Abstract
The calibrated application of limited-duration, cyclic, moderately intense hypoxia-reoxygenation increases cardiac resistance to ischemia-reperfusion stress. These intermittent hypoxic conditioning (IHC) programs consistently produce striking reductions in myocardial infarction and ventricular tachyarrhythmias after coronary artery occlusion and reperfusion and, in many cases, improve contractile function and coronary blood flow. These IHC protocols are fundamentally different from those used to simulate sleep apnea, a recognized cardiovascular risk factor. In clinical studies, IHC improved exercise capacity and decreased arrhythmias in patients with coronary artery or pulmonary disease and produced robust, persistent, antihypertensive effects in patients with essential hypertension. The protection afforded by IHC develops gradually and depends on β-adrenergic, δ-opioidergic, and reactive oxygen-nitrogen signaling pathways that use protein kinases and adaptive transcription factors. In summary, adaptation to intermittent hypoxia offers a practical, largely unrecognized means of protecting myocardium from impending ischemia. The myocardial and perhaps broader systemic protection provided by IHC clearly merits further evaluation as a discrete intervention and as a potential complement to conventional pharmaceutical and surgical interventions.
Keywords: enkephalin, glycolysis, mitochondrial permeability transition, myocardial ischemia, nitric oxide, protein kinase, reactive oxygen species, sarcoplasmic reticulum
INTRODUCTION
Western medicine associates “intermittent hypoxia” with the repeated, often severe, arterial desaturation observed in obstructive sleep apnea (OSA). Since OSA is an accepted risk factor for cardiac disease (9, 183), the notion that other forms of intermittent hypoxia are cardioprotective may seem counterintuitive. The original observation that prior ischemia could protect the myocardium during subsequent ischemia may have once seemed equally surprising (133). Now, ischemic preconditioning is an accepted concept, although its practical applications thus far have been limited.
Even before ischemic preconditioning was discovered, Meerson and colleagues (122, 124) demonstrated that weeks of moderate intermittent hypoxic conditioning (IHC) mobilized mechanisms that defended myocardium from acute or prolonged ischemia and prevented life-threatening arrhythmias. Experimental animals similarly exposed to 3 wk of IHC were almost completely protected from infarct during a subsequent coronary occlusion-reperfusion protocol (107, 229). Despite these remarkable observations, the cardioprotective benefits of IHC have generated far less attention than the less practical ischemic preconditioning. IHC has safely been used clinically to protect subjects with developing coronary disease or those awaiting cardiac procedures (27, 46, 58, 178). Moreover, the fact that IHC impacts the entire body may offer important advantages over treatments targeting a single organ. Understanding how IHC achieves these remarkable results should generate new therapeutic approaches and perhaps a broader application, for instance, to reduce postsurgical hospital stays and/or the cognitive impairment associated with cardiac bypass surgery.
This article reviews the literature documenting the direct cardioprotective benefits of IHC on myocardial infarction, cardiac function, arrhythmias, and coronary flow. In addition, evidence of indirect cardioprotective benefits of IHC on hypertension, exercise tolerance, particularly in patients with cardiac or respiratory diseases, and glucose homeostasis in patients with diabetes are considered. After addressing potential mechanisms of IHC-mediated cardioprotection, we conclude with a discussion of apparent impediments to widespread applications of IHC and suggestions for future IHC research. Studies investigating IHC use a variety of related conditioning programs. This article does not address details of individual programs unless these aspects impact the outcomes.
DIRECT CARDIOPROTECTIVE EFFECTS OF IHC
Hypoxia Conditioning Programs
Myriad hypoxia programs have been found to elicit cardioprotection (163). Ascent to altitude, or lowering the pressure in a chamber, produces hypobaric hypoxia, in which Po2 is decreased, whereas the fraction of inspired O2 () approximates 21%. In normobaric hypoxia, is lowered at constant barometric pressure. The daily hypoxia sessions may consist of several hours of continuous exposure to normobaric or hypobaric hypoxia or multiple cycles of several minutes of normobaric hypoxia and reoxygenation. Table 1 categorizes these programs and shows reports exemplifying the cardioprotective capabilities of each.
Table 1.
Representative intermittent hypoxia conditioning programs and outcomes
| Program | Acute Challenge | Outcomes | Reference(s) |
|---|---|---|---|
| Hypobaric hypoxia: one daily bout | |||
| Rat | Occlusion-reperfusion | ↓Infarct, ↓arrhythmias | 81, 121, 140 |
| Rat | Isolated heart: occlusion-reperfusion | ↓Ventricular arrhythmias | 3, 4 |
| Rat | Isolated heart: I/R | ↑Left ventricular function | 23, 141, 193 |
| Rat | Isolated heart: I/R | ↓Infarct | 194 |
| Rat | Isolated mitochondria: anoxia-reoxygenation | ↑Bcl-2/Bax, ↓mitochondrial permeability transition pore | 105 |
| Rat | Isolated heart: I/R | ↓DNA fragmentation | 44 |
| Rat | Isolated heart: Ca2+ paradox | ↑Left ventricular contractile function | 187, 205 |
| Normobaric hypoxia: one daily bout | |||
| Rat | Isolated heart: I/R | ↓Infarct, ↓enzyme release | 214 |
| Normobaric hypoxia: multiple daily cycles | |||
| Mouse | Occlusion-reperfusion | ↓Infarct, ↑Left ventricular function | 127 |
| Rat | Occlusion-reperfusion | ↓Infarct, ↓arrhythmias, ↑endothelial function | 110 |
| Rat | Occlusion-reperfusion | ↓Infarct, ↓contracture | 13 |
| Dog | Occlusion-reperfusion | ↓Infarct, ↓arrhythmias | 49, 107, 229 |
| Human (chronic obstructive pulmonary disease) | Exercise | ↑Exercise capacity | 12, 26, 156 |
| Human (coronary artery disease) | Exercise | ↓Arrhythmias, ↑exercise tolerance | 27, 46, 58, 101, 178 |
| Human (hypertension) | 24-h Blood pressure monitoring | ↓Systolic and diastolic arterial pressure | 100 |
| Human (prediabetic) | Glucose tolerance test; hypoxia | ↑Glycemic control, ↑hypoxia tolerance | 56, 160 |
Representative reports for each intermittent hypoxia program category are shown. Hearts were studied in situ unless otherwise specified. Occlusion-reperfusion, coronary artery occlusion and reperfusion; I/R, global ischemia-reperfusion of isolated, perfused hearts; ↑, increase in the outcome variable; ↓, decrease in the outcome variable.
IHC Reduces Infarct Size
In 1973, Meerson et al. reported in the American Journal of Cardiology (122) a seminal observation that, 2 days after coronary artery ligation, rats adapted to hypobaric hypoxia had 35% less ischemic necrosis and 85% fewer deaths than nonadapted rats. Despite the growing interest in protecting the heart, three decades elapsed before the next reports of IHC-mediated reduction of infarct size (29, 141). Infarcts were decreased from 67% to 50% of the ischemic myocardium, and arrhythmias were attenuated to a similar extent in hypoxia-conditioned versus control rats subjected to coronary artery occlusion-reperfusion (141). Isolated hearts from acutely conditioned mice were subjected to global ischemia-reperfusion (I/R) beginning 30 min or 24 h after IHC (29). Infarct size was ~40% in both unconditioned controls and 30 min after IHC. However, infarcts in animals challenged 24 h afterward were reduced by half, demonstrating that the protective adaptations associated with IHC require time to develop.
In dogs conditioned by a 20-day IHC program, the infarcts resulting from coronary artery occlusion-reperfusion 24 h later were reduced more than 95% (49, 107, 229). When the conditioning program was shortened, no protection was observed after 1 day, but after 10 days infarct sizes were intermediate, approximately one-third those in unconditioned dogs (107). Protection also failed to develop if the reoxygenation intervals were omitted. If β1-adrenergic or δ-opioid receptor antagonists or antioxidants were administered daily before each conditioning session, the protection was abrogated (49, 107). These last outcomes exemplify the consistent observation that IHC uses a spectrum of interdependent signaling pathways.
IHC protocols generally improve outcomes after I/R in rodents but to a degree that can differ among protocols. No reduction in infarct size was observed after 6 wk of IHC hypobaric conditioning at 5,000 m for 6 h/day, but infarcts were reduced when the intensity and daily duration were increased to 7,000 m for 8 h/day (83). Interestingly, a shorter (4 wk), less-intense (5,000 m, 4 h/day) hypobaric program applied to juvenile rats reduced the damage by half (194). This protection was confirmed and extended (192) in adult rats conditioned for programs as short as 1 day. IHC programs consisting of multiple daily hypoxia-reoxygenation cycles, each hypoxia exposure lasting only 2–10 min, also decreased infarcts by ~50% after myocardial I/R in rats (110, 127). The remarkable reduction of infarct size observed in canine hearts (49, 107, 229) exceeded that found in similar studies in small animals. Given the numerous species differences, these observations may indicate that coronary occlusion and reperfusion is a more serious insult for the hypermetabolic rodent heart.
IHC may reduce damage even when applied after the ischemic challenge. Rats were subjected to permanent coronary artery occlusion 1 wk before starting 14- or 28-day IHC. Echocardiography provided evidence for sustained improvements at 14 and 28 days of IHC including reduced left ventricular (LV) dilation, increased LV pressure development including faster contractions and relaxations, decreased cardiomyocyte apoptosis, and increased coronary blood flow (208). Scar volume and fibrosis were reduced 30% and 40%, respectively, at 14 and 28 days of IHC, whereas LV capillary density was increased by ~60% versus non-IHC controls. When IHC was initiated 2 wk after I/R, no benefit was observed (125), suggesting a critical window of opportunity in the postinjury remodeling process. The practical prospect of improving the functional recovery of postinfarct patients would seem to merit immediate attention.
IHC Improves Postischemic Cardiac Mechanical Function
In addition to reducing myocardial damage, IHC consistently improves cardiac function (87, 122, 125). IHC appears to preserve contractile function even in isolated cells. When cardiomyocytes from IHC-programmed rats were ischemically challenged in vitro, they shortened more rapidly and to a greater extent than those from unconditioned rats (31, 226). The preservation or recovery of function was also observed in isolated LV papillary muscles from hypoxia-conditioned rats (191, 220). When Po2 was reduced, the contractile function of muscles from rats conditioned for 28 and 42 days was significantly better preserved than that of the controls or of rats conditioned for 14 days (220). The better contractile function was associated with an extended action potential, suggesting that IHC moderates Ca2+ transport.
Much like isolated cardiac cells and muscle, isolated perfused hearts from IHC mice recovered almost twice the developed pressure during reperfusion as their nonconditioned counterparts (29). Similar results were obtained in hearts from IHC rats (220, 225). After 30 min of zero-flow ischemia, LV dP/dtmax and the heart rate × LV developed pressure product in IHC hearts were again nearly double those of control hearts. IHC hearts also exhibited improved diastolic function evident in a greater LV dP/dtmin and lower LV end-diastolic pressure. While most studies of IHC have been conducted in adult animals, IHC initiated in neonatal rodents demonstrated a similar degree of protection (23, 102, 219). The developing heart was, however, less hypoxia tolerant since the protection observed at 3,000 m was absent at 5,000 m.
IHC Reduces Cardiac Arrhythmias and Fibrillation
Ischemia reduces the fibrillation threshold in rats, yet IHC prevents this shift without altering the resting electrocardiogram (120, 125) and restores the fibrillation threshold when applied after myocardial infarction (182). IHC stabilizes the electrical activity (182) and reduces arrhythmias during acute ischemia as well as during reperfusion (121, 124). These initial electrocardiac findings have been expanded to encompass a spectrum of model systems including human subjects. Patients with arrhythmias sensitive to emotional distress were treated with normobaric IHC for 10 or 20 days (101). Heart rhythm disorders improved progressively in all 23 participants between 10- and 20-day IHC. Premature ventricular complexes ceased in 11 patients, decreased by 75% in 6 patients, and decreased by 50% in the final 6 patients.
Cardiac myocytes isolated from IHC rats display a complex of antiarrhythmic changes (187). IHC at both 5,000 and 7,000 m produced progressive antiarrhythmic effects in intact open-chest animals during ischemia (3). When the same protocols were applied to isolated hearts, a biphasic response was observed analogous to the neonatal studies described earlier; thus, an appreciable antiarrhythmic effect was observed in the 5,000-m group but a proarrhythmic effect in the 7,000-m group. Comparison of the in situ and isolated heart results suggests a beneficial neural or humoral adaptation in situ (4).
A significant antiarrhythmic effect was reported during 15-min ischemia in in situ rat hearts after 21 and 28 days of hypobaric IHC. The arrhythmias were likewise reduced during the subsequent 120-min reperfusion. Perhaps most impressively, the protection persisted for 2 wk (220, 223). The antiarrhythmic effect was potentiated by estrogen, suggesting that sex and sexual maturity should also be considered moderating factors (221).
A marked reduction in cardiac arrhythmias was observed in in situ canine hearts after 20 days of normobaric IHC (107, 229). Although premature ventricular complexes (PVCs) were observed in all nonconditioned and two-thirds of IHC dogs, ventricular tachycardia and fibrillation were observed only in non-IHC dogs. The 80% reduction in reperfusion arrhythmia scores was abolished by daily pretreatment with metoprolol, naltrindole, or N-acetylcysteine before each IHC session (49, 107), suggesting mediation by adrenergic, opioid, and free radical signals. The electrocardiac protection was absent if the hypoxia lacked the intermittent reoxygenations.
IHC Improves Coronary Blood Flow
Mild hypobaric IHC improved LV perfusion in rats (11). However, cardiac output was elevated in these IHC rat hearts as well, making it difficult to distinguish the effects of IHC per se from the functional hyperemic response to increased myocardial metabolic demand. Coronary blood flow was higher in isolated perfused hearts from conditioned rats before and after ischemia, but, as observed above, the higher flow was associated with greater function, making the assignment of cause and effect difficult (42). Coronary blood flow followed a similar pattern in neonatal animals, with higher flow consistently observed after 3,000-m conditioning. However, as observed with other measures of protection in this model, coronary flow declined when hypoxia was increased to 5,000 m (23, 102, 219). Thus, it appears that the developing heart is less adaptable than its adult counterparts.
As described earlier, IHC initiated 7 days postinfarction reduced infarct volume at 21 and 35 days (208). This effect was also accompanied by a sustained increase in coronary flow, but, again, the greater flow correlated with improved function. Closer inspection of the myocardium surrounding the infarcted area identified a 57% increase in capillary density in IHC hearts, providing the first clear structural basis of IHC-mediated enhancement of coronary flow, which may have contributed to the less-extensive fibrotic scars in those hearts.
Angina, a symptom of inadequate coronary blood flow was evaluated in 47 patients with ischemic heart disease (46). After 25 daily normobaric IHC sessions with 10% O2, the frequency of angina declined by half, both in mild disease and in severe disease that had required continued medication. Exercise perfusion imaging is a noninvasive method for assessing myocardial perfusion in human subjects. Six patients were conditioned in a hypobaric chamber at 4,200 m in 14 weekly sessions during the 6 mo after coronary bypass surgery (37). Regional coronary perfusion was improved in all subjects after 6 mo, and, importantly, none of the subjects experienced serious symptomatic distress, including angina, during hypoxia.
In summary, multiple reports have demonstrated that IHC reduces infarct size, improves contractile recovery, and reduces arrhythmias in small and large animal models of I/R. The improved function is associated with increased blood flow and limited evidence of compensatory vascular remodeling. The tissue salvaged varies from ~50% in rodents to near 100% in dogs. The duration of the postischemic improvements is unclear as well as how long the post-IHC protections persist and whether they can be boosted at intervals. One encouraging study demonstrated a lasting reduction in damage when IHC was administered 1 wk after myocardial infarction (208). The animal studies are compelling, and since IHC appears safe, even for patients with coronary disease (27), detailed clinical studies are clearly warranted.
