Abstract
Poor prenatal development, followed by rapid childhood growth, conveys greater cardiometabolic risk in later life. Microswine offspring exposed to perinatal maternal protein restriction [MPR; “low protein offspring” (LPO)] grow poorly in late-fetal/neonatal stages. After weaning to an ad libitum (AL) diet, LPO-AL exhibit accelerated growth and fat deposition rates with low adiponectin mRNA, despite low-normal body fat and small intra-abdominal adipocytes. We examined effects of caloric restriction (CR) on growth and metabolic status in LPO and normal protein offspring (NPO) randomized to AL or CR diets from weaning. CR transiently reduced growth in both LPO and NPO, delaying recovery in female LPO-CR. Over 7.5–12.5 weeks, linear growth rates in LPO-CR were slower than LPO-AL (P < 0.001) but exceeded NPO-AL; body weight growth rates fell but were lower in LPO-CR versus NPO-CR. Linear acceleration ceased after 12 weeks. At 16 weeks, percent catch-up in LPO-CR was reduced versus LPO-AL (P < 0.001). Plasma growth hormone was low in LPO (P < 0.02). CR normalized fat deposition rate, yet adiponectin mRNA remained low in LPO-CR (P < 0.001); plasma adiponectin was low in all LPO-AL and in female LPO-CR. Insulin sensitivity improved during CR. We conclude that in LPO: 1) CR delays onset of, but does not abolish, accelerated linear growth, despite low growth hormone; 2) CR yields stunting via delayed onset, plus a finite window for linear growth acceleration; 3) MPR lowers adiponectin mRNA independently of growth, adiposity, or adipocyte size; and 4) MPR reduces circulating adiponectin in LPO-AL and female LPO-CR, potentially enhancing cardiometabolic risk.
Keywords: adiponectin, adipose tissue, catch-up growth, fetal programming, growth hormone
INTRODUCTION
Detrimental effects of a poor intrauterine environment on later health have been consistently observed in humans and in multiple animal models across a variety of species and prenatal exposures. These exposures include over- and undernutrition (35), psychosocial stressors (20), and drugs/toxins (39). In humans, postnatal growth rates that upwardly cross weight centiles (“catch-up growth”) exacerbate risk for adverse outcomes, such as hypertension (22), obesity (9), type 2 diabetes (32), and coronary-related mortality (28, 33). Children born small who experience catch-up growth are especially at risk for these adverse cardiometabolic outcomes. The mechanism(s) of exacerbated disease risk are not yet clear but have been commonly attributed to the obesity that often accompanies or follows catch-up growth.
We have previously described a microswine model of perinatal maternal protein restriction (MPR) in which pregnant sows are exposed to an isocaloric low protein diet from 0.7 gestation through 2 weeks postnatally, a period chosen to accommodate delayed (relative to humans) development of adipose tissue (AT) and kidney in swine and thus to approximate the third trimester of human gestation. These low protein (LP) offspring (LPO) develop asymmetric intrauterine growth restriction (IUGR) with continued poor growth until sows resume a normal diet at 2 weeks. IUGR in these offspring, when on an ad libitum (AL) diet after weaning, is followed by accelerated postweaning growth, sustained both by enhanced feed utilization efficiency (gram tissue gained/gram feed intake/week) and by increased relative feed intake (gram feed/kilogram body weight/meal). Unexpectedly, accelerated growth was not associated with excess adiposity, yet LPO-AL offspring accumulated both fat and lean tissues at an accelerated rate (as a fold increase from 6 to 11 weeks of age) (19). LPO-AL offspring, as juveniles, also exhibited reduced adiponectin mRNA in both intra-abdominal (ABD) and subcutaneous (SC) fat depots (18) despite the absence of adiposity excess or adipocyte hypertrophy. We considered the possibility that as with obesity, the accelerated rate of tissue and/or fat accrual might drive reduced adiponectin levels and thus contribute to increased cardiometabolic risk. A critical question in LPO is whether the limiting of the rate of growth acceleration will be harmful or beneficial to overall growth and metabolic health. We hypothesized that reduction of growth acceleration via postweaning moderate caloric restriction (CR) would restore adiponectin and other metabolic abnormalities to levels similar to sex-matched normal protein (NP) offspring (NPO)-AL controls.
Therefore, we examined effects of moderate postweaning CR in LPO on growth and efficacy of catch-up, on rate of fat and lean tissue accrual, and on adiponectin mRNA, each as compared with previously reported AL conditions (18, 19). Furthermore, we assessed for the first time levels of adiponectin protein and growth hormone/insulin-like growth factor (IGF)-1 axis hormones in both AL and CR conditions.
MATERIALS AND METHODS
Animal Care and Experimental Design
With the use of a microswine model of perinatal MPR, LPO and NPO controls exposed to AL feed postweaning [reported previously (18, 19)] are here compared with concurrently studied offspring exposed to postweaning CR, specifically to examine CR effects on the accelerated growth, degree of catch-up, rate of fat deposition/body composition, adipocyte size, and hormonal indices of growth and adipokine function. The experimental design is summarized in Fig. 1. Experiments were approved by the Oregon Health & Science University (OHSU) Institutional Animal Care and Use Committee under protocol A439. AL offspring have been previously described (18, 19); these data are used with permission as controls to examine the effect of MPR on offspring responses to CR. New outcomes from the same AL animals (growth hormone, IGF-1, plasma adiponectin), as well as all data from CR animals, are described here for the first time.
Fig. 1.
Experimental design and time course of studies. Time-mated microswine sows were randomized to either normal protein (NP) or low protein (LP) diets during the last ¼ gestation and first 2 wk of lactation. Offspring of NP sows are termed NPO; offspring of LP sows are termed LPO. Studies were performed in sets of 2 litters each: 1 NPO and 1 LPO; a total of 5 such 2-litter study sets were carried out. Offspring were weaned at ~4 wk of age to ad libitum (AL) or calorie-restricted (CR) diets. Prospectively age-matched growth indices were measured from 4 to 12 wk in all animals. Offspring body composition was assessed by the dual-energy X-ray absorptiometry (DEXA) scan at weeks 6 and 11. Beginning at 12 wk, a series of 3–4, 3-wk-long protocols was initiated, each including 4 mixed-group offspring, to assess renal physiology and blood pressure (reported separately), culminating in terminal tissue harvest. The number of sequential 3-wk-long protocols required to complete each 2-litter study set thus varied with the total number of animals in the set. Because of the staggered protocols, age at initiation of the 3-wk-long protocol ranged from 12 to 23 wk, with harvest ages ranging from 14 to 25 wk.
Yucatan microswine sows (Charles River Laboratories, Wilmington, MA; and later, Sinclair BioResources, Auxvasse, MO) and their offspring were housed at the Department of Comparative Medicine, OHSU, with free access to water and a 12:12-h light:dark cycle. Sows and weaned offspring were offered two meals per day (see details below for offspring feeding). Temperature was maintained at 25°C; before birth, heat lamps and farrowing gates were provided to keep piglets warm and to prevent accidental crushing by the sow. Upon being weaned at 4 weeks of age, LPO and NPO (controls) were grouped by sex and then randomly assigned to either AL or CR diets. Animals were housed in adjacent individual cages to allow visual, auditory, olfactory, and limited physical contact among these social animals while eliminating competition for feed and permitting accurate feed-intake data collection.
Timed pregnant primiparous sows were randomized to an NP or LP diet from gestation day 80 (of 113) through the first 2 weeks of lactation, a period developmentally approximating the third trimester of human pregnancy with respect to renal and adipose development. There was no significant difference between number of live offspring born between NP and LP sows (NP 6.6 ± 0.5 per litter, n = 5 litters; LP 6.4 ± 0.6 per litter, n = 5 litters; P = 0.82), so no culling or fostering was performed. As expected, birth weights were lower in piglets born to sows on the LP diet compared with piglets born to sows on the NP diet (P < 0.02) and lower in females (P = 0.01): male NP, 0.985 ± 0.029 kg, n = 16 offspring; male LP, 0.955 ± 0.036 kg, n = 15 offspring; female NP, 0.920 ± 0.026 kg, n = 17 offspring; female LP, 0.832 ± 0.024, n = 17 offspring (P < 0.05). Birth lengths were not affected by maternal diet or sex. As reported, LPO exhibit asymmetric growth restriction, with maximal growth deficits at 2 weeks postnatally, the end point of MPR, compared with NPO (19). Studies were carried out in sets of two litters each: one NPO litter and one LPO litter (Fig. 1). The first two study sets of paired NPO and LPO litters were assigned to an AL postweaning diet to establish baselines for growth and normal feed intake in preparation for adding the CR protocol. In subsequent study sets, LPO and NPO were randomized within sex to AL or CR diets after 4 weeks of age. All healthy offspring were studied. However, some animal losses and some data losses occurred over the timeframe of experiments. Thus the number of animals (n) is given in figures and text if it differs from the maximum n for each group listed in Table 1. A total of 51 offspring were studied, from 10 sows (five NP and five LP).
Table 1.
Numbers of offspring studied in each group
| Maternal diet: | Normal Protein |
Low Protein |
||
|---|---|---|---|---|
| Postweaning diet: | Ad libitum | Calorie restricted | Ad libitum | Calorie restricted |
| Male | 8 | 7 | 10 | 4 |
| Female | 9 | 5 | 7 | 4 |
These are maximum numbers in each group. In measures that differ from these, the number of animals (n) is given in text, tables, and/or figures as appropriate.
Serial Measurements: Whole Body Growth
Offspring weights and crown-to-rump lengths were measured 3 days/week from weaning. Growth rates were assessed across weeks 5–11 (see Statistical Methods for details.)
General overview of protocol.
Five study sets were performed, each consisting of two litters (Fig. 1). During the period from weaning (3–4 weeks) to harvest for each two-litter set, two categories of data were collected: 1) from weaning to 12 weeks of age, serially and prospectively age-adjusted measurements were made of body size (growth, three times per week), feed intake (three times per week), and body composition [dual-energy X-ray absorptiometry (DEXA), at 6 and at 12 weeks of age], and 2) from 12 weeks to harvest, for each study set, we sequentially performed 3-week-long preharvest/harvest protocols, each accommodating four pigs from each experimental group where possible, during which cross-sectional data were collected. (This staggered design was logistically necessary, given the time and labor intensity of the protocol: multidisciplinary staffing/personnel, surgical procedure, and complex physiological measurements were involved.) To complete these protocols for a typical two-litter study set of 12–14 animals, three or four, 3-wk-long time blocks were required, across harvest ages ranging from 12 to 25 wk. These cross-sectional data included the following: 1) the single last-measured body-size values (used to compare degree of catch-up growth and measured just before initiation of the 3-week protocol to avoid effects of surgical stress); 2) harvest-linked plasma measurements (e.g., growth hormone, IGF-1, adiponectin); and 3) harvest-linked tissue collections (e.g., mRNAs, adipocyte size). Because of the wide age distribution of the cross-sectional data, each measurement was independently evaluated for age effects within each group and overall and age adjusted if appropriate. We did not find any case of group-specific age effects; thus parameters affected by age were adjusted using age as a covariate within the ANOVA.
