Abstract
In this paper, we propose and verify a theoretical model of the development of dispersion quality of aqueous carbon nanotube (CNT) colloid as a function of sonochemical yield of the sonication process. Four different surfactants; Triton X-100, Pluronic F-127, CTAB and SDS were studied. From these four SDS had the lowest dispersion performance which was surprising. Optical dispersion quality results fits well with proposed theoretical model.
Keywords: Physical chemistry, Materials science
1. Introduction
There is one significant feature requiring attention when it comes to CNT-nanocomposites and colloids; a dispersion quality. Dispersion quality, i.e. dispersed nanotubes divided by the total number of nanotubes, has a huge impact on the effective surface area of the interaction between the matrix and the filler. In order to optimize the performance one needs to know how to control the dispersion quality during each step of the manufacturing process.
CNTs forming large agglomerates creates challenges to process and stabilize colloids made of them. To optimize a dispersion process, enough energy density needs to be generated to overcome internal forces holding the aggregates together. Typical methods are shear-mixing [1] and sonication [2]. Of these two methods, sonication is superior especially for low viscosity systems where conventional mixing methods cannot create the required high strains rates. The dispersion process using sonication is based on inertial cavitation where imploding microscopic cavities generate intensive streams of molecules with high energy densities inside the liquid. Cavities are known to preferably exist at the boundaries of different materials [3] which makes sonication a very effective and precise method for dispersing nanotubes. Prolonged sonication however can cause damages to the tubes and must be avoided [4].
After the CNTs have been detached from aggregates, there is a possibility of re-agglomeration. To stabilize the system in water based dispersions, different types of surfactants such as ionic (anionic and cationic), non-ionic, polymer based and their combinations have been used comprising current state-of-the-art [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]. The basic idea is to enable surfactant molecules to be adsorbed on the surface of CNTs via hydrophobic interactions, π-π bonds, hydrogen bonds or electrostatic interactions [29], [30].
The nanotube dispersing efficiency of surfactants is linked to the length of an alkyl chain of the surfactant, presence of benzene ring, and the functional (terminal) group [10], concentration [22] and charge [31]. An optimum surfactant-CNT weight ratio has been reported to vary, ranging from 1:1 to 1:10 [10], [32]. It has been reported that an efficient CNT dispersion is possible only when the surfactant concentration is above the CMC value [27], [33], [34], [35]. It has also been reported that dispersing agents can form stable dispersions below and equal to their CMC limit [5], [7], [12], [36]. Moreover, it has been noted that the best result can be reached with a concentration of 0.5 CMC, and that any further increases in the concentration of the surfactant has only a minor effect [36]. Even with a absence of consensus using too high surfactant concentration may affect the properties of CNT network in the end product, using too low surfactant concentration can cause re-aggregation in colloid since a sufficient amount is needed to cover all CNT surfaces [32].
During the sonication, there is a dynamic equilibrium of concentrations between individual, surfactant coated nanotubes and nanotube agglomerates. As more energy is brought into the system, more nanotubes are being detached from the agglomerates and a dispersion quality is approaching unity. There are number of methods available for studying the quality of CNT-dispersions and they include; atomic force microscopy (AFM) [10], transmission electron microscopy (TEM) [32], Raman spectroscopy [37] and UV–Vis spectroscopy [38]. Of these methods, UV–Vis spectroscopy has been shown to represent the most accessible and versatile method to determine the dispersion quality of CNT dispersions especially for liquid systems. In the method, the light passing through a sample of colloid experiences scattering and absorbance. Both of these phenomena scale linearly with the concentration of colloidal particles and, therefore, the opacity α of the dispersion can be used to measure the number of nanotubes (individual tubes or dispersed small aggregates) in the supernatant [23]. At a fixed wavelength, UV–Vis spectroscopy can be used to determine the onset point of α as a function of the applied acoustic sonication energy. At this point, the system is close to its optimal dispersion state and further sonication would only damage the nanotubes without improving the quality of the dispersion.
In the previous studies the parameters to describe sonication have been total energy and time [38], [39]. These parameters work well with specific processes but are insufficient for comparing different studies since different sonication systems have different yields of transforming electrical energy to acoustic energy and individual systems also produce different amounts of inertial cavitation (vs. non-inertial). Inertial cavitation is mainly responsible of exfoliation of nanotubes whereas non-inertial cavitation is related to surface damages of the tubes [40]. In order to generalize all types of sonication systems parameter of sonochemical yield should be used; like first proposed by Koda et al. [41].