INDIRECT CARDIOPROTECTIVE EFFECTS OF IHC
Antihypertensive Actions of IHC
IHC is therapeutic, whereas OSA is a major risk factor for cardiovascular disease and hypertension (143). Cardiovascular responses to intermittent hypoxia depend on the degree of hypoxia, the duration of individual episodes, their frequency, and the length of sessions whether natural or programmed (51, 52). Each bout of OSA imposes brief, intense hypoxia stress; accordingly, studies of the mechanisms of OSA typically use cyclic intermittent hypoxia-reoxygenation protocols approximating the frequency and intensity of OSA (137, 161). Hypoxia periods modeling OSA are typically brief (e.g., 30–60 s), sufficiently intense ( < 0.08) to produce appreciable arterial O2 desaturation during the brief hypoxia exposures, and numerous, i.e., 30–120 exposures/h for 8–12 h/day (161). In contrast, cardioprotective IHC programs use either one daily prolonged (e.g., 4–8 h) bout or a limited number (e.g., 5–10/day) of shorter (e.g. 4–10 min) bouts of moderately intense (e.g. : 0.10–0.12) hypoxia.
These programs produce strikingly disparate blood pressure responses (161). Both IHC and OSA increase sympathetic activity (107, 115), but that associated with OSA persists during wakefulness and results in hypertension (35, 36, 94, 131, 161, 199). While intermittent hypoxia modeling OSA typically increases systemic arterial pressure (136, 201), cardioprotective IHC reduces blood pressure in rodents (146) and patients with hypertension (78, 100, 132, 186). Clinically hypertensive patients exposed to a 20-day normobaric IHC program had their arterial pressure restored to near normal, with the majority remaining normotensive 3 mo later without the assistance of antihypertensive medication (100). IHC does not raise arterial pressure during hypoxic intervals in healthy human subjects (97). Similarly, the arterial blood pressure in patients with mild chronic obstructive pulmonary disease was unaffected during the hypoxic intervals of a 3-wk IHC program (51). The hypoxia associated with IHC did produce a modest 4–6 beat/min increase in heart rate.
IHC Improves Exercise Tolerance in Patients with Cardiac or Respiratory Disease
IHC improved exercise tolerance and increased exercise threshold power by 25% in patients with ischemic heart disease and stable angina after myocardial infarction (46). The frequency of angina and spontaneous arrhythmias were both reduced. A similar increase in exercise tolerance was reported in patients with chronic obstructive pulmonary disease (26). These subjects had more hemoglobin, reached the anaerobic threshold later, and produced less lactate, suggesting improved O2 delivery or O2 utilization efficiency.
After a program of alternating IHC and hyperoxia, patients with stable coronary disease had lower blood pressure, increased ejection fraction, less angina, better glycemic control, and better exercise tolerance. The protocol was well tolerated, and patients related a better quality of life with less anxiety and depression (58, 178). Many of the benefits remained in force 1 mo later. Other studies in elderly subjects again found the protocol well tolerated with improved exercise performance and better cognition (12, 156). When IHC was administered before coronary artery bypass surgery, the combined IHC-hyperoxia protocol reduced circulating troponin and lactate concentrations, common measures of myocardial distress (181).
IHC Produces a Spectrum of Benefits in Patients With Diabetes
IHC preserved islet cell function and insulin secretion in chemically induced diabetes (85). The common diabetic sequelae of oxidative stress, declining nitric oxide (NO), endothelial dysfunction, and myocardial and vascular damage were also moderated (64, 149). In the insulin-resistant type 2 model of diabetes, IHC improved glucose tolerance, insulin signaling, and glucose trapping while moderating the gluconeogenesis pathway (179, 180). IHC also exerted an antihypertensive effect in type 2 diabetic rats (50).
IHC improves glycemic control in patients with type 2 diabetes, reducing blood glucose and postprandial glucose tolerance after one session (45). These findings were confirmed with longer IHC protocols in glucose-intolerant subjects (56, 160). Importantly, the improvements in glycemic control persisted and were still apparent 1 mo later. The increases in hypoxia-inducible factor (HIF)-1α paralleled glycemic control. Leone and Lalande (93) suggested that IHC might serve as a therapeutic substitute for exercise in sedentary patients with diabetes and noted the IHC-associated increases in hemoglobin, blood volume, stroke volume, and O2 consumption. In this regard, some (26, 27) but not all (166) human studies have reported increases in hemoglobin after IHC.
IHC Protects the Brain and Kidney
IHC impacts the entire body and can protect other organs in addition to the heart. There is extensive evidence of IHC-induced protection of the brain from ischemic stroke (6, 71, 173) and ethanol intoxication-withdrawal (75, 153) and improved motoneuron function in the hemisected spinal cord (6, 98, 138). IHC also protects the kidneys. In a rat model of type 2 diabetes mellitus, a 4-wk program of daily 6-h hypobaric (5,000 m) exposures decreased albuminuria, glomerular hyperplasia, and renal corpuscle fibrosis and preserved activity of the antioxidant enzyme superoxide dismutase (SOD) (180). A 7-day program of 15 h/day hypobaric hypoxia suppressed renal I/R-induced apoptosis, autophagy, leukocyte infiltration, and superoxide formation of the renal parenchyma while preventing the increased blood urea nitrogen and serum creatinine indicative of renal failure (212). Systemic disorders including heart failure, diabetes mellitus, hypertension, and metabolic syndrome threaten multiple organs. IHC may offer a simple, noninvasive intervention to simultaneously treat multiorgan dysfunction and damage.
CARDIOPROTECTIVE MECHANISMS OF INTERMITTENT HYPOXIA
Research conducted over the last two decades has delineated a complex array of cardioprotective mechanisms mobilized by IHC. The complexity reflects the global nature of the stimulus and the wide selection of protocols, model systems, and target end points for studying and evaluating IHC (Table 1). This section attempts to summarize the potential mechanisms with the most experimental support. Figure 1 shows the relationships between these mechanisms. Figure 2 shows the numerous mediators and effectors of IHC while emphasizing the integrated character of the mechanisms due to the extensive cross-talk between their signaling cascades. Some, but not all, of these mechanisms are in common with those of the extensively studied ischemic preconditioning. Interestingly, nonischemic myocardial hypoxia achieved by regional coronary perfusion with deoxygenated blood (168) or crystalloid (126) was equally cardioprotective as preconditioning ischemia.
Fig. 1.
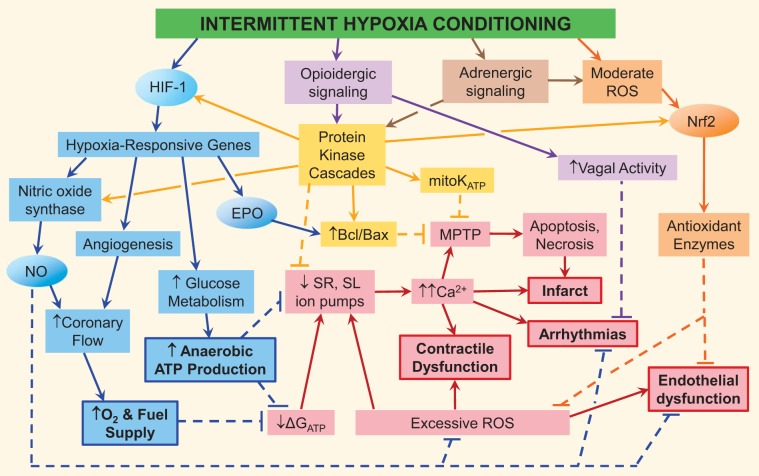
Cardioprotective signaling cascades mobilized by intermittent hypoxia conditioning (IHC). Intermittent hypoxia-induced cardioprotective mechanisms include the hypoxia-inducible factor (HIF)-1-activated gene program (blue), adrenergically induced cardioprotective signaling (brown), ROS induction of the program of genes of nuclear factor erythroid-2 related factor 2 (Nrf2) encoding antioxidant enzymes (orange) and nitric oxide (NO) synthase (NOS; blue), enkephalinergic signaling cascade (purple), and protein kinase activation of mitoprotection, NOS, and cardioprotective genes (yellow). The ischemia-reperfusion (I/R)-triggered injury cascade is shown in red; dashed lines represent the suppression of this cascade by IHC-induced mechanisms. The bold font and borders indicate the principal manifestations of I/R injury and the favorable adaptations produced by the gene program of HIF-1. Ovals represent IHC-inducible transcription factors and cytoprotective molecules. EPO, erythropoietin; ΔGATP, Gibbs free energy of ATP hydrolysis; MPTP, mitochondrial permeability transition pore; mitoKATP, mitochondrial ATP-sensitive K+ channel; SR, sarcoplasmic reticulum; SL, sarcolemma.
Fig. 2.
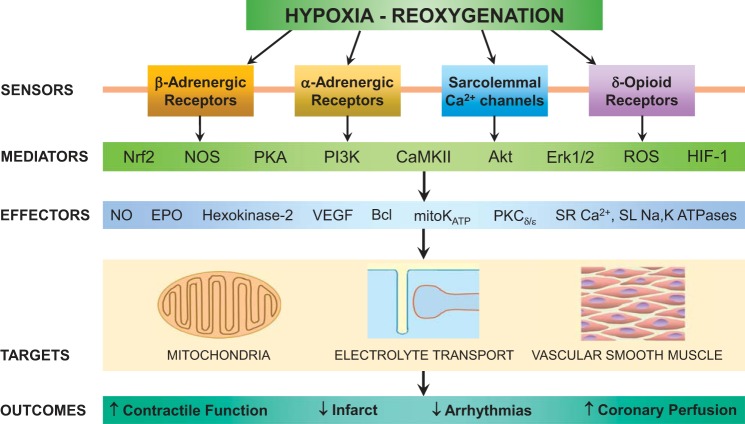
Integrated cardioprotection by intermittent hypoxia conditioning-activated mechanisms. Sarcolemmal receptors and Ca2+ channels respond to neurohumoral signals by mobilizing signaling cascades mediated by protein kinases, transcription factors, nitric oxide (NO) synthase (NOS), and ROS. These cascades mobilize various effectors that suppress ischemia-reperfusion (I/R)-induced injury mechanisms targeting mitochondria, membrane Ca2+, Na+ transport, and coronary artery vasoactivity. Protection of these I/R-vulnerable components culminates in preservation of myocardial and coronary function and the prevention of cardiac rhythm disturbances and irreversible tissue damage. Nrf2, nuclear factor erythroid-2 related factor 2; PI3K, phosphatidylinositol 3-kinase, CaMKII, Ca2+/calmodulin-dependent kinase II; HIF-1, hypoxia-inducible factor 1; EPO, erythropoietin; mitoKATP, mitochondrial ATP-sensitive K+ channel; SR, sarcoplasmic reticulum; SL, sarcolemma.
Enhancement of Intermediary Metabolism and ATP Production
Myocardial ischemia interrupts the ATP synthesis required to sustain contractile function and cardiomyocyte survival (38). However, hypoxic exposures trigger adaptations, including augmented carbohydrate metabolism and mitochondrial respiratory capacity, that increase ATP production and lower ATP demand during subsequent ischemic threats (47). These adjustments pave the way for rapid restoration of myocardial ATP and phosphocreatine after acute anoxia (87) or I/R (193).
Any increase in O2-independent glycolytic capacity should extend the critical window of cellular survival during sustained ischemia. Hypoxia-conditioned cardiomyocytes increase their glycogen stores, which enables them to later delay lethal rigor during ATP-depleting hypoxia (169). In rats, extended daily hypoxia augmented hexokinase and preserved mitochondrial integrity, positioning the animal to function better in low-O2 environments (80, 195). Glycolysis serves as the preferred energy source for intracellular Ca2+ management (20, 207); thus, increased glycolytic enzymes and glycogen may sustain cytosolic Ca2+ transients and sarcoplasmic reticulum (SR) Ca2+ sequestration in hypoxia-conditioned cardiomyocytes (202). Glucose and glycogen are more O2 efficient than other fuels, and the hypoxia-induced metabolic remodeling may increase mechanical efficiency in hypoxia-adapted hearts (123).
Electrolyte Transport and Ca2+ Management
Cardiac contractile function is orchestrated by SR Ca2+ release and extracellular influx during systole and the reuptake and extracellular extrusion during diastole. I/R threatens these systems either by depriving them of ATP or by generating reactive oxygen species (ROS) that disable the transporters and channels (Fig. 1) (67, 76, 228). In this regard, hypoxia conditioning in neonatal rat cardiomyocytes doubled calreticulin, a key SR Ca2+-binding protein, and protected cells from apoptosis and necrosis during severe hypoxia (227). When subjected to an in vitro model of I/R, conditioned cardiomyocytes demonstrated larger systolic Ca2+ transients and more rapid and complete diastolic Ca2+ sequestration versus cardiomyocytes from nonconditioned rats. SR Ca2+ release and sarcolemmal Na+/Ca2+ exchange both functioned better in cardiomyocytes from IHC rats (214). Hypoxia conditioning of intact rats also doubled SR calreticulin and preserved SR Ca2+ uptake during a later left anterior descending coronary artery (LAD) occlusion. This adaptation was abrogated by inhibition of p38 MAPK (202). Preceding hypobaric IHC reduced ischemic contracture and increased LV recovery in parallel with better preserved SR Ca2+-ATPase activity and phospholamban function (205, 215). Also in rats, hypobaric IHC limited Ca2+ overload during myocardial ischemia and hastened the postischemic disposal of excess cytosolic Ca2+ (105, 227). These Ca2+ management adjustments were associated with reduced infarct size after LAD occlusion and reperfusion (214). An IHC program in guinea pigs increased the sarcolemmal Na+-K+-ATPase current density by 52% and its protein content by 76%. Cardiomyocytes from these animals resisted inactivation of the pump current during anoxia-reoxygenation, and the associated improvements in contractile recovery were sensitive to the Na+-K+ pump inhibitor ouabain (62).
In summary, IHC augments SR and sarcolemmal Ca2+ and Na+ transport mechanisms and SR Ca2+ storage capacity. Reinforcing cation homeostasis before I/R presumably limits the subsequent impairment and may serve to minimize intracellular Ca2+ overload and maintain diastolic membrane potential. These adjustments may explain the smaller infarcts, fewer arrhythmias, and improved contractile function observed in the IHC-programmed, postischemic myocardium (Fig. 2).
Mitochondrial Mechanisms of Cardioprotection
Mitochondrial ATP-sensitive K+ (mitoKATP) channels, key mediators of ischemic preconditioning (213), have also been implicated in IHC cardioprotection (Figs. 1 and 2). In dogs subjected to coronary artery occlusion-reperfusion, intracoronary perfusion with hypoxic buffer strikingly decreased the subsequent tissue injury (126), whereas blockade of sarcolemmal KATP and mitoKATP channels blunted the protection. Hearts from IHC rats had significantly fewer arrhythmic PVCs during ischemia than controls, and, again, KATP channel blockade increased arrhythmias in both groups. Pharmacologically opening these same channels decreased arrhythmias only in the control group, suggesting the channels were already open in the IHC group (4). In support of this thesis, the mitoKATP channel antagonist 5-hydroxydecanoate abolished the IHC cardioprotection without expanding infarcts in control rats, whereas two different channel openers decreased infarcts only in controls (141). MCC-134 {1-[4-(H-imidazol-1-yl)benzoyl]-N-methylcyclobutane-carbothioamide}, which inhibits mitoKATP channels but also opens sarcolemmal KATP channels, abolished the antiarrhythmic and anti-infarct protection in IHC rats (82, 83), suggesting differing final pathways for metabolic and electrical protection. In another study, IHC and control hearts were injured by Ca2+-free perfusion and Ca2+ reintroduction. The resulting depression in LV contractile function and subsequent hypercontracture was observed only in control rat hearts. However, the robust preservation of contractile performance by IHC and cardiomyocyte morphology were again abrogated by pharmacological blockade of the mitoKATP channel (204), suggesting a pivotal mitochondrial role in the cardioprotection afforded by IHC against infarct and arrhythmias.
