Abstract
The G protein-coupled receptor APJ is a promising therapeutic target for heart failure. Constitutive deletion of APJ in the mouse is protective against the hypertrophy-heart failure transition via elimination of ligand-independent, β-arrestin-dependent stretch transduction. However, the cellular origin of this stretch transduction and the details of its interaction with apelin signaling remain unknown. We generated mice with conditional elimination of APJ in the endothelium (APJendo−/−) and myocardium (APJmyo−/−). No baseline difference was observed in left ventricular function in APJendo−/−, APJmyo−/−, or control (APJendo+/+, APJmyo+/+) mice. After exposure to transaortic constriction, APJendo−/− mice displayed decreased left ventricular systolic function and increased wall thickness, whereas APJmyo−/− mice were protected. At the cellular level, carbon fiber stretch of freshly isolated single cardiomyocytes demonstrated decreased contractile responses to stretch in APJ−/− cardiomyocytes compared with APJ+/+ cardiomyocytes. Ca2+ transients did not change with stretch in either APJ−/− or APJ+/+ cardiomyocytes. Application of apelin to APJ+/+ cardiomyocytes resulted in decreased Ca2+ transients. Furthermore, hearts of mice treated with apelin exhibited decreased phosphorylation in cardiac troponin I NH2-terminal residues (Ser22 and Ser23) consistent with increased Ca2+ sensitivity. These data establish that APJ stretch transduction is mediated specifically by myocardial APJ, that APJ is necessary for stretch-induced increases in contractility, and that apelin opposes APJ’s stretch-mediated hypertrophy signaling by lowering Ca2+ transients while maintaining contractility through myofilament Ca2+ sensitization. These findings underscore apelin’s unique potential as a therapeutic agent that can simultaneously support cardiac function and protect against the hypertrophy-heart failure transition.
NEW & NOTEWORTHY These data address fundamental gaps in our understanding of apelin-APJ signaling in heart failure by localizing APJ’s ligand-independent stretch sensing to the myocardium, identifying a novel mechanism of apelin-APJ inotropy via myofilament Ca2+ sensitization, and identifying potential mitigating effects of apelin in APJ stretch-induced hypertrophic signaling.
Keywords: apelin/APJ, cardiomyocyte contractility, cardiomyocyte hypertrophy, heart failure
INTRODUCTION
Heart failure is a common cause of morbidity and mortality, accounting for more than $30 billion of health care spending per year in the United States alone. Therapies for heart failure currently target compensatory mechanisms that become maladaptive over time. Although mortality is reduced, the burden of disease remains significant. In addition, the utility of these therapies in the acute heart failure setting is lacking. The search for molecular targets that improve both acute and chronic disease continues.
Apelin was identified (26, 46) as one of the endogenous ligands for APJ, a G protein-coupled receptor cloned over a decade ago (31). Since its discovery, apelin has been noted to have many actions in the cardiovascular system, ranging from inotropy to vasodilatory properties, both directly in a nitric oxide- and endothelium-dependent manner (1, 4, 28, 30, 47) and indirectly through control of vasopressin release in the central nervous system (16, 57, 59). Apelin was first noted to play a role in cardiovascular disease through a “reverse heart failure” model in patients implanted with ventricular assist devices who later went on to transplantation (8). Apelin-null mice display depression in cardiac systolic function at rest and significant detriments in exercise capacity (8, 10, 24). Furthermore, administration of apelin can rescue multiple heart failure phenotypes (1, 14). Apelin acts both to inhibit hypertrophy and to support contraction in the myocardium (1). While the physiology of these findings is well established, the underlying cellular basis of these effects remains incompletely understood.
Consistent with findings in zebrafish (32, 40, 56), homozygous elimination of APJ in mice leads to frequent embryonic mortality and significant cardiac developmental abnormalities (56). Surviving APJ-null mice show no difference in baseline blood pressure, although APJ interacts directly with ANG II type 1 receptors (AT1Rs) to antagonize its effects on blood pressure (2, 7, 10). Additionally, surviving APJ-null mice show modest decrements in baseline cardiac contractility as well as severe exercise limitations, similar to apelin-null mice (7, 24). However, elimination of APJ confers protection against hypertrophy and heart failure in response to transaortic constriction (TAC) (39). This counterintuitive finding may be due to a second function of APJ as a stretch sensor. When cells expressing APJ are stretched, it leads to increased β-arrestin signaling and a consequent hypertrophic response. This signaling pathway is antagonized by apelin-APJ binding (39).
One explanation for the differences observed in embryonic lethality, developmental effects, and differential myocardial responses to APJ versus apelin elimination may be the presence of multiple stimulators of APJ signaling, including multiple ligands and stretch transduction. For example, ELABELA/Toddler, described nearly a decade after apelin, binds APJ and is critical for vasculogenesis and normal development in zebrafish, independent of apelin (9, 25, 33). Additionally, stretch-induced, prohypertrophic APJ signaling through β-arrestins is induced by stretch, potentially leading to a paradoxical protective effect of APJ elimination in the myocardium (39), even as apelin itself is antihypertrophic via a non-β-arrestin-based APJ signaling mechanism. Although a downstream pathway for this surprising finding has been proposed (39), it remains to be determined whether this stretch function of APJ is localized in the myocardium or endothelium. Furthermore, the downstream mechanism of increased contractility due to apelin-APJ binding remains to be elucidated. Here, we used inducible tissue-specific elimination of APJ as well as in vitro cellular physiology to address these questions. We hypothesized that myocardial APJ is solely responsible for the observed stretch-induced prohypertrophic signaling, whereas endothelial APJ is not itself prohypertrophic. We also elucidate one mechanism by which downstream apelin-APJ signaling leads to increased Ca2+ sensitivity in the sarcomere without increasing Ca2+ transients, explaining the complex effects of apelin in supporting inotropy and countersignaling hypertrophy.
METHODS
Generation of tissue-specific APJ elimination.
All procedures involving animal use, housing, and surgeries were approved by the Stanford Animal Administrative Panel on Laboratory Animal Care. Animal care and interventions were provided in accordance with the Laboratory Animal Welfare Act.
All mouse strains were maintained in C57BL/6 background. The APJ−/− (7), floxed APJ allele, APJF (7) (Fig. 1A), α-MHC-MerCreMer [purchased from the Jackson Laboratory (Bar Harbor, ME), termed α-MHC-Cre], and Tie2Cre (20, 45) strains were as previously described. For tissue-specific deletions, α-MHC-MerCreMer; APJF/F mice and Tie2Cre; APJF/F mice were generated to eliminate APJ in cardiomyocytes (termed APJmyo−/−) and in endothelial cells (termed APJendo−/−), respectively. Robust description and confirmation of the α-myosin heavy chain (αMHC) and Tie2 promoters as cardiomyocyte- and endothelium-specific drivers of Cre expression, respectively, were first published in 2001 [myocardial α-MHC-MerCreMer (44) and endothelial Tie2Cre (20)]. These strains have been used successfully for the cardiomyocyte- and endothelium-specific knockout of many genes, as we demonstrate here as well (Fig. 1, B and C). Of note, given previously observed transient cardiomyopathy caused by tamoxifen induction (23), great care was taken to avoid confounding: we used tamoxifen-treated, α-MHC-Cre+ animals as APJmyo+/+ controls, treated with low-dose tamoxifen over 12 days, and compared cardiac function measurements after ample recovery time (TAC was performed at least 4 wk after tamoxifen treatment).
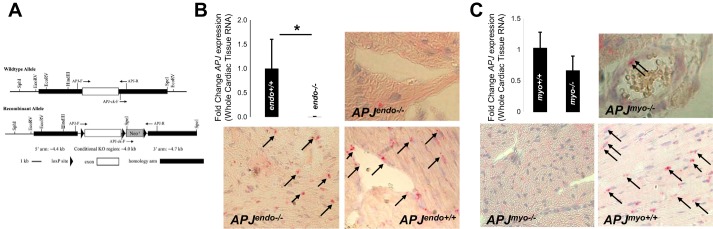
Fig. 1.