Serial Measurements: Feed Intake and Feed Utilization Efficiency
All animals were fed two weighed meals per day of standard piglet growth chow, on 7 days/wk from weaning to harvest, and were observed for the two, 1-h meal periods; no other feed was consumed outside of these meal periods. Feed intake was measured at both meals on 3 days/wk. For those on AL diets, piglets were offered preweighed meals in an amount larger than they could eat in the 1-h meal period. Preweighed meals were based on current body weight (see below). Feed remaining after 1 h or after pigs lost interest in eating and began playing with feeders was weighed. The difference between pre- and postmeal feed weight was recorded as the amount ingested. Meal weight was not recorded if feed spilled beyond the perimeter of the catch bin.
CR offspring were offered 75–80% of the amount of feed consumed by sex-matched NPO-AL relative to current body weight. As the study was designed to reduce growth acceleration, and specifically, to normalize fractional growth rates, if an animal was still growing quickly on 80% feed, then its offering was reduced to 75%. We considered feed below 75% too severe so did not restrict more than that. Not all CR animals consumed all of the feed offered at every meal; thus some animals were voluntarily more CR than others, especially as time progressed. Actual mean relative feed intake is shown in Fig. 2.
Fig. 2.
Effect of calorie restriction (CR) on feed intake and feed utilization efficiency. Relative feed intake [grams per kilogram body weight (Bwt) per meal] is shown for females (A) and males (B); feed utilization efficiency (weight gained per kilogram feed consumed) is shown for females (C) and males (D). Normal protein offspring (NPO)-ad libitum (AL): n = 5–9 females, 3–8 males; low protein offspring (LPO)-AL: n = 6–8 females, 6–10 males; NPO-CR: n = 4–5 females, 5 males; LPO-CR: n = 4 females, 4 males. See text for analysis.
Serial Measurements: Body Composition and Relative Fat and Lean Deposition Rates
Body fat and lean tissue amounts were measured using DEXA scanning, as previously described (19). In brief, scans were performed at 6 and 11 wk of age under isoflurane anesthesia. Head and forelimbs were excluded from analysis due to constraints imposed by software and porcine anatomy. Grams of fat or lean tissue, expressed here relative to body length, were reported directly by the software; percent body fat was automatically calculated by the DEXA software. Relative fat (or lean) deposition rate from 6 to 11 wk of age was calculated as fold change in total body fat (or lean) mass (grams) relative to the initial 6-wk value: total body fat11 weeks/total body fat6 weeks.
Cross-Sectional Measurements: Final Body Size as Degree of Catch-up
To estimate CR impacts on catch-up growth, a one-time, last-measured “preharvest” length and weight were collected for each animal, just before initiation of the final 3-wk harvest protocol, thus varying across a 12- to 23-wk age range. Measurements taken after this date were not used in body-size analyses to avoid confounding by surgical stress. Group-specific regression analyses indicated similar rates of growth among the four groups (NPO-AL, NPO-CR, LPO-AL, and LPO-CR) for body length and body weight over the range of ages at harvest. Thus all raw preharvest data were age-adjusted within the analysis; adjusted group means averaged 16 ± 4 wk of age. The effect of CR on degree of catch-up growth in LPO was estimated by expressing age-adjusted values as percentage of the average sex-matched NPO-AL value and comparing LPO-CR versus LPO-AL.
Cross-Sectional Measurements: Plasma and Tissue Collection
On day of harvest, fasted animals were anesthetized with isoflurane, as previously described. Venous blood was collected into EDTA- or sodium heparin-containing tubes and then centrifuged, and the collected plasma was stored at −80°C until use (18). After plasma and vital organ collection, aliquots of ABD fat and SC fat were collected, cleaned, snap frozen in liquid nitrogen, and stored at −80°C until use in PCR studies. Separate biopsies of ABD and SC fat were formalin fixed and paraffin embedded for sectioning and histological assessment of adipocyte size.
Cross-Sectional Measurements: Plasma Analyses
As previously reported for AL animals (18), plasma leptin levels were measured in samples anticoagulated with EDTA by RIA using the Multi-Species Leptin RIA kit (MilliporeSigma, Burlington, MA; formerly Linco Research, St. Charles, MO); values are reported as “human equivalents” (HE), as human leptin was used as the standard. The antibody detects pig leptin with 67% efficiency versus human leptin, according to kit instructions. The minimum detection level is 1.0 ng/ml HE. Plasma insulin levels were measured by RIA in heparin-anticoagulated plasma using the Porcine Insulin RIA kit (MilliporeSigma). Minimum detection level is 2 μU/ml. Plasma glucose levels in samples anticoagulated with sodium heparin were measured by the OHSU Clinical Laboratory by autoanalyzer, according to standard procedure. Quantitative insulin-sensitivity check index (QUICKI) values were calculated as 1/[(log fasting plasma insulin) + (log fasting plasma glucose)] (54). Plasma growth hormone, IGF-1, and IGF-binding protein (IGFBP)-3 levels, not previously reported in AL, were measured using ELISA kits in plasma (EDTA) samples, collected under isoflurane anesthesia. Plasma growth hormone was measured using the Pig Growth Hormone ELISA kit (Kamiya Biomedical, Seattle, WA), according to the kit instructions. The minimum detection level is 0.051 ng/ml. Intra-assay coefficient of variation (CV) was 5.0%; interassay CV was 7.8%. Plasma IGF-1 was measured using the IGF-1 ELISA (ALPCO, Salem, NH). Intra-assay CV was 11.1%; interassay CV was 9.5%. The minimum detection level is 0.09 ng/ml. Plasma IGFBP-3 was measured using the porcine-specific IGFBP-3 ELISA kit (MyBioSource, San Diego, CA). Intra-assay CV was 11.9%; interassay CV was 23.3%. The minimum detection level is 0.138 ng/ml.
Total plasma adiponectin was measured by dot-blot assay (no commercial ELISA or RIA is available that can detect porcine adiponectin). The antibody was optimized, first using Western blot on two samples (see Fig. 10C); bands corresponding to the trimer molecular mass (at ~75 kDa) were observed, with no nonspecific bands observed. This corresponds with the higher molecular mass bands observed in other pig adiponectin studies (16). To avoid the need to compare across multiple Western blots, the dot blot was used to analyze all samples on a single membrane. Plasma (2 μl) was spotted onto a nitrocellulose membrane and allowed to dry. Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline-Tween 20 (0.1%) and then incubated for 1 h at room temperature in rabbit anti-porcine adiponectin antibody (XenoDiagnostics, Indianapolis, IN) at 1:500. Membranes were washed three times in Tris-buffered saline-Tween 20, blocked again, and then incubated in horseradish peroxidase-conjugated anti-rabbit antibody (Cell Signaling Technology, Danvers, MA) at 1:1,000 for 1 h at room temperature. Spots were detected using chemiluminescence and film. Scanned images were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD). All samples were analyzed on a single blot and each spot density reported as percentage of the reference group mean. Male NPO-AL was used as the reference group. Values from duplicate assays were averaged.
Fig. 10.
Effect of calorie restriction (CR) on adiponectin mRNA and protein. Levels of adiponectin mRNA in intra-abdominal (ABD; A) and subcutaneous (SC; B) adipose tissue (AT) depots and total plasma adiponectin protein as percent of reference group mean measured in dot blots (D) are shown as means ± SE for juvenile offspring on ad libitum (AL) or CR diets. Western blot used in antibody optimization shows clean bands in 2 distinct porcine plasma samples at ~75 kDa, expected size for adiponectin trimers (C). Normal protein offspring (NPO)-AL: n = 8–9 females, 6–7 males; low protein offspring (LPO)-AL: n = 7 females, 9–10 males; NPO-CR: n = 5 females, 7 males; LPO-CR: n = 3 females, 4 males. *P < 0.05; **P < 0.01 for maternal diet effect in AL only; ***P < 0.001 [includes AL, previously reported (§), and CR in pooled analysis; shaded boxes designate data reported previously (18)]; #P < 0.03 for maternal diet by sex interaction in CR only. Adiponectin mRNA is significantly lower in SC than in ABD-AT (P < 0.001).
Cross-Sectional Measurements: Gene Expression in AT
Primer sequences for adiponectin mRNA, TNF-α mRNA, and 18S rRNA (endogenous control) and other details for quantitative (real-time) RT-PCR procedures were as previously described (18). Reactions using the SYBR Green method were run in triplicate and compared with a standard curve composed of a mix of all samples. There was no difference in mean 18S rRNA threshold cycle value between groups.
Cross-Sectional Measurements: Adipocyte Size
Methods for adipocyte-size measurements are as described in DuPriest et al. (18). In brief, 5-μm sections were stained with hematoxylin and photographed. Images were analyzed for cross-sectional area of adipocytes by an individual blinded to the experimental group. All adipocytes completely contained within a field of view using a ×20 objective lens were included. Four separate ×20 fields per section from two sections per tissue block from two separate tissue blocks per tissue biopsy per animal were measured. The mean adipocyte cross-sectional area per tissue biopsy (ABD and SC) was reported for each animal.
Statistical Methods
Serial measurements.
For body weight and crown-rump body length, values within a given week (birth = day 0) were averaged for each animal and designated as a midweek weight or length. Average weekly values over 5–12 weeks, plotted at midweek, were analyzed by regression analysis for slope differences. LPO-CR females exhibited biphasic length curves (see Fig. 5), and all groups exhibited biphasic body weight curves (slower from 5.5 to 7.5 wk than 7.5 to 12.5 wk); we analyzed slopes separately within each of the two phases. To detect sensitively acute growth rate changes more with CR, we additionally analyzed fractional growth trajectories [see below; PRISM 6 (GraphPad Software, La Jolla, CA) and General Univariate Regression analysis (SPSS v16; IBM, Armonk, NY)]. Weekly fractional body weight and fractional body length growth rates were calculated by dividing the change between two consecutive average weekly values by the mean value across the period. [Because fractional body-size growth rates were calculated as the change across two midweek values (e.g., 5.5–6.5 wk), the fractional rate values are plotted as e.g., week 6.] Finally, to demonstrate visually (but not for analysis) the impact of CR on the trajectory of catch-up growth in LPO, we additionally expressed serial (over 7.5–12.5 wk), prospectively age-matched body-size data for each individual animal as age of average NPO-AL values (see Fig. 6). Separately from serial data, single body-size values, one per animal, were collected just before initiation of each preharvest protocol, thus at staggered ages, spanning 12–22 wk of age. These values were also each expressed as percentage of sex-matched NPO-AL controls, age adjusted, and plotted on the same graph to show the CR effect on the degree of catch-up at an average of 16 ± 4 wk of age. [Note that the 16-wk data in Fig. 6 are age-adjusted, cross-sectional data, thus derived differently than the prospectively age-matched, serial growth data over 4–12 wk in the same animals (see materials and methods)].