Another mitochondrial channel, the large-conductance Ca2+-activated K+ (BKCa) channel, has been implicated in ischemic preconditioning, where cardioprotection was abrogated by the BKCa inhibitor paxilline (30) and by transgenic ablation of BKCa expression (171). Borchert et al. (21) examined the role of BKCa in cardioprotection afforded by continuous exposure of rats to 10% O2 for 3 wk. Ventricular cardiomyocytes isolated from these rats showed increased tolerance to metabolic inhibition by sodium cyanide, the BKCa antagonist paxilline blocked the hypoxia-induced cytoprotection, and the BKCa activator NS-1619 increased ischemic resistance of cardiomyocytes from nonhypoxic rats. The role of BKCa channels in IHC cardioprotection is not known but merits investigation.
Preventing Mitochondrial Permeability Transition and Apoptosis
ROS and excess Ca2+ open large pores spanning the mitochondrial membranes. The resultant uncontrolled influx of material through these mitochondrial permeability transition pores (MPTP) threatens the cardiomyocyte (Fig. 1) by collapsing the mitochondrial membrane potential (Δψmito), thereby compromising ATP synthesis. The concurrent release of cytochrome c and added ROS may perpetuate the process, culminating in cell death (7). In rats, IHC delayed MPTP opening, cytochrome c release, and the onset of contracture induced by I/R (226), excess Ca2+ (105), and chemical ROS generation (226). From the opposite perspective, opening the MPTP abolished the IHC-mediated protection from ischemic Ca2+ overload (226). In isolated hearts from neonatal rats, IHC protected mitochondria during I/R by preserving KATP channel function and preventing MPTP opening. Drugs that reversed these specific actions blunted the mitochondrial protection (23).
IHC shifts the balance of apoptosis mediators in favor of survival. Hexokinase activity reduces the incorporation of proapoptotic Bax into the outer mitochondrial membrane, restricting MPTP opening and the resultant apoptosis (5, 145). IHC increases the mitochondria-associated hexokinase-2 isoform in parallel with MPTP suppression (195). An intense IHC protocol ameliorated ischemically initiated cardiomyocyte apoptosis (192). Antiapoptotic Bcl-2 content rose fourfold, whereas markers of cellular damage, DNA fragmentation, and lipid peroxidation declined. Similar IHC protocols in mice reduced infarct size in combination with an increase in the survival protein heme oxygenase-1 and a decline in the apoptotic mediator C/EBP homologous protein (CHOP) (127). Hearts isolated from male IHC rats had an elevated prosurvival Bcl-2-to-Bax ratio and, as a result, less postischemic DNA damage (44). Newer studies have suggested that the favorable antiapoptotic Bcl-2-to-Bax ratio is a consistent finding in IHC-programmed hearts (80, 105). Potential hypoxia-induced mediators include decreased proapoptotic caspase-9 and increases in the pro-Bcl transcription factor GATA4 (105, 144).
ROS as Cardioprotectants
Excessive ROS production during I/R is cytotoxic (Fig. 1), but lower concentrations trigger a spectrum of adaptive responses, including redox-sensitive protein kinases and transcription factors that increase myocardial resistance to subsequent, more severe stress (54, 89). Conversely, antioxidant supplementation during IHC interrupts this low-level signaling and can blunt cardioprotection (49, 81).
To interrogate the cardioprotective role of IHC-induced ROS, rats were treated with the antioxidant N-acetylcysteine (81). A decrease in myocardial glutathione/glutathione disulfide during IHC suggested moderately intense ROS formation. IHC alone decreased infarct size by half, but when combined with the ROS scavenger, the infarct increased again. N-acetylcysteine treatment decreased infarct size in the nonhypoxic group such that the infarcts in the two antioxidant-treated groups were now equal. Pretreatment of dogs daily with N-acetylcysteine likewise prevented both anti-infarct and antiarrhythmia cardioprotection afforded by the IHC program (49). In another report (194), another ROS scavenger, mercaptopropionylglycine, administered during reperfusion abolished the cardioprotection. Combined, these observations suggest that ROS signaling is needed for both development and execution of the cardioprotective mechanisms.
When hypoxia in dogs was continuous, lacking periodic reoxygenations, cardioprotection failed to develop, suggesting that the continuous hypoxia eliminated multiple opportunities for low-level ROS signaling (49). Similar studies in rats found that cyclic hypoxia-reoxygenation was protective but that continuous hypoxia was not (14). IHC decreased the duration of ventricular arrhythmias after I/R, whereas continuous hypoxia actually increased arrhythmias (119, 121, 124). Furthermore, 6 h/day of IHC increased postischemic LV contractile recovery versus controls, whereas nearly continuous 23.5 h/day hypoxia worsened LV recovery (216). Cellular DNA damage increased 10-fold, and caspase activity doubled during I/R in hearts from rats conditioned to continuous hypoxia. Simply reoxygenating rats for 60 min each day attenuated the damage, blunted apoptotic caspase activity, improved contractile performance, and reduced infarcts compared with continuous hypoxia (128, 129). In striking opposition, two other rat studies have reported that continuous hypoxia reduced ischemia-induced infarct size and arrhythmias and that 60 min daily of reoxygenation abrogated the protection (77, 139). The reasons for these contradictory effects of once-daily reoxygenation are unclear and merit investigation.
Adrenergic and Opioidergic Signaling
Although protracted hyperadrenergic activity is cytotoxic and a central factor in chronic cardiovascular disease, transitory adrenergic activation by IHC may elicit cardioprotective adaptations (Figs. 1 and 2). Adrenergic receptor function was evaluated in electrically stimulated papillary muscles from IHC-conditioned rats (191). The α1-adrenoceptor agonist phenylephrine produced greater increases in developed tension in muscles from conditioned animals. Contractile force was also better preserved in IHC muscles during simulated ischemia, an effect blunted by the α-adrenoceptor blocker prazosin. In a later study in rats (57), IHC-enhanced recovery of postischemic LV function and reductions in infarct size were mimicked by phenylephrine and abolished by prazosin, by a more selective α1B-adrenoceptor antagonist and by a PKC-ε inhibitor (57). Although IHC strikingly reduced ischemic tissue damage and reperfusion arrhythmias in dogs, the development of this protection was abrogated by daily β1-adrenoceptor blockade with metoprolol (107).
Endogenous opioids have been implicated in a variety of cardioprotective strategies, including aerobic exercise (40, 130), ischemic preconditioning (157, 158) and postconditioning (218), and IHC. Rats conditioned with continuous hypoxia had elevated amounts of a variety of opioid peptides in both the circulation and myocardium (117). In these rats, the final expression of the hypoxia-induced anti-infarct cardioprotection was blocked by acute preischemic injection of several different μ- and δ-selective antagonists, whereas the δ1-antagonist and, less surprisingly, the κ-antagonist were conspicuously ineffective. In dogs, daily δ-opioid receptor blockade before each IHC session prevented the development of myocardial resistance to ischemic infarct and arrhythmias (49). Opioidergic blockade also blunted IHC-mediated increases in parasympathetic transmission and local parasympathetic innervation, suggesting that opioid-mediated cardioprotection may use cholinergic mechanisms (48). Also, IHC increased the content of the neurotrophic monosialoganglioside GM-1, which favors vagotonic δ1-receptors over vagolytic δ2-receptors (91), in cholinergic neurites (48). These conflicting reports indicate the potentially critical differences in the mechanisms responsible for the development of cardioprotection versus those involved in the acute delivery of that protection.
Intermittent Hypoxia Activation of Cardioprotective Gene Expression
Cyclic hypoxia-reoxygenation activates two powerful transcription factors, HIF-1 and nuclear factor erythroid-2 related factor 2 (Nrf2), which promote expression of a spectrum of cytoprotective proteins (Fig. 1). HIF-1 triggers the expression of glycogen-synthesizing and glycolytic enzymes, which foster hypoxemic tolerance, VEGF, which promotes angiogenesis and tissue perfusion, and erythropoietin, which exerts anti-infarct and antiapoptotic effects (24, 63, 147, 164). Recent studies have demonstrated IHC induction of myocardial VEGF (148, 166) and erythropoietin synthesis (18).
Hypoxia activates the HIF-1-driven gene program by dampening prolyl hydroxylation and degradation of the α-subunit of HIF-1 (15). Continuous hypoxia increases myocardial HIF-1α, and, importantly, both erythropoietin and VEGF (170). Hypoxia also promoted a number of Nrf2-responsive genes for key antioxidant enzymes and proteins (170). An IHC program that reduced ischemic infarct size and cellular DNA damage also increased mRNA for myocardial SOD, VEGF, and HIF-1α. HIF-1α and VEGF content were likewise increased along with the pro-survival Bcl-2 (192). Adrenergic activity and ROS may facilitate the HIF-1 gene program by phosphorylating HIF-1α, making it resistant to proteasomal degradation (25). The two signals may be synergistic, with PKA increasing HIF-1α phosphorylation and ROS suppressing its dephosphorylation (19).
The transcription factor Nrf2 activates a gene program encoding an extensive panel of antioxidant enzymes (70, 165, 209) and has been implicated in cardioprotection resulting from ischemic preconditioning (33, 43). Under normoxic conditions, Nrf2 is sequestered, bound to proteins that promote its ubiquitinylation and proteasomal degradation (43, 118). ROS oxidize this complex and release Nrf2, which is then free to promote its portfolio of antioxidant enzymes (10, 43). Like HIF-1α, Nrf2 also is stabilized by phosphorylation and may benefit from IHC-mediated moderation of a variety of protein kinases and phosphatases.
A single 10-min hypoxic challenge reduced cell death in cultured H9c2 rat cardiomyoblasts during simulated I/R the next day. The initial hypoxia triggered Nrf2 translocation to the nucleus, binding to the antioxidant response element and the expression of its antioxidant portfolio during the secondary anoxic challenge 24 h later (70, 209, 210). The protection just described was abrogated when siRNAs were used to interrupt the Nrf2-mediated expression of antioxidant proteins or the related Nrf2 stabilizer DJ-1 (32, 150, 209, 210). Whether DJ-1 responds to IHC or is simply an essential cofactor in the Nrf2 pathway is unclear. In a recent study in rats subjected to four cycles of alternating 4-day hypobaric hypoxia and 4-day recovery to model sojourns at altitude, MnSOD and glutathione peroxidase, antioxidant enzyme products of the gene program of Nrf2, were increased in the LV, in association with increased LV contractile performance (2).
Protein Kinase Signaling Activated by Intermittent Hypoxia
Several protein kinases have been implicated in IHC during both conditioning and the ischemic challenge (Figs. 1 and 2). In two models, cardioprotection was blunted by a Ca2+-calmodulin dependent protein kinase II (CaMKII) inhibitor (204, 205). IHC-evoked improvements in many aspects of Ca2+ management discussed above were likewise abolished by inhibition of PKA, PKC, and/or CaMKII (205, 214, 215). The protection and coincident adjustments in Ca2+ management observed in conditioned neonatal cardiomyocytes were all attenuated by p38 MAPK inhibition delivered before conditioning (202, 227). These results support a mechanism in which IHC improves the capacity for Ca2+ sequestration as a defense against Ca2+ overload.
Protein kinase activation in conditioned hearts is a common theme, with their inhibition abolishing or attenuating protection (13). In some cases, kinase inhibition was effective when applied before IHC (p38 MAPK and ERK1/2) and other cases only when applied before I/R (PKC) (13). These results indicate again that the acquisition of protection and its ultimate execution are distinct processes with different participants and temporal relationships.
PKC is a key participant in Ca2+ homeostasis and hypoxia induces four isoforms, of which one, PKC-δ, initially appears pivotal in cardioprotective signaling (99, 140). IHC altered the intracellular distribution of PKC-δ and both nonselective and PKC-δ-selective inhibitors, administered just before coronary occlusion, attenuated the subsequent infarct reduction. Hypoxia-conditioned rats consuming an n-3 fatty acid-enriched diet had smaller infarcts than unconditioned rats, whereas those consuming an n-6 diet had large infarcts similar to unconditioned rats. PKC-δ was elevated in n-3-treated hearts but not in n-6-treated hearts (66). IHC also facilitated PKC-δ activation and translocation. Attenuating these key adjustments with N-acetylcysteine abrogated the cardioprotection, suggesting again that ROS are involved and at least moderate ROS signaling is required (81). Despite the absence of effect in these studies, PKC-ε may also serve a critical role in IHC cardioprotection. Overexpression of constitutively active PKC-ε improved key aspects of mitochondrial respiration and membrane potential during 2 wk of uninterrupted hypoxic stress (117). Hearts had higher HIF-1α, which was colocalized with active PKC-ε.
The coupled protein kinases phosphatidylinositol-3-kinase (PI3K) and Akt have been implicated in cardioprotection induced by β-adrenergic agonists (155, 211). Activated PKA initiates sequential phosphorylation of PI3K and its Akt substrate. In mice, Akt activation accompanied the reduction in I/R-induced myocardial infarct afforded by 14-day IHC, and the Akt inhibitor wortmannin blunted the protection (127). Similarly, in intermittent hypoxia-conditioned rats, Akt activation was associated with improved postischemic contractile performance, again inhibited by wortmannin (194). Akt phosphorylates several targets, thereby activating endothelial NO synthase (eNOS), promoting PKC-ε translocation to the mitochondrial membrane, and inactivating the MPTP-promoting kinase glycogen synthase kinase (GSK)-3β. When kinase activity was evaluated in a protective IHC program, Akt, PKC-ε, and GSK-3β were all phosphorylated during ischemic challenge, and PKC-ε associated strongly with the membrane fraction (194). In that study, inhibition of PI3K or PKC-ε before the final ischemia abolished the cardioprotective effects of IHC, the phosphorylation of Akt, GSK-3β, and PKC-ε, and the translocation of PKC-ε. In confirmation, ischemia resistance in IHC hearts was abolished by PKC-ε inhibition (57). Cardiomyocytes from conditioned hearts expressed elevated PKC-ε, and the resulting ischemic resistance was attenuated by inhibition of PKC-ε (68). Although hypoxia moderates multiple protein kinases, their role in cardioprotection remains controversial, with the best support for PKC-δ and PKC-ε.
Role of NO in IHC-Mediated Cardioprotection
NO exerts a variety of salutary effects during myocardial I/R (16, 53). Stimulation of NO synthesis, inhalation of gaseous NO, or administration of NO donors reduces infarct size in mice (134), rats (142), rabbits (200), cats (198), and dogs (135). NOS inhibition (69, 200) or suppression of eNOS (73, 177) worsened I/R injury. NO reduces arrhythmogenesis in dogs (184), rats (17), and pigs (188). NO activates cGMP production to mobilize the cardioprotective signaling of PKG (88). As a potent coronary vasodilator, NO may reduce infarct size by increasing collateral inflow from the tissues adjacent to the area at risk (65, 79). NO also limits infiltration by neutrophils, reducing again the opportunity for both occlusive and oxidant damage during I/R (159). Aside from these major influences, NO also activates a variety of antistress (109), antioxidant (39, 56), and antiapoptotic adaptations (104, 197).
IHC increases HIF-1, which promotes expression of all three NOS isoforms, inducible NOS (iNOS) (152), neural NOS (95), and eNOS (34), increasing the capacity for cardiac NO synthesis. Hypoxia increases Ca2+ influx through L-type Ca2+ channels (103). The added Ca2+ activates eNOS (203) and stabilizes HIF-1α (217), thereby sustaining a beneficial feedback loop. IHC improves NO production in a variety of model systems, including cells, blood vessels, muscles, and isolated hearts (72, 90, 174–176). iNOS appears to be the primary source of cardioprotective NO (92) during IHC (41). IHC increased myocardial iNOS expression (61, 84), and inhibition of iNOS abolished the associated cardioprotection (15, 41, 172). Some reports have suggested that eNOS expression is similarly elevated in IHC-exposed animals (8, 90, 167). The direct reduction of nitrite to NO is also enhanced by hypoxia (106).
IHC reduces coronary endothelial dysfunction during I/R (110). That dysfunction arises primarily from oxidative stress (28), which NO may moderate by promoting antioxidant systems (39, 55). Itself a highly soluble antioxidant, NO may scavenge free radicals directly (151), a concept supported by reciprocal changes in NO and ROS in the hypoxic rat heart (170). IHC-mediated NO may also reduce myocardial Ca2+ overload by improving Ca2+ reuptake (108) and by protecting membrane transporters from oxidative stress (206).