Tissue-specific elimination of APJ. A: floxed APJ construct for APJ elimination. B: RNA expression in whole heart (APJendo+/+: 1 ± 0.6 vs. APJendo−/−: 0.003 ± 0.007, n = 8 and 6, P = 0.002) and in situ hybridization to APJ transcripts depicting expression of APJ in cardiomyocytes of APJendo−/− and APJendo+/+ hearts. Red stain indicated by black arrows indicates RNA expression; hematoxylin stained cardiomyocyte nuclei blue. C: RNA expression in whole heart (APJmyo+/+: 1.0 ± 0.3 vs. APJmyo−/−: 0.66 ± 0.2, n = 7 and 4, P = 0.05) and in situ hybridization to APJ transcripts depicting expression of APJ in cardiomyocytes of APJmyo−/− and APJmyo+/+ hearts. Red stain indicated by black arrows indicates RNA expression; hematoxylin stained nuclei blue.
Primers used for genotyping were as follows: APJ flox forward: 5′-CTGTCCAAAGCGTCCTCATT-3′ and APJ floxed reverse: 5′CCCATTGTTATGTGGTTTCC-3′, α-MHC-Cre forward: 5′-AGGTGGACCTGATCATGGAG-3′ and α-MHC-Cre reverse: 5′ATACCGGAGATCATGCAAGC-3′, and Cre reverse: 5′-TCCATGAGTGAACGAACCTG-3′ and Cre forward: 5′-TCGATGCAACGAGTGATGAG-3′.
Additional experiments were performed using C57BL/6 mice (Jackson Laboratory) with and without constitutive APJ knockout [APJ+/+, APJ−/− (18)]. These were used to generate primary cardiomyocytes for carbon fiber experiments, as described below (39).
Animal surgery and phenotyping.
For tamoxifen induction of the cardiomyocyte-specific CreER lines, male mice were intraperitoneally injected with tamoxifen at 4 wk of age (25 mg/kg wt daily for 12 days).
Mice underwent TAC as previously described (42) at 12–16 wk of age. Tissue-specific APJ knockout and control animals were divided into two groups: TAC and sham controls. Briefly, mice were anesthetized using an isoflurane inhalation chamber, intubated, and ventilated. After surgical exposure of the thoracic aorta, a 6.0 silk suture was placed between the innominate and left carotid arteries to induce a constriction diameter of ∼0.4 mm. In sham control mice, an identical procedure was conducted except that the aorta was constricted.
In vivo left ventricular (LV) systolic function was evaluated by echocardiography in the short-axis view, as previously described (42). Measurements occurred at 1 day before surgery (baseline), 7 days, and 14 days after surgery and then every 14 days before euthanasia and tissue collection. Male APJendo−/− and APJendo+/+ mice were followed until 10 wk after TAC, as a significant proportion of control mice had not progressed to heart failure until that time. APJmyo−/− and APJmyo+/+ mice were followed until 4 wk after TAC, as APJmyo+/+ mice had started to display heart failure in that group at that time.
Upon euthanasia, heart weight, body weight, and tibia length were measured by standard methods. Hearts were paraffin fixed, sectioned, and mounted on slides. Trichrome staining as well as wheat germ agglutinin (WGA; rhodamine labeled) staining (1:200 in PBS, Vector Laboratories, Burlingame, CA) was performed. Fibrosis quantification and cell size measurements were performed using ImageJ after image capture at ×20.
Cardiomyocyte isolation.
Adult LV cardiac myocytes were isolated from 8- to 12-wk-old male C57BL6 [APJ+/+ and APJ−/− (18)] using collagenase type II (Worthington Biochemical, Lakewood, NJ). Experiments were performed immediately after isolation, with myocytes resuspended in a HEPES-buffered solution containing (in mM) 1 CaCl2, 137 NaCl, 5.4 KCl, 15 dextrose, 1.3 MgSO4, 1.2 NaH2PO4, and 20 HEPES (pH 7.4).
Cardiomyocyte sretch by the two-carbon fiber technique.
Cardiomyocytes were isolated, prepared, and mounted on the inverted microscope. A pair of micro carbon fibers each attached to miniature hydraulic manipulators (SM-28, Narishige, Tokyo, Japan) and computer controlled with a piezoelectric translator (P-621.1CL, Physik Instrument, Karlsruhe/Palmbach, Germany) mounted on a custom-made railing system (IonOptix, Milton, MA) were attached to single isolated cardiomyocytes robustly twitching in response to field stimulation (IonOptix, Milton, MA). Cells were axially stretched by the piezoelectric translator movement of the carbon fibers using custom software (MATLAB).
Single cardiomyocyte contraction and Ca2+ dynamics.
Cardiomyocytes were loaded with 0.2 µM of fluo-5f-acetoxymethyl ester (Molecular Probes, Eugene, OR) for 30 min and then allowed to incubate in dye-free HEPES-buffered saline with or without pyroglutamylated ([Pyr1])apelin-13 (10 nM) for an additional 30 min to allow for deesterification of the Ca2+ dye. Cardiomyocytes were electrically field stimulated at 1 Hz, and spatially averaged Ca2+ transients and sarcomere length were measured using a standard FITC cube (Chroma) using the HyperSwitch system (IonOptix). Fractional sarcomeric shortening (FSS) and fluorescence transients were normalized to ΔF/F units by taking the average proportional increase in fluorescence across 10 beats per condition per cell. The dye was assumed to be at near equilibrium with the Ca2+ transient. For Ca2+ transient and unloading sarcomeric shortening experiments, data were analyzed in a blinded fashion. [Pyr1]apelin-13 was used in all experiments and was obtained from American Peptide (Sunnyvale, CA).
Apelin infusion.
Apelin was dissolved in distilled, autoclaved, degassed water, frozen at −20°C at 100 µM, and aliquoted on the morning of use. Minipumps contained either apelin or saline. The pumps infused 2 ml over 7 days, after which mice were euthanized according to Animal Administrative Panel on Laboratory Animal Care guidelines.
Immunoblot analysis and in situ hybridization.
LV tissue was homogenized in 400 μl RIPA buffer, and the resultant protein concentration was measured by BCA assay (Thermo Scientific, Waltham, MA). Western blot analysis was performed using the following primary antibodies: anti-cardiac troponin I (cTnI; phospho-Thr143, ab58546, Abcam, Cambridge, MA), anti-cTnI (phospho-Ser22 + Ser23, ab58545, Abcam) at a dilution of 1:1,000, and anti-GAPDH (sc-25778, Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibody was horseradish peroxidase-conjugated anti-rabbit IgG antibody (1:5,000, no. 18-8816-33, Rockland Antibodies, Limerick, PA). For confirmation of tissue specificity of APJ elimination, in situ hybridization was performed on formalin-fixed, paraffin-embedded sections mounted on glass slides using RNAscope technology, with the probe specific to mouse Aplnr (ACDbio no. 436171 and RNAscope 2.5 HD Assay-RED).
Statistics.
Continuous variables were compared using Student’s t-test for two sample comparisons and ANOVA for multiple groups. Echocardiography data were analyzed using a paired Student’s t-test comparing fractional shortening (FS) within each animal before and after TAC.
RESULTS
Endothelium- and cardiomyocyte-specific APJ-null mice display normal cardiac size and function at baseline but exhibit differential responses to pressure overload.
At baseline, neither APJendo−/− nor APJmyo−/− lines showed a difference in LV size or systolic function from APJendo+/+ and APJmyo+/+ lines, respectively. APJmyo−/− mice displayed a mean FS of 37 ± 2.8% (means ± SD, n = 8), and APJmyo+/+ mice (α-MHC-Cre+, also tamoxifen treated) displayed a mean FS of 39 ± 5.7% (n = 8, P = 0.59). APJendo−/− mice displayed a mean FS of 39 ± 3.9% (n = 15), and APJendo+/+ mice displayed a mean FS of 40 ± 3.3% (n = 11, P = 0.41). There was also no difference in LV internal dimension at diastole, interventricular septal thickness at diastole, or posterior wall thickness at diastole at baseline (Table 1).
Table 1.
