Abstract
Large, elastic arteries are composed of cells and a specialized extracellular matrix that provides reversible elasticity and strength. Elastin is the matrix protein responsible for this reversible elasticity that reduces the workload on the heart and dampens pulsatile flow in distal arteries. Here, we summarize the elastin protein biochemistry, self-association behavior, cross-linking process, and multistep elastic fiber assembly that provide large arteries with their unique mechanical properties. We present measures of passive arterial mechanics that depend on elastic fiber amounts and integrity such as the Windkessel effect, structural and material stiffness, and energy storage. We discuss supravalvular aortic stenosis and autosomal dominant cutis laxa-1, which are genetic disorders caused by mutations in the elastin gene. We present mouse models of supravalvular aortic stenosis, autosomal dominant cutis laxa-1, and graded elastin amounts that have been invaluable for understanding the role of elastin in arterial mechanics and cardiovascular disease. We summarize acquired diseases associated with elastic fiber defects, including hypertension and arterial stiffness, diabetes, obesity, atherosclerosis, calcification, and aneurysms and dissections. We mention animal models that have helped delineate the role of elastic fiber defects in these acquired diseases. We briefly summarize challenges and recent advances in generating functional elastic fibers in tissue-engineered arteries. We conclude with suggestions for future research and opportunities for therapeutic intervention in genetic and acquired elastinopathies.
Keywords: aorta, compliance, elasticity, extracellular matrix, stiffness
INTRODUCTION
Elastin is an extracellular matrix (ECM) protein with a unique biochemical structure that provides entropic elasticity, allowing the large arteries to reversibly expand and relax with every cardiac cycle. Elastin is the main component of elastic fibers that provide the large artery elasticity necessary for proper cardiovascular function in vertebrate animals. Insufficiency of elastin or disorganization, improper assembly, fragmentation, and biochemical modifications of elastic fibers change the passive mechanical behavior of the large arteries and affect cardiovascular mechanics at multiple length scales. Both genetic and acquired cardiovascular diseases are associated with elastin and elastic fiber defects and the resulting changes in arterial mechanics. Tropoelastin, the soluble precursor to elastin, and the degradation products of fragmented elastic fibers also have important chemical signaling properties.
Elastin is highly hydrophobic, extensively cross-linked, and is assembled into elastic fibers in a dynamic process involving cells, cell surface receptors, and numerous elastic fiber associated proteins. Mutations in the elastin gene cause supravalvular aortic stenosis (SVAS), associated with elastin insufficiency, and autosomal dominant cutis laxa-1 (ADCL1), associated with tissue-specific defects in elastic fiber assembly. SVAS mutations appear throughout the gene, but ADCL1 mutations are clustered near the last few exons, suggesting a role for that region in tissue-specific elastic fiber assembly. Since elastin synthesis and elastic fiber assembly are primarily restricted to development and maturation, it is critical to understand the assembly mechanisms for designing interventions in elastinopathies and to recreate elastic fiber structure and function in tissue-engineered arteries. Acquired diseases are generally associated with elastic fiber degradation or biochemical modification. This degradation initiates a destructive arterial remodeling cascade that is difficult to stop and may be impossible to reverse. As modern medicine has extended life expectancy beyond elastin’s 70-yr half-life, it is imperative to understand how degradation and modification of elastic fibers within the arterial wall contribute to the pathogenesis of acquired diseases.
In this review, we summarize the biochemistry of elastin and elastic fiber assembly process. We discuss measures of passive arterial mechanics that are affected by elastin amounts, elastic fiber integrity, and cross-linking. When elastin amounts are reduced or elastic fiber integrity is compromised, the vascular wall cells compensate by producing other ECM proteins and remodeling arterial geometry that can further alter the mechanical behavior. Additionally, circulating elastin fragments produced during elastic fiber degradation can activate cytokine and inflammatory signaling pathways that further degrade elastic fibers in a positive feedback cycle. We summarize the results of human and animal studies that have advanced our understanding of genetic elastinopathies and the cardiovascular effects of varying elastin gene dosage. We address acquired cardiovascular diseases that are associated with elastic fiber degradation or modification. We briefly discuss advances in generating elastic fibers within tissue-engineered arteries and conclude with suggestions for future research.
ELASTIN
Elastin Characterization
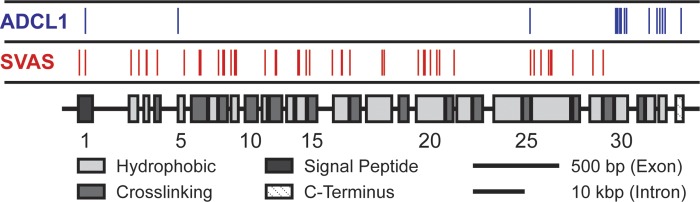
Elastin is an elastic protein polymer that is highly resilient due to extensive posttranslational cross-linking of the monomeric precursor tropoelastin. Reviews focusing on tropoelastin and/or elastin have been written by Vrhovski and Weiss (191) and Kozel et al. (87). Due to the numerous cross-links at lysine residues and having a composition of nearly 75% hydrophobic residues, elastin is extremely hydrophobic (191). The precursor tropoelastin is a 70-kDa protein transcribed from a single elastin gene in the mammalian genome (51, 128, 158). The human elastin gene is 45 kb, located on chromosome 7, and contains 34 exons of which each exon encodes either a hydrophobic or cross-linking domain in an alternating pattern (Fig. 1) (9, 138, 180, 206). Elastin is only present in vertebrate animals, and there is extensive homology among human, chick, bovine, and rat species at the nucleic and amino acid levels (191). During an organism’s lifespan, elastin gene expression and protein synthesis occur within a narrow timeframe initiating late in embryonic development and terminating in adolescence with essentially no de novo elastin production throughout adult life of the animal (15, 26, 34, 119, 133, 142, 145, 163). The narrow timeframe of elastin expression and synthesis is unusual for an ECM component, as generally ECM production is dynamic throughout the organism’s lifespan. Because of elastin’s resilience and a half-life of nearly 70 yr, the limited time period of expression and synthesis is sufficient for the protein to last a lifetime in most species (151, 165).
Fig. 1.
Domain structure of the human elastin gene. Alternating hydrophobic and cross-linking domains are generally transcribed by individual exons. The locations of 64 unique polymorphisms (Human Gene Mutation Database, www.hgmd.cf.ac.uk) associated with either supravalvular aortic stenosis (SVAS; red, 49 mutations not including large deletions) or autosomal dominant cutis laxa-1 (ADCL1; blue, 15 mutations) are indicated.
Regulation of Tropoelastin Expression
Immunohistochemistry studies in the developing chicken lung (71) and aorta (68) have shown that tropoelastin expression correlates with smooth muscle differentiation markers and suggest that transcription of elastin is developmentally regulated. Tropoelastin mRNA levels are decreased in adult compared with neonatal rat lungs, but tropoelastin pre-mRNA levels are similar, suggesting that posttranscriptional regulation turns off tropoelastin expression in adulthood (176). An element in the open reading frame specifically binds a cytosolic protein that destabilizes tropoelastin mRNA. Binding affinity increases in adult compared with neonatal lung fibroblast cells, mediating rapid tropoelastin mRNA decay and decreasing overall mRNA levels. Binding affinity is decreased by transforming growth factor-β1 (TGF- β1) (212), which stimulates tropoelastin expression in human fetal lung fibroblasts by stabilizing its mRNA (89). Active Smads and de novo tropoelastin expression are required for TGF-β1-mediated tropoelastin mRNA stabilization in cell culture (90). TGF-β1 immunoreactivity is present at the sites of arterial elastin expression as detected by in situ hybridization, supporting a role for TGF-β1 regulation of tropoelastin expression in vivo (159). TGF-β1 may additionally regulate tropoelastin expression through interactions with the promoter region in a tissue-specific manner (109). IGF-1, vitamin D, and IL-1β also modulate tropoelastin expression at either the promoter level or through increased mRNA stability (191). Cytokine regulation of tropoelastin expression has been reviewed by Sproul and Argraves (171).
Coacervation and Cross-Linking
In the arterial wall, tropoelastin is primarily produced from vascular smooth muscle cells (VSMCs) in the media (46, 68), although it can also be produced by endothelial cells (ECs) in the intima (19, 113) and fibroblasts in the adventitia (152, 187). After secretion to the extracellular space, tropoelastin simultaneously undergoes coacervation and cross-linking. Cross-linking is facilitated by enzymes from the lysyl oxidase (LOX) family, in particular LOX and LOX-like-1 (LOXL-1). The role of LOX in elastic fiber assembly has been reviewed by Lucero and Kagan (105). Coacervation is a reversible and thermodynamically driven self-assembly of tropoelastin into globular aggregates that occurs spontaneously at physiological temperatures and is a consequence of the numerous hydrophobic regions in tropoelastin (22, 23, 134, 183, 186, 190). LOX activity appears to depend on coacervation, as LOX demonstrates higher affinity for insoluble, coacervated tropoelastin and is less effective at cross-linking tropoelastin at temperatures below the threshold for spontaneous coacervation (75, 120). While accepted that coacervation is a critical step to cross-linking, it is unclear whether this is due to induced conformational changes in tropoelastin or to tropoelastin sequestering (80, 120, 191).
Through the activity of LOX, an oxidative deamination reaction converts many lysine residues into allysine (143). It is suspected that the presence of an aromatic residue adjacent to and on the COOH-terminal end of lysine sterically inhibits LOX activity, preserving several lysine residues for downstream reactions in the cross-linking process (43). After side chain oxidation, a sequence of spontaneous condensation reactions occurs between the newly formed allysine residues and unreacted lysine residues to form covalent cross-links called desmosine and isodesmosine between tropoelastin units. Desmosine and isodesmosine are unique to elastin and serve as a basis for quantification of elastin amounts in tissues (173).
Elastic Fiber Assembly
Elastin forms the core of elastic fibers and is the most abundant component of the mature structure. The other major component is a scaffold of microfibrils, which are composed mostly of fibrillin-1 and -2 (156, 210). Elastic fiber assembly involves nearly 30 different proteins that are tightly coordinated spatially and temporally (80). The function of only a handful of these associated proteins is understood and elucidation of the function of others is an active area of investigation. Reviews focusing on elastic fiber assembly have been written by Kielty et al. (80), Wagenseil and Mecham (195), and Yanagisawa and Davis (205). Spontaneous tropoelastin coacervation and the cross-linking catalyzed by LOX at the cell surface initiate the assembly process. There is evidence that some of the associated assembly proteins, namely, fibulin (FBLN)4 and/or FBLN5, serve to modulate the size and conformation of the developing elastin aggregates (131). These elastin aggregates are then shuttled to the microfibrils that are anchored to the cell surface through integrins. Finally, further cross-linking occurs, again through LOX, to develop the mature elastic fiber that provides elasticity to the large, elastic arteries.
Elastolytic Enzymes
Proteases capable of degrading elastin or elastic fibers are known as elastases. In total, 12 elastases have been identified; however, they are nonspecific to elastin. Neutrophil elastase (Ela-2), cathepsin G (CatG), and proteinase-3 (Pr-3) are serine-class proteases that may have a stronger affinity for tropoelastin than cross-linked elastin. They have been found in atherosclerotic plaques, are secreted from neutrophils, and likely contribute to atherosclerosis progression (111). Ela-2 and CatG are also secreted from VSMCs in the pulmonary artery (83). Four matrix metalloproteinases (MMP-2, MMP-7, MMP-9, and MMP-12) have elastolytic activity. All four MMPs are secreted by macrophages, linking elastic fiber degradation and inflammation. MMP-2 and MMP-9 are also produced by VSMCs and are able to activate latent TGF-β (207). There are three levels of posttranslational regulation of MMP activity. MMPs are initially secreted as inactive pro-MMPs that require proteolytic cleavage of the propeptide, activity is also turned on by the deactivation of a cysteine switch, and MMPs are regulated by specialized inhibitors called tissue inhibitors of metalloproteinase (TIMPs) (189). Another four elastases, also secreted by macrophages, belong to the cysteine class: cathepsin L, S, K, and V (CatL, CatS, CatK, and CatV). CatL, CatS, and CatK are also expressed by VSMCs (111). Pepsin A is the final known elastase, but as a digestive enzyme released in the stomach it is unlikely to play a role in vascular remodeling.
ARTERIAL MECHANICS
Arterial Wall Structure
The structure of the arterial wall is divided into three layers: intima, media, and adventitia. The intima is a single layer of ECs directly adjacent to the arterial lumen that sits on a basement membrane constructed of collagen type IV, laminin, and proteoglycans (208). The middle and largest layer, the media, is further sublayered into lamellar units defined by fenestrated sheets of elastic fibers (Fig. 2), or elastic laminae. These laminae are separated by a region composed of VSMCs, thin elastic fibers, collagen (mostly types I and III), and proteoglycans (27, 32, 64, 123). An internal elastic lamina separates the media from the intima. Elastic fibers and collagen interconnect the elastic laminae forming a continuous network with three-dimensional helical structure in which the fibers are oriented in a near-circumferential direction (123). The number of lamellar units decreases along the arterial tree with larger elastic arteries, such as the descending thoracic aorta, having ~60 units in humans (203) and muscular arteries closer to the periphery having fewer than 3 units. The number of lamellar units scales with physiological pressure and arterial size across mammalian species, providing a constant wall tension/lamellar unit (204). Cross-sections of a large and small artery from a mouse, demonstrating the number and organization of elastic laminae in the wall, are shown in Fig. 3. An external elastic lamina exists between the media and the outermost layer, the adventitia, in large arteries but is absent in the smallest muscular arteries. The adventitia contains fibroblasts, fibrocytes, and a collagen-rich matrix. Large ateries in large animals contain a vasa vasorum in the adventitia to supply blood to the outer wall cells that are too far from the arterial lumen for diffusion of oxygen and nutrients across the wall.
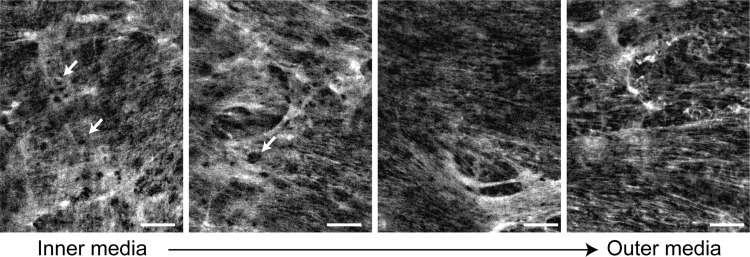
Fig. 2.
Structure of the elastin network in arterial elastic laminae. En face images of elastin in a wild-type mouse ascending aorta obtained using nonlinear fluorescence microscopy are shown (166). A dense, fenestrated (arrows) elastin network can be observed in elastic laminae near the inner media (left). The network appears more fibrous toward the outer media (right). Circumferential direction is horizontal; axial direction is vertical. Scale bars = 20 μm.
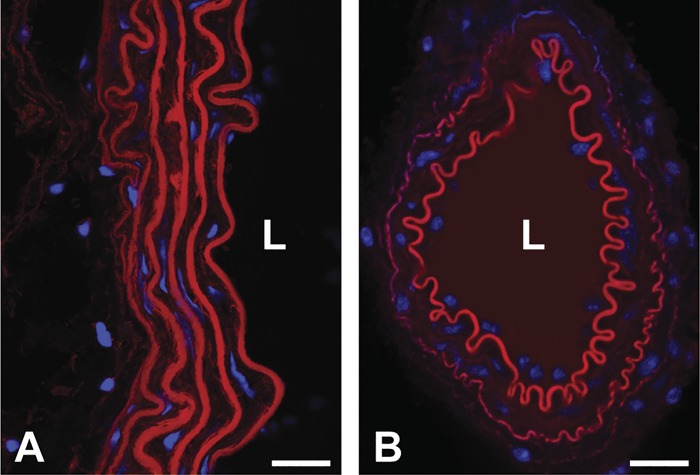
Fig. 3.