This article introduces theoretical framework for controlling the dispersion quality of aqueous carbon nanotube colloids during a sonication process. This framework is indifferent towards the sonication system, used energies and times.
2. Theory
We propose that for an ultrasound system, where the re-agglomeration of carbon nanotubes is inhibited by using surfactants the rate of opacity increase is related to effective acoustic energy in a following way:
| (1) |
where E is the effective acoustic energy divided by CNT mass, is the maximum achievable opacity of the system and f is a shape function. By separation we arrive to
| (2) |
Integration, rearrangement, and then using both sides as exponents leads to
| (3) |
For a system where f is a positive constant, and by applying boundary conditions, and , α can be expressed as
| (4) |
where, κ is a system-specific constant related to the types and quantities of the chemical components in the colloid.
In order to generalize (4) for all types of sonication systems, sonochemical yield is used instead of energy. In our experiments we used the concentration of iodine-ions divided by CNT mass, as a parameter [42].
It is known that sonolysis of water produces hydrogen peroxide via hydroxyl and hydrogen radicals and it causes oxidation of to from dissolved potassium iodide. then reacts with to produce , which has a peak absorbance at 355 nm and which can be detected by using UV–vis spectroscopy. The method, also known as Weissler reaction, has been proposed to be used as a standard method for the calibration of sonication systems [41]. The chain of chemical reactions is induced only by inertial cavitation, which is mainly responsible of the de-agglomeration of CNT aggregates. Therefore, Weissler reaction can be used to measure and compare effective dispersive processes of different sonication systems.
Thus, using with Equation (4), it leads to
| (5) |
where the value of is determined based on an experimental graph of electrical energy versus concentration of iodine-ions.
For determination of sonochemical yield of versus electrical energy consumed by the sonicator concentrations of were analyzed using the Beer–Lambert law
| (6) |
where α is the absorbance, ϵ is the molar attenuation coefficient of , b is the length of the optical path (in cm), and c is the concentration of . Linear fitting was used to interpolate production as a function of electrical energy and slope of the fitting was used to calculate values for the sonochemical yield.
3. Materials & methods
3.1. Materials
In this study, we used Nanocyl7000 multiwall carbon nanotubes (Nanocyl SA., Sambreville, Belgium) and four different surfactants: octyl phenol ethoxylate (Triton X-100), polyoxyethylene-polyoxypropylene block co-polymer (Pluronic F-127), sodium dodecyl sulfate (SDS) and cetyltrimethylammonium bromide (CTAB), all from Sigma Alrdich (Merck KGaA, Darmstadt, Germany). For the Weissler reaction potassium iodide (KI) (Merck KGaA, Darmstadt, Germany) was used.
3.2. Weissler reaction
For determination of sonochemical yield of versus electrical energy consumed by the sonicator, 200 ml of 0.1M KI solution was sonicated using different total energies and corresponding concentrations of were detected by measuring specific absorbance with Shimadzu UV-1800 spectrophotometer (Shimadzu Corp., Kyoto, Japan).
3.3. Dispersion of carbon nanotubes
160 samples with g of Nanocyl NC 7000 multiwall carbon nanotubes, four different surfactants (Triton X100, Pluronic F-127, CTAB, and SDS) with four different surfactant masses ( g, g, g and g) and deionized water were weighed in 100 ml glass beakers so that all samples weighted g. Dispersions were sonicated using 10 different electrical energies with QSonica Q700 sonicator (Qsonica L.L.C, Newtown, USA). A 12.7 mm diameter titanium probe was used and the vibration amplitude of a sonotrode was set to 60 μm. To guarantee identical sample preparation throughout the series, the tip was always placed in the same position inside the beaker (15 mm ± 2 mm from the bottom) and an external cooling bath with c. 200 W cooling capacity was used to limit the temperature variations during the sonication. The applied acoustic energy was varied by controlling the sonication time and it was monitored by an internal calorimeter of the QSonica Q700 sonicator. A power reading given by the sonicator remained between 100–120 W for all the sonications. An opacity at 500 nm, directly related to the concentration of carbon nanotubes in the dispersed state, was used to measure the quality of the sonicated dispersions. A portion of each dispersion was collected, let settle for five days, and its supernatant was diluted with to 1:300 with deionized water to get the solutions transparent. The opacity of the diluted dispersions were measured by using Shimadzu UV-1800 spectrophotometer and plastic cuvettes with 1 cm optical path length.
