Abstract
Signaling proteins, including bone morphogenetic proteins (BMPs), specifically interact with heparan sulfate (HS). These interactions regulate protein distribution and function and are largely mediated by domains rich in basic amino acids. The N-terminal region of BMP2 and BMP4 contains one such domain with a typical Cardin-Weintraub (CW) motif, but it is unclear whether the same occurs in BMP5, BMP6, and BMP7 that constitute a separate evolutionary subgroup. Peptides spanning the N-terminal domain of BMP2/4 interacted with substrate-bound HS with nanomolar affinity, but peptides spanning BMP5/6/7 N-terminal domain did not. We re-examined the entire BMP5/6/7 sequences and identified a novel CW-like motif at their C terminus. Peptides spanning this domain displayed high-affinity HS binding, but corresponding BMP2/4 C-terminal peptides did not, likely because of acidic or noncharged residue substitutions. Peptides pre-assembled into NeutrAvidin tetramers displayed the same exact binding selectivity of respective monomers but bound HS with greater affinity. Tests of possible peptide biological activities showed that the HS-binding N-terminal BMP2/4 and C-terminal BMP5/6/7 peptides stimulated chondrogenesis in vitro, potentially by freeing endogenous BMPs. Thus, HS interactions appear largely ascribable to domains at opposite ends of BMP2/4 versus BMP5/6/7, reiterating the evolutionary distance of these BMP subgroups and possible functional diversification.
Keywords: bone morphogenetic protein (BMP), heparan sulfate, cartilage biology, cell signaling, heparin-binding protein, chondrogenesis, Cardein-Weintraub motif, heparin-binding domain, hyaluronic acid
Introduction
The bone morphogenetic proteins (BMPs)2 are members of the transforming growth factor-β (TGF-β) superfamily and comprise an evolutionary diverse group of about 15 signaling proteins (1–3). The BMPs are initially synthesized as large precursor proteins while entering the secretory pathway, undergo proteolytic processing and glycosylation, and are secreted as active dimers (4–6). Dimerization is needed for signaling function and is stabilized by seven highly conserved Cys residues that interact to form cysteine-knot motifs (6–9). Active BMPs interact with type I and type II cell-surface receptors that have serine/threonine kinase activity and assemble into tetrameric signaling complexes (10, 11). The type II receptors are constitutively active, whereas the type I receptors contain a Gly/Ser-rich domain that is phosphorylated by a type II receptor within the complex to activate kinase activity. These steps lead to recruitment of downstream canonical signaling effectors referred to as receptor-activated SMADs (SMAD1/5/8) that interact with SMAD4, translocate to the nucleus, and modulate expression of target genes (10, 11). These basic processes and steps are shared by all BMPs, but the proteins exert diverse functions and affect a large number of distinct developmental, homeostatic, and pathological processes (3, 12, 13). Such a diversity of roles and action is largely ascribable to the distinct binding affinities by the BMPs for various combinations of type I and type II receptors and to the patterns of ligands, receptors, and endogenous inhibitors expressed in distinct biological contexts and tissues and at different developmental and growth stages (14–16).
A long-known but still intriguing feature of BMPs is that they interact with the heparan sulfate (HS) chains of cell-surface and matrix-bound proteoglycans (17–19), a macromolecular family that includes syndecans, glypicans, and perlecan (20). The ability to interact with HS is actually shared by several other growth factors and signaling proteins, including hedgehogs and fibroblast growth factors (FGFs) (21, 22), and is assignable to the presence of HS-binding domains present in these proteins (17). The interactions with HS are thought to be important in regulating protein distribution, turnover, diffusion, and availability and in turn interactions with and signaling by cognate receptors (19, 23). Cardin and Weintraub (24) were among the very first to carry out detailed studies to identify and characterize protein domain(s) responsible for interactions with HS. Comparative analyses on vitronectin, platelet factor-4, apoliprotein A, and apoliprotein B led to the identification of two HS-binding motifs, XBBXBX and XBBBXXBX, where B represents a basic residue (Arg or Lys), and X represents a noncharged residue. Subsequent studies on many other proteins have verified and greatly extended those original findings (25–27). The HS-binding domains in BMP2 and BMP4 have been characterized in previous studies (18, 28). The domains reside immediately upstream of the first conserved cysteine and are thus near the N terminus of the mature protein, and their sequences in human BMP2 and BMP4 are QAKHKQRKRLKSSC and SPKHHSQRARKKKNKNC, respectively, with the first cysteine of the knot serving as a reference point (29). These distinct sequences are highly conserved (Tables S1 and S3), and experimental mutations of their basic residues were shown to alter HS binding and biological function (18, 30). Notably, these sequences reiterate a fundamental and yet largely unexplained feature of HS-binding domains characterized in these and many other proteins, namely that the amino acid sequence and organization of each domain vary greatly from protein to protein (25).
In an attempt to address this and related puzzles, we focused here on BMP5, BMP6, and BMP7 and did so for several reasons. First, their HS-binding domains have not been well defined compared with those of BMP2 and BMP4 (31–33). Based on sequence homologies, the three proteins are classified as a separate evolutionary subgroup distinct from the BMP2/BMP4 subgroup within the TGF-β superfamily (34). Of relevance to our own field of research, the three BMPs have been found to have roles in skeletal development and growth (35–37) different from those of BMP2 and BMP4 (38, 39). Intriguingly, their N-terminal regions from the first conserved Cys are much longer than those of BMP2 and BMP4, and an initial perusal of possible HS-binding domains within that region revealed some unexpected anomalies as detailed below. Those initial insights were systematically explored in this study, and the results presented here reveal that the domain with highest HS-binding affinity is actually located at the C-terminal portion of mature BMP5, -6, and -7. We also present evidence that synthetic peptides corresponding to HS-binding domains have biological activity in chondrogenic and cell-signaling assays in vitro.
Results
N-terminal regions have distinct sequences and binding properties
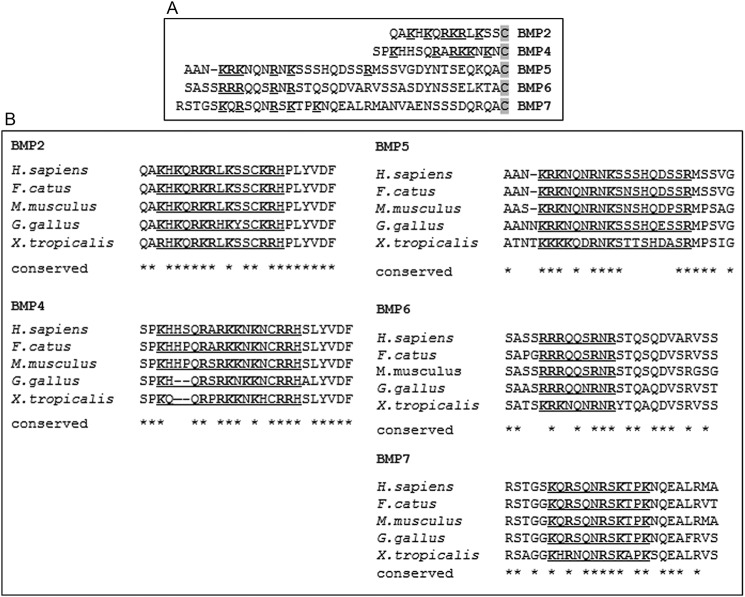
As indicated above, the N-terminal regions of mature BMP5, BMP6, and BMP7 upstream of the cysteine knot are much longer than those in BMP2 and BMP4 and are currently thought to contain a major HS-binding domain (31–33). To ask what may lie behind such length difference, we aligned the N-terminal regions from each BMP protein and compared the putative HS-binding domains within them, using the first conserved cysteine as a reference mark (Fig. 1A). As expected, the HS-binding domains of BMP2 and BMP4 exhibited typical Cardin-Weintraub (CW) motifs with XBBXBX and XBBBXBX arrangements, respectively (Fig. 1A), and their amino acid sequences are highly conserved from Xenopus to humans (Fig. 1B and Fig. S2). In comparison, the putative HS-binding domains in BMP5, BMP6, and BMP7 were not only further upstream of the first cysteine (Fig. 1A) but also had unusual sequence features. The domains in BMP5 and BMP6 consisted of three basic residues separated from the next single basic residue by three noncharged amino acids, and the domain in BMP7 lacked a doublet or triplet of basic residues altogether (Fig. 1A). These considerations raised the question whether these domains in BMP5, BMP6, and BMP7 were actually able to interact with HS. To investigate this, we synthesized 20–25-amino acid–long peptides spanning the predicted HS-binding domain of each of the five BMPs (Fig. 2A), and we tested them in solid-phase binding assays with immobilized HS. The BMP2- and BMP4-derived peptides readily bound to HS and exhibited saturable binding curves, yielding calculated Kd values of about 100 nm (Fig. 3A). However, the peptides derived from BMP5, BMP6, and BMP7 did not bind appreciably (Fig. 3A), and the same outcome was observed when the microwell plates were coated with heparin instead of HS (data not shown).