Organization of the elastic laminae in large and small arteries. Cross-sections of a mouse descending aorta (A) and mesenteric artery (B) stained for elastin (red) and cell nuclei (blue) are shown (93). The descending aorta has multiple layers of elastic laminae. The mesenteric artery has an internal elastic lamina at the luminal surface and a thin external elastic lamina at the adventitial surface. L, lumen. Scale bars = 20 μm.
The Windkessel Effect
Elastin is only present in vertebrate animals with a closed circulatory system and pulsatile pressure and flow (155). The amount of elastin is highest in the large, elastic arteries closest to the heart and decreases as one moves distally in the cardiovascular system (16). Elastic fibers provide reversible elasticity to the large, elastic arteries. This allows the aorta to deform elastically under an applied hemodynamic load, with no permanent deformation and no energy dissipation when the load is removed. Stephen Hales in 1733 recognized the importance of arterial elasticity in cardiovascular function and Otto Frank in 1895 formalized Hales’ ideas into a mathematical model of the Windkessel effect (132). A Windkessel is an air chamber that was used in fire engines during the 18th century to convert pulsatile pumping into a constant flow of water. In the cardiovascular system, the Windkessel effect dampens the pulsatile flow from the left ventricle (LV), so that the distal vasculature receives almost constant perfusion. Blood ejected from the LV during systole distends the aortic wall and during diastole the aorta reversibly returns to its original configuration. The strain energy stored during systolic deformation is returned as work to further pump blood downstream during diastole. If the Windkessel effect is compromised, for example, by increased arterial stiffness in aged or diseased arteries, the microvasculature of downstream organs, especially in the brain and kidney, can be damaged (125). Furthermore, the Windkessel effect reduces cardiac afterload and perfuses the coronary arteries during diastole; hence, cardiac function can deteriorate when the Windkessel effect is compromised (126).
The Windkessel effect can be compromised through genetic or acquired elastinopathies that alter elasticity of the arterial wall. Elasticity can be altered by increasing wall stiffness or increasing energy dissipation so that less stored strain energy is available to do work on circulating blood. Increased wall stiffness also increases the pulse wave velocity (PWV) of traveling pressure waves in the cardiovascular system. Reflected waves from arterial branch points normally augment diastolic pressure, but increased PWV causes the reflected waves to return earlier in the cardiac cycle and augments systolic blood pressure, leading to systolic hypertension, decreased diastolic perfusion, and increased pulse pressure, which can further damage downstream organs (52).
Arterial Stiffness and Passive Mechanical Behavior
Arterial stiffness is a structural mechanical property that depends on the material properties and geometry of the arterial wall. Common measurements of stiffness include Peterson’s modulus, distensibility, and PWV (50) and almost always refer to deformation and loading in the circumferential direction. We will call these measurements “structural stiffness” values. Peterson’s modulus and distensibility can be measured in vivo or in vitro, while PWV is usually measured in vivo. PWV can be determined simply and reproducibly in the clinic by recording the time it takes for a blood pressure pulse wave to travel a specified distance in the arterial tree (usually from the carotid to the femoral artery) (124). PWV is a measure of structural stiffness because the value is related to Young’s modulus of the arterial wall (a material property), wall thickness, arterial diameter, and density of blood according to the Moens-Korteweg equation (50). The derivation of the Moens-Korteweg equation assumes that the aorta is a perfect cylinder with no tapering or branches, made up of a linear elastic, isotropic material that experiences small deformations during the cardiac cycle, none of which are precisely true. While PWV is a useful measure of structural arterial stiffness, the assumptions accompanying its use must be kept in mind.
Arterial wall material properties are nonlinear and vary with the direction of deformation and loading (i.e., circumferential vs. axial); hence, they cannot be quantified by a single measurement, such as Young’s modulus in the Moens-Korteweg equation. However, estimates of arterial material properties can be obtained by linearizing the mechanical behavior within a given deformation or loading range in a specified direction to provide an incremental Young’s modulus for comparison across samples (50). Material stiffness values can also be obtained by fitting constitutive models to detailed in vitro mechanical testing data and calculating derivatives of the conjugate stress and strain measurements (41). Fitting constitutive models allows comparison of material behavior and stored strain energy for any loading condition in any direction but requires multiple experimental protocols for each sample and is dependent on the suitability of the chosen constitutive equation.
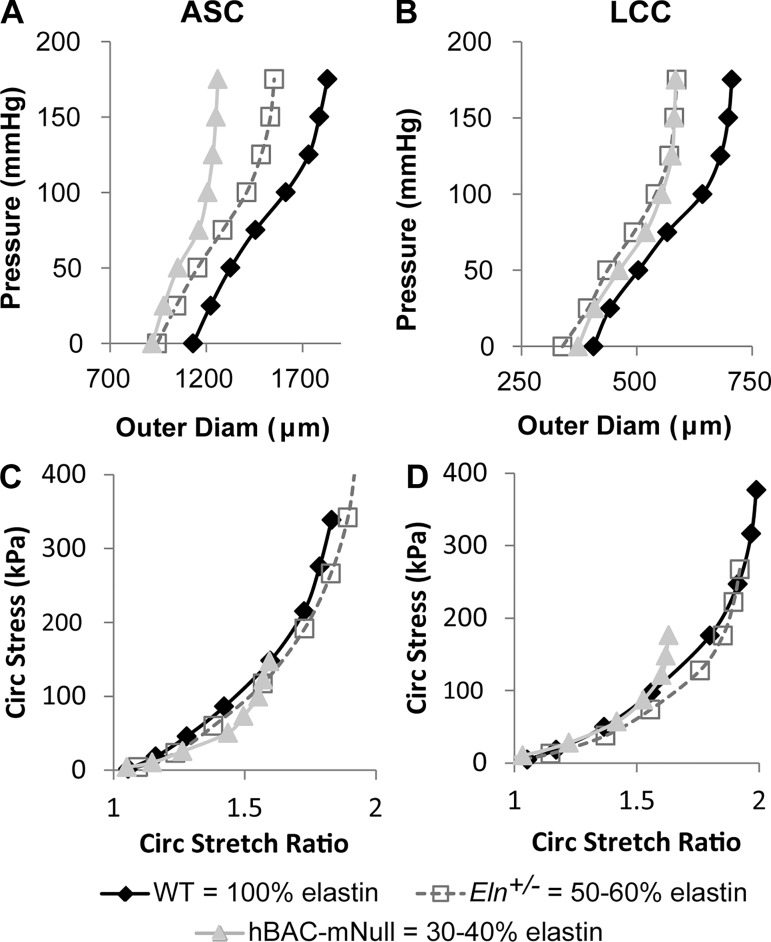
The passive mechanical behavior of large, elastic arteries is determined mostly by the amount and organization of elastic fibers and collagen fibers in the wall. VSMCs contribute to arterial mechanics through active contraction or relaxation and by producing the surrounding ECM proteins, but they do not contribute substantially to the passive mechanical behavior (38). Although there is only one elastin protein, there are multiple collagen proteins. The most abundant arterial collagens are types I and III, with lesser amounts of types IV, V, and VI. The amounts and transmural organization of collagen types depend on location in the cardiovascular tree (64). Elastic arteries show nonlinear behavior with maximum distensibililty near physiological pressures and reduced distensibility at high pressures (150), presumably to protect the cells and protein components from irreversible damage due to excessive deformation. Selective digestion of elastic fibers or collagen fibers with proteases indicates that the resistance to stretch in elastic arteries at low pressures is due to elastic fibers, while the resistance to stretch at high pressures is due to collagen fibers (33, 42, 146, 164). Experiments on the aorta from mice lacking elastin (Eln−/−) have demonstrated a reduction in the circumferential material stiffness at low pressure, consistent with the observed role of elastic fibers in protease experiments (82). However, experiments on arteries from elastin haploinsufficient mice (Eln+/−) with 50–60% of normal elastin levels show similar circumferential stress-stretch behavior to wild-type (WT) mice (Fig. 4), which has been attributed to remodeling of the cardiovascular system during development and maturation (39, 197).
Fig. 4.
Effects of elastin amounts on arterial mechanical behavior. A−D: representative pressure-outer diameter (A and B) and circumferential Cauchy stress-stretch ratio (C and D) curves (197) for the ascending aorta (ASC; A and C) and left common carotid (LCC; B and D) from wild-type (WT; 100% elastin), Eln+/− (50–60% elastin), and hBAC-mNull (30–40% elastin) mice. Decreased elastin amounts shift the pressure-diameter behavior for both artery locations, but the difference between Eln+/− and hBAC-mNull pressure-diameter behavior for the LCC is negligible. For both artery locations, the stress-stretch behavior is similar between WT and Eln+/− mice but becomes more nonlinear at lower stretch ratios for hBAC-mNull mice. The data highlight the importance of examining structural (pressure-diameter) and material (stress-stretch) mechanical behavior as well as differences between artery locations in the structural response to reduced elastin amounts. The results suggest that mouse arteries can remodel to maintain WT material behavior when elastin amounts are 50–60% of normal but cannot fully remodel and adapt the material behavior when elastin amounts are 30–40% of normal.
Remodeling of the arterial wall in response to genetic or acquired elastinopathies is important for determining the physiologic mechanical behavior. Production and reorganization of collagens (198) or other ECM components, such as proteoglycans (70), are often observed in response to defects in or degradation of the elastic fibers. Additional collagen deposition or a shift in the ratio of different types of collagens in the wall may increase stiffness of the wall and affect the Windkessel function and strain energy storage capacity (194). Additional proteoglycans may pool in the wall and provide a catalyst for arterial dissections (148).
Energy Storage and Return
Because elastin is a highly resilient protein, it allows the wall of the large arteries to deform with little energy loss on loading and unloading. Energy loss is small but finite at 15–20%, and this value appears to be consistent across various species (48). Energy loss is attributed to viscous losses from VSMCs (201) or proteoglycans (200). The relative contributions of different components to the viscoelastic behavior of arteries have been determined using protease digestion in a similar manner to the elastic contributions for different pressure ranges. Recent experiments on newborn mouse aortae lacking elastin (Eln−/−), lysyl oxidase (Lox−/−), or fibulin-4 (Fbln4−/−) have shown that properly assembled and cross-linked elastic fibers are necessary for low energy loss (82). Many investigations do not include energy loss in mechanical studies on large elastic arteries, but this may be an important consideration in diseases with highly fragmented elastic fibers.
ELASTIN AS A SIGNALING MOLECULE
Tropoelastin
While we have focused so far on the mechanical effects of structural changes in elastic fibers, elastin and its degradation products have chemical signaling effects as well. For example, soluble tropoelastin can regulate the cell phenotype. The addition of tropoelastin to the culture media of VSMCs from Eln−/− mice inhibits proliferation (76) and increases stiffness as measured by atomic force microscopy (37). Karnik et al. (77) showed that a specific domain within tropoelastin, VGVAPG, induces actin polymerization in VSMCs through a G protein-coupled pathway. The VGVAPG peptide is a potent chemoattractant for VSMCs (77), fibroblasts, and monocytes (162). Sites have been identified at the COOH terminus and central region of tropoelastin that mediate binding to αvβ3- and αvβ5-integrins, which are involved in numerous signaling pathways (96, 149). A nonintegrin-mediated interaction between tropoelastin and the cell membrane has also been identified through the binding of fractionated tropoelastin to heparin sulfated proteoglycans (14). Tropoelastin expression is detectable in the adult human aorta at decreasing levels with aging (44), implying that reduced tropoelastin as well as degradation and modification of existing elastic fibers may modulate age-related changes in the arterial wall.
Elastin-Derived Peptides
Elastic fibers can be degraded and fragmented by mechanical fatigue, calcification, glycation, lipid peroxidation, and protease digestion (35). The resulting elastin-derived peptides (EDPs) are bioactive and retain the VGVAPG peptide sequence found in tropoelastin, rendering similar signaling capability. EDP levels in the blood are increased in humans with obliterative arteriosclerosis of the legs, type IIb hyperlipidemia, hypertension, diabetes, and ischemic heart disease (45). Whether the increased levels of EDPs are a cause or consequence of the disease is unknown. Recent studies in mice have suggested that while elastic fiber fragmentation may be secondary to the genetic or acquired disease, circulating EDPs contribute to further disease progression. Chronic doses of EDPs, as well as VGVAPG peptides, increase the size of atherosclerotic plaques at the aortic root in two different atherosclerosis susceptible mouse models (47). EDPs are capable of binding the lactose-insensitive receptor found on the surface of macrophages, implicating a role for EDPs in macrophage migration in inflammation and atherosclerosis progression (107). A single dose of EDPs, as well as VGVAPG peptides, causes hyperglycemia in mice (162). Rat aortic VSMCs incubated with EDPs show increased expression of typical bone proteins, which may contribute to arterial calcification (167). An antibody to VGVAPG blocks EDP signaling to inflammatory cells and protects against elastase-induced emphysema in mice (63). VGVAPG antibody also reduces aortic elastic fiber fragmentation, macrophage infiltration, and TGF-β1 activity in a mouse model of aortic aneurysm due to reduced fibrillin-1 levels (54). Finally, VGVAPG sequence-carrying EDPs have been shown to interact with the galectin-3 receptor, which is involved in melanoma chemotaxis and expression of MMP-2 and MMP-3 (141). A review summarizing the effects of elastin degradation and EDPs in cardiovascular aging and disease has been written by Duca et al. (35).
The Elastin Receptor Complex
Many of the chemical signaling effects of tropoelastin and/or EDPs are thought to be mediated by the elastin receptor complex (ERC) (111). The ERC is composed of two membrane-bound subunits, protective protein/cathepsin A (PPCA) and neuraminidase-1 (Neu-1), plus elastin-binding protein (EBP). EBP (also known as S-Gal) is a splice variant of lysosomal β-galactosidase that retains the ability to bind galactosugars but has lost enzymatic activity. In addition, the splice variant has a 32-peptide sequence capable of binding tropoelastin (59). In elastogenesis, EBP is thought to be responsible for shuttling tropoelastin to the cell surface, and antagonistic interaction between EBP and galactose or lactose may release tropoelastin to the extracellular space (58). Neu-1 appears to control signal transduction by ERC through the catalytic conversion of ganglioside to lactosylceramide (153). Downstream signaling of the ERC diverges depending on cell type but reconvenes at the activation of ERK1/2 (35).
TGF-β Sequestration
TGF-β is secreted from cells in the form of a large latent complex (LLC) that includes a latency-associated peptide (LAP) and a latent TGF-β-binding protein (LTBP). LTBP localizes latent TGF-β to the ECM. LTBP1 and LTBP3 bind well to all three TGF-β isoforms (147). LTBP1 has specific interactions with fibrillin-1 microfibrils (129). Although direct interactions of LTBP3 with fibrillin-1 have not been shown, LTBP3 protein is reduced in the aorta of fibrillin-1 knockout (Fbln1−/−) mice (213). In the early stages of elastic fiber assembly, microfibrils are abundant and there is little elastin. Throughout late embryonic development and early maturation, elastin synthesis increases and eventually obscures the microfibrils (196). It is possible that elastin accumulation indirectly regulates TGF-β availability by modulating LLC binding to microfibrils. TGF-β1 immunoreactivity and tropoelastin expression colocalize in the developing rat aorta and decrease with age (159). This has been interpreted as evidence of TGF-β1-regulated expression of tropoelastin in vivo, but it could also be interpreted as tropoelastin-regulated availability of TGF-β1 as elastin accumulates on microfibrils. TGF-β1 signaling is altered when arterial elastic fibers are not assembled properly and through proteolytic cleavage of elastic fiber associated proteins, indicating bidirectional effects between TGF-β1 activity and elastic fiber integrity (61). Elastinopathies that alter elastin amounts or elastic fiber integrity may then affect TGF-β activity (185).
