Abstract
Juvenile rainbow trout (Oncorhynchus mykiss) confined in pairs form social hierarchies in which socially subordinate fish display characteristic traits, including reduced growth rates and altered glucose metabolism. These effects are, in part, mediated by chronically elevated cortisol levels and/or reduced feeding. To determine the effects of social status on lipid metabolism, trout were held in pairs for 4 days, following which organismal and liver-specific indexes of lipid metabolism were measured. At the organismal level, circulating triglycerides were elevated in dominant trout, whereas subordinate trout exhibited elevated concentrations of circulating free fatty acids (FFAs) and lowered plasma total cholesterol levels. At the molecular level, increased expression of lipogenic genes in dominant trout and cpt1a in subordinate trout was identified, suggesting a contribution of increased de novo lipogenesis to circulating triglycerides in dominant trout and reliance on circulating FFAs for β-oxidation in the liver of subordinates. Given the emerging importance of microRNAs (miRNA) in the regulation of hepatic lipid metabolism, candidate miRNAs were profiled, revealing increased expression of the lipogenic miRNA-33 in dominant fish. Because the Akt-TOR-S6-signaling pathway is an important upstream regulator of hepatic lipid metabolism, its signaling activity was quantified. However, the only difference detected among groups was a strong increase in S6 phosphorylation in subordinate trout. In general, the changes observed in lipid metabolism of subordinates were not mimicked by either cortisol treatment or fasting alone, indicating the existence of specific, emergent effects of subordinate social status itself on this fuel.
Keywords: behavior; cell signaling; energy metabolism; gene expression; hierarchy; liver, microRNA; salmonid
INTRODUCTION
Among juvenile salmonid fish, linear dominance hierarchies form as a result of competition for feeding territories (1, 23). The dominant fish within these hierarchies monopolize resources, such as food or shelter, displaying high levels of aggression toward their subordinate counterparts (1, 60). These differences in behavior are accompanied by a range of physiological effects, including changes in energy metabolism (21, 33, 35). Previous studies largely have focused on changes in carbohydrate metabolism, revealing an increased potential for hepatic glucose liberation in subordinates. For example, subordinate trout display increased mobilization of stored glycogen compared with dominant trout, as evidenced by lower hepatic glycogen concentrations and higher glycogen phosphorylase activity (35). As well, subordinate trout display enhanced gluconeogenic potential (35), which is further supported by increased hepatic phosphoenol pyruvate carboxykinase (PEPCK) activity and decreased pyruvate kinase activity (21). These changes are largely dependent on cortisol (21, 62, 83), with chronically elevated cortisol levels in subordinate trout leading to increased circulating glucose concentrations (14). However, while circulating glucose represents an important fuel source for specific rainbow trout tissues, such as the brain, gill, and erythrocytes (70), glucose utilization for global energy metabolism is limited in rainbow trout (63).
In contrast, it is well established that lipid metabolism plays a key role in energy homeostasis in rainbow trout. Indeed, basal lipolytic rates in this species are so high that only 13% of liberated free fatty acids (FFAs) are sufficient to support total energy expenditure with 87% being reesterified (52). Another index supporting the important contribution of lipid metabolism to total energy expenditure in rainbow trout is the resting rate of triglyceride (TG) turnover, which is sufficiently high to fuel red muscle during endurance exercise (53). Rainbow trout store excess energy in the form of TGs, which, once synthesized in the liver, are stored in white adipose tissue (WAT) (2, 81). Conversely, in times of energy demand, FFAs are mobilized from WAT and are shuttled by lipoproteins to fuel metabolically active target tissues (2, 81). In line with their high reported lipid turnover rates, high basal and exercise-induced lipoprotein lipase activity in rainbow trout allows lipids to be liberated from shuttling proteins for use in muscle (52, 53). As important contributors to overall energy expenditure at rest, as well as during locomotion, it is not surprising that lipids play a key role in energetically demanding life-history events, such as migration and sexual maturation (10, 11, 31).
Given the importance of lipids in rainbow trout energy metabolism, there is a need to investigate the consequences of social interactions on lipid metabolism. To determine these effects, size-matched juvenile rainbow trout were confined in pairs for 4 days, after which organismal and liver-specific indexes of lipid metabolism of dominant and subordinate fish were compared with those of sham-treated fish that were handled in the same fashion as paired fish but housed individually. Both chronic elevation of cortisol and reduced feeding are key traits of subordinate status in rainbow trout that could mediate changes in lipid metabolism. Therefore, the contribution of each factor individually was examined in a second experiment, where fish were either cortisol-treated or fasted for 4 days.
We tested the hypothesis that social status affects organismal and hepatic lipid metabolism, with the specific prediction that dominant trout would upregulate anabolic pathways, while subordinate trout would upregulate catabolic pathways. A secondary hypothesis was that changes in lipid metabolism were mediated by endocrine and physiological correlates of social status, specifically, cortisol elevation and fasting. While most previous studies have focused on consequences of long-term fasting on lipid metabolism parameters in rainbow trout (71), previous studies revealing a decrease in serum TG after a 3-day fast (44), a decrease in total liver lipid content (26) after a 2-day fast, and decreases in hepatic mRNA abundance of lipogenic genes and increases in hepatic mRNA abundance of genes involved in fatty acid β-oxidation (57) after a 2-day fast led us to formulate the prediction that fasting would significantly inhibit lipid anabolism and stimulate lipid catabolism in our experimental time period.
Both hypotheses and their specific predictions were addressed at the organismal level and, given its importance in regulating organismal lipid metabolism, also at the level of the liver. Specifically, to address effects of social status and associated endocrine and physiological factors on organismal lipid metabolism, plasma concentrations of three classes of lipids, TG, FFAs, and total cholesterol (TC), were determined. To gain insight into hepatic metabolic pathways, which are key mediators in organismal lipid homeostasis, transcript abundance of components involved in lipogenesis [sterol regulatory element-binding protein 1c (srebp1c), fatty acid synthase (fasn), and ATP citrate lyase (acly)], β-oxidation [(carnitine palmitoyltransferase 1A (cpt1a)], and cholesterol biosynthesis and degradation pathways [(hydroxymethylglutaryl-CoA synthase (hmgcs), liver X receptor (lxr), UDP glucuronosyltransferase family 1 member A3 (ugt1a3), and sterol regulatory element-binding protein 2 (srebp2)] were measured. Given the emerging importance of microRNAs (miRNA) in the regulation of hepatic and organismal lipid metabolism in rainbow trout (55–59), the potential involvement of miRNAs was first investigated by measuring hepatic mRNA abundance of drosha. Drosha is a necessary factor in the miRNA biogenesis pathway (48) that exists as a single paralogue in rainbow trout (9). We also measured the hepatic abundance of two specific miRNAs, miRNA-122 and miRNA-33, owing to their roles in teleost lipid metabolism (55–58, 85). Liver-specific miRNA-122 has been shown to stimulate serum triglyceride and cholesterol concentrations in rainbow trout (56), in line with previously reported roles in mammals (24, 25). While miRNA-122 remains the only miRNA to have been studied functionally in rainbow trout (56), we also profiled the hepatic abundance of miRNA-33, first because of its evolutionarily conserved genomic location within the introns of srebp2 suggesting a supporting function in its host gene’s role as a transcription factor mediating cholesterol biosynthesis (65, 72), and second, because a functional role for miRNA-33 in fatty acid biosynthesis was suggested recently in a marine fish species, Siganus canaliculatus (85).
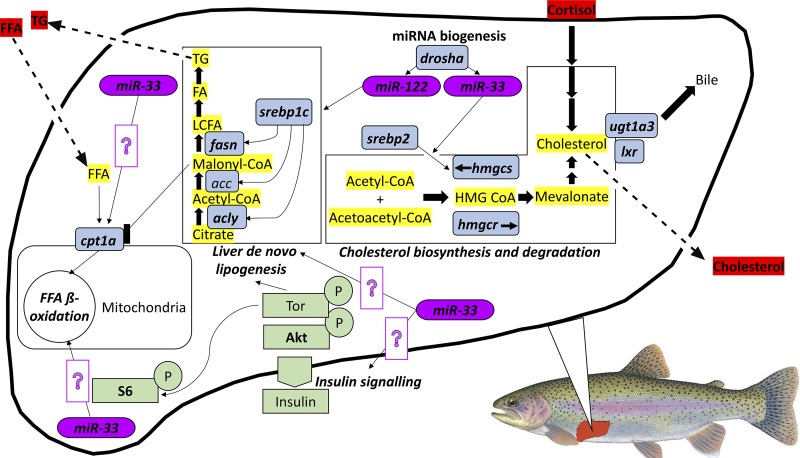
Although the phospholipase C/protein kinase C (PLC/PKC), mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK/ERK) and the Janus tyrosine kinase/signal transducer and activator of transcription (JAK-STAT) pathways recently have been shown to correlate with (6), and indeed functionally regulate lipolytic pathways in the liver (7, 8), the protein kinase B/target of rapamycin/ribsosomal protein S6 (Akt/Tor/S6) pathway remains the only hepatic cell pathway in rainbow trout that has been directly linked to the acute regulation of mRNA abundance and enzyme activity of hepatic fasn/Fasn and cpt1a/Cpt1 in studies employing rapamycin, a Tor inhibitor (15–17, 50). In light of the demonstrated functional importance of hepatic Akt-Tor-S6 cell signaling at the transcriptional level of lipogenic (srebp1c, fasn) and β-oxidation (cpt1a) pathways, also probed in this study, we quantified Akt/Tor/S6 activity to determine its possible contribution to social status-dependent differences in lipid metabolism. An integrative overview of the relevant indexes of hepatic and organismal lipid metabolism profiled in this study is presented in Fig. 1.
Fig. 1.
Schematic of hepatic lipid metabolic pathways investigated in this study. Protein kinase B/target of rapamycin/ribsosomal protein S6 (Akt/Tor/S6) pathway components are highlighted in green, key metabolic enzymes in blue and microRNAs are in purple. Circulating metabolites are highlighted in red, while metabolites present within the liver are highlighted in yellow. In all cases, bold letters indicate variables measured in this study. Established functional relationships between measured parameters are based on previous literature (15, 17, 57), and question mark symbols indicate predicted interactions. FFA, free fatty acid; srebp1c, sterol regulatory element-binding protein 1c; fasn, fatty acid synthase; acly, ATP citrate lyase; cpt1a, carnitine palmitoyltransferase 1A; hmgcs, hydroxymethylglutaryl-CoA synthase; lxr, liver X receptor; ugt1a3, UDP glucuronosyltransferase family 1 member A3; srebp2, sterol regulatory element-binding protein 2.
MATERIALS AND METHODS
Experimental Animals
Female rainbow trout, Oncorhynchus mykiss, purchased from Linwood Acres Trout Farm (Campbellcroft, ON, Canada) were held at the University of Ottawa in 1,275-liter fiberglass tanks. Tanks were supplied with flowing, aerated, dechloraminated city of Ottawa tap water at a temperature of 13°C. Fish were fed a ration of 0.5% body mass daily by scattering commercial trout pellets on the water’s surface. A 12:12-h light-dark photoperiod was maintained. Trout were acclimated to these holding conditions, which acted to minimize hierarchy formation (e.g., use of scatter feeding, homogenous tanks with a mild current), for at least 2 wk before experimentation. All experimental protocols complied with the guidelines of the Canadian Council on Animal Care for the use of animals in research and teaching and were approved by the University of Ottawa’s Animal Care Committee (Protocol No. BL-2118).