Figure 1.
Sequence of N-terminal region is distinct in different BMPs. A, amino acid alignment of the entire N-terminal region of BMP2, BMP4, BMP5, BMP6, and BMP7 using the first conserved cysteine in the cysteine knot as a reference point (shaded in gray). The basic amino acids Arg and Lys are boldface and underlined. Note that the predicted HS-binding domain in BMP2 and BMP4 lies directly upstream of the first cysteine, whereas the predicted site in BMP5, BMP6, and BMP7 is further upstream. B, evolutionary N-terminal amino acid sequence alignment of the five BMPs from Xenopus tropicalis to Homo sapiens. All five proteins are highly conserved. Predicted HS-binding domains are boldface and underlined.
Figure 2.
Synthetic peptides from N- and C-terminal regions. A, amino acid sequences of the synthesized N-terminal peptides that span the predicted HS-binding domains of BMP2 to BMP7. Peptides were N-terminally linked to a biotin molecule via a triple glycine linker. Kd values (in nanomolar) and pI values of synthetic peptides are shown, as well as the accession numbers for the full-length proteins from which the respective peptides were derived. B, amino acid sequences of the synthesized C-terminal peptides spanning putative HS-binding domains of BMP4, BMP5, and BMP6/7. Note that the C-terminal regions of BMP6 and BMP7 are completely identical. C, schematic of a representative BMP protein. The encoded and newly synthesized protein consists of a signal peptide (SP, green), a prodomain (yellow), and the mature biologically-active ligand (blue). A furin cleavage site separates the prodomain from the mature ligand, which contains seven conserved cysteines forming three intramolecular disulfide bonds and one intermolecular disulfide bond.
Figure 3.
N-terminal peptides have diverse HS-binding properties compared with full-length proteins. A, N-terminal peptides from BMP2, BMP4, BMP5, BMP6, and BMP7 (designated as 2N, 4N, 5N, 6N, and 7N, respectively) were each incubated on plates coated with immobilized HS. Binding was measured using NA-HRP at an absorbance of 450 nm. Note that the N-terminal peptides from BMP2 and BMP4 interacted with HS with saturation kinetics and high affinity (Kd ∼100 nm), whereas those from BMP5, BMP6, and BMP7 did not. B, binding assays for rhBMP2, rhBMP4, and rhBMP5 to HS-coated plates. Bound proteins were detected using their respective antibodies conjugated to HRP. Note that BMP2 and BMP5 displayed saturable binding in the conditions used, whereas BMP4 binding exhibited slower kinetics. Inset shows a double-reciprocal plot for rhBMP2 and rhBMP5 with calculated Kd values of 37 and 16 nm, respectively. C, binding assays for rhBMP6 and rhBMP7. Because specific antibodies for these BMPs could not be obtained, the proteins were biotinylated using EZ-link Sulfo-NHS-LC-Biotin (ThermoFisher Scientific) prior to binding assays, and the bound proteins were then detected using NA-HRP. Inset shows a double-reciprocal plot with calculated Kd values of 56 and 40 nm for the two BMPs, respectively. Note that NA-HRP by itself elicited no signal. Each binding curve is representative of a minimum of five independent experiments.
It is possible that when free in solution, the BMP5/6/7 peptides might have acquired abnormal conformations that precluded or inhibited their natural interactions with HS. To address this question, the peptides from the five proteins were preassembled and tethered into tetrameric complexes by incubation with NeutrAvidin-horseradish peroxidase (NA-HRP) (Fig. 4A) and tested in solid-phase HS-binding assays (Fig. 4B). Interestingly, the tetrameric peptide complexes from BMP2 or BMP4 not only bound to HS but did so with higher affinity (Kd ∼6 nm) (Fig. 4B) than their respective monomeric peptides (Fig. 3A); their binding was fully prevented by addition of soluble heparin (Fig. 4B). However, the tetrameric peptide complexes from BMP5, BMP6, or BMP7 were still unable to appreciably bind to HS (Fig. 4C). As expected, full-length recombinant human (rh) BMP2 and -4–7 readily interacted with immobilized HS and did so with comparable affinities (Fig. 3, B and C).
Figure 4.
N-terminal tetrameric complexes display differential binding properties. A, architecture of a tetrameric complex assembled with NA-HRP and biotinylated peptides. Each NA-HRP molecule interacts with four peptide monomers to form a tetrameric binding complex. B, solid-phase binding assays of N-terminal peptide tetrameric complexes from BMP2 and BMP4 (designated 2N and 4N, respectively) to immobilized HS. Note that both peptide complexes bind to substrate-bound HS with saturable kinetics and are fully competed by soluble heparin (H). NA-HRP by itself elicited no signal. C, binding assays of N-terminal peptide tetrameric complexes from BMP5, BMP6, and BMP7 (designated 5N, 6N, and 7N) to substrate-bound HS. Note that all three complexes exhibit very poor binding. Each binding curve is representative of a minimum of five independent experiments.
C-terminal region of BMP5/6/7 contains a CW motif
The ability of full-length rhBMP5, rhBMP6, and rhBMP7 to bind to immobilized HS in contrast to the very poor binding by monomeric or tetrameric peptides from their N-terminal regions led us to consider whether the main HS-binding domain in these proteins may reside elsewhere. Thus, we aligned the entire amino acid sequences of these BMPs (Fig. S2) and searched for possible additional CW-like motifs. Indeed, we noted that their C-terminal region did contain one such motif with an XBBXBX configuration (Fig. 5A). Intriguingly, in the corresponding C-terminal region in BMP2 and BMP4, the motif was different and contained noncharged residues in place of Lys and Arg (Fig. 5A), thus likely minimizing its potential ability to interact with HS. To test these predictions, we prepared monomeric and tetrameric peptides spanning the C-terminal region of BMP4–7 (Fig. 2B) and tested them for HS binding in solid-phase assays. We chose the C-terminal peptide from BMP4 as a representative for the BMP2/4 subfamily, as their sequences are nearly identical. In line with our reasoning above, the peptides from BMP5–7 did in fact bind to HS with high affinity (Kd ∼9 nm) and were competed out by soluble heparin (Fig. 5, B and C), whereas the peptide from BMP4 bound poorly (Fig. 5B).
Figure 5.

C-terminal region of BMP5, BMP6, and BMP7 has high HS-binding affinity A, amino acid alignment of the C-terminal region of BMP2 and BMP4 versus BMP5, BMP6, and BMP7. Basic residues are boldface and underlined. Note that the region in BMP5, BMP6, and BMP7 contains a XBBXBX sequence that fully matches a typical CW motif, whereas the corresponding region in BMP2 and BMP4 contains nonconservative substitutions with Asn and Gln replacing a Lys and an Arg. In addition, note that the BMP2 and BMP4 regions also contain two negatively charged acidic residues (Glu and Asp) that would likely interfere with HS binding. B, solid-phase binding assays of synthetic C-terminal peptides from BMP4, BMP5, and BMP6/7 (designated 4C, 5C, and 6/7C) to substrate-bound HS. The C-terminal sequences of BMP6 and BMP7 are identical. Note that although the BMP4 peptide failed to bind HS, the BMP5 and BMP6/7 peptides did bind. C, solid-phase binding assays of tetrameric C-terminal peptide complexes from BMP4, BMP5, and BMP6/7 to HS. The BMP5 and BMP6/7 complexes bind to HS with saturable kinetics and were competed out by soluble heparin (H), whereas the tetrameric BMP4 peptide still failed to bind. Inset shows a double-reciprocal plot for BMP5 and BMP6/7 peptide tetramers. Each binding curve is representative of a minimum of five independent experiments.