Echocardiographic characteristics
| APJendo−/− TAC | APJendo−/− Sham | APJendo+/+ TAC | APJendo+/+Sham | |
|---|---|---|---|---|
| n | 8 | 7 | 6 | 6 |
| Baseline | ||||
| FS, % | 39.1 ± 6.2 | 37.9 ± 3.7 | 39.0 ± 6.2 | 41.0 ± 4.2 |
| LVIDd, mm | 3.5 ± 0.3 | 3.5 ± 0.5 | 3.7 ± 0.3 | 3.4 ± 0.4 |
| IVSd, mm | 1.0 ± 0.1 | 1.0 ± 0.2 | 1.2 ± 0.2 | 1.1 ± 0.1 |
| PWd, mm | 1.0 ± 0.1 | 1.0 ± 0.2 | 1.2 ± 0.3 | 1.1 ± 0.2 |
| Heart rate, beats/min | 532 ± 58 | 559 ± 76 | 531 ± 38 | 492 ± 66 |
| Week 4 | ||||
| FS, % | 31.0 ± 12.5 | 42.6 ± 6.7 | 34.5 ± 10.1 | 37.8 ± 6.6 |
| LVIDd, mm | 3.9 ± 0.6 | 3.4 ± 0.4 | 3.7 ± 0.3 | 3.7 ± 0.9 |
| IVSd, mm | 1.0 ± 0.2 | 0.83 ± 0.2 | 1.1 ± 0.1* | 0.9 ± 0.05* |
| PWd, mm | 1.1 ± 0.2 | 1.0 ± 0.4 | 1.1 ± 0.2 | 0.7 ± 0.2 |
| Heart rate, beats/min | 538 ± 28 | 534 ± 38 | 535 ± 88 | 522 ± 54 |
| Heart weight/body weight (10 wk) | 7.3 ± 2.3 | 4 ± 0.45 | 5.8 ± 1.2 | 4.6 ± 0.45 |
| Heart weight/tibia length (10 wk)† | 11.8 ± 3.2* | 6.3 ± 0.8* | 9.8 ± 1.6* | 7.5 ± 1.0* |
| APJmyo−/− TAC | APJmyo−/− Sham | APJmyo+/+ TAC | APJmyo+/+ Sham | |
| n | 3 | 4 | 5 | 3 |
| Baseline | ||||
| FS, % | 36.1 ± 4.0 | 38.0 ± 3.3 | 37.6 ± 5.9 | 36.9 ± 3.1 |
| LVIDd, mm | 3.5 ± 0.1 | 3.6 ± 0.1 | 3.5 ± 0.5 | 3.4 ± 0.2 |
| IVSd, mm | 0.9 ± 0.1 | 0.65 ± 0.05 | 0.9 ± 0.2 | 0.73 ± 0.03 |
| PWd, mm | 0.9 ± 0.1 | 0.87 ± 0.09 | 1.1 ± 0.1 | 0.8 ± 0.1 |
| Heart rate, beats/min | 413 ± 15 | 529 ± 38 | 435 ± 70 | 508 ± 12 |
| Week 4 | ||||
| FS, % | 40.1 ± 10.6 | 38 ± 5.6 | 29.7 ± 8.2 | 37 ± 1.9 |
| LVIDd, mm | 3.5 ± 0.2 | 3.3 ± 0.3 | 3.4 ± 0.4 | 3.5 ± 0.2 |
| IVSd, mm | 0.9 ± 0.2 | 0.7 ± 0.08 | 1.1 ± 0.3 | 0.7 ± 0.06 |
| PWd, mm | 1.1 ± 0.2 | 0.8 ± 0.09 | 1.3 ± 0.2* | 0.9 ± 0.09* |
| Heart rate, beats/min | 444 ± 35 | 498 ± 70 | 421 ± 69 | 466 ± 35 |
| Heart weight/body weight† | 6.7 ± 0.7 | 5.5 ± 0.7 | 9.2 ± 1.8 | 5.3±0.4 |
| Heart weight/tibia length† | 9.0 ± 0.9 | 7.3 ± 0.8 | 12.2 ± 1.3* | 8.3±0.4* |
Values are expressed as means ± SD. TAC, transaortic constriction; FS, fractional shortening; LVIDd, left ventricular internal dimension at diastole; IVSd, interventricular septal thickness at diastole; PWd, posterior wall thickness at diastole.
P < 0.01 by post hoc Studentʼs t-test;
P < 0.05 by ANOVA.
Four weeks after TAC, APJmyo+/+ mice treated with TAC had significantly reduced FS (37.6 ± 5.9% before vs. 29.7 ± 8.2% after TAC, means ± SD, n = 3, P = 0.01; Fig. 2A), whereas the systolic function of APJmyo−/− mice was preserved (36.1 ± 4.0% before vs. 40.9 ± 10.6% after TAC, n = 4, P = 0.6; Fig. 2A). Posterior wall thickness was also greater in hearts of APJmyo+/+ mice that underwen TAC compared with sham-treated APJmyo+/+ mice (P = 0.004), whereas no difference in wall thickness was detected in APJmyo−/− mice between TAC and sham-treated groups (Table 1). At 4 wk after TAC, the heart weight-to-tibia length ratio was significantly greater in APJmyo+/+ TAC mice than in any other group (APJmyo+/+ TAC: 12.2 ± 1.3 vs. APJmyo+/+ sham: 8.3 ± 0.4, APJmyo−/− TAC: 9.0 ± 0.9, and APJmyo−/− sham: 7.3 ± 0.8, P < 0.01 by ANOVA; Table 1). This indicates that APJmyo−/− mice were protected from the hypertrophy and LV dysfunction caused by TAC in APJmyo+/+ mice.
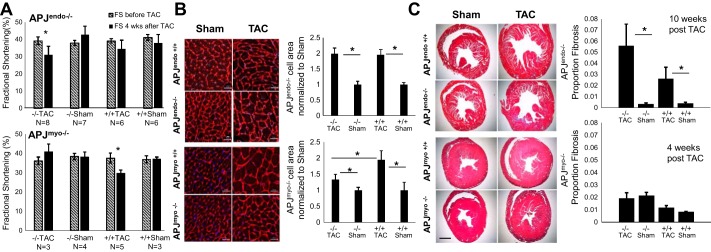
Fig. 2.
Endothelium- and cardiomyocyte-specific APJ elimination differentially affect the cardiac response to pressure overload. A: 4 wk after transaortic constriction (TAC), APJendo−/− mice displayed a significant decrease in fractional shortening (FS; n = 8, P = 0.02), where APJendo+/+ mice did not (n = 6, P = 0.6). APJmyo−/− mice did not display a decrease in FS at this time point (n = 3, P = 0.6), whereas APJmyo+/+ mice did display decreased FS (n = 5, P = 0.01). Error bars show means ± SE. B: APJendo−/− mice displayed a significant increase in cardiomyocyte size 10 wk after TAC compared with sham-treated control mice (P < 0.01), and this difference was not greater than APJendo+/+ mice treated with TAC. Four weeks after TAC, APJmyo−/− mice displayed a significant decrease in cardiomyocyte size compared with APJmyo+/+ TAC-treated mice (P < 0.05). C: APJendo−/− mice displayed a trend toward increased interstitial fibrosis compared with APJendo+/+ mice 10 wk after TAC, whereas no significant change was seen in fibrosis in APJmyo−/− or APJmyo+/+ mice.