3.4. Imaging
The CNT agglomerate size was evaluated by drying a droplet of the dispersions on a metal plate and characterizing them by scanning electron microscopy (FIB-SEM, Zeiss Crossbeam 540).
4. Results
4.1. Weissler reaction
Figure 1 shows the development of concentration as a function of electrical energy consumed by the sonicator. It can be observed that the production rate was higher in the beginning of the sonication. This is caused by the dissolved gases, which accelerate the hydrogen peroxide production by participating in the chemical reactions (oxygen) and by lowering the inertial cavitation threshold [43]. After dissolved gases have fully diffused and consumed by the process, the rate of conversion slightly slows down. Linear fitting still gives a good approximation for versus and was used in all future calculations.
Figure 1.
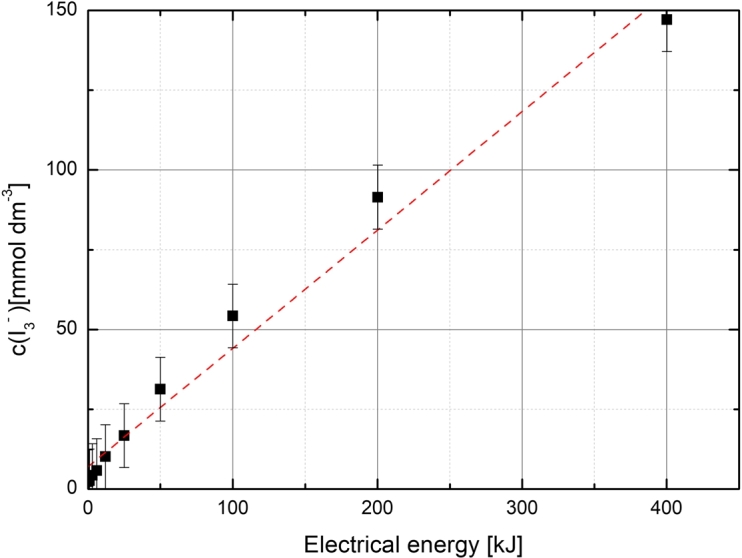
Concentration of as a function of electrical energy Eel used by the sonicator. The sonochemical yield/energy for this particular sonication system was 0.371 mmol dm−3 kJ−1. An error from sample preparation and measurements were estimated to be ± 10 mmol dm−3.
4.2. Dispersion of CNTs
As the applied sonication energy gets higher, the supernatant of the dispersion becomes darker indicating an increase in the concentration of CNTs in dispersed state (Figure 2). The dark appearance was found to be stable up to several weeks.
Figure 2.
Series of diluted supernatants of CNT-dispersions corresponding different acoustic energies of sonication. Evolution of the dispersion quality can be seen from samples a to j. As solutions get darker, more individual nanotubes are being dispersed in to the liquid.
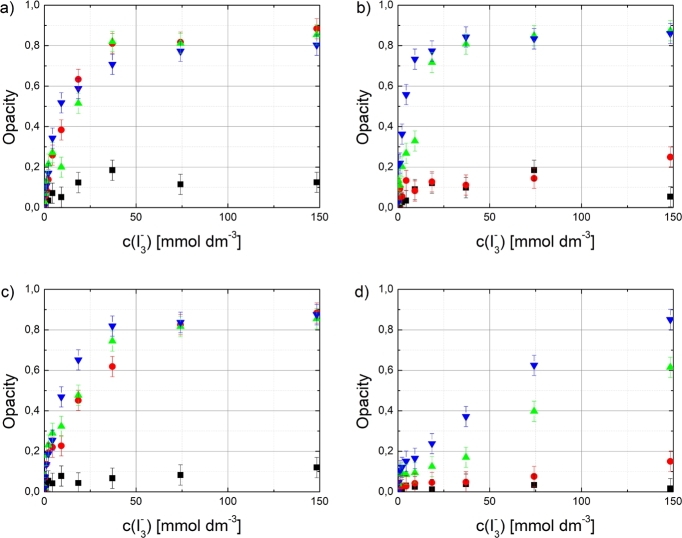
Figure 3 shows that the opacity at 500 nm follows the proposed dependence on sonochemical yield (5). It can be seen that Triton X-100 and CTAB can disperse CNTs close to the maximum dispersion quality at a lower surfactant/CNT mass ratios compared to Pluronic F-127 and SDS. It is also evident that the acoustic energy required to disperse CNTs with SDS is significantly higher compared to others, and that the rate of development of dispersion quality is respectively lower. This is somewhat surprising since SDS is widely used in many of the reported studies and yet it seems to be more difficult to use in order to optimize the dispersion quality.