CARDIOVASCULAR DISEASE
Several genetic and acquired cardiovascular diseases are associated with elastin and elastic fiber defects. The defects include altered amounts of elastin and improper assembly, fragmentation, and modification of elastic fibers. Additional reviews on cardiovascular diseases associated with elastin or elastic fiber defects have been written by Baldwin et al. (7) and Milewicz et al. (115).
Genetic Elastinopathies
Supravalvular aortic stenosis.
SVAS (OMIM no. 185500) is a rare (~1:20,000 births) congenital obstruction of the LV outflow tract. The defining defect is a lesion in the aorta at the sinotubular junction (STJ) as a result of VSMC hypertrophy, increased collagen, and reduced/disorganized elastic fibers in the medial layer (29, 136). Often there are additional morphological defects in other elastic arteries, but the arteries affected and severity of the defects are patient specific. There are three forms of SVAS: autosomal dominant familial, sporadic, and Williams-Beuren syndrome (WBS; OMIM no. 194050) associated. The genetic origin defines the form of SVAS. Familial and sporadic SVAS arise from loss-of-function mutations to the elastin gene that are either inherited or de novo, respectively. A variety of mutations have been reported, including translocations, partial deletions, and point mutations (Fig. 1), often resulting in a truncated elastin protein. In WBS-associated SVAS, the entire elastin gene, along with 25–27 other genes, is encompassed in a large deletion on chromosome 7 (140). In all cases, one functional elastin gene remains and sporadic mutations are more common than inherited mutations (5).
SVAS can present as one of two different types. Type I (discrete) is defined by a localized narrowing at the STJ. Type II (diffuse) shows uniform narrowing beginning at the STJ and extending as far as the aortic arch or even the descending aorta (97). The ratio of the type I to type II incidence is ~7:3 (202). Due to the rarity of SVAS, data correlating type I or II with specific elastin mutations are not available (30). In addition to the primary STJ lesion, vascular defects occur in other arteries. Often the aortic valve leaflets are hypertrophic, resulting in partial fusion of the leaflets to the STJ (118). Thirty-nine percent of patients with SVAS have bicuspid aortic valve (30). Pulmonary artery stenosis is present in 83% of patients with SVAS (112, 202). Lesions in the right ventricular outflow tract are also common (30). Coronary arteries can exhibit fibrodysplasia and may have stenotic lesions (160). Because coronaries are muscular arteries, it is unclear whether the lesions are a direct result of the elastinopathy or are a secondary pathology.
Hypertension is common in patients with WBS even at early ages (139) and may be linked to increased arterial structural stiffness due to reduced elastin amounts. Kozel et al. (85) measured PWV in 77 patients with WBS and found an increase versus healthy control subjects. However, the increased PWV was independent of whether or not the patient was hypertensive (85). While hypertension and increases in arterial structural stiffness generally develop coincidentally in acquired cardiovascular disease, their dissociation in WBS may indicate that reduced elastin amounts are a common causal factor. Patients with SVAS have an increased risk of sudden cardiac death, with patients with WBS having the highest risk (202). The most common cause of sudden cardiac death is myocardial ischemia, likely resulting from coronary obstruction and LV hypertrophy (11). Ischemia could also be caused by reduced coronary perfusion during diastole due to a compromised Windkessel effect. Compromised arterial Windkessel function would also increase cardiac afterload and could contribute to LV hypertrophy.
Autosomal dominant cutis laxa-1.
ADCL1 (OMIM no. 123700) is a rare connective tissue disorder in which the skin is loose, is hyperextensible, and has redundant folds. Patients may have cardiovascular pathologies including bicuspid aortic valve, dilation of the aorta or pulmonary artery, and pulmonary emphysema (55).The majority of reported ADCL1 cases have a frame shift mutation within the last five exons of the ELN gene (Fig. 1). The result of the frame shift is usually an elongation of tropoelastin protein that is believed to form larger aggregates during coacervation and impair the ability of elastin to bind to the microfibrillar scaffold during elastic fiber assembly. Callewaert et al. (17) demonstrated impaired tropoelastin deposition onto microfibrils, increased TGF-β signaling, and indications of endoplasmic reticulum stress in cultured fibroblasts from patients with ADCL1. In nearly 50% of ADCL1 cases, aneurysms are present in the aortic root, aortic arch, or pulmonary artery. Aneurysm development is likely a combined result of reduced elastic fiber synthesis and increased susceptibility to elastic fiber degradation (36).
Mouse Models of Genetic Elastinopathies
Targeted deletion of the Eln gene in mice was first reported by Li et al. in 1998 (98). Eln−/− mice represent an extreme elastinopathy and provide insights into the role of elastin in arterial mechanics, VSMC phenotype modulation, and cardiovascular disease. The same year, Li et al. showed that Eln+/− mice demonstrate some of the cardiovascular phenotypes observed in patients with SVAS, such as increased arterial structural stiffness and an increased number of arterial lamellar units but do not have the local aortic lesions observed in SVAS (99). A mouse model of WBS with a large deletion that includes the Eln gene (100) has a similar cardiovascular phenotype to Eln+/− mice (49). Eln+/− mice have been used to study cardiovascular effects of reduced elastin amounts and the associated increases in arterial structural stiffness.
Due to the insertion of a short exon after exon 4 in the mouse and the loss of two exons (34, 35) in the human during primate evolution, the mouse Eln gene has 37 exons, whereas the human ELN gene has 34 exons (178). The human ELN gene also shows unique alternative splicing patterns (40). To create a mouse model to study ELN mutations in human disease, Hirano et al. expressed ELN gene in a bacterial artificial chromosome (BAC) in Eln−/− mice (60). These hBAC-mNull mice only express human elastin and have total elastin amounts at 30–40% of normal (60). hBAC-mNull mice also show VSMC hypertrophy and aortic luminal narrowing representative of SVAS lesions (72). Sugitani et al. engineered a single base deletion associated with ADCL1 within the ELN gene in a BAC and expressed it in mice (175). The mutant elastin was incorporated into elastic fibers in the mouse skin and lung and compromised tissue function. The mutant elastin was incorporated at low levels into the mouse aorta and did not affect tissue function. The results show tissue specific differences in elastic fiber assembly and highlight the utility of “humanized” mice for studying human disease (175).
Effects of graded elastin amounts in mice.
Eln−/−, Eln+/−, and hBAC-mNull mice allow investigation of how graded amounts of elastin affect cardiovascular function. They are relevant for determining threshold amounts of elastin necessary for restoring cardiovascular function in genetic and acquired elastinopathies, as well as in tissue-engineered arteries. Each mouse model is discussed in additional detail below. Comparisons of arterial mechanics, wall structure, and morphology for WT mice with 100% of normal elastin amounts, Eln+/− mice with 50–60% of normal elastin amounts, and hBAC-mNull mice with 30–40% of normal elastin amounts are shown in Figs. 4, 5, and 6, respectively.
Fig. 5.
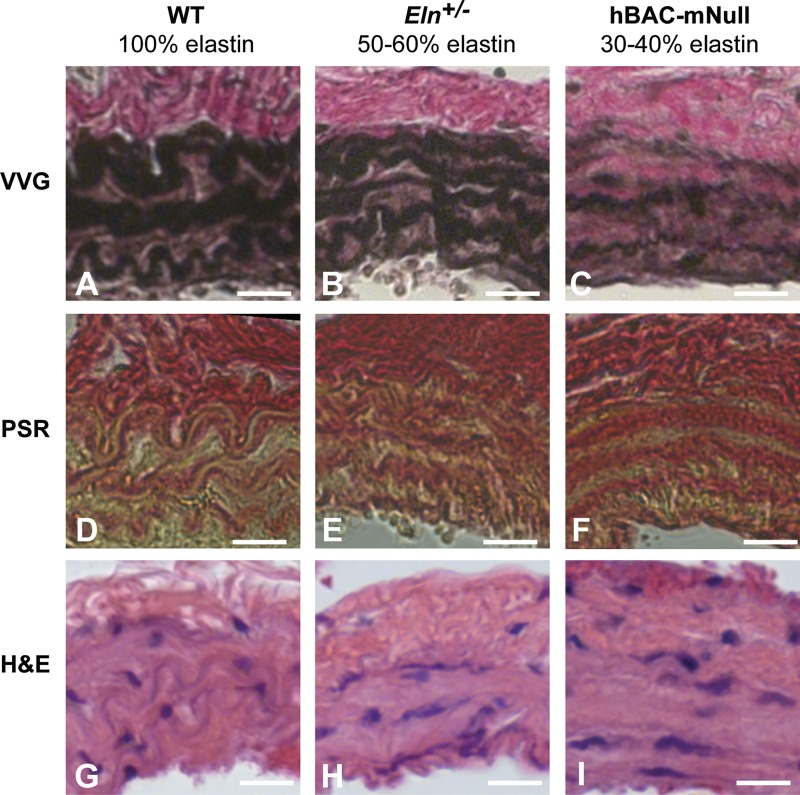
Effects of elastin amounts on arterial wall structure and composition. A−I: Histological sections (93) of the left common carotid artery from wild-type (WT) mice with 100% elastin (A, D, and G), Eln+/− mice with 50–60% elastin (B, E, and H), and hBAC-mNull mice with 30–40% elastin (C, F, and I). A–C: Verhoeff-Van Gieson (VVG)-stained sections show black elastic laminae, brown muscle tissue, and pink collagen. The elastic laminae are thinner, more numerous, and have more diffuse staining as elastin amounts decrease. D−F: picrosirius red (PSR)-stained sections show collagen fibers in red and other material in yellow. In WT arteries, red collagen clearly outlines the yellow elastic laminae. In Eln+/− and hBAC-mNull arteries, the collagen staining overlaps with the elastic laminae. G−I: hematoxylin and eosin (H&E)-stained sections show cell nuclei in purple and other material in pink. Scale bars = 20 μm.
Fig. 6.
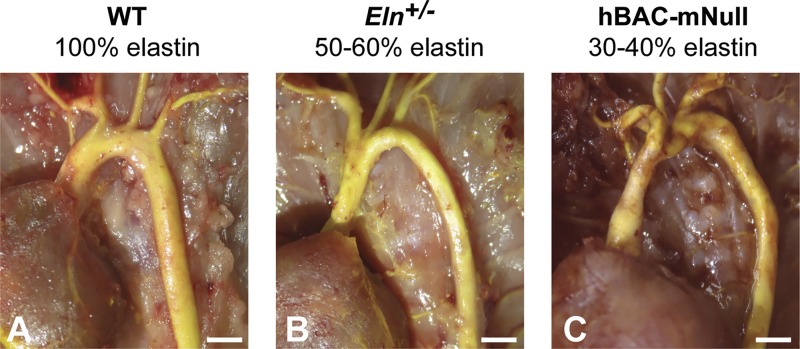
Effects of elastin amounts on arterial morphology. Yellow latex (172) was injected for contrast into the ascending aorta, major branches, and descending thoracic aorta in wild-type (WT; 100% elastin), Eln+/− (50–60% elastin), and hBAC-mNull (30–40% elastin) mice. With decreasing elastin amounts, the aortic diameter decreases and length increases. There are also changes in branching morphology with reduced elastin amounts. Scale bars = 1 mm.
0% elastin.
Eln−/− mice die within a few days of birth due to VSMC overproliferation in the aortic wall leading to luminal occlusion that is similar to the aortic root lesion observed in SVAS (98). Eln−/− mice have normal blood pressure and aortic structural stiffness at embryonic day 18 (192) but high blood pressure and increased aortic structural stiffness at birth (193). Aortas from Eln−/− mice have decreased aortic material stiffness compared with WT mice in the low pressure range but similar aortic material stiffness to WT mice in the high pressure range (82). VSMCs from Eln−/− mice have higher proliferation rates (76) and lower material stiffness values (37), and these phenotypes can be partially reversed by the addition of tropoelastin to the cell culture medium. Treatment with the mechanistic target of rapamycin (mTOR) inhibitor rapamycin decreases VSMC overproliferation in Eln−/− mice but does not extend their lifespan (101). β3-Integrin expression is upregulated in the Eln−/− aorta, and genetically reducing β3-integrin dosage extends the lifespan of Eln−/− mice by ~2 days (116). Pharmacological inhibition of β3-integrin with cilengitide reduces VSMC overproliferation in the Eln−/− aorta and may be a novel treatment to prevent SVAS (116).
50–60% elastin.
Eln+/− mice live a normal lifespan but have hypertension, increased arterial structural stiffness (Fig. 4), and an increased number of lamellar units in the arterial wall (Fig. 5) (39, 99). Treatment with the mTOR inhibitor rapamycin prevents the formation of increased lamellar units in Eln+/− mice (101). Eln+/− arteries show reduced in vivo axial stretch, residual torsion (197), and increased length (Fig. 6). Increased arterial structural stiffness precedes increases in blood pressure compared with WT mice during maturation, indicating that it may be a causative factor in the development of hypertension in Eln+/− mice (94). Eln+/− mice have indications of diastolic dysfunction, which may be linked to compromised arterial Windkessel function and decreased cardiac perfusion during diastole (95). Eln+/− mice have impaired glucose metabolism (28) and altered susceptibility to atherosclerotic plaque accumulation (108, 174). Genetic modifiers of the Eln+/− cardiovascular phenotype have been identified by outcrossing Eln+/− mice. Several genes, including Ren1, Ncf1, and Nos1, were identified that may synergize with elastin insufficiency to predispose an individual to hypertension and increased arterial structural stiffness (86). Treatment of Eln+/− mice with anti-hypertensive medications improves pulse pressure and subsequently physiologic structural stiffness due to the shift in operating pressures but does not change material stiffness of the arteries (56). Treatment of Eln+/− mice with minoxidil, a KATP channel opener and vasodilator, also improves physiologic structural stiffness (84). Eln+/− mice have reduced susceptibility to vascular calcification (Fig. 7) (79). Mice with reduced elastin due to a large genetic deletion that mimics the heterozygous deletion observed in WBS (100) have hypertension and increased arterial stiffness but do not have the increased number of lamellar units observed in Eln+/− mice (49).
Fig. 7.
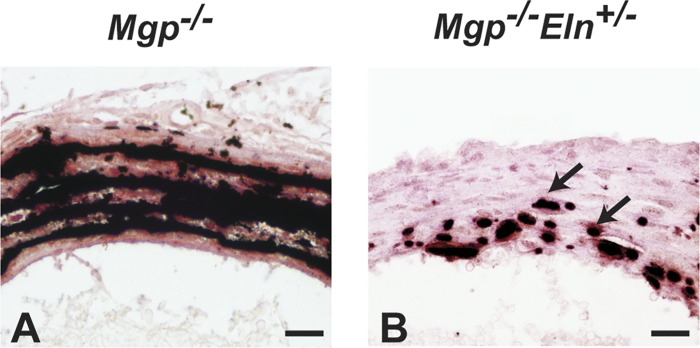
Effects of elastin amounts on medial calcification caused by matrix Gla protein (MGP) deficiency. Mgp−/− mouse arteries show extensive mineral deposition (black) along the elastic laminae (A), which is markedly reduced (arrows) in Mgp−/−Eln+/− mice (B). Thin plastic sections of the thoracic aortae from 2-wk-old mice were stained with von Kossa and van Gieson (79). Scale bars = 20 μm.