Fig. 5.
Absolute growth rates. Absolute body length values (meters) for females (A) and males (B) and absolute body weight values (kilograms) for females (D) and males (E) are shown across 5.5–12.5 wk as means ± SE for offspring on ad libitum (AL) or calorie restriction (CR) postweaning diets. Early and late phases were based on phasic fractional growth patterns (Figs. 3 and 4) and on 2-segment patterns in absolute growth (this figure; see text). Regression lines are shown separately for each phase. Slopes of late-phase regression lines represent absolute growth rates and are reported as means ± SE for length (meters/wk; C) and weight (kilograms/wk; F). Normal protein offspring (NPO)-AL: n = 9 females, 7 males; low protein offspring (LPO)-AL: n = 7 females, 10 males; NPO-CR: n = 5 females, 7 males; LPO-CR: n = 3 females, 4 males. See text for statistical significance for A, B, D, and E. *P < 0.05 (C); **P < 0.02 (C and F); ***P < 0.01 (C and F); ****P < 0.001 (C and F).
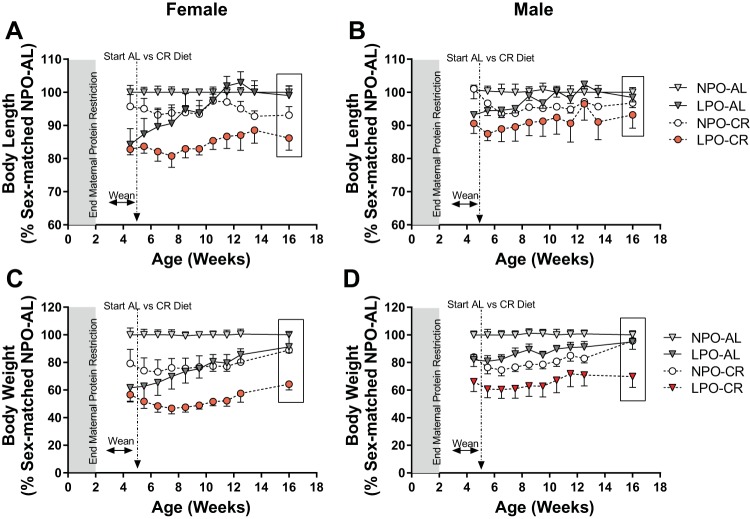
Fig. 6.
Body length and weight growth as percent of sex-matched controls. Body length in females (A) and males (B) and body weight in females (C) and males (D), plotted as percentage of sex-matched normal protein offspring (NPO)-ad libitum (AL), are shown as means ± SE for juvenile offspring on AL or calorie restriction (CR) postweaning diets. NPO-AL: n = 7–9 females, 5–8 males; low protein offspring (LPO)-AL: n = 6–7 females, 6–10 males; NPO-CR: n = 5 females, 7 males; LPO-CR: n = 3–4 females, 4 males. See text for analysis. Shaded regions designate postnatal continuation of maternal protein restriction period.
Cross-sectional measurements and DEXA.
Measurements of plasma adiponectin, growth hormone, IGF-1, and IGFBP-3 are newly reported here in both AL and CR groups. We have previously reported results of growth, body composition, adipocyte size, plasma leptin, and adipose-tissue adiponectin mRNA (18, 19) in LPO and NPO weaned to the AL diet. In the present study, we have combined those previously reported AL data (same animals) and new CR results (new animals) in a single analysis to assess effects of CR on LPO animals and to compare the responses of LPO versus NPO groups with chronic CR. In figures, previously reported results are designated and shown alongside new results under CR conditions. Data were analyzed using maternal diet, postweaning diet, and sex as factors via the Generalized Linear Model/Univariate and Regression programs in SPSS (v. 16.0 for Windows; IBM). For AT-based mRNA studies and adipocyte size, AT depot (ABD vs. SC) was also included as a factor in a four-way ANOVA. All cross-sectional data reported here were collected in a 3-wk-long protocol, applied to matched sets of animals, as described above; age at harvest thus varied. Therefore, each variable was first assessed by linear-regression analysis and adjusted for age dependence when relevant. Raw mRNA values were natural log transformed before analysis (untransformed data reported in text and figures). Data are reported as means ± SE; P < 0.05 was considered significant.
As noted above, previously published data from animals on an AL diet (18, 19) have been included to compare the responses with CR in LPO versus NPO. These data are included with permission from the publisher (Cambridge University Press and the International Society for Developmental Origins of Health and Disease 2012).
RESULTS
Feed Intake and Feed Utilization Efficiency over 5–12 Wk of Age
By experimental design, relative feed intake (gram feed consumed per gram body weight per meal) over weeks 5–12 was reduced in CR offspring in both NPO and LPO (P < 0.001; Fig. 2, A and B); relative feed intakes in NPO-CR and LPO-CR were statistically similar (P = 0.23). Thus the CR diet eliminated the previously reported increase in relative food intake in LPO-AL compared with NPO-AL (19).
CR caused an increase in feed utilization efficiency (gram body weight gain per gram feed consumed) in a sex and maternal diet-dependent manner (Fig. 2, C and D). LPO females, which alone exhibited increased feed utilization efficiency on an AL diet [previously reported in DuPriest et al. (19)], experienced under CR conditions a 3-wk delay before reaching levels seen in LPO-AL (Fig. 2C). In contrast, all other groups responded to CR with an early and sustained increase in their feed utilization efficiency and to comparable degrees above NPO-AL levels (P = 0.002). The significant progressive decline in feed utilization efficiency with increasing age, previously observed in all AL offspring (19), was also apparent in CR offspring (P < 0.001; Fig. 2, C and D).
Effect of Postweaning CR on LPO Growth
Body length and body weight responses to CR are presented as fractional growth rates (Figs. 3 and 4), as absolute growth rates (Fig. 5), and as percentage of sex-matched NPO-AL (Fig. 6). Fractional body weight and fractional linear growth trajectories for all groups exhibited an overall curvilinear fall with age (Fig. 3, A and B, and Fig. 4, A and B) and further delineated a biphasic response following initiation of CR: 1) early phase with nadir and a 1- to 3-wk rebound period (5–8 wk) and 2) a late-phase postrebound period (8–12 wk). Absolute growth trajectories (Fig. 5) were also biphasic in all groups for body weight (with slope shift at 7.5 wk) and for body length in LPO-CR; thus absolute growth was also analyzed separately for early and late phases. The final degree of catch-up growth was assessed using the final preharvest set of body-size measurements and represents a third growth phase spanning 12–22 wk (average 16.4 wk) of age.
Fig. 3.
Effect of calorie restriction (CR) on fractional body length (Lgth) trajectories. Fractional growth in body length is shown from 3 to 12 wk of age in females (A) and males (B). Normal protein offspring (NPO)-ad libitum (AL) trajectory defines the normal curvilinear age-dependent decline, interrupted by the weaning process at 4 wk. Phases designated by boxed areas are based on low protein offspring (LPO) responses to CR: the left box on each graph indicates the transient early-phase response with nadir, followed by a rebound of variable duration; the right box on each graph, from 8 to 12 wk, indicates the post-rebound late phase. Weekly fractional growth rates from late phase (8–12 wk), age adjusted (P < 0.001) and pooled across time, are shown for females (C) and males (D) as means ± SE. NPO-AL: n = 7–9 females, 6–7 males; LPO-AL: n = 6–7 females, 6–10 males; NPO-CR: n = 5 females, 7 males; LPO-CR: n = 3–4 females, 4 males. *P < 0.05 for postweaning diet effect; ***P < 0.001 for maternal diet effect.
Fig. 4.
Effect of calorie restriction (CR) on fractional body weight (Wt) trajectories. Fractional growth in body weight is shown from 3 to 12 wk of age in females (A) and males (B). The normal protein offspring (NPO)-ad libitum (AL) trajectory defines the normal curvilinear age-dependent decline, interrupted by the weaning process at 4 wk. Phases designated by boxed areas are based on low protein offspring (LPO) responses to CR: the left box on each graph indicates the transient early-phase response with nadir, followed by a rebound of variable duration; the right box on each graph, from 8 to 12 wk, indicates the post-rebound late phase. Weekly fractional growth rates from 8 to 12 wk, age adjusted (P < 0.001) and pooled across time, are shown for females (C) and males (D) as means ± SE. NPO-AL: n = 7–9 females, 6–7 males; LPO-AL: n = 6–7 females, 6–10 males; NPO-CR: n = 5 females, 7 males; LPO-CR: n = 3–4 females, 4 males. ***P < 0.001 for maternal diet effect pooled across both sexes. Maternal diet by sex effect, P = 0.014.
Effect of CR on Accelerated Linear Growth in LPO
Early-phase linear growth response to CR.
As previously reported (19), the rate of increase in body length over 5.5–12.5 wk in LPO on an AL diet was higher than NPO-AL in both absolute (centimeters per week; Fig. 5, A–C) and relative terms (Figs. 3, A and B). In the early-phase response to CR, fractional linear growth trajectories in LPO-CR males exhibited nadir and rebound responses that were similar to those of NPO-CR males, rebounding within 1 wk to then parallel the normal (NPO-AL) age-dependent decline (Fig. 3B). In contrast, fractional length growth in LPO-CR females exhibited a premature nadir and a delayed recovery compared with NPO-CR females (Fig. 3A), thus delaying onset of linear growth acceleration for a full 4 wk beyond that seen in LPO-AL females (Fig. 3A and Fig. 6A).
Late-phase linear growth response to CR.
Notably, during the late phase, NPO-CR offspring rebounded to attain absolute (Fig. 5C) and fractional (Fig. 3, C and D) linear growth trajectories similar to NPO-AL. In contrast, over the same period, LPO-CR of both sexes had significantly reduced absolute (Fig. 5C) linear growth rates compared with accelerated LPO-AL levels (P < 0.001), but both absolute and fractional rates remained significantly increased above both sex-matched NPO-CR and NPO-AL levels (P < 0.001; Fig. 3, C and D). Thus in LPO, CR delayed the onset of (in early-phase females), but did not eliminate (in either sex), accelerated fractional linear growth (late phase), whereas still slowing absolute linear growth to near-NPO-AL levels (Fig. 5C).
Effect of CR Diet on Accelerated Body Weight Gain in LPO
Early-phase (4–8 wk) body weight response to CR.