Figure 3.
The development of opacity as a function of with different surfactant/CNT ratios: ■ 1:4, ● 1:2, ▲ 1:1 and ▼ 2:1 for a) Triton X-100, b) Pluronic F-127, c) CTAB and d) SDS.
In Figure 4, a fitted opacity function (5) gives a theoretical asymptote for the maximum opacity, , with a theoretical infinite yield. Possible deviations from (5) with higher yields are due to the fracture (damage) of CNTs as the sonication progresses causing additional opacity, which is not related to the surfactant-assisted exfoliation of the aggregates.
Figure 4.
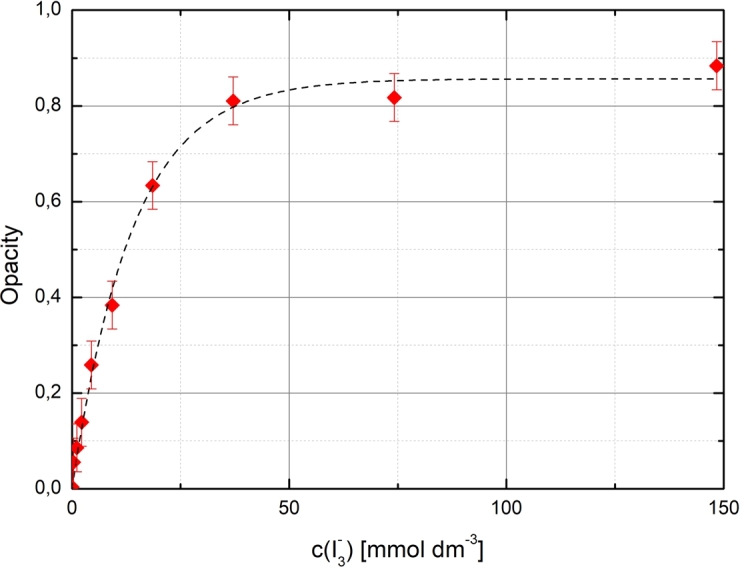
Measured opacity of sonicated 1:2 Triton X-100/CNT dispersion as a function of sonochemical yield of the sonicator together with a fitted opacity function (5).
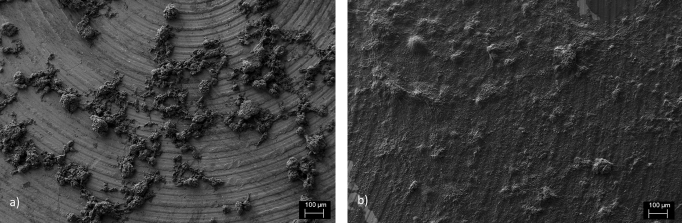
In Figure 5 it can be seen that investigating dispersion quality with dried samples is rather challenging. One can say that larger agglomerates do disappear as a function of sonochemical yield, but calculating a number describing the dispersion quality is basically impossible. Dried sample for FIB microscopy is not an accurate 2D presentation of the 3D situation. During the drying process the surface tension of water affects how 2D-structure is being formed. Therefore SEM or FIB microscopy is not optimal for studying dispersion quality of water based CNT colloids.
Figure 5.
FIB images of dried 1:2 Triton X-100/CNT dispersions. a) Non-sonicated and b) sonicated with maximum opacity. It can be seen that most of the large agglomerates have been dispersed and the metal plate is coated with individual CNTs. Still some larger particles exists.
Variation of maximum absorbance can be seen for different surfactant/CNT ratios depending on the surfactant type (Figure 6). It is noticeable that there is a sharp change in the development of the maximum opacity as the surfactant/CNT ratio increases. Below this threshold value of surfactant/CNT ratio, the highest reachable opacity (indicating the maximum dispersion quality) is to be low. On the other hand, when the ratio matches the threshold value, the maximum opacity gets also rapidly reached and further increase in the ratio does not improve the dispersion. For applications where surfactant assisted dispersions are necessary, the determination of an optimum surfactant ratio is critical since excess surfactant remaining, for example in a nanocomposite matrix, will diminish the physical properties. SDS is not included in Figure 6 since the applied acoustic energy range was not high enough to reach the saturation in the opacity. SDS was the only surfactant which did not reach saturation point of the opacity with used energies even with highest concentrations. All sonications except the lowest concentration of SDS were above critical micelle concentration.