Experimental Protocols
Two experimental series were conducted. The first experiment used juvenile trout (mass = 97.2 ± 2.4 g; fork length = 20.3 ± 0.2 cm; means ± SE; n = 37) that had been confined in pairs or handled identically but held individually (sham-treated), for 4 days (n = 12 pairs, 13 shams). The second experiment used groups of 10–12 trout (mass = 187.0 ± 16.4 g; fork length = 25.8 ± 0.6 cm; means ± SE; n = 17). These trout were cortisol treated, fasted, or controls and were held under these conditions for 4 days. These treatments were included to assess the effects of subordinate-associated traits, specifically, elevated cortisol and reduced feeding.
Experiment 1: social interactions and behavioral phenotyping.
Fish were lightly anesthetized to the point of losing equilibrium in a solution of benzocaine (0.05 g/l ethyl-p-aminobenzoate; Sigma-Aldrich, Oakville, ON, Canada), initial mass and fork length were measured, and fin damage was scored. When existing physical differences did not allow for identification of individual fish, a pectoral fin clip was used for identification. Fish were paired based on fork length, with differences not exceeding 5% (fork length difference averaged 33 ± 9 mm or 1.6% of fork length; n = 12 pairs). After initial assessment, members of a pair were placed into a 40-liter flow-through Plexiglas observation tank, separated from one another by an opaque, perforated divider. Tanks were supplied with flowing, aerated 13°C water.
The following morning, the divider was removed and fish were allowed to interact for 4 days. A shelter (t-shaped PVC tube, 11 × 13 cm long, 6-cm diameter) was added at the end of the first day of interaction. Behavioral observations were carried out twice per day, at 900–1100 and 1500–1700, for 5 min per observation period. Sham-treated trout underwent the same handling and treatment as paired fish but were held individually. Fish were offered 0.5% fish mass per tank daily following the final observation period (except on the initial day of interaction), and the mass of food consumed as well as the fish that consumed the food was noted.
Social status was assessed by assigning points to each fish for position within the tank, food acquisition, aggressive acts, and fin damage acquired during the interaction period (as previously described by Refs. 20, 21, 78). This scoring system awards more points for more dominant behaviors; specifically, patrolling the water column in the tank, aggressive behavior, first to feed, and absence of fin damage. A principle components analysis (SigmaPlot v13.0; Systat software, San Jose, CA) was used to calculate behavior scores for each fish based on the mean scores across observation periods of each parameter. Within a pair, the fish with the higher score was assigned dominant status, while the fish with the lower score was subordinate. Behavior scores as well as parameters associated with social status (plasma cortisol levels, specific growth rate, and food intake) are listed in Table 1.
Table 1.
Characterization of dominant, subordinate, and sham-treated rainbow trout (Oncorhynchus mykiss)
| Dominant (n = 12) | Subordinate (n = 12) | Sham (n = 13) | P Value | |
|---|---|---|---|---|
| Behavior score | 1.6 ± 0.1 | −1.6 ± 0.2 | — | — |
| Plasma cortisol concentration, ng/ml | 4.0 ± 0.9A | 111.4 ± 25.2B | 2.5 ± 0.5A | <0.001 |
| Food intake, g/day | 1.12 ± 0.13A | 0.04 ± 0.04B | 0.62 ± 0.15A | <0.001 |
| SGR, %/day | 0.48 ± 0.14A | −0.50 ± 0.24B | −0.15 ± 0.16A,B | 0.003 |
Values are means ± SE. Data were analyzed by ANOVA and ANOVA on ranks for food intake, and treatment groups that share a letter are not significantly different from one another. Specific growth rate (SGR) was calculated as (ln final mass – ln initial mass)/interaction period (day) × 100%.
After the interaction period, both fish in a pair were rapidly euthanized via terminal anesthesia (0.5 g/l ethyl-p-aminobenzoate). Mass and fork length were measured, fin damage was scored, and blood samples were collected via caudal venipuncture into heparin-coated syringes (2,500 IU/ml heparin sodium salt; Sigma-Aldrich). Blood samples were centrifuged (10,000 g for 2 min), and plasma was extracted. Plasma samples were flash frozen in liquid nitrogen before being stored at −80°C. Liver tissue was collected, freeze clamped, and stored at −80°C for later analysis.
Experiment 2: control, cortisol-treated, and fasted groups.
Trout were randomly allocated into one of three 115-liter holding tanks in groups of 10–12 fish per tank. This group size (in combination with the use of scatter-feeding, where appropriate, and homogenous tanks with a mild current) acted to minimize unwanted hierarchy formation. One set of fish was not fed for 4 days (fasted treatment group). Fish in a second group (cortisol-treated) were lightly anesthetized and given an intraperitoneal implant of cocoa butter (5 ml/kg body mass) containing hydrocortisone 21-hemisuccinate (22 mg/ml; Sigma-Aldrich). In previous studies, this dosage (110 mg/kg body mass) raised circulating cortisol levels to those of subordinate fish (33, 45). Lastly, an untreated group of fish was used to control for effects of handling (control treatment group). Sham-implanted fish (fish treated only with cocoa butter) were not used because the injection itself has unpredictable effects on cortisol levels (20). A random subset of five to six fish from each set was sampled as described above (see Experiment 1: social interactions and behavioral phenotyping).
Plasma Cortisol and Metabolite Concentrations
Plasma cortisol levels were measured using a commercially available radioimmunoassay (MP Biomedicals, Santa Ana, CA) that had previously been validated for trout plasma (30). The kit has a detection limit of 0.17 μg/dl. Intra-assay variation was 9.6% and interassay variation was 11.3% (% coefficient variation). All lipid metabolites were assayed in duplicate using commercially available kits according to the manufacturer’s protocols (Cayman Chemical, Ann Arbor, MI). Plasma TG levels were measured using a colorimetric assay with absorbance measurements at 538 nm. The detection limit of this assay was 3.125 mg/dl. Plasma FFA levels were measured by means of a fluorometric assay using an excitation wavelength of 535 nm and an emission wavelength of 590 nm. The detection limit of this assay was 7.04 mg/dl. Plasma TC levels were measured using a fluorometric assay with an excitation wavelength of 535 nm and an emission wavelength of 590 nm. The detection limit for this assay was 77.34 μg/dl. Colorimetric and fluorometric assays were carried out using SpectraMax Plus 384 or SpectraMAX GeminiXS plate readers, respectively (Molecular Devices, Sunnyvale, CA).
Analysis of mRNA Transcript Abundance
Total RNA was extracted from 20 to 100 mg of liver using TRIzol reagent (Invitrogen, Burlington, ON, Canada) following the manufacturer’s protocol. Tissues were homogenized by forcing the solution of TRIzol and tissue through 18- and 23-G needles using a syringe until the solution passed easily through the needle. Extracted RNA was quantified using a NanoDrop 2000c UV-Vis Spectrophotometer (Thermo-Fisher Scientific, Ottawa, ON, Canada). Next, cDNA was generated using a QuantiTech Reverse Transcription Kit (Qiagen, Toronto, ON, Canada) following the manufacturer’s protocol.
Two-step semiquantitative real-time RT-PCR assays were performed on a BioRad CFX96 instrument (Bio-Rad, Mississauga, ON, Canada) to quantify fold changes in relative hepatic mRNA abundances of key transcripts involved in lipogenesis (srebp1, fasn, and acly), fatty acid β-oxidation (cpt1a), TC biosynthesis (srebp2 and hmgcs), TC degradation (lxr, ugt1a3), and miRNA biogenesis (drosha), as well as two reference genes (ef1a and 18s). Briefly, a standard curve consisting of serial dilutions of pooled cDNA, a negative no-RT control consisting of cDNA generated in a reaction that did not include reverse transcriptase, and individual samples were run in duplicate for each experiment. For each individual reaction, the total volume was 20 µl, which consisted of 4 µl of diluted cDNA template, 0.5 µl of 10 nM specific forward and 0.5 µl of 10 nM specific reverse primer (Table 2), 10 µl of SsoAdvanced Universal Inhibitor-Tolerant SYBR Green Supermix (Bio-Rad), and 5 µl of H2O. For each assay, cycling parameters were a 5-min activation step at 95°C, followed by 40 cycles consisting of a 20-s denaturation step at 95°C and a combined 30-s annealing and extension step at primer specific temperatures (Table 2). After each run, melting curves were produced by gradually increasing temperature and the final curves were monitored for single peaks to confirm the specificity of the reaction and the absence of primer dimers. In cases where primers were newly designed (drosha), pooled samples were sent for sequencing (Ottawa Hospital Research Institute, Ottawa, ON, Canada), followed by BLAST search (National Center for Biotechnology Information), to confirm amplicon specificity. The acceptable range for amplification efficiency calculated from serially diluted standard curves was 90–110%, with R2 values >0.95. Assays were subsequently normalized using the NORMA-Gene approach as described by Heckman et al. (39). Finally, mRNA fold changes were calculated relative to the sham group for the social hierarchy experiment (experiment 1), and relative to the control group for the experiment investigating fasting and cortisol treatment (experiment 2).
Table 2.
Primer sequences and annealing temperatures used for mRNA and miRNA quantification by real-time RT-PCR
| Target | Forward Primer Sequence (5′–3′) | Reverse Primer Sequences (3′–5′) | Annealing Temperature, °C | Reference No. |
|---|---|---|---|---|
| srebp1c | GACAAGGTGGTCCCAGTTGCT | CACACGTTAGTCCGCATCAC | 58 | (68a) |
| fasn | TGATCTGAAGGCCCGTGTCA | GGGTGACGTTGCGTGGTAT | 58 | (58) |
| acly | GCTTTTGCCACGGTGGTCTC | GCTTCCGCTACGCCAATGTC | 60 | (76a) |
| cpt1a | TCGATTTTCAAGGGTCTTCG | CACAACGATCAGCAAAGTGG | 57 | (76a) |
| srebp2 | TAGGCCCCAAAGGGATAAG | TCAGACACGACGAGCACAA | 58 | (58) |
| hmgcs | AGTGGCAAAGAGAGGGTGTG | TTCTGGTTGGAGACGAGGAG | 60 | (58) |
| lxr | TGCAGCAGCCGTATGTGGA | GCGGCGGGAGCTTCTTGTC | 60 | (14a) |
| ugt1a3 | CCACCAGCAAGACAGTCTCA | CAACAGCACAGTGGCTGACT | 61 | (58) |
| ef1a | CATTGACAAGAGAACCATTGA | CCTTCAGCTTGTCCAGCAC | 56 | (1a) |
| 18S | GGCGGCGTTATTCCCATGA | TGCCCTTCCGTCAATTCCTTTA | 60 | (45) |
| drosha | GAGGAGTCGGTGAAGGAATG | CATGTGGGAGAAGAGGGAGA | 60 | Newly designed |
| miR-122 | TGGAGTGTGACAATGGTGTTTG | Universal reverse primer | 60 | (58) |
| miR-33 | GTGCATTGTAGTTGCATTGCAT | Universal reverse primer | 58 | (58) |
| Sno-U23 | GCCCATGTCTGCTGTGAAACAAT | Universal reverse primer | 60 | (76) |
srebp1c, Sterol regulatory element-binding protein 1c; fasn, fatty acid synthase; acly, ATP citrate lyase; cpt1a, carnitine palmitoyltransferase 1A; hmgcs, hydroxymethylglutaryl-CoA synthase; lxr, liver X receptor; ugt1a3, UDP glucuronosyltransferase family 1 member A3; srebp2, sterol regulatory element-binding protein 2.
