Abstract
Objective
To establish whether a novel biomaterial scaffold with tunable degradation profile will aid in cartilage repair of chondral defects versus microfracture alone in vitro and in a rat model in vivo.
Design
In vitro—Short- and long-term degradation scaffolds were seeded with culture expanded articular chondrocytes or bone marrow mesenchymal stem cells. Cell growth and differentiation were evaluated with cell morphological studies and gene expression studies. In vivo—A microfracture rat model was used in this study to evaluate the repair of cartilage and subchondral bone with the contralateral knee serving as the empty control. The treatment groups include (1) empty osteochondral defect, (2) polycaprolactone copolymer–based polyester polyurethane–urea (PSPU-U) caffold short-term degradative profile, and (3) PSPU-U scaffold long-term degradative profile. After placement of the scaffold, the rats were then allowed unrestricted activity as tolerated, and histological analyses were performed at 4, 8, and 16 weeks. The cartilage defect was measured and compared with the contralateral control side.
Results
In vitro—Long-term scaffolds showed statistically significant higher levels of aggrecan and type II collagen expression compared with short-term scaffolds. In vivo—Within 16 weeks postimplantation, there was new subchondral bone formation in both scaffolds. Short-term scaffolds had a statistically significant increase in defect filling and better qualitative histologic fill compared to control.
Conclusions
The PSPU short-term degradation scaffold may aid in cartilage repair by ultimately incorporating the scaffold into the microfracture procedure.
Keywords: Cartilage Repair, Microfracture, Rat Model, Biodegradable Scaffold
Introduction
The treatment of articular cartilage defects of the knee remains an ongoing challenge facing an orthopedic surgeon. These lesions are highly prevalent; Curl et al.1 reported 53,569 hyaline cartilage lesions in a review of 19,827 patients undergoing knee arthroscopy. Correspondingly, another investigation demonstrated evidence of articular cartilage abnormality in 66% of 993 consecutive knee arthroscopies.2 Lesions in articular cartilage can cause considerable musculoskeletal morbidity including pain and loss of function. With that comes significant economic implications, especially when considering its progression to osteoarthritis.3 Since these lesions have a poor spontaneous repair potential, they present a clinical treatment dilemma, particularly in young and active individuals.4-6
Several modalities of treatment have been described with varying results.6-9 A frequently used method of treatment is microfracture (MFX): An awl is used to create several holes 3 to 4 mm apart that allow clot to form in the defect without the deleterious thermal effects of a drill.10-13 This clot contains marrow-derived mesenchymal stem cells (MSC), which then produces a fibrocartilage repair with varying amounts of type II collagen content.10-12 Its widespread use as a first-line treatment option can be explained by its technical simplicity, low morbidity, and cost-effectiveness.14
Potential problems with MFX include an uneven repair fill and a mixed quality of resultant tissue.13,14 Consistently restoring hyaline cartilage instead of fibrocartilage after injury and/or MFX continues to remain an elusive challenge that is in need of further investigation. The inferior fibrous quality of resultant tissue post MFX may be due to lack of appropriate mechanical substrate or scaffold for differentiation of MSCs into articular chondrocytes, thus limiting the deposition of neocartilage. Mechanical environment plays a significant role in stem cell differentiation, illustrating that scaffold stiffness should be taken into consideration when design engineering implantable scaffolds.15-17 The scaffolds tested in this report are designed to mimic cartilage substrate stiffness. Our approach was to augment the procedure of MFX by placement of a novel scaffold that (1) is designed to provide structural features and mechanical properties to facilitate cartilage formation, (2) is conducive to cell infiltration by MSC’s with a high void content morphology, and (3) exhibits a degradation profile coordinated with de novo matrix deposition.
Current available synthetic resorbable scaffolds made from thermoplastic polyesters18-21 and polyurethanes22-25 are suboptimal. They suffer from slow elastic recovery and low resilience, low interconnected porosity and low permeability for fluids or passage for tissue ingrowth, and low surface area leading to possibly inadequate amount of loading for active cellular agents at time of implantation. Cross-linked, biointegrative, degradable elastomeric and resilient polycaprolactone copolymer–based polyester polyurethane-urea (PSPU-U) matrix scaffolds used in this study represent a new class of biomaterials. These scaffolds are designed to mimic load-bearing abilities of cartilage, not only by having a similar equilibrium compressive stiffness but also with excellent resilience, recovery from deformation (viscoelasticity), and controlled porosity. They consist of fully interconnected and accessible open-cells with high void content (>95%) morphology which gives it the crucial elements for effective and efficient cell culture and organized tissue ingrowth. All these facets of design make this unique scaffold the ideal platform for potential cartilage growth/repair.26 Our aim was to determine the optimal degradation profile for this novel tunable scaffold with potential cartilage growth/repair.
