Abstract
Labile heme, as opposed to heme that is tightly bound within proteins, is thought to require a chaperone to be trafficked within the cell due to its cytotoxicity, but the identity of this chaperone was not known. A new study reveals that an unlikely protein, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), is a heme chaperone that binds and transfers labile heme to downstream target proteins. These results provide a new framework for understanding heme homeostasis and raise intriguing questions regarding the intersection of heme transport, carbohydrate metabolism, and intracellular signaling.
Introduction
Heme is one of the most important biological cofactors. Much of the cellular heme is tightly sequestered in proteins that function to bind and transport oxygen, catalyze oxygen-dependent reactions, and perform electron transfer. Cells also contain a pool of exchange-labile heme (LH),2 which includes proteins that bind heme with lower affinity at sites such as heme regulatory motifs (1), which play a role in cellular signaling. It has been long proposed that cells also contain a heme chaperone that can interface with the LH pool and transfer heme to its target proteins. However, such a protein has never been identified. In this issue, Sweeny et al. (2) describe experiments performed in vitro and in mammalian cells and yeast demonstrating that the glycolytic enzyme GAPDH moonlights in this role, shuttling heme from its site of synthesis to target proteins.
Cellular heme can be acquired from exogenous sources or newly synthesized, with the final synthetic step occurring in the mitochondria prior to transport to the cytosol (Fig. 1). There is growing interest in studying the resultant LH pool to gain a more complete understanding of how heme levels are set and maintained. Recent results have suggested that the LH pool responds to overall cellular heme levels, as different compartments maintain different concentrations of LH to reflect the binding affinities of hemeproteins (∼20–40 nm in the cytosol and below 2.5 nm in the nucleus in Saccharomyces cerevisiae, measured using a ratiometric fluorescent heme sensor) (3). Furthermore, the LH pool is dynamic: Nitric oxide (NO) rapidly mobilizes heme within the LH pool (3). However, one of the most basic questions about this pool is how the toxicity of free heme is controlled. Heme needs to be sequestered and escorted by protein chaperones as it travels from its source to its final destination in a tightly controlled manner. Several proteins have been predicted to play such a role based on their heme-binding affinity (4), including GAPDH (3, 5); however, none of these had been directly shown to bind heme or to be involved in delivering heme to its downstream protein targets.
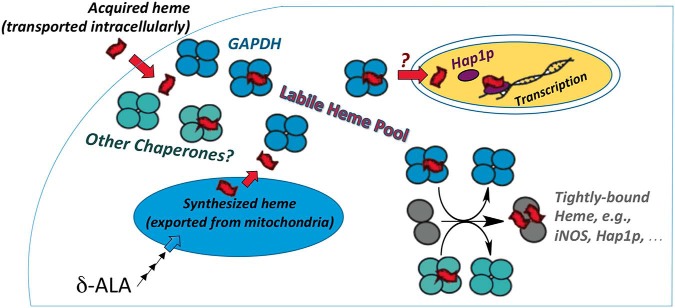
Figure 1.
Model of GAPDH as a heme chaperone that sequesters and transfers bioavailable heme to target hemeproteins. Heme can be synthesized endogenously from δ-ALA (aminolevulinic acid) or imported from exogenous sources through the plasma membrane. Sweeny et al. (2) demonstrate that GAPDH can bind both exogenous and endogenously generated heme and make heme bioavailable to target proteins. They also show that GAPDH-bound heme can be delivered to soluble proteins such as iNOS or to nuclear proteins such as the transcription factor Hap1p. There are likely heme chaperones other than GAPDH to interact with the many cellular target proteins.
GAPDH is best known for its function within glycolysis, converting glyceraldehyde 3-phosphate to 1,3-bisphospho-d-glycerate, and thus a role in energy metabolism and in the production of ATP and pyruvate. As a result, GAPDH is a “most unlikely protein,” as termed by Sweeny et al. (2), to be involved in heme trafficking. However, various nonglycolytic functions have been assigned to GAPDH, and studies have suggested that the GAPDH subcellular location is dynamic, which seems unnecessary for GAPDH's function in glycolysis (6). Stuehr's group began tracking a potential heme-trafficking role for GAPDH around 2010 when they knocked down GAPDH in mammalian cells and found reduced heme content and activity of heme-dependent inducible nitric oxide synthase (iNOS) (5). Then, Hamza and Reddi and colleagues (3) found that knocking down the yeast homologue of GAPDH, TDH3, led to increased LH, yet reduced transcriptional activity of the nuclear, heme-dependent transcription factor Hap1p. The question that remained then is whether GAPDH directly binds and chaperones heme to target proteins or assists in heme trafficking in a more passive way, for example, by limiting the cytotoxicity of heme as proposed by another group (7).
To address these questions, Sweeny et al. (2) started with the premise that a histidine residue should be involved in heme binding; they then used computer modeling, site-directed mutagenesis, and binding experiments to identify the GAPDH heme-binding site, including His-53 as an axial ligand. Addition of a 14C-labeled heme precursor to promote intracellular synthesis of 14C-heme coupled with a tagged form of WT GAPDH and a H53A variant that binds heme 10-fold less tightly confirmed that the affinity-purified GAPDH contained labeled heme, whereas the His-53 variant did not. Thus, based on these and other experiments, it was clear that GAPDH has a specific binding site for heme.
