Abstract
This review summarizes the opening keynote presentation overview of the American Physiological Society Conference on Cardiovascular Aging: New Frontiers and Old Friends held in Westminster, CO, in August 2017. Age is the primary risk factor for cardiovascular diseases (CVDs). Without effective intervention, future increases in the number of older adults will translate to a greater prevalence of CVDs and related disorders. Advancing age increases the risk of CVDs partly via direct effects on the heart and through increases in blood pressure; however, much of the risk is mediated by vascular dysfunction, including large elastic artery stiffening and both macro- and microvascular endothelial dysfunction. Although excessive superoxide-related oxidative stress and chronic low-grade inflammation are the major processes driving cardiovascular aging, the upstream mechanisms involved represent new frontiers of investigation and potential therapeutic targets. Lifestyle practices, including aerobic exercise, energy intake (caloric) restriction, and healthy diet composition, are the most evidence-based strategies (old friends) for optimal cardiovascular aging, but adherence is poor in some groups. Healthy lifestyle “mimicking” approaches, including novel forms of physical training, intermittent fasting paradigms, exercise/healthy diet-inspired nutraceuticals (functional foods and natural supplements), as well as controlled environmental stress exposure (e.g., heat therapy), may hold promise but are unproven. Mitigating the adverse effects of aging on cardiovascular function and health is a high biomedical priority.
Keywords: caloric restriction, energy sensing, mitochondrial dysfunction, nitric oxide
INTRODUCTION
The combination of increasing life expectancy and low birth rates is remodeling the age demographics of both developed and developing nations, resulting in unprecedented numbers of older adults. Meanwhile, cardiovascular diseases (CVDs) remain the leading cause of morbidity (illness) and mortality in most societies, and aging is by far the strongest independent risk factor for CVDs (3). This intersection of age-associated CVD risk and an increasing pipeline of older adults is the key factor driving predictions of a significant rise in future CVD prevalence (17).
In this summary of the opening keynote presentation of the 2017 American Physiological Society Conference on Cardiovascular Aging: New Frontiers and Old Friends, we provide a brief overview of several key issues in the field of cardiovascular aging based largely on our three-plus decades of work in the area. We attempt to emphasize both what has been and continues to be investigated (old friends) as well as emerging areas of investigative interest (new frontiers).
WHY AGING INCREASES THE RISK OF CVDs
The projected increases in age-associated CVDs and the need to establish effective interventions lead to the question of why aging is associated with such a marked increase in CVD risk. The answer is multifactorial. Some of the increase in risk is mediated by the direct effects of aging on the heart, which include left ventricular fibrosis, myocyte apoptosis (and hypertrophy of the remaining myocytes), impaired calcium homeostasis, dysregulation of cardiac stem cells, and blunted β-adrenergic signaling/responsiveness (23). Age-related increases in systolic blood pressure (SBP) are another major factor in the increase in CVD risk observed with aging (3). However, much of the striking effect of age on CVD risk is transduced through the development of vascular dysfunction (22). Several changes to arteries contribute to this “vascular aging” phenotype, but stiffening of the large elastic arteries (aorta and carotid arteries) and diffuse impairments in macro- and microvascular endothelial function, assessed as reduced dilation to endothelium-derived nitric oxide (NO), are among the most important clinically (22). These changes in arterial function also contribute to the progressive rise in SBP with aging (and widening of arterial pulse pressure) as well cardiac dysfunction, including left ventricular hypertrophy and diastolic dysfunction (22, 23).
MECHANISMS OF CARDIOVASCULAR AGING
A complex array of dynamically interacting cellular and molecular mechanisms underlie the effects of aging on cardiovascular function and health. The major established processes involved (old friends) are excessive superoxide-driven oxidative stress and chronic low-grade inflammation featuring increased expression of proinflammatory cytokines (21, 39). These are mutually reinforcing processes, each sustaining and amplifying the other.
Oxidative stress and proinflammatory signaling stimulate collagen deposition in the walls of arteries (fibrosis), cause fragmentation and degradation of elastin filaments, and induce the formation of advanced glycation end products that cross-link structural proteins, all of which lead to stiffening of the heart and large elastic arteries (13, 21–23). Vascular oxidative stress with aging is largely related to elevated superoxide levels, created by excessive superoxide production from dysregulated cellular respiration (4, 44), increased expression and activity of the oxidant enzyme NADPH oxidase (10, 12), and uncoupling of the endothelial isoform of the (normally) NO-producing enzyme endothelial NO synthase (45) in combination with either the absence of an appropriate compensatory upregulation of antioxidant enzymes such as superoxide dismutase (12, 39) or an actual age-related decrease in the expression and/or activity of antioxidant enzymes (14, 16).
