Abstract
Objective
This study aimed to compare microfracture and application of adipose-derived stem cells (ADSCs) by local adherent technique enhanced by platelet-rich plasma (PRP) to provide a new approach for the repair of cartilage defect.
Design
Full-thickness cylindrical defects were created in the medial femoral condyle in 9 New Zealand White rabbits (5 months old, 4.65 ± 0.20 kg). Two groups of rabbits (n = 3) were either treated with ADSCs (Group 1) or the microfracture technique (Group 2) following intraarticular injection of PRP 3 times in weekly intervals. Rabbits in control group (n = 3) remained untreated. The outcome was assessed macroscopically, histologically, and immunohistochemically.
Results
At the end of week 12, Group 1 showed better defect filling compared with Group 2. Specimens treated with the combination of ADSCs and PRP exhibited significant differences from the other groups in all criteria of International Cartilage Repair Society macroscopic scoring system.
Conclusions
Intraarticular injection of autologous PRP in combination with transplantation of autologous ADSCs by local adherent technique enhances the quality of cartilage defect repair with better results in comparison with microfracture surgery in a rabbit model.
Keywords: adipose-derived stem cells, microfracture, platelet-rich plasma, chondral defect
Introduction
Self-renewal capacity and complete regeneration of injured cartilage is inhibited due to its slow metabolism and avascular nature. Ongoing efforts to search for effective biological therapy for cartilage defects have led to the discovery of natural products with bioactive properties. Platelet-rich plasma (PRP) is thought to stimulate the proliferation, migration, and differentiation of cells through the presence of high quantity of bioactive factors in platelets.1 The safety and efficacy of autologous growth factors to treat knee osteoarthritis (OA) by means of PRP injections was demonstrated in our previous clinical study.2 However, the microfracture technique was frequently applied during the past 20 years to treat damaged cartilage in order to support tissue regeneration, but it has some limitations including the fact that the lesion is repaired with fibrocartilage and there are no long-term satisfactory results.3,4 In order to recruit more cells into the defective area and enhance the quality of repair, application of autologous or syngeneic ex vivo expanded mesenchymal stem cells (MSCs) by local adherent technique was described by Koga et al.5 The use of adipose-derived stem cells (ADSCs) as an autologous and self-replenishing source of tissue provides much promise in regenerative medicine due to their pluripotency and high renewal capacity.6,7 We propose that application of autologous PRP following one of the described technique might create an environment more appropriate for hyaline-like cartilage tissue formation and could improve the quality of treatment option with the goal of accelerating and promoting healing. The aim of the present study was to investigate the effect of ADSCs administered by local adherent technique in combination with PRP for repairing articular chondral defect in the rabbit knee joint and compare the results with the results of microfracture in combination with PRP.
Methods
This study was approved by institutional ethical committee of the University of Veterinary Medicine and Pharmacy in Kosice. All experimental procedures involving animals were performed in accordance to guidelines for the care and use of laboratory animals approved by State Veterinary and Food Administration of the Slovak Republic. Experiments were carried out on New Zealand white rabbits weighing 4.65 ± 0.20 kg, divided into 3 groups with 3 rabbits in each group. Ketamine (1%) and xylasin hydrochlorid (2%) were administered by intramuscular injection for anesthesia. Adipose tissue was removed from the dorsomedial line by lipectomy and transported to the cultivation laboratory in sterile transport medium containing high-glucose Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich, St Louis, MO) and 2% (v/v) antibiotic/antimycotic solution (10,000 units penicillin, 10 mg streptomycin, and 25 µg amphotericin B per milileter) (Sigma-Aldrich). The adipose tissue (2 g) was washed with sterile phosphate-buffered saline (Sigma-Aldrich) and cut into small pieces (1-2 mm). Rabbit ADSCs were finally isolated by enzymatic digestion of adipose pieces with collagenase type I (Thermo Fisher Scientific, Waltham, MA) in DMEM supplemented with 1% (v/v) antibiotic/antimycotic solution for 2 hours at 37°C under gentle agitation. Undigested tissue was removed using a 40 µm nylon cell strainer (Falcon, Becton Dickinson, Franklin Lakes, NJ) and cells were collected by centrifugation at 150g for 7 minutes at 4°C, rinsed twice with DMEM and resuspended in complete culture medium containing Minimum Essential Medium Eagle (Alpha modification, without L-glutamine; Sigma-Aldrich) supplemented with 10% (v/v) fetal bovine serum (FBS) (Sigma-Aldrich) and 1% (v/v) antibiotic/antimycotic solution, seeded at a density of 2.0 × 103 cells/cm2 and allowed to become adherent. Nonadherent cells were removed by replacement with fresh culture medium, and the attached cells were cultured in complete culture medium at 37°C in a humidified 5% CO2 atmosphere to reach confluence.