Hypothesis
The goals of this study were to determine the optimal time for degradation of a cartilage repair scaffold. The two competing processes of matrix half-life and migration of host cells into a scaffold and commencing load bearing have yet to be fully defined. We attempted to bracket the time span of when healing would occur (4 months) initially, and one that would provide a longer half-life (12 months) to determine if a more mature cartilage repair could be achieved by a longer lasting scaffold. We hypothesized that the short-term degradation profile scaffold tested in this report will significantly improve the cartilage repair quality, and result in a more complete filling of the defect volume with repair tissue compared with MFX alone by supporting optimal remodeling of the tissue with mature bone and cartilage.
Materials and Methods
In Vitro Design
Two in vitro experiments were conducted to mimic the cellular environment that the scaffold may encounter post–MFX implantation: chondrocyte and MSC growth and differentiation. While chondrocytes may terminally migrate in, most important are the MSCs that have a potential to differentiate into chondrocytes.
Chondrocyte Isolation and Culture Expansion
Bilateral femurs and tibias were harvested from 8-week-old male inbred Fischer-344 rats with aseptic techniques. Articular chondrocytes were harvested from the femoral head, patella-femoral surface, and bilateral tibial plateaus. Soft tissues were removed carefully under dissection microscope. The bones were then washed with phosphate-buffered saline (PBS) with 2% antibiotic-antimycotic (Fisher Scientific, Pittsburgh, PA) 4 times. The articular cartilage from the femoral head, patella-femoral surface, and bilateral tibial plateaus were shaved off using a No. 15 surgical blade under the microscope. The cartilage fragments were transferred to a 50-mL tube with PBS and 0.05% hyaluronidase (Fisher Scientific, Pittsburgh, PA). The tube was kept at 37°C in a water bath with shaking for 10 minutes. After washing with PBS, the cartilage fragments were cut into about 1-mm slices before being transferred to another 50-mL tube with PBS and 0.2% trypsin (Fisher Scientific, Pittsburgh, PA). The tube was shaken in a water bath at 37°C for 5 minutes. After all cartilage fragments were washed with PBS 3 times, Dulbecco’s modified Eagle medium (DMEM)/F12 medium with 0.5% clostridium histolyticum collagenase (type II) (Sigma-Aldrich, St. Louis, MO) was added to the tube. The tube was shaken in a water bath at 37°C for 1 hour, and was then centrifuged at 1000 rpm for 10 minutes. All supernatants were removed. The pellet on the bottom of the tube was resuspended in DMEM/F12 medium with 10% fetal bovine serum (FBS). Cells were maintained at 37°C, 5% CO2, and 95% humidity with the medium changed every 3 days until use.
Scaffold Procurement and Degradation Determination
The unique PSPU-U matrix scaffolds tested in this study were used with permission from Biomerix Corp. (Somerset, NJ). Datta et al.26,27 demonstrated that by varying chain extension and cross-linking in the finely dispersed biocompatible nonhydrolytically degradable hard segment of the PSPU-U scaffolds, they were able to control the degradation, a characteristic which is not available or attainable in thermoplastic polyesters or polyester urethanes. A wide range of in vitro degradation profiles were obtained varying from 4 to 13 months; both upper and lower limit scaffolds were used for the purposes in this study.