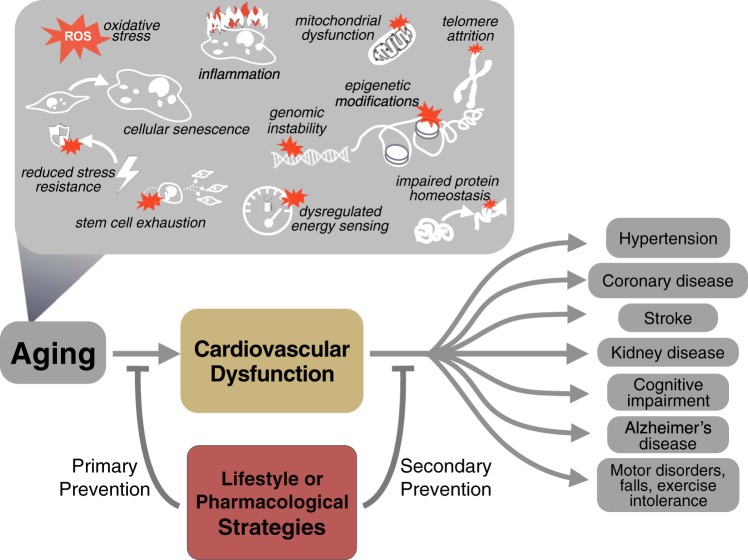
One of the new frontiers of cardiovascular aging research is to establish the upstream, interacting, and/or reinforcing molecular events driving cellular oxidative stress and inflammation. Presently, there is evidence for mitochondrial dysfunction, impaired autophagy/mitophagy, altered signaling of energy-sensing pathways (e.g., 5′-AMP-activated protein kinase and NAD+-dependent sirtuin activation), and sex hormone deficiency playing important roles (40). However, other fundamental mechanisms of biological aging, including cellular senescence, reduced stress resistance, genomic instability, telomere attrition, reduced proteostasis, stem cell dysfunction, and/or epigenetic modifications, along with dysbiosis of the gut and/or oral microbiomes (35, 40), also may contribute. Interestingly, all but one of these putative mechanisms (gut dysbiosis) are among those recently described as “hallmarks of aging,” i.e., fundamental biological processes implicated in the development of cellular aging phenotypes in multiple tissues (27). It is likely that in the future, many if not all of these mechanisms also will be viewed as hallmarks (molecular transducers) of cardiovascular aging (Fig. 1, top).
Fig. 1.
Top: established and putative cellular and molecular mechanisms driving cardiovascular dysfunction and common clinical disorders of aging. Bottom: lifestyle and pharmacological strategies may protect against the development of cardiovascular dysfunction with aging (primary prevention) and/or improve existing dysfunction to slow progression to clinical disorders (secondary prevention).
Presently, most of the work in this area is being conducted on wild-type and genetically modified mice, but new efforts are underway using high-throughput molecular analyses of accessible biological samples from humans that may add complementary translational insights (9). These analyses may also yield novel biomarkers that discriminate individual cardiovascular aging status in a more sensitive manner than chronological age or assessment of conventional CVD risk factors.
STRATEGIES FOR OPTIMAL CARDIOVASCULAR AGING
Strategies for achieving optimal cardiovascular aging may be viewed as lifestyle or pharmacological approaches aimed at primary or secondary prevention of CVDs and other clinical consequences of age-related cardiovascular dysfunction, including chronic kidney disease, cognitive impairment, Alzheimer’s disease, and exercise intolerance (Fig. 1, bottom) (40). Primary prevention efforts focus on slowing/delaying, minimizing, or completely preventing the adverse effects of aging on cardiovascular function, whereas secondary prevention strategies seek to exert the same effects on the progression of age-associated cardiovascular dysfunction to clinically relevant end points. Successful strategies often demonstrate benefits for both primary and secondary prevention.
