similarly, the subsequent performance of renal clearance studies showed that the ratio between free empagliflozin plasma concentrations or the filtered rate of empagliflozin and fractional urinary glucose excretion was not significantly different in Oat1 knockout mice compared with their WT littermates. Nevertheless, the acute glucosuric effect in response to a single oral dose of empagliflozin, as studied in metabolic cages, was similarly reduced in mice lacking Oat1 vs. WT mice as observed in Oat3 knockout mice. The explanation for these different findings is not fully clear. Previous studies in Xenopus oocytes with heterologous expression of rat or mouse Oat1 appeared not to show evidence for a role of OAT1 in empagliflozin transport (10, 17). One possibility is that empagliflozin can also be transported by OAT1, which becomes more evident in the natural transporter setting and in the acute response to the drug. If both OAT1 and OAT3 contributed to basolateral empagliflozin uptake into proximal tubule cells and ultimately to tubular secretion, then it would be expected that one transporter can compensate at least in part for the other when the latter is inhibited. This may explain why renal empagliflozin excretion was largely intact in both knockout models. Studies in a double knockout model may resolve this issue. Unfortunately, due to the fact that the genes for OAT1 and OAT3 are localized directly next to each other on the same chromosome, cross breeding of the two individual knockout lines is unlikely to generate a double knockout mouse, but this would require the generation of a completely new mouse model. In addition, other transport pathways may contribute to the renal secretion of empagliflozin. Our immunohistochemical analysis suggested that in the S1 segment of the proximal tubule the localization of OAT3 and thus the colocalization with SGLT2 was more evident than for OAT1. Such a difference could explain differences in the effect of Oat3 vs. Oat1 knockout on the glucosuric response to empagliflozin while the effect on overall tubular secretion could be similar. Validated antibodies against human OAT1 or OAT3 immunostained the BLM of proximal tubules in the human kidney cortex with the pattern of S1 = S2 > S3 in medullary rays, whereas S3 in the outer stripe and other nephron segments remained unstained (2). Thus, also in the human early proximal tubule, secretion of empagliflozin may involve basolateral uptake by OAT3 and/or OAT1 to reach its target SGLT2 in the brush border. Potential candidates for the transport and secretion of empagliflozin across the apical brush border include MRP2/4 or OAT4 (13), but no studies have specifically tested this issue.
In summary, in mice the SGLT2 inhibitor empagliflozin is excreted into the urine to a similar extent by glomerular filtration and tubular secretion. On a whole kidney level, tubular secretion of empagliflozin can occur largely independent of OAT3, which may include compensation by OAT1 in the absence of OAT3. However, the presence of OAT3 enhances the glucosuric effect of empagliflozin, which may be related to the partial overlap of its expression with SGLT2 in the proximal tubule S1 and S2 segments, where OAT3-mediated tubular secretion of empagliflozin may contribute to its concentrations close to SGLT2 in the BBM. Further studies will determine the impact of OAT3 on the antihyperglycemic effect of empagliflozin in the diabetic setting.
GRANTS
We were supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants R01-DK-112042 and R01-DK-106102, University of Alabama at Birmingham/University of California, San Diego O’Brien Center of Acute Kidney Injury NIDDK Grant P30-DK-079337, Department of Veterans Affairs, and Boehringer Ingelheim Pharma (Biberach an der Riss, Germany). D. Breljak and I. Sabolic were supported by Croatian Science Foundation Grant IP-11-2013-1481 (AGEMETAR).
DISCLOSURES
Over the past 36 mo, V. Vallon has served as a consultant and received honoraria from Bayer, Boehringer Ingelheim, Intarcia Therapeutics, Astra-Zeneca, Janssen Pharmaceutical, Eli Lilly, and Merck and received grant support for investigator-initiated research from Astra-Zeneca, Bayer, Boehringer Ingelheim, Fresenius, and Janssen.
AUTHOR CONTRIBUTIONS
Y.F., D.B., H.K., N.A., S.K.N., I.S., and V.V. established conception and design of research; Y.F., D.B., A.O., F.B., R.P., W.H., P.S., B.F., I.S., and V.V. performed experiments; Y.F., D.B., I.S., and V.V. analyzed data; Y.F., D.B., I.S., and V.V. interpreted results of experiments; D.B., I.S., and V.V. prepared Figs.; D.B., I.S., and V.V. drafted manuscript; Y.F., D.B., A.O., F.B., R.P., W.H., P.S., B.F., E.M., H.K., N.A., S.K.N., I.S., and V.V. edited and revised manuscript; Y.F., D.B., A.O., F.B., R.P., W.H., P.S., B.F., E.M., H.K., N.A., S.K.N., I.S., and V.V. approved final version of manuscript.