Conversely, APJendo−/− mice that underwent TAC showed reduced FS after 4 wk (39.1 ± 6.2% before vs. 31.0 ± 12.4% after TAC, n = 8, P = 0.03; Fig. 2A), whereas APJendo+/+ mice that underwent TAC did not have significantly reduced FS at this time point (39.0 ± 3.4% before vs. 34.5 ± 10% after TAC, n = 6, P = 0.6; Fig. 2A). A small number of APJendo+/+ TAC animals had failed at the 4-wk time point compared with most APJmyo+/+ TAC animals. For this reason, APJendo animals were followed until 10 wk after TAC, at which time five of six APJendo+/+ TAC mice and five of eight APJendo−/− TAC mice displayed a FS of <30% (data not shown). There was an increase in interventricular septal thickness at diastole in APJendo+/+ TAC animals compared with sham animals at 4 wk after TAC (P = 0.02), although no difference was observed in the APJendo−/− groups (Table 1). At 10 wk after TAC, the heart weight-to-tibia length ratio was significantly greater in APJendo+/+ TAC mice versus APJendo+/+ sham mice and in APJendo−/− TAC mice versus APJendo−/− sham mice but not across genotypes (APJendo+/+ TAC: 9.8 ± 1.6 vs. APJendo+/+ sham: 7.5 ± 1.0, P < 0.01; APJendo−/− TAC: 11.8 ± 3.2 vs. APJendo−/− sham: 6.3 ± 0.8, P = 0.01; Table 1). This indicates that APJendo−/− show a more rapid worsening of FS and wall thickening in response to TAC but that end-stage measures of cardiac size and function did not differ between APJendo−/− and APJendo+/+ mice exposed to TAC.
APJmyo−/− mice were clearly protected from cellular hypertrophy 4 wk after TAC, as measured by WGA cell membrane stain. In response to TAC, the increase in cardiomyocyte size in APJmyo−/− compared with sham-treated animals was significantly smaller compared with APJmyo+/+ animals [APJmyo−/− (n = 4): 1.32 ± 0.03-fold increase over sham (n = 5, 1 ± 0.05-fold, P = 0.006) vs. APJmyo+/+ (n = 5): 1.94 ± 06-fold increase over sham (n = 3, 1 ± 0.2-fold, P = 0.008), and APJmyo−/− vs. APJmyo+/+ after TAC; P = 0.01; Fig. 2B]. This indicates that APJ expression in the myocardium is necessary for TAC-induced cardiomyocyte hypertrophy. Neither APJmyo−/− mice nor APJmyo+/+ mice displayed significant fibrosis on trichrome staining 4 wk after TAC. APJmyo−/− mice after TAC displayed only 2 ± 0.4% total fibrosis area (n = 4), similar to sham APJmyo−/− mice that displayed 2.2 ± 0.2% fibrosis (n = 5). APJmyo+/+ mice after TAC displayed 1.1 ± 0.2% fibrosis (n = 5), whereas sham APJmyo+/+ control mice showed 0.8 ± 0.02% fibrosis (n = 3). Fibrosis in APJmyo−/− and APJmyo+/+ mice after TAC was significantly different, but the biological meaning of this is uncertain, given overall fibrosis is low in both groups (P = 0.03; Fig. 2C).
Both APJendo−/− and APJendo+/+ mice displayed significantly increased cardiomyocyte size compared with sham-treated control mice [APJendo−/− (n = 6): 1.98 ± 0.18-fold increase over sham (n = 6, 1 ± 0.1-fold, P < 0.01) vs. APJendo+/+ (n = 8): 1.95 ± 0.16-fold increase over sham (n = 4, 1 ± 0.06-fold, P < 0.01; APJendo−/− vs. APJendo+/+ after TAC: P = 0.9; Fig. 2B]. Ten weeks after TAC, both APJendo−/− and APJendo+/+ mice displayed increased fibrosis compared with sham mice [APJendo−/− (n = 8): 5.6 ± 1.9% total fibrosis area vs. 0.3 ± 0.09% fibrosis in sham (n = 8), P = 0.03; APJendo+/+ (n = 6): 2.2 ± 1.1% total fibrosis vs. 0.4 ± 0.08% fibrosis in sham (n = 6), P = 0.04; Fig. 2C], with a higher mean fibrosis area in APJendo−/− mice compared with APJendo+/+ mice after TAC, although this did not reach statistical significance (P = 0.22; Fig. 2B). Therefore, in accordance with the cardiac function data above, while myocardial expression of APJ drives hypertrophy, conditional tissue-specific elimination of APJ from the endothelium does not protect from pressure overload hypertrophy and cardiac dysfunction.
APJ is necessary for stretch-induced augmentation of contraction in single cardiomyocytes.
Having established that the previously observed stretch-sensing role of APJ is specific to APJ expression in the myocardium and further that endothelial loss of APJ is associated with a poor ventricular response to pressure overload, we went on to examine potential mechanisms downstream of apelin-APJ signaling in single isolated cardiomyocytes (1). The G protein-independent stretch transduction of APJ has been previously attributed to β-arrestin-dependent signaling in APJ−/− mice (39). This lack of hypertrophic signaling is thought to prevent the hypertrophy-heart failure transition in APJ−/− mice in response to pressure overload. We chose to further investigate the mechanism of increased contractility observed downstream of apelin-APJ signaling.
We first asked whether APJ is necessary for increased contractility due to stretch at the cellular level as reported in APJ+/+ single cardiomyocytes stretched with carbon fibers (41). To measure contractility, we used a metric of FSS, which is defined as the change in sarcomere length with contraction divided by its baseline length. We then sequentially stretched cardiomyocytes and determined the slope of increase in FSS per percent increase from initial sarcomere length [referred to here as stretch-augmented FSS (SAFSS)]. We found that APJ−/− cardiomyocytes augmented their contractility significantly less with stretch than APJ+/+ cardiomyocytes [APJ−/− SAFSS = 0.37 ± 0.04 and APJ+/+ = 0.84 ± 0.05, P = 0.04 (mean slope ± SE; n = 3 mice/group); Fig. 3A].
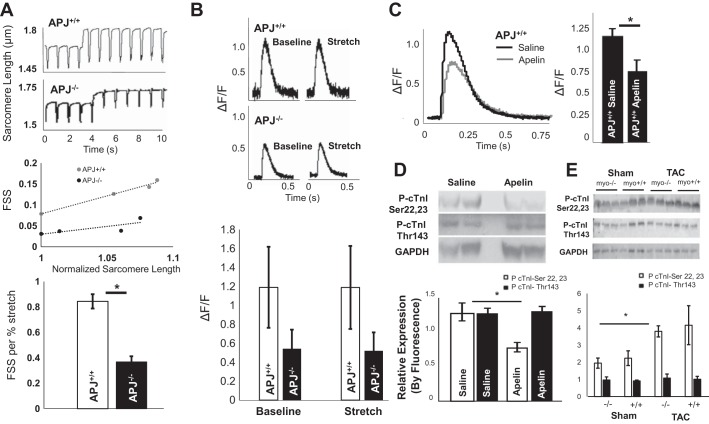
Fig. 3.
Apelin lowers Ca2+ transients in isolated cardiomyocytes and simultaneously increases myofilament sensitivity, whereas length-dependent activation by APJ is independent of Ca2+ transients. A: representative sarcomere length tracings and slopes of fractional sarcomere shortening (FSS) per increase in diastolic sarcomere length (stretch) [APJ−/− FSS/%: 0.37 ± 0.04/% sarcomere length (SL) and APJ+/+: 0.84 ± 0.05/% SL, P = 0.04 (mean slopes ± SE, n = 3 mice/group]. B: change in Ca2+-induced Ca2+ release in APJ−/− compared with APJ+/+ cardiomyocytes [APJ−/− ΔF/F: 1.2 ± 0.43 and APJ+/+ ΔF/F: 0.54 ± 0.2, P = 0.2 (means ± SE, n = 3 mice/group)]. No difference in ΔF/F was found within genotype between baseline and stretch. C: apelin significantly decreased peak Ca2+ transients in APJ+/+ cardiomyocytes (49% reduction in ΔF/F in apelin-treated animals, apelin: n = 39 cells from four animals and saline: n = 23 cardiomyocytes from four animals in the control group, P = 3 × 10−4). D: immunoblot analysis of cardiac troponin I (cTnI) phosphorylation in apelin- and saline-treated mice using anti-phospho (Ser22 and Ser23) antibody against the PKA-mediated site demonstrated decreased phosphorylation in the presence of apelin (P < 0.01). No difference was found in PKC-phosphorylated residue Thr143 (n = 6 per group). E: immunoblot showing fluorescence detection of antibodies to phospho-cTnI (Ser22 and Ser23), phospho-cTnI (Thr143), and GAPDH in cardiac tissue of APJmyo+/+ and APJmyo−/− mice before and after transaortic constriction (TAC). Fluorescence quantification of phospho-cTnI (Ser22 and Ser23, open bars) and phospho-cTnI (Thr143, solid bars) normalized to GAPDH fluorescence is shown (bars show means ± SD of arbitrary units). *P < 0.01.
