Abstract
This article is a review of the current understanding of the etiology, pathogenesis, and how to diagnose and treat knee osteochondritis dissecans (OCD) followed by an analysis of and outcomes of the treatments available. OCD is seen in children and adolescents with open growth plates (juvenile OCD) and adults with closed growth plates (adult OCD). The etiology of OCD lesions remains unclear and is characterized by an aseptic necrosis in the subchondral bone area. Mechanical factors seem to play an important role. Clinical symptoms are unspecific. Thus, imaging techniques are most important. Regarding treatment, a tremendous number of publications exist. Spontaneous healing is expected unless there is an unstable fragment, and treatment involves rest and different degrees of immobilization until healing. Patients with open physes and low-grade lesions have good results with conservative therapy. When surgery is necessary, the procedure depends on the stage and on the state of the cartilage. With intact cartilage, retrograde procedures are favorable. When the cartilage is damaged, several techniques can be used. While techniques such as drilling and microfracturing produce reparative cartilage, other techniques reconstruct the defect with additional osteochondral grafts or cell-based procedures such as chondrocyte transplantation. There is a tendency toward better results when using procedures that reconstruct the bone and the cartilage and there is also a trend toward better long-term results when comorbidities are treated. Severe grades of osteoarthrosis are rare.
Keywords: osteochondritis dissecans, etiology, pathology, general, knee joint, imaging
Introduction
Osteochondritis dissecans (OCD) is a common cause of knee disorder among skeletally immature and adult patients and it occurs when a small piece of subchondral bone begins to separate from its surrounding area due to a disturbance of the local blood supply. Finally, a small fragment of bone and the cartilage covering it may begin to crack and get loosened. It was Ambroise Paré and not Paget, as was previously assumed, who was the first (in 1870) to describe such loose bodies found in a joint,1 The term osteochondritis dissecans was initially mentioned in 1888 by König1 who suggested 3 possible causes of the development of loose bodies:
Direct trauma with acute osteochondral fracture
Minimal trauma that develops into osteonecrosis and consecutive fragmentation
No evidence of trauma with a spontaneous development, which König called “osteochondritis dissecans” (OCD).1
The exact prevalence of OCD is unknown but rates of between 15 and 29 per 100,000 have been reported.2,3 Kessler et al.4 have shown that the incidence of OCD of the knee in patients aged 6 to 19 years was 9.5 per 100,000 and 15.4 and 3.3 per 100,000 for male and female patients, respectively ( Table 1 ). Patients aged 12 to 19 years represented the majority of OCD, with an incidence of 11.2 per 100,000 versus 6.8 per 100,000 for those aged 6 to 11 years. In summary, male patients had much greater incidence of OCD and almost 4 times the risk of OCD compared with female patients.4
Table 1.
Osteochondritis Dissecans (OCD) Epidemiology.
| Peak incidence: age of 15 years5 |
| Prevalence in Sweden: 6/10 0005 |
| Incidence between ages of 2 to 5 years: 0 |
| Incidence between ages of 6 to 19 years: 9.5/100,000 Males: 15.4/100,000 Females: 3.3/100,000 |
| Incidence between ages 6 and 11 years: 6.8/100,000 Males: 11.1/100,000 Females: 2.3/100,000 |
| Incidence between ages of 12 and 19 years: 11.2/100,000 Males: 18.1/100,000 Females: 3.9/100,0004 |
| Risk for OCD at the knee Ages 6-11 vs 12-19 years: 1:3.3 |
| Risk for OCD at the knee Males: 3.8 Females: 14 |
OCD in this article means a chronic disease of the involved joint that has not resulted from an acute trauma. It is consisting of a fresh osteochondral or chondral lesion (OCL) and with or without a loose osteochondral fragment. OCD is usually regarded as either juvenile OCD (=JOCD) (occurring with an open epiphyseal plate) or adult OCD (=AOCD) (after the physis has closed). These definitions suggest a greater chance of a successful nonsurgical management in patients where the physes are still open than in adult patients with OCD lesions where the physes are already closed.6
This article is a review on what is known about OCD and with a special focus on the largest joint affected; the knee joint. In a future article, the elbow and the ankle joint will be addressed.
Etiology
One may divide the OCD etiology into 4 different possible causes; traumatic, ischemic, hereditary, and idiopathic7,8 ( Table 2 ). However, etiology of multifactorial origin is the most probable cause.
Table 2.
Etiological Factors in Osteochondritis Dissecans.a
| Etiological Factor | Knee Joint |
|---|---|
| Predominant location | Medial condyle and lateral condyle |
| Trauma Microtrauma |
++ +++ |
| General genetics | + |
| Infection | + Exclusion |
| Vascularity | + |
| Constitutional | Medial condyle: Varus malalignment Lateral condyle: Valgus malalignment Discoid lateral meniscus |
| Metabolic | +++ |
Evidence: ++++ = very high; +++ = high; ++ = clinical observation + = assumption.
Trauma: Probably caused by indirect trauma as seen on the most common OCD lesion, the posteromedial medial femoral condylar position.7 Repetitive stress to immature knees and on the tibial spine on the lateral aspect of the medial femoral condyle during internal rotation of the tibia may contribute to the development of human OCD. Such a subchondral stress reaction probably interferes with bony trabecular healing and impedes the ability of the bone to heal. Owing to the lack of underlying support of the cartilage, later stages can lead to a separation of the articular cartilage bone connection with partial loosening of the involved osteochondral region.
Ischemia: Poor vascularity and induced ischemia have been described as a potential cause of OCD.8 Some studies have shown difference in vascular pattern that has been seen at the OCD-positioned sites. Such a joint morphology combined with focal repeated trauma on this site with a unique vascular architecture may trigger ischemic events and subsequent OCD.9
Genetics: Several authors have investigated a potential genetic link for OCD but still genetic and developmental factors in the development of OCD remain relatively unstudied. Skagen et al.10 propose that OCD lesions are caused by an alteration in chondrocyte matrix synthesis causing an endoplasmic reticulum storage disease phenotype, which disturbs or abrupt endochondral ossification. Furthermore, cases of identical twins presenting with a similar disease process are highly suggestive of a genetic component.11
General OCD Pathogenesis
Although the etiology is not fully clear, the pathogenesis of OCD is relatively well understood. Independent from the etiology, at least 4 stages can be described.
Stage 1
OCD lesions start in the subchondral bone with intraosseous subchondral osteopenia, which is only detectable with magnetic resonance imaging (MRI) or bone scans.
Stage 2
The lesions are associated with an intraosseous edema of the subchondral bone.12-14 A bone bruise is probably the initial stage and subchondral trabecular microfractures might be the morphological correlate of the bone marrow edema.15-20
Stage 3
The continuing, natural course is characterized by a radiologically detectable sclerotic ring, which demarcates the lesions from the surrounding healthy bone. The center of the lesions is thought to be an osteonecrosis (see section “OCD Histology”). At this stage, the cartilage still seems to appear intact in imaging techniques such as MRI and computed tomography (CT).12
Stage 4
A “softening phenomenon and alteration in the mechanical properties of cartilage”19 promotes a reaction of the bone at the border of the necrosis toward the healthy surrounding bone. Still remaining mechanical loads are probably responsible for the cartilage now being involved and showing signs of separation. Finally, the ongoing natural course leads to a loosening of an osteochondral fragment resulting in a single loose body or the occurrence of multiple fragments (the so-called “malicious variant” first described by Wagner.21,22
There are several biomechanically orientated analyses concerning the suggestion of a biomechanical etiology. Rehbein23 in 1950 was able to experimentally produce loose bodies in knee joints of dogs by artificially produced repetitive stress. The specimens histologically resembled those findings described below, which were obtained from loose bodies in the knee joints of humans.
An experimental trial using plane and stereoscopic knee models made from epoxy resins,24 as well as a finite elements analysis of the distal femur,25 revealed peak stresses in the region where an OCD lesion occurs. Using photosensitive foils in the knee mimicking the clinically obvious factors, such as varus or valgus malalignment (knee) with stable and unstable ligaments, exhibited a significant stress concentration in those areas well-known for the clinical development of OCD lesions.26,27
Seen clinically, bone bruises following a bone contusion of the knee are assumed to be primary lesions of the subchondral trabecular bone, which probably initiates an OCD.17,18
OCD Histology
Histology of an advanced lesion is presented in Figure 1 . Green and Banks28,29 were, to our knowledge, the first to describe a subchondral osteonecrosis as the initial lesion with still intact overlying cartilage.
Figure 1.
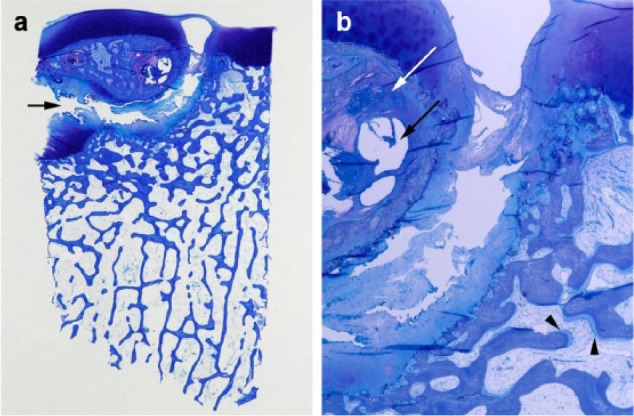
(a) Histology of an advanced osteochondritis dissecans (OCD) lesion (knee joint, toluidine blue) showing a partially loosened “joint mouse” with a broad cleft (arrow) between the partial loose body (top) and the normal subchondral bone (bottom). In the lesion, subchondral bone cysts are visible (C) and necrotic areas (N). (b) Magnification of fig, 1a: The lesion exhibits an area of osteonecrosis (white arrow), subchondral bone cysts (black arrow), and in the “mouse bed,” the sublesional bone a thickened osteoid layer as a sign of enlarged endostal activity (arrowheads).
Owing to the loss of the mechanical support of the bone for the cartilage, the ongoing process results in secondary damage to the cartilage layer.30 The authors suggested that healing might be possible by creeping substitution provided that the overlying cartilage is still intact. Histological examinations of loose bodies revealed that hypertrophy was common and laminar calcification was found in 53%.31,32
Chiroff and Cooke33 detected fibrocartilaginous tissue at the level of separation and in the bony part of the loose bodies, an increased osteoblastic and osteolytic activity under the almost normal cartilage was found. Furthermore, Milgram34 found no bone in half of the loose bodies. Koch et al.35 analyzed 30 specimens from patients aged 16 to 44 years who had advanced stages of OCD and observed a decreased toluidine staining of PH 1 in the cartilage. A reduced number of chondrocytes could be seen as well as fractured areas in the subchondral bone plate and in the cancellous bone. Furthermore, they found areas of enhanced bone resorption and necrotic subchondral bone surrounded by fatty bone marrow.
Uozumi et al20 have described 3 types of histopathological features:
OCD with necrotic subchondral trabeculae
OCD with viable subchondral trabeculae
OCD cartilage without bone trabeculae.
They summarized that “the initial change in the subchondral area is bone necrosis or subchondral fracture; the necrotic bone is then absorbed and replaced by viable subchondral trabeculae or cartilage without bone trabeculae.”20 In contrast, osteonecrosis could not be detected in any of 8 needle biopsies from the center of stable JOCD lesions in the medial femoral condyles without any degenerative changes.36 Only a thick cartilage layer and fibrous tissue, or thin cartilage with mixed cartilage underneath were found, as were subchondral trabeculae and fibrous and fibrocartilage at the areas of separation.