After reaching 80% to 90% confluence, cells were washed with DMEM and detached by trypsin-EDTA (Sigma-Aldrich) for 4 minutes, washed twice with DMEM, centrifuged, and then seeded at 2.0 × 103 cells/cm2. Cell number was calculated with TC20 Automated Cell Counter (Bio-Rad, Hercules, CA) before in vivo application. Cells from passage 2 were characterized by induction toward adipogenic, osteogenic, and chondrogenic lineages in vitro. Calcium deposits characteristic for osteogenic differentiation in cultures were visualized by Alizarin red staining. Alcian blue staining was used to confirm chondrogenic differentiation. Cytoplasmic inclusions of neutral lipids after adipogenic differentiation were stained with Oil Red O. To determine ADSCs’ viability and proliferation in vitro, MTS assay was used according to the manufacturer’s recommendations (CellTiter96 AQueous Assay, Promega, Madison, WI). Right lower extremity of every rabbit was shaved. The knee joint of rabbits was approached by means of a medial parapatellar incision under sterile conditions. The patella was dislocated laterally to expose the articular surface. Full-thickness cylindrical defects (3-3.5 mm in diameter, 1.5-2 mm in depth) were created in the medial femoral condyle without traumatizing subchondral bony structures in all experimental groups. Treatment interventions in Groups 1 and 2 were performed immediately after generation of chondral defect. Transplantation of autologous ADSCs from passage 2 (0.97 × 107 ± 0.23 in 100 µL) was used for the treatment of rabbits in Group 1 using a low invasive local adherent technique described previously by Koga et al.5 Rabbits in Group 2 underwent microfracture using a 0.035-in. (0.5 mm) Kirschner wire. Defects in control group (Group 3) was left untreated without an additional intervention. Fluorescent-labeled ADSCs with PKH26 (Sigma-Aldrich) were used to confirm the adherence of the cells into the joint defect 1 week after implantation. Four milliliters of blood was obtained from the superficial ear vein of each rabbit once a week and collected into the sodium citrate tube to prepare fresh autologous PRP for each application. The sample of blood (100 µL) before and after preparation was saved for determination of platelet concentration. PRP was prepared by multiple centrifugation as previously described.2 The precipitated platelets were collected to yield PRP in a volume of 1 mL and administered intraarticularly 3 times in a weekly interval in Groups 1 and 2 starting with first injection 1 week postsurgery. After surgeries, the rabbits were allowed free movement in their cages and their limbs were not immobilized. At 12 weeks after treatment the rabbits were sacrificed using an intravenous overdose of pentobarbital and the right condyles were harvested and subjected to macroscopic, histological, and immunohistochemical analysis. For histologic evaluation, the samples of condyle containing the defect (1 × 1 × 1 cm) were fixed in formalin, decalcified in Chelaton III (Centralchem, Bratislava, Slovakia), embedded in paraffin and then 4 µm thick sections were cut sagittally. The sections were stained with hematoxylin and eosin and Alcian blue. Immunohistochemical staining was performed on the same serial sections used in the histochemical staining. Collagen type II alpha1 chain antibody (Acris Antibodies, Rockville, MD) was used in this analysis according to the manufacturer’s recommendations. All images were obtained using a high throughput Aperio AT2 Digital Pathology Scanner automated digital image system (Leica, Germany). The samples were evaluated independently and a total of 5 factors were observed, and graded according to International Cartilage Repair Society macroscopic evaluation of cartilage repair.8 The highest score (24) was assigned to the ideal repair result (i.e., truly regenerated tissue), and the lowest score (1) was assigned to the poorest repair result in evaluated parameters. Results were calculated as mean ± standard deviation (SD). Statistical significance was determined by t test, and results were considered to be significant if P < 0.01 (**) and P < 0.001 (***).