Preparation and Seeding of Scaffolds
Rat Chondrocytes
Rat chondrocytes at passage 3 were used for the test of biocompatibility of the scaffold in vitro. A short-term degradation scaffold and a long-term degradation scaffold that will structurally degrade after 4 and 13 months, respectively, were used. Scaffolds were sterilized with 70% ethanol (EtOH) without any pretreatment. In polytetrafluoroethylene dishes (Fisher Scientific, Pittsburgh, PA), both scaffold types (dimensions 16-mm diameter by 2-mm thick) were loaded with 1 × 104 cells in 10 µL of medium and cultured at 37°C for 4 hours. Then enough medium was added to the dish allow thorough permeation of the scaffolds to saturation. Medium was changed every 3 days. Samples were harvested at 3 and 8 days after seeding, then fixed, dehydrated, and critical point dried for observation. The morphological appearance of cells on the scaffold were observed by scanning electron microscope (SEM) (JEOL, Peabody, MA). Cell proliferation was detected with Quant-iT PicoGreen dsDNA Assay Kit (Life Technologies, Grand Island, NY). RNA was extracted and purified with the RNeasy mini kit (Qiagen, Germantown, MD). The RNA was reverse transcripted to cDNA with the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). The gene expression analysis used primers for GAPDH, collagen type I, collagen type II, aggrecan, and p16INK4a. These were measured by real-time polymerase chain reaction (PCR) study with the SYBR green method. The differences were analyzed with a 2-tailed t test.
Rat MSCs
The differentiation of stem cells on the scaffold was evaluated by using rat MSCs. Rat MSCs at passage 3 were loaded in cell culture plastic plate or on the scaffold with 1 × 105 per well or on each scaffold. After 24 hours, the cells were separated into 2 groups for each culture materials. Cells in the control group were cultured with DMEM/F12 medium containing 1% FBS, 1% insulin-transferrin-sodium selenite supplement (ITS), 200 µM sodium l-ascorbate, and 100 nM dexamethasone. Cells in the experiment groups were cultured with the same medium in addition to 100 ng/mL growth and differentiation factor 5 (GDF5), which has been shown to have chondrogenic differentiation properties.28 Media were changed every 3 days. Cells were harvested after 6 days. Gene expressions of type I collagen and type II collagen were compared with real-time PCR study with the SYBR green method.
PCR Primer Sequences
Rat GAPDH TGCCACTCAGAAGACTGTGG, GGATGCAGGGATGATGTTCT.
Rat Collagen-I GCTGAATCCTTCCGTGTT, AGGGAGGGGACTTATCTG.
Rat Collagen-II AGAGCGGAGACTACTGGATTG, TCTGGACGTTAGCGGTGTT.
Rat Aggrecan ACCCGACAATTTCTTTGC, GGTCTCATCGTCCGCTTC.
Rat P16INK4a TTCTCCTTGGCTTCACTTCTG, ATAGTCCACTCTGTCCCTCC.
Scanning Electrical Microscopy Observation
Cell seeded scaffolds were fixed in 2.5% glutaraldehyde (Sigma-Aldrich, St. Louis, MO) for 2 hours. After washing with buffer, samples were postfixed in 2% osmium tetroxide (Sigma-Aldrich, St. Louis, MO) for secondary 2 hours. After fixation, samples were dehydrated with increasing gradient ethanol solutions until 100% ethanol. Ethanol was replaced by hexamethyldisilazane (HMDS, Sigma-Aldrich, St. Louis, MO) for 3 changes and then critical point dried. Samples were sputter coated with aurum and observed by SEM (JEOL, Peabody, MA).
In Vivo Design
Surgical Method
Thirty 350- to 400-g male Sprague-Dawley rats were anesthetized and maintained with isoflurane, and then placed in the supine position. Standard sterile technique was employed. A medial parapatellar approach was used to enter the knee joint. The patella was dislocated laterally to expose the articular surface of the distal femur. The medial femoral condyle was identified and a 1.5-mm diameter osteochondral defect was created with a custom drill bit down into the subchondral plate without destabilization ( Fig. 5a ). A scaffold was then press fit into the defect site with forceps with the mechanical properties of the scaffold allowing for elastic recoil that secured the scaffold in place ( Fig. 5b ).
Figure 5. (A).
Type I collagen expression in the short- and long-term scaffolds at 3 and 8 days in the rat mesenchymal stem cell (MSC) culture. Cells cultured on both short- and long-term scaffolds had statistically significant less amounts of type I collagen expression than monolayer cultured cells from day 3 to day 8. (*P < 0.05; **P < 0.01). (B) Type II collagen expression in the short- and long-term scaffolds at 3 and 8 days in the rat MSC culture. Statistically significant differences of type II collagen expression were found between the short-term scaffold and the control (both treated with growth factor). There was a statistically significant increase in type II collagen expression in the long-term degradation scaffold versus the control (both without growth factor) (*P < 0.05).