Most recently, an analysis of loose bodies showed that the chondrocytes from the loose bodies displayed a normal behavior and the cells were regarded to be usable for autologous chondrocyte implantation (ACI).37
A meta-analysis of the already published data on histological analyses38 resulted in inconsistent findings: In 7 out of 10 studies, which included the subchondral bone, signs of a bony necrosis had been reported; in 2 out of 11 publications, degenerative or irregular cartilage was mentioned. Regarding the possible underlying etiology, 5 out of 11 articles suggested one major or multiple repetitive microtraumata as the etiological factor. In conclusion, the histological results suggest a focal alteration of cartilage matrix originating from the deep layers of the joint cartilage, potentially the mineralized layer or the subchondral bone.37
Diagnosis of OCD
OCD-related Symptoms
Symptoms are often vague and poorly localized. Different degrees of pain and stiffness may be present; swelling and effusion of the joint and “giving way,” “catching,” or “blocking” of the joint might occur. There are no typical clinical signs for an OCD in any joint.12,30,39,40 The Wilson test, recommended as a clinical diagnostic test at the knee joint is not reliable.41-43
Table 3 presents the OCD classification schemes.
Table 3.
Classification Schemes.
| General | Knee Joint | ||
|---|---|---|---|
| ICRS classification (arthroscopically)44 | Arcq45: 3 radiological stages | ||
| Stage I: stable lesion, continuous, softened cartilage | Rodegerdts and Gleissner46: 5 radiological stages | ||
| Stage II: lesion with partial discontinuity, but stable | |||
| Stage III: lesion with complete continuity, not dislocated | |||
| Stage IV: empty defects or dislocated fragments or loose fragment within the bed | |||
| Bruns classification12 | |||
| X-ray | MRI | ||
| Stage I: | No changes | Bone bruise, edema | |
| Stage II: | Sclerosis | Osteolysis, sclerosis | |
| Stage IIIa: | Partial | Partial loosening, | |
| Loosening | Fluid subchondral | ||
| Stage IVa: | Complete | Joint mouse | |
| Dissection | Empty defect | ||
| Loose body | Loose body | ||
| Bone scans47,48 | Bone scans47,48 | ||
| CT49 | CT49 | ||
| MRI50-53 | MRI50,51,53 | ||
| Open vs closed epiphyses6,54 | Open vs closed epiphyses6,54 | ||
| Arthroscopy50,55 | Arthroscopy50,56 | ||
| Stability57-59 | Stable or unstable57,58,60 | ||
CT = computed tomography; ICRS = International Cartilage Repair Society; MRI = magnetic resonance imaging.
Stages III and IV can be subdivided into the malicious (M) and dissected form (D).
OCD localization schemes are presented in Table 4 .
Table 4.
Localization Schemes.
| Aichroth-Lindholm scheme61,62 |
| Anterior-posterior view: medial condyle: localization “central,” “centrolateral,” and “inferolateral” |
| Anterior-posterior view: lateral condyle: “inferocentral,” “anterior,” and “inferolateral for the lateral condyle |
| Hughston scheme63: |
| Anterior-posterior view: 5 zones from medial to lateral: meniscal, nonmeniscal (medial condyle), intercondylar, nonmeniscal, meniscal (lateral condyle) |
| Lateral view: divided into 2 parts in relation tangent at the dorsal femoral cortex: direct distal or posterior64
or |
| Lateral view: A = Anterior of the Blumensaat’s line, B = Posterior of the Blumensaat’s line C = Most posterior third |
Imaging Techniques for OCD Evaluation
Plain X-rays
Before the use of MRI started, initial changes could only be detected with bone scans, or suspected on conventional radiographs. With the introduction of MRI, it was possible to differentiate stages more easily. However, it is still difficult to estimate reliably the mechanical properties of the cartilage layer.
The initial diagnostic schedule when an OCD lesion is suspected starts with an X-ray in 2 orthogonal planes. The standard series include a standing anterior-posterior (AP) view ( Fig. 2 ), a lateral view with the knee flexed 35°, and a 45°patella sunrise view. Additional special X-ray views could be useful such as a tunnel view bringing the area with the lesion more in line with the imaging plane up.65
Figure 2.
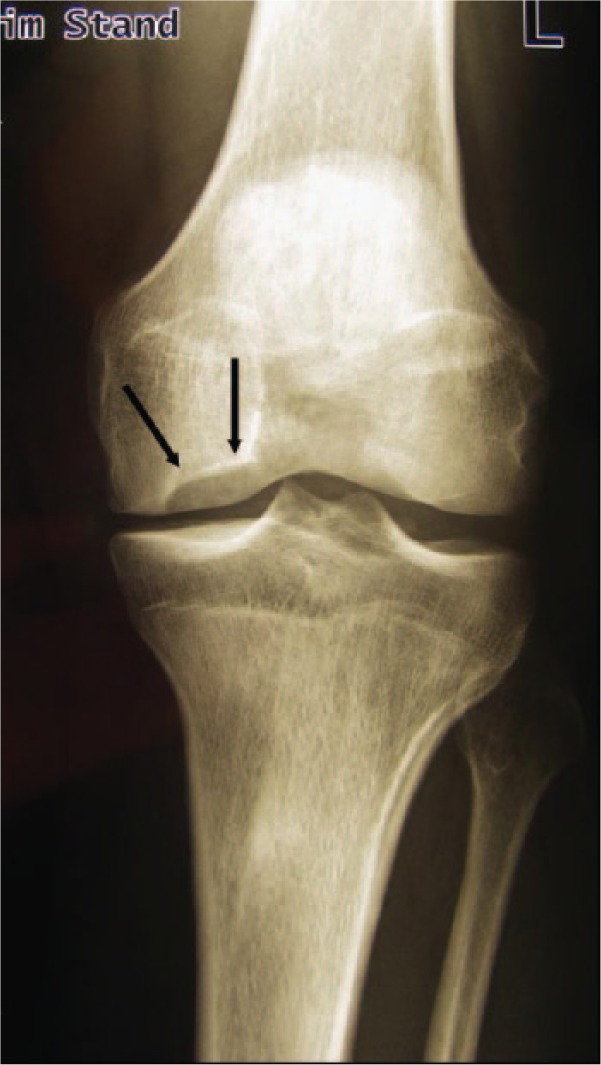
X-ray of the knee. Anterior-posterior view showing an osteochondritis dissecans lesion at the typical location (medial condyle), black arrows indicate stage IV with an empty lacuna. The loose body is not visible.
Magnetic Resonance Imaging
MRI is the method of choice as the second step in an imaging workup ( Fig. 3a and b ). Since the availability of MR systems has increased in the past 10 years, the lack of radiation, the sudden development of higher field strengths (1.5 and 3 T), dedicated coil settings, and high-resolution sequences saved the way for the advance of MRI in musculoskeletal imaging. The regular MR approach uses T1- and T2-weighted images in all 3 spatial directions. The maximal slice thickness should be 3 mm, offering a sensitivity of 96% (specificity 0.96) for detecting osteochondral defects at the talus. Diapola et al.50 have developed a useful MRI system for OCD evaluation with 4 gradings:
Figure 3.
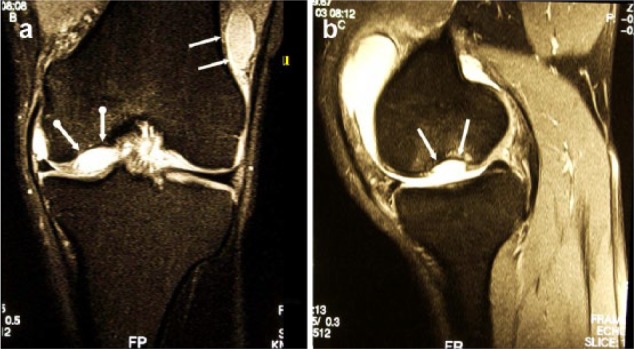
(a) Magnetic resonance imaging (MRI) (proton density fat saturation) of the same knee joint exhibiting the empty lacuna at the medial condyle (dotted white arrows) and the loose body located at the lateral recessus (white arrows), coronal plane. (b) MRI (proton density fat saturation) of the same knee joint exhibiting the empty lacuna at the medial condyle (dotted white arrows) and the loose body located at the lateral recessus (white arrows), sagittal plane.
Stage 1: Thickening of articular cartilage and low signal changes.
Stage 2: Articular cartilage breached, low signal rim behind fragment indicating fibrous attachment.
Stage 3: Articular cartilage breached, high signal changes behind fragment, indicating synovial fluid between fragments and underlying bone
Stage 4: Loose body.
It is also possible to use the arthroscopic International Cartilage Repair Society (ICRS) OCD classification and Guhl’s classsifiaction44,56 when evaluating OCD on MR images (see Arthroscopic Classifications).
To improve the evaluation, MRI can also be performed by injecting gadolinium MR contrast material into the examined joint shortly before the examination. Such a dGemeric MRI gives information about the matrix quality. Using T2-weighted sequences, the presence of a high signal line or a cyst below an osteochondral lesion indicates the presence of fluid and suggests the presence of an unstable osteochondral defect, even though this signal can reflect vascular granulation tissue representing a healing reaction. Proton density images and 3-dimensional T1-weighted sequences with fat saturation using isotropic voxels below 1 mm with a dedicated field of view (14-20 cm) and intravenous contrast material offer a brilliant image impression and can also differentiate subtle changes.65 Using these sequences as well, MRI offers excellent diagnostic capabilities in detecting even unstable osteochondral lesions. Consequently, routinely intra-articular administration is not necessary for evaluating osteochondral lesions.
Using newly implemented high-resolution sequences to differentiate different types of osteochondral defects offers an overall accuracy of more than 90%.66
In the daily clinical routine, 1.5- and 3-T systems are available. Comparing dedicated coil settings on both systems, the image impression might be better using higher field strength (3-T systems). However, 3-T systems have not yet proved to offer better diagnostic results with regard to cartilage lesions.67
Computed Tomography
The 2 important shortcomings of CT are the applied radiation, especially with regard to the age of examined patients, and the lack of visualization of the cartilage. The lack of cartilage visualization can be overcome by using intra-articular contrast material, which can be applied by a direct puncture of the joint and offers an indirect visualization of the cartilage. CT scans can be used to assess the osseous integration after refixation of OCD loose fragments.68
Scintigraphic Examination
Paletta and colleagues69 found that quantitative bone scanning had a 100% predictive value for the prognosis in OCD patients with open physes, but for those with closed physes the predictive value was less.
Cahill and Berg47 have developed a classification useful when to evaluate scintigraphic results of juvenile OCD patients:
0.Normal radiographic and scintigraphic appearance.
1.The lesion is visible on plain radiographs, but bone scans reveal normal findings.
2.The scan reveals increased uptake in the area of the lesion.
3.In addition, there is increased isotopic uptake in the entire femoral condyle.
4.In addition, there is uptake in the tibial plateau opposite the lesion.
Treatment of OCD of the Knee Joint
OCD lesions in the knee joint are located predominantly in the medial femoral condyle and are often associated with a varus malalignment. A minority of OCD lesions are located in the lateral condyle and is associated with valgus malalignment.61,70-72
Lesion Location
Lesions at the lateral femoral condyle can also occur in association with discoid menisci. A lesion at the lateral condyle can develop either primarily with a discoid meniscus or secondarily, after a total resection of a discoid lateral meniscus.73-78 It has been assumed that the altered biomechanics of the knee with a discoid meniscus, or after total lateral meniscectomy, are responsible for the development of an OCD lesion.73-78 The prominence ratio of the lateral condyles of patients with a discoid meniscus is significantly larger than that of controls.78
Only a small number of lesions are located in the patellofemoral joint.12,79,80
Lesion Stability
For both JOCD and AOCD, the indication to follow a conservative therapy or go for a surgical approach depends on the stability of the osteochondral fragment (Table 5). However, what is a stable lesion? Wall et al.81 stated, “A stable OCD was defined as one showing no breach in the articular or the subchondral bone-lesion interface.” Trinh et al.82 realized that their review contained varying definitions for a stable or unstable lesion and adapted them to those used by De Smet et al.51
Table 5.