Although NO is clearly cardioprotective, its overproduction is detrimental; thus, under select circumstances, both NOS inhibitors and NO donors have proven cardioprotective (111, 196). Excess NO condenses with superoxide to form toxic peroxynitrite, a powerful oxidant and nitrating agent (74). During I/R, excess NO has been attributed to both leukocyte iNOS (190) and to myocardial eNOS activated by excess Ca2+ (222). Modest increases in plasma nitrite and nitrate suggest an equally modest IHC-mediated increase in NO synthesis (111, 114). IHC effectively prevents NO overproduction during I/R (59, 154) and after acute myocardial infarction without reperfusion (114). Moderation of NO synthesis during I/R in conditioned animals is supported by lower myocardial iNOS and eNOS expression (60, 154), lower myocardial peroxynitrite contents during reperfusion (59), enhanced postischemic coronary endothelial function (110), and increased resistance to acute hypotension after myocardial infarction (114).
Despite increasing NO synthesis, IHC can limit NO overproduction by restricting available NOS substrate arginine (224), by chelating Fe2+, a NOS cofactor (1), and by increasing capacitive storage for NO by sequestration. IHC facilitates nontoxic storage by binding NO in S-nitrosothiols and dinitrosyl iron complexes (112, 114) and by increasing NO storage capacity (112, 113, 185). Concordant with this mechanism, administration of NO complexes was cardioprotective during myocardial infarction and cardioplegic arrest (86, 148).
In summary, IHC increases cardiac NO production, which is cardioprotective during myocardial ischemia, primarily by improving coronary endothelial function, coronary perfusion, and perhaps collateral inflow to the area at risk (Figs. 1 and 2). At the same time, IHC increases the capacity to absorb the toxic overflow of NO, e.g., from activated neutrophils converging on the injury during reperfusion.
POTENTIAL CLINICAL APPLICATIONS OF IHC AND IMPEDIMENTS TO THEIR IMPLEMENTATION
As reviewed here, IHC has proved consistently beneficial in a wide variety of preclinical models and has been safely implemented in at-risk patients with hypertension, coronary artery disease, resistant arrhythmias, and lung disease. In Eastern Europe, successful cardioprotective applications of IHC have been recently reported (58, 178). It also should be noted that airline flight and cabin crews experience an occupational form of intermittent hypoxia as frequently as several times daily, ascending to and returning from a barometric equivalent of 2,500 m with each flight. One study reported: “. . . flying personnel have low all-cause mortality. . . mostly due to a reduced cardiovascular mortality reflecting a low (acute myocardial infarction) incidence during the working life as well as after retirement” (96). Despite this favorable evidence, there are no reports adopting IHC in Western clinical practice. The factors limiting the clinical use of IHC merit discussion.
For clinical conditions potentially treatable with IHC, pharmacological therapy is currently available. To what extent IHC could complement or reduce the need for medications must be determined. There are also many practical gaps. Can IHC be made more economical and convenient? What is the optimal IHC regimen? How frequently should IHC be administered, how long do its effects persist, and can it be boosted periodically? Do these parameters differ depending on the condition being treated? Importantly, would funding be available for the rather mundane but critical research required to answer these questions, and to what extent would preclinical findings translate to the clinical setting?
To our knowledge, sex-specific responses to IHC have not been reported. Estrogens have been proposed to afford protection against oxidative stress associated with OSA in premenopausal women (22). Whether women and men would respond differently to IHC, and whether those differences might disappear after menopause, merits investigation. Another topic for future study is the impact of maternal IHC during gestation on fetal development and the long-term consequences for the offspring’s cardiovascular health.
Perhaps the most vital clinical benefit of IHC would be for patients with or at risk of ischemic heart disease. Experimental findings suggest that a prophylactic program might delay the onset of myocardial infarction by the antiarrhythmic action of IHC and by its ability to improve coronary circulatory function. Even in the event of acute myocardial infarction, the antiarrhythmic benefits of prior IHC could be lifesaving. Furthermore, upon coronary reperfusion, prior IHC should lessen dangerous arrhythmias and improve cardiac function. It might even be possible that IHC would limit deleterious postinfarction ventricular remodeling. However, impediments to implementing a prophylactic program of IHC are daunting, including the above-mentioned paucity of practical information necessary for implementing any clinical IHC program. Could patients be persuaded to continue a long-term program of IHC if the benefits had not been proven clinically? Could IHC be safely self-administered unsupervised? In this regard, there are already available a variety of devices for self-administration of IHC to enhance athletic performance (56, 163). Finally, the cost of such a long-term clinical trial would be significant.
Physicians might perceive that exposing heart patients to low O2 is far too risky, yet these are precisely the patient populations who might benefit most. One might expect that supervised pretreatment before elective heart surgery would reduce risk and tissue damage, hasten recovery, shorten hospital stays, and perhaps lessen the postsurgical cognitive impairments. One consistent impediment to clinical applications of IHC is its mistaken link to OSA and associated cardiovascular disorders. In this review, we have attempted to demonstrate that IHC is not only distinct from OSA but that the resulting outcomes are diametrically opposite.
Few studies have examined the pro- versus anti-inflammatory character of moderate IHC. In sedentary men, an 8-wk regimen of daily 60-min exposures to moderate hypoxia (15% O2) dampened intense exercise-induced leukocyte-platelet aggregation and circulating IL-1β but did not alter other pro- and anti-inflammatory cytokines (189). Exposure of healthy young adult men to four cycles of 5-min 10% O2 and 5-min room air breathing decreased circulating TNF-α and IL-4 by over 90% (162). The comparative impact of moderate IHC versus OSA on systemic inflammation warrants further study.
The growing understanding of the mechanisms of IHC may foster the development of pharmacological interventions to mobilize these mechanisms, yet the complexity of the cardioprotective signaling cascade of IHC likely precludes monotherapy. Conversely, high dosages of antioxidants (49, 81) and antagonists of β-adrenergic (107) and opioid receptors (49, 116) have been shown to abrogate IHC cardioprotection. It is not yet known whether IHC or IHC mimetics would afford cardioprotection in the presence of clinically calibrated dosages of β-blockers, opioid antagonists, and antioxidants.
IHC appears safe, is consistently beneficial, noninvasive, practical, and potentially inexpensive and thus may merit serious consideration as a therapeutic modality. The most powerful argument for applying IHC in clinical settings is the mounting evidence of the safety and efficacy of IHC in patients with chronic illnesses (27, 46, 56, 58, 100, 101, 160, 178). We hope this review will broaden the perspective of researchers, clinicians, and stakeholders to reconsider the clinical potential of IHC.
GRANTS
Work by the authors summarized in this manuscript was funded by research grants from the Netherlands Organization for Scientific Research (047-014-016), the Russian Foundation for Basic Research (03-04-49065, 07-04-00650, 10-04-00980), the Russian Science Foundation (17-15-013418), and the US National Center for Complementary and Alternative Medicine (R21 AT-003598), National Heart, Lung and Blood Institute (R01 HL-064785, R01 HL-071684), and National Institute for Neurological Disorders and Stroke (R01 NS-076975).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.T.M. prepared figures; R.T.M., E.B.M., S.S.R., J.L.C., and H.F.D. drafted manuscript; R.T.M., E.B.M., S.S.R., J.L.C., and H.F.D. edited and revised manuscript; R.T.M., E.B.M., S.S.R., J.L.C., and H.F.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the many outstanding trainees and technical staff who contributed to this research.
REFERENCES
- 1.Abu-Soud HM, Wang J, Rousseau DL, Fukuto JM, Ignarro LJ, Stuehr DJ. Neuronal nitric oxide synthase self-inactivates by forming a ferrous-nitrosyl complex during aerobic catalysis. J Biol Chem 270: 22997–23006, 1995. doi: 10.1074/jbc.270.39.22997. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar M, González-Candia A, Rodríguez J, Carrasco-Pozo C, Cañas D, García-Herrera C, Herrera EA, Castillo RL. Mechanisms of cardiovascular protection associated with intermittent hypobaric hypoxia exposure in a rat model: role of oxidative stress. Int J Mol Sci 19: 366, 2018. doi: 10.3390/ijms19020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asemu G, Neckár J, Szárszoi O, Papousek F, Ostádal B, Kolar F. Effects of adaptation to intermittent high altitude hypoxia on ischemic ventricular arrhythmias in rats. Physiol Res 49: 597–606, 2000. [PubMed] [Google Scholar]
- 4.Asemu G, Papousek F, Ostádal B, Kolár F. Adaptation to high altitude hypoxia protects the rat heart against ischemia-induced arrhythmias. Involvement of mitochondrial KATP channel. J Mol Cell Cardiol 31: 1821–1831, 1999. doi: 10.1006/jmcc.1999.1013. [DOI] [PubMed] [Google Scholar]
- 5.Azoulay-Zohar H, Israelson A, Abu-Hamad S, Shoshan-Barmatz V. In self-defence: hexokinase promotes voltage-dependent anion channel closure and prevents mitochondria-mediated apoptotic cell death. Biochem J 377: 347–355, 2004. doi: 10.1042/bj20031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baillieul S, Chacaroun S, Doutreleau S, Detante O, Pépin JL, Verges S. Hypoxic conditioning and the central nervous system: a new therapeutic opportunity for brain and spinal cord injuries? Exp Biol Med (Maywood) 242: 1198–1206, 2017. doi: 10.1177/1535370217712691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baines CP. The cardiac mitochondrion: nexus of stress. Annu Rev Physiol 72: 61–80, 2010. doi: 10.1146/annurev-physiol-021909-135929. [DOI] [PubMed] [Google Scholar]
- 8.Baker JE, Holman P, Kalyanaraman B, Griffith OW, Pritchard KA Jr. Adaptation to chronic hypoxia confers tolerance to subsequent myocardial ischemia by increased nitric oxide production. Ann N Y Acad Sci 874: 236–253, 1999. doi: 10.1111/j.1749-6632.1999.tb09239.x. [DOI] [PubMed] [Google Scholar]
- 9.Baltzis D, Bakker JP, Patel SR, Veves A. Obstructive sleep apnea and vascular diseases. Compr Physiol 6: 1519–1528, 2016. doi: 10.1002/cphy.c150029. [DOI] [PubMed] [Google Scholar]
- 10.Barančík M, Grešová L, Barteková M, Dovinová I. Nrf2 as a key player of redox regulation in cardiovascular diseases. Physiol Res 65, Suppl 1: S1–S10, 2016. [DOI] [PubMed] [Google Scholar]
- 11.Barta E, Brveník P, Kolesár J, Babusíková F. Resting values of left ventricular work to coronary blood flow ratio in rats exposed to intermittent high altitude hypoxia and swimming. Eur J Appl Physiol Occup Physiol 39: 173–179, 1978. doi: 10.1007/BF00421344. [DOI] [PubMed] [Google Scholar]
- 12.Bayer U, Likar R, Pinter G, Stettner H, Demschar S, Trummer B, Neuwersch S, Glazachev O, Burtscher M. Intermittent hypoxic-hyperoxic training on cognitive performance in geriatric patients. Alzheimers Dement (N Y) 3: 114–122, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Béguin PC, Belaidi E, Godin-Ribuot D, Lévy P, Ribuot C. Intermittent hypoxia-induced delayed cardioprotection is mediated by PKC and triggered by p38 MAP kinase and Erk1/2. J Mol Cell Cardiol 42: 343–351, 2007. doi: 10.1016/j.yjmcc.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Béguin PC, Joyeux-Faure M, Godin-Ribuot D, Lévy P, Ribuot C. Acute intermittent hypoxia improves rat myocardium tolerance to ischemia. J Appl Physiol 99: 1064–1069, 2005. doi: 10.1152/japplphysiol.00056.2005. [DOI] [PubMed] [Google Scholar]
- 15.Belaïdi E, Béguin PC, Ribuot C, Godin-Ribuot D. [Hypoxic preconditioning: role of transcription factor HIF-1alpha]. Ann Cardiol Angeiol (Paris) 55: 70–73, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Bice JS, Jones BR, Chamberlain GR, Baxter GF. Nitric oxide treatments as adjuncts to reperfusion in acute myocardial infarction: a systematic review of experimental and clinical studies. Basic Res Cardiol 111: 23, 2016. doi: 10.1007/s00395-016-0540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilińska M, Maczewski M, Beresewicz A. Donors of nitric oxide mimic effects of ischaemic preconditioning on reperfusion induced arrhythmias in isolated rat heart. Mol Cell Biochem 160–161: 265–271, 1996. doi: 10.1007/BF00240058. [DOI] [PubMed] [Google Scholar]
- 18.Bin-Jaliah I, Ammar HI, Mikhailidis DP, Dallak MA, Al-Hashem FH, Haidara MA, Yassin HZ, Bahnasi AA, Rashed LA, Isenovic ER. Cardiac adaptive responses after hypoxia in an experimental model. Angiology 61: 145–156, 2010. doi: 10.1177/0003319709352486. [DOI] [PubMed] [Google Scholar]
- 19.Biswas S, Gupta MK, Chattopadhyay D, Mukhopadhyay CK. Insulin-induced activation of hypoxia-inducible factor-1 requires generation of reactive oxygen species by NADPH oxidase. Am J Physiol Heart Circ Physiol 292: H758–H766, 2007. doi: 10.1152/ajpheart.00718.2006. [DOI] [PubMed] [Google Scholar]
- 20.Boehm E, Ventura-Clapier R, Mateo P, Lechène P, Veksler V. Glycolysis supports calcium uptake by the sarcoplasmic reticulum in skinned ventricular fibres of mice deficient in mitochondrial and cytosolic creatine kinase. J Mol Cell Cardiol 32: 891–902, 2000. doi: 10.1006/jmcc.2000.1130. [DOI] [PubMed] [Google Scholar]