Results
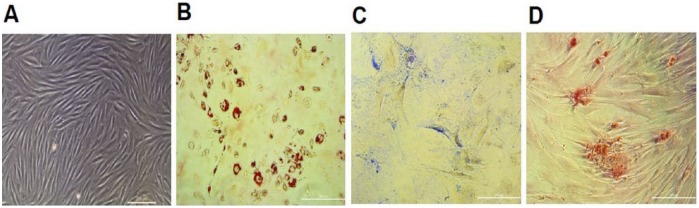
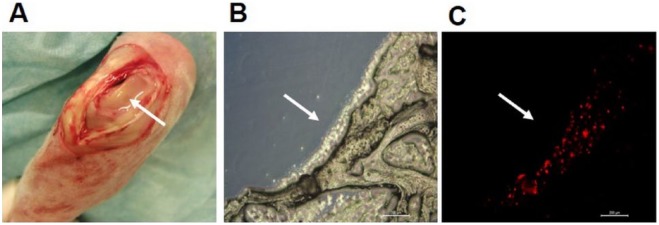
Successfully isolated and ex vivo expanded ADSCs from rabbit adipose tissue showed typical morphology of fibroblast-like MSCs ( Fig. 1A ) and maintained their proliferation capacity without signs of senescence during all passages. According to MTS proliferation assay, a significant increase in proliferation was determined for ADSCs from the second passage during 7 days of cultivation in vitro (data not shown). Differentiation capacity of cultivated ADSCs into 3 mesenchymal cell lines were also confirmed by adequate staining ( Fig. 1B-D ). ADSCs at passage 2 were labeled with PKH26 and this cell suspension was used in an open arthrotomy to fill the cartilage defect in joint faced upward and held stationary for 10 minutes ( Fig. 2A ). The result of this control experiment confirmed the presence of transplanted ADSCs directly in the defect area in cryosections 7 days postimplantation ( Fig. 2B and C ). The platelet level in autologous PRP prepared by our simple and cost-effective method was increased 3.48 ± 0.20 times over the baseline level. This result corresponds to our previously published data,9 in which positive effects of PRP on proliferation and migration of human MSCs and chondrocytes were demonstrated in vitro.
Figure 1.
Phase contrast of cultured living rabbit ADSCs (A) and differential potential of rabbit ADSCs in vitro after passage 2: staining with Oil red O (B), Alcian blue (C), and Alizarin red (D). Scale bar = 200 µm.
Figure 2.
Transplantation of ADSCs into a chondral defect in a rabbit knee by the local adherent technique (A). Images of cryosection demonstrating the presence of PKH26-labeled ADSCs in the chondral defect under light (B) and fluorescent (C) microscopy 7 days postoperatively. Scale bar = 200 µm. Arrows indicate the defect filled with cell suspension.
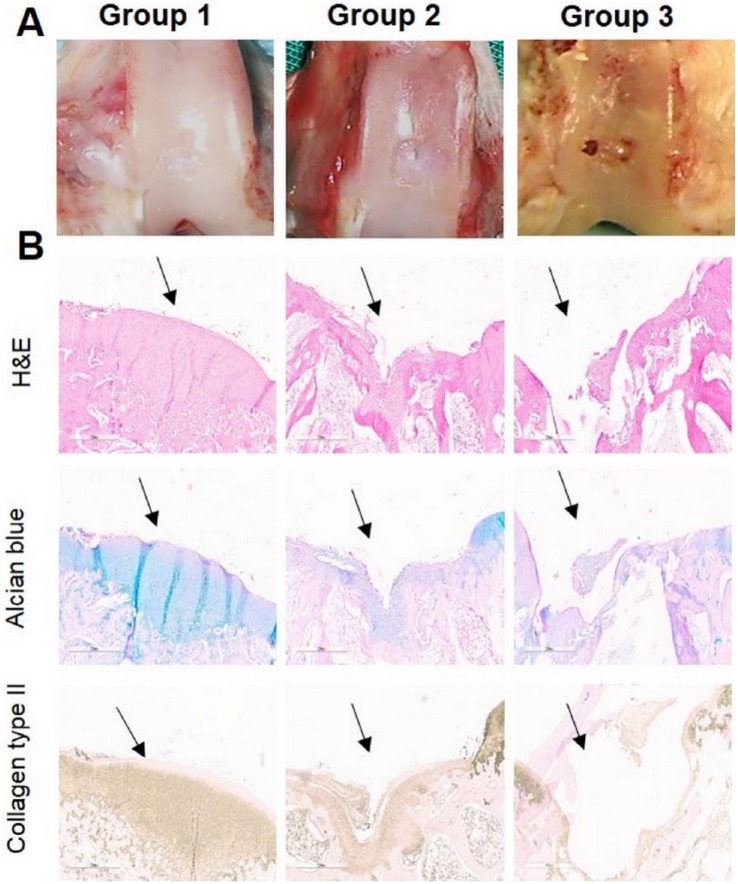
At the end of our in vivo study, macroscopic observation showed irregularities with a surface color different from the surrounding cartilage in Group 2 ( Fig. 3A ). The surface of defect in ADSC-treated Group 1 showed greater regularity with smooth surface and the demarcation between repaired tissue and neighboring cartilage was less distinct ( Fig. 3A ). Histological analysis supported our findings during macroscopic observation. At week 12, histological analysis of Group 2 showed no hyaline cartilage–like tissue formation, and disorganized cells were embedded in a fibrous and poorly organized