In the early phase, fractional body weight slowing in LPO-CR males compared with NPO-CR males responded similarly to CR in both nadir level and rebound time (Fig. 4B). In contrast, LPO-CR females were more sensitive to initial slowing (Fig. 4A), with fractional body weight growth rate exhibiting a significantly prolonged recovery time (4 wk vs. 2 wk in NPO-CR females).
Late-phase (8–12 wk) body weight response to CR.
Over the late-phase CR period, average fractional body weight growth rate in LPO-CR of both sexes recovered to levels significantly increased over sex-matched NPO-CR (P < 0.001; Fig. 4, C and D); thus the capacity for accelerated fractional body weight growth, reported previously under AL conditions, re-emerged in LPO-CR by 8 wk, despite ongoing CR. Absolute body weight growth rates in late-phase CR were, for both LPO-CR and NPO-CR, lower than the respective AL levels in both sexes (P < 0.001; Fig. 5F). However, also in both sexes, LPO-CR rates were slower than NPO-CR during CR late phase (P < 0.02; Fig. 5F). Thus body weight growth rates in LPO were slowed to a greater degree by sustained CR exposure than rates in NPO.
Effect of CR on Pattern and Degree of Catch-up Growth in LPO
Final body-size indices, collected just before initiation of the preharvest protocol, include one set of values per animal; ages at measurement ranged over 3–5 mo of age (average 16 ± 4 wk). Group-specific regression analyses on age indicated similar slopes (as estimates of rates of growth) over this period among all LPO and NPO groups for length, body weight, and weight-to-length ratio. Because the NPO-CR group was coincidentally smaller in length and weight before initiation of CR, its lower average values at 16 wk do not reflect a CR effect. Thus to estimate the degree of catch-up growth in LPO-CR compared with LPO-AL (Fig. 6), their age-adjusted preharvest values were each referenced to sex-matched average NPO-AL values and plotted as percent of sex-matched control. [As noted, the age-adjusted 16-wk data in Fig. 6 are derived differently from the prospectively age-matched serial growth data collected over 4–12 wk in the same animals. (See materials and methods).]
As previously reported, catch-up in body length was already complete in LPO-AL by 11–12 wk (103 ± 6% of sex-matched NPO-AL; Fig. 6, A and B), with linear growth rates then returning to normal NPO-AL levels at subsequent times (at 99 ± 2% in preharvest set; Fig. 6, A and B) (19). In contrast, LPO-CR body length by 11–12 wk had attained only 87 ± 9% of sex-matched NPO-AL in females and 91 ± 11% in males (P < 0.001 vs. LPO-AL), despite the still-accelerated fractional linear growth rate in LPO-CR, up to 12 wk. Thereafter, as in LPO-AL, there is an apparent cessation of linear growth acceleration in LPO-CR, especially clear in females, with linear growth continuing but returning to rates comparable with sex-matched NPO-AL controls (Fig. 6, A and B). In preharvest data (16 ± 4 wk), body length remained significantly reduced in LPO-CR of both sexes compared with LPO-AL (P = 0.001): in females, 86 ± 7% of the sex-matched NPO-AL average (Fig. 6A) and in males, 93 ± 8% (Fig. 6B). These relative levels were unchanged from those at 11–12 wk, further supporting cessation of growth acceleration after 12 wk of age.
As previously shown, catch-up in body weight (Fig. 4, C and D) progressed more slowly in LPO-AL but closely approached sex-matched NPO-AL levels by 16 wk (19): 91 ± 12% in females; 95 ± 8% in males. During CR, preharvest body weight catch-up was impaired in LPO-CR to an even greater degree than body length, with body weight at 64 ± 8% of sex-matched NPO-AL in females and 70 ± 15% in males (P < 0.001 vs. LPO-AL). The weight-to-length ratio closely followed the body weight pattern, exhibiting similarly impaired catch-up at 16 wk in LPO-CR of both sexes (data not shown).
Growth Hormone–IGF-1 Axis
Plasma growth hormone, adjusted for a significant fall with age (P = 0.002), was significantly reduced in all LPO, independently of postweaning diet and sex (5.79 ± 0.73 ng/ml in LPO, n = 24) compared with all NPO (8.27 ± 0.61 ng/ml in NPO, n = 28; P = 0.01; Fig. 7A). Plasma IGF-1 was not affected by either maternal diet or postweaning diet nor was it related to age. Males had higher plasma IGF-1 (524 ± 27.2 ng/ml, n = 28) than females (318 ± 17.4 ng/ml, n = 24; P < 0.001; Fig. 7B). Age-adjusted (P = 0.008) plasma IGFBP-3 was also unaffected by maternal diet or postweaning diet, although again, males (67.4 ± 3.8 ng/ml; n = 28) had higher plasma IGFBP-3 than females (53.6 ± 4.1 ng/ml, n = 24; P = 0.02; Fig. 7C). The age-adjusted (P = 0.008) IGF-1-to-IGFBP-3 ratio, an index of free IGF-1, revealed a significant maternal diet by sex interaction (P = 0.040): female LPO (5.16 ± 1.05, n = 10) had a lower IGF-1-to-IGFBP-3 ratio than female NPO (7.73 ± 0.85, n = 14; Fig. 7D), whereas male LPO (9.26 ± 0.91, n = 14) were similar to male NPO (7.97 ± 0.82, n = 14; P > 0.05).
Fig. 7.
Effects of maternal diet and postweaning calorie restriction (CR) on growth hormone/insulin-like growth factor (IGF)-1 axis. Plasma levels of growth hormone (age adjusted, P = 0.002; A), IGF-1 (B), and IGF-binding protein (IGFBP)-3 (age adjusted, P = 0.008; C) and ratio of IGF-1 to IGFBP-3 (age adjusted, P = 0.008; D) are shown as means ± SE for juvenile offspring on ad libitum (AL) or CR postweaning diets. Normal protein offspring (NPO)-AL: n = 9 females, 7 males; low protein offspring (LPO)-AL: n = 7 females, 9–10 males; NPO-CR: n = 5 females, 7 males; LPO-CR: n = 3 females, 4 males. *P < 0.05 for sex effect in IGFBP-3 or IGF-1:IGFBP-3 ratio and for maternal diet by postweaning diet interaction in IGF-1:IGFBP-3 ratio; **P < 0.01 for maternal diet effect; ***P < 0.001 for sex effect.
Effect of CR on Body Composition at 6 and 11 Wk of Age
At 6 wk (only 2 wk after weaning and CR initiation) in both NPO and LPO, CR had no significant effect on percent body fat (data not shown).
In females at 11 wk of age, CR caused greater reduction in percent body fat in LPO-CR, both compared with NPO-CR (P < 0.05) and with LPO-AL females (P < 0.02; Fig. 8A). A similar difference was not observed in LPO-CR males. As percent body fat includes lean tissue in its calculation, we also analyzed body fat (grams) normalized to length to avoid confounding by lower lean mass in CR offspring versus NPO. Length-normalized body fat is reduced by maternal diet (P = 0.003) and by postweaning CR (P = 0.001) in an additive manner in both sexes (Fig. 8B). Furthermore, at 11 wk, length-normalized lean mass was reduced in both sexes by postweaning CR to comparable degrees in both NPO and LPO (P < 0.0001; Fig. 8C), thus maintaining during CR the significantly lower lean mass in LPO versus NPO seen in AL [P < 0.01 (19)]. Note, however, that body length at 11 wk is significantly reduced in LPO-CR, whereas body length in NPO-CR is not altered by CR. Thus the referencing of fat and lean mass to length in the shorter LPO-CR underestimates the fat or lean deficits by failing to include the reduction in fat or lean mass due to loss of body length.
Fig. 8.
Effect of calorie restriction (CR) on body composition. Body composition at 11 wk of age: percent body fat (A), fat mass to length (B), lean mass to length (C), fold change in fat mass (grams fat at 11 wk/g fat at 6 wk; D), and fold change in lean mass (E) are shown as means ± SE for offspring on ad libitum (AL) or CR postweaning diets. Normal protein offspring (NPO)-AL: n = 9 females, 6 males; low protein offspring (LPO)-AL: n = 6 (5 for fold increases) females, 8 (7 for fold increases) males; NPO-CR: n = 5 females, 7 males; LPO-CR: n = 4 (3 for fold increases) females, 4 (3 for fold increases) males. *P < 0.02; **P < 0.01; ***P < 0.001. §Significant data previously reported. Shaded regions designate data reported previously (19).
Accrual rates of fat (Fig. 8D) and lean (Fig. 8E) tissue mass were previously reported to be elevated in LPO-AL versus NPO-AL (19). These differences were eliminated by postweaning CR. However, male offspring on a CR diet had greater fold increases in fat mass over 6–11 wk compared with females on a CR diet (P < 0.01; Fig. 8D).
Adipocyte Size
In ABD-AT, adipocyte size was strongly correlated with age (P < 0.001). After age adjusting, ABD-AT adipocyte size was reduced by both postweaning CR (P < 0.017) and by maternal diet (P < 0.017; Fig. 9A); the effect was similar in both sexes, and there were no effects of sex per se. Age was also strongly correlated with adipocyte size in SC-AT (P < 0.006). After age adjustment, SC-AT adipocyte size was higher in females than in males (P < 0.001), but there were no maternal diet or postweaning diet effects (Fig. 9B).
Fig. 9.

Response of adipocyte size to calorie restriction (CR). Age-adjusted adipocyte size in intra-abdominal (ABD) adipose tissue (AT; A) and subcutaneous (SC)-AT (B) is shown as means ± SE for offspring on ad libitum (AL) or CR diets. Normal protein offspring (NPO)-AL: n = 9 (8 for SC-AT) females, 6 (5 for SC-AT) males; low protein offspring (LPO)-AL: n = 6 females, 7 males; NPO-CR: n = 5 females, 7 males; LPO-CR: n = 3 females, 4 males. *P < 0.05 for both maternal diet effect and postweaning diet effect; ***P < 0.001 for sex effect. Age covariate significance was P < 0.001 for ABD-AT and P = 0.006 for SC-AT. §Significant data previously reported (maternal diet effect for ABD-AT; maternal diet by sex interaction for SC-AT). Shaded boxes designate data reported previously (18).
Adipokines
Adiponectin mRNA in ABD- and SC-AT.
