Abstract
Apamin-sensitive small-conductance Ca2+-activated K+ (SK) current (IKAS) is encoded by Ca2+-activated K+ channel subfamily N (KCNN) genes. IKAS importantly contributes to cardiac repolarization in conditions associated with reduced repolarization reserve. To test the hypothesis that IKAS inhibition contributes to drug-induced long QT syndrome (diLQTS), we screened for KCNN variants among patients with diLQTS, determined the properties of heterologously expressed wild-type (WT) and variant KCNN channels, and determined if the 5-HT3 receptor antagonist ondansetron blocks IKAS. We searched 2,306,335 records in the Indiana Network for Patient Care and found 11 patients with diLQTS who had DNA available in the Indiana Biobank. DNA sequencing discovered a heterozygous KCNN2 variant (p.F503L) in a 52-yr-old woman presenting with corrected QT interval prolongation at baseline (473 ms) and further corrected QT interval lengthening (601 ms) after oral administration of ondansetron. That patient was also heterozygous for the p.S38G and p.P2835S variants of the QT-controlling genes KCNE1 and ankyrin 2, respectively. Patch-clamp experiments revealed that the p.F503L KCNN2 variant heterologously expressed in human embryonic kidney (HEK)-293 cells augmented Ca2+ sensitivity, increasing IKAS density. The fraction of total F503L-KCNN2 protein retained in the membrane was higher than that of WT KCNN2 protein. Ondansetron at nanomolar concentrations inhibited WT and p.F503L SK2 channels expressed in HEK-293 cells as well as native SK channels in ventricular cardiomyocytes. Ondansetron-induced IKAS inhibition was also demonstrated in Langendorff-perfused murine hearts. In conclusion, the heterozygous p.F503L KCNN2 variant increases Ca2+ sensitivity and IKAS density in transfected HEK-293 cells. Ondansetron at therapeutic (i.e., nanomolar) concentrations is a potent IKAS blocker.
NEW & NOTEWORTHY We showed that ondansetron, a 5-HT3 receptor antagonist, blocks small-conductance Ca2+-activated K+ (SK) current. Ondansetron may be useful in controlling arrhythmias in which increased SK current is a likely contributor. However, its SK-blocking effects may also facilitate the development of drug-induced long QT syndrome.
Keywords: drug-induced long QT syndrome, Ca2+-activated K+ channel subfamily N variants, ondansetron, small-conductance Ca2+-activated K+ channel
INTRODUCTION
Various classes of drugs have been shown to induce arrhythmogenic prolongation of the QT interval, including different types of antiarrhythmic drugs and noncardiac agents. Besides its profound clinical implications, drug-induced long QT syndrome (diLQTS) has been a major cause for drug withdrawal and relabeling, complicating drug development and approval (22). Identification of factors predisposing to diLQTS, therefore, is highly desirable. Electrolyte disturbances, female sex, and heart failure are all known to increase susceptibility to diLQTS. Recent studies have also identified genetic factors. Screening genes that are commonly associated with the congenital LQTS has revealed mutations in 5−40% of subjects with diLQTS (18). Another study identified numerous single-nucleotide polymorphisms in key genes controlling cardiac electrical properties that were associated with an increased risk of diLQTS (15). No studies, however, have reported susceptibility alleles in the Ca2+-activated K+ channel subfamily N (KCNN) gene family, which encodes for small-conductance Ca2+-activated K+ (SK) channels. SK channels possess a relatively small unitary conductance (~10 pS in symmetrical K+ concentration), are gated solely by nanomolar concentrations of intracellular Ca2+ (apparent Kd: ~0.5 μM), and are selectively inhibited by pico- to nanomolar concentrations of the bee venom apamin (1). Three SK channel subtypes (SK1, SK2, and SK3, which are encoded by KCNN1, KCNN2, and KCNN3, respectively) are expressed in the mammalian heart and assemble to form homo- or heterotetramers (12, 28). Under physiological conditions, SK channels generate very little or no outward current in the ventricle. However, ventricular SK channels elaborate sizable currents in the failing heart and during hypokalemia (4, 5, 13, 31) to maintain a normal repolarization reserve. Here, we identified a rare c.1509C>G (p.F503L) variant in one KCNN2 allele in a 52-yr-old patient who developed reversible QT interval prolongation after treatment with the 5-HT3 receptor antagonist ondansetron, a commonly prescribed antiemetic. The patient was also heterozygous for variations in the QT interval-controlling genes KCNE1 and ANK2, which encode for the β-subunit of the slowly activating voltage-gated K+ channel and the ion channel-anchoring protein ankyrin-2, respectively. These findings suggested the possibility that the genetic variations acted individually or synergistically to increase the susceptibility to ondansetron-induced QT prolongation. Interactions of any of the three protein variants with ondansetron have not been described to date. The present study focused on the characterization of p.F503L SK2 channel properties as well as the modulation of wild-type (WT) and p.F503L SK channels by therapeutic (i.e., nanomolar) concentrations of ondansetron. We found that the polymorphism in KCNN2 resulted in an increase in the apparent Ca2+ sensitivity of SK2 (gain of function). Additional experiments revealed that ondansetron at nanomolar concentrations inhibited heterologously expressed WT and p.F503L SK2 channels as well as native cardiac SK channels. These results are compatible with a role of ondansetron-induced SK channel inhibition in diLQTS. They further enable subsequent studies that test the anti-/proarrhythmic potential of SK current blockade by ondansetron in animal models and in humans.
METHODS
The present study was approved by the Institutional Biosafety Committee, the Institutional Animal Care and Use Committee, the Institutional Review Board of the Indiana University, and the Methodist Research Institute in Indianapolis, IN. All procedures were performed in accordance with the ethical standards of the responsible committees (institutional and national) on human experimentation. Mice were maintained on a 12:12-h light-dark cycle with lights off at 7:00 PM in a temperature- and humidity-controlled vivarium with food and water available ad libitum.
Genetic Screening
We screened 2,306,335 records in the Indiana Network for Patient Care electronic database for ECGs with a corrected QT (QTc) of >500 ms in patients who had received at least one of the QT-prolonging drugs that are listed on the Indiana University Health Methodist formulary (25). The patients identified by the search were then matched with the blood samples in the Indiana Biobank. The initial results showed that 29 patients with diLQTS had blood samples stored in the Indiana Biobank. A manual review of the medical records confirmed that 11 of 29 patients had a normal or borderline QTc before and prolonged QTc within 7 or 60 days, respectively, after taking antibiotics or nonantibiotics. The remaining patients were excluded because of ventricular pacing or incorrect QT measurements.
Genomic DNA from patients was amplified by PCR using Hotstar Taq DNA Polymerase (Qiagen, Valencia, CA) and laboratory-designed primers from Integrated DNA Technologies (Coralville, IA) with M13 tails used for BigDye (Sanger, Applied Biosystems, Foster City, CA) sequencing (primer sequences available upon request). A 25-µl reaction was set up containing 50 ng DNA, 1 µM primer pairs, 2 mM dNTP, 2.5 µl of 10 × buffer, 5 µl of 5× Q-solution, and 1 µl of 25 mM MgCl2. Touchdown PCR was performed using the following conditions: 95°C for 15 min; 14 cycles of 94°C for 45 s, 65°C with a −0.5°C change per cycle for 30 s, and 72°C for 1 min; 24 cycles of 94°C for 45 s, 58°C for 30 s, and 72°C for 1 min; and 72°C for 10 min. Small aliquots of the PCR products were run by electrophoresis on a 1% agarose gel with ethidium bromide to confirm appropriate amplification. Products were then sequenced using an Applied Biosystems 3130xl or 3500xl Genetic Analyzer in conjunction with the ABI BigDye Terminator v3.1 Cycle Sequencing kit chemistry and protocol (Applied Biosystems). Sequences where aligned to each gene and analyzed using Mutation Surveyor software (version 4.0.7, Softgenetics, State College, PA).
Next-generation sequencing was performed using a custom panel containing probes for human DNA to sequence the coding and splicing regions of 246 genes associated with cardiovascular disorders, including arrhythmias, cardiomyopathies, congenital heart defects, aortopathy, connective tissue disorders, Noonan spectrum disorders, pulmonary arterial hypertension, metabolic disorders that afflict the heart, and lipid disorders, as previously described (3). Variants were annotated based on available data from human studies as previously described (3). Selected variants were confirmed by Sanger sequencing.
Cell Culture and Transfection
Human embryonic kidney (HEK)-293 cells (American Type Culture Collection, Manassas, VA) were cultured in Iscove’s modified Dulbecco's medium (ThermoFisher Scientific, Waltham, MA) supplemented with 10% FBS and 1% penicillin-streptomycin in a 95% O2-5% CO2 incubator at 37°C. HEK-293 cells were transfected with 1 µg of a plasmid encoding WT KCNN2 or mutant p.F503L KCNN2 and internal ribosome entry site-enhanced green fluorescent protein (IRES-eGFP) (pKCNN2-IRES-eGFP and pF503LKCNN2-IRES-eGFP, respectively) using Effectene Transfection Reagent (Qiagen). Different cell cultures were transfected at the same time with either plasmid and incubated for 48–72 h. Cells expressing either KCNN2 gene were identified by virtue of their green fluorescence and used for patch-clamp experiments. Whole cell current recordings were obtained alternately from cells expressing WT KCNN2 or p.F503L KCNN2. Cells of either genotype were studied alternately on the same day to minimize the effect of different incubation intervals on KCNN2 expression levels.
For experiments assessing the effect of ondansetron on whole cell apamin-sensitive SK current (IKAS), HEK-293 cells stably expressing SK2 (hereafter designated SK2 cells) were used as previously described (27, 32). In brief, HEK-293 cells were transfected with 2 µg of a plasmid encoding KCNN2 and an IRES-dependent hygromycin resistance gene (pKCNN2-IRES-hyg). Single cells were picked and propagated in selection media containing hygromycin at 200 µg/ml.
Immunoblot Assay
Cultures of HEK-293 cells transfected with WT SK2 or F503L SK2 were grown in six-well plates. To obtain whole cell lysates, cells were harvested in 100 µl PBS and homogenized. To obtain the membrane portions of the cells, cells were homogenized in 10 mM NaHCO3, pelleted, and resuspended in 40 µl PBS. Five microliters each of whole cell homogenates and membrane vesicles were subjected to SDS-PAGE and transferred to a nitrocellulose membrane. The blot was probed with anti-SK2 polyclonal antibody (1:500, APC-028, Alomone Labs, Jerusalem, Israel). Antibody-binding protein bands were visualized and quantified by horseradish peroxidase epifluorescence intensities. Expression of SK2 for each sample was normalized to GAPDH levels and expressed as arbitrary units. The mean intensity value from five independent experiments was used.