ECM in areas corresponding to microfracture holes ( Fig. 3B ). Repaired tissue in Group 1 was integrated with the surrounding healthy cartilage, and a great amount of small rounded cells was observed ( Fig. 3B ). In the negative control group (Group 3), the chondral defects were not filled with new tissue during the study; furthermore, cartilage defects were further enlarged. As seen in Figure 3B , the expression of collagen type II was observed except the untreated Group 3. Group 2 showed a reduced staining intensity in collagen type II, and Group 1 showed a slightly reduced staining intensity in the newly formed tissue than in the normal cartilage. When compared with untreated Group 3, both experimental groups (Groups 1 and 2) showed a significant increasing in scores in all parameters P < 0.001 (***) and P < 0.01 (**) ( Fig. 4 ). The differences between Groups 1 and 2 were also significant P < 0.01 (**) according to macroscopic scoring system used in our study.
Figure 3.
Representative images of macroscopic (A), histological and immunohistochemical observation (B) of chondral defects at 12 weeks postoperatively repaired by microfracture + PRP (Group 1), transplantation of autologous ADSCs + PRP (Group 2), and without any treatment (Group 3). Arrows show the defect. Scale bar = 600 µm (magnification 20×).
Figure 4.
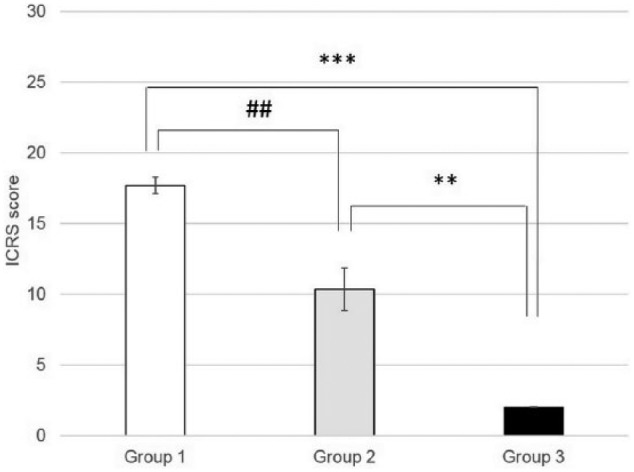
International Cartilage Repair Society macroscopic assessment scale. Values are expressed as mean ± SD (n = 3). **P < 0.01, ***P < 0.001 versus the control group; ##P < 0.01, Group 1 versus Group 2.
Discussion
In the present study, we found that injection of autologous PRP intraarticularly may enhance cartilage repair in the treatment of focal chondral defect with ADSCs transplanted by local adherent technique (Group 1). Huh et al. demonstrated that microfracture following PRP injection resulted in repaired cartilage that was more histologically differentiated than that from microfracture alone.10 These findings coincide with those reported previously, in which microfracture was tested as a treatment option.11-13
In order to enhance the number of stem cells and their adherence directly into the cartilage defect without disrupting subchondral structures, the application of ex vivo expanded ADSCs by local adherent technique followed by PRP injection was used to improve the healing process. The concentration of growth factors and the number of MSCs used in this approach is unambiguously higher than that derived via bleeding from the bone marrow.