We went on to investigate whether this difference in contractile response to stretch is associated with changes in the peak Ca2+ transient in APJ−/− and APJ+/+ cardiomyocytes. We measured the Ca2+ transient as the change in fluorescence with contraction as a proportion of baseline fluorescence of calcium dyes (ΔF/F). We found a decrease in the Ca2+ transient in APJ−/− cardiomyocytes compared with APJ+/+ cardiomyocytes [APJ−/− ΔF/F: 1.2 ± 0.43, APJ+/+ ΔF/F: 0.54 ± 0.2, P = 0.2 (means ± SE, n = 3 mice/group); Fig. 3B]. No difference was discovered in ΔF/F within genotype between baseline and stretch (Fig. 3B).
Apelin-APJ binding decreases the Ca2+ transient in spontaneously beating cardiomyocytes and alters TnI phosphorylation of Ser22 and Ser23, residues known to control myofilament Ca2+ sensitivity.
Given this lack of change in Ca2+ transients with stretch, we hypothesized that the documented inotropic effect of apelin-APJ signaling might also be independent of changes in cytoplasmic Ca2+ concentration (7, 14). Further supporting such a hypothesis, APJ is necessary for apelin’s effect on cardiomyocyte contractility (35) and it does not activate Gs (3, 55) but stimulates extracellular acidification (46, 51). To investigate this, we assessed Ca2+ transients in freely contracting APJ+/+ cardiomyocytes with and without apelin. Surprisingly, apelin significantly decreased peak Ca2+ transients in APJ+/+ cardiomyocytes (49% reduction in ΔF/F in apelin-treated animals, apelin: n = 39 cells from four animals, saline: n = 23 cardiomyocytes from four animals in the control group, P = 3 × 10−4; Fig. 3C).
Since apelin decreases cardiomyocyte Ca2+ transients, its observed inotropic effect remains to be explained. One way in which force generation can be augmented without increasing prohypertrophic Ca2+ transients is myofilament sensitization. During β-adrenergic stimulation, PKA phosphorylates cTnI at its NH2 terminus (human Ser23 and Ser24; mouse Ser22 and Ser23), leading to decreased myofilament Ca2+ sensitivity and increased lusitropy (37). Furthermore, cTnI phosphorylation at these and other residues is increased in hypertensive heart failure, perhaps representing a maladaptive mechanism of disease (13, 58). Apelin-APJ signaling leads to decreased PKA activation via the inhibition of adenylyl cyclase and cAMP production, and we have recently reported increased diastolic relaxation and an increase in FSS in single cardiomyocytes exposed to apelin (35). Thus, we hypothesized that apelin-APJ binding leads to decreased PKA-dependent phosphorylation of cTnI at Ser22 and Ser23, increasing myofilament Ca2+ sensitivity and thus also contractile capability in the absence of increased Ca2+ transient.
To test this hypothesis, we treated APJ+/+ C57BL/6 mice with apelin infusion for 7 days and examined cardiac tissue by phospho-Western blot analysis. We found that, unlike Thr143, a residue phosphorylated by PKC (a protein kinase canonically activated by apelin-APJ signaling), treatment with apelin reduced phosphorylation at PKA phosphorylated at Ser22 and Ser23 compared with saline treatment [n = 6 mice/group, apelin-treated density at PKA residues (cTnI-Ser22+ Ser23): 0.76 ± 0.03, saline- treated density: 1.27 ± 0.06 (means ± SE, arbitrary units of density normalized to GAPDH expression, P = 0.01); Fig. 3D]. APJmyo−/− mice did not display increased cTnI phosphorylation at Ser22 and Ser23 at baseline or after TAC (Fig. 3E), indicating that endogenous levels of apelin do not overcome maladaptive phosphorylation at this residue in heart failure.
DISCUSSION
Here, we present data demonstrating that 1) cardiomyocyte-specific elimination of APJ is protective against pressure-overload heart failure, 2) this protection extends to abrogation of cellular hypertrophy and fibrosis, 3) APJ is necessary for the stretch-dependent force increase in single cardiomyocytes, 4) the presence of apelin reduces Ca2+ transients regardless of preload, and 5) apelin may preserve inotropy through myofilament Ca2+ sensitization via reduced cTnI Ser23 and Ser24 phosphorylation.
These findings add to our understanding of the fundamental mechanisms of cardiac hypertrophy and failure. They confirm that afterload-induced stretch signaling is mediated by cardiac, not endothelial, APJ. They further underscore apelin’s therapeutic potential as an agent capable of antagonizing this stretch-APJ-β-arrestin-based hypertrophy signaling. Prior data have shown that constitutive deletion of APJ during development was frequently lethal and leads to marked developmental cardiac abnormalities (7). Given the importance of APJ for cardiac development, a question arose over the extent to which the function of these remaining mice reflected a developmental abnormality or adult loss of APJ. Later data showed a protective effect of APJ elimination, but the cellular basis of that effect remained speculative at that time (39). Here, we show that elimination of myocardial APJ in the adult is also protective, which underlines the likelihood of a developmentally driven explanation for cardiac decrements in the original APJ−/− mice. It is also particularly important in light of recent findings that endothelial progenitor populations expressing APJ can compensate for one another during heart development (43).
Here, we provide mechanistic insights clarifying these findings, in particular, with respect to contractile response to stretch and associated changes in Ca2+ handling in the presence or absence of APJ. We demonstrate that loss of APJ significantly reduces stretch-augmented FS and show that no increase in Ca2+ transients is observed with stretch in either these cells or APJ+/+ cardiomyocytes. These findings complement our recent work (35), which demonstrates that the increase in Frank-Starling gain observed with apelin treatment is mediated by a lusitropic effect on carbon fiber-stretched cardiomyocytes. Here, we show that APJ−/− cells have a significantly smaller increase in FSS in response to stretch, suggesting that that increases in Frank-Starling gain are mediated by APJ’s stretch-sensing function. Our data showing decreased phosphorylation of cTnI at Ser22 and Ser23 in response to apelin treatment are consistent with increased Ca2+ sensitivity and therefore reduced lusitropy, indicating that apelin’s lusitropic effect is mediated via a different mechanism and potentially relies on a combinatorial effect of myofilament residue phosphorylation to increase contractility and lusitropy simultaneously (36, 58).
Given the preserved ejection fraction of APJmyo−/− mice after TAC, the loss of contractile response to stretch associated with APJ elimination in cardiomyocytes requires some consideration. First, it must be noted that single cell contractility assessments test the response of single myocytes outside of the tissue context of the hypertrophy-heart failure transition; they are not exposed to chronic pressure-induced hypertrophy, fibrosis, or cell death. It is possible, for example, that although a single cell exhibits less contractile response to stretch without APJ, this does not translate to an organ level measure of ejection fraction at one preload (as we observed in APJ−/− mice). Further, it is possible that, because of decreased contractility, APJ−/− cardiomyocytes are actually protected in the long term from the high basal cytoplasmic Ca2+ accumulation associated with the hypertrophy-heart failure transition. Finally, the small loss in contractility may not outweigh the detrimental effect of β-arrestin signaling downstream of APJ-stretch transduction, leading in sum to protection against the hypertrophy-heart failure transition.