We previously reported AT adiponectin mRNA to be reduced in LPO-AL of both sexes and in both AT depots compared with NPO-AL (18). Here, we show that this difference persists unchanged in LPO-CR offspring (Fig. 10, A and B; pooled across depots, maternal diet effect for AL and CR combined: P = 0.001). In ABD-AT, adiponectin mRNA, pooled across postweaning diet and sex, was lower in LPO (1.024 ± 0.092, n = 24) than in NPO (1.897 ± 0.288, n = 28, P < 0.015). Likewise, in SC-AT, adiponectin mRNA was lower in LPO (0.400 ± 0.063, n = 24) than in NPO (0.933 ± 0.179, n = 27). Fat depot and maternal diet-, postweaning diet-, and sex-specific values are shown in Fig. 10, A and B. CR had no effect on AT adiponectin mRNA in any group. Adiponectin mRNA values were higher in the ABD-AT depot (1.452 ± 0.134, n = 50) compared with the SC-AT depot (0.638 ± 0.134, n = 50; P < 0.001). In SC (but not ABD)-AT, males (0.74 ± 0.1, n = 27) had higher adiponectin mRNA than females (0.64 ± 0.1, n = 23; P = 0.04).
TNF-α mRNA in ABD- and SC-AT.
Levels of TNF-α mRNA were assessed as an index of inflammation within AT depots. In ABD-AT, TNF-α mRNA was reduced by a CR diet (0.53 ± 0.15, n = 19) compared with an AL diet (1.72 ± 0.38, n = 32; P = 0.04). The same pattern was observed in the SC depot (CR: 0.20 ± 0.04, n = 19; AL: 1.03 ± 0.26, n = 31; P = 0.01; CR effect for the combined depots: P < 0.001). However, there were no significant differences related to maternal diet or sex. In all groups, TNF-α mRNA was higher in the ABD depot than in the SC depot (P = 0.003).
Plasma adiponectin levels.
In offspring weaned to an AL diet, plasma adiponectin protein (expressed as a percentage of reference group mean) was significantly lower in LPO-AL of both sexes compared with male NPO-AL (LPO-AL: 69.8 ± 4.6%, n = 17; NPO-AL: 88.6 ± 5.3%, n = 16; P = 0.003; Fig. 10C). Postweaning CR did not affect plasma adiponectin in NPO. However, LPO responded differently to CR based on sex (three-way interaction, P = 0.009). Male LPO-CR had higher plasma adiponectin (90.5 ± 11.3, n = 4) than male LPO-AL (75.4 ± 5.4%, n = 10), but female LPO-CR showed low plasma adiponectin (54.6 ± 5.1%, n = 3) compared with female LPO-AL (61.7 ± 7.4).
Plasma leptin levels.
Plasma leptin levels were not significantly affected by maternal diet, postweaning diet, or sex, regardless of whether values were unadjusted or adjusted for body fat (Table 2).
Table 2.
Plasma metabolic parameters
| Maternal diet: | Normal Protein |
Low Protein |
||||||
|---|---|---|---|---|---|---|---|---|
| Postwean diet: | Ad libitum |
Calorie restricted |
Ad libitum |
Calorie restricted |
||||
| Sex (n): | Male (7) | Female (9) | Male (7) | Female (5) | Male (10) | Female (7) | Male (4) | Female (3) |
| Plasma leptin, ng/ml (HE) | 1.50 ± 0.10 | 1.93 ± 0.19 | 1.53 ± 0.17 | 1.41 ± 0.14 | 1.25 ± 0.05 | 1.97 ± 0.32 | 1.95 ± 0.55 | 1.55 ± 0.18 |
| Plasma glucose, mg/dl | 98.0 ± 5.01 | 114.7 ± 11.7 | 83.6 ± 6.8 | 79.4 ± 4.8 | 108.0 ± 5.5* | 84.3 ± 8.1* | 101.3 ± 16.9† | 84.7 ± 12.3 |
| Plasma insulin, μU/ml | 4.39 ± 1.35 | 3.97 ± 0.92 | 4.43 ± 1.13 | 2.30 ± 0.07 | 3.82 ± 0.42 | 4.16 ± 1.13 (6) | 3.31 ± 0.41 | 3.13 ± 0.16 |
| QUICKI index | 0.394 ± 0.014 | 0.391 ± 0.012†‡ | 0.404 ± 0.016§ | 0.443 ± 0.006†‡§ | 0.388 ± 0.009 | 0.398 ± 0.025‡ | 0.402 ± 0.016§ | 0.415 ± 0.013†‡§ |
Values shown as means ± SE. Where n differs from column heading, it is given in parentheses. Leptin RIA used human insulin in standard curve; antibody efficiency may be different for pig leptin. Log fasting plasma insulin/log fasting plasma glucose × 100; higher values reflect higher sensitivity. HE, human equivalent; QUICKI, qualitative insulin-sensitivity check index.
Significant data previously published (18).
P < 0.05, glucose, maternal diet effect in males only; QUICKI, postweaning diet effect in females only.
P < 0.05, QUICKI, sex effect.
P < 0.05, QUICKI, postweaning diet effect.
CR Effects on Glucose Homeostasis
Basal fasting plasma glucose and insulin levels and the QUICKI insulin-sensitivity values are shown in Table 2. In brief, plasma glucose levels were increased in male LPO compared with male NPO; females were unaffected by maternal diet. Postweaning CR tended to reduce plasma glucose, although this was not significant (P = 0.053). Plasma insulin was not affected by maternal diet, postweaning diet, or sex. Insulin sensitivity was increased by postweaning CR (P = 0.02), an effect that was greater in females than in males (sex by postweaning diet interaction, P = 0.058). Overall, females had greater insulin sensitivity than males (P = 0.04).
DISCUSSION
Growth patterns during early life, both prenatal and postnatal, have been shown to reflect developmental exposures and to influence risk of cardiovascular and metabolic disease later in life (12, 13, 22–30, 63). However, the mechanisms involved are not well delineated. In epidemiological studies from the Helsinki cohorts, low birthweight children, who later developed cardiometabolic diseases, exhibited, on average, accelerated growth (22, 28); however, not all cohort subjects fit this pattern, and data were not available to distinguish the degree to which prenatal exposures versus postnatal factors (appetite, food access, diet quality/quantity, stressors) influenced childhood growth rates. Our prior work has shown that perinatal MPR in microswine (0.75 gestation through 2 wk postnatally) causes asymmetric growth restriction (IUGR) and, on an AL postweaning diet, consistently yields accelerated growth over 4–12 wk and an increased rate of fat tissue accrual without excess adiposity (19). The accelerated growth in LPO-AL offspring is associated with a sustained, increased appetite in both males and females and in female LPO-AL only, with elevated feed utilization efficiency (19). LPO-AL also exhibits reduced adiponectin gene expression in both ABD- and SC-AT depots and subtly dysregulated glucose metabolism (18), features suggestive of vulnerability to cardiometabolic disease risk with aging. To address the hypothesis that reduction of accelerated growth would restore metabolic functions, especially adiponectin levels, to normal, we report here responses of LPO to moderate postweaning CR, specifically on growth rates and degree of catch-up growth, on rates of fat and lean tissue accrual and on adiponectin mRNA, each compared with NPO responses to CR and to levels reported previously in LPO-AL (18, 19). Moreover, we newly assess plasma levels of adiponectin protein and growth hormone/IGF-1 axis hormones in LPO under both AL and CR conditions.
Summary of Major Findings
To address our hypothesis, we consider first, whether postweaning CR successfully reduced accelerated growth in LPO and second, whether adiponectin levels and other metabolic outcomes were restored to normal levels. Major findings in the LPO growth response to CR include the following: 1) enhanced susceptibility to sustained slowing of linear growth rate during late-phase CR; 2) increased magnitude of sustained body weight growth slowing during late-phase CR; 3) capacity to resume still-accelerated late-phase fractional linear and body weight growth rates despite ongoing CR after transient initial slowing; 4) impaired length and body weight catch-up in LPO-CR in the final preharvest period, reflecting prolonged early-phase slowing (females), delayed onset of growth acceleration (all LPO-CR), larger growth-rate reductions during late-phase CR, and an apparently finite time point (11–12 wk of age) beyond which linear growth acceleration (but not linear growth per se) ceases in LPO. New findings of reduced growth hormone with normal IGF-1 and IGFBP-3 in all LPO, in the face of accelerated (LPO-AL) or normal (LPO-CR) absolute linear growth rates, suggest the potential for enhanced tissue sensitivity to growth hormone and/or IGF-1. Thus CR successfully reduced absolute but did not completely prevent fractional growth acceleration in LPO. Regarding metabolic outcomes, the low adiponectin gene expression [previously reported in LPO-AL (18)] was not corrected by CR, despite normalization of the excess fat accrual rate and hyperphagia, continued low adiposity, and persistence of small abdominal fat cell size. Adiponectin protein levels were significantly reduced in LPO-AL of both sexes compared with male NPO-AL, concordant with the low mRNA; however, plasma adiponectin during CR remained low only in female LPO-CR.
Enhanced Susceptibility of LPO to Growth Slowing During Postweaning CR
During the early-phase response to CR, LPO-CR females (but not males) exhibited a prolonged post-nadir rebound time in both fractional length and body weight growth rates compared with sex-matched NPO-CR (Figs. 3A vs. Fig. 4A). This delay corresponds to the unexpectedly delayed onset of increased feed utilization efficiency in LPO-CR females (Fig. 2) and may reflect issues of prolonged gut immaturity (14) and/or more severe programming effects stemming from less vigorous competition for suckling/feeding in the growth-restricted females. During the late phase, male and female LPO both exhibited significant and sustained slowing of both fractional (Figs. 3 and 4) and absolute (Figs. 5 and 6) linear and body weight growth rates compared with the accelerated levels in LPO-AL, whereas NPO-CR was minimally affected. Thus all LPO were uniquely susceptible to sustained slowing of both linear growth and body weight gain during late-phase CR.
These exaggerated reductions in both linear and body weight growth rates in LPO during the transient early-phase (LPO-CR females only) and during the sustained late-phase CR are relevant to current understanding of the “thrifty phenotype” concept (41) and to its limitations in real life. Whereas the phenomenon has been viewed as protective [“predictive adaptive” (37)] in the face of nutrient-poor postnatal environments, our findings instead suggest heightened vulnerability of LPO to both linear and body weight growth impairment with a moderate postweaning CR. Our results do not address whether a less restrictive CR or an altered macronutrient profile might better preserve growth.
Is There a Finite Window for Linear Growth Acceleration?