N- and C-terminal domains display different configurations
The differential ability of N- or C-terminal BMP domains to interact with HS raised the question whether there may be differences in their 3D configuration and spatial arrangement, possibly providing further insights into the basis of protein–HS interactions. Thus, we utilized the I-TASSER server at the University of Michigan that allows for protein structural and functional predictions (40). Focusing on BMP2, BMP4, and BMP5 as representatives of the two BMP subgroups above, we found that their N-terminal domains displayed a helical structure, more prominent in BMP2 and BMP4 than BMP5 (Fig. 6, A–C). We subjected the domains to helical wheel projection analysis. Quite interestingly, the basic residues in BMP2 and BMP4 aligned to form a large cationic cluster along one face of the helix (Fig. 6, D and E), whereas the basic residues in BMP5 were separated by noncharged amino acids seemingly preventing the assembly of a large cationic surface (Fig. 6F). The latter provides an additional explanation for poor HS binding of the BMP5 N-terminal peptide (see Fig. 3A).
Figure 6.
N-terminal region of BMP2 and BMP4 displays a continuous electropositive surface. A–C, I-TASSER–based models of the N-terminal regions of BMP2, BMP4, and BMP5, spanning the predicted HS-binding motifs and designated as BMP2 N, BMP4 N, and BMP5 N. The N-terminal amino acid is designated by a blue dot, and the C-terminal amino acid is designated by a red dot; the backbone is in black; and Lys and Arg residues are in purple and cyan, respectively. Note that all the regions display some degree of helical structure. D–F, helical wheel diagrams of the regions shown in A–C. The wheel diagrams for BMP2 and BMP4 reveal continuous positive charge on the surface of the helix (D and E), and the BMP5 diagram presents with an unorganized and discontinuous arrangement of positive charge (F). G–I, I-TASSER–based models of the C-terminal regions of BMP2, BMP4, and BMP5 spanning the putative HS-binding motifs and designated as BMP2 C, BMP4 C, and BMP5 C. Symbols are as in A–C, but note that the region in BMP2 and BMP4 contains negatively charged residues (Asp in orange and Glu in yellow) that are inconsistent with a typical HS-binding domain and that are absent in the BMP5 region.
We carried out a similar I-TASSER analysis of the C-terminal domains of BMP2, BMP4, and BMP5, but they turned out to have largely unstructured configurations with no obvious pattern (Fig. 6, G–I). This indicates that the strong HS-binding properties of the C-terminal domain in BMP5 (and by extension BMP6 and BMP7) are mainly due to its specific amino acid sequence and spacing.
Peptides have differential cell surface–binding abilities
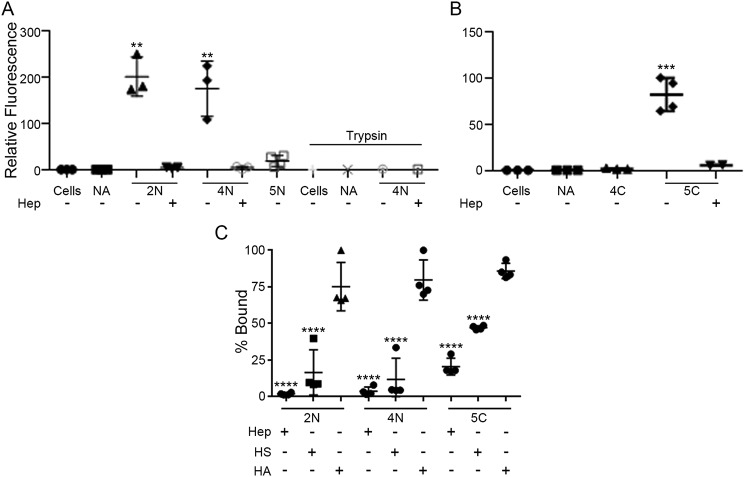
Next, we asked whether the differential ability of N- and C-terminal peptides from the five BMPs to interact with immobilized HS was also displayed in their binding to the cell surface, thus providing insights into the interactive behaviors of mature BMPs. To address this question, we carried out FACS analyses using K562 cells as a convenient in vitro model system. Cells were briefly fixed and incubated for 2 h on ice with N- or C-terminal peptides from BMP2, BMP4, BMP5, BMP6, or BMP7 that had been pre-assembled into tetramers by incubation with fluorescent NA-488. After incubation, the cells were washed, and FACS was used to assess the levels of bound peptides. The N-terminal peptides from BMP2 and BMP4 prominently bound to the cells, whereas those derived from BMP5 did not (Fig. 7A). BMP2/4 peptide binding was inhibited by addition of soluble heparin or pretreatment of the cells with trypsin (Fig. 7A). Virtually opposite results were obtained with the C-terminal peptides. The BMP5 peptide readily bound to the cells, whereas the BMP4 peptide did not (Fig. 7B); binding was prevented by soluble heparin.
Figure 7.
Peptides are able to interact with the cell surface. A, fluorescent N-terminal peptide tetramers from BMP2, BMP4, and BMP5 (designated 2N, 4N, and 5N, respectively) were allowed to interact with K562 cells in vitro, and binding was assessed by flow cytometry. Note that the 2N and 4N peptides vigorously interacted with the cell surface and were competed out by soluble heparin (Hep). However, the peptide from BMP5 produced minimal if any binding. The fluorescent NA backbone produced no signal on its own as did the cells (A, far left). As an additional control for binding specificity, cells were trypsinized prior to incubation with peptides, and this treatment fully prevented binding. B, fluorescent C-terminal peptide tetramers from BMP4 and BMP5 (designated 4C and 5C, respectively) were allowed to interact with K562 cells in vitro, and binding levels were assessed as above. Note that the 5C peptide did bind to the cell surface and was competed out by soluble heparin, but the 4C peptide did not bind. C, K562 cells were incubated with tetrameric complexes containing BMP2N, BMP4N, and BMP5C peptides in the absence or presence of soluble heparin (Hep), heparan sulfate (HS), or hyaluronic acid (HA) as competitors. Following incubation, the cells were washed, and the amount of bound peptide was assessed by FACS. **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
To further evaluate peptide-binding specificity to the cell surface, the tetrameric peptide complexes were preincubated with soluble heparin, HS, or hyaluronic acid (HA), a nonsulfated glycosaminoglycan, and were then incubated with K562 cells for 2 h as above. Cells were washed, and levels of peptide binding were assessed by FACS. As shown in Fig. 7C, peptide binding was prevented by heparin and HS, albeit to a slightly lesser extent. In contrast, HA was not able to prevent binding in a significant manner. The data indicate that binding of the BMP2N, BMP4N, and BMP5C peptides to the cell surface was specific and competed out by soluble heparin and HS.
Peptides can stimulate chondrogenic cell differentiation
A previous study indicated that a peptide spanning the N-terminal HS-binding domain of BMP4 (residues 15–24 within the mature protein) had biological activity on its own and stimulated osteogenic cell differentiation in cultures of human mesenchymal stem cells (28). To investigate the potential biological activity of the BMP peptides characterized in our study, we determined their effects on chondrogenic differentiation, using micromass cultures of mouse embryo limb bud mesenchymal cells (41). Indeed, treatment with N-terminal tetrameric peptide complexes from BMP2 or BMP4 or C-terminal peptide complex from BMP5 stimulated chondrogenesis appreciably (Fig. 8, D–F). As expected, full-length rhBMP2 stimulated chondrogenesis as well (Fig. 8C). This stimulation was evident by Alcian blue staining of cartilage nodules (Fig. 8, D–F) and computer-assisted image quantification of the nodules (Fig. 8G) compared with untreated control cultures (Fig. 8, A and G) or cultures treated with empty NA backbone (Fig. 8, B and G). To verify these data, we carried out quantitative PCR analyses of chondrogenic genes (42) and found that expression of Sox9, collagen 2, and aggrecan was stimulated in peptide-treated cultures compared with controls (Fig. 8, H and J). The peptides were also able to stimulate the expression of Id1, a direct target of canonical BMP signaling (Fig. 8K) (43).
Figure 8.