Adipose tissue is now considered an attractive source of MSCs because of the large numbers of cells that can be harvested with relatively little donor morbidity. Compared with bone marrow–derived mesenchymal stem cells (BMSCs), ADSCs are more easily cultured and grow more rapidly. The main benefits of ADSCs are that their proliferation and differentiation potentials and telomerase are less affected by age than those of BMSCs.14 ADSCs, like BMSCs, are negative for MHC class II molecules, CD80-B7, and CD40. By contrast, ADSCs prohibit B cell proliferation, decrease immunoglobulin production, and restrict B cell functions more significantly than BMSCs.15
Interestingly, some experimental in vivo studies show only limited cartilage formation by chondrogenic differentiation of the injected MSCs.16,17 Probably the mode of action of MSCs in OA modifying mechanism is different and more complex, including paracrine actions on microenvironment, stimulation of locally present progenitor cells to proliferate and repair OA damage, or attraction of circulating endogenous progenitor cells.18
MSCs have been injected intraarticularly in many preclinical studies as a treatment for OA in animal models including sheep, rabbit, horses, rats, mouse, and guinea pig.19 In a rabbit model documented by ter Huurne et al., a single injection of green fluorescent protein-labeled ADSCs into the knee joints of mice with early-stage collagenase-induced OA inhibits synovial thickening, formation of enthesophytes associated with ligaments, and cartilage destruction on day 42.20 In another study rabbits showed lower degree of cartilage degeneration, osteophyte formation, and subchondral sclerosis than control group at 20 week after intraarticular injection of MSCs isolated from infrapatellar fat pad.21 Animal studies have shown beneficial effects of MSCs on cartilage morphology and histology in these OA models.22 These successful preclinical studies led to the initiation of many clinical trials.
The number of preclinical studies demonstrating the efficacy of co-transplantation of ADSCs and PRP in cartilage injury model system is limited. Van Pham et al. transplanted ADSCs cultured with 15% PRP into the articular cartilage injury model of NOD/SCID mice and their results showed that PRP-pretreated ADSCs improved healing of injured articular cartilage more effectively than untreated ADSCs.23 Xie et al. in their comparative study described the effect of PRP scaffold on chondrogenic differentiation and cartilage regeneration in vivo of rabbit BMSCs and ADSCs.24 Results of this study showed the ability of ADSC seeded constructs to developed into functional chondrocytes secreting cartilaginous matrix in rabbits at 9 weeks postimplantation. Although BMSCs showed higher proliferation rate, expression of cartilage-specific genes and proteins, as well as subchondral bone regeneration than ADSCs.
Results of our study showed a superior role of autologous ADSCs transplantation following with PRP injections to microfracture with PRP injection in the treatment of chondral lesions. There are some shortcomings of our preliminary study, but they do not decrease the significance of this study. The follow-up period was too short and the number of animals was limited. Also creating another control groups to follow the effect of treatment options alone would increase the strength of the study. Further studies with well-designed randomized controlled trials will advance and extend the clinical application of PRP as a coadjuvant to stem cell therapy to achieve successful human cartilage injury treatment with long-term effect.
Footnotes
Acknowledgment and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Slovak Research and Development Agency under Contract No. APVV-0684-12 and by VEGA Grant 1/0217/16.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by the institutional ethical committee of the University of Veterinary Medicine and Pharmacy in Kosice (ID: 14/2014).
Animal Welfare: All experimental procedures involving animals were performed in accordance to guidelines for the care and use of laboratory animals approved by State Veterinary and Food Administration of the Slovak Republic.