Experimental Design
Rats were allowed unrestricted activity. Each knee was randomized to 1 of 2 treatment groups. The contralateral knee served as the empty control. The arms of the experiment included (1) empty osteochondral defect serving as control (n = 30), (2) short-term degradative profile PSPU-U scaffold (n = 15), and (3) long-term degradative profile PSPU-U scaffold (n = 15).
Histological Preparation and Analysis
Rats were sacrificed at 4, 8, and 16 weeks postimplantation and the femurs were harvested and placed in formalin. Samples were then decalcified, dehydrated, and embedded in paraffin and cut into 7 µm sections for histologic examination with Safranin O/Fast green staining. Immunochemical staining for collagen II was performed using a collagen staining kit (Chondrex, Redmond, WA) following the manufacturer’s instructions. The cartilage defect was measured histologically for percent surface and volume fill between implantation side and contralateral control side at each time point. The differences were analyzed with a 2-tailed t test.
Results
In Vitro
Microscopic and Proliferation Findings
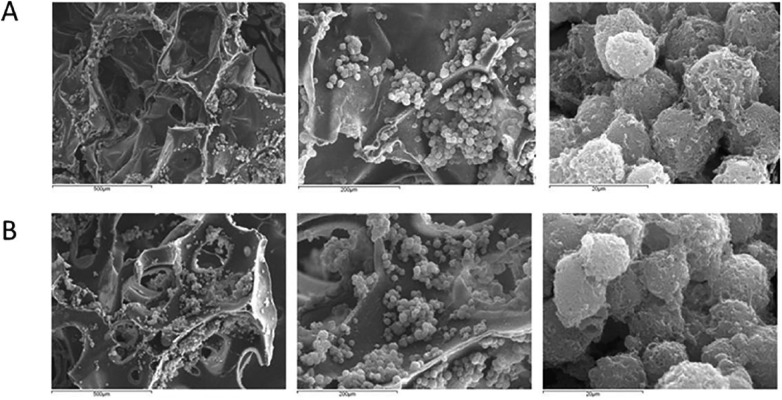
SEM observation showed large cartilage nodule formation at eight days with cells seen on the surface and on the inside of the short- and long-term scaffolds ( Figs. 1 and 2 ). The scaffolds have a reticulated structure reminiscent of trabecular bone. The pore size for the short-term scaffold is 125 to 400 µm in diameter whereas the long-term scaffold has an increased range up to 900 µm. Pico green detection of rat chondrocyte proliferation in the short- and long-term scaffolds at 3 and 8 days showed cell number increased at day 8 compared with day 3 in both kinds of scaffolds. After the same amount cells were loaded on all the scaffolds, the short-term scaffolds had more cells when compared with long-term scaffolds. The proliferation was seen faster in long-term scaffolds than in short-term scaffolds (Figure 3).
Figure 1.
Scanning electron microscopy observation of cells at 8 days. Cells were seen growing on the surface and inside of the (A) short-term and (B) long-term degradation scaffolds.
Figure 2. (A).
Phase contrast photomicrograph of chondrocytes growing on a polycaprolactone copolymer–based polyester polyurethane-urea (PSPU-U) scaffold (40× original magnification) (B) 100× magnification of original image.
Figure 3.

Pico green detection of rat chondrocyte proliferation in the short- and long-term scaffolds at 3 and 8 days. After the same amount cells were loaded on all the scaffolds, the short-term degradation scaffolds had more cells when compared to long-term degradation scaffolds. The proliferation was seen faster in long-term degradation scaffolds than short-term degradation scaffolds (*P < 0.05; **P < 0.01).