REFERENCES
- 1.Breljak D, Brzica H, Sweet DH, Anzai N, Sabolic I. Sex-dependent expression of Oat3 (Slc22a8) and Oat1 (Slc22a6) proteins in murine kidneys. Am J Physiol Renal Physiol 304: F1114–F1126, 2013. doi: 10.1152/ajprenal.00201.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breljak D, Ljubojević M, Hagos Y, Micek V, Balen Eror D, Vrhovac Madunić I, Brzica H, Karaica D, Radović N, Kraus O, Anzai N, Koepsell H, Burckhardt G, Burckhardt BC, Sabolić I. Distribution of organic anion transporters NaDC3 and OAT1-3 along the human nephron. Am J Physiol Renal Physiol 311: F227–F238, 2016. doi: 10.1152/ajprenal.00113.2016. [DOI] [PubMed] [Google Scholar]
- 3.Burckhardt BC, Burckhardt G. Transport of organic anions across the basolateral membrane of proximal tubule cells. Rev Physiol Biochem Pharmacol 146: 95–158, 2003. doi: 10.1007/s10254-002-0003-8. [DOI] [PubMed] [Google Scholar]
- 4.Burckhardt G. Drug transport by organic anion transporters (OATs). Pharmacol Ther 136: 106–130, 2012. doi: 10.1016/j.pharmthera.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Emami Riedmaier A, Nies AT, Schaeffeler E, Schwab M. Organic anion transporters and their implications in pharmacotherapy. Pharmacol Rev 64: 421–449, 2012. doi: 10.1124/pr.111.004614. [DOI] [PubMed] [Google Scholar]
- 6.Eraly SA, Vallon V, Vaughn DA, Gangoiti JA, Richter K, Nagle M, Monte JC, Rieg T, Truong DM, Long JM, Barshop BA, Kaler G, Nigam SK. Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in OAT1 knock-out mice. J Biol Chem 281: 5072–5083, 2006. doi: 10.1074/jbc.M508050200. [DOI] [PubMed] [Google Scholar]
- 7.Ghezzi C, Hirayama BA, Gorraitz E, Loo DD, Liang Y, Wright EM. SGLT2 inhibitors act from the extracellular surface of the cell membrane. Physiol Rep 2: e12058, 2014. doi: 10.14814/phy2.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grempler R, Thomas L, Eckhardt M, Himmelsbach F, Sauer A, Sharp DE, Bakker RA, Mark M, Klein T, Eickelmann P. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab 14: 83–90, 2012. doi: 10.1111/j.1463-1326.2011.01517.x. [DOI] [PubMed] [Google Scholar]
- 9.Kato Y, Kuge K, Kusuhara H, Meier PJ, Sugiyama Y. Gender difference in the urinary excretion of organic anions in rats. J Pharmacol Exp Ther 302: 483–489, 2002. doi: 10.1124/jpet.102.033878. [DOI] [PubMed] [Google Scholar]
- 10.Macha S, Koenen R, Sennewald R, Schöne K, Hummel N, Riedmaier S, Woerle HJ, Salsali A, Broedl UC. Effect of gemfibrozil, rifampicin, or probenecid on the pharmacokinetics of the SGLT2 inhibitor empagliflozin in healthy volunteers. Clin Ther 36: 280–90.e1, 2014. doi: 10.1016/j.clinthera.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Nigam SK. What do drug transporters really do? Nat Rev Drug Discov 14: 29–44, 2015. doi: 10.1038/nrd4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nigam SK, Bush KT, Martovetsky G, Ahn SY, Liu HC, Richard E, Bhatnagar V, Wu W. The organic anion transporter (OAT) family: a systems biology perspective. Physiol Rev 95: 83–123, 2015. doi: 10.1152/physrev.00025.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nigam SK, Wu W, Bush KT, Hoenig MP, Blantz RC, Bhatnagar V. Handling of drugs, metabolites, and uremic toxins by kidney proximal tubule drug transporters. Clin J Am Soc Nephrol 10: 2039–2049, 2015. doi: 10.2215/CJN.02440314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rieg T, Masuda T, Gerasimova M, Mayoux E, Platt K, Powell DR, Thomson SC, Koepsell H, Vallon V. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol 306: F188–F193, 2014. doi: 10.1152/ajprenal.00518.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabolic I, Vrhovac I, Eror DB, Gerasimova M, Rose M, Breljak D, Ljubojevic M, Brzica H, Sebastiani A, Thal SC, Sauvant C, Kipp H, Vallon V, Koepsell H. Expression of Na+-d-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am J Physiol Cell Physiol 302: C1174–C1188, 2012. doi: 10.1152/ajpcell.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sweet DH, Miller DS, Pritchard JB, Fujiwara Y, Beier DR, Nigam SK. Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat3 (Slc22a8)) knockout mice. J Biol Chem 277: 26934–26943, 2002. doi: 10.1074/jbc.M203803200. [DOI] [PubMed] [Google Scholar]
- 17.Taub ME, Ludwig-Schwellinger E, Ishiguro N, Kishimoto W, Yu H, Wagner K, Tweedie D. Sex-, species-, and tissue-specific metabolism of empagliflozin in male mouse kidney forms an unstable hemiacetal metabolite (M466/2) that degrades to 4-hydroxycrotonaldehyde, a reactive and cytotoxic species. Chem Res Toxicol 28: 103–115, 2015. doi: 10.1021/tx500380t. [DOI] [PubMed] [Google Scholar]
- 18.Vallon V, Eraly SA, Rao SR, Gerasimova M, Rose M, Nagle M, Anzai N, Smith T, Sharma K, Nigam SK, Rieg T. A role for the organic anion transporter OAT3 in renal creatinine secretion in mice. Am J Physiol Renal Physiol 302: F1293–F1299, 2012. doi: 10.1152/ajprenal.00013.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 22: 104–112, 2011. doi: 10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia 60: 215–225, 2017. doi: 10.1007/s00125-016-4157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vrhovac I, Balen Eror D, Klessen D, Burger C, Breljak D, Kraus O, Radović N, Jadrijević S, Aleksic I, Walles T, Sauvant C, Sabolić I, Koepsell H. Localizations of Na(+)-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch 467: 1881–1898, 2015. doi: 10.1007/s00424-014-1619-7. [DOI] [PubMed] [Google Scholar]