30–40% elastin.
Eln−/− mice can be rescued by genetic insertion of a BAC expressing the human elastin gene (60). These hBAC-mNull mice have 35% of normal elastin amounts in the ascending aorta, have extreme hypertension, and have increased arterial structural and material stiffness (Fig. 4) (60). Increased arterial structural stiffness has been confirmed in vivo using ultrasound imaging and may be caused by additional collagen amounts in the aortic media (Fig. 5) and increased wall thickness (72). While lesion development in SVAS is attributed to VSMC overproliferation in the aortic media, Jiao et al. (72) showed that deficient circumferential growth that limits outward expansion of the aorta, combined with normal VSMC proliferation, is a mechanism for luminal narrowing in hBAC-mNull mice. Inhibition of mTOR with rapalogs does not prevent luminal narrowing in hBAC-mNull mice but does prevent the observed collagen fibrosis and arterial structural stiffening (73). hBAC-mNull mice have a longer aorta than WT mice (Fig. 6), and the axial growth may be a compensation mechanism for the deficient circumferential growth (67).
Acquired Cardiovascular Diseases
Due to the limited timeframe of elastin synthesis, elastic fibers that are damaged or degraded with aging or disease are generally not repaired. Elastic fibers are then replaced by collagens and proteoglycans that stiffen the arterial wall and lead to hypertension and damage to downstream organs. The ratio of elastin to collagen in the human aorta decreases 30–40% with age from the second to the ninth decade of life (62). In aging primates, it was recently observed that the elastin-to-collagen ratio changes with age, but disarray of the remaining elastic and collagen fibers may be an even more important contributor to increased arterial stiffness with aging (211). Many acquired diseases represent accelerated or exacerbated versions of the arterial remodeling observed with aging. The cardiovascular diseases discussed in this review, with a focus on elastic fibers and arterial mechanics, include hypertension and arterial stiffening, diabetes, obesity, atherosclerosis, calcification, and aneurysms and dissections.
Hypertension and arterial stiffening.
Hypertension is frequently observed in conjunction with diseases associated with defects in elastin or elastic fibers (4). While hypertension is typically associated with aging, the coincidental occurrence of hypertension in infants with SVAS indicates that age-related factors are not required for SVAS-associated hypertension (85, 139) and that elastin insufficiency likely plays a key role. Modulation of TGF-β availability by emilin-1, an elastic fiber-associated protein, determines systolic blood pressure in mice, supporting a role for elastic fiber integrity and TGF-β signaling in hypertension (209). TGF-β is an important regulator of ECM remodeling in the arterial wall (57). Inflammatory signaling stimulated by EDPs from elastic fiber degradation may also play a role in hypertension. Reduced amounts of elastin or compromised elastic fiber integrity and the subsequent alterations in ECM composition and arterial geometry lead to increased material and/or structural arterial stiffness. While hypertension has long been associated with changes in resistance of the small, muscular arteries, it is now appreciated that increased stiffness of the large arteries can contribute to hypertension. The role of large artery stiffness in hypertension has been reviewed by Greenwald (52), Wagenseil and Mecham (194), and Mitchell (117), among others. Targeting ECM remodeling and the resulting changes in arterial stiffness represent a challenging but potentially effective treatment for hypertension (137).
Spontaneous hypertensive rats (SHRs) are an animal model for essential hypertension developed through selective breeding (127). In young and adult SHRs, systolic blood pressure is increased over Wistar-Kyoto (WKY) rats, but material stiffness of the thoracic aorta is not different (110), indicating that increased stiffness of the large arteries does not precede hypertension in SHRs. SHRs have reduced elastin amounts, smaller arterial diameter, and increased structural stiffness of the mesenteric resistance arteries compared with WKY rats, suggesting that increased resistance of the distal arteries contributes to hypertension development in SHRs (13). With aging, aortic material stiffness dramatically increases in SHRs compared with WKY rats (110). The increased stiffness has been attributed to endothelial dysfunction (154), increases in advanced glycation end products (6), and decreases in the elastin-to-collagen ratio (20) in the aged SHR compared with WKY aorta. These results imply that the SHR is not an appropriate model to investigate large artery stiffness in the development of hypertension but is an appropriate model to investigate the mechanisms of ECM remodeling and increased arterial stiffness in aging coupled with hypertension.
Genetic factors associated with selective breeding of inbred rat strains are linked to changes in amount and organization of the aortic ECM. Behmoaras et al. (10) found that aortic, blood pressure, elastin, collagen and cytokine amounts, and elastic laminae structure are different among seven inbred rat strains. Of the seven strains, the Brown-Norway rat (BNR) has the lowest aortic elastin amount and lowest elastin-to-collagen ratio, leading to increased structural stiffness of the aorta compared with young LOU control rats (130). BNRs are normotensive, suggesting that they are a good model for investigating interactions between elastin and arterial stiffness that are independent of changes in blood pressure. Treatment of cultured BNR VSMCs with K+ channel openers (minoxidil or diazoxide) increases tropoelastin transcription and mRNA stability. Treatment of BNRs with minoxidil or diazoxide increases aortic elastic fiber content (169). In contrast, treatment of Eln+/− mice with minoxidil increases arterial size and stimulates differential expression of 127 matrix or matrix-associated genes but does not show an elastin specific effect (84). Therapeutic options for increasing elastic fiber content may depend on the primary cause of the elastinopathy.
Diabetes.
In diabetes, the body does not produce or respond to insulin appropriately, leading to increased blood glucose levels. In vitro studies have examined the effects of increased glucose on VSMCs, isolated arterial elastin, and arterial mechanical behavior. High-glucose media, with the addition of EDPs and TGF-β1, increase expression of osteogenic genes in cultured VSMCs, and may lead to arterial calcification associated with diabetes (168). Incubation of isolated arterial elastin in high-glucose solution increases the storage and loss modulus, indicating an increase in material stiffness and viscous energy loss (102). After 48 h of incubation in a high-glucose solution, Zou and Zhang (214) found an increase in the material stiffness of isolated arterial elastin in the longitudinal direction but not in the circumferential direction. With incubation times up to 28 days, Wang et al. (199) found an increased material stiffness in both longitudinal and circumferential directions as well as increased hysteresis (or energy loss). The mechanical changes in isolated arterial elastin have been attributed to the dehydrating effects of glycation (102), but it is unclear how these results translate in vivo for an intact arterial wall with ECs acting as an impermeable barrier. Assuming that the glucose can reach the medial layers, increased glucose may lead to glycation of elastic fibers that alters stiffness and protease susceptibility.
In a rat diabetic model, glycation increases over time in arterial samples as diabetes develops but is unchanged in control rats (182). Diabetic rats also have reduced elastin protein levels in aortic extracts as well as an increase in elastolytic activity (91). A decreased elastin-to-collagen mRNA ratio and increased structural stiffness of the aorta are observed in the leptin receptor-deficient mouse model for type 2 diabetes (db/db). Interestingly, the same study (78) found opposite trends in the elastin-to-collagen mRNA ratio and structural stiffness for the db/db coronary artery. Human patients with diabetes show a similar decrease in elastin-to-collagen protein ratios in aortic tissues (179) and an increase in circulating CatS levels (103). There are increased EDP levels in children with diabetes (122), indicating that degradation of elastic fibers may occur in diabetes that contributes to arterial stiffening and inflammatory signaling. EDPs may also contribute to insulin resistance and the onset of diabetes with aging (12). These results suggest that high glucose associated with diabetes may physically stiffen elastic fibers as well as make them more susceptible to degradation, altering passive arterial mechanics and initiating a chemical signaling cascade through EDPs.
Obesity.
Obesity is a risk factor for increased cardiovascular-related mortality, but it is typically associated with additional cardiovascular risk factors including hypertension and diabetes. Rider et al. (144) observed an increase in aortic PWV in obese patients absent other cardiovascular risk factors, providing evidence for a direct correlation between obesity and aortic structural stiffness. Elastin is decreased in adipose tissue of obese patients (170); however, investigations on elastin and elastic fiber changes in large arteries from obese patients have yet to be performed. TGF-β1 levels positively correlate with body mass index in humans (184) and are upregulated in adipose tissue from obese humans (2) and mice (157). Cathepsin elastases, CatK, CatL, and CatS have increased activity with increased body mass index in humans and in the leptin-deficient (ob/ob) mouse model of obesity (92). In ob/ob mice, aortic stiffening was observed by an increase in PWV, an increase in abdominal aorta structural stiffness in vitro, and an increase in thoracic aorta material stiffness as measured by atomic force microscopy. Elastic fiber remodeling in the ob/ob aorta was evidenced by a decrease in LOX protein levels and activity, a decrease in desmosine cross-links, an increase in elastase activity, and a higher degree of fragmentation in the elastic lamellae (21). The observed increase in aortic stiffness with obesity may arise from elastic fiber remodeling through LOX downregulation and an upregulation of elastolytic activity.
Atherosclerosis.
Both the synthesis of tropoelastin and degradation of elastic fibers are increased in human atherosclerotic lesions (1). This may be due to the production of tropoelastin (88), metalloproteinases (121), and cathepsins (92) by macrophages involved in atherosclerosis. Elastic fiber degradation allows infiltration of lipids and immune cells into the aortic wall for plaque formation and rupture in mice (188). EDPs resulting from elastic fiber fragmentation further enhance atherogenesis (47). TGF-β1 activity, which may be modulated by elastic fiber degradation, has a wide range of effects in atherosclerosis (181). Elastin insufficiency and the associated increase in arterial structural stiffness do not increase atherosclerotic plaque deposition in mice (108, 174). Together, these results suggest that elastic fiber degradation and the resulting circulating peptides are key mechanisms linking elastin to atherosclerosis progression.
Calcification.
Elastic fiber degradation has been linked to ectopic calcium phosphate mineral deposition (calcification) in the arterial walls, particularly in the medial layer. As demonstrated by Basalyga et al. (8), periadventitial treatment of surgically exposed rat aortas with calcium chloride leads to ectopic mineral deposition along the elastic laminae. Calcified aortas of treated rats show the presence of degraded ECM proteins, including elastin. Later, Khavandgar et al. (79) showed that medial calcification in mice lacking matrix Gla protein (MGP), a potent inhibitor of soft tissue calcification, is elastin dependent. The amount of deposited minerals in Mgp−/− arteries scales with elastin amounts in the cardiovascular tree, with mineral deposition and elastin amounts decreasing from the thoracic to the abdominal aorta. Khavandgar et al. (79) further showed that Mgp−/−Eln+/− mice have markedly reduced arterial mineral deposition compared with Mgp−/− mice (Fig. 7). This genetic experiment provides evidence that elastin content, and not just elastic fiber degradation, is a critical determinant of arterial medial calcification.
Currently, the mechanism of mineral accumulation by elastin and how this process is inhibited by MGP are not well understood. Also, the role of elastin in intimal calcification associated with atherosclerotic plaques is still unknown. Future mechanistic work focusing on these aspects of elastin biology may lead to new therapeutic approaches to treat vascular calcification, for which no treatment is currently available
Aneurysms and dissections.
Degraded and fragmented elastic fibers in the arterial wall are a common histopathology in thoracic and abdominal aortic aneurysms (18, 114). It appears that fragmented elastic fibers or improper elastic fiber deposition is critical to aneurysm formation rather than reduced elastin amounts. ADCL1, characterized by improper elastic fiber deposition, is generally associated with aneurysms, whereas SVAS, characterized by elastin insufficiency, is generally not associated with aneurysms. Additionally, genetic mutations that affect proteins necessary for elastic fiber assembly, such as FBLN4 (66) and LOX (53), cause aneurysms. Loss-of-function mutations in genes that affect TGF-β1 sequestration in the ECM, such as fibrillin-1, are associated with aberrant TGF-β1 activity and aortic aneurysms (31). Fragmented, degraded, or improperly assembled elastic fibers may be more susceptible to protease digestion, allowing aneurysmal dilation over time and stimulating cytokine and inflammatory signaling. Homogenates of abdominal aortic aneurysms show high elastinolytic activity (18). Degraded or improperly assembled elastic fibers allow inflammatory cell infiltration and affect TGF-β1 sequestration, and the released EDPs can enhance macrophage activation (24) and TGF-β1 activity (54).
Researchers have capitalized on the relationship among elastic fiber degradation, inflammatory cell activation, and chemokine signaling to generate a variety of chemically stimulated models of abdominal aortic aneurysms and dissections in animals (3, 25, 106). The models include intraluminal or periadventitial application of elastase, periadventitial incubation with calcium chloride, and subcutaneous infusion of angiotensin II. Several of the models have been extended to create thoracic aortic aneurysms and dissections (69, 74). It is important to note that many of the chemically induced animal models demonstrate aortic dilation and aneurysm formation but do not progress to aortic rupture or dissection (161). Models that progress to rupture and dissection will be critical for translating the results of animal studies to treatment of human disease.
TISSUE ENGINEERING
Increasing elastin amounts in genetic elastinopathies or repairing fragmented elastic fibers in acquired cardiovascular diseases are challenging problems. Tissue engineering, however, encounters the most challenging problem, recreating functional elastic fibers de novo. Elastogenesis has been called the “holy grail” of arterial tissue engineering, as the lack of mature elastic fibers in tissue-engineered constructs results in inferior mechanical properties compared with native arteries (135). Long and Tranquillo (104) showed that the type of scaffold and cells chosen affects elastogenesis in tissue-engineered arteries, with increased elastin amounts secreted from neonatal compared with adult cells and on fibrin compared with collagen scaffolds. They also used chemokines known to increase steady-state elastin mRNA expression, but the resulting elastic fiber structure in their constructs does not resemble arterial elastic laminae. Trying to replicate in vivo loading conditions, Kim et al. (81) showed that long-term application of circumferential cyclic strain increases tropoelastin mRNA and protein expression along with increasing the material stiffness and ultimate tensile strength of tissue-engineered arteries, although the mechanical properties are still inferior to native aorta. Huang et al. (65) recently showed that application of biaxial cyclic strain, more representative of the in vivo loading environment, produces tissue-engineered arteries with evidence of mature elastic fibers and improved mechanical properties compared with circumferentially strained constructs or constructs grown without cyclic strain. A tissue-engineered graft implanted as a pulmonary artery replacement in 8-wk-old lambs exposed to cytokines and mechanical loading present in early maturation and growth showed abundant elastic fiber deposition and ~50% of the insoluble elastin levels of the native pulmonary artery when evaluated 42 wk after implantation (177). Overall, these results demonstrate the importance of reproducing the biochemical and biomechanical environment of developing arteries to encourage elastogenesis in tissue engineering.
SUMMARY AND FUTURE DIRECTIONS
We have summarized genetic and acquired cardiovascular diseases linked to elastin and elastic fiber defects with a focus on large artery mechanics. Elastin and elastic fibers play a critical mechanical role at multiple length scales from pulse wave reflections in the cardiovascular system, to local material properties of the arterial wall, to interactions of tropoelastin with VSMCs. Elastin is a unique protein due to its limited time period of synthesis, long half-life, multistep assembly process, and high degree of cross-linking that provides mechanical elasticity to the large arteries. Acquired damage to the elastic fibers is especially problematic due to the inability of the organism to generate de novo elastic fibers postadolescence. Despite the increasing therapeutic options for controlling diseases such as hypertension, obesity, and diabetes, elastic fiber damage is currently irreparable, meaning that the cardiovascular risks associated with arterial stiffness may persist even when other disease aspects are controlled. The process of elastic fiber assembly, especially inducing assembly in vitro or in vivo, is an active and important area of investigation for treating elastinopathies and the associated changes in cardiovascular mechanics, as well as advancing tissue engineering.