Healthy Lifestyle Strategies
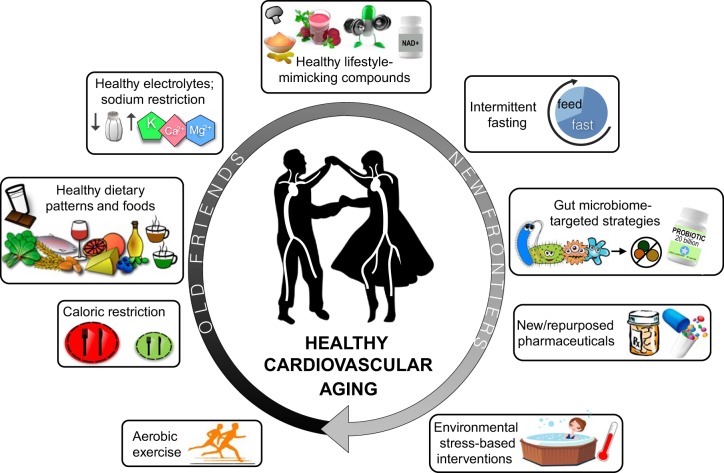
By far, healthy lifestyle strategies have the strongest established evidence for efficacy in the primary and secondary prevention of adverse cardiovascular aging (24, 28, 38, 40, 41) and thus can be considered “old friends” in this context (Fig. 2).
Fig. 2.
Healthy lifestyle- and pharmacological-based approaches for achieving healthy cardiovascular aging, from old friends (the most evidence-based strategies) to new frontiers (promising interventions that remain unproven).
Aerobic exercise.
Among healthy lifestyle factors, regular aerobic exercise has the most extensive evidence base. Habitual aerobic exercise is associated with enhanced cardiac function, lower SBP and large elastic artery stiffness, and greater macro- and microvascular endothelial function during aging compared with less active states and exerts significant therapeutic effects in older animals and humans with existing cardiovascular dysfunction (2, 8, 12, 15, 25, 26, 34, 38). The mechanisms underlying these benefits of aerobic exercise include suppression of oxidative stress and inflammation as well as increased mitochondrial quality control (12, 15, 25, 38). Aerobic exercise also increases resistance to stressors in aging arteries (38), essentially shielding them from a variety of chronic adverse influences, including a Western diet (15, 26) and suboptimal CVD risk factors (8). Novel forms of aerobic exercise such as high-intensity interval training are presently understudied strategies in this area (new frontiers), although continuous, moderate-intensity aerobic exercise remains a highly effective and well-established option (7).
Energy intake (caloric) restriction.
Another highly effective lifestyle strategy for preserving cardiovascular function with aging is reduced energy intake (calorie restriction) without malnutrition (43). In rodents, lifelong caloric restriction prevents age-related cardiac changes, increases in SBP, arterial stiffening, and endothelial dysfunction (1, 11), whereas shorter-term calorie restriction initiated later in life reverses several features of cardiovascular aging (36). Consistent with the latter findings in mice, short-term (12-wk) caloric restriction-based weight loss lowers SBP and improves both large elastic artery stiffness and macro- and microvascular endothelial function in overweight and obese middle-aged and older humans (6, 33). However, sustained caloric restriction is not feasible from a public health perspective due to poor adherence, and the accompanying weight loss and reductions in muscle and bone mass would have negative implications for normal-weight older adults (24, 28, 40). As a result, currently there is great interest in alternatives to chronic caloric restriction involving intermittent fasting (fasting ≥2 days/wk or daily time-restricted feeding) or periodic fasting (fasting several days/month) paradigms (24, 28). However, the feasibility, efficacy, and safety of these approaches remain to be determined in humans and represent new investigative frontiers (24).
Diet composition.
Diet composition also exerts an important influence on cardiovascular aging. There is good evidence that broad healthy dietary patterns featuring unprocessed, plant-based foods, and sources of fat (e.g., olive oil), such as the Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets, as well as general diets that are high in fruit, vegetable, fatty fish, and fiber intake, are associated with better preservation of cardiovascular function and health with aging (24, 32). In preclinical models (rodents and nonhuman primates), “Western” style diets (high in saturated fat and refined sugar, low in fiber) exacerbate the effects of aging on cardiovascular dysfunction (24, 26), but more research is needed in humans.