In light of these findings, it is particularly interesting that in the absence of stretch, apelin decreases the peak Ca2+ transient (Fig. 3C). While stretch-dependent APJ signaling likely activates the β-arrestin and ERK1/2 pathways to induce prohypertrophic transcriptional program in cardiomyocytes (39), this is a Ca2+-independent mechanism that, to our knowledge, would not be expected to directly activate calcineurin signaling. As phasic Ca2+ concentration changes are well known to be associated with eccentric cardiomyocyte hypertrophy via calcineurin activation (11, 51), this represents a contributory explanation for the antihypertrophic effect of apelin-APJ binding previously described (39). Potentially, this effect of apelin-APJ binding could bypass β-arrestin-ERK1/2-mediated concentric-hypertrophic signaling to ameliorate the hypertrophy associated with hypertensive remodeling (11). In addition, apelin-APJ binding leads to decreased PKA activation via Gi. PKA-dependent phosphorylation of ryanodine receptor 2 leads to increased Ca2+ transients (29). Thus, we expected and confirmed here that apelin should reduce Ca2+ transients due to its deactivation of cAMP/PKA signaling. One prior research group showed increased Ca2+ transients with apelin application to isolated rat cardiomyocytes (48). The discrepancy may be explained by the use of a different apelin isoform (apelin-16), which is not the predominantly active form in human hearts [as is [Pyr1]apelin-13, used here (28)] or may be due to different pacing frequency (0.5 Hz in the referenced report compared with 1 Hz here). We also used low-affinity fluorescent Ca2+ dyes to avoid stacking of Ca2+ transients with repeated stimulation.
Although our finding of apelin-induced decrease in Ca2+ transients is consistent with the known antihypertrophic effects of apelin-APJ binding (1, 39, 54) and PKA control of ryanodine receptor 2 phosphorylation (29), apelin’s observed inotropic effect remains to be explained (1, 8, 14). Although some data have implicated PKC-ε and increased ERK1/2 phosphorylation in apelin-mediated increased contractility (34), our understanding of the underlying mechanism at the level of Ca2+ handling and the sarcomere remains limited. Here, we examined myofilament phosphorylation as a potential intermediary of apelin’s inotropic effect, showing reduced Ser22 and Ser23 phosphorylation of cTnI, consistent with increased Ca2+ sensitivity that could explain increased contractility despite lower Ca2+ transients.
Certainly, the control of myofilament Ca2+ sensitivity by phosphorylation is complex. Multiple phosphorylation sites on TnI control contractility and Ca2+ sensitivity. A significant body of in vitro and in silico work exists to suggest that TnI phosphorylation is dynamic between disease states and that unique residues confer different and sometimes opposing effects on contractility (27, 38, 50, 58). For Ser22 and Ser23 specifically, phosphorylation at this site has consistently been found to decrease myofilament Ca2+ sensitivity (12, 13, 37), suggesting that apelin, in fact, increases myofilament Ca2+ sensitivity by reducing phosphorylation at this site. That the immediate inotropic effect of apelin is not mediated by increased Ca2+ transients but is due to differential myofilament Ca2+ sensitization is novel. It is also consistent with the known extracellular acidification and intracellular alkalinization resulting from apelin binding that leads to a leftward shift in the Ca2+-contractility curve (15, 22). This would make apelin one of only a handful potential inotropic therapeutics to work through nonadrenergic signaling by directly affecting myofilament Ca2+ sensitivity, the other major therapeutics being pimobendan, levosimendan, and omecamtiv mecarbil. Apelin is unique in this class of potential therapeutics, as, on top of its inotropic, antihypertrophic, and vasodilatory effects, it has been implicated in protection against vascular remodeling and diabetes as well as myocardial infarction and ischemic stroke (5, 6, 17, 49, 52, 53, 59).
Overall, these data address fundamental gaps in our understanding of apelin-APJ signaling in heart failure and cardiac hypertrophy. We have developed tissue-specific elimination of APJ to localize the ligand-independent stretch-sensing function of this receptor to the myocyte. Consistent with this, we show loss of the APJ receptor decreases the contractile response to stretch. We offer evidence for a novel mechanism of apelin-APJ-induced contractility via cTnI phosphorylation-mediated Ca2+ sensitivity in the face of reduced phasic Ca2+ release, helping to explain mitigating effects of apelin in APJ stretch-based signaling and providing a rationale for the antiarrhythmic effect. Taken together, these results confirm the fundamental role of APJ as a stretch sensor in both acute and chronic increased afterload, localize its stretch transduction to the myocardium, and further support the rare therapeutic combination afforded by apelin-APJ signaling: increased cardiac output due to improved contractility and decreased afterload and simultaneous protection against the deleterious consequences of chronically raised phasic Ca2+ in the hypertrophy-heart failure transition.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants F32-HL-134233-02 (to V. N. Parikh) and R01-HL-105993-03 (to E. A. Ashley).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
E. A. Ashley is a Founder of Personalis and DeepCell, Inc., and an advisor for SequenceBio and Genome Medical.
AUTHOR CONTRIBUTIONS
J.L., C.S., C.W., D.B., T.Q., and E.A.A. conceived and designed research; V.N.P., J.L., C.S., C.W., M.Z., D.N.C., Z.G., and Y.H. performed experiments; V.N.P., J.L., C.S., C.W., M.Z., K.S., and E.A.A. analyzed data; V.N.P., J.L., C.S., C.W., A.C.Y.C., P.S.T., P.R.-L., T.Q., and E.A.A. interpreted results of experiments; V.N.P. and J.L. prepared figures; V.N.P. drafted manuscript; V.N.P., A.C.Y.C., K.S., D.B., P.R.-L., T.Q., and E.A.A. edited and revised manuscript; V.N.P., J.L., C.S., P.R.-L., T.Q., and E.A.A. approved final version of manuscript.
REFERENCES
- 1.Ashley EA, Powers J, Chen M, Kundu R, Finsterbach T, Caffarelli A, Deng A, Eichhorn J, Mahajan R, Agrawal R, Greve J, Robbins R, Patterson AJ, Bernstein D, Quertermous T. The endogenous peptide apelin potently improves cardiac contractility and reduces cardiac loading in vivo. Cardiovasc Res 65: 73–82, 2005. doi: 10.1016/j.cardiores.2004.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashley E, Chun HJ, Quertermous T. Opposing cardiovascular roles for the angiotensin and apelin signaling pathways. J Mol Cell Cardiol 41: 778–781, 2006. doi: 10.1016/j.yjmcc.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Bai B, Tang J, Liu H, Chen J, Li Y, Song W. Apelin-13 induces ERK1/2 but not p38 MAPK activation through coupling of the human apelin receptor to the Gi2 pathway. Acta Biochim Biophys Sin (Shanghai) 40: 311–318, 2008. doi: 10.1111/j.1745-7270.2008.00403.x. [DOI] [PubMed] [Google Scholar]
- 4.Berry MF, Pirolli TJ, Jayasankar V, Burdick J, Morine KJ, Gardner TJ, Woo YJ. Apelin has in vivo inotropic effects on normal and failing hearts. Circulation 110, Suppl 1: II187–II193, 2004. doi: 10.1161/01.CIR.0000138382.57325.5c. [DOI] [PubMed] [Google Scholar]
- 5.Boucher J, Masri B, Daviaud D, Gesta S, Guigné C, Mazzucotelli A, Castan-Laurell I, Tack I, Knibiehler B, Carpéné C, Audigier Y, Saulnier-Blache J-S, Valet P. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 146: 1764–1771, 2005. doi: 10.1210/en.2004-1427. [DOI] [PubMed] [Google Scholar]