Analysis of Mature miRNA Abundance
With the use of the extracted total RNA, small RNAs <17 nucleotides in length were removed using a miRNeasy kit (Qiagen) according to the manufacturer’s instructions. After quantification by NanoDrop (Thermo-Fisher Scientific), 144 ng of the purified RNAs were used to synthesize cDNA with HiFlex buffer using the miScript II RT kit (Qiagen) according to the manufacturer’s instructions. Specific miRNAs were then quantified using the miRScript SYBR Green PCR kit (Qiagen) with miRNA-specific forward primers and a universal reverse primer (Table 2). Reactions were run in duplicate on a CFX96 instrument (Bio-Rad) with a total volume of 25 µl containing 2.5 µl cDNA, 2.5 µl of 10 nM miRNA specific primer, 2.5 µl miScript Universal Primer, 12.5 µl 2× QuantiTect SYBR Green PCR Master Mix (Qiagen), and 5 µl H2O, according to the manufacturer’s instructions. For each assay, cycling parameters were an initial 15 min 95°C activation step, followed by 40 cycles of 15-s incubation at 94°C, 30 s at 60°C, and 30 s at 70°C. After each run, melting curves were produced by a gradual increase in temperature and the final curves were monitored for single peaks to confirm the specificity of the reaction and the absence of primer dimers. Standard curves and no-RT controls were used to assess efficiency and specificity of amplifications as previously described. Because the abundance of fewer than five miRNAs was analyzed, the NORMA-Gene method was not used. Rather, the ∆∆CT (67) method for normalization was adopted using SnoU23 as a reference gene as previously described for rainbow trout (76). The miRNA fold changes were then calculated relative to sham and control groups, for experiments 1 and 2, respectively.
Analysis of Cell Signaling Pathways
Frozen liver samples (~100 mg) were homogenized on ice with a sonicator model 100 (Thermo-Fisher Scientific). Tissues were homogenized in a buffer (pH 7.4) containing 150 mmol/l NaCl, 10 mmol/l Tris, 1 mmol/l EGTA, 1 mmol/l EDTA, 100 mmol/l sodium fluoride, 4 mmol/l sodium pyrophosphate, 2 mmol/l sodium orthovanadate, 1% (vol/vol) Triton X-100, 0.5% (vol/vol) NP40-IGEPAL (all Sigma-Aldrich), and Pierce protease inhibitor (Thermo-Fisher Scientific). Homogenates were centrifuged at 15,000 g for 30 min at 4°C, and supernatants were stored at −80°C. Protein concentrations were quantified using a BCA (Sigma-Aldrich) assay with BSA standards and a SpectraMax Plus 384 plate reader (Molecular Devices) at a wavelength of 562 nm. Aliquots of 50 μg protein per sample were subjected to SDS-PAGE and Western blotting, using the appropriate primary antibodies (Table 3). All primary antibodies used for analysis of the insulin signaling pathway were obtained from Cell Technologies (Ozyme, Saint Quentin Yvelines, France) and previously have been shown to cross react successfully with rainbow trout proteins of interest and to be regulated in physiological responses to nutritional and endocrine factors (56, 57, 68, 77). Also, activation of downstream components of this pathway has been shown to be inhibited with concurrent utilization of the Tor-specific inhibitor rapamycin (15–17, 50). All primary antibodies used were raised in rabbit, and after final washing, membranes were incubated with an IRDye infrared secondary anti-rabbit antibody raised in goat (LI-COR Biotechnology, Lincoln, NE). Bands were visualized and quantified by infrared fluorescence using the Odyssey Imaging System (LI-COR Biotechnology). Active phosphorylated protein levels were expressed relative to total protein level.
Table 3.
Primary antibody sources and dilutions used for cell signaling quantification by Western blot analysis
| Target | Reference ID No. | Supplier | Dilution Factor | Band Size, kDa |
|---|---|---|---|---|
| Akt-P | 9271 | Cell Signaling/New England Biolabs | 1:10,000 | 60 |
| Akt | 9272 | Cell Signaling/New England Biolabs | 1:10,000 | 60 |
| S6-P | 2211 | Cell Signaling/New England Biolabs | 1:10,000 | 32 |
| S6 | 2217 | Cell Signaling/New England Biolabs | 1:10,000 | 32 |
Statistical Analysis
All data were tested for normality and homoscedasticity using the Shapiro-Wilk test and Bartlett’s test, respectively. If these assumptions were met, data were tested for single outliers using Grubb’s test and subsequently analyzed using a one-way ANOVA, followed by Tukey’s post hoc test for multiple comparisons. When data did not meet these assumptions, data were either transformed to meet the assumptions or were analyzed using the nonparametric Kruskal-Wallis test followed by Bonferroni-adjusted Mann-Whitney U-tests. To facilitate statistical analyses that included paired and sham fish, the members of a pair were considered to be independent of one another. With the use of this conservative approach, it may be more difficult to reject the null hypothesis of no difference stemming from social status than when the members of a pair are not considered to be independent of one another (13). All statistical analysis and graphing were carried out using Prism, Version 7 (Graphpad software, La Jolla, CA).
RESULTS
Behavioral and Endocrine Correlates of Social Status
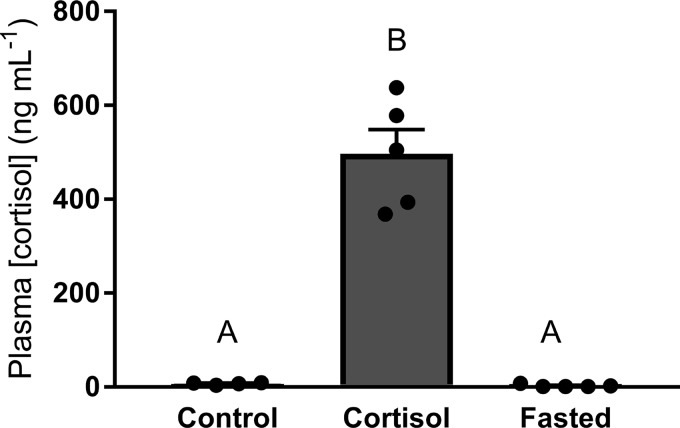
The social pairing experiment generated dominant and subordinate phenotypes (Table 1). Plasma cortisol concentration (Table 1, df = 2; F = 75.90, P < 0.01) was significantly higher in subordinate trout compared with both dominant and sham-treated trout (P < 0.01). The elevation of circulating cortisol levels in subordinate trout was accompanied by significantly lower food intake (Table 1, df = 2; F = 7.02, P < 0.01) compared with dominant fish (P < 0.01). Food intake was marginally elevated in dominant fish compared with sham fish (P = 0.052). Specific growth rates (Table 1, df = 2; H = 23.17, P < 0.01) in subordinate fish were significantly lower compared with both dominant and sham fish (P < 0.01 and P < 0.05, respectively). In experiment 2, plasma cortisol concentration (Fig. 2, df = 2; H = 11.93, P < 0.01) was significantly elevated in cortisol-treated fish compared with control and fasted fish (P < 0.01).
Fig. 2.
Plasma cortisol concentrations of control, cortisol-treated, and fasted rainbow trout (Oncorhynchus mykiss). Values are presented as means ± SE with n = 5–7 for all groups; values for individuals included in the means are indicated by the symbols. Bars that share a letter are not significantly different from one another (see text for details).
Circulating Lipid Metabolites
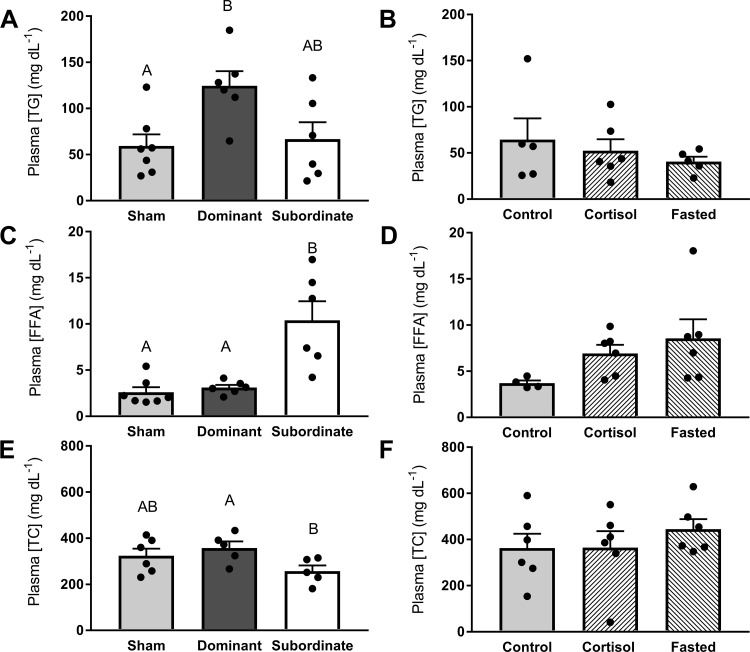
Plasma TG concentrations differed significantly with social status (Fig. 3A, df = 2; F = 5.24, P < 0.05), with plasma TG concentrations in dominant fish being elevated compared with sham fish (P < 0.05) and marginally higher than those of subordinate fish (P = 0.0501). Circulating plasma TG concentrations did not differ among control, cortisol-treated, and fasted fish (Fig. 3B, df = 2; F = 0.58, P > 0.05). Plasma FFA concentrations were significantly influenced by social status (Fig. 3C, df = 2; F = 17.65, P < 0.01), with elevated concentrations in subordinate fish compared with dominant and sham fish (P < 0.05). No differences in plasma FFAs were observed among control, cortisol-treated, and fasted fish (Fig. 3D, df = 2; F = 2.37, P > 0.05). Plasma TC concentration was significantly (Fig. 3E, df = 2; F = 2.99, P < 0.05) lower in subordinate compared with dominant fish (P < 0.05). Circulating TC concentrations were not significantly different among control, cortisol-treated, or fasted trout (Fig. 3F, df = 2; F = 0.60, P > 0.05).
Fig. 3.
Plasma concentrations of triglycerides (TG; A and B), free fatty acids (FFAs; C and D), and total cholesterol (TC; E and F) in sham, dominant, and subordinate (A, C, and E), and control, cortisol-treated, and fasted (B, D, and F) rainbow trout (Oncorhynchus mykiss). Values are presented as means ± SE with n = 5–7 for all groups; values for individuals included in the means are indicated by the symbols. Bars that share a letter are not significantly different from one another (see text for details).
Hepatic mRNA Abundance of Genes Involved in Lipid Metabolism
mRNA abundance of lipogenic genes.
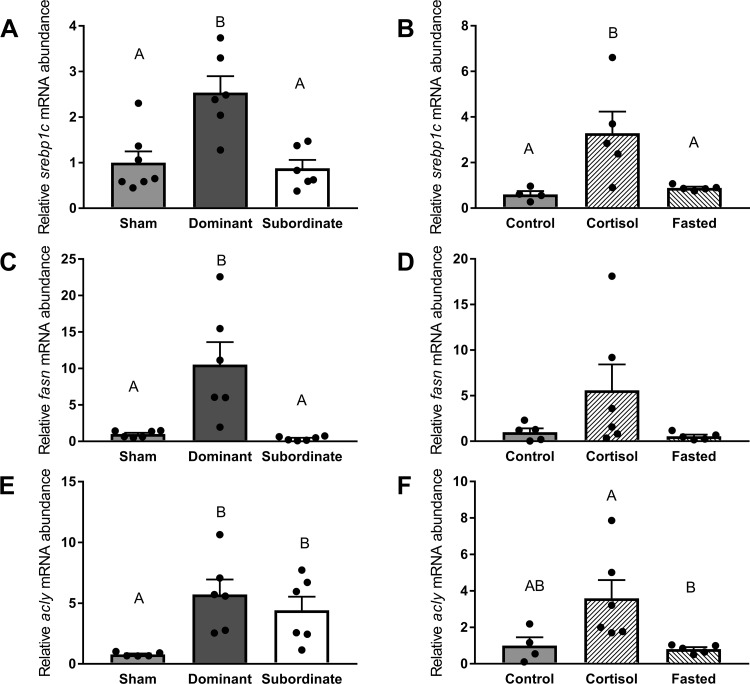
The mRNA abundance of srebp1c was significantly (Fig. 4A, df = 2; F = 11.20, P < 0.01) higher in dominant fish compared with subordinates and shams (P < 0.01). In experiment 2, srebp1c mRNA abundance was significantly elevated (Fig. 4B, df = 2; F = 6.24, P < 0.05) in cortisol-treated fish compared with control and fasted fish (P < 0.05). The mRNA abundance of fasn was affected by social status (Fig. 4C, df = 2; F = 30.02, P < 0.01), with elevated levels in dominant fish compared with subordinates and shams (P < 0.01) but did not differ significantly among control, cortisol-treated and fasted fish (Fig. 4D, df = 2; F = 2.34, P > 0.05). The mRNA abundance of acly was significantly (Fig. 4E, df = 2, F = 16.74, P < 0.01) increased in both dominant and subordinate trout compared with shams (P < 0.01). In addition, a higher abundance of acly mRNA was detected in cortisol-treated fish compared with fasted fish (Fig. 4F, df = 2, F = 4.88, P < 0.05).