Treatment options (for references see the text).
| General | Knee Joint | |
|---|---|---|
| Stage I: | ||
| JOCD | Conservative | Conservative |
| AOCD | Conservative | Conservative |
| Stage II: | ||
| JOCD | Conservative, drilling | Conservative, drilling |
| AOCD | Conservative, drilling | Conservative, drilling |
| Stage III: | ||
| JOCD + AOCD | (removal), REFIX, MFX, ACI, AMIC, MOPLA, OAT, BMS Bone + cells |
(removal), REFIX, MFX, ACI, AMIC, MOPLA, OAT, BMS Bone + cells |
| Stage IV: | ||
| JOCD + AOCD | (removal, REFIX, MFX), ACI, AMIC, MOPLA, OAT, BMS Bone + cells allografts |
(removal, REFIX, MFX), ACI, AMIC, MOPLA, OAT, BMS Bone + cells Allografts |
ACI = autologous chondrocyte implantation; AMIC = autologous matrix-induced chondrogenesis; AOCD = adult osteochondritis dessicans; BMS = bone marrow stimulation; JOCD = juvenile osteochondritis dissecans; MFX = microfracture; MOPLA = mosaicplasty ; OAT = osteochondral autograft transfer; REFIX = refixation.
Lesion instability is said to exists if
A line of high-signal deep to the fragment is seen on T2-weighted image on MRI.
An articular fracture, indicated by a high signal, passes through the subchondral bone plate.
A focal, osteochondral defect is present.
A 5-mm diameter, fluid-filled cyst is deep to the lesion.
Conservative Treatment
A few articles have been published that differentiate between JOCD and AOCD advocating conservative treatment but with different treatment regimes
JOCD
Most children suffering from JOCD can be successfully treated conservatively.6,19,48,56,83 Restrictions on weightbearing and sports activities have been suggested or simply limitation of daily activities and immobilisation.5,6,33,48,51,62-64,84,85 A common treatment suggestion is that the patient has a brace for 6 to 12 weeks with partial weightbearing and follows regularly with physiotherapy training. If the patient is pain free at 12 weeks and if the imaging shows healing, the patient could start running activities but more aggressive activities should be restricted until the patient have been followed for more months of symptom free activities in sport and leisure such as jumping, twisting and impact loading.
In a recently published, retrospective study on 42 JOCD patients, two-thirds (66%) of the stable lesions healed after an initial treatment with plaster-cast immobilization followed by bracing and limitation of activity for up to 6 months.81 However, the authors experienced failure of treatment in 34% of the patients. Large lesions did significantly worse than the smaller ones (relatively and absolutely), but all the lateral lesions healed.
Prospective factors such as size of a lesion, condyle or noncondyle localization, age, and gender of the patient are still being controversially discussed.5,6,48,63,84-89
Of interest is a European multicenter study6 with the largest number of patients up to now (452 patients with 509 affected knee joints). In 452 patients with a minimal follow-up of 1 year, they differentiated a group A of 276 patients with open physes, for example, males up to 14 years of age and females up to the age of 13 years, from a group B of so-called “premature” patients, for example, over 14 years of age for males and over 13 years for females. A total of 154 patients received conservative treatment while 355 patients needed surgery. Significantly better results were seen in patients from group A than from group B. Those whose situation was favorable (no gross dissection, size < 20 cm2) did significantly better than those with an already detectable dissection (so-called “unfavorable conditions”). Application of a plaster-cast did not influence the result of the conservative treatment in comparison to treatment without a cast (normal and near-normal knees 69.2% vs 72%, respectively). In contrast, those patients with an unfavorable condition had significantly worse results after conservative treatment (abnormal knees in 44%) when compared with surgical therapy (abnormal knees in 33.1%).6
AOCD
Regarding AOCD patients, little knowledge exists. Meanwhile, the question is as to whether OCD in AOCD patients occur de novo or whether it is already present prior to epiphyseal closure but, owing to a failed treatment, is still there after epiphyseal closure. The question as to conservative therapy is “How are those persons with an AOCD affected?” In general, to our knowledge there is no explicit answer. Only 1 study has compared patients up to an age of 13 (girls) or 14 years (boys) with those in a premature stage (girls older than 13 or boys older than 14 years) and presented some reliable data. The results for JOCD patients were better after any type of treatment than for any patient in a premature stage.
For AOCD, successful conservative treatment is less likely.6 Lindén5 noted excellent results, regardless of the conservative therapeutic regime, and that children with open physes display no degenerative changes. Hughston et al.63 recommended normal activity and strengthening of the muscles rather than immobilization. The rate of healing following nonoperative treatment ranged from 50% to 94%.5,6,29,33,48,63,64,81,84,85,90
Surgical Treatment
Arthroscopic evaluation and treatment is used as next step when conservative treatment has failed. Accepted, general indications for surgical treatment are4,47,54,64,91
Unstable lesions with already-visible loose bodies
Detachment that occurs during observation or nonoperative treatment when a physeal closure is predicted to occur within 6 to 12 months
When juvenile lesions remain symptomatic despite adequate nonoperative treatment
When an established nonunion of a fragment is detectable
There exist several different classification systems for the arthroscopic evaluation of an OCD lesion. The most well-known is the arthroscopic classification according to Guhl56:
Stage 1: Stable lesion
Stage 2: Lesions showing signs of early separation
Stage 3: Partially detached lesions
Stage 4: Craters with loose bodies.
ICRS has developed a system for evaluating of cartilage lesions and also a system for OCD evaluations.2 The ICRS OCD classification is a modified Guhl classification to adjust cartilage evaluation of OCD lesions to the common ICRS evaluation system44
ICRS OCD 0: Stable, normal intact overlying cartilage
ICRS OCD I: Stable with continuous but softened area with intact cartilage
ICRS OCD II: Stable with partial discontinuity
ICRS OCD III: In situ lesion with complete discontinuity
ICRS OCD IV: Empty defect with dislocated or loose fragments
General Remarks of Operative Treatment
However, indications for surgery are controversial and unclear.82 In a recent review article,82 30 studies (only 1 level-I) on 783 subjects with 862 knees were evaluated. The mean postoperative follow-up was 77 months, minimum 2 years. Nearly all patients demonstrated significant clinical and radiographic improvements in surgically treated JOCD at short-, mid-, and long-term follow-up. Excision of weightbearing OCD lesions led to poorer clinical and radiographic results than other surgical techniques. Outcomes were significantly better for JOCD versus AOCD.
Different surgical techniques, such as retrograde or anterograde drilling (alone or in combination with cancellous bone grafting),54,57,92-95 should only be indicated for low-grade lesions preferably JOCD.54,96 The anterograde technique is easier than the retrograde approach, but perforation of the cartilage layer is necessary in order to reach the involved subchondral bone.
The retrograde approach is more difficult owing to the open physes but it does leave the cartilage layer intact. Imaging techniques, such as fluoroscopy, MRI, ultrasound, or arthroscopy are recommended in order to be able to navigate the drills toward the defect.95,97-100 The goal of both variants is either to perforate the subchondral sclerosis or to promote blood supply to the subchondral necrotic area.
The most important prognostic factor is age. It was observed radiographically that the lesions had healed within 6 weeks to 2 years postoperatively in up to 100% of the JOCD patients but in only 25% of the AOCD cases.56,57,82,91,101,102
Large lesions need a longer time to heal than small ones.101
JOCD
A summary of the most recent review of 25 articles, all on JOCD,103 showed that the most common techniques were transarticular drilling for stable lesions and the use of bioabsorbable pin-fixation for fragment refixation. The key findings were that the vast majority of lesions healed postoperatively, regardless of technique, and that high-quality trials are required to more appropriately compare the effectiveness of techniques.103 A similar résumé was published after reviewing anterograde and retrograde drilling.97
AOCD
Nearly nothing is known about drilling stable lesions in AOCD. Unstable AOCD lesions are mostly treated surgically. For several years, in cases of damage to the cartilage layer, removal of the loose cartilage or osteochondral fragments was recommended, possibly in combination with a debridement procedure. Nowadays, however, this is no longer done owing to poor results, with up to 71% rate of osteoarthritic (OA) changes.2,6,47,55,58,63,83,87,91,104-115 For these reasons, fragment refixation of partially or completely loose bodies—as far as is possible—is recommended (see example in Fig. 4 ). Histologically, these fragments contain mostly viable cartilage.35,37 Either combining fragment refixation with drilling of the subchondral bone, in order to perforate the subchondral sclerosis, or removal of the sclerosis followed by cancellous bone grafting followed by fragment refixation4,6,64,91,103 is recommended.
Figure 4.
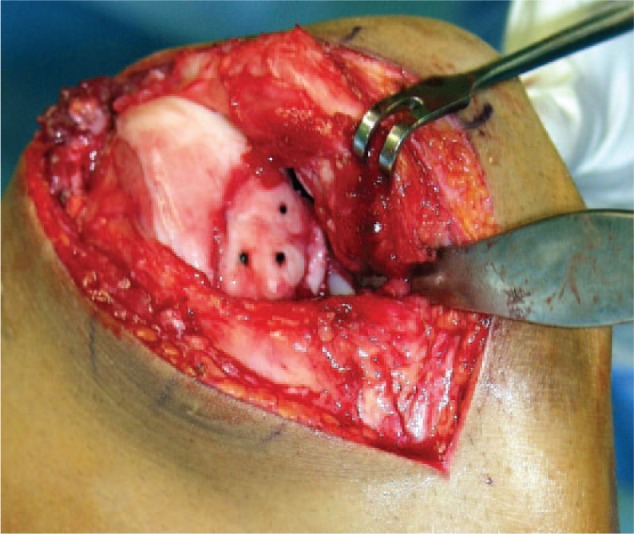
Example of a refixated loose body in the medial condyle of the knee. Prior to the refixation, the subchondral sclerosis had been removed, cancellous bone taken from the iliac crest transplanted into the defect, followed by the refixation using fibrin glue (Tissucol, Baxter, Unterschleißheim, Germany) and resorbable pins (Ethipin, Ethicon, Hamburg, Germany).
Techniques for Fragment Refixation
Several methods have been used for OCD fragment refixation, such as osteochondral pins, plugs or pegs, metallic screws or pins, or resorbable screws, anchors, arrows or pins, all probably in combination with fibrin glue.2,64,82,103,110,112,114,116 The success rate reported has been between 91.7% and 100%, depending on the imaging technique or definition of success.103,116 However, degenerative joint-space narrowing has been radiographically detectable in 75%.110 The optimal fragment refixation technique is still under discussion. It has been observed experimentally that screw-fixation gave the best results117 but that resorbable material can initiate allergic and/or synovial reactions and cartilage damage.118
The authors’ opinion is that successful fragment refixation depends on the existence of a substantial amount of bone on the fragment to allow bony consolidation with the subchondral defect bottom. In cases where fragment refixation is not possible because the loose body is too fragmented, or shows the so-called “malicious form,”21 reconstructive techniques are indicated.
Alternative Techniques for Knee OCD Treatment when Fragment Refixation Is Not Possible
There are numerous reports on these various operative procedures but almost all the articles are case series, that is, level-IV reports; although with a prospective character but without comparison with other procedures.
Only a few level-I/II publications119-126 are available. Even these articles have not always differentiated distinctly between an OCL124 and a typical OCD.119-121 Clear differentiation between JOCD and AOCD has not been made and scoring systems and follow-up criteria have not been consistently adhered to.
With the mixture of different methods of surgery and conservative treatment described in the literature, and with all the different definitions, profound comparisons are nearly impossible.