Isolation of Cardiomyocytes
Mouse left ventricular myocytes were isolated enzymatically by a previously described protocol (20). Only female C57BL/6J mice between 3 and 6 mo of age were used to avoid possible confounding effects of sex on the experimental outcome. After inhalation of anesthesia with 5% isoflurane-95% O2, mice were euthanized by cervical dislocation and hearts were removed immediately via thoracotomy. After retrograde perfusion with nominally Ca2+-free, oxygenated Tyrode solution for 5 min, the heart was perfused with the same solution containing 200 U/ml collagenase (Worthington type II, Worthington Biochemical, Lakewood, NJ) for ~10 min. Next, the heart was transferred to a dish filled with 0.1 mmol CaCl2-Tyrode solution, the right ventricular free wall and atria were removed, and the left ventricle was minced into small pieces, triturated, and filtered through a 100-μm nylon mesh. The filtered cells were centrifuged at 500 revolutions/min for 1 min. The supernatant was removed, and the cell pellet was resuspended in 0.2 mmol/l CaCl2-Tyrode solution. Centrifugation was repeated, and the resuspended cells were stored in 0.5 mmol/l CaCl2-Tyrode solution and used within 6–8 h. Only Ca2+-tolerant, quiescent, and rod-shaped cells were studied.
Electrophysiology
Measurement of whole cell IKAS.
Whole cell IKAS was recorded using the voltage-clamp technique in the ruptured patch configuration. The extracellular solution contained (in mmol/l) 140 NaCl, 4 KCl, 1 MgCl2, 10 HEPES, 1.5 CaCl2, and 10 glucose, pH 7.4 (adjusted with NaOH). The bath solution for used to measure whole cell IKAS in ventricular myocytes contained (in mmol/l) 140 N-methylglucamine, 4 KCl, 1 MgCl2, 10 HEPES, 1.5 CaCl2, and 10 glucose, pH 7.4 (adjusted with HCl). The internal solution consisted of (in mmol/l) 144 K-gluconate, 1.15 MgCl2, 1 EGTA, 10 HEPES, 2 MgATP, and 0.0005 free Ca2+ (Ca-EGTA Calculator v1.3), pH 7.25 (adjusted with KOH). All experiments were carried out at room temperature. Pipette resistances ranged from 3 to 5 MΩ when filled with the pipette solution. After a gigaohm seal had been achieved, the test pulse current was nulled by adjusting the pipette capacitance compensation. After break in, the whole cell charging transient was nulled by adjusting whole cell capacitance and series resistance. Series resistance was electronically compensated by 70–80%. Voltage-clamp commands were generated with an Axopatch 200B amplifier/Digidata 1440A acquisition system using pCLAMP10 software (Molecular Devices). The electrical signal was filtered with a built-in four-pole low-pass Bessel filter at 1 kHz and digitized at 5 kHz. Whole cell recordings were analyzed using Clampfit 10.2 (Molecular Devices).
Whole cell currents were elicited using a voltage ramp from 40 to −100 mV (0.35 mV/ms) from a holding potential of −50 mV. Voltage ramps were repeated every 5 s. Dose-response curves for the blocking effect of ondansetron on whole cell IKAS were obtained by adding ascending concentrations of the drug into the bath solution and by measuring the corresponding change in whole cell current magnitude. After exposure to the highest ondansetron concentration, a saturating apamin concentration (100 nmol/l) was applied cumulatively into the bath solution to block any remaining currents carried by SK2 channels. The sum of the ondansetron- and apamin-sensitive current was defined as total IKAS carried by SK2 channels. Ondansetron-sensitive currents (Iondan) were normalized to total IKAS, and data were fitted with the following Hill equation: Iondan/IKAS = 1/(1 + ([C]/IC50)n, where IC50 is the ondansetron concentration ([C]) that produces 50% inhibition of total IKAS and n is the Hill coefficient.
Ca2+ sensitivity of heterologously expressed SK2 channels.
The Ca2+ sensitivity of SK2 channel activity was determined in inside-out patches. The same amplifier and AD/DA interface as for whole cell recordings were used. Signals were low-pass filtered at 5 kHz using the built-in filter of the amplifier and sampled at 1 kHz. SK currents were elicited by a voltage ramp from 40 to −100 mV (0.35 mV/ms). Current levels at 0 mV were measured for each free Ca2+ concentration and normalized to the maximal current level to determine the Ca2+ dependence of SK channel activity. Experiments were performed at room temperature. Data analyses, fitting, and plotting were performed with Clampfit and Sigmaplot software.
Extracellular (pipette) solution contained (in mM) 116 K-gluconate, 4 KCl, and 10 HEPES, pH 7.2. The internal (bath) solution contained (in mM) 116 K-Gluconate, 4 KCl, and 10 HEPES, pH 7.2. Ca2+ chelators were used to control free Ca2+ concentration in internal solutions. For this purpose, 5 mmol/l chelators (EGTA for Ca2+ concentrations of 500 nmol/l and below and hydroxyethylethylenediaminetriacetic acid for > 500 nmol/l) were added to the standard internal solution. Different amounts of CaCl2 were added based on calculations of the WEBMAX program using experimentally determined dissociation constants of Ca2+ for the chelator (2). For solutions containing free Ca2+ ≥ 500 nmol/l, the actual free Ca2+ concentrations were determined by measurements with a Ca2+-sensitive electrode (Mettler-Toledo, Columbus, OH) and reported. We then deduced the affinities of chelators from these measurements and used them to calculate free Ca2+ concentrations for other solutions with <500 nM expected free Ca2+, which cannot be accurately measured with Ca2+ electrodes. Solution with 5 mM EGTA and no added Ca2+ was considered as nominally Ca2+ free. Because the single SK channel open probability is extremely low in this solution, it was used to estimate leak current level for subtraction from currents recorded in various Ca2+ concentrations.
Optical voltage mapping of isolated mouse hearts.
Optical voltage mapping of Langendorff-perfused mouse hearts was used to determine whether ondansetron prolongs ventricular action potential duration (APD) through inhibition of IKAS and was performed as previously described (32). Hearts were harvested from female C57BL/6J mice between 3 and 6 mo of age. The perfused Tyrode solution (pH 7.4 when gassed with 95% O2 and 5% CO2) was maintained at 37.5°C with a constant flow rate of ~1 ml/min at an aortic pressure of 70 cm H2O. After 20 min of stabilization, hearts were stained with 2 μl of the voltage-sensitive dye di-4-ANEPPS (2 mmol/l). Hearts were then washed with dye-free solution for 10 min followed by the addition of blebbistatin to uncouple contraction from excitation (10 µmol/l, Tocris Bioscience, Minneapolis, MN). Stained hearts were illuminated with a laser at a 532-nm wavelength, and fluorescence was collected by a MiCAMUltima-L CMOS camera (SciMedia, Costa Mesa, CA) through a 715-nm long-pass filter. Fluorescence was recorded at a 1-ms frame rate in a 100 × 100-pixel grid with a spatial resolution of 0.35 × 0.35 mm2/pixel. Optical signals were processed with both spatial (3 × 3-pixel Gaussian filter) and temporal (3 frames moving average) filtering.
SK channels conduct little to no outward current in normal murine ventricles. We have previously shown that the late Na+ current enhancer, Anemonia sulcata II (ATX-II; 15 nmol/l), acutely upregulates IKAS in mouse ventricles (32). We exploited this property of ATX-II to examine whether ondansetron prolongs ventricular repolarization via IKAS blockade. Hearts were randomly divided into the following four experimental groups (drugs or their respective vehicles were applied cumulatively into the perfusate at 15-min intervals): 15 nmol/l ATX-II, 100 nmol/l apamin, and 100 nmol/l ondansetron (group 1), 15 nmol/l ATX-II, 100 nmol/l ondansetron, and 100 nmol/l apamin (group 2), 15 nmol/l ATX-II, ondansetron vehicle, and 100 nmol/l apamin (group 3), and ATX-II vehicle, ondansetron vehicle, and 100 nmol/l apamin (group 4). Blebbistatin (10 µmol/l) was present in the perfusate throughout the duration of the experiments. Optical maps were acquired at baseline and at the end of each consecutive treatment step while hearts were paced at the right ventricular base at cycle lengths of 100, 150, and 200 ms. Signals were averaged across the entire map, and these spatially averaged signals were used to measure APD at 80% repolarization (APD80).
Drugs and Reagents
Apamin was purchased from Tocris Bioscience. All other chemicals and drugs were purchased from Sigma-Aldrich (St. Louis, MO). Blebbistatin was dissolved in a mixture of 10% double-distilled H2O and 90% DMSO to obtain a 40 mmol/l stock solution. Apamin, ondansetron, and ATX-II were dissolved in double-distilled H2O to make stock solutions at concentrations of 10 μM/l, 10 mM/l, and 10 μM/l, respectively.
Statistical Analyses
Data are presented as mean ± SE. Nonnormally distributed data are shown as box plots with median and percentiles. A Student’s t-test and the Wilcoxon rank-sum test were used for comparisons of normally and non-normally distributed data, respectively. Two-tailed P values of ≤0.05 were considered significant. Linear mixed effect models were used to analyze APD80 optical mapping data, with group, phase, and pacing cycle length as the fixed effects and mouse as the random effect. Nominal P values are reported without adjustment of multiple comparisons. QT intervals were corrected for heart rate using Bazett’s formula.