We would like to highlight the differential effects of reduced elastin amounts compared with improper assembly or degradation of elastic fibers. Reduced elastin amounts lead to altered arterial wall structure, narrowed arterial lumen, increased arterial structural stiffness, and hypertension in humans and mice. In mice, the structural, mechanical, and functional phenotypes scale with elastin amounts. For the most part, mice appear to adapt reasonably well to 50–60% elastin, with more severe phenotypes appearing when elastin amounts drop to 30–40% of normal. The results of the mouse studies suggest that a threshold amount of elastin may provide near-normal cardiovascular function in genetic diseases associated with elastin insufficiency. Hence, therapies that moderately increase elastin amounts, rather than completely restoring them to normal levels, may be sufficient for improved cardiovascular outcomes.
Improperly assembled or degraded elastic fibers alter the passive mechanical properties of the arterial wall. The consequences extend far beyond physical changes to the elastic fibers to the initiation of several arterial remodeling events including VSMC phenotype modulation, ECM deposition, inflammatory cell infiltration, and the release of EDPs. EDPs stimulate cytokine signaling and activate inflammatory cells. The inflammatory cells secrete proteases that contribute to additional elastic fiber degradation in a positive feedback cycle. It is unclear whether the arterial remodeling signaling cascade is similar for genetic diseases with improperly assembled elastic fibers, such as ADCL1, and acquired diseases with elastic fiber degradation, such as diabetes or abdominal aortic aneurysms. However, elastic fiber fragmentation, inflammatory signaling, and arterial stiffening are common factors in many of the cardiovascular diseases discussed in this review. Better understanding of the biochemical and biomechanical signaling associated with improperly assembled or degraded elastic fibers may allow therapeutic interventions that halt or slow down the arterial remodeling cascade and prevent disease progression.
GRANTS
This work was partially supported by National Heart, Lung, and Blood Institute Grants HL-115560 and HL-105314.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.Z.H., M.C.S., and E.O.J. performed experiments. A.J.C., J.Z.H., M.C.S., E.O.J., M.M., and J.E.W. prepared figures; A.J.C., M.M., and J.E.W. drafted manuscript; A.J.C. and J.E.W. edited and revised manuscript; A.J.C., J.Z.H., M.C.S., E.O.J., M.M., and J.E.W. approved final version of manuscript.
REFERENCES
- 1.Akima T, Nakanishi K, Suzuki K, Katayama M, Ohsuzu F, Kawai T. Soluble elastin decreases in the progress of atheroma formation in human aorta. Circ J 73: 2154–2162, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Alessi MC, Bastelica D, Morange P, Berthet B, Leduc I, Verdier M, Geel O, Juhan-Vague I. Plasminogen activator inhibitor 1, transforming growth factor-beta1, and BMI are closely associated in human adipose tissue during morbid obesity. Diabetes 49: 1374–1380, 2000. doi: 10.2337/diabetes.49.8.1374. [DOI] [PubMed] [Google Scholar]
- 3.Anidjar S, Salzmann JL, Gentric D, Lagneau P, Camilleri JP, Michel JB. Elastase-induced experimental aneurysms in rats. Circulation 82: 973–981, 1990. doi: 10.1161/01.CIR.82.3.973. [DOI] [PubMed] [Google Scholar]
- 4.Arribas SM, Hinek A, González MC. Elastic fibres and vascular structure in hypertension. Pharmacol Ther 111: 771–791, 2006. doi: 10.1016/j.pharmthera.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Ashkenas J. Williams syndrome starts making sense. Am J Hum Genet 59: 756–761, 1996. [PMC free article] [PubMed] [Google Scholar]
- 6.Atanasova M, Dimitrova A, Ruseva B, Stoyanova A, Georgieva M, Konova E.. Quantification of elastin, collagen, and advanced glycation end products as a function of age and hypertension. In: Senescence, edited by Nagata T. Rijeka, Croatia: In Tech, 2012, p. 519−530. [Google Scholar]
- 7.Baldwin AK, Simpson A, Steer R, Cain SA, Kielty CM. Elastic fibres in health and disease. Expert Rev Mol Med 15: e8, 2013. doi: 10.1017/erm.2013.9. [DOI] [PubMed] [Google Scholar]
- 8.Basalyga DM, Simionescu DT, Xiong W, Baxter BT, Starcher BC, Vyavahare NR. Elastin degradation and calcification in an abdominal aorta injury model: role of matrix metalloproteinases. Circulation 110: 3480–3487, 2004. doi: 10.1161/01.CIR.0000148367.08413.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bashir MM, Indik Z, Yeh H, Ornstein-Goldstein N, Rosenbloom JC, Abrams W, Fazio M, Uitto J, Rosenbloom J. Characterization of the complete human elastin gene. Delineation of unusual features in the 5′-flanking region. J Biol Chem 264: 8887–8891, 1989. [PubMed] [Google Scholar]
- 10.Behmoaras J, Osborne-Pellegrin M, Gauguier D, Jacob MP. Characteristics of the aortic elastic network and related phenotypes in seven inbred rat strains. Am J Physiol Heart Circ Physiol 288: H769−H777, 2005. doi: 10.1152/ajpheart.00544.2004. [DOI] [PubMed] [Google Scholar]
- 11.Bird LM, Billman GF, Lacro RV, Spicer RL, Jariwala LK, Hoyme HE, Zamora-Salinas R, Morris C, Viskochil D, Frikke MJ, Jones MC. Sudden death in Williams syndrome: report of ten cases. J Pediatr 129: 926–931, 1996. doi: 10.1016/S0022-3476(96)70042-2. [DOI] [PubMed] [Google Scholar]
- 12.Blaise S, Romier B, Kawecki C, Ghirardi M, Rabenoelina F, Baud S, Duca L, Maurice P, Heinz A, Schmelzer CE, Tarpin M, Martiny L, Garbar C, Dauchez M, Debelle L, Durlach V. Elastin-derived peptides are new regulators of insulin resistance development in mice. Diabetes 62: 3807–3816, 2013. doi: 10.2337/db13-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briones AM, González JM, Somoza B, Giraldo J, Daly CJ, Vila E, González MC, McGrath JC, Arribas SM. Role of elastin in spontaneously hypertensive rat small mesenteric artery remodelling. J Physiol 552: 185–195, 2003. doi: 10.1113/jphysiol.2003.046904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broekelmann TJ, Kozel BA, Ishibashi H, Werneck CC, Keeley FW, Zhang L, Mecham RP. Tropoelastin interacts with cell-surface glycosaminoglycans via its COOH-terminal domain. J Biol Chem 280: 40939–40947, 2005. doi: 10.1074/jbc.M507309200. [DOI] [PubMed] [Google Scholar]
- 15.Burnett W, Finnigan-Bunick A, Yoon K, Rosenbloom J. Analysis of elastin gene expression in the developing chick aorta using cloned elastin cDNA. J Biol Chem 257: 1569–1572, 1982. [PubMed] [Google Scholar]
- 16.Burton AC. Relation of structure to function of the tissues of the wall of blood vessels. Physiol Rev 24: 619−642, 1954. [DOI] [PubMed] [Google Scholar]
- 17.Callewaert B, Renard M, Hucthagowder V, Albrecht B, Hausser I, Blair E, Dias C, Albino A, Wachi H, Sato F, Mecham RP, Loeys B, Coucke PJ, De Paepe A, Urban Z. New insights into the pathogenesis of autosomal-dominant cutis laxa with report of five ELN mutations. Hum Mutat 32: 445–455, 2011. doi: 10.1002/humu.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campa JS, Greenhalgh RM, Powell JT. Elastin degradation in abdominal aortic aneurysms. Atherosclerosis 65: 13–21, 1987. doi: 10.1016/0021-9150(87)90003-7. [DOI] [PubMed] [Google Scholar]
- 19.Cantor JO, Keller S, Parshley MS, Darnule TV, Darnule AT, Cerreta JM, Turino GM, Mandl I. Synthesis of crosslinked elastin by an endothelial cell culture. Biochem Biophys Res Commun 95: 1381–1386, 1980. doi: 10.1016/S0006-291X(80)80050-7. [DOI] [PubMed] [Google Scholar]
- 20.Chamiot-Clerc P, Renaud JF, Safar ME. Pulse pressure, aortic reactivity, and endothelium dysfunction in old hypertensive rats. Hypertension 37: 313–321, 2001. doi: 10.1161/01.HYP.37.2.313. [DOI] [PubMed] [Google Scholar]
- 21.Chen JY, Tsai PJ, Tai HC, Tsai RL, Chang YT, Wang MC, Chiou YW, Yeh ML, Tang MJ, Lam CF, Shiesh SC, Li YH, Tsai WC, Chou CH, Lin LJ, Wu HL, Tsai YS. Increased aortic stiffness and attenuated lysyl oxidase activity in obesity. Arterioscler Thromb Vasc Biol 33: 839–846, 2013. doi: 10.1161/ATVBAHA.112.300036. [DOI] [PubMed] [Google Scholar]
- 22.Clarke AW, Arnspang EC, Mithieux SM, Korkmaz E, Braet F, Weiss AS. Tropoelastin massively associates during coacervation to form quantized protein spheres. Biochemistry 45: 9989–9996, 2006. doi: 10.1021/bi0610092. [DOI] [PubMed] [Google Scholar]
- 23.Cox BA, Starcher BC, Urry DW. Communication: coacervation of tropoelastin results in fiber formation. J Biol Chem 249: 997–998, 1974. [PubMed] [Google Scholar]
- 24.Dale MA, Xiong W, Carson JS, Suh MK, Karpisek AD, Meisinger TM, Casale GP, Baxter BT. Elastin-derived peptides promote abdominal aortic aneurysm formation by modulating M1/M2 macrophage polarization. J Immunol 196: 4536–4543, 2016. doi: 10.4049/jimmunol.1502454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 24: 429–434, 2004. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- 26.Davidson JM, Smith K, Shibahara S, Tolstoshev P, Crystal RG. Regulation of elastin synthesis in developing sheep nuchal ligament by elastin mRNA levels. J Biol Chem 257: 747–754, 1982. [PubMed] [Google Scholar]
- 27.Davis EC. Smooth muscle cell to elastic lamina connections in developing mouse aorta. Role in aortic medial organization. Lab Invest 68: 89–99, 1993. [PubMed] [Google Scholar]
- 28.DeMarsilis AJ, Walji TA, Maedeker JA, Stoka KV, Kozel BA, Mecham RP, Wagenseil JE, Craft CS. Elastin Insufficiency Predisposes Mice to Impaired Glucose Metabolism. J Mol Genet Med 8: 129, 2014. doi: 10.4172/1747-0862.1000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denie JJ, Verheugt AP. Supravalvular aortic stenosis. Circulation 18: 902–908, 1958. doi: 10.1161/01.CIR.18.5.902. [DOI] [PubMed] [Google Scholar]
- 30.Deo SV, Burkhart HM, Dearani JA, Schaff HV. Supravalvar aortic stenosis: current surgical approaches and outcomes. Expert Rev Cardiovasc Ther 11: 879–890, 2013. doi: 10.1586/14779072.2013.811967. [DOI] [PubMed] [Google Scholar]
- 31.Dietz HC, Loeys B, Carta L, Ramirez F. Recent progress towards a molecular understanding of Marfan syndrome. Am J Med Genet C Semin Med Genet 139C: 4–9, 2005. doi: 10.1002/ajmg.c.30068. [DOI] [PubMed] [Google Scholar]
- 32.Dingemans KP, Teeling P, Lagendijk JH, Becker AE. Extracellular matrix of the human aortic media: an ultrastructural histochemical and immunohistochemical study of the adult aortic media. Anat Rec 258: 1–14, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 33.Dobrin PB, Canfield TR. Elastase, collagenase, and the biaxial elastic properties of dog carotid artery. Am J Physiol Heart Circ Physiol 247: H124–H131, 1984. doi: 10.1152/ajpheart.1984.247.1.H124. [DOI] [PubMed] [Google Scholar]
- 34.Dubick MA, Rucker RB, Cross CE, Last JA. Elastin metabolism in rodent lung. Biochim Biophys Acta 672: 303–306, 1981. doi: 10.1016/0304-4165(81)90297-X. [DOI] [PubMed] [Google Scholar]
- 35.Duca L, Blaise S, Romier B, Laffargue M, Gayral S, El Btaouri H, Kawecki C, Guillot A, Martiny L, Debelle L, Maurice P. Matrix ageing and vascular impacts: focus on elastin fragmentation. Cardiovasc Res 110: 298–308, 2016. doi: 10.1093/cvr/cvw061. [DOI] [PubMed] [Google Scholar]
- 36.Duz MB, Kirat E, Coucke PJ, Koparir E, Gezdirici A, Paepe A, Callewaert B, Seven M. A novel case of autosomal dominant cutis laxa in a consanguineous family: report and literature review. Clin Dysmorphol 26: 142–147, 2017. doi: 10.1097/MCD.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 37.Espinosa MG, Gardner WS, Bennett L, Sather B, Yanagisawa H, Wagenseil JE. The effects of elastic fiber protein insufficiency and treatment on the modulus of arterial smooth muscle cells. J Biomech Eng 136: 021030, 2014. doi: 10.1115/1.4026203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faury G, Maher GM, Li DY, Keating MT, Mecham RP, Boyle WA. Relation between outer and luminal diameter in cannulated arteries. Am J Physiol Heart Circ Physiol 277: H1745–H1753, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, Minkes RK, Blumer KJ, Kovacs A, Kelly DP, Li DY, Starcher B, Mecham RP. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest 112: 1419–1428, 2003. doi: 10.1172/JCI19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fazio MJ, Olsen DR, Kauh EA, Baldwin CT, Indik Z, Ornstein-Goldstein N, Yeh H, Rosenbloom J, Uitto J. Cloning of full-length elastin cDNAs from a human skin fibroblast recombinant cDNA library: further elucidation of alternative splicing utilizing exon-specific oligonucleotides. J Invest Dermatol 91: 458–464, 1988. doi: 10.1111/1523-1747.ep12476591. [DOI] [PubMed] [Google Scholar]
- 41.Ferruzzi J, Bersi MR, Humphrey JD. Biomechanical phenotyping of central arteries in health and disease: advantages of and methods for murine models. Ann Biomed Eng 41: 1311–1330, 2013. doi: 10.1007/s10439-013-0799-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fonck E, Prod’hom G, Roy S, Augsburger L, Rüfenacht DA, Stergiopulos N. Effect of elastin degradation on carotid wall mechanics as assessed by a constituent-based biomechanical model. Am J Physiol Heart Circ Physiol 292: H2754–H2763, 2007. doi: 10.1152/ajpheart.01108.2006. [DOI] [PubMed] [Google Scholar]
- 43.Foster JA, Rubin L, Kagan HM, Franzblau C, Bruenger E, Sandberg LB. Isolation and characterization of cross-linked peptides from elastin. J Biol Chem 249: 6191–6196, 1974. [PubMed] [Google Scholar]
- 44.Fritze O, Romero B, Schleicher M, Jacob MP, Oh DY, Starcher B, Schenke-Layland K, Bujan J, Stock UA. Age-related changes in the elastic tissue of the human aorta. J Vasc Res 49: 77–86, 2012. doi: 10.1159/000331278. [DOI] [PubMed] [Google Scholar]
- 45.Fülöp T Jr, Wei SM, Robert L, Jacob MP. Determination of elastin peptides in normal and arteriosclerotic human sera by ELISA. Clin Physiol Biochem 8: 273–282, 1990. [PubMed] [Google Scholar]