Bone Marrow Stimulation Techniques
Microfracture (MFX) alone, or other bone marrow stimulations combined with a supportive matrix so-called “autologous matrix-induced chondrogenesis” (AMIC) are other possible alternatives.127-129 However, failures can be expected beyond 5 years following MFX.120,130 In a comparison between MFX and AMIC in the treatment of small non-OCD lesions, no significant differences for small cartilage lesions were found.131
Osteochondral Autologous Plug Implants (Osteochondral Autograft Transfer [OAT] and Mosaicplasty)
One of the first studies on OAT was reported by Wirth et al.132 with favorable results in almost all of the 12 patients suffering from OCD. First long-term results were published by Laprell and Petersen.133 In their case series, they reported good and excellent results (ICRS score) in 26 out of 29 patients (mostly OCD lesions) at a follow-up of 6 and 12 years (mean 8.1 years). They had used the dorsal medial condyle as the donor region but did not fill up the remaining defect. At the follow-up, they observed cystic lesions in the harvest area in 26 patients.133
Hangody et al.134 reported good or excellent results in 89% of 76 patients, all of whom were suffering from OCD. In another study using the mosaicplasty, in which not all the lesions were caused by OCD (33%), the authors stated that better results were achieved in patients who had a condylar lesion (92% good and excellent results) than in those with a tibial resurfacing (87%) or a patellar or trochlear lesion (79%).135
An overview of the literature on osteochondral transplantation techniques shows that a lot of papers do not differentiate between OAT and mosaicplasty, although there is a substantial difference. In contrast to the original OAT technique, in 2 studies the mosaicplasty filled defects consisted of only 60% to 70% hyaline cartilage (see example in Fig. 5 ). The rest (30%-49%) were fibrocartilage tissues.135,136 The varying use of the nomenclature makes exact comparison difficult.116,121,122,136-140
Figure 5.
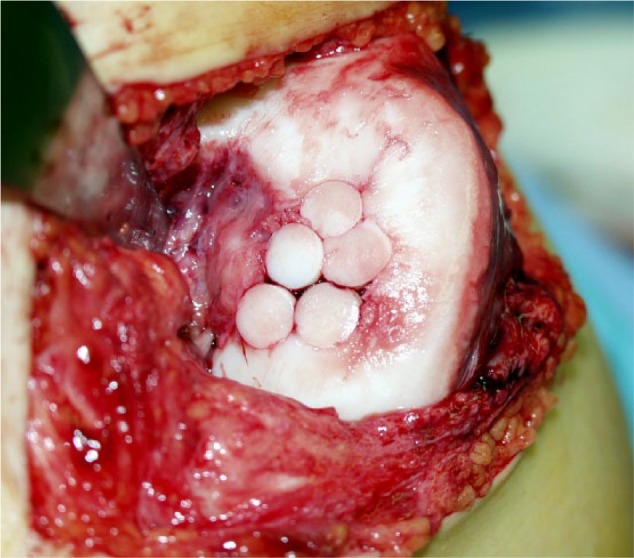
Adult osteochondritis dissecans (AOCD) stage IV lesion at the medial condyle of the knee with already visible secondary arthritic changes treated with 5 osteochondral plugs implanted as a mosaicplasty with small clefts between the plugs.
One of the very few level-I articles122 compared MFX with mosaicplasty in exclusively JOCD patients up to an age of 18 years. While up to 1 year postoperative, there was no significant difference between the 2 techniques, at the second follow-up after 4.2 years, MFX patients exhibited a significant deterioration (41% failure) while those treated by mosaicplasty remained stable with 91% excellent or good results.
Another follow-up study analyzed 57 athletes after either MFX or mosaicplasty, including 43% OCD lesions. Ninety-two of the mosaicplasty patients had excellent or good results while 52% of the patients who were treated with MFX were significantly worse at a maximum of 37.1 months postoperation.121 Similar results were observed after a long-term follow-up of 10 years where there was a 25% failure rate with mosaicplasty as opposed to 75% with MFX.137
These 2 independent studies showed that MFX does not seem to be a surgical alternative in the treatment of OCD lesions. In principle, this can be expected since the lesion is an osteochondral and not a solely chondral lesion with an intact subchondral bone plate.
Both osteochondral plug techniques (OAT, mosaicplasty) can be applied via an arthrotomy, mini-arthrotomy, or arthroscopically.133-135 With both techniques, the open variant allows a precise positioning of the transplant, enabling it to adapt in height and shape to the surrounding, healthy, articular surface. A disadvantage is the disturbed proprioception and prolonged rehabilitation period after arthrotomy than after arthroscopy.135,141,142 In contrast, arthroscopic techniques require a very experienced surgeon.
Mega-OATS
In cases with fairly large lesions, the mega-OAT procedure is an alternative. This technique uses large osteochondral plugs explanted from the dorsal condyles and was inaugurated by Imhoff et al.143 However, well before that, the posterior condyle was described as a potential donor site.21,22,144 First results on operated knee joints with a mean follow-up of 9.8 months (range 2-26 months) showed a distinct postoperative improvement in 93.8% of patients (15/16).143 The authors also treated malalignments of the involved leg but did not observe an influence on the results. Another article on this technique145 reported satisfactory results for 26 out of 29 patients after a follow-up of up to 18 months. Furthermore, a high tibial osteotomy did not significantly influence the results. Altogether 26 out of 29 patients (89.7%) were subjectively satisfied. Sixteen patients (55.2%) were able to return to their preoperative level of sports activities. Neither donor site morbidity nor problems at the rim of the explant region were observed.
Mega-OAT has the advantage that the transplants are fixed without having to hammer them into place. This means that chondrocyte death in the transplants can be avoided. Results of mega-OAT in 16 patients (4 laterals, 12 medial lesions) after 5 years showed a significant improvement in 15 patients (93.8%). No donor site morbidity was detected but the authors mentioned newly-formed tissue in the region from where the transplants had been taken.146 Regarding allogenic mega-OAT transplants, only 1 report on 5 patients has been published.147
Autologous Chondrocyte Implantation (ACI)
Since the first publications on ACI,148 several articles of mostly level-IV quality have been published. Today, there are several generations of this technique mostly with cell suspension seeded under a periosteal membrane or seeded into or on scaffolding matrices.
The scientific situation of ACI is the same as for OAT/mosaicplasty or fragment refixation. There are only a few level-I and -II studies. Peterson et al.149 reported successful treatment in 58 patients with OCD, 35 with JOCD, and 23 with AOCD. After a mean follow-up of 5.6 years, 91% of the patients had a good or excellent overall result; 93% reported a self-assessed improvement.
Taking into consideration that OCD is not only a chondral but also an OCL, some of the patients received additional bone grafts. However, unfortunately, no differentiation was made between those with and those without bone grafts.
Another level-IV study150 reported similar results in 40 exclusively JOCD patients. A follow-up in 80% after the classic ACI treated patients a success rate of 85% was found while the failure rate was 19%. Ferruzzi et al.151 compared ACI via an arthrotomy (n = 48) with an arthroscopic procedure (n = 50) using a cell seeded matrix. Twenty-five of the patients were suffering from OCD. They observed a significant improvement in both groups but the failure rate after an open procedure was 19%, distinctly higher than after the arthroscopic technique (4%). In addition, they noted a faster rehabilitation following arthroscopy-mediated treatment.
One level-I study on 80 patients comparing ACI (n = 40) with MFX (n = 40),119,120 including 65% traumatic lesions, 28% OCD lesions and 7% with unspecified diagnoses revealed no significant differences between both groups. At a follow-up of 2 and 5 years, a success rate of 77% and a failure rate of 23% were reported for each group.
Two comparisons of ACI with mosaicplasty were made by Bentley et al. the first in 2003 with a mean follow-up of 1.7 years125 and the second in 2012126 with a minimum follow-up of 10 years. At the first follow-up, 9 out of 42 mosaicplasty patients (21%) exhibited an excellent result in contrast to 23 out of 58 (40%) in the ACI group. Furthermore, the rate of poor results for the mosaicplasty patients was distinctly higher (17%) than in the ACI group (0%). Arthroscopy at 1 year postoperatively demonstrated excellent or good repairs in 82% after ACI. Following mosaicplasty, 34% had good results, no “excellent” outcome. At a minimum of 10 years’ follow-up,126 the repair had failed in 10 out of 58 ACI patients (17%) and 23 out of 42 (55%) from the mosaicplasty group.
Assuming that the grafts of patients who could no longer be traced were intact (“best-case scenario”), grafts of patients lost to follow-up were not intact (“worst-case scenario”), comparison of the Kaplan-Meier curves revealed distinctly better results after ACI than after mosaicplasty. Deterioration of the results after mosaicplasty started at approximately 2 years postoperatively.126
Basad et al.152 analyzed the results of a 2-step procedure using autologous bone grafts implanted into the defect prior to the cell seeded scaffold procedure with a double-layer technique. All their patients had a distinct mean improvement 24 months postoperatively. Two other studies,153,154 both using a 1-step procedure and cell-seeded collagen scaffold or chondrocytes in a gel (CaReS, example in Fig. 6 ), demonstrated a significant improvement after a follow-up of up to 36 months in all of the OCD patients. Steinhagen et al.153 showed a continual improvement from preoperative to 3 months postoperatively and longer (up to 36 months postoperatively) in all of the OCD patients. The size of the lesions measured up to 12 cm2 and 9 cm2 in the studies by Steinhagen et al.153 and Ochs et al.,154 respectively.
Figure 6.
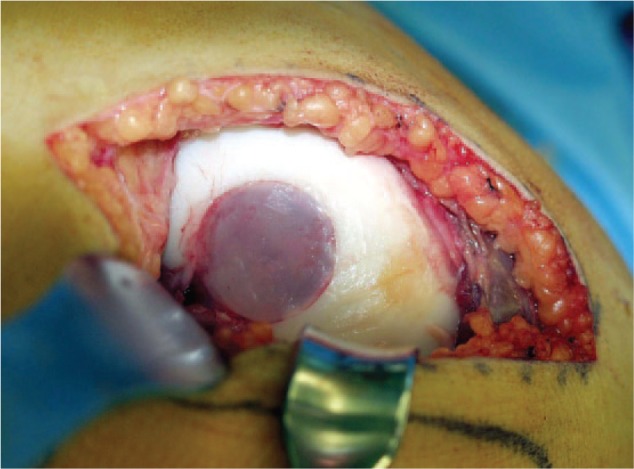
Adult osteochondritis dissecans (AOCD) stage-IV lesion at the lateral femoral condyle treated with removal of the subchondral sclerosis, transplantation of cancellous bone taken from the iliac crest and implantation of an autologous chondrocyte implantation (ACI) of the second generation using a gel as carrier for the chondrocytes (CaReS, Arthro Kinetics, Bebenhausen, Germany).
To our knowledge, there is only 1 level-I study comparing more than 2 techniques.123 These authors described a very interesting, prospective, randomized trial on JOCD and AOCD patients. The trial compared the following procedures:
Massive autologous osteochondral transplants
Autologous bone-cartilage-paste grafts,
Autologous chondrocyte transplantation (second generation) in combination with a bone graft
Biomimetic osteochondral scaffolds
Bone marrow–derived cell transplantation.