- 21.Borchert GH, Hlaváčková M, Kolář F. Pharmacological activation of mitochondrial BK(Ca) channels protects isolated cardiomyocytes against simulated reperfusion-induced injury. Exp Biol Med (Maywood) 238: 233–241, 2013. doi: 10.1177/1535370212474596. [DOI] [PubMed] [Google Scholar]
- 22.Boukari R, Laouafa S, Ribon-Demars A, Bairam A, Joseph V. Ovarian steroids act as respiratory stimulant and antioxidant against the causes and consequences of sleep-apnea in women. Respir Physiol Neurobiol 239: 46–54, 2017. doi: 10.1016/j.resp.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Bu HM, Yang CY, Wang ML, Ma HJ, Sun H, Zhang Y. KATP channels and MPTP are involved in the cardioprotection bestowed by chronic intermittent hypobaric hypoxia in the developing rat. J Physiol Sci 65: 367–376, 2015. doi: 10.1007/s12576-015-0376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bullard AJ, Govewalla P, Yellon DM. Erythropoietin protects the myocardium against reperfusion injury in vitro and in vivo. Basic Res Cardiol 100: 397–403, 2005. doi: 10.1007/s00395-005-0537-4. [DOI] [PubMed] [Google Scholar]
- 25.Bullen JW, Tchernyshyov I, Holewinski RJ, DeVine L, Wu F, Venkatraman V, Kass DL, Cole RN, Van Eyk J, Semenza GL. Protein kinase A-dependent phosphorylation stimulates the transcriptional activity of hypoxia-inducible factor 1. Sci Signal 9: ra56, 2016. doi: 10.1126/scisignal.aaf0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burtscher M, Haider T, Domej W, Linser T, Gatterer H, Faulhaber M, Pocecco E, Ehrenburg I, Tkatchuk E, Koch R, Bernardi L. Intermittent hypoxia increases exercise tolerance in patients at risk for or with mild COPD. Respir Physiol Neurobiol 165: 97–103, 2009. doi: 10.1016/j.resp.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Burtscher M, Pachinger O, Ehrenbourg I, Mitterbauer G, Faulhaber M, Pühringer R, Tkatchouk E. Intermittent hypoxia increases exercise tolerance in elderly men with and without coronary artery disease. Int J Cardiol 96: 247–254, 2004. doi: 10.1016/j.ijcard.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 28.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000. doi: 10.1161/01.RES.87.10.840. [DOI] [PubMed] [Google Scholar]
- 29.Cai Z, Manalo DJ, Wei G, Rodriguez ER, Fox-Talbot K, Lu H, Zweier JL, Semenza GL. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation 108: 79–85, 2003. doi: 10.1161/01.CIR.0000078635.89229.8A. [DOI] [PubMed] [Google Scholar]
- 30.Cao CM, Xia Q, Gao Q, Chen M, Wong TM. Calcium-activated potassium channel triggers cardioprotection of ischemic preconditioning. J Pharmacol Exp Ther 312: 644–650, 2005. doi: 10.1124/jpet.104.074476. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Lu XY, Li J, Fu JD, Zhou ZN, Yang HT. Intermittent hypoxia protects cardiomyocytes against ischemia-reperfusion injury-induced alterations in Ca2+ homeostasis and contraction via the sarcoplasmic reticulum and Na+/Ca2+ exchange mechanisms. Am J Physiol Cell Physiol 290: C1221–C1229, 2006. doi: 10.1152/ajpcell.00526.2005. [DOI] [PubMed] [Google Scholar]
- 32.Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci USA 103: 15091–15096, 2006. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cominacini L, Mozzini C, Garbin U, Pasini A, Stranieri C, Solani E, Vallerio P, Tinelli IA, Fratta Pasini A. Endoplasmic reticulum stress and Nrf2 signaling in cardiovascular diseases. Free Radic Biol Med 88: 233–242, 2015. doi: 10.1016/j.freeradbiomed.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 34.Coulet F, Nadaud S, Agrapart M, Soubrier F. Identification of hypoxia-response element in the human endothelial nitric-oxide synthase gene promoter. J Biol Chem 278: 46230–46240, 2003. doi: 10.1074/jbc.M305420200. [DOI] [PubMed] [Google Scholar]
- 35.Cutler MJ, Swift NM, Keller DM, Wasmund WL, Burk JR, Smith ML. Periods of intermittent hypoxic apnea can alter chemoreflex control of sympathetic nerve activity in humans. Am J Physiol Heart Circ Physiol 287: H2054–H2060, 2004. doi: 10.1152/ajpheart.00377.2004. [DOI] [PubMed] [Google Scholar]
- 36.Cutler MJ, Swift NM, Keller DM, Wasmund WL, Smith ML. Hypoxia-mediated prolonged elevation of sympathetic nerve activity after periods of intermittent hypoxic apnea. J Appl Physiol 96: 754–761, 2004. doi: 10.1152/japplphysiol.00506.2003. [DOI] [PubMed] [Google Scholar]
- 37.del Pilar Valle M, García-Godos F, Woolcott OO, Marticorena JM, Rodríguez V, Gutiérrez I, Fernández-Dávila L, Contreras A, Valdivia L, Robles J, Marticorena EA. Improvement of myocardial perfusion in coronary patients after intermittent hypobaric hypoxia. J Nucl Cardiol 13: 69–74, 2006. doi: 10.1016/j.nuclcard.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Depre C, Vanoverschelde JL, Taegtmeyer H. Glucose for the heart. Circulation 99: 578–588, 1999. doi: 10.1161/01.CIR.99.4.578. [DOI] [PubMed] [Google Scholar]
- 39.Dhakshinamoorthy S, Porter AG. Nitric oxide-induced transcriptional up-regulation of protective genes by Nrf2 via the antioxidant response element counteracts apoptosis of neuroblastoma cells. J Biol Chem 279: 20096–20107, 2004. doi: 10.1074/jbc.M312492200. [DOI] [PubMed] [Google Scholar]
- 40.Dickson EW, Hogrefe CP, Ludwig PS, Ackermann LW, Stoll LL, Denning GM. Exercise enhances myocardial ischemic tolerance via an opioid receptor-dependent mechanism. Am J Physiol Heart Circ Physiol 294: H402–H408, 2008. doi: 10.1152/ajpheart.00280.2007. [DOI] [PubMed] [Google Scholar]
- 41.Ding HL, Zhu HF, Dong JW, Zhu WZ, Yang WW, Yang HT, Zhou ZN. Inducible nitric oxide synthase contributes to intermittent hypoxia against ischemia/reperfusion injury. Acta Pharmacol Sin 26: 315–322, 2005. doi: 10.1111/j.1745-7254.2005.00046.x. [DOI] [PubMed] [Google Scholar]
- 42.Ding HL, Zhu HF, Dong JW, Zhu WZ, Zhou ZN. Intermittent hypoxia protects the rat heart against ischemia/reperfusion injury by activating protein kinase C. Life Sci 75: 2587–2603, 2004. doi: 10.1016/j.lfs.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Done AJ, Traustadóttir T. Nrf2 mediates redox adaptations to exercise. Redox Biol 10: 191–199, 2016. doi: 10.1016/j.redox.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong JW, Zhu HF, Zhu WZ, Ding HL, Ma TM, Zhou ZN. Intermittent hypoxia attenuates ischemia/reperfusion induced apoptosis in cardiac myocytes via regulating Bcl-2/Bax expression. Cell Res 13: 385–391, 2003. doi: 10.1038/sj.cr.7290184. [DOI] [PubMed] [Google Scholar]
- 45.Duennwald T, Gatterer H, Groop PH, Burtscher M, Bernardi L. Effects of a single bout of interval hypoxia on cardiorespiratory control and blood glucose in patients with type 2 diabetes. Diabetes Care 36: 2183–2189, 2013. doi: 10.2337/dc12-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ehrenbourg IV, Gorbachenkov AA. Interval hypoxic training: development of an anti-anginal effect in patients with different functional classes of stable angina. Hypox Med J 1: 12–15, 1993. [Google Scholar]
- 47.Essop MF. Cardiac metabolic adaptations in response to chronic hypoxia. J Physiol 584: 715–726, 2007. doi: 10.1113/jphysiol.2007.143511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Estrada JA, Barlow MA, Yoshishige D, Williams AG Jr, Downey HF, Mallet RT, Caffrey JL. δ-Opioid receptors: pivotal role in intermittent hypoxia-augmentation of cardiac parasympathetic control and plasticity. Auton Neurosci 198: 38–49, 2016. doi: 10.1016/j.autneu.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Estrada JA, Williams AG Jr, Sun J, Gonzalez L, Downey HF, Caffrey JL, Mallet RT. δ-Opioid receptor (DOR) signaling and reactive oxygen species (ROS) mediate intermittent hypoxia induced protection of canine myocardium. Basic Res Cardiol 111: 17, 2016. doi: 10.1007/s00395-016-0538-5. [DOI] [PubMed] [Google Scholar]
- 50.Faramoushi M, Amir Sasan R, Sari Sarraf V, Karimi P. Cardiac fibrosis and down regulation of GLUT4 in experimental diabetic cardiomyopathy are ameliorated by chronic exposures to intermittent altitude. J Cardiovasc Thorac Res 8: 26–33, 2016. doi: 10.15171/jcvtr.2016.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faulhaber M, Gatterer H, Haider T, Linser T, Netzer N, Burtscher M. Heart rate and blood pressure responses during hypoxic cycles of a 3-week intermittent hypoxia breathing program in patients at risk for or with mild COPD. Int J Chron Obstruct Pulmon Dis 10: 339–345, 2015. doi: 10.2147/COPD.S75749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Faulhaber M, Gatterer H, Haider T, Patterson C, Burtscher M. Intermittent hypoxia does not affect endurance performance at moderate altitude in well-trained athletes. J Sports Sci 28: 513–519, 2010. doi: 10.1080/02640410903581588. [DOI] [PubMed] [Google Scholar]
- 53.Ferdinandy P, Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Br J Pharmacol 138: 532–543, 2003. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Finkel T. Redox-dependent signal transduction. FEBS Lett 476: 52–54, 2000. doi: 10.1016/S0014-5793(00)01669-0. [DOI] [PubMed] [Google Scholar]
- 55.Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest 105: 1631–1639, 2000. doi: 10.1172/JCI9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fuller NR, Courtney R. A case of remission from pre-diabetes following intermittent hypoxic training. Obes Res Clin Pract 10: 487–491, 2016. doi: 10.1016/j.orcp.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 57.Gao L, Chen L, Lu ZZ, Gao H, Wu L, Chen YX, Zhang CM, Jiang YK, Jing Q, Zhang YY, Yang HT. Activation of α1B-adrenoceptors contributes to intermittent hypobaric hypoxia-improved postischemic myocardial performance via inhibiting MMP-2 activation. Am J Physiol Heart Circ Physiol 306: H1569–H1581, 2014. doi: 10.1152/ajpheart.00772.2013. [DOI] [PubMed] [Google Scholar]
- 58.Glazachev O, Kopylov P, Susta D, Dudnik E, Zagaynaya E. Adaptations following an intermittent hypoxia-hyperoxia training in coronary artery disease patients: a controlled study. Clin Cardiol 40: 370–376, 2017. doi: 10.1002/clc.22670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.goriacheva AV, Belkina LM, Terekhina OL, Dawney HF, Mallet RT, Smirin BV, Smirnova EA, Mashina SI, Manukhina EB. [Role of restricted nitric oxide overproduction in the cardioprotective effect of adaptation to intermittent hypoxia]. Patol Fiziol Eksp Ter 1: 23–28, 2012. [PubMed] [Google Scholar]
- 60.Goryacheva AV, Terekhina OL, Abramochkin DV, Budanova OP, Belkina LM, Smirin BV, Downey HF, Malyshev IY, Manukhina EB. [Effect of adaptation to hypoxia on expression of NO synthase isoforms in rat myocardium]. Patol Fiziol Eksp Ter 59: 73–77, 2015. [PubMed] [Google Scholar]
- 61.Grilli A, De Lutiis MA, Patruno A, Speranza L, Cataldi A, Centurione L, Taccardi AA, Di Napoli P, De Caterina R, Barbacane R, Conti P, Felaco M. Effect of chronic hypoxia on inducible nitric oxide synthase expression in rat myocardial tissue. Exp Biol Med (Maywood) 228: 935–942, 2003. doi: 10.1177/153537020322800809. [DOI] [PubMed] [Google Scholar]
- 62.Guo HC, Guo F, Zhang LN, Zhang R, Chen Q, Li JX, Yin J, Wang YL. Enhancement of Na/K pump activity by chronic intermittent hypobaric hypoxia protected against reperfusion injury. Am J Physiol Heart Circ Physiol 300: H2280–H2287, 2011. doi: 10.1152/ajpheart.01164.2010. [DOI] [PubMed] [Google Scholar]
- 63.Guven Bagla A, Ercan E, Asgun HF, Ickin M, Ercan F, Yavuz O, Bagla S, Kaplan A. Experimental acute myocardial infarction in rats: HIF-1α, caspase-3, erythropoietin and erythropoietin receptor expression and the cardioprotective effects of two different erythropoietin doses. Acta Histochem 115: 658–668, 2013. doi: 10.1016/j.acthis.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 64.Güzel D, Dursun AD, Fıçıcılar H, Tekin D, Tanyeli A, Akat F, Topal Çelikkan F, Sabuncuoğlu B, Baştuğ M. Effect of intermittent hypoxia on the cardiac HIF-1/VEGF pathway in experimental type 1 diabetes mellitus. Anatol J Cardiol 16: 76–83, 2016. doi: 10.5152/akd.2015.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hare JM, Colucci WS. Role of nitric oxide in the regulation of myocardial function. Prog Cardiovasc Dis 38: 155–166, 1995. doi: 10.1016/S0033-0620(05)80004-0. [DOI] [PubMed] [Google Scholar]
- 66.Hlavácková M, Neckár J, Jezková J, Balková P, Stanková B, Nováková O, Kolár F, Novák F. Dietary polyunsaturated fatty acids alter myocardial protein kinase C expression and affect cardioprotection induced by chronic hypoxia. Exp Biol Med (Maywood) 232: 823–832, 2007. [PubMed] [Google Scholar]
- 67.Holmberg SR, Williams AJ. The calcium-release channel from cardiac sarcoplasmic reticulum: function in the failing and acutely ischaemic heart. Basic Res Cardiol 87, Suppl 1: 255–268, 1992. [DOI] [PubMed] [Google Scholar]
- 68.Holzerová K, Hlaváčková M, Žurmanová J, Borchert G, Neckář J, Kolář F, Novák F, Nováková O. Involvement of PKCε in cardioprotection induced by adaptation to chronic continuous hypoxia. Physiol Res 64: 191–201, 2015. [DOI] [PubMed] [Google Scholar]
- 69.Hoshida S, Yamashita N, Igarashi J, Nishida M, Hori M, Kamada T, Kuzuya T, Tada M. Nitric oxide synthase protects the heart against ischemia-reperfusion injury in rabbits. J Pharmacol Exp Ther 274: 413–418, 1995. [PubMed] [Google Scholar]
- 70.Huang XS, Chen HP, Yu HH, Yan YF, Liao ZP, Huang QR. Nrf2-dependent upregulation of antioxidative enzymes: a novel pathway for hypoxic preconditioning-mediated delayed cardioprotection. Mol Cell Biochem 385: 33–41, 2014. doi: 10.1007/s11010-013-1812-6. [DOI] [PubMed] [Google Scholar]
- 71.Jackman KA, Zhou P, Faraco G, Peixoto PM, Coleman C, Voss HU, Pickel V, Manfredi G, Iadecola C. Dichotomous effects of chronic intermittent hypoxia on focal cerebral ischemic injury. Stroke 45: 1460–1467, 2014. doi: 10.1161/STROKEAHA.114.004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang C, Collins P. Inhibition of hypoxia-induced relaxation of rabbit isolated coronary arteries by NG-monomethyl-L-arginine but not glibenclamide. Br J Pharmacol 111: 711–716, 1994. doi: 10.1111/j.1476-5381.1994.tb14795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones SP, Girod WG, Palazzo AJ, Granger DN, Grisham MB, Jourd’Heuil D, Huang PL, Lefer DJ. Myocardial ischemia-reperfusion injury is exacerbated in absence of endothelial cell nitric oxide synthase. Am J Physiol Heart Circ Physiol 276: H1567–H1573, 1999. [DOI] [PubMed] [Google Scholar]