RESULTS
Clinical Background
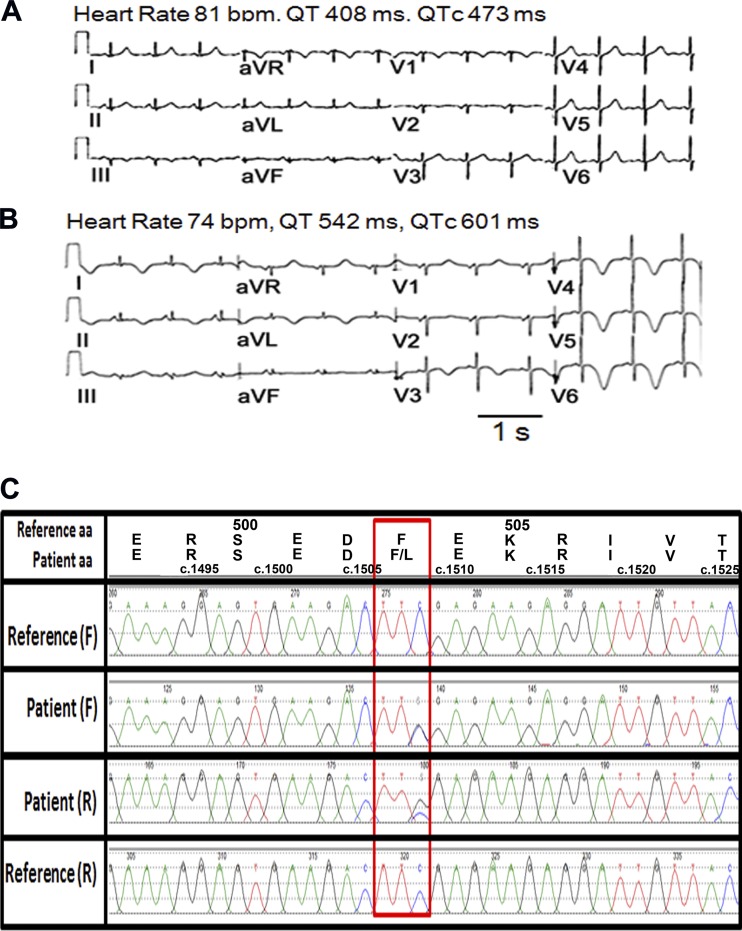
The patient was a 52-yr-old Caucasian woman with a history of adult onset respiratory distress syndrome, hypertension, gastroesophageal reflux disease, and atypical chest pain. Previous stress ECGs showed normal left ventricular ejection fractions at baseline, ranging from 58% to 76%, and normal left ventricular wall thickness and volumes. There were no wall motion abnormalities either at baseline or during exercise. Her baseline ECGs within 1 yr before admission showed variable QTc intervals ranging from 440 to 500 ms. There was no history of cardiac arrhythmia. She presented to the emergency room with nausea the day before admission. An ECG showed sinus rhythm with minimal QTc prolongation (Fig. 1A). She returned the next day because of repeated vomiting. She was given ondansetron (4 mg sublingually every 8 h) and admitted for evaluation. She was also given hydromorphone, metoclopramide, vancomycin, and a piperacillin-tazobactam injection in the emergency room. Among those, ondansetron is a QT-prolonging drug, whereas metoclopramide is a drug with conditional long QT risk, i.e., it prolongs the QT interval when combined with other QT-prolonging drugs. An ECG taken on the day of admission showed T wave inversion in anterolateral leads and QTc prolongation to 601 ms (Fig. 1B). Three additional ECGs in the hospital showed heart rates ranging from 57 to 74 beats/min and QTc intervals ranging from 490 ms to 560 ms. Serum K+ levels were within normal limits (3.9−4.4 mmol/l within 1 day of ECGs). She was discharged 7 days later with diagnoses that included adrenal insufficiency, urinary tract infection, hypertension, and gastroesophageal reflux disease. Because of an absence of cardiovascular symptoms and repeatedly negative stress tests, the patient did not have another ECG taken until 3 yr later, showing normal sinus rhythm with upright T waves.
Fig. 1.
Ondansetron causes corrected QT (QTc) prolongation in a patient heterozygous for the p.F503L Ca2+-activated K+ channel subfamily N member 2 (KCNN2) variant. A and B: 12-lead ECGs at rest in a 52-yr-old woman the day before ondansetron (A) and after ondansetron (B). C: chromatogram showing heterozygous C to G variant c.1509 C>G (p.F503L) in exon 8 of KCNN2 compared with reference genome NM_021614, chr5:113,831,648 (human GRCh37/hg19 assembly), and single-nucleotide polymorphism database rs150495473. Overlapping peaks in the chromatogram present in both the forward (F) and reverse (R) directions are indicative of a nucleotide change. AA, amino acid; bpm, beats/min.
Identification of a Rare KCNN2 Variant in a Patient With diLQTS.
Sanger sequencing revealed a heterozygous variation c.1509C>G (GenBank NM_021614.3) in exon 8 of SK2-encoding KCNN2 (Fig. 1C) in this patient. This variation results in a change from phenylalanine to leucine in position 503 (p.F503L) within the distal region of the intracellular COOH-terminus of SK2. This rare variation was seen in 4:10,400 (0.038%) alleles from African individuals but not in any other ethnicities (http://exac.broadinstitute.org, accessed on 19 September 2016]. We also sequenced 11 age-, sex-, and ethnicity-matched control subjects and found no KCNN2 variants. In addition to KCNN2 c.1509C>G, a custom next-generation sequencing with targeted enrichment of 246 cardiovascular disease-causing genes showed that the patient was also heterozygous for the KCNE1 p.S38G variation and ANK2 p.P2835S variation. The latter has been associated with drug-induced QT prolongation (15), whereas the KCNE1 p.S38G variant has been linked to rate-dependent repolarization abnormalities with risk to develop arrhythmia (30). We next sought to characterize the impact of the F503L KCNN2 variation on properties of SK2-mediated currents using the patch-clamp technique.
The p.F503L KCNN2 Variant Increases Apparent Ca2+ Sensitivity of SK2 Channels
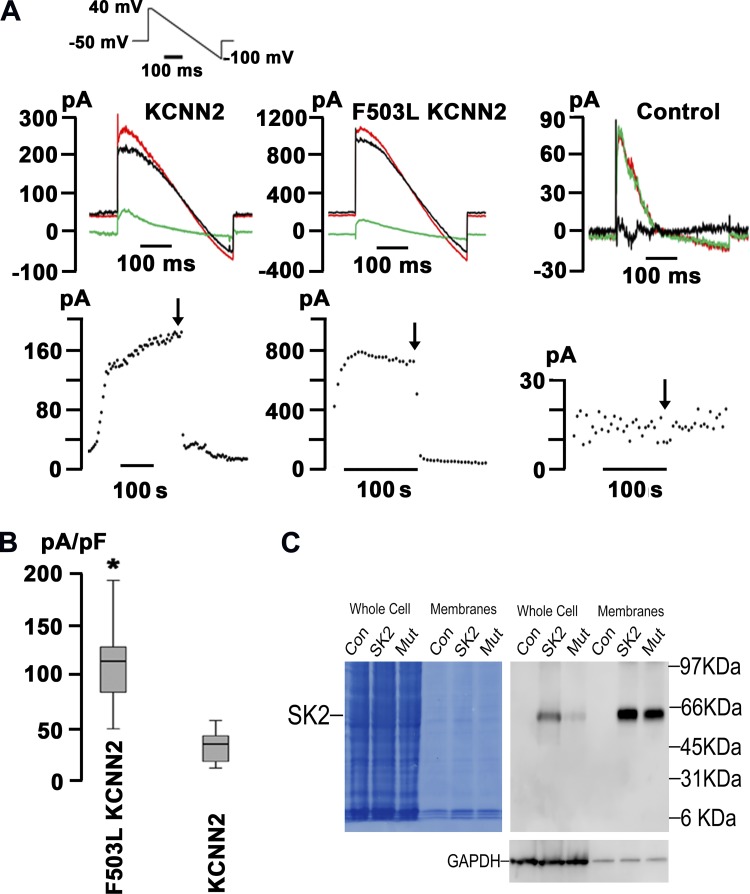
HEK-293 cells transiently transfected with either WT or p.F503L KCNN2 were dialyzed with 500 nmol/l free Ca2+ to activate SK2 channels using the conventional whole cell configuration of the patch-clamp technique. Representative time series of whole cell current traces are shown in Fig. 2A. Currents were elicited by a 400-ms voltage ramp protocol from 40 to −100 mV applied every 5 s from a holding potential of −50 mV (protocol shown in the inset of Fig. 2A, top left). After membrane rupture, ramp currents gradually increased over time and eventually reached a steady state. Application of 100 nM apamin into the bath solution largely suppressed ramp currents in KCNN2- and p.F503L KCNN2- transfected cells (Fig. 2A, left and middle, respectively). Apamin-sensitive currents reversed near the K+ potential (EK; black traces in Fig. 2A), indicating that they were largely carried by K+. Figure 2A, bottom, shows whole cell current amplitudes measured at a potential of 0 mV as a function of time for the same cells shown at the top, demonstrating that apamin-sensitive currents contributed 95% and 92% to the total membrane current in WT- and variant-expressing cells, respectively. In comparison, genetically naïve HEK-293 cells did not exhibit an apamin-sensitive current component (Fig. 2A, right). Levels of IKAS density measured at 0 mV varied widely among individual cells transfected with p.F503L KCNN2, but their average level was ~1.5-fold larger than in WT KCNN2-transfected cells (Fig. 2B). Immunoblot analyses revealed that relative to WT SK2, variant F503L-SK2 expressed only poorly in HEK-293 whole cell lysates (Fig. 2C). When normalized to GAPDH expression levels, the ratio of WT to variant SK2 in whole cell lysates was 3.5 ± 1.6 (mean ± SE, n = 5), whereas it decreased to 1.6 ± 0.8 in the membrane portions because of an increase in the fraction of F503L-SK2 that was retained in the membrane. These findings support the idea that increased trafficking rates of F503L-SK2 protein to and/or decreased removal rates from the outer membrane contribute to the higher IKAS density in p.F503L KCNN2-transfected HEK293 cells.
Fig. 2.
Larger apamin-sensitive small-conductance Ca2+-activated K+ current (IKAS) density in human embryonic kidney (HEK)-293 cells transfected with p.F503L Ca2+-activated K+ channel subfamily N member 2 (KCNN2). A, top: representative whole cell current traces obtained from HEK-293 cells transiently transfected with human wild-type KCNN2 (left) or p.F503L KCNN2 (middle) and from a genetically naïve HEK-293 cell (right). The inset in B shows the voltage-clamp protocol. The free Ca2+ concentration in the pipette solution was 500 nmol/l. Red and green traces were recorded before and after exposure to 100 nM apamin, respectively. Black traces represent apamin-sensitive currents that were obtained by digitally subtracting the currents recorded in the presence of apamin from those in its absence. Note differences in y-axis scales between graphs. A, bottom: plots of current amplitudes at 0 mV recorded from the respective cells shown in the top as a function of time. Voltage ramps were applied every 5 s. The start of apamin exposure is indicated by arrows. B: box plot comparing IKAS density at 0 mV in HEK-293 cells expressing wild-type KCNN2 or p.F503L KCNN2. The ends of each box mark the first and third quartile, the median is indicated by the horizontal line in the box interior, and the 10th/90th percentiles are marked by the ends of the whiskers. Values are from 9 cells/genotype, distributed among 9 transfections. *P < 0.001 vs. wild-type KCNN2 by Wilcoxon rank-sum test. C: immunoblot analysis of small-conductance Ca2+-activated K+ (SK)2 protein levels in HEK-293 cells. Representative Western blots show the expression of wild-type (SK2) and F503L mutant (Mut) SK2 proteins in whole cell homogenates and NaHCO3-extracted membrane vesicles. Con, untransfected HEK-293 cells. Nitrocellulose was first stained with Amido black and then probed with anti-SK2 antibody and anti-GAPDH antibody.