In a total of 60 patients, they did not find significant differences but there was a tendency toward better results in JOCD patients.123 Overall, the IKDC (International Knee Documentation Committee) objective score increased from 37% preoperatively to 97% at the last follow-up. However, the follow-up time varied from 2.3 years (bone marrow–derived cells) to 12.2 years (massive osteochondral grafts) and the number of patients in a particular group from 7 (bone-derived cell implantation) to 28 (chondrocytes with bone grafts). The only difference among the results of the different techniques was a trend toward better results following ACI (0.06).123
Allografts
For many years fresh, fresh-frozen or stored allografts have also been used in advanced knee OCD lesions.154-161 Fresh, refrigerated allografts are the standard choice for osteochondral allografts since frozen and freeze-dried cartilage has insufficient viable cartilage cells.142,162 When the refrigerated allograft is fresh, up to 98% of the chondrocytes are viable for 7 days; this decreases to 70% by 28 days.65,163 The decreased viability is accompanied by diminished cell density and decreased metabolic activity.65,164 The matrix and chondrocytes have been shown to survive in long-term recovery studies.142
However, extensive serological, bacterial, and viral testing of grafts is necessary prior to allograft transplantation until negative test results have been ensured. Donors must be screened. A round-the-clock transplantation service must be available.142,165 Furthermore, the immunogenicity and unplanned transfer of diseases has not yet been fully eliminated. However, the risk of HIV transmission is estimated to be as low as approximately 1 in 1.6 million, and there have been no reports of this route of disease transmission since the late 1980s.142,165
While chondrocytes are preserved against immunological reactions by the matrix cells, cells in the bony part of the graft should be removed to a great extent. In contrast to the cartilage, which seems to be completely integrated, bony integration can be a cause of failure.142
There are 2 studies reporting exclusively on OCD; other publications include up to 45% OCD patients. On one hand, a relatively high success rate is described for OCD patients with a survival rate of between 72% after 7.7 years159 and, at 10 years, 82%. The survival rate decreases to 66% after 20 years (45% OCD lesions).160 On the other hand, in 15% to 47%, there is a high rate of failure and/or the necessity of further operations.154,158 The use of allografts in JOCD was reported by Lyon et al.161; after surgery, patients (mean age 15.2 years) had returned within 6 months without difficulty to the activities of daily living and, between the 9th and 12th month to full sports activities.
A retrieval analysis of 26 specimens from 14 patients revealed 82% viable chondrocytes after a survival of 42 months. Histologically, all specimens showed some cartilage fibrillation but no signs of transplant rejection.166
Long-term Results after Different Treatments
There are a large number of articles with a mean follow-up of between 5 and 34 years.5,14,59-61,87,107-109,111,133,135,166-170 The articles included at least 2 longitudinal studies in which patients were examined twice.107,109 However, all the articles are only level IV, which means that the interpretation of the long-term results is difficult, particularly since the authors may be biased.107,108,112
Regarding excision or removal of OCD fragments, results revealed a clear tendency toward poor or fair results after a period of 10 to 20 years.166,170 Michael et al.108 observed excellent and good results in only 35% after 28 years mostly following excision with a rate of OA of 92%. Similar data were mentioned by Twyman et al.109 One report on exclusively lateral condylar OCD105 described a moderate OA but better clinical results 14 years after arthroscopic excision and subchondral drilling in most of the patients (22/28 knee joints). In contrast, results after fragment refixation were excellent or good in 85% to 92% of patients after 5 to 15 years.57 It seems that refixation results in a distinctly lower rate of OA and at a follow-up of 34 years, a rate of 35% of moderate OA was seen.111,112
So far as comparison is possible, reconstructive therapies have a tendency to better long-term results with a lower rate of OA, as is described for OAT133: Most of the patients (48%) exhibit the same postoperative grade of OA after 8.1 years when compared with preoperatively, and 34% exhibited an impairment of one grade.
Peterson et al.148 referred to excellent or good clinical results after ACI in 91% of 58 patients with a mean follow-up of 5.6 years but also mentioned signs of OA in nearly 50%.
Long-term results after repair with allografts showed a relatively high rate of success with a survival rate of 72% after 7.7 years (all OCD lesions) and 82% after 10 years.158 However, it was only 66% after 20 years (45% OCD)160 and there was a high failure rate and/or reoperations between 15% up to 47%.155,159,160,171
Comorbidities
Malalignment
Several authors have reported on the relation between medial OCD lesions in the knee joint and varus malalignment as well as between lateral lesions and valgus malalignment.26,96,135,142,172-176 Jacobi et al.173 analyzed the bilateral full-leg radiographs of their patients and found that OCD lesions and deviation of the mechanical axis in the varus or valgus were correlated significantly with medial (varus) and lateral lesions (valgus), respectively. The difference between affected and unaffected legs was also significant for lateral but not medial lesions. Subsequently, correction of the malalignment should be considered as an additional therapeutic goal, more for varus than for valgus malalignment. Slawski176 reported on 6 AOCD patients suffering from a varus malalignment in 7 of their knees with a high-tibial osteomy and achieved a distinct improvement of the postoperative Lysholm score.
ACL Instability and Meniscal Lesions
ACL instability or meniscal lesions should also be therapeutically addressed.133,135,142,143 Hangody et al.135 reported a rate of 85% concomitant surgical interventions. The majority of these procedures were ACL reconstructions, realignment osteotomy, meniscal surgery, or patellofemoral realignment.
There are reports on the combination of OCD lesions at the lateral femoral condyle with a discoid meniscus, and the development of an OCD lesion after a total meniscectomy of a discoid meniscus.75 Subsequently, in our opinion, discoid menisci should be surgically reduced to the size of a normal meniscus. However, a total meniscectomy of discoid menisci can also result in the development of an ipsilateral OCD lesion.76
Conclusion
OCD remains an etiological, histological, and therapeutic mystery. There is much confusion regarding the classification and definition of OCD lesions and their differentiation from others, as well as with regard to a clear definition of JOCD and AOCD. Furthermore, there are no clear and scientifically well-based recommendations as to which therapeutic strategy should be used. In addition, a clear and uniformly used definition of the clinical and radiographical success and/or healing is still missing.
Although there are a tremendous number of publications on all aspects regarding OCD in different joints, there is a great lack of scientifically reliable prospective randomized studies.
Confusion still remains, at least for OCD lesions in the knee, and is expressed in the “Summary of Recommendations” in the publication “The Diagnosis and Treatment of Osteochondritis Dissecans” elaborated by a working group of the “American Academy of Orthopaedic Surgeons” and published by Chambers et al.2,177 They found that the strength of recommendations regarding 16 different aspects was inconclusive in 10 and weak in 2. Only in 4 aspects did the group find consensus.
In the future, it should be an international aim of institutions dealing with osteoarticular diseases to develop a protocol for providing more satisfactory data than those obtained from level-IV studies, these being of little scientific worth.
Footnotes
Acknowledgments and Funding: We thank Mrs. H. Dawson for her help in correcting the manuscript. The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. König F. Ueber freie Körper in den Gelenken. Dtsch Z Chir. 1888;27:90-109. [Google Scholar]
- 2. Chambers HG, Shea KG, Carey JL. AAOS Clinical practice guideline: diagnosis and treatment of osteochondritis dissecans. J Am Acad Orthop Surg. 2011;19:307-9. [DOI] [PubMed] [Google Scholar]
- 3. Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current concepts review. Am J Sports Med. 2006;34:1181-91. [DOI] [PubMed] [Google Scholar]
- 4. Kessler J, Nikizad H, Shea KG, Jacobs JC, Jr, Bebchuk JD, Weiss JM. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med. 2014;42:320-6. [DOI] [PubMed] [Google Scholar]
- 5. Lindén B: Osteochondritis dissecans of the femoral condyles. A long-term follow-up study. J Bone Joint Surg Am. 1977;59:769-76. [PubMed] [Google Scholar]
- 6. Hefti F, Beguiristain J, Krauspe R, Moller-Madsen B, Riccio V, Tschauner C, et al. Osteochondritis dissecans: a multicenter study of the European Pediatric Orthopedic Society. J Pediatr Orthop. 1999;8B:231-45. [PubMed] [Google Scholar]
- 7. Robertson W, Kelly BT, Green DW. Osteochondritis dissecans of the knee in children. Curr Opin Pediatr. 2003;15:38-44. [DOI] [PubMed] [Google Scholar]
- 8. Tóth F, Nissi MJ, Ellermann JM, Wang L, Shea KG, Polousky J, et al. Novel application of magnetic resonance imaging demonstrates characteristic differences in vasculature at predilection sites of osteochondritis dissecans. Am J Sports Med. 2015;43:2522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martel G, Kiss S, Gilbert G, Anne-Archard N, Richard H, Moser T, et al. Differences in the vascular tree of the femoral trochlear growth cartilage at osteochondrosis-susceptible sites in foals revealed by SWI 3T MRI. J Orthop Res. 2016;34:1539-46. [DOI] [PubMed] [Google Scholar]
- 10. Skagen PS, Horn T, Kruse HA, Staergaard B, Rapport MM, Nicolaisen T. Osteochondritis dissecans (OCD), an endoplasmic reticulum storage disease? A morphological and molecular study of OCD fragments. Scand J Med Sci Sports. 2011;21:e17-33. [DOI] [PubMed] [Google Scholar]
- 11. Richie LB, Sytsma MJ. Matching osteochondritis dissecans lesions in identical twin brothers. Orthopedics. 2013;36:e1213-6. [DOI] [PubMed] [Google Scholar]
- 12. Bruns J. Osteochondrosis dissecans. Stuttgart, Germany: Enke; 1996. [Google Scholar]
- 13. Kusumi T, Ishibashi Y, Tsuda E, Kusumi A, Tanaka M, Sato F, et al. Osteochondritis dissecans of the elbow: histopathological assessment of the articular cartilage and subchondral bone with emphasis on their damage and repair. Pathology Int. 2006;56:604-12. [DOI] [PubMed] [Google Scholar]
- 14. Hughes JA, Cook JV, Churchill MA, Warren ME. Juvenile osteochondritis dissecans: a 5-year review of the natural history using clinical and MRI evaluation. Pediatr Radiol. 2003;33:410-7. [DOI] [PubMed] [Google Scholar]