The authors next demonstrated that GAPDH directly chaperones heme delivery to two target proteins, cytosolic murine iNOS and nuclear yeast Hap1, using 55Fe-heme to determine heme incorporation and a ratiometric fluorescent heme sensor (3) to measure LH levels. 55Fe-heme was incorporated into iNOS only when induced in heme-depleted mouse macrophages containing WT GAPDH, but not its H53A variant. Further, the heme-dependent activity of iNOS was significantly suppressed when expressed in cells containing the H53A variant as compared with WT GAPDH. As for Hap1, its heme-dependent activity decreased by 50% even as intracellular LH concentrations increased by 50% upon deletion of TDH3 (the yeast homologue of GAPDH) in yeast strains. Both Hap1 activity and LH concentrations returned to normal levels when the TDH3 deletion strain was complemented with TDH3 or human GAPDH, but not with the His-to-Ala variants of either protein.
Sweeny et al. (2) make a convincing argument for GAPDH as a heme chaperone that plays a critical role in heme trafficking, increasing the bioavailability of heme to its protein targets as a part of a dynamic network. Of course, this finding raises multiple new questions about how the system works. For example, does heme binding by GAPDH also protect the cell from cytotoxicity, as previously proposed (7)? Does GAPDH play a role in the dynamic modulation of LH caused by NO? Is there any functional interplay between GAPDH's known glycolytic role and this chaperone activity? What other proteins are involved as targets and chaperones? Given that intracellular LH increased by ∼50% upon deletion of TDH3 in yeast, GAPDH appears to sequester a significant amount of LH. Thus, many other unidentified proteins must obtain heme from GAPDH. Sweeny et al. mention soluble guanylate cyclase, hemoglobin, cytochrome P450, and catalase as potential GAPDH target proteins. Similarly, there are likely other chaperones for LH, highlighting the possibility that other unlikely proteins can “moonlight” in this role, and possibly chaperones of chaperones, as heat shock protein 90 may play a supporting role (8, 9). Finally, how does GAPDH recognize and transfer heme to target proteins? These answers and more will further our understanding of heme trafficking.
This work was supported by NIGMS, National Institutes of Health Grant GM R01–123513 (to S. W. R.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- LH
- labile heme
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- iNOS
- inducible nitric oxide synthase
- δ-ALA
- δ-5-aminolevulinic acid.
References
- 1. Fleischhacker A. S., Carter E. L., and Ragsdale S. W. (2018) Redox regulation of heme oxygenase-2 and the transcription factor, Rev-Erb, through heme regulatory motifs. Antioxid. Redox Signal. 10.1089/ars.2017.7368 10.1089/ars.2017.7368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sweeny E. A., Singh A. B., Chakravarti R., Martinez-Guzman O., Saini A., Haque M. M., Garee G., Dans P. D., Hannibal L., Reddi A. R., and Stuehr D. J. (2018) Glyceraldehyde-3-phosphate dehydrogenase is a chaperone that allocates labile heme in cells. J. Biol. Chem. 293, 14557–14568 10.1074/jbc.RA118.004169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanna D. A., Harvey R. M., Martinez-Guzman O., Yuan X., Chandrasekharan B., Raju G., Outten F. W., Hamza I., and Reddi A. R. (2016) Heme dynamics and trafficking factors revealed by genetically encoded fluorescent heme sensors. Proc. Natl. Acad. Sci. U.S.A. 113, 7539–7544 10.1073/pnas.1523802113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reddi A. R., and Hamza I. (2016) Heme mobilization in animals: A metallolipid's journey. Acc. Chem. Res. 49, 1104–1110 10.1021/acs.accounts.5b00553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chakravarti R., Aulak K. S., Fox P. L., and Stuehr D. J. (2010) GAPDH regulates cellular heme insertion into inducible nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 107, 18004–18009 10.1073/pnas.1008133107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sirover M. A. (2011) On the functional diversity of glyceraldehyde-3-phosphate dehydrogenase: Biochemical mechanisms and regulatory control. Biochim. Biophys. Acta 1810, 741–751 10.1016/j.bbagen.2011.05.010 [DOI] [PubMed] [Google Scholar]
- 7. Huang Y., Zhang P. F., Yang Z., Wang P. P., Li H., and Gao Z. (2017) Interaction of glyceraldehyde-3-phosphate dehydrogenase and heme: The relevance of its biological function. Arch. Biochem. Biophys. 619, 54–61 10.1016/j.abb.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 8. Ghosh A., Stasch J. P., Papapetropoulos A., and Stuehr D. (2014) Nitric oxide and heat shock protein 90 activate soluble guanylate cyclase by driving rapid change in its subunit interactions and heme content. J. Biol. Chem. 289, 15259–15271 10.1074/jbc.M114.559393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghosh A., Garee G., Sweeny E. A., Nakamura Y., and Stuehr D. J. (2018) Hsp90 chaperones hemoglobin maturation in erythroid and nonerythroid cells. Proc. Natl. Acad. Sci. U.S.A. 115, E1117–E1126 10.1073/pnas.1717993115 [DOI] [PMC free article] [PubMed] [Google Scholar]