- 74.Jugdutt BI. Nitric oxide and cardioprotection during ischemia-reperfusion. Heart Fail Rev 7: 391–405, 2002. doi: 10.1023/A:1020718619155. [DOI] [PubMed] [Google Scholar]
- 75.Jung ME, Simpkins JW, Wilson AM, Downey HF, Mallet RT. Intermittent hypoxia conditioning prevents behavioral deficit and brain oxidative stress in ethanol-withdrawn rats. J Appl Physiol 105: 510–517, 2008. doi: 10.1152/japplphysiol.90317.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaplan P, Matejovicova M, Herijgers P, Flameng W. Effect of free radical scavengers on myocardial function and Na+,K+-ATPase activity in stunned rabbit myocardium. Scand Cardiovasc J 39: 213–219, 2005. doi: 10.1080/14017430510035871. [DOI] [PubMed] [Google Scholar]
- 77.Kasparova D, Neckar J, Dabrowska L, Novotny J, Mraz J, Kolar F, Zurmanova J. Cardioprotective and nonprotective regimens of chronic hypoxia diversely affect the myocardial antioxidant systems. Physiol Genomics 47: 612–620, 2015. doi: 10.1152/physiolgenomics.00058.2015. [DOI] [PubMed] [Google Scholar]
- 78.Katiukhin VN, Shliakhto EV, Shuĭskaia GA. [Effect of discontinuous high-altitude barotherapy on the hemodynamics in arterial hypertension]. Kardiologiia 19: 107–108, 1979. [PubMed] [Google Scholar]
- 79.Kitakaze M, Node K, Minamino T, Kosaka H, Shinozaki Y, Mori H, Inoue M, Hori M, Kamada T. Role of nitric oxide in regulation of coronary blood flow during myocardial ischemia in dogs. J Am Coll Cardiol 27: 1804–1812, 1996. doi: 10.1016/0735-1097(96)00064-2. [DOI] [PubMed] [Google Scholar]
- 80.Kolar D, Gresikova M, Waskova-Arnostova P, Elsnicova B, Kohutova J, Hornikova D, Vebr P, Neckar J, Blahova T, Kasparova D, Novotny J, Kolar F, Novakova O, Zurmanova JM. Adaptation to chronic continuous hypoxia potentiates Akt/HK2 anti-apoptotic pathway during brief myocardial ischemia/reperfusion insult. Mol Cell Biochem 432: 99–108, 2017. doi: 10.1007/s11010-017-3001-5. [DOI] [PubMed] [Google Scholar]
- 81.Kolář F, Jezková J, Balková P, Breh J, Neckár J, Novák F, Nováková O, Tomásová H, Srbová M, Ost’ádal B, Wilhelm J, Herget J. Role of oxidative stress in PKC-δ upregulation and cardioprotection induced by chronic intermittent hypoxia. Am J Physiol Heart Circ Physiol 292: H224–H230, 2007. doi: 10.1152/ajpheart.00689.2006. [DOI] [PubMed] [Google Scholar]
- 82.Kolár F, Neckár J, Ostádal B. MCC-134, a blocker of mitochondrial and opener of sarcolemmal ATP-sensitive K+ channels, abrogates cardioprotective effects of chronic hypoxia. Physiol Res 54: 467–471, 2005. [PubMed] [Google Scholar]
- 83.Kolar F, Nekar J, Ostadal B, Maslov LN, Stakheev DL, Tayurskaya AS, Lishmanov YB. Role of ATP-sensitive K+-channels in antiarrhythmic and cardioprotective action of adaptation to intermittent hypobaric hypoxia. Bull Exp Biol Med 145: 418–421, 2008. doi: 10.1007/s10517-008-0106-6. [DOI] [PubMed] [Google Scholar]
- 84.Kolář F, Szárszoi O, Neckář J, Pecháňová O, Miková D, Hampl V, Ošťádal B. Role of nitric oxide and reactive oxygen species in reperfusion-induced arrhythmias and cardioprotection in chronically hypoxic rat hearts. Physiol Res 52: 52P, 2003. [Google Scholar]
- 85.Kolesnyk IU, Orestenko IM, Seredenko MM, Abramov AV. [The effect of intermittent hypoxic training on pancreatic endocrine function in animals with diabetes mellitus]. Fiziol Zh 40: 87–95, 1994. [PubMed] [Google Scholar]
- 86.Konorev EA, Tarpey MM, Joseph J, Baker JE, Kalyanaraman B. S-nitrosoglutathione improves functional recovery in the isolated rat heart after cardioplegic ischemic arrest-evidence for a cardioprotective effect of nitric oxide. J Pharmacol Exp Ther 274: 200–206, 1995. [PubMed] [Google Scholar]
- 87.Kopylov IN, Golubeva LI. [Effect of adaptation to periodic hypoxia on the resistance of the indicators of energy metabolism and myocardial contraction in acute anoxia and reoxygenation]. Biull Eksp Biol Med 111: 22–25, 1991. doi: 10.1007/BF00841231. [DOI] [PubMed] [Google Scholar]
- 88.Korkmaz S, Radovits T, Barnucz E, Hirschberg K, Neugebauer P, Loganathan S, Veres G, Páli S, Seidel B, Zöllner S, Karck M, Szabó G. Pharmacological activation of soluble guanylate cyclase protects the heart against ischemic injury. Circulation 120: 677–686, 2009. doi: 10.1161/CIRCULATIONAHA.109.870774. [DOI] [PubMed] [Google Scholar]
- 89.Lander HM. An essential role for free radicals and derived species in signal transduction. FASEB J 11: 118–124, 1997. doi: 10.1096/fasebj.11.2.9039953. [DOI] [PubMed] [Google Scholar]
- 90.La Padula PH, Etchegoyen M, Czerniczyniec A, Piotrkowski B, Arnaiz SL, Milei J, Costa LE. Cardioprotection after acute exposure to simulated high altitude in rats. Role of nitric oxide. Nitric Oxide 73: 52–59, 2018. doi: 10.1016/j.niox.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 91.Ledeen RW, Wu G. The multi-tasked life of GM1 ganglioside, a true factotum of nature. Trends Biochem Sci 40: 407–418, 2015. doi: 10.1016/j.tibs.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 92.Lefer DJ. Induction of HIF-1α and iNOS with siRNA: a novel mechanism for myocardial protection. Circ Res 98: 10–11, 2006. doi: 10.1161/01.RES.0000200398.52220.cc. [DOI] [PubMed] [Google Scholar]
- 93.Leone RJ, Lalande S. Intermittent hypoxia as a means to improve aerobic capacity in type 2 diabetes. Med Hypotheses 100: 59–63, 2017. doi: 10.1016/j.mehy.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 94.Leuenberger UA, Brubaker D, Quraishi SA, Hogeman CS, Imadojemu VA, Gray KS. Effects of intermittent hypoxia on sympathetic activity and blood pressure in humans. Auton Neurosci 121: 87–93, 2005. [Erratum in Auton Neurosci 183: 120, 2014.] 10.1016/j.autneu.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 95.Li G, Zhao Y, Li Y, Lu J. Up-regulation of neuronal nitric oxide synthase expression by cobalt chloride through a HIF-1α mechanism in neuroblastoma cells. Neuromolecular Med 17: 443–453, 2015. doi: 10.1007/s12017-015-8373-7. [DOI] [PubMed] [Google Scholar]
- 96.Linnersjö A, Brodin LÅ, Andersson C, Alfredsson L, Hammar N. Low mortality and myocardial infarction incidence among flying personnel during working career and beyond. Scand J Work Environ Health 37: 219–226, 2011. doi: 10.5271/sjweh.3134. [DOI] [PubMed] [Google Scholar]
- 97.Liu X, Xu D, Hall JR, Ross S, Chen S, Liu H, Mallet RT, Shi X. Enhanced cerebral perfusion during brief exposures to cyclic intermittent hypoxemia. J Appl Physiol 123: 1689–1697, 2017. doi: 10.1152/japplphysiol.00647.2017. [DOI] [PubMed] [Google Scholar]
- 98.Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, Mitchell GS. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci 32: 3591–3600, 2012. doi: 10.1523/JNEUROSCI.2908-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lu MJ, Chen YS, Huang HS, Ma MC. Hypoxic preconditioning protects rat hearts against ischemia-reperfusion injury via the arachidonate12-lipoxygenase/transient receptor potential vanilloid 1 pathway. Basic Res Cardiol 109: 414, 2014. doi: 10.1007/s00395-014-0414-0. [DOI] [PubMed] [Google Scholar]
- 100.Lyamina NP, Lyamina SV, Senchiknin VN, Mallet RT, Downey HF, Manukhina EB. Normobaric hypoxia conditioning reduces blood pressure and normalizes nitric oxide synthesis in patients with arterial hypertension. J Hypertens 29: 2265–2272, 2011. doi: 10.1097/HJH.0b013e32834b5846. [DOI] [PubMed] [Google Scholar]
- 101.Lyamina NP, Pilyavsky BG. Interval hypoxic training in treatment of heart rhythm disorders in patients with neurocirculatory dystonia. Hyp Med J 3: 18–19, 1995. [Google Scholar]
- 102.Ma HJ, Li Q, Ma HJ, Guan Y, Shi M, Yang J, Li DP, Zhang Y. Chronic intermittent hypobaric hypoxia ameliorates ischemia/reperfusion-induced calcium overload in heart via Na/Ca2+ exchanger in developing rats. Cell Physiol Biochem 34: 313–324, 2014. doi: 10.1159/000363001. [DOI] [PubMed] [Google Scholar]
- 103.Macdonald WA, Hool LC. The effect of acute hypoxia on excitability in the heart and the L-type calcium channel as a therapeutic target. Curr Drug Discov Technol 5: 302–311, 2008. doi: 10.2174/157016308786733546. [DOI] [PubMed] [Google Scholar]
- 104.Maejima Y, Adachi S, Ito H, Nobori K, Tamamori-Adachi M, Isobe M. Nitric oxide inhibits ischemia/reperfusion-induced myocardial apoptosis by modulating cyclin A-associated kinase activity. Cardiovasc Res 59: 308–320, 2003. doi: 10.1016/S0008-6363(03)00425-5. [DOI] [PubMed] [Google Scholar]
- 105.Magalhães J, Gonçalves IO, Lumini-Oliveira J, Marques-Aleixo I, Passos E, Rocha-Rodrigues S, Machado NG, Moreira AC, Rizo D, Viscor G, Oliveira PJ, Torrella JR, Ascensão A. Modulation of cardiac mitochondrial permeability transition and apoptotic signaling by endurance training and intermittent hypobaric hypoxia. Int J Cardiol 173: 40–45, 2014. doi: 10.1016/j.ijcard.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 106.Maher AR, Milsom AB, Gunaruwan P, Abozguia K, Ahmed I, Weaver RA, Thomas P, Ashrafian H, Born GV, James PE, Frenneaux MP. Hypoxic modulation of exogenous nitrite-induced vasodilation in humans. Circulation 117: 670–677, 2008. doi: 10.1161/CIRCULATIONAHA.107.719591. [DOI] [PubMed] [Google Scholar]
- 107.Mallet RT, Ryou MG, Williams AG Jr, Howard L, Downey HF. β1-Adrenergic receptor antagonism abrogates cardioprotective effects of intermittent hypoxia. Basic Res Cardiol 101: 436–446, 2006. doi: 10.1007/s00395-006-0599-y. [DOI] [PubMed] [Google Scholar]
- 108.Malyshev IY, Aymasheva NP, Malenyuk EB, Manukhina EB, Khaspekov GL, Mikoyan VD, Kubrina LN, Vanin AF. Nitric oxide increases gene expression of Ca2+-ATPase in myocardial and skeletal muscle sarcoplasmic reticulum: physiological implications. Med Sci Monit 6: 480–485, 2000. [PubMed] [Google Scholar]
- 109.Malyshev IYu, Malugin AV, Manukhina EB, Larionov NP, Malenyuk EB, Malysheva EV, Mikoyan VD, Vanin AF. Is HSP70 involved in nitric oxide-induced protection of the heart? Physiol Res 45: 267–272, 1996. [PubMed] [Google Scholar]
- 110.Manukhina EB, Belkina LM, Terekhina OL, Abramochkin DV, Smirnova EA, Budanova OP, Mallet RT, Downey HF. Normobaric, intermittent hypoxia conditioning is cardio- and vasoprotective in rats. Exp Biol Med (Maywood) 238: 1413–1420, 2013. doi: 10.1177/1535370213508718. [DOI] [PubMed] [Google Scholar]
- 111.Manukhina EB, Downey HF, Mallet RT. Role of nitric oxide in cardiovascular adaptation to intermittent hypoxia. Exp Biol Med (Maywood) 231: 343–365, 2006. doi: 10.1177/153537020623100401. [DOI] [PubMed] [Google Scholar]
- 112.Manukhina EB, Downey HF, Mallet RT, Goryacheva AV, Malyshev IY, Vanin AF. Role of nitric oxide (NO) stores in adaptive defense against NO overproduction. In: Adaptation Biology and Medicine. Cell Adaptations and Challenges, edited by Wang P, Kuo CH, Takeda N, Singal PK. Delhi, India: Narosa, 2011, vol. 6, p. 293–315. [Google Scholar]
- 113.Manukhina EB, Jasti D, Vanin AF, Downey HF. Intermittent hypoxia conditioning prevents endothelial dysfunction and improves nitric oxide storage in spontaneously hypertensive rats. Exp Biol Med (Maywood) 236: 867–873, 2011. doi: 10.1258/ebm.2011.011023. [DOI] [PubMed] [Google Scholar]
- 114.Manukhina EB, Mashina SYu, Smirin BV, Lyamina NP, Senchikhin VN, Vanin AF, Malyshev IYu. Role of nitric oxide in adaptation to hypoxia and adaptive defense. Physiol Res 49: 89–97, 2000. [PubMed] [Google Scholar]
- 115.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365: 1046–1053, 2005. doi: 10.1016/S0140-6736(05)74229-X. [DOI] [PubMed] [Google Scholar]
- 116.Maslov LN, Naryzhnaia NV, Tsibulnikov SY, Kolar F, Zhang Y, Wang H, Gusakova AM, Lishmanov YB. Role of endogenous opioid peptides in the infarct size-limiting effect of adaptation to chronic continuous hypoxia. Life Sci 93: 373–379, 2013. doi: 10.1016/j.lfs.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 117.McCarthy J, Lochner A, Opie LH, Sack MN, Essop MF. PKCε promotes cardiac mitochondrial and metabolic adaptation to chronic hypobaric hypoxia by GSK3β inhibition. J Cell Physiol 226: 2457–2468, 2011. doi: 10.1002/jcp.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem 278: 21592–21600, 2003. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 119.Meerson FZ, Arkhipenko IVb, Rozhitskaia II, Didenko VV, Sazontova TG. [Opposite effects on antioxidant enzymes of adaptation to continuous and intermittent hypoxia]. Bull Exp Biol Med 114: 920–922, 1992. doi: 10.1007/BF00790042. [DOI] [PubMed] [Google Scholar]
- 120.Meerson FZ, Belkina LM, Diusenov SS, Ustinova EE, Shabunina EV. [Prevention of cardiac fibrillation using antioxidants and preliminary adaptation of animals to the effects of stress]. Kardiologiia 25: 29–34, 1985. [PubMed] [Google Scholar]
- 121.Meerson FZ, Beloshitskiĭ PV, Vorontsova EI, Ustinova EE, Rozhitskaia II. [Effect of adaptation to continuous and intermittent hypoxia on heart resistance to ischemic and reperfusion arrhythmias]. Patol Fiziol Eksp Ter: 48–50, 1989. [PubMed] [Google Scholar]
- 122.Meerson FZ, Gomzakov OA, Shimkovich MV. Adaptation to high altitude hypoxia as a factor preventing development of myocardial ischemic necrosis. Am J Cardiol 31: 30–34, 1973. doi: 10.1016/0002-9149(73)90806-0. [DOI] [PubMed] [Google Scholar]
- 123.Meerson FZ, Larionov NP, Makarova EK, Bogomolov AF. [Contractile function and the effectiveness of oxygen utilization by the heart during adaptation to hypoxia]. Kardiologiia 15: 70–78, 1975. [PubMed] [Google Scholar]
- 124.Meerson FZ, Ustinova EE, Manukhina EB. Prevention of cardiac arrhythmias by adaptation to hypoxia: regulatory mechanisms and cardiotropic effect. Biomed Biochim Acta 48: S83–S88, 1989. [PubMed] [Google Scholar]
- 125.Meerson FZ, Ustinova EE, Orlova EH. Prevention and elimination of heart arrhythmias by adaptation to intermittent high altitude hypoxia. Clin Cardiol 10: 783–789, 1987. doi: 10.1002/clc.4960101202. [DOI] [PubMed] [Google Scholar]