HS-binding peptides stimulate chondrogenesis. A–F, day 3 mouse embryo limb bud cell micromass cultures stained with Alcian blue on day 3 following treatment with the following: vehicle control (A); NA backbone (B); rhBMP2 (C); N-terminal BMP2 peptide tetramer designated 2N (D); N-terminal BMP4 peptide tetramer designated 4N (E); and C-terminal BMP5 peptide tetramer designated 5C (F). Note that the peptides stimulated chondrogenesis as indicated by an increase in Alcian blue-positive nodules (D–F, compared with controls, A and B). G, scatter plots of levels of Alcian blue staining in A–F quantified by ImageJ. Data confirm that treatment with peptides 2N, 4N, or 5C or with rhBMP2 increased chondrogenesis over control levels (Con and NA). H–J, scatter plots of expression levels of Sox9, Col2, and aggrecan in day 3 limb bud cell cultures treated with peptide tetramers or left untreated (Con). Note that the 2N, 4N, and 5C peptides significantly increased expression of Sox9 and Col2 (H and I), whereas the strongest stimulation of aggrecan expression occurred with the 4N peptide (J). Data are averages of three independent experiments. K, scatter plots of ID1 expression in AD293 cells after treatment with vehicle (Con), NA backbone (NA), or tetrameric 2N, 4N, or 5C peptides. Each peptide stimulated ID1 expression with respect to controls. Data are averages of five independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Peptides exhibit binding-competition ability toward their respective proteins
The stimulatory effects of the peptides on chondrogenesis could be due to a variety of mechanisms. One possibility is that the peptides competed with binding of endogenous BMPs to cell-surface HS and rendered the proteins available for further biological action. To gain insights into this possibility, we carried out solid-phase competition assays using peptides and full-length rhBMP2 and rhBMP5. HS-coated microplates were incubated with rhBMP2 (Fig. 9A) or rhBMP5 (Fig. 9B) in the presence of increasing amounts of N-terminal BMP2, BMP4, or BMP5 peptide or C-terminal BMP5 peptide. Binding of rhBMP2 to HS was readily competed out by the BMP2 or BMP4 peptides but minimally by both BMP5 peptides (Fig. 9A). In good agreement, rhBMP5 binding to HS was competed by its HS-binding C-terminal peptide but not the nonbinding N-terminal peptide (Fig. 9B). Interestingly, however, rhBMP5 binding was also inhibited by the BMP2/4 peptides (Fig. 9B).
Figure 9.

Peptides compete with respective BMPs for HS binding. A, competition binding assays in which rhBMP2 (4 μm) was co-incubated with increasing concentrations of monomeric 2N, 4N, 5N, or 5C peptides and tested for binding to substrate-bound HS. Note that both the 2N and 4N peptides competed with rhBMP2 binding to HS (blue and black lines), and both 5N and 5C peptides had minimal effects (red lines). B, competition binding assays in which rhBMP5 (4 μm) was co-incubated with increasing concentrations of monomeric 2N, 4N, 5N, or 5C peptides and tested for binding to substrate-bound HS. Note that the 5C peptide did compete for binding (red lines with open circle), but the 5N peptide did not (red line with filled red circles). Note also that both 2N and 4N peptides also competed with rhBMP5 binding. Each binding curve is representative of three independent experiments.
Discussion
The data in our study revealed that the C-terminal regions of mature BMP5, BMP6, and BMP7 contain a domain characterized by strong HS-binding ability. The amino acid sequence of this domain is virtually identical in the three BMPs and is highly conserved through evolution (see Fig. S2). As indicated by the solid-phase assays, peptides encompassing this C-terminal domain bind to HS with nanomolar affinities comparable with those exhibited by peptides of the N-terminal domains of BMP2 and BMP4. Our solid-phase assays show that the different peptides also possess significant specificity in their ability to compete with BMP binding, indicating that their respective amino acid sequences are functionally distinctive and discriminatory. Previous studies found that mutations of basic residues within the N-terminal domain of BMP2 and BMP4 reduced HS binding and also altered the bioactivity of the mutant proteins (18, 30). Interestingly, however, mutations of basic residues within the N-terminal domain of BMP7 were found to have no major effect on HS binding (44), hinting at the possibility that the major HS-binding domain may actually reside elsewhere along the protein as in fact shown here. In sum, our data and previous studies provide strong evidence that the C-terminal domain in BMP5/6/7 and the N-terminal domain in BMP2/4 represent major and selective mediators of HS binding.
The HS-binding C- and N-terminal domains of these BMPs contain a motif conforming to typical CW structures. Cardin and Weintraub (24) originally proposed that the positively charged amino acids in the XBBBXXBX motif would be arrayed on one face of an α-helix, and they would be aligned on one side of a β-strand in the XBBXBX motif, providing a suitable surface for interaction with sulfated sugar clusters along the HS chains in each case (19). Subsequent studies have emphasized the importance of spacing among positively charged amino acids, irrespective of whether they are arranged in an α-helix or a β-strand (26, 45). Protein structure predictions and mapping of electrostatic surfaces have suggested that the mature BMP2 dimer exhibits two electropositive surfaces, whereas the BMP7 dimer exhibits a single one (33). A major conclusion of this work was that the BMP2 dimer would possess two major HS-binding domains in line with previous protein–HS binding kinetics data (18), whereas BMP7 would possess one only. The authors speculated that the two surfaces in the BMP2 dimer were provided by the N-terminal α-helical CW domain of each monomer, with the two domains located on opposite sides of the dimer fingers held together by the central wrist/palm region (29, 33). However, no explanation was given as to why the BMP7 dimer would display one electropositive surface only. Based on our data indicating that the major HS-binding motif in BMP7 is in the C terminus, it is possible that the single surface could originate from two C-terminally unstructured domains arranged in proximity within the wrist/palm region of the dimer as suggested by structural predictions with the I-TASSER server (Fig. S1).
It is important to point out that this server carries out protein/peptide structure predictions by searching the Protein Data Bank (PDB) and relating the input sequence to known protein structures with similar or identical sequences (40). Thus, the predicted structure of the peptides characterized here may not fully mimic their native configurations within the intact protein. Given their biological activities, however, it is likely that the peptides did possess functionally relevant configurations. This was indicated by their selective ability to interact with substrate-bound HS, bind to the cell surface, stimulate chondrogenesis, and compete with binding of their respective full-length proteins.
Deciphering the exact 3D organization and features of BMP dimers will require further analysis, and the contribution of single or groups of basic residues, distant from the CW motifs, will need to be taken into consideration to precisely delineate the overall structural basis of BMP–HS interactions (25, 46, 47). It will also be necessary to clarify the spatial orientation of different BMPs with respect to the HS chain backbone given that their electropositive surfaces differ in location and structure as suggested above. In addition, the HS chains themselves are endowed with a remarkable degree of structural diversity and complexity they acquire during their biosynthesis in the Golgi and by action of extracellular sulfatases (19, 23). The synthesis of HS chains initiates with the assembly of a tetrasaccharide linkage region to prescribed serine residues along the proteoglycan core proteins. Chain polymerization continues with the addition of an N-acetyl-d-glucosamine (GlcNAc) residue and then proceeds with alternating addition of glucuronic acid (GlcA) and GlcNAc residues by EXT1/EXT2 glycosyl polymerase complexes, producing HS chains of about 20–25 kDa in size. Although these steps are ongoing, the elongating chains undergo a series of concurrent structural modifications that start with N-deacetylation and N-sulfation of GlcNAc residues by members of the N-deacetylase–N-sulfotransferase family. Modifications continue with epimerization of certain d-glucuronic acid residues to l-iduronic acid and with O-sulfation at positions C2, C6, or C3 around glucosamine and glucuronic/iduronic rings by O-sulfotransferase family members. Such multiple serial biosynthetic steps result in chains with highly diverse sulfation and sugar modification patterns within 6–12 sugar residue–long segments flanked by largely unmodified and unsulfated segments (19, 23). These features are thought to represent the basis for the ability of HS chains to strongly and specifically interact with proteins (23, 25, 48). Some proteins such as FGF2 and antithrombin require specific modifications of HS for optimal binding, including 3-O sulfation (49–51), whereas other proteins such as IL-8 and thrombin mainly rely on HS domain structure or charge density, respectively (52, 53). At present, relatively little is known about HS structural features mediating BMP interactions and whether different BMPs require distinct HS modifications and segments for optimal binding (48, 54), although the BMP antagonist Noggin preferentially binds to HS carrying N-, 6-O-, and 2-O-sulfates (55). Nonetheless, it is interesting to note that whereas HS is a positive regulator of the biological function of certain signaling proteins such as FGF2 (56, 57), it appears to normally restrain and limit the function and activity of BMPs (18, 30). Together, the above studies underline the striking diversity and subtleties of HS–protein interactions that are of essential importance to numerous developmental and physiological processes (13, 54, 58) and can cause pathologies when deranged (59).