- 46.Gadson PF Jr, Rossignol C, McCoy J, Rosenquist TH. Expression of elastin, smooth muscle alpha-actin, and c-jun as a function of the embryonic lineage of vascular smooth muscle cells. In Vitro Cell Dev Biol Anim 29A: 773–781, 1993. doi: 10.1007/BF02634344. [DOI] [PubMed] [Google Scholar]
- 47.Gayral S, Garnotel R, Castaing-Berthou A, Blaise S, Fougerat A, Berge E, Montheil A, Malet N, Wymann MP, Maurice P, Debelle L, Martiny L, Martinez LO, Pshezhetsky AV, Duca L, Laffargue M. Elastin-derived peptides potentiate atherosclerosis through the immune Neu1-PI3Kγ pathway. Cardiovasc Res 102: 118–127, 2014. doi: 10.1093/cvr/cvt336. [DOI] [PubMed] [Google Scholar]
- 48.Gibbons CA, Shadwick RE. Functional similarities in the mechanical design of the aorta in lower vertebrates and mammals. Experientia 45: 1083–1088, 1989. doi: 10.1007/BF01950164. [DOI] [PubMed] [Google Scholar]
- 49.Goergen CJ, Li HH, Francke U, Taylor CA. Induced chromosome deletion in a Williams-Beuren syndrome mouse model causes cardiovascular abnormalities. J Vasc Res 48: 119–129, 2011. doi: 10.1159/000316808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gosling RG, Budge MM. Terminology for describing the elastic behavior of arteries. Hypertension 41: 1180–1182, 2003. doi: 10.1161/01.HYP.0000072271.36866.2A. [DOI] [PubMed] [Google Scholar]
- 51.Gray WR, Sandberg LB, Foster JA. Molecular model for elastin structure and function. Nature 246: 461–466, 1973. doi: 10.1038/246461a0. [DOI] [PubMed] [Google Scholar]
- 52.Greenwald SE. Ageing of the conduit arteries. J Pathol 211: 157–172, 2007. doi: 10.1002/path.2101. [DOI] [PubMed] [Google Scholar]
- 53.Guo DC, Regalado ES, Gong L, Duan X, Santos-Cortez RL, Arnaud P, Ren Z, Cai B, Hostetler EM, Moran R, Liang D, Estrera A, Safi HJ, Leal SM, Bamshad MJ, Shendure J, Nickerson DA, Jondeau G, Boileau C, Milewicz DM; University of Washington Center for Mendelian Genomics . LOX mutations predispose to thoracic aortic aneurysms and dissections. Circ Res 118: 928–934, 2016. doi: 10.1161/CIRCRESAHA.115.307130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo G, Muñoz-García B, Ott CE, Grünhagen J, Mousa SA, Pletschacher A, von Kodolitsch Y, Knaus P, Robinson PN. Antagonism of GxxPG fragments ameliorates manifestations of aortic disease in Marfan syndrome mice. Hum Mol Genet 22: 433–443, 2013. doi: 10.1093/hmg/dds439. [DOI] [PubMed] [Google Scholar]
- 55.Hadj-Rabia S, Callewaert BL, Bourrat E, Kempers M, Plomp AS, Layet V, Bartholdi D, Renard M, De Backer J, Malfait F, Vanakker OM, Coucke PJ, De Paepe AM, Bodemer C. Twenty patients including 7 probands with autosomal dominant cutis laxa confirm clinical and molecular homogeneity. Orphanet J Rare Dis 8: 36, 2013. doi: 10.1186/1750-1172-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Halabi CM, Broekelmann TJ, Knutsen RH, Ye L, Mecham RP, Kozel BA. Chronic antihypertensive treatment improves pulse pressure but not large artery mechanics in a mouse model of congenital vascular stiffness. Am J Physiol Heart Circ Physiol 309: H1008–H1016, 2015. doi: 10.1152/ajpheart.00288.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harvey A, Montezano AC, Lopes RA, Rios F, Touyz RM. Vascular fibrosis in aging and hypertension: molecular mechanisms and clinical implications. Can J Cardiol 32: 659–668, 2016. doi: 10.1016/j.cjca.2016.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hinek A, Pshezhetsky AV, von Itzstein M, Starcher B. Lysosomal sialidase (neuraminidase-1) is targeted to the cell surface in a multiprotein complex that facilitates elastic fiber assembly. J Biol Chem 281: 3698–3710, 2006. doi: 10.1074/jbc.M508736200. [DOI] [PubMed] [Google Scholar]
- 59.Hinek A, Wrenn DS, Mecham RP, Barondes SH. The elastin receptor: a galactoside-binding protein. Science 239: 1539–1541, 1988. doi: 10.1126/science.2832941. [DOI] [PubMed] [Google Scholar]
- 60.Hirano E, Knutsen RH, Sugitani H, Ciliberto CH, Mecham RP. Functional rescue of elastin insufficiency in mice by the human elastin gene: implications for mouse models of human disease. Circ Res 101: 523–531, 2007. doi: 10.1161/CIRCRESAHA.107.153510. [DOI] [PubMed] [Google Scholar]
- 61.Horiguchi M, Ota M, Rifkin DB. Matrix control of transforming growth factor-β function. J Biochem 152: 321–329, 2012. doi: 10.1093/jb/mvs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hosoda Y, Kawano K, Yamasawa F, Ishii T, Shibata T, Inayama S. Age-dependent changes of collagen and elastin content in human aorta and pulmonary artery. Angiology 35: 615–621, 1984. doi: 10.1177/000331978403501001. [DOI] [PubMed] [Google Scholar]
- 63.Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM, Shapiro SD. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest 116: 753–759, 2006. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Howard PS, Macarak EJ. Localization of collagen types in regional segments of the fetal bovine aorta. Lab Invest 61: 548–555, 1989. [PubMed] [Google Scholar]
- 65.Huang AH, Balestrini JL, Udelsman BV, Zhou KC, Zhao L, Ferruzzi J, Starcher BC, Levene MJ, Humphrey JD, Niklason LE. Biaxial stretch improves elastic fiber maturation, collagen arrangement, and mechanical properties in engineered arteries. Tissue Eng Part C Methods 22: 524–533, 2016. doi: 10.1089/ten.tec.2015.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hucthagowder V, Sausgruber N, Kim KH, Angle B, Marmorstein LY, Urban Z. Fibulin-4: a novel gene for an autosomal recessive cutis laxa syndrome. Am J Hum Genet 78: 1075–1080, 2006. doi: 10.1086/504304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Humphrey JD, Eberth JF, Dye WW, Gleason RL. Fundamental role of axial stress in compensatory adaptations by arteries. J Biomech 42: 1–8, 2009. doi: 10.1016/j.jbiomech.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hungerford JE, Owens GK, Argraves WS, Little CD. Development of the aortic vessel wall as defined by vascular smooth muscle and extracellular matrix markers. Dev Biol 178: 375–392, 1996. doi: 10.1006/dbio.1996.0225. [DOI] [PubMed] [Google Scholar]
- 69.Ikonomidis JS, Gibson WC, Gardner J, Sweterlitsch S, Thompson RP, Mukherjee R, Spinale FG. A murine model of thoracic aortic aneurysms. J Surg Res 115: 157–163, 2003. doi: 10.1016/S0022-4804(03)00193-8. [DOI] [PubMed] [Google Scholar]
- 70.Jain D, Dietz HC, Oswald GL, Maleszewski JJ, Halushka MK. Causes and histopathology of ascending aortic disease in children and young adults. Cardiovasc Pathol 20: 15–25, 2011. doi: 10.1016/j.carpath.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.James MF, Rich CB, Trinkaus-Randall V, Rosenbloom J, Foster JA. Elastogenesis in the developing chick lung is transcriptionally regulated. Dev Dyn 213: 170–181, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 72.Jiao Y, Li G, Korneva A, Caulk AW, Qin L, Bersi MR, Li Q, Li W, Mecham RP, Humphrey JD, Tellides G. Deficient circumferential growth is the primary determinant of aortic obstruction attributable to partial elastin deficiency. Arterioscler Thromb Vasc Biol 37: 930–941, 2017. doi: 10.1161/ATVBAHA.117.309079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiao Y, Li G, Li Q, Ali R, Qin L, Li W, Qyang Y, Greif DM, Geirsson A, Humphrey JD, Tellides G. mTOR (mechanistic target of rapamycin) inhibition decreases mechanosignaling, collagen accumulation, and stiffening of the thoracic aorta in elastin-deficient mice. Arterioscler Thromb Vasc Biol 37: 1657–1666, 2017. doi: 10.1161/ATVBAHA.117.309653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnston WF, Salmon M, Pope NH, Meher A, Su G, Stone ML, Lu G, Owens GK, Upchurch GR Jr, Ailawadi G. Inhibition of interleukin-1β decreases aneurysm formation and progression in a novel model of thoracic aortic aneurysms. Circulation 130, Suppl 1: S51–S59, 2014. doi: 10.1161/CIRCULATIONAHA.113.006800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kagan HM, Vaccaro CA, Bronson RE, Tang SS, Brody JS. Ultrastructural immunolocalization of lysyl oxidase in vascular connective tissue. J Cell Biol 103: 1121–1128, 1986. doi: 10.1083/jcb.103.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, Keating MT, Li DY. A critical role for elastin signaling in vascular morphogenesis and disease. Development 130: 411–423, 2003. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- 77.Karnik SK, Wythe JD, Sorensen L, Brooke BS, Urness LD, Li DY. Elastin induces myofibrillogenesis via a specific domain, VGVAPG. Matrix Biol 22: 409–425, 2003. doi: 10.1016/S0945-053X(03)00076-3. [DOI] [PubMed] [Google Scholar]
- 78.Katz PS, Trask AJ, Souza-Smith FM, Hutchinson KR, Galantowicz ML, Lord KC, Stewart JA Jr, Cismowski MJ, Varner KJ, Lucchesi PA. Coronary arterioles in type 2 diabetic (db/db) mice undergo a distinct pattern of remodeling associated with decreased vessel stiffness. Basic Res Cardiol 106: 1123–1134, 2011. doi: 10.1007/s00395-011-0201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khavandgar Z, Roman H, Li J, Lee S, Vali H, Brinckmann J, Davis EC, Murshed M. Elastin haploinsufficiency impedes the progression of arterial calcification in MGP-deficient mice. J Bone Miner Res 29: 327–337, 2014. doi: 10.1002/jbmr.2039. [DOI] [PubMed] [Google Scholar]
- 80.Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci 115: 2817–2828, 2002. [DOI] [PubMed] [Google Scholar]
- 81.Kim BS, Nikolovski J, Bonadio J, Mooney DJ. Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nat Biotechnol 17: 979–983, 1999. doi: 10.1038/13671. [DOI] [PubMed] [Google Scholar]
- 82.Kim J, Staiculescu MC, Cocciolone AJ, Yanagisawa H, Mecham RP, Wagenseil JE. Crosslinked elastic fibers are necessary for low energy loss in the ascending aorta. J Biomech 61: 199–207, 2017. doi: 10.1016/j.jbiomech.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim YM, Haghighat L, Spiekerkoetter E, Sawada H, Alvira CM, Wang L, Acharya S, Rodriguez-Colon G, Orton A, Zhao M, Rabinovitch M. Neutrophil elastase is produced by pulmonary artery smooth muscle cells and is linked to neointimal lesions. Am J Pathol 179: 1560–1572, 2011. doi: 10.1016/j.ajpath.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Knutsen R, Beeman SC, Broekelmann TJ, Liu D, Tsang KM, Kovacs A, Ye L, Danback J, Watson A, Wardlaw A, Wagenseil J, Garbow JR, Shoykhet M, Kozel BA. Minoxidil improves vascular compliance, restores cerebral blood flow and alters extracellular matrix gene expression in a model of chronic vascular stiffness. Am J Physiol Heart Circ Physiol. doi: 10.1152/ajpheart.00683.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kozel BA, Danback JR, Waxler JL, Knutsen RH, de Las Fuentes L, Reusz GS, Kis E, Bhatt AB, Pober BR. Williams syndrome predisposes to vascular stiffness modified by antihypertensive use and copy number changes in NCF1. Hypertension 63: 74–79, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kozel BA, Knutsen RH, Ye L, Ciliberto CH, Broekelmann TJ, Mecham RP. Genetic modifiers of cardiovascular phenotype caused by elastin haploinsufficiency act by extrinsic noncomplementation. J Biol Chem 286: 44926–44936, 2011. doi: 10.1074/jbc.M111.274779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kozel BA, Mecham RP, Rosenbloom J. Elastin. In: The Extracellular Matrix: an Overview, edited by Mecham RP. Berlin, Germany: Spring-Verlag, 2011, p. 267–301. [Google Scholar]
- 88.Krettek A, Sukhova GK, Libby P. Elastogenesis in human arterial disease: a role for macrophages in disordered elastin synthesis. Arterioscler Thromb Vasc Biol 23: 582–587, 2003. doi: 10.1161/01.ATV.0000064372.78561.A5. [DOI] [PubMed] [Google Scholar]
- 89.Kucich U, Rosenbloom JC, Abrams WR, Bashir MM, Rosenbloom J. Stabilization of elastin mRNA by TGF-beta: initial characterization of signaling pathway. Am J Respir Cell Mol Biol 17: 10–16, 1997. doi: 10.1165/ajrcmb.17.1.2816. [DOI] [PubMed] [Google Scholar]
- 90.Kucich U, Rosenbloom JC, Abrams WR, Rosenbloom J. Transforming growth factor-beta stabilizes elastin mRNA by a pathway requiring active Smads, protein kinase C-delta, and p38. Am J Respir Cell Mol Biol 26: 183–188, 2002. doi: 10.1165/ajrcmb.26.2.4666. [DOI] [PubMed] [Google Scholar]
- 91.Kwan CY, Wang RR, Beazley JS, Lee RM. Alterations of elastin and elastase-like activities in aortae of diabetic rats. Biochim Biophys Acta 967: 322–325, 1988. doi: 10.1016/0304-4165(88)90027-X. [DOI] [PubMed] [Google Scholar]
- 92.Lafarge JC, Naour N, Clément K, Guerre-Millo M. Cathepsins and cystatin C in atherosclerosis and obesity. Biochimie 92: 1580–1586, 2010. doi: 10.1016/j.biochi.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 93.Le VP, Cheng JK, Kim J, Staiculescu MC, Ficker SW, Sheth SC, Bhayani SA, Mecham RP, Yanagisawa H, Wagenseil JE. Mechanical factors direct mouse aortic remodelling during early maturation. J R Soc Interface 12: 20141350, 2015. doi: 10.1098/rsif.2014.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Le VP, Knutsen RH, Mecham RP, Wagenseil JE. Decreased aortic diameter and compliance precedes blood pressure increases in postnatal development of elastin-insufficient mice. Am J Physiol Heart Circ Physiol 301: H221–H229, 2011. doi: 10.1152/ajpheart.00119.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Le VP, Wagenseil JE. Echocardiographic characterization of postnatal development in mice with reduced arterial elasticity. Cardiovasc Eng Technol 3: 424–438, 2012. doi: 10.1007/s13239-012-0108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee P, Bax DV, Bilek MM, Weiss AS. A novel cell adhesion region in tropoelastin mediates attachment to integrin αVβ5. J Biol Chem 289: 1467–1477, 2014. doi: 10.1074/jbc.M113.518381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lev M. The pathologic anatomy of cardiac complexes associated with transposition of arterial trunks. Lab Invest 2: 296–311, 1953. [PubMed] [Google Scholar]