- 15. Krappel FA, Bauer E, Harland U. Are bone bruises a possible cause of osteochondritis dissecans of the capitellum? A case report and review of the literature. Arch Orthop Trauma Surg. 2005;125:545-9. doi: 10.1007/s00402-005-0018-0. [DOI] [PubMed] [Google Scholar]
- 16. Nishimura G, Yamato M, Togawa M. Trabecular trauma of the talus and medial malleolus concurrent with lateral collateral ligamentous injuries of the ankle: evaluation with MR imaging. Skeletal Radiol. 1996;25:49-54. [DOI] [PubMed] [Google Scholar]
- 17. Nakamae A, Engebretsen L, Bahr R, Krosshaug T, Ochi M. Natural history of bone bruises after acute knee injury: clinical outcome and histopathological findings. Knee Surg Sports Traumatol Arthrosc. 2006;14:1252-8. [DOI] [PubMed] [Google Scholar]
- 18. Shea K, Jacobs JC, Grimm NL, Pfeiffer RP. Osteochondritis dissecans development after bone contusion of the knee in the skeletally immature: a case series. Knee Surg Sports Traumatol Arthrosc. 2013;21:403-7. [DOI] [PubMed] [Google Scholar]
- 19. Crawford D, Safran MR. Osteochondritis dissecans of the knee. J Am Acad Orthop Surg. 2006;14:90-100. [DOI] [PubMed] [Google Scholar]
- 20. Uozumi H, Sugita T, Aizawa T, Takahashi A, Ohnuma M, Itoi E. Histologic findings and possible causes of osteochondritis dissecans of the knee. Am J Sports Med. 2009;37:2003-8. [DOI] [PubMed] [Google Scholar]
- 21. Wagner H. Operative Behandlung der Osteochondrosis dissecans des Kniegelenkes. Z Orthop. 1964;98:333-55. [Google Scholar]
- 22. Wagner H. Surgical treatment of osteochondritis dissecans, a cause of arthritis deformans of the knee. Rev Chir Orthop Reparat Apparat Mot 1964;50:335-52. [PubMed] [Google Scholar]
- 23. Rehbein F. Die Entstehung der Osteochondritis dissecans. Langenbecks Arch Chir. 1950;265:69-114. [PubMed] [Google Scholar]
- 24. Kolp W, Fethke K. Spannungsoptische Untersuchungen eines belasteten Kniegelenkes als Beitrag zur Ätiologie der Osteochondrosis dissecans. Beitr Orthop Traumatol. 1982;29:493-500. [PubMed] [Google Scholar]
- 25. Nambu T, Gasser B, Schneider E, Bandi W, Perren SM. Deformation of the distal femur: a contribution towards the pathogenesis of osteochondrosis dissecans in the knee Joint. J Biomech. 1991;24:421-33. [DOI] [PubMed] [Google Scholar]
- 26. Bruns J, Volkmer M, Luessenhop S. Pressure distribution at the knee joint. Influence of varus-valgus malalignment without and with ligament dissection. Arch Orthop Trauma Surg. 1993;113:12-19. [DOI] [PubMed] [Google Scholar]
- 27. Bruns J, Volkmer M, Luessenhop S. Pressure distribution at the knee joint. Influence of flexion without and with ligament dissection. Arch Orthop Trauma Surg. 1994;113:204-9. [DOI] [PubMed] [Google Scholar]
- 28. Green W, Banks HH. Osteochondritis dissecans in children. J Bone Joint Surg Am. 1953;35:26-47. [PubMed] [Google Scholar]
- 29. Green WT, Banks HH. Osteochondritis dissecans in children. Clin Orthop. 1990;255:3-12. [PubMed] [Google Scholar]
- 30. Clanton T, DeLee JC. Osteochondritis dissecans. History, pathophysiology and current treatment concepts. Clin Orthop Relat Res. 1982;167:50-64. [PubMed] [Google Scholar]
- 31. Barrie HJ. Hypertrophy and laminar calcification of cartilage in loose bodies as probable evidence of an ossification abnormality. J Pathol. 1980;132:161-8. [DOI] [PubMed] [Google Scholar]
- 32. Barrie H. Hypothesis—a diagram of the form and origin of loose bodies in osteochondritis dissecans. J Rheumatol. 1984;11:512-3. [PubMed] [Google Scholar]
- 33. Chiroff R, Cooke CP., 3rd Osteochondritis dissecans: a histologic and microradiographic analysis of surgically excised lesions. J Trauma. 1975;15:689-96. [PubMed] [Google Scholar]
- 34. Milgram J. Radiological and pathological manifestations of osteochondritis dissecans of the distal femur. A study of 50 cases. Radiology. 1978;126:305-11. [DOI] [PubMed] [Google Scholar]
- 35. Koch S, Kampen WU, Laprell H. Cartilage and bone morphology in osteochondritis dissecans. Knee Surg Sport Traumatol Arthrosc. 1997;5:42-5. [DOI] [PubMed] [Google Scholar]
- 36. Yonetani Y, Nakamura N, Natsuume T, Shiozaki Y, Tanaka Y, Horibe S. Histological evaluation of juvenile osteochondritis dissecans of the knee: a case series. Knee Surg Sports Traumatol Arthrosc. 2010;18:723-30. [DOI] [PubMed] [Google Scholar]
- 37. Aurich M, Hofmann GO, Mückley T, Mollenhauer J, Rolauffs B. In vitro phenotypic modulation of chondrocytes from knees of patients with ostechondritis dissceans: implications for chondrocyte implantation procedures. J Bone Joint Surg Br. 2012;94:62-7. [DOI] [PubMed] [Google Scholar]
- 38. Shea K, Jacobs JC, Jr, Carey JL, Anderson AF, Oxford JT. Osteochondritis dissecans knee histology studies have variable findings and theories of etiology. Clin Orthop Relat Res. 2013;471:1127-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. O’Loughlin P, Heyworth BE, Kennedy JG. Current concepts in the diagnosis and treatment of osteochondral lesions of the ankle. Am J Sports Med. 2010;38:392-404. [DOI] [PubMed] [Google Scholar]
- 40. Penttilä P, Liukkonen J, Joukainen A, Virén T, Jurvelin JS, Töyräs J, et al. Diagnosis of knee osteochondral lesions with ultrasound imaging. Arthrosc Tech. 2015;4:e429-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wilson JN. A diagnostic sign in osteochondritis dissecans of the knee. J Bone Joint Surg Am. 1967;49:471-80. [PubMed] [Google Scholar]
- 42. Conrad J, Stanitski CL. Osteochondritis dissecans: Wilson’s sign revisited. Am J Sports Med. 2003;31:777-8. [DOI] [PubMed] [Google Scholar]
- 43. Zaremski JL, Herman DC, Vincent KR. Clinical utility of Wilson test for osteochondral lesions at the knee. Curr Sports Med Rep. 2015;14:430. [DOI] [PubMed] [Google Scholar]
- 44. Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85-A(suppl. 2):58-69. [DOI] [PubMed] [Google Scholar]
- 45. Arcq M. Behandlung der Osteochondrosis dissecans durch Knochenspanbolzung. Arch Orthop Unfall-Chir. 1974;79:297-312. [DOI] [PubMed] [Google Scholar]
- 46. Rodegerdts U, Gleissner S. Langzeiterfahrungen mit der operativen Therapie der Osteochon-drosis dissecans des Kniegelenkes. Orthop Praxis. 1979;15:612-22. [Google Scholar]
- 47. Cahill BR, Berg BC. 99m-Technetium phosphate compound joint scintigraphy in the management of juvenile osteochondritis dissecans of the femoral condyles. Am J Sports Med. 1983;11:329-35. [DOI] [PubMed] [Google Scholar]
- 48. Cahill BR, Phillips MR, Navarro R. The results of conservative management of juvenile osteochondritis dissecans using joint scintigraphy: a prospective study. Am J Sports Med. 1989;17:601-6. [DOI] [PubMed] [Google Scholar]
- 49. Verhagen R, Maas M, Dijkgraaf MG, Tol JL, Krips R, van Dijk CN. Prospective study on diagnostic strategies in osteochondral lesions of the talus. Is MRI superior to helical CT? J Bone Joint Surg Br. 2005;87:41-6. [PubMed] [Google Scholar]
- 50. Dipaola JD, Nelson DW, Colville MR. Characterizing osteochondral lesions by magnetic resonance imaging. Arthroscopy. 1991;7:101-4. [DOI] [PubMed] [Google Scholar]
- 51. De Smet AA, Ilahi OA, Graf BK. Untreated osteochondrosis dissecans of the femoral condyles: prediction of patient outcome using radiographic and MR findings. Skeletal Radiol. 1997;26:463-7. [DOI] [PubMed] [Google Scholar]
- 52. Kramer J, Stiglbauer R, Engel A, Prayer L, Imhof H. MR contrast arthrography (MRA) in osteochondrosis dissecans. J Comput Assist Tomogr. 1992;16:254-60. [DOI] [PubMed] [Google Scholar]
- 53. Moktassi A, Popkin CA, White LM, Murnaghan ML. Imaging of osteochondritis dissecans. Orthop Clin North Am. 2012;43:201-11, v-vi. doi: 10.1016/j.ocl.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 54. Smillie I. Treatment of osteochondritis dissecans. J Bone Joint Surg Br. 1957;39:248-60. [DOI] [PubMed] [Google Scholar]
- 55. Ewing J, Voto SJ. Arthroscopic surgical management of osteochondritis dissecans of the knee. Arthroscopy. 1988;4:37-40. [DOI] [PubMed] [Google Scholar]
- 56. Guhl JF. Arthroscopic treatment of osteochondritis dissecans. Clin Orthop Relat Res. 1982;167:65-74. [PubMed] [Google Scholar]
- 57. Anderson A, Lipscomb AB, Coulam C. Antegrade curettement, bone grafting and pinning of osteochondritis dissecans in the skeletally mature knee. Am J Sports Med. 1990;18:254-61. [DOI] [PubMed] [Google Scholar]
- 58. Anderson A, Richards DB, Pagnani MJ, Hovis WD. Antegrade drilling for osteochondritis dissecans of the knee. Arthroscopy. 1997;13:319-24. [DOI] [PubMed] [Google Scholar]
- 59. Anderson A, Pagnani MJ. Osteochondritis dissecans of the femoral condyles. Am J Sports Med. 1997;25:830-4. [DOI] [PubMed] [Google Scholar]
- 60. Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Rel Res. 2000;374:212-34. [DOI] [PubMed] [Google Scholar]
- 61. Aichroth P. Osteochondritis dissecans of the knee. J Bone Joint Surg Br. 1971;53:440-7. [PubMed] [Google Scholar]
- 62. Lindholm T. Osteochondritis dissecans of the knee. A clinical study. Ann Chir Gynaecol Fenn. 1974;63:69-76. [PubMed] [Google Scholar]
- 63. Hughston J, Hergenroeder PT, Courtenay BG. Osteohcondritis dissecans of the femoral condyles. J Bone Joint Surg Am. 1984;66:1340-8. [PubMed] [Google Scholar]
- 64. Schenck R, Goodnight JM. Osteochondritis dissecans. J Bone Joint Surg Am. 1996;78:439-56. [PubMed] [Google Scholar]
- 65. Williams S, Amiel D, Ball ST, Allen RT, Wong VW, Chen AC, et al. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85:2111-20. [DOI] [PubMed] [Google Scholar]
- 66. Chen C, Liu YS, Choud PH, Hsieha CC, Wanga CK. MR grading system of osteochondritis dissecans lesions: comparison with arthroscopy. Eur J Radiol. 2013;82:518-25. [DOI] [PubMed] [Google Scholar]
- 67. Saupe N, Pfirrmann CWA, Schmid MR, Schertler T, Manestar M, Weishaupt D. MR imaging of cartilage in cadaveric wrists: comparison between imaging at 1.5 and 3.0 T and gross pathologic inspection. Radiology. 2007;243:180-7. [DOI] [PubMed] [Google Scholar]
- 68. Brown D, Shirzad K, Lavigne SA, Crawford DC. Osseous integration after fresh osteochondral allograft transplantation to the distal femur: a prospective evaluation using computed tomography. Cartilage. 2011;2:337-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Paletta GA, Jr, Bednarz PA, Stanitski CL, Sandman GA, Stanitski DF, Kottamasu S. The prognostic value of quantitative bone scan in knee osteochondritis dissecans. A preliminary experience. Am J Sports Med. 1998;26:7-14. [DOI] [PubMed] [Google Scholar]
- 70. Tobin WJ. Familial osteochondritis dissecans with associated tibia vara. J Bone Joint Surg Am. 1957;39:1091-105. [PubMed] [Google Scholar]
- 71. Brückl R, Rosemeyer B, Thiermann G. Osteochondrosis dissecans of the knee. Results of operative treatment in juveniles. Arch Orthop Trauma Surg. 1984;102:221-4. [DOI] [PubMed] [Google Scholar]
- 72. Jacobi M, Wahl P, Bouaicha S, Jakob RP, Gautier E. Association between mechanical axis of the leg and osteochondritis dissecans of the knee: radiographic study on 103 knees. Am J Sports Med. 2010;38:1425-8. [DOI] [PubMed] [Google Scholar]