Individual micronutrients in the diet also appear to influence cardiovascular aging. Greater natural intake of potassium, magnesium, and calcium as well as lower sodium intake in the diet are positively related to cardiovascular health with aging (18, 24, 41); moreover, dietary sodium restriction markedly reduces SBP and improves vascular function in older adults (19). There also is evidence that whey protein, dairy, and cocoa products, dark chocolate, seeds, whole grains, nuts, legumes, and coffee are among the foods associated with favorable cardiovascular aging (24). A future research priority will be to determine if the cardiovascular benefits of established healthy dietary patterns (e.g., DASH diet) are mediated by specific foods within those diets or rely on the interactive (holistic) effects of multiple bioactive components.
Healthy Lifestyle-Mimicking Compounds
There are many well-established barriers to meeting current public health guidelines for physical activity and consuming a healthy diet, including time, motivation, access (to facilities and healthy foods), safety, and expense (31, 37); as a result, adherence to these recommendations is notoriously low, particularly in groups with lower income and education. This situation has driven recent interest in the development of pharmacological compounds intended to “mimic” or recapitulate at least some of the benefits of healthy lifestyle practices (Fig. 2, top). One investigative approach to identifying promising compounds has been to establish the molecular and cellular mechanisms underlying positive effects of exercise and/or healthy diet and then to view these pathways as putative targets for primary and secondary prevention of cardiovascular aging (24, 28). Mitochondria-specific antioxidants, anti-inflammatory agents, NO bioavailability-boosting molecules, autophagy-enhancing compounds, and activators of energy-sensing signaling pathways are among the many mechanisms identified using this approach (28, 40, 41).
Because strategies for healthy cardiovascular aging are aimed at preventing rather than treating clinical disease, much of the focus for testing compounds has centered on potential “nutraceuticals,” i.e., natural food ingredients with bioactive properties that may exert health benefits, including dietary supplements and functional foods, as opposed to prescription pharmaceuticals (24, 40, 41). As summarized in more detail in recent reviews (24, 28), several nutraceutical compounds have been assessed for possible beneficial effects on cardiovascular aging in mice and/or humans. Presently, the strongest translational evidence for beneficial effects on cardiovascular function with aging is associated with ω-3 fish oils and curcumin (antioxidant/endogenous antioxidant-inducing and anti-inflammatory), nitrite/dietary nitrate supplementation (NO-boosting), trehalose administration (autophagy activator/antioxidant), and resveratrol (sirtuin-1 activation); emerging support also exits for mitochondria-specific antioxidants (MitoQ) and NAD+-boosting compounds targeting activation of sirtuin energy-sensing cellular pathways (nicotinamide mononucleotide and nicotinamide riboside) (16, 24, 28, 40, 41). Establishing safety and efficacy in clinical trials, inconsistencies among formulations, and possible negative interactions with healthy lifestyle practices are among the challenges for future research in this area (24, 40, 41).
Other Strategies
Many other strategies for promoting healthy cardiovascular aging hold promise and are presently in the early stages of preclinical or clinical testing (new frontiers; Fig. 2). These include oral formulations targeting gut dysbiosis (prebiotics, probiotics) or gut-derived metabolites [e.g., trimethylamine lyase inhibitors (35, 42)], environmental stress-based interventions [e.g., heat therapy (5)], and repurposing of existing pharmaceuticals/development of new pharmaceuticals aimed at other fundamental mechanisms (hallmarks) of cardiovascular aging [e.g., anti-cell senescence therapies or “senolytics” (20)].
SEX DIFFERENCES IN CARDIOVASCULAR AGING
Sex differences in cardiovascular aging was the focus of another lecture in this American Physiological Society conference. However, it is important to emphasize in the present overview that cardiovascular changes driven by primary aging processes (i.e., hallmarks of cardiovascular aging) are strongly modulated by changes in the production and circulating concentrations of sex hormones as women progress through the premenopausal, perimenopausal, and postmenopausal stages of life (29). Moreover, it is crucial that putative strategies for enhancing cardiovascular health throughout the lifespan be rigorously investigated in both men and women because of potential sex differences in responsiveness to specific interventions (30).
CONCLUSIONS
Cardiovascular dysfunction not only plays a central role in the marked age-associated increase in CVD risk but also in the etiology of many other common disorders of aging. As such, identifying the key biological mechanisms driving cardiovascular aging (therapeutic targets) and establishing strong translational evidence for safe, feasible, and efficacious strategies for preventing/treating cardiovascular dysfunction with advancing age should be among our highest biomedical research priorities.