Gene Expression Analysis
Rat Chondrocytes
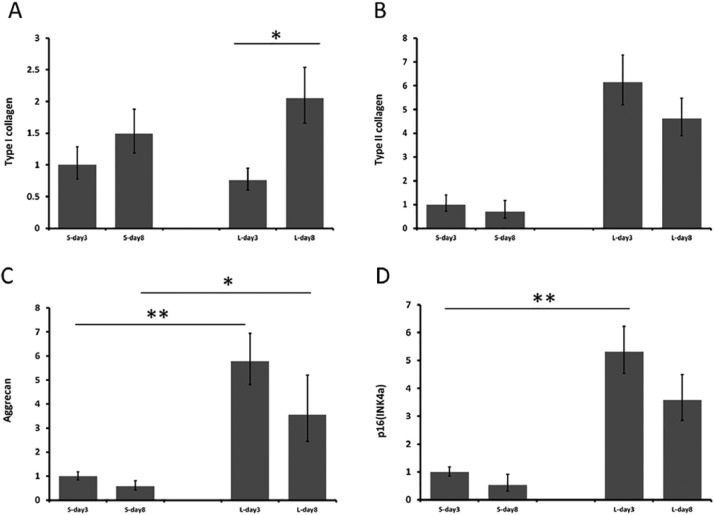
A significant increase of type I collagen expression was observed in long-term degradation scaffolds from day 3 to day 8 ( Fig. 4a ). There was an increased type II collagen expression in the long-term degradation scaffolds compared with short-term ones; however, the differences did not reach statistical significance ( Fig. 4b ). Long-term degradation scaffolds showed significantly higher levels of aggrecan expression than short-term degradation scaffolds ( Fig. 4c ). However, the expressions of both type II collagen and aggrecan were maintained at a similar level between day 3 and day 8 on both scaffolds.
Figure 4. (A).
Type I collagen expression in the short- and long-term scaffolds at 3 and 8 days. A significant increase of type I collagen expression is seen in long-term degradation scaffolds from day 3 to day 8. (B) Type II collagen expression in the short- and long-term scaffolds at 3 and 8 days. Long-term degradation scaffolds had higher levels of type II collagen expression than short-term degradation scaffolds but the differences did not reach statistical significance. (C) Aggrecan expression in the short and long term scaffolds at 3 and 8 days. Long-term degradation scaffolds showed significantly higher levels of aggrecan expression than short-term degradation scaffolds. (D) p16INK4a expression in the short- and long-term scaffolds at 3 and 8 days. Cells on long-term degradation scaffolds showed higher level of p16INK4a. (*P < 0.05; **P < 0.01).
The expression of p16INK4a expression decelerates the cell cycling from G1 phase to S phase. Thus, p16INK4a has the capacity to arrest cells in the G1-phase of the cell cycle and plays a role in irreversible growth arrest termed cellular senescence.29 Cells on long-term scaffolds showed higher level of p16INK4a. The short-term scaffold had more cells with a lower level of expression of p16INK4a ( Fig. 4d ).
Rat MSC Culture
Gene expression of type I collagen and type II collagen were compared among MSCs cultured with a monolayer condition on the cell culture dishes or on the 2 types of scaffolds. Cells cultured on both short- and long-term scaffolds had statistically significant less amounts of type I collagen expression than monolayer cultured cells (P < 0.01) ( Fig. 5a ). In contrast, cells on both types scaffolds had higher levels of type II collagen than cells cultured monolayer. Statistically significant differences of type II collagen expression were found between the short-term scaffold and the control (both treated with growth factor) (P < 0.05). In addition, there was a statistically significant increase in type II collagen expression in the long-term degradation scaffold versus the control (both not treated with growth factor) (P < 0.05) ( Fig. 5b ).
In Vivo
Macroscopic Findings
At 4 weeks, repair tissue is sparse in the control specimen, but appears to increase by 8 and 16 weeks while remaining less than both experimental scaffolds. In the short- and long-term scaffolds, repair tissue had formed in the defect at 4 and 8 weeks at relatively similar rates. By 16 weeks, however, there was a more complete defect filling seen in the short-term scaffolds compared with the long-term scaffolds ( Figs. 6 and 7 ).
Figure 6. (A).
Macroscopic image showing intraoperative image of a control knee after microfracture (MFX) (B) Macroscopic image showing intraoperative image of an experimental knee after MFX and implantation of scaffold.
Figure 7.
Macroscopic images showing postoperative images of both of the experimental knees containing the short- and long-term scaffold at 16 weeks.