- 6.Castan-Laurell I, Dray C, Knauf C, Kunduzova O, Valet P. Apelin, a promising target for type 2 diabetes treatment? Trends Endocrinol Metab 23: 234–241, 2012. doi: 10.1016/j.tem.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Charo DN, Ho M, Fajardo G, Kawana M, Kundu RK, Sheikh AY, Finsterbach TP, Leeper NJ, Ernst KV, Chen MM, Ho YD, Chun HJ, Bernstein D, Ashley EA, Quertermous T. Endogenous regulation of cardiovascular function by apelin-APJ. Am J Physiol Heart Circ Physiol 297: H1904–H1913, 2009. doi: 10.1152/ajpheart.00686.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen MM, Ashley EA, Deng DXF, Tsalenko A, Deng A, Tabibiazar R, Ben-Dor A, Fenster B, Yang E, King JY, Fowler M, Robbins R, Johnson FL, Bruhn L, McDonagh T, Dargie H, Yakhini Z, Tsao PS, Quertermous T. Novel role for the potent endogenous inotrope apelin in human cardiac dysfunction. Circulation 108: 1432–1439, 2003. doi: 10.1161/01.CIR.0000091235.94914.75. [DOI] [PubMed] [Google Scholar]
- 9.Chng SC, Ho L, Tian J, Reversade B. ELABELA: a hormone essential for heart development signals via the apelin receptor. Dev Cell 27: 672–680, 2013. doi: 10.1016/j.devcel.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Chun HJ, Ali ZA, Kojima Y, Kundu RK, Sheikh AY, Agrawal R, Zheng L, Leeper NJ, Pearl NE, Patterson AJ, Anderson JP, Tsao PS, Lenardo MJ, Ashley EA, Quertermous T. Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. J Clin Invest 118: 3343–3354, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis J, Davis LC, Correll RN, Makarewich CA, Schwanekamp JA, Moussavi-Harami F, Wang D, York AJ, Wu H, Houser SR, Seidman CE, Seidman JG, Regnier M, Metzger JM, Wu JC, Molkentin JD. A tension-based model distinguishes hypertrophic versus dilated cardiomyopathy. Cell 165: 1147–1159, 2016. doi: 10.1016/j.cell.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong WJ, Chandra M, Xing J, Solaro RJ, Cheung HC. Conformation of the N-terminal segment of a monocysteine mutant of troponin I from cardiac muscle. Biochemistry 36: 6745–6753, 1997. doi: 10.1021/bi962226d. [DOI] [PubMed] [Google Scholar]
- 13.Dong X, Sumandea CA, Chen Y-C, Garcia-Cazarin ML, Zhang J, Balke CW, Sumandea MP, Ge Y. Augmented phosphorylation of cardiac troponin I in hypertensive heart failure. J Biol Chem 287: 848–857, 2012. doi: 10.1074/jbc.M111.293258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernst KV, Ashley EA, Charo D, Kawana M, Fajardo G, Bernstein D, Quertermous T. Apelin regulates cardiac contractility and rescues neurohormonal heart failure. J Card Fail 12: S1, 2006. doi: 10.1016/j.cardfail.2006.06.009. [DOI] [Google Scholar]
- 15.Farkasfalvi K, Stagg MA, Coppen SR, Siedlecka U, Lee J, Soppa GK, Marczin N, Szokodi I, Yacoub MH, Terracciano CMN. Direct effects of apelin on cardiomyocyte contractility and electrophysiology. Biochem Biophys Res Commun 357: 889–895, 2007. doi: 10.1016/j.bbrc.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Flahault A, Couvineau P, Alvear-Perez R, Iturrioz X, Llorens-Cortes C. Role of the vasopressin/apelin balance and potential use of metabolically stable apelin analogs in water metabolism disorders. Front Endocrinol (Lausanne) 8: 120, 2017. doi: 10.3389/fendo.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hegab II. Ameliorative effect of apelin on streptozotocin-induced diabetes and its associated cardiac hypertrophy. Alexandria J Med 54: 119–127, 2017. doi: 10.1016/j.ajme.2017.05.006. [DOI] [Google Scholar]
- 18.Ishida J, Hashimoto T, Hashimoto Y, Nishiwaki S, Iguchi T, Harada S, Sugaya T, Matsuzaki H, Yamamoto R, Shiota N, Okunishi H, Kihara M, Umemura S, Sugiyama F, Yagami K, Kasuya Y, Mochizuki N, Fukamizu A. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem 279: 26274–26279, 2004. doi: 10.1074/jbc.M404149200. [DOI] [PubMed] [Google Scholar]
- 20.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol 230: 230–242, 2001. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 22.Kohmoto O, Spitzer KW, Movsesian MA, Barry WH. Effects of intracellular acidosis on [Ca2+]i transients, transsarcolemmal Ca2+ fluxes, and contraction in ventricular myocytes. Circ Res 66: 622–632, 1990. doi: 10.1161/01.RES.66.3.622. [DOI] [PubMed] [Google Scholar]
- 23.Koitabashi N, Bedja D, Zaiman AL, Pinto YM, Zhang M, Gabrielson KL, Takimoto E, Kass DA. Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced MerCreMer gene deletion models. Circ Res 105: 12–15, 2009. doi: 10.1161/CIRCRESAHA.109.198416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuba K, Zhang L, Imai Y, Arab S, Chen M, Maekawa Y, Leschnik M, Leibbrandt A, Markovic M, Schwaighofer J, Beetz N, Musialek R, Neely GG, Komnenovic V, Kolm U, Metzler B, Ricci R, Hara H, Meixner A, Nghiem M, Chen X, Dawood F, Wong KM, Sarao R, Cukerman E, Kimura A, Hein L, Thalhammer J, Liu PP, Penninger JM. Impaired heart contractility in apelin gene-deficient mice associated with aging and pressure overload. Circ Res 101: e32–e42, 2007. [Erratum in Circ Res 102: e36, 2008]. doi: 10.1161/CIRCRESAHA.107.158659. [DOI] [PubMed] [Google Scholar]
- 25.LaFlamme B. ELABELA, a peptide hormone for heart development. Nat Genet 46: 7, 2013. [Google Scholar]
- 26.Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, Osmond DH, George SR, O’Dowd BF. Characterization of apelin, the ligand for the APJ receptor. J Neurochem 74: 34–41, 2000. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- 27.Li MX, Wang X, Lindhout DA, Buscemi N, Van Eyk JE, Sykes BD. Phosphorylation and mutation of human cardiac troponin I deferentially destabilize the interaction of the functional regions of troponin I with troponin C. Biochemistry 42: 14460–14468, 2003. doi: 10.1021/bi035408y. [DOI] [PubMed] [Google Scholar]
- 28.Maguire JJ, Kleinz MJ, Pitkin SL, Davenport AP. [Pyr1]apelin-13 identified as the predominant apelin isoform in the human heart: vasoactive mechanisms and inotropic action in disease. Hypertension 54: 598–604, 2009. doi: 10.1161/HYPERTENSIONAHA.109.134619. [DOI] [PubMed] [Google Scholar]
- 29.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101: 365–376, 2000. doi: 10.1016/S0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 30.El Messari S, Iturrioz X, Fassot C, De Mota N, Roesch D, Llorens-Cortes C. Functional dissociation of apelin receptor signaling and endocytosis: implications for the effects of apelin on arterial blood pressure. J Neurochem 90: 1290–1301, 2004. doi: 10.1111/j.1471-4159.2004.02591.x. [DOI] [PubMed] [Google Scholar]
- 31.O’Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, Shi X, Petronis A, George SR, Nguyen T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 136: 355–360, 1993. doi: 10.1016/0378-1119(93)90495-O. [DOI] [PubMed] [Google Scholar]
- 32.Paskaradevan S, Scott IC. Agtrl1b acts non-cell-autonomously for proper cell migration during myocardial progenitor development. Dev Biol 344: 502, 2010. doi: 10.1016/j.ydbio.2010.05.317. [DOI] [Google Scholar]