- 126.Mei DA, Gross GJ. Evidence for the involvement of the ATP-sensitive potassium channel in a novel model of hypoxic preconditioning in dogs. Cardiovasc Res 30: 222–230, 1995. doi: 10.1016/S0008-6363(95)00023-2. [DOI] [PubMed] [Google Scholar]
- 127.Milano G, Abruzzo PM, Bolotta A, Marini M, Terraneo L, Ravara B, Gorza L, Vitadello M, Burattini S, Curzi D, Falcieri E, von Segesser LK, Samaja M. Impact of the phosphatidylinositide 3-kinase signaling pathway on the cardioprotection induced by intermittent hypoxia. PLoS One 8: e76659, 2013. doi: 10.1371/journal.pone.0076659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Milano G, Bianciardi P, Rochemont V, Vassalli G, von Segesser LK, Corno AF, Guazzi M, Samaja M. Phosphodiesterase-5 inhibition mimics intermittent reoxygenation and improves cardioprotection in the hypoxic myocardium. PLoS One 6: e27910, 2011. [Erratum in PLoS One 7: 2012.] 10.1371/journal.pone.0027910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Milano G, von Segesser LK, Morel S, Joncic A, Bianciardi P, Vassalli G, Samaja M. Phosphorylation of phosphatidylinositol-3-kinase-protein kinase B and extracellular signal-regulated kinases 1/2 mediate reoxygenation-induced cardioprotection during hypoxia. Exp Biol Med (Maywood) 235: 401–410, 2010. doi: 10.1258/ebm.2009.009153. [DOI] [PubMed] [Google Scholar]
- 130.Miller LE, McGinnis GR, Peters BA, Ballmann CG, Nanayakkara G, Amin R, Quindry JC. Involvement of the δ-opioid receptor in exercise-induced cardioprotection. Exp Physiol 100: 410–421, 2015. doi: 10.1113/expphysiol.2014.083436. [DOI] [PubMed] [Google Scholar]
- 131.Morgan BJ, Crabtree DC, Palta M, Skatrud JB. Combined hypoxia and hypercapnia evokes long-lasting sympathetic activation in humans. J Appl Physiol 79: 205–213, 1995. doi: 10.1152/jappl.1995.79.1.205. [DOI] [PubMed] [Google Scholar]
- 132.Mukharliamov FI, Smirnova MI, Bedritskiĭ SA, Liadov KV. [Interval hypoxic training in arterial hypertension]. Vopr Kurortol Fizioter Lech Fiz Kult 2: 5–6, 2006. [PubMed] [Google Scholar]
- 133.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136, 1986. doi: 10.1161/01.CIR.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 134.Nagasaka Y, Fernandez BO, Garcia-Saura MF, Petersen B, Ichinose F, Bloch KD, Feelisch M, Zapol WM. Brief periods of nitric oxide inhalation protect against myocardial ischemia-reperfusion injury. Anesthesiology 109: 675–682, 2008. doi: 10.1097/ALN.0b013e318186316e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nakanishi K, Vinten-Johansen J, Lefer DJ, Zhao Z, Fowler WC III, McGee DS, Johnston WE. Intracoronary l-arginine during reperfusion improves endothelial function and reduces infarct size. Am J Physiol Heart Circ Physiol 263: H1650–H1658, 1992. [DOI] [PubMed] [Google Scholar]
- 136.Nanduri J, Peng YJ, Wang N, Khan SA, Semenza GL, Kumar GK, Prabhakar NR. Epigenetic regulation of redox state mediates persistent cardiorespiratory abnormalities after long-term intermittent hypoxia. J Physiol 595: 63–77, 2017. doi: 10.1113/JP272346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Navarrete-Opazo A, Mitchell GS. Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol 307: R1181–R1197, 2014. doi: 10.1152/ajpregu.00208.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Navarrete-Opazo A, Vinit S, Dougherty BJ, Mitchell GS. Daily acute intermittent hypoxia elicits functional recovery of diaphragm and inspiratory intercostal muscle activity after acute cervical spinal injury. Exp Neurol 266: 1–10, 2015. doi: 10.1016/j.expneurol.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Neckár J, Borchert GH, Hlousková P, Mícová P, Nováková O, Novák F, Hroch M, Papousek F, Ost’ádal B, Kolár F. Brief daily episode of normoxia inhibits cardioprotection conferred by chronic continuous hypoxia. Role of oxidative stress and BKCa channels. Curr Pharm Des 19: 6880–6889, 2013. doi: 10.2174/138161281939131127115154. [DOI] [PubMed] [Google Scholar]
- 140.Neckář J, Marková I, Novák F, Nováková O, Szárszoi O, Ost’ádal B, Kolár F. Increased expression and altered subcellular distribution of PKC-δ in chronically hypoxic rat myocardium: involvement in cardioprotection. Am J Physiol Heart Circ Physiol 288: H1566–H1572, 2005. doi: 10.1152/ajpheart.00586.2004. [DOI] [PubMed] [Google Scholar]
- 141.Neckár J, Szárszoi O, Koten L, Papousek F, Ost’ádal B, Grover GJ, Kolár F. Effects of mitochondrial KATP modulators on cardioprotection induced by chronic high altitude hypoxia in rats. Cardiovasc Res 55: 567–575, 2002. doi: 10.1016/S0008-6363(02)00456-X. [DOI] [PubMed] [Google Scholar]
- 142.Neye N, Enigk F, Shiva S, Habazettl H, Plesnila N, Kuppe H, Gladwin MT, Kuebler WM. Inhalation of NO during myocardial ischemia reduces infarct size and improves cardiac function. Intensive Care Med 38: 1381–1391, 2012. doi: 10.1007/s00134-012-2605-1. [DOI] [PubMed] [Google Scholar]
- 143.Parish JM, Somers VK. Obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc 79: 1036–1046, 2004. doi: 10.4065/79.8.1036. [DOI] [PubMed] [Google Scholar]
- 144.Park AM, Nagase H, Vinod Kumar S, Suzuki YJ. Acute intermittent hypoxia activates myocardial cell survival signaling. Am J Physiol Heart Circ Physiol 292: H751–H757, 2007. doi: 10.1152/ajpheart.01016.2006. [DOI] [PubMed] [Google Scholar]
- 145.Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem 277: 7610–7618, 2002. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- 146.Perim RR, Amorim MR, Bonagamba TLLGH, Machado BH. Previous exposure to chronic intermittent hypoxia blunts the development of one-kidney, one-clip hypertension in rats. Exp Physiol 103: 473–482, 2018. doi: 10.1113/EP086734. [DOI] [PubMed] [Google Scholar]
- 147.Pescador N, Villar D, Cifuentes D, Garcia-Rocha M, Ortiz-Barahona A, Vazquez S, Ordoñez A, Cuevas Y, Saez-Morales D, Garcia-Bermejo ML, Landazuri MO, Guinovart J, del Peso L. Hypoxia promotes glycogen accumulation through hypoxia inducible factor (HIF)-mediated induction of glycogen synthase 1. PLoS One 5: e9644, 2010. doi: 10.1371/journal.pone.0009644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pisarenko OI, Serebriakova LI, Tskitishvili OV, Studneva IM, Vanin AF. [Myocardial infarction remodeling in rats with dinitrosyl iron complex with glutathione]. Ross Fiziol Zh Im I M Sechenova 95: 465–475, 2009. [PubMed] [Google Scholar]
- 149.Prysiazhna OD, Kotsiuruba AV, Talanov SO, Sahach VF. [Normalizing effect of intermittent hypoxic training on the function of endothelium in experimental diabetes mellitus]. Fiziol Zh 53: 3–7, 2007. [PubMed] [Google Scholar]
- 150.Raninga PV, Di Trapani G, Tonissen KF. The multifaceted roles of DJ-1 as an antioxidant. Adv Exp Med Biol 1037: 67–87, 2017. doi: 10.1007/978-981-10-6583-5_6. [DOI] [PubMed] [Google Scholar]
- 151.Ritchie RH, Drummond GR, Sobey CG, De Silva TM, Kemp-Harper BK. The opposing roles of NO and oxidative stress in cardiovascular disease. Pharmacol Res 116: 57–69, 2017. doi: 10.1016/j.phrs.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 152.Robinson MA, Baumgardner JE, Otto CM. Oxygen-dependent regulation of nitric oxide production by inducible nitric oxide synthase. Free Radic Biol Med 51: 1952–1965, 2011. doi: 10.1016/j.freeradbiomed.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 153.Ryou M-G, Mallet RT, Metzger DB, Jung ME. Intermittent hypoxia training blunts cerebrocortical presenilin 1 overexpression and amyloid-β accumulation in ethanol-withdrawn rats. Am J Physiol Regul Integr Comp Physiol 313: R10–R18, 2017. doi: 10.1152/ajpregu.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ryou M-G, Sun J, Oguayo KN, Manukhina EB, Downey HF, Mallet RT. Hypoxic conditioning suppresses nitric oxide production upon myocardial reperfusion. Exp Biol Med (Maywood) 233: 766–774, 2008. doi: 10.3181/0710-RM-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Salie R, Moolman JA, Lochner A. The mechanism of beta-adrenergic preconditioning: roles for adenosine and ROS during triggering and mediation. Basic Res Cardiol 107: 281, 2012. doi: 10.1007/s00395-012-0281-5. [DOI] [PubMed] [Google Scholar]
- 156.Schega L, Peter B, Brigadski T, Leßmann V, Isermann B, Hamacher D, Törpel A. Effect of intermittent normobaric hypoxia on aerobic capacity and cognitive function in older people. J Sci Med Sport 19: 941–945, 2016. doi: 10.1016/j.jsams.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 157.Schultz JE, Rose E, Yao Z, Gross GJ. Evidence for involvement of opioid receptors in ischemic preconditioning in rat hearts. Am J Physiol Heart Circ Physiol 268: H2157–H2161, 1995. [DOI] [PubMed] [Google Scholar]
- 158.Schulz R, Gres P, Heusch G. Role of endogenous opioids in ischemic preconditioning but not in short-term hibernation in pigs. Am J Physiol Heart Circ Physiol 280: H2175–H2181, 2001. doi: 10.1152/ajpheart.2001.280.5.H2175. [DOI] [PubMed] [Google Scholar]
- 159.Seal JB, Gewertz BL. Vascular dysfunction in ischemia-reperfusion injury. Ann Vasc Surg 19: 572–584, 2005. doi: 10.1007/s10016-005-4616-7. [DOI] [PubMed] [Google Scholar]
- 160.Serebrovska TV, Portnychenko AG, Drevytska TI, Portnichenko VI, Xi L, Egorov E, Gavalko AV, Naskalova S, Chizhova V, Shatylo VB. Intermittent hypoxia training in prediabetes patients: Beneficial effects on glucose homeostasis, hypoxia tolerance and gene expression. Exp Biol Med (Maywood) 242: 1542–1552, 2017. doi: 10.1177/1535370217723578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Serebrovskaya TV, Manukhina EB, Smith ML, Downey HF, Mallet RT. Intermittent hypoxia: cause of or therapy for systemic hypertension? Exp Biol Med (Maywood) 233: 627–650, 2008. doi: 10.3181/0710-MR-267. [DOI] [PubMed] [Google Scholar]
- 162.Serebrovskaya TV, Nikolsky IS, Nikolska VV, Mallet RT, Ishchuk VA. Intermittent hypoxia mobilizes hematopoietic progenitors and augments cellular and humoral elements of innate immunity in adult men. High Alt Med Biol 12: 243–252, 2011. doi: 10.1089/ham.2010.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Serebrovskaya TV, Xi L. Intermittent hypoxia training as non-pharmacologic therapy for cardiovascular diseases: Practical analysis on methods and equipment. Exp Biol Med (Maywood) 241: 1708–1723, 2016. doi: 10.1177/1535370216657614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shan X, Xu X, Cao B, Wang Y, Guo L, Zhu Q, Li J, Que L, Chen Q, Ha T, Li C, Li Y. Transcription factor GATA-4 is involved in erythropoietin-induced cardioprotection against myocardial ischemia/reperfusion injury. Int J Cardiol 134: 384–392, 2009. doi: 10.1016/j.ijcard.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 165.Shanmugam G, Narasimhan M, Tamowski S, Darley-Usmar V, Rajasekaran NS. Constitutive activation of Nrf2 induces a stable reductive state in the mouse myocardium. Redox Biol 12: 937–945, 2017. doi: 10.1016/j.redox.2017.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shatilo VB, Korkushko OV, Ischuk VA, Downey HF, Serebrovskaya TV. Effects of intermittent hypoxia training on exercise performance, hemodynamics, and ventilation in healthy senior men. High Alt Med Biol 9: 43–52, 2008. doi: 10.1089/ham.2007.1053. [DOI] [PubMed] [Google Scholar]
- 167.Shi Y, Baker JE, Zhang C, Tweddell JS, Su J, Pritchard KA Jr. Chronic hypoxia increases endothelial nitric oxide synthase generation of nitric oxide by increasing heat shock protein 90 association and serine phosphorylation. Circ Res 91: 300–306, 2002. doi: 10.1161/01.RES.0000031799.12850.1E. [DOI] [PubMed] [Google Scholar]
- 168.Shizukuda Y, Mallet RT, Lee SC, Downey HF. Hypoxic preconditioning of ischaemic canine myocardium. Cardiovasc Res 26: 534–542, 1992. doi: 10.1093/cvr/26.5.534. [DOI] [PubMed] [Google Scholar]
- 169.Silverman HS, Wei S, Haigney MC, Ocampo CJ, Stern MD. Myocyte adaptation to chronic hypoxia and development of tolerance to subsequent acute severe hypoxia. Circ Res 80: 699–707, 1997. doi: 10.1161/01.RES.80.5.699. [DOI] [PubMed] [Google Scholar]
- 170.Singh M, Thomas P, Shukla D, Tulsawani R, Saxena S, Bansal A. Effect of subchronic hypobaric hypoxia on oxidative stress in rat heart. Appl Biochem Biotechnol 169: 2405–2419, 2013. doi: 10.1007/s12010-013-0141-2. [DOI] [PubMed] [Google Scholar]
- 171.Soltysinska E, Bentzen BH, Barthmes M, Hattel H, Thrush AB, Harper ME, Qvortrup K, Larsen FJ, Schiffer TA, Losa-Reyna J, Straubinger J, Kniess A, Thomsen MB, Brüggemann A, Fenske S, Biel M, Ruth P, Wahl-Schott C, Boushel RC, Olesen SP, Lukowski R. KCNMA1 encoded cardiac BK channels afford protection against ischemia-reperfusion injury. PLoS One 9: e103402, 2014. doi: 10.1371/journal.pone.0103402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Southan GJ, Szabó C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol 51: 383–394, 1996. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- 173.Stowe AM, Altay T, Freie AB, Gidday JM. Repetitive hypoxia extends endogenous neurovascular protection for stroke. Ann Neurol 69: 975–985, 2011. doi: 10.1002/ana.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Strijdom H, Chamane N, Lochner A. Nitric oxide in the cardiovascular system: a simple molecule with complex actions. Cardiovasc J Afr 20: 303–310, 2009. [PMC free article] [PubMed] [Google Scholar]
- 175.Strijdom H, Jacobs S, Hattingh S, Page C, Lochner A. Nitric oxide production is higher in rat cardiac microvessel endothelial cells than ventricular cardiomyocytes in baseline and hypoxic conditions: a comparative study. FASEB J 20: 314–316, 2006. doi: 10.1096/fj.05-4225fje. [DOI] [PubMed] [Google Scholar]
- 176.Strijdom H, Muller C, Lochner A. Direct intracellular nitric oxide detection in isolated adult cardiomyocytes: flow cytometric analysis using the fluorescent probe, diaminofluorescein. J Mol Cell Cardiol 37: 897–902, 2004. doi: 10.1016/j.yjmcc.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 177.Sumeray MS, Rees DD, Yellon DM. Infarct size and nitric oxide synthase in murine myocardium. J Mol Cell Cardiol 32: 35–42, 2000. doi: 10.1006/jmcc.1999.1050. [DOI] [PubMed] [Google Scholar]