- 73. Garrett J, Kress KJ, Mudano M. Osteochondritis dissecans of the lateral femoral condyle in the adult. J Arthrosc. 1992;8:474-81. [DOI] [PubMed] [Google Scholar]
- 74. Räber DA, Friederich NF, Hefti F. Discoid lateral meniscus in children. J Bone Joint Surg Am. 1998;80:1579-86. [DOI] [PubMed] [Google Scholar]
- 75. Mizuta H, Nakamura E, Otsuka Y, Kudo S, Takagi K. Osteochondritis dissecans of the lateral femoral condyle following total resection of the discoid lateral meniscus. Arthroscopy. 2001;17:608-12. [DOI] [PubMed] [Google Scholar]
- 76. Deie M, Ochi M, Sumen Y, Kawasaki K, Adachi N, Yasunaga Y, et al. Relationship between osteochondritis dissecans of the lateral femoral condyle and lateral menisci types. J Pediatr Orthop. 2006;26:79-82. [DOI] [PubMed] [Google Scholar]
- 77. Camathias C, Rutz E, Gaston MS. Massive osteochondritis of the lateral femoral condyle associated with discoid meniscus: management with meniscoplasty, rim stabilization and bioabsorbable screw fixation. J Pediatr Orthop B. 2012;21:421-4. [DOI] [PubMed] [Google Scholar]
- 78. Kamei G, Adachi N, Naakamae A, Nakasa T, Shibuya H, Okuhara A, et al. Characteristic shape of the lateral femoral condyle in patients with osteochondritis dissecans accompanied by a discoid lateral meniscus. J Orthop Sci. 2012;17:124-8. doi: 10.1007/s00776-0190-8. [DOI] [PubMed] [Google Scholar]
- 79. Bruns J, Luessenhop S, Lehmann L. Etiological aspects in osteochondritis dissecans patellae. Knee Surg Sports Traumatol Arthrosc. 1999;7:356-9. [DOI] [PubMed] [Google Scholar]
- 80. Peters T, McLean ID. Osteochondritis dissecans of the patellofemoral joint. Am J Sports Med. 2000;28:63-7. [DOI] [PubMed] [Google Scholar]
- 81. Wall E, Vourazeris J, Myer GD, Emery KH, Divine JG, Nick TG, et al. The healing potential of stable juvenile osteochondritis dissecans knee lesions. J Bone Joint Surg Am. 2008;90:2655-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Trinh T, Harris JD, Flanigan DC. Surgical management of juvenile osteochondritis dissecans of the knee. Knee Surg Sports Traumatol Arthrosc. 2012;20:2419-29. [DOI] [PubMed] [Google Scholar]
- 83. Cahill BR. Osteochondritis dissecans of the knee: treatment of juvenile and adult forms. J Am Acad Orthop Surg. 1995;3:237-47. [DOI] [PubMed] [Google Scholar]
- 84. Yoshida S, Ikata T, Takai H, Kashiwaguchi S, Katoh S, Takeda Y. Osteochondritis dissecans of the femoral head in the growth stage. Clin Orthop Relat Res. 1998;346:162-70. [PubMed] [Google Scholar]
- 85. Jürgensen I, Bachmann G, Schleicher I, Haas H. Arthroscopic versus conservative treatment of osteochondritis dissecans of the knee: value of magnetic resonance imaging in therapy planning and follow-up. Arthroscopy. 2002;18:378-86. [DOI] [PubMed] [Google Scholar]
- 86. Wall E, Vourazeris J, Myer GD, Emery KH, Divine JG, Nick TG, Hewett TE. The healing potential of stable juvenile osteochondritis dissecans knee lesions. J Bone Joint Surg Am. 2008;90:2655-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Aglietti P, Ciardullo A, Giron F, Ponteggia F. Results of arthroscopic excision of the fragment in the treatment of osteochondritis dissecans of the knee. Arthroscopy. 2001;17:741-6. [DOI] [PubMed] [Google Scholar]
- 88. Crawford E, Emery RJ, Aichroth PM. Stable osteochondritis dissecans—does the lesion unite? J Bone Joint Surg Br. 1990;72:320. [DOI] [PubMed] [Google Scholar]
- 89. Mesgarzadeh M, Sapega AA, Bonakdarpour A, Revesz G, Moyer RA, Maurer AH, et al. Osteochondritis dissecans: analysis of mechanical stability with radiography, scintigraphy, and MR imaging. Radiology. 1987;165:775-780. [DOI] [PubMed] [Google Scholar]
- 90. Glancy GL. Juvenile osteochondritis dissecans. Am J Knee Surg. 1999;12:120-4. [PubMed] [Google Scholar]
- 91. Edmonds E, Polousky J. A review of knowledge in osteochondritis dissecans: 123 years of minimal evolution from König to the ROCK study group. Clin Orthop Relat Res. 2013;471:1118-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Adachi N, Deie M, Nakamae A, Ishikawa M, Motoyama M, Ochi M. Functional and radiographic outcome of stable juvenile osteochondritis dissecans of the knee treated with retroarticular drilling without bone grafting. Arthroscopy. 2009;25:145-52. [DOI] [PubMed] [Google Scholar]
- 93. Kocher MS, Micheli LJ, Yaniv M, Zurakowski D, Ames A, Adrignolo AA. Functional and radiographic outcome of juvenile osteochondritis dissecans of the knee treated with transarticular arthroscopic drilling. Am J Sports Med. 2001;29:562-6. [DOI] [PubMed] [Google Scholar]
- 94. Lebolt J, Wall EJ. Retroarticular drilling and bone grafting of juvenile osteochondritis dissecans of the knee. Arthroscopy. 2007;23:794.e1-4. [DOI] [PubMed] [Google Scholar]
- 95. Yonetani Y, Tanaka Y, Shiozaki Y, Kanamoto T, Kusano M, Tsujii A, et al. Transarticular drilling for stable juvenile osteocchondritis dissecans of the medial femorala condyle. Knee Surg Sports Traumatol Arthrosc. 2012;20:1528-32. [DOI] [PubMed] [Google Scholar]
- 96. Pascual-Garrido C, McNickle G, Cole BJ. Surgical treatment options for osteochondritis dissecans of the knee. Sports Health. 2009;1:326-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gunton M, Carey JL, Shaw CR, Murnaghan ML. Drilling juvenile osteochondritis dissecans: retro- or transarticular? Clin Orthop Relat Res. 2013;471:1144-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Berná-Serna J, Martinez F, Reus M, Berná-Mestre JD. Osteochondritis dissecans of the knee. J Ultrasound Med. 2008;27:255-9. [DOI] [PubMed] [Google Scholar]
- 99. Ojala R, Kerimaa P, Lakovaara M, Hyvönen P, Lehenkari P, Tervonen O, et al. MRI- guided percutaneous retrograde drilling of osteochondritis dissecans of the knee. Skeletal Radiol. 2011;40:765-70. [DOI] [PubMed] [Google Scholar]
- 100. Hoffmann M, Schroeder M, Petersen JP, Spiro AS, Kammal M, Lehmann W, et al. Arthroscopically assisted retrograde drilling for osteochondritis dissecans (OCD) lesions of the knee. Knee Surg Sports Traumatol Arthrosc. 2012;20:2257-62. [DOI] [PubMed] [Google Scholar]
- 101. Edmonds E, Albright J, Bastrom T, Chambers HG. Outcomes of extra-articular, intra-epiphyseal drilling for osteochondritis dissecans of the knee. J Pediatr Orthop. 2010;30:870-8. [DOI] [PubMed] [Google Scholar]
- 102. Louisia S, Beufils P, Katabi M, Robert H. Transchondral drilling for osteochondritis dissecans of the medial condyle of the knee. Knee Surg Sport Traumatol Arthrosc. 2003;11:33-9. [DOI] [PubMed] [Google Scholar]
- 103. Abouassaly M, Peterson D, Salci L, Farrokhyar F, D’Souza J, Bhandari M, et al. Surgical management of osteochondritis dissecans of the knee in the paediatric population: a systematic review addressing surgical techniques. Knee Surg Sport Traumatol Arthrosc. 2013;2014:1216-24. [DOI] [PubMed] [Google Scholar]
- 104. Green J. Osteochondritis dissecans of the knee. J Bone Joint Surg Br. 1966;48:82-91. [PubMed] [Google Scholar]
- 105. Lim H, Bae JH, Park YE, Park YH, Park JH, Park JW, et al. Long-term results of arthroscopic excision of unstable osteochondral lesions of the lateral femoral condyle. J Bone Joint Surg Br. 2012;94:185-9. [DOI] [PubMed] [Google Scholar]
- 106. Murray J, Chitnavis J, Dixon P, Hogan NA, Parker G, Parish EN, et al. Osteochondritis dissecans of the knee; long-term clinical outcome following arthroscopic debridement. Knee 2007;14:94-8. doi: 10.1016/j.knee.2006.1011.1011. [DOI] [PubMed] [Google Scholar]
- 107. Bruns J, Rayf M, Steinhagen J. Longitudinal long-term results of surgical treatment in patients with osteochondritis dissecans of the femoral condyles. Knee Surg Sports Traumatol Arthrosc. 2008;16:436-41. [DOI] [PubMed] [Google Scholar]
- 108. Michael J, Wurth A, Eysel P, König DP. Long-term results after operative treatment of osteochondritis dissecans of the knee joint—30 year results. Int Orthop. 2008;32:217-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Twyman RS, Desai K, Aichroth PM. Osteochondritis dissecans of the knee. A long-term study. J Bone Joint Surg Br. 1991;73:461-4. [DOI] [PubMed] [Google Scholar]
- 110. Fonseca F, Balaco I. Fixation with autogenous osteochondral grafts for the treatment of osteochondritis dissecans (stages III and IV). Int Orthop. 2009;33:139-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Havulinna J, Jokio P, Lindholm TS, Viljanen V, Savilahti S. Long-term results of Smillie pin fixation of osteochondritis dissecans in the femoral condyles. Ann Chir Gynaecol. 1995;84:71-80. [PubMed] [Google Scholar]
- 112. Magnussen R, Carey JL, Spindler KP. Does operative refixation of an osteochondritis loose body result in healing and long-term maintenance of knee function? Am J Sports Med. 2009;37:754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lipscomb PR, Jr, Lipscomb PR, Bryan RS. Osteochondritis dissecans of the knee with loose fragments. Treatment by replacement and fixation with readily removed pins. J Bone Joint Surg Am. 1978;60:235-40. [PubMed] [Google Scholar]
- 114. Tabaddor R, Banffy MB, Andersen JS, McFeely E, Ogunwole O, Micheli LJ, et al. Fixation of juvenile osteochondritis dissecans lesions of the knee using poly 96L/4D-lactide copolymer bioabsorbable implants. J Pediatr Orthop. 2010;30:14-20. [DOI] [PubMed] [Google Scholar]
- 115. Uematsu K, Habata T, Hasegawa Y, Hattori K, Kasanami R, Takakura Y, et al. Osteochondritis dissecans of the knee: long-term results of excision of the osteochondral fragment. Knee. 2005;12:205-8. [DOI] [PubMed] [Google Scholar]
- 116. Miniaci A, Tytherleigh-Strong G. Fixation of unstable osteochondritis dissecans lesions of the knee using arthroscopic autogenous osteochondral grafting (mosaicplasty). Arthroscopy. 2007;23:845-51. [DOI] [PubMed] [Google Scholar]
- 117. Morelli M, Poitras P, Grimes V, Backman D, Dervin G. Comparison of the stability of various internal fixators used in the treatment of osteochondritis dissecans—a mechanical model. J Orthop Res. 2007;25:495-500. [DOI] [PubMed] [Google Scholar]
- 118. Wouters DB, Bos RR, van Horn JR, van Luyn MJ. Should in the treatment of osteochondritis dissecans biodegradable or metallic fixation devices be used? A comparative study in goat knees. J Biomed Mater Res B Appl Biomater. 2008;84:154-64. [DOI] [PubMed] [Google Scholar]
- 119. Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grøntvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A:455-64. [DOI] [PubMed] [Google Scholar]
- 120. Knutsen G, Droset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. J Bone Joint Surg Am. 2007;89:2105-12. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]
- 121. Gudas R, Stankevicius E, Monastyreckiene E, Pranys D, Kalesinskas R. Osteochondral autologous transplantation versus microfracture for the treatment of articular defects in the knee joint in athletes. Knee Surg Sport Traumatol Arthrosc. 2006;14:834-42. [DOI] [PubMed] [Google Scholar]
- 122. Gudas R, Simonaityte R, Cekanauskas E, Tamosiunas R. A prospective, randomized clinical study of osteochondral autologous transplantation versus microfracture for the treatment of osteochondritis dissecans in the knee joint in children. J Pediatr Orthop. 2009;29:741-8. [DOI] [PubMed] [Google Scholar]