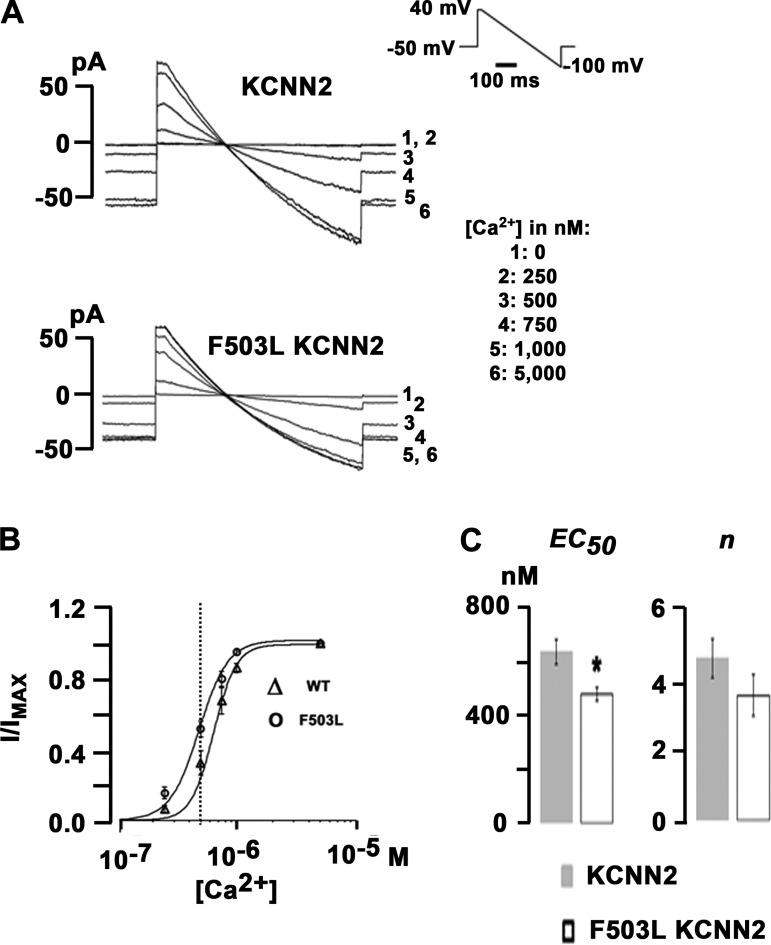
Next, we examined the Ca2+ sensitivity of SK2 channels using the inside-out configuration of the patch-clamp technique. Representative current traces from HEK-293 cells transfected with WT KCNN2 or p.F503L KCNN2 are shown in Fig. 3A. Currents were elicited by a 400-ms voltage ramp protocol from 40 to −100 mV applied every 5 s from a holding potential of −50 mV (protocol shown in the inset of Fig. 3A, top right). Solutions containing different free Ca2+ concentrations were applied to the patches, resulting in SK2 current activation in a Ca2+ concentration-dependent manner. Current levels at 0 mV were measured at each Ca2+ concentration, normalized to the maximal value, and plotted as a function of free Ca2+ concentration, as shown in Fig. 3B. p.F503L KCNN2 expression led to an ~22% decrease in the average EC50 for Ca2+ activation of SK2 channels (WT EC50: 610 ± 47 nmol/l and F503L EC50: 478 ± 25 nmol/l, P < 0.05 by t-test; Fig. 3C) but had no significant effect on the Hill coefficient (WT: 4.2 ± 0.6 and p.F503L: 3.8 ± 0.6, P > 0.05 by t-test). When these values were inserted into the Hill equation, we found an ~1.25-fold increase in SK2 current activation in p.F503L KCNN2-transfected cells at 500 nmol/l free Ca2+ concentration. These results suggest that the F503L mutation increases the apparent Ca2+ sensitivity, which contributes to the larger IKAS density seen in p.F503L KCNN2-transfected cells.
Fig. 3.
p.F503L small-conductance Ca2+-activated K+ (SK)2 channels have increased apparent Ca2+ affinity. A: representative current traces recorded under voltage clamp with inside-out patches excised from human embryonic kidney (HEK)-293 cells transfected with cDNA for wild-type or p.F503L Ca2+-activated K+ channel subfamily N member 2 (KCNN2) channels. Bath solutions containing free Ca2+ concentrations as indicated were sequentially applied to the patches. The inset shows the voltage-clamp protocol. B: average current levels at 0 mV were measured at each Ca2+ concentration for each patch, normalized to the maximal value, and plotted as a function of Ca2+ concentration. The concentration-response relationships were fitted with the following Hill equation: I/Imax = 1/[1 + ([Ca2+])/EC50)n], where I/Imax is current/maximal current and n is the Hill coefficient. Values are mean ± SE from five and six patches excised from HEK-293 cells transfected with KCNN2 or p.F503L KCNN2 cDNA, respectively. A vertical dotted line was drawn to highlight the large differences of SK current densities at 500 nM of intracellular Ca2+. C: average values for EC50 and Hill coefficient n from experiments shown in B. Dose-response relationships were individually fitted for each experiment. Error bars represent SEs. EC50 values for p.F503L KCNN2 and KCNN2 were significantly different from each other (*P < 0.05 by t-test), whereas n values were not (P > 0.05).
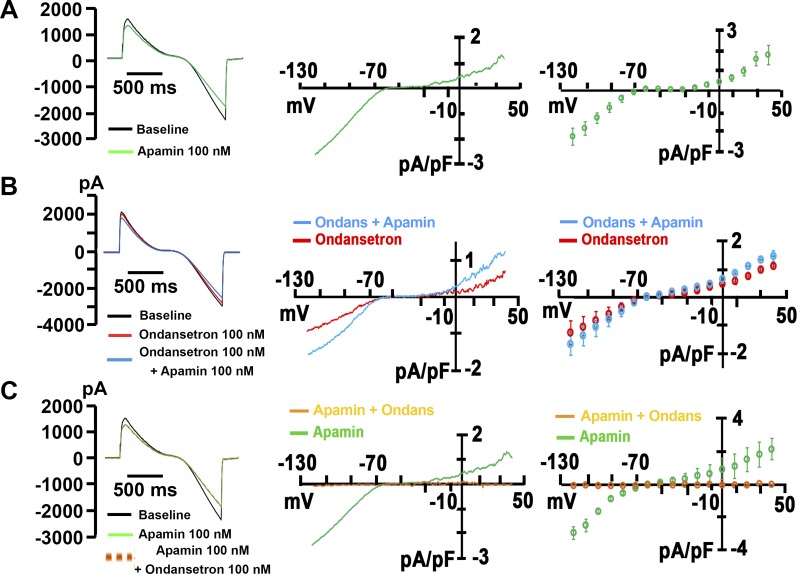
Ondansetron Inhibits Heterologously Expressed SK2 Channels
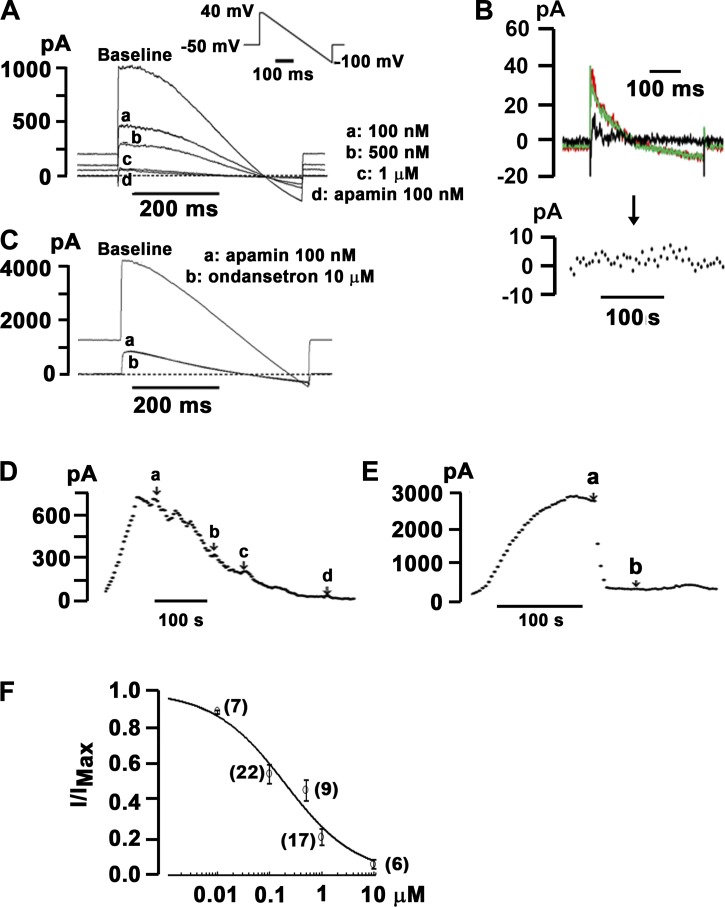
Inhibition of the cardiac voltage-gated K+ channels HERG and voltage-gated K+ channel subfamily Q member 1/minK has been implicated in the pathogenesis of ondansetron-induced LQTS. However, the concentrations needed to obtain 50% block (IC50) exceed peak plasma levels ~4- to ~25-fold (9, 17). Accordingly, we next sought to examine whether ondansetron at therapeutic concentrations inhibits heterologously expressed SK2 channels. HEK-293 cells stably transfected with human KCNN2 (SK2 cells) (32) were dialyzed with 500 nmol/l free Ca2+ to activate SK2 channels using the conventional whole cell configuration of the patch-clamp technique. Representative current traces obtained from a voltage-clamped SK2 cell at baseline and during cumulative exposure to ascending concentrations of ondansetron are shown in Fig. 4A. Currents were evoked by the same voltage ramp protocol shown in the inset of Fig. 2A, top left. Control experiments confirmed that ondansetron (10 μmol/l) had no significant effect on whole cell currents in genetically naïve HEK-293 cells (Fig. 4B). Ondansetron concentration dependently decreased whole cell currents at all voltages. The addition of apamin (100 nmol/l) in the continual presence of 1 µmol/l ondansetron only minimally reduced current amplitude, indicating that virtually all current through SK2 channels was blocked by ondansetron. Exposure of SK2 cells to 10 µmol/l ondansetron in the continuing presence of apamin (100 nmol/l) had no significant effect on current size, suggesting that ondansetron selectively blocks IKAS under the experimental conditions used (Fig. 4C). Identical results were found in three SK2 cells exposed to excess concentrations of ondansetron in the presence of 100 nmol/l apamin. Time plots of current levels at 0 mV from the same cells in Fig. 4, A and C, revealed that the time course of ondansetron inhibition was slower than that by apamin (Fig. 4, D and E). Blockade of SK2 channels by ondansetron was only partially reversible (not shown). To examine the dose dependency of ondansetron inhibition of IKAS, stepwise ascending concentrations of ondansetron were applied cumulatively into the bath solution after steady-state IKAS was achieved using a repetitive voltage ramp protocol. The ondansetron-sensitive current component was normalized to total IKAS (i.e., the sum of apamin- and ondansetron-sensitive currents) and plotted as a function of ondansetron concentration (Fig. 4F). Fitting the Hill equation to the dose-response curve yielded an IC50 value of 196 nmol/l and a Hill coefficient value of 0.63. Overall, these results suggest that ondansetron at clinically relevant (i.e., submicromolar) concentrations is a potent SK2 channel blocker. Furthermore, ondansetron binding to SK2 channels exhibits negative cooperativity, suggesting involvement of more than one binding site.