Fig. 4.
Abundance of mRNA markers of hepatic lipogenesis pathways. Transcript levels of sterol regulatory element-binding protein 2 (srebp1c; A and B), fatty acid synthase (fasn; C and D), and ATP citrate lyase (acly; E and F) in sham, dominant, and subordinate (A, C, and E) and control, cortisol-treated, and fasted (B, D, and F) rainbow trout (Oncorhynchus mykiss). Values are presented as means ± SE with n = 4–7 for all groups; values for individuals included in the means are indicated by the symbols. Bars that share a letter are not significantly different from one another (see text for details).
mRNA abundance of β-oxidation genes.
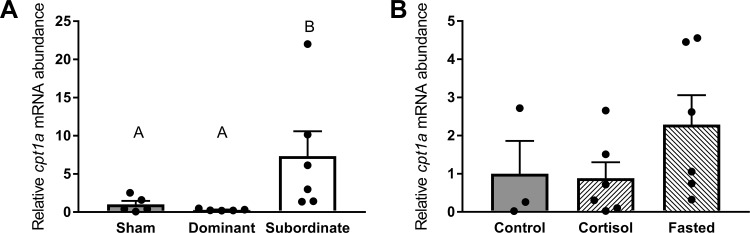
The mRNA abundance of cpt1a differed significantly with social status (Fig. 5A, df = 2; F = 10.25, P < 0.01), with increased mRNA abundance in subordinates compared with both sham (P < 0.05) and dominant fish (P < 0.01). Conversely, no significant differences in cpt1a mRNA abundance were observed among control, cortisol-treated, and fasted fish (Fig. 5B, df = 2; F = 1.48, P > 0.05).
Fig. 5.
mRNA abundance of the β-oxidation marker carnitine palmitoyltransferase 1A (cpt1a) in sham, dominant, and subordinate (A) and control, cortisol-treated, and fasted (B) rainbow trout (Oncorhynchus mykiss). Values are presented as means ± SE with n = 3–7 for all groups; values for individuals included in the means are indicated by the symbols. Bars that share a letter are not significantly different from one another (see text for details).
mRNA abundance of TC biosynthesis genes.
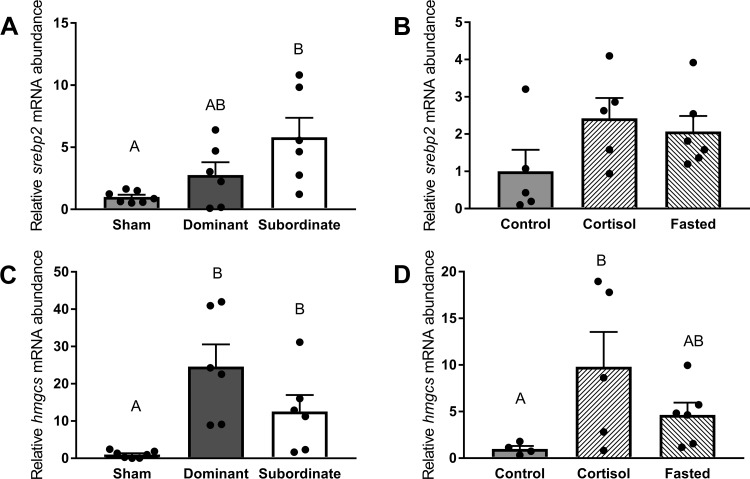
Increased mRNA abundance of srebp2 (Fig. 6A, df = 2; H = 6.38, P < 0.05) was detected in subordinate fish compared with sham fish (P < 0.05), while no significant differences among control, cortisol-treated, and fasted trout were observed (Fig. 6B, df = 2, F = 2.02, P < 0.05). The mRNA abundance of hmgcs differed significantly with social status (Fig. 6C, df = 2; F = 16.29, P < 0.01), with dyad-housed trout (dominant and subordinate) exhibiting increased mRNA abundance compared with sham fish (P < 0.01). In addition, hmgcs mRNA abundance was significantly (Fig. 6D, df = 2; F = 4.441, P < 0.05) increased in cortisol-treated trout relative to control trout (P < 0.05).
Fig. 6.
Abundance of mRNA markers of total cholesterol (TC) biosynthesis pathways. Transcript levels of sterol regulatory element-binding protein 2 (srebp2; A and B) and hydroxymethylglutaryl-CoA synthase (hmgcs; C and D) in sham, dominant, and subordinate (A and C) and control, cortisol-treated, and fasted (B and D) rainbow trout (Oncorhynchus mykiss). Values are presented as means ± SE with n = 4–7 for all groups; values for individuals included in the means are indicated by the symbols. Bars that share a letter are not significantly different from one another (see text for details).
mRNA abundance of TC degradation genes.
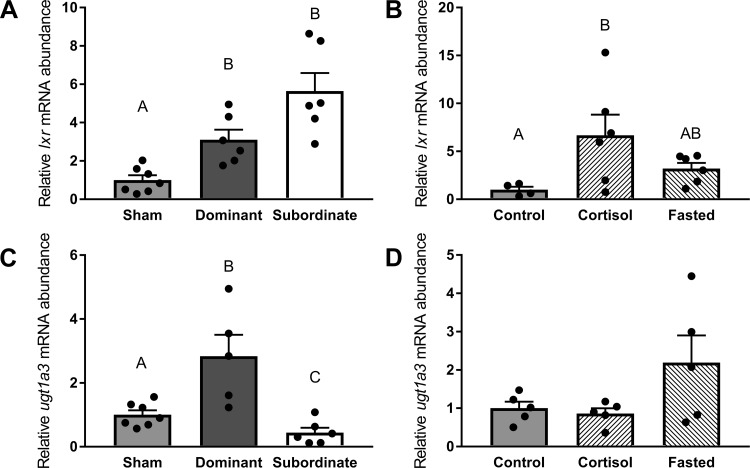
The mRNA abundance of lxr was significantly (Fig. 7A, df = 2; F = 19.35, P < 0.01) increased in paired fish (dominant and subordinate) compared with shams (P < 0.01). When we investigated cortisol and fasting as factors in regulating lxr abundance (Fig. 7B, df = 2; F = 4.28, P < 0.05), a significantly higher mRNA abundance in cortisol-treated fish compared with control fish (P < 0.05) was detected. Social status also differentially affected ugt1a3 mRNA abundance (Fig. 7C, df = 2; F = 14.05, P < 0.01), with transcript abundances differing significantly among all treatment groups (dominant > sham > subordinate, P < 0.05). Conversely, no significant differences among control, cortisol-treated, and fasted trout were observed (Fig. 7D, df = 2; F = 2.94, P > 0.05).
Fig. 7.
Abundance of mRNA markers of total cholesterol (TC) degradation pathways. Transcript levels of liver X receptor (lxr; A and B) and UDP glucuronosyltransferase family 1 member A3 (ugt1a3; C and D) in sham, dominant, and subordinate (A and C) and control, cortisol-treated, and fasted (B and D) rainbow trout (Oncorhynchus mykiss). Values are presented as means ± SE with n = 4–7 for all groups; values for individuals included in the means are indicated by the symbols. Bars that share a letter are not significantly different from one another (see text for details).
Hepatic mRNA Abundance of Drosha and Abundance of miRNAs Involved in Lipid Metabolism
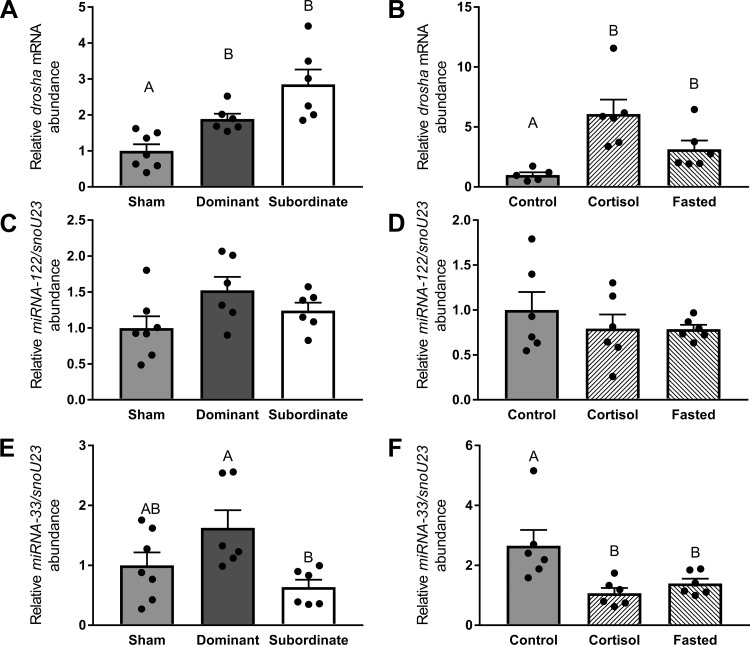
The mRNA abundance of drosha was significantly elevated (Fig. 8A, df = 2; F = 14.23, P < 0.01) in both dominant (P < 0.05) and subordinate (P < 0.01) trout compared with shams. Similarly, both cortisol treatment and fasting induced a significant increase in drosha mRNA abundance (Fig. 8B, df = 2; F = 20.16, P < 0.01) compared with the control group (P < 0.01).
Fig. 8.
Abundance of mRNA of the rate-limiting enzyme in miRNA biogenesis drosha (A and B) and lipid metabolism-related miRNA-122 (C and D) and miRNA-33 (E and F) in sham, dominant, and subordinate (A, C, and E) and control, cortisol-treated, and fasted (B, D, and F) rainbow trout (Oncorhynchus mykiss). Values are presented as means ± SE with n = 5–7 for all groups; values for individuals included in the means are indicated by the symbols. Bars that share a letter are not significantly different from one another (see text for details).
The abundance of miRNA-122 was unaffected by social status (Fig. 8C, df = 2; F = 2.77, P > 0.05) or cortisol and fasting treatments (Fig. 8D, df = 2; F = 0.65, P > 0.05). However, social interactions (Fig. 8E, df = 2; F = 4.48, P < 0.05) significantly increased miRNA-33 in dominant compared with subordinate fish (P < 0.05). In addition, miRNA-33 abundance was significantly (Fig. 8F, df = 2; F = 9.42, P < 0.01) lower in both cortisol-treated (P < 0.01) and fasted fish (P < 0.05) relative to control fish.