Figure 6.
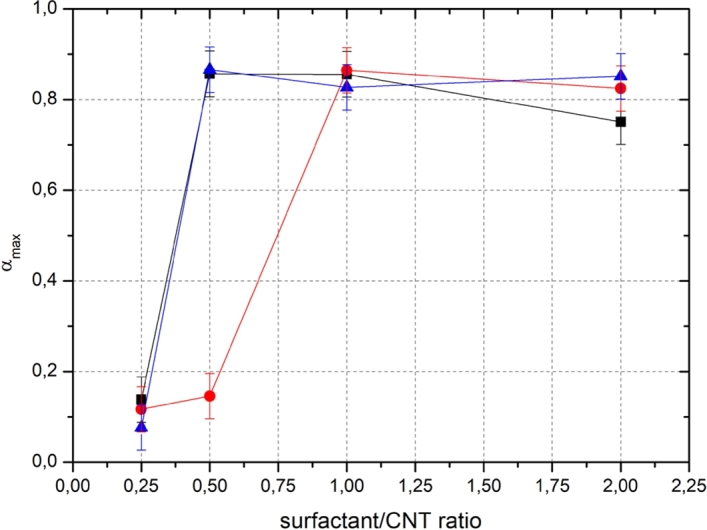
Measured maximum opacities as a function of surfactant/CNT ratio for three different surfactants; ■ Triton X-100, ● Pluronic F-127 and ▲ CTAB.
The factor κ in Equation (5), indicating the rate of opacity increase as a function of acoustic energy, is implicitly dependent on the surfactant/CNT ratio (Figure 7). κ can be observed to have a different local minimum for different surfactants, which is most likely related to the adsorption mechanism of the surfactant on the CNT surface. Depending on surfactant concentration, the assembly on the CNT surface is different. The tendency to improve the dispersion along with the increase in the acoustic energy is weaker the stronger is the surfactant layers internal binding on the initial agglomerates. Also, the response in the acoustic energy transfer by the surfactant layer can hinder the CNT agglomerate dispersion yet it is challenging to theoretically verify the difference in this response between different surfactants.
Figure 7.
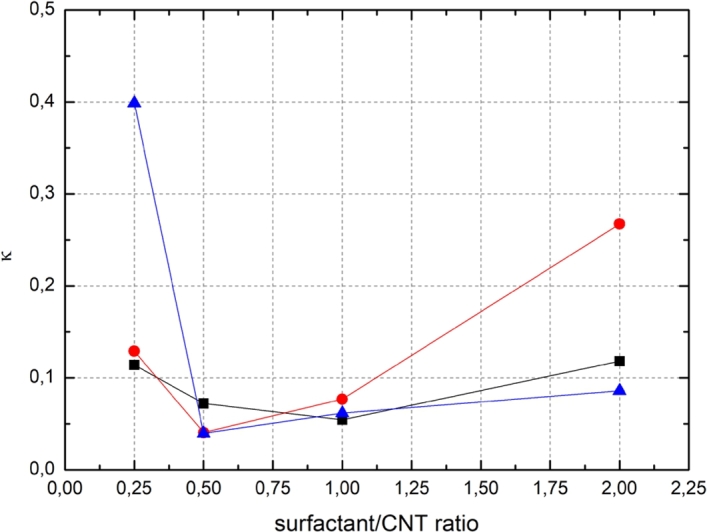
The factor κ, indicating a growth speed of the opacity as a function of surfactant/CNT for ■ Triton X-100, ● Pluronic F-127 and ▲ CTAB.
5. Conclusions
A theoretical equation for a development of the dispersion quality of aqueous CNT colloid as a function of sonochemical yield was proposed. Sonication experiments with four different surfactant types and different surfactant/CNT ratios and inertial cavitation activities were performed. The inertial cavitation activity was determined using the Weissler reaction. It was shown that the proposed equation fits well with the performed measurements and that the maximum opacities, received via fitting the equations, follows an S-curve as a function of surfactant/CNT ratio. The revealed sonochemical yield-dispersion (SCY-D) relation indicates that there is a threshold for the minimum surfactant/CNT ratio to achieve the optimal dispersion quality for a CNT-surfactant system. Here, we determined the lower and upper values of the threshold region of surfactant/CNT ratios for three different surfactants, namely Triton X-100, Pluronic F-127, and CTAB.