- 123. Kon E, Vannini F, Buda R, Filardo G, Cavallo M, Ruffilli A, et al. How to treat osteochondritis dissecans of the knee: surgical techniques and new trends: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94:e1(1-8). [DOI] [PubMed] [Google Scholar]
- 124. Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85:185-92. [DOI] [PubMed] [Google Scholar]
- 125. Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223-30. [DOI] [PubMed] [Google Scholar]
- 126. Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. J Bone Joint Surg Br. 2012;94:504-9. doi: 10.1302/0301-620X.94B4.27495. [DOI] [PubMed] [Google Scholar]
- 127. Gille J, Schuseil E, Wimmer J, Gellissen J, Schulz AP, Behrens P. Mid-term results of autologous matrix-induced chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2010;18:1456-64. [DOI] [PubMed] [Google Scholar]
- 128. Gille J, Behrens P, Volpi P, de Girolamo L, Reiss E, Zoch W, et al. Outcome of autologous matrix induced chondrogenesis (AMIC) in cartilage knee surgery: data of the AMIC Registry. Arch Orthop Trauma Surg. 2013;133:87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Goyal D, Keyhani S, Lee EH, Hui JH. Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy. 2013;29:1579-88. [DOI] [PubMed] [Google Scholar]
- 130. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37:2053-63. [DOI] [PubMed] [Google Scholar]
- 131. Anders S, Volz M, Frick H, Gellissen J. A randomized, controlled trial comparing autologous matrix-induced chondrogenesis (AMIC®) to microfracture: analysis of 1- and 2-year follow-up data of 2 centers. Open Orthop J. 2013;7:133-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wirth T, Rauch G, Schuler P, Griss P. Das autologe Knorpel-Knochen-Transplantat zur Therapie der Osteochondrosis dissecans des Kniegelenkes. Z Orthop. 1991;129:80-4. [DOI] [PubMed] [Google Scholar]
- 133. Laprell H, Petersen W. Autologous osteochondral transplantation using the diamond bone-cutting system (DBCS): 6-12 years’ follow-up of 35 patients with osteochondral defects at the knee joint. Arch Orthop Trauma Surg. 2001;121:248-53. [DOI] [PubMed] [Google Scholar]
- 134. Hangody L, Kish G, Karpati Z. Mosaicplasty for the treatment of osteochondritis dissecans of the knee. J Sports Traumatol Relat Res. 1998;2:126-33. [Google Scholar]
- 135. Hangody L, Füles P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85(suppl. 2):25-32. [DOI] [PubMed] [Google Scholar]
- 136. Hangody L, Kish G, Kárpáti Z, Szerb I, Udvarhelyi I. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. A preliminary report. Knee Surg Sport Traumatol Arthrosc. 1997;5:262-7. [DOI] [PubMed] [Google Scholar]
- 137. Gudas R, Gudaite A, Pocius A, Gudiene A, Cekanauskas E, Monastyreckiene E, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40:2499-508. [DOI] [PubMed] [Google Scholar]
- 138. Jakob R, Franz T, Gautier E, Mainil-Varlet P. Autologous osteochondral grafting in the knee: indication, results, and reflections. Clin Orthop Relat Res. 2002;401:170-84. [DOI] [PubMed] [Google Scholar]
- 139. Koulalis D, Schultz W, Heyden M, König F. Autologous osteochondral grafts in the treatment of cartilagedefects of the knee joint. Knee Surg Sports Traumatol Arthrosc. 2004;12:329-34. [DOI] [PubMed] [Google Scholar]
- 140. Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85:17-24. [DOI] [PubMed] [Google Scholar]
- 141. Chow J, Hantes ME, Houle JB, Zalavras CG. Arthroscopic autogenous osteochondral transplantation for treating knee cartilage defects: a 2- to 5-year follow-up study. Arthroscopy. 2004;20:681-90. [DOI] [PubMed] [Google Scholar]
- 142. Gomoll A, Farr J, Gillogly SD, Kercher J, Minas T. Surgical management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:2470-90. [PubMed] [Google Scholar]
- 143. Imhoff A, Burkart A, Öttl GM. Der posteriore Femurkondylentransfer. Orthopäde. 1999;28:45-51. [DOI] [PubMed] [Google Scholar]
- 144. Müller W. Ostechondrosis dissecans. In: Hastings D, editor. Progress in orthopaedic surgery. Vol. 3 New York, NY: Springer; 1978. p. 135-42. [Google Scholar]
- 145. Agneskirchner J, Brucker P, Burkart A, Imhoff AB. Large osteochondral defects of the femoral condyle: press-fit transplantation of the posterior femoral condyle (MEGA-OATS). Knee Surg Sport Traumatol Arthrosc. 2002;10:160-8. [DOI] [PubMed] [Google Scholar]
- 146. Minzlaff P, Braun S, Haller B, Wörtler K, Imhoff AB. Autologous transfer of the posterior femoral condyle for large osteochondral lesions of the knee. 5-year results of the Mega-OATS technique. Orthopade. 2010;39:631-6. [DOI] [PubMed] [Google Scholar]
- 147. Karataglis D, Learmonth DJ. Management of big osteochondral defects of the knee using osteochondral allografts with the MEGA-OATS technique. Knee. 2005;12:389-93. [DOI] [PubMed] [Google Scholar]
- 148. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-95. [DOI] [PubMed] [Google Scholar]
- 149. Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85:17-24. [DOI] [PubMed] [Google Scholar]
- 150. Cole B, DeBerardino T, Brewster R, Farr J, Levine DW, Nissen C, et al. Outcomes of the autologous chondrocyte implantation in Study of the Treatment of Articular Repair (STAR) patients with osteochondritis dissecans. Am J Sports Med. 2012;40:2015-22. [DOI] [PubMed] [Google Scholar]
- 151. Ferruzzi A, Buda R, Faldini C, Vannini F, Di Caprio F, Luciani D, et al. Autologous chondrocyte implantation in the knee joint: open compared with arthroscopic technique. J Bone Joint Surg Am. 2008;90(Suppl. 4):90-101. [DOI] [PubMed] [Google Scholar]
- 152. Basad E, Stürz H, Steinmeyer J. Treatment of osteochondral defects of the knee with autologous bone graft and chondrocyte transplantation: an overview together with our results. Acta Orthop Traumatol Turc. 2007;41(Suppl. 2):79-86. [PubMed] [Google Scholar]
- 153. Steinhagen J, Bruns J, Deuretzbacher G, Ruether W, Fuerst M, Niggemeyer O. Treatment of osteochondritis dissecans of the femoral condyle with autologous bone grafts and matrix-supported autologous chondrocytes. Int Orthop. 2010;34:819-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ochs B, Müller-Horvat C, Rolauffs B, Fritz J, Weise K, Schewe B. Treatment of osteochondritis dissecans of the knee: one-step procedure with bone grafting and matrix-supported autologous chondrocyte transplantation. Z Orthop Unfallchir. 2007;145:146-51. [DOI] [PubMed] [Google Scholar]
- 155. Oakeshott R, Farine I, Pritzker KP, Langer F, Gross AE. A clinical and histologic analysis of failed fresh osteochondral allografts. Clin Orthop Relat Res. 1988;233:283-94. [PubMed] [Google Scholar]
- 156. Tomford W, Springfield DS, Mankin HJ. Fresh and frozen articular cartilage allografts. Orthopedics. 1992;15:1183-8. [DOI] [PubMed] [Google Scholar]
- 157. Salai M, Ganel A, Horoszowski H. Fresh osteochondral allografts at the knee joint: good functional results in a follow-up study of more than 15 years. Arch Orthop Trauma Surg. 1997;116:423-5. [DOI] [PubMed] [Google Scholar]
- 158. Bakay A, Csonge L, Papp G, Fekete L. Osteochondral resurfacing of the knee joint with allograft. Clinical analysis of 33 cases. Int Orthop. 1998;22:277-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Emmerson B, Görtz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35:907-14. doi: 10.1177/0363546507299932. [DOI] [PubMed] [Google Scholar]
- 160. Levy Y, Görtz S, Pulido PA, McCauley JC, Bugbee WD. Do fresh osteochondral allografts successfully treat femoral condyle lesions? Clin Orthop Relat Res. 2013;471:231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Lyon R, Nissen C, Liu XC, Curtin B. Can fresh osteochondral allografts restore function in juveniles with osteochondritis dissecans of the knee? Clin Orthop Relat Res. 2013;471:1166-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. LaPrade R, Botker J, Herzog M, Agel J. Refrigerated osteoarticular allografts to treat articular cartilage defects of the femoral condyles. A prospective outcomes study. J Bone Joint Surg Am. 2009;91:805-11. [DOI] [PubMed] [Google Scholar]
- 163. Pearsall AT, Tucker JA, Hester RB, Heitman RJ. Chondrocyte viability in refrigerated osteochondral allografts used for transplantation within the knee. Am J Sports Med. 2004;32:125-31. [DOI] [PubMed] [Google Scholar]
- 164. Ball S, Amiel D, Williams SK, Tontz W, Chen AC, Sah RL, et al. The effects of storage on fresh human osteochondral allografts. Clin Orthop Relat Res. 2004;418:246-52. [DOI] [PubMed] [Google Scholar]
- 165. Mroz T, Joyce MJ, Steinmetz MP, Lieberman IH, Wang JC. Musculoskeletal allograft risks and recalls in the United States. J Am Acad Orthop Surg. 2008;16:559-65. [DOI] [PubMed] [Google Scholar]
- 166. Williams S, Amiel D, Ball ST, Allen RT, Tontz WL, Jr, Emmerson BC, et al. Analysis of cartilage tissue on a cellular level in fresh osteochondral allograft retrievals. Am J Sports Med. 2007;35:2022-32. [DOI] [PubMed] [Google Scholar]
- 167. Gurtner P, Lauber P, Bamert P. Langzeitresultate bei operativ behandelter Osteochondrosis dissecans am Kniegelenk. In: Debrunner AM, editor. Langzeitresultate in der Orthopädie. Stuttgart, Germany: Enke; 1990. p. 271-80. [Google Scholar]
- 168. Imhoff A, Minotti O, Schreiber A. 15 Jahresresultate nach konservativer und operativer Behandlung der Osteochondrosis dissecans am Knie. Arthroskopie. 1992;5:10-18. [Google Scholar]
- 169. Outerbridge HK, Outerbridge RE, Smith DE. Osteochondral defects in the knee: a treatment using lateral patella autografts. Clin Orthop Rel Res. 2000;377:145-51. [PubMed] [Google Scholar]
- 170. Wright R, McLean M, Matava MJ, Shively RA. Osteochondritis dissecans of the knee. Clin Orthop Rel Res. 2004;424:239-43. [PubMed] [Google Scholar]
- 171. Chahal J, Gross AE, Gross C, Mall N, Dwyer T, Chahal A, et al. Outcomes of osteochondral allograft transplantation in the knee. Arthroscopy. 2013;29:575-88. [DOI] [PubMed] [Google Scholar]
- 172. Brückl R, Rosemeyer B, Thiermann G. Osteochondrosis dissecans of the knee. Results of operative treatment in juveniles. Arch Orthop Trauma Surg. 1984;102:221-4. [DOI] [PubMed] [Google Scholar]
- 173. Jacobi M, Wahl P, Bouaicha S, Jakob RP, Gautier E. Association between mechanical axis of the leg and osteochondritis dissecans of the knee: radiographic study on 103 knees. Am J Sports Med. 2010;38:1425-8. [DOI] [PubMed] [Google Scholar]
- 174. Bruns J, Klima H. Osteochondrosis dissecans genus. Z Orthop. 1993;131:413-9. [DOI] [PubMed] [Google Scholar]
- 175. Bruns J, Klima H. Osteochondrosis dissecans of the knee and sports. Sportverletz Sportschaden. 1993;7:68-72. [DOI] [PubMed] [Google Scholar]
- 176. Slawski D. High tibial osteotomy in treatment of adult osteochondritis dissecans. Clin Orthop Relat Res. 1997;341:155-61. [PubMed] [Google Scholar]
- 177. Chambers H, Shea KG, Anderson AF, Jojo Brunelle TJ, Carey JL, Ganley TJ, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: the diagnosis and treatment of osteochondritis dissecans. J Bone Joint Surg Am. 2012;94:1322-4. [DOI] [PubMed] [Google Scholar]