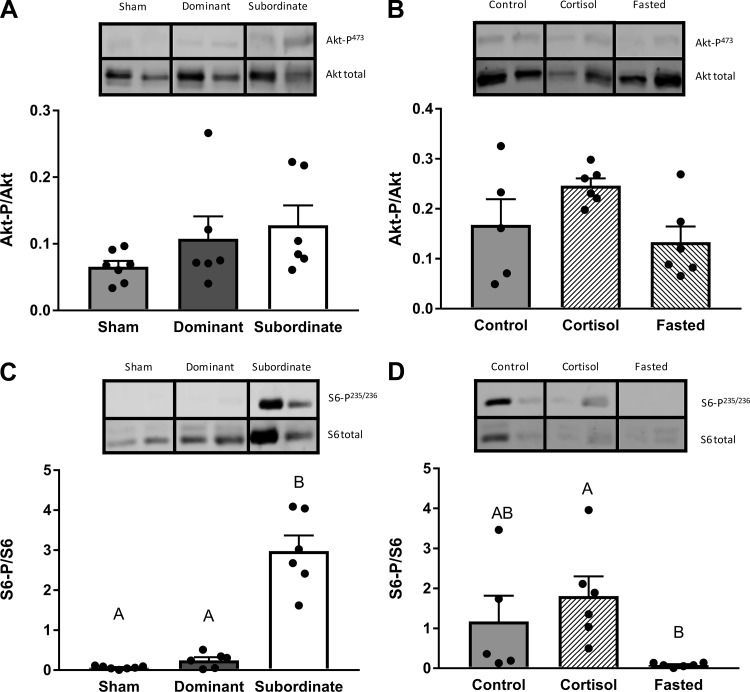
Hepatic Cell Signaling
The ratio of Akt-p/Akt did not differ with social status (Fig. 9A, df = 2; F = 2.14, P > 0.05) or in response to cortisol treatment or fasting (Fig. 9B, df = 2; F = 3.15, P > 0.05). However, social status significantly affected the S6-p /S6 ratio (Fig. 9C, df = 2; H = 12.13, P < 0.01), with a significantly higher ratio in subordinate fish compared with sham (P < 0.01) and dominant fish (P < 0.05). A significant effect of treatment on S6-p/S6 ratio was also identified in experiment 2 (Fig. 9D, df = 2; H = 10.66, P < 0.01), with a lower S6-p/S6 ratio in fasted fish compared with cortisol-treated fish (P < 0.01).
Fig. 9.
Ratio of phosphorylated protein to total protein for Akt (A and B) and S6 (C and D) pathways in sham, dominant, and subordinate (A and C) and control, cortisol-treated, and fasted (B and D) rainbow trout (Oncorhynchus mykiss). Values are presented as means ± SE with n = 5–7 for all groups; values for individuals included in the means are indicated by the symbols. Bars that share a letter are not significantly different from one another (see text for details). Representative images of Western blots are included.
DISCUSSION
Confirmation of Social Status Phenotypes in Juvenile Rainbow Trout
Behavioral observations over a 4-day period allowed differentiation of dominant and subordinate phenotypes as described in previous studies (1, 14, 21, 34, 78). These behavioral distinctions were corroborated by clear differences in endocrine indexes of stress, evidenced by elevated plasma cortisol concentrations in subordinate fish, again as previously described (22, 66, 78). Clear physiological differences were identified between the subordinates and dominants, as evidenced by differences in food intake and specific growth rates (Table 1), again similar to previously reported studies (14, 21, 60). Collectively, these differences distinguish dominant and subordinate phenotypes, allowing the hypothesis that social status affects lipid metabolism to be tested. Because subordinates are characterized by increased circulating cortisol levels coupled with reduced food intake, cortisol-treated and fasted fish were used to address the possible contribution of these individual factors to changes in indexes of lipid metabolism.
Lipid Metabolism Indexes in Dominant Fish Suggest a Shift Toward Energy Storage
Dominant fish displayed elevated levels of circulating TG compared with shams and marginally elevated levels compared with subordinate fish. The molecular signature of increased hepatic mRNA abundance of the lipogenic genes srebp1c and fasn in dominant fish as well as acly relative to shams suggests that hepatic de novo lipogenesis contributes to this effect, at least in part. The srebp1c gene is a well-characterized lipogenic transcription factor that stimulates the hepatic de novo lipogenesis pathway by inducing fasn and acly transcription in response to physiological signals via insulin signaling (19, 42, 43). The enzyme products of fasn and acly catalyze palmitate synthesis and its building block, cytosolic acetyl-CoA, respectively (43). In trout, coinduction of hepatic srebp1c, fasn, and acly has been observed in fish selected for increased lipid content (77), during feeding after a short-term fast (57), and following insulin treatment (50). Because dominant trout monopolize the majority of the food offered to each pair, the increase in molecular markers of de novo lipogenesis suggests that selective activation of this pathway in dominants contributes to the observed increase in circulating TG. However, measurement of enzyme activities would be necessary to extrapolate our findings from mRNA abundance to the functional protein level. In addition to energetically costly de novo lipogenesis (79), other factors, especially intestinal absorption of dietary lipids and release of resynthesized dietary lipids, likely contribute to an increase in circulating TG (4). Indeed, a postprandial time-course study in rainbow trout suggested a peak in plasma TG concentration 12 h after the ingestion of a meal (5), timing that corresponds in the present study to the last feeding in the evening before animals were collected the following morning. Investigation of metabolic and molecular markers of intestinal lipid metabolism in rainbow trout (47) may provide insight into the specific contribution of lipid absorption. Similarly, study of metabolic and molecular changes in WAT (49) would be useful in confirming whether the pattern of changes in hepatic transcript abundance reflects a shift toward lipid synthesis for lipid storage in dominant trout.
Subordinate and sham trout were similar in terms of plasma TG levels and lipogenic mRNA abundances, and neither cortisol-treated nor fasted fish exhibited significant decreases in these variables from control fish. Collectively, these data support the hypothesis of a dominant phenotype in which diet-induced stimulation of circulating TG levels allows the initiation of energetically costly de novo lipogenesis. A comparable effect was proposed to explain elevation of hepatic glycogen stores in dominant trout above those in control fish (35).
Lipid Metabolism Indexes in Subordinate Fish Suggest a Reliance on Circulating FFAs for Hepatic β-Oxidation Pathways
In contrast to TG, circulating FFAs were elevated in subordinate rainbow trout compared with dominant and sham fish, and this increase coincided with increased mRNA abundance of the cpt1a isoform in the liver. Cpt1 is the rate-limiting enzyme of mitochondrial FFA β-oxidation in that it regulates FFA import into mitochondria (64). Together, these responses suggest increased reliance on FFAs for energy metabolism in the liver of subordinate rainbow trout. FFAs increase in response to fasting in rainbow trout within a week or two of cessation of feeding (26, 71), an effect generally attributed to liberation of FFAs from storage in WAT (46). However, although FFAs tended to increase in response to fasting and cortisol treatment, neither factor alone was sufficient to significantly elevate either circulating FFAs or the mRNA abundance of cpt1a. These differences between subordinate fish and fasted or cortisol-treated fish suggest that it is either the combination of both factors, or additional differences related to low social status, that cause FFAs to increase in subordinate trout. Investigation of molecular markers involved in FFA mobilization from WAT and/or muscle tissue is warranted.
Plasma TC is Lowered in Subordinate Rainbow Trout
In subordinate rainbow trout, TC, which serves among other roles as the precursor for cortisol synthesis, was significantly lower than in dominant trout. Examination of the mRNA abundances of genes involved in hepatic TC biosynthesis and degradation pathways did not reveal any significant differences between dominant and subordinate trout, with the exception of ugt1a3. Transcript abundance of ugt1a3 was elevated in dominant fish and reduced in subordinate fish relative to sham-treated trout. In humans, the UGT1A3 enzyme transforms hydrophobic TC metabolites into polar metabolites for biliary excretion (3), so these differences in ugt1a3 mRNA abundance might suggest a counterregulatory response to maintain TC homeostasis, particularly in subordinate fish where presumably there is an increase in TC demand for cortisol synthesis. The decrease in hepatic ugt1a3 mRNA abundance elicited by subordinate social status was not mimicked by cortisol treatment or fasting, suggesting again that additional factors are responsible for the differential regulation of ugt1a3 mRNA abundance observed in dominant vs. subordinate fish.
Hepatic miRNAs as Mediators of Social Status-Dependent Regulation of Lipid Metabolism
Lipid metabolism differs between dominant and subordinate rainbow trout, and while many factors may be responsible for these differences, we examined the role of hepatic miRNAs, because studies in rainbow trout have shown their regulation by nutritional status on the one hand (55, 57, 58) and their function to regulate hepatic and organismal lipid metabolism on the other (56), similar to findings from mammalian models (28, 73). The present study reports, for the first time, social status-dependent regulation of the rate-limiting miRNA biogenesis component Drosha (48), of which a single paralogue exists in rainbow trout (9). Social interaction increased drosha mRNA abundance in both dominant and subordinate trout compared with shams, suggesting that hepatic miRNA biogenesis plays a role in shaping social status-dependent hepatic metabolism in rainbow trout. Both cortisol treatment and fasting increased drosha mRNA abundance compared with controls, suggesting that both factors may contribute to the observed elevation of drosha mRNA abundance in subordinate trout. The factors responsible for increased drosha transcript abundance in dominant fish remain to be determined. In keeping with the focus of the present study on lipid metabolism, the liver-specific miRNA-122 was subsequently targeted because it previously had been shown to be an important regulator of hepatic de novo lipogenesis and circulating TG and TC concentrations in trout (56), as in mice (24, 25, 82) in vivo. However, no significant differences in miRNA-122 abundance were detected with respect to social status or cortisol or fasting treatments, suggesting that miRNA-122 is not a major contributor to the changes in lipid metabolism observed in the present study.
The hepatic mRNA abundance of miRNA-33 was suppressed in subordinate fish relative to dominant fish. Moreover, two hallmarks of the subordinate phenotype, fasting and elevation of cortisol, also significantly reduced miRNA-33 abundance, suggesting that these factors may contribute to the observed decrease in hepatic miRNA-33 abundance in subordinate rainbow trout. In fish as in mammals, miRNA-33 is located in srebp host genes and once transcribed supports the regulatory role of its host gene in hepatic and organismal lipid metabolism (18, 32, 36, 40–43, 65, 72). Functionally, several studies in mammalian models provide evidence for a role of srebp2 intron-encoded miRNA-33 in stimulating hepatic cholesterol abundance by limiting cholesterol efflux via abc transport proteins (32, 36, 41, 65, 72), thus augmenting the principal role of its srebp2 host gene as a key transcription factor in the cholesterol biogenesis pathway (42, 43). These and other studies in mammalian models have also shown inhibitory roles for miRNA-33 in hepatic β-oxidation by targeting enzymes involved in FA oxidation, including cpt1a (18, 32, 36). More recently, studies in mammalian model systems have revealed hepatic de novo lipogenesis as being regulated by miRNA-33 via inhibition of its target srebp1 (40). Conversely, evidence from what is currently the only study to have explored functional roles of miRNA-33 in a teleost, the marine species Siganus canaliculatus (85), points to a stimulatory role of miRNA-33 in Srebp1-mediated de novo lipogenesis by targeting insig1, a repressor blocking Srebp proteolytic activation. With regard to cholesterol metabolism, miRNA-33-dependent regulation of abca1 suggests a conserved role in the regulation of hepatic cholesterol metabolism between teleost fish and mammals (85). Although miRNA-33-dependent regulation of lipid metabolism in conjunction with its srebp host genes appears to be evolutionarily deeply conserved, as evidenced by its role in the negative regulation of β-oxidation in fat bodies of the fruit fly Drosophila melanogaster (18), the extrapolation of its functional role in rainbow trout is currently difficult, particularly because miRNA-33 can be differentially located in introns of srebp1 or srebp2 in teleost fish and other species (85) and because miRNA-target networks between mammals and fish can vary considerably (55). To extrapolate possible roles for miRNA-33 in the absence of functional studies of miRNA-33 in rainbow trout, we determined the genomic location of miRNA-33 in the National Center for Biotechonology Institute-deposited rainbow trout genome (www.salmobase.org) and then used rainbow trout-specific in silico predictions (59) to determine whether characterized miRNA-33 target genes involved in lipid metabolism in mammals are conserved predicted targets in rainbow trout.