GRANTS
This research was supported by National Institutes of Health Grants AG-013038 (MERIT), HL-134887, AG-049451, AG-053009, HL-007822, AG-000279, HL-107120, HL-107105, AG-042795, AG-006537, and RR-000051/TR-001082.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.R.S., V.E.B., and M.J.R. prepared figures; D.R.S., V.E.B., and M.J.R. drafted manuscript; D.R.S., V.E.B., and M.J.R. edited and revised manuscript; D.R.S., V.E.B., and M.J.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Erzsebet Nagy and Dr. Thomas LaRocca for contributions to the figures.
REFERENCES
- 1.Ahmet I, Tae HJ, de Cabo R, Lakatta EG, Talan MI. Effects of calorie restriction on cardioprotection and cardiovascular health. J Mol Cell Cardiol 51: 263–271, 2011. doi: 10.1016/j.yjmcc.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation 110: 1799–1805, 2004. doi: 10.1161/01.CIR.0000142863.71285.74. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 135: e146–e603, 2017. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown KA, Didion SP, Andresen JJ, Faraci FM. Effect of aging, MnSOD deficiency, and genetic background on endothelial function: evidence for MnSOD haploinsufficiency. Arterioscler Thromb Vasc Biol 27: 1941–1946, 2007. doi: 10.1161/ATVBAHA.107.146852. [DOI] [PubMed] [Google Scholar]
- 5.Brunt VE, Howard MJ, Francisco MA, Ely BR, Minson CT. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J Physiol 594: 5329–5342, 2016. doi: 10.1113/JP272453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dengo AL, Dennis EA, Orr JS, Marinik EL, Ehrlich E, Davy BM, Davy KP. Arterial destiffening with weight loss in overweight and obese middle-aged and older adults. Hypertension 55: 855–861, 2010. doi: 10.1161/HYPERTENSIONAHA.109.147850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. doi: 10.1161/01.CIR.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 8.DeVan AE, Eskurza I, Pierce GL, Walker AE, Jablonski KL, Kaplon RE, Seals DR. Regular aerobic exercise protects against impaired fasting plasma glucose-associated vascular endothelial dysfunction with aging. Clin Sci (Lond) 124: 325–331, 2013. doi: 10.1042/CS20120291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeVan AE, Johnson LC, Brooks FA, Evans TD, Justice JN, Cruickshank-Quinn C, Reisdorph N, Bryan NS, McQueen MB, Santos-Parker JR, Chonchol MB, Bassett CJ, Sindler AL, Giordano T, Seals DR. Effects of sodium nitrite supplementation on vascular function and related small metabolite signatures in middle-aged and older adults. J Appl Physiol 120: 416–425, 2016. doi: 10.1152/japplphysiol.00879.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100: 1659–1666, 2007. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 11.Donato AJ, Walker AE, Magerko KA, Bramwell RC, Black AD, Henson GD, Lawson BR, Lesniewski LA, Seals DR. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell 12: 772–783, 2013. doi: 10.1111/acel.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol 587: 3271–3285, 2009. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleenor BS. Large elastic artery stiffness with aging: novel translational mechanisms and interventions. Aging Dis 4: 76–83, 2012. [PMC free article] [PubMed] [Google Scholar]
- 14.Fleenor BS, Seals DR, Zigler ML, Sindler AL. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell 11: 269–276, 2012. doi: 10.1111/j.1474-9726.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gioscia-Ryan RA, Battson ML, Cuevas LM, Zigler MC, Sindler AL, Seals DR. Voluntary aerobic exercise increases arterial resilience and mitochondrial health with aging in mice. Aging (Albany NY) 8: 2897–2914, 2016. doi: 10.18632/aging.101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol 592: 2549–2561, 2014. doi: 10.1113/jphysiol.2013.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research . Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123: 933–944, 2011. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 18.Jablonski KL, Gates PE, Pierce GL, Seals DR. Low dietary sodium intake is associated with enhanced vascular endothelial function in middle-aged and older adults with elevated systolic blood pressure. Ther Adv Cardiovasc Dis 3: 347–356, 2009. doi: 10.1177/1753944709345790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol 61: 335–343, 2013. doi: 10.1016/j.jacc.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ, Robbins PD. The clinical potential of senolytic drugs. J Am Geriatr Soc 65: 2297–2301, 2017. doi: 10.1111/jgs.14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation 107: 490–497, 2003. doi: 10.1161/01.CIR.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 22.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 23.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation 107: 346–354, 2003. doi: 10.1161/01.CIR.0000048893.62841.F7. [DOI] [PubMed] [Google Scholar]