- 33.Pauli A, Norris ML, Valen E, Chew G-L, Gagnon JA, Zimmerman S, Mitchell A, Ma J, Dubrulle J, Reyon D, Tsai SQ, Joung JK, Saghatelian A, Schier AF. Toddler: an embryonic signal that promotes cell movement via apelin receptors. Science 343: 1248636, 2014. doi: 10.1126/science.1248636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perjés Á, Skoumal R, Tenhunen O, Kónyi A, Simon M, Horváth IG, Kerkelä R, Ruskoaho H, Szokodi I. Apelin increases cardiac contractility via protein kinase Cε- and extracellular signal-regulated kinase-dependent mechanisms. PLoS One 9: e93473, 2014. doi: 10.1371/journal.pone.0093473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peyronnet R, Bollensdorff C, Capel RA, Rog-Zielinska EA, Woods CE, Charo DN, Lookin O, Fajardo G, Ho M, Quertermous T, Ashley EA, Kohl P. Load-dependent effects of apelin on murine cardiomyocytes. Prog Biophys Mol Biol 130, Pt B: 333–343, 2017. doi: 10.1016/j.pbiomolbio.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pi Y, Kemnitz KR, Zhang D, Kranias EG, Walker JW. Phosphorylation of troponin I controls cardiac twitch dynamics: evidence from phosphorylation site mutants expressed on a troponin I-null background in mice. Circ Res 90: 649–656, 2002. doi: 10.1161/01.RES.0000014080.82861.5F. [DOI] [PubMed] [Google Scholar]
- 37.Ramirez-Correa GA, Cortassa S, Stanley B, Gao WD, Murphy AM. Calcium sensitivity, force frequency relationship and cardiac troponin I: critical role of PKA and PKC phosphorylation sites. J Mol Cell Cardiol 48: 943–953, 2010. doi: 10.1016/j.yjmcc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rüegg JC, Caspar Rüegg J, Zeugner C, van Eyk JE, Hodges RS, Trayer IP. Myosin and troponin peptides affect calcium sensitivity of skinned muscle fibres. In: Peptides as Probes in Muscle Research. Berlin, Germany: Springer-Verlag, 1991, p. 95–109. [Google Scholar]
- 39.Scimia MC, Hurtado C, Ray S, Metzler S, Wei K, Wang J, Woods CE, Purcell NH, Catalucci D, Akasaka T, Bueno OF, Vlasuk GP, Kaliman P, Bodmer R, Smith LH, Ashley E, Mercola M, Brown JH, Ruiz-Lozano P. APJ acts as a dual receptor in cardiac hypertrophy. Nature 488: 394–398, 2012. doi: 10.1038/nature11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott IC, Masri B, D’Amico LA, Jin S-W, Jungblut B, Wehman AM, Baier H, Audigier Y, Stainier DYR. The G protein-coupled receptor Agtrl1b regulates early development of myocardial progenitors. Dev Cell 12: 403–413, 2007. doi: 10.1016/j.devcel.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Seo K, Inagaki M, Hidaka I, Fukano H, Sugimachi M, Hisada T, Nishimura S, Sugiura S. Relevance of cardiomyocyte mechano-electric coupling to stretch-induced arrhythmias: optical voltage/calcium measurement in mechanically stimulated cells, tissues and organs. Prog Biophys Mol Biol 115: 129–139, 2014. doi: 10.1016/j.pbiomolbio.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Serpooshan V, Sivanesan S, Huang X, Mahmoudi M, Malkovskiy AV, Zhao M, Inayathullah M, Wagh D, Zhang XJ, Metzler S, Bernstein D, Wu JC, Ruiz-Lozano P, Rajadas J. [Pyr1]-apelin-13 delivery via nano-liposomal encapsulation attenuates pressure overload-induced cardiac dysfunction. Biomaterials 37: 289–298, 2015. doi: 10.1016/j.biomaterials.2014.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma B, Ho L, Ford GH, Chen HI, Goldstone AB, Woo YJ, Quertermous T, Reversade B, Red-Horse K. Alternative progenitor cells compensate to rebuild the coronary vasculature in Elabela- and Apj-deficient hearts. Dev Cell 42: 655–666.e3, 2017. doi: 10.1016/j.devcel.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res 89: 20–25, 2001. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 45.Stankunas K, Hang CT, Tsun Z-Y, Chen H, Lee NV, Wu JI, Shang C, Bayle JH, Shou W, Iruela-Arispe ML, Chang C-P. Endocardial Brg1 represses ADAMTS1 to maintain the microenvironment for myocardial morphogenesis. Dev Cell 14: 298–311, 2008. doi: 10.1016/j.devcel.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun 251: 471–476, 1998. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 47.Tatemoto K, Takayama K, Zou MX, Kumaki I, Zhang W, Kumano K, Fujimiya M. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul Pept 99: 87–92, 2001. doi: 10.1016/S0167-0115(01)00236-1. [DOI] [PubMed] [Google Scholar]
- 48.Wang C, Du J-F, Wu F, Wang H-C. Apelin decreases the SR Ca2+ content but enhances the amplitude of [Ca2+]i transient and contractions during twitches in isolated rat cardiac myocytes. Am J Physiol Heart Circ Physiol 294: H2540–H2546, 2008. doi: 10.1152/ajpheart.00046.2008. [DOI] [PubMed] [Google Scholar]
- 49.Wang W, McKinnie SMK, Patel VB, Haddad G, Wang Z, Zhabyeyev P, Das SK, Basu R, McLean B, Kandalam V, Penninger JM, Kassiri Z, Vederas JC, Murray AG, Oudit GY. Loss of apelin exacerbates myocardial infarction adverse remodeling and ischemia-reperfusion injury: therapeutic potential of synthetic apelin analogues. J Am Heart Assoc 2: e000249, 2013. doi: 10.1161/JAHA.113.000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wijnker PJM, Li Y, Zhang P, Foster DB, dos Remedios C, Van Eyk JE, Stienen GJM, Murphy AM, van der Velden J. A novel phosphorylation site, serine 199, in the C-terminus of cardiac troponin I regulates calcium sensitivity and susceptibility to calpain-induced proteolysis. J Mol Cell Cardiol 82: 93–103, 2015. doi: 10.1016/j.yjmcc.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu X, Chang B, Blair NS, Sargent M, York AJ, Robbins J, Shull GE, Molkentin JD. Plasma membrane Ca2+-ATPase isoform 4 antagonizes cardiac hypertrophy in association with calcineurin inhibition in rodents. J Clin Invest 119: 976–985, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xin Q, Cheng B, Pan Y, Liu H, Yang C, Chen J, Bai B. Neuroprotective effects of apelin-13 on experimental ischemic stroke through suppression of inflammation. Peptides 63: 55–62, 2015. doi: 10.1016/j.peptides.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 53.Ye J, Ni P, Kang L, Xu B. Apelin and vascular endothelial growth factor are associated with mobilization of endothelial progenitor cells after acute myocardial infarction. J Biomed Res 26: 400–409, 2012. doi: 10.7555/JBR.26.20120052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye L, Ding F, Zhang L, Shen A, Yao H, Deng L, Ding Y. Serum apelin is associated with left ventricular hypertrophy in untreated hypertension patients. J Transl Med 13: 290, 2015. doi: 10.1186/s12967-015-0635-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yue P, Jin H, Xu S, Aillaud M, Deng AC, Azuma J, Kundu RK, Reaven GM, Quertermous T, Tsao PS. Apelin decreases lipolysis via Gq, Gi, and AMPK-dependent mechanisms. Endocrinology 152: 59–68, 2011. doi: 10.1210/en.2010-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeng X-XIXI, Wilm TP, Sepich DS, Solnica-Krezel L. Apelin and its receptor control heart field formation during zebrafish gastrulation. Dev Cell 12: 391–402, 2007. doi: 10.1016/j.devcel.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 57.Zhang F, Sun H-J, Xiong X-Q, Chen Q, Li Y-H, Kang Y-M, Wang J-J, Gao X-Y, Zhu G-Q. Apelin-13 and APJ in paraventricular nucleus contribute to hypertension via sympathetic activation and vasopressin release in spontaneously hypertensive rats. Acta Physiol (Oxf) 212: 17–27, 2014. doi: 10.1111/apha.12342. [DOI] [PubMed] [Google Scholar]
- 58.Zhang P, Kirk JA, Ji W, dos Remedios CG, Kass DA, Van Eyk JE, Murphy AM. Multiple reaction monitoring to identify site-specific troponin I phosphorylated residues in the failing human heart. Circulation 126: 1828–1837, 2012. doi: 10.1161/CIRCULATIONAHA.112.096388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhong J-C, Yu X-Y, Huang Y, Yung L-M, Lau C-W, Lin S-G. Apelin modulates aortic vascular tone via endothelial nitric oxide synthase phosphorylation pathway in diabetic mice. Cardiovasc Res 74: 388–395, 2007. doi: 10.1016/j.cardiores.2007.02.002. [DOI] [PubMed] [Google Scholar]