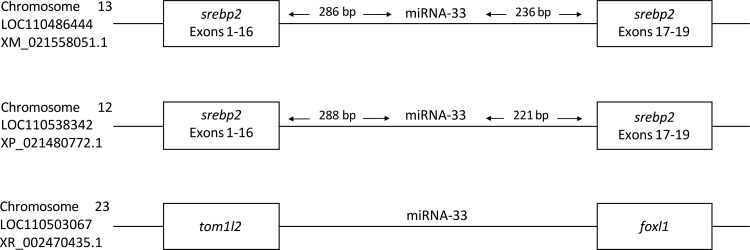
With regard to its genomic location, we identified mature miRNA-33 sequences in the intron between exons 16/17 in two srebp2 gene loci, suggesting a synergistic function of miRNA-33 and srebp2 and, additionally, in an intergenic region (Fig. 10). With regard to predicted target genes involved in hepatic lipid metabolism, miRNA-33 targets in rainbow trout include few directly conserved targets identified in mammals, but nevertheless contain several target genes whose Gene Ontology annotation is enriched for lipid metabolism (Supplemental Table 1; Supplemental Material for this article is available online at the Journal website). This observation suggests that the regulation of hepatic lipid metabolism by miRNA-33 in rainbow trout is likely mediated via different target genes compared with mammalian models (54) and functional studies are clearly needed to establish the role of miRNA-33 in the regulation of hepatic lipid metabolism in rainbow trout before its role in mediating effects of social status on lipid metabolism in rainbow trout can be firmly established.
Fig. 10.
Schematic illustrating the genomic loci of miRNA-33 in rainbow trout (Oncorhynchus mykiss) derived from Salmobase (75).
Akt-Tor-S6 Signaling Cascade is Differentially Affected by Social Status
Because the insulin-responsive Akt-Tor-S6 cell signaling cascade upregulates hepatic lipogenesis while downregulating β-oxidation in rainbow trout (15, 50), the potential involvement of these upstream regulators in shaping differences in lipid metabolism between dominant and subordinate rainbow trout was investigated. There are multiple pathways involved in lipid metabolism; however, the Akt-Tor-S6 cell pathway has previously been functionally characterized and been directly linked to the acute regulation of mRNA abundance and enzyme activity of hepatic fas/Fasn and cpt1a/Cpt1 in studies employing rapamycin, a Tor inhibitor (15, 50). No significant differences in the phosphorylation status of Akt (indicative of Akt activity) were observed with social status, cortisol treatment, or fasting, suggesting that other metabolic signaling pathways that have been characterized in rainbow trout, such as PLC/PKC, MEK/ERK, and JAK–STAT (6–8), may be involved in this response. Additionally, it is relevant to note that fish were sampled in the morning, ~16 h after feeding, which may have masked differences that were previously reported to occur soon after feeding, as documented in a previous time-course study (57).
Interestingly, S6 phosphorylation status was elevated in subordinate trout. The regulation of S6P is mainly via S6K, which is also termed p70 RSK (29). In turn, S6K is prominently activated by the Akt-Tor pathway in trout (15) in response to metabolic hormones, such as insulin, as well as macronutrients, especially amino acids (16, 17, 50). Although not investigated in trout, a second Tor-independent pathway utilizing p90 RSK has been described as phosphorylating S6 serine residues 235/236 in mammals, albeit to a lesser extent (61). Given the lack of significant differences in Akt activation among treatment groups, the observed difference in S6 phosphorylation status is unlikely to have been mediated by endocrine factors, such as insulin. Fasted trout in the present study did not exhibit an increase in S6P compared with controls, suggesting that the increase in S6 phosphorylation in subordinate trout was not related to fasting. Future studies should investigate Tor activation status to clarify whether branched-chain AA-dependent p70 RSK or p90 RSK signaling is involved.
The paradigm that S6 phosphorylation is a global regulator of translation recently has been called into question, following pharmacological inhibition and genetic ablation experiments that inhibited S6 phosphorylation yet reported a lack of effect on translation in various conditions (61, 74, 80). Attention has returned to a role for S6 postulated in a pioneer study, which identified S6 as the only ribosomal protein that was phosphorylated in liver regeneration (37). In line with this possibility, recent evidence suggested a role for S6 phosphorylation in cell cycle progression, cell proliferation, and cell size determination (12, 61, 74, 80, 84). Speculatively, the strong activation of S6 in subordinate trout may reflect a physiological response to sustain liver function in the face of reductions in hepatosomatic index (12).
Perspectives and Significance
The current study revealed, for the first time, that social status in juvenile rainbow trout results in clear differences in lipid metabolism, reflected at the organismal level by increased plasma TG in dominant fish versus increased FFAs as well as decreased TC in the plasma of subordinate trout. Molecular signatures in the liver, a key hub of organismal lipid metabolism (69), provided insight into contributions of hepatic de novo lipogenesis to plasma TG concentrations in dominant rainbow trout and, conversely, increased β-oxidation pathways likely to utilize increased circulating FFAs in subordinate rainbow trout. Overall, these trends are suggestive of diet-dependent changes as reported in short-term fasted rainbow trout after acute refeeding (5, 57). In particular, increased TG and lipogenic gene expression in dominant compared with sham fish suggests a physiological signal, because dominant trout monopolize the ration offered to the paired fish, thus resulting in increased caloric intake compared with sham fish that were fed a single ration. Neither fasting nor cortisol treatment, two factors strongly associated with subordinate status, independently mimicked the circulating FFAs and hepatic cpt1a mRNA induction detected in subordinate trout. This observation suggests that either both factors together or additional factors are required to elicit these responses. Given the strong effects of social status on organismal and hepatic lipid metabolism in juvenile rainbow trout described in the present study, two areas of investigation will be of interest for future studies.
First, at the molecular level in the liver, the increase in paired trout of mRNA abundance of drosha, the rate-limiting enzyme in miRNA biogenesis (38), emphasizes a need for further study of possible roles of hepatic miRNAs in rainbow trout in social hierarchies. Our current study investigated the possible involvement of two specific miRNAs, miRNA-122 and miRNA-33, because of their importance in hepatic lipid metabolism in teleost fish (55), but combinatorial effects of miRNAs on lipid metabolism gene mRNAs in the liver can only be assessed by transcriptome level approaches (55) in conjunction with species-specific in silico prediction algorithms (59). Together, transcriptomic and bioinformatic approaches will help to uncover novel rainbow trout miRNA-target networks involved in regulating lipid metabolism (current study) and other metabolic pathways, such as glucose metabolism (35), in dominant and subordinate trout. In addition, the lower abundance of hepatic miRNA-33 in subordinate and cortisol-treated fish warrants investigation, as the role of hepatic miRNAs as possible mediators of metabolic effects of cortisol remains uncharacterized (27). Equally at the molecular level, the contribution of extrahepatic tissues, including the intestine, WAT, and muscle to social status-dependent changes in organismal lipid metabolism is warranted, and such studies should use a combination of lipid flux analysis (52, 53) and measurements of molecular markers of lipid metabolic pathways at the mRNA and enzymatic activity levels (16, 47, 49). With regard to the observed social status-dependent changes in hepatic cell signaling, and the significant activation of S6 in subordinate trout, future studies should address the upstream involvement of cell signaling pathway components, especially p70 RSK or p90 RSK and mTOR. At the tissue level, possible downstream effects S6P on liver plasticity warrant further investigation, as they contribute to the sustainability of altered metabolic demands in subordinate fish. Second, the central role of lipids in trout energy metabolism suggests that social status-dependent changes in lipid metabolism in rainbow trout may have functional consequences at physiological and ecological levels. For example, a fall in FFAs was linked to increased food intake in rainbow trout (51), raising the question of whether the sustained inhibition of feeding observed in subordinate rainbow trout, even following separation from the dominant fish (14), may be mediated at least in part by increased circulating FFAs. The subtle yet significant decrease in circulating TC in subordinate rainbow trout may reflect increased cortisol biogenesis and/or may impact cortisol biogenesis. At the ecological level, lipid metabolism in juvenile trout is predictive of migration strategy (11), and whether social status-dependent changes in lipid metabolism in juvenile fish translate into different life-history strategies warrants further study.
GRANTS
This work was supported by funds from Natural Sciences and Engineering Research Council Discovery (to K. M. Gilmour and J. A. Mennigen) and Research Tools & Instruments (to K. M. Gilmour) grants, John R. Evans Leader Fund from the Canadian Foundation for Innovation (to J. A. Mennigen), and Ontario Research Fund-Research Infrastructure from the Ministry of Research, Innovation and Science (to J. A. Mennigen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.A.M. and K.M.G. conceived and designed research; D.J.K. and B.M.C. performed experiments; D.J.K., B.M.C., J.A.M., and K.M.G. analyzed data; D.J.K., B.M.C., J.A.M., and K.M.G. interpreted results of experiments; D.J.K. prepared figures; D.J.K. and J.A.M. drafted manuscript; D.J.K., B.M.C., J.A.M., and K.M.G. edited and revised manuscript; D.J.K., B.M.C., J.A.M., and K.M.G. approved final version of manuscript.
Supplemental Data
ACKNOWLEDGMENTS
We thank Bill Fletcher for help with animal care and Carol Best for experimental help.