Among the latter, a case in point is hereditary multiple exostoses, a rare congenital pediatric disorder that is caused by loss-of-function mutations in EXT1 or EXT2 and involves significant decreases in HS levels (60, 61). HME is characterized by cartilaginous tumors (called exostoses or osteochondromas) that develop within perichondrium flanking the growth plates of long bones, ribs, and other elements, but the underlying mechanisms had long remained unclear (62, 63). Using mouse models, we found that conditional Ext1 ablation in perichondrial cells (and concurrent drop in HS levels) caused a sharp decrease in local ERK/FGF signaling and a reciprocal increase in canonical BMP signaling (64, 65). These opposing signaling changes were followed by the development of cartilaginous tumors in very good correlation with the fact that normally the BMP signaling is pro-chondrogenic, whereas ERK/FGF signaling is anti-chondrogenic (66, 67). We do not know yet which BMP(s) is/are the main culprits in inducing ectopic chondrogenesis and osteochondroma formation in HME, although the preferential expression and function of BMP2 in perichondrium point to this protein as a pathogenic candidate (38). Nonetheless, the studies stress the point that HS plays a critical role in fine-tuning the local activities, range of action, and developmental effects of different HS-binding proteins (23) and that severe consequences can ensue when these balances are not maintained.
Given their pro-skeletogenic properties, recombinant human BMP2 and BMP7 are currently used clinically to stimulate fracture repair and spine fusion (68, 69). However, because the proteins are very potent and are used in large excess over endogenous levels in these clinical conditions, they exert broad and unrestrained action and can elicit side effects, at times severe (68, 69). One possible way to alleviate these problems would be to use peptides that mimic properties of their full-length counterparts (70) and/or induce similar biological responses. One example is the study cited above in which a peptide spanning the N-terminal HS-binding domain of BMP4 was found to stimulate osteogenic cell differentiation in vitro and bone repair in vivo in a manner similar to full-length rhBMP4 (28). Our data complement well and extend those observations and show that peptides spanning the HS-binding domain of BMP2 and BMP4 at the N terminus and of BMP5 at the C terminus stimulate chondrogenesis and chondrogenic gene expression in primary mouse embryo limb bud cells in micromass culture. Our solid-phase binding assays indicate that the peptides can compete with their full-length counterparts for HS binding, demonstrating also a degree of selectivity and specificity. In recent studies, we demonstrated the presence of endogenous BMPs bound to the cell surface (71) and also observed that treatment with heparitinase (in the absence of exogenous BMPs) greatly and rapidly stimulated canonical BMP signaling and chondrogenic cell differentiation (41, 64). Therefore, it is likely that the peptides were able to exert pro-chondrogenic effects by the following: dislodging endogenous HS-bound BMPs; reducing the normal restraining effects of HS on BMP availability and action; and allowing the proteins to exert greater biological activity. By relying on endogenous BMPs and stimulating their action, the peptides may prove to be a safer and more versatile strategy for treatment of fracture repair, spine fusion, and related clinical problems. In addition, the peptides could be used in combination with rhBMP2 or rhBMP7 to reduce the amounts of these proteins needed for treatment, thus likely rendering their use much safer.
Experimental procedures
Reagents
Antibodies against human BMP2/BMP4 (BMP-2/4 (H-1): sc-137087) and BMP5 (AF6176 and MAB7151) were obtained from Santa Cruz Biotechnology (Dallas, TX) and R&D Systems (Minneapolis, MN), respectively. NeutrAvidin (NA), NA-HRP, NA-DyLight 488 (NA-488), and anti-mouse HRP secondary antibody conjugates were obtained from ThermoFisher Scientific and Cell Signaling Technology (Danvers, MA). Heparan sulfate (Sigma catalog no. H7640) isolated from bovine kidney and hyaluronic acid (Sigma catalog no. 53747) from Streptococcus equisimilis were obtained from Millipore-Sigma. Full-length human BMP2 and BMP4–7 proteins were obtained from R&D and ProSpec (East Brunswick, NJ).
Protein modifications and peptide synthesis
Where indicated, full-length BMPs were biotinylated using EZ-Link Sulfo-NHS-LC-Biotin (ThermoFisher Scientific) in PBS in a total volume of 150 μl on ice for 1 h. Reactions were quenched by the addition of 5 μl of Tris-buffered saline containing 50 μg of BSA. Peptides encompassing the HS-binding domain from each BMP contained the putative CW motif and also a minimum of three flanking amino acids on the N- and C-terminal sides of the domain. Three glycine residues were added to the N terminus of each peptide to provide a flexible linker between an N-terminal biotin tag and the peptide itself. Peptides were purified by reverse-phase HPLC (C18 column), and peptide mass was confirmed by MALDI-TOF MS. All peptides were synthesized and purified by Peptide 2 (Chantilly, VA). When indicated, biotinylated peptides were oligomerized into tetramers by incubation with NA or NA-HRP at a molar ratio of 50:1 of peptide to NA-HRP, with each avidin molecule binding four biotins (72) and referred to as tetrameric complexes. Because the C-terminal peptides from BMP5 and BMP7 contain two Cys residues, they were prepared in water containing 1 mm dithiothreitol (DTT). Peptides were used within 2 weeks after being dissolved.
In silico modeling
Amino acid sequence alignments were performed using the T-coffee alignment tool. Helical wheel projections were constructed using DrawCoil 1.0. Secondary structure predictions made use of the structural information of BMP2 (PDB code 3BMP) and BMP7 (PDB code 1BMP) and were carried out using the I-TASSER server for protein structure and function prediction (40). Resulting structures were visualized using Chimera. All peptides have isoelectric points (pI) >10, with the exception of the BMP4 C-terminal peptide, and are predicted to contain some α-helix, except the C-terminal domain of BMP5–7.
Solid-phase binding assays
Nunc MaxiSorp flat bottom 96-well plates were coated with HS (5 μg/ml) in 50 mm carbonate buffer (pH 9.4) overnight at 4 °C. Unless otherwise indicated, all binding assays were carried out in 1× PBS, 0.1% Tween 20 (PBST) containing 1% BSA (PBSTB). Plates were incubated with peptides for a minimum of 2 h at room temperature with gentle shaking. At the termination of the binding assay, the plates were washed three times with PBST and a final rinse with PBS. Plates were developed by addition of HRP substrate O-phenylenediamine dihydrochloride (OPD) in phosphate/citrate buffer (50 mm sodium phosphate and 25 mm citric acid (pH 5)) and read at 450 nm. Very low levels of background binding were exhibited by NA-HRP alone or when complexed with biotinylated BSA.
To assess binding of full-length rhBMP2, rhBMP4, and rhBMP5, proteins were applied to 96-well HS-coated plates in PBST at indicated concentrations for 2 h at 4 °C. Plates were rinsed three times with PBST, and bound proteins were assessed with BMP-specific antibodies. To assess binding of full-length rhBMP6 and rhBMP7, the proteins were first labeled with biotin and applied to 96-well HS-coated plates in PBST for 2 h. The plates were washed, incubated with NA-HRP, washed again, and developed with OPD substrate. Similar results were obtained with heparin-coated plates.
Competition assays
HS plates were incubated with full-length rhBMP2 or rhBMP5 (4 μm) in the presence of increasing amounts of peptides in PBST for 2 h with gentle shaking. The plates were washed, and bound BMP2 and BMP5 were assessed using anti-BMP2 antibody (1:2000) and anti-BMP5 antibody (1:2000) followed by anti-mouse HRP secondary antibody conjugate (1:5000). Plates were developed by addition of OPD substrate.