- 24.LaRocca TJ, Martens CR, Seals DR. Nutrition and other lifestyle influences on arterial aging. Ageing Res Rev 39: 106–119, 2017. doi: 10.1016/j.arr.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ, Seals DR. Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol 301: H1025–H1032, 2011. doi: 10.1152/ajpheart.01276.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lesniewski LA, Zigler ML, Durrant JR, Nowlan MJ, Folian BJ, Donato AJ, Seals DR. Aging compounds western diet-associated large artery endothelial dysfunction in mice: prevention by voluntary aerobic exercise. Exp Gerontol 48: 1218–1225, 2013. doi: 10.1016/j.exger.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 153: 1194–1217, 2013. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martens CR, Seals DR. Practical alternatives to chronic caloric restriction for optimizing vascular function with ageing. J Physiol 594: 7177–7195, 2016. doi: 10.1113/JP272348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreau KL, Hildreth KL. Vascular aging across the menopause transition in healthy women. Adv Vasc Med 2014: 204390, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 98: 4507–4515, 2013. doi: 10.1210/jc.2013-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morland K, Wing S, Diez Roux A. The contextual effect of the local food environment on residents’ diets: the atherosclerosis risk in communities study. Am J Public Health 92: 1761–1767, 2002. doi: 10.2105/AJPH.92.11.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation 133: 187–225, 2016. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension 52: 72–79, 2008. doi: 10.1161/HYPERTENSIONAHA.108.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci (Lond) 120: 13–23, 2011. doi: 10.1042/CS20100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raizada MK, Joe B, Bryan NS, Chang EB, Dewhirst FE, Borisy GG, Galis ZS, Henderson W, Jose PA, Ketchum CJ, Lampe JW, Pepine CJ, Pluznick JL, Raj D, Seals DR, Gioscia-Ryan RA, Tang WHW, Oh YS. Report of the National Heart, Lung, and Blood Institute Working Group on the role of microbiota in blood pressure regulation: current status and future directions. Hypertension. In press. doi: 10.1161/HYPERTENSIONAHA.117.09699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell 9: 304–312, 2010. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sallis JF, Hovell MF. Determinants of exercise behavior. Exerc Sport Sci Rev 18: 307–330, 1990. doi: 10.1249/00003677-199001000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Seals DR. Edward F. Adolph Distinguished Lecture: the remarkable anti-aging effects of aerobic exercise on systemic arteries. J Appl Physiol 117: 425–439, 2014. doi: 10.1152/japplphysiol.00362.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 120: 357–375, 2011. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seals DR, Justice JN, LaRocca TJ. Physiological geroscience: targeting function to increase healthspan and achieve optimal longevity. J Physiol 594: 2001–2024, 2016. doi: 10.1113/jphysiol.2014.282665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seals DR, Kaplon RE, Gioscia-Ryan RA, LaRocca TJ. You’re only as old as your arteries: translational strategies for preserving vascular endothelial function with aging. Physiology (Bethesda) 29: 250–264, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 163: 1585–1595, 2015. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss EP, Fontana L. Caloric restriction: powerful protection for the aging heart and vasculature. Am J Physiol Heart Circ Physiol 301: H1205–H1219, 2011. doi: 10.1152/ajpheart.00685.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wenzel P, Schuhmacher S, Kienhöfer J, Müller J, Hortmann M, Oelze M, Schulz E, Treiber N, Kawamoto T, Scharffetter-Kochanek K, Münzel T, Bürkle A, Bachschmid MM, Daiber A. Manganese superoxide dismutase and aldehyde dehydrogenase deficiency increase mitochondrial oxidative stress and aggravate age-dependent vascular dysfunction. Cardiovasc Res 80: 280–289, 2008. doi: 10.1093/cvr/cvn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol 297: H1829–H1836, 2009. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]