Fig. 4.
Ondansetron is a potent inhibitor of heterologously expressed small-conductance Ca2+-activated K+ (SK)2 channels. A: representative whole cell current traces sequentially recorded from voltage-clamped human embryonic kidney (HEK)-293 cells stably transfected with human wild-type Ca2+-activated K+ channel subfamily N member 2 (KCNN2). Individual traces show baseline recordings, steady-state responses to ascending concentrations of ondansetron (traces a, b, and c), and steady-state response to 100 nmol/l apamin in the continuing presence of 1 µmol/l ondansetron (trace d). The dashed line indicates the zero current level. The free Ca2+ concentration in the pipette solution was 500 nmol/l. The inset shows the voltage-clamp protocol. B, top:representative superimposed whole cell current traces recorded from a naïve HEK-293 cell before (red trace) and 2 min after (green trace) exposure to external ondansetron (10 µmol/l). The black trace represents ondansetron-sensitive current. B, bottom: time plot of whole cell current amplitude measured at 0 mV. The black arrow indicates the start of apamin administration. C: representative whole cell currents sequentially recorded from a wild-type KCNN2-expressing HEK-293 cell at baseline, in the presence of 100 nmol/l apamin (trace a), and in the presence of 100 nmol/l apamin plus 10 µmol/l ondansetron (trace b). The dashed line indicates the zero current level. The free Ca2+ concentration in the pipette solution was 500 nmol/l. D: current amplitudes at 0 mV recorded from the cell in A as a function of time. Voltage ramps were applied every 5 s. a–d correspond to the same experimental conditions as in A. Apamin at 100 nmol/l had no significant effect on whole cell current in the continuing presence of 1 µmol/l ondansetron. E: current amplitudes at 0 mV recorded from the cell in C as a function of time. Apamin (100 nmol/l) was started at the time point labeled a. Ondansetron at 10 µmol/l (labeled b) did not alter whole cell currents in the presence of 100 nmol/l apamin. F: ondansetron inhibited heterologously expressed SK2 currents in a concentration-dependent manner. Normalized apamin-sensitive SK current (IKAS) was plotted as a function of extracellular ondansetron concentration. Values are means ± SE. Numbers of cells per each concentration are shown in parentheses. The solid line represents best fit of the data to the Hill equation with an IC50 value of 196 nmol/l and a Hill coefficient n value of 0.63 (R2 = 0.97).
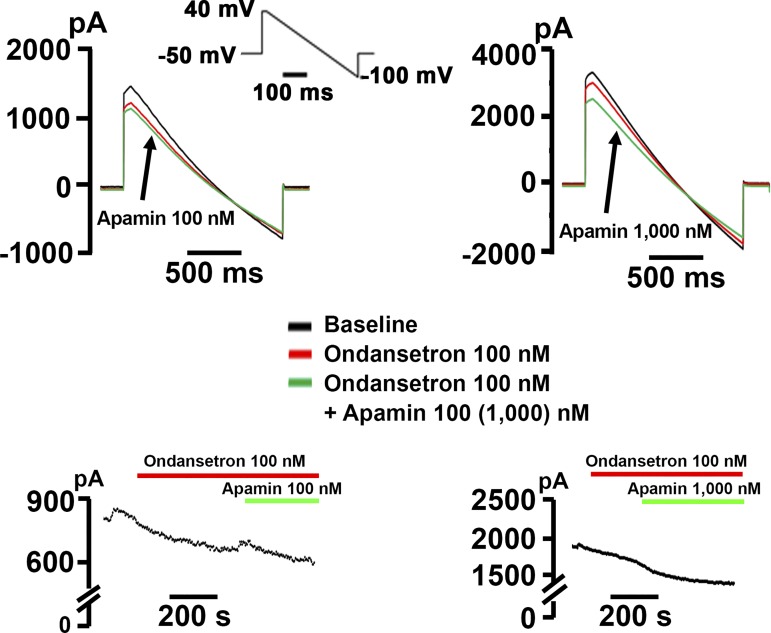
We subsequently tested whether ondansetron inhibits p.F503L KCNN2 channels expressed in HEK-293 cells. Ondansetron at a concentration of 100 nM partially blocked both inward and outward currents (Fig. 5, left). The subsequent addition of apamin (100 nM) had only a small effect, even though apamin applied alone at the same concentration produces >90% inhibition of the current in p.F503L KCNN2-transfected HEK-293 cells (Fig. 2). Increasing the concentration of apamin to 1 μM enhanced current inhibition in ondansetron-pretreated p.F503L KCNN2-transfected cells (Fig. 5, right), but inhibition remained incomplete, indicating that the effects of ondansetron and apamin were not additive. Assuming that 92% of the outward current at 0 mV is carried by p.F503L KCNN2 channels (Fig. 2), we estimated that ondansetron at a concentration of 100 nM on average reduces this current by 16.7 ± 2.3% (n = 8 cells) compared with ~39.5% in WT KCNN2-transfected cells (see concentration-response curve shown in Fig. 4F).
Fig. 5.
p.503L Ca2+-activated K+ channel subfamily N member 2 (KCNN2) channels that have already bound ondansetron only poorly respond to apamin. Top: representative ramp currents recorded in p. KCNN2-transfected human embryonic kidney (HEK)-293 cells in control (black), in ondansetron alone (red), and in apamin + ondansetron (green). Concentrations of apamin and ondansetron are as shown. Bottom: plots of currents at 0 mV as a function of time for the same cells shown in the top.
Ondansetron Selectively Blocks IKAS in Intact Mouse Hearts
We next determined the efficacy of ondansetron in inhibiting native SK channels in single murine ventricular myocytes. Initial measurements confirmed the presence of robust IKAS (Fig. 6A) in these cells. Exposure of myocytes to 100 nM ondansetron unveiled a current with IKAS-typical properties, i.e., a reversal potential close to EK and an inwardly-rectifying current-voltage relationship (Fig. 6B). The same concentration of ondansetron applied in the continuing presence of 100 nM apamin was without effect (Fig. 6C), indicating that ondansetron selectively reduced IKAS under the experimental conditions used. The percentage of total IKAS at 0 mV that was blocked by ondansetron (~40%) was almost identical with that predicted from the dose-response curve (39.5%; Fig. 4F), suggesting similar sensitivity of native and heterologously expressed SK currents to inhibition by the 5-HT3 receptor blocker.
Fig. 6.
Ondansetron (Ondans) inhibits native small-conductance Ca2+-activated K+ (SK) currents in murine ventricular myocytes. A, left: representative ramp currents in a ventricular myocyte in the absence (black) and presence (green) of 100 nM apamin. The inset shows the voltage-clamp protocol. The free intracellular Ca2+ concentration was 500 nM. A, middle: plot of apamin-sensitive current as a function of voltage for the same cell on the left. A, right: summary of current-voltage relationship for apamin-sensitive current. Values were means ± SE of 7 cells from 3 mice. B, left: representative ramp currents in control (black), in the presence of 100 nM ondansetron (red), and after addition of 100 nM apamin (blue) in the continual presence of ondansetron. B, middle: current-voltage relationships for current inhibited by ondansetron and for current inhibited by cumulatively applied apamin for the same cell as on the left. B, right: summary of current-voltage relationships for current inhibited by ondansetron and for current inhibited by apamin in the continuing presence of ondansetron. Values are means ± SE of 6 cells from 2 mice. C, left: exemplar ramp currents in control (black), in the presence of 100 nM apamin (green), and after addition of 100 nM ondansetron (dashed beige) in the continual presence of apamin. C, middle: current-voltage relationships for current inhibited by apamin and for current inhibited by cumulatively applied ondansetron for the same cell as on the left. C, right: summary of current-voltage relationships for current inhibited by apamin and for current inhibited by ondansetron in the continuing presence of apamin. Values are means ± SE of 4 cells from 2 mice.