Cells and cell culture
K562 human myelogenous leukemia cells (73) and AD293 cells were grown in DMEM containing 10% fetal calf serum. Micromass cultures were prepared from E11 CD-1 mouse embryo limb buds (74). Briefly, limb bud mesenchyme was dissociated in 0.5% trypsin/EDTA at 37 °C. The dissociated cells were suspended at a concentration of 10 × 106 cells/ml in DMEM containing 3% fetal bovine serum and antibiotics. Micromass cultures were initiated by spotting 15 μl of cell suspensions (1.5 × 105 cells) onto the surface of 24-well tissue culture plates. After a 90-min incubation at 37 °C in a humidified CO2 incubator to allow for cell attachment, the cultures were given 0.5 ml of medium. After 24 h, cultures were treated with full-length BMP proteins or tetrameric peptide complexes in the same medium. Fresh reagents, including BMP proteins or peptides, were given with medium change every 3rd day. Cultures were stained with Alcian blue (pH 1.0) after 3 days to monitor chondrogenic cell differentiation or processed for PCR analysis of gene expression (74). Images were taken with a Nikon SMZ-U microscope equipped with a SPOT insight camera (Diagnostic Instruments, Inc.; Sterling Heights, MI) and acquired with SPOT 4.0 software. Micromass analysis was performed using ImageJ. Images were made binary under an RGB threshold, and “Particle Analysis” was utilized to measure the Alcian blue positive area and nodule number (75).
FACS analysis of peptide binding
K562 cells were washed with PBS and fixed with 2% buffered formalin for 20 min on ice. The cells were washed with PBS and blocked by incubation in PBS, 1% BSA for 20 min on ice. Approximately 106 cells (100 μl) were incubated with peptide tetramers containing fluorescent NA-488. Following incubation for 2 h on ice, the cells were washed and analyzed on an Accuri flow cytometer (BD Biosciences) located in the Flow Cytometry Core Laboratory at our institution.
Gene expression analysis
Total RNA was isolated using TRIzol Reagent (ThermoFisher Scientific) following the manufacturer's protocol. Five μg of glycogen (ThermoFisher Scientific catalog no. AM9510) was added to the aqueous phase prior to the addition of isopropyl alcohol to facilitate RNA precipitation. RNA quantification was determined using a Nanodrop spectrophotometer. cDNA was prepared from 2 μg of purified RNA using a Verso cDNA synthesis kit (ThermoFisher Scientific catalog no. AB1453A) following the manufacturer's protocol. Gene expression was determined by quantitative real-time PCR using SYBR Green PCR Master Mix in an ABI 7500 real-time PCR system, located in the NAPCore facility in our institution. GAPDH was used as the endogenous control, and relative expression was calculated using the ΔΔCt method. All PCR primers were obtained from Integrated DNA Technologies and are listed in Table S2.
Statistical analysis
All statistical analysis was performed using GraphPad Prism software.
Animal studies
E11 CD-1 mouse embryo limb buds for micromass cultures were obtained from The Jackson Laboratory (Bar Harbor, ME). All animal studies were approved under protocol no. 0952 by the Institutional Animal Care and Use Committee (IACUC).
Author contributions
P. C. B. and M. P. conceptualization; P. C. B., E. Y., and C. M. data curation; P. C. B., E. Y., and C. M. formal analysis; P. C. B., E. Y., and C. M. investigation; P. C. B. and E. Y. methodology; P. C. B., E. Y., and M. P. writing-original draft; P. C. B. and M. P. project administration; P. C. B., E. Y., C. M., and M. P. writing-review and editing; E. Y. software; E. Y. visualization; C. M. validation; M. P. resources; M. P. supervision; M. P. funding acquisition.
Supplementary Material
Acknowledgments
We thank Hal Bradford and Dr. Sriram Krishnaswamy in the Department of Pediatrics at The Children's Hospital of Philadelphia for helpful discussions and assistance with data analysis. We are grateful to Dr. Florin Tuluc, Jennifer Murray, and Lily Wu in the Flow Cytometry Core Laboratory at The Children's Hospital of Philadelphia for invaluable assistance.
This work was supported by National Institutes of Health Grant RO1AR061758. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S3 and Tables S1–S3.
- BMP
- bone morphogenetic protein
- HA
- hyaluronic acid
- HS
- heparan sulfate
- CW
- Cardin-Weintraub
- HME
- hereditary multiple exostoses
- NA
- NeutrAvidin
- NA-HRP
- NeutrAvidin-horseradish peroxidase
- PDB
- Protein Data Bank
- rh
- recombinant human
- OPD
- O-phenylenediamine dihydrochloride
- DMEM
- Dulbecco's modified Eagle's medium
- ERK
- extracellular signal-regulated kinase
- FGF
- fibroblast growth factor
- TGF-β
- transforming growth factor-β.
References
- 1. Huminiecki L., Goldovsky L., Freilich S., Moustakas A., Ouzounis C., and Heldin C.-H. (2009) Emergence, development and diversification of the TGF-β signaling pathway within the animal kingdom. BMC Evol. Biol. 9, 28 10.1186/1471-2148-9-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Massagué J. (1998) TGF-β signal transduction. Annu. Rev. Biochem. 67, 753–791 10.1146/annurev.biochem.67.1.753 [DOI] [PubMed] [Google Scholar]
- 3. Wang R. N., Green J., Wang Z., Deng Y., Qiao M., Peabody M., Zhang Q., Ye J., Yan Z., Denduluri S., Idowu O., Li M., Shen C., Hu A., Haydon R. C., et al. (2014) Bone morphogenetic protein (BMP) signaling in development and human diseases. Genes Dis. 1, 87–105 10.1016/j.gendis.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Constam D. B., and Robertson E. J. (1999) Regulation of bone morphogenetic protein activity by prodomains and proprotein convertases. J. Cell Biol. 144, 139–149 10.1083/jcb.144.1.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Degnin C., Jean F., Thomas G., and Christian J. L. (2004) Cleavages within the prodomain direct intracellular trafficking and degradation of mature bone morphogenetic protein-4. Mol. Biol. Cell 15, 5012–5020 10.1091/mbc.e04-08-0673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun P. D., and Davies D. R. (1995) The cysteine-knot growth-factor superfamily. Annu. Rev. Biophys. Biomol. Struct. 24, 269–291 10.1146/annurev.bb.24.060195.001413 [DOI] [PubMed] [Google Scholar]
- 7. Avsian-Kretchmer O., and Hsueh A. J. (2004) Comparative genomic analysis of the eight-membered ring cysteine knot-containing bone morphogenetic protein antagonists. Mol. Endocrinol. 18, 1–12 10.1210/me.2003-0227 [DOI] [PubMed] [Google Scholar]
- 8. Scheufler C., Sebald W., and Hülsmeyer M. (1999) Crystal structure of human bone morphogenetic protein-2 at 2.7A resolution. J. Mol. Biol. 287, 103–115 [DOI] [PubMed] [Google Scholar]
- 9. Iyer S., and Acharya K. R. (2011) Tying the knot–the cysteine signature and molecular-recognition processes of the vascular endothelial growth factor family of angiogenic cytokines. FEBS J. 278, 4304–4322 10.1111/j.1742-4658.2011.08350.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi Y., and Massagué J. (2003) Mechanisms of TFG-β signaling from cell membrane to the nucleus. Cell 113, 685–700 10.1016/S0092-8674(03)00432-X [DOI] [PubMed] [Google Scholar]
- 11. Heldin C. H., Miyazono K., and ten Dijke P. (1997) TGF-β signaling from cell membrane to nucleus through SMD proteins. Nature 390, 465–471 10.1038/37284 [DOI] [PubMed] [Google Scholar]
- 12. Hogan B. L. (1996) Bone morphogenetic proteins in development. Curr. Opin. Genet. Dev. 6, 432–438 10.1016/S0959-437X(96)80064-5 [DOI] [PubMed] [Google Scholar]
- 13. Salazar V. S., Gamer L. W., and Rosen V. (2016) BMP signaling in skeletal development, disease and repair. Nat. Rev. Endocrinology 12, 203–221 10.1038/nrendo.2016.12 [DOI] [PubMed] [Google Scholar]
- 14. Balemans W., and Van Hul W. (2002) Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev. Biol. 250, 231–250 10.1006/dbio.2002.0779 [DOI] [PubMed] [Google Scholar]