REFERENCES
- 1.Abbott JC, Dill LM. Patterns of aggressive attack in juvenile steelhead trout (Salmo gairdneri). Can J Fish Aquat Sci 42: 1702–1706, 1985. doi: 10.1139/f85-213. [DOI] [Google Scholar]
- 1a.Aluru N, Leatherland JF, Vijayan MM. Bisphenol A in oocytes leads to growth suppression and altered stress performance in juvenile rainbow trout. PLoS One 5: e10741, 2010. doi: 10.1371/journal.pone.0010741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babin PJ, Vernier JM. Plasma lipoproteins in fish. J Lipid Res 30: 467–489, 1989. [PubMed] [Google Scholar]
- 3.Barbier O, Trottier J, Kaeding J, Caron P, Verreault M. Lipid-activated transcription factors control bile acid glucuronidation. Mol Cell Biochem 326: 3–8, 2009. doi: 10.1007/s11010-008-0001-5. [DOI] [PubMed] [Google Scholar]
- 4.Bauermeister AE, Sargent JR. Biosynthesis of triacylglycerols in the intestines of rainbow trout (Salmo gairdnerii) fed marine zooplankton rich in wax esters. Biochim Biophys Acta 575: 358–364, 1979. doi: 10.1016/0005-2760(79)90104-8. [DOI] [PubMed] [Google Scholar]
- 5.Belghit I, Panserat S, Sadoul B, Dias K, Skiba-Cassy S, Seiliez I. Macronutrient composition of the diet affects the feeding-mediated down regulation of autophagy in muscle of rainbow trout (O. mykiss). PLoS One 8: e74308, 2013. doi: 10.1371/journal.pone.0074308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergan HE, Kittilson JD, Sheridan MA. Nutrition-regulated lipolysis in rainbow trout (Oncorhynchus mykiss) is associated with alterations in the ERK, PI3K-Akt, JAK-STAT, and PKC signaling pathways. Gen Comp Endocrinol 176: 367–376, 2012. doi: 10.1016/j.ygcen.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Bergan HE, Kittilson JD, Sheridan MA. PKC and ERK mediate GH-stimulated lipolysis. J Mol Endocrinol 51: 213–224, 2013. doi: 10.1530/JME-13-0039. [DOI] [PubMed] [Google Scholar]
- 8.Bergan HE, Kittilson JD, Sheridan MA. Nutritional state modulates growth hormone-stimulated lipolysis. Gen Comp Endocrinol 217–218: 1–9, 2015. doi: 10.1016/j.ygcen.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Best C, Ikert H, Kostyniuk DJ, Craig PM, Navarro-Martin L, Marandel L, Mennigen JA. Epigenetics in teleost fish: From molecular mechanisms to physiological phenotypes. Comp Biochem Physiol B Biochem Mol Biol S1096–4959: 30012–30015, 2018. [DOI] [PubMed] [Google Scholar]
- 10.Black D, Skinner ER. Changes in plasma lipoproteins and tissue lipoprotein lipase and salt-resistant lipase activities during spawning in the rainbow trout (Salmo gairdnerii R.). Comp Biochem Physiol B 88: 261–267, 1987. doi: 10.1016/0305-0491(87)90111-8. [DOI] [PubMed] [Google Scholar]
- 11.Boel M, Aarestrup K, Baktoft H, Larsen T, Søndergaard Madsen S, Malte H, Skov C, Svendsen JC, Koed A. The physiological basis of the migration continuum in brown trout (Salmo trutta). Physiol Biochem Zool 87: 334–345, 2014. doi: 10.1086/674869. [DOI] [PubMed] [Google Scholar]
- 12.Boylan JM, Anand P, Gruppuso PA. Ribosomal protein S6 phosphorylation and function during late gestation liver development in the rat. J Biol Chem 276: 44457–44463, 2001. doi: 10.1074/jbc.M103457200. [DOI] [PubMed] [Google Scholar]
- 13.Briffa M, Elwood RW. Repeated measures analysis of contests and other dyadic interactions: problems of semantics, not statistical validity. Anim Behav 80: 583–588, 2010. doi: 10.1016/j.anbehav.2010.06.009. [DOI] [Google Scholar]
- 14.Culbert BM, Gilmour KM. Rapid recovery of the cortisol response following social subordination in rainbow trout. Physiol Behav 164: 306–313, 2016. doi: 10.1016/j.physbeh.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 14a.Cruz-Garcia L, Sánchez-Gurmaches J, Gutiérrez J, Navarro I. Role of LXR in trout adipocytes: target genes, hormonal regulation, adipocyte differentiation and relation to lipolysis. Comp Biochem Physiol A Mol Integr Physiol 163: 120–126, 2012. doi: 10.1016/j.cbpa.2012.05.193. [DOI] [PubMed] [Google Scholar]
- 15.Dai W, Panserat S, Kaushik S, Terrier F, Plagnes-Juan E, Seiliez I, Skiba-Cassy S. Hepatic fatty acid biosynthesis is more responsive to protein than carbohydrate in rainbow trout during acute stimulations. Am J Physiol Regul Integr Comp Physiol 310: R74–R86, 2016. doi: 10.1152/ajpregu.00281.2015. [DOI] [PubMed] [Google Scholar]
- 16.Dai W, Panserat S, Mennigen JA, Terrier F, Dias K, Seiliez I, Skiba-Cassy S. Post-prandial regulation of hepatic glucokinase and lipogenesis requires the activation of TORC1 signalling in rainbow trout (Oncorhynchus mykiss). J Exp Biol 216: 4483–4492, 2013. doi: 10.1242/jeb.091157. [DOI] [PubMed] [Google Scholar]
- 17.Dai W, Panserat S, Plagnes-Juan E, Seiliez I, Skiba-Cassy S. Amino acids attenuate insulin action on gluconeogenesis and promote fatty acid biosynthesis via mTORC1 signaling pathway in trout hepatocytes. Cell Physiol Biochem 36: 1084–1100, 2015. doi: 10.1159/000430281. [DOI] [PubMed] [Google Scholar]
- 18.Dávalos A, Goedeke L, Smibert P, Ramírez CM, Warrier NP, Andreo U, Cirera-Salinas D, Rayner K, Suresh U, Pastor-Pareja JC, Esplugues E, Fisher EA, Penalva LOF, Moore KJ, Suárez Y, Lai EC, Fernández-Hernando C. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc Natl Acad Sci USA 108: 9232–9237, 2011. doi: 10.1073/pnas.1102281108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol Rev 86: 465–514, 2006. doi: 10.1152/physrev.00025.2005. [DOI] [PubMed] [Google Scholar]
- 20.DiBattista JD, Anisman H, Whitehead M, Gilmour KM. The effects of cortisol administration on social status and brain monoaminergic activity in rainbow trout Oncorhynchus mykiss. J Exp Biol 208: 2707–2718, 2005. doi: 10.1242/jeb.01690. [DOI] [PubMed] [Google Scholar]
- 21.DiBattista JD, Levesque HM, Moon TW, Gilmour KM. Growth depression in socially subordinate rainbow trout Oncorhynchus mykiss: more than a fasting effect. Physiol Biochem Zool 79: 675–687, 2006. doi: 10.1086/504612. [DOI] [PubMed] [Google Scholar]
- 22.Doyon C, Gilmour KM, Trudeau VL, Moon TW. Corticotropin-releasing factor and neuropeptide Y mRNA levels are elevated in the preoptic area of socially subordinate rainbow trout. Gen Comp Endocrinol 133: 260–271, 2003. doi: 10.1016/S0016-6480(03)00195-3. [DOI] [PubMed] [Google Scholar]
- 23.Elliott JM. Mechanisms responsible for population regulation in young migratory trout, Salmo trutta. III. The role of territorial behaviour. J Anim Ecol 59: 803–818, 1990. doi: 10.2307/5015. [DOI] [Google Scholar]
- 24.Elmén J, Lindow M, Schütz S, Lawrence M, Petri A, Obad S, Lindholm M, Hedtjärn M, Hansen HF, Berger U, Gullans S, Kearney P, Sarnow P, Straarup EM, Kauppinen S. LNA-mediated microRNA silencing in non-human primates. Nature 452: 896–899, 2008. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 25.Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R, Subramaniam A, Propp S, Lollo BA, Freier S, Bennett CF, Bhanot S, Monia BP. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab 3: 87–98, 2006. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Farbridge KJ, Leatherland JF. Temporal changes in plasma thyroid hormone, growth hormone and free fatty acid concentrations, and hepatic 5′-monodeiodinase activity, lipid and protein content during chronic fasting and re-feeding in rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 10: 245–257, 1992. doi: 10.1007/BF00004518. [DOI] [PubMed] [Google Scholar]
- 27.Faught E, Vijayan MM. Mechanisms of cortisol action in fish hepatocytes. Comp Biochem Physiol B Biochem Mol Biol 199: 136–145, 2016. doi: 10.1016/j.cbpb.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Fernández-Hernando C, Suárez Y, Rayner KJ, Moore KJ. MicroRNAs in lipid metabolism. Curr Opin Lipidol 22: 86–92, 2011. doi: 10.1097/MOL.0b013e3283428d9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frödin M, Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol 151: 65–77, 1999. doi: 10.1016/S0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 30.Gamperl AK, Vijayan MM, Boutilier RG. Experimental control of stress hormone levels in fishes: techniques and applications. Rev Fish Biol Fish 4: 215–255, 1994. doi: 10.1007/BF00044129. [DOI] [Google Scholar]
- 31.Gauthey Z, Freychet M, Manicki A, Herman A, Lepais O, Panserat S, Elosegi A, Tentelier C, Labonne J. The concentration of plasma metabolites varies throughout reproduction and affects offspring number in wild brown trout (Salmo trutta). Comp Biochem Physiol A Mol Integr Physiol 184: 90–96, 2015. doi: 10.1016/j.cbpa.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 32.Gerin I, Clerbaux LA, Haumont O, Lanthier N, Das AK, Burant CF, Leclercq IA, MacDougald OA, Bommer GT. Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J Biol Chem 285: 33652–33661, 2010. doi: 10.1074/jbc.M110.152090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilmour KM, Craig PM, Dhillon RS, Lau GY, Richards JG. Regulation of energy metabolism during social interactions in rainbow trout: a role for AMP-activated protein kinase. Am J Physiol Regul Integr Comp Physiol 313: R549–R559, 2017. doi: 10.1152/ajpregu.00341.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilmour KM, Dibattista JD, Thomas JB. Physiological causes and consequences of social status in salmonid fish. Integr Comp Biol 45: 263–273, 2005. doi: 10.1093/icb/45.2.263. [DOI] [PubMed] [Google Scholar]
- 35.Gilmour KM, Kirkpatrick S, Massarsky A, Pearce B, Saliba S, Stephany C-É, Moon TW. The influence of social status on hepatic glucose metabolism in rainbow trout Oncorhynchus mykiss. Physiol Biochem Zool 85: 309–320, 2012. doi: 10.1086/666497. [DOI] [PubMed] [Google Scholar]
- 36.Goedeke L, Vales-Lara FM, Fenstermaker M, Cirera-Salinas D, Chamorro-Jorganes A, Ramírez CM, Mattison JA, de Cabo R, Suárez Y, Fernández-Hernando C. A regulatory role for microRNA 33* in controlling lipid metabolism gene expression. Mol Cell Biol 33: 2339–2352, 2013. doi: 10.1128/MCB.01714-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gressner AM, Wool IG. The phosphorylation of liver ribosomal proteins in vivo. Evidence that only a single small subunit protein (S6) is phosphorylated. J Biol Chem 249: 6917–6925, 1974. [PubMed] [Google Scholar]
- 38.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15: 509–524, 2014. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 39.Heckmann LH, Sørensen PB, Krogh P, Sørensen JG. NORMA-Gene: a simple and robust method for qPCR normalization based on target gene data. BMC Bioinformatics 712: 250, 2011. doi: 10.1186/1471-2105-12-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horie T, Nishino T, Baba O, Kuwabara Y, Nakao T, Nishiga M, Usami S, Izuhara M, Sowa N, Yahagi N, Shimano H, Matsumura S, Inoue K, Marusawa H, Nakamura T, Hasegawa K, Kume N, Yokode M, Kita T, Kimura T, Ono K. MicroRNA-33 regulates sterol regulatory element-binding protein 1 expression in mice. Nat Commun 4: 2883, 2013. doi: 10.1038/ncomms3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horie T, Ono K, Horiguchi M, Nishi H, Nakamura T, Nagao K, Kinoshita M, Kuwabara Y, Marusawa H, Iwanaga Y, Hasegawa K, Yokode M, Kimura T, Kita T. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc Natl Acad Sci USA 107: 17321–17326, 2010. doi: 10.1073/pnas.1008499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horton JD, Goldstein JL, Brown MS. SREBPs: transcriptional mediators of lipid homeostasis. Cold Spring Harb Symp Quant Biol 67: 491–498, 2002. doi: 10.1101/sqb.2002.67.491. [DOI] [PubMed] [Google Scholar]