Declarations
Author contribution statement
Pasi Keinänen – Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Sanna Siljander – Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Mikko Koivula, Essi Sarlin – Performed the experiments.
Jatin Sethi, Jyrki Vuorinen, Mikko Kanerva: Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Andrews R., Jacques D., Minot M., Rantell T. Fabrication of carbon multiwall nanotube/polymer composites by shear mixing. Macromol. Mater. Eng. 2002;287(6):395–403. [Google Scholar]
- 2.Huang Y., Terentjev E. Dispersion and rheology of carbon nanotubes in polymers. Int. J. Mater. Forming. 2008;1(2):63–74. [Google Scholar]
- 3.Lavilla I., Bendicho C. Water Extraction of Bioactive Compounds. Elsevier; 2018. Fundamentals of ultrasound-assisted extraction; pp. 291–316. [Google Scholar]
- 4.Yang D.-Q., Rochette J.-F., Sacher E. Functionalization of multiwalled carbon nanotubes by mild aqueous sonication. J. Phys. Chem. B. 2005;109(16):7788–7794. doi: 10.1021/jp045147h. [DOI] [PubMed] [Google Scholar]
- 5.Bonard J.-M., Stora T., Salvetat J.-P., Maier F., Stöckli T., Duschl C., Forró L., de Heer W.A., Châtelain A. Purification and size-selection of carbon nanotubes. Adv. Mater. 1997;9(10):827–831. [Google Scholar]
- 6.Vaisman L., Marom G., Wagner H.D. Dispersions of surface-modified carbon nanotubes in water-soluble and water-insoluble polymers. Adv. Funct. Mater. 2006;16(3):357–363. [Google Scholar]
- 7.Geng Y., Liu M.Y., Li J., Shi X.M., Kim J.K. Effects of surfactant treatment on mechanical and electrical properties of CNT/epoxy nanocomposites. Composites, Part A, Appl. Sci. Manuf. 2008;39(12):1876–1883. [Google Scholar]
- 8.Rausch J., Zhuang R.-C., Mäder E. Surfactant assisted dispersion of functionalized multi-walled carbon nanotubes in aqueous media. Composites, Part A, Appl. Sci. Manuf. 2010;41(9):1038–1046. [Google Scholar]
- 9.Javadian S., Motaee A., Sharifi M., Aghdastinat H., Taghavi F. Dispersion stability of multi-walled carbon nanotubes in catanionic surfactant mixtures. Colloids Surf. A, Physicochem. Eng. Asp. 2017;531:141–149. [Google Scholar]
- 10.Islam M., Rojas E., Bergey D., Johnson A., Yodh A. High weight fraction surfactant solubilization of single-wall carbon nanotubes in water. Nano Lett. 2003;3(2):269–273. [Google Scholar]
- 11.McDonald T.J., Engtrakul C., Jones M., Rumbles G., Heben M.J. Kinetics of PL quenching during single-walled carbon nanotube rebundling and diameter-dependent surfactant interactions. J. Phys. Chem. B. 2006;110(50):25339–25346. doi: 10.1021/jp065281x. [DOI] [PubMed] [Google Scholar]
- 12.Bystrzejewski M., Huczko A., Lange H., Gemming T., Büchner B., Rümmeli M. Dispersion and diameter separation of multi-wall carbon nanotubes in aqueous solutions. J. Colloid Interface Sci. 2010;345(2):138–142. doi: 10.1016/j.jcis.2010.01.081. [DOI] [PubMed] [Google Scholar]
- 13.Vigolo B., Penicaud A., Coulon C., Sauder C., Pailler R., Journet C., Bernier P., Poulin P. Macroscopic fibers and ribbons of oriented carbon nanotubes. Science. 2000;290(5495):1331–1334. doi: 10.1126/science.290.5495.1331. [DOI] [PubMed] [Google Scholar]
- 14.O'connell M.J., Bachilo S.M., Huffman C.B., Moore V.C., Strano M.S., Haroz E.H., Rialon K.L., Boul P.J., Noon W.H., Kittrell C. Band gap fluorescence from individual single-walled carbon nanotubes. Science. 2002;297(5581):593–596. doi: 10.1126/science.1072631. [DOI] [PubMed] [Google Scholar]