- 15. Brazil D. P., Church R. H., Surae S., Godson C., and Martin F. (2015) BMP signaling: agony and antagony in the family. Trends Cell Biol. 25, 249–264 10.1016/j.tcb.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 16. Massagué J. (2012) TGFβ signaling in context. Nat. Rev. Mol. Cell Biol. 13, 616–630 10.1038/nrm3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rider C. C. (2006) Heparin/heparan sulphate binding in the TGF-β cytokine superfamily. Biochem. Soc. Trans. 34, 458–460 10.1042/BST0340458 [DOI] [PubMed] [Google Scholar]
- 18. Ruppert R., Hoffmann E., and Sebald W. (1996) Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur. J. Biochem. 237, 295–302 10.1111/j.1432-1033.1996.0295n.x [DOI] [PubMed] [Google Scholar]
- 19. Sarrazin S., Lamanna W. C., and Esko J. D. (2011) Heparan sulfate proteoglycans. Cold Spring Harb. Prospect. Biol. 3, a004952 10.1101/cshperspect.a004952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iozzo R. V. (2001) Heparan sulfate proteoglycans: intricate molecules with intriguing functions. J. Clin. Invest. 108, 165–167 10.1172/JCI200113560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ohlig S., Pickhinke U., Sirko S., Bandari S., Hoffmann D., Dreier R., Farshi P., Götz M., and Grobe K. (2012) An emerging role of sonic hedgehog shedding as a modulator of heparan sulfate interactions. J. Biol. Chem. 287, 43708–43719 10.1074/jbc.M112.356667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu Z. L., Zhang L., Yabe T., Kuberan B., Beeler D. L., Love A., and Rosenberg R. D. (2003) The involvement of heparan sulfate (HS) in FGF1/HS/FGR1 signaling complex. J. Biol. Chem. 278, 17121–17129 10.1074/jbc.M212590200 [DOI] [PubMed] [Google Scholar]
- 23. Bishop J. R., Schuksz M., and Esko J. D. (2007) Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446, 1030–1037 10.1038/nature05817 [DOI] [PubMed] [Google Scholar]
- 24. Cardin A. D., and Weintraub H. J. (1989) Molecular modeling of protein–glycosaminoglycan interactions. Arterioscler. Thromb. Vasc. Biol. 9, 21–32 10.1161/01.ATV.9.1.21 [DOI] [PubMed] [Google Scholar]
- 25. Billings P. C., and Pacifici M. (2015) Interactions of signaling proteins, growth factors and other proteins with heparan sulfate: mechanisms and mysteries. Connect. Tissue Res. 56, 272–280 10.3109/03008207.2015.1045066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fromm J. R., Hileman R. E., Caldwell E. E., Weiler J. M., and Linhardt R. J. (1997) Pattern and spacing of basic amino acids in heparin binding sites. Arch. Biochem. Biophys. 343, 92–100 10.1006/abbi.1997.0147 [DOI] [PubMed] [Google Scholar]
- 27. Hileman R. E., Fromm J. R., Weiler J. M., and Linhardt R. J. (1998) Glycosaminoglycan-protein interactions: definition of consensus sites in glycosaminoglycan binding proteins. Bioessays 20, 156–167 10.1002/(SICI)1521-1878(199802)20:2%3C156::AID-BIES8%3E3.0.CO%3B2-R [DOI] [PubMed] [Google Scholar]
- 28. Choi Y. J., Lee J. Y., Park J. H., Park J. B., Suh J. S., Choi Y. S., Lee S. J., Chung C.-P., and Park Y. J. (2010) The identification of a heparin binding domain peptide from bone morphogenetic protein-4 and its role in osteogenesis. Biomaterials 31, 7226–7238 10.1016/j.biomaterials.2010.05.022 [DOI] [PubMed] [Google Scholar]
- 29. Rider C. C., and Mulloy B. (2017) Heparin, heparan sulphate and the TGF-β cytokine superfamily. Molecules 22, 713 10.3390/molecules22050713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ohkawara B., Iemura S., ten Dijke P., and Ueno N. (2002) Action range of BMP is defined by its N-terminal basic amino acid core. Curr. Biol. 12, 205–209 10.1016/S0960-9822(01)00684-4 [DOI] [PubMed] [Google Scholar]
- 31. Brkljacic J., Pauk M., Erjavec I., Cipcic A., Grgurevic L., Zadro R., Inman G. J., and Vukicevic S. (2013) Exogenous heparin binds and inhibits bone morphogenetic protein 6 biological activity. Int. Orthop. 37, 529–541 10.1007/s00264-012-1714-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Irie A., Habuchi H., Kimata K., and Sanai Y. (2003) Heparan sulfate is required for bone morphogenetic protein-7 signaling. Biochem. Biophys. Res. Commun. 308, 858–865 10.1016/S0006-291X(03)01500-6 [DOI] [PubMed] [Google Scholar]
- 33. Gandhi N. S., and Mancera R. L. (2012) Prediction of heparin binding sites in bone morphogenetic proteins (BMPs). Biochim. Biophys. Acta 1824, 1374–1381 10.1016/j.bbapap.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 34. Hinck A. P. (2012) Structural studies of the TFG-βs and their receptors–insights into evaluation of the TGF-β superfamily. FEBS Lett. 586, 1860–1870 10.1016/j.febslet.2012.05.028 [DOI] [PubMed] [Google Scholar]
- 35. Mikić B., van der Meulen M. C., Kingsley D. M., and Carter D. R. (1995) Long bone geometry and strength in adult BMP-5 deficient mice. Bone 16, 445–454 [PubMed] [Google Scholar]
- 36. Perry M. J., McDougall K. E., Hou S.-C., and Tobias J. H. (2008) Impaired growth plate function in bmp-6 null mice. Bone 42, 216–225 10.1016/j.bone.2007.09.053 [DOI] [PubMed] [Google Scholar]
- 37. Luo G., Hofmann C., Bronckers A. L., Sohocki M., Bradley A., and Karsenty G. (1995) BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 9, 2808–2820 10.1101/gad.9.22.2808 [DOI] [PubMed] [Google Scholar]
- 38. Tsuji K., Bandyopadhyay A., Harfe B. D., Cox K., Kakar S., Gerstenfeld L., Einhorn T., Tabin C. J., and Rosen V. (2006) BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat. Genet. 38, 1424–1429 10.1038/ng1916 [DOI] [PubMed] [Google Scholar]
- 39. Selever J., Liu W., Lu M.-F., Behringer R. R., and Martin J. F. (2004) Bmp4 in limb bud mesoderm regulates digit pattern by controlling AER development. Dev. Biol. 276, 268–279 10.1016/j.ydbio.2004.08.024 [DOI] [PubMed] [Google Scholar]
- 40. Yang J., Yan R., Roy A., Xu D., Poisson J., and Zhang Y. (2015) The I-TASSER suite: protein structure and function prediction. Nat. Methods 12, 7–8 10.1038/nmeth.3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huegel J., Enomoto-Iwamoto M., Sgariglia F., Koyama E., and Pacifici M. (2015) Heparanase stimulates chondrogenesis and is up-regulated in human ectopic cartilage. A mechanism possibly involved in hereditary multiple exostoses. Am. J. Pathol. 185, 1676–1685 10.1016/j.ajpath.2015.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lefebvre V., and Bhattaram P. (2010) Vertebrate skeletogenesis. Curr. Top. Dev. Biol. 90, 291–317 10.1016/S0070-2153(10)90008-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Katagiri T., Imada M., Yanai T., Suda T., Takahashi N., and Kamijo R. (2002) Identification of a BMP-responsive element in Id1, the gene for inhibition of myogenesis. Genes Cells 7, 949–960 10.1046/j.1365-2443.2002.00573.x [DOI] [PubMed] [Google Scholar]
- 44. McClarence D. (2011) An Investigation into the Location of the Heparan Sulphate/Heparin-binding Site of Human Morphogenetic Protein-7. Ph.D. thesis, University of London School of Biological Sciences, London [Google Scholar]
- 45. Margalit H., Fischer N., and Ben-Sasson S. A. (1993) Comparative analysis of structurally defined heparin binding sequences reveals a distinct spatial distribution of basic residues. J. Biol. Chem. 268, 19228–19231 [PubMed] [Google Scholar]