Histological Evaluation: Safranin O/Fast Green
In the short-term scaffold group, at 4 weeks the resilient conformal scaffold interfaced tightly with the surrounding tissue. At 8 weeks, chondrocytes (unclear whether they are newly migrated chondrocytes or bone marrow cells that have differentiated into chondrocyte-like cells) were seen at the defect–subchondral bone interface. At 16 weeks, the defect showed new subchondral bone formation ( Fig. 8a ). By comparison, the control specimen exhibited fibrous tissue ingrowth at the cartilage defect at all 3 time points ( Fig. 8b ). The long-term scaffold also incorporated with chondrocytes and new subchondral bone formation, but at 16 weeks there was less cartilage repair than the short-term degradation scaffold ( Fig. 8c ). Again, by comparison, the control specimen exhibited fibrous tissue ingrowth at the cartilage defect at all 3 time points ( Fig. 8d ). The quality of tissue in the scaffold groups varied with the shorter term scaffold having a higher percentage of hyaline like tissue present earlier in the repair process compared with the longer term scaffolds, which exhibited a predominantly a fibrocartilaginous repair tissue.
Figure 8.
Photomicrograph images after Safranin O staining showing defect area after 16 weeks in (A) short-term scaffold with microfracture (MFX) (40× original magnification). (B) Corresponding control with MFX alone. (C) Long-term scaffold with MFX. (D) Corresponding control with MFX alone (the arrow bar in each photo showing unhealed surface of the joint).
Histological Evaluation: Type II Collagen Staining
At 4 weeks, both scaffolds along with control groups demonstrated positive type II collagen staining on the surface of the adjacent intact cartilage as wells as at the surface of the defect ( Fig. 9 ). At 16 weeks in the control groups most of the staining occurred deep in the defects. In contrast, the short-term scaffold demonstrated a positive staining area that was still seen in the top half of the defect. The long-term scaffold showed a positive stained area at the edge of the cartilage defect as well as in the deep area among the struts of the scaffold within the area that between newly formed bone tissue and the surface of the defect ( Fig. 10 ).
Figure 9.
Photomicrograph images after type II collagen staining showing defect area after 4 weeks in (A) short-term scaffold corresponding control with microfracture (MFX) alone (40× original magnification). (B) Short-term scaffold with MFX. (C) Long-term scaffold in corresponding control with MFX alone. (D) Long-term scaffold with MFX.
Figure 10.
Photomicrograph images after type II collagen staining showing defect area after 16 weeks in (A) Short-term scaffold corresponding control with MFX alone (40× original magnification). (B) Short-term scaffold with MFX. (C) Long-term scaffold in corresponding control with MFX alone. (D) Long-term scaffold with MFX.
Quantitative Analysis of Defect Filling
The volume filling of the cartilage defect was greater in both of experimental scaffolds compared with that of the control. At 8 and 16 weeks in the long-term scaffold, there was a smaller quantitative cartilage defect compared with the control. Similarly, the short-term scaffold had an increase in cartilage defect filling compared with the control at all 3 time points with a statistically significant increase seen at 8 and 16 weeks ( Fig. 11 ).
Figure 11.
Bar graphs showing quantitative decrease in defect size at 4, 8, and 16 weeks in both the short- and long-term degradation scaffolds. A significant decrease in defect size is seen in the short term at 8 and 16 weeks (*P < 0.05).
Discussion
Our preliminary studies demonstrated that our tunable degradation profile scaffolds allow cartilage-like matrix deposition in an in vivo osteochondral defect repair in the rat with no significant inflammatory mediated or foreign body reaction. The scaffold used in this study is innovative because of (1) its unique total interconnected pore structure with void volumes more than 95% facilitating tissue regeneration across the entire defect; (2) its cartilage-like compressive and stiffness properties facilitating load bearing immediately after implantation and promoting differentiation of marrow stem cells into articular chondrocytes; (3) its fast elastic recovery and durable tight interface ensuring conformal press fit to the cartilage implantation site; (4) a controllable degradation profile that can be engineered for various structural and biomechanical requirements; and (5) its capability of being manufactured in a well-controlled automated process that is faster and more cost effective than the standard fabrication processes for existing degradable scaffolds.
In this study, we were able to demonstrate that chondrocytes will proliferate on both kinds of scaffolds. PSPU-U cartilage repair scaffolds supported early migration of chondrocytes and MSCs into the scaffolds on placement into articular cartilage defects. The growth of cartilage and subchondral bone progressed in a simultaneous fashion due to the porous structure of the scaffolds. In vitro, the short-term scaffold had more cells with a lower level of expression of p16INK4a. This indicates cell proliferation on the short-term scaffold is more active at the early stage of the cell cycle. While the long-term scaffold showed higher levels of type I collagen, type II collagen, and aggrecan expression. In addition, both type II collagen and aggrecan were maintained at a similar level between day 3 and day 8 on both scaffolds, indicating that no significant dedifferentiation of the cells occurred.