- 15.Poulin P., Vigolo B., Launois P. Films and fibers of oriented single wall nanotubes. Carbon. 2002;40(10):1741–1749. [Google Scholar]
- 16.Jiang L., Gao L., Sun J. Production of aqueous colloidal dispersions of carbon nanotubes. J. Colloid Interface Sci. 2003;260(1):89–94. doi: 10.1016/s0021-9797(02)00176-5. [DOI] [PubMed] [Google Scholar]
- 17.Moore V.C., Strano M.S., Haroz E.H., Hauge R.H., Smalley R.E., Schmidt J., Talmon Y. Individually suspended single-walled carbon nanotubes in various surfactants. Nano Lett. 2003;3(10):1379–1382. [Google Scholar]
- 18.Yurekli K., Mitchell C.A., Krishnamoorti R. Small-angle neutron scattering from surfactant-assisted aqueous dispersions of carbon nanotubes. J. Am. Chem. Soc. 2004;126(32):9902–9903. doi: 10.1021/ja047451u. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee T., Yurekli K., Hadjiev V.G., Krishnamoorti R. Single-walled carbon nanotube dispersions in poly (ethylene oxide) Adv. Funct. Mater. 2005;15(11):1832–1838. [Google Scholar]
- 20.Hertel T., Hagen A., Talalaev V., Arnold K., Hennrich F., Kappes M., Rosenthal S., McBride J., Ulbricht H., Flahaut E. Spectroscopy of single-and double-wall carbon nanotubes in different environments. Nano Lett. 2005;5(3):511–514. doi: 10.1021/nl050069a. [DOI] [PubMed] [Google Scholar]
- 21.Grossiord N., Loos J., Van Laake L., Maugey M., Zakri C., Koning C.E., Hart A.J. High-conductivity polymer nanocomposites obtained by tailoring the characteristics of carbon nanotube fillers. Adv. Funct. Mater. 2008;18(20):3226–3234. [Google Scholar]
- 22.Blanch A.J., Lenehan C.E., Quinton J.S. Optimizing surfactant concentrations for dispersion of single-walled carbon nanotubes in aqueous solution. J. Phys. Chem. B. 2010;114(30):9805–9811. doi: 10.1021/jp104113d. [DOI] [PubMed] [Google Scholar]
- 23.Clark M.D., Subramanian S., Krishnamoorti R. Understanding surfactant aided aqueous dispersion of multi-walled carbon nanotubes. J. Colloid Interface Sci. 2011;354(1):144–151. doi: 10.1016/j.jcis.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Ryabenko A., Fokeeva L., Dorofeeva T. Spectroscopic study of suspensions of single-wall carbon nanotubes in polyaniline solutions in N-methylpyrrolidone in UV–Vis–NIR regions. Russ. Chem. Bull. 2004;53(12):2695–2699. [Google Scholar]
- 25.Jiang M.-J., Dang Z.-M., Yao S.-H., Bai J. Effects of surface modification of carbon nanotubes on the microstructure and electrical properties of carbon nanotubes/rubber nanocomposites. Chem. Phys. Lett. 2008;457(4):352–356. [Google Scholar]
- 26.Rastogi R., Kaushal R., Tripathi S., Sharma A.L., Kaur I., Bharadwaj L.M. Comparative study of carbon nanotube dispersion using surfactants. J. Colloid Interface Sci. 2008;328(2):421–428. doi: 10.1016/j.jcis.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Bai Y., Lin D., Wu F., Wang Z., Xing B. Adsorption of triton x-series surfactants and its role in stabilizing multi-walled carbon nanotube suspensions. Chemosphere. 2010;79(4):362–367. doi: 10.1016/j.chemosphere.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 28.Madni I., Hwang C.-Y., Park S.-D., Choa Y.-H., Kim H.-T. Mixed surfactant system for stable suspension of multiwalled carbon nanotubes. Colloids Surf. A, Physicochem. Eng. Asp. 2010;358(1):101–107. [Google Scholar]
- 29.Bai Y., Park I.S., Lee S.J., Bae T.S., Watari F., Uo M., Lee M.H. Aqueous dispersion of surfactant-modified multiwalled carbon nanotubes and their application as an antibacterial agent. Carbon. 2011;49(11):3663–3671. [Google Scholar]