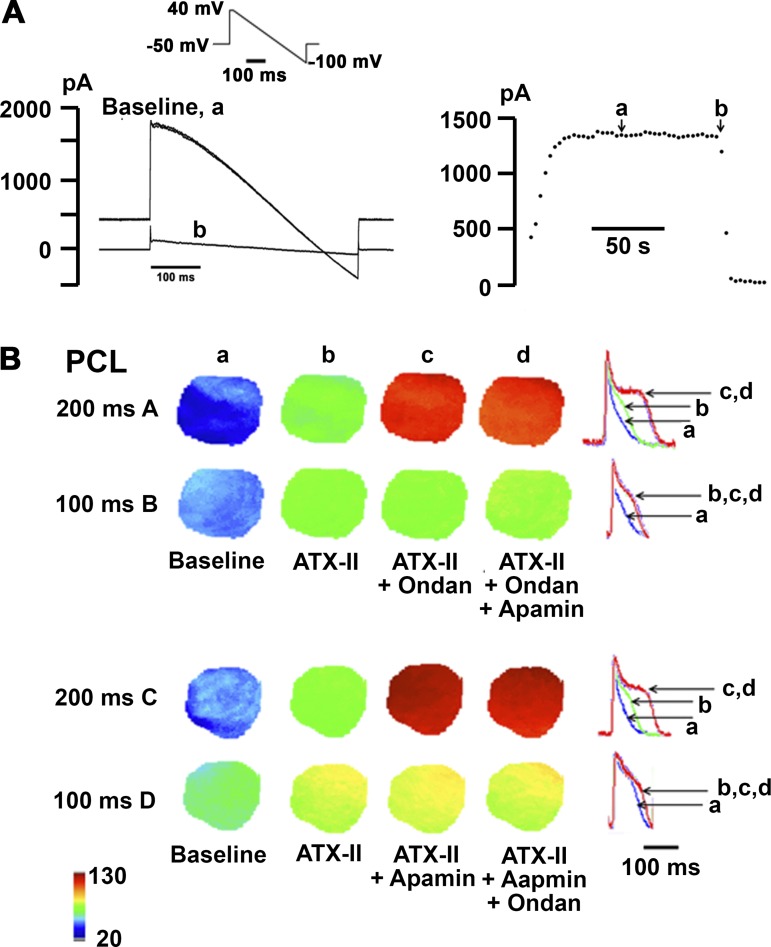
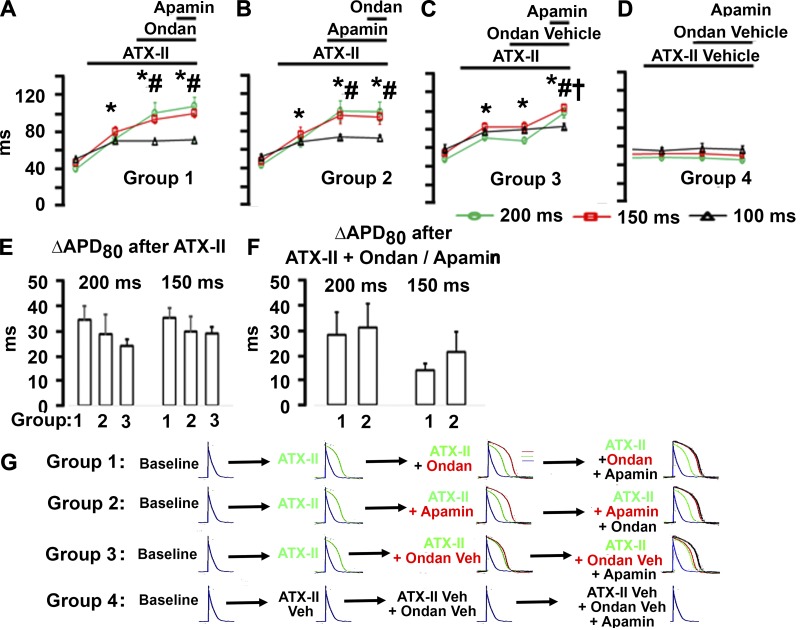
To investigate whether ondansetron prolongs ventricular repolarization via IKAS inhibition, we performed optical voltage mapping on Langendorff-perfused mouse hearts pretreated with ATX-II (15 nM). We have previously shown that ATX-II in low nanomolar concentrations stably upregulates IKAS in isolated mouse hearts (33). Additional voltage-clamp experiments confirmed that ATX-II did not affect IKAS in KCNN2-transfected HEK-293 cells (Fig. 7A). Representative APD80 distributions across the left ventricular epicardium that were generated from optical maps obtained during right ventricular pacing at cycle lengths of 100 ms and 200 ms are shown in Fig. 7B. ATX-II at a concentration of 15 nmol/l prolonged APD80 compared with baseline (which was obtained in the presence of 10 μmol/l blebbistatin only; Fig. 7Bb). Subsequent addition of ondansetron (100 nmol/l; Fig. 7, Ac and Bc) or apamin (100 nmol/l; Fig. 7, Cc and Dc) in the continuing presence of ATX-II in the perfusate further lengthened APD80 at the long, but not short, pacing cycle lengths (Fig. 7B). Finally, 100 nmol/l apamin (Fig. 7, Ad and Bd) or 100 nmol/l ondansetron (Fig. 7, Cd and Dd) cumulatively applied into the perfusion buffer had no additional effect on APD80 at either pacing cycle length (Fig. 7B). Optical action potentials were aligned by the peak and superimposed to illustrate the changes in APD as shown in Fig. 7, right (top and bottom rows). Numerical results of the optical mapping experiments are shown in Fig. 8. Intragroup comparisons (Fig. 8, A–D) revealed significant APD80 prolongation during ATX-II exposure at all three pacing cycle lengths in groups 1–3 (Fig. 8, A–C), whereas the ATX-II vehicle had no significant effect on APD80 (Fig. 8D). Ondansetron significantly prolonged APD80 in ATX-II-pretreated hearts at long, but not short, pacing cycle lengths, and subsequent addition of apamin in the continuing presence of both ATX-II and ondansetron had no additional effect on APD80 (Fig. 8A). Identical results were obtained when the sequence of ondansetron and apamin was reversed (Fig. 8B). Control experiments confirmed that APD80 was not significantly altered by ondansetron vehicle in ATX-II-pretreated hearts (Fig. 8C) or by 100 nmol/l apamin in untreated hearts (Fig. 8D). Intergroup comparisons (Fig. 8, E and F) demonstrated that ATX-II equally prolonged APD80 in groups 1–3 (Fig. 8E), and the changes in APD80 evoked by ondansetron or apamin in ATX-II-pretreated hearts were of similar magnitude (Fig. 8F). Overall, the findings, which are schematically shown in Fig. 8G, support the notion that ondansetron at the concentrations tested here is a potent and selective inhibitor of cardiac IKAS under the experimental conditions used.
Fig. 7.
Ondansetron (Ondan) unmasks apamin-sensitive small-conductance Ca2+-activated K+ current (IKAS) upregulation in Anemonia sulcata II (ATX-II)-pretreated mouse hearts. A: ATX-II did not affect heterologously expressed small-conductance Ca2+-activated K+ (SK)2 current. A, left: representative steady-state whole cell current responses sequentially recorded from a wild-type Ca2+-activated K+ subfamily N member 2 (KCNN2)-transfected human embryonic kidney (HEK)-293 cell in the presence of 15 nmol/l ATX-II (trace a) and during subsequent incubation with 100 nmol/l apamin (trace b). The voltage-clamp protocol is shown in the inset. A, right: plot of whole cell current amplitude at 0 mV as a function of time for the cell on the left. Exposure to 15 nmol/l ATX-II (arrow a) did not alter current amplitude measured at a potential of 0 mV, whereas subsequent exposure to 100 nmol/l apamin (arrow b) instantaneously and almost completely suppressed whole cell outward current. Identical results were obtained in another SK2 cell. B: rows A–D show representative sequences of action potential duration at 80% repolarization (APD80) distribution maps obtained from one heart each at baseline (a) and across three consecutive 15-min sessions (b−d) during which hearts were exposed to the drugs indicated. Right graphs show superimpositions of representative optical action potentials. Blebbistatin (10 µmol/l) was present throughout all experiments. PCL, pacing cycle length.
Fig. 8.
Ondansetron (Ondan) inhibits apamin-induced action potential duration at 80% repolarization (APD80) prolongation in Anemonia sulcata II (ATX-II)-treated mouse hearts. A–D: intragroup comparisons of the effects of different experimental interventions on APD80 at the pacing cycle lengths (PCLs) indicated. Data are means ± SE from 5 hearts in groups 1–3 and 3 hearts in group 4. In A, *P < 0.001 vs. baseline for all three PCLs; #P < 0.001 for PCL of 200 and 150 ms vs. PCL of 100 ms. In B, *P < 0.001 vs. baseline for all three PCLs; #P = 0.048 for PCL of 200 and 150 ms vs. PCL of 100 ms. In C, *P < 0.001 vs. baseline for all three PCLs; #P < 0.001 for PCL of 200 and 150 ms vs. PCL of 100 ms; †P < 0.001 vs. ondansetron vehicle + ATX-II for PCLs of 200 and 150 ms. E and F: intergroup comparisons of the effects of different experimental interventions on ∆APD80 during the conditions indicated. Only values for PCLs of 150 and 200 ms are shown. E: magnitudes of ∆APD80 induced by ATX-II alone were not significantly different between groups 1–3. F: ∆APD80 sizes after exposure to ondansetron or apamin in the continuing presence of ATX-II (groups 1 and 2) were similar. Linear mixed models were used for statistical analyses with group, phase, and PCL as the fixed effects and mouse as the random effect. G: schematic presentation of the changes in APD in each experimental group.
DISCUSSION
Major Findings
Here, we report a novel gain-of-function variation in the KCNN2 gene encoding for the pore-forming α-subunit of the SK2 channel. This variation results in a change from phenylalanine to leucine in position 503 (p.F503L) distal to the calmodulin-binding domain within the intracellular COOH-terminus, leading to an increase in the apparent Ca2+ sensitivity of SK2. We also found that ondansetron at nanomolar concentrations potently blocks heterologously expressed WT and p.F503L SK2 channels as well as native cardiac SK channels.
Possible Mechanisms Underlying the Gain of Function of p.F503L SK2 Channels
The ~1.5-fold difference in the average magnitudes of IKAS densities between KCNN2- and p.F503L KCNN2-expressing HEK-293 cells (Fig. 2B) largely overlaps with that calculated from the Ca2+ concentration-response curve (1.25-fold; Fig. 3, B and C) using the same Ca2+ concentration that was used in the whole cell current measurements (500 nM). This result strongly supports the notion that an increase in the apparent Ca2+ sensitivity of p.F503L SK2 channels contributes to a larger IKAS density in cells transfected with p.F503L KCNN2. SK2 channels are solely gated by intracellular Ca2+. They form complexes of four pore-forming α-subunits, each constitutively bound to calmodulin, which mediates Ca2+ gating (1). Thus, the increase in Ca2+ sensitivity may result from changes in the ability of Ca2+-bound calmodulin to initiate channel gating and/or from changes in the phosphorylation status of calmodulin because of altered activity of coassembled casein kinase 2 and/or protein phosphatase 2A (1). Also, our immunoblot analyses support the notion that increased trafficking of F503L KCNN2 to the outer membrane and/or its reduced ubiquination underlie, at least partially, the gain of function phenotype. Finally, enhanced unitary conductance may also contribute to the increase in IKAS density.
Ondansetron Is a Potent Cardiac SK Channel Blocker
Peak free plasma levels of 68−102 nmol/l are achieved at around 1.5 h after a single 8-mg oral dose of ondansetron, whereas peak plasma concentration is 136 nmol/l during repeat dosing of an 8-mg tablet every 8 h for 6 days (10, 23). These therapeutic concentrations overlap with those shown here to effectively block heterologously expressed human WT and p.F503L SK2 channels as well as native cardiac SK channels, indicating that ondansetron at therapeutic concentrations is a potent inhibitor of cardiac IKAS. Ondansetron, when applied in the continuing presence of a saturating apamin concentration, did not significantly alter whole cell currents in ventricular myocytes, nor did it prolong ventricular repolarization in Langendorff-perfused mouse hearts pretreated with ATX-II. Also, we have recently demonstrated that the effects of apamin (100 nM) and ondansetron (100 nM) on ventricular repolarization of isolated perfused rabbit hearts exposed to low extracellular K+ concentration are mutually occlusive (31). Collectively, we infer from these findings that ondansetron at nanomolar concentrations selectively targets SK channels under the experimental conditions used. It is possible, however, that ondansetron concentrations seen at the cellular level in vivo exceed peak plasma concentrations, as has been previously demonstrated for other drugs, e.g., naproxen (14), leading to modulation of other ion channels/transporters.