The short-term degradative profile scaffold had a statistically significant increase of volume filling of the cartilage defect than MFX alone. It also had a better qualitative cartilage repair than the long-term degradative profile and MFX alone at 16 weeks. The short-term scaffold facilitated the differentiation of MSCs and promoted chondrogenesis. Of note, it was unclear whether the chondrocytes seen are newly migrated chondrocytes or bone marrow cells that have differentiated into chondrocyte-like cells. The degradation of the short-term scaffold promoted the remodeling of the tissue with mature bone and cartilage at early time points postsurgery and this more closely matched the repair kinetics for articular cartilage.
While MFX is successful in the regeneration of small cartilage defects, it has a wide variance in the quality of the cartilage fill. Our study showed a statistically significant decrease in defect size with a better qualitative histologic fill of the defect at 16 weeks in the short-term degradation scaffold compared to MFX alone in an in vivo model. In addition, the relatively small size of our scaffold allows for easy implantation arthroscopically making it ideal for combination treatment with MFX. A recent prospective cohort study showed that patients undergoing MFX with a “good” grade fill of the defect had a significant improvement in knee function scores, including activities of daily living scores, Short Form–36 physical component subscale, and subjective rating. The “grade” was based on the percentage of volume filling of the defect.14 Similarly another study showed that at 36 months after MFX, patients’ clinical symptoms were correlated best with the defect filling and the overall MRI score.30 Based on the results of our study, combining MFX with our novel short term degradation scaffold, in an in vivo model, will induce bone marrow MSCs to undergo directed differentiation resulting in better cartilage repair than MFX alone.
Articular cartilage repair remains a challenge to the orthopaedic surgeon. The potential of an off the shelf option that can easily be implanted during arthroscopy, such as our scaffold, has the potential to aid significantly in combating the difficulties with repair of cartilage defects by potentially increasing the number of patients that have a consistent good defect fill.
Conclusions
By significantly decreasing the defect size with a better qualitative histologic fill compared with control, a PSPU short-term degradation scaffold may aid in cartilage repair after chondral injury by ultimately incorporating the scaffold arthroscopically into the MFX procedure.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this project was provided by the Division of Research in the Department of Orthopaedic Surgery at Northwell Health.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Ethical approval for this study was obtained from our Institutional Animal Care and Use Committee (IACUC).
Animal Welfare: The present study followed international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation.
References
- 1. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-60. [DOI] [PubMed] [Google Scholar]
- 2. Arøen A, Løken S, Heir S, Alvik E, Ekeland A, Granlund OG, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32(1):211-5. [DOI] [PubMed] [Google Scholar]
- 3. Jackson DW, Simon TM, Aberman HM. Symptomatic articular cartilage degeneration: the impact in the new millennium. Clin Orthop Relat Res. 2001;(391 Suppl):S14-25. [PubMed] [Google Scholar]
- 4. Jackson DW, Lalor PA, Aberman HM, Simon TM. Spontaneous repair of full-thickness defects of articular cartilage in a goat model. A preliminary study. J Bone Joint Surg Am. 2001;83-A(1):53-64. [DOI] [PubMed] [Google Scholar]
- 5. Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487-504. [PubMed] [Google Scholar]
- 6. Buckwalter JA. Evaluating methods of restoring cartilaginous articular surfaces. Clin Orthop Relat Res. 1999;(367 Suppl):S224-38. [DOI] [PubMed] [Google Scholar]
- 7. Minas T, Nehrer S. Current concepts in the treatment of articular cartilage defects. Orthopedics. 1997;20(6):525-38. [DOI] [PubMed] [Google Scholar]