References
- 1. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;47:489-96. [DOI] [PubMed] [Google Scholar]
- 2. Spakova T, Rosocha J, Lacko M, Harvanova D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91:411-7. [DOI] [PubMed] [Google Scholar]
- 3. Kreuz P, Steinwachs M, Erggelet C, Krause S, Konrad G, Uhl M, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14:1119-25. [DOI] [PubMed] [Google Scholar]
- 4. Steadman J, Rodkey W, Briggs K. Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg. 2002;15:170-6. [PubMed] [Google Scholar]
- 5. Koga H, Shimaya M, Muneta T, Nimura A, Morito T, Hayashi M, et al. Local adherent technique for transplanting mesenchymal stem cells as a potential treatment of cartilage defect. Arthritis Res Ther. 2008;10:R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu L, Cai X, Zhang S, Karperien M, Lin Z. Regeneration of articular cartilage by adipose tissue derived mesenchymal stem cells: perspectives from stem cell biology and molecular medicine. J Cell Physiol. 2013;228:938-44. [DOI] [PubMed] [Google Scholar]
- 7. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211-28. [DOI] [PubMed] [Google Scholar]
- 8. van den Borne MPJ, Raijmakers NJH, Vanlauwe J, Victor J, de Jong SN, Bellemans J, et al. International Cartilage Repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in autologous chondrocyte implantation (ACI) and microfracture. Osteoarthritis Cartilage. 2007;15:1397-402. [DOI] [PubMed] [Google Scholar]
- 9. Amrichova J, Spakova T, Rosocha J, Harvanova D, Bacenkova D, Lacko M, et al. Effect of PRP and PPP on proliferation and migration of human chondrocytes and synoviocytes in vitro. Cent Eur J Biol. 2014;9:139-48. [Google Scholar]
- 10. Huh SW, Shetty AA, Kim SJ, Kim YJ, Choi NY, Jun YJ, et al. The effect of platelet rich plasma combined with microfracture for the treatment of chondral defect in a rabbit knee. Tissue Eng Regen Med. 2014;11:178-85. [Google Scholar]
- 11. Garcia-Alvarez F, Castiella T, Grasa JM, Monzón M, Laclériga A, Palanca D. Autologous platelets and articular surface repair in an experimental model. J Orthop Sci. 2005;10:237-9. [DOI] [PubMed] [Google Scholar]
- 12. Karakaplan M, Elmalı N, Mirel E, Şahin N, Ergen E, Elmalı C. Effect of microfracture and autologous-conditioned plasma application in the focal full-thickness chondral defect of the knee: an experimental study on rabbits. J Orthop Surg Res. 2015;10:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Milano G, Deriu L, Sanna Passino E, Masala G, Manunta A, Postacchini R, et al. Repeated platelet concentrate injections enhance reparative response of microfractures in the treatment of chondral defects of the knee: an experimental study in an animal model. Arthroscopy. 2012;28:688-701. [DOI] [PubMed] [Google Scholar]
- 14. Mirsaidi A, Kleinhans KN, Rimann M, Tiaden AN, Stauber M, Rudolph KL, et al. Telomere length, telomerase activity and osteogenic differentiation are maintained in adipose-derived stromal cells from senile osteoporotic samp6 mice. J Tissue Eng Regen Med. 2012;6:378-90. [DOI] [PubMed] [Google Scholar]
- 15. Saka Y, Furuhashi K, Katsuno T, Kim H, Ozaki T, Iwasaki K, et al. Adipose-derived stromal cells cultured in a low-serum medium, but not bone marrow-derived stromal cells, impede xenoantibody production. Xenotransplantation. 2011;18:196-208. [DOI] [PubMed] [Google Scholar]
- 16. Matsumoto T, Cooper GM, Gharaibeh B, Meszaros LB, Li G, Usas A, et al. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum. 2009;60:1390-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464-74. [DOI] [PubMed] [Google Scholar]
- 18. Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wyles CC, Houdek MT, Behfar A, Sierra RJ. Mesenchymal stem cell therapy for osteoarthritis: current perspectives. Stem Cells Cloning. 2015;8:117-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. ter Huurne M, Schelbergen R, Blattes R, Blom A, de Munter W, Grevers LC, et al. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012;64:3604-13. [DOI] [PubMed] [Google Scholar]
- 21. Toghraie FS, Chenari N, Gholipour MA, Faghih Z, Torabinejad S, Dehghani S, et al. Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in rabbit. Knee. 2011;18:71-5. [DOI] [PubMed] [Google Scholar]
- 22. Nadia SK, Mona MA, Amr N, Hnaa A, Irene RA, Naglaa S, et al. Effect of intra-articular injection of mesenchymal stem cells in cartilage repair in experimental animals. Egyptian Rheumatologist. 2014;36:179-86. [Google Scholar]
- 23. Van Pham P, Bui KH-T, Ngo DQ, Vu NB, Truong NH, Phan NL, et al. Activated platelet-rich plasma improves adipose-derived stem cell transplantation efficiency in injured articular cartilage. Stem Cell Res Ther. 2013;4:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xie X, Wang Y, Zhao C, Guo S, Liu S, Jia W, et al. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials. 2012;33:7008-18. [DOI] [PubMed] [Google Scholar]