Our results emphasize the detrimental impact on body-size catch-up of even a transient delay in onset of growth acceleration, even if brief. Of particular relevance is the evidence that a limited window of time, ending at ~11–12 wk of age in this model, may exist, not for linear growth per se but specifically for linear growth acceleration, beyond which linear growth continued in LPO at normal NPO-AL rates (compare Fig. 5, A and B, with Fig. 6, A and B). In our prior paper describing LPO on an AL diet (19), we interpreted the cessation of accelerated linear growth as reflecting completion of catch-up to the sex-matched normal level, implying an unidentified mechanism for recognition of “normal.” However, the linear growth trajectory of the LPO-CR group (Fig. 6, A and B) suggests that linear growth acceleration ceases at the same time point in LPO of both AL and CR groups, suggesting instead some limiting factor operating independently of the actual crown-rump length achieved. In contrast to linear growth, body weight trajectories in LPO-AL groups appear to sustain accelerated rates beyond 12 wk, but body weight growth trajectories in LPO-CR (Fig. 6, C and D) preclude firm conclusions. Pubertal changes are well known to inhibit linear growth. We cannot definitively establish age of pubertal onset in our animals; however, testosterone levels and testicular histopathology examined at harvest (E. A. DuPriest and T. K. Morgan, unpublished observations) were similar in LPO and NPO and indicate clear prepubertal status in all males <90 days (<13 wk) of age (testosterone levels <6 ng/ml; no visible sperm or germ-cell activation), making puberty an unlikely reason for the end of the window of linear growth acceleration at 11 wk. Regardless of etiology, a biological mechanism that limits time available specifically for accelerated (but not general) linear growth in LPO has critical implications for understanding the programming process, for understanding catch-up growth, and for strategic interventions that can maximize skeletal growth and avoid obesity.
From a different perspective, sacrifice of growth rate during CR does not necessarily preclude, and may even be an essential component of, other beneficial adaptations in response to CR. Specifically, since catch-up growth is independently linked with chronic disease risk, some degree of stunting may limit other risks, e.g., by reducing metabolic load (via lower body size and calorie intake) to a level more compatible with prenatally limited organ capacity (2), protecting kidney, heart, and pancreas from organ failure. Specifically in the microswine model, in addition to the CR-induced increase in insulin sensitivity shown here, we have found that CR is associated with mitigation of vascular hyperreactivity to norepinephrine in isolated mesenteric small arteries of LPO (3).
Discrete Periods of Growth Acceleration in LPO Can Occur Despite CR-Induced Growth Slowing
Paradoxically, slower growth rates and smaller body size in LPO during CR did not preclude discrete periods of accelerated fractional growth rates despite ongoing CR, albeit at growth rates of lesser magnitude than those of LPO on an AL diet. This was particularly notable with fractional linear growth in LPO-CR (Fig. 3, A and B), where an initial marked growth slowing was followed by rebound to a late-phase linear growth rate, which although less than that of LPO-AL, remained greater than NPO-AL. Likewise, body weight gain in LPO-CR females, although low as an absolute rate (Fig. 5F), rebounded to a lower fractional rate (vs. LPO-AL) but still elevated compared with NPO-AL during late-phase CR (Fig. 4C). These observations underscore the potential for discrete periods of accelerated growth (with potential later-life risks) despite limited postnatal nutrients, even in those programmed individuals who at maturity, remain small and/or stunted. Findings also indicate that, whereas excess calorie intake—in part, a reflection of a nutritionally programmed increase in appetite (51, 66)—modulates the intensity of accelerated growth in LPO, it is not alone sufficient to account fully for the phenomenon.
Paradoxical Growth-Promoting Hormone Levels in LPO on AL and CR Diets
The accelerated growth observed in LPO-AL (19) was expected to reflect increased plasma levels of growth hormone and/or IGF-1, hormones classically responsible for supporting growth of soft and skeletal tissues. Paradoxically, circulating growth hormone levels were significantly decreased in all LPO groups, whereas IGF-1 and IGFBP-3 levels were similar to normal controls. In female LPO, the IGF-1-to-IGFBP-3 ratio was also reduced. This pattern pertained to LPO, regardless of postweaning diet, and suggests that growth hormone and IGF-1 target tissues may be hypersensitive to these hormones. Specifically, growth hormone stimulates IGF-1 transcription in both liver and adipocytes (17, 68); the fact of low growth hormone with normal plasma IGF-1 may indicate hypersensitivity in these key tissues. This concept is supported by normal liver and AT IGF-1 mRNA expression in LPO versus NPO (E. A. DuPriest, unpublished observations); the mechanism by which this occurs is as-yet undetermined. Concomitantly, the normal IGF-1 in the face of accelerated whole body growth may also indicate hypersensitivity of multiple peripheral tissues to IGF-1. Altered components of homeostatic regulatory systems are a common feature of developmental programming, including renin-angiotensin, sympathetic nervous system, and hypothalamic–pituitary–adrenal axis agonists and receptors (64). In the microswine model, we have observed increased mesenteric vascular reactivity to ANG II in both neonatal and juvenile LPO (3). Although other circulating or local factors may contribute to growth acceleration, the possibility that accelerated growth may, in part, reflect hypersensitivity to growth-promoting hormones deserves further exploration.
Adiponectin Responses to CR
Adiponectin is a protective adipokine with effects on multiple target tissues throughout the body. Broadly viewed, its cardiometabolically protective actions across vascular tissue, liver, and other metabolically relevant organs convey antidiabetic, anti-inflammatory, antioxidant, anti-apoptotic, antiatherogenic, antithrombotic, antiproliferative, and vasodilatory impacts via endocrine, autocrine, and paracrine actions (15, 61).
In the adult obesity setting, plasma adiponectin levels are related inversely to body composition measures (1, 21, 31, 36, 45) and adipocyte size (71). At 10 years of age, plasma adiponectin levels were lower in IUGR children than those born appropriate for gestational age (11); they also had higher body mass index and waist circumference. The general implication has been that catch-up growth is accompanied by disproportionate visceral fat deposition (72) even in the absence of increased adiposity (49), thus accounting for adiponectin suppression. However, the degree to which IUGR mediates lowered plasma adiponectin via adiposity-related factors versus other mechanisms has not been rigorously addressed. In LPO in our study, chronic postweaning CR eliminated or minimized multiple factors typically associated with reduced adiponectin gene expression and/or plasma levels as intended, without actually correcting adiponectin expression: postweaning CR failed to restore adiponectin mRNA levels to those matching controls, and plasma adiponectin levels are low in LPO-AL of both sexes and in female LPO-CR. (Low n in male LPO-CR reduces our confidence in a true sex-specific difference.) Specifically, in LPO, postweaning CR reduced growth rates, stunted weight and length catch-up growth, normalized fat-tissue accrual rate, enhanced body fat deficits, reduced adipocyte size in ABD fat, and equalized calorie intake versus NPO-CR. Additionally, indirect support for the absence of visceral adiposity exists in that AT mRNA levels of TNF-α, a proinflammatory marker (56), were normal in LPO-AL, with lower levels during CR. Together, these findings suggest that adiponectin suppression in this developmental programming model is not dependent on the features typically implicated in adult obesity settings. Our observations suggest that suppression of adiponectin gene expression may be programmed perinatally and may precede other manifestations of metabolic dysfunction. Systemic hyperreactivity to norepinephrine (5) and/or ANG II in nutritionally programmed offspring (6, 7, 77) could potentially mediate adiponectin gene suppression, as each has been shown to regulate adiponectin levels (34, 42) and to be altered in maternal nutrient restriction models (74, 76, 77). Alternatively, adiponectin gene expression may be altered more directly by epigenetic or other mechanisms.
The concurrent observations of accelerated growth and reduced adiponectin in LPO may not be merely coincidental. In human infants born small for gestational age, plasma adiponectin levels at birth or in early childhood have been reported to be either increased (10, 38, 52, 55, 59, 62) or decreased (8, 46, 49, 53, 65), with no obvious explanation resolving the differences. However, in children born small for gestational age, plasma adiponectin levels were inversely related to catch-up growth, with the effect persisting into adulthood (10, 47–50, 57, 60), suggesting a possible growth-inhibitory effect of adiponectin. Adiponectin has recently been linked to growth in two additional contexts. First, maternal adiponectin knockout has recently been shown to enhance mouse fetal growth (67). Second, adiponectin released by perivascular AT was shown to bind and sequester heparin-binding EGF, reducing access to EGF receptors on vascular smooth muscle (75). Reduced adiponectin may thus enhance the growth effects of agonists that act via heparin-binding EGF release, including IGF-1 (69), as well as growth and contractile effects of ANG II (70). It is thus of interest to consider whether reduced adiponectin in nutritionally programmed offspring could contribute both to accelerated growth and in parallel, to metabolic dysfunction with aging.
It is noteworthy that adiponectin levels did not correlate with indices of insulin sensitivity (plasma glucose, plasma insulin, QUICKI). It is possible that the effect of low adiponectin is limited in young animals but that aging and/or dietary challenge may subsequently reveal impaired glucose homeostasis. In human studies, offspring of maternal undernutrition do not typically develop excess adiposity or overt glucose intolerance in youth, both instead emerging in adulthood (23, 25, 29, 32). This is consistent with the concept of suppressed adiponectin in healthy LPO juveniles as a predictive risk factor for development of cardiometabolic disease, rather than a consequence of disease.
Strengths and Limitations
The major strengths of our work include the following: 1) the physiological and pathophysiological similarities of porcine and human species in their cardiovascular, metabolic, and nutritional systems; 2) longitudinal design spanning midgestation to 4 mo; and 3) measurement of body fat stores and adipocyte cross-sectional area in both ABD- and SC-AT depots in coordination with adiponectin indices. Limitations include the following: 1) relatively small numbers in CR groups for variables with sex-dependent responses to CR; 2) the inability to determine the degree to which apparent sex effects reflect sex-specific vulnerability to MPR; and 3) the inability to distinguish molecular sizes of circulating adiponectin protein (swine proteins are resistant to enzymatic reagents typically used).
Perspectives and Significance
Accelerated growth following early developmental growth restriction has been thought of as a double-edged sword: on the one hand, it is necessary for overall health during childhood, especially for neurological and immunological development. On the other hand, it has been assumed that the rapid growth in later childhood was causally responsible, even if indirectly, for poor cardiometabolic outcomes later in life. Although our data do not formally exclude that cause-and-effect relationship, they do open the door to the possibility that the correlation between the two phenomena derives instead from a common source. Thus with the development of new therapeutic strategies, it may become possible to encourage childhood growth without also necessarily increasing long-term cardiometabolic risks. What is more clear is that LPO are more vulnerable than controls to the effects of postweaning CR with regards to growth outcomes, and as such, childhood CR is not a useful technique to manage long-term risk at the expense of short-term health in children born small. In addition, the present findings are consistent with the hypothesis that AT adiponectin mRNA levels are, directly or indirectly, programmed perinatally by MPR. The apparent absence of metabolic dysfunction suggests that reduced adiponectin after MPR may represent an early biomarker predictive of future metabolic disease in programmed offspring.
GRANTS
Support for this work was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grants 1P01 HD34430 and R01 HD042570); Medical Research Foundation of Oregon; Eagles Foundation of Spokane, WA; and M. J. Murdock Charitable Trust. Support for E. A. DuPriest was provided by American Heart Association Pacific Mountain Affiliate Predoctoral Fellowship Award no. 0415530Z and by the Oregon Health & Science University Foundation Tartar Trust Fellowship. This material is the result of work supported with resources and the use of facilities at the Veterans Affairs Portland Health Care System in Portland, OR.