- 30.Yang K., Xing B. Adsorption of organic compounds by carbon nanomaterials in aqueous phase: Polanyi theory and its application. Chem. Rev. 2010;110(10):5989–6008. doi: 10.1021/cr100059s. [DOI] [PubMed] [Google Scholar]
- 31.Wenseleers W., Vlasov I.I., Goovaerts E., Obraztsova E.D., Lobach A.S., Bouwen A. Efficient isolation and solubilization of pristine single-walled nanotubes in bile salt micelles. Adv. Funct. Mater. 2004;14(11):1105–1112. [Google Scholar]
- 32.Yu J., Grossiord N., Koning C.E., Loos J. Controlling the dispersion of multi-wall carbon nanotubes in aqueous surfactant solution. Carbon. 2007;45(3):618–623. [Google Scholar]
- 33.Utsumi S., Kanamaru M., Honda H., Kanoh H., Tanaka H., Ohkubo T., Sakai H., Abe M., Kaneko K. RBM band shift-evidenced dispersion mechanism of single-wall carbon nanotube bundles with NaDDBs. J. Colloid Interface Sci. 2007;308(1):276–284. doi: 10.1016/j.jcis.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 34.Sun Z., Nicolosi V., Rickard D., Bergin S.D., Aherne D., Coleman J.N. Quantitative evaluation of surfactant-stabilized single-walled carbon nanotubes: dispersion quality and its correlation with zeta potential. J. Phys. Chem. C. 2008;112(29):10692–10699. [Google Scholar]
- 35.Maillaud L., Zakri C., Ly I., Pénicaud A., Poulin P. Conductivity of transparent electrodes made from interacting nanotubes. Appl. Phys. Lett. 2013;103(26) [Google Scholar]
- 36.Angelikopoulos P., Gromov A., Leen A., Nerushev O., Bock H., Campbell E.E. Dispersing individual single-wall carbon nanotubes in aqueous surfactant solutions below the cmc. J. Phys. Chem. C. 2009;114(1):2–9. [Google Scholar]
- 37.Shen K., Curran S., Xu H., Rogelj S., Jiang Y., Dewald J., Pietrass T. Single-walled carbon nanotube purification, pelletization, and surfactant-assisted dispersion: a combined TEM and resonant micro-Raman spectroscopy study. J. Phys. Chem. B. 2005;109(10):4455–4463. doi: 10.1021/jp045046j. [DOI] [PubMed] [Google Scholar]
- 38.Grossiord N., Regev O., Loos J., Meuldijk J., Koning C.E. Time-dependent study of the exfoliation process of carbon nanotubes in aqueous dispersions by using UV-visible spectroscopy. Anal. Chem. 2005;77(16):5135–5139. doi: 10.1021/ac050358j. [DOI] [PubMed] [Google Scholar]
- 39.Alafogianni P., Dassios K., Farmaki S., Antiohos S., Matikas T., Barkoula N.-M. On the efficiency of UV–vis spectroscopy in assessing the dispersion quality in sonicated aqueous suspensions of carbon nanotubes. Colloids Surf. A, Physicochem. Eng. Asp. 2016;495:118–124. [Google Scholar]
- 40.Sesis A., Hodnett M., Memoli G., Wain A.J., Jurewicz I., Dalton A.B., Carey J.D., Hinds G. Influence of acoustic cavitation on the controlled ultrasonic dispersion of carbon nanotubes. J. Phys. Chem. B. 2013;117(48):15141–15150. doi: 10.1021/jp410041y. [DOI] [PubMed] [Google Scholar]
- 41.Koda S., Kimura T., Kondo T., Mitome H. A standard method to calibrate sonochemical efficiency of an individual reaction system. Ultrason. Sonochem. 2003;10(3):149–156. doi: 10.1016/S1350-4177(03)00084-1. [DOI] [PubMed] [Google Scholar]
- 42.Weissler A., Cooper H.W., Snyder S. Chemical effect of ultrasonic waves: oxidation of potassium iodide solution by carbon tetrachloride. J. Am. Chem. Soc. 1950;72(4):1769–1775. [Google Scholar]
- 43.Petrier C., Lamy M.-F., Francony A., Benahcene A., David B., Renaudin V., Gondrexon N. Sonochemical degradation of phenol in dilute aqueous solutions: comparison of the reaction rates at 20 and 487 kHz. J. Phys. Chem. 1994;98(41):10514–10520. [Google Scholar]