Normal ventricles generate little IKAS. It is therefore difficult to demonstrate that either apamin or ondansetron blocks IKAS and prolongs APD under physiological conditions. Here, we used ATX-II to acutely and stably upregulate IKAS, enabling us to measure the effects of either molecule on cardiac SK channels. A recent study has shown that ATX-II causes rate-dependent Na+ overload in cardiomyocytes, which, in turn, gives rise to graded elevations of cytosolic free Ca2+ (16). Because ATX-II at the concentration used in the present study did not significantly affect heterologously expressed SK2 currents, ATX-II likely activated cardiac IKAS indirectly through an increase in Ca2+.
Both apamin and ondansetron similarly prolonged APD80 at long, but not short, pacing cycle lengths in ATX-II-pretreated mouse hearts, whereas they did not significantly alter ventricular repolarization at any cycle length in the absence of ATX-II. Several mechanisms acting alone or in combination can be responsible for the loss of the effect of apamin and ondansetron on APD at high heart rates. First, heart rate may negatively regulate SK channel activity through changes in the phosphorylation of SK-bound calmodulin by associated casein kinase 2 and/or protein phosphatase 2A, reducing the Ca2+ sensitivity of SK channels as the rate increases (1). Second, levels of cytosolic Ca2+ in the vicinity of SK channels may decline as heart rates increase, progressively diminishing their activation. Third, inhibition of SK channels by apamin and ondansetron may be state dependent, with stronger inhibition in the open state (i.e., at low rates) and weaker inhibition in the closed state (i.e., at high rates). Intriguingly, we have previously demonstrated that inhibition of heterologously expressed SK2 channels by amiodarone increases with an increase in cytosolic Ca2+, suggesting stronger binding of the drug to the open state (27).
A saturating concentration of apamin was equally effective in inhibiting WT and p.F503L SK2 channels. However, its efficacy was drastically reduced for variant but not WT SK2 channels that had already bound ondansetron. These findings suggest that ondansetron binds to p.F503L SK2 channels and that substitution of phenylalanine with leucine in the 503 position alters the interaction of ondansetron with SK2 in such a way as to hinder apamin from reaching its binding site and/or from displacing ondansetron from a common binding site. The loss of the additive effects of ondansetron and apamin prevented quantitation of total SK current magnitudes in p.F503L KCNN2-transfected HEK-293 cells and thus generation of an ondansetron dose-inhibition curve. Nevertheless, in normalizing the ondansetron-sensitive current to the total outward current in p.F503L KCNN2-transfected cells, we estimated that variant SK channels are ~57% less sensitive to inhibition by 100 nM ondansetron than their WT counterparts.
Possible Rescue Function of the p.F503L KCNN2 Variant
Our patient was also heterozygous for the p.S38G single-nucleotide polymorphism in the KCNE1 gene, which encodes the β-subunit (minK) of the slowly activated cardiac K+ channel, and the p.P2835S single-nucleotide polymorphism in the ANK2 gene, which elaborates the cytoskeletal protein ankyrin 2. Whereas the latter gene variation has been previously shown to confer increased susceptibility to diLQTS (15), the KCNE1 p.S38G variant has been associated with rate-dependent repolarization abnormalities predisposing to arrhythmias (30). It is thus possible that APD prolongation caused by either gene variation alone or their combination was effectively opposed by enhanced IKAS carried by gain-of-function p.F503L SK2 channels and blockade of variant SK2 and possibly variant voltage-gated K+ channel subfamily Q member 1/minK channels by ondansetron unveils a “forme fruste” long QT phenotype. Alternatively, ondansetron-induced QT prolongation in our patient may have resulted from inhibition of repolarizing currents other than IKAS [e.g., rapid component of the delayed rectifier K+ current (IKr)]. This appears unlikely, however, because ondansetron in the continual presence of a saturating apamin concentration had no significant effect on APD in low K+ concentration-perfused rabbit hearts, in which IKr is known to significantly contribute to ventricular repolarization (31). Finally, it is also possible that mechanisms not involving KCNE1 and/or ANK2 cause QT prolongation in our patient. For example, the patient could have undiagnosed heart diseases that resulted in an upregulation of IKAS.
In addition to QT interval prolongation, our patient also had significant T wave inversion after taking ondansetron. Cowan et al. (8) showed that there are epicardial repolarization gradients in humans and that these are related to the configuration of the T wave. In patients with upright T waves, an inverse relation between APD and activation time exists, whereas in patients with inverted T waves this relation was no longer found. It is possible that our patient similarly had a heterogeneous upregulation of IKAS density in response to reduced repolarization reserve, leading to the maintenance of upright T wave morphology and QT interval at baseline. By blocking IKAS, ondansetron changed not only the QT interval but also T wave morphology because of heterogeneous responses of APD to IKAS blockade by ondansetron.
Although it is possible that ondansetron has contributed to diLQTS, this particular patient was also given other drugs that can potentially prolong QT interval. It is not possible to tell whether or not ondansetron has played a major role in causing QT prolongation and T wave changes in this particular patient. More studies will be needed to document that SK current-blocking effects of ondansetron are causally related to diLQTS.
Clinical Implications
The first potential clinical implication is that ondansetron may be useful as an antiarrhythmic agent. Previous studies in animal models have shown that apamin is potentially useful in the management of electrical storm (7) and atrial fibrillation (6, 24, 26). However, because apamin is a neurotoxin, it cannot be used as a drug for humans. Ondansetron, on the other hand, has already been approved for human use and is widely available. Its IKAS-blocking action may potentially be useful in managing patients with cardiac arrhythmias in certain clinical conditions, although its proarrhythmic potential may limit the clinical applications. A second potentially important clinical implication is that IKAS blockade is a new mechanism for diLQTS. Because IKAS is not an important repolarization current in normal ventricles (7, 8, 29), ondansetron is proarrhythmic only in patients with organic heart diseases (11, 19, 21). Potential exceptions could include chronic ventricular pacing, hypokalemia (4), bradycardia, and heart block (34) when IKAS is upregulated to compensate for reduced repolarization reserve. This information is important to the clinicians who prescribe ondansetron.
Limitations
We did not study the effects of heterozygous KCNE1 p.S38G variation and ANK2 p.P2835S variation on cardiac ionic currents. The contribution of these two variants to the long QT interval after ondansetron remains unclear. Because of a complex clinical presentation that included chest pain, hypotension, and the use of metoclopramide, it is difficult to attribute the QT prolongation entirely to ondansetron. The possibility that ondansetron concentrations occurring at the cellular level in vivo exceeded the in vitro concentrations used here and resulted in modulation of QT-controlling mechanisms other than SK channel activity also makes it difficult to establish a clear causal link between ondansetron treatment and LQTS. However, these limitations do not invalidate the conclusion that ondansetron is an IKAS blocker and that IKAS blockade may contribute to the development of diLQTS.
Conclusions
Our results show that heterozygous p.F503L KCNN2 variant increases the Ca2+ sensitivity of heterologously expressed SK2 channels. Ondansetron at nanomolar concentrations blocks WT SK2 and p.F503L SK2 channels expressed in HEK-293 cells as well as native SK channels in ventricular myocytes.
GRANTS
This work was supported, in part, by National Institutes of Health Grants P01-HL-78931, R01-HL-71140, R01-HL-139829, UL1-TR-001108, and RR-020128, a Medtronic-Zipes Endowment, the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative, the Lilly Endowment, the Indiana Biobank, and a Charles Fisch Cardiovascular Research Award endowed by Dr. Suzanne B. Knoebel of the Krannert Institute of Cardiology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.V. and M.R. conceived and designed research; J.-S.K., S.G., J.H., P.C.-S., T.C.L., J.E.T., J.J.Z., and S.E.T. performed experiments; J.-S.K., S.G., J.H., P.C.-S., T.C.L., J.E.T., J.J.Z., S.E.T., M.D.M., and X.L. analyzed data; J.J.Z., S.E.T., M.D.M., X.L., S.-F.L., M.V., P.-S.C., and M.R. interpreted results of experiments; J.-S.K. and S.G. prepared figures; S.E.T., T.F., M.D.M., R.J.K., X.L., S.-F.L., Z.C., M.V., P.-S.C., and M.R. edited and revised manuscript; J.-S.K., J.H., P.C.-S., T.C.L., J.E.T., J.J.Z., S.E.T., T.F., M.D.M., R.J.K., X.L., S.-F.L., Z.C., M.V., P.-S.C., and M.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Nicole Courtney, Glen Schmeisser, and Jessica Kincaid for support.