- 46. Chang S.-C., Mulloy B., Magee A. I., and Couchman J. R. (2011) Two distinct sites in sonic hedgehog combine for heparan sulfate interactions and cell signaling functions. J. Biol. Chem. 286, 44391–44402 10.1074/jbc.M111.285361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Whalen D. M., Malinauskas T., Gilbert R. J., and Siebold C. (2013) Structural insights into proteoglycan-shaped hedgehog signaling. Proc. Natl. Acad. Sci. U.S.A. 110, 16420–16425 10.1073/pnas.1310097110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xu D., and Esko J. D. (2014) Demystifying heparan sulfate-protein interactions. Annu. Rev. Biochem. 83, 129–157 10.1146/annurev-biochem-060713-035314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Richard B., Swanson R., and Olson S. T. (2009) The signature 3-O-sulfo group of the anticoagulant heparin sequence is critical for heparin binding to antithrombin but is not required for allosteric activation. J. Biol. Chem. 284, 27054–27064 10.1074/jbc.M109.029892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Turnbull J. E., Fernig D. G., Ke Y., Wilkinson M. C., and Gallagher J. T. (1992) Identification of the basic fibroblast growth factor binding sequence in fibroblast heparan sulfate. J. Biol. Chem. 267, 10337–10341 [PubMed] [Google Scholar]
- 51. Schultz V., Suflita M., Liu X., Zhang X., Yu Y., Li L., Green D. E., Xu Y., Zhang F., DeAngelis P. L., Liu J., and Linhardt R. J. (2017) Heparan sulfate domains required for fibroblast growth factor 1 and 2 signaling through fibroblast growth factor receptor 1c. J. Biol. Chem. 292, 2495–2509 10.1074/jbc.M116.761585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lortat-Jacob H., Turnbull J. E., and Grimaud J. A. (1995) Molecular organization of the interferon γ-binding domain of heparan sulphate. Biochem. J. 310, 497–505 10.1042/bj3100497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Spillmann D., Witt D., and Lindahl U. (1998) Defining the interleukin-8-binding domain of heparan sulfate. J. Biol. Chem. 273, 15487–15493 10.1074/jbc.273.25.15487 [DOI] [PubMed] [Google Scholar]
- 54. Matsuo I., and Kimura-Yoshida C. (2014) Extracellular distribution of diffusible growth factors controlled by heparan sulfate proteoglycans during mammalian embryogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130545 10.1098/rstb.2013.0545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Viviano B. L., Paine-Saunders S., Gasiunas N., Gallagher J., and Saunders S. (2004) Domain-specific modification of heparan sulfate by Qsulf1 modulates the binding of the bone morphogenetic protein antagonist Noggin. J. Biol. Chem. 279, 5604–5611 10.1074/jbc.M310691200 [DOI] [PubMed] [Google Scholar]
- 56. Ornitz D. M., and Itoh N. (2015) The fibroblast growth factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 4, 215–266 10.1002/wdev.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shimokawa K., Kimura-Yoshida C., Nagai N., Mukai K., Matsubara K., Watanabe H., Matsuda Y., Mochida K., and Matsuo I. (2011) Cell surface heparan sulfate chains regulate local reception of FGF signaling in the mouse embryo. Dev. Cell 21, 257–272 10.1016/j.devcel.2011.06.027 [DOI] [PubMed] [Google Scholar]
- 58. Lin X. (2004) Functions of heparan sulfate proteoglycans in cell signaling during development. Development 131, 6009–6021 10.1242/dev.01522 [DOI] [PubMed] [Google Scholar]
- 59. Lindahl U., and Kjellén L. (2013) Pathophysiology of heparan sulphate: many diseases, few drugs. J. Intern. Med. 273, 555–571 10.1111/joim.12061 [DOI] [PubMed] [Google Scholar]
- 60. Cheung P. K., McCormick C., Crawford B. E., Esko J. D., Tufaro F., and Duncan G. (2001) Etiological point mutations in the hereditary multiple exostoses gene EXT1: a functional analysis of heparan sulfate polymerase activity. Am. J. Hum. Genet. 69, 55–66 10.1086/321278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hecht J. T., Hogue D., Strong L. C., Hansen M. F., Blanton S. H., and Wagner M. (1995) Hereditary multiple exostosis and chondrosarcoma: linkage to chromosome 11 and loss of heterozygosity for EXT-linked markers on chromosome 11 and 8. Am. J. Hum. Genet. 56, 1125–1131 [PMC free article] [PubMed] [Google Scholar]
- 62. Hecht J. T., Hayes E., Haynes R., Cole W. G., Long R. J., Farach-Carson M. C., and Carson D. D. (2005) Differentiation-induced loss of heparan sulfate in human exostosis derived chondrocytes. Differentiation 73, 212–221 10.1111/j.1432-0436.2005.00025.x [DOI] [PubMed] [Google Scholar]
- 63. Jones K. B. (2011) Glycobiology and the growth plate: current concepts in multiple hereditary exostoses. J. Pediatr. Orthop. 31, 577–586 10.1097/BPO.0b013e31821c7738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huegel J., Mundy C., Sgariglia F., Nygren P., Billings P. C., Yamaguchi Y., Koyama E., and Pacifici M. (2013) Perichondrium phenotype and border function are regulated by Ext1 and heparan sulfate in developing long bones: a mechanism likely deranged in hereditary multiple exostoses. Dev. Biol. 377, 100–112 10.1016/j.ydbio.2013.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sinha S., Mundy C., Bechtold T., Sgariglia F., Ibrahim M. M., Billings P. C., Carroll K., Koyama E., Jones K. B., and Pacifici M. (2017) Unsuspected osteochondroma-like outgrowths in the cranial base of hereditary multiple exostoses patients and modeling and treatment with a BMP antagonist in mice. PLoS Genet. 13, e1006742 10.1371/journal.pgen.1006742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Buckland R. A., Collinson J. M., Graham E., Davidson D. R., and Hill R. E. (1998) Antagonistic effects of FGF4 on BMP induction of apoptosis and chondrogenesis in the chick limb bud. Mech. Dev. 71, 143–150 10.1016/S0925-4773(98)00008-2 [DOI] [PubMed] [Google Scholar]
- 67. Yoon B. S., Pogue R., Ovchinnikov D. A., Yoshii I., Mishina Y., Behringer R. R., and Lyons K. M. (2006) BMPs regulate multiple aspects of growth plate chondrogenesis through opposing actions of FGF pathways. Development 133, 4667–4678 10.1242/dev.02680 [DOI] [PubMed] [Google Scholar]
- 68. James A. W., LaChaud G., Shen J., Asatrian G., Nguyen V., Zhang X., Ting K., and Soo C. (2016) A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part B Rev. 22, 284–297 10.1089/ten.teb.2015.0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lissenberg-Thunnissen S. N., de Gorter D. J., Sier C. F., and Schipper I. B. (2011) Use and efficacy of bone morphogenetic proteins in fracture healing. Int. Orthop. 35, 1271–1280 10.1007/s00264-011-1301-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Janda C. Y., Dang L. T., You C., Chang J., de Lau W., Zhong Z. A., Yan K. S., Marecic O., Siepe D., Li X., Moody J. D., Williams B. O., Clevers H., Piehler J., Baker D., et al. (2017) Surrogate Wnt agonists that phenocopy canonical Wnt and β-catenin signalling. Nature 545, 234–237 10.1038/nature22306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mundy C., Yang E., Takano H., Billings P. C., and Pacifici M. (2018) Heparan sulfate antagonism alters bone morphogenetic protein signaling and receptor dynamics, suggesting a mechanism in hereditary multiple exostoses. J. Biol. Chem. 293, 7703–7716 10.1074/jbc.RA117.000264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dundas C. M., Demonte D., and Park S. (2013) Streptavidin-biotin technology: improvements and innovations in chemical and biological applications. Appl. Microbiol. Biotechnol. 97, 9343–9353 10.1007/s00253-013-5232-z [DOI] [PubMed] [Google Scholar]
- 73. Lozzio B. B., and Lozzio C. B. (1979) Properties and usefulness of the original K-562 human myelogenous leukemia cell line. Leuk. Res. 3, 363–370 10.1016/0145-2126(79)90033-X [DOI] [PubMed] [Google Scholar]
- 74. Mundy C., Bello A., Sgariglia F., Koyama E., and Pacifici M. (2016) HhAntag, a hedgehog signaling antagonist, suppresses chondrogenesis and modulates canonical and non-canonical BMP signaling. J. Cell. Physiol. 231, 1033–1044 10.1002/jcp.25192 [DOI] [PubMed] [Google Scholar]
- 75. Gutierrez M. A., Guevara J., and Barrera L. A. (2012) Semi-automatic grading system in histologic and immunohistochemistry analysis to evaluate in vitro chondrogenesis. Universitas Scientiarum 17, 167–178 10.11144/javeriana.SC17-2.sags [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.