- 98.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature 393: 276–280, 1998. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 99.Li DY, Faury G, Taylor DG, Davis EC, Boyle WA, Mecham RP, Stenzel P, Boak B, Keating MT. Novel arterial pathology in mice and humans hemizygous for elastin. J Clin Invest 102: 1783–1787, 1998. doi: 10.1172/JCI4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li HH, Roy M, Kuscuoglu U, Spencer CM, Halm B, Harrison KC, Bayle JH, Splendore A, Ding F, Meltzer LA, Wright E, Paylor R, Deisseroth K, Francke U. Induced chromosome deletions cause hypersociability and other features of Williams-Beuren syndrome in mice. EMBO Mol Med 1: 50–65, 2009. doi: 10.1002/emmm.200900003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li W, Li Q, Qin L, Ali R, Qyang Y, Tassabehji M, Pober BR, Sessa WC, Giordano FJ, Tellides G. Rapamycin inhibits smooth muscle cell proliferation and obstructive arteriopathy attributable to elastin deficiency. Arterioscler Thromb Vasc Biol 33: 1028–1035, 2013. doi: 10.1161/ATVBAHA.112.300407. [DOI] [PubMed] [Google Scholar]
- 102.Lillie MA, Gosline JM. Swelling and viscoelastic properties of osmotically stressed elastin. Biopolymers 39: 641–652, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 103.Liu J, Ma L, Yang J, Ren A, Sun Z, Yan G, Sun J, Fu H, Xu W, Hu C, Shi GP. Increased serum cathepsin S in patients with atherosclerosis and diabetes. Atherosclerosis 186: 411–419, 2006. doi: 10.1016/j.atherosclerosis.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 104.Long JL, Tranquillo RT. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol 22: 339–350, 2003. doi: 10.1016/S0945-053X(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 105.Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci 63: 2304–2316, 2006. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lysgaard Poulsen J, Stubbe J, Lindholt JS. Animal models used to explore abdominal aortic aneurysms: a systematic review. Eur J Vasc Endovasc Surg 52: 487–499, 2016. doi: 10.1016/j.ejvs.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 107.Maeda I, Mizoiri N, Briones MP, Okamoto K. Induction of macrophage migration through lactose-insensitive receptor by elastin-derived nonapeptides and their analog. J Pept Sci 13: 263–268, 2007. doi: 10.1002/psc.845. [DOI] [PubMed] [Google Scholar]
- 108.Maedeker JA, Stoka KV, Bhayani SA, Gardner WS, Bennett L, Procknow JD, Staiculescu MC, Walji TA, Craft CS, Wagenseil JE. Hypertension and decreased aortic compliance due to reduced elastin amounts do not increase atherosclerotic plaque accumulation in Ldlr-/- mice. Atherosclerosis 249: 22–29, 2016. doi: 10.1016/j.atherosclerosis.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marigo V, Volpin D, Bressan GM. Regulation of the human elastin promoter in chick embryo cells. Tissue-specific effect of TGF-beta. Biochim Biophys Acta 1172: 31–36, 1993. doi: 10.1016/0167-4781(93)90265-F. [DOI] [PubMed] [Google Scholar]
- 110.Marque V, Kieffer P, Atkinson J, Lartaud-Idjouadiene I. Elastic properties and composition of the aortic wall in old spontaneously hypertensive rats. Hypertension 34: 415–422, 1999. doi: 10.1161/01.HYP.34.3.415. [DOI] [PubMed] [Google Scholar]
- 111.Maurice P, Blaise S, Gayral S, Debelle L, Laffargue M, Hornebeck W, Duca L. Elastin fragmentation and atherosclerosis progression: the elastokine concept. Trends Cardiovasc Med 23: 211–221, 2013. doi: 10.1016/j.tcm.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 112.McDonald AH, Gerlis LM, Somerville J. Familial arteriopathy with associated pulmonary and systemic arterial stenoses. Br Heart J 31: 375–385, 1969. doi: 10.1136/hrt.31.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mecham RP, Madaras J, McDonald JA, Ryan U. Elastin production by cultured calf pulmonary artery endothelial cells. J Cell Physiol 116: 282–288, 1983. doi: 10.1002/jcp.1041160304. [DOI] [PubMed] [Google Scholar]
- 114.Milewicz DM, Guo DC, Van TF, Lafont AL, Papke CL, Inamoto S, Kwartler CS, Pannu H. Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu Rev Genomics Hum Genet 9: 283–302, 2008. doi: 10.1146/annurev.genom.8.080706.092303. [DOI] [PubMed] [Google Scholar]
- 115.Milewicz DM, Urbán Z, Boyd C. Genetic disorders of the elastic fiber system. Matrix Biol 19: 471–480, 2000. doi: 10.1016/S0945-053X(00)00099-8. [DOI] [PubMed] [Google Scholar]
- 116.Misra A, Sheikh AQ, Kumar A, Luo J, Zhang J, Hinton RB, Smoot L, Kaplan P, Urban Z, Qyang Y, Tellides G, Greif DM. Integrin β3 inhibition is a therapeutic strategy for supravalvular aortic stenosis. J Exp Med 213: 451–463, 2016. doi: 10.1084/jem.20150688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mitchell GF. Arterial stiffness and hypertension: chicken or egg? Hypertension 64: 210–214, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Morrow AG, Waldhausen JA, Peters RL, Blood-Well RD, Braunwald E. Supravalvular aortic stenosis: clinical, hemodynamic and pathologic observations. Circulation 20: 1003–1010, 1959. doi: 10.1161/01.CIR.20.6.1003. [DOI] [PubMed] [Google Scholar]
- 119.Myers B, Dubick M, Last JA, Rucker RB. Elastin synthesis during perinatal lung development in the rat. Biochim Biophys Acta 761: 17–22, 1983. doi: 10.1016/0304-4165(83)90357-4. [DOI] [PubMed] [Google Scholar]
- 120.Narayanan AS, Page RC, Kuzan F, Cooper CG. Elastin cross-linking in vitro. Studies on factors influencing the formation of desmosines by lysyl oxidase action on tropoelastin. Biochem J 173: 857–862, 1978. doi: 10.1042/bj1730857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Newby AC. Metalloproteinase production from macrophages−a perfect storm leading to atherosclerotic plaque rupture and myocardial infarction. Exp Physiol 101: 1327–1337, 2016. doi: 10.1113/EP085567. [DOI] [PubMed] [Google Scholar]
- 122.Nicoloff G, Petrova C, Christova P, Nikolov A. Detection of free elastin-derived peptides among diabetic children. Atherosclerosis 192: 342–347, 2007. doi: 10.1016/j.atherosclerosis.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 123.O’Connell MK, Murthy S, Phan S, Xu C, Buchanan J, Spilker R, Dalman RL, Zarins CK, Denk W, Taylor CA. The three-dimensional micro- and nanostructure of the aortic medial lamellar unit measured using 3D confocal and electron microscopy imaging. Matrix Biol 27: 171–181, 2008. doi: 10.1016/j.matbio.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol 50: 1–13, 2007. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 125.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 46: 200–204, 2005. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 126.Ohtsuka S, Kakihana M, Watanabe H, Sugishita Y. Chronically decreased aortic distensibility causes deterioration of coronary perfusion during increased left ventricular contraction. J Am Coll Cardiol 24: 1406–1414, 1994. doi: 10.1016/0735-1097(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 127.Okamoto K. Spontaneous hypertension in rats. Int Rev Exp Pathol 7: 227–270, 1969. [PubMed] [Google Scholar]
- 128.Olliver L, Luvalle PA, Davidson JM, Rosenbloom J, Mathew CG, Bester AJ, Boyd CD. The gene coding for tropoelastin is represented as a single copy sequence in the haploid sheep genome. Coll Relat Res 7: 77–89, 1987. doi: 10.1016/S0174-173X(87)80022-5. [DOI] [PubMed] [Google Scholar]
- 129.Ono RN, Sengle G, Charbonneau NL, Carlberg V, Bächinger HP, Sasaki T, Lee-Arteaga S, Zilberberg L, Rifkin DB, Ramirez F, Chu ML, Sakai LY. Latent transforming growth factor beta-binding proteins and fibulins compete for fibrillin-1 and exhibit exquisite specificities in binding sites. J Biol Chem 284: 16872–16881, 2009. doi: 10.1074/jbc.M809348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Osborne-Pellegrin M, Labat C, Mercier N, Challande P, Lacolley P. Changes in aortic stiffness related to elastic fiber network anomalies in the Brown Norway rat during maturation and aging. Am J Physiol Heart Circ Physiol 299: H144–H152, 2010. doi: 10.1152/ajpheart.00040.2010. [DOI] [PubMed] [Google Scholar]
- 131.Papke CL, Yanagisawa H. Fibulin-4 and fibulin-5 in elastogenesis and beyond: Insights from mouse and human studies. Matrix Biol 37: 142–149, 2014. doi: 10.1016/j.matbio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Parker KH. A brief history of arterial wave mechanics. Med Biol Eng Comput 47: 111–118, 2009. doi: 10.1007/s11517-009-0440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Parks WC, Secrist H, Wu LC, Mecham RP. Developmental regulation of tropoelastin isoforms. J Biol Chem 263: 4416–4423, 1988. [PubMed] [Google Scholar]
- 134.Pasquali-Ronchetti I, Baccarani-Contri M. Elastic fiber during development and aging. Microsc Res Tech 38: 428–435, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 135.Patel A, Fine B, Sandig M, Mequanint K. Elastin biosynthesis: The missing link in tissue-engineered blood vessels. Cardiovasc Res 71: 40–49, 2006. doi: 10.1016/j.cardiores.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 136.Perou ML. Congenital supravalvular aortic stenosis. A morphological study with attempt at classification. Arch Pathol 71: 453–466, 1961. [PubMed] [Google Scholar]
- 137.Pierce GL. Mechanisms and subclinical consequences of aortic stiffness. Hypertension 70: 848–853, 2017. doi: 10.1161/HYPERTENSIONAHA.117.08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pierce RA, Deak SB, Stolle CA, Boyd CD. Heterogeneity of rat tropoelastin mRNA revealed by cDNA cloning. Biochemistry 29: 9677–9683, 1990. doi: 10.1021/bi00493a024. [DOI] [PubMed] [Google Scholar]
- 139.Pober BR, Johnson M, Urban Z. Mechanisms and treatment of cardiovascular disease in Williams-Beuren syndrome. J Clin Invest 118: 1606–1615, 2008. doi: 10.1172/JCI35309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pober BR. Williams-Beuren syndrome. N Engl J Med 362: 239–252, 2010. doi: 10.1056/NEJMra0903074. [DOI] [PubMed] [Google Scholar]
- 141.Pocza P, Süli-Vargha H, Darvas Z, Falus A. Locally generated VGVAPG and VAPG elastin-derived peptides amplify melanoma invasion via the galectin-3 receptor. Int J Cancer 122: 1972–1980, 2008. doi: 10.1002/ijc.23296. [DOI] [PubMed] [Google Scholar]
- 142.Pollock J, Baule VJ, Rich CB, Ginsburg CD, Curtiss SW, Foster JA. Chick tropoelastin isoforms. From the gene to the extracellular matrix. J Biol Chem 265: 3697–3702, 1990. [PubMed] [Google Scholar]
- 143.Reiser K, McCormick RJ, Rucker RB. Enzymatic and nonenzymatic cross-linking of collagen and elastin. FASEB J 6: 2439–2449, 1992. doi: 10.1096/fasebj.6.7.1348714. [DOI] [PubMed] [Google Scholar]
- 144.Rider OJ, Tayal U, Francis JM, Ali MK, Robinson MR, Byrne JP, Clarke K, Neubauer S. The effect of obesity and weight loss on aortic pulse wave velocity as assessed by magnetic resonance imaging. Obesity (Silver Spring) 18: 2311–2316, 2010. doi: 10.1038/oby.2010.64. [DOI] [PubMed] [Google Scholar]
- 145.Ritz-Timme S, Laumeier I, Collins MJ. Aspartic acid racemization: evidence for marked longevity of elastin in human skin. Br J Dermatol 149: 951–959, 2003. doi: 10.1111/j.1365-2133.2003.05618.x. [DOI] [PubMed] [Google Scholar]
- 146.Roach MR, Burton AC. The reason for the shape of the distensibility curves of arteries. Can J Biochem Physiol 35: 681–690, 1957. doi: 10.1139/y57-080. [DOI] [PubMed] [Google Scholar]
- 147.Robertson IB, Rifkin DB. Regulation of the Bioavailability of TGF-β and TGF-β-Related Proteins. Cold Spring Harb Perspect Biol 8: a021907, 2016. doi: 10.1101/cshperspect.a021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Roccabianca S, Ateshian GA, Humphrey JD. Biomechanical roles of medial pooling of glycosaminoglycans in thoracic aortic dissection. Biomech Model Mechanobiol 13: 13–25, 2014. doi: 10.1007/s10237-013-0482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rodgers UR, Weiss AS. Integrin alpha v beta 3 binds a unique non-RGD site near the C-terminus of human tropoelastin. Biochimie 86: 173–178, 2004. doi: 10.1016/j.biochi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 150.Roy CS. Elastic properties of the arterial wall. J Physiol 3: 125–159, 1881. doi: 10.1113/jphysiol.1881.sp000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rucker RB, Tinker D. Structure and metabolism of arterial elastin. Int Rev Exp Pathol 17: 1–47, 1977. [PubMed] [Google Scholar]
- 152.Ruckman JL, Luvalle PA, Hill KE, Giro MG, Davidson JM. Phenotypic stability and variation in cells of the porcine aorta: collagen and elastin production. Matrix Biol 14: 135–145, 1994. doi: 10.1016/0945-053X(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 153.Rusciani A, Duca L, Sartelet H, Chatron-Colliet A, Bobichon H, Ploton D, Le Naour R, Blaise S, Martiny L, Debelle L. Elastin peptides signaling relies on neuraminidase-1-dependent lactosylceramide generation. PLoS One 5: e14010, 2010. doi: 10.1371/journal.pone.0014010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Safar M, Chamiot-Clerc P, Dagher G, Renaud JF. Pulse pressure, endothelium function, and arterial stiffness in spontaneously hypertensive rats. Hypertension 38: 1416–1421, 2001. doi: 10.1161/hy1201.096538. [DOI] [PubMed] [Google Scholar]
- 155.Sage H, Gray WR. Studies on the evolution of elastin−I. Phylogenetic distribution. Comp Biochem Physiol B 64: 313–327, 1979. doi: 10.1016/0305-0491(79)90277-3. [DOI] [PubMed] [Google Scholar]
- 156.Sakai LY, Keene DR, Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol 103: 2499–2509, 1986. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Samad F, Yamamoto K, Pandey M, Loskutoff DJ. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol Med 3: 37–48, 1997. [PMC free article] [PubMed] [Google Scholar]
- 158.Sandberg LB, Weissman N, Gray WR. Structural features of tropoelastin related to the sites of cross-links in aortic elastin. Biochemistry 10: 52–56, 1971. doi: 10.1021/bi00777a008. [DOI] [PubMed] [Google Scholar]