- 43.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 109: 1125–1131, 2002. doi: 10.1172/JCI0215593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoseini SM, Yousefi M, Rajabiesterabadi H, Paktinat M. Effect of short- term (0–72 h) fasting on serum biochemical characteristics in rainbow trout Oncorhynchus mykiss. J Appl Ichthyology 30: 569–573, 2014. doi: 10.1111/jai.12221. [DOI] [Google Scholar]
- 45.Jeffrey JD, Esbaugh AJ, Vijayan MM, Gilmour KM. Modulation of hypothalamic-pituitary-interrenal axis function by social status in rainbow trout. Gen Comp Endocrinol 176: 201–210, 2012. doi: 10.1016/j.ygcen.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 46.Jezierska B, Hazel JR, Gerking SD. Lipid mobilization during starvation in the rainbow trout, Salmo gairdneri Richardson, with attention to fatty acids. J Fish Biol 21: 681–692, 1982. doi: 10.1111/j.1095-8649.1982.tb02872.x. [DOI] [Google Scholar]
- 47.Kamalam BS, Médale F, Larroquet L, Corraze G, Panserat S. Metabolism and fatty acid profile in fat and lean rainbow trout lines fed with vegetable oil: effect of carbohydrates. PLoS One 8: e76570, 2013. doi: 10.1371/journal.pone.0076570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim YK, Kim B, Kim VN. Re-evaluation of the roles of DROSHA, Export in 5, and DICER in microRNA biogenesis. Proc Natl Acad Sci USA 113: E1881–E1889, 2016. doi: 10.1073/pnas.1602532113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolditz CI, Langin D. Adipose tissue lipolysis. Curr Opin Clin Nutr Metab Care 13: 377–381, 2010. doi: 10.1097/MCO.0b013e32833bed6a. [DOI] [PubMed] [Google Scholar]
- 50.Lansard M, Panserat S, Plagnes-Juan E, Seiliez I, Skiba-Cassy S. Integration of insulin and amino acid signals that regulate hepatic metabolism-related gene expression in rainbow trout: role of TOR. Amino Acids 39: 801–810, 2010. doi: 10.1007/s00726-010-0533-3. [DOI] [PubMed] [Google Scholar]
- 51.Librán-Pérez M, Velasco C, López-Patiño MA, Míguez JM, Soengas JL. Counter-regulatory response to a fall in circulating fatty acid levels in rainbow trout. Possible involvement of the hypothalamus-pituitary-interrenal axis. PLoS One 9: e113291, 2014. doi: 10.1371/journal.pone.0113291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Magnoni L, Vaillancourt E, Weber JM. In vivo regulation of rainbow trout lipolysis by catecholamines. J Exp Biol 211: 2460–2466, 2008. doi: 10.1242/jeb.018143. [DOI] [PubMed] [Google Scholar]
- 53.Magnoni L, Weber JM. Endurance swimming activates trout lipoprotein lipase: plasma lipids as a fuel for muscle. J Exp Biol 210: 4016–4023, 2007. doi: 10.1242/jeb.007708. [DOI] [PubMed] [Google Scholar]
- 54.Marquart TJ, Allen RM, Ory DS, Baldán A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci USA 107: 12228–12232, 2010. doi: 10.1073/pnas.1005191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mennigen JA. Micromanaging metabolism-a role for miRNAs in teleost energy metabolism. Comp Biochem Physiol B Biochem Mol Biol 199: 115–125, 2016. doi: 10.1016/j.cbpb.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Mennigen JA, Martyniuk CJ, Seiliez I, Panserat S, Skiba-Cassy S. Metabolic consequences of microRNA-122 inhibition in rainbow trout, Oncorhynchus mykiss. BMC Genomics 15: 70, 2014. doi: 10.1186/1471-2164-15-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mennigen JA, Panserat S, Larquier M, Plagnes-Juan E, Medale F, Seiliez I, Skiba-Cassy S. Postprandial regulation of hepatic microRNAs predicted to target the insulin pathway in rainbow trout. PLoS One 7: e38604, 2012. doi: 10.1371/journal.pone.0038604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mennigen JA, Plagnes-Juan E, Figueredo-Silva CA, Seiliez I, Panserat S, Skiba-Cassy S. Acute endocrine and nutritional co-regulation of the hepatic omy-miRNA-122b and the lipogenic gene fas in rainbow trout, Oncorhynchus mykiss. Comp Biochem Physiol B Biochem Mol Biol 169: 16–24, 2014. doi: 10.1016/j.cbpb.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Mennigen JA, Zhang D. MicroTrout: A comprehensive, genome-wide miRNA target prediction framework for rainbow trout, Oncorhynchus mykiss. Comp Biochem Physiol Part D Genomics Proteomics 20: 19–26, 2016. doi: 10.1016/j.cbd.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 60.Metcalfe NB. Intraspecific variation in competitive ability and food intake in salmonids: consequences for energy budgets and growth rates. J Fish Biol 28: 525–531, 1986. doi: 10.1111/j.1095-8649.1986.tb05190.x. [DOI] [Google Scholar]
- 61.Meyuhas O. Ribosomal protein S6 phosphorylation: four decades of research. Int Rev Cell Mol Biol 320: 41–73, 2015. doi: 10.1016/bs.ircmb.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 62.Mommsen TP, Vijayan MM, Moon TW. Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fish 9: 211–268, 1999. doi: 10.1023/A:1008924418720. [DOI] [Google Scholar]
- 63.Moon TW. Glucose intolerance in teleost fish: fact or fiction? Comp Biochem Physiol B Biochem Mol Biol 129: 243–249, 2001. doi: 10.1016/S1096-4959(01)00316-5. [DOI] [PubMed] [Google Scholar]
- 64.Morash AJ, McClelland GB. Regulation of carnitine palmitoyltransferase (CPT) I during fasting in rainbow trout (Oncorhynchus mykiss) promotes increased mitochondrial fatty acid oxidation. Physiol Biochem Zool 84: 625–633, 2011. doi: 10.1086/662552. [DOI] [PubMed] [Google Scholar]
- 65.Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Näär AM. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 328: 1566–1569, 2010. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Øverli O, Harris CA, Winberg S. Short-term effects of fights for social dominance and the establishment of dominant-subordinate relationships on brain monoamines and cortisol in rainbow trout. Brain Behav Evol 54: 263–275, 1999. doi: 10.1159/000006627. [DOI] [PubMed] [Google Scholar]
- 67.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Plagnes-Juan E, Lansard M, Seiliez I, Médale F, Corraze G, Kaushik S, Panserat S, Skiba-Cassy S. Insulin regulates the expression of several metabolism-related genes in the liver and primary hepatocytes of rainbow trout (Oncorhynchus mykiss). J Exp Biol 211: 2510–2518, 2008. doi: 10.1242/jeb.018374. [DOI] [PubMed] [Google Scholar]
- 68a.Polakof S, Médale F, Skiba-Cassy S, Corraze G, Panserat S. Molecular regulation of lipid metabolism in liver and muscle of rainbow trout subjected to acute and chronic insulin treatments. Domest Anim Endocrinol 39: 26–33, 2010. doi: 10.1016/j.domaniend.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 69.Polakof S, Moon TW, Aguirre P, Skiba-Cassy S, Panserat S. Glucose homeostasis in rainbow trout fed a high-carbohydrate diet: metformin and insulin interact in a tissue-dependent manner. Am J Physiol Regul Integr Comp Physiol 300: R166–R174, 2011. doi: 10.1152/ajpregu.00619.2010. [DOI] [PubMed] [Google Scholar]
- 70.Polakof S, Panserat S, Soengas JL, Moon TW. Glucose metabolism in fish: a review. J Comp Physiol B 182: 1015–1045, 2012. doi: 10.1007/s00360-012-0658-7. [DOI] [PubMed] [Google Scholar]
- 71.Pottinger TG, Rand-Weaver M, Sumpter JP. Overwinter fasting and re-feeding in rainbow trout: plasma growth hormone and cortisol levels in relation to energy mobilisation. Comp Biochem Physiol B Biochem Mol Biol 136: 403–417, 2003. doi: 10.1016/S1096-4959(03)00212-4. [DOI] [PubMed] [Google Scholar]
- 72.Rayner KJ, Suárez Y, Dávalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernández-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science 328: 1570–1573, 2010. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rottiers V, Näär AM. MicroRNAs in metabolism and metabolic disorders. Nat Rev Mol Cell Biol 13: 239–250, 2012. doi: 10.1038/nrm3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ruvinsky I, Sharon N, Lerer T, Cohen H, Stolovich-Rain M, Nir T, Dor Y, Zisman P, Meyuhas O. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev 19: 2199–2211, 2005. doi: 10.1101/gad.351605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Samy JKA, Mulugeta TD, Nome T, Sandve SR, Grammes F, Kent MP, Lien S, Våge DI. SalmoBase: an integrated molecular data resource for Salmonid species. BMC Genomics 18: 482, 2017. doi: 10.1186/s12864-017-3877-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schyth BD, Bela-Ong DB, Jalali SAH, Kristensen LBJ, Einer-Jensen K, Pedersen FS, Lorenzen N. Two virus-induced MicroRNAs known only from teleost fishes are orthologues of MicroRNAs involved in cell cycle control in Humans. PLoS One 10: e0132434, 2015. doi: 10.1371/journal.pone.0132434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76a.Skiba-Cassy S, Geurden I, Panserat S, Seiliez I. Dietary methionine imbalance alters the transcriptional regulation of genes involved in glucose, lipid and amino acid metabolism in the liver of rainbow trout (Oncorhynchus mykiss). Aquaculture 454: 56–65, 2016. doi: 10.1016/j.aquaculture.2015.12.015. [DOI] [Google Scholar]
- 77.Skiba-Cassy S, Lansard M, Panserat S, Médale F. Rainbow trout genetically selected for greater muscle fat content display increased activation of liver TOR signaling and lipogenic gene expression. Am J Physiol Regul Integr Comp Physiol 297: R1421–R1429, 2009. doi: 10.1152/ajpregu.00312.2009. [DOI] [PubMed] [Google Scholar]
- 78.Sloman KA, Metcalfe NB, Taylor AC, Gilmour KM. Plasma cortisol concentrations before and after social stress in rainbow trout and brown trout. Physiol Biochem Zool 74: 383–389, 2001. doi: 10.1086/320426. [DOI] [PubMed] [Google Scholar]
- 79.Solinas G, Borén J, Dulloo AG. De novo lipogenesis in metabolic homeostasis: More friend than foe? Mol Metab 4: 367–377, 2015. doi: 10.1016/j.molmet.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tang H, Hornstein E, Stolovich M, Levy G, Livingstone M, Templeton D, Avruch J, Meyuhas O. Amino acid-induced translation of TOP mRNAs is fully dependent on phosphatidylinositol 3-kinase-mediated signaling, is partially inhibited by rapamycin, and is independent of S6K1 and rpS6 phosphorylation. Mol Cell Biol 21: 8671–8683, 2001. doi: 10.1128/MCB.21.24.8671-8683.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tocher DR. Metabolism and functions of lipids and fatty acids in teleost fish. Rev Fish Sci 11: 107–184, 2003. doi: 10.1080/713610925. [DOI] [Google Scholar]
- 82.Tsai WC, Hsu SD, Hsu CS, Lai TC, Chen SJ, Shen R, Huang Y, Chen HC, Lee CH, Tsai TF, Hsu MT, Wu JC, Huang HD, Shiao MS, Hsiao M, Tsou AP. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest 122: 2884–2897, 2012. doi: 10.1172/JCI63455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vijayan MM, Raptis S, Sathiyaa R. Cortisol treatment affects glucocorticoid receptor and glucocorticoid-responsive genes in the liver of rainbow trout. Gen Comp Endocrinol 132: 256–263, 2003. doi: 10.1016/S0016-6480(03)00092-3. [DOI] [PubMed] [Google Scholar]
- 84.Volarević S, Thomas G. Role of S6 phosphorylation and S6 kinase in cell growth. Prog Nucleic Acid Res Mol Biol 65: 101–127, 2001. doi: 10.1016/S0079-6603(00)65003-1. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Q, You C, Wang S, Dong Y, Monroig Ó, Tocher DR, Li Y. The miR-33 gene is identified in a marine teleost: a potential role in regulation of LC-PUFA biosynthesis in Siganus canaliculatus. Sci Rep 6: 32909, 2016. doi: 10.1038/srep32909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.