- 8. Gill TJ. The treatment of articular cartilage defects using microfracture and debridement. Am J Knee Surg. 2000;13(1):33-40. [PubMed] [Google Scholar]
- 9. Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212-34. [DOI] [PubMed] [Google Scholar]
- 10. Steadman JR, Miller BS, Karas SG, Schlegel TF, Briggs KK, Hawkins RJ. The microfracture technique in the treatment of full-thickness chondral lesions of the knee in National Football League players. J Knee Surg. 2003;16(2):83-6. [PubMed] [Google Scholar]
- 11. Steadman JR, Rodkey WG, Briggs KK. Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg. 2002;15(3):170-6. [PubMed] [Google Scholar]
- 12. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;(391 Suppl):S362-9. [DOI] [PubMed] [Google Scholar]
- 13. Falah M, Nierenberg G, Soudry M, Hayden M, Volpin G. Treatment of articular cartilage lesions of the knee. Int Orthop. 2010;34(5):621-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87(9):1911-20. [DOI] [PubMed] [Google Scholar]
- 15. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677-89. [DOI] [PubMed] [Google Scholar]
- 16. Evans ND, Minelli C, Gentleman E, LaPointe V, Patankar SN, Kallivretaki M, et al. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur Cell Mater. 2009;18:1-13. [DOI] [PubMed] [Google Scholar]
- 17. Lee J, Abdeen AA, Zhang D, Kilian KA. Directing stem cell fate on hydrogel substrates by controlling cell geometry, matrix mechanics and adhesion ligand composition. Biomaterials. 2013;34(33):8140-8. [DOI] [PubMed] [Google Scholar]
- 18. Widmer MS, Gupta PK, Lu L, Meszlenyi RK, Evans GR, Brandt K, et al. Manufacture of porous biodegradable polymer conduits by an extrusion process for guided tissue regeneration. Biomaterials. 1998;19(21):1945-55. [DOI] [PubMed] [Google Scholar]
- 19. Sikavitsas VI, Bancroft GN, Mikos AG. Formation of three-dimensional cell/polymer constructs for bone tissue engineering in a spinner flask and a rotating wall vessel bioreactor. J Biomed Mater Res. 2002;62(1):136-48. [DOI] [PubMed] [Google Scholar]
- 20. Mikos AG, Temenoff JS. Formation of highly porous biodegradable scaffolds for tissue engineering. Electron J Biotechnol. 2000;3(2). doi: 10.2225/vol3-issue2-fulltext-5 [DOI] [Google Scholar]
- 21. Derwin KA, Codsi MJ, Milks RA, Baker AR, McCarron JA, Iannotti JP. Rotator cuff repair augmentation in a canine model with use of a woven poly-l-lactide device. J Bone Joint Surg Am. 2009;91(5):1159-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang J, Doll BA, Beckman EJ, Hollinger JO. A biodegradable polyurethane-ascorbic acid scaffold for bone tissue engineering. J Biomed Mater Res A. 2003;67(2):389-400. [DOI] [PubMed] [Google Scholar]
- 23. Guelcher SA, Srinivasan A, Dumas JE, Didier JE, McBride S, Hollinger JO. Synthesis, mechanical properties, biocompatibility, and biodegradation of polyurethane networks from lysine polyisocyanates. Biomaterials. 2008;29(12):1762-75. [DOI] [PubMed] [Google Scholar]
- 24. Guelcher SA. Biodegradable polyurethanes: synthesis and applications in regenerative medicine. Tissue Eng Part B Rev. 2008;14(1):3-17. [DOI] [PubMed] [Google Scholar]
- 25. Guan J, Fujimoto KL, Sacks MS, Wagner WR. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials. 2005;26(18):3961-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Datta A, Lavelle LP, Friedman C, Haridas B, inventors; Biomerix Corporation, assignee. At least partially resorbable reticulated elastomeric matrix elements and methods of making same. US9050176 B2. June 9, 2015. [Google Scholar]
- 27. Datta A, Lavelle L, Andrade K, Grande D. Novel biointegrative cross-linked degradable polyurethane scaffold matrix. Paper presented at: Society for Biomaterials Annual Meeting and Exposition; April 10-13, 2013; Boston, MA. [Google Scholar]
- 28. Coleman CM, Vaughan EE, Browe DC, Mooney E, Howard L, Barry F. Growth differentiation factor-5 enhances in vitro mesenchymal stromal cell chondrogenesis and hypertrophy. Stem Cells Dev. 2013;22(13):1968-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ohtani N, Yamakoshi K, Takahashi A, Hara E. The p16INK4a-RB pathway: molecular link between cellular senescence and tumor suppression. J Med Invest. 2004;51(3-4):146-53. [DOI] [PubMed] [Google Scholar]
- 30. Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119-25. [DOI] [PubMed] [Google Scholar]