- 159.Sauvage M, Hinglais N, Mandet C, Badier C, Deslandes F, Michel JB, Jacob MP. Localization of elastin mRNA and TGF-beta1 in rat aorta and caudal artery as a function of age. Cell Tissue Res 291: 305–314, 1998. doi: 10.1007/s004410051000. [DOI] [PubMed] [Google Scholar]
- 160.Schmidt RE, Gilbert EF, Amend TC, Chamberlain CR Jr, Lucas RV Jr. Generalized arterial fibromuscular dysplasia and myocardial infarction in familial supravalvular aortic stenosis syndrome. J Pediatr 74: 576–584, 1969. doi: 10.1016/S0022-3476(69)80041-7. [DOI] [PubMed] [Google Scholar]
- 161.Sénémaud J, Caligiuri G, Etienne H, Delbosc S, Michel JB, Coscas R. Translational Relevance and Recent Advances of Animal Models of Abdominal Aortic Aneurysm. Arterioscler Thromb Vasc Biol 37: 401–410, 2017. doi: 10.1161/ATVBAHA.116.308534. [DOI] [PubMed] [Google Scholar]
- 162.Senior RM, Griffin GL, Mecham RP, Wrenn DS, Prasad KU, Urry DW. Val-Gly-Val-Ala-Pro-Gly, a repeating peptide in elastin, is chemotactic for fibroblasts and monocytes. J Cell Biol 99: 870–874, 1984. doi: 10.1083/jcb.99.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sephel GC, Buckley A, Davidson JM. Developmental initiation of elastin gene expression by human fetal skin fibroblasts. J Invest Dermatol 88: 732–735, 1987. doi: 10.1111/1523-1747.ep12470403. [DOI] [PubMed] [Google Scholar]
- 164.Shadwick RE. Mechanical design in arteries. J Exp Biol 202: 3305–3313, 1999. [DOI] [PubMed] [Google Scholar]
- 165.Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest 87: 1828–1834, 1991. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shih CC, Oakley DM, Joens MS, Roth RA, Fitzpatrick JAJ. Nonlinear optical imaging of extracellular matrix proteins. Methods Cell Biol 143: 57–78, 2018. doi: 10.1016/bs.mcb.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 167.Simionescu A, Philips K, Vyavahare N. Elastin-derived peptides and TGF-beta1 induce osteogenic responses in smooth muscle cells. Biochem Biophys Res Commun 334: 524–532, 2005. doi: 10.1016/j.bbrc.2005.06.119. [DOI] [PubMed] [Google Scholar]
- 168.Sinha A, Vyavahare NR. High-glucose levels and elastin degradation products accelerate osteogenesis in vascular smooth muscle cells. Diab Vasc Dis Res 10: 410–419, 2013. doi: 10.1177/1479164113485101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Slove S, Lannoy M, Behmoaras J, Pezet M, Sloboda N, Lacolley P, Escoubet B, Buján J, Jacob MP. Potassium channel openers increase aortic elastic fiber formation and reverse the genetically determined elastin deficit in the BN rat. Hypertension 62: 794–801, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01379. [DOI] [PubMed] [Google Scholar]
- 170.Spencer M, Unal R, Zhu B, Rasouli N, McGehee RE Jr, Peterson CA, Kern PA. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab 96: E1990–E1998, 2011. doi: 10.1210/jc.2011-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sproul EP, Argraves WS. A cytokine axis regulates elastin formation and degradation. Matrix Biol 32: 86–94, 2013. doi: 10.1016/j.matbio.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Staiculescu MC, Kim J, Mecham RP, Wagenseil J. Mechanical behavior and matrisome gene expression in aneurysm-prone thoracic aorta of newborn lysyl oxidase knockout mice. Am J Physiol Heart Circ Physiol 313: H446−H456, 2017. doi: 10.1152/ajpheart.00712.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Stoilov I, Starcher BC, Mecham RP, Broekelmann TJ. Measurement of elastin, collagen, and total protein levels in tissues. Methods Cell Biol 143: 133–146, 2018. doi: 10.1016/bs.mcb.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 174.Stoka KV, Maedeker JA, Bennett L, Bhayani SA, Gardner WS, Procknow JD, Cocciolone AJ, Walji TA, Craft CS, Wagenseil JE. Effects of increased arterial stiffness on atherosclerotic plaque amounts. J Biomech Eng 140: 051007, 2018. doi: 10.1115/1.4039175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sugitani H, Hirano E, Knutsen RH, Shifren A, Wagenseil JE, Ciliberto C, Kozel BA, Urban Z, Davis EC, Broekelmann TJ, Mecham RP. Alternative splicing and tissue-specific elastin misassembly act as biological modifiers of human elastin gene frameshift mutations associated with dominant cutis laxa. J Biol Chem 287: 22055–22067, 2012. doi: 10.1074/jbc.M111.327940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Swee MH, Parks WC, Pierce RA. Developmental regulation of elastin production. Expression of tropoelastin pre-mRNA persists after down-regulation of steady-state mRNA levels. J Biol Chem 270: 14899–14906, 1995. doi: 10.1074/jbc.270.25.14899. [DOI] [PubMed] [Google Scholar]
- 177.Syedain Z, Reimer J, Lahti M, Berry J, Johnson S, Tranquillo RT. Tissue engineering of acellular vascular grafts capable of somatic growth in young lambs. Nat Commun 7: 12951, 2016. doi: 10.1038/ncomms12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Szabó Z, Levi-Minzi SA, Christiano AM, Struminger C, Stoneking M, Batzer MA, Boyd CD. Sequential loss of two neighboring exons of the tropoelastin gene during primate evolution. J Mol Evol 49: 664–671, 1999. doi: 10.1007/PL00006587. [DOI] [PubMed] [Google Scholar]
- 179.Tanno T, Yoshinaga K, Sato T. Alteration of elastin in aorta from diabetics. Atherosclerosis 101: 129–134, 1993. doi: 10.1016/0021-9150(93)90109-8. [DOI] [PubMed] [Google Scholar]
- 180.Tassabehji M, Metcalfe K, Donnai D, Hurst J, Reardon W, Burch M, Read AP. Elastin: genomic structure and point mutations in patients with supravalvular aortic stenosis. Hum Mol Genet 6: 1029–1036, 1997. doi: 10.1093/hmg/6.7.1029. [DOI] [PubMed] [Google Scholar]
- 181.Toma I, McCaffrey TA. Transforming growth factor-β and atherosclerosis: interwoven atherogenic and atheroprotective aspects. Cell Tissue Res 347: 155–175, 2012. doi: 10.1007/s00441-011-1189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tomizawa H, Yamazaki M, Kunika K, Itakura M, Yamashita K. Association of elastin glycation and calcium deposit in diabetic rat aorta. Diabetes Res Clin Pract 19: 1–8, 1993. doi: 10.1016/0168-8227(93)90138-U. [DOI] [PubMed] [Google Scholar]
- 183.Toonkool P, Jensen SA, Maxwell AL, Weiss AS. Hydrophobic domains of human tropoelastin interact in a context-dependent manner. J Biol Chem 276: 44575–44580, 2001. doi: 10.1074/jbc.M107920200. [DOI] [PubMed] [Google Scholar]
- 184.Torun D, Ozelsancak R, Turan I, Micozkadioglu H, Sezer S, Ozdemir FN. The relationship between obesity and transforming growth factor beta on renal damage in essential hypertension. Int Heart J 48: 733–741, 2007. doi: 10.1536/ihj.48.733. [DOI] [PubMed] [Google Scholar]
- 185.Urban Z, Davis EC. Cutis laxa: intersection of elastic fiber biogenesis, TGFβ signaling, the secretory pathway and metabolism. Matrix Biol 33: 16–22, 2014. doi: 10.1016/j.matbio.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Urry DW. Entropic elastic processes in protein mechanisms. I. Elastic structure due to an inverse temperature transition and elasticity due to internal chain dynamics. J Protein Chem 7: 1–34, 1988. doi: 10.1007/BF01025411. [DOI] [PubMed] [Google Scholar]
- 187.Ushiki T, Murakumo M. Scanning electron microscopic studies of tissue elastin components exposed by a KOH-collagenase or simple KOH digestion method. Arch Histol Cytol 54: 427–436, 1991. doi: 10.1679/aohc.54.427. [DOI] [PubMed] [Google Scholar]
- 188.Van der Donckt C, Van Herck JL, Schrijvers DM, Vanhoutte G, Verhoye M, Blockx I, Van Der Linden A, Bauters D, Lijnen HR, Sluimer JC, Roth L, Van Hove CE, Fransen P, Knaapen MW, Hervent AS, De Keulenaer GW, Bult H, Martinet W, Herman AG, De Meyer GR. Elastin fragmentation in atherosclerotic mice leads to intraplaque neovascularization, plaque rupture, myocardial infarction, stroke, and sudden death. Eur Heart J 36: 1049–1058, 2015. doi: 10.1093/eurheartj/ehu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92: 827–839, 2003. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 190.Volpin D, Urry DW, Cox BA, Gotte L. Optical diffraction of tropoelastin and alpha-elastin coacervates. Biochim Biophys Acta 439: 253–258, 1976. doi: 10.1016/0005-2795(76)90181-1. [DOI] [PubMed] [Google Scholar]
- 191.Vrhovski B, Weiss AS. Biochemistry of tropoelastin. Eur J Biochem 258: 1–18, 1998. doi: 10.1046/j.1432-1327.1998.2580001.x. [DOI] [PubMed] [Google Scholar]
- 192.Wagenseil JE, Ciliberto CH, Knutsen RH, Levy MA, Kovacs A, Mecham RP. The importance of elastin to aortic development in mice. Am J Physiol Heart Circ Physiol 299: H257–H264, 2010. doi: 10.1152/ajpheart.00194.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wagenseil JE, Ciliberto CH, Knutsen RH, Levy MA, Kovacs A, Mecham RP. Reduced vessel elasticity alters cardiovascular structure and function in newborn mice. Circ Res 104: 1217–1224, 2009. doi: 10.1161/CIRCRESAHA.108.192054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wagenseil JE, Mecham RP. Elastin in large artery stiffness and hypertension. J Cardiovasc Transl Res 5: 264–273, 2012. doi: 10.1007/s12265-012-9349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wagenseil JE, Mecham RP. New insights into elastic fiber assembly. Birth Defects Res C Embryo Today 81: 229–240, 2007. doi: 10.1002/bdrc.20111. [DOI] [PubMed] [Google Scholar]
- 196.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev 89: 957–989, 2009. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wagenseil JE, Nerurkar NL, Knutsen RH, Okamoto RJ, Li DY, Mecham RP. Effects of elastin haploinsufficiency on the mechanical behavior of mouse arteries. Am J Physiol Heart Circ Physiol 289: H1209–H1217, 2005. doi: 10.1152/ajpheart.00046.2005. [DOI] [PubMed] [Google Scholar]
- 198.Wan W, Yanagisawa H, Gleason RL Jr. Biomechanical and microstructural properties of common carotid arteries from fibulin-5 null mice. Ann Biomed Eng 38: 3605–3617, 2010. doi: 10.1007/s10439-010-0114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang Y, Zeinali-Davarani S, Davis EC, Zhang Y. Effect of glucose on the biomechanical function of arterial elastin. J Mech Behav Biomed Mater 49: 244–254, 2015. doi: 10.1016/j.jmbbm.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang Z, Lakes RS, Golob M, Eickhoff JC, Chesler NC. Changes in large pulmonary arterial viscoelasticity in chronic pulmonary hypertension. PLoS One 8: e78569, 2013. doi: 10.1371/journal.pone.0078569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wells SM, Langille BL, Lee JM, Adamson SL. Determinants of mechanical properties in the developing ovine thoracic aorta. Am J Physiol Heart Circ Physiol 277: H1385–H1391, 1999. [DOI] [PubMed] [Google Scholar]
- 202.Wessel A, Pankau R, Kececioglu D, Ruschewski W, Bürsch JH. Three decades of follow-up of aortic and pulmonary vascular lesions in the Williams-Beuren syndrome. Am J Med Genet 52: 297–301, 1994. doi: 10.1002/ajmg.1320520309. [DOI] [PubMed] [Google Scholar]
- 203.Wolinsky H. Comparison of medial growth of human thoracic and abdominal aortas. Circ Res 27: 531–538, 1970. doi: 10.1161/01.RES.27.4.531. [DOI] [PubMed] [Google Scholar]
- 204.Wolinsky H, Glagov S. A lamellar unit of aortic medial structure and function in mammals. Circ Res 20: 99–111, 1967. doi: 10.1161/01.RES.20.1.99. [DOI] [PubMed] [Google Scholar]
- 205.Yanagisawa H, Davis EC. Unraveling the mechanism of elastic fiber assembly: The roles of short fibulins. Int J Biochem Cell Biol 42: 1084–1093, 2010. doi: 10.1016/j.biocel.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yeh H, Anderson N, Ornstein-Goldstein N, Bashir MM, Rosenbloom JC, Abrams W, Indik Z, Yoon K, Parks W, Mecham R, Rosenbloom J. Structure of the bovine elastin gene and S1 nuclease analysis of alternative splicing of elastin mRNA in the bovine nuchal ligament. Biochemistry 28: 2365–2370, 1989. doi: 10.1021/bi00432a003. [DOI] [PubMed] [Google Scholar]
- 207.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev 14: 163–176, 2000. [PMC free article] [PubMed] [Google Scholar]
- 208.Yurchenco PD, O’Rear JJ. Basal lamina assembly. Curr Opin Cell Biol 6: 674–681, 1994. doi: 10.1016/0955-0674(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 209.Zacchigna L, Vecchione C, Notte A, Cordenonsi M, Dupont S, Maretto S, Cifelli G, Ferrari A, Maffei A, Fabbro C, Braghetta P, Marino G, Selvetella G, Aretini A, Colonnese C, Bettarini U, Russo G, Soligo S, Adorno M, Bonaldo P, Volpin D, Piccolo S, Lembo G, Bressan GM. Emilin1 links TGF-beta maturation to blood pressure homeostasis. Cell 124: 929–942, 2006. doi: 10.1016/j.cell.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 210.Zhang H, Apfelroth SD, Hu W, Davis EC, Sanguineti C, Bonadio J, Mecham RP, Ramirez F. Structure and expression of fibrillin-2, a novel microfibrillar component preferentially located in elastic matrices. J Cell Biol 124: 855–863, 1994. doi: 10.1083/jcb.124.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhang J, Zhao X, Vatner DE, McNulty T, Bishop S, Sun Z, Shen YT, Chen L, Meininger GA, Vatner SF. Extracellular matrix disarray as a mechanism for greater abdominal versus thoracic aortic stiffness with aging in primates. Arterioscler Thromb Vasc Biol 36: 700–706, 2016. doi: 10.1161/ATVBAHA.115.306563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang M, Pierce RA, Wachi H, Mecham RP, Parks WC. An open reading frame element mediates posttranscriptional regulation of tropoelastin and responsiveness to transforming growth factor beta1. Mol Cell Biol 19: 7314–7326, 1999. doi: 10.1128/MCB.19.11.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zilberberg L, Todorovic V, Dabovic B, Horiguchi M, Couroussé T, Sakai LY, Rifkin DB. Specificity of latent TGF-β binding protein (LTBP) incorporation into matrix: role of fibrillins and fibronectin. J Cell Physiol 227: 3828–3836, 2012. doi: 10.1002/jcp.24094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zou Y, Zhang Y. The orthotropic viscoelastic behavior of aortic elastin. Biomech Model Mechanobiol 10: 613–625, 2011. doi: 10.1007/s10237-010-0260-4. [DOI] [PubMed] [Google Scholar]