- 178.Syrkin AL, Glazachev OS, Kopylov FY, Dudnik EN, Zagaynaya EE, Tuter DS. Adaptation to intermittent hypoxia-hyperoxia in the rehabilitation of patients with ischemic heart disease: exercise tolerance and quality of life. Kardiologiia 57: 10–16, 2017. [PubMed] [Google Scholar]
- 179.Tian YM, Guan Y, Li N, Ma HJ, Zhang L, Wang S, Zhang Y. Chronic intermittent hypobaric hypoxia ameliorates diabetic nephropathy through enhancing HIF1 signaling in rats. Diabetes Res Clin Pract 118: 90–97, 2016. doi: 10.1016/j.diabres.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 180.Tian YM, Liu Y, Wang S, Dong Y, Su T, Ma HJ, Zhang Y. Anti-diabetes effect of chronic intermittent hypobaric hypoxia through improving liver insulin resistance in diabetic rats. Life Sci 150: 1–7, 2016. doi: 10.1016/j.lfs.2016.02.053. [DOI] [PubMed] [Google Scholar]
- 181.Tuter T, Kopylov P. Intermittent hypoxia-hyperoxic training as a new method of cardioprotection during coronary artery bypass surgery (Abstract). Eur Heart J 38, Suppl 1: ehx502.P2478, 2017. doi: 10.1093/eurheartj/ehx502.P2478. [DOI] [Google Scholar]
- 182.Ustinova EE, Saltykova VA, Didenko VV, Beloshitskiĭ PV, Meerson FZ. [Effect of adaptation to periodic and continuous hypoxia in disorders of electrical stability of the heart in postinfarction cardiosclerosis]. Biull Eksp Biol Med 105: 636–638, 1988. doi: 10.1007/BF00841515. [DOI] [PubMed] [Google Scholar]
- 183.Uyar M, Davutoglu V. An update on cardiovascular effects of obstructive sleep apnoea syndrome. Postgrad Med J 92: 540–544, 2016. doi: 10.1136/postgradmedj-2016-134093. [DOI] [PubMed] [Google Scholar]
- 184.Vegh A, Szekeres L, Parratt J. Preconditioning of the ischaemic myocardium; involvement of the l-arginine nitric oxide pathway. Br J Pharmacol 107: 648–652, 1992. doi: 10.1111/j.1476-5381.1992.tb14501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vlasova MA, Smirin BV, Pokidyshev DA, Mashina SY, Vanin AF, Malyshev IY, Manukhina EB. Mechanism of adaptation of the vascular system to chronic changes in nitric oxide level in the organism. Bull Exp Biol Med 142: 670–674, 2006. doi: 10.1007/s10517-006-0447-y. [DOI] [PubMed] [Google Scholar]
- 186.Vorob’ev GF, Engelgardt GN. Normo- and hypobaric hypoxytherapy in chronic diseases. Physiother Balneol Rehabil 6: 9–13, 2004. [Google Scholar]
- 187.Vovc E. The antiarrhythmic effect of adaptation to intermittent hypoxia. Folia Med (Plovdiv) 40, Suppl 3: 51–54, 1998. [PubMed] [Google Scholar]
- 188.Wainwright CL, Martorana PA. Pirsidomine, a novel nitric oxide donor, suppresses ischemic arrhythmias in anesthetized pigs. J Cardiovasc Pharmacol 22, Suppl 7: S44–S50, 1993. doi: 10.1097/00005344-199300221-00009. [DOI] [PubMed] [Google Scholar]
- 189.Wang JS, Lin HY, Cheng ML, Wong MK. Chronic intermittent hypoxia modulates eosinophil- and neutrophil-platelet aggregation and inflammatory cytokine secretion caused by strenuous exercise in men. J Appl Physiol 103: 305–314, 2007. doi: 10.1152/japplphysiol.00226.2007. [DOI] [PubMed] [Google Scholar]
- 190.Wang XL, Liu HR, Tao L, Liang F, Yan L, Zhao RR, Lopez BL, Christopher TA, Ma XL. Role of iNOS-derived reactive nitrogen species and resultant nitrative stress in leukocytes-induced cardiomyocyte apoptosis after myocardial ischemia/reperfusion. Apoptosis 12: 1209–1217, 2007. doi: 10.1007/s10495-007-0055-y. [DOI] [PubMed] [Google Scholar]
- 191.Wang YP, Cui F, Zhang LP, Yang CY, Guan Y, Zhou ZN, Zhang Y. Effect of chronic intermittent hypobaric hypoxia on α(1)-adrenergic receptor of myocardium participates in the cardioprotection. Sheng Li Xue Bao 61: 21–26, 2009. [PubMed] [Google Scholar]
- 192.Wang Z, Si LY. Hypoxia-inducible factor-1α and vascular endothelial growth factor in the cardioprotective effects of intermittent hypoxia in rats. Ups J Med Sci 118: 65–74, 2013. doi: 10.3109/03009734.2013.766914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang ZH, Cai XL, Wu L, Yu Z, Liu JL, Zhou ZN, Liu J, Yang HT. Mitochondrial energy metabolism plays a critical role in the cardioprotection afforded by intermittent hypobaric hypoxia. Exp Physiol 97: 1105–1118, 2012. doi: 10.1113/expphysiol.2012.065102. [DOI] [PubMed] [Google Scholar]
- 194.Wang ZH, Chen YX, Zhang CM, Wu L, Yu Z, Cai XL, Guan Y, Zhou ZN, Yang HT. Intermittent hypobaric hypoxia improves postischemic recovery of myocardial contractile function via redox signaling during early reperfusion. Am J Physiol Heart Circ Physiol 301: H1695–H1705, 2011. doi: 10.1152/ajpheart.00276.2011. [DOI] [PubMed] [Google Scholar]
- 195.Waskova-Arnostova P, Elsnicova B, Kasparova D, Hornikova D, Kolar F, Novotny J, Zurmanova J. Cardioprotective adaptation of rats to intermittent hypobaric hypoxia is accompanied by the increased association of hexokinase with mitochondria. J Appl Physiol 119: 1487–1493, 2015. doi: 10.1152/japplphysiol.01035.2014. [DOI] [PubMed] [Google Scholar]
- 196.Weerateerangkul P, Chattipakorn S, Chattipakorn N. Roles of the nitric oxide signaling pathway in cardiac ischemic preconditioning against myocardial ischemia-reperfusion injury. Med Sci Monit 17: RA44–RA52, 2011. doi: 10.12659/MSM.881385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Weiland U, Haendeler J, Ihling C, Albus U, Scholz W, Ruetten H, Zeiher AM, Dimmeler S. Inhibition of endogenous nitric oxide synthase potentiates ischemia-reperfusion-induced myocardial apoptosis via a caspase-3 dependent pathway. Cardiovasc Res 45: 671–678, 2000. doi: 10.1016/S0008-6363(99)00347-8. [DOI] [PubMed] [Google Scholar]
- 198.Weyrich AS, Ma XL, Lefer AM. The role of l-arginine in ameliorating reperfusion injury after myocardial ischemia in the cat. Circulation 86: 279–288, 1992. doi: 10.1161/01.CIR.86.1.279. [DOI] [PubMed] [Google Scholar]
- 199.White SG, Fletcher EC, Miller CC III. Acute systemic blood pressure elevation in obstructive and nonobstructive breath hold in primates. J Appl Physiol 79: 324–330, 1995. doi: 10.1152/jappl.1995.79.1.324. [DOI] [PubMed] [Google Scholar]
- 200.Williams MW, Taft CS, Ramnauth S, Zhao ZQ, Vinten-Johansen J. Endogenous nitric oxide (NO) protects against ischaemia-reperfusion injury in the rabbit. Cardiovasc Res 30: 79–86, 1995. doi: 10.1016/S0008-6363(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 201.Wu Q, Cunningham JT, Mifflin S. Transcription factor ΔFosB acts within the nucleus of the solitary tract to increase mean arterial pressure during exposures to intermittent hypoxia. Am J Physiol Heart Circ Physiol 314: H270–H277, 2018. doi: 10.1152/ajpheart.00268.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wu X, Liu X, Zhu X, Tang C. Hypoxic preconditioning induces delayed cardioprotection through p38 MAPK-mediated calreticulin upregulation. Shock 27: 572–577, 2007. doi: 10.1097/01.shk.0000246901.58068.a8. [DOI] [PubMed] [Google Scholar]
- 203.Wyatt AW, Steinert JR, Mann GE. Modulation of the L-arginine/nitric oxide signalling pathway in vascular endothelial cells. Biochem Soc Symp 71: 143–156, 2004. doi: 10.1042/bss0710143. [DOI] [PubMed] [Google Scholar]
- 204.Xie Y, Zhu WZ, Zhu Y, Chen L, Zhou ZN, Yang HT. Intermittent high altitude hypoxia protects the heart against lethal Ca2+ overload injury. Life Sci 76: 559–572, 2004. doi: 10.1016/j.lfs.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 205.Xie Y, Zhu Y, Zhu WZ, Chen L, Zhou ZN, Yuan WJ, Yang HT. Role of dual-site phospholamban phosphorylation in intermittent hypoxia-induced cardioprotection against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 288: H2594–H2602, 2005. doi: 10.1152/ajpheart.00926.2004. [DOI] [PubMed] [Google Scholar]
- 206.Xu KY, Kuppusamy SP, Wang JQ, Li H, Cui H, Dawson TM, Huang PL, Burnett AL, Kuppusamy P, Becker LC. Nitric oxide protects cardiac sarcolemmal membrane enzyme function and ion active transport against ischemia-induced inactivation. J Biol Chem 278: 41798–41803, 2003. doi: 10.1074/jbc.M306865200. [DOI] [PubMed] [Google Scholar]
- 207.Xu KY, Zweier JL, Becker LC. Functional coupling between glycolysis and sarcoplasmic reticulum Ca2+ transport. Circ Res 77: 88–97, 1995. doi: 10.1161/01.RES.77.1.88. [DOI] [PubMed] [Google Scholar]
- 208.Xu WQ, Yu Z, Xie Y, Huang GQ, Shu XH, Zhu Y, Zhou ZN, Yang HT. Therapeutic effect of intermittent hypobaric hypoxia on myocardial infarction in rats. Basic Res Cardiol 106: 329–342, 2011. doi: 10.1007/s00395-011-0159-y. [DOI] [PubMed] [Google Scholar]
- 209.Yan YF, Chen HP, Huang XS, Qiu LY, Liao ZP, Huang QR. DJ-1 mediates the delayed cardioprotection of hypoxic preconditioning through activation of Nrf2 and subsequent upregulation of antioxidative enzymes. J Cardiovasc Pharmacol 66: 148–158, 2015. doi: 10.1097/FJC.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 210.Yan YF, Yang WJ, Xu Q, Chen HP, Huang XS, Qiu LY, Liao ZP, Huang QR. DJ-1 upregulates anti-oxidant enzymes and attenuates hypoxia/re-oxygenation-induced oxidative stress by activation of the nuclear factor erythroid 2-like 2 signaling pathway. Mol Med Rep 12: 4734–4742, 2015. doi: 10.3892/mmr.2015.3947. [DOI] [PubMed] [Google Scholar]
- 211.Yano N, Suzuki D, Endoh M, Tseng A, Stabila JP, McGonnigal BG, Zhao TC, Padbury JF, Tseng Y-T. β-adrenergic receptor mediated protection against doxorubicin-induced apoptosis in cardiomyocytes: the impact of high ambient glucose. Endocrinology 149: 6449–6461, 2008. doi: 10.1210/en.2008-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yeh CH, Hsu SP, Yang CC, Chien CT, Wang NP. Hypoxic preconditioning reinforces HIF-alpha-dependent HSP70 signaling to reduce ischemic renal failure-induced renal tubular apoptosis and autophagy. Life Sci 86: 115–123, 2010. doi: 10.1016/j.lfs.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 213.Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev 83: 1113–1151, 2003. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 214.Yeung HM, Kravtsov GM, Ng KM, Wong TM, Fung ML. Chronic intermittent hypoxia alters Ca2+ handling in rat cardiomyocytes by augmented Na+/Ca2+ exchange and ryanodine receptor activities in ischemia-reperfusion. Am J Physiol Cell Physiol 292: C2046–C2056, 2007. doi: 10.1152/ajpcell.00458.2006. [DOI] [PubMed] [Google Scholar]
- 215.Yu Z, Wang ZH, Yang HT. Calcium/calmodulin-dependent protein kinase II mediates cardioprotection of intermittent hypoxia against ischemic-reperfusion-induced cardiac dysfunction. Am J Physiol Heart Circ Physiol 297: H735–H742, 2009. doi: 10.1152/ajpheart.01164.2008. [DOI] [PubMed] [Google Scholar]
- 216.Yuan F, Guo Z, Xu Y, Wang X, Bu HM, Zhong N, Zhang Y, Zhou ZN. Comparison of the effects of chronic intermittent hypobaric hypoxia and continuous hypobaric hypoxia on hemodynamics in rats. Sheng Li Xue Bao 60: 687–694, 2008. [PubMed] [Google Scholar]
- 217.Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1α expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol 217: 674–685, 2008. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zatta AJ, Kin H, Yoshishige D, Jiang R, Wang N, Reeves JG, Mykytenko J, Guyton RA, Zhao ZQ, Caffrey JL, Vinten-Johansen J. Evidence that cardioprotection by postconditioning involves preservation of myocardial opioid content and selective opioid receptor activation. Am J Physiol Heart Circ Physiol 294: H1444–H1451, 2008. doi: 10.1152/ajpheart.01279.2006. [DOI] [PubMed] [Google Scholar]
- 219.Zhang H, Yang CY, Wang YP, Wang X, Cui F, Zhou ZN, Zhang Y. Effects of different modes of intermittent hypobaric hypoxia on ischemia/reperfusion injury in developing rat hearts. Sheng Li Xue Bao 59: 660–666, 2007. [PubMed] [Google Scholar]
- 220.Zhang Y, Zhong N, Zhou ZN. Effects of intermittent hypoxia on action potential and contraction in non-ischemic and ischemic rat papillary muscle. Life Sci 67: 2465–2471, 2000. doi: 10.1016/S0024-3205(00)00832-8. [DOI] [PubMed] [Google Scholar]
- 221.Zhang Y, Zhong N, Zhou ZN. Estradiol potentiates antiarrhythmic and antioxidative effects of intermittent hypoxic rat heart. Acta Pharmacol Sin 21: 609–612, 2000. [PubMed] [Google Scholar]
- 222.Zhao X, Chen YR, He G, Zhang A, Druhan LJ, Strauch AR, Zweier JL. Endothelial nitric oxide synthase (NOS3) knockout decreases NOS2 induction, limiting hyperoxygenation and conferring protection in the postischemic heart. Am J Physiol Heart Circ Physiol 292: H1541–H1550, 2007. doi: 10.1152/ajpheart.00264.2006. [DOI] [PubMed] [Google Scholar]
- 223.Zhong N, Zhang Y, Fang QZ, Zhou ZN. Intermittent hypoxia exposure-induced heat-shock protein 70 expression increases resistance of rat heart to ischemic injury. Acta Pharmacol Sin 21: 467–472, 2000. [PubMed] [Google Scholar]
- 224.Zhou J, Kim DD, Peluffo RD. Nitric oxide can acutely modulate its biosynthesis through a negative feedback mechanism on l-arginine transport in cardiac myocytes. Am J Physiol Cell Physiol 299: C230–C239, 2010. doi: 10.1152/ajpcell.00077.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhu HF, Dong JW, Zhu WZ, Ding HL, Zhou ZN. ATP-dependent potassium channels involved in the cardiac protection induced by intermittent hypoxia against ischemia/reperfusion injury. Life Sci 73: 1275–1287, 2003. doi: 10.1016/S0024-3205(03)00429-6. [DOI] [PubMed] [Google Scholar]
- 226.Zhu WZ, Xie Y, Chen L, Yang HT, Zhou ZN. Intermittent high altitude hypoxia inhibits opening of mitochondrial permeability transition pores against reperfusion injury. J Mol Cell Cardiol 40: 96–106, 2006. doi: 10.1016/j.yjmcc.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 227.Zhu XM, Liu XH, Cai LR, Xu FF. [Hypoxic preconditioning induces endoplasmic reticulum stress-related cardioprotection mediated by p38 mitogen-activated protein kinase]. Sheng Li Xue Bao 58: 463–470, 2006. [PubMed] [Google Scholar]
- 228.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res 71: 310–321, 2006. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 229.Zong P, Setty S, Sun W, Martinez R, Tune JD, Ehrenburg IV, Tkatchouk EN, Mallet RT, Downey HF. Intermittent hypoxic training protects canine myocardium from infarction. Exp Biol Med (Maywood) 229: 806–812, 2004. doi: 10.1177/153537020422900813. [DOI] [PubMed] [Google Scholar]