REFERENCES
- 1.Allen D, Fakler B, Maylie J, Adelman JP. Organization and regulation of small conductance Ca2+-activated K+ channel multiprotein complexes. J Neurosci 27: 2369–2376, 2007. doi: 10.1523/JNEUROSCI.3565-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bers DM. A simple method for the accurate determination of free [Ca] in Ca-EGTA solutions. Am J Physiol Cell Physiol 242: C404–C408, 1982. doi: 10.1152/ajpcell.1982.242.5.C404. [DOI] [PubMed] [Google Scholar]
- 3.Celestino-Soper PB, Doytchinova A, Steiner HA, Uradu A, Lynnes TC, Groh WJ, Miller JM, Lin H, Gao H, Wang Z, Liu Y, Chen PS, Vatta M. Evaluation of the genetic basis of familial aggregation of pacemaker implantation by a large next generation sequencing panel. PLoS One 10: e0143588, 2015. [Erratum in PLoS One 11: e0147455, 2016.] doi: 10.1371/journal.pone.0143588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan YH, Tsai WC, Ko JS, Yin D, Chang P-C, Rubart M, Weiss JN, Everett TH IV, Lin SF, Chen PS. Small conductance calcium-activated potassium current is activated during hypokalemia and masks short term cardiac memory induced by ventricular pacing. Circulation 132: 1377–1386, 2015. doi: 10.1161/CIRCULATIONAHA.114.015125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang PC, Hsieh YC, Hsueh CH, Weiss JN, Lin SF, Chen PS. Apamin induces early afterdepolarizations and torsades de pointes ventricular arrhythmia from failing rabbit ventricles exhibiting secondary rises in intracellular calcium. Heart Rhythm 10: 1516–1524, 2013. doi: 10.1016/j.hrthm.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen WT, Chen YC, Lu YY, Kao YH, Huang JH, Lin YK, Chen SA, Chen YJ. Apamin modulates electrophysiological characteristics of the pulmonary vein and the Sinoatrial Node. Eur J Clin Invest 43: 957–963, 2013. doi: 10.1111/eci.12125. [DOI] [PubMed] [Google Scholar]
- 7.Chua SK, Chang PC, Maruyama M, Turker I, Shinohara T, Shen MJ, Chen Z, Shen C, Rubart-von der Lohe M, Lopshire JC, Ogawa M, Weiss JN, Lin SF, Ai T, Chen PS. Small-conductance calcium-activated potassium channel and recurrent ventricular fibrillation in failing rabbit ventricles. Circ Res 108: 971–979, 2011. doi: 10.1161/CIRCRESAHA.110.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowan JC, Hilton CJ, Griffiths CJ, Tansuphaswadikul S, Bourke JP, Murray A, Campbell RW. Sequence of epicardial repolarisation and configuration of the T wave. Br Heart J 60: 424–433, 1988. doi: 10.1136/hrt.60.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Lorenzi FG, Bridal TR, Spinelli W. Block of the delayed rectifier current (IK) by the 5-HT3 antagonists ondansetron and granisetron in feline ventricular myocytes. Br J Pharmacol 113: 527–535, 1994. doi: 10.1111/j.1476-5381.1994.tb17021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.GlaxoSmithKline Product Monograph, Zofran Tablets, Oral Solution and Injection (Online) https://www.novartis.ca/sites/www.novartis.ca/files/zofran_scrip_e.pdf [20 July 2017].
- 11.Hafermann MJ, Namdar R, Seibold GE, Page RL II. Effect of intravenous ondansetron on QT interval prolongation in patients with cardiovascular disease and additional risk factors for torsades: a prospective, observational study. Drug Healthc Patient Saf 3: 53–58, 2011. doi: 10.2147/DHPS.S25623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hancock JM, Weatherall KL, Choisy SC, James AF, Hancox JC, Marrion NV. Selective activation of heteromeric SK channels contributes to action potential repolarization in mouse atrial myocytes. Heart Rhythm 12: 1003–1015, 2015. doi: 10.1016/j.hrthm.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh YC, Chang PC, Hsueh CH, Lee YS, Shen C, Weiss JN, Chen Z, Ai T, Lin SF, Chen PS. Apamin-sensitive potassium current modulates action potential duration restitution and arrhythmogenesis of failing rabbit ventricles. Circ Arrhythm Electrophysiol 6: 410–418, 2013. doi: 10.1161/CIRCEP.111.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huntjens DR, Spalding DJ, Danhof M, Della Pasqua OE. Correlation between in vitro and in vivo concentration-effect relationships of naproxen in rats and healthy volunteers. Br J Pharmacol 148: 396–404, 2006. doi: 10.1038/sj.bjp.0706737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kääb S, Crawford DC, Sinner MF, Behr ER, Kannankeril PJ, Wilde AA, Bezzina CR, Schulze-Bahr E, Guicheney P, Bishopric NH, Myerburg RJ, Schott JJ, Pfeufer A, Beckmann BM, Martens E, Zhang T, Stallmeyer B, Zumhagen S, Denjoy I, Bardai A, Van Gelder IC, Jamshidi Y, Dalageorgou C, Marshall V, Jeffery S, Shakir S, Camm AJ, Steinbeck G, Perz S, Lichtner P, Meitinger T, Peters A, Wichmann HE, Ingram C, Bradford Y, Carter S, Norris K, Ritchie MD, George AL Jr, Roden DM. A large candidate gene survey identifies the KCNE1 D85N polymorphism as a possible modulator of drug-induced torsades de pointes. Circ Cardiovasc Genet 5: 91–99, 2012. doi: 10.1161/CIRCGENETICS.111.960930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kornyeyev D, El-Bizri N, Hirakawa R, Nguyen S, Viatchenko-Karpinski S, Yao L, Rajamani S, Belardinelli L. Contribution of the late sodium current to intracellular sodium and calcium overload in rabbit ventricular myocytes treated by anemone toxin. Am J Physiol Heart Circ Physiol 310: H426–H435, 2016. doi: 10.1152/ajpheart.00520.2015. [DOI] [PubMed] [Google Scholar]
- 17.Kuryshev YA, Brown AM, Wang L, Benedict CR, Rampe D. Interactions of the 5-hydroxytryptamine 3 antagonist class of antiemetic drugs with human cardiac ion channels. J Pharmacol Exp Ther 295: 614–620, 2000. [PubMed] [Google Scholar]
- 18.Lehtonen A, Fodstad H, Laitinen-Forsblom P, Toivonen L, Kontula K, Swan H. Further evidence of inherited long QT syndrome gene mutations in antiarrhythmic drug-associated torsades de pointes. Heart Rhythm 4: 603–607, 2007. doi: 10.1016/j.hrthm.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Lu Z, Kamiya K, Opthof T, Yasui K, Kodoma I. Density and kinetics of IKr and IKs in guinea pig and rabbit ventricular myocytes explain different efficay of IKs blockade at high heart rate in guinea pig and rabbit. Circulation 104: 951–956, 2001. doi: 10.1161/hc3401.093151. [DOI] [PubMed] [Google Scholar]
- 20.Maruyama M, Li BY, Chen H, Xu X, Song LS, Guatimosim S, Zhu W, Yong W, Zhang W, Bu G, Lin SF, Fishbein MC, Lederer WJ, Schild JH, Field LJ, Rubart M, Chen PS, Shou W. FKBP12 is a critical regulator of the heart rhythm and the cardiac voltage-gated sodium current in mice. Circ Res 108: 1042–1052, 2011. doi: 10.1161/CIRCRESAHA.110.237867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moeller JR, Gummin DD, Nelson TJ, Drendel AL, Shah BK, Berger S. Risk of ventricular arrhythmias and association with ondansetron. J Pediatr 179: 118–123.e1, 2016. doi: 10.1016/j.jpeds.2016.08.058. [DOI] [PubMed] [Google Scholar]
- 22.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med 350: 1013–1022, 2004. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 23.Simpson KH, Hicks FM. Clinical pharmacokinetics of ondansetron. A review. J Pharm Pharmacol 48: 774–781, 1996. doi: 10.1111/j.2042-7158.1996.tb03973.x. [DOI] [PubMed] [Google Scholar]
- 24.Skibsbye L, Diness JG, Sørensen US, Hansen RS, Grunnet M. The duration of pacing-induced atrial fibrillation is reduced in vivo by inhibition of small conductance Ca2+-activated K+ channels. J Cardiovasc Pharmacol 57: 672–681, 2011. doi: 10.1097/FJC.0b013e318217943d. [DOI] [PubMed] [Google Scholar]
- 25.Tisdale JE, Jaynes HA, Kingery JR, Mourad NA, Trujillo TN, Overholser BR, Kovacs RJ. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes 6: 479–487, 2013. [Erratum in Circ Cardiovasc Qual Outcomes 6, e57, 2013.] doi: 10.1161/CIRCOUTCOMES.113.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai WC, Chan YH, Hsueh CH, Everett TH IV, Chang PC, Choi EK, Olaopa MA, Lin SF, Shen C, Kudela MA, Rubart-von der Lohe M, Chen Z, Jadiya P, Tomar D, Luvison E, Anzalone N, Patel VV, Chen PS. Small conductance calcium-activated potassium current and the mechanism of atrial arrhythmia in mice with dysfunctional melanocyte-like cells. Heart Rhythm 13: 1527–1535, 2016. doi: 10.1016/j.hrthm.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turker I, Yu CC, Chang PC, Chen Z, Sohma Y, Lin SF, Chen PS, Ai T. Amiodarone inhibits apamin-sensitive potassium currents. PLoS One 8: e70450, 2013. doi: 10.1371/journal.pone.0070450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuteja D, Xu D, Timofeyev V, Lu L, Sharma D, Zhang Z, Xu Y, Nie L, Vázquez AE, Young JN, Glatter KA, Chiamvimonvat N. Differential expression of small-conductance Ca2+-activated K+ channels SK1, SK2, and SK3 in mouse atrial and ventricular myocytes. Am J Physiol Heart Circ Physiol 289: H2714–H2723, 2005. doi: 10.1152/ajpheart.00534.2005. [DOI] [PubMed] [Google Scholar]
- 29.Xu Y, Tuteja D, Zhang Z, Xu D, Zhang Y, Rodriguez J, Nie L, Tuxson HR, Young JN, Glatter KA, Vázquez AE, Yamoah EN, Chiamvimonvat N. Molecular identification and functional roles of a Ca2+-activated K+ channel in human and mouse hearts. J Biol Chem 278: 49085–49094, 2003. doi: 10.1074/jbc.M307508200. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi Y, Mizumaki K, Hata Y, Sakamoto T, Nakatani Y, Kataoka N, Ichida F, Inoue H, Nishida N. Latent pathogenicity of the G38S polymorphism of KCNE1 K+ channel modulator. Heart Vessels 32: 186–192, 2017. doi: 10.1007/s00380-016-0859-1. [DOI] [PubMed] [Google Scholar]
- 31.Yin D, Yang N, Tian Z, Wu AZ, Xu D, Chen M, Wang Z, Chan Y, Chen Z, Lin SF, Chen PS, Everett TH. Ondansetron inhibits apamin-sensitive small conductance Ca2+ activated K+ currents in pacing induced failing rabbit heart. Circulation 134: A16251, 2016. [Google Scholar]
- 32.Yu CC, Ai T, Weiss JN, Chen PS. Apamin does not inhibit human cardiac Na+ current, L-type Ca2+ current or other major K+ currents. PLoS One 9: e96691, 2014. [Erratum in PLoS One 9: e104445, 2014.] doi: 10.1371/journal.pone.0096691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu CC, Ko JS, Ai T, Tsai WC, Chen Z, Rubart M, Vatta M, Everett TH IV, George AL Jr, Chen PS. Arrhythmogenic calmodulin mutations impede activation of small-conductance calcium-activated potassium current. Heart Rhythm 13: 1716–1723, 2016. doi: 10.1016/j.hrthm.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang XD, Lieu DK, Chiamvimonvat N. Small-conductance Ca2+-activated K+ channels and cardiac arrhythmias. Heart Rhythm 12: 1845–1851, 2015. doi: 10.1016/j.hrthm.2015.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]