DISCLOSURES
The content does not represent the views of the U.S. Department of Veterans Affairs or the U.S. government. No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.A.D., T.K.M., and S.P.B. conceived and designed research; E.A.D., B.L., P.K., K.S., A.B., A.Q., A.C., and S.P.B. performed experiments; E.A.D. and S.P.B. analyzed data; E.A.D., T.K.M., J.Q.P., and S.P.B. interpreted results of experiments; E.A.D. and S.P.B. prepared figures; E.A.D. and S.P.B. drafted manuscript; E.A.D. and S.P.B. edited and revised manuscript; E.A.D., B.L., P.K., K.S., A.B., A.Q., A.C., T.K.M., J.Q.P., and S.P.B. approved final version of manuscript.
REFERENCES
- 1.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257: 79–83, 1999. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 2.Bagby SP. Maternal nutrition, low nephron number, and hypertension in later life: pathways of nutritional programming. J Nutr 137: 1066–1072, 2007. doi: 10.1093/jn/137.4.1066. [DOI] [PubMed] [Google Scholar]
- 3.Bagby SP, Roullet CM, Xue H, Saunders K. Endothelium-independent mesenteric vascular hyperreactivity to pressors in hypertensive prepubertal microswine offspring exposed to maternal protein restriction (Abstract). 3rd International Congress on Developmental Origins of Health & Disease Toronto, ON, Canada, November 16–19, 2005, p. 1107 https://www.nature.com/articles/pr2005811.pdf [Google Scholar]
- 5.Boguszewski MC, Johannsson G, Fortes LC, Sverrisdóttir YB. Low birth size and final height predict high sympathetic nerve activity in adulthood. J Hypertens 22: 1157–1163, 2004. doi: 10.1097/00004872-200406000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Cambonie G, Comte B, Yzydorczyk C, Ntimbane T, Germain N, Lê NL, Pladys P, Gauthier C, Lahaie I, Abran D, Lavoie JC, Nuyt AM. Antenatal antioxidant prevents adult hypertension, vascular dysfunction, and microvascular rarefaction associated with in utero exposure to a low-protein diet. Am J Physiol Regul Integr Comp Physiol 292: R1236–R1245, 2007. doi: 10.1152/ajpregu.00227.2006. [DOI] [PubMed] [Google Scholar]
- 7.Ceravolo GS, Franco MC, Carneiro-Ramos MS, Barreto-Chaves ML, Tostes RC, Nigro D, Fortes ZB, Carvalho MH. Enalapril and losartan restored blood pressure and vascular reactivity in intrauterine undernourished rats. Life Sci 80: 782–787, 2007. doi: 10.1016/j.lfs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Challa AS, Evagelidou EN, Cholevas VI, Kiortsis DN, Giapros VI, Drougia AA, Andronikou SK. Growth factors and adipocytokines in prepubertal children born small for gestational age: relation to insulin resistance. Diabetes Care 32: 714–719, 2009. doi: 10.2337/dc08-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho WK, Suh BK. Catch-up growth and catch-up fat in children born small for gestational age. Korean J Pediatr 59: 1–7, 2016. doi: 10.3345/kjp.2016.59.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cianfarani S, Martinez C, Maiorana A, Scirè G, Spadoni GL, Boemi S. Adiponectin levels are reduced in children born small for gestational age and are inversely related to postnatal catch-up growth. J Clin Endocrinol Metab 89: 1346–1351, 2004. doi: 10.1210/jc.2003-031704. [DOI] [PubMed] [Google Scholar]
- 11.Crume TL, Scherzinger A, Stamm E, McDuffie R, Bischoff KJ, Hamman RF, Dabelea D. The long-term impact of intrauterine growth restriction in a diverse U.S. cohort of children: the EPOCH study. Obesity (Silver Spring) 22: 608–615, 2014. doi: 10.1002/oby.20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curhan GC, Chertow GM, Willett WC, Spiegelman D, Colditz GA, Manson JE, Speizer FE, Stampfer MJ. Birth weight and adult hypertension and obesity in women. Circulation 94: 1310–1315, 1996. doi: 10.1161/01.CIR.94.6.1310. [DOI] [PubMed] [Google Scholar]
- 13.Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation 94: 3246–3250, 1996. doi: 10.1161/01.CIR.94.12.3246. [DOI] [PubMed] [Google Scholar]
- 14.D’Inca R, Gras-Le Guen C, Che L, Sangild PT, Le Huërou-Luron I. Intrauterine growth restriction delays feeding-induced gut adaptation in term newborn pigs. Neonatology 99: 208–216, 2011. doi: 10.1159/000314919. [DOI] [PubMed] [Google Scholar]
- 15.Dadson K, Liu Y, Sweeney G. Adiponectin action: a combination of endocrine and autocrine/paracrine effects. Front Endocrinol (Lausanne) 2: 62, 2011. doi: 10.3389/fendo.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniele A, Cammarata R, Masullo M, Nerone G, Finamore F, D’Andrea M, Pilla F, Oriani G. Analysis of adiponectin gene and comparison of its expression in two different pig breeds. Obesity (Silver Spring) 16: 1869–1874, 2008. doi: 10.1038/oby.2008.275. [DOI] [PubMed] [Google Scholar]
- 17.Doglio A, Dani C, Fredrikson G, Grimaldi P, Ailhaud G. Acute regulation of insulin-like growth factor-I gene expression by growth hormone during adipose cell differentiation. EMBO J 6: 4011–4016, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DuPriest EA, Kupfer P, Lin B, Sekiguchi K, Morgan TK, Saunders KE, Chatkupt TT, Denisenko ON, Purnell JQ, Bagby SP. Altered adipocyte structure and function in nutritionally programmed microswine offspring. J Dev Orig Health Dis 3: 198–209, 2012. doi: 10.1017/S2040174412000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DuPriest EA, Kupfer P, Lin B, Sekiguchi K, Purnell JQ, Saunders KE, Chatkupt TT, Bagby SP. Accelerated growth without prepubertal obesity in nutritionally programmed microswine offspring. J Dev Orig Health Dis 3: 92–102, 2012. doi: 10.1017/S2040174412000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emack J, Kostaki A, Walker CD, Matthews SG. Chronic maternal stress affects growth, behaviour and hypothalamo-pituitary-adrenal function in juvenile offspring. Horm Behav 54: 514–520, 2008. doi: 10.1016/j.yhbeh.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 21.Engeli S, Feldpausch M, Gorzelniak K, Hartwig F, Heintze U, Janke J, Möhlig M, Pfeiffer AF, Luft FC, Sharma AM. Association between adiponectin and mediators of inflammation in obese women. Diabetes 52: 942–947, 2003. doi: 10.2337/diabetes.52.4.942. [DOI] [PubMed] [Google Scholar]
- 22.Eriksson J, Forsén T, Tuomilehto J, Osmond C, Barker D. Fetal and childhood growth and hypertension in adult life. Hypertension 36: 790–794, 2000. doi: 10.1161/01.HYP.36.5.790. [DOI] [PubMed] [Google Scholar]
- 23.Eriksson J, Forsén T, Tuomilehto J, Osmond C, Barker D. Size at birth, childhood growth and obesity in adult life. Int J Obes Relat Metab Disord 25: 735–740, 2001. doi: 10.1038/sj.ijo.0801602. [DOI] [PubMed] [Google Scholar]
- 24.Eriksson J, Forsén T, Tuomilehto J, Osmond C, Barker D. Size at birth, fat-free mass and resting metabolic rate in adult life. Horm Metab Res 34: 72–76, 2002. doi: 10.1055/s-2002-20518. [DOI] [PubMed] [Google Scholar]
- 25.Eriksson JG, Forsén T, Tuomilehto J, Jaddoe VW, Osmond C, Barker DJ. Effects of size at birth and childhood growth on the insulin resistance syndrome in elderly individuals. Diabetologia 45: 342–348, 2002. doi: 10.1007/s00125-001-0757-6. [DOI] [PubMed] [Google Scholar]
- 26.Eriksson JG, Forsén T, Tuomilehto J, Osmond C, Barker DJ. Early growth, adult income, and risk of stroke. Stroke 31: 869–874, 2000. doi: 10.1161/01.STR.31.4.869. [DOI] [PubMed] [Google Scholar]
- 27.Eriksson JG, Forsén T, Tuomilehto J, Osmond C, Barker DJ. Early growth and coronary heart disease in later life: longitudinal study. BMJ 322: 949–953, 2001. doi: 10.1136/bmj.322.7292.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eriksson JG, Forsén T, Tuomilehto J, Winter PD, Osmond C, Barker DJ. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ 318: 427–431, 1999. doi: 10.1136/bmj.318.7181.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eriksson JG, Forsen TJ, Osmond C, Barker DJ. Pathways of infant and childhood growth that lead to type 2 diabetes. Diabetes Care 26: 3006–3010, 2003. doi: 10.2337/diacare.26.11.3006. [DOI] [PubMed] [Google Scholar]
- 30.Fall CH, Osmond C, Barker DJ, Clark PM, Hales CN, Stirling Y, Meade TW. Fetal and infant growth and cardiovascular risk factors in women. BMJ 310: 428–432, 1995. doi: 10.1136/bmj.310.6977.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filippi E, Sentinelli F, Trischitta V, Romeo S, Arca M, Leonetti F, Di Mario U, Baroni MG. Association of the human adiponectin gene and insulin resistance. Eur J Hum Genet 12: 199–205, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Forsén T, Eriksson J, Tuomilehto J, Reunanen A, Osmond C, Barker D. The fetal and childhood growth of persons who develop type 2 diabetes. Ann Intern Med 133: 176–182, 2000. doi: 10.7326/0003-4819-133-3-200008010-00008. [DOI] [PubMed] [Google Scholar]
- 33.Forsén T, Eriksson JG, Tuomilehto J, Osmond C, Barker DJ. Growth in utero and during childhood among women who develop coronary heart disease: longitudinal study. BMJ 319: 1403–1407, 1999. doi: 10.1136/bmj.319.7222.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu L, Isobe K, Zeng Q, Suzukawa K, Takekoshi K, Kawakami Y. beta-Adrenoceptor agonists downregulate adiponectin, but upregulate adiponectin receptor 2 and tumor necrosis factor-alpha expression in adipocytes. Eur J Pharmacol 569: 155–162, 2007. doi: 10.1016/j.ejphar.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Ganu RS, Harris RA, Collins K, Aagaard KM. Maternal diet: a modulator for epigenomic regulation during development in nonhuman primates and humans. Int J Obes Suppl 2, Suppl 2: S14–S18, 2012. doi: 10.1038/ijosup.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gavrila A, Chan JL, Yiannakouris N, Kontogianni M, Miller LC, Orlova C, Mantzoros CS. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab 88: 4823–4831, 2003. doi: 10.1210/jc.2003-030214. [DOI] [PubMed] [Google Scholar]
- 37.Gluckman PD, Hanson MA, Low FM. The role of developmental plasticity and epigenetics in human health. Birth Defects Res C Embryo Today 93: 12–18, 2011. doi: 10.1002/bdrc.20198. [DOI] [PubMed] [Google Scholar]
- 38.Gohlke BC, Bartmann P, Fimmers R, Huber A, Hecher K, Roth CL. Fetal adiponectin and resistin in correlation with birth weight difference in monozygotic twins with discordant growth. Horm Res 69: 37–44, 2008. doi: 10.1159/000111794. [DOI] [PubMed] [Google Scholar]
- 39.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol 13: 330–338, 2014. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull 60: 5–20, 2001. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 42.Hasan AU, Ohmori K, Hashimoto T, Kamitori K, Yamaguchi F, Ishihara Y, Ishihara N, Noma T, Tokuda M, Kohno M. Valsartan ameliorates the constitutive adipokine expression pattern in mature adipocytes: a role for inverse agonism of the angiotensin II type 1 receptor in obesity. Hypertens Res 37: 621–628, 2014. doi: 10.1038/hr.2014.51. [DOI] [PubMed] [Google Scholar]
- 45.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 271: 10697–10703, 1996. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 46.Huang Y, Li Y, Chen Q, Chen H, Ma H, Su Z, Du M. Low serum adiponectin levels are associated with reduced insulin sensitivity and lipid disturbances in short children born small for gestational age. Clin Endocrinol (Oxf) 83: 78–84, 2015. doi: 10.1111/cen.12663. [DOI] [PubMed] [Google Scholar]
- 47.Ibáñez L, Lopez-Bermejo A, Diaz M, Angulo M, Sebastiani G, de Zegher F. High-molecular-weight adiponectin in children born small- or appropriate-for-gestational-age. J Pediatr 155: 740–742, 2009. doi: 10.1016/j.jpeds.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 48.Ibáñez L, Lopez-Bermejo A, Diaz M, de Zegher F. Catch-up growth in girls born small for gestational age precedes childhood progression to high adiposity. Fertil Steril 96: 220–223, 2011. doi: 10.1016/j.fertnstert.2011.03.107. [DOI] [PubMed] [Google Scholar]
- 49.Ibáñez L, López-Bermejo A, Díaz M, Marcos MV, Casano P, de Zegher F. Abdominal fat partitioning and high-molecular-weight adiponectin in short children born small for gestational age. J Clin Endocrinol Metab 94: 1049–1052, 2009. doi: 10.1210/jc.2008-2176. [DOI] [PubMed] [Google Scholar]
- 50.Ibáñez L, Lopez-Bermejo A, Suárez L, Marcos MV, Díaz M, de Zegher F. Visceral adiposity without overweight in children born small for gestational age. J Clin Endocrinol Metab 93: 2079–2083, 2008. doi: 10.1210/jc.2007-2850. [DOI] [PubMed] [Google Scholar]
- 51.Ikenasio-Thorpe BA, Breier BH, Vickers MH, Fraser M. Prenatal influences on susceptibility to diet-induced obesity are mediated by altered neuroendocrine gene expression. J Endocrinol 193: 31–37, 2007. doi: 10.1677/joe.1.07017. [DOI] [PubMed] [Google Scholar]
- 52.Jaquet D, Deghmoun S, Chevenne D, Czernichow P, Lévy-Marchal C. Low serum adiponectin levels in subjects born small for gestational age: impact on insulin sensitivity. Int J Obes 30: 83–87, 2006. doi: 10.1038/sj.ijo.0803106. [DOI] [PubMed] [Google Scholar]
- 53.Kalampokas E, Vrachnis N, Samoli E, Rizos D, Iliodromiti Z, Sifakis S, Kalampokas T, Vitoratos N, Creatsas G, Botsis D. Association of adiponectin and placental growth factor in amniotic fluid with second trimester fetal growth. In Vivo 26: 327–333, 2012. [PubMed] [Google Scholar]
- 54.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85: 2402–2410, 2000. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 55.Laudes M, Oberhauser F, Bilkovski R, Schubert M, Udelhoven M, Wassmer G, Roth B, Krone W. Human fetal adiponectin and retinol-binding protein (RBP)-4 levels in relation to birth weight and maternal obesity. Exp Clin Endocrinol Diabetes 117: 146–149, 2009. doi: 10.1055/s-0028-1100379. [DOI] [PubMed] [Google Scholar]
- 56.Lee H, Lee IS, Choue R. Obesity, inflammation and diet. Pediatr Gastroenterol Hepatol Nutr 16: 143–152, 2013. doi: 10.5223/pghn.2013.16.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.López-Bermejo A, Casano-Sancho P, Fernández-Real JM, Kihara S, Funahashi T, Rodríguez-Hierro F, Ricart W, Ibañez L. Both intrauterine growth restriction and postnatal growth influence childhood serum concentrations of adiponectin. Clin Endocrinol (Oxf) 61: 339–346, 2004. doi: 10.1111/j.1365-2265.2004.02102.x. [DOI] [PubMed] [Google Scholar]
- 59.Martos-Moreno GA, Barrios V, Sáenz de Pipaón M, Pozo J, Dorronsoro I, Martínez-Biarge M, Quero J, Argente J. Influence of prematurity and growth restriction on the adipokine profile, IGF1, and ghrelin levels in cord blood: relationship with glucose metabolism. Eur J Endocrinol 161: 381–389, 2009. doi: 10.1530/EJE-09-0193. [DOI] [PubMed] [Google Scholar]
- 60.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Kuint J, Yinon Y, Schiff E, Sivan E. Cord blood adiponectin and infant growth at one year. J Pediatr Endocrinol Metab 24: 411–418, 2011. doi: 10.1515/jpem.2011.181. [DOI] [PubMed] [Google Scholar]
- 61.Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem 50: 1511–1525, 2004. doi: 10.1373/clinchem.2004.032482. [DOI] [PubMed] [Google Scholar]
- 62.Meral C, Cekmez F, Pirgon O, Asya Tanju I, Metin Ipcioglu O, Karademir F, Gocmen I. The relationship between serum visfatin, adiponectin, and insulin sensitivity markers in neonates after birth. J Matern Fetal Neonatal Med 24: 166–170, 2011. doi: 10.3109/14767058.2010.482604. [DOI] [PubMed] [Google Scholar]
- 63.Mi J, Law C, Zhang KL, Osmond C, Stein C, Barker D. Effects of infant birthweight and maternal body mass index in pregnancy on components of the insulin resistance syndrome in China. Ann Intern Med 132: 253–260, 2000. doi: 10.7326/0003-4819-132-4-200002150-00002. [DOI] [PubMed] [Google Scholar]
- 64.Nuyt AM. Mechanisms underlying developmental programming of elevated blood pressure and vascular dysfunction: evidence from human studies and experimental animal models. Clin Sci (Lond) 114: 1–17, 2008. doi: 10.1042/CS20070113. [DOI] [PubMed] [Google Scholar]
- 65.Palcevska-Kocevska S, Aluloska N, Krstevska M, Shukarova-Angelovska E, Kojik L, Zisovska E, Kocevski D, Kocova M. Correlation of serum adiponectin and leptin concentrations with anthropometric parameters in newborns. Srp Arh Celok Lek 140: 595–599, 2012. doi: 10.2298/SARH1210595P. [DOI] [PubMed] [Google Scholar]
- 66.Plagemann A, Harder T, Melchior K, Rake A, Rohde W, Dörner G. Elevation of hypothalamic neuropeptide Y-neurons in adult offspring of diabetic mother rats. Neuroreport 10: 3211–3216, 1999. doi: 10.1097/00001756-199910190-00016. [DOI] [PubMed] [Google Scholar]
- 67.Qiao L, Wattez JS, Lee S, Guo Z, Schaack J, Hay WW Jr, Zita MM, Parast M, Shao J. Knockout maternal adiponectin increases fetal growth in mice: potential role for trophoblast IGFBP-1. Diabetologia 59: 2417–2425, 2016. doi: 10.1007/s00125-016-4061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roberts CT Jr, Brown AL, Graham DE, Seelig S, Berry S, Gabbay KH, Rechler MM. Growth hormone regulates the abundance of insulin-like growth factor I RNA in adult rat liver. J Biol Chem 261: 10025–10028, 1986. [PubMed] [Google Scholar]
- 69.Roudabush FL, Pierce KL, Maudsley S, Khan KD, Luttrell LM. Transactivation of the EGF receptor mediates IGF-1-stimulated shc phosphorylation and ERK1/2 activation in COS-7 cells. J Biol Chem 275: 22583–22589, 2000. doi: 10.1074/jbc.M002915200. [DOI] [PubMed] [Google Scholar]
- 70.Shah BH, Yesilkaya A, Olivares-Reyes JA, Chen HD, Hunyady L, Catt KJ. Differential pathways of angiotensin II-induced extracellularly regulated kinase 1/2 phosphorylation in specific cell types: role of heparin-binding epidermal growth factor. Mol Endocrinol 18: 2035–2048, 2004. doi: 10.1210/me.2003-0476. [DOI] [PubMed] [Google Scholar]
- 71.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 92: 1023–1033, 2007. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 72.Suzuki M, Shibanuma M, Kimura S. Effect of severe maternal dietary restriction on growth and intra-abdominal adipose tissue weights in offspring rats. J Nutr Sci Vitaminol (Tokyo) 56: 293–298, 2010. doi: 10.3177/jnsv.56.293. [DOI] [PubMed] [Google Scholar]
- 74.Vehaskari VM, Stewart T, Lafont D, Soyez C, Seth D, Manning J. Kidney angiotensin and angiotensin receptor expression in prenatally programmed hypertension. Am J Physiol Renal Physiol 287: F262–F267, 2004. doi: 10.1152/ajprenal.00055.2004. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, Xu A. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem 280: 18341–18347, 2005. doi: 10.1074/jbc.M501149200. [DOI] [PubMed] [Google Scholar]
- 76.Woods LL, Ingelfinger JR, Nyengaard, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res 49: 460–467, 2001. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 77.Yzydorczyk C, Gobeil F Jr, Cambonie G, Lahaie I, Lê NL, Samarani S, Ahmad A, Lavoie JC, Oligny LL, Pladys P, Hardy P, Nuyt AM. Exaggerated vasomotor response to ANG II in rats with fetal programming of hypertension associated with exposure to a low-protein diet during gestation. Am J Physiol Regul Integr Comp Physiol 291: R1060–R1068, 2006. doi: 10.1152/ajpregu.00798.2005. [DOI] [